Lateral pararectal versus transrectal stoma placement for prevention of parastomal herniation
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009487.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 24 abril 2019see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Colorrectal
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Design, data extraction, analysis, interpretation, and drafting of review: JH, FH, MIM.
Design, resolution of discrepancies, interpretation, drafting, and supervision of review: JM, PK, SP.
Design and execution of search strategies, search documentation: MIM.
Sources of support
Internal sources
-
University Medicine Mannheim, Germany.
The authors received their regular salaries while they worked on the review update.
-
Institute for Evidence in Medicine (for Cochrane Germany Foundation), Germany.
The authors received their regular salaries while they worked on the review update.
-
Cochrane Metabolic and Endocrine Disorders Group, Institute of General Practice, Medical Faculty of the Heinrich‐Heine‐University Düsseldorf, Germany.
The authors received their regular salaries while they worked on the review update.
External sources
-
There was no external funding available., Other.
Declarations of interest
The PATRASTOM trial was undertaken by some of the authors of this review (JH, FH, PK, SP). In order to prevent confounding and to guarantee transparency and objectivity in regard to the evaluation of the PATRASTOM trial, the data extraction and risk of bias assessment of this trial was checked by an external clinician not involved in the trial.
Acknowledgements
Thanks to:
-
Gerta Rücker, Institute of Medical Biometry and Medical Informatics of the University Medical Center Freiburg, Freiburg i. Br., Germany, for statistical advice and methodological support;
-
Lasse T. Krogsbøll, Nordic Cochrane Center, Rigshospitalet, Copenhagen, Denmark, for the translation of Eldrup 1982 and the valuable discussion on how to review observational studies;
-
the Cochrane Colorectal Cancer Group (CCCG), Copenhagen, Denmark, especially to Henning K. Andersen and Marija Barbateskovic;
-
Gerd Antes and the team at the German Cochrane Center, Freiburg i. Br., Germany.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Apr 24 | Lateral pararectal versus transrectal stoma placement for prevention of parastomal herniation | Review | Julia Hardt, Joerg J Meerpohl, Maria‐Inti Metzendorf, Peter Kienle, Stefan Post, Florian Herrle | |
| 2013 Nov 22 | Lateral pararectal versus transrectal stoma placement for prevention of parastomal herniation | Review | Julia Hardt, Joerg J Meerpohl, Maria‐Inti Metzendorf, Peter Kienle, Stefan Post, Florian Herrle | |
| 2011 Dec 07 | Lateral pararectal stoma placement versus transrectal stoma siting for prevention of parastomal herniation | Protocol | Julia Hardt, Florian Herrle, Peter Kienle | |
Differences between protocol and review
We concretised the inclusion criteria for studies. Studies had to compare lateral pararectal versus transrectal enterostomy placement with regard to the incidence of parastomal herniation.
Only one of the planned subgroup analyses could be conducted (see Subgroup analysis and investigation of heterogeneity and Analysis 2.1). None of the planned sensitivity analyses were conducted (see Sensitivity analysis).
Embase was not searched for this update of the review because of the following reasons and based on the following rationale:
1. Embase was no longer available to the author team. Since we still included Web of Science, another large biomedical database, in addition to PubMed and CENTRAL (searches in both databases are mandatory according to MECIR C24, whereas the recommendation to search Embase is facultative), our electronic searches are still in compliance with the MECIR standards (Higgins 2016).
2. RCTs from Embase are now regularly and prospectively included in CENTRAL, approximately four weeks after publication in Embase (see: www.cochranelibrary.com/help/central‐creation‐details.html), so we are confident that we did not miss a RCT that was only indexed in Embase.
3. All of the included studies in the previous and the present review version were indexed in MEDLINE.
Taking these aspects into account, it is very unlikely that searching Embase would have identified further studies for inclusion, especially further RCTs.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO

Funnel plot of comparison 1. Lateral pararectal versus transrectal enterostomy placement, outcome 1.1. parastomal hernia. Only non‐randomized studies

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
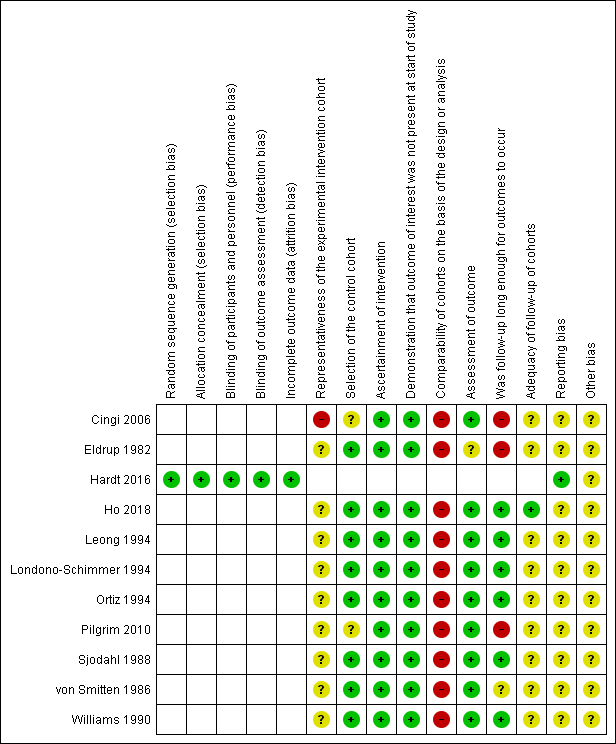
Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Trial flow diagram
RCT: randomized controlled trial; NRS: non‐randomized study

Comparison 1 Lateral pararectal versus transrectal enterostomy placement, Outcome 1 parastomal hernia (RCT).

Comparison 1 Lateral pararectal versus transrectal enterostomy placement, Outcome 2 parastomal hernia (NRS).
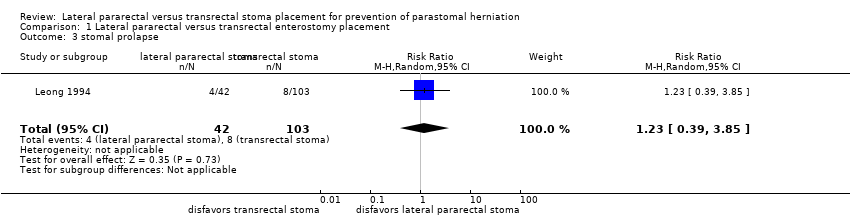
Comparison 1 Lateral pararectal versus transrectal enterostomy placement, Outcome 3 stomal prolapse.
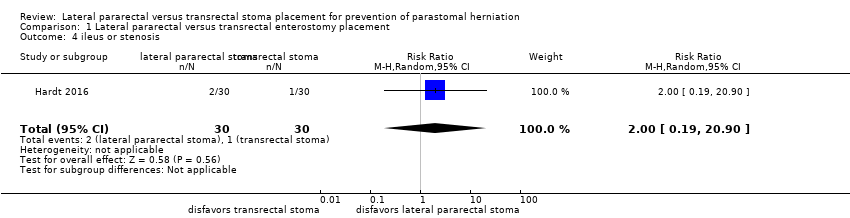
Comparison 1 Lateral pararectal versus transrectal enterostomy placement, Outcome 4 ileus or stenosis.

Comparison 1 Lateral pararectal versus transrectal enterostomy placement, Outcome 5 skin irritation.

Comparison 2 Subgroup analyses ‐ ileostomy versus colostomy, Outcome 1 parastomal hernia.
| Lateral pararectal versus transrectal enterostomy placement for prevention of parastomal herniation | ||||||
| Patient or population: people undergoing enterostomy placement for any reason | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Transrectal enterostomy placement | Lateral pararectal enterostomy placement | |||||
| parastomal hernia (NRS) clinical examination, CT scan, or botha Follow‐up: 2 to 240 months | Study population | RR 1.22 | 864 | ⊕⊝⊝⊝ | ||
| 295 per 1000 | 360 per 1000 | |||||
| Moderate | ||||||
| 348 per 1000 | 425 per 1000 | |||||
| parastomal hernia (RCT) clinical exam, sonography, intraoperatively, or combination, during stoma reversal Follow‐up: 0.5 to 21.4 months (15 to 642 days) | Study population | RR 1.34 | 56 | ⊕⊕⊝⊝ | ||
| 138 per 1000 | 185 per 1000 | |||||
| Moderate | ||||||
| 138 per 1000 | 185 per 1000 | |||||
| stomal prolapse clinical exam, documented on a standard pro forma Follow‐up: mean 110.4 months | Study population | RR 1.23 | 145 | ⊕⊝⊝⊝ | ||
| 78 per 1000 | 96 per 1000 | |||||
| Moderate | ||||||
| 78 per 1000 | 96 per 1000 | |||||
| ileus or stenosis clinical exam, sonography Follow‐up: 0.5 to 21.4 months (15 to 642 days) | Study population | RR 2 | 60 | ⊕⊕⊝⊝ | ||
| 33 per 1000 | 67 per 1000 | |||||
| Moderate | ||||||
| 33 per 1000 | 66 per 1000 | |||||
| skin irritation clinical exam, intraoperative exam, or both, during stoma reversal Follow‐up: 0.5 to 21.4 months (15 to 642 days) | Study population | RR 0.67 | 60 | ⊕⊕⊕⊝ | ||
| 200 per 1000 | 134 per 1000 | |||||
| Moderate | ||||||
| 200 per 1000 | 134 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aIn four studies the presence of parastomal hernia was assessed by clinical examination and CT scan, or CT scan alone. In the remaining studies, participants were clinically examined, e.g. in stoma therapy or outpatient clinic at follow‐up visits, and the findings were documented in the patient chart. | ||||||
| Representativeness of the experimental intervention cohort | Selection of the control cohort | Ascertainment of intervention | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow‐up long enough for outcomes to occur | Adequacy of follow‐up of cohorts | |
| ★ | ★ | ★ | ||||||
| ★ | ★ | ★ | ★ | |||||
| ★ | ★ | ★ | ★ | ★ | ★ | |||
| ★ | ★ | ★ | ★ | ★ | ||||
| ★ | ★ | ★ | ★ | ★ | ||||
| ★ | ★ | ★ | ★ | ★ | ||||
| ★ | ★ | ★ | ||||||
| ★ | ★ | ★ | ★ | ★ | ||||
| ★ | ★ | ★ | ★ | |||||
| ★ | ★ | ★ | ★ | ★ | ||||
| A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for Comparability. A maximum of nine stars can be awarded overall. | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 parastomal hernia (RCT) Show forest plot | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.40, 4.48] |
| 2 parastomal hernia (NRS) Show forest plot | 10 | 864 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.84, 1.75] |
| 3 stomal prolapse Show forest plot | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.39, 3.85] |
| 4 ileus or stenosis Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.19, 20.90] |
| 5 skin irritation Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.21, 2.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 parastomal hernia Show forest plot | 7 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.81, 1.65] |
| 1.1 ileostomy | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.21, 2.29] |
| 1.2 colostomy | 5 | 451 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.80, 2.11] |

