Ácidos grasos poliinsaturados(AGPI) para los niños con trastornos específicos de aprendizaje
Resumen
Antecedentes
Alrededor del 5% de los niños en edad escolar presenta un trastorno específico de aprendizaje, definido como el fracaso en la adquisición de capacidades adecuadas en la lectura, la escritura o las matemáticas que no es resultado de una capacidad intelectual reducida, de una enseñanza inadecuada o de la marginación social. De estos eventos, el 80% está relacionado con la lectura. Los ácidos grasos poliinsaturados (AGPI), en particular los ácidos grasos omega 3 y omega 6, que se encuentran normalmente en abundancia en el cerebro y la retina, son importantes para el aprendizaje. Se ha encontrado que algunos niños con trastornos específicos de aprendizaje presentan deficiencias en estos ácidos grasos poliinsaturados, y se sostiene que los suplementos de ácidos grasos poliinsaturados pueden ayudar a estos niños a mejorar las capacidades de aprendizaje.
Objetivos
1. Evaluar los efectos sobre los resultados de aprendizaje de la administración de suplementos de ácidos grasos poliinsaturados (AGPI) en niños con trastornos específicos de aprendizaje.
2. Determinar si se informan efectos adversos de la administración de suplementos AGPI en estos niños.
Métodos de búsqueda
En noviembre 2015, se hicieron búsquedas en CENTRAL, Ovid MEDLINE, Embase, PsycINFO, en otras 10 bases de datos y en dos registros de ensayos. Además, se buscaron las listas de referencias de artículos pertinentes.
Criterios de selección
Ensayos controlados aleatorizados (ECA) o cuasialeatorizados que comparan AGPI con placebo o ningún tratamiento en niños menores de 18 años con trastornos específicos de aprendizaje, diagnosticados de acuerdo con la quinta (o anterior) edición del Manual Diagnóstico y Estadístico de los Trastornos Mentales (DSM‐5), o la décima (o anterior) revisión de la Clasificación Internacional de Enfermedades (CIE‐10) o criterios equivalentes. Se incluyeron niños con trastornos del desarrollo coexistentes como trastorno de déficit de atención e hiperactividad (TDAH) o autismo.
Obtención y análisis de los datos
Dos autores de la revisión (MLT y KHT) seleccionaron de forma independiente los títulos y los resúmenes de artículos identificados por la búsqueda y eliminaron todos los estudios que no cumplían con los criterios de inclusión. Cuando fue necesario, se contactó con los autores de los estudios para pedir información y aclaraciones que faltaban. Se utilizaron los criterios GRADE para evaluar la calidad de la evidencia.
Resultados principales
Dos estudios pequeños con 116 niños,principalmente chicos de entre 10 y 18 años, cumplieron con los criterios de inclusión. Un estudio se realizó en un contexto escolar y el otro en una clínica especializada. Ambos estudios utilizaron durante tres meses una combinación de cápsulas suplementos de omega‐3 y omega‐6 como intervención comparada con placebo. Aunque ambos estudios tuvieron en general un bajo riesgo de sesgo, se consideró que el riesgo de sesgo de informe fue incierto en un estudio y alto en el otro. Además, uno de los estudios fue financiado por la industria e informó de la participación activa de la empresa en el estudio.
Ninguno de los estudios informó de datos sobre los resultados primarios de las puntuaciones de lectura, escritura, ortografía y matemáticas, evaluados mediante pruebas estandarizadas.
La evidencia de baja calidad indica que la administración de suplementos de AGPI no aumentó el riesgo de trastornos gastrointestinales (riesgo relativo 1,43, intervalo de confianza del 95%: 0,25 a 8,15; dos estudios, 116 niños). Los investigadores no informaron otros efectos adversos.
Ambos estudios informaron resultados de conductas relacionadas con el trastorno de déficit de atención e hiperactividad (TDAH). No se puedo combinar los resultados en un metanálisis porque un estudio informó de los resultados como un resultado continuo y el otro como un resultado dicotómico. No se informó sobre otros resultados secundarios.
Se excluyó un estudio porque utilizaba una cointervención (carnosina), y otros cinco estudios porque no proporcionaban un diagnóstico sólido de un trastorno específico del aprendizaje. Se identificó un estudio en curso y se encontraron tres estudios que están a la espera de clasificación.
Conclusiones de los autores
No hay evidencia suficiente para establecer conclusiones acerca del efecto de los AGPI sobre las capacidades de aprendizaje de niños con trastornos específicos de aprendizaje. Se necesitan ECA bien diseñados con poblaciones claramente definidas de niños con trastornos específicos de aprendizaje que hayan sido diagnosticados por criterios diagnósticos estandarizados.
PICO
Resumen en términos sencillos
Ácidos grasos poliinsaturados(AGPI) para los niños con trastornos específicos de aprendizaje
Pregunta de la revisión
Se revisó la evidencia sobre los efectos de los ácidos grasos poliinsaturados (AGPI), comparados con placebo o ningún tratamiento, en la lectura, la escritura o las habilidades matemáticas de los niños de hasta 18 años de edad que tienen trastornos específicos de aprendizaje.
Antecedentes
Los niños con trastornos específicos de aprendizaje son aquellos cuyas capacidades de lectura, ortografía, escritura y habilidades matemáticas se encuentran considerablemente por debajo de lo esperado para su edad y cuyos problemas no son el resultado de una menor inteligencia, de una enseñanza insuficiente ni de la marginación social. Un niño puede tener un solo trastorno, como un trastorno de lectura, o un trastorno combinado, como un trastorno de lectura y matemáticas. Los niños podrían presentar otros trastornos también, como trastorno de déficit de atención e hiperactividad (TDAH).
Se sabe que los ácidos grasos poliinsaturados son necesarios para el desarrollo y funcionamiento normal del cerebro. Los AGPI más conocidos son los ácidos grasos omega‐3, que incluyen el ácido docosahexaenoico (comúnmente conocido como DHA), pero los ácidos grasos omega‐6 también son conocidos. Los ácidos grasos poliinsaturados deben obtenerse de alimentos o suplementos, ya que el cuerpo humano no puede fabricarlos a partir de otros tipos de grasa. Debido a que son necesarios para el crecimiento y desarrollo normal del cerebro, los AGPI pueden ayudar a los niños con trastornos específicos de aprendizaje.
Características de los estudios
La evidencia se actualizó hasta noviembre de 2015.
Se encontraron dos estudios pequeños con 116 niños que cumplieron con los criterios de inclusión. Ambos estudios dieron a los niños una combinación de cápsulas de omega‐3 y omega‐6 como intervención durante tres meses. La mayoría de estos estudios implicaron a niños de entre 10 y 18 años de edad; uno fue realizado en un ambiente escolar, y el otro en una clínica especializada. Uno de los estudios fue financiado por la empresa que suministró los suplementos de omega 3 y omega 6.
El otro estudio no se pudo incluir en esta revisión porque los investigadores agregaron carnosina (un aminoácido altamente concentrado en el cerebro) a los AGPI. La carnosina y los AGPI podrían tener efectos similares, por lo que no sería posible separar los efectos de los dos ingredientes. Los autores de la revisión excluyeron cinco estudios porque no se confirmó que se diagnosticara un trastorno específico del aprendizaje en estos niños.
Resultados clave
Ninguno de los estudios incluidos informó de los efectos de los AGPI en la lectura, la escritura, la ortografía o las capacidades matemáticas de los niños.
La evidencia de baja calidad (porque los estudios incluyeron pocos participantes y mostraron evidencia de sesgo) sugieren que el uso de los AGPI no aumentó el riesgo de trastornos menores del aparato digestivo. Los estudios incluidos no informaron ningún otro tipo de efectos adversos.
Ambos estudios informaron sobre la conducta relacionada con el TDAH. Sin embargo, el formato de los datos disponibles no permitió combinarlos fácilmente para sacar conclusiones. Los estudios incluidos no informaron ningún otro resultado secundario.
Conclusión
No hay evidencia suficiente para apoyar ni refutar el uso de AGPI en niños con trastornos específicos de aprendizaje.
Authors' conclusions
Summary of findings
| PUFAs vs placebo | ||||||
| Patient or population: children with specific learning disorders | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with PUFAs | |||||
| Standardised tests of reading, writing, spelling or mathematics skills (overall) | None of the included studies assessed effects on standardised tests of reading, writing, spelling or mathematics skills | Not estimable | ‐ | ‐ | ‐ | |
| Adverse effect (gastrointestinal disturbance) | Study population | RR 1.43 | 116 | ⊕⊕⊝⊝ | ‐ | |
| 35 per 1000 | 50 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSmall sample size with few events. Extremely wide CIs. | ||||||
Background
Description of the condition
Specific learning disorders are a heterogenous group of disorders that can be broadly defined as unexpected, specific and persistent failure to acquire adequate academic skills despite conventional teaching, adequate intelligence and ample sociocultural opportunities (Lagae 2008). These specific academic skills have been categorised as listening (receptive language), speaking (expressive language), basic reading, reading comprehension, written expression, mathematical calculation and mathematical reasoning (Lyons 1996). In general, about 5% of school children have a specific learning disorder (Lagae 2008), 80% of which are reading disorders (Shaywitz 1998).
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) (APA 2013) and the International Classification of Diseases, 10th Revision (ICD‐10) (WHO 1992) classify and describe specific learning disorders similarly. They point out two important characteristics. First, learning disorders are developmental disorders that begin in early childhood and do not result from inadequate opportunities for learning nor from the presence of physical abnormalities. Second, researchers have noted a discrepancy between expected achievement for the level of intelligence and actual achievement on individually administered, standardised tests of reading, writing and mathematics.
Specific learning disorders present as a reading disorder (dyslexia), a writing disorder (dysgraphia), a mathematical skills disorder (dyscalculia) or a combination of any of the three. The specific learning disorder that occurs most often as an independent disorder is reading disorder, or dyslexia. Dyslexia affects between 5% and 17.5% of school‐aged children (Shaywitz 1998). The next most common disorder is dyscalculia, which affects between 3% and 6.5% of school‐aged children (Shalev 2004). Other types of specific learning disorders, such as difficulties with writing or spelling, are usually accompanied by a reading disorder (APA 2000; Dirks 2008). It is not unusual for individuals with a specific learning disorder to have other coexistent neurodevelopmental problems such as attention deficit hyperactivity disorder (ADHD), developmental co‐ordination disorder, conduct disorder, oppositional defiant disorder, major depressive disorder and dysthymic disorder (APA 2000). A recent review found that up to 45% of children have a specific learning disorder coexisting with ADHD (DuPaul 2013). DSM‐5 provides a more inclusive definition and states that it is no longer necessary to divide specific learning disorders into the categories above (APA 2013).
Researchers who assess learning outcomes or academic achievement in children with specific learning disorders should measure the component skills of reading, spelling and mathematics by administering various achievement tests (Beitchman 1997). Tests of reading should assess accuracy, fluency and comprehension (Beitchman 1997), and tests of mathematics should assess numerical concepts, number facts and arithmetic procedures (Shalev 2004). Writing is sometimes assessed as spelling (Angelelli 2004), but specific tests are also available to assess handwriting. These assessments are best done by means of standardised tests, namely, tests that can be conducted under exact and repeatable conditions and yield scores that can be interpreted uniformly (Domino 2006).
Children with specific learning disorders may have poor academic achievement, as well as problems with self‐esteem (Terras 2009) and school failure (Lagae 2008). In the United States in 2006, about 25% of school‐aged children and adolescents with specific learning disorders failed to complete school (USDE 2011).
Description of the intervention
Polyunsaturated fatty acids (PUFAs) are essential fatty acids that must be obtained through the diet because the human body is incapable of producing them (Innis 2008). Polyunsaturated fatty acids are classified according to the position of double bonds from the methyl end of the molecule. The main classes of PUFAs studied in children with neurodevelopmental problems are omega‐3 and omega‐6 fatty acids (Schuchardt 2010). Little is known about the use of other PUFAs, such as omega‐9 (oleic acid), in these children.
The omega‐3 fatty acids include alpha‐linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Simopoulos 1991). Among these, DHA is most abundantly found in the human central nervous system and is concentrated in the membrane lipids of cerebral gray matter and in the retina (Innis 2008). Omega‐6 fatty acids comprise linoleic acid (LA), gamma‐linolenic acid (GLA) and arachidonic acid (AA) (Simopoulos 1991). Common dietary sources of omega‐3 fatty acids are oily fish (Whelan 2009), and omega‐6 fatty acids are commonly found in grains such as wheat, rice and maize (Simopoulos 1991). Alternative sources of omega‐3 fatty acids include marine microbiota and transgenic oilseeds (Sayanova 2011).
Conversion of the omega‐3 fatty acid precursor ALA to EPA and DHA requires the same enzyme as is required for conversion of the omega‐6 fatty acid precursor LA to GLA and AA. Therefore, an increase in the supply of LA will lead to competitive reduction in production of EPA and DHA (Schuchardt 2010). The importance of this competition is that both AA (omega‐6) and EPA (omega‐3) are parent derivatives of eicosanoids, but they have different physiological functions (Simopoulos 2002). The ideal omega‐6/omega‐3 ratio is 1:1 to 4:1, but ratios in the modern diet may be as high as 20:1 (Simopoulos 2002). The ratio of omega‐6 to omega‐3 fatty acids is thus important, and abnormalities in this ratio are seen in adults with dyslexia (Cyhlarova 2007; Laasonen 2009) and in children with ADHD and learning disabilities (Milte 2011).
Polyunsaturated fatty acid supplements are given as omega‐3 fatty acids alone (Richardson 2005), or as omega‐3 fatty acids in combination with omega‐6 fatty acids (Johnson 2009). Most PUFA supplements contain some vitamin E (usually 0.6 mg of alpha‐tocopherol to 1 gram PUFA) to prevent oxidation of PUFAs in tissues (Valk 2000). The duration of PUFA supplementation is an important factor. Kinetic studies show that it takes about four to six months for DHA to achieve a steady state in red blood cell membranes (Arterburn 2006). Therefore, any duration of intervention less than three months is likely to be ineffective.
How the intervention might work
Polyunsaturated fatty acids have two major functions in the central nervous system: maintaining the fluidity of the cell membrane and sustaining the integrity of the myelin sheath (Yehuda 2005). Neuronal cell membrane fluidity is necessary for exchange of neuronal information. Both ALA (omega‐3) and LA (omega‐6) can directly increase the fluidity index of the cell membrane (Yehuda 2005). Deficiency in omega‐3 fatty acids, especially during the early years of development, can result in delayed myelination of neurons and may manifest as learning, motor, visual and auditory abnormalities (Yehuda 2005). In an animal study model, the addition of an omega‐6‐rich diet reversed the learning disability of omega‐3‐deficient rats (Ikemoto 2001).
Children with neurodevelopmental disorders, such as dyslexia, dyspraxia, ADHD and autism, have a higher incidence of omega‐3 fatty acid deficiency and imbalance of omega‐3 and omega‐6 fatty acids (Richardson 2000a). The earliest suggestion that children with a specific learning disorder may have an imbalance or deficiency in fatty acids came in a case report in 1985 (McDonald Baker 1985). Subsequently, two studies demonstrated that individuals with dyslexia were more likely to have fatty acid deficiency signs (FADS), which include excessive thirst, frequent urination, dry skin, dry hair, brittle nails, dandruff and follicular keratosis (Richardson 2000b; Stevens 1996). However, to date, no studies have specifically compared omega‐3 fatty acid levels in children with a specific learning disorder versus those in the general population. One study measuring cheek cell omega‐3 fatty acid levels in children attending mainstream school found no correlation of omega‐3 fatty acid level with reading or spelling abilities (Kirby 2010). Another randomised controlled trial (RCT) showed a significant improvement in the reading ability of children with developmental co‐ordination disorder whose diet was supplemented with omega‐3 fatty acids (Richardson 2005).
The exact mechanism by which PUFAs may work in children with specific learning disorders remains unknown. Most available evidence pertains to reading disorders only. Below, we present three possible explanations of how supplementation of PUFAs may help children with a specific reading disorder.
Improving the visual magnocellular pathway
The visual neural pathway responsible for transmitting signals from the retina to the brain can be divided into large, heavily myelinated cells ‐ the magnocellular pathway ‐ and smaller cells ‐ the parvocellular pathway (Stein 1997). The magnocellular pathway is responsible for visual motion sensitivity, which is strongly related to reading ability (Stein 2000). Dyslexic individuals have been found to have poor motion sensitivity and abnormalities in binocular stability, both of which are linked to the visual magnocellular pathway (Stein 2000). The role of PUFAs in specific reading disorders or dyslexia may be linked to both early development of the magnocellular pathway (Taylor 2000) and its dysfunction (Stein 2000).
Improving working memory
In animal studies, rats raised with a diet deficient in omega‐3 fatty acids were found to have poorer reference and working memory (Chung 2008). Children with specific learning disorders ‐ reading or mathematics disorder alone or in combination (Geary 2011; Schuchardt 2008) ‐ also have impairments in their working memory. Therefore, PUFA supplementation could help to improve their learning abilities.
Improving dark adaptation
Vision in low light is regulated mainly by rod cells in the retina, which are rich in omega‐3 fatty acids, in particular, DHA (Innis 2008). Poor dark adaptation or reduced scotopic sensitivity has been associated with dyslexia, although not consistently (Carroll 1994; Greatrex 2000). If such an association is substantiated, this adds to the biological plausibility of why omega‐3 might be involved in dyslexia. In a small study of a group of dyslexic individuals, supplementation with DHA‐rich fish oil improved their dark adaptation (Stordy 2000).
Safety concerns of PUFA supplementation
Researchers have expressed two major concerns regarding PUFA supplements. The first arises from the sources of PUFAs, especially omega‐3 fatty acids, which often are derived from oily fish. Concerns surround consumption of oily fish and toxic environmental contamination from mercury, polychlorinated biphenyls (PCBs) and dioxin (Bays 2007; USFDA 1995). Strict adherence to good manufacturing guidelines is the key factor in the production of omega‐3 fatty acid supplements that are free from these contaminants (Bays 2007). The second concern involves adverse effects resulting directly from omega‐3 fatty acids, specifically, increased risk of bleeding and reduced immunity (USFDA 2000). High doses of omega‐3 fatty acids are thought to impair platelet aggregation and reduce the individual's immune response (Lien 2009). However, a review of preclinical toxicology studies and intervention studies showed that omega‐3 fatty acid supplementation is generally safe (Lien 2009). A narrative review on the safety of PUFAs in high‐risk individuals taking up to three grams of PUFAs per day did not show increased risk of bleeding (Hamazaki 2014). Other minor adverse effects include hypersensitivity to fish or seafood and gastrointestinal disturbances (Abramowicz 2014; Prester 2016).
Why it is important to do this review
The shift in the burden of health care for children from acute, life‐threatening problems to chronic, non‐communicable diseases has resulted in increased recognition of specific learning disorders (Liu 2012; Lopez 2006). At present, the mainstay of treatment for a child with a specific learning disorder is educational intervention because learning disorders are viewed essentially as language‐based disorders (Handler 2011). To be effective, educational interventions require long‐term commitment from the child and his or her family (Alexander 2004). During childhood, intervention is focused on remediation, but in adulthood, intervention is targeted at adjustment and compensation strategies (Lagae 2008). Therefore, it is not surprising that individuals with this disability and their families look for unconventional methods of treatment, such as visual training, optometric exercise, tinted glasses and cerebellar exercises (Lagae 2008). Most of these methods are costly and have not been scientifically proven (NPG 2007).
Nutritional supplementation is a popular alternative treatment because of its ease of administration, relative safety, availability and affordability. Two Cochrane systematic reviews recently examined the use of PUFAs in children with ADHD (Gillies 2012) and autism spectrum disorders (James 2011). Review authors concluded that little or no evidence indicates that PUFAs are beneficial for children with ADHD or autism. This review will add to our understanding of the potential therapeutic role of PUFAs.
Objectives
-
To assess effects on learning outcomes of supplementation of polyunsaturated fatty acids (PUFAs) for children with specific learning disorders.
-
To determine whether adverse effects of supplementation of PUFAs are reported in these children.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or quasi‐RCTs.
Types of participants
Children under 18 years of age with specific learning disorders, as diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) (APA 2013) or earlier editions; the International Classification of Diseases, 10th Revision (ICD‐10) (WHO 1992) or earlier revisions; or similar criteria. Specific learning disorders may include:
-
reading disorder (developmental dyslexia);
-
mathematics disorder (developmental dyscalculia);
-
writing disorder (dysgraphia); and
-
spelling disorder.
The specific learning disorder may occur in conjunction with other neurodevelopmental disorders such as attention deficit hyperactivity disorder (ADHD) and autism spectrum disorders (ASDs).
Types of interventions
Polyunsaturated fatty acids (PUFAs) compared with placebo or no treatment. Any other nutritional supplements and educational interventions could have been used but must have been given to both intervention and control groups.
('Nutritional supplements' refers to interventions that may directly affect the outcome and does not include antioxidants, such as vitamin E, added to PUFA supplements for the purpose of preventing oxidation.)
Types of outcome measures
Primary outcomes
-
Reading, writing, spelling or mathematics scores measured by standardised tests such as:
-
Weschsler Objective Reading Dimensions (Weschler 1993);
-
Woodcock Reading Mastery Tests (Woodcock 2011);
-
Gray Oral Reading Test (Bryant 2011);
-
Wide Range Achievement Test (Robertson 2006);
-
Young's Group Mathematics Test (Young 1996);
-
Neuropsychological Test Battery for Number Processing and Calculation in Children (von Aster 2006);
-
British Ability Scales (BAS3 2011);
-
Test of Handwriting Skills ‐ Revised (Milone 2007); and
-
other similar tests, provided they are standardised for the population intended.
-
-
Reports of adverse effects, including:
-
bleeding manifestation such as prolonged bleeding time or haemorrhagic stroke;
-
reduction of immunity such as increased incidence of infection; and
-
other minor adverse effects such as hypersensitivity or gastrointestinal disturbances.
-
Secondary outcomes
Only results of validated scales (if used) were to be included.
-
Parent‐ or teacher‐reported outcomes such as academic performance, school dropout or behaviour.
-
Self‐reported outcomes such as self‐esteem.
Search methods for identification of studies
We ran searches for this review update in October 2014 and November 2015 (see Appendix 1). When possible, we limited updated searches to the period following the previous search. Review authors conducted searches for the original review (Tan 2012) in July 2011 and April 2012 (see Appendix 2), with no restrictions on language or date.
Electronic searches
We searched the electronic databases and trials registers listed below.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 10) in the Cochrane Library, which includes the Cochrane Developmental, Psychosocial and Learning Problems Group Specialised Register.
-
Ovid MEDLINE (1946 to November week 2 2015).
-
Embase Ovid (1980 to 2015 week 46).
-
PsycINFO Ovid (1967 to November week 2 2015).
-
ERIC ProQuest (Education Resources Information Center; 1966 to current).
-
Science Citation Index ‐ Expanded Web of Science (1970 to 19 November 2015).
-
Social Science Citation Index Web of Science (1970 to 19 November 2015).
-
Conference Proceedings Citation Index ‐ Science Web of Science (1990 to 19 November 2015).
-
Conference Proceedings Citation Index ‐ Social Sciences & Humanites Web of Science (1990 to 19 November 2015).
-
Cochrane Database of Systematic Reviews (CDSR; 2015, Issue 11 of 12) in the Cochrane Library.
-
Database of Abstracts of Reviews of Effects (DARE; 2015, Issue 2 of 4) in the Cochrane Library.
-
ZETOC (all available years; zetoc.jisc.ac.uk/wzgw?db=etoc).
-
WorldCat (limited to theses and dissertations; all available years; worldcat.org).
-
Networked Digital Library of Theses and Dissertations (NDLTD; all available years; ndltd.org).
-
ClinicalTrials.gov (all available years; clinicaltrials.gov).
-
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; all available years; apps.who.int/trialsearch).
Searching other resources
We also searched the references of relevant reports identified by the searches.
Data collection and analysis
Selection of studies
Two review authors (MLT and KHT) independently screened the titles and abstracts of articles identified by the search and eliminated all studies that clearly did not meet the inclusion criteria. We retrieved full‐text reports for the remaining papers, and two review authors (MLT and KHT) screened them for inclusion. We resolved all disagreements by discussion and by including a third review author as arbitrator (JJH). Review authors were not blinded to the names of the study authors, nor to institutions or journals of publication. We documented all reasons for excluding studies assessed in full text (see Characteristics of excluded studies).
Data extraction and management
MLT and KHT independently extracted data from included studies onto a specially designed data extraction form. We extracted data on study setting and methods, participant characteristics, interventions and placebo, and on outcome measures. We contacted study authors by email to seek clarification of data or to request further information about the study, when required. We resolved disagreements by discussion and by including a third review author as arbitrator (JJH).
Assessment of risk of bias in included studies
We used the Cochrane risk of bias tool in assessing risk of bias in included studies (Higgins 2011a). Two review authors (MLT and KHT) independently assessed each study for risk of bias in sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other sources of bias. For each of these risk of bias elements, we rated studies as having low, unclear or high risk of bias. We resolved disagreements by discussion and by including a third review author as arbitrator (JJH).
Sequence generation
We rated studies as having low risk of bias if the method of sequence generation consisted of random allocating of participants by random number generator or table, toss of a coin, roll of a dice or drawing of lots. We considered studies that used non‐random sequence generation, including methods such as alternation or date of birth, as having high risk of bias. When information was insufficient to permit a judgement, we rated the study as having unclear risk of bias.
Allocation concealment
We looked to see whether allocation to the intervention was concealed and could not be predicted by participants or investigator by methods such as central allocation, sequentially numbered identical drug containers or sequentially numbered sealed, opaque envelopes. We rated studies that used these methods of allocation concealment as having low risk of bias. We rated studies as having high risk of bias if investigators used open random allocation or unsealed or non‐opaque envelopes. When information was insufficient to permit a judgement, we rated the study as having unclear risk of bias.
Blinding of participants and personnel
All participants and personnel can be blinded in studies examined for this review. In addition to the identical appearance of pills or capsules of PUFAs, the smell and taste should be similar, as PUFAs derived from fish sources have a distinct taste and smell. This has been shown to be detectable even when provided in capsular form (Makrides 2010). We rated blinding of participants and personnel as introducing low risk of bias if blinding was described adequately and knowledge of the allocated intervention was prevented throughout the study. We rated the study as having high risk of performance bias if researchers reported no blinding or incomplete blinding. When information was insufficient to permit a judgement, we rated the study as having unclear risk of performance bias.
Blinding of outcome assessments
We first considered if the outcome was likely (such as self‐reported or parent‐reported outcomes) or not likely (such as results from standardised tests of learning abilities and some teacher‐reported outcomes) to be influenced by blinding. We then rated the study as having low risk of detection bias if blinding was described adequately and knowledge of the allocated intervention was prevented throughout the study. We rated the study as having high risk of detection bias if researchers reported no blinding, or incomplete blinding, and the outcome was likely to be influenced by this. When information was insufficient to permit a judgement, we rated the study as having unclear risk of bias.
Incomplete outcome data
For each outcome of included studies, we looked for reports of attrition and exclusion, including reasons, for each intervention group. We rated studies that addressed incomplete outcome data adequately ‐ all intervention groups had similar reasons for missing data (not related to the true outcome) and numbers in all groups were balanced ‐ as having low risk of bias. We rated studies with imbalanced numbers or different reasons for missing data across groups, or that performed 'as treated' analysis with substantial departure of the intervention received from that assigned at randomisation, as having high risk of bias. When information was insufficient to permit a judgement, we rated the study as having unclear risk of bias.
Selective outcome reporting
We looked for selective outcome reporting by determining whether all prespecified outcomes from the study's protocol (if available) were reported or all expected outcomes from the study were reported; in such cases, we rated studies as having low risk of bias. We rated studies that did not report all of their prespecified outcomes or did not report an expected key outcome as having high risk of bias. When information was insufficient to permit a judgement, we rated the study as having unclear risk of bias.
Other sources of bias
We looked at studies to identify other sources of potential bias, such as inappropriate design, premature stopping of the study, extreme baseline imbalance or suspicion of fraud. We rated studies with such biases as having high risk of bias. We rated studies that were free of these biases as having low risk of bias. If information on which to base our judgement was insufficient, we rated the study as having unclear risk of bias.
Measures of treatment effect
Continuous data
Treatment effects were likely to use a variety of standardised tests. For this review, as only one study provided continuous data (and therefore one test), we presented the mean difference (MD) with 95% confidence interval (CI). Our strategy for continuous data obtained through different tests or scales was set out in our protocol (Tan 2011) and is presented in Table 1.
| Measures of treatment effect |
| Continuous data Treatment effects are likely to require a variety of standardised tests. If we encounter the use of different tests for the same outcome, and we judge these to be similar enough, we will calculate the standardised mean difference (SMD) and the 95% confidence interval (CI) for each. |
| Unit of analysis Issues |
| Cross‐over studies In the analysis of studies with cross‐over design, we will first consider the possibility of carry‐over effect, whether only first period data are available, if any analysis is incorrect and if results are comparable with those of parallel‐group studies. We will use the methods suggested in the Cochrane Handbook for Systematic Reviews of Interventions when analysing and incorporating the trials for meta‐analysis (Higgins 2011b, section 16.4). As a carry‐over effect is likely, given the persistence of PUFAs in tissues (Katan 1997), when data are available for the first period, we will use these in the analyses of learning and behavioural outcomes. However, as adverse effects are readily reversible outcomes, we could use data from both phases. Cluster‐randomised studies We do not anticipate finding any cluster‐randomised trials for this review. However, if we do find them, we will analyse them in the unit to which they were randomised and we will adjust the effective sample size using an intracluster correlation coefficient (ICC), which we will obtain from the studies themselves, if given, or will estimate from similar studies. If we use ICCs from other sources, we will report this and will conduct sensitivity analyses to investigate the effects of variation in the ICC, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b, section16.3). Studies with multiple intervention groups For studies with more than 2 intervention groups, we will combine the intervention (if they are similar, e.g. different dose of PUFAs or different combination of PUFAs), and control groups to create a single pair‐wise comparison. If any of the intervention groups is completely different (e.g. visual exercises, music therapy), we will exclude that particular intervention group from the analysis. |
| Dealing with missing data |
| If more studies are included in the future, we will explore by sensitivity analysis the impact of including studies with high levels of missing data in the overall assessment of treatment effect. We will exclude from the analysis studies with missing data that have a high impact on the final results. For all outcomes, we will carry out analyses, as far as possible, on an intention‐to‐treat basis (i.e. we will attempt to include in the analyses all participants randomised to each group). Missing standard deviations (SDs) may be imputed according to the methods provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). |
| Assessment of reporting biases |
| If we identify 10 or more studies, we will use funnel plots to assess the relationships of treatment effect size and standard error. Asymmetry in the funnel plot may be a result of reporting bias, but could also be a true reflection of heterogeneity among intervention effects. We will further examine clinical heterogeneity of the studies as a possible explanation. We will compare results obtained from published studies and results from other sources (e.g. correspondence) to detect publication bias. |
| Subgroup analysis and investigation of heterogeneity |
| We intend to perform subgroup analyses for the primary outcome only. We will investigate the following subgroups.
|
| Sensitivity analysis |
| If we identify enough studies, we will perform a sensitivity analysis to assess how much the quality of the studies affects meta‐analysis results. We will conduct sensitivity analyses to find out if excluding studies with high risk of bias (as assessed by using the Cochrane tool for assessing risk of bias) has an impact on the final results. These will include studies with cross‐over design, inadequate sequence allocation and concealment, missing data or use of any imputed values. |
ADHD: attention deficit hyperactivity disorder.
ALA: alpha‐lipoic acid.
DHA: docosahexaenoic acid.
EPA: eicosapentaenoic acid.
PUFAs: polyunsaturated fatty acids.
Dichotomous data
When dichotomous data were presented, we used the risk ratio (RR) with 95% CI.
Unit of analysis issues
Because we considered that a carry‐over effect was likely, we treated the cross‐over study included in this review as a parallel‐group study and therefore used only data from the first period. We identified no cluster‐randomised studies and no studies with multiple intervention groups for inclusion in this review. Our strategy for managing cross‐over studies, cluster‐randomised studies and studies with multiple intervention groups in future updates of this review was set out in our protocol (Tan 2011) and is presented in Table 1.
Dealing with missing data
We described details of participant dropout for each of the included studies in 'Risk of bias' tables (beneath the Characteristics of included studies tables). We contacted the original authors of a particular study to request unreported summary data. We encountered no other missing data issues. Our strategy for dealing with missing data in future updates of this review was set out in our protocol (Tan 2011) and is presented in Table 1.
Assessment of heterogeneity
Variation across selected studies may be a result of participant factors (e.g. different specific learning disorders) or study factors (e.g. study design, dropout rates, types and combinations of PUFAs used). We assessed heterogeneity across studies by visually inspecting the forest plots for overlapping CIs. We assessed for statistical heterogeneity by using the Chi² test or the I² statistic. The I² statistic describes the percentage of variability that is due to heterogeneity rather than to chance. A rough estimate for interpretation of I² follows (Deeks 2011).
-
I² = 0% to 40%: might not be important.
-
I² = 30% to 60%: may represent moderate heterogeneity.
-
I² = 50% to 90%: may represent substantial heterogeneity.
-
I² = 75% to 100%: represents considerable heterogeneity.
The threshold for interpreting the I² value can be misleading. Therefore, we determined the importance of the observed I² by looking at the magnitude and direction of the effect, as well as the strength of evidence for clinical heterogeneity.
Assessment of reporting biases
We did not have enough studies to assess for reporting bias. Our strategy for assessing reporting biases in future updates of this review was set out in our protocol (Tan 2011) and is presented in Table 1.
Data synthesis
When we identified two or more homogenous studies for each outcome, we performed a meta‐analysis using Review Manager 5 (RevMan 5) (RevMan 5 2014) and the fixed‐effect model. We did not combine for meta‐analysis studies that were clinically distinct ‐ different treatment combinations or different measurements of learning abilities ‐ and instead presented a narrative description of study results. This narrative description included the general direction, size, consistency and strength of evidence of effect for each individual study but did not compare the effects of each study and did not present an overall conclusion.
Assessment of the quality of the evidence using the GRADE approach
For this update, we specified how we intended to use the GRADE approach, as outlined in the GRADE Handbook, to assess the quality of the body of evidence related to the following outcomes.
-
Reading, writing, spelling or mathematics scores measured by standardised tests.
-
Reports of adverse effects.
We used GRADEpro GDT to import data from RevMan 5 2014 when creating 'Summary of findings' tables. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes using the GRADE approach. The GRADE approach considers five factors (study limitations, consistency of effect, imprecision, indirectness and publication bias) in assessing the quality of the body of evidence for each outcome. Evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
We downgraded the quality of evidence for adverse effects to low because of imprecision (small sample size and wide CIs) and study limitations (selective reporting in one study). Reading, writing, spelling and mathematics scores on standardised tests were not reported in the included studies.
Subgroup analysis and investigation of heterogeneity
We did not have enough studies to perform a subgroup analysis. Our strategies for subgroup analysis and investigation of heterogeneity in future updates of this review were set out in our protocol (Tan 2011) and are presented in Table 1.
Sensitivity analysis
We did not have enough studies to perform a sensitivity analysis. Our strategy for sensitivity analysis in future updates of this review was set out in our protocol (Tan 2011) and is presented in Table 1.
Results
Description of studies
Results of the search
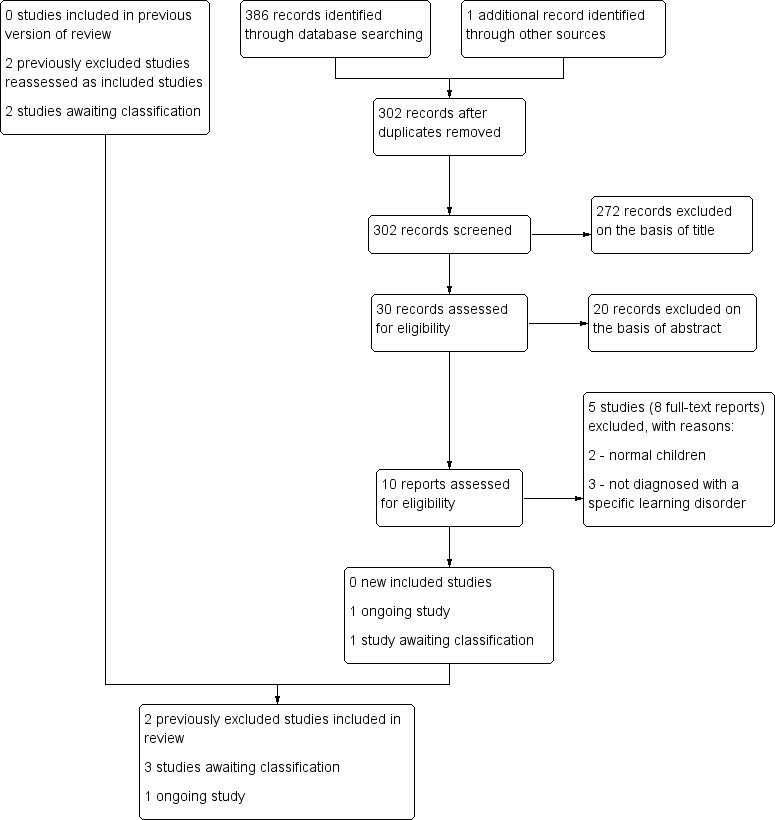
The original search for the previous version of this review identified 795 unique records (794 from the search of databases and one record from another source) (Tan 2012). This record (Anon 2004) was an unpublished study identified from a narrative review on the effects of PUFAs (Richardson 2004). It was not cited, and attempts to contact the author of the narrative review failed. In addition, one paper could not be obtained even after we attempted to contact the publisher and the study author (Richardson 2000).
Searches for this update yielded 302 unique records, including one record discovered in the reference list of a study (Soerensen 2013). We excluded 272 irrelevant records upon reviewing titles alone, and an additional 20 records after reading the abstracts. We retrieved eight full‐text articles, which reported five studies, all of which we excluded with reasons (Milte 2012; Parletta 2013; Perera 2012; Soerensen 2013; Richardson 2012). We found no new studies for inclusion, but we identified one ongoing study (ISRCTN48803273) and one study awaiting classification (ISRCTN54901093), both of which may be relevant to this review.
To date, therefore, this review has two included studies, nine excluded studies, three studies awaiting classification and one ongoing study. See Figure 1.

Study selection flow chart.
Included studies
We have included in this update two studies (Johnson 2009; Richardson 2002), which we identified in our original search and previously excluded. Neither study reports on the primary outcome for this review, but MECIR (Methodological Expectations of Cochrane Intervention Reviews) guidance (Chandler 2013) indicates that reported outcomes should not be considered among the inclusion criteria for a review. (See Differences between protocol and review.)
Study population
Johnson 2009 included 75 children, all with a diagnosis of ADHD, out of which 32 (43% of the entire study population: 12 out of 37 in the intervention group and 20 out of 38 in the control group) had a combination reading and writing disorder, which was diagnosed on the basis of criteria from the fourth edition of the DSM (DSM‐IV) (APA 2000). Richardson 2002 included 41 children with a specific reading disorder, which was diagnosed on the basis of "usual diagnostic criteria for specific developmental dyslexia". Although the criteria were not named, the description of the diagnostic criteria used would fit DSM‐IV (APA 2000). These children had higher than normal scores on the Conners' Parent Rating Scale (CPRS) (Conners 1997) but were given no formal diagnosis of ADHD. Most of the children in both studies were boys from 10 to 18 years of age.
Settings
Both studies were conducted in high‐income countries ‐ Richardson 2002 in a school setting, and Johnson 2009 in a clinic setting.
Intervention and comparators
Both studies used a combination of omega‐3 and omega‐6 supplements given as capsules, although the doses were slightly different; Richardson 2002 used a higher omega‐6 dose compared with Johnson 2009. Investigators in both studies used olive oil as the placebo and provided no additional interventions.
Study design
Johnson 2009 was described as a cross‐over study. The first period was an RCT, but the second period included no randomisation, and all participants received the intervention treatment. Therefore, we used first period data and treated this as a parallel‐group study. Richardson 2002 was a parallel‐group study.
Duration of intervention
Both studies gave the intervention for three months.
Outcomes
Neither study reported the results of standardised tests of reading, writing, spelling or mathematics skills.
Both studies reported adverse effects as the number of participants in each group who developed the effects. The main adverse effects reported were dyspepsia, diarrhoea, vomiting and "digestive upset".
Both studies measured behaviour by using validated scales; Richardson 2002 used Conners' Parent Rating Scale ‐ Long (Conners 1997), and Johnson 2009 used the ADHD Rating Scale ‐ Fourth Version (ADHD‐RS‐IV) (DuPaul 1998) and the Clinical Global Impression (CGI) ‐ Severity scale (CGI‐S) (Guy 1976). Johnson 2009 used several other outcome assessment tools but did not report this (see Characteristics of included studies). Richardson 2002 reported mean scores, but Johnson 2009 reported mean change in scores for all participants, irrespective of whether they had a learning disorder. Study authors also reported the number of participants who had a reduction in score of at least 25% on the ADHD‐RS‐IV (called 'responders' in the study) and provided data for the subgroup of participants with specific learning disorders.
Both studies measured outcomes at three months.
Funding source
Johnson 2009 was funded by the company that produced the PUFA supplement used in the study. Richardson 2002 was funded by The Dyslexia Trust.
Excluded studies
We excluded nine studies (12 reports) after assessing the full text. One was an open‐label study that included no comparison group (Lindmark 2007). One was a review that included reports of unpublished, non‐randomised studies (Portwood 2006). One study involved normal children (Soerensen 2013). We excluded five studies because the children were not diagnosed with specific learning disorders (Milte 2012; Parletta 2013; Perera 2012; Richardson 2005; Richardson 2012), and another study because the intervention contained another active ingredient (carnosine) (Kairaluoma 2009). Study authors stated that carnosine had its own potential enhancing effects on cognition, and, although it can be used to provide an antioxidant effect for PUFAs, we judged the addition of carnosine as a cointervention, which was not given to the control group. See Characteristics of excluded studies.
Studies awaiting classification
We could not obtain one abstract even after we attempted to contact the publisher and the study author (Richardson 2000). In addition, in a narrative review on the effects of PUFAs (Richardson 2004), we found a reference to an anonymous, unpublished and uncited study (Anon 2004). It is not clear whether Anon 2004 refers to a completely unpublished study or is a duplicate of Richardson 2000. We also found a record of a completed study (ISRCTN54901093) on the WHO ICTRP. The contact author of this study has since retired, and our attempts to contact the author's institution have yielded no further information about the study.
Ongoing studies
We found one ongoing study (ISRCTN48803273), which was completed in 2015 but has not yet published study results.
Risk of bias in included studies
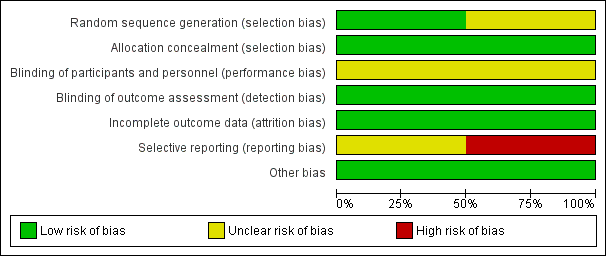
We present our assessment of risk of bias in included studies in the 'Risk of bias' tables found beneath the Characteristics of included studies tables and summarise this in Figure 2.
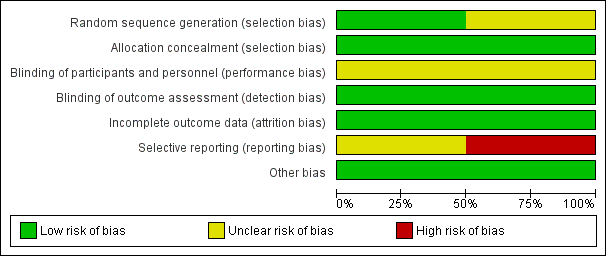
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
Richardson 2002 used a computer‐generated sequence, and so we rated this study as having low risk of selection bias. We rated Johnson 2009 as having unclear risk of selection bias, as it was not clear how the randomisation code was generated.
Allocation concealment
As both studies used sequentially numbered bottles for allocation, we rated both as having low risk of selection bias related to allocation concealment.
Blinding
Blinding of participants and personnel
Although both studies described their placebos as identical‐looking, investigators did not mention the taste. Polyunsaturated fatty acids were derived from fish in Johnson 2009, but the source is not clear in Richardson 2002. Difficulty in masking the taste led to our judgement that both studies had unclear risk of performance bias.
Blinding of outcome assessment
We judged both studies to be at low risk of detection bias because it is unlikely that taste would have become known to the assessors.
Incomplete outcome data
Although both studies reported a substantial number of dropouts, the numbers and reasons for dropouts were similar in both groups, and in each study, analysis was based only on participants who had complete data. Consequently, we rated both studies as having low risk of attrition bias.
Selective reporting
The expected outcome in both studies was the result of reading tests. However, neither study reported this information. The study protocols of both studies were not available for review authors to assess whether this outcome was predetermined. Consequently, we rated Richardson 2002 as having unclear risk of reporting bias. Johnson 2009 reported that the study administered other outcome measures (including reading‐writing tests) to study participants, but some of these outcomes would be reported in a separate publication. We found no other publication of this study in our search. Therefore, we rated Johnson 2009 as having high risk of reporting bias.
Other potential sources of bias
We detected no other potential sources of bias. Although Johnson 2009 was funded by the manufacturer of the PUFAs, and the company was clearly involved in the randomisation and allocation concealment process, we did not consider funding to be an additional source of bias (Higgins 2011a, section 8.15.15). We assessed independently the specific aspects of methods (sequence generation and allocation concealment) that might have been influenced by vested interests. Thus, we rated both studies as having low risk of other sources of bias.
Effects of interventions
See: Summary of findings for the main comparison Summary of findings table
Comparison: PUFAs versus placebo
The summary of effects of the intervention can be found in summary of findings Table for the main comparison.
Primary outcomes
Reading, writing, spelling and mathematics scores
No studies reported data on this outcome.
Reporting of adverse effects
The adverse effects reported in both studies were gastrointestinal disturbances. For Johnson 2009, we used data from the first period for all participants, irrespective of the diagnosis, when analysing adverse effects because these effects would not be expected to be specific only to those with specific learning disorders. There was no difference in the risk of gastrointestinal disturbances when comparing the PUFA group with the placebo group (RR 1.43, 95% CI 0.25 to 8.15; I² = 0%; two studies, 116 children; low‐quality evidence; see Figure 3). We downgraded the evidence to low quality because of imprecision and study limitations.

Forest plot of comparison: 1 PUFAs versus placebo, outcome: 1.1 Adverse effects (gastrointestinal disturbances).
Secondary outcomes
Parent‐ or teacher‐reported outcomes
Both studies reported the results of ADHD symptom scores. Richardson 2002 used the CPRS ‐ Long (Conners 1997), and Johnson 2009 used the ADHD‐RS‐IV, Parent Version (DuPaul 1998). Richardson 2002 reported mean ADHD symptom scores and revealed no differences in PUFA and placebo groups after 12 weeks of intervention (MD ‐5.20, 95% CI ‐12.26 to 1.86; 29 children). However, Johnson 2009 reported the number of responders (those with > 25% reduction in ADHD scores) for the subgroup with specific learning disorders, revealing more responders in the PUFA group than in the placebo group. We have contacted the authors of Johnson 2009 to request summary data for the subgroup with specific learning disorders but have not yet received a response. Therefore, for this outcome, no meta‐analysis was possible because one study reported continuous data, and the other dichotomous data.
Self‐reported outcomes
No studies reported data on this outcome.
Discussion
Summary of main results
Two small studies met the eligibility criteria for this review, but neither measured the primary learning outcomes. Therefore, to date, no information is available to guide us in the use of polyunsaturated fatty acids (PUFAs) for children with specific learning disorders.
The little evidence that we have reveals that PUFAs did not increase the risk of developing gastrointestinal disturbance compared with placebo, when taken for three months. Included studies did not report other adverse effects.
Overall completeness and applicability of evidence
We were unable to find any studies that directly assessed the use of PUFAs and their effects on learning outcomes of children with specific learning disorders. The two included studies reported only attention deficit hyperactivity disorder (ADHD)‐related behavioural outcomes. Given the extensive overlap of specific learning disorders and ADHD, it is important to know whether PUFAs have any effects on learning outcomes ‐ the core problem associated with specific learning disorders. Improvement in behaviour does not necessarily result in improvement in reading, spelling, writing or mathematics skills.
When other active elements are added to the PUFA supplement, such as carnosine in Kairaluoma 2009, and no comparative placebos are given, it is not possible to attribute any effects of the intervention to PUFAs. Our search found many studies of PUFAs given to help children with unspecified learning problems or to improve learning in normal children. Our review excluded studies that examined children without a diagnosis of specific learning disorders. Therefore, this review is unable to address the question of whether PUFAs have any effect on poorly performing or normal children in mainstream education.
Quality of the evidence
We used the GRADE approach to assess the quality of the evidence for adverse effects. We downgraded the evidence by two levels to low quality because of small sample size and few events (imprecision), as well as high risk of reporting bias (study limitations), in Johnson 2009.
Potential biases in the review process
For three studies, it remains unclear whether they meet our inclusion criteria (see Characteristics of studies awaiting classification). We were able to retrieve neither the abstract nor the full text for one study that might qualify for inclusion in this review (Richardson 2000). This article is no longer available from the publisher, and our attempts to contact the study author were unsuccessful. Our handsearch of the citations of other studies revealed that this study is often cited together with Richardson 2002; therefore, it is possible that the two citations may apply to the same study. Also, in a review of clinical studies on PUFAs in children with ADHD and dyslexia (Richardson 2004), we found one report of an unpublished randomised controlled trial (RCT) of 102 children eight to 12 years of age who had reading difficulties (Anon 2004). No citation is available for this unpublished report, and we could not contact the author of the review to request further information. We could not find the results of another completed study, retrospectively registered on the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (ISRCTN54901093), which may have met our inclusion criteria. The contact author has retired, and we could obtain no further information about the study from the contact institution.
For this update, we reviewed our eligibility criteria and made a decision to leave as they were the prespecified strict criteria for the diagnosis of specific learning disorders. We avoided taking a pragmatic approach to the definition of learning disorders because children might not learn for a variety of other reasons, including other nutritional and environmental factors, such as socioeconomic status and access to school, and the presence of other neurodevelopmental disorders, especially ADHD (DuPaul 2013; Hamilton 2006). We believe our strict criteria strengthen this review.
Agreements and disagreements with other studies or reviews
One of the excluded studies that involved children with dyslexia reported that supplementation with PUFAs had a positive effect on speed of reading and decoding fluency (Lindmark 2007). However, this study was small (17 children) and was not randomised, controlled or blinded. Another excluded study, which was an RCT (Kairaluoma 2009), examined effects of PUFAs and carnosine on 61 children with specific learning disorders and found no evidence of benefit for reading, spelling or mathematics outcomes.
No systematic reviews have examined PUFAs and specific learning disorders, but two narrative reviews combined clinical studies on dyslexia, dyspraxia and ADHD (Richardson 2004; Schuchardt 2010). These reviews concluded that PUFAs may be beneficial for common neurodevelopmental conditions such as these.
Study selection flow chart.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
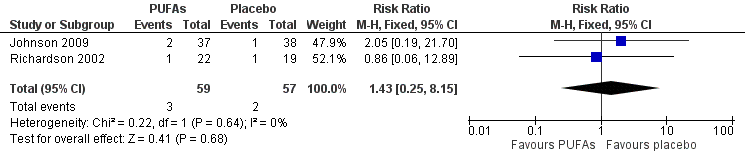
Forest plot of comparison: 1 PUFAs versus placebo, outcome: 1.1 Adverse effects (gastrointestinal disturbances).
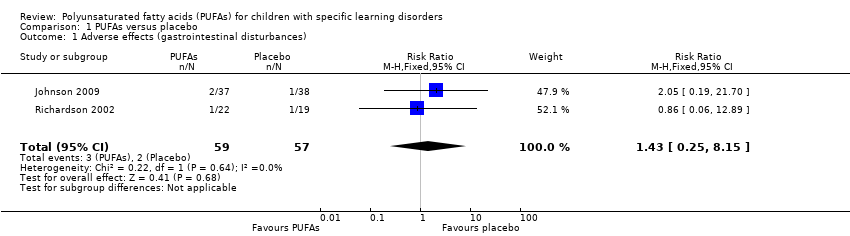
Comparison 1 PUFAs versus placebo, Outcome 1 Adverse effects (gastrointestinal disturbances).
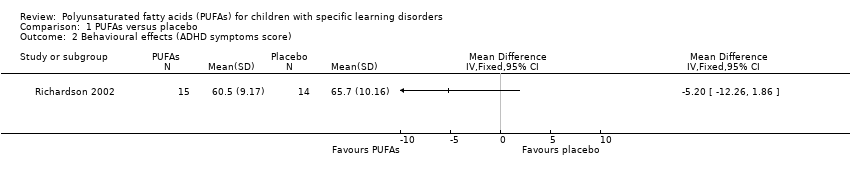
Comparison 1 PUFAs versus placebo, Outcome 2 Behavioural effects (ADHD symptoms score).
| PUFAs vs placebo | ||||||
| Patient or population: children with specific learning disorders | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with PUFAs | |||||
| Standardised tests of reading, writing, spelling or mathematics skills (overall) | None of the included studies assessed effects on standardised tests of reading, writing, spelling or mathematics skills | Not estimable | ‐ | ‐ | ‐ | |
| Adverse effect (gastrointestinal disturbance) | Study population | RR 1.43 | 116 | ⊕⊕⊝⊝ | ‐ | |
| 35 per 1000 | 50 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSmall sample size with few events. Extremely wide CIs. | ||||||
| Measures of treatment effect |
| Continuous data Treatment effects are likely to require a variety of standardised tests. If we encounter the use of different tests for the same outcome, and we judge these to be similar enough, we will calculate the standardised mean difference (SMD) and the 95% confidence interval (CI) for each. |
| Unit of analysis Issues |
| Cross‐over studies In the analysis of studies with cross‐over design, we will first consider the possibility of carry‐over effect, whether only first period data are available, if any analysis is incorrect and if results are comparable with those of parallel‐group studies. We will use the methods suggested in the Cochrane Handbook for Systematic Reviews of Interventions when analysing and incorporating the trials for meta‐analysis (Higgins 2011b, section 16.4). As a carry‐over effect is likely, given the persistence of PUFAs in tissues (Katan 1997), when data are available for the first period, we will use these in the analyses of learning and behavioural outcomes. However, as adverse effects are readily reversible outcomes, we could use data from both phases. Cluster‐randomised studies We do not anticipate finding any cluster‐randomised trials for this review. However, if we do find them, we will analyse them in the unit to which they were randomised and we will adjust the effective sample size using an intracluster correlation coefficient (ICC), which we will obtain from the studies themselves, if given, or will estimate from similar studies. If we use ICCs from other sources, we will report this and will conduct sensitivity analyses to investigate the effects of variation in the ICC, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b, section16.3). Studies with multiple intervention groups For studies with more than 2 intervention groups, we will combine the intervention (if they are similar, e.g. different dose of PUFAs or different combination of PUFAs), and control groups to create a single pair‐wise comparison. If any of the intervention groups is completely different (e.g. visual exercises, music therapy), we will exclude that particular intervention group from the analysis. |
| Dealing with missing data |
| If more studies are included in the future, we will explore by sensitivity analysis the impact of including studies with high levels of missing data in the overall assessment of treatment effect. We will exclude from the analysis studies with missing data that have a high impact on the final results. For all outcomes, we will carry out analyses, as far as possible, on an intention‐to‐treat basis (i.e. we will attempt to include in the analyses all participants randomised to each group). Missing standard deviations (SDs) may be imputed according to the methods provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). |
| Assessment of reporting biases |
| If we identify 10 or more studies, we will use funnel plots to assess the relationships of treatment effect size and standard error. Asymmetry in the funnel plot may be a result of reporting bias, but could also be a true reflection of heterogeneity among intervention effects. We will further examine clinical heterogeneity of the studies as a possible explanation. We will compare results obtained from published studies and results from other sources (e.g. correspondence) to detect publication bias. |
| Subgroup analysis and investigation of heterogeneity |
| We intend to perform subgroup analyses for the primary outcome only. We will investigate the following subgroups.
|
| Sensitivity analysis |
| If we identify enough studies, we will perform a sensitivity analysis to assess how much the quality of the studies affects meta‐analysis results. We will conduct sensitivity analyses to find out if excluding studies with high risk of bias (as assessed by using the Cochrane tool for assessing risk of bias) has an impact on the final results. These will include studies with cross‐over design, inadequate sequence allocation and concealment, missing data or use of any imputed values. |
| ADHD: attention deficit hyperactivity disorder. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse effects (gastrointestinal disturbances) Show forest plot | 2 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.25, 8.15] |
| 2 Behavioural effects (ADHD symptoms score) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

