نقش آسپرین خوراکی برای درمان زخمهای وریدی پا
چکیده
پیشینه
زخمهای وریدی پا (venous leg ulcers; VLUs) یا زخمهای واریسی، مرحله نهایی نارسایی وریدی مزمن (chronic venous insufficiency; CVI)، و شایعترین نوع زخم پا هستند. پیشرفت VLUها در مچ پا و قسمت پائینتر پاها میتواند به صورت خودبهخودی یا پس از یک ترومای کوچک به وجود بیاید. زخمها اغلب دردناک و حاوی ترشحات بوده، التیام غالبا طولانیمدت و عود هم شایع است. این چرخه از التیام و عود، تاثیر قابل توجهی بر سلامت و کیفیت زندگی افراد، و کارکنان بخش مراقبت سلامت و هزینههای اجتماعیاقتصادی دارد. VLUها یک مشکل شایع و پُر‐هزینه در سراسر جهان است؛ شیوع آن در جهان غرب بین 1.65% تا 1.74% تخمین زده شده و در بزرگسالان 65 سال و بالاتر شایعتر است. درمان اصلی برای VLU، پانسمان فشرده محکم است. کمپرس کردن با استفاده از کاهش هیپرتانسیون وریدی، افزایش بازگشت وریدی و کاهش ادم محیطی به درمان این زخمها کمک میکند. با این حال، مطالعات نشان میدهد که این کار فقط تاثیرات متوسطی بر التیام دارد و تا 50% از زخمهای وریدی پا پس از دو سال درمان فشردهسازی التیام نمییابند. عدم پایبندی به درمان ممکن است علت اصلی این نتایج ضعیف باشد، اما وجود التهاب و تورم در افراد مبتلا به CVI، ممکن است عامل دیگری باشد، بنابراین درمانی که مانع از التهاب شود (درمان زخمها با سرعت بیشتر) و تکرار عود زخم را کاهش دهد (در نتیجه زمان طولانی بین اپیزودهای عود)، میتواند یک مداخله ارزشمند برای تکمیل درمانهای فشردهسازی باشد. آسپرین (aspirin) خوراکی ممکن است تاثیر قابل توجهی در عملکرد بالینی VLU در سراسر جهان داشته باشد. شواهدی مبنی بر اثربخشی آسپرین در بهبود زخم و عود در RCTهای با کیفیت بالا در حال حاضر وجود ندارد.
اهداف
ارزیابی مزایا و مضرات مصرف آسپرین در درمان و عود زخمهای وریدی پا.
روشهای جستوجو
در ماه می 2015 ما منابع زیر جستوجو شد: پایگاه ثبت تخصصی گروه زخمها در کاکرین (Cochrane Wounds Specialised Register)؛ پایگاه ثبت مرکزی کارآزماییهای کنترل شده کاکرین (CENTRAL) (کتابخانه کاکرین (The Cochrane Library))؛ Ovid MEDLINE؛ Ovid MEDLINE (استنادات نمایه نشده در حال انجام و سایذ استنادات نمایه نشده)؛ Ovid EMBASE و EBSCO CINAHL. در پایگاههای ثبت کارآزمایی و فهرست منابع نشریات مرتبط با کارآزماییهای منتشر شده یا در حال انجام، جستوجوهای دیگری انجام شد. هیچ گونه محدودیتی از نظر زبان و یا تاریخ انتشارات وجود نداشت.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و کنترل شدهای (randomised controlled trials; RCTs) را وارد کردیم که آسپرین را با دارونما (placebo) یا عدم مداخله با دارو (در حضور یا عدم حضور درمان فشردهسازی) برای درمان افراد مبتلا به زخمهای وریدی پا مقایسه کردند. پیامدهای اصلی ما، زمان سپری شده تا تکمیل بهبود زخم، نرخ تغییرات در ناحیه زخم، نسبت التیام زخم طی کارآزمایی، خونریزی عمده، درد، مورتالیتی، عوارض جانبی و عود زخم (زمان سپری شده تا عود و نسبت عود) بود.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم مطالعات را برای ورود انتخاب کرده، دادهها را استخراج، خطر سوگیری (bias) را برای هر کارآزمایی وارد شده ارزیابی و کیفیت کلی شواهد را برای پیامدهای اصلی در جدول «خلاصهای از یافتهها» ارزیابی کردند.
نتایج اصلی
جستوجوی الکترونیکی بین 62 مطالعه انجام شد. ما دو RCT را درباره آسپرین خوراکی (300 میلیگرم/روز) را وارد کردیم که فشردهسازی را با فشردهسازی و دارونما، یا فشردهسازی به تنهایی مقایسه کرده بودند. تا به امروز، تاثیر آسپرین در VLUها، فقط در دو کارآزمایی بالینی تصادفیسازی شده، که هر دو تعداد کمی شرکتکننده داشتند، بررسی شده است. اولین RCT در انگلستان (20 = n) انجام شد و گزارش داد که استفاده روزانه از آسپرین (300 میلیگرم) همراه با بانداژ فشردهسازی، نرخ بهبودی و تعداد شرکتکنندگان التیام یافته را در مقایسه با دارونما به علاوه بانداژ فشردهسازی در یک دوره چهار ماهه افزایش میدهد. سیوهشت درصد از شرکتکنندگانی که آسپرین دریافت کردند در مقایسه با 0% در گروه دارونما بهبودی کامل را گزارش کردند. در 52% از شرکتکنندگان مصرف کننده آسپرین در مقایسه با 26% از کسانی که دارونما دریافت کردند، بهبود رخ داده است (ارزیابی شده بوسیله کاهش اندازه زخم). این مطالعه مزایای بالقوه مصرف آسپرین را به عنوان مکمل فشردهسازی شناسایی کرد، اما حجم نمونه کوچک بود، و مکانیسمی که آسپرین در آن التیام را بهبود بخشید و تاثیرات آن بر عود، بررسی نشد.
در سال 2012 یک RCT در اسپانیا (51 = n) مصرف روزانه آسپرین (300 میلیگرم) را علاوه بر بانداژ فشردهسازی با فشردهسازی به تنهایی در یک دوره پنج ماهه مقایسه کرد. تفاوت کمی در نرخ بهبودی کامل بین گروهها وجود داشت (21 از 28 مورد در گروه آسپرین و 17 از 23 مورد در گروه بانداژ فشردهسازی به تنهایی)، اما به طور میانگین زمان سپری شده تا التیام کوتاهتر بود (12 هفته در گروه درمان شده در برابر 22 هفته در گروه بانداژ فشردهسازی تنها) و میانگین زمان تا عود در گروه آسپرین طولانیتر بود (39 روز: (SD: 6.0) با 16.3 روز (SD: 7.5) در گروه فشردهسازی به تنهایی مقایسه شد). اگرچه این کارآزمایی دادههای محدودی را در مورد استفاده بالقوه از آسپریندرمانی فراهم میکند، حجم نمونه (فقط 20 بیمار) برای نتیجهگیریهای معنیدار در مرور حاضر بیش از حد کوچک بود. علاوه بر این، بیماران فقط برای مدت 4 ماه پیگیری شدند و هیچ اطلاعاتی در مورد گروه دارونما گزارش نشد.
نتیجهگیریهای نویسندگان
شواهد با کیفیت پائین به دست آمده از دو کارآزمایی نشان میدهد که در حال حاضر شواهد کافی برای نتیجهگیری قطعی در مورد مزایا و مضرات آسپرین در درمان و عود زخم وریدی پا وجود ندارد. به دلیل سوگیری بالقوه انتخاب و عدم‐دقت به دلیل حجم کوچک نمونه، کیفیت شواهد را پائین ارزیابی کردیم. تعداد کم شرکتکنندگان ممکن است مزایای واقعی پنهان داشته باشد و یا افزایشی در مضرات آن داشته باشند. با توجه به فقدان شواهد قابل اطمینان، قادر به نتیجهگیری در مورد مزایا و مضرات مصرف روزانه آسپیرین خوراکی به عنوان مکمل باندهای فشردهسازی در بهبود VLU در پا یا عود آنها نیستیم. مطالعات بیشتری با کیفیت بالا در این زمینه مورد نیاز است.
PICOs
خلاصه به زبان ساده
آسپرین برای زخمهای وریدی پا
پیشینه
زخمهای وریدی پا (venous leg ulcers; VLU) شایعترین نوع زخمهای پا (جراحت) هستند و توسط جریان ضعیف خون در سیاهرگهای پا (نارسایی مزمن وریدی) ایجاد میشوند. نارسایی مزمن وریدی منجر به فشار خون بالا در وریدها (هیپرتانسیون وریدی) میشوند، که باعث بسیاری از تغییرات در پوست پا میشوند. زخمهای پا مرحله نهایی این تغییرات هستند. VLUها میتوانند به صورت خودبهخودی یا پس از یک صدمه کوچک رخ دهند، اغلب دردناک هستند و تولید تراوشات سنگین میکنند (از دست دادن مایع). VLUها یک مشکل عمده سلامت هستند زیرا بسیار شایع هستند، تمایل به تبدیل شدن به حالت مزمن دارند (طولانیمدت) و همچنین تمایل زیادی به عود نشان میدهند. آنها افراد مسن را بیشتر تحت تاثیر قرار میدهند، هزینههای بالای مراقبتی دارند و برای کسانی که تحت تاثیر قرار گرفتهاند، بار (burden) فردی و اجتماعی بالایی دارد.
درمان فشردهسازی، در قالب یک باند محکم روی پا، که به جریان خون در وریدها کمک میکند، درمان خوب و تثبیت شدهای برای VLUها است. با این حال، مطالعات نشان میدهد که کمپرس فقط تاثیرات متوسطی بر التیام دارد، بیشتر از 50% از VLUها احتمالا به علت فرایند التهاب طولانیمدت، پس از دو سال کمپرس به صورت التیام نیافته باقی ماندند. درک بهتر از تغییرات از بین برنده پوست پا در افراد مبتلا به VLUها و روند التهاب مزمن در آنها، باعث شده که محققان داروهای مختلفی را که میتوانند درمان این بیماری را بهبود بخشند، تست کنند. آسپرین (aspirin) برخی خواص شناخته شده دارد، از جمله کاهش درد (آنالژزیک)، کاهش التهاب و تب، و توقف تجمع سلولهای خون با هم، که از تشکیل لخته خونی پیشگیری میکند. آسپیریندرمانی ممکن است زمان تا التیام را بهبود بخشیده و تعداد اپیزودهای عود VLU را کاهش دهد. اگر این درمان موثر باشد، هزینه پائین آسپیریندرمانی به عنوان درمان کمکی فشردهسازی، آن را یک عامل پیشگیرانه مقرون به صرفه برای افراد مبتلا به VLUها در همه کشورها تبدیل میکند.
سوال مطالعه مروری
مزایا و مضرات آسپرین خوراکی در درمان و عود زخمهای وریدی پا چیست؟
آنچه ما به دست آوردیم
ما فقط دو کارآزمایی تصادفیسازی و کنترل شده را شناسایی کردیم که آسپرین خوراکی (300 میلیگرم در روز) را به علاوه فشردهسازی با فشردهسازی و دارونما (placebo) یا فشردهسازی به تنهایی مقایسه کرد. یک مطالعه انجام شده در بریتانیا 20 شرکتکننده را انتخاب کرد (ده نفر در گروه آسپرین و ده نفر در گروه کنترل) و افراد به مدت چهار ماه پیگیری شدند. این کارآزمایی گزارش داد که ناحیه زخم در گروه آسپرین (با 6.5 سانتیمتر مربع، کاهش 39.4%) در مقایسه با گروه کنترل که کاهشی نداشت، کاهش یافت و نسبت بالاتری از زخمها (38%) در گروه آسپرین در مقایسه با گروه کنترل که کاهشی نداشت، به طور کامل التیام یافت. عود در این مطالعه بررسی نشد. مطالعه دیگری که در اسپانیا انجام شد شامل 51 شرکتکننده بود (23 نفر در گروه آسپرین و 28 نفر در گروه کنترل) و تا زمان التیام زخمها افراد را پیگیری کرد. این مطالعه گزارش داد که میانگین زمان التیام، 12 هفته در گروه آسپرین و 22 هفته در گروه کنترل بود، و هیچ تفاوت واقعی بین نسبت افرادی که زخم آنها التیام یافته بود؛ وجود نداشت (17 نفر (74%) از 23 نفر در گروه آسپرین و 21 نفر (75%) از 28 نفر در گروه کنترل). میانگین زمان تا عود در گروه آسپرین (39 روز) در مقایسه با گروه فشردهسازی به تنهایی (16.3 روز) طولانیتر بود. عوارض جانبی در هیچ یک از کارآزماییها گزارش نشد.
این دو مطالعه خیلی کوچک و با کیفیت پائین را برای خود برای نتیجهگیری قطعی در مورد مزایا و مضرات آسپرین خوراکی در التیام و عود زخمهای وریدی پا در نظر گرفتیم. مطالعهای در انگلستان فقط دادههای محدودی را درباره مزایای روزانه آسپرین درمانی خوراکی همراه با فشردهسازی، به دلیل حجم کوچک نمونه با فقط 20 شرکتکننده و پیگیری کوتاهمدت، فراهم کرد. مطالعهای در اسپانیا دادههای محدود به دست آمده را از 51 شرکتکننده برای مقایسه آسپرین و فشردهسازی در گروه کنترل فراهم کرد. واقعیت این است که هیچ اطلاعاتی در مورد دارونما در گروه کنترل گزارش نشد این بدان معنی است که تخمین این تاثیر نامطمئن است. مطالعات بیشتری با کیفیت بالا در این زمینه مورد نیاز است.
این خلاصه به زبان ساده تا 27 می 2015 بهروز است.
Authors' conclusions
Summary of findings
| Oral aspirin for venous leg ulcers | ||||||
| Patient or population: patients with venous leg ulcers | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral aspirin | |||||
| Average time for ulcer healing | 22 weeks | 12 weeks | Not estimable | 51 (1 study) | ⊕⊕⊝⊝ | P values and confidence intervals were not reported |
| Reduction of ulcer area (median) | 0 cm² | 6.5cm² | Not estimable | 20 | ⊕⊕⊝⊝ | P value < 0.002 Follow‐up: 4 months |
| Proportion of healed ulcers in the trial period | No healed ulcers | 38% of healed ulcers | Not estimable | 20 | ⊕⊕⊝⊝ | P value < 0.007 Follow‐up: 4 months |
| Major bleeding | See comment | See comment | Not estimable | 20 | ⊕⊕⊝⊝ | No events were observed in either group, follow‐up: 4 months Another study reported 2 hospitalisations for unknown reasons, intervention group not specified |
| Average time of ulcer recurrence | 16.33 days SD: 7.5 | 39 days SD: 6.0 | Not estimable | 51 | ⊕⊕⊝⊝ | P value = 0.007 Post hoc assessment not pre‐specified in protocol |
| Mortality | See comment | See comment | Not estimable | See comment | See comment | Mortality not reported |
| Other adverse events | See comment | See comment | Not estimable | 71 | See comment | No events were observed in either group. del Río Solá reported 2 hospitalisations for unknown reasons, the group of these patients were not specified and they were removed from the study |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Allocation concealment and blinding of outcome assessment were not described. Participants and personnel were not blinded. There was a high risk of bias from incomplete outcome data.There were some inconsistencies in the reporting of the data | ||||||
Background
Description of the condition
Venous leg ulcers (VLUs; also known as varicose ulcers or stasis ulcers) occur as results of the chronic venous insufficiency (CVI), which is a functional disorder of the venous system of the leg. The venous leg system is a compound of superficial, deep and perforating veins (perforating veins connect the superficial and deep veins). Damage in any of these veins causes an impairment of venous return, this impairment causes increased venous pressure also known as venous hypertension Ballard 2000). Chronic venous hypertension (CVH) leads to an inflammatory response by leukocytes (white blood cells involved in the inflammation process) that causes cellular and tissue dysfunction which in turn results in varicose veins (veins unnaturally and permanently distended) and dermal changes called lipodermatosclerois characterised by the presence of oedema, hyperpigmentation, induration and eczema of the skin. Ulceration is the final stage of these alterations. (Appendix 1; Thomas 1988; De Araujo 2003; Barron 2007; Rafetto 2009).
Clinically, a VLU is characterised by erosion of the skin usually in the gaiter area of the lower leg (between the knee and the ankle; Dorland 2007). Ulcers vary in size and number. Usually, they have a shallow base, flat margin with the surrounding skin showing features of CVH (Gilliland 1991; Valencia 2001; Raju 2009). Pain that impairs quality of life is present in 75% of people with this condition (Friedman 1990; Philips 1994); in some cases the ulcer has an associated odour that can result in social isolation and depression (Gilliland 1991;Jones 2008).
Venous leg ulceration has a tendency to become chronic and recurrent; one estimate suggests that 30% of healed ulcers recur in the first year, rising to 78% after two years (Mayer 1994). Around 80% of lower extremity leg ulcers presenting in general practice are VLUs; the remaining 20% are as a result of arterial insufficiency, neuropathy, trauma, inflammatory or metabolic conditions, malignancy and infections (Falabella 1998; Sibbald 1998; Valencia 2001; Moloney 2004; Dealey 2005). Diagnosis of a VLU is based on a clinical assessment and the presence of symptoms that are consistent with venous hypertension (i.e. an ulcer located in the medial gaiter area; presence of varicose veins, eczema, pigmentation, induration and oedema, in any combination). In a few cases the diagnosis can be complemented by non‐invasive methods such as ultrasonography.
VLUs are a major health problem because of their frequent occurrence and associated high cost of care. The disease mainly affects people between 60 and 80 years of age, women are affected three times more frequently than men. The rate of occurrence of VLUs is likely to increase as the average age of the population increases (Callam 1987; Margolis 2002). Estimates of its occurrence rate vary by country. For example, in Europe, including countries such as Denmark, Czechoslovakia and Switzerland, the rates of occurrence have been reported at 1% to 5.5% in women and 0.9% to 1.9% in men (Bobek 1966; Arnoldi 1968; Kamber 1978); in the USA the rates were reported as 0.2% in women and 0.1% in men (Coon 1973); and in Brazil as approximately 1.5% for open or healed VLU (Maffei 1986). The cost of treating VLUs is estimated to be one billion USD per year in the USA, and the average cost for one person over a lifetime has been estimated to exceed USD 40,000 (Valencia 2001). Another study of people with VLUs estimated that the average duration of follow‐up was 119 days and the average total medical cost per person was USD 9685 (Olin 1999).
Description of the intervention
The goals of treatment for people with venous leg ulcers include: reduction of oedema, relief of pain and lipodermatosclerosis, ulcer healing, and prevention of recurrence (De Araujo 2003). Different modalities of treatment are used for treating VLUs and these are sometimes used in combination (Blankensteijn 2009). The most common form of treatment is compression therapy (covering the leg with a firm bandage or socks, to apply an external force which aids the flow of blood in the veins). Whilst compression has the potential to heal approximately 50% of VLUs (Weller 2012), rest with elevation of the affected leg, venous surgery, and oral medication with drugs (such as pentoxifylline that aim to improve blood flow and reduce clotting (Jull 2007)) are also used to treat this condition. A treatment that can suppress inflammation would be useful. Oral aspirin is a widely used drug that may have the potential to exert a beneficial influence in the treatment of VLUs. However, until now, no comprehensive summary of the available evidence has been conducted.
How the intervention might work
Classical signs of inflammation have been observed in biopsies and plasma samples in experimental models of venous disease. The cascade starts with increased vascular permeability (increased leakage of plasma and cells through the vein wall) and progression to adhesion of leukocytes (white blood cell involved in the inflammation process) and platelets (very small structures shaped like a discus, present in the blood with important role in the coagulation) to endothelium (the cells lining the lumen of the veins). Over time the disease progresses to vascular restructuring of venous varicosities (veins which are unnaturally and permanently distended). Unlike acute wound healing, chronic VLUs remain at an prolonged inflammatory stage with formation of granulation tissue (newly formed tissue which repairs damaged areas) (Bergan 2007).
Aspirin or acetylsalicylic acid was introduced as a medication in 1899 by Dreser (Burke 2006). Aspirin has analgesic, anti‐inflammatory and antipyretic (fever‐reducing) properties (Winter 1966). It inhibits platelet aggregation, and acts as an inhibitor of cyclooxygenase (substance involved in the synthesis prostaglandins), resulting in the inhibition of the biosynthesis (physiologic production of a substance into the body) of prostaglandins (substances involved in the inflammatory process causing venous dilatation and inhibition the platelet aggregation) (Salzman 1971; Vane 1971). Prostaglandins are released during the inflammatory phase, and are thought to increase blood vessel permeability, manifested by venous oedema and capillary leakage. Aspirin stimulates the biosynthesis of other anti‐inflammatory compounds by inhibiting the cyclooxygenase pathway. The precise mechanism by which aspirin is thought to mediate effects on VLU healing is unclear, although inhibition of platelet activation and reduction of inflammation and pain have been suggested (Ibbotson 1995; De Araujo 2003). However, aspirin is known to have adverse effects, most commonly gastric ulceration and other gastrointestinal effects, as well as hepatotoxicity (liver damage), exacerbation of asthma, skin rashes and renal toxicity (Cappelleri 1995; Burke 2006).
Why it is important to do this review
Chronic VLU healing remains a complex clinical problem and requires the intervention of skilled, but costly, multidisciplinary wound care teams. Recurrence is often an ongoing issue for people who experience venous ulcers. Aspirin is an inexpensive and widely available treatment currently used in several other clinical situations. Oral aspirin is potentially one of the most effective preventive agents for use in people with VLUs. It has the potential to improve healing rates, shorten time to healing and decrease the number of recurrent episodes after healing. If proved effective, the low cost of aspirin therapy as an adjunct to compression would make it an affordable preventive agent for people with VLUs in all countries. Despite its potential benefits, there are limited data available about the effectiveness of aspirin in people with VLUs. Additionally, with the number of people with VLUs expected to rise significantly in the coming decades, development of new safe ways of healing and reducing recurrence are high priorities in health research.
Objectives
To assess the benefits and harms of oral aspirin on the healing and recurrence of venous leg ulcers.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) of oral aspirin to treat people with venous leg ulcers.
Types of participants
Adults (as defined in trials) undergoing treatment for venous leg ulceration or prevention of recurrence of venous leg ulcers.
Types of interventions
Oral aspirin compared with placebo or any other therapy in the presence or absence of compression therapy.
To be eligible for inclusion, treatment with oral aspirin must be the only systematic difference between treatment arms, therefore a study in which one group received compression and one did not would not be eligible for inclusion unless within a factorial design.
Types of outcome measures
Primary outcomes
-
Time to complete ulcer healing.
-
Rate of changes in the area of the ulcer in the trial period.
-
Proportion of ulcers healed in the trial period.
-
Proportions of people with ulcers completely healed in the trial period.
-
Major bleeding (haemorrhagic stroke, gastric bleeding, any bleeding requiring blood transfusion, any bleeding causing hospitalisation).
Secondary outcomes
-
Pain relief (as measured by a valid pain scale).
-
Mortality from any cause.
-
Any adverse events (e.g. renal failure, neutropenia, low platelets level, gastric complaints, diarrhoea, skin rash, minor bleeding).
-
Ulcer recurrence (time to recurrence and proportion of people with recurrence).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of randomised controlled trials:
-
The Cochrane Wounds Specialised Register (searched 27 May 2015);
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2015, Issue 4);
-
Ovid MEDLINE (1946 to 26 May 2015);
-
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (searched 26 May 2015);
-
Ovid EMBASE (1974 to 26 May 2015);
-
EBSCO CINAHL (1982 to 27 May 2015).
The search strategies we used can be found in Appendix 2. The Ovid MEDLINE searches were combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision) (Lefebvre 2011). We combined the EMBASE search with the Ovid EMBASE trial filter terms developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL search with the trial filter developed by the Scottish Intercollegiate Guidelines Network (SIGN 2011). There was no restriction on the basis of date, or language of publication.
We also searched the following clinical trials registries:
ClinicalTrials.gov (https://clinicaltrials.gov/);
WHO International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/default.aspx).
Searching other resources
We searched the references of all identified studies in order to find any further relevant trials. We also contacted experts in the field.
Data collection and analysis
Selection of studies
Two reviews authors (NGM and RFA) selected studies as described in Chapter 7 of theCochrane Handbook for Systematic Reviews of Interventions as follows (Higgins 2011a):
-
We merged search results using reference management software, and removed duplicate records of the same report.
-
We examined titles and abstracts to remove obviously irrelevant reports.
-
We retrieved full text of the potentially relevant reports.
-
We linked multiple reports of the same study.
-
We examined full‐text reports for compliance of studies with eligibility criteria.
-
We clarified study eligibility (where appropriate) by corresponding with investigators.
-
We made final decisions on study inclusion to allow data collection to proceed.
Any disagreements were solved by discussion. If this did not result in agreement, the opinion of the third review author (PEdOC) was decisive. We documented the reasons for exclusion of any article.
Data extraction and management
Two reviews authors (NGM and RFA) independently extracted details of eligible studies and summarised them using a data extraction sheet specific to this review that was constructed according Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). The data extracted included the baseline characteristics of the intervention and control group participants, and included (where available): participant numbers, age, gender, ethnicity, the main outcome measures, length of follow‐up, and numbers of drop‐outs (Table 1).
| Study identification | Layton | del Río Solá | ||||
| Country | United Kingdom | Spain | ||||
| Period | Not reported | 2001 to 2005 | ||||
| Centres | Academic Unit of Dermatology, General Infirmary at Leeds, West Yorkshire | University Hospital of Valladolid | ||||
| Source of funding | Not specified | Not specified | ||||
| Method | ||||||
| Study design | Prospective randomized, double‐blind | Prospective randomized trial | ||||
| Power calculation | Not described | Yes | ||||
| Method of randomisation | Not described | Generated by computer program | ||||
| Concealment of allocation | Not described | Not described | ||||
| Number of participants randomized | 20 | 51 | ||||
| Number of participants analyzed | 20 | 47 | ||||
| Number of participants excluded after randomizations | 0 | 0 | ||||
| Number of participant withdrawals and reasons | 0 | 4 people; 2 people needed hospitalisation and left the study and 2 people opted for treatment in another service | ||||
| Intention‐to‐treat analysis | Yes | Yes | ||||
| Participants | ||||||
| Inclusion criteria | People with chronic venous leg ulcer | Venous leg ulcer ≥ 2 cm Ankle‐brachial rate < 0.9 No contraindication to taking aspirin | ||||
| Exclusion criteria | Ulcer diameter < 2 cm Already taking aspirin, anticoagulants or non‐steroidal anti‐inflammatory Doppler flowmetry ankle‐brachial rate < 0.9 | People with diabetes mellitus, rheumatoid arthritis, peripheral arterial disease and neurologic disease Previous or concomitant therapy with aspirin | ||||
| Aspirin | Control | P value | Aspirin | Control | P value | |
| Number of participants | 10 | 10 |
| 23 | 28 |
|
| Age (years) | 62.2 years (mean) (48‐81) | 66 years (mean) (46 ‐ 85) |
| 60.50 years (SD:12.07) | 58.59 years (SD:16.55) | reported as non significant |
| Sex | 3 female, 7 male | 5 female, 5 male |
| 10 female, 13 male | 19 female, 9 male | reported as non significant |
| Ulcer duration before the study | 11.4 years (mean) (1‐24) | 10.5 years (mean) (2‐22) |
| 6‐12 months | > 12 months |
|
| 1 Number of ulcers | Not reported | Not reported | Not reported | Not reported | reported as non significant | |
| Initial ulcer surface area (cm²) | 16.5 cm² (mean) (2.5‐39.5) | 14.25 cm² (mean) (1.5‐48.5) | 25.15 cm² | 24.87 cm² | P=0.944 | |
| Signs of ulcer infection | Not reported | Not reported | Yes, 20 patients | Yes, 22 patients | P=0.094 | |
| Any comorbidity | Not reported | Not reported | 9 patients | 10 patients | ||
| Previously treated | \not reported | Not reported | 10 patients | 20 patients | ||
| Interventions | Aspirin 300 mg/day | Placebo | Aspirin 300 mg/day | No drug treatment | ||
| Outcomes | ||||||
| Follow‐up (months) | 4 months | 4 months | 42 months mean (24‐61) | 42 months mean (24‐61) | ||
| 2 Withdrawals | 0 | 0 | 2 people | 2 people | ||
| Duration of the study to complete ulcer healing | Not reported | Not reported | Not reported | 12.4 weeks | 16.5 weeks | P=0.07 Mann‐Whitney |
| Healing period | Not reported | Not reported | Not reported | Reported as short in the aspirin group | Reported as short in the aspirin group | P=0.04 log‐rank test = 3.90 OR = 0.93 95% CI 0.25‐3.5 |
| Average time to complete ulcer healing (weeks) | Not reported | Not reported | Not reported | 12 | 22 | Not reported |
| Number of participants with complete ulcer healing in the trial period | Not reported | Not reported | Not reported | 17 (74%) | 21 (75%) | reported as non significant |
| Proportion of ulcers healed in the trial period | 38% of the ulcers | 0% of the ulcers | < 0.007 (x² test) | Not reported | Not reported | Not reported |
| Change in ulcer areas in the trial period (second month; ulcer area cm²) | 15.5 cm² (median) 1 cm² of reduction (6.07% of reduction) | No reduction | < 0.01 (x² test) | Not reported | Not reported | Not reported |
| Reduction in ulcer size in the trial period (fourth month; ulcer area cm²) | 10.0 cm² (median) 6.5 cm² of reduction (39.4% of reduction) | No reduction | < 0.002 (x² test) | Not reported | Not reported | Not reported |
| Improvement assessed by reduction in ulcer size | 52% of the ulcers | 26% of the ulcers | < 0.007 (x² test) | Not reported | Not reported | Not reported |
| Increase in ulcer size in the trial period | 10% of the ulcers | 26% of the ulcers | < 0.004 (x² test) | Not reported | Not reported | Not reported |
| Ulcers size unchanged in the trial period | 0% of the ulcers | 48% of the ulcers | < 0.001 (x2 test) | Not reported | Not reported | Not reported |
| Proportion of participants with ulcers healed in the trial period | Not reported | Not reported | Not reported | 17 (74%) | 21 (75%) | reported as non significant |
| Proportion of participants with ulcer recurrence | Not reported | Not reported | Not reported | 25% | 33.33% | 0.74 |
| Average time for ulcer recurrence (days) | Not reported | Not reported | Not reported | 39 (SD 6) | 16.33 (SD 7.5) | P=0.007 Kaplan‐Meier |
| adverse effects | 0 | 0 | 0 | 0 | Not reported | |
-
Layton reported 12 people (60%) and del Rio 28 people (54%) with multiples ulcers but they did not specified the number in each group
-
del Rio Solá reported that two people were hospitalised and were withdrawn from the study, but did not specify the cause of hospitalisation or their trial group
Assessment of risk of bias in included studies
We used the Cochrane tool for assessing risk of bias to present a summary of the risk of bias for each included study . This tool addresses seven specific domains Higgins 2011b:
-
Was the allocation sequence adequately generated?
-
Was the allocation adequately concealed?
-
Was the blinding of participants and personnel adequately provided?
-
Was the blinding of outcome assessors adequately provided?
-
Were incomplete outcome data adequately addressed?
-
Were reports of the study free of suggestion of selective outcome reporting?
-
Was the study apparently free of other problems that could put it at a high risk of bias?
Two authors (NGM and RFA) assessed each study independently; disagreements were solved by consultation with the third review author (PEdOC). Additionally, we presented a 'Risk of bias' summary figure, reporting all our judgements in a cross‐tabulation of study by entry (Figure 1). This display of internal validity shows the weight given to the results of the particular studies.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
For binary outcomes (e.g. recurrence, improvement), we planned to present risk ratios (RR); risk differences (RD), the number needed to treat for an additional beneficial outcome (NNTB), and the number needed to treat for an additional harmful outcome (NNTH), all with corresponding 95% confidence intervals (CI).
For continuous data (e.g. ulcer area), we planned to present differences as mean differences (MD) with corresponding 95% CIs, or standardised mean differences (SMD) when the studies assessed the same outcome but measured it in a variety of ways (e.g. different questionnaires for pain assessment).
For time to complete ulcer healing, that is time‐to‐event data, we planned to use the most appropriate way of summarising this type of data using survival analysis methods and to express the intervention effect as a hazard ratio (HR).
Unit of analysis issues
We only considered simple parallel‐group designs for clinical trials (Deeks 2011). If healing outcomes were reported at several time points, we planned to perform the analysis using the time‐points reported (for example, 30 or 60 days or the time for ulcer healing). We planned to consider the number of ulcers per patient and the ulcer area per patient, which means, for patients with more than one ulcer, calculating the total area of the ulcers.
Dealing with missing data
We contacted the trial authors to obtain relevant missing data and investigated attrition rates (for example, drop‐outs, losses to follow‐up and withdrawals). Authors did not respond to our requests. We address the potential impact of missing data on the findings of the review in the discussion section (Higgins 2011c).
Assessment of heterogeneity
We planned to assess statistical heterogeneity using the I² statistic to examine the percentage of total variation across studies due to heterogeneity rather than to chance (Higgins 2003). Values of I² under 40% indicate a low level of heterogeneity and justify the use of a fixed‐effect model for meta‐analysis. Values of I² between 30% and 60% are considered to indicate moderate heterogeneity and a random‐effects model would have been used. Values of I² higher than 60% indicate a high level of heterogeneity; in which case meta‐analysis would not be appropriate. We planned to report whether statistical, methodological and clinical heterogeneity were present (Deeks 2011).
Assessment of reporting biases
If a sufficient number of studies had been eligible for inclusion (more than 10), we planned to use funnel plots to assess for the potential existence of small study bias. There are a number of explanations for the asymmetry of a funnel plot and we planned to interpret the results (Egger 1997).
Data synthesis
In the absence of heterogeneity, we planned to use a fixed‐effect model for meta‐analysis. If statistical heterogeneity was moderate (I² between 30% and 60%), we planned to use a random‐effects model. In the presence of substantial statistical heterogeneity between studies, we planned to present a narrative summary (O'Rourke 1989).
'Summary of findings' tables
We planned to present the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined and the sum of available data for the main outcomes (Schunemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach. The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schunemann 2011b). We planned to present the following outcomes in the 'Summary of findings' tables.
-
Time to complete ulcer healing.
-
Rate of changes in the area of the ulcer in the trial period.
-
Proportion of ulcers healed in the trial period.
-
Major bleeding (haemorrhagic stroke, gastric bleeding, any bleeding requiring blood transfusion, any bleeding causing hospitalisation).
-
Mortality from any cause.
-
Any adverse events (e.g. renal failure, neutropenia, low platelets level, gastric complaints, diarrhoea, skin rash, minor bleeding).
-
Ulcer recurrence (time to recurrence and proportion of people with recurrence).
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors on the effect of aspirin:
-
repeating the analysis excluding unpublished studies;
-
repeating the analysis taking into account risk of bias: we planned to exclude those studies at high risk of bias (i.e. those lacking adequate sequence generation, unclear allocation concealment and no blinding of outcome assessment).
We also planned to test the robustness of the results by repeating the analysis using different measures of effect size (risk ratio, odds ratio, etc.) and different statistical models (fixed‐effect and random‐effects models; (Deeks 2011).
Results
Description of studies
Results of the search
The electronic search identified 62 studies; no studies were found through additional searching strategies (such as contact with experts and checking the references of studies). After the analysis of title and abstracts, we excluded 59 studies as they did not meet the inclusion criteria.Three RCTs were selected for full‐text analysis: Layton 1994; Ibbotson 1995 and del Río Solá 2012. After the full‐text analysis, two RCTs were included in the review (Layton 1994; del Río Solá 2012). Ibbotson 1995 used the same people and data as the Layton RCT but compared them with a control group of healthy people, so we excluded this trial (Figure 2).
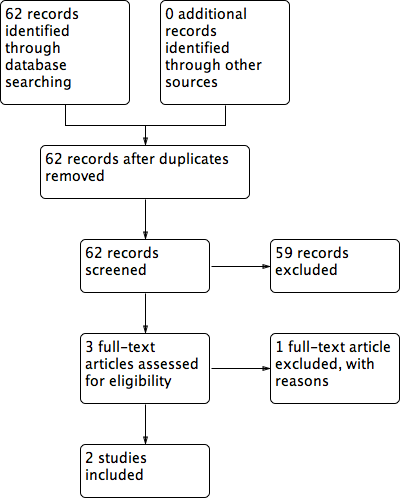
Flow diagram of included and excluded studies
Included studies
Details of the included studies are summarised in Characteristics of included studiesTable 1.
Layton 1994 reported a single‐centre parallel RCT (20 participants) that evaluated daily administration of oral aspirin (300 mg) in addition to compression, compared to placebo and compression. Participants were recruited from the Academic Unit of Dermatology, General Infirmary at Leeds, West Yorkshire, UK. del Río Solá 2012 reported a single‐centre parallel RCT (51 participants) that evaluated daily administration of oral aspirin (300 mg) in addition to compression compared to compression only and recruited from the Department of Angiology and Vascular Surgery, University Hospital of Valladolid, Spain, from December 2001 to September 2005.
Layton 1994 was a prospective trial that included 20 people with venous leg ulcers 2 cm or larger, divided into two parallel groups: 10 participants received oral aspirin (300 mg/day) plus compression therapy compared with 10 who received placebo plus compression therapy.
The del Río Solá 2012 study was a prospective trial that included 51 people with venous leg ulcers 2 cm or larger, divided into two parallel groups: 23 participants received oral aspirin (300 mg/day) plus compression therapy compared with 28 who received compression therapy alone.
The source of funding was not reported for either trial.
Both RCTs used the same intervention (oral aspirin 300 mg/day). The Layton 1994 study randomized participants to a placebo as an adjunct to compression therapy in the control group, while the del Río Solá 2012 study randomized those in the control group to compression therapy alone (no placebo). Compression therapy consisted of high compression (Setopress) in the Layton 1994 RCT. The del Río Solá 2012 RCT did not specify what type of compression was used, although the trialists reported the application of a two‐layer system consisting of one layer of padding and one layer of compression bandage.
The Layton 1994 trial followed participants for four months and then stopped the trial. The results were expressed in terms of reduction of the ulcers' size (surface area cm²) and proportions of ulcers completely healed in the trial period.
The del Río Solá 2012 trial followed participants until their ulcers had healed completely. The results were expressed in terms of proportion of healed ulcers in each group and average time for ulcer healing. . After healing, they continued to follow participants to analyse the proportion of ulcer recurrence and time until ulcer recurrence.
Excluded studies
We excluded the Ibbotson 1995 trial. This trial used the same participants reported in Layton 1994 and compared them with a control group of healthy people without venous leg ulcers. The objective was to evaluate some haemostatic parameters in people with venous leg ulcers taking oral aspirin (see Characteristics of excluded studies).
Ongoing studies
We identified three ongoing studies which evaluate the benefits and harms of aspirin in people with VLUs. (Characteristics of ongoing studies).
Low dose aspirin for venous leg ulcers in NZ (Aspirin4VLU) (NCT02158806) is a prospective, randomised, double blinded, 2 groups in parallel study that will evaluate whether aspirin (150 mg) once daily for up to 24 weeks improves time to healing when compared to placebo once daily for up to 24 weeks as an adjunct to compression. NCT02333123 is leading a phase II randomised, double blind, parallel group, placebo‐controlled efficacy trial taking place in the UK. The Aspirin for Venous Ulcer Randomised Trial (AVURT) will evaluate if aspirin 300 mg once daily for up to 27 weeks added to compression improves time to healing when compared to placebo once daily for up to 27 weeks. ACTRN12614000293662 is investigating the clinical effectiveness of aspirin as an adjunct to compression therapy in healing chronic venous leg ulcers: a randomised double‐blinded placebo‐controlled trial in Australia. The ASPiVLU study will compare if a daily dose of 300 mg enteric coated aspirin as an adjunct to compression for 24 weeks improves time to healing when compared to a placebo. Recruitment has commenced for all three trials.
Risk of bias in included studies
See Figure 1 and summary of findings Table for the main comparison.
Allocation
The Layton 1994 trial was described as 'randomized' but did not report the method of randomizations or the concealment of allocation. In del Río Solá 2012, randomization was undertaken by an independent researcher using a computer program, but allocation concealment was not described.
Blinding
The Layton 1994 trial compared oral aspirin with placebo, and the trial was described as 'double‐blinded', but the trialists did not describe their methods. The evaluation of ulcer size was conducted by planimetry of photographs taken in standardized conditions, so we judged this as low risk of bias.
The del Río Solá 2012 trial did not use a placebo but compared intervention (aspirin) with no intervention. In this scenario, blinding of participants and personnel was not possible. The study reported information about ulcer development (size, epithelization) weekly in a specific data sheet, but they did not describe who did what and how this work was done, so we judged this study as high risk of bias.
Incomplete outcome data
The Layton 1994 trial did not have any withdrawals. The del Río Solá 2012 trial reported four withdrawals, two from each group. Two of these four participants were withdrawn because they needed to be in hospital, but the cause and group assignments were not reported.
Selective reporting
As there were several inconsistencies in the del Río Solá 2012 report, we assigned the study a low risk of reporting bias. Time for complete ulcer healing in the control group, which was stated as 22 weeks in the text but as 16.5 weeks in the table (Table I of the study); errors with the numbers of people in each group, which were reported as 23 for the aspirin group and 28 for the control group in the main text, but appeared as 20 and 31 in the table; and the proportion of people completely healed was reported as 0.73% rather than 75% (21 out of 28 people) for the control group and 0.75% rather than 74% (17 out of 23 people) for the treatment group.
As the results of both prespecified primary outcomes were reported in Layton 1994, we judged it to be free of selective reporting.
Other potential sources of bias
The Layton 1994 trial was stopped in the fourth month. In that time, 38% of ulcers had healed in the aspirin group, but no ulcers had healed in the placebo group. However, the time period could be considered too short, as the assumption that all ulcers could heal in this period may lead to misinterpretation (i.e. some ulcers may have healed in the control group given more time).
In the del Río Solá 2012 trial, despite an appropriate method of randomizations (an independent researcher and computer program), the generated groups were different in relation to the length of time that people had ulcers before treatment. 'Young' ulcers predominated in the aspirin group, and may have had a tendency to heal faster than chronic or 'older' ulcers. Also, the trial authors did not determine a specific trial period. To calculate the size of the study, they used an expected difference of 45% between groups, but did not specify whether the difference was in the proportion of people with healed ulcers, the area of the ulcers or the time for healing.
Effects of interventions
See: Summary of findings for the main comparison Oral aspirin for venous leg ulcers
Both included RCTs analysed the ulcer healing time, but reported it using different outcome measures. The interventions that were studied in the two trials that reported relevant outcomes for this review were too heterogenous to allow pooling of outcomes data. We therefore reported the trial results for each trial separately.
Primary outcomes
Time to complete ulcer healing
The del Río Solá 2012 trial followed the people until the healing of their ulcers and reported the average time for healing was 12 weeks in the aspirin group and 22 weeks in the control group, P values and confidence intervals were not reported Table 1.
Rate of changes in the area of the ulcer in the trial period
The Layton 1994 stopped the trial in the fourth month and reported this outcome: by four months, ulcer area had reduced (by 6.5 cm², a 39.4% reduction) in the aspirin group compared with no reduction in ulcer area in the control group (P value < 0.002; summary of findings Table for the main comparison).
Proportion of ulcers healed in the trial period
The Layton 1994 trial reported this outcome: a higher proportion of the ulcers (38%) in the aspirin group had completely healed in the trial period compared with none in the control group (P value < 0.007; summary of findings Table for the main comparison).
Proportion of people with ulcers healed in the trial period
The del Río Solá 2012 trial reported that there was no real difference between the proportion of people with ulcers healed in the aspirin group (74%, 17 out of 23 people) and in the control group (75%, 21 out of 28 people; Table 1), (RR 0.99, 95% CI 0.71 to 1.36; Analysis 1.1).
Major bleeding (haemorrhagic stroke, gastric bleeding, any bleeding requiring blood transfusion, any bleeding causing hospitalisation)
The del Río Solá 2012 trial reported two hospitalisations, in addition to two other withdrawals, but did not specify the cause or the group assignment of the participants who withdrew (Table 1).
The Layton 1994 trial reported that no side effects were experienced (Table 1).
Secondary outcomes
Pain relief
Neither study reported pain relief.
Mortality
The Layton 1994 trial reported that no side effects were experienced.
The del Río Solá 2012 trial reported that two people needed hospitalisation and had to be withdrawn from the trial (in addition to two other withdrawals), but they didn't report the reason for hospitalisation or their group assignment. The authors did not report other adverse events.
Adverse events
The Layton 1994 trial reported that participants had no adverse effects, but did not explain how this was established. The del Río Solá 2012 trial did not report adverse events.
Ulcer recurrence
The del Río Solá 2012 trial reported the average time of ulcer recurrence was 16.33 days (SD: 7.5) in the control group and 39 days (SD: 6.0) in the aspirin group (P = 0.007).
Layton 1994 did not report recurrence.
Discussion
Summary of main results
Aspirin has been used as medication since 1899 and its pharmacodynamic properties as an anti‐inflammatory, analgesic, antipyretic, and inhibitor of both platelet aggregation and biosynthesis of prostaglandins, have been well studied. VLUs are associated with venous stasis, inflammation and necrosis; so some of the properties of aspirin may be useful for patients with VLUs. We searched extensively in seven different databases, using an appropriate search strategy for each one, to identify studies that had analysed the effect of aspirin on healing of VLUs. This review intended to summarize the effect of oral aspirin in treatment of VLUs; our searches identified no previous reviews and only two clinical trials that could be included, del Río Solá 2012 with 51 participants, and Layton 1994 with 20 participants. Both studies used the same intervention (oral aspirin 300 mg/day) and had the objective of evaluating the effects of oral aspirin on the healing of VLUs, however they used different outcome measurements. The Layton 1994 trial compared the average reduction in ulcer area in the second and fourth months, while the del Río Solá 2012 trial compared the average time for complete healing of the ulcer. The conclusions of both studies favoured the aspirin group: Layton 1994 showed a significant reduction in ulcer area in the fourth month of treatment and del Río Solá 2012 showed a significant reduction in the time required for complete healing of ulcers. However the two studies included very few participants, a total of 71, and the differences in the outcomes measurements prevented meta‐analysis. The evidence was downgraded to low quality due to the potential for selection bias and imprecision in the results, thus there is uncertainty around the effect estimates.
Overall completeness and applicability of evidence
There was scant evidence available to assess the benefits of 300,mg aspirin to heal people with venous leg ulcers.The small number, small size and differing outcome measurements of the two included trials meant that there was insufficient evidence for us to draw meaningful conclusions about the use of oral aspirin in the treatment of VLUs.
The benefits and harms of aspirin varies with its plasmatic level. Aspirin blood concentration from 50 to 300 mcg/ml are sufficient for most of its therapeutic effects and doses greater than 200 mcg/ml increase the risks for adverse events. Nowadays aspirin is used to treat many vascular conditions such as, ischemic stroke, angina pectoris, myocardial infarction, revascularization procedures, etc, with doses that vary from 50 to 325 mg/day. Both studies included in this review used 300 mg aspirin daily, however, the ideal dose for each clinical condition remains in debate (Cappelleri 1995; Dalen 2006).
Layton 1994, did not report any characteristics of the population included in his study; del Río Solá 2012 included 51 patients, 29 women and 22 men with mean age of 60 years ranging from 36‐86 years old. The study excluded patients with co morbidities such as diabetes mellitus, rheumatoid arthritis, peripheral arterial disease and neurologic disease. The characteristics of this population is in concordance with the populations described in the epidemiologic studies of chronic venous insufficiency (Beebe‐Dimmer 2005).
Quality of the evidence
Only low quality evidence was available from two trials and both the RCTs that were eligible for inclusion in the review had an unclear or high risk of bias for most domains (allocation concealment, blinding of participants and personnel, incomplete outcome data and other biases). Due to the lack of reliable evidence, we are unable to draw conclusions about the benefits and harms of oral daily aspirin as an adjunct to compression in VLU healing or recurrence. We graded the overall quality of the evidence as low, indicating that future research is likely to have an important influence on the effect estimates.
Potential biases in the review process
We are confident that the broad literature search used in this review has captured most of the relevant literature, and minimised the likelihood that we have missed any relevant trials. Two review authors independently selected trials, extracted data, and assessed risk of bias, in order to minimise bias. Due to the differences in reporting of outcome measures between the trials, we could not conduct the planned meta‐analysis.
Agreements and disagreements with other studies or reviews
This is the first review of randomised studies that address this question, as far as we are aware. The treatment of VLUs can be frustrating for physicians and people with ulcers because these kinds of ulcers have a tendency to recur and become chronic. Some therapies are already well established, such as compression and rest with elevated legs (O'Meara 2012). On the basis of recent research that showed the importance of inflammation in the development of ulcers, new therapies using anti‐inflammatory drugs have been evaluated, including pentoxifylline (Jull 2007), prostaglandins (Rudofsky 1989), and prostacyclin analogues (Werner‐Schlenzka 1994). These studies often describe benefits, but like the studies included in this review, they have only included small numbers of participants.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Flow diagram of included and excluded studies

Comparison 1 Oral aspirin versus control, Outcome 1 Number of people with healed ulcer.

Comparison 1 Oral aspirin versus control, Outcome 2 Time to recurrence (days).
| Oral aspirin for venous leg ulcers | ||||||
| Patient or population: patients with venous leg ulcers | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral aspirin | |||||
| Average time for ulcer healing | 22 weeks | 12 weeks | Not estimable | 51 (1 study) | ⊕⊕⊝⊝ | P values and confidence intervals were not reported |
| Reduction of ulcer area (median) | 0 cm² | 6.5cm² | Not estimable | 20 | ⊕⊕⊝⊝ | P value < 0.002 Follow‐up: 4 months |
| Proportion of healed ulcers in the trial period | No healed ulcers | 38% of healed ulcers | Not estimable | 20 | ⊕⊕⊝⊝ | P value < 0.007 Follow‐up: 4 months |
| Major bleeding | See comment | See comment | Not estimable | 20 | ⊕⊕⊝⊝ | No events were observed in either group, follow‐up: 4 months Another study reported 2 hospitalisations for unknown reasons, intervention group not specified |
| Average time of ulcer recurrence | 16.33 days SD: 7.5 | 39 days SD: 6.0 | Not estimable | 51 | ⊕⊕⊝⊝ | P value = 0.007 Post hoc assessment not pre‐specified in protocol |
| Mortality | See comment | See comment | Not estimable | See comment | See comment | Mortality not reported |
| Other adverse events | See comment | See comment | Not estimable | 71 | See comment | No events were observed in either group. del Río Solá reported 2 hospitalisations for unknown reasons, the group of these patients were not specified and they were removed from the study |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Allocation concealment and blinding of outcome assessment were not described. Participants and personnel were not blinded. There was a high risk of bias from incomplete outcome data.There were some inconsistencies in the reporting of the data | ||||||
| Study identification | Layton | del Río Solá | ||||
| Country | United Kingdom | Spain | ||||
| Period | Not reported | 2001 to 2005 | ||||
| Centres | Academic Unit of Dermatology, General Infirmary at Leeds, West Yorkshire | University Hospital of Valladolid | ||||
| Source of funding | Not specified | Not specified | ||||
| Method | ||||||
| Study design | Prospective randomized, double‐blind | Prospective randomized trial | ||||
| Power calculation | Not described | Yes | ||||
| Method of randomisation | Not described | Generated by computer program | ||||
| Concealment of allocation | Not described | Not described | ||||
| Number of participants randomized | 20 | 51 | ||||
| Number of participants analyzed | 20 | 47 | ||||
| Number of participants excluded after randomizations | 0 | 0 | ||||
| Number of participant withdrawals and reasons | 0 | 4 people; 2 people needed hospitalisation and left the study and 2 people opted for treatment in another service | ||||
| Intention‐to‐treat analysis | Yes | Yes | ||||
| Participants | ||||||
| Inclusion criteria | People with chronic venous leg ulcer | Venous leg ulcer ≥ 2 cm Ankle‐brachial rate < 0.9 No contraindication to taking aspirin | ||||
| Exclusion criteria | Ulcer diameter < 2 cm Already taking aspirin, anticoagulants or non‐steroidal anti‐inflammatory Doppler flowmetry ankle‐brachial rate < 0.9 | People with diabetes mellitus, rheumatoid arthritis, peripheral arterial disease and neurologic disease Previous or concomitant therapy with aspirin | ||||
| Aspirin | Control | P value | Aspirin | Control | P value | |
| Number of participants | 10 | 10 |
| 23 | 28 |
|
| Age (years) | 62.2 years (mean) (48‐81) | 66 years (mean) (46 ‐ 85) |
| 60.50 years (SD:12.07) | 58.59 years (SD:16.55) | reported as non significant |
| Sex | 3 female, 7 male | 5 female, 5 male |
| 10 female, 13 male | 19 female, 9 male | reported as non significant |
| Ulcer duration before the study | 11.4 years (mean) (1‐24) | 10.5 years (mean) (2‐22) |
| 6‐12 months | > 12 months |
|
| 1 Number of ulcers | Not reported | Not reported | Not reported | Not reported | reported as non significant | |
| Initial ulcer surface area (cm²) | 16.5 cm² (mean) (2.5‐39.5) | 14.25 cm² (mean) (1.5‐48.5) | 25.15 cm² | 24.87 cm² | P=0.944 | |
| Signs of ulcer infection | Not reported | Not reported | Yes, 20 patients | Yes, 22 patients | P=0.094 | |
| Any comorbidity | Not reported | Not reported | 9 patients | 10 patients | ||
| Previously treated | \not reported | Not reported | 10 patients | 20 patients | ||
| Interventions | Aspirin 300 mg/day | Placebo | Aspirin 300 mg/day | No drug treatment | ||
| Outcomes | ||||||
| Follow‐up (months) | 4 months | 4 months | 42 months mean (24‐61) | 42 months mean (24‐61) | ||
| 2 Withdrawals | 0 | 0 | 2 people | 2 people | ||
| Duration of the study to complete ulcer healing | Not reported | Not reported | Not reported | 12.4 weeks | 16.5 weeks | P=0.07 Mann‐Whitney |
| Healing period | Not reported | Not reported | Not reported | Reported as short in the aspirin group | Reported as short in the aspirin group | P=0.04 log‐rank test = 3.90 OR = 0.93 95% CI 0.25‐3.5 |
| Average time to complete ulcer healing (weeks) | Not reported | Not reported | Not reported | 12 | 22 | Not reported |
| Number of participants with complete ulcer healing in the trial period | Not reported | Not reported | Not reported | 17 (74%) | 21 (75%) | reported as non significant |
| Proportion of ulcers healed in the trial period | 38% of the ulcers | 0% of the ulcers | < 0.007 (x² test) | Not reported | Not reported | Not reported |
| Change in ulcer areas in the trial period (second month; ulcer area cm²) | 15.5 cm² (median) 1 cm² of reduction (6.07% of reduction) | No reduction | < 0.01 (x² test) | Not reported | Not reported | Not reported |
| Reduction in ulcer size in the trial period (fourth month; ulcer area cm²) | 10.0 cm² (median) 6.5 cm² of reduction (39.4% of reduction) | No reduction | < 0.002 (x² test) | Not reported | Not reported | Not reported |
| Improvement assessed by reduction in ulcer size | 52% of the ulcers | 26% of the ulcers | < 0.007 (x² test) | Not reported | Not reported | Not reported |
| Increase in ulcer size in the trial period | 10% of the ulcers | 26% of the ulcers | < 0.004 (x² test) | Not reported | Not reported | Not reported |
| Ulcers size unchanged in the trial period | 0% of the ulcers | 48% of the ulcers | < 0.001 (x2 test) | Not reported | Not reported | Not reported |
| Proportion of participants with ulcers healed in the trial period | Not reported | Not reported | Not reported | 17 (74%) | 21 (75%) | reported as non significant |
| Proportion of participants with ulcer recurrence | Not reported | Not reported | Not reported | 25% | 33.33% | 0.74 |
| Average time for ulcer recurrence (days) | Not reported | Not reported | Not reported | 39 (SD 6) | 16.33 (SD 7.5) | P=0.007 Kaplan‐Meier |
| adverse effects | 0 | 0 | 0 | 0 | Not reported | |
|
| ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of people with healed ulcer Show forest plot | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.71, 1.36] |
| 2 Time to recurrence (days) Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 22.67 [18.96, 26.38] |

