Tratamiento complementario con sultiame para la epilepsia
Appendices
Appendix 1. Search strategy for CRS Web
1. (sulthiame OR sultiame OR Ospolot) AND CENTRAL:TARGET
2. MESH DESCRIPTOR Epilepsy EXPLODE ALL AND CENTRAL:TARGET
3. MESH DESCRIPTOR Seizures EXPLODE ALL AND CENTRAL:TARGET
4. (epilep* OR seizure* OR convuls*):AB,KW,MC,MH,TI AND CENTRAL:TARGET
5. #2 OR #3 OR #4 AND CENTRAL:TARGET
6. #1 AND #5
Appendix 2. Search strategy for MEDLINE Ovid
This strategy is based on the Cochrane highly sensitive search strategy for identifying randomised trials published in Lefebvre 2011.
1. (sulthiame or sultiame or Ospolot).tw.
2. exp Epilepsy/
3. exp Seizures/
4. (epilep$ or seizure$ or convuls$).tw.
5. 2 or 3 or 4
6. exp *Pre‐Eclampsia/ or exp *Eclampsia/
7. 5 not 6
8. (randomized controlled trial or controlled clinical trial or pragmatic clinical trial).pt. or (randomi?ed or placebo or randomly).ab.
9. clinical trials as topic.sh.
10. trial.ti.
11. 8 or 9 or 10
12. exp animals/ not humans.sh.
13. 11 not 12
14. 1 and 7 and 13
15. remove duplicates from 14
Appendix 3. ClinicalTrials.gov search strategy
Interventional Studies | Epilepsy | sulthiame or sultiame or ospolot
Appendix 4. ICTRP search strategy
Condition: epilepsy
Intervention: sulthiame or sultiame or ospolot
Appendix 5. SCOPUS search strategy
(TITLE‐ABS‐KEY(sulthiame OR sultiame OR ospolot)) AND (((((TITLE‐ABS‐KEY(epilep* OR "infantile spasm" OR "ring chromosome 20" OR "R20" OR "myoclonic encephalopathy" OR "pyridoxine dependency")) OR (TITLE‐ABS‐KEY(syndrome W/2 (aicardi OR angelman OR doose OR dravet OR janz OR jeavons OR "landau kleffner" OR "lennox gastaut" OR ohtahara OR panayiotopoulos OR rasmussen OR rett OR "sturge weber" OR tassinari OR "unverricht lundborg" OR west)))) OR (TITLE(seizure OR convuls*))) OR ((TITLE‐ABS‐KEY(lafora* W/4 (disease OR epilep*))) AND NOT (TITLE(dog OR canine) OR INDEXTERMS(dog OR canine)))) AND NOT (TITLE(*eclampsia) OR INDEXTERMS(*eclampsia)) AND NOT INDEX(medline)) AND (TITLE((randomiz* OR randomis* OR controlled OR placebo OR blind* OR unblind* OR "parallel group" OR crossover OR "cross over" OR cluster OR "head to head") PRE/2 (trial OR method OR procedure OR study)) OR ABS((randomiz* OR randomis* OR controlled OR placebo OR blind* OR unblind* OR "parallel group" OR crossover OR "cross over" OR cluster OR "head to head") PRE/2 (trial OR method OR procedure OR study)))

Study flow diagram
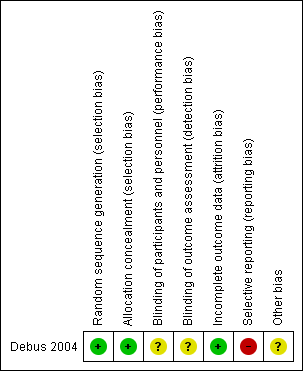
Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Sulthiame add‐on versus placebo, Outcome 1 Complete cessation of seizures.
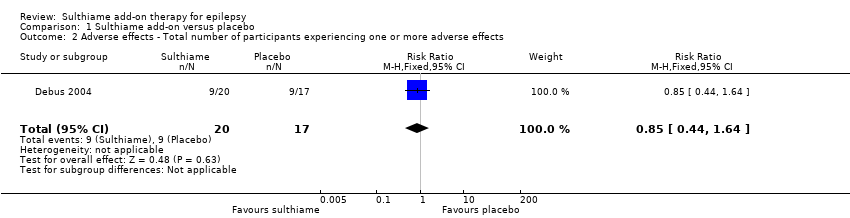
Comparison 1 Sulthiame add‐on versus placebo, Outcome 2 Adverse effects ‐ Total number of participants experiencing one or more adverse effects.

Comparison 1 Sulthiame add‐on versus placebo, Outcome 3 Adverse effects ‐ Somnolence.
| Sulthiame add‐on compared to placebo for epilepsy | ||||||
| Patient or population: patients between 3 to 15 months of age with West syndrome | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with Sulthiame add‐on | |||||
| 50% or greater reduction in seizure frequency | Outcome was not reported by Debus 2004 | |||||
| Complete cessation of seizures during follow‐up Follow‐up: 9 days | Study population | RR 11.14 | 37 | ⊕⊝⊝⊝ | ||
| 0 per 1,000 | 0 per 1000 | |||||
| Mean seizure frequency | Outcome was not reported by Debus 2004 | |||||
| Time‐to‐treatment withdrawal | Outcome was not reported by Debus 2004 | |||||
| Adverse effects: total number of participants experiencing one or more adverse effects Follow‐up: 9 days | Study population | RR 0.85 | 37 | ⊕⊝⊝⊝ | ||
| 529 per 1,000 | 450 per 1000 | |||||
| Adverse effects: somnolence Follow‐up: 9 days | Study population | RR 3.40 | 37 | ⊕⊝⊝⊝ | ||
| 59 per 1,000 | 200 per 1000 | |||||
| Quality of life | Outcome was not reported by Debus 2004 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once for risk of bias: Debus 2004 did not describe how blinding was achieved or maintained, and we suspected reporting bias for the adverse effect outcomes. bDowngraded twice for imprecision: small study population, therefore, the number of events failed to suffice optimal information size. cEvidence was not upgraded for effect size: normally, the evidence would be upgraded twice when a RR is greater than 5.00, however, due to the concerns regarding risk of bias and small sample size, we were unable to upgrade the evidence. dEvidence was not upgraded for effect size: normally, the evidence would be upgraded once when a RR is greater than 2.00, however, due to the concerns regarding risk of bias and small sample size, we were unable to upgrade the evidence. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete cessation of seizures Show forest plot | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.14 [0.67, 184.47] |
| 2 Adverse effects ‐ Total number of participants experiencing one or more adverse effects Show forest plot | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.44, 1.64] |
| 3 Adverse effects ‐ Somnolence Show forest plot | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.4 [0.42, 27.59] |

