رسپریدون در درمان رفتار تهاجمی (aggression) یا تحریکپذیری و بیقراری (agitation) ایجاد شده توسط سایکوز (برقراری سریع آرامش)
چکیده
پیشینه
رفتار تهاجمی (aggressive) تحریکپذیر (agitated) یا خشونتآمیز (violent) به دلیل سایکوز یک اورژانس روانپزشکی است که نیازمند مداخلات سریعالاثر است. رسپریدون (risperidone) یک آنتیسایکوتیک با دسترسی گسترده است که میتواند برای مدیریت رفتار تهاجمی یا تحریکپذیری ایجاد شده توسط سایکوز استفاده شود.
اهداف
بررسی اینکه رسپریدون خوراکی به تنهایی برای درمان رفتار تهاجمی یا تحریکپذیری ایجاد شده توسط سایکوز درمان موثری است یا خیر.
روشهای جستوجو
ما پایگاه ثبت کارآزماییهای مطالعهمحور گروه اسکیزوفرنی در کاکرین را جستوجو کردیم (تا اپریل 2017)، این پایگاه ثبت، مرکز گردآوری پژوهشهای سیستماتیک منابع اصلی (شامل AMED؛ BIOSIS CINAHL؛ Embase؛ MEDLINE؛ PsycINFO؛ PubMed و پایگاههای ثبت کارآزماییهای بالینی) و نسخههای بهروز شده ماهانه آنها، جستوجوهای دستی (handsearches)، منابع علمی خاکستری و خلاصه مقالات کنفرانسها است. هیچ محدودیت زبانی، تاریخی، نوع مقاله یا وضعیت انتشار برای انتخاب رکوردها درون ثبتها لحاظ نکردیم.
معیارهای انتخاب
معیار انتخاب ما کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) بود که در آنها این مقایسه صورت گرفته بود: مقایسه استفاده سریع از رسپریدون با داروهای دیگر، ترکیبی از داروها یا دارونما (placebo) در افرادی که رفتارهای تهاجمی یا تحریکپذیر (یا هردو) را در جریان سایکوز دارند.
گردآوری و تجزیهوتحلیل دادهها
ما بهطور مستقل از یکدیگر، همه استنادهای پژوهشها را بازرسی کردیم، سپس چکیدههای مرتبط را شناسایی و بهطور مستقل از یکدیگر دادهها را از مطالعات وارد شده استخراج کردیم. خطر نسبی (RR) را برای دادههای باینری و تفاوت میانگین (MD) را برای دادههای پیوسته با 95% فواصل اطمینان (CI) و با استفاده از مدل اثر‐ثابت محاسبه کردیم. خطر سوگیری (bias) را برای مطالعات وارد شده ارزیابی کرده و از رویکرد درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE) برای ایجاد جدول «خلاصه یافتهها» استفاده کردیم.
نتایج اصلی
این مرور شامل گزارش دادههایی از نه کارآزمایی (جمعا 582 نفر) بر اساس پنج مقایسه است. به دلیل خطر سوگیری، اندازه کوچک کارآزماییها، غیر‐مستقیم بودن معیارهای پیامد و کم بودن پیامدهای عملی (pragmatic) گزارش شده شواهد با کیفیت بسیار پائین بود. هیچ یک از مطالعات وارد شده دادههای قابل استفاده را برای پیامد اولیه ما یعنی «آرامشبخشی یا خوابیدن» (tranquillisation or asleep) در عرض 30 دقیقه، نیاز به تکرار آرامشبخشی یا هر پیامد اقتصادی دیگر نداشتند. دادهها برای پیامدهای اصلی دیگر مانند تحریکپذیری یا رفتار تهاجمی، نیاز به کنترلکنندهها (needing restraint) برای پیشگیری از آسیب زدن و بروز عوارض جانبی موجود بودند.
رسپریدون در برابر هالوپریدول (تا 24 ساعت پیگیری)
برای پیامد رفتار خاص تحریکپذیری هیچ تفاوت آشکاری بین رسپریدون و هالوپریدول از نظر اثربخشی به صورت اندازهگیری کاهش حداقل 50% در مقیاس سندرم منفی و مثبت با زیر‐مقیاس تحریکپذیری سایکوتیک (Positive and Negative Syndrome Scale ‐ Psychotic Agitation Sub‐score; PANSS‐PAS) مشاهده نشد (RR: 1.04؛ 95% CI؛ 0.86 تا 1.26؛ 124 شرکتکننده؛ 1 مطالعه؛ شواهد با کیفیت بسیار پائین) و همچنین هیچ تاثیری برای نیاز به استفاده از کنترلکنندهها (restraints) مشاهده نشد (RR: 2.00؛ 95% CI؛ 0.43 تا 9.21؛ 28 شرکتکننده؛ 1 مطالعه؛ شواهد با کیفیت بسیار پائین). بروز عوارض جانبی بین گروههای درمانی مشابه بود (RR: 0.94؛ 95% CI؛ 0.54 تا 1.66؛ 124 شرکتکننده؛ 1 مطالعه؛ شواهد با کیفیت بسیار پائین).
رسپریدون در برابر اولانزاپین (olanzapine)
یک مطالعه کوچک (29 نفر)، دادههای قابل استفادهای را در مقایسه رسپریدون در برابر اولانزپین در برداشت. هیچ تاثیری در این موارد مشاهده نشد: تحریکپذیری با اندازهگیری نمره نقطه پایان PANSS‐PAS در دو ساعت (MD: 2.50؛ 95% CI؛ 2.46‐ تا 7.46؛ شواهد با کیفیت بسیار پائین)، نیاز به کنترل کنندهها در چهار روز (RR: 1.43؛ 95% CI؛ 0.39 تا 5.28؛ شواهد با کیفیت بسیار پائین)، و اختلالات حرکتی خاص بر اساس نمره نقطه پایانی اندازهگیری «مقیاس درجهبندی فعالیت رفتاری» (Behavioural Activity Rating Scale; BARS) در چهار روز (MD: 0.20؛ 95% CI؛ 0.43‐ تا 0.83؛ شواهد با کیفیت بسیار پائین).
رسپریدون در برابر کوئتیاپین (quetiapine)
یک مطالعه (40 = n) دادههای قابل استفادهای را در مقایسه رسپریدون با کوئتیاپین دربرداشت. رفتار تهاجمی با استفاده از نمره نقطه پایانی مقیاس تهاجم آشکار تغییر یافته (Modified Overt Aggression Scale; MOAS) در دو هفته اندازهگیری شد. تفاوت آشکاری به نفع کوئتیاپین مشاهده شد (MD: 1.80؛ 95% CI؛ 0.20 تا 3.40؛ شواهد با کیفیت بسیار پائین). هیچ شواهدی برای تفاوت میان گروههای درمانی از نظر بروز آکاتیژیا (akathisia) بعد از 24 ساعت وجود نداشت (RR: 1.67؛ 95% CI؛ 0.46 تا 6.06؛ شواهد با کیفیت بسیار پائین). دو شرکتکننده که رسپریدون دریافت میکردند و یک شرکتکننده که کوئتیاپین میگرفت، در جریان کارآزمایی، دچار ایسکمی قلبی شدند.
رسپریدون در برابر رسپریدون + اکسکاربازپین (oxcarbazepine)
در یک کارآزمایی، تحریکپذیری با استفاده از نمره نقطه پایانی مقیاس سندرم مثبت و منفی – مولفه برانگیخته (Positive and Negative Syndrome Scale ‐ Excited Component) اندازهگیری شد و یک تفاوت آشکار به نفع ترکیب درمانی (رسپریدون + اکسکاربازپین) در یک هفته مشاهده شد (MD: 2.70؛ 95% CI؛ 0.42 تا 4.98؛ شواهد با کیفیت بسیار پائین) اما هیچ تاثیری برای وضعیت کلی با استفاده از نمره نقطه پایانی بهبود در احساس کلی بالینی مشاهده نشد (MD: ‐0.20؛ 95% CI؛ 0.61‐ تا 0.21؛ شواهد با کیفیت بسیار پائین). بروز نشانههای اکستراپیرامیدال (extrapyramidal) بعد از 24 ساعت بین گروههای درمانی مشابه بود (RR: 1.59؛ 95% CI؛ 0.49 تا 5.14؛ شواهد با کیفیت بسیار پائین).
رسپریدون در برابر رسپریدون + والپروئیک اسید (valproic acid)
در دو کارآزمایی رسپریدون با ترکیب رسپریدون و والپروئیک اسید مقایسه شده بود. هیچ تفاوت آشکاری بین گروههای درمانی از نظر رفتار تهاجمی (نمره نقطه پایانی MOAS در سه روز: MD: 1.07؛ 95% CI؛ 0.20‐ تا 2.34؛ 54 شرکتکننده؛ 1 مطالعه؛ شواهد با کیفیت بسیار پائین) یا بروز آکاتیژیا بعد از 24 ساعت: (RR: 0.75؛ 95% CI؛ 0.28 تا 2.03؛ 122 شرکتکننده؛ 2 مطالعه؛ شواهد با کیفیت بسیار پائین) مشاهده نشد.
نتیجهگیریهای نویسندگان
به طور کلی، نتایج برای پیامدهای اصلی نشان میدهد که رسپریدون هیچ تاثیر واقعی ندارد. تنها دادههای موجود برای استفاده در این مرور، از نه کارآزمایی با تعداد نمونه کم به دست آمد و شواهد موجود کیفیت بسیار پائینی داشتند. این نکته نشان دهنده عدم قطعیت درباره نقش رسپریدون در آرامشبخشی سریع در افراد مبتلا به رفتار تهاجمی ناشی از سایکوز است. RCTهای عملی با کیفیت بالا امکانپذیر است و باید پیش از توصیه برای استفاده از رسپریدون در مدیریت تحریکپذیری یا رفتار تهاجمی ناشی از سایکوز انجام شوند.
PICOs
خلاصه به زبان ساده
رسپریدون به مثابه آرامش دهنده افراد مبتلا به رفتار تهاجمی یا تحریکپذیری ناشی از سایکوز (psychosis)
پیشینه
افراد مبتلا به سایکوز دچار وضعیت شنیدن صدا (توهم) یا تفکرات غیر‐نرمال میشوند، این نشانهها میتواند افراد را وحشتزده (frightened)، پریشان (distressed) و تحریکپذیر کند. تجربه چنین هیجاناتی میتواند گاهی موجب رفتار تهاجمی شود. این موضوع، چالش و وضع دشواری را برای پزشکان ایجاد میکند. متخصصین سلامت روان باید تشخیص دهند و بهترین درمان موجود را برای پیشگیری از خطر آسیب به بیمار و/یا دیگران به کارببرند، درمان سریعتر بهتر است. رسپریدون (risperidone) یک داروی خوراکی است و به طور گستردهای برای درمان و مدیریت نشانههای سایکوز استفاده میشود. این دارو به مثابه یک آنتیسایکوتیک (پیشگیری از سایکوز) باعث آرامش افراد میشود یا کمک میکند آنها به خواب روند.
هدف مطالعه مروری
این مرور به بررسی این موضوع پرداخت که رسپریدون میتواند یک درمان سریع و موثر در افراد مبتلا به رفتارهای تهاجمی یا تحریکپذیر ناشی از سایکوز باشد یا خیر.
جستوجوها
متخصص اطلاعات گروه اسکیزوفرنی در کاکرین جستوجو را در پایگاه ثبت تخصصی برای یافتن کارآزماییهای تصادفیسازی شده انجام داد، در این کارآزماییها رسپریدون به تنهایی، با دارونما (placebo) یا درمانهای دیگر در افراد مبتلا به رفتار تهاجمی یا تحریکپذیر ناشی از سایکوز بررسی شده بود. آخرین زمان جستوجو اپریل 2017 بود.
نتایج
نه مطالعه با 582 شرکتکننده در این مرور وارد شدند، اما اطلاعات به دست آمده کیفیت ضعیفی داشتند و فقط اطلاعاتی را به طور نسبی برای هدف اصلی این مرور فراهم میکردند، خصوصا فقدان اطلاعات مربوط به تاثیرات آرامبخشی سریع (یعنی زیر یک ساعت بعد از درمان) و نیاز به تکرار آرامبخشی مشهود بود. دادههای اقتصادی نیز گزارش نشده بود. در کارآزماییها رسپریدون با آنتیسایکوتیکهای دیگر مقایسه شده بود، این آنتیسایکوتیکها شامل هالوپریدول (haloperidol)، الانزاپین (olanzapine) و کوئتیاپین (quetiapine) بود. در این مرور مشخص شد که برای آرام کردن رفتار تهاجمی، رسپریدون بهتر یا بدتر از هالوپریدول درون 24 ساعت و دو هفته بعد از درمان نیست، افرادی که رسپریدون دریافت میکردند بر اساس مقیاسهای سنجش رفتار تهاجمی نمره بالاتری از افراد مصرف کننده کوئتیاپین داشتند. با این حال، هر دوی این نتایج دارای شواهد با کیفیت بسیار پائین رتبهبندی شدند. در یک مطالعه کوچک مشخص شد که ترکیب رسپریدون و اکسکاربازپین (oxcarbazepine) در کاهش تحریکپذیری بهتر از رسپریدون به تنهایی است، اما گردآوری این دادهها بعد از یک هفته بوده و باز هم شواهد کیفیت بسیار پائین داشتند. هیچ تفاوت آشکاری در بروز عوارض جانبی مانند اختلالات حرکتی مشاهده نشد.
نتیجهگیریها
نویسندگان این مرور نتیجه گرفتند که تا این لحظه شواهد نامشخص و ضعیف درباره استفاده از رسپریدون در آرام کردن افراد مبتلا به رفتار تهاجمی ناشی از سایکوز وجود دارد و نمیتوان به نتیجهگیریهای قطعی دست یافت. بنابراین هیچ نتیجه محکم و استواری نمیتوان گرفت و راهنمای واضح و مبتنی بر شواهد برای متخصصین سلامت و افراد مبتلا به مشکلات سلامت روان وجود ندارد. با این حال، کارآزماییهای با کیفیت خوب امکانپذیر است و تحقیقات بیشتر نیاز است تا به افراد مبتلا به رفتار تهاجمی ناشی از سایکوز برای یافتن داروی بهتر برای آرامبخشی کمک کند؛ دارویی که سریع عمل میکند و عوارض جانبی کمتری داشته باشد.
Authors' conclusions
Summary of findings
| RISPERIDONE compared to OTHER ANTIPSYCHOTIC: a. HALOPERIDOL for psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Patient or population: psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTIC: a. HALOPERIDOL | Risk with RISPERIDONE | |||||
| Tranquillisation or asleep by 30 minutes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Repeated need for tranquillisation within 24 hours ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Specific behaviour: agitation, up to 24 hours (PANSS‐PAS response) | Study population | RR 1.04 | 124 | ⊕⊝⊝⊝ | ||
| 758 per 1.000 | 788 per 1.000 | |||||
| Global outcome: need for additional measures | Study population | RR 2.00 | 28 | ⊕⊝⊝⊝ | ||
| 143 per 1.000 | 286 per 1.000 | |||||
| Adverse effects: up to 24 hours | Study population | RR 0.94 | 124 | ⊕⊝⊝⊝ | ||
| 290 per 1.000 | 273 per 1.000 | |||||
| Economic outcomes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias ‐ rated 'very serious': high risk of performance and 'selective reporting' bias. Definition of the outcomes are not consistent between the multiple publications. 2 Imprecision ‐ rated 'serious': Optimal Information Size (OIS) criterion not met. 3 Only one study available. 4 Risk of bias ‐ rated 'very serious': high risk of attrition bias and 'selective reporting' bias. 5 Indirectness ‐ rated 'serious': provided outcome is at a time point (4 days) different from those of primary importance in this setting. | ||||||
| RISPERIDONE compared to OTHER ANTIPSYCHOTIC: b. OLANZAPINE for psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Patient or population: psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTIC: b. OLANZAPINE | Risk with RISPERIDONE | |||||
| Tranquillisation or asleep by 30 minutes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Repeated need for tranquillisation within 24 hours ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Specific behaviour: agitation, up to 2 hours | MD 2.5 higher | ‐ | 29 | ⊕⊝⊝⊝ | ||
| Global outcome: need for additional measures | Study population | RR 1.43 | 29 | ⊕⊝⊝⊝ | ||
| 200 per 1.000 | 286 per 1.000 | |||||
| Adverse effects: movement disorder | MD 0.20 higher | ‐ | 29 | ⊕⊝⊝⊝ | ||
| Economic outcomes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias ‐ rated 'very serious': high risk of attrition bias and ‘selective reporting’ bias. 2 Only one study available. 3 Imprecision ‐ rated 'very serious': Optimal Information Size (OIS) criterion not met. 4 Indirectness ‐ rated 'serious': provided outcome is at a time point (4 days) different from those of primary importance in this setting. | ||||||
| RISPERIDONE compared to OTHER ANTIPSYCHOTIC: c. QUETIAPINE for psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Patient or population: psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTIC: c. QUETIAPINE | Risk with RISPERIDONE | |||||
| Tranquillisation or asleep by 30 minutes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Repeated need for tranquillisation within 24 hours ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Specific behaviour ‐ aggression, over 24 hours | MD 1.80 higher | ‐ | 40 | ⊕⊝⊝⊝ | ||
| Global outcome ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse effects: movement disorders over 24 hours | Study population | RR 1.67 | 40 | ⊕⊝⊝⊝ | ||
| 150 per 1.000 | 251 per 1.000 | |||||
| Economic outcomes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Only one study available. 2 Indirectness ‐ rated 'serious': provided outcome is at a time point (2 weeks) different from those of primary importance in this setting. 3 Imprecision ‐ rated 'very serious': Optimal Information Size (OIS) criterion not met. | ||||||
| RISPERIDONE compared to COMBINATION: a. RISPERIDONE + OXCARBAZEPINE for psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Patient or population: psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATION: a. RISPERIDONE + OXCARBAZEPINE | Risk with RISPERIDONE | |||||
| Tranquillisation or asleep by 30 minutes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Repeated need for tranquillisation within 24 hours ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Specific behaviour: agitation, over 24 hours | MD 2.70 higher | ‐ | 68 | ⊕⊝⊝⊝ | ||
| Global Outcome: average scores, over 24 hours | MD 0.20 lower | ‐ | 68 | ⊕⊝⊝⊝ | ||
| Adverse effects: movement disorders, over 24 hours | Study population | RR 1.59 | 68 | ⊕⊝⊝⊝ | ||
| 114 per 1.000 | 182 per 1.000 | |||||
| Economic outcomes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias ‐ rated 'very serious': high risk for performance bias and detection bias. 2 Only one study available. 3 Indirectness ‐ rated 'serious': provided outcome is at a time point (1 week) different from those of primary importance in this setting. 4 Imprecision ‐ rated 'very serious': Optimal Information Size (OIS) criterion not met. | ||||||
| RISPERIDONE compared to COMBINATION: b. RISPERIDONE + VALPROIC ACID for psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Patient or population: psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATION: b. RISPERIDONE + VALPROIC ACID | Risk with RISPERIDONE | |||||
| Tranquillisation or asleep by 30 minutes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Repeated need for tranquillisation within 24 hours ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Specific behaviour ‐ aggression, over 24 hours | The mean specific behaviour ‐ aggression, over 24 hours was 0 | MD 1.07 higher | ‐ | 54 | ⊕⊝⊝⊝ | |
| Global outcome ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse effects: movement disorders, over 24 hours | Study population | RR 0.75 | 122 | ⊕⊝⊝⊝ | ||
| 66 per 1.000 | 49 per 1.000 | |||||
| Economic outcomes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Only one study available. 2 Indirectness ‐ rated 'serious': provided outcome is at a time point (3 days) different from those of primary importance in this setting. 3 Imprecision ‐ rated 'very serious': Optimal Information Size (OIS) criterion not met. | ||||||
Background
Description of the condition
Aggression has been defined by NICE 2005 as a willingness to inflict harm, whether behavioural or verbally expressed, and regardless of whether physical harm is sustained. Violence has been described as the intentional use of physical force whether threatened or actual, against oneself, another person, a group, or community, that results in, or is likely to result in injury, death, psychological harm, maldevelopment or deprivation (WHO 2002; Wright 2002). Aggression is not a diagnosis in itself, but can be a feature of several mental health conditions. There is a well‐established significant relationship between psychosis and violence (Arseneault 2000; Brennan 2000; Fazel 2006). Agitated or violent behaviour constitutes roughly 10% of all emergency psychiatric treatment (Tardiff 1982). Overall, the prevalence of violence in people who have schizophrenia, major depression or manic/bipolar disorder is about 11% to 13%. An even higher percentage of people with alcoholism (25%) or substance misuse (35%) have, at some stage, presented with violence or aggression. Even when additional factors such as alcohol and drug use are taken into account, psychotic symptoms such as delusions or hallucinations are significantly and strongly associated with aggressive and violent behaviour (Swanson 1990). Low GABA (gamma‐aminobutyric acid) and serotonin levels in various parts of the brain have been suggested to be associated with aggressive behaviour whilst enhanced norepinephrine and dopamine levels with increased aggression (Bazire 2009).
Description of the intervention
There are many guidelines that describe the management of people with aggression and violence. (APA 2004; Addington 2005; NICE 2015). NICE guidelines recommend preventative measures such as observation, de‐escalation and use of p.r.n. (i.e.: pro re nata, as needed) medication should initially be used. If these measures fail to calm the agitated individual, restrictive measures including seclusion and manual/mechanical restraint, may be pursued. Individuals unable or unwilling to consent to treatment may require rapid tranquillisation with lorazepam on its own or intramuscular haloperidol combined with intramuscular promethazine.
Risperidone was the first novel second‐generation antipsychotic and has been widely available since the 1990s (C23H27FN4O2, Figure 1). The main pharmacological activities of risperidone include serotonin 5‐HT2 receptor blockade and dopamine D2 antagonism (Megens 1994), and it has therefore been suggested that atypical antipsychotics could have an anti‐aggressive effect (Buckley 1999). Risperidone is licensed in the UK for the treatment of psychotic conditions in which positive or negative symptoms are prominent, to maintain clinical improvement during continuation therapy in patients who have shown an initial treatment response, and for the treatment of mania in bipolar disorder (BNF 2017).

Risperidone structure
Risperidone and aripiprazole are the only two antipsychotics having FDA (Food and Drug Administration) approval for irritability associated with autistic disorder in children (Mathis 2009). Risperidone is also licensed in the UK for psychosis, persistent aggression in conduct disorder and severe aggression in autism in children (BNF 2017). A Cochrane review on atypical antipsychotics for aggression and psychosis in Alzheimer's disease found that the adverse events associated with risperidone may outweigh the benefits and suggested that risperidone should only be used for treating aggression in those with dementia when there is severe distress or risk of physical harm (Ballard 2006). Risperidone is now licensed in the UK at a low dose for the short‐term management of aggression in Alzheimer dementia which is unresponsive to non‐pharmacological interventions (BNF 2017). Katz 1999 also found that 1 mg/day of risperidone was useful in controlling aggression in severe dementia.
Risperidone has atypical properties especially at lower doses but can become more 'conventional' at high doses (Stahl 2008). Risperidone has been found to be associated with more adverse effects such as extrapyramidal side effects, hyperprolactinaemia and sexual dysfunction than other antipsychotics (Tran 1997). A double‐blind study looking at risperidone use in schizophrenia found that although hyperprolactinaemia is significantly associated with long‐term risperidone use, symptoms related to high prolactin levels are rare (Conley 2001). Risperidone has been found to cause weight gain, but the link is not as significant as with olanzapine (Conley 2001) and clozapine (Wirshing 1999). Respiridone may cause a disproportionate increase in weight gain in adolescents compared to adults, which is a key area in non‐compliance in this group (Fleischhaker 2007). Risperidone was the first atypical antipsychotic that became available in a long‐term depot injectable formulation lasting for two weeks. Such dosage formulations may improve compliance, and if compliance is enhanced, may lead to better long‐term outcomes. The difficulty is patient volunteers are generally co‐operative and studies therefore do not address efficacy of depot injection in the non‐compliant patient population. (Sampson 2016).
How the intervention might work
Among the atypical antipsychotics, risperidone has one of the simplest pharmacological profiles and comes closest to a serotonin‐dopamine antagonist. Risperidone is a benzisoxazole derivative which blocks dopamine2 receptors and 5HT2 receptors (with a high ratio of serotonin to D2 receptor blockade). It also blocks alpha1 and alpha2 adrenoceptors, H1 receptors, and has no effect on beta adrenoceptors, muscarinic cholinoceptors or peptidergic receptors (Janssen 1988). Psychosis is considered to be associated with disturbances in the activity of neurotransmitters, dopamine in particular, in the brain. Risperidone has therefore been suggested to work by blocking the receptors in the brain that dopamine acts on, which prevents the excessive activity of dopamine and helps to control aggression or agitation. Czobor 1995 suggested that the combination of risperidone on the serotonergic and dopaminergic systems may underlie risperidone's effect on hostility.
Aleman 2001 found some evidence that risperidone is useful in reducing aggression in schizophrenia; although there is some conflicting evidence, risperidone may have less of a sedative effect than conventional antipsychotics, which in turn suggests the anti‐aggressive effects of risperidone is not mediated by sedation. Compared with conventional antipsychotics such as haloperidol, risperidone produces some significantly better results according to Positive and Negative Syndrome Scale (PANSS) scores (Hunter 2003).
Why it is important to do this review
Mental health problems impose a significant burden in developing countries (Shah 2000). As about 1% of any population suffers from schizophrenia (Sartorius 1972), and around 80% of the world live in developing countries (CIA 2008), most care of people with serious mental illnesses such as schizophrenia must take place in these low‐ and middle‐income country situations. There is no evidence that the prevalence of psychiatric emergencies differ across the globe and it seems reasonable to assume that most episodes of severe aggression and agitation in people with severe mental health problems will be taking place in low‐ and middle‐income countries. In many of these countries expensive antipsychotic drugs may be available, but they are generally not affordable (WPA 2008).
Aronson 1997 conducted a review looking at the cost‐effectiveness and quality of life of patients before and after commencing risperidone treatment and found that risperidone improved symptoms of psychosis, decreased the need for hospitalisation and improved quality of life. Viale 1997 also investigated the cost‐effectiveness of risperidone before and after commencing treatment in patients with schizophrenia and found days in hospital were reduced by 26%, but there was a 3.4% increase in total psychiatric healthcare costs.
The fast‐dissolving risperidone tablet formulation may be useful to ensure administration. These 'orodispersible' tablets are an option in acutely agitated psychosis (Normann 2006), and can be as effective as an alternative to intramuscular antipsychotics (Currier 2001). Despite being regularly used for the management of psychosis‐induced agitation, we know of no systematic reviews on the use of risperidone in the emergency situation. This is one of a series of linked reviews (Table 1).
| Focus of review | Reference |
| Completed and maintained reviews | |
| Aripiprazole (intramuscular) for psychosis‐induced aggression or agitation (rapid tranquillisation) | |
| Benzodiazepines for psychosis‐induced aggression or agitation | |
| Chlorpromazine for psychosis‐induced aggression or agitation | |
| Containment strategies for people with serious mental illness | |
| De‐escalation techniques for psychosis‐induced aggression or agitation | |
| Droperidol for psychosis‐induced aggression or agitation | |
| Haloperidol for psychosis‐induced aggression or agitation (rapid tranquillisation) | |
| Haloperidol plus promethazine for psychosis‐induced aggression | |
| Olanzapine IM or velotab for acutely disturbed/agitated people with suspected serious mental illnesses | |
| Zuclopenthixol acetate for acute schizophrenia and similar serious mental illnesses | |
| Reviews in the process of being completed | |
| Loxapine inhaler for psychosis‐induced aggression | |
| Quetiapine for psychosis‐induced aggression |
Objectives
To examine whether risperidone is an effective treatment for psychosis‐induced aggression or agitation.
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomised controlled trials (RCTs). If a trial had been described as 'double‐blind' but had implied randomisation and the demographic details of each group had been similar, we would have included it. We excluded quasi‐randomised studies, such as those allocated by using alternate days of the week.
Types of participants
People exhibiting aggression or agitation (or both) thought to be due to psychosis, regardless of age and sex. Studies that also involved people with other diagnoses, such as drug or alcohol intoxication, organic problems including dementia, non‐psychotic mental illnesses or learning disabilities, were included as long as the majority of participants (> 50%) were experiencing psychosis.
We are interested in making sure that information is as relevant to the current care of people with schizophrenia as possible, so, if reported, would clearly highlight the current clinical state (acute, early post‐acute, partial remission, remission) as well as the stage (prodromal, first episode, early illness, persistent). In addition, where possible, we would report whether the studies primarily focused on people with particular problems (for example, negative symptoms, treatment‐resistant illnesses).
Types of interventions
1. Risperidone
Given alone, any dose and mode of administration.
2. Other antipsychotic medications
Given alone, any dose and mode of administration.
3. Placebo
Active or non‐active.
Types of outcome measures
Where possible, we grouped outcomes by time: by 30 minutes, up to two hours, up to four hours, up to 24 hours, and over 24 hours.
Primary outcomes
1. Not tranquil or asleep
1.1 Not tranquil or asleep ‐ by up to 30 minutes
2. Adverse events
Secondary outcomes
1. Tranquillisation or asleep
1.1 Not tranquil
1.2 Not asleep
1.3 Time to tranquillisation/sleep
1.4 Time to tranquillisation
1.5 Time to sleep
2. Specific behaviours
2.1 Self‐harm, including suicide
2.2 Injury to others
2.3 Agitation
2.3.1 Another episode of agitation by 24 hours
2.3.2 No clinically important change in agitation
2.3.3 Any change in agitation
2.4 Aggression
2.4.1 Another episode of aggression by 24 hours
2.4.2 No clinically important change in aggression
2.4.3 No change in aggression
2.4.4 Average endpoint aggression score
2.4.5 Average change in aggression scores
3. Global outcomes
3.1 No overall improvement
3.2 Use of additional medication
3.3 Use of restraints/seclusion
3.4 Relapse ‐ as defined by each study
3.5 Recurrence of violent incidents
3.6 Needing extra visits from the doctor
3.7 Refusing oral medication
3.8 Not accepting treatment
3.9 Average endpoint score
3.10 Average change score
3.11 Average dose of drug
4. Service outcomes
4.1 Duration of hospital stay
4.2 Re‐admission
4.3 No clinically important engagement with services
4.4 No engagement with services
4.5 Average endpoint engagement score
4.6 Average change in engagement scores
5. Mental state
5.1 No clinically important change in general mental state
5.2 No change in general mental state
5.3 Average endpoint general mental state score
5.4 Average change in general mental state scores
6. Adverse effects
6.1 Death
6.2 Any general adverse effects
6.3 Any serious specific adverse effects
6.4 Average endpoint general adverse effect score
6.5 Average change in general adverse effect scores
6.6 Clinically important change in specific adverse effects
6.7 Any change in specific adverse effects
6.8 Average endpoint specific adverse effects
6.9 Average change in specific adverse effects
7. Leaving the study early
7.1 For specific reasons
7.2 For general reasons
8. Satisfaction with treatment
8.1 Recipient of treatment not satisfied with treatment
8.2 Recipient of treatment average satisfaction score
8.3 Recipient of treatment average change in satisfaction scores
8.4 Informal treatment provider not satisfied with treatment
8.5 Informal treatment providers' average satisfaction score
8.6 Informal treatment providers' average change in satisfaction scores
8.7 Professional providers not satisfied with treatment
8.8 Professional providers' average satisfaction score
8.9 Professional providers' average change in satisfaction scores
9. Acceptance of treatment
9.1 Not accepting treatment
9.2 Average endpoint acceptance score
9.3 Average change in acceptance score
10. Quality of life
10.1 No clinically important change in quality of life
10.2 Not any change in quality of life
10.3 Average endpoint quality of life score
10.4 Average change in quality of life scores
10.5 No clinically important change in specific aspects of quality of life
10.6 No change in specific aspects of quality of life
10.7 Average endpoint specific aspects of quality of life
10.8 Average change in specific aspects of quality of life
11. Economic outcomes
11.1 Direct costs
11.2 Indirect costs
Outcomes used for 'Summary of findings' table
We used the GRADE approach to interpret findings (Schünemann 2011) and used GRADEpro GDT to create 'Summary of findings' tables. These tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient‐care and decision making. We included the following outcomes in the 'Summary of findings' table:
-
tranquillisation or asleep ‐ by 30 minutes;
-
repeated need for rapid tranquillisation ‐ within 24 hours;
-
specific behaviours ‐ agitation or aggression;
-
global state ‐ needing restraints or seclusion;
-
adverse events ‐ serious adverse effects (not death);
-
economic outcomes.
For assessments of the overall quality of evidence for each outcome that included pooled data from RCTs only, we downgraded the evidence from 'high quality' by one level for 'serious' (or by two for 'very serious') study limitations (risk of bias), indirectness of evidence, inconsistency, imprecision of effect estimates or potential publication bias.
Search methods for identification of studies
No language restriction was applied within the limitations of the search tools.
Electronic searches
Cochrane Schizophrenia Group Study‐Based Register of Trials
The Information Specialist searched the register (up to 12 April 2017) using the following phrase:
(*rispe* or *9‐OH‐risperid* or *r 64766* in intervention of STUDY) AND (*aggress* or *violen* or *agitation* or *tranq* in title, abstract, index terms of REFERENCE or intervention of STUDY)
In such a study‐based register, searching the major concept retrieves all the synonyms and relevant studies because all the studies have already been organised based on their interventions and linked to the relevant topics.
This register is compiled by systematic searches of major resources (including AMED, BIOSIS, CINAHL, Embase, MEDLINE, PsycINFO, PubMed, and registries of clinical trials) and their monthly updates, handsearches, grey literature, and conference proceedings (see Group’s Module). There are no language, date, document type, or publication status limitations for inclusion of records into the register.
Searching other resources
1. Reference searching
We inspected references of all included studies for further relevant studies.
2. Personal contact
Where necessary, we contacted the first author of each included study for information regarding unpublished trials.
Data collection and analysis
Selection of studies
1. 2017 search
Review authors EGO and MH independently inspected all abstracts of studies identified as above to identify potentially relevant reports. Where disagreement occurred, we resolved it by discussion, or where there was still doubt, we acquired the full article for further inspection and further discussion with CEA. We acquired the full articles of relevant reports for re‐assessment and to make a final decision on inclusion (see Criteria for considering studies for this review for this review). Once we had obtained the full articles, EGO and MH independently inspected all reports and independently decided whether they met the inclusion criteria. EGO and MH were not blinded to the names of the authors, institutions or journal of publication. Where difficulties or disputes arose, we discussed them with CEA and if a decision could not be reached, we added these studies to those awaiting assessment and contacted the authors of the papers for clarification.
2. 2015 search
Review author KM independently inspected all records identified in the search for potential relevance. Where difficulties or disputes arose, KM discussed them with CEA .
3. 2011 and 2013 searches
Review authors UA and FR independently inspected all abstracts of studies identified as above to identify potentially relevant reports. In addition, to ensure reliability, HJ (see Acknowledgements) inspected a random sample of these abstracts, comprising 10% of the total. Where disagreement occurred, we resolved it by discussion, or where there was still doubt, we acquired the full article for further inspection and further discussion with CEA. We acquired the full articles of relevant reports for re‐assessment and to make a final decision on inclusion (see Criteria for considering studies for this review for this review). Once we had obtained the full articles, UA and FR independently inspected all reports and independently decided whether they met the inclusion criteria. UA and FR were not blinded to the names of the authors, institutions or journal of publication. Where difficulties or disputes arose, we discussed them with HJ and CEA and if we had been unable to reach a decision, we would have added these studies to those awaiting assessment and contacted the authors of the papers for clarification.
Data extraction and management
1. Extraction
1.1 2017 search
Review authors EGO and MH independently extracted data from all included studies. Again, any disagreement was discussed, decisions documented and, if necessary, we contacted the authors of studies for clarification. If there had been any remaining problems, we would have consulted with CEA to help clarify issues and these final decisions would have been documented. We extracted data presented only in graphs and figures whenever possible, but we only included the data if both EGO and MH independently had the same result; we used Plot Digitizer open source software for data extraction from figures, following instructions provided by Kadic 2016. We attempted to contact authors through an open‐ended request in order to obtain missing information or for clarification whenever necessary. If studies had been multi‐centre, where possible, we would have extracted data relevant to each component centre separately.
1.2 2011 and 2013 searches
Review authors UA and FR independently extracted data from all included studies. In addition, to ensure reliability, HJ independently extracted data from a random sample of these studies. Again, we discussed any disagreement, documented decisions and, if necessary, we contacted the authors of studies for clarification. With any remaining problems, CA helped to clarify issues and we documented these final decisions. The need did not arise, but we had planned to extract data presented only in graphs and figures whenever possible while only including the data if two review authors independently had the same result. We had also planned to attempt to contact authors through an open‐ended request in order to obtain missing information or for clarification whenever necessary, and to extract data relevant to each component of multi‐centre studies separately if we had found such studies.
2. Management
2.1 Forms
We extracted data onto standard, pre‐designed, simple forms.
2.2 Scale‐derived data
We included continuous data from rating scales only if:
a) the psychometric properties of the measuring instrument have been described in a peer‐reviewed journal (Marshall 2000);
b) the measuring instrument has not been written or modified by one of the trialists for that particular trial; and
c) the instrument should be a global assessment of an area of functioning and not sub‐scores which are not, in themselves, validated or shown to be reliable. However there are exceptions, we included sub‐scores from mental state scales measuring positive and negative symptoms of schizophrenia.
Ideally, the measuring instrument should either be i. a self‐report or ii. completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly; in Description of studies we noted if this was the case or not.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data: change data can remove a component of between‐person variability from the analysis; however, calculation of change needs two assessments (baseline and endpoint), which can be difficult to obtain in unstable and difficult‐to‐measure conditions such as schizophrenia. We decided primarily to use endpoint data, and only use change data if the former were not available. If necessary, we combined endpoint and change data in the analysis, as we preferred to use mean differences (MDs) rather than standardised mean differences (SMDs) throughout (Deeks 2011).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to relevant continuous data before inclusion.
For endpoint data from studies including fewer than 200 participants:
a) when a scale started from the finite number zero, we subtracted the lowest possible value from the mean, and divided this by the standard deviation (SD). If this value was lower than one, it strongly suggests that the data are skewed. We excluded these data and entered them as 'other data'. If this ratio was higher than one but less than two, there is suggestion that the data are skewed: we entered these data and tested whether their inclusion or exclusion would change the results substantially. If such data changed the results we entered them as 'other data'; if they did not change the results substantially, we used these data in the analyses. Finally, if the ratio was larger than two, we included these data, because it is less likely that they are skewed (Altman 1996; Higgins 2011).
b) if a scale starts from a positive value (such as the Positive and Negative Syndrome Scale (PANSS), which can have values from 30 to 210 (Kay 1986)), we modified the calculation described above to take the scale starting point into account. In these cases skewed data are present if 2 SD > (S − S min), where S is the mean score and 'S min' is the minimum score.
Please note: we planned to enter all relevant data from studies of more than 200 participants in the analysis irrespective of the above rules, because skewed data pose less of a problem in large studies. We entered all relevant change data, as when continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether or not data are skewed.
2.5 Common measurement
To facilitate comparison between trials we aimed, where relevant, to convert variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, we made efforts to convert outcome measures to dichotomous data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS) (Overall 1962), or the PANSS (Kay 1986), this could be considered as a clinically significant response (Leucht 2005; Leucht 2005a). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicates a favourable outcome for risperidone. Where keeping to this made it impossible to avoid outcome titles with double‐negatives (e.g. 'Not un‐improved'), we reported data where the left of the line indicated an unfavourable outcome. If needed in order to improve readability, we switched labels on the X axis and stated it in a note accompanying the graph.
Assessment of risk of bias in included studies
For the 2017 search review authors EGO and MH independently assessed risk of bias within the included studies by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions to assess trial quality (Higgins 2011a). This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting.
If the raters had disagreed, we would have made the final rating by consensus, with the involvement of CEA. Where inadequate details of randomisation and other characteristics of trials were provided, we attempted to contact authors of the studies in order to obtain further information. If non‐concurrence had occurred, we would have reported this.
We noted the level of risk of bias in the text of the review and in the text and 'Summary of findings' tables.
For the 2011 and 2013 searches, UA and FR undertook assessment of risk of bias as above.
Measures of treatment effect
1. Binary data
For binary outcomes we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI), as it has been shown that RR is more intuitive than odds ratios (Boissel 1999); and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). Although the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH), with their CIs are intuitively attractive to clinicians, they are problematic to calculate and interpret in meta‐analyses (Hutton 2009). For binary data presented in the 'Summary of findings' tables we, where possible, we calculated illustrative comparative risks.
2. Continuous data
For continuous outcomes we estimated the mean difference (MD) between groups. We preferred not to calculate effect size measures (standardised mean difference (SMD)). However if scales of very considerable similarity were used, we presumed there was a small difference in measurement, and we calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra‐class correlation (ICC) in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, CIs unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
If clustering had not been accounted for in primary studies, we would have presented data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. We would have attempted to contact the first authors of studies to obtain intra‐class correlation coefficients (ICCs) for their clustered data and to adjust for this by using accepted methods (Gulliford 1999). If clustering had been incorporated into the analysis of primary studies, we would have presented these data as if from a non‐cluster randomised study, but adjusted for the clustering effect. We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a ’design‐effect’. This is calculated using the mean number of participants per cluster (m) and the ICC [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999). If cluster studies had been appropriately analysed taking into account ICCs and relevant data documented in the report, synthesis with other studies would have been possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, if we had included cross‐over studies, we would only have used data from the first phase of cross‐over studies.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for we would not reproduce these data or use them within analyses. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we would address this within the 'Summary of findings' tables by down‐rating quality. Finally, we would also downgraded quality within the 'Summary of findings' tables should the loss be 25% to 50% in total.
2. Binary
In the case where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat (ITT) analysis). Those leaving the study early were all assumed to have the same rates of negative outcome as those who completed. We used the rate of those who stayed in the study ‐ in that particular arm of the trial ‐ and applied this also to those who did not. We undertook a sensitivity analysis to test how prone the primary outcomes were to change when data only from people who completed the study to that point were compared to the ITT analysis using the above assumptions.
3. Continuous
3.1 Attrition
We used data where attrition for a continuous outcome was between 0% and 50%, and data only from people who completed the study to that point were reported.
3.2 Standard deviations
If standard deviations (SDs) were not reported, we tried to obtain the missing values from the authors. If these were not available, where there were missing measures of variance for continuous data, but an exact standard error (SE) and CIs available for group means, and either P value or t value available for differences in mean, we could calculate SDs according to the rules described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When only the SE is reported, SDs are calculated by the formula SD = SE * √(n). The Cochrane Handbook for Systematic Reviews of Interventions presents detailed formulae for estimating SDs from P, t or F values, CIs, ranges or other statistics (Higgins 2011). If these formulae do not apply, we could calculate the SDs according to a validated imputation method which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. Nevertheless, we would have examined the validity of the imputations in a sensitivity analysis that excludes imputed values.
3.3 Assumptions about participants who left the trials early or were lost to follow‐up
Various methods are available to account for participants who left the trials early or were lost to follow‐up. Some trials just present the results of study completers; others use the method of last observation carried forward (LOCF); while more recently, methods such as multiple imputation or mixed‐effects models for repeated measurements (MMRM) have become more of a standard. While the latter methods seem to be somewhat better than LOCF (Leon 2006), we feel that the high percentage of participants leaving the studies early and differences between groups in their reasons for doing so is often the core problem in randomised schizophrenia trials. We therefore determined not to exclude studies based on the statistical approach used. However, by preference we planned to use the more sophisticated approaches, i.e. we would have used MMRM or multiple‐imputation to LOCF, and would only have presented completer analyses if some kind of ITT data were not available at all. Moreover, we planned to address this issue in the item 'Incomplete outcome data' of the 'Risk of bias' tool.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying situations or people which we had not predicted would arise. Where such situations or participant groups arose, we fully discussed these.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise. Where such methodological outliers arose, we fully discussed these.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
We investigated heterogeneity between studies by considering the I2 statistic alongside the Chi2 P value. The I2 statistic provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on i. magnitude and direction of effects and ii. strength of evidence for heterogeneity (e.g. P value from Chi2 test, or a CI for I2). An I2 estimate greater than or equal to 50% accompanied by a statistically significant Chi2 statistic, was interpreted as evidence of substantial levels of heterogeneity (Deeks 2011). Where substantial levels of heterogeneity were found in the primary outcome, we explored reasons for the heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results ( Egger 1997). These are described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011).
1. Protocol versus full study
We tried to locate protocols of included randomised trials. If the protocol was available, we compared outcomes in the protocol and in the published report. If the protocol was not available, we compared outcomes listed in the methods section of the trial report with actually reported results.
2. Funnel plot
We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We decided not to use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar size. In future versions of this review, if funnel plots are possible, we will seek statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model. It puts added weight onto small studies which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We chose the fixed‐effect model for analyses.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
We did not anticipate any subgroup analyses.
2. Investigation of heterogeneity
If inconsistency was high, we reported it. First, we investigated whether data were entered correctly. Second, if data were correct, we visually inspected the graph and successively removed outlying studies to see if homogeneity was restored. For this review, we decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, we would present the data. If not, we would not pool data but would discuss the issues. We know of no supporting research for this 10% cut‐off but are investigating the use of prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity were obvious, we stated hypotheses regarding these for future reviews or versions of this review. We did not anticipate undertaking analyses relating to these.
Sensitivity analysis
We planned that if there were substantial differences in the direction or precision of effect estimates in any of the sensitivity analyses listed below, we would not add data from the lower‐quality studies to the results of the higher‐quality trials, but would present these data within a subcategory. If their inclusion did not result in a substantive difference, they would remain in the analyses.
1. Implication of randomisation
We aimed to include trials in a sensitivity analysis if they had been described in some way as to imply randomisation. For the primary outcomes we would have included these studies, and if there was no substantive difference when these implied randomised studies were added to those with better description of randomisation, then we would have used all data from the implied studies.
2. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data), we compared the findings of the primary outcomes where we used our assumption compared with completer data only. If there was a substantial difference, we reported results and discussed them but continued to employ our assumption.
Where assumptions had to be made regarding missing SDs data (see Dealing with missing data), we compared the findings on primary outcomes where we used our assumption compared with complete data only. We undertook a sensitivity analysis to test how prone results were to change when completer data only were compared with the imputed data using the above assumption. If there was a substantial difference, we reported results and discussed them but continued to employ our assumption.
3. Risk of bias
We analysed the effects of excluding trials that were judged to be at high risk of bias across one or more of the domains of randomisation (see Assessment of risk of bias in included studies) for the meta‐analysis of the primary outcome/s. If the exclusion of trials at high risk of bias did not substantially alter the direction of effect or the precision of the effect estimates, then we included data from these trials in the analysis.
4. Imputed values
We also would have undertaken a sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect in cluster‐randomised trials if included. If substantial differences had been noted in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we would not pool data from the excluded trials with the other trials contributing to the outcome, but would have presented them separately.
5. Fixed and random effects
We synthesised data using a fixed‐effect model; however, we also synthesised data for the primary outcomes using a random‐effects model to evaluate whether this altered the significance of the result.
Results
Description of studies
For substantive descriptions of studies please see the Characteristics of included studies, Characteristics of studies awaiting classification, and Characteristics of excluded studies.
Results of the search
Electronic searches up to April 2017 identified 850 records of potentially eligible studies. After an initial screening of these records (checking titles and abstracts), the full‐text articles of 92 records (referring to 70 studies) were obtained. From these 70 studies, 57 (76 records) were excluded with reasons, four studies (four records were placed in awaiting assessment) and nine studies (12 records) were included. Please see Figure 2 for summary of the searches up to April 2017.
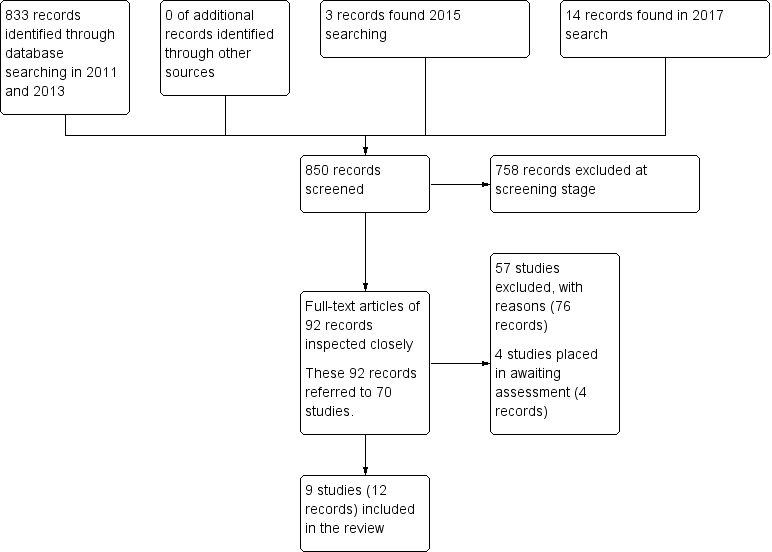
Search results ‐ up to April 2017
Included studies
Details of the included studies in the review are provided in the Characteristics of included studies table. Searches undertaken between 2011 and 2015 identified three studies for inclusion (Lim 2010; Walther 2014; Yao 2010). In 2017, we identified six additional studies for inclusion (Dai 2012; Jin 2013; Li 2013; Wang 2012; Wang 2013; Zhou 2013).
1. Length of studies
The duration of the studies ranged from 24 hours (Lim 2010) to eight weeks (Dai 2012; Li 2013; Yao 2010).
2. Participants
2.1 Clinical state
Participants presented with acute exacerbation of psychotic symptoms. All the studies focused on people whose psychosis had primarily triggered agitation (Lim 2010; Walther 2014; Wang 2012; Zhou 2013) or aggressive behaviour (Dai 2012; Jin 2013; Li 2013; Wang 2013; Yao 2010).
2.2 Diagnosis
Eighty‐nine per cent of participants (n = 520) had a diagnosis of schizophrenia. Other diagnosis included schizoaffective disorder (n = 6, 1%), bipolar I disorder with or without psychotic symptoms (n = 43, 7%), schizophreniform disorder (n = 10, 2%), and psychotic disorder not otherwise specified (n = 3, 1%).
2.3 Exclusions
Reported exclusion criteria included pregnant or lactating women, people with serious medical illnesses, people who had used certain medications (e.g. antipsychotics, long‐acting antipsychotics, benzodiazepines) within a specified period prior to enrolment, people with a known allergy or hypersensitivity to the study drugs, and people with alcohol or psychoactive substance use disorder.
2.4 Age
Six studies reported age range and a mean age (Dai 2012; Jin 2013; Li 2013; Walther 2014; Wang 2012; Yao 2010), one study reported an age range (Lim 2010), and two studies reported a mean age (Wang 2013; Zhou 2013). Age ranges varied from the narrowest being 20‐30 years (Dai 2012) to the largest one being of 18‐65 (Jin 2013; Lim 2010); mean ages varied from the lowest being of 25.3 (Dai 2012) years to the highest 39.4 years (Jin 2013).
2.5 Sex
The studies included a total of 353 male participants and 229 female participants.
3. Study size
The study sizes varied with the smallest study having 40 participants (Dai 2012) and the largest randomising 124 people (Lim 2010).
4. Setting
In all the included studies, participants presented at psychiatric emergency departments and were newly admitted inpatients.
5. Interventions
A total of five comparisons were identified in the included studies: three comparisons involved a single drug whilst the other two comparisons involved a combination. Unfortunately, only two comparisons ('risperidone versus haloperidol', and 'risperidone versus valproic acid') could benefit from more than one study as source of data; moreover, as for the first comparison this held true for a single outcome only ('leaving the study early').
Involved daily doses of risperidone started from 1 mg (Dai 2012; Li 2013; Wang 2013; Yao 2010) or 2 mg (Lim 2010; Walther 2014) to a maximum of 4 mg (Zhou 2013) or 6 mg (Dai 2012; Li 2013; Lim 2010; Walther 2014; Wang 2013; Yao 2010). In one study (Wang 2012), authors declared a titration without a specification on the intended doses, reporting only a mean daily administered dose (4.2 mg ± 0.35mg). In Jin 2013 dosages were not specified.
5.1 Versus antipsychotics
Haloperidol intramuscular (IM) dose was in the 5 mg to 15 mg range (Lim 2010), whilst as for the oral formulation it was a fixed dose of 15 mg (Walther 2014). Olanzapine was given as a fixed dose of 20 mg, oral (Walther 2014). Quetiapine flexible dose started from 100 mg/day and then increased to 400 mg to 500 mg/day with a maximum dose of 750 mg/day (Dai 2012).
5.2 Versus combinations
In the risperidone + oxcarbazepine comparison (Wang 2012), daily administered dose of risperidone and oxcarbazepine were, respectively, of 4.1 mg ± 0.4 mg (oral formulation) and 1.20 g ± 0.42 g (oral formulation).
As for the risperidone + valproic acid comparison, magnesium valproate was administered at 500 mg/day (Yao 2010), within the 750 mg to 1000 mg/day range (Jin 2013; Li 2013), or with a mean dose of 800 mg ± 50 mg/day (Li 2013); sodium valproate daily administered dose was in the 600 mg to 1200 mg/day range (Wang 2013) or of 400 mg twice daily (intravenous formulation (Zhou 2013).
6. Outcomes
The majority of the included studies provided binary data with respect to "specific behaviour ‐ agitation" outcome, "global state" outcome, "mental state" outcome, "adverse effects" outcome, and "leaving the study early" outcome. The majority of trials that employed continuous scales measured "specific behaviour ‐ agitation" outcome, "specific behaviour ‐ aggression" outcome, "mental state" outcome, and "adverse effects ‐ movement disorders" outcome.
The various rating scales, from which we were able to obtain usable data, are listed below.
6.1 Specific behaviour ‐ agitation
a. Positive and Negative Syndrome Scale ‐ Excited Component (PANSS‐EC)
The PANSS‐EC is a five‐item scale (excitement, tension, hostility, uncooperativeness, and poor impulse control). The items are rated from one (not present) to seven (extremely severe). Scores range from five to 35, with mean scores ≥ 20 indicating agitation. A high score indicates high levels of agitation (Montoya 2011).
b. Positive and Negative Syndrome Scale ‐ Psychotic Agitation Subscale (PANSS‐PAS)
The PANSS‐PAS is a five‐item scale (excitement, hallucinatory behaviour, hostility, uncooperativeness, and poor impulse control). The items are rated from one (not present) to seven (extremely severe), with total scores ranging from five to 35. A high score indicates high levels of psychotic agitation (Currier 2000).
6.2 Specific behaviour ‐ aggression
a. Modified Overt Aggression Scale (MOAS)
The OAS (Yudofsky 1986) is a 16‐item rating scale which aims to measure the intensity of verbal and physical aggression. Clinicians are required to comment on the duration of the aggressive incident as well as the intervention required to control it. High scores are indicative of higher levels of aggression.
6.3 Global state
a. Clinical Global Impression (CGI)
The CGl (Guy 1976) is not a diagnostic tool but rather, enables clinicians to quantify the severity of symptoms of any mental health problem at one point in time. Clinicians are then able to use this to track whether there has been any improvement or worsening of symptoms over time. A seven‐point rating scale is used with high scores indicating increased severity or less recovery.
b. Clinical Global Impression ‐ Improvement (CGI‐I)
The CGI‐I (Guy 1976) enables clinicians to assess whether a per‐ son’s symptoms have improved or worsened following an intervention. Based on the clinicians judgement, a rating on a seven‐ point scale is given from one (very much improved) to seven (very much worse). Low scores indicate greater improvement.
c. Clinical Global Impression ‐ Severity (CGI‐S)
The CGI‐S (Guy 1976) requires clinicians to consider the severity of a person’s symptoms in relation to the clinicians past experience of people with the same diagnosis. Clinicians then have to give a rating from one (normal) to seven (extremely ill). High scores indicate increased severity.
6.4 Mental state
a. Brief Psychiatric Rating Scale (BPRS)
The Brief Psychiatric Rating Scale was originally developed by Overall and Gorham (Overall 1962) as a 14‐item scale to measure the severity of a range of psychiatric symptoms, including psychosis. This rating scale items evolved over time and now consists of 24 items which can be rated on a seven‐point scale from ‘not present’ to ‘extremely severe’. A high score would suggest poor mental health. It is not clear for the majority of the studies included in this review, which version of the BPRS was used.
b. Positive and Negative Syndrome Scale (PANSS)
The PANSS was developed and published by Kay, Flszbein and Opler (Kay 1986). The PANSS is designed as a brief interview, whereby the severity of 30 symptoms of schizophrenia can be assessed on a scale of one to seven. A high score would indicate more severe symptoms. The PANSS can be divided into separate sub scales by focusing on the statements relating to positive symptoms (e.g. hallucinations), negative symptoms (e.g. social withdrawal) or general psychopathology (e.g. anxiety and uncooperativeness).
6.5 Adverse effects
a. Abnormal Involuntary Movement Scale (AIMS)
The AIMS (Guy 1976) is a 12‐items scale which records the occurrence of tardive dyskinesia. The first 10 items are rated from one to five, whilst the last two items are dichotomous items (yes, no).
b. Barnes Akathisia Scale (BAS)
The BAS scale was developed by Barnes 1989 and includes items which aim to rate both the observable symptoms which characterise akathisia such as restless movements and also the person’s subjective experience, including any distress. The items are rated from zero = normal to three = severe. There is also an item for rating global severity from zero (absent) to five (severe). A high score indicates high levels of akathisia.
c. Simpson‐Angus Scale (SAS)
The SAS (Simpson 1970) is a 10‐item scale which measures drug induced parkinsonism (extrapyramidal side effects). Each item is scored from zero to four. A high score would indicate increased levels of parkinsonism.
d. Treatment Emergent Symptom Scale (TESS)
The TESS (Guy 1976) is a six‐item scale which is used to assess the occurrence and intensity of treatment‐related adverse effects. A high score indicates worse symptoms.
7. Missing outcomes
Not one of the studies evaluated tranquillisation within 30 minutes, satisfaction with care, acceptance of treatment, quality of life, or economic outcomes.
8. Funders
One of the nine included studies received sponsorship from a pharmaceutical company.
Excluded studies
In total 59 studies had to be excluded: 12 studies were excluded based on their method of allocation (Beck 1997; Buckley 1997; Currier 2000; Greenspan 2005; Hatta 2008; Hovens 2005; Lewis 2006; Li 2014; Pei 2009; Potkin 2005; Schooler 2003; Villari 2008); 35 studies were excluded based on the characteristics of their participants, who were not experiencing a psychosis‐induced aggression or agitation (Belenkaya 2005; Citrome 2001; Briken 2002; Buitelaar 2001; Citrome 2004; Chan 2013; Czobor 1995; Francey 2007; Han 2005; He 2005; Huaqiang 2009; ISRCTN11736448 2003; Kane 2003; Kirwan 2002; Kolivakis 2002; Lieberman 2001; Liu 2010; NCT00174200 2005; NCT00203775 2005; NCT00205699 2005; Citrome 2007; NCT00485498 2003; Ou 2007; Peng 2009; Swanson 2008; Tang 2007; Temputrn 2007; Tosic Golubovic Suzana 2009; Veser 2006; Wan 2005; Wang 2004; Wang 2006; Wei 2010; Xi 2010; Xuan 2007). For 11 studies the intervention under investigation did not meet our inclusion criteria (Conde 2011; Currier 2004; Fang 2012; Hong 2014; Hou 2011; Jiang 2012; Liu 2012; Wang 2015; Zhang 2012; Zheng 2010; Zhou 2012); one study was excluded because the study terminated too early due to difficulties in recruiting participants (NCT00418873 2007).
Risk of bias in included studies
Please see the relevant 'Risk of bias' tables in the Characteristics of included studies, Figure 3 and Figure 4.
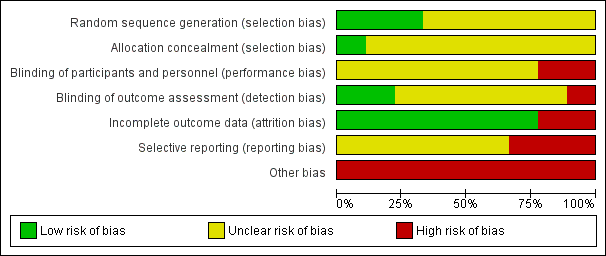
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All nine studies were reported as randomised. In Dai 2012, Jin 2013, Li 2013, Wang 2013, Yao 2010, and Zhou 2013 randomisation is stated but no informations on randomisation procedures and allocation process were given. For the studies that did report further details, methods are described as random number sequence (Wang 2012), pre‐defined randomisation code (Lim 2010), and randomisation in blocks (Walther 2014).
Blinding
None of the studies were double‐blind with two studies (Lim 2010 and Wang 2012) reporting to be open‐label . Only two studies reported being rater‐blind (Lim 2010; Walther 2014).
Incomplete outcome data
There was no evidence of attrition bias for seven studies (Dai 2012; Li 2013; Lim 2010; Wang 2012; Wang 2013; Yao 2010; Zhou 2013). In Jin 2013, two participants left the study early due to adverse effects (without any procedure in order to take into account attrition bias), whilst in Walther 2014, nine people were excluded due to refusal to provide post‐hoc consent.
Selective reporting
In Lim 2010, Walther 2014 and Yao 2010, there was evidence of selective reporting, since several outcomes ‐ despite being stated in the methods ‐ are not reported or reported only partially.
Other potential sources of bias
A study being sponsored by a pharmaceutical company does not automatically indicate bias, but indicates a level of risk for uncertainty. One study was sponsored by Janssen Phamaceutica Korea (Lim 2010). Although Walther 2014 stated that their study was unfunded, several authors were affiliated with Novartis, AstraZeneca, Bristol‐Myers Squibb, Janssen, Servier, Eli Lilly, Zeller Medical, and Sandoz. Eight out of the nine studies had a very small sample size (Dai 2012; Jin 2013; Li 2013; Walther 2014; Wang 2012; Wang 2013; Yao 2010; Zhou 2013).
Effects of interventions
See: Summary of findings for the main comparison RISPERIDONE compared to OTHER ANTIPSYCHOTIC: a. HALOPERIDOL for psychosis‐induced aggression or agitation (rapid tranquillisation); Summary of findings 2 RISPERIDONE compared to OTHER ANTIPSYCHOTIC: b. OLANZAPINE for psychosis‐induced aggression or agitation (rapid tranquillisation); Summary of findings 3 RISPERIDONE compared to OTHER ANTIPSYCHOTIC: c. QUETIAPINE for psychosis‐induced aggression or agitation (rapid tranquillisation); Summary of findings 4 RISPERIDONE compared to COMBINATION: a. RISPERIDONE + OXCARBAZEPINE for psychosis‐induced aggression or agitation (rapid tranquillisation); Summary of findings 5 RISPERIDONE compared to COMBINATION: b. RISPERIDONE + VALPROIC ACID for psychosis‐induced aggression or agitation (rapid tranquillisation)
See: summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4; summary of findings Table 5.
1. COMPARISON 1: RISPERIDONE versus OTHER ANTIPSYCHOTIC: a. HALOPERIDOL
Two studies (n = 152) compared risperidone with haloperidol (Lim 2010; Walther 2014).
1.1 Specific behaviour ‐ agitation
There were no clear differences in terms of Positive And Negative Syndrome Scale ‐ Psychotic Agitation Sub‐score (PANSS‐PAS) response rate up to 24 hours (risk ratio (RR) 1.04, 95% confidence interval (CI) 0.86 to 1.26; participants = 124; studies = 1; Analysis 1.1) and PANSS‐PAS rating scales up to two hours (mean difference (MD) 0.40, 95% CI ‐4.42 to 5.22; participants = 28; studies = 1; Analysis 1.2), up to 24 hours (MD 0.20, 95% CI ‐3.96 to 4.36; participants = 28; studies = 1; Analysis 1.3), and over 24 hours (at 48 hours, MD 1.50, 95% CI ‐1.36 to 4.36; participants = 28; studies = 1; at 72 hours, MD 1.40, 95% CI ‐1.62 to 4.42; participants = 28; studies = 1; at 96 hours, MD 2.90, 95% CI ‐0.34 to 6.14; participants = 28; studies = 1; Analysis 1.4).
1.2 Global outcome
No clear differences were identified in terms of need for benzodiazepines (RR 0.88, 95% CI 0.34 to 2.27; participants = 124; studies = 1), need for seclusion room (RR 0.33, 95% CI 0.01 to 7.55; participants = 28; studies = 1), and use of restraints (RR 2.00, 95% CI 0.43 to 9.21; participants = 28; studies = 1; Analysis 1.5).
1.3 Adverse effects
A similar proportion of participants experienced one or more adverse effects, thus resulting in no clear differences between treatment groups (RR 0.94, 95% CI 0.54 to 1.66; participants = 124; studies = 1; Analysis 1.7).
More people allocated to risperidone experienced insomnia, without clear differences (RR 13.00, 95% CI 0.75 to 225.90; participants = 124; studies = 1), while a similar number of patients experienced somnolence (RR 1.29, 95% CI 0.51 to 3.24; participants = 124; studies = 1; Analysis 1.8).
No clear differences resulted between risperidone and haloperidol in terms of movement disorders, both when considering the proportion of people experiencing extrapyramidal symptoms up to 24 hours (RR 0.63, 95% CI 0.22 to 1.80; participants = 124; studies = 1; Analysis 1.9), or continuous scale scores such as Behavioural Activity Rating Scale (BARS) (MD ‐0.60, 95% CI ‐1.56 to 0.36; participants = 28; studies = 1) and SAS (MD ‐0.40, 95% CI ‐3.00 to 2.20; participants = 28; studies = 1; Analysis 1.11).
Risperidone and haloperidol were similar for adverse events such as headache (RR 0.75, 95% CI 0.18 to 3.21; participants = 124; studies = 1) and dizziness (RR 1.00, 95% CI 0.26 to 3.82; participants = 124; studies = 1) up to 24 hours (Analysis 1.12).
1.4 Leaving the study early
A comparable proportion of participants left the study early due to any reason (RR 2.20, 95% CI 0.51 to 9.48; participants = 152; studies = 2; I2 = 59%), due to adverse effects (RR 0.50, 95% CI 0.05 to 5.37; participants = 124; studies = 1), or due to lack of efficacy (RR 9.00, 95% CI 0.53 to 152.93; participants = 28; studies = 1; Analysis 1.13).
2. COMPARISON 2: RISPERIDONE versus OTHER ANTIPSYCHOTIC: b. OLANZAPINE
One study (n = 29) compared risperidone with olanzapine (Walther 2014). Unsurprisingly with such a small study, no difference between the compared drugs for any of the considered outcomes could be observed.
2.1 Specific behaviour ‐ agitation
There were no clear differences in PANSS‐PAS endpoint scores up to two hours (MD 2.50, 95% CI ‐2.46 to 7.46; participants = 29; studies = 1; Analysis 2.1), up to 24 hours (MD 0.90, 95% CI ‐3.40 to 5.20; participants = 29; studies = 1; Analysis 2.2), and over 24 hours (at 48 hours, MD ‐1.20, 95% CI ‐5.15 to 2.75; participants = 29; studies = 1; at 72 hours, MD ‐0.30, 95% CI ‐4.47 to 3.87; participants = 29; studies = 1; at 96 hours, MD 2.10, 95% CI ‐1.41 to 5.61; participants = 29; studies = 1; Analysis 2.3).
2.2 Global outcome
No clear differences were found when comparing risperidone and olanzapine in terms of need for seclusion room (RR 0.36, 95% CI 0.02 to 8.07; participants = 29; studies = 1) and use of restraints (RR 1.43, 95% CI 0.39 to 5.28; participants = 29; studies = 1; Analysis 2.4).
2.3 Adverse effects
Comparison in terms of BARS (MD 0.20, 95% CI ‐0.43 to 0.83; participants = 29; studies = 1) and SAS scores (MD 1.80, 95% CI ‐0.63 to 4.23; participants = 29; studies = 1) resulted in no clear difference (Analysis 2.6).
2.4 Leaving the study early
More people allocated to risperidone left the study early due to lack of efficacy, but there was no clear difference between treatment groups for this outcome (RR 2.14, 95% CI 0.46 to 9.93; participants = 29; studies = 1; Analysis 2.7).
3. COMPARISON 3: RISPERIDONE versus OTHER ANTIPSYCHOTIC: c. QUETIAPINE
One study (n = 40) compared risperidone with quetiapine (Dai 2012).
3.1 Specific behaviour ‐ aggression
A clear difference at Modified Overt Aggression Scale (MOAS) endpoint score could be seen at two weeks favouring participants allocated to quetiapine (MD 1.80, 95% CI 0.20 to 3.40; participants = 40; studies = 1), whilst this was not the case at four weeks (MD 0.90, 95% CI ‐0.44 to 2.24; participants = 40; studies = 1), at six weeks (MD 0.70, 95% CI ‐0.29 to 1.69; participants = 40; studies = 1), and at eight weeks (MD 0.55, 95% CI ‐0.40 to 1.50; participants = 40; studies = 1). (Analysis 3.1).
3.2 Mental state
There was no clear difference observed in the 'no response' outcome of the PANSS rating scale at eight weeks (RR 1.00, 95% CI 0.16 to 6.42; participants = 40; studies = 1; Analysis 3.2). No clear differences were found as for the other dichotomous outcome (Analysis 3.3).
PANSS total endpoint scores and sub‐scale endpoint scores (positive symptoms, negative symptoms, general psychopathology) were collected at two, four, six and eight weeks. Only PANSS positive symptoms sub‐scale endpoint scores at four weeks resulted in a clear difference in favour of quetiapine (MD 1.70, 95% CI 0.01 to 3.39; participants = 40; studies = 1; Analysis 3.4).
3.3 Adverse effects
No clear differences could be observed between risperidone and quetiapine for a list of different adverse effects: blurred vision (RR 0.50, 95% CI 0.10 to 2.43; participants = 40; studies = 1; Analysis 3.5), somnolence (RR 0.60, 95% CI 0.17 to 2.18; participants = 40; studies = 1; Analysis 3.6), tachycardia (RR 4.00, 95% CI 0.49 to 32.72; participants = 40; studies = 1; Analysis 3.7), nausea and vomiting (RR 1.00, 95% CI 0.07 to 14.90; participants = 40; studies = 1; Analysis 3.8), akathisia (RR 1.67, 95% CI 0.46 to 6.06; participants = 40; studies = 1), hypermyotonia (RR 7.00, 95% CI 0.95 to 51.80; participants = 40; studies = 1; Analysis 3.9), headache (RR 1.00, 95% CI 0.16 to 6.42; participants = 40; studies = 1), liver function tests (LFTs) elevation (RR 1.00, 95% CI 0.07 to 14.90; participants = 40; studies = 1), weight gain (RR 4.00, 95% CI 0.49 to 32.72; participants = 40; studies = 1), and agitation (RR 3.50, 95% CI 0.83 to 14.83; participants = 40; studies = 1; Analysis 3.10).
4. COMPARISON 4: RISPERIDONE versus COMBINATION: a. RISPERIDONE + OXCARBAZEPINE
One study (n = 68) compared risperidone with the 'risperidone + oxcarbazepine' combination (Wang 2012).
4.1 Specific behaviour ‐ agitation
There was a clear difference in the Positive And Negative Syndrome Scale ‐ Excited Component (PANSS‐EC) endpoint scores at one week (MD 2.70, 95% CI 0.42 to 4.98; participants = 68; studies = 1; Analysis 4.1) in favour of the combination.
4.2 Global outcome
Comparison at one week in terms of Clinical Global Impression ‐ Improvement (CGI‐I) (MD ‐0.20, 95% CI ‐0.61 to 0.21; participants = 68; studies = 1) and Clinical Global Impression ‐ Severity (CGI‐S) endpoint scores (MD 0.20, 95% CI ‐0.25 to 0.65; participants = 68; studies = 1) resulted in no clear difference (Analysis 4.2).
4.3 Mental state
There was a clear difference between Brief Psychiatric Rating Scale (BPRS) endpoint scores at one week (MD 5.20, 95% CI 1.04 to 9.36; participants = 68; studies = 1) in favour of the combination (Analysis 4.5).
4.4 Adverse effects
In the combination group a total number of 47 adverse effects were registered compared to a total of 40 adverse effects in participants allocated to risperidone. (Analysis 4.6).
There was a clear difference in the number of participants experiencing excessive sedation in favour of risperidone. (RR 0.06, 95% CI 0.00 to 0.92; participants = 68; studies = 1; Analysis 4.8).
Risperidone and combination groups were comparable in terms of the number of participants that were experiencing various adverse effects at four weeks: dry mouth (RR 2.12, 95% CI 0.81 to 5.55; participants = 68; studies = 1) and constipation at four weeks (RR 1.30, 95% CI 0.62 to 2.72; participants = 68; studies = 1) (Analysis 4.7); tachycardia (RR 1.24, 95% CI 0.46 to 3.30; participants = 68; studies = 1; Analysis 4.9); nausea (RR 2.12, 95% CI 0.20 to 22.31; participants = 68; studies = 1; Analysis 4.10), extrapyramidal symptoms (EPS) (RR 1.59, 95% CI 0.49 to 5.14; participants = 68; studies = 1) and tremor (RR 0.85, 95% CI 0.25 to 2.89; participants = 68; studies = 1) (Analysis 4.11); headache (RR 0.07, 95% CI 0.00 to 1.19; participants = 68; studies = 1) and skin rash (RR 0.35, 95% CI 0.01 to 8.37; participants = 68; studies = 1) (Analysis 4.12).
5. COMPARISON 5: RISPERIDONE versus COMBINATION: b. RISPERIDONE + VALPROIC ACID
Five studies (n = 307) compared risperidone with the 'risperidone + valproic acid' combination (Jin 2013; Li 2013; Wang 2013; Yao 2010; Zhou 2013).
5.1 Specific behaviour ‐ agitation
PANSS‐EC endpoint scores were comparable between risperidone and combination at three days (MD ‐0.11, 95% CI ‐2.98 to 2.76; participants = 54; studies = 1); a clear difference in favour of the combination was identified at five days (MD 5.47, 95% CI 2.64 to 8.30; participants = 54; studies = 1) and seven days (MD 5.11, 95% CI 2.51 to 7.71; participants = 54; studies = 1; Analysis 5.1).
Endpoint scores at PANSS‐EC at two weeks (MD 0.09, 95% CI ‐0.90 to 1.08; participants = 63; studies = 1) and at four weeks (MD 0.17, 95% CI ‐0.85 to 1.19; participants = 63; studies = 1) could not show a difference between the compared drugs (Analysis 5.2).
5.2 Specific behaviour ‐ aggression
Risperidone and 'risperidone + valproic acid' combination were comparable both at three days (MD 1.07, 95% CI ‐0.20 to 2.34; participants = 54; studies = 1) and five days (MD 0.38, 95% CI ‐0.83 to 1.59; participants = 54; studies = 1) in terms of Modified Overt Aggression Scale (MOAS) endpoint scores. A clear difference in favour of the combination could be identified at seven days (MD 3.32, 95% CI 2.07 to 4.57; participants = 54; studies = 1), at two weeks (MD 1.13, 95% CI 0.23 to 2.02; participants = 128; studies = 2; I2 = 25%), at four weeks (MD 1.57, 95% CI 0.75 to 2.39; participants = 128; studies = 2; I2 = 0%), and at six weeks (MD 1.47, 95% CI 0.83 to 2.11; participants = 68; studies = 1), whilst being comparable at eight weeks (MD 1.00, 95% CI ‐0.11 to 2.11; participants = 60; studies = 1;Analysis 5.2).
5.3 Mental state
Five participants allocated to risperidone and three participants allocated to the combination showed no clinical response based on the PANSS score (< 30% reduction from baseline score) at eight weeks (RR 1.67, 95% CI 0.44 to 6.38; participants = 62; studies = 1; Analysis 5.3).
However, when looking at PANSS endpoint scores, a clear difference favouring the combination could be seen at two weeks (MD 3.48, 95% CI 1.88 to 5.08; participants = 253; studies = 4; I2 = 80%), at four weeks (MD 5.45, 95% CI 3.81 to 7.08; participants = 253; studies = 4; I2 = 70%), at six weeks (MD 9.90, 95% CI 7.42 to 12.37; participants = 130; studies = 2; I2 = 92%) and at eight weeks (MD 5.83, 95% CI 4.12 to 7.54; participants = 122; studies = 2; I2 = 58%).
High I2 values that should be translated into inconsistency of findings between the studies; data at two, four and six weeks had Chi2 P values statistically significant, showing evidence of substantial levels of heterogeneity (Assessment of heterogeneity; Section 9.5.2, Higgins 2011). We investigated for input errors and found that data were entered correctly. At the visual inspection of the forest plots we identified Wang 2013 as an outlying study for the two and four weeks pooled data analyses and removed it to see if homogeneity was restored. PANSS endpoint scores at two weeks (MD 2.50, 95% CI 0.78 to 4.21; participants = 185; studies = 3; I2 = 62%, Chi2 = 5.23, P = 0.07) showed a I2 value > 50% with a Chi2 P value non statistically significant, whilst at four weeks (MD 4.52, 95% CI 2.76 to 6.29; participants = 185; studies = 3; I2 = 21%, Chi2 = 2.55, P = 0.28) I2 value resulted being <50% accompanied by a non statistically significant Chi2 P value. Given the concordance of direction of effects, the summary effect resulting statistically significant even by removal of the outlying study, and that the relative weights of this last were 13.3% and 14.2%, (respectively, two and four weeks), we decided to present the pooled data (Subgroup analysis and investigation of heterogeneity).
Sub‐scale continuous data showed a clear difference at PANSS positive symptoms sub‐scale in favour of the combination at four weeks (MD 2.75, 95% CI 1.86 to 3.64; participants = 191; studies = 3; I2 = 76%), six weeks (MD 4.40, 95% CI 1.40 to 7.40; participants = 68; studies = 1), and eight weeks (MD 1.70, 95% CI 0.71 to 2.69; participants = 60; studies = 1), despite being comparable at two weeks (MD 0.64, 95% CI ‐0.24 to 1.53; participants = 191; studies = 3; I2 = 81%; Analysis 5.4).
Again, we investigated for input errors and that data were entered correctly. At the visual inspection of the graphs, Li 2013 could be identified as an hypothetical outlying study. By removing it, I2 values resulted < 50% and Chi2 P values resulted non statistically significant (two weeks: I2 = 2%, Chi2 = 1.02, P = 0.31; four weeks: I2 = 0%, Chi2 = 0.36, P = 0.55). Given the relative weights of the outlying study being 68.8% at two weeks and 69.8% at four weeks, we decided not to consider pooled data and wait for more studies and data.
PANSS negative symptoms sub‐scale could not show clear differences at two weeks (MD 0.29, 95% CI ‐0.57 to 1.15; participants = 128; studies = 2; I2 = 65%), four weeks (MD 1.23, 95% CI ‐0.16 to 2.62; participants = 128; studies = 2; I2 = 59%), and eight weeks (MD 1.30, 95% CI ‐0.02 to 2.62; participants = 60; studies = 1); a clear difference was observed at six weeks only, favouring the combination (MD 3.80, 95% CI 1.07 to 6.53; participants = 68; studies = 1).
PANSS general psychopathology sub‐scale showed clear differences in favour of the combination at four weeks (MD 1.14, 95% CI 0.04 to 2.23; participants = 128; studies = 2; I2 = 50%), six weeks (MD 6.50, 95% CI 4.06 to 8.94; participants = 68; studies = 1), and eight weeks (MD 1.60, 95% CI 0.43 to 2.77; participants = 60; studies = 1); at two weeks data did not show a clear difference between the compared drugs (MD 0.93, 95% CI ‐0.07 to 1.93; participants = 128; studies = 2; I2 = 66%) (Analysis 5.4).
5.4 Adverse effects
In the combination group a total number of 20 adverse effects were registered, compared to the 16 total adverse effects experienced by participants allocated to risperidone (Analysis 5.5).
Two participants allocated to risperidone and one allocated to the combination suffered from myocardial ischaemia (RR 2.00, 95% CI 0.19 to 20.93; participants = 62; studies = 1; Analysis 5.6).
Risperidone and combination groups resulted in comparable in terms of probability of experiencing blurred vision (RR 1.00, 95% CI 0.37 to 2.68; participants = 122; studies = 2; I2 = 0%), dry mouth (RR 1.64, 95% CI 0.76 to 3.54; participants = 129; studies = 2; I2 = 78%; Analysis 5.7); insomnia (RR 2.00, 95% CI 0.19 to 20.77; participants = 54; studies = 1), somnolence (RR 0.85, 95% CI 0.44 to 1.63; participants = 121; studies = 2; I2 = 0%; Analysis 5.8); decreased blood pressure (RR 0.75, 95% CI 0.18 to 3.10; participants = 68; studies = 1), tachycardia (RR 1.48, 95% CI 0.83 to 2.67; participants = 251; studies = 4; I2 = 35%), T‐wave changes in ECG (RR 2.00, 95% CI 0.19 to 20.77; participants = 54; studies = 1; Analysis 5.9); constipation (RR 1.11, 95% CI 0.70 to 1.76; participants = 189; studies = 3; I2 = 6%), nausea (RR 0.70, 95% CI 0.29 to 1.71; participants = 122; studies = 2; I2 = 0%; Analysis 5.10); EPS (RR 1.35, 95% CI 0.76 to 2.39; participants = 121; studies = 2; I2 = 91%), akathisia (RR 0.75, 95% CI 0.28 to 2.03; participants = 122; studies = 2; I2 = 0%), tremor (RR 1.20, 95% CI 0.40 to 3.56; participants = 68; studies = 1; Analysis 5.11); headache (RR 0.94, 95% CI 0.53 to 1.68; participants = 243; studies = 4; I2 = 0%), weight gain (RR 1.50, 95% CI 0.47 to 4.78; participants = 60; studies = 1), oedema (RR 0.34, 95% CI 0.01 to 8.13; participants = 63; studies = 1), leukopenia (RR 0.34, 95% CI 0.01 to 8.13; participants = 63; studies = 1), and liver function tests elevation (RR 0.60, 95% CI 0.15 to 2.40; participants = 116; studies = 2; I2 = 0%; Analysis 5.13).
Treatment Emergent Symptom Scale (TESS) endpoint scores did not find a clear difference in movement disorders between the compared groups at three days (MD ‐0.04, 95% CI ‐1.35 to 1.27; participants = 54; studies = 1), five days (MD ‐0.11, 95% CI ‐1.55 to 1.33; participants = 54; studies = 1), and at seven days (MD ‐0.51, 95% CI ‐1.88 to 0.86; participants = 54; studies = 1; Analysis 5.12).
5.5 Leaving the study early
Two participants allocated to the combination and none of those allocated to risperidone left the study early due to adverse effects (RR 0.21, 95% CI 0.01 to 4.13; participants = 63; studies = 1; Analysis 5.14).
Discussion
Summary of main results
1. COMPARISON 1: RISPERIDONE versus OTHER ANTIPSYCHOTIC: a. HALOPERIDOL
Two studies were included (Lim 2010; Walther 2014), with a total number of 152 participants (risperidone, n = 76; haloperidol, n = 76); although two potential sources of data were available, only one outcome could benefit from both ('leaving the study early'). We did expect to find more relevant studies since haloperidol has an historical role in drug development research and still could be indicated as the 'common comparator' when designing randomised controlled trials (RCTs). Moreover, available outcomes were rated of very low quality (summary of findings Table for the main comparison), due to high risk of attrition and 'selective reporting' bias, small sample size and indirectness of outcome measures. No data concerning 'tranquillisation or asleep', repeated need for tranquillisation and economic outcomes were available.
1.1 Agitation, global outcome
Risperidone and haloperidol showed similar results in agitation measurement scales and global outcomes such as need for benzodiazepine, need for seclusion room or use of restraints.
Interestingly, dichotomous data on efficacy outcomes provided no strong evidence of a difference between oral risperidone and intramuscular administration of haloperidol; even if higher‐quality evidence is needed to draw any firm conclusion, patients preference and best interest should be taken into consideration in treatment decision making when delivering a tailored therapy.
1.2 Adverse effects
Analyses provides no strong evidence that the intervention yielded any differences in terms of adverse effects.
1.3 Leaving the study early
A comparable number of participants allocated to either risperidone or haloperidol left the study early.
2. COMPARISON 2: RISPERIDONE versus OTHER ANTIPSYCHOTIC: b. OLANZAPINE
Only one study was included (Walther 2014), with a total number of 29 participants (risperidone, n = 14; olanzapine, n = 15). Due to high risk of attrition and 'selective reporting' bias, the small sample size and the indirectness of the outcome measures, available outcomes were rated of very low quality (summary of findings Table 2). No data concerning 'tranquillisation or asleep', repeated need for rapid tranquillisation and economic outcomes were available.
Analyses provides no strong evidence that risperidone has an effect any different than olanzapine for any of the considered outcomes: 'specific behaviour ‐ agitation', 'global outcome', 'adverse effects', 'leaving the study early'.
3. COMPARISON 3: RISPERIDONE versus OTHER ANTIPSYCHOTIC: c. QUETIAPINE
Only one study was included (Dai 2012), with a total number of 40 participants (risperidone, n = 20; quetiapine, n = 20). Due to small sample size and the indirectness of the outcome measures, available outcomes were rated of very low quality (summary of findings Table 3). No data concerning 'tranquillisation or asleep', repeated need for rapid tranquillisation, 'global state' and economic outcomes were available. No data were available on the 'rapid tranquillisation' topic: the first time point at which data were collected was at two weeks.
Data analyses provide no strong evidence that risperidone has an effect clinically significantly different than quetiapine for almost all the outcomes: 'specific behaviour ‐ aggression', 'mental state', 'adverse effects'.
4. COMPARISON 4: RISPERIDONE versus COMBINATION: a. RISPERIDONE + OXCARBAZEPINE
Only one study was included (Wang 2012), with a total number of 68 participants (risperidone, n = 33; risperidone + oxcarbazepine, n = 35). Due to high risk of performance and detection bias, small sample size and indirectness of outcome measures, available outcomes were rated of very low quality (summary of findings Table 4); no data concerning 'tranquillisation or asleep', repeated need for tranquillisation and economic outcomes were available.
4.1 Agitation, global outcome, mental state
As for the available 'agitation' and 'mental state' outcomes (PANSS‐EC and BPRS endpoint scores, respectively), we could find evidences of differences in favour of the combination, with no strong evidence of any difference for the 'global outcome'. However, it has to be noted that available data were collected at seven days, a timing far too late to be informative for the 'rapid tranquillisation' topic. Moreover, the quality of evidence was very low due to aforementioned risks.
4.2 Adverse effects
Very low quality of evidence was found that 'risperidone + oxcarbazepine' combination yielded to higher number of 'excessive sedation' adverse events (zero events occurred in the risperidone group, whilst nine events were reported for the combination group; Analysis 4.8). Interestingly, since we do not have further details on data and the latter have not been collected to multiple time points, we could not exclude that this evidence is just another way to read the higher efficacy one (Analysis 4.1; Analysis 4.5).
The ideal concept of 'tranquillisation' is to find a way to get people calm without causing sedation. Even if this last could be necessary within the first hours, it could not be justified in the later days (if no other aggression or agitation episodes occurred).
No strong evidence of any difference could be observed as for the other collected adverse events.
5. COMPARISON 5: RISPERIDONE versus COMBINATION: b. RISPERIDONE + VALPROIC ACID
Five studies were included (Jin 2013; Li 2013; Wang 2013; Yao 2010; Zhou 2013), with a total number of 307 participants (risperidone, n = 153; risperidone + valproic acid, n = 154). Available outcomes were rated of very low quality (summary of findings Table 5), due to small sample size and indirectness of outcome measures. No data concerning 'tranquillisation or asleep', repeated need for tranquillisation and economic outcomes were available.
5.1 Agitation, aggression
As for available efficacy outcomes on agitation, continuous data analyses provides no strong evidence of different efficacy between risperidone and the 'risperidone + valproic acid' combination at three days; when focusing on later time points, a clear difference in favours of the combination at five and seven days, but not at two and four weeks, could be seen.
Concerning continuous data on aggression, evidence of a clear difference could be observed in favour of the combination at five and seven7 days, two, four, and six weeks. However, mean difference (MD) and confidence interval (CI) values should be taken into account to evaluate clinically significance. At eight weeks a strong evidence of any difference between the compared drugs could not be provided.
5.2 Mental state
Very low quality of evidence of a clear difference in PANSS endpoint scores favouring the combination could be observed (subgroup analyses were carried out for PANSS endpoint scores data at two and four weeks); this held true for the later time points (mainly six and eight weeks) of positive symptoms, negative symptoms and general psychopathology sub‐scales; PANSS positive symptoms sub‐scale endpoint scores data at two and four weeks could not be pooled due to high heterogeneity.
It has to be highlighted, however, that available data were measured starting from the second week: no evidence could be found concerning time points relevant to the 'rapid tranquillisation' topic.
5.3 Adverse effects
Data analyses provide no strong evidence of any difference in terms of adverse effects between risperidone and the 'risperidone + valproic acid' combination. However, in one study (Yao 2010), three episodes of myocardial ischaemia were observed: an association between the drug administration and the event could not be found throughout the relevant paper, nor more information about their cardiovascular baseline risk (presumably not high since age range of participants recruited in the study was between 17 and 42 years, with a mean age of 26.9 years).
5.4 Leaving the study early
No strong evidence of any difference in terms of 'leaving the study early' outcome could be observed.
Overall completeness and applicability of evidence
1. Completeness
We identified nine relevant studies and all reported only a few outcomes: only two comparisons could benefit from more than one study as data source and ‐ even more surprisingly ‐ as for the haloperidol comparison this held true only for a single outcome; the most represented comparison is the one comparing risperidone with 'risperidone + valproic acid' combination (five studies). No study reported data regarding the 'tranquillisation or asleep' outcome, service outcomes, satisfaction with treatment, acceptance of treatment, quality of life, or economic outcomes. Three included studies suffered from 'selective reporting' bias, thus further limiting available data. Protocols were not available for all the included studies.
2. Applicability
All of the included studies were more of the explanatory type of study rather the practical/pragmatic and easily applicable to real‐world practice one (Thorpe 2009).
Included studies were conducted in three different countries (China, seven; Korea, one; Switzerland, one) and in psychiatric inpatient and emergency admission settings.
Rapid tranquillisation is often used to achieve a fast resolution of this clinical state that could otherwise result in injury to self or others, hence preventing prolonged physical restraint of people, which should be seen as the very last available resource. It was therefore surprising and disappointing that few studies measured agitation and aggression outcomes at time points that one would associate with 'rapid tranquillisation'. The first available time point is at two hours (Walther 2014), with seven studies having set time points greater than 24 hours (three days, Zhou 2013; one week, Wang 2012; two weeks, Dai 2012; Jin 2013; Li 2013; Wang 2013; Yao 2010). In Lim 2010, outcomes are stated to be measured as early as 30 minutes but, unfortunately, only 24 hours measurements were provided.
Quality of the evidence
1. General
All of the included studies were published after the first CONSORT guidance (1996); no studies complied with this internationally agreed standard. Disappointingly, a large proportion of data was rendered unusable, which could have been avoided had the trials been better reported.
2. Specific
All nine studies were randomised, but details were vaguely reported and none was double‐blinded. Three studies suffered from 'selective reporting' bias. Data from more than one source were available for two comparisons only. These factors strongly limit the quality of the available evidence and this can be evinced in the 'Risk of bias' and 'Summary of findings' tables; all of the selected outcomes to be included in th 'Summary of findings' tables were rated as 'very low' quality.
Potential biases in the review process
We followed the protocol and conducted a thorough search and adhered to our pre‐specified inclusion and exclusion criteria when inspecting citations. It is possible that we have failed to identify small relevant studies, but we feel that it is unlikely that we would have missed large studies that would make a substantial difference to the findings from this review. Given the small number of included studies, review authors EGO and MH independently inspected all citations obtained from the search and extracted the data to minimise bias.
In order to further limit the risk for potential biases and improve the overall quality of the work, we followed the recommendations of the Cochrane Editorial Unit (CEU) Screening Programme by Dr Nuala Livingstone and the Methodological Expectations of Cochrane Intervention Reviews (MECIR; Higgins 2016); the systematic review was checked and verified by using the CEU Screening Tool items. Previously excluded studies were checked for NROD exclusion bias (no relevant outcome data exclusion bias; Higgins 2016; Kirkham 2010): one study was retrieved.
Agreements and disagreements with other studies or reviews
The review authors are not aware of any other studies or systematic reviews that have focused solely on use of risperidone alone for people who are agitated or aggressive due to serious mental illnesses.

Search results ‐ up to April 2017

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 RISPERIDONE vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL, Outcome 1 Specific behaviour: 1a. Agitation ‐ Various measures.

Comparison 1 RISPERIDONE vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL, Outcome 2 Specific behaviour: 1b. Agitation ‐ Average scores ‐ i. up to 2 hours.

Comparison 1 RISPERIDONE vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL, Outcome 3 Specific behaviour: 1c. Agitation ‐ Average scores ‐ ii. up to 24 hours.

Comparison 1 RISPERIDONE vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL, Outcome 4 Specific behaviour: 1d. Agitation ‐ Average scores ‐ iii. over 24 hours.

Comparison 1 RISPERIDONE vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL, Outcome 5 Global outcome: 1a. General ‐ Need for additional measures.
| Study | Intervention | Mean | SD | N |
| Walther 2014 | Risperidone | 92.9 | 86.8 | 14 |
| Walther 2014 | Haloperidol | 71.1 | 70.4 | 14 |
Comparison 1 RISPERIDONE vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL, Outcome 6 Global outcome: 1b. General ‐ Need for additional medication (skewed data).

Comparison 1 RISPERIDONE vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL, Outcome 7 Adverse effects: 1. General.

Comparison 1 RISPERIDONE vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL, Outcome 8 Adverse effects: 2a. Specific ‐ Arousal level.

Comparison 1 RISPERIDONE vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL, Outcome 9 Adverse effects: 2b. Specific ‐ Movement disorder ‐ i. Various.
| Study | Intervention | Mean | SD | N |
| Walther 2014 | Risperidone | 4.1 | 7.3 | 14 |
| Walther 2014 | Haloperidol | 5.2 | 8.3 | 14 |
Comparison 1 RISPERIDONE vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL, Outcome 10 Adverse effects: 2b. Specific ‐ Movement disorder ‐ ii. Need for biperiden.

Comparison 1 RISPERIDONE vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL, Outcome 11 Adverse effects: 2b. Specific ‐ Movement disorder ‐ iii. Average scores (skewed data).

Comparison 1 RISPERIDONE vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL, Outcome 12 Adverse effects: 2c. Specific ‐ Miscellaneous.

Comparison 1 RISPERIDONE vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL, Outcome 13 Leaving the study early.

Comparison 2 RISPERIDONE vs OTHER ANTIPSYCHOTIC: b. OLANZAPINE, Outcome 1 Specific behaviour: 1. Agitation ‐ Average scores ‐ i. Up to 2 hours.
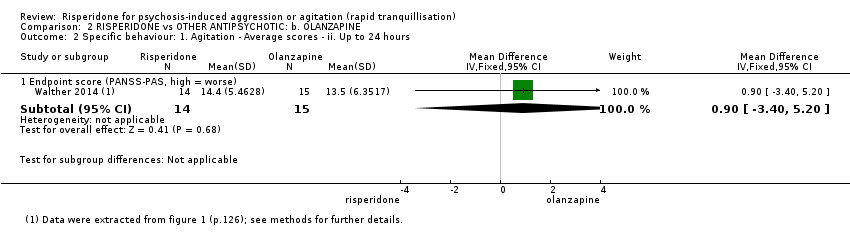
Comparison 2 RISPERIDONE vs OTHER ANTIPSYCHOTIC: b. OLANZAPINE, Outcome 2 Specific behaviour: 1. Agitation ‐ Average scores ‐ ii. Up to 24 hours.
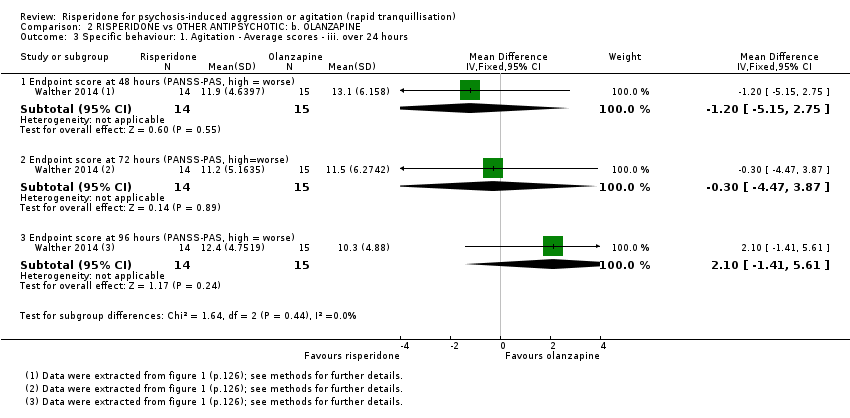
Comparison 2 RISPERIDONE vs OTHER ANTIPSYCHOTIC: b. OLANZAPINE, Outcome 3 Specific behaviour: 1. Agitation ‐ Average scores ‐ iii. over 24 hours.

Comparison 2 RISPERIDONE vs OTHER ANTIPSYCHOTIC: b. OLANZAPINE, Outcome 4 Global outcome: 1a. General ‐ Need for additional measures.
| Study | Intervention | Mean | SD | N |
| Walther 2014 | Risperidone | 92.9 | 86.8 | 14 |
| Walther 2014 | Olanzapine | 114.0 | 79.5 | 15 |
Comparison 2 RISPERIDONE vs OTHER ANTIPSYCHOTIC: b. OLANZAPINE, Outcome 5 Global outcome: 1b. General ‐ Need for additional medication (skewed data).
| Study | Intervention | Mean | SD | N |
| Walther 2014 | Risperidone | 4.1 | 7.3 | 14 |
| Walther 2014 | Olanzapine | 0.7 | 2.6 | 15 |
Comparison 2 RISPERIDONE vs OTHER ANTIPSYCHOTIC: b. OLANZAPINE, Outcome 6 Adverse effects: 1a. Specific ‐ Movement disorder ‐ i. Meed for biperiden.

Comparison 2 RISPERIDONE vs OTHER ANTIPSYCHOTIC: b. OLANZAPINE, Outcome 7 Adverse effects: 1b. Specific ‐ Movement disorder ‐ ii. Average scores (skewed data).

Comparison 2 RISPERIDONE vs OTHER ANTIPSYCHOTIC: b. OLANZAPINE, Outcome 8 Leaving the study early.

Comparison 3 RISPERIDONE vs OTHER ANTIPSYCHOTIC: c. QUETIAPINE, Outcome 1 Specific behaviour: 1. Aggression ‐ Average scores ‐ i. Over 24 hours.

Comparison 3 RISPERIDONE vs OTHER ANTIPSYCHOTIC: c. QUETIAPINE, Outcome 2 Mental state: 1a. No change in general mental state.
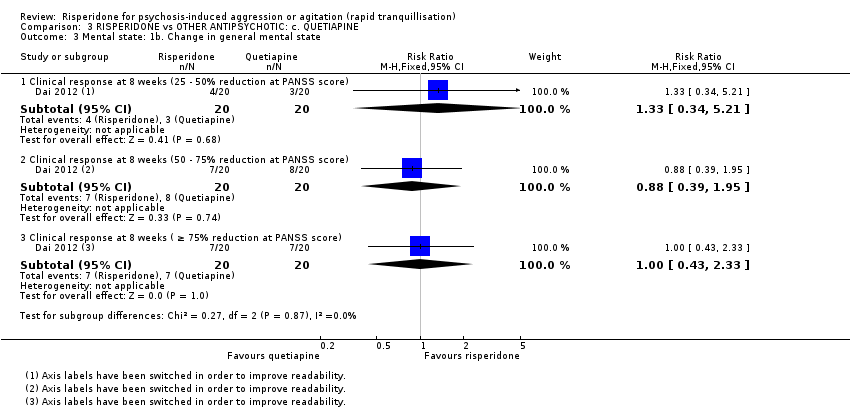
Comparison 3 RISPERIDONE vs OTHER ANTIPSYCHOTIC: c. QUETIAPINE, Outcome 3 Mental state: 1b. Change in general mental state.
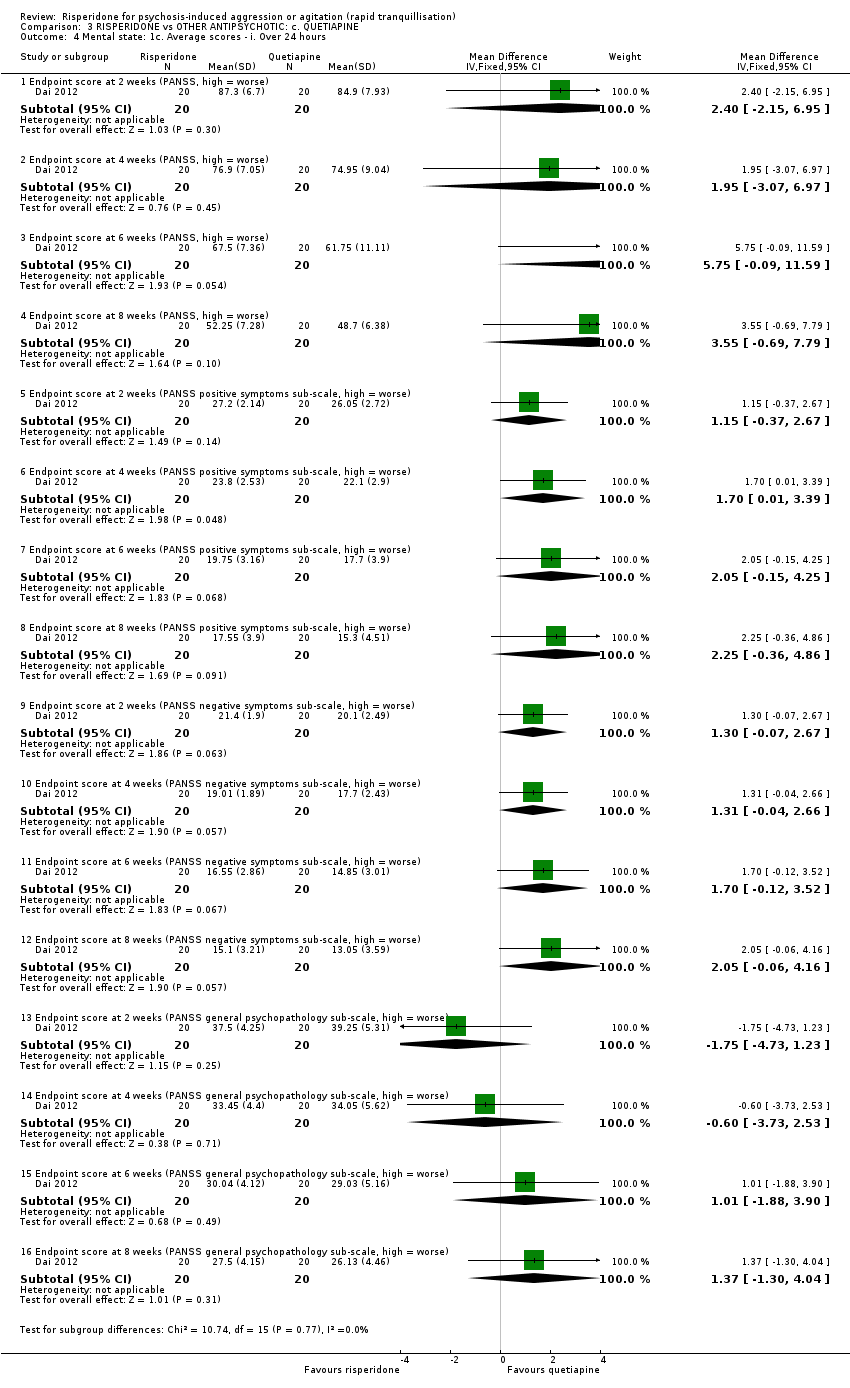
Comparison 3 RISPERIDONE vs OTHER ANTIPSYCHOTIC: c. QUETIAPINE, Outcome 4 Mental state: 1c. Average scores ‐ i. Over 24 hours.

Comparison 3 RISPERIDONE vs OTHER ANTIPSYCHOTIC: c. QUETIAPINE, Outcome 5 Adverse effects: 1a. Specific ‐ Anticholinergic.

Comparison 3 RISPERIDONE vs OTHER ANTIPSYCHOTIC: c. QUETIAPINE, Outcome 6 Adverse effects: 1b. Specific ‐ Arousal.

Comparison 3 RISPERIDONE vs OTHER ANTIPSYCHOTIC: c. QUETIAPINE, Outcome 7 Adverse effects: 1c. Specific ‐ Cardiovascular.

Comparison 3 RISPERIDONE vs OTHER ANTIPSYCHOTIC: c. QUETIAPINE, Outcome 8 Adverse effects: 1d. Specific ‐ Gastrointestinal.

Comparison 3 RISPERIDONE vs OTHER ANTIPSYCHOTIC: c. QUETIAPINE, Outcome 9 Adverse effects: 1e. Specific ‐ Movement disorders.

Comparison 3 RISPERIDONE vs OTHER ANTIPSYCHOTIC: c. QUETIAPINE, Outcome 10 Adverse effects: 1f. Specific ‐ Miscellaneous.

Comparison 4 RISPERIDONE vs COMBINATION: a. RISPERIDONE + OXCARBAZEPINE, Outcome 1 Specific behaviour: 1. Agitation ‐ Average scores ‐ i. over 24 hours.

Comparison 4 RISPERIDONE vs COMBINATION: a. RISPERIDONE + OXCARBAZEPINE, Outcome 2 Global Outcome: 1. Average scores ‐ i. Over 24 hours.

Comparison 4 RISPERIDONE vs COMBINATION: a. RISPERIDONE + OXCARBAZEPINE, Outcome 3 Mental state: 1a. No change in general mental state.

Comparison 4 RISPERIDONE vs COMBINATION: a. RISPERIDONE + OXCARBAZEPINE, Outcome 4 Mental state: 1b. Change in general mental state.

Comparison 4 RISPERIDONE vs COMBINATION: a. RISPERIDONE + OXCARBAZEPINE, Outcome 5 Mental state: 1c. Average scores ‐ i. Over 24 hours.
| Study | AEs (n), risperidone | patients (n), risperidone | AEs (n), combination | patients (n), combination |
| Wang 2012 | 40 | 33 | 47 | 35 |
Comparison 4 RISPERIDONE vs COMBINATION: a. RISPERIDONE + OXCARBAZEPINE, Outcome 6 Adverse effects: 1. General ‐ Total number of AEs.

Comparison 4 RISPERIDONE vs COMBINATION: a. RISPERIDONE + OXCARBAZEPINE, Outcome 7 Adverse effects: 2a. Specific ‐ Anticholinergic.

Comparison 4 RISPERIDONE vs COMBINATION: a. RISPERIDONE + OXCARBAZEPINE, Outcome 8 Adverse effects: 2b. Specific ‐ Arousal.

Comparison 4 RISPERIDONE vs COMBINATION: a. RISPERIDONE + OXCARBAZEPINE, Outcome 9 Adverse effects: 2c. Specific ‐ Cardiovascular.

Comparison 4 RISPERIDONE vs COMBINATION: a. RISPERIDONE + OXCARBAZEPINE, Outcome 10 Adverse effects: 2d. Specific ‐ Gastrointestinal.

Comparison 4 RISPERIDONE vs COMBINATION: a. RISPERIDONE + OXCARBAZEPINE, Outcome 11 Adverse effects: 2e. Specific ‐ Movement disorders.

Comparison 4 RISPERIDONE vs COMBINATION: a. RISPERIDONE + OXCARBAZEPINE, Outcome 12 Adverse effects: 2f. Specific ‐ Miscellaneous.
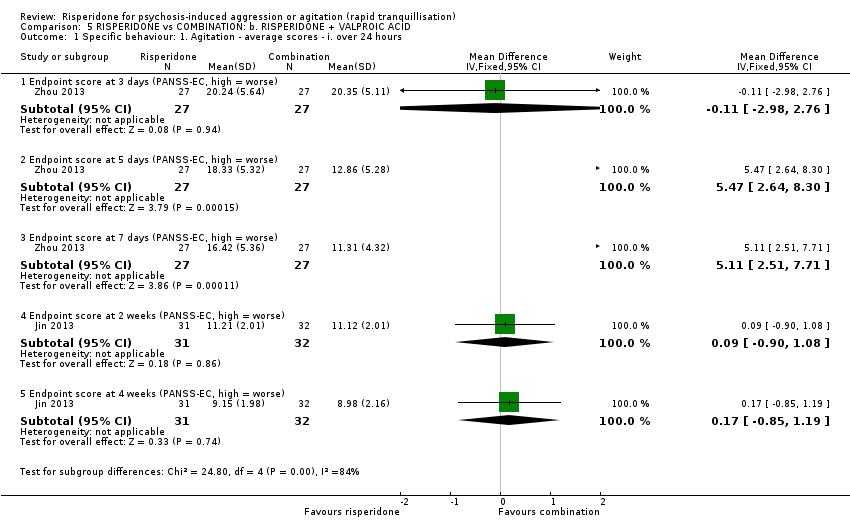
Comparison 5 RISPERIDONE vs COMBINATION: b. RISPERIDONE + VALPROIC ACID, Outcome 1 Specific behaviour: 1. Agitation ‐ average scores ‐ i. over 24 hours.

Comparison 5 RISPERIDONE vs COMBINATION: b. RISPERIDONE + VALPROIC ACID, Outcome 2 Specific behaviour: 1. Aggression ‐ Average scores ‐ i. over 24 hours.

Comparison 5 RISPERIDONE vs COMBINATION: b. RISPERIDONE + VALPROIC ACID, Outcome 3 Mental state: 1. No change in general mental state.

Comparison 5 RISPERIDONE vs COMBINATION: b. RISPERIDONE + VALPROIC ACID, Outcome 4 Mental state: 2. Average scores ‐ i. over 24 hours.
| Study | AEs (n), risperidone | patients (n), risperidone | AEs (n), combination | patients (n), combination |
| Wang 2012 | 16 | 27 | 20 | 27 |
Comparison 5 RISPERIDONE vs COMBINATION: b. RISPERIDONE + VALPROIC ACID, Outcome 5 Adverse effects: 1a. General ‐ Total number of AEs.

Comparison 5 RISPERIDONE vs COMBINATION: b. RISPERIDONE + VALPROIC ACID, Outcome 6 Adverse effects: 1b. General ‐ Serious.

Comparison 5 RISPERIDONE vs COMBINATION: b. RISPERIDONE + VALPROIC ACID, Outcome 7 Adverse effects: 2a. Specific ‐ Anticholinergic.

Comparison 5 RISPERIDONE vs COMBINATION: b. RISPERIDONE + VALPROIC ACID, Outcome 8 Adverse effects: 2b. Specific ‐ Arousal.

Comparison 5 RISPERIDONE vs COMBINATION: b. RISPERIDONE + VALPROIC ACID, Outcome 9 Adverse effects: 2c. Specific ‐ Cardiovascular.

Comparison 5 RISPERIDONE vs COMBINATION: b. RISPERIDONE + VALPROIC ACID, Outcome 10 Adverse effects: 2d. Specific ‐ Gastrointestinal.

Comparison 5 RISPERIDONE vs COMBINATION: b. RISPERIDONE + VALPROIC ACID, Outcome 11 Adverse effects: 2e. Specific ‐ Movement disorders.

Comparison 5 RISPERIDONE vs COMBINATION: b. RISPERIDONE + VALPROIC ACID, Outcome 12 Adverse effects: 2f. Specific ‐ Movement disorders ‐ Average scores ‐ i. Over 24 hours.
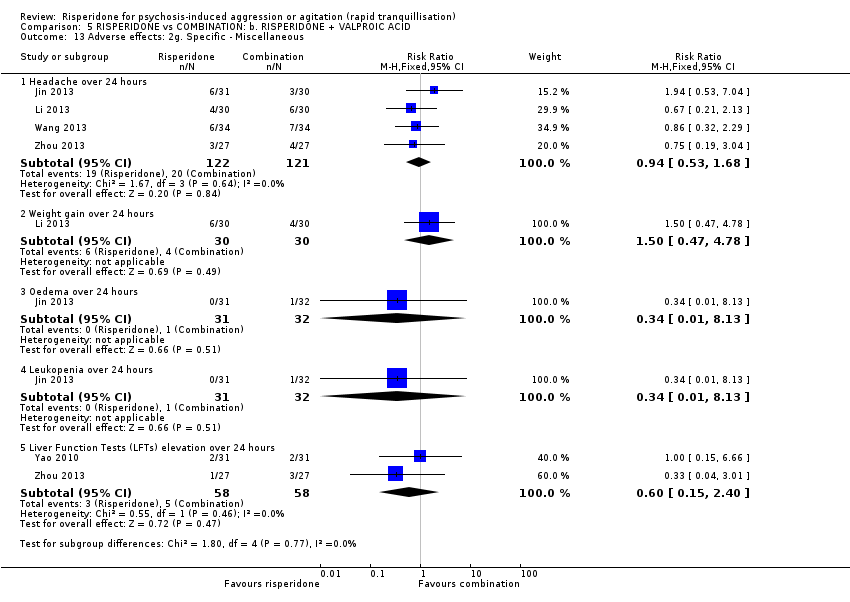
Comparison 5 RISPERIDONE vs COMBINATION: b. RISPERIDONE + VALPROIC ACID, Outcome 13 Adverse effects: 2g. Specific ‐ Miscellaneous.

Comparison 5 RISPERIDONE vs COMBINATION: b. RISPERIDONE + VALPROIC ACID, Outcome 14 Leaving the study early.
| Methods | Allocation: randomised, clearly described and concealed. |
| Participants | Diagnosis: thought to be psychosis. |
| Interventions | 1. Risperidone: dose flexible within recommended limits. N = 150. |
| Outcomes | All outcomes are grouped by time: by 30 minutes, up to two hours, up to four hours, up to 24 hours, and over 24 hours. First outcome of interest could be as early as 20 minutes. Tranquillisation or asleep ‐ tranquil; asleep. Repeated need for tranquillisation ‐ needing additional injections. Specific behaviours ‐ self‐harm, including suicide, injury to others. Global outcomes ‐ patient satisfaction; use of restraint or seclusion. Service outcomes ‐ length of hospitalisation; readmission rate. Mental state ‐ effect of medication on mental state. Adverse effects ‐ medication significant side effects. Leaving the study early ‐ detailed reasons provided. Quality of life outcomes. Economic outcomes. |
| Notes | * Enough power to be able to identify a difference of ˜20% between groups for primary outcome with adequate degree of certainty. |
| RISPERIDONE compared to OTHER ANTIPSYCHOTIC: a. HALOPERIDOL for psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Patient or population: psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTIC: a. HALOPERIDOL | Risk with RISPERIDONE | |||||
| Tranquillisation or asleep by 30 minutes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Repeated need for tranquillisation within 24 hours ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Specific behaviour: agitation, up to 24 hours (PANSS‐PAS response) | Study population | RR 1.04 | 124 | ⊕⊝⊝⊝ | ||
| 758 per 1.000 | 788 per 1.000 | |||||
| Global outcome: need for additional measures | Study population | RR 2.00 | 28 | ⊕⊝⊝⊝ | ||
| 143 per 1.000 | 286 per 1.000 | |||||
| Adverse effects: up to 24 hours | Study population | RR 0.94 | 124 | ⊕⊝⊝⊝ | ||
| 290 per 1.000 | 273 per 1.000 | |||||
| Economic outcomes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias ‐ rated 'very serious': high risk of performance and 'selective reporting' bias. Definition of the outcomes are not consistent between the multiple publications. 2 Imprecision ‐ rated 'serious': Optimal Information Size (OIS) criterion not met. 3 Only one study available. 4 Risk of bias ‐ rated 'very serious': high risk of attrition bias and 'selective reporting' bias. 5 Indirectness ‐ rated 'serious': provided outcome is at a time point (4 days) different from those of primary importance in this setting. | ||||||
| RISPERIDONE compared to OTHER ANTIPSYCHOTIC: b. OLANZAPINE for psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Patient or population: psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTIC: b. OLANZAPINE | Risk with RISPERIDONE | |||||
| Tranquillisation or asleep by 30 minutes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Repeated need for tranquillisation within 24 hours ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Specific behaviour: agitation, up to 2 hours | MD 2.5 higher | ‐ | 29 | ⊕⊝⊝⊝ | ||
| Global outcome: need for additional measures | Study population | RR 1.43 | 29 | ⊕⊝⊝⊝ | ||
| 200 per 1.000 | 286 per 1.000 | |||||
| Adverse effects: movement disorder | MD 0.20 higher | ‐ | 29 | ⊕⊝⊝⊝ | ||
| Economic outcomes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias ‐ rated 'very serious': high risk of attrition bias and ‘selective reporting’ bias. 2 Only one study available. 3 Imprecision ‐ rated 'very serious': Optimal Information Size (OIS) criterion not met. 4 Indirectness ‐ rated 'serious': provided outcome is at a time point (4 days) different from those of primary importance in this setting. | ||||||
| RISPERIDONE compared to OTHER ANTIPSYCHOTIC: c. QUETIAPINE for psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Patient or population: psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTIC: c. QUETIAPINE | Risk with RISPERIDONE | |||||
| Tranquillisation or asleep by 30 minutes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Repeated need for tranquillisation within 24 hours ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Specific behaviour ‐ aggression, over 24 hours | MD 1.80 higher | ‐ | 40 | ⊕⊝⊝⊝ | ||
| Global outcome ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse effects: movement disorders over 24 hours | Study population | RR 1.67 | 40 | ⊕⊝⊝⊝ | ||
| 150 per 1.000 | 251 per 1.000 | |||||
| Economic outcomes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Only one study available. 2 Indirectness ‐ rated 'serious': provided outcome is at a time point (2 weeks) different from those of primary importance in this setting. 3 Imprecision ‐ rated 'very serious': Optimal Information Size (OIS) criterion not met. | ||||||
| RISPERIDONE compared to COMBINATION: a. RISPERIDONE + OXCARBAZEPINE for psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Patient or population: psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATION: a. RISPERIDONE + OXCARBAZEPINE | Risk with RISPERIDONE | |||||
| Tranquillisation or asleep by 30 minutes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Repeated need for tranquillisation within 24 hours ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Specific behaviour: agitation, over 24 hours | MD 2.70 higher | ‐ | 68 | ⊕⊝⊝⊝ | ||
| Global Outcome: average scores, over 24 hours | MD 0.20 lower | ‐ | 68 | ⊕⊝⊝⊝ | ||
| Adverse effects: movement disorders, over 24 hours | Study population | RR 1.59 | 68 | ⊕⊝⊝⊝ | ||
| 114 per 1.000 | 182 per 1.000 | |||||
| Economic outcomes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias ‐ rated 'very serious': high risk for performance bias and detection bias. 2 Only one study available. 3 Indirectness ‐ rated 'serious': provided outcome is at a time point (1 week) different from those of primary importance in this setting. 4 Imprecision ‐ rated 'very serious': Optimal Information Size (OIS) criterion not met. | ||||||
| RISPERIDONE compared to COMBINATION: b. RISPERIDONE + VALPROIC ACID for psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Patient or population: psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATION: b. RISPERIDONE + VALPROIC ACID | Risk with RISPERIDONE | |||||
| Tranquillisation or asleep by 30 minutes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Repeated need for tranquillisation within 24 hours ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Specific behaviour ‐ aggression, over 24 hours | The mean specific behaviour ‐ aggression, over 24 hours was 0 | MD 1.07 higher | ‐ | 54 | ⊕⊝⊝⊝ | |
| Global outcome ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse effects: movement disorders, over 24 hours | Study population | RR 0.75 | 122 | ⊕⊝⊝⊝ | ||
| 66 per 1.000 | 49 per 1.000 | |||||
| Economic outcomes ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Only one study available. 2 Indirectness ‐ rated 'serious': provided outcome is at a time point (3 days) different from those of primary importance in this setting. 3 Imprecision ‐ rated 'very serious': Optimal Information Size (OIS) criterion not met. | ||||||
| Focus of review | Reference |
| Completed and maintained reviews | |
| Aripiprazole (intramuscular) for psychosis‐induced aggression or agitation (rapid tranquillisation) | |
| Benzodiazepines for psychosis‐induced aggression or agitation | |
| Chlorpromazine for psychosis‐induced aggression or agitation | |
| Containment strategies for people with serious mental illness | |
| De‐escalation techniques for psychosis‐induced aggression or agitation | |
| Droperidol for psychosis‐induced aggression or agitation | |
| Haloperidol for psychosis‐induced aggression or agitation (rapid tranquillisation) | |
| Haloperidol plus promethazine for psychosis‐induced aggression | |
| Olanzapine IM or velotab for acutely disturbed/agitated people with suspected serious mental illnesses | |
| Zuclopenthixol acetate for acute schizophrenia and similar serious mental illnesses | |
| Reviews in the process of being completed | |
| Loxapine inhaler for psychosis‐induced aggression | |
| Quetiapine for psychosis‐induced aggression |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1a. Agitation ‐ Various measures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 PANSS‐PAS response up to 24 hours ( ≥ 50% reduction at PANSS‐PAS score) | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.86, 1.26] |
| 2 Specific behaviour: 1b. Agitation ‐ Average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Endpoint score (PANSS‐PAS, high = worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐4.42, 5.22] |
| 3 Specific behaviour: 1c. Agitation ‐ Average scores ‐ ii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Endpoint score (PANSS‐PAS, high = worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐3.96, 4.36] |
| 4 Specific behaviour: 1d. Agitation ‐ Average scores ‐ iii. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Endpoint score at 48 hours (PANSS‐PAS, high = worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [‐1.36, 4.36] |
| 4.2 Endpoint score at 72 hours (PANSS‐PAS, high = worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐1.62, 4.42] |
| 4.3 Endpoint score at 96 hours (PANSS‐PAS, high = worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 2.90 [‐0.34, 6.14] |
| 5 Global outcome: 1a. General ‐ Need for additional measures Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Need for benzodiazepine up to 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.34, 2.27] |
| 5.2 Need for seclusion room | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.55] |
| 5.3 Use of restraints | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.43, 9.21] |
| 6 Global outcome: 1b. General ‐ Need for additional medication (skewed data) Show forest plot | Other data | No numeric data | ||
| 7 Adverse effects: 1. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 One or more AEs up to 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.54, 1.66] |
| 8 Adverse effects: 2a. Specific ‐ Arousal level Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Insomnia up to 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.0 [0.75, 225.90] |
| 8.2 Somnolence up to 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.51, 3.24] |
| 9 Adverse effects: 2b. Specific ‐ Movement disorder ‐ i. Various Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 EPS up to 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.22, 1.80] |
| 10 Adverse effects: 2b. Specific ‐ Movement disorder ‐ ii. Need for biperiden Show forest plot | Other data | No numeric data | ||
| 11 Adverse effects: 2b. Specific ‐ Movement disorder ‐ iii. Average scores (skewed data) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Endpoint scores at 96 hours (AIMS, high = worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Endpoint scores at 96 hours (BARS, high = worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.56, 0.36] |
| 11.3 Endpoint scores at 96 hours (SAS, high = worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.00, 2.20] |
| 12 Adverse effects: 2c. Specific ‐ Miscellaneous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Headache up to 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.18, 3.21] |
| 12.2 Dizziness up to 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.26, 3.82] |
| 13 Leaving the study early Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 For any reason | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.2 [0.51, 9.48] |
| 13.2 Due to adverse effects | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.37] |
| 13.3 Lack of efficacy | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.53, 152.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1. Agitation ‐ Average scores ‐ i. Up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Endpoint score (PANSS‐PAS, high = worse) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [‐2.46, 7.46] |
| 2 Specific behaviour: 1. Agitation ‐ Average scores ‐ ii. Up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Endpoint score (PANSS‐PAS, high = worse) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐3.40, 5.20] |
| 3 Specific behaviour: 1. Agitation ‐ Average scores ‐ iii. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Endpoint score at 48 hours (PANSS‐PAS, high = worse) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐5.15, 2.75] |
| 3.2 Endpoint score at 72 hours (PANSS‐PAS, high=worse) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐4.47, 3.87] |
| 3.3 Endpoint score at 96 hours (PANSS‐PAS, high = worse) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [‐1.41, 5.61] |
| 4 Global outcome: 1a. General ‐ Need for additional measures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Need for seclusion room | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.07] |
| 4.2 Use of restraints | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.39, 5.28] |
| 5 Global outcome: 1b. General ‐ Need for additional medication (skewed data) Show forest plot | Other data | No numeric data | ||
| 6 Adverse effects: 1a. Specific ‐ Movement disorder ‐ i. Meed for biperiden Show forest plot | Other data | No numeric data | ||
| 7 Adverse effects: 1b. Specific ‐ Movement disorder ‐ ii. Average scores (skewed data) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Endpoint score (BARS, high = worse) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.43, 0.83] |
| 7.2 Endpoint score (SAS, high = worse) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [‐0.63, 4.23] |
| 8 Leaving the study early Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Lack of efficacy | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.46, 9.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1. Aggression ‐ Average scores ‐ i. Over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Endpoint score at 2 weeks (MOAS, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [0.20, 3.40] |
| 1.2 Endpoint score at 4 weeks (MOAS, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.44, 2.24] |
| 1.3 Endpoint score at 6 weeks (MOAS, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.29, 1.69] |
| 1.4 Endpoint score at 8 weeks (MOAS, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.55 [‐0.40, 1.50] |
| 2 Mental state: 1a. No change in general mental state Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 No response at 8 weeks ( ≤ 25% reduction at PANSS score) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.16, 6.42] |
| 3 Mental state: 1b. Change in general mental state Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Clinical response at 8 weeks (25 ‐ 50% reduction at PANSS score) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.34, 5.21] |
| 3.2 Clinical response at 8 weeks (50 ‐ 75% reduction at PANSS score) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.39, 1.95] |
| 3.3 Clinical response at 8 weeks ( ≥ 75% reduction at PANSS score) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.43, 2.33] |
| 4 Mental state: 1c. Average scores ‐ i. Over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Endpoint score at 2 weeks (PANSS, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [‐2.15, 6.95] |
| 4.2 Endpoint score at 4 weeks (PANSS, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.95 [‐3.07, 6.97] |
| 4.3 Endpoint score at 6 weeks (PANSS, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 5.75 [‐0.09, 11.59] |
| 4.4 Endpoint score at 8 weeks (PANSS, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 3.55 [‐0.69, 7.79] |
| 4.5 Endpoint score at 2 weeks (PANSS positive symptoms sub‐scale, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.15 [‐0.37, 2.67] |
| 4.6 Endpoint score at 4 weeks (PANSS positive symptoms sub‐scale, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [0.01, 3.39] |
| 4.7 Endpoint score at 6 weeks (PANSS positive symptoms sub‐scale, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 2.05 [‐0.15, 4.25] |
| 4.8 Endpoint score at 8 weeks (PANSS positive symptoms sub‐scale, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 2.25 [‐0.36, 4.86] |
| 4.9 Endpoint score at 2 weeks (PANSS negative symptoms sub‐scale, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐0.07, 2.67] |
| 4.10 Endpoint score at 4 weeks (PANSS negative symptoms sub‐scale, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.31 [‐0.04, 2.66] |
| 4.11 Endpoint score at 6 weeks (PANSS negative symptoms sub‐scale, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐0.12, 3.52] |
| 4.12 Endpoint score at 8 weeks (PANSS negative symptoms sub‐scale, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 2.05 [‐0.06, 4.16] |
| 4.13 Endpoint score at 2 weeks (PANSS general psychopathology sub‐scale, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐1.75 [‐4.73, 1.23] |
| 4.14 Endpoint score at 4 weeks (PANSS general psychopathology sub‐scale, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐3.73, 2.53] |
| 4.15 Endpoint score at 6 weeks (PANSS general psychopathology sub‐scale, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.01 [‐1.88, 3.90] |
| 4.16 Endpoint score at 8 weeks (PANSS general psychopathology sub‐scale, high = worse) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.37 [‐1.30, 4.04] |
| 5 Adverse effects: 1a. Specific ‐ Anticholinergic Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Blurred vision over 24 hours | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.43] |
| 6 Adverse effects: 1b. Specific ‐ Arousal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Somnolence over 24 hours | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.17, 2.18] |
| 7 Adverse effects: 1c. Specific ‐ Cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Tachycardia over 24 hours | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.49, 32.72] |
| 8 Adverse effects: 1d. Specific ‐ Gastrointestinal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Nausea and vomiting over 24 hours | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.90] |
| 9 Adverse effects: 1e. Specific ‐ Movement disorders Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Akathisia over 24 hours | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.46, 6.06] |
| 9.2 Hypermyotonia over 24 hours | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.95, 51.80] |
| 10 Adverse effects: 1f. Specific ‐ Miscellaneous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Headache over 24 hours | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.16, 6.42] |
| 10.2 Liver Function Tests (LFTs) elevation over 24 hours | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.90] |
| 10.3 Weight gain over 24 hours | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.49, 32.72] |
| 10.4 Agitation over 24 hours | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.5 [0.83, 14.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1. Agitation ‐ Average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Endpoint score at 1 week (PANSS‐EC, high = worse) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [0.42, 4.98] |
| 1.2 Endpoint score at 2 weeks (PANSS‐EC, high = worse) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [0.32, 4.48] |
| 1.3 Endpoint score at 4 weeks (PANSS‐EC, high = worse) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [0.53, 4.27] |
| 2 Global Outcome: 1. Average scores ‐ i. Over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Endpoint score at 1 week (CGI‐I, high = worse) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.61, 0.21] |
| 2.2 Endpoint score at 2 weeks (CGI‐I, high = worse) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [0.07, 0.93] |
| 2.3 Endpoint score at 4 weeks (CGI‐I, high = worse) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [0.14, 0.86] |
| 2.4 Endpoint score at 1 week (CGI‐S, high = worse) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.25, 0.65] |
| 2.5 Endpoint score at 2 weeks (CGI‐S, high = worse) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [0.07, 0.93] |
| 2.6 Endpoint score at 4 weeks (CGI‐S, high = worse) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [0.14, 0.86] |
| 3 Mental state: 1a. No change in general mental state Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 No response at 4 weeks ( < 50% reduction BPRS score) | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.61 [1.44, 78.36] |
| 4 Mental state: 1b. Change in general mental state Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Clinical response at 4 weeks (50 ‐ 75% reduction at BPRS score) | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.50, 0.97] |
| 4.2 Clinical response at 4 weeks ( ≥ 75% reduction at BPRS score) | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.25, 2.89] |
| 5 Mental state: 1c. Average scores ‐ i. Over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Endpoint score at 1 week (BPRS, high = worse) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 5.20 [1.04, 9.36] |
| 5.2 Endpoint score at 2 weeks (BPRS, high = worse) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 6.20 [2.48, 9.92] |
| 5.3 Endpoint score at 4 weeks (BPRS, high = worse) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 5.40 [1.84, 8.96] |
| 6 Adverse effects: 1. General ‐ Total number of AEs Show forest plot | Other data | No numeric data | ||
| 7 Adverse effects: 2a. Specific ‐ Anticholinergic Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Dry mouth over 24 hours | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.81, 5.55] |
| 7.2 Constipation over 24 hours | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.62, 2.72] |
| 8 Adverse effects: 2b. Specific ‐ Arousal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Excessive sedation over 24 hours | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 0.92] |
| 9 Adverse effects: 2c. Specific ‐ Cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Tachycardia over 24 hours | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.46, 3.30] |
| 10 Adverse effects: 2d. Specific ‐ Gastrointestinal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Nausea over 24 hours | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.20, 22.31] |
| 11 Adverse effects: 2e. Specific ‐ Movement disorders Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 EPS over 24 hours | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.49, 5.14] |
| 11.2 Tremor over 24 hours | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.25, 2.89] |
| 12 Adverse effects: 2f. Specific ‐ Miscellaneous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Headache over 24 hours | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.19] |
| 12.2 Skin rash over 24 hours | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1. Agitation ‐ average scores ‐ i. over 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Endpoint score at 3 days (PANSS‐EC, high = worse) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐2.98, 2.76] |
| 1.2 Endpoint score at 5 days (PANSS‐EC, high = worse) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 5.47 [2.64, 8.30] |
| 1.3 Endpoint score at 7 days (PANSS‐EC, high = worse) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 5.11 [2.51, 7.71] |
| 1.4 Endpoint score at 2 weeks (PANSS‐EC, high = worse) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.90, 1.08] |
| 1.5 Endpoint score at 4 weeks (PANSS‐EC, high = worse) | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.85, 1.19] |
| 2 Specific behaviour: 1. Aggression ‐ Average scores ‐ i. over 24 hours Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Endpoint score at 3 days (MOAS, high = worse) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 1.07 [‐0.20, 2.34] |
| 2.2 Endpoint score at 5 days (MOAS, high = worse) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [‐0.83, 1.59] |
| 2.3 Endpoint score at 7 days (MOAS, high = worse) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 3.32 [2.07, 4.57] |
| 2.4 Endpoint score at 2 weeks (MOAS, high = worse) | 2 | 128 | Mean Difference (IV, Fixed, 95% CI) | 1.13 [0.23, 2.02] |
| 2.5 Endpoint score at 4 weeks (MOAS, high = worse) | 2 | 128 | Mean Difference (IV, Fixed, 95% CI) | 1.57 [0.75, 2.39] |
| 2.6 Endpoint score at 6 weeks (MOAS, high = worse) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 1.47 [0.83, 2.11] |
| 2.7 Endpoint score at 8 weeks (MOAS, high = worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐0.11, 2.11] |
| 3 Mental state: 1. No change in general mental state Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 No clinical response at 8 weeks ( < 30% reduction at PANSS score) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.44, 6.38] |
| 4 Mental state: 2. Average scores ‐ i. over 24 hours Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Endpoint score at 2 weeks (PANSS, high = worse) | 4 | 253 | Mean Difference (IV, Fixed, 95% CI) | 3.48 [1.88, 5.08] |
| 4.2 Sub‐group analysis: Endpoint score at 2 weeks (PANSS, high = worse) | 3 | 185 | Mean Difference (IV, Fixed, 95% CI) | 2.50 [0.78, 4.21] |
| 4.3 Endpoint score at 4 weeks (PANSS, high = worse) | 4 | 253 | Mean Difference (IV, Fixed, 95% CI) | 5.45 [3.81, 7.08] |
| 4.4 Sub‐group analysis: Endpoint score at 4 weeks (PANSS, high = worse) | 3 | 185 | Mean Difference (IV, Fixed, 95% CI) | 4.52 [2.76, 6.29] |
| 4.5 Endpoint score at 6 weeks (PANSS, high = worse) | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | 9.90 [7.42, 12.37] |
| 4.6 Endpoint score at 8 weeks (PANSS, high = worse) | 2 | 122 | Mean Difference (IV, Fixed, 95% CI) | 5.83 [4.12, 7.54] |
| 4.7 Endpoint score at 2 weeks (PANSS positive symptoms sub‐scale, high = worse) | 3 | 191 | Mean Difference (IV, Fixed, 95% CI) | 0.64 [‐0.24, 1.53] |
| 4.8 Endpoint score at 4 weeks (PANSS positive symptoms sub‐scale, high = worse) | 3 | 191 | Mean Difference (IV, Fixed, 95% CI) | 2.75 [1.86, 3.64] |
| 4.9 Endpoint score at 6 weeks (PANSS positive symptoms sub‐scale, high = worse) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 4.4 [1.40, 7.40] |
| 4.10 Endpoint score at 8 weeks (PANSS positive symptoms sub‐scale, high = worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [0.71, 2.69] |
| 4.11 Endpoint score at 2 weeks (PANSS negative symptoms sub‐scale, high = worse) | 2 | 128 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.57, 1.15] |
| 4.12 Endpoint score at 4 weeks (PANSS negative symptoms sub‐scale, high = worse) | 2 | 128 | Mean Difference (IV, Fixed, 95% CI) | 1.23 [‐0.16, 2.62] |
| 4.13 Endpoint score at 6 weeks (PANSS negative symptoms sub‐scale, high = worse) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [1.07, 6.53] |
| 4.14 Endpoint score at 8 weeks (PANSS negative symptoms sub‐scale, high = worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐0.02, 2.62] |
| 4.15 Endpoint score at 2 weeks (PANSS general psychopathology sub‐scale, high = worse) | 2 | 128 | Mean Difference (IV, Fixed, 95% CI) | 0.93 [‐0.07, 1.93] |
| 4.16 Endpoint score at 4 weeks (PANSS general psychopathology sub‐scale, high = worse) | 2 | 128 | Mean Difference (IV, Fixed, 95% CI) | 1.14 [0.04, 2.23] |
| 4.17 Endpoint score at 6 weeks (PANSS general psychopathology sub‐scale, high = worse) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 6.5 [4.06, 8.94] |
| 4.18 Endpoint score at 8 weeks (PANSS general psychopathology sub‐scale, high = worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [0.43, 2.77] |
| 5 Adverse effects: 1a. General ‐ Total number of AEs Show forest plot | Other data | No numeric data | ||
| 6 Adverse effects: 1b. General ‐ Serious Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 myocardial ischaemia (at 8 weeks) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.93] |
| 7 Adverse effects: 2a. Specific ‐ Anticholinergic Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Blurred vision over 24 hours | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.37, 2.68] |
| 7.2 Dry mouth over 24 hours | 2 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.76, 3.54] |
| 8 Adverse effects: 2b. Specific ‐ Arousal Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Insomnia over 24 hours | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.77] |
| 8.2 Somnolence over 24 hours | 2 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.44, 1.63] |
| 9 Adverse effects: 2c. Specific ‐ Cardiovascular Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Decreased blood pressure over 24 hours | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.18, 3.10] |
| 9.2 Tachycardia over 24 hours | 4 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.83, 2.67] |
| 9.3 T‐wave changes in ECG over 24 hours | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.77] |
| 10 Adverse effects: 2d. Specific ‐ Gastrointestinal Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Constipation over 24 hours | 3 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.70, 1.76] |
| 10.2 Nausea over 24 hours | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.7 [0.29, 1.71] |
| 11 Adverse effects: 2e. Specific ‐ Movement disorders Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 EPS over 24 hours | 2 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.76, 2.39] |
| 11.2 Akathisia over 24 hours | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.28, 2.03] |
| 11.3 Tremor over 24 hours | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.40, 3.56] |
| 12 Adverse effects: 2f. Specific ‐ Movement disorders ‐ Average scores ‐ i. Over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 Endpoint score at 3 days (TESS, high = worse) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐1.35, 1.27] |
| 12.2 Endpoint score at 5 days (TESS, high = worse) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐1.55, 1.33] |
| 12.3 Endpoint score at 7 days (TESS, high = worse) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐1.88, 0.86] |
| 13 Adverse effects: 2g. Specific ‐ Miscellaneous Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Headache over 24 hours | 4 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.53, 1.68] |
| 13.2 Weight gain over 24 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.47, 4.78] |
| 13.3 Oedema over 24 hours | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 13.4 Leukopenia over 24 hours | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 13.5 Liver Function Tests (LFTs) elevation over 24 hours | 2 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.15, 2.40] |
| 14 Leaving the study early Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 For any reason | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.13] |
| 14.2 Due to adverse effects | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.13] |

