Tratamiento complementario con sultiame para la epilepsia
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Double‐blind, randomised, placebo‐controlled parallel study | |
| Participants | 37 participants (sulthiame: N = 20; placebo: N = 17) Mean age (range): 7.7 months (3 to 15 months) Type of epilepsy: West syndrome | |
| Interventions | All participants received PDX (150 to 300 mg/kg/day) only, during the first 3 days of the study On day 4, sulthiame 5 mg/kg/day was added to the intervention group, and placebo was added to the control On day 7, the dose of sulthiame and placebo were doubled in participants who had not achieved complete cessation of seizures ('non‐responders') | |
| Outcomes | Complete cessation of seizure activity during 9‐day study Adverse effects for entire trial population Adverse effects for entire population ‐ somnolence | |
| Notes | Trial funded by DESITIN Pharma (Hamburg, Germany) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by roll of dice. |
| Allocation concealment (selection bias) | Low risk | Allocation concealed in sealed envelope for duration of study. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Method not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method not stated. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis performed. |
| Selective reporting (reporting bias) | High risk | Incomplete data reported for time to treatment withdrawal and adverse effects. |
| Other bias | Unclear risk | Baseline period was not defined. Limited participant demographic data provided. |
PDX: pyridoxine
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Sulthiame used as monotherapy in intervention group | |
| Sulthiame used as monotherapy in intervention group | |
| Sulthiame used as monotherapy in intervention group | |
| Not a randomised controlled trial | |
| Not a randomised controlled trial | |
| Sulthiame used as monotherapy in intervention group | |
| Sulthiame used as monotherapy in intervention group | |
| Not a randomised controlled trial | |
| Study assessing the effects of sulthiame on aggressive behaviour in both epileptic and non‐epileptic participants | |
| Sulthiame used as monotherapy in the intervention group | |
| Study on the effect of sulthiame as monotherapy on axonal excitability of cortical neurons in subjects with no history of epilepsy |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete cessation of seizures Show forest plot | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.14 [0.67, 184.47] |
| Analysis 1.1  Comparison 1 Sulthiame add‐on versus placebo, Outcome 1 Complete cessation of seizures. | ||||
| 2 Adverse effects ‐ Total number of participants experiencing one or more adverse effects Show forest plot | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.44, 1.64] |
| Analysis 1.2  Comparison 1 Sulthiame add‐on versus placebo, Outcome 2 Adverse effects ‐ Total number of participants experiencing one or more adverse effects. | ||||
| 3 Adverse effects ‐ Somnolence Show forest plot | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.4 [0.42, 27.59] |
| Analysis 1.3  Comparison 1 Sulthiame add‐on versus placebo, Outcome 3 Adverse effects ‐ Somnolence. | ||||

Study flow diagram
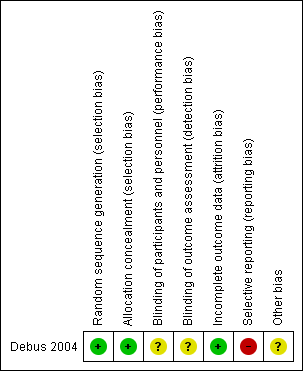
Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Sulthiame add‐on versus placebo, Outcome 1 Complete cessation of seizures.
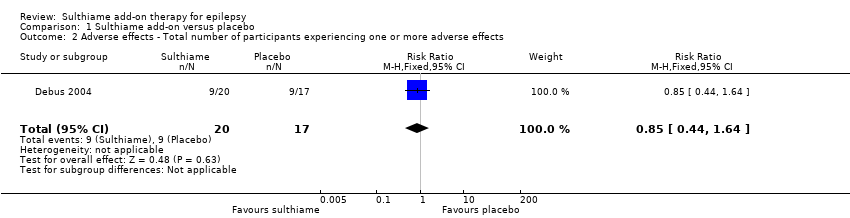
Comparison 1 Sulthiame add‐on versus placebo, Outcome 2 Adverse effects ‐ Total number of participants experiencing one or more adverse effects.

Comparison 1 Sulthiame add‐on versus placebo, Outcome 3 Adverse effects ‐ Somnolence.
| Sulthiame add‐on compared to placebo for epilepsy | ||||||
| Patient or population: patients between 3 to 15 months of age with West syndrome | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with Sulthiame add‐on | |||||
| 50% or greater reduction in seizure frequency | Outcome was not reported by Debus 2004 | |||||
| Complete cessation of seizures during follow‐up Follow‐up: 9 days | Study population | RR 11.14 | 37 | ⊕⊝⊝⊝ | ||
| 0 per 1,000 | 0 per 1000 | |||||
| Mean seizure frequency | Outcome was not reported by Debus 2004 | |||||
| Time‐to‐treatment withdrawal | Outcome was not reported by Debus 2004 | |||||
| Adverse effects: total number of participants experiencing one or more adverse effects Follow‐up: 9 days | Study population | RR 0.85 | 37 | ⊕⊝⊝⊝ | ||
| 529 per 1,000 | 450 per 1000 | |||||
| Adverse effects: somnolence Follow‐up: 9 days | Study population | RR 3.40 | 37 | ⊕⊝⊝⊝ | ||
| 59 per 1,000 | 200 per 1000 | |||||
| Quality of life | Outcome was not reported by Debus 2004 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once for risk of bias: Debus 2004 did not describe how blinding was achieved or maintained, and we suspected reporting bias for the adverse effect outcomes. bDowngraded twice for imprecision: small study population, therefore, the number of events failed to suffice optimal information size. cEvidence was not upgraded for effect size: normally, the evidence would be upgraded twice when a RR is greater than 5.00, however, due to the concerns regarding risk of bias and small sample size, we were unable to upgrade the evidence. dEvidence was not upgraded for effect size: normally, the evidence would be upgraded once when a RR is greater than 2.00, however, due to the concerns regarding risk of bias and small sample size, we were unable to upgrade the evidence. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete cessation of seizures Show forest plot | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.14 [0.67, 184.47] |
| 2 Adverse effects ‐ Total number of participants experiencing one or more adverse effects Show forest plot | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.44, 1.64] |
| 3 Adverse effects ‐ Somnolence Show forest plot | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.4 [0.42, 27.59] |

