Haloperidol za psihozom induciranu agresiju ili uznemirenost (brzo umirivanje)
Abstract
Background
Haloperidol used alone is recommended to help calm situations of aggression or agitation for people with psychosis. It is widely accessible and may be the only antipsychotic medication available in limited‐resource areas.
Objectives
To examine whether haloperidol alone is an effective treatment for psychosis‐induced aggression or agitation, wherein clinicians are required to intervene to prevent harm to self and others.
Search methods
We searched the Cochrane Schizophrenia Group's Study‐Based Register of Trials (26th May 2016). This register is compiled by systematic searches of major resources (including AMED, BIOSIS CINAHL, Embase, MEDLINE, PsycINFO, PubMed, and registries of clinical trials) and their monthly updates, handsearches, grey literature, and conference proceedings, with no language, date, document type, or publication status limitations for inclusion of records into the register.
Selection criteria
Randomised controlled trials (RCTs) involving people exhibiting aggression and/or agitation thought to be due to psychosis, allocated rapid use of haloperidol alone (by any route), compared with any other treatment. Outcomes of interest included tranquillisation or asleep by 30 minutes, repeated need for rapid tranquillisation within 24 hours, specific behaviours (threat or injury to others/self), adverse effects. We included trials meeting our selection criteria and providing useable data.
Data collection and analysis
We independently inspected all citations from searches, identified relevant abstracts, and independently extracted data from all included studies. For binary data we calculated risk ratio (RR), for continuous data we calculated mean difference (MD), and for cognitive outcomes we derived standardised mean difference (SMD) effect sizes, all with 95% confidence intervals (CI) and using a fixed‐effect model. We assessed risk of bias for the included studies and used the GRADE approach to produce 'Summary of findings' tables which included our pre‐specified main outcomes of interest.
Main results
We found nine new RCTs from the 2016 update search, giving a total of 41 included studies and 24 comparisons. Few studies were undertaken in circumstances that reflect real‐world practice, and, with notable exceptions, most were small and carried considerable risk of bias. Due to the large number of comparisons, we can only present a summary of main results.
Compared with placebo, more people in the haloperidol group were asleep at two hours (2 RCTs, n=220, RR 0.88, 95%CI 0.82 to 0.95, very low‐quality evidence) and experienced dystonia (2 RCTs, n=207, RR 7.49, 95%CI 0.93 to 60.21, very low‐quality evidence).
Compared with aripiprazole, people in the haloperidol group required fewer injections than those in the aripiprazole group (2 RCTs, n=473, RR 0.78, 95%CI 0.62 to 0.99, low‐quality evidence). More people in the haloperidol group experienced dystonia (2 RCTs, n=477, RR 6.63, 95%CI 1.52 to 28.86, very low‐quality evidence).
Four trials (n=207) compared haloperidol with lorazepam with no significant differences with regard to number of participants asleep at one hour (1 RCT, n=60, RR 1.05, 95%CI 0.76 to 1.44, very low‐quality of evidence) or those requiring additional injections (1 RCT, n=66, RR 1.14, 95%CI 0.91 to 1.43, very low‐quality of evidence).
Haloperidol's adverse effects were not offset by addition of lorazepam (e.g. dystonia 1 RCT, n=67, RR 8.25, 95%CI 0.46 to 147.45, very low‐quality of evidence).
Addition of promethazine was investigated in two trials (n=376). More people in the haloperidol group were not tranquil or asleep by 20 minutes (1 RCT, n=316, RR 1.60, 95%CI 1.18 to 2.16, moderate‐quality evidence). Acute dystonia was too common in the haloperidol alone group for the trial to continue beyond the interim analysis (1 RCT, n=316, RR 19.48, 95%CI 1.14 to 331.92, low‐quality evidence).
Authors' conclusions
Additional data from new studies does not alter previous conclusions of this review. If no other alternative exists, sole use of intramuscular haloperidol could be life‐saving. Where additional drugs are available, sole use of haloperidol for extreme emergency could be considered unethical. Addition of the sedating promethazine has support from better‐grade evidence from within randomised trials. Use of an alternative antipsychotic drug is only partially supported by fragmented and poor‐grade evidence. Adding a benzodiazepine to haloperidol does not have strong evidence of benefit and carries risk of additional harm.
After six decades of use for emergency rapid tranquillisation, this is still an area in need of good independent trials relevant to real‐world practice.
PICOs
Laički sažetak
Haloperidol kao sredstvo smirivanja ljudi koji su agresivni ili uznemireni zbog psihoze
Cilj pregleda
Ovaj Cochrane sustavni pregled literature je analizirao je li haloperidol učinkovit pri liječenju ljudi koji su uznemireni ili agresivni zbog psihoze
Dosadašnje spoznaje
Ljudi sa psihozom mogu čuti (različite) glasove (halucinacije) ili doživjeti abnormalne misli (deluzije), koje takvu osobu mogu prestrašiti, zabrinuti i uznemiriti (nemir, razdražljivost) ponekad vodeći prema agresivnom ponašanju. To postavlja značajni izazov za stručnjake za mentalno zdravlje koji moraju brzo odabrati najbolje dostupno liječenje kako bi spriječili rizik od daljnje štete i za pacijenta i/ili druge osobe.
Haloperidol je lijek koji se koristi za liječenje ljudi s psihozom, a koji se može uzimati na usta ili putem injekcije. Osim što je i antipsihotik (sprječava psihozu), on također umiruje ljude ili im pomaže da zaspu.
Pretraživanje
U 2011. i 2016., informacijski stručnjak Cochraneove grupe za shizofreniju pretraživao je posebni registar istraživanja koja su promatrala učinke liječenja haloperidolom u usporedbi s ili placebo ili drugim tretmanima kod agresivnih ili uznemirenih ljudi što je uzrokovano psihozom.
Rezultati
U pregled je uključena 41 studija, ali informacije u tim studijama nisu baš kvalitetne. Glavni rezultati pokazuju kako u usporedbi s placebo ili bez liječenja, oni koji su bili liječeni haloperidolom češće su zaspali nakon dva sata. Međutim, dokazi nisu čvrsti. Rezultati su složeniji zbog velike raznolikosti dostupnih terapija (24 usporedbe).
Zaključci
Autori zaključuju da postoje tek slabi dokazi kako haloperidol smiruje ljude i pomaže im u nošenju s teškim situacijama. Međutim, ti rezultati nisu utemeljeni na kvalitetnim istraživanjima i stoga medicinski stručnjaci i ljudi s mentalnim problemima ostaju bez jasnijeg i na dokazima utemeljenoga zaključka. U ponekim situacijama, haloperidol može biti jedinim izborom, ali to je daleko od idealnoga jer iako je haloperidol učinkovit za smirivanje ljudi ima popratne efekte (npr. nemir, treskanje glave, ruku i tijela, srčani problemi). Te nuspojave mogu jednako onesposobiti pacijenta kao i psihoza i mogu biti smetnja zbog koje se pacijenti više neće odlučiti na ponovni dolazak na liječenje. Potrebna su daljnja istraživanja koja bi pomogla analizirati i razumjeti koji je lijek bolji za umirivanje ljudi; koji ima manje neželjenih učinaka; koji djeluje brže; i koji se može koristiti u manjim količinama (ili rjeđe davati u obliku injekcije).
Ovaj laički sažetak u engleskom originalu napisao je Ben Gray.
Authors' conclusions
Summary of findings
| HALOPERIDOL compared with PLACEBO/NIL for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PLACEBO/NIL | HALOPERIDOL | |||||
| Tranquillisation or asleep | Low1 | RR 0.88 | 220 | ⊕⊝⊝⊝ | ||
| 965 per 1000 | 849 per 1000 | |||||
| Moderate1 | ||||||
| 980 per 1000 | 862 per 1000 | |||||
| High1 | ||||||
| 995 per 1000 | 876 per 1000 | |||||
| Repeated need for tranquillisation | Low1 | RR 0.51 | 660 | ⊕⊕⊝⊝ | ||
| 400 per 1000 | 204 per 1000 | |||||
| Moderate1 | ||||||
| 600 per 1000 | 306 per 1000 | |||||
| High1 | ||||||
| 800 per 1000 | 408 per 1000 | |||||
| Specific behaviour ‐ threat or injury to self or others | The mean specific behaviour ‐ threat or injury to self or others in the intervention groups was | 474 | ⊕⊝⊝⊝ | |||
| Global outcome ‐ overall improvement (not any improvement) | Low1 | RR 0.28 | 40 | ⊕⊕⊝⊝ | ||
| 200 per 1000 | 56 per 1000 | |||||
| Moderate1 | ||||||
| 350 per 1000 | 98 per 1000 | |||||
| High1 | ||||||
| 500 per 1000 | 140 per 1000 | |||||
| Adverse effects: Specific ‐ dystonia within 24 hours | Moderate1 | RR 7.49 | 207 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| High1 | ||||||
| 10 per 1000 | 75 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control risk ‐ moderate risk is roughly equal to that of the control group. | ||||||
| HALOPERIDOL compared with OTHER ANTIPSYCHOTICS: a. ARIPIPRAZOLE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTICS: a. ARIPIPRAZOLE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for rapid tranquillisation ‐ needing additional injection | Low1 | RR 0.78 | 473 | ⊕⊕⊝⊝ | ||
| 200 per 1000 | 156 per 1000 | |||||
| Moderate1 | ||||||
| 400 per 1000 | 312 per 1000 | |||||
| High1 | ||||||
| 650 per 1000 | 507 per 1000 | |||||
| Specific behaviours ‐ agitation: CABS average change score at 2 hours | The mean specific behaviours ‐ agitation: average change score at 2 hours in the intervention groups was | 470 | ⊕⊝⊝⊝ | |||
| Global outcomes: need for benzodiazepine 2 | Low1 | RR 1.26 | 477 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 63 per 1000 | |||||
| Moderate1 | ||||||
| 100 per 1000 | 126 per 1000 | |||||
| High1 | ||||||
| 200 per 1000 | 252 per 1000 | |||||
| Adverse effects: any serious, specific ‐ dystonia during 24 hours | Low6 | RR 6.63 | 477 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate6 | ||||||
| 50 per 1000 | 332 per 1000 | |||||
| High6 | ||||||
| 100 per 1000 | 663 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk roughly equates with that of the trial control group. | ||||||
| HALOPERIDOL compared with OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE | HALOPERIDOL | |||||
| Tranquillisation or asleep | Low1 | RR 1.93 | 39 | ⊕⊝⊝⊝ | ||
| 200 per 1000 | 386 per 1000 | |||||
| Moderate1 | ||||||
| 500 per 1000 | 965 per 1000 | |||||
| High1 | ||||||
| 700 per 1000 | 1000 per 1000 | |||||
| Repeated need for rapid tranquillisation ‐ more that 1 injection | Low1 | RR 1.07 | 30 | ⊕⊝⊝⊝ | ||
| 800 per 1000 | 856 per 1000 | |||||
| Moderate1 | ||||||
| 900 per 1000 | 963 per 1000 | |||||
| High1 | ||||||
| 990 per 1000 | 1000 per 1000 | |||||
| Specific behaviour ‐ threat or injury of harm to self or others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome ‐ not any improvement | Low1 | RR 0.15 | 89 | ⊕⊝⊝⊝ | ||
| 1000 per 1000 | 150 per 1000 | |||||
| Moderate1 | ||||||
| 300 per 1000 | 45 per 1000 | |||||
| High1 | ||||||
| 500 per 1000 | 75 per 1000 | |||||
| Adverse effects: specific ‐ cardiovascular ‐ hypotension | Low1 | RR 0.51 | 30 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 10 per 1000 | |||||
| Moderate1 | ||||||
| 150 per 1000 | 30 per 1000 | |||||
| High1 | ||||||
| 250 per 1000 | 50 per 1000 | |||||
| Adverse effects: specific ‐ central nervous system ‐ seizures | Low1 | RR 0.33 | 30 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 3 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 17 per 1000 | |||||
| High1 | ||||||
| 150 per 1000 | 50 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control risk ‐ moderate risk roughly equates to that of the control group | ||||||
| HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: c. DROPERIDOL for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTIC: c. DROPERIDOL | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not asleep up to 2 hours | Study population | RR 1.07 | 228 | ⊕⊕⊕⊝ | ||
| 82 per 1.000 | 88 per 1.000 | |||||
| Repeated need for rapid tranquillisation ‐ more than 1 injection | Low | RR 2.38 | 255 | ⊕⊕⊕⊝ | ||
| 50 per 1.000 | 119 per 1.000 | |||||
| Moderate | ||||||
| 70 per 1.000 | 167 per 1.000 | |||||
| High | ||||||
| 360 per 1.000 | 857 per 1.000 | |||||
| Specific behaviour ‐ threat or injury to self or others ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Global outcome ‐ need for benzodiazepine | Study population | RR 0.31 | 228 | ⊕⊕⊕⊝ | ||
| 59 per 1.000 | 18 per 1.000 | |||||
| Adverse effects: specific ‐ dystonia | Study population | RR 0.86 | 255 | ⊕⊕⊕⊝ | ||
| 8 per 1.000 | 7 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 CI include no effect 2 Risk of bias: rated 'serious' ‐ method of randomisation not reported, described as double blind but no further information given regarding rater blinding, small short study. 3 Publication bias: rated 'strongly suspected' ‐ small study. 4 Moderate risk roughly equates with that of the trial control group, 5 Adverse effects ‐ imprecision ‐ 95% confidence interval. 6 Adverse effects ‐ publication bias ‐ small study. | ||||||
| HALOPERIDOL compared with OTHER ANTIPSYCHOTICS: d. LOXAPINE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients to psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTICS: d. LOXAPINE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not asleep by 12 hours | Low1 | RR 4.31 | 54 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 43 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 215 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 431 per 1000 | |||||
| Global outcome: specific | Low1 | RR 3.50 | 30 | ⊕⊝⊝⊝ | * data for prespecified outcome Repeated need for rapid tranquillisation were not reported | |
| 10 per 1000 | 57 per 1000 | |||||
| Moderate1 | ||||||
| 100 per 1000 | 569 per 1000 | |||||
| High1 | ||||||
| 200 per 1000 | 1000 per 1000 | |||||
| Specific behaviour ‐ aggression ‐ no overall improvement | Low1 | RR 1.10 | 30 | ⊕⊝⊝⊝ | ||
| 500 per 1000 | 550 per 1000 | |||||
| Moderate1 | ||||||
| 650 per 1000 | 715 per 1000 | |||||
| High1 | ||||||
| 800 per 1000 | 880 per 1000 | |||||
| Global outcome: no change at 48 hours | Low1 | RR 0.93 | 56 | ⊕⊕⊝⊝ | ||
| 10 per 1000 | 9 per 1000 | |||||
| Moderate1 | ||||||
| 70 per 1000 | 65 per 1000 | |||||
| High1 | ||||||
| 140 per 1000 | 130 per 1000 | |||||
| Adverse effects: Specific ‐ dystonia between 0‐3 days (IM phase) | Low1 | RR 0.94 | 35 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 9 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 47 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 94 per 1000 | |||||
| Adverse effects: Specific ‐ rigidity between 0‐3 days (IM phase) | Low1 | RR 0.88 | 35 | ⊕⊝⊝⊝ | ||
| 750 per 1000 | 660 per 1000 | |||||
| Moderate1 | ||||||
| 850 per 1000 | 748 per 1000 | |||||
| High1 | ||||||
| 950 per 1000 | 836 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the control group. | ||||||
| HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: e. OLANZAPINE for psychosis induced aggression or agitation | ||||||
| Patient or population: psychosis induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTIC: e. OLANZAPINE | Risk with HALOPERIDOL | |||||
| Tranquilisation or asleep | Low | RR 1.16 | 257 | ⊕⊝⊝⊝ | ||
| 200 per 1.000 | 232 per 1.000 | |||||
| Moderate | ||||||
| 700 per 1.000 | 812 per 1.000 | |||||
| High | ||||||
| 900 per 1.000 | 1000 per 1.000 | |||||
| Repeated need for tranquillisation | Low | RR 1.06 | 392 | ⊕⊝⊝⊝ | ||
| 50 per 1.000 | 53 per 1.000 | |||||
| Moderate | ||||||
| 200 per 1.000 | 212 per 1.000 | |||||
| High | ||||||
| 350 per 1.000 | 371 per 1.000 | |||||
| Specific behaviour ‐ threat or injury to self or others | Low | RR 0.96 | 45 | ⊕⊝⊝⊝ | ||
| 200 per 1.000 | 192 per 1.000 | |||||
| Moderate | ||||||
| 400 per 1.000 | 384 per 1.000 | |||||
| High | ||||||
| 600 per 1.000 | 576 per 1.000 | |||||
| Global outcome ‐ need for benzodiazepine during 24 hours | Low | RR 1.05 | 343 | ⊕⊝⊝⊝ | ||
| 50 per 1.000 | 53 per 1.000 | |||||
| Moderate | ||||||
| 150 per 1.000 | 158 per 1.000 | |||||
| High | ||||||
| 250 per 1.000 | 263 per 1.000 | |||||
| Adverse effects: Specific ‐ dystonia during 24 hours | Low | RR 12.92 | 343 | ⊕⊝⊝⊝ | ||
| 0 per 1.000 | 0 per 1.000 | |||||
| Moderate | ||||||
| 0 per 1.000 | 0 per 1.000 | |||||
| High | ||||||
| 5 per 1.000 | 65 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the control group. 2 Risk of bias: rated 'very serious' ‐ method of randomisation not reported, allocation concealment not stated, described as double blind but no further details are given regarding blinding, selective reporting, sponsored by drug company. 3 Indirectness: rated 'serious' ‐ tranquil at 30 minutes is not measured, therefore used not asleep at 2 hour. 4 Publication bias: rated 'strongly suspected' ‐ sponsored by drug company. 5 Indirectness: rated 'serious' ‐ not threat or injury to self or others as stated in protocol, therefore inferred further aggressive behaviour by less than a 40% reduction in PANSS‐EC score. 6 Risk of bias: rated 'serious' ‐ method of randomisation is not reported, allocation concealment not stated, described as double blind but no further information given, incomplete outcome data, selective reporting, sponsored by drug company. 7 Imprecision: rated 'serious' ‐ 95% confidence interval is wide. | ||||||
| HALOPERIDOL compared with OTHER ANTIPSYCHOTIC: f. PERPHENAZINE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTIC: f. PERPHENAZINE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for rapid tranquillisation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Specific behaviour ‐ injury or threat of self to others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome: general ‐ no improvement | Low1 | RR 0.46 | 44 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 5 per 1000 | |||||
| Moderate1 | ||||||
| 100 per 1000 | 46 per 1000 | |||||
| High1 | ||||||
| 200 per 1000 | 92 per 1000 | |||||
| Adverse effect: Specific ‐ hypotensive episode | Low1 | RR 0.31 | 44 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 3 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 15 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 31 per 1000 | |||||
| Adverse effect ‐ discontinued due to EPS | Low1 | RR 1.83 | 44 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 18 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 92 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 183 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the trial control group. | ||||||
| HALOPERIDOL compared to OTHER ANTIPSYCHOTICS: g. QUETIAPINE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTICS: g. QUETIAPINE | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Repeated need for tranquillisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Specific behaviour ‐ agitation | MD 0.10 higher | ‐ | 80 | ⊕⊕⊝⊝ | ||
| Global outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Adverse effects | Low | RR 1.81 | 80 | ⊕⊕⊝⊝ | ||
| 200 per 1.000 | 362 per 1.000 | |||||
| Moderate | ||||||
| 400 per 1.000 | 724 per 1.000 | |||||
| High | ||||||
| 600 per 1.000 | 1000 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Imprecision: rated 'very serious' ‐ small study, 95% CI are wide. | ||||||
| HALOPERIDOL compared with OTHER ANTIPSYCHOTIC: g. RISPERIDONE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTIC: g. RISPERIDONE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not asleep | Low1 | RR 0.84 | 162 | ⊕⊕⊕⊝ | ||
| 700 per 1000 | 588 per 1000 | |||||
| Moderate1 | ||||||
| 900 per 1000 | 756 per 1000 | |||||
| High1 | ||||||
| 1000 per 1000 | 840 per 1000 | |||||
| Repeated need for rapid tranquillisation ‐ needing additional benzodiazepine | Low | RR 0.98 | 286 | ⊕⊕⊝⊝ | ||
| 100 per 1000 | 98 per 1000 | |||||
| Moderate | ||||||
| 200 per 1000 | 196 per 1000 | |||||
| High | ||||||
| 300 per 1000 | 294 per 1000 | |||||
| Specific behaviours ‐ agitation | Low1 | RR 1.15 | 124 | ⊕⊕⊝⊝ | ||
| 50 per 1000 | 58 per 1000 | |||||
| Moderate1 | ||||||
| 200 per 1000 | 230 per 1000 | |||||
| High1 | ||||||
| 350 per 1000 | 402 per 1000 | |||||
| Global outcome ‐ rated as severe ‐ CGI‐S Follow‐up: 1 days | Low1 | RR 0.89 | 162 | ⊕⊝⊝⊝ | ||
| 100 per 1000 | 89 per 1000 | |||||
| Moderate1 | ||||||
| 200 per 1000 | 178 per 1000 | |||||
| High1 | ||||||
| 300 per 1000 | 267 per 1000 | |||||
| Adverse effects: Specific ‐ EPS during 24 hours | Low1 | RR 1.6 | 124 | ⊕⊕⊝⊝ | ||
| 10 per 1000 | 16 per 1000 | |||||
| Moderate1 | ||||||
| 100 per 1000 | 160 per 1000 | |||||
| High1 | ||||||
| 200 per 1000 | 320 per 1000 | |||||
| Adverse effects: Specific ‐ acute dystonia during 24 hours | Low1 | RR 5 | 286 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 50 per 1000 | |||||
| Moderate1 | ||||||
| 20 per 1000 | 100 per 1000 | |||||
| High1 | ||||||
| 30 per 1000 | 150 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the trial control group. | ||||||
| HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: h. THIOTHIXENE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTIC: h. THIOTHIXENE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for rapid tranquillisation | Low1 | RR 1.07 | 30 | ⊕⊝⊝⊝ | ||
| 850 per 1000 | 910 per 1000 | |||||
| Moderate1 | ||||||
| 900 per 1000 | 963 per 1000 | |||||
| High1 | ||||||
| 950 per 1000 | 1000 per 1000 | |||||
| Specific behaviour ‐ threat or injury to self or others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome | Low1 | RR 0.50 | 44 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 25 per 1000 | |||||
| Moderate1 | ||||||
| 250 per 1000 | 125 per 1000 | |||||
| High1 | ||||||
| 450 per 1000 | 225 per 1000 | |||||
| Adverse effects: specific ‐ hypotensive episode | Low | Not estimable | 30 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Adverse effects: specific ‐ tachycardia | Low1 | RR 0.28 | 44 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 3 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 14 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 28 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control risk ‐ moderate risk roughly equates to that of the trial control group. | ||||||
| HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: i. ZIPRASIDONE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTIC: i. ZIPRASIDONE | Risk with HALOPERIDOL | |||||
| Tranquilisation or asleep ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No trial reported this outcome. |
| Repeated need for tranquilisation | Study population | RR 1.64 | 60 | ⊕⊝⊝⊝ | ||
| 467 per 1.000 | 765 per 1.000 | |||||
| Specific behaviour ‐ agitation | MD 0.06 higher | ‐ | 231 | ⊕⊕⊝⊝ | ||
| Global outcome | MD 0.34 higher | ‐ | 132 | ⊕⊝⊝⊝ | ||
| Adverse effects: Specific ‐ dystonia during 72 hours | Low | RR 10.26 | 508 | ⊕⊝⊝⊝ | ||
| 2 per 1.000 | 21 per 1.000 | |||||
| Moderate | ||||||
| 4 per 1.000 | 41 per 1.000 | |||||
| High | ||||||
| 10 per 1.000 | 103 per 1.000 | |||||
| Adverse effects: Specific ‐ clinically significant abnormal ECG during 72 hours | Study population | RR 1.01 | 376 | ⊕⊝⊝⊝ | ||
| 127 per 1.000 | 128 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No trial reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'serious' ‐ method of allocation concealment not stated, blinding of participants uneffective 2 Imprecision: rated 'very serious' ‐ less than 100 patients, 95% CI extends beyond the no effect point 3 Risk of bias: rated 'serious' ‐ method of randomisation is not described, allocation concealment is not stated, single‐blind. 4 Indirectness: rated 'serious' ‐ not threat or injury to self or others, therefore had to use scale derived data for agitation. 5 Risk of bias: rated 'very serious' ‐ allocation of concealment not stated, open trial, adverse effects only reported where they occurred in ≥10% of people, sponsored by drug company. 6 Publication bias: rated 'strongly suspected' ‐ sponsored by drug company. 7 Imprecision: rated 'serious' ‐ 95% confidence intervals are wide. 8 Moderate risk roughly is roughly equal to that of the control group. 9 Indirectness: rated 'serious' ‐ abnormal ECG ‐ not necessarily a serious adverse effect. | ||||||
| HALOPERIDOL compared with OTHER ANTIPSYCHOTICS: j. ZUCLOPENTHIXOL ACETATE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTICS: j. ZUCLOPENTHIXOL ACETATE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for rapid tranquillisation ‐ more than 3 injections | Low1 | RR 2.54 | 70 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 127 per 1000 | |||||
| Moderate1 | ||||||
| 200 per 1000 | 508 per 1000 | |||||
| High1 | ||||||
| 350 per 1000 | 889 per 1000 | |||||
| Specific behaviour ‐ threat or injury to self or others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Adverse effects: specific ‐ tremor by 7 days | Low1 | RR 4.16 | 70 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 42 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 208 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 416 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the control group. | ||||||
| HALOPERIDOL compared with BENZODIAZEPINE: a. FLUNITRAZEPAM for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| BENZODIAZEPINE: a. FLUNITRAZEPAM | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for tranquillisation within 24 hours ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Specific behaviours ‐ aggression | Low1 | RR 1.15 | 28 | ⊕⊝⊝⊝ | ||
| 650 per 1000 | 747 per 1000 | |||||
| Moderate1 | ||||||
| 800 per 1000 | 920 per 1000 | |||||
| High1 | ||||||
| 950 per 1000 | 1000 per 1000 | |||||
| Global outcome ‐ need for restraint or seclusion | See comment | See comment | Not estimable | 28 | ⊕⊝⊝⊝ | No events in either group. |
| Adverse effects: specific ‐ EPS | See comment | See comment | Not estimable | 28 | ⊕⊝⊝⊝ | No events in either group. |
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the control group. | ||||||
| HALOPERIDOL compared with BENZODIAZEPINE: b. LORAZEPAM for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| BENZODIAZEPINE: b. LORAZEPAM | HALOPERIDOL | |||||
| Tranquillisation or asleep | Low1 | RR 1.05 | 60 | ⊕⊝⊝⊝ | ||
| 500 per 1000 | 525 per 1000 | |||||
| Moderate1 | ||||||
| 700 per 1000 | 735 per 1000 | |||||
| High1 | ||||||
| 900 per 1000 | 945 per 1000 | |||||
| Repeated need for rapid tranquillisation | Low1 | RR 1.14 | 66 | ⊕⊝⊝⊝ | ||
| 500 per 1000 | 570 per 1000 | |||||
| Moderate1 | ||||||
| 750 per 1000 | 855 per 1000 | |||||
| High1 | ||||||
| 900 per 1000 | 1000 per 1000 | |||||
| Specific behaviour ‐ threat or injury to self or others within 24 hours ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome: no overall improvement ‐ at 60 minutes | Low1 | RR 1.64 | 44 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 82 per 1000 | |||||
| Moderate1 | ||||||
| 150 per 1000 | 246 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 164 per 1000 | |||||
| Adverse effects: specific ‐ dystonia (only reported if occurred in ≥9% during 24 hours) | Low1 | RR 3.54 | 66 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 35 per 1000 | |||||
| Moderate1 | ||||||
| 30 per 1000 | 106 per 1000 | |||||
| High1 | ||||||
| 50 per 1000 | 177 per 1000 | |||||
| Adverse effects: specific ‐ hypertonia/rigidity (only reported if occurred in ≥9%) during 24 hours | Low7 | RR 6.22 | 66 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate7 | ||||||
| 50 per 1000 | 311 per 1000 | |||||
| High7 | ||||||
| 100 per 1000 | 622 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the trial control group. | ||||||
| HALOPERIDOL compared with BENZODIAZEPINE: c. MIDAZOLAM for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| BENZODIAZEPINE: c. MIDAZOLAM | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for rapid tranquillisation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Specific behaviour ‐ threat or injury to self or others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome | Low1 | RR 1.14 | 84 | ⊕⊕⊝⊝ | ||
| 50 per 1000 | 57 per 1000 | |||||
| Moderate1 | ||||||
| 150 per 1000 | 171 per 1000 | |||||
| High1 | ||||||
| 250 per 1000 | 285 per 1000 | |||||
| Adverse effects ‐ general ‐ one or more drug‐related adverse effect | Low4 | RR 5.00 | 84 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate4 | ||||||
| 50 per 1000 | 250 per 1000 | |||||
| High4 | ||||||
| 100 per 1000 | 500 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Medium risk is roughly equal to that of the trial control group. | ||||||
| HALOPERIDOL compared with 1. COMBINATIONS ‐ HALOPERIDOL + LEVOMEPROMAZINE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| COMBINATIONS: a. HALOPERIDOL + LEVOMEPROMAZINE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for tranquillisation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Specific behaviours ‐ threat or injury to self or others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome: general ‐ no overall improvement | Low1 | RR 8.18 | 19 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate1 | ||||||
| 200 per 1000 | 1000 per 1000 | |||||
| High1 | ||||||
| 400 per 1000 | 1000 per 1000 | |||||
| Adverse effect: any serious, specific ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Economic outcome ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Low risk is roughly equal to that of the trial control group. | ||||||
| HALOPERIDOL compared with COMBINATIONS: b. HALOPERIDOL + LORAZEPAM for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| COMBINATIONS: b. HALOPERIDOL + LORAZEPAM | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not asleep by 3 hours | Low1 | RR 1.83 | 67 | ⊕⊝⊝⊝ | ||
| 200 per 1000 | 366 per 1000 | |||||
| Moderate1 | ||||||
| 400 per 1000 | 732 per 1000 | |||||
| High1 | ||||||
| 600 per 1000 | 1000 per 1000 | |||||
| Repeated need for rapid tranquillisation ‐ needing additional injection during 24 hours | Low1 | RR 1.05 | 67 | ⊕⊝⊝⊝ | ||
| 750 per 1000 | 788 per 1000 | |||||
| Moderate1 | ||||||
| 850 per 1000 | 892 per 1000 | |||||
| High1 | ||||||
| 950 per 1000 | 997 per 1000 | |||||
| Specific behaviour ‐ threat or injury to self or others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Global outcome: no overall improvement ‐ at 30 minutes | Low1 | RR 2.67 | 45 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 134 per 1000 | |||||
| Moderate1 | ||||||
| 250 per 1000 | 668 per 1000 | |||||
| High1 | ||||||
| 450 per 1000 | 1000 per 1000 | |||||
| Adverse effects: Specific ‐ dystonia (only reported if occurred in ≥9%) during 24 hours | Low7 | RR 8.25 | 67 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate7 | ||||||
| 50 per 1000 | 412 per 1000 | |||||
| High7 | ||||||
| 100 per 1000 | 825 per 1000 | |||||
| Adverse effects: Specific ‐ hypertonia/rigidity (only reported if occurred in ≥9%) during 24 hours | Low1 | RR 2.74 | 67 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 27 per 1000 | |||||
| Moderate1 | ||||||
| 30 per 1000 | 82 per 1000 | |||||
| High1 | ||||||
| 50 per 1000 | 137 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the control group. | ||||||
| HALOPERIDOL compared to COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Repeated need for tranquillisation | Study population | RR 0.88 | 60 | ⊕⊝⊝⊝ | ||
| 867 per 1.000 | 763 per 1.000 | |||||
| Specific behaviour ‐ agitation | MD 11.20 lower | ‐ | 60 | ⊕⊝⊝⊝ | ||
| Global outcome | Low | RR 0.29 | 60 | ⊕⊝⊝⊝ | ||
| 350 per 1.000 | 102 per 1.000 | |||||
| Moderate | ||||||
| 700 per 1.000 | 203 per 1.000 | |||||
| High | ||||||
| 950 per 1.000 | 275 per 1.000 | |||||
| Adverse effects | Study population | RR 1.67 | 60 | ⊕⊝⊝⊝ | ||
| 100 per 1.000 | 167 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'very serious' ‐ method of allocation not stated, patients instructed to not reveal their allocation although the trial is described as double blind, selective reporting, small study. 2 Imprecision: rated 'very serious' ‐ small study, 95% CI cross the no effect line. | ||||||
| HALOPERIDOL compared to COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep | Study population | RR 1.60 | 316 | ⊕⊕⊕⊝ | ||
| 281 per 1.000 | 450 per 1.000 | |||||
| Repeated need for tranquillisation | Low | RR 0.78 | 376 | ⊕⊝⊝⊝ | ||
| 50 per 1.000 | 39 per 1.000 | |||||
| Moderate | ||||||
| 120 per 1.000 | 94 per 1.000 | |||||
| High | ||||||
| 450 per 1.000 | 351 per 1.000 | |||||
| Specific behaviours ‐ aggression | Study population | RR 1.06 | 316 | ⊕⊕⊕⊝ | ||
| 194 per 1.000 | 205 per 1.000 | |||||
| Global outcomes | Study population | RR 1.21 | 316 | ⊕⊕⊕⊝ | ||
| 238 per 1.000 | 287 per 1.000 | |||||
| Adverse effects: specific ‐ acute dystonia | Low | RR 19.48 | 316 | ⊕⊕⊝⊝ | ||
| 0 per 1.000 | 0 per 1.000 | |||||
| Moderate | ||||||
| 50 per 1.000 | 974 per 1.000 | |||||
| High | ||||||
| 100 per 1.000 | 1000 per 1.000 | |||||
| Adverse effects: specific ‐ seizure | Study population | RR 2.05 | 316 | ⊕⊕⊝⊝ | ||
| 6 per 1.000 | 13 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No trial reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'serious' ‐ open trial. 2 Moderate risk is roughly equal to that of the trial control group. 3 Inconsistency: rated 'very serious' ‐ I2 86%. 4 Publication bias: rated 'strongly suspected' ‐ visual inspection of the funnel plot. 5 Indirectness: overall improvement not reported, therefore global improvement inferred from need for restraints at by 120 minutes. 6 Imprecision: rated 'serious' ‐ 95% confidence intervals are wide. 7 Low risk is roughly equal to that of the trial control group. | ||||||
| HALOPERIDOL compared to COMBINATIONS: e. HALOPERIDOL + RISPERIDONE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATIONS: e. HALOPERIDOL + RISPERIDONE | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Repeated need for tranquillisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Specific behaviour ‐ agitation | MD 3.82 higher | ‐ | 100 | ⊕⊕⊝⊝ | ||
| Global outcome assessed with: no improvement at 72 hours | Study population | RR 0.38 | 100 | ⊕⊕⊝⊝ | ||
| 160 per 1.000 | 61 per 1.000 | |||||
| Adverse effects: specific assessed with akathisia during 14 days | Study population | RR 0.88 | 100 | ⊕⊕⊝⊝ | ||
| 320 per 1.000 | 282 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'serious' ‐ allocation concealment procedures not stated, blinding procedures not stated. 2 Imprecision: rated 'serious' ‐ 95% CIs are wide. | ||||||
| HALOPERIDOL compared to COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Repeated need for tranquillisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Specific behaviour ‐ agitation | MD 0.04 lower | ‐ | 64 | ⊕⊝⊝⊝ | ||
| Global outcome | Study population | RR 1.06 | 64 | ⊕⊝⊝⊝ | ||
| 182 per 1.000 | 193 per 1.000 | |||||
| Adverse effects | Low | RR 3.06 | 64 | ⊕⊝⊝⊝ | ||
| 100 per 1.000 | 306 per 1.000 | |||||
| Moderate | ||||||
| 240 per 1.000 | 734 per 1.000 | |||||
| High | ||||||
| 500 per 1.000 | 1000 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'serious' ‐ allocation concealment procedures not stated, blinding procedures not stated, small study. 2 Imprecision: rated 'very serious' ‐ small study, 95% CI cross the no effect area. | ||||||
| HALOPERIDOL compared with COMBINATIONS: d. QUETIAPINE + MAGNESIUM VALPROATE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| COMBINATIONS: d. QUETIAPINE + MAGNESIUM VALPROATE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for tranquillisation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Specific behaviours ‐ agitation at 3 days | The mean specific behaviours ‐ agitation at 3 days in the intervention groups was | 60 | ⊕⊝⊝⊝ | |||
| Global effect | Low3 | RR 1.17 | 60 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 58 per 1000 | |||||
| Moderate3 | ||||||
| 200 per 1000 | 234 per 1000 | |||||
| High3 | ||||||
| 350 per 1000 | 409 per 1000 | |||||
| Adverse effects ‐ any specific, serious ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | 0 | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'very serious' ‐ method of randomisation is not reported, allocation concealment not stated, open‐label, selective reporting. | ||||||
| HALOPERIDOL compared to COMBINATIONS: h. RISPERIDONE + CLONAZEPAM for psychosis induced aggression or agitation | ||||||
| Patient or population: psychosis induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATIONS: h. RISPERIDONE + CLONAZEPAM | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep | Study population | RR 0.82 | 205 | ⊕⊝⊝⊝ | ||
| 673 per 1.000 | 552 per 1.000 | |||||
| Repeated need for tranquillisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Specific behaviour ‐ agitation | MD 0.50 lower | ‐ | 108 | ⊕⊝⊝⊝ | ||
| Global outcome | Study population | RR 0.60 | 100 | ⊕⊕⊝⊝ | ||
| 100 per 1.000 | 60 per 1.000 | |||||
| Adverse effects | Low | RR 1.63 | 205 | ⊕⊝⊝⊝ | ||
| 150 per 1.000 | 244 per 1.000 | |||||
| Moderate | ||||||
| 300 per 1.000 | 489 per 1.000 | |||||
| High | ||||||
| 500 per 1.000 | 815 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'very serious' ‐ open study, evidence of reporting bias, sponsored trial. 2 Imprecision: rated 'serious' ‐ 95% CI cross the no effect area. 3 Risk of bias: rated 'serious' ‐ allocation concealment and blinding procedures not stated. 4 Imprecision: rated 'serious' ‐ 95% CI are wide. 5 Imprecision: rated 'serious' ‐ 95% CI are wide. | ||||||
| HALOPERIDOL compared to COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Repeated need for tranquillisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Specific behaviour ‐ agitation | MD 0.28 lower | ‐ | 71 | ⊕⊝⊝⊝ | ||
| Global outcome ‐ no improvement ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Adverse effects ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'very serious' ‐ randomisation, allocation and blinding procedures not stated, evidence of attrition bias, small study. 2 Imprecision: rated 'very serious' ‐ small study, 95% CI are wide. | ||||||
Background
Description of the condition
Psychosis is associated with a number of mental disorders, including schizophrenia and bipolar disorder. Symptoms of psychosis include delusions and hallucinations, which can lead some people to become confused, frightened or agitated. Often they can suffer a combination of these distressing emotions (APA 2006). Agitation is characterised by restlessness, excitability and irritability, and for some people, this can result in verbal and physical aggressive behaviour (Mohr 2005). Agitation and aggression within the psychiatric setting imposes a significant challenge to clinicians who, while attempting to make an accurate diagnosis and formulation (Schleifer 2011), have to intervene quickly in order to manage the risk that the service user may present to themselves, other service users and staff (NICE 2005, NICE 2015).
Description of the intervention
Haloperidol was developed in 1958 by the Belgian company Janssen Pharmaceutica, and with the earlier development of chlorpromazine was considered a "psychopharmacological revolution" for the treatment of schizophrenia (López‐Munoz 2009). Newer anti‐psychotic medication has been developed for the day‐to‐day management of symptoms of schizophrenia, however, haloperidol continues to be in wide use, particularly for the management of psychosis‐induced agitation. In clinical practice, where de‐escalation techniques have not been appropriate nor sufficient in managing a person’s agitation and where that person poses a threat to themselves or others, it may be necessary to use medication for rapid tranquillisation (Pratt 2008). Although there may be occasions where it is appropriate to induce sleep, the aim of rapid tranquillisation should usually be to sedate agitated or aggressive people to a point where they are still able to engage in further assessment and be responsive to communication (Parker 2010). If clinically feasible, oral administration is preferred. However, if the person refuses medication or there is no improvement after 30 to 60 minutes, haloperidol in single doses of 5 mg, administered intramuscularly (IM) is a commonly used treatment option (Pereira 2007).
Cardiac problems and extrapyramidal side effects (EPS) such as dystonia and akathisia are associated with conventional antipsychotics such as haloperidol. Experiencing distressing adverse effects may act as a barrier for future engagement with services or treatment (Pratt 2008). The UK’s NICE guidelines recommend that when using haloperidol IM, an antimuscarinic agent such as procyclidine or benzatropine should be administered to counter the potential for EPS. Subsequent to the publication of these guidelines, the manufacturers of Haldol (Janssen‐Cilag Ltd 2010) recommended that a baseline electrocardiogram (ECG) should be undertaken prior to treatment with haloperidol. In addition, they have discontinued the license for intravenous (IV) use and reduced the recommended maximum dose from 90 mg/day to 30 mg/day orally, and 18 mg/day IM (Parker 2010). When Haldol is prescribed outside of these recommendations, this would be considered ‘off label’ and may increase the prescriber’s professional responsibility and potential liability (Pratt 2008). Despite these adverse effects, haloperidol alone or combined with a benzodiazepine was recommended by the NICE guidelines (NICE 2005) and by the American Psychiatric Association (APA 2006), and haloperidol remains on the World Health Organization's Essential Medicine list (WHO 2011, WHO 2015). With the last updated NICE guidelines, recommendation changed indicating either the use of haloperidol combined with promethazine or lorazepam alone for rapid tranquillisation (NICE 2015).
How the intervention might work
Haloperidol is mainly indicated for schizophrenia or other psychoses, mania, violent or dangerously impulsive behaviour, excitement and for short‐term adjunctive management of psychomotor agitation (BNF 2011). Haloperidol is from the butyrophenone family of antipsychotics (neuroleptics) (López‐Munoz 2009). It is thought that haloperidol prevents the occurrence of delusions and hallucinations by blocking the dopamine D2 receptors in the meso‐cortico‐limbic system. In one study, while the D2 dopamine receptor binding was high in the temporal cortex for both haloperidol and atypical antipsychotics, haloperidol induced a significantly higher binding index in the thalamus and striatum than atypical antipsychotics. It is thought that this antidopaminergic activity in the dorsolateral striatum may contribute to the extra pyramidal effects that are associated with typical antipsychotics such as haloperidol (Xiberas 2001).
Why it is important to do this review
The UK's 2005 NICE guidelines recommended that where haloperidol is administered via injection it should be combined with lorazepam when given IM. However, these guidelines also recommended, that, in exceptional circumstances, haloperidol can be given alone by IV injection (NICE 2005). Despite this recommendation, it is difficult to administer IV injections safely in restraint situations and there is evidence that haloperidol alone continues to be given via IM injection (Choudhury 2011). Surveys undertaken with consultant psychiatrists have shown that following the withdrawal of droperidol, haloperidol was the preferred alternative for rapid tranquillisation (Reid 2003) and that over 33% of respondents would be prepared to use doses above the BNF limits (Pereira 2007). Further, since the NICE guideline on the management of disturbed and violent behaviour was published, Janssen‐Cilag Ltd has withdrawn the license for IV use. With the increased prescribing of haloperidol in the UK by 16% (excluding depot injections) between 2004 and 2009 (NHS 2009), the authors felt it timely and justified to examine the evidence regarding the efficacy and safety of prescribing haloperidol alone for treating psychosis‐induced agitation and aggression. This is one of a series of linked completed and maintained reviews and other reviews that are underway (Table 1) that will create a body of evidence assessing the effectiveness of various drugs and their preferred routes of administration for both short‐ and long‐term psychosis‐induced aggression or agitation.
In the UK’s 2015 NICE guidelines, either use of haloperidol combined with promethazine or lorazepam alone is now recommended, with the latter preferred when in presence of cardiovascular disease or in case a recent ECG is unavailable (NICE 2015).
| Focus of review | Reference |
| Completed and maintained reviews | |
| 'As required' medication regimens for seriously mentally ill people in hospital | |
| Benzodiazepines for psychosis‐induced aggression or agitation | |
| Chlorpromazine for psychosis induced aggression or agitation | |
| Clotiapine for acute psychotic illnesses | |
| Containment strategies for people with serious mental illness | |
| Droperidol for acute psychosis | |
| Haloperidol plus promethazine for psychosis‐induced aggression | |
| Olanzapine IM or velotab for acutely disturbed/agitated people with suspected serious mental illnesses | |
| Seclusion and restraint for serious mental illnesses | |
| Zuclopenthixol acetate for acute schizophrenia and similar serious mental illnesses | |
| Reviews in the process of being completed | |
| Risperidone for psychosis induced aggression or agitation | |
| Haloperidol for long term aggression in psychosis | |
| Loxapine inhaler for psychosis‐induced aggression | |
| Quetiapine for psychosis‐induced aggression | |
| De‐escalation techniques for psychosis‐induced aggression | |
Objectives
To examine whether haloperidol alone, administered orally, intramuscularly or intravenously, is an effective treatment for psychosis‐induced aggression or agitation, wherein clinicians are required to intervene to prevent harm to self and others.
Methods
Criteria for considering studies for this review
Types of studies
All relevant randomised controlled trials. If a trial was described as 'double‐blind' but implied randomisation, we included such trials in a sensitivity analysis (see Sensitivity analysis). If their inclusion did not result in a substantive difference, they remained in the analyses. If their inclusion resulted in statistically significant differences, we did not add the data from these lower‐quality studies to the results of the better trials, but presented such data within a subcategory. We excluded quasi‐randomised studies, such as those allocating by alternate days of the week.
Types of participants
People exhibiting agitation or aggression (or both) thought to be due to psychotic illness, regardless of age or sex. We included studies that involved participants with other diagnoses, such as drug or alcohol intoxication, organic problems including dementia, learning disability and non‐psychotic mental illness, providing that the number of participants in these groups did not exceed the number of participants with psychosis.
Types of interventions
1. Rapid use of haloperidol
Alone, any dose via any route of administration; compared with rapid use of the following.
a. Placebo or no intervention
We are aware that, in a situation where haloperidol is available, the option of not giving treatment would be problematic. These are, however, valuable and pioneering studies which give us some insight to the absolute value of haloperidol in the emergency situation.
b. Other antipsychotic
Any dose via any route of administration.
c. Benzodiazepine
Any dose via any route of administration.
d. Anticonvulsant alone
Any dose via any route of administration.
e. Combination of drugs
Any dose via any route of administration.
Rapid use of the interventions is defined as administration deemed necessary to calm behaviour or prevent harm to the participant or others.
Types of outcome measures
All outcomes grouped by time: by 30 minutes, up to two hours, up to four hours, up to 24 hours and over 24 hours.
Primary outcomes
1. Tranquilisation or asleep
1.1 Not tranquil or asleep ‐ by up to 30 minutes
1.2. Repeated need for rapid tranquillisation
Secondary outcomes
1. Tranquillisation or asleep
1.1 Not tranquil ‐ over 30 minutes
1.2 Not asleep ‐ over 30 minutes
1.3 Time to tranquillisation/sleep
1.4 Time to tranquillisation
1.5 Time to sleep
2. Specific behaviours
2.1 Self‐harm, including suicide
2.2 Injury to others
2.3 Agitation
2.3.1 Another episode of agitation by 24 hours
2.3.2 No clinically important change in agitation
2.3.3 No change in agitation
2.3.4 Average endpoint in agitation score
2.3.5 Average change in agitation scores
2.4 Aggression
2.4.1 Another episode of aggression by 24 hours
2.4.2 No clinically important change in aggression
2.4.3 No change in aggression
2.4.4 Average endpoint in aggression score
2.4.5 Average change in aggression scores
3. Global outcomes
3.1 No overall improvement
3.2 Use of restraints/seclusion
3.3 Relapse ‐ as defined by each study
3.4 Recurrence of violent incidents
3.5 Needing extra visits from the doctor
3.6 Refusing oral medication
3.7 Not accepting treatment
3.8 Average endpoint score
3.9 Average change score
3.10 Average dose of drug
4. Service outcomes
4.1 Duration of hospital stay
4.2 Re‐admission
4.3 No clinically important engagement with services
4.4 No engagement with services
4.5 Average endpoint engagement score
4.6 Average change in engagement scores
5. Mental state
5.1 No clinically important change in general mental state
5.2 No change in general mental state
5.3 Average endpoint general mental state score
5.4 Average change in engagement scores
6. Adverse effects
6.1 Death
6.2 Any non‐serious general adverse effects
6.3 Any serious, specific adverse effects
6.4 Average endpoint general adverse effect score
6.5 Average change in general adverse effect scores
6.6 No clinically important change in specific adverse effects
6.7 No change in specific adverse effects
6.8 Average endpoint specific adverse effects
6.9 Average change in specific adverse effects
7. Leaving the study early
7.1 For specific reasons
7.2 For general reasons
8. Satisfaction with treatment
8.1 Recipient of treatment not satisfied with treatment
8.2 Recipient of treatment average satisfaction score
8.3 Recipient of treatment average change in satisfaction scores
8.4 Informal treatment provider not satisfied with treatment
8.5 Informal treatment providers' average satisfaction scores
8.6 Informal treatment providers' average change in satisfaction scores
8.7 Professional providers' not satisfied with treatment
8.8 Professional providers' average satisfaction score
8.9 Professional providers' average change in satisfaction scores
9. Acceptance of treatment
9.1 Not accepting treatment
9.2 Average endpoint acceptance score
9.3 Average change in acceptance scores
10. Quality of life
10.1 No clinically important change in quality of life
10.2 Not any change in quality of life
10.3 Average endpoint quality of life score
10.4 Average change in quality of life score
10.5 No clinically important change in specific aspects of quality of life
10.6 No change in specific aspects of quality of life
10.7 Average endpoint specific aspects of quality of life
10.8 Average change in specific aspects of quality of life
11. Economic outcomes
11.1 Direct costs
11.2 Indirect costs
Outcomes used for 'Summary of findings' table
We used the GRADE approach to interpret findings (Schünemann 2008) and used the GRADE profiler (GRADE PRO) to import data from RevMan 5 (Review Manager) to create 'Summary of findings' tables. These tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes that we rated as important to patient‐care and decision making. We selected the following main outcomes for inclusion in the 'Summary of findings' tables.
-
Tranquillisation or asleep ‐ not tranquil or asleep by 30 minutes.
-
Tranquillisation or asleep ‐ repeated need for rapid tranquillisation ‐ within 24 hours.
-
Specific behaviours ‐ threat or injury to others/self.
-
Global outcomes ‐ overall improvement.
-
Adverse effects ‐ any serious, specific adverse effects.
-
Economic outcomes.
Search methods for identification of studies
Electronic searches
Cochrane Schizophrenia Group’s Study‐Based Register of Trials
On May 26, 2016, the information specialist searched the register using the following search strategy which has been developed based on literature review and consulting with the authors of the review:
(*Agitation* OR *Aggression*) in Healthcare Condition AND (*Haloperidol*) in Intervention of STUDY
In such study‐based register, searching the major concept retrieves all the synonym and relevant studies because all the studies have already been organised based on their interventions and linked to the relevant topics.
This register is compiled by systematic searches of major resources (including AMED, BIOSIS CINAHL, Embase, MEDLINE, PsycINFO, PubMed, and registries of clinical trials) and their monthly updates, handsearches, grey literature, and conference proceedings (see Group’s Module). There is no language, date, document type, or publication status limitations for inclusion of records into the register.
For previous searches, please see Appendix 1.
Searching other resources
1. Reference searching
We inspected references of all included studies for further relevant studies.
2. Personal contact
We attempted to contact the first author of each included study for information regarding unpublished trials.
Data collection and analysis
Selection of studies
Review author MJP inspected citations from the searches and identified relevant abstracts. A random 20% sample was independently re‐inspected by CEA to ensure reliability. Where disputes arose, we acquired the full report for more detailed scrutiny. We obtained full reports of the abstracts that met the review criteria and these were inspected by MJP. Again, a random 20% of reports were re‐inspected by CEA, in order to ensure reliable selection. Where it was not possible to resolve disagreement by discussion, we attempted to contact the authors of the study for clarification.
Review authors EGO and XL screened results from 2016 search to identify relevant abstracts. We obtained and inspected full reports of the abstracts meeting the review criteria.
Data extraction and management
1. Extraction
Review author MJP (original search), EGO (2016 search) extracted data from all included studies. In addition, to ensure reliability, CEA independently extracted data from a random sample of these studies, comprising 10% of the total. Again, any disagreement was discussed, decisions documented and, if necessary, we contacted the authors of studies for clarification. If there were any remaining problems, HJ helped clarify issues and these final decisions were documented. We extracted data presented only in graphs and figures whenever possible, but we only included the data if two review authors independently had the same result. We attempted to contact authors through an open‐ended request in order to obtain missing information or for clarification whenever necessary. If studies were multi‐centre, where possible, we extracted data relevant to each component centre separately.
2. Management
2.1 Forms
We extracted data onto standard, simple forms.
2.2 Scale‐derived data
We included continuous data from rating scales only if:
a. the psychometric properties of the measuring instrument have been described in a peer‐reviewed journal (Marshall 2000); and
b. the measuring instrument has not been written or modified by one of the trialists for that particular trial.
Ideally, the measuring instrument should either be i. a self‐report or ii. completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly, therefore, in Description of studies, we recorded whether or not this was the case.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint), which can be difficult in unstable and difficult to measure conditions such as schizophrenia. We decided primarily to use endpoint data, and only use change data if the former were not available. We combined endpoint and change data in the analysis and we used mean differences (MD) rather than standardised mean differences (SMD) throughout (Higgins 2011).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we aimed to apply the following standards to all data before inclusion: a) standard deviations (SDs) and means are reported in the paper or obtainable from the authors; b) when a scale starts from the finite number zero, the SD, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, (Altman 1996); c) if a scale started from a positive value (such as the Positive and Negative Syndrome Scale (PANSS), (Kay 1986)), which can have values from 30 to 210), the calculation described above was modified to take the scale starting point into account. In these cases skew is present if 2 SD > (S‐S min), where S is the mean score and 'S min' is the minimum score. Endpoint scores on scales often have a finite start and end point and these rules can be applied. Skewed data pose less of a problem when looking at means if the sample size is large (> 200) and we entered these into the syntheses. We present skewed data from studies of less than 200 participants in ‘Additional tables’ rather than in analyses.
When continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. These were entered into analyses.
2.5 Common measure
To facilitate comparison between trials, if required, we converted variables that can be reported in different metrics, such as time to tranquillisation (e.g. minutes, hours) to a common metric (e.g. minutes).
2.6 Conversion of continuous to binary
Where possible, efforts were made to convert outcome measures to dichotomous data. This was done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the PANSS scale (Kay 1986), this could be considered as a clinically significant response (Leucht 2005; Leucht 2005a). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for haloperidol for psychosis‐induced agitation. Where keeping to this made it impossible to avoid outcome titles with clumsy double‐negatives (e.g. 'Not improved'), we reported data where the left of the line indicates an unfavourable outcome. This was noted in the relevant graphs.
Assessment of risk of bias in included studies
Review authors MJP and EGO assessed risk of bias by using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to assess trial quality. This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting. HJ re‐rated 10% of included studies.
If the raters disagreed, or if either rater was in doubt, we decided the final rating by consensus, with the involvement of another member of the review group. Where inadequate details of randomisation and other characteristics of trials were provided, we contacted authors of the studies in order to obtain further information. We reported non‐concurrence in quality assessment, but if disputes arose as to which category a trial was to be allocated, again, we resolved these by discussion.
We noted the level of risk of bias in both the text of the review and in the 'Summary of findings' tables.
Measures of treatment effect
1. Binary data
For binary outcomes, we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000).
2. Continuous data
For continuous outcomes, we estimated mean difference (MD) between groups. We preferred not to calculate effect size measures (SMD). However, if scales of very considerable similarity were used, we presumed there was a small difference in measurement, and we calculated the effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992), whereby P values are spuriously low, CIs unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
Where clustering was not accounted for in primary studies, we presented data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. We attempted to contact the first authors of studies to obtain intra‐class correlation coefficients (ICCs) for their clustered data and to adjust for this by using accepted methods (Gulliford 1999). Where clustering had been incorporated into the analysis of primary studies, we presented these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC [Design effect=1+(m‐1)*ICC] (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999).
Where cluster studies had been appropriately analysed taking into account ICCs and relevant data documented in the report, synthesis with other studies was possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, if we had included cross‐over trials, we would only have used data from the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, where relevant, the additional treatment arms were presented in comparisons. If data were binary these were simply added and combined within the two‐by‐two table. If data were continuous, we combined data following the formula in section 7.7.3.8 (Combining groups) of the Cochrane Handbook for Systematic Reviews of Interventions. Where the additional treatment arms were not relevant, we did not reproduce these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for, we did not reproduce these data or use them within analyses, (except for the outcome 'leaving the study early'). Where more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we marked such data with (*) to indicate that such a result may well be prone to bias.
2. Binary
In the case where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat (ITT) analysis). Those leaving the study early were all assumed to have the same rates of negative outcome as those who completed, with the exception of the outcome of death and adverse effects. For these outcomes, the rate of those who stayed in the study ‐ in that particular arm of the trial ‐ were used for those who did not. We undertook a sensitivity analysis to test how prone the primary outcomes were to change when 'completer' data only were compared with the ITT analysis using the above assumptions.
3. Continuous
3.1 Attrition
In the case where attrition for a continuous outcome was between 0% and 50% and completer‐only data were reported, we reproduced these.
3.2 Standard deviations (SDs)
If SDs were not reported, we first tried to obtain the missing values from the authors. If not available, where there were missing measures of variance for continuous data, but an exact standard error (SE) and confidence intervals (CIs) were available for group means, and either a P value or 't' value were available for differences in mean, we calculated them according to the rules described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): When only the SE was reported, SDs were calculated by the formula SD = SE * square root (n). Chapters 7.7.3 and 16.1.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) present detailed formulae for estimating SDs from P values, t or F values, CIs, ranges or other statistics. If these formulae did not apply, we calculated the SDs according to a validated imputation method which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. Nevertheless, we examined the validity of the imputations in a sensitivity analysis excluding imputed values.
3.3 Last observation carried forward (LOCF)
We anticipated that in some studies the method of LOCF would be employed within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results (Leucht 2007). Therefore, where LOCF data were used in the trial, if less than 50% of the data had been assumed, we reproduced these data and indicated that they were the product of LOCF assumptions.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We inspected all studies for clearly outlying people or situations which we had not predicted would arise. When such situations or participant groups arose, these were discussed.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We inspected all studies for clearly outlying methods which we had not predicted would arise. When such methodological outliers arose, these were discussed.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
We investigated heterogeneity between studies by considering the I2 statistic alongside the Chi2 P value. The I2 statistic provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on i. magnitude and direction of effects and ii. strength of evidence for heterogeneity (e.g. P value from Chi2 test, or a CI for I2). We interpreted an I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistic, as evidence of substantial levels of heterogeneity (Section 9.5.2 ‐ Higgins 2011). When substantial levels of heterogeneity were found in the primary outcome, we explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
1. Protocol versus full study
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. These are described in section 10.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We tried to locate protocols of included randomised trials. If the protocol was available, outcomes in the protocol and in the published report were compared. If the protocol was not available, outcomes listed in the methods section of the trial report were compared with actual reported results.
2. Funnel plot
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are again described in Section 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar sizes. In other cases, where funnel plots were possible, we sought statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model. It puts added weight onto small studies which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We chose the fixed‐effect model for all analyses. The reader is, however, able to choose to inspect the data using the random‐effects model.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
We did not anticipate any subgroup analyses.
2. Investigation of heterogeneity
If inconsistency was high, we reported it. First, we investigated whether data were entered correctly. Second, if data were correct, we visually inspected the graph and successively removed outlying studies to see if homogeneity was restored. For this review, we decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, we would present the data. If not, we would not pool data but would discuss the issues. We know of no supporting research for this 10% cut‐off but are investigating the use of prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity were obvious, we stated hypotheses regarding these for future reviews or versions of this review. We did not anticipate undertaking analyses relating to these.
Sensitivity analysis
1. Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in some way as to imply randomisation. For the primary outcomes we included these studies, and if there was no substantive difference when the implied randomised studies were added to those with better description of randomisation, then we used all data from these studies.
2. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumption compared with 'completer' data only. Where there was a substantial difference, we reported results and discussed them, but continued to employ our assumption.
Where assumptions had to be made regarding missing SDs data (see Dealing with missing data), we compared the findings on primary outcomes when we used our assumption compared with 'completer' data only. We undertook a sensitivity analysis to test how prone results were to change when 'completer' data only were compared with the imputed data using the above assumption. If there was a substantial difference, we reported results and discussed them, but continued to employ our assumption.
3. Risk of bias
We analysed the effects of excluding trials that were judged to be at high risk of bias across one or more of the domains of randomisation (implied as randomised with no further details available) allocation concealment, blinding and outcome reporting for the meta‐analysis of the primary outcome. If the exclusion of trials at high risk of bias did not substantially alter the direction of effect or the precision of the effect estimates, then we included data from these trials in the analysis.
4. Imputed values
We also undertook a sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect in cluster‐randomised trials.
If substantial differences were noted in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we did not pool data from the excluded trials with the other trials contributing to the outcome, but presented them separately.
5. Fixed and random effects
All data was be synthesised using a fixed‐effect model, however, we also synthesised data for the primary outcome using a random‐effects model to evaluate whether this altered the significance of the result.
Results
Description of studies
For substantive descriptions of studies please see the Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification and Characteristics of ongoing studies.
Results of the search
The electronic search (June 2011) yielded 665 citations of potentially eligible studies. After checking titles and abstracts, 209 full‐text papers were obtained for a second assessment. These publications related to 100 different studies (29 included studies, 55 excluded, 15 awaiting classification and one ongoing). The reference lists of the 29 studies to be included were checked, through which we identified another three potential included studies. Again, full‐text papers were obtained and on closer inspection, Reschke 1974 and Fitzgerald 1969 were included and Goldstein 1966 excluded because, although participants, were experiencing psychosis, they were not specifically aggressive or agitated. The reference lists of related Cochrane systematic reviews were also searched, from which we identified Bailine 1987 for inclusion (2011 search, Figure 1).

Study flow diagram.
The update search run in 2016 yielded 34 citations, of which nine new studies were included (Figure 2).
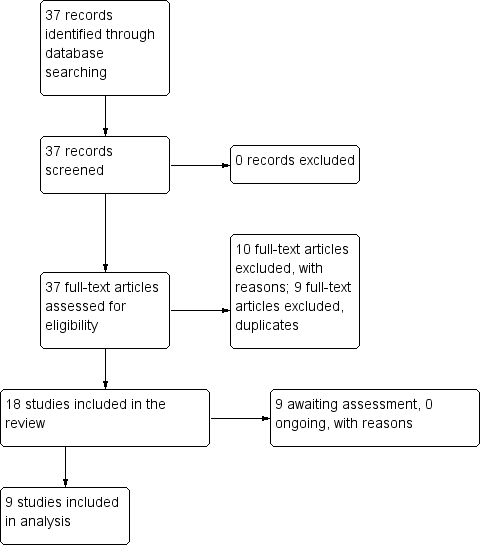
Study flow diagram.
Included studies
Details of the 41 studies included in the review are provided in the Characteristics of included studies table.
1. Length of studies
The duration of the studies ranged from two hours (Calver 2015; Dorevitch 1999) to eight weeks (Higashima 2004). The majority were ≦ 72 hours, apart from Garza‐Trevino 1989 and Nobay 2004 which did not report the duration of the study.
2. Participants
2.1 Clinical state
Participants in the studies presented with acute exacerbation of psychotic symptoms. Some studies focused on people whose psychosis had primarily triggered agitation (Battaglia 2002; Breier 2002; Bristol Myers 2004a; Bristol‐Myers 2005b; Brook 2000; Eli Lilly 2004; Fang 2012; Garza‐Trevino 1989; Guo 2007, Higashima 2004; Kinon 2001d; Liang 2013; Lim 2010; Liu 2012; Resnick 1984; Shu 2010; Walther 2014; Yin 2012; Zhou 2014), and other trials included people with both agitation and aggression (Bailine 1987; Baldacara 2011; Battaglia 1997; Calver 2015; Currier 2004; Dorevitch 1999; Fitzgerald 1969; Foster 1997; Fruensgaard 1977; Huf 2007; Kewala 1984; Li 2006; Man 1973; Nobay 2004; Paprocki 1977; Reschke 1974; Ritter 1972; Salzman 1991; Shi 2010; Stotsky 1977; Taymeeyapradit 2002; Tuason 1986). All participants were so disturbed that clinicians felt that they required rapid tranquillisation.
2.2 Diagnosis
Over 80% of participants had a diagnosis of schizophrenia. Other diagnoses included schizoaffective disorder, schizophreniform disorder, bipolar disorder, and 'psychosis not otherwise specified'. A small minority of participants had a diagnosis of organic mental disorder and substance‐induced psychosis, but in no trial was this subgroup the majority or the focus of the study.
2.3. Exclusions
Where exclusion criteria were reported, these often included pregnant or lactating women, people with serious medical illnesses, people who had used certain medications (e.g. anti‐psychotics/anti‐depressants) within a specified period prior to enrolment, people with a known allergy/hypersensitivity to the study drugs and people with alcohol or drug dependency.
2.4 Age
In the 23 studies that reported an age range, participants ranged from 18 to 73 years. Nine studies reported a mean age (mostly around 30), with Garza‐Trevino 1989 being the only study not to report the age of participants.
2.5 Sex
There were 2236 male participants and 1310 participants were women; the sex of 1387 people was not reported.
3. Study size
The study sizes varied with the smallest study having only 19 participants (Higashima 2004) and the largest randomising 448 people (Bristol Myers 2004a).
4. Setting
Where reported, the majority of participants presented at psychiatric emergency departments and were newly admitted inpatients. Interestingly, one study included outpatients (Stotsky 1977).
5. Interventions
It is unsurprising that this old and well‐established drug has been compared with many other interventions. For all but one study (Kinon 2001d ‐ see below), haloperidol and the other drugs were administered by the intramuscular (IM) route. What was surprising, however, was how poorly reported the true doses were. Dose ranges were commonly reported but to stipulate their mean or median was the exception rather than the rule. In several comparisons we have had to make educated guesses and the likely end doses.
5.1 Versus placebo
Trials comparing haloperidol with placebo largely used around 10 mg of the drug given by IM injection.
5.2 Versus other antipsychotics
The aripiprazole comparison involved around 10 mg of haloperidol compared with up to 45 mg of the control drug. The older trials involved in the chlorpromazine comparison used larger doses of haloperidol (10 mg to 20 mg with one trial allowing the option of up to 100 mg/day) with between 100 mg and up to 500 mg of chlorpromazine. In the droperidol trial 10 mg of haloperidol was compared with around 15 mg of droperidol. Although one loxapine versus haloperidol trial did allow for up to 100 mg of haloperidol, more commonly used doses across trials were in the 10 mg to 20 mg range compared with 50 mg to 250 mg of loxapine. The olanzapine dose tended to be around 10 mg to 15 mg, and in these trials haloperidol was given around the 10 mg dose. The Kinon 2001d trial (olanzapine versus haloperidol) is notable for it being the only one that used oral drugs rather then IM route of administration. The perphenazine comparison used doses of both drugs in the mid 20’s mg given by IM injection. The quetiapine trial compared a maximum of 12 mg of haloperidol with up to 750 mg of quetiapine. In the risperidone comparison, haloperidol was used at between about 5 mg and 15 mg and the risperidone between 2 mg and 6 mg oral liquid and oral velotab. The thiothixene trial used higher doses of both drugs (˜30 mg) given by IM injection. The ziprasidone studies used rather high dose haloperidol (20 mg up to 40 mg) and compared this with around 40 mg of ziprasidone oral and IM. Haloperidol dose in the zuclopenthixol acetate trial was also high (˜30 mg) compared with up to ˜100 mg IM of the longer‐acting compound).
5.3 Versus benzodiazepine
The one trial comparing IM flunitrazepam with Im haloperidol used about 1 mg of the benzodiazepine and 5 mg of the antipsychotic. The lorazepam trials, using a combination of oral and IM administration employed doses of around 4 mg to 6 mg of the drug. The comparison dose was around 10 mg of haloperidol. The single midazolam study compared 5 mg with 5 mg (both IM).
5.4 Versus combinations
The haloperidol versus haloperidol plus levomepromazine study were oral treatments, but we remain unclear as to the doses. These seem to be around 5 mg for each compound. For the haloperidol versus haloperidol plus lorazepam comparison, the antipsychotic tended to be given at around the 5 mg level for both groups and the benzodiazepine at 4 mg each. In the haloperidol versus haloperidol plus midazolam study, the antipsychotic at 5 mg was compared with the adjunction of 15 mg of midazolam. In the haloperidol versus haloperidol plus promethazine trial, all treatments were IM. The haloperidol was up to 10 mg and the promethazine up to 50 mg. The haloperidol versus haloperidol plus risperidone study compared 10 mg to 20 mg of haloperidol with 3 mg to 6 mg of risperidone. For the haloperidol versus olanzapine plus magnesium valproate comparison, the first treatment tended to be given at around 15 mg and the second one at respectively 10 mg plus 1.25 mg. In the haloperidol versus quetiapine (IM) plus magnesium valproate (oral) comparison, the dose of haloperidol was up to 30 mg/day and the quetiapine's 800 mg/day. The additional magnesium valproate was dosed at about 0.5 g to 1 g/orally/day. In the haloperidol versus risperidone plus clonazepam comparison, haloperidol was administered at 10 mg to 20, mg whilst risperidone plus clonazepam doses ranged from, respectively, 2 mg to 6 mg plus 0 mg to 8 mg. Finally, the haloperidol versus ziprasidone plus clonazepam study compared up to 30 mg of haloperidol versus up to 160 mg of ziprasidone and up to 6 mg of clonazepam.
6. Outcomes
For the majority of trials, binary data were available with respect to repeated need for tranquillisation, not tranquil or asleep, need for adjunctive medication, leaving the study early and adverse effects. The majority of trials employed continuous scales to measure other clinical outcomes. However, the results of these scale‐derived findings were often presented in graphs, as P values alone, or as means without standard deviations. Much data were unusable for synthesis. Requests for further information from the majority of authors remain unanswered. Dr Dubin (Dubin 1985) kindly answered our request, however was unable to provide us with the standard deviation or standard error.
The various rating scales, from which we were able to obtain usable data are listed below.
6.1 Specific behaviour ‐ Agitation
a. Agitated Behavior Scale (ABS/CABS)
The ABS (Corrigan 1989) is a 14‐item scale used for measuring agitation levels. The ABS was originally designed to monitor agitated behaviour in the recovery period after stroke and there are three factor‐based sub‐scores: i. aggression, ii. disinhibition; and iii. lability. High scores indicated higher levels of aggression.
b. Agitation‐Calmness Evaluation Scale (ACES)
The ACES is a single‐item rating scale developed by Eli Lilly and company (Breier 2002). On this scale, 1 = marked agitation, 4 = normal, 9 = unable to be aroused.
c. Positive and Negative Syndrome Scale Excited Component (PANSS‐EC)
The PANSS‐EC is a five‐item scale (excitement, tension, hostility, uncooperativeness, and poor impulse control. The items are rated from one (not present) to seven (extremely severe). Scores range from five to 35, with mean scores ≥ 20 indicating agitation. A high score indicates high levels of agitation (Montoya 2011).
d. Overt Agitation Severity scale (OASS)
The OASS (Yudofsky 1997) is a 12‐item scale used for identification and measurement of agitated behaviours, rated with a intensity score (ranging from one to four); the items are rated in terms of frequency from zero (not present) to four (always present). Total OASS score is the sum of all the Severity Scores (intensity*frequency).
6.2 Specific behaviour ‐ Aggression
a. Overt Aggression Scale (OAS)
The OAS (Yudofsky 1986) is a 16‐item rating scale which aims to measure the intensity of verbal and physical aggression. Clinicians are required to comment on the duration of the aggressive incident as well as the intervention required to control it. High scores are indicative of higher levels of aggression.
6.3 Global state
a. Clinical Global Impression (CGI)
The CGl (Guy 1976) is not a diagnostic tool but rather, enables clinicians to quantify the severity of symptoms of any mental health problem at one point in time. Clinicians are then able to use this to track whether there has been any improvement or worsening of symptoms over time. A seven‐point rating scale is used with high scores indicating increased severity or less recovery.
b. Clinical Global Impression ‐ Severity (CGI‐S)
The CGI‐S (Guy 1976) requires clinicians to consider the severity of a person's symptoms in relation to the clinicians past experience of people with the same diagnosis. Clinicians then have to give a rating from one = normal to seven = extremely ill. High scores indicate increased severity.
c. Clinical Global Impression ‐ Improvement (CGI‐I)
The CGI‐I (Guy 1976) enables clinicians to assess whether a person's symptoms have improved or worsened following an intervention. Based on the clinicians judgement, a rating on a seven‐point scale is given from one (= very much improved) to seven (= very much worse). Therefore, low scores indicate greater improvement.
6.4 Mental state
a. Brief Psychiatric Rating Scale (BPRS)
The Brief Psychiatric Rating Scale was originally developed by Overall and Gorham (Overall 1962) as a 14‐item scale to measure the severity of a range of psychiatric symptoms, including psychosis. This rating scale items evolved over time and now consists of 24 items which can be rated on a seven‐point scale from ‘not present’ to ‘extremely severe’. A high score would suggest poor mental health. It is not clear for the majority of the studies included in this review, which version of the BPRS was used.
b. Positive and Negative Syndrome Scale (PANSS)
The PANSS was developed and published by Kay, Flszbein and Opler (Kay 1987). The PANSS is designed as a brief interview, whereby the severity of 30 symptoms of schizophrenia can be assessed on a scale of one to seven. A high score would indicate more severe symptoms. The PANSS can be divided into separate sub‐scales by focusing on the statements relating to positive symptoms (e.g. hallucinations), negative symptoms (e.g. social withdrawal) or general psychopathology (e.g. anxiety and uncooperativeness).
6.5 Adverse effects
a. Barnes Akathisia Scale (BAS)
The BAS scale was developed by Barnes 1989 and includes items which aim to rate both the observable symptoms which characterise akathisia such as restless movements and also the person's subjective experience, including any distress. The items are rated from zero = normal to three = severe. There is also an item for rating global severity from zero = absent to five = severe. A high score indicates high levels of akathisia.
b. Simpson‐Angus Scale (SAS)
The SAS (Simpson 1970) is a 10‐item scale which measures drug‐induced parkinsonism (extrapyramidal side effects). Each item is scored from zero to four. A high score would indicate increased levels of parkinsonism.
c. Treatment Emergent Symptom Scale (TESS)
The TESS (Guy 1976) is a six‐item scale which is used to assess the occurrence and intensity of treatment‐related adverse effects. A high score indicates worse symptoms. This scale was not reported as a continuous score but studies did glean binary data using this measure (e.g. Li 2006).
7. Missing outcomes
Not one of the studies evaluated satisfaction with care, acceptance of treatment, quality of life or economic outcomes.
8. Funders
Fifteen of the 41 included studies received sponsorship from pharmaceutical companies.
Excluded studies
In total we excluded 66 studies.
Of these, 20 studies had to be excluded based on their method of allocation. NCT00189995 2005 was not conducted because ethical approval was not granted and Daniel 2000, Jones 2001 and Mendelowitz 2004 were literature reviews. Chen 2004, Chen 2008, Fan 2012; Huang 2004, Jiang 2009; Li 2007, Liang 2003; Pan 2005, Pei 2009; Wang 2004 and Wang 2005 were all quasi‐randomised whereby randomisation was based on methods such as allocating to group by hospital number. It is possible that these studies were randomised if allocation of hospital numbers were somehow, designated by a procedure involving chance alone. We were, however, not reassured by the text of the trials, thus, we have excluded this quasi‐randomised group. Currier 2000, Freeman 2009, Kinon 2003, Mantua 2006 and Pedros 2004 used research methods other than a randomised controlled trial, for example a naturalistic observational study or cohort study.
We had to exclude 34 studies on the basis of the characteristics of participants. Singh 1981 and Zapletalek 1986 were not explicitly described as randomised, however, they were reported to be double‐blind and thus remained included on the basis of allocation. They were eventually excluded because although participants were suspected to have psychosis, these people were not overtly agitated or aggressive requiring rapid tranquillisation. We excluded a group of clearly randomised trials for the same reason (Addington 1996; Allen 2007; Beasley 1998; Brook 1998; Chouinard 1991; Colonna 1998; Crandall 2005; Daniel 2003; Daniel 2004; Goldstein 1966; Kane 2000; Kinon 2001a; Mandel 2008; McEvoy 1994; NCT00631722 2008; Pathiraja 1995; Puech 1998; Smith 1974; Teja 1975; Ungvari 1982; Veser 2006). Participants included in Harvey 2004 had mild symptoms of schizophrenia and, again, were not overtly aggressive. We identified two studies where participants were children. We excluded these because either, participants did not display aggression (Faretra 1970), or because less than half of participants were suspected to have psychosis (Asadollahi 2015; Campbell 1982).
In Citrome 2001, Krakowski 2006, Krakowski 2008 and Lewis 2006, participants had a diagnosis of schizophrenia and displayed aggressive behaviour. However, the studies had to be excluded because participants displayed long‐term aggression, rather than aggression within an emergency situation requiring rapid tranquillisation. Participants in Romeo 2009 and Vaisanen 1981 were people with intellectual disability who presented with challenging behaviour rather than psychosis. Thomas 1992 focused on rapid tranquillisation in the emergency setting, but the majority of participants were intoxicated and therefore did not necessarily have psychosis‐induced aggression.
For 10 studies the intervention under investigation did not meet our inclusion criteria. Alexander 2004, Bieniek 1998, Conde 2011, TREC CG, NCT00797277 2008, Simpson 2010 and Srinath 2010 did not include a comparison group which involved giving of haloperidol alone; Vives 2015 did not include a comparison group which involved giving a drug out of haloperidol; Liu 2012b compared haloperidol with a non‐drug methodology of intervention; in Li 2005 there was no specific outcome for agitation.
Finally, two relevant studies were excluded because we found no usable data. Participants were randomised and had a diagnosis of schizophrenia with agitation/aggression. However, in Wan 2005 the scales that were used to assess outcome were not validated and results for haloperidol and chlorpromazine were not presented separately. In Wyant 1990 they used a "modified CGI" for which we could find no evidence of validation and then reported mean data graphically rather than numerically.
Risk of bias in included studies
Although each 'Summary of findings' table describes bias by outcome, an overview is reported here and a graphical impression is seen in Figure 3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All but two of the included studies were said to be randomised. Man 1973 and Salzman 1991 were not explicitly described as randomised, however they were described as double‐blind and we felt randomisation was implied. Few of the randomised studies reported information regarding the method of allocation. For the studies that did report further details, methods are described as computer‐generated randomisation (Battaglia 1997; Brook 2000; Huf 2007; Nobay 2004), random number sequence (Bailine 1987; Dorevitch 1999; Liang 2013; Liu 2012; Shi 2010; Zhou 2014), pre‐defined randomisation code (Lim 2010) and randomisation in blocks (Stotsky 1977; Walther 2014).
Blinding
Twenty‐two studies described that they were 'double‐blind' but the variations on how this was carried out were almost as many (Table 2); some studies were reported to be 'single‐blind' (Baldacara 2011; Yin 2012), whilst in five studies it was not stated (Liang 2013; Liu 2012; Shi 2010; Walther 2014; Zhou 2014). Trialists were more or less concerned with issues of performance and detection bias and most 'double‐blind' studies are likely not to have been in reality. Open studies (Brook 2000; Fang 2012; Garza‐Trevino 1989; Guo 2007; Higashima 2004; Huf 2007) recognised that blinded giving of drugs added the possibility that findings may be problematic to apply in routine care. These studies also attempted to use outcomes that were less prone to rater bias.
| Description | Study |
| Explicitly referred to how participants, study personnel and raters were blinded + tested blinding. | |
| Explicitly referred to how participants, study personnel and raters were blinded. | |
| Report that drugs had identical appearance ‐ no further details regarding rater blinding. | Fitzgerald 1969, Fruensgaard 1977, Paprocki 1977, Resnick 1984, Ritter 1972 |
| Explicitly state that rater was blinded ‐ no reference to participant blinding. Not all study personnel could have been blinded because person preparing drug (not blinded), often administered drug. | |
| Study personnel and raters blinded, not clear if attempts made to blind participants. | |
| Raters blinded ‐ not clear if study personnel and participants were blinded. | |
| "Double blind" ‐ no further information regarding blinding of participants, study personnel and raters. | |
| Described as double‐blind, but during oral phase haloperidol described as being in capsule form, and aripiprazole in tablet form, therefore unclear whether drugs had identical appearance. Not explicit if raters were blinded. | |
| Described as double‐blind during IM phase ‐ open during oral phase ‐ no further information given regarding blinding. | |
| Described as "modified double", whereby staff administering drugs were not blinded, but raters were ‐ not clear if participants were blinded. | |
| Single‐blind studies ‐ "open label, rater blind". | |
| Reported to be single‐blind, but unclear who was blind. | |
| Open‐label and rater blinding for some outcomes (BPRS). | |
| Rater blinding for assessment of BPRS, but unclear regarding rater blinding for other outcomes and whether participants and study personnel were blind. | |
| Open studies | Brook 2000, Fang 2012, Garza‐Trevino 1989, Guo 2007, Higashima 2004, Huf 2007 |
| Not stated |
BPRS ‐ Brief Psychiatric Rating Scale
Incomplete outcome data
There was no evidence of attrition bias for approximately two thirds of the studies with all participants being accounted for (Baldacara 2011; Brook 2000; Calver 2015; Dorevitch 1999; Fang 2012; Foster 1997; Fruensgaard 1977; Garza‐Trevino 1989; Guo 2007; Higashima 2004; Huf 2007; Kewala 1984; Kinon 2001d; Li 2006; Lim 2010; Liu 2012; Nobay 2004; Reschke 1974; Resnick 1984; Ritter 1972; Shi 2010; Stotsky 1977; Taymeeyapradit 2002; Yin 2012).
In the Bristol‐Myers 2005b, Currier 2004 and Shu 2010 studies, the reasons why participants left early are clearly presented in a table, but the numbers of participants completing different assessments at the same time point vary and reasons are not given. Other studies where attrition is not clearly described are Bailine 1987, Fitzgerald 1969 and Paprocki 1977.
In Battaglia 1997, two people were excluded from the efficacy analysis post randomisation; in Walther 2014 nine people were excluded due to refusal to provide post‐hoc consent and in three studies (Man 1973; Liang 2013; Zhou 2014), although reasons are given for why participants discontinued, results are only presented for those who completed and missing data have not been imputed. Reasons why participants left the study early were not reported in Battaglia 2002, Breier 2002, Bristol Myers 2004a, Eli Lilly 2004, Salzman 1991 and Tuason 1986, Also, attrition approached 50% at 24 hours in Salzman 1991, and was over 50% in Tuason 1986 at 10 days for some outcomes.
Selective reporting
Overall, there was much redundancy in reporting. It is difficult to judge whether this is bias or inadvertent but even‐handed poor reproduction of data. For continuous data, reasons for data being rendered unusable where often that the mean was presented but no variance could be extracted. Also, inexact P values were often presented alone and several studies presented their findings in graphs from which we could not estimate numbers.
Even simple issues were not reported well. For example, use of additional medication can indicate how treatments are not effective and need to be supplemented or that adverse effects are prevalent. The amount of additional benzodiazepine in the Foster 1997 study, the amount of anticholinergic and hypnotic medication in Tuason 1986, and the amount of antiparkinson drugs administered in Lim 2010 and Walther 2014 are not reported. Further, there is inconsistent reporting regarding additional medications in Brook 2000 and Tuason 1986.
In general, there was poor reporting with regard to adverse effects. In many studies it is not clear whether all side effects are reported (Breier 2002; Dorevitch 1999; Fitzgerald 1969; Garza‐Trevino 1989; Higashima 2004; Ritter 1972). Further, in other trials adverse effects are only reported where they occurred in a particular percentage of participants (Battaglia 1997; Bristol Myers 2004a; Bristol‐Myers 2005b; Brook 2000; Currier 2004; Fang 2012; Fitzgerald 1969; Kinon 2001d; Shu 2010). This further undermines methodology that is already weak to pick up adverse effect that may not be common, but could well be important.
Other potential sources of bias
That a study is small or sponsored by industry (or both) is not indicative of bias in itself. There are, however, associations between small trials tending to be positive (Easterbrook 1991), and those sponsored by industry favouring the drug for which they have the pecuniary interest (Lexchin 2003). Many studies in this review were small (Bailine 1987; Dorevitch 1999; Foster 1997; Fruensgaard 1977; Higashima 2004; Man 1973; Paprocki 1977; Resnick 1984; Stotsky 1977; Walther 2014), many were sponsored by industry (Battaglia 1997; Battaglia 2002; Breier 2002; Bristol Myers 2004a; Bristol‐Myers 2005b; Brook 2000; Currier 2004; Eli Lilly 2004; Fang 2012; Kewala 1984; Kinon 2001d; Lim 2010; Salzman 1991; Shu 2010;Stotsky 1977), and one was both (Stotsky 1977).
Effects of interventions
See: Summary of findings for the main comparison HALOPERIDOL compared to PLACEBO/NIL for psychosis‐induced aggression or agitation; Summary of findings 2 HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE for psychosis‐induced aggression or agitation; Summary of findings 3 HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE for psychosis‐induced aggression or agitation; Summary of findings 4 HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: c. DROPERIDOL for psychosis‐induced aggression or agitation; Summary of findings 5 HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: d. LOXAPINE for psychosis‐induced aggression or agitation; Summary of findings 6 HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: e. OLANZAPINE for psychosis induced aggression or agitation; Summary of findings 7 HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: f. PERPHENAZINE for psychosis‐induced aggression or agitation; Summary of findings 8 HALOPERIDOL compared to OTHER ANTIPSYCHOTICS: g. QUETIAPINE for psychosis‐induced aggression or agitation; Summary of findings 9 HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: h. RISPERIDONE for psychosis‐induced aggression or agitation; Summary of findings 10 HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: i. THIOTHIXENE for psychosis‐induced aggression or agitation; Summary of findings 11 HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE for psychosis‐induced aggression or agitation; Summary of findings 12 HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: k. ZUCLOPENTHIXOL ACETATE for psychosis‐induced aggression or agitation; Summary of findings 13 HALOPERIDOL compared to BENZODIAZEPINE: a. FLUNITRAZEPAM for psychosis‐induced aggression or agitation; Summary of findings 14 HALOPERIDOL compared to BENZODIAZEPINE: b. LORAZEPAM for psychosis‐induced aggression or agitation; Summary of findings 15 HALOPERIDOL compared to BENZODIAZEPINE: c. MIDAZOLAM for psychosis‐induced aggression or agitation; Summary of findings 16 HALOPERIDOL compared to COMBINATIONS: a. HALOPERIDOL + LEVOMEPROMAZINE for psychosis‐induced aggression or agitation; Summary of findings 17 HALOPERIDOL compared to COMBINATIONS: b. HALOPERIDOL + LORAZEPAM for psychosis‐induced aggression or agitation; Summary of findings 18 HALOPERIDOL compared to COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM for psychosis‐induced aggression or agitation; Summary of findings 19 HALOPERIDOL compared to COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE for psychosis‐induced aggression or agitation; Summary of findings 20 HALOPERIDOL compared to COMBINATIONS: e. HALOPERIDOL + RISPERIDONE for psychosis‐induced aggression or agitation; Summary of findings 21 HALOPERIDOL compared to COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE for psychosis‐induced aggression or agitation; Summary of findings 22 HALOPERIDOL compared to COMBINATIONS: g. QUETIAPINE + MAGNESIUM VALPROATE for psychosis‐induced aggression or agitation; Summary of findings 23 HALOPERIDOL compared to COMBINATIONS: h. RISPERIDONE + CLONAZEPAM for psychosis induced aggression or agitation; Summary of findings 24 HALOPERIDOL compared to COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM for psychosis‐induced aggression or agitation
In the following section, the summary statistic for binary outcome results is reported as risk ratio (RR) and for continuous outcomes, we used mean difference (MD). In all instances 95% confidence intervals (CIs) are reported.
1. COMPARISON 1. HALOPERIDOL versus PLACEBO
Five studies (total n = 700) compared haloperidol with placebo (Battaglia 2002; Breier 2002; Bristol Myers 2004a; Bristol‐Myers 2005b; Reschke 1974).
1.1 Tranquillisation or asleep ‐ asleep
Significantly more people in the haloperidol group were asleep at two hours compared with those in the placebo group (2 RCTs, n = 220, RR 0.88, 95% CI 0.82 to 0.95, I2 = 76%, Analysis 1.1).
1.2 Repeated need for tranquillisation
More people in the placebo group required repeated rapid tranquillisation compared with those in the haloperidol group (4 RCTs, n = 660, RR 0.51, 95% CI 0.42 to 0.62, Analysis 1.2).
1.3 Specific behaviour ‐ agitation
Two studies (n = 395) found that significantly more people in the haloperidol group were reported as demonstrating at least a 40% reduction in agitation scores at two hours according to the PANSS‐EC response at two hours (RR 1.62, 95% CI 1.28 to 2.07, Analysis 1.3).
Studies used a series of measures at different time periods. By about two hours, participants in the haloperidol group were reported to have experienced a significantly greater reduction in agitation than those in the placebo group according to the Agitated Behavior Scale (ABS) (CABS) rating scale (3 RCTs, n = 474, MD ‐4.48, 95% CI ‐5.78 to ‐3.18). In the same way, again at about two hours, people allocated to placebo had significantly smaller average change scores on the Agitation‐Calmness Evaluation Scale (ACES) compared with those in the haloperidol group (2 RCTs, n = 390, MD 0.83, 95% CI 0.39 to 1.26). One study (n = 199) examined the difference in the Positive and Negative Syndrome Scale Excited Component (PANSS‐EC) scores between the haloperidol and placebo groups in the schizophrenia subgroup, in non‐sedated people based on the ACES and in non‐sedated people. People who received haloperidol had significantly improved scores for agitation when compared with placebo in all three of these subgroups (MD ‐2.57, 95% CI ‐4.05 to ‐1.09, MD ‐2.64, 95% CI ‐3.95 to ‐1.33, and MD ‐2.97, 95% CI ‐4.27 to ‐1.67, respectively, Analysis 1.4). Again, according to average change and endpoint combined scores on the PANSS‐EC, participants in the haloperidol group had significantly reduced scores for agitation (3 RCTs, n = 514, MD ‐3.67, 95% CI ‐4.75 to ‐2.59, Analysis 1.4).
By 24 hours, average change on the ABS (CABS) rating scale was significantly changed in favouring haloperidol (1 RCT, n = 85, MD ‐2.40, 95% CI ‐4.13 to ‐0.67). This was not found for the PANSS‐EC average change scores (1 RCT, n = 85, MD ‐1.40, 95% CI ‐2.97 to 0.17, Analysis 1.5).
1.4 Global outcome
Significantly more people in the placebo group exhibited 'not marked improvement' than those who received haloperidol (1 RCT, n = 40, RR 0.61, 95% CI 0.44 to 0.84, Analysis 1.6). Further, people in the placebo group were more likely to be rated as showing 'not any improvement' in global functioning than those receiving haloperidol, however this difference was not statistically significant (1 RCT, n = 40, RR 0.28, 95% CI 0.08 to 1.07, Analysis 1.6).
Four studies found that more people in the placebo group required benzodiazepines (n = 660, RR 0.44, 95% CI 0.31 to 0.62, Analysis 1.7).
Global rating took place around two hours and 24 hours. By two hours people who received haloperidol were reported to have had significantly more symptom improvement according to their average endpoint scores on the Clinical Global Impression ‐ Improvement (CGI‐I), when compared with placebo (2 RCTs, n = 390, MD ‐0.73, 95% CI ‐0.96 to ‐0.51), and in the schizophrenia subgroup (1 RCT, n = 199, MD ‐0.64, 95% CI ‐0.98 to ‐0.30). When the same outcome was measured using average change score on the Clinical Global Impression ‐ Severity (CGI‐S), participants in the haloperidol group were rated as having significantly less severe symptoms than those in the placebo group (2 RCTs, n = 390, MD ‐0.48, 95% CI ‐0.78 to ‐0.18, Analysis 1.8).
By 24 hours, average change scores on the CGI‐I remained significantly improved (1 RCT, n = 169, MD ‐0.40, 95% CI ‐0.62 to ‐0.18). Using the CGI‐S scale to rate symptom severity at 24 hours, one trial (n = 85) found that although those in the haloperidol group were rated as less severe, the difference was not statistically significant (MD ‐0.20, 95% CI ‐0.46 to 0.06, Analysis 1.9).
1.5 Mental state
Participants in the haloperidol group had significantly improved average change scores on the Brief Psychiatric Rating Scale (BPRS) positive sub‐scale when rated at two (3 RCTs, n = 372, MD ‐1.04, 95% CI ‐1.54 to ‐0.54, Analysis 1.10) and 24 hours (2 RCTs, n = 264, MD ‐1.61, 95% CI ‐2.34 to ‐0.89,Analysis 1.11). On the BPRS Total sub‐scale, participants in the haloperidol group had significantly improved scores when rated at two (3 RCTs, n = 371, MD ‐5.69, 95% CI ‐7.38 to ‐4.00, Analysis 1.10) and 24 hours (2 RCTs, n = 264, MD ‐4.81, 95% CI ‐6.82 to ‐2.81, Analysis 1.11).
1.6 Adverse effects
1.6.1 General
Bristol Myers 2004a (n = 273) specifically reported that no deaths occurred. Significantly more people in the haloperidol group experienced one or more adverse reactions than those in the placebo group (2 RCTs, n = 395, RR 1.64, 95% CI 1.22 to 2.20). One trial (n = 273) found that people in the haloperidol group were significantly more likely than those in the placebo group to report overall adverse effects at 72 hours (RR 1.78, 95% CI 1.23 to 2.59) and to experience increased severity of adverse effects after the second injection (RR 3.25, 95% CI 1.88 to 5.63, Analysis 1.12).
Bristol‐Myers 2005b reported that of all adverse effects reported, only one was rated as 'serious' ‐ this was related to a participant in the placebo group. This difference was not statistically significant (n = 122, RR 0.34, 95% CI 0.01 to 8.29, Analysis 1.13).
1.6.2 Specific
a. Arousal
Insomnia was reported by people in both the haloperidol and placebo groups and the difference between them was not statistically significant (1 RCT, n = 273, RR 1.31, 95% CI 0.61 to 2.82). Somnolence was reported in both the placebo and haloperidol group and the difference between the groups was not statistically significant (4 RCTs, n = 615, RR 2.28, 95% CI 0.97 to 5.36, Analysis 1.14). Significantly more people in the haloperidol group than the placebo group were reported to be "over sedated" (2 RCTs, n = 313, RR 3.36, 95% CI 1.42 to 7.99. Analysis 1.14).
b. Cardiac
We included dizziness in 'cardiac' adverse effects (2 RCTs, n = 392, RR 1.33, 95% CI 0.48 to 3.65). More specific cardiac effects also did not suggest an effect for the active drug (QTc abnormality, 1 RCT, n = 122, RR 3.10, 95% CI 0.33 to 28.98; sinus tachycardia, 1 RCT, n = 122, RR 3.10, 95% CI 0.33 to 28.98; tachycardia, 1 RCT, n = 122, RR 1.03, 95% CI 0.07 to 16.15, Analysis 1.15). Although in favour of placebo, there were no statistically significant differences between the haloperidol and placebo groups with regards to QTc abnormality according to the average change score (2 RCTs, n = 265, MD 3.63, 95% CI ‐2.67 to 9.93), when rated at 24 hours (Analysis 1.16).
c. Movement disorders
In the haloperidol group, six people reported experiencing akathisia within 24 hours, compared with none in the placebo group. This difference between groups was not statistically significant (1 RCT, n = 122, RR 13.43, 95% CI 0.77 to 233.23). Dystonia was reported in the haloperidol group within 24 hours by six people compared with none in the placebo group; the difference between groups was not statistically significant (2 RCTs, n = 207, RR 7.49, 95% CI 0.93 to 60.21). Significantly more people in the haloperidol group required antiparkinson drugs due to Extrapyramidal Side Effects (EPS) (1 RCT, n = 180, RR 5.57, 95% CI 1.37 to 22.65) with significantly more people experiencing EPS during 24 hours (3 RCTs, n = 398, RR 6.79, 95% CI 2.19 to 21.07) , Analysis 1.17).
There was no statistically significant difference between the average change scores on the Barnes Akathisia Scale (BAS) for participants in the haloperidol group when compared with the placebo group (1 RCT, n = 168, MD 0.09, 95% CI ‐0.17 to 0.35, Analysis 1.18). In one study (n = 167) participants in the haloperidol group experienced significantly higher levels of EPS compared with participants in the placebo group according to their average change scores on the Simpson‐Angus Scale (SAS) (MD 1.89, 95% CI 0.77 to 3.01, Analysis 1.18).
d. Miscellaneous
Two studies (total n = 395) listed a range of effects including agitation (2 RCTs, RR 0.80, 95% CI 0.29 to 2.19), headache (2 RCTs, RR 1.28, 95% CI 0.55 to 3.00), nausea (2 RCTs, RR 0.69, 95% CI 0.13 to 3.67) and vomiting (1 RCT, n = 122, RR 1.03, 95% CI 0.07 to 16.15). Although not statistically significant, two people in the placebo group reported pain at the injection site compared with none in the haloperidol group (1 RCT, n = 122, RR 0.21, 95% CI 0.01 to 4.22). This changed to significantly more people in the haloperidol group complaining of pain at the injection site following the second injection, when compared with the placebo group (44% versus 14%, 1 RCT, n = 273, RR 3.25, 95% CI 1.88 to 5.63, Analysis 1.19).
1.7 Leaving the study early
In three studies (total n = 435), although more people in the haloperidol group left the study early (for any reason) when compared with the placebo group, the difference was not statistically significant (RR 1.06, 95% CI 0.53 to 2.11, I2 = 83%). There were significant differences between groups for number of participants who left early due to lack of efficacy (3 RCTs, n = 435, RR 0.12, 95% CI 0.03 to 0.49), but not for reasons of consent (2 RCTs, n = 395, RR 6.20, 95% CI 0.77 to 49.98), adverse effects (3 RCTs, n = 435, RR 1.78, 95% CI 0.21 to 15.39), being asleep (1 RCT, n = 40, RR 1.20, 95% CI 0.05 to 27.44), or 'other' reasons (2 RCTs, n = 395, RR 1.81, 95% CI 0.27 to 12.07). In one study (n = 273), three people in the haloperidol group were withdrawn from the study compared with none in the placebo, although the difference was not statistically significant (RR 3.35, 95% CI 0.17 to 64.15, Analysis 1.20).
2. COMPARISON 2. HALOPERIDOL versus OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE
We found two studies comparing haloperidol with aripiprazole (total n = 599, Bristol Myers 2004a; Bristol‐Myers 2005b).
2.1 Repeated need for rapid tranquillisation
In both studies, people in the haloperidol group required fewer injections than those in the aripiprazole, the difference between the groups being statistically significant (2 RCTs, n = 473, RR 0.78, 95% CI 0.62 to 0.99, Analysis 2.1).
2.2 Specific behaviours ‐ agitation
In both studies, more people in the aripiprazole group demonstrated at least a 40% reduction in agitation scores (PANSS‐EC) at two hours than in the haloperidol group, though the difference between the groups was not statistically significant (2 RCTs, n = 477, RR 1.07, 95% CI 0.92 to 1.26, Analysis 2.2).
At two hours, participants showed similar average change scores according to the Agitation‐Calmness Evaluation Scale (ACES) rating scale (2 RCTs, n = 470, MD 0.12, 95% CI ‐0.30 to 0.55). Also, at two hours, participants showed similar average change scores according to the CABS rating scale (2 RCTs, n = 470, MD ‐0.55, 95% CI ‐2.10 to 1.01). One study found that no differences between the haloperidol and aripiprazole groups in average change scores on the PANSS‐EC (n = 357, MD ‐0.48, 95% CI ‐2.12 to 1.16). This study also examined whether there were differences in various subgroups (schizophrenia subgroup, non‐sedated people based on the ACES and non‐sedated. There were no significant differences between haloperidol and aripiprazole within any of these subgroups (Analysis 2.3).
2.3 Global outcomes
Neither those allocated haloperidol or aripiprazole required additional benzodiazepines (2 RCTs, n = 477, RR 1.26, 95% CI 0.74 to 2.16, Analysis 2.4).
At two hours, participants in both groups showed similar levels of severity of symptoms according to their average change scores on the CGI‐S rating scale (2 RCTs, n = 470, MD 0.07, 95% CI ‐0.22 to 0.36). On another scale, at the same time, participants showed similar levels of symptom improvement according to their average endpoint scores on the CGI‐I rating scale (2 RCTs, n = 470, MD ‐0.02, 95% CI ‐0.23 to 0.19) . Again, at two hours, people in the haloperidol group (schizophrenia subgroup), were not rated as more improved on the CGI‐I than those in the aripiprazole group (1 RCT, n = 257, MD ‐0.02, 95% CI ‐0.30 to 0.26, Analysis 2.5).
2.4 Mental state
One study (n = 103) found no difference for average change scores for participants on the BPRS (positive sub‐scale MD ‐0.23, 95% CI ‐1.28 to 0.82 and total score n = 102, MD ‐2.03, 95% CI ‐5.76 to 1.70, Analysis 2.6).
2.5 Adverse effects
2.5.1 General
Studies found no difference between groups for 'adverse effects within 24 hours' (2 RCTs, n = 477, RR 1.18, 95% CI 0.95 to 1.46). However, significantly more people in the haloperidol group suffered with increased severity of adverse effects after the second injection (1 RCT, n = 360, RR 1.34, 95% CI 1.03 to 1.74), and overall adverse effects at 72 hours (1 RCT, n = 360, RR 1.33, 95% CI 1.04 to 1.70, Analysis 2.7).
There were no significant differences between the groups with respect to 'serious' adverse effects (Analysis 2.8). In the Bristol Myers 2004a study, it was reported that no participants in either group died during the study. Four people developed severe adverse effects; three in the aripiprazole group compared with none in the haloperidol group (2 RCTs, n = 477, RR 0.57, 95% CI 0.18 to 1.81). In the Bristol Myers 2004a study, one person in the haloperidol group developed severe dystonia and in the Bristol‐Myers 2005b study, one person in the aripiprazole group had a tonic‐clonic seizure.
2.5.2 Specific
a. Arousal
Significantly more people in the haloperidol group complained of insomnia during the intramuscular (IM) phase (1 RCT, n = 360, RR 2.08, 95% CI 1.01 to 4.27). There were no clear differences between aripiprazole and haloperidol for insomnia during the oral phase (1 RCT, n = 360, RR 0.82, 95% CI 0.47 to 1.44), and for people being recorded as being "over sedated" during the study (1 RCT, n = 360, RR 1.66, 95% CI 0.93 to 2.95). With regards to the various ways of reporting somnolence, again, no difference was apparent (e.g. 1 RCT, n = 360, RR somnolence ‐ between zero to one days 0.71, 95% CI 0.25 to 2.00, Analysis 2.9).
b. Cardiac
Similar numbers of people in each group experienced dizziness (2 RCTs, n = 477, RR 0.69, 95% CI 0.33 to 1.48), sinus tachycardia (1 RCT, n = 117, RR 6.66, 95% CI 0.35 to 126.06) and tachycardia (1 RCT, n = 117, RR 0.24, 95% CI 0.03 to 2.06, Analysis 2.10).
c. Gastrointestinal problems
Participants in the haloperidol groups complained of significantly more dyspepsia (1 RCT, n = 360, RR 10.41, 95% CI 1.36 to 79.76), and similar problems with diarrhoea (1 RCT, n = 360, RR 0.95, 95% CI 0.28 to 3.21), nausea (1 RCT, n = 360, RR 0.14, 95% CI 0.02 to 1.09), and vomiting (1 RCT, n = 360, RR 0.38, 95% CI 0.07 to 1.92) between three and seven days (Analysis 2.11). .
d. Movement disorder
These all tended to be more common in the group allocated to haloperidol (e.g. 2 RCTs, n = 477, RR akathisia 2.85, 95% CI 0.94 to 8.61). More people in the haloperidol group were reported to have experienced dystonia (2 RCTs, n = 477, RR 6.63, 95% CI 1.52 to 28.86). Significantly more people in the haloperidol group experienced EPS during both the IM (1 RCT, n = 360, RR 9.46, 95% CI 1.22 to 73.13) and oral phase (1 RCT, n = 360, RR 7.09, 95% CI 1.65 to 30.58), and required antiparkinson medication during the oral phase (1 RCT, n = 360, RR 4.94, 95% CI 2.50 to 9.78, Analysis 2.12).
e. Miscellaneous
In both studies, participants in both the haloperidol and aripiprazole groups were reported to have agitation (2 RCTs, n = 477, RR 1.22, 95% CI 0.46 to 3.22), and headache (2 RCTs, n = 477, RR 0.85, 95% CI 0.45 to 1.59) between days zero and one. Although not statistically significant, more people in the aripiprazole group reported pain at the injection site following the first injection (1 RCT, n = 360, RR 0.32, 95% CI 0.06 to 1.54, Analysis 2.13).
2.6 Leaving the study early
In both studies, more people left the study early in the haloperidol group than in the aripiprazole group, however the difference between groups was not statistically significant (2 RCTs, n = 480, RR 2.07, 95% CI 0.86 to 4.98). When reasons were given these data were also equivocal (e.g. 2 RCTs, n = 480, RR leaving because of adverse effects 0.97, 95% CI 0.17 to 5.59, Analysis 2.14).
3. COMPARISON 3. HALOPERIDOL versus OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE
3.1 Tranquillisation or asleep ‐ asleep
One small study (n = 39) reported that more people allocated to chlorpromazine did experience sleep by two hours (RR 1.93, 95% CI 1.04 to 3.60, Analysis 3.1).
3.2 Repeated need for rapid tranquillisation
In the Man 1973 study, all participants in the haloperidol group and all but one participant in the chlorpromazine study required more than one injection, with several in both groups requiring more than three (1 RCT, n = 30, 1.50, 95% CI 0.53 to 4.26, Analysis 3.2).
3.3 Global outcome ‐ not marked improvement
For the outcome of "not marked improvement" there were no clear differences between groups (2 RCTs, n = 89, RR 0.79, 95% CI 0.61 to 1.02). More people in the chlorpromazine group were rated as showing "not any improvement" (2 RCTs, n = 89, RR 0.15, 95% CI 0.05 to 0.49, Analysis 3.3).
3.4 Adverse effects ‐ specific
Reporting of adverse effects was sporadic. One person developed status epilepticus (1 RCT, n = 30, RR 0.33, 95% CI 0.01 to 7.58), and two people had hypotension (3 RCTs, n = 69, RR 0.51, 95% CI 0.10 to 2.60). In the Man 1973 study (n = 30), there were no reported incidences of EPS or irritation at the injection site in either group.
One person in the chlorpromazine group had an elevated SGPT (serum glutamic pyruvic transaminase) level (1 RCT, n = 30, RR 0.33, 95% CI 0.01 to 7.81) and another in the haloperidol group developed mild leucopenia (1 RCT, n = 50, RR 3.00, 95% CI 0.13 to 70.30, Analysis 3.4).
3.5 Leaving the study early ‐ for any reason
Few people left these studies early (5%) with little, but significant, difference between groups (4 RCTs, n = 153, RR 0.21, 95% CI 0.07 to 0.71, Analysis 3.5 ).
4. COMPARISON 4. HALOPERIDOL versus OTHER ANTIPSYCHOTICS: c. DROPERIDOL
We found two relevant studies (n = 255) (Calver 2015; Resnick 1984).
4.1 Tranquillisation or asleep
There was no statistical significant difference in terms of being not asleep at two hours between the compared groups (1 RCT, n = 228, RR 1.07, 95% CI 0.44 to 2.60, Analysis 4.1). People with haloperidol or droperidol experienced no different time to sleep (1 RCT, n = 114, MD 5.20, 95% CI ‐6.05 to 16.45, Analysis 4.2).
4.2 Repeated need for rapid tranquillisation
More people in the haloperidol group required more than one injection compared with those allocated to droperidol (2 RCTs, n = 255, RR 2.38, 95% CI 1.27 to 4.47); however, this was not statistically significant for the outcome of ‘required more than three injections’ (1 RCT, n = 27, RR 2.12, 95% CI 0.09 to 47.68, Analysis 4.3).
4.3 Global outcome ‐ need for benzodiazepine
There was no statistical significant difference in terms of need for adjunctive benzodiazepine between the two comparison groups (1 RCT, n = 228, RR 0.31, 95% CI 0.07 to 1.44, Analysis 4.4).
4.4 Adverse effects
4.4.1 General
In the haloperidol group there was only one adverse effects up to two hours, compared with seven in the droperidol group (1 RCT, n = 228, RR 0.15, 95% CI 0.02 to 1.23), involving one person in the haloperidol group and six in the droperidol group (1 RCT, n = 228, Analysis 4.5).
4.4.2 Specific
a. Arousal
There were few differences between groups as for being over‐sedated (1 RCT, n = 228, RR 0.36, 95% CI 0.01 to 8.68, Analysis 4.6).
b. Cardiovascular
One person in the haloperidol group experienced hypotension, compared with four in the droperidol group (1 RCT, n = 228, RR 0.27, 95% CI 0.03 to 2.36, Analysis 4.7).
c. Movement disorder
One person in the haloperidol group experienced dystonia, compared with one in the droperidol group (2 RCTs, n = 255, RR 0.86, 95% CI 0.11 to 6.56, Analysis 4.8).
d. Miscellaneous
There was no difference in terms of desaturation events between the two compared groups (1 RCT, n = 228, RR 0.36, 95% CI 0.01 to 8.68, Analysis 4.9).
5. COMPARISON 5. HALOPERIDOL versus OTHER ANTIPSYCHOTICS: d. LOXAPINE
We found three relevant studies (total n = 119, Fruensgaard 1977; Paprocki 1977; Tuason 1986). Fruensgaard 1977, unlike the other two studies also made sure that both groups of participants were given biperiden, an anticholinergic drug which offsets adverse effects such as movement disorders.
5.1 Tranquillisation or asleep – not asleep at about 12 hours
Loxapine does seem more sedating than haloperidol although the difference was not statistically significant (1 RCT, n = 54, RR 4.31, 95% CI 0.54 to 34.48, Analysis 5.1).
5.2 Specific behaviour
There were very few differences between groups for improvement in levels of agitation (1 RCT, n = 30, RR 1.17, 95% CI 0.51 to 2.66) or levels of aggression (1 RCT, n = 30, RR 1.10, 95% CI 0.69 to 1.76, Analysis 5.2).
5.3 Global outcome
Studies found no differences found between groups at any time point in regards to the number of people who were reported as having ‘no change in global outcome’ (1 RCT, n = 56, RR at 48 hours 0.93, 95% CI 0.14 to 6.15; 1 RCT, n = 54, RR at 72 hours 0.39, 95% CI 0.04 to 3.49 and 1 RCT, n = 35, RR up to 4 weeks 0.40, 95% CI 0.12 to 1.32, Analysis 5.3).
Although never statistically significant, more people in the loxapine group were sedated at 30 minutes (1 RCT, n = 30, RR 1.29, 95% CI 0.65 to 2.54), 60 minutes (1 RCT, n = 30, RR 3.50, 95% CI 0.86 to 14.18) and at 120 minutes (1 RCT, n = 30, RR 7.00, 95% CI 0.98 to 50.16) than in the haloperidol group (Analysis 5.4).
5.4 Mental state
One trial found no clear differences between the haloperidol and loxapine in average endpoint scores on the BPRS (n = 52, MD 6.10, 95% CI 4.48 to 7.72, Analysis 5.6).
5.5 Adverse effects
Over 20 different adverse effects are reported including anticholinergic disorders (Analysis 5.8), arousal levels (Analysis 5.9), cardiovascular effects (Analysis 5.10), movement disorders (Analysis 5.11) and several that were difficult to classify (Analysis 5.12), but these are probably best summarised by the general impression from Analysis 5.7 where eight people in the haloperidol group and 10 people in the loxapine group experienced one or more drug‐related adverse effects (1 RCT, n = 30, RR 0.80, 95% CI 0.44 to 1.45).
5.6 Leaving the study early
a. General reasons
At no point at anytime did statically more people leave one group than the other (e.g. 1 RCT, n = 35, RR leaving around 72 hours 0.63, 95% CI 0.21 to 1.85). The Tuason 1986 study found no significant differences between the groups for the number of people who discontinued with the study by 10 days (n = 54, RR 1.18, 95% CI 0.67 to 2.07, Analysis 5.13).
b. Specific reasons
In Paprocki 1977 they withdrew one person because of “toxicity” during the IM phase (n = 35, RR 2.84, 95% CI 0.12 to 65.34, Analysis 5.14).
6. COMPARISON 6. HALOPERIDOL versus OTHER ANTIPSYCHOTICS: e. OLANZAPINE
We found six studies (total n = 720) that compared haloperidol with olanzapine (Baldacara 2011; Battaglia 2002; Breier 2002; Eli Lilly 2004; Kinon 2001d; Walther 2014).
6.1 Tranquillisation or asleep
By two hours significantly more people in the olanzapine group were asleep compared with those allocated haloperidol (1 RCT, n = 257, RR 1.16, 95% CI 1.02 to 1.32, Analysis 6.1).
6.2 Repeated need for tranquillisation
The need for additional injection results were statistically significant between the two groups in favour of olanzapine at 12 hours (1 RCT, n = 60, RR 3.29, 95% CI 1.67 to 6.47); however, the two groups results are similar at two hours (1 RCT, n = 60, RR 4.00, 95% CI 0.47 to 33.73) and at 24 hours (3 RCTs, n = 392, RR 1.06, 95% CI 0.75 to 1.51, Analysis 6.2).
6.3 Specific behaviour
a. Agitation
At two hours, a similar number of participants in both the haloperidol and olanzapine group were rated as having at least a 40% reduction according to the PANSS‐EC (1 RCT, n = 45, RR 0.96, 95% CI 0.58 to 1.58). Also agitation was rated as an ‘adverse effect’ (1 RCT, n = 49, RR 1.04, 95% CI 0.07 to 15.73), and again reported by 21 days (1 RCT, n = 100, RR 1.08, 95% CI 0.33 to 3.51, Analysis 6.3).
By 30 minutes haloperidol and olanzapine showed similar findings at average change scores of ACES (15 minutes: 1 RCT, n = 46, MD 0.50, 95% CI ‐0.35 to 1.35; 30 minutes: 1 RCT, n = 46, MD 0.90, 95% CI ‐0.06 to 1.86) and PANSS‐EC (15 minutes: 1 RCT, n = 46, MD 0.30, 95% CI ‐1.64 to 2.24; 30 minutes: 1 RCT, n = 46, MD 1.30, 95% CI ‐1.16 to 3.76, Analysis 6.4).
By about two hours, continuous measures of agitation showed similar findings. The ABS change score did favour olanzapine (1 RCT, n = 85, MD 2.70, 95% CI 0.38 to 5.02) and so did the ACES change score at one hour (1 RCT, n = 46, MD 1.20, 95% CI 0.06 to 2.34) and the Overt Agitation Severity Scale (OASS) endpoint score at one hour (1 RCT, n = 60, MD 2.00, 95% CI 1.18 to 2.82) and two hours (1 RCT, n = 60, MD 4.20, 95% CI 3.54 to 4.86). However, the others measurements did not show similar findings (for example, average change and endpoint combined scores PANSS‐EC: 3 RCTs, n = 332, MD ‐0.31, 95% CI ‐1.49 to 0.86, Analysis 6.5).
At four hours, continuous measures of agitation showed similar findings. The average endpoint score on the OASS was statistically significant (1 RCT, n = 60, MD 0.20, 95% CI ‐0.05 to 0.45, Analysis 6.6).
When it came to 24 hours, continuous measures of agitation showed similar findings. For example, the average change score on the ABS (1 RCT, n = 86, MD 2.40, 95% CI 0.01 to 4.79) and the average endpoint score on the OASS at six hours (1 RCT, n = 60, MD 0.20, 95% CI 0.05 to 0.35) and 12 hours (1 RCT, n = 60, MD 0.90, 95% CI 0.82 to 0.98) were statistically significant, but on the PANSS‐EC it was not (1 RCT, n = 86, MD 1.40, 95% CI ‐0.55 to 3.35, Analysis 6.7).
b. Aggression
The two groups showed no statistical significant difference on the average endpoint OASS score at one hour (1 RCT, n = 60, MD 0.90, 95% CI 0.13 to 1.67) and at two hours (1 RCT, n = 60, MD 0.50, 95% CI ‐0.41 to 1.41, Analysis 6.8).
At four hours, the average endpoint score on the OASS showed similar findings (1 RCT, n = 60, MD 0.10, 95% CI ‐0.11 to 0.31, Analysis 6.9).
When it came to 24 hours, the continuous measures of aggression at six hours showed similar findings at the average endpoint OASS score (1 RCT, n = 60, MD ‐0.10, 95% CI ‐0.26 to 0.06); however, at 12 hours the average endpoint OASS score resulted in favour of haloperidol (1 RCT, n = 60, MD ‐0.20, 95% CI ‐0.39 to ‐0.01, Analysis 6.10).
c. Hostility
This was recorded – again as an adverse effect – but there was not a clear difference between groups (1 RCT, n = 49, RR 0.35, 95% CI 0.01 to 8.12, Analysis 6.11)
6.4 Global outcome
a. General
There were no significant differences between the groups with regards to the number of people who required additional benzodiazepines during 24 hours (2 RCTs, n = 343, RR 1.05, 95% CI 0.63 to 1.74). This applied up to seven days (1 RCT, n = 100, RR 1.00, 95% CI 0.79 to 1.27), and use of additional restraint, seclusion or special observation (2 RCTs, n = 160, RR 1.49, 95% CI 0.70 to 3.17, Analysis 6.12). In one study (n = 100), there was a significant difference between the haloperidol and olanzapine group for time until the person discontinued the study – it was significantly earlier in the olanzapine group (MD ‐3.48, 95% CI ‐6.28 to ‐0.68). In the same study, the dose of adjunctive lorazepam needed was very similar (MD 0.34, 95% CI ‐0.14 to 0.82, Analysis 6.13).
People in the haloperidol and olanzapine group were rated as demonstrating similar levels of improvement in global functioning according to average endpoint scores using the CGI‐I when rated at two hours (1 RCT, n = 42, MD ‐0.10, 95% CI ‐0.65 to 0.45, Analysis 6.14), and their average change scores at 24 hours (1 RCT, n = 243, MD 0.00, 95% CI ‐0.20 to 0.20). There was no difference by 24‐72 hours according to average change scores using the CGI‐S (1 RCT, n = 86, MD 0.00, 95% CI ‐0.24 to 0.24, Analysis 6.15). Kinon 2001d used a non‐parametric test and reported that at 21 days, those in the olanzapine group did have a significantly greater change in the CGI‐I score (n = 100, ‐0.38 ± 1.31 and ‐0.2 ± 1 .44, P = 0.25, F test 1.71 df). Further, one study demonstrated similar levels of improvement according to average change scores when using the CGI‐I by 21 days (1 RCT, n= 100, MD 0.36, 95% CI ‐0.18 to 0.90, Analysis 6.15).
b. Specific
One study repeatedly recorded levels of arousal (alert to asleep) across several periods of time (one to 24 hours). There were no convincing differences between the two drugs (Analysis 6.16).
6.5 Service use
There was no difference between groups for the outcome of average time to discharge (about 13 days) (1 RCT, n = 100, MD ‐0.60, 95% CI ‐1.85 to 0.65, Analysis 6.28). Time was also measured for the average number of patient‐hours used. These data were skewed and therefore presented in a table (Analysis 6.29).
6.6 Mental state
Several adverse effects seem to be better classified in ‘mental state’. Two different studies reported anxiety (n = 49, RR 0.35, 95% CI 0.01 to 8.12), or anxiety by 21 days (n = 100, RR 0.36, 95% CI 0.08 to 1.70). Delusions were also reported as an adverse effect (1 RCT, n = 49, RR 0.35, 95% CI 0.01 to 8.12), as was nervousness (1 RCT, n = 100, RR 2.17, 95% CI 0.70 to 6.74, Analysis 6.30). There were no significant differences between olanzapine and haloperidol with respect to improved mental state according to average change scores on the PANSS Total sub‐scale (1 RCT, n = 100, MD 4.70, 95% CI ‐0.18 to 9.58, Analysis 6.34). There was also few differences according to average change scores on positive sub‐scale of the BPRS at any time period (1 RCT, n = 46) nor on BPRS totals (Analysis 6.31).
6.7 Adverse effects
6.7.1 General
In three studies significantly more people in the haloperidol group experienced a drug‐related adverse event (3 RCTs, n = 209, RR 1.38, 95% CI 1.09 to 1.76, Analysis 6.35).
6.7.2 Serious
There was no clear difference in risk of treatment emergent adverse events between groups (1 RCT, n = 49, RR 0.81, 95% CI 0.36 to 1.83). Only a few of these were attributed to the use of the drug. No serious adverse effects nor death were experienced by any participant who received haloperidol or olanzapine (Analysis 6.36).
6.7.3 Specific
a. Arousal level
Despite being reported in a way that may have underestimated the overall prevalence, complaints of insomnia and/or somnolence were not different between groups (Analysis 6.38).
b. Cardiovascular
Binary and continuous measures of any cardiovascular change did not show differences between groups (e.g. hypotension 2 RCTs, n = 146, RR 0.27, 95% CI 0.03 to 2.38, Analysis 6.42; change in QT interval 1 RCT, n = 257, MD 1.80, 95% CI ‐3.81 to 7.41, Analysis 6.43).
c. Movement disorders
Studies reported different movement disorders in several different ways. There was the impression that more people who received haloperidol complained of these effects (e.g. EPS at 24 hours, 3 RCTs, n = 403, RR 8.35, 95% CI 2.27 to 30.63, Analysis 6.45).
This finding is supported by the continuous measure of SAS – again, this tends to favour olanzapine at 24 hours (1 RCT, n = 242, MD 1.31, 95% CI 0.56 to 2.06, Analysis 6.46). Other skewed SAS endpoint data are not so favourable, but these are presented only in tabular form (Analysis 6.47). This also applies to the akathisia endpoint measure (see Analysis 6.47) and, finally, change scores on the same scale (BAS) at 24 hours favoured olanzapine (1 RCT, n = 241, MD 0.28, 95% CI 0.09 to 0.47, Analysis 6.46).
d. Miscellaneous
Reports recorded several other adverse effects, none of which were different in the two groups. These included anticholinergic effects (increased salivation, 1 RCT, n = 100, RR 9.73, 95% CI 0.54 to 176.18, Analysis 6.37), gastric problems (e.g. abdominal pain 1 RCT, n =49, RR 3.12, 95% CI 0.13 to 73.04, Analysis 6.44), and nose bleeds (1 RCT, n = 49, RR 3.12, 95% CI 0.13 to 73.04, Analysis 6.49).
No participants in either group were reported to have had a clinically relevant change in laboratory test results.
6.8 Leaving the study early
About 20% of each group left the study early but slightly fewer in the olanzapine group (2 RCTs, n = 148, RR 1.66, 95% CI 1.04 to 2.65). Power to investigate specific reasons was low but eight people in the haloperidol group, compared with one in the olanzapine group left the study early due to adverse events (1 RCT, n = 100, RR 8.67, 95% CI 1.13 to 66.75, Analysis 6.50).
7. COMPARISON 7. HALOPERDIOL versus OTHER ANTIPSYCHOTICS: f. PERPHENAZINE
We found one relevant study (n = 44, Fitzgerald 1969).
7.1 Global outcome ‐ no improvement
One person in the haloperidol group and two people in the perphenazine were reported to have shown no global improvement (n = 44, RR 0.46, 95% CI 0.04 to 4.68, Analysis 7.1).
7.2 Adverse effects
7.2.1 General
Ten people in the haloperidol group and seven in the perphenazine group experienced one or more adverse effect, however, this difference is not statistically significant (n = 44, RR 1.30, 95% CI 0.61 to 2.80, Analysis 7.2). No participants in either group developed clinically significant laboratory changes.
7.2.2 Specific
Six people in the haloperidol group and two people in the perphenazine group required antiparkinson medication, but again, this result was not statistically significant (n = 44, RR 2.74, 95% CI 0.62 to 12.12). One person in the perphenazine group developed a hypotensive episode compared with none in the haloperidol group (n = 44, RR 0.31, 95% CI 0.01 to 7.12).
Two people in the haloperidol group and one person in the perphenazine discontinued with the study because they developed EPS (n = 44, RR 1.83, 95% CI 0.18 to 18.70). Also one person was withdrawn from the haloperidol group due to drowsiness/tension and no therapeutic effect (n = 44, RR 2.75, 95% CI 0.12 to 64.04, Analysis 7.3).
7.3 Leaving the study early
We felt that we should add these specific reasons into the data on adverse effects (Analysis 7.3), but there were no differences between groups (Analysis 7.4).
8. COMPARISON 8. HALOPERIDOL versus OTHER ANTIPSYCHOTICS: g. QUETIAPINE
We found one relevant study (n = 80, Liang 2013).
8.1 Specific behaviour ‐ agitation
There was no difference between the two groups at the average change score of the PANSS‐EC at 24 hours (MD 0.10, 95% CI ‐0.56 to 0.76, Analysis 8.1).
Continuous measures over 24 hours showed similar findings, from 72 hours (MD 0.20, 95% CI ‐0.87 to 1.27) to 28 days (MD 0.00, 95% CI ‐1.87 to 1.87, Analysis 8.2).
8.2 Mental state
No statistically significant difference was found at 28 days in average change score of PANSS (MD ‐0.40, 95% CI ‐7.04 to 6.24), PANSS positive sub‐scale (MD 2.00, 95% CI ‐0.87 to 4.87), and PANSS negative sub‐scale (MD ‐0.60, 95% CI ‐2.27 to 1.07, Analysis 8.3).
8.3 Adverse effects
8.3.1 General
People in the haloperidol group experienced more adverse events in 28 days when comparing with the quetiapine group (RR 1.81, 95% CI 1.19 to 2.77, Analysis 8.4).
8.3.2 Specific ‐ movement disorder
Average endpoint SAS scores at 28 days were higher in the haloperidol group (MD 1.30, 95% CI 0.57 to 2.03, Analysis 8.5).
8.4 Leaving the study early
There were no differences between groups (RR 1.25, 95% CI 0.36 to 4.32, Analysis 8.6).
9. COMPARISON 9. HALOPERIDOL versus OTHER ANTIPSYCHOTICS: h. RISPERIDONE
We found three relevant studies (n = 314), one of which ‐ Currier 2004 – randomised haloperidol versus risperidone but both groups were given lorazepam (2 mg).
9.1 Tranquillisation or asleep
One trial reported on the outcome of ‘not asleep’ across different time periods (30‐120 minutes). In the company of lorazepam, haloperidol caused more people to be asleep than risperidone (e.g. awake at 30 minutes: 1 RCT, n = 162, RR 0.84, 95% CI 0.74 to 0.95, Analysis 9.1).
9.2 Specific behaviour
About 80% of participants in both groups had at least a 50% reduction in agitation scores according to the PANNS‐EC (n = 124, RR 0.96, 95% CI 0.79 to 1.16). By 24 hours, one person in the risperidone group was withdrawn from the study early due to agitation, compared with no one in the haloperidol group (n = 124, RR 0.33, 95% CI 0.01 to 8.03, Analysis 9.3).
Continuous measures showed similar findings, confirming the absence of a difference between the two groups (1 RCT, n = 28, MD ‐0.40, 95% CI ‐5.22 to 4.42, Analysis 9.4)
9.3 Global outcome ‐ need for benzodiazepine
Oddly, in the study that used lorazepam, the great majority of both groups were given additional benzodiazepine. This did not apply to the other trial where benzodiazepine was not part of the routine treatment. There was no difference between groups (Analysis 9.7).
9.4 Adverse effects
9.4.1 General
There were no statistically significant differences between the groups with regard to the number of people who reported adverse effects (e.g. one or more adverse effects during 24 hours, 2 RCTs, n = 286, RR 1.01, 95% CI 0.84 to 1.23, Analysis 9.9).
9.4.2 Specific
Again, levels of arousal suggested that haloperidol was more sedating than risperidone (e.g. sedated at 30 minutes 1 RCT, n = 162, RR 1.90, 95% CI 1.39 to 2.59, Analysis 9.10). Cardiovascular measures were not different except that risperidone seems to marginally increase pulse and haloperidol decrease it (Analysis 9.12).
Although not statistically significant, eight people the haloperidol group compared with five allocated risperidone suffered EPS (n = 124, RR 1.60, 95% CI 0.55 to 4.62). There was no difference in the levels of acute dystonia between the old and the new drug (about 2%, 2 RCTs, n = 286, RR 1.44, 95% CI 0.28 to 7.28, Analysis 9.14). Continuous measures of akathisia and movement disorders did, however, favour risperidone within 24 hours (Analysis 9.15), finding no difference at 96 hours (Analysis 9.16).
Other adverse effects were no different between groups (Analysis 9.17).
9.5 Leaving the study early
Again, few people left these studies early with no clear differences between groups (Analysis 9.18).
10. COMPARISON 10. HALOPERIDOL versus OTHER ANTIPSYCHOTICS: i. THIOTHIXENE
We found two relevant studies (n = 74, Kewala 1984; Stotsky 1977).
10.1 Repeated need for rapid tranquillisation
There were no significant differences between haloperidol and thiothixene with respect to the number of participants who required more than one injection (1 RCT, n = 30, RR 1.07, 95% CI 0.89 to 1.28) and more than three (1 RCT, n = 30, RR 2.50, 95% CI 0.57 to 10.93, Analysis 10.1).
10.2 Specific behaviours
Although agitation was rated as ‘adverse effect’, we have reported it here as it also reflects how we have done so with other comparisons. The study found no difference between those allocated to haloperidol and those given thiothixine (1 RCT, n = 44, RR 0.28, 95% CI 0.01 to 6.52, Analysis 10.2).
10.3 Global outcome
In one study six people who received haloperidol compared with two people who received thiothixene exhibited no response to the first injection (n = 44, RR 2.50, 95% CI 0.57 to 11.05). This one trial continued to rate people hourly for five hours and never found a clear difference (Analysis 10.3).
10.4 Adverse effects
10.4.1 General
There were no differences between the groups in terms of the number of participants who experienced one or more adverse effects (2 RCTs, n = 74, RR 1.47, 95% CI 0.97 to 2.22, Analysis 10.4).
10.4.2 Specific
There were no clear differences between the two drugs in terms of movement disorders (e.g. ataxia, 1 RCT, n = 44, RR 0.83, 95% CI 0.06 to 12.49, Analysis 10.5) and a series of other adverse effect outcomes (Analysis 10.6).
11. COMPARISON 11. HALOPERIDOL versus OTHER ANTIPSYCHOTICS: j. ZIPRASIDONE
We found five relevant studies (total n = 829, Baldacara 2011; Brook 2000; Li 2006; Shu 2010; Yin 2012).
11.1 Repeated need for tranquillisation
There were no significant differences between haloperidol and ziprasidone in terms of additional drugs for tranquillisation up to two hours (1 RCT, n = 60, RR 0.36, 95% CI 0.13 to 1.01); however, the same outcome up to 24 hours revealed a significant difference in favour of ziprasidone (1 RCT, n = 60, RR 1.64, 95% CI 1.07 to 2.53, Analysis 11.1).
11.2 Specific behaviour
11.2.1 Agitation
In one study (n = 231), there were no significant differences between groups in their average endpoint scores on the PANSS‐EC across any time period from two hours (MD 0.06, 95% CI ‐1.13 to 1.25), right on to 72 hours (MD 0.62, 95% CI ‐0.45 to 1.69, Analysis 11.2).
However, in another study (n = 60), there were significant differences between groups in their average endpoint scores on the OASS: in favour of haloperidol at one hour (MD ‐7.70, 95% CI ‐9.41 to ‐5.99), and at two hours (MD ‐3.90, 95% CI ‐6.38 to ‐1.42, Analysis 11.2), in favour of ziprasidone at six hours (MD 0.20, 95% CI 0.05 to 0.35), and at 12 hours (MD 0.90, 95% CI 0.82 to 0.98, Analysis 11.4).
11.2.2 Aggression
There were no significant differences between groups in their average endpoint scores on the OAS until six hours, when there was a significant difference in favour of ziprasidone (1 RCT, n = 60, MD 0.70, 95% CI 0.60 to 0.80), confirmed at 12 hours (MD 0.20, 95% CI 0.07 to 0.33, Analysis 11.8).
11.3 Global outcome
In one study (n = 132), there were no significant differences between the groups with regard to the need for anxiolytic drugs (RR 1.11, 95% CI 0.84 to 1.48) or hypnotic medications (RR 0.71, 95% CI 0.20 to 2.50) up to seven days.
Also, there were no significant differences between groups in terms of additional restraint (1 RCT, n = 60, RR 0.60, 95% CI 0.25 to 1.44, Analysis 11.9).
Using the CGI‐S, this same study found people in the ziprasidone group had significantly improved average change scores for symptom severity when rated at three days (n = 132, MD 0.34, 95% CI 0.13 to 0.55) and seven days (n = 132, MD 0.51, 95% CI 0.07 to 0.95, Analysis 11.10).
11.4 Mental state
In one trial (n = 60), people were rated with BPRS at two hours (MD ‐4.25, 95% CI ‐10.16 to 1.66, Analysis 11.11), four hours (MD ‐4.60, 95% CI ‐9.60 to 0.40, Analysis 11.12), 24 hours (MD ‐4.00, 95% CI ‐9.09 to 1.09, Analysis 11.13), and 48 hours (MD ‐2.05, 95% CI ‐6.45 to 2.35, Analysis 11.14); the BPRS was also rated at 72 hours (3 RCTs, n = 511, MD ‐0.43, 95% CI ‐1.93 to 1.07), and at seven days (1 RCT, n = 132, MD 2.93, 95% CI ‐0.81 to 6.67, Analysis 11.14).
Another trial (n = 231) rated people at 72 hours on the PANSS. There were no differences between the groups on the positive sub‐scale (MD 1.11, 95% CI ‐0.45 to 2.67), general pathology score (MD 0.00, 95% CI ‐2.27 to 2.27) or total score (MD 2.45, 95% CI ‐2.19 to 7.09). However, there was a significant difference in favour of haloperidol between groups on the negative sub‐scale (MD ‐4.19, 95% CI ‐5.71 to ‐2.67, Analysis 11.14).
11.5 Adverse effects
For several of the studies reporting of adverse effects only occurred when > 2% or 5% or even 10% of people suffered the effect. This results in under‐reporting.
11.5.1 General
No significant differences were found between the groups in terms of experiencing one or more adverse effects at 12 hours (1 RCT, n = 60, RR 1.38, 95% CI 0.64 to 2.93, Analysis 11.15). At 72 hours, significantly more people in the haloperidol group had experienced one or more adverse effects (4 RCTs, n = 799, RR 1.74, 95% CI 1.47 to 2.06). However, by seven days, the difference between the groups was not statistically significant (1 RCT, n = 132, RR 1.31, 95% CI 0.93 to 1.83, Analysis 11.16).
No one experienced an adverse effect that was thought to be ‘severe’ (Analysis 11.17).
11.5.2 Specific
a. Anticholinergic
More people with haloperidol experienced episodes of blurred vision (1 RCT, n = 231, RR 3.97, 95% CI 1.15 to 13.68), dry mouth (1 RCT, n = 231, RR 2.97, 95% CI 1.22 to 7.22) and hypersalivation (1 RCT, n = 231, RR 2.55, 95% CI 1.11 to 5.87); no significant differences were found concerning constipation (1 RCT, n = 376, RR 0.40, 95% CI 0.08 to 2.06, Analysis 11.18).
b. Arousal
There were no differences between haloperidol and ziprasidone using both dichotomous assessments of excessive sedation (1 RCT, n = 60, RR 1.00, 95% CI 0.22 to 4.56), insomnia (1 RCT, n = 376, RR 0.51, 95% CI 0.05 to 5.53), lethargy (1 RCT, n = 231, RR 1.26, 95% CI 0.67 to 2.35) and somnolence (1 RCT, n = 376, RR 1.16, 95% CI 0.43 to 3.12, Analysis 11.19), and continuous rating scales (Analysis 11.20, Analysis 11.21, Analysis 11.22).
b. Cardiovascular
Cardiac adverse effects seemed more common in the ziprasidone group (e.g. ECG abnormal by 72 hours 2 RCTs, n = 607, RR 0.38, 95% CI 0.17 to 0.84; tachycardia by 72 hours 3 RCTs, n = 739, RR 0.49, 95% CI 0.23 to 1.04, Analysis 11.23).
c. Gastrointestinal
This also seems to apply to gastric upset – although not to a level that is statistically significant (e.g. vomiting between zero and three days 1 RCT, n = 132, RR 0.30, 95% CI 0.02 to 5.72; between zero and seven days 1 RCT, n = 132, RR 0.16, 95% CI 0.01 to 2.82, Analysis 11.24).
d. Haematological
Abnormal laboratory results were recorded for participants in both groups, but there were no significant differences across a range of measures (e.g. abnormal lab tests – by three days, 2 RCTs, n = 508, RR 0.90, 95% CI 0.69 to 1.17, Analysis 11.25).
e. Movement disorders
Over a wide range of measures, ziprasidone caused fewer movement disorders than haloperidol (e.g. akathisia by 72 hours 3 RCTs, n = 739, RR 2.32, 95% CI 1.34 to 4.01; acute dystonia by 72 hours 2 RCTs, n = 508, RR 10.26, 95% CI 1.67 to 63.17). Overall, more people in the haloperidol group were reported to suffer EPS during 12 hours (1 RCT, n = 60, RR 11.00, 95% CI 0.64 to 190.53) and at a significant level during 72 hours (2 RCTs, n = 508, RR 19.13, 95% CI 7.59 to 48.21) and at seven days (1 RCT, n = 132, RR 34.29, 95% CI 4.70 to 250.02, Analysis 11.26).
Continuous measures of movement disorders partially supported these findings (Analysis 11.27): a higher incidence of EPS and akathisia in the haloperidol group compared with the ziprasidone group according to the average score on the SAS at three days (2 RCTs, n = 191, MD 3.59, 95% CI 2.15 to 5.03), average change scores at seven days (n = 131, MD 6.10, 95% CI 3.91 to 8.29), and the average change score on the BAS at three days (n = 131, MD 0.47, 95% CI 0.18 to 0.76) and seven days (n = 131, MD 0.90, 95% CI 0.51 to 1.29).
11.6 Leaving the study early
There were no significant differences with regard to the number of participants who left the study early for any reasons during 72 hours (1 RCT, n = 376, RR 1.77, 95% CI 0.53 to 5.94) or within seven days (1 RCT, n = 132, RR 2.14, 95% CI 0.86 to 5.32, Analysis 11.29).
There were also no differences between the groups with regards to the number of participants who left studies early because of adverse effects (by 72 hours, 2 RCTs, n = 508, RR 2.40, 95% CI 0.45 to 12.75; seven days 1 RCT, n = 132, RR 0.54, 95% CI 0.06 to 4.65). There were no differences between number of people who withdrew consent (1 RCT, n = 376, RR 5.05, 95% CI 0.24 to 104.55) or left for any other reason (1 RCT, n = 376, RR 0.20, 95% CI 0.01 to 4.18) by 72 hours (Analysis 11.30).
12. COMPARISON 12. HALOPERIDOL versus OTHER ANTIPSYCHOTICS: k. ZUCLOPENTHIXOL ACETATE
We found one study (n = 70, Taymeeyapradit 2002).
12.1 Repeated need for tranquillisation
More people in the haloperidol group required more than three injections (n = 70, RR 2.54, 95% CI 1.19 to 5.46, Analysis 12.1).
12.2 Adverse effects
One person in the haloperidol group suffered a reaction at the injection site, whereas no one allocated zuclopenthixol experienced this effect (n = 70, RR 3.55, 95% CI 0.15 to 84.14, Analysis 12.3). Seven people allocated to haloperidol and two in the zuclopenthixol acetate group developed a tremor during seven days (n = 70, RR 4.16, 95% CI 0.93 to 18.62, Analysis 12.2).
12.3 Leaving the study early
No participants in either group left the study early (Analysis 12.4).
13. HALOPERIDOL versus BENZODIAZEPINE: a. FLUNITRAZEPAM
We found one relevant study (n = 28, Dorevitch 1999).
13.1 Specific behaviours ‐ aggression
The Dorevitch 1999 study found no differences in the number of participants in the haloperidol and flunitrazepam groups who had a ≥ 50% reduction on the OAS by 90 minutes (n = 28, RR 1.15, 95% CI 0.86 to 1.55, Analysis 13.1).
13.2 Global outcome ‐ need for seclusion or restraint
No participants in either the haloperidol or flunitrazepam group required further seclusion or restraint (Analysis 13.2).
13.3 Adverse effects ‐ EPS
No participants in either the haloperidol or flunitrazepam group have experienced extrapyramidal symptoms (Analysis 13.3).
14. COMPARISON 14. HALOPERIDOL versus BENZODIAZEPINE: b. LORAZEPAM
We found four relevant studies (n = 207, Battaglia 1997; Foster 1997; Garza‐Trevino 1989; Salzman 1991).
14.1 Tranquillisation or asleep ‐ not asleep
There were no significant differences between the haloperidol and lorazepam groups with regard to the number of participants asleep at one hour (1 RCT, n = 60, RR 1.05, 95% CI 0.76 to 1.44). However, by three hours, significantly more people were asleep in the lorazepam group compared with the haloperidol group (1 RCT, n = 66, RR 1.93, 95% CI 1.14 to 3.27, Analysis 14.1).
14.2 Repeated need for rapid tranquillisation
There were no differences in numbers requiring more than one and more than three injections (1 RCT, n = 66, RR 1.14, 95% CI 0.91 to 1.43 and RR 1.11, 95% CI 0.50 to 2.45, respectively, Analysis 14.2).
14.3 Global outcome ‐ no overall improvement
One trial (n = 44) found no difference between groups for the outcome of ‘showing no improvement at 30 minutes’ (RR 1.10, 95% CI 0.70 to 1.71). By 60 minutes this had not changed (1 RCT, n = 44, RR 1.64, 95% CI 0.54 to 5.03). All participants in both groups had reportedly exhibited global improvement beyond 60 minutes (Analysis 14.3).
14.4 Mental state ‐ average endpoint score
No differences were found between the haloperidol and lorazepam groups for improvement in mental state as measured by BPRS at one hour (1 RCT, n = 37, MD 3.26, 95% CI ‐4.13 to 10.65), and several times up to four hours (Analysis 14.4).
14.5 Adverse effects
14.5.1 General
Approximately a third of the participants in both groups experienced one or more drug‐related adverse effect (1 RCT, n = 66, RR 1.13, 95% CI 0.60 to 2.10). No participants in either group were reported to have experienced serious adverse effects (Analysis 14.6).
14.5.2 Specific
There were no differences between those allocated haloperidol and lorazepam in terms of dry mouth (1 RCT, n = 66, RR 0.53, 95% CI 0.14 to 2.04, Analysis 14.8).
In terms of movement disorders and the emergence of ataxia (1 RCT, n = 66, RR 0.44, 95% CI 0.04 to 4.65), dystonia (1 RCT, n = 66, RR 3.54, 95% CI 0.42 to 30.03), speech disorder (1 RCT,n = 66, RR 1.77, 95% CI 0.35 to 9.01), rigidity (1 RCT, n = 66, RR 6.22, 95% CI 0.33 to 115.91), tremor (1 RCT, n = 66, RR 1.77, 95% CI 0.17 to 18.60) and the need for benztropine (1 RCT, n = 66, RR 1.99, 95% CI 0.68 to 5.83). One study (n = 60) reported that significantly more participants experienced EPS in the haloperidol group compared with the lorazepam group (RR 15.00, 95% CI 2.11 to 106.49, Analysis 14.11).
Some other adverse effects are reported. One study (n = 60) found no differences between the groups in the number of participants asleep at one hour (RR 0.89, 95% CI 0.40 to 1.99). Clonidine was used for hypertension but not significantly more in one group or the other (1 RCT, n = 66, RR 2.67, 95% CI 0.11 to 63.17). Dizziness was not more prevalent in either group (1 RCT, n = 66, RR 0.89, 95% CI 0.19 to 4.07, Analysis 14.9).
15. HALOPERIDOL versus BENZODAIZEPINE: c. MIDAZOLAM
We found one relevant study (n = 84, Nobay 2004).
15.1 Tranquillisation or asleep ‐ average time
Significant differences favouring midazolam over haloperidol were reported for the average time to sedation, however these data were skewed and are presented in tabular form only (Analysis 15.1).
15.2 Global outcome
There were no clear differences between the groups for the number of people requiring a rescue drug (n = 84, RR 1.14, 95% CI 0.46 to 2.87, Analysis 15.2).
15.3 Adverse effects
Two participants were reported to have experienced adverse effects. These were both in the haloperidol group with one person experiencing hypotension (n = 84, RR 3.00, 95% CI 0.13 to 71.61) and the other, apnoea (n = 84, RR 3.00, 95% CI 0.13 to 71.61, Analysis 15.3).
16. HALOPERIDOL versus COMBINATIONS: a. HALOPERIDOL + LEVOMEPROMAZINE
We found one study (n = 19, Higashima 2004).
16.1. Global outcome
There was no clear difference for the outcome of ‘not improved’ (1 RCT, n = 19, RR 8.18, 95% CI 0.50 to 133.66, Analysis 16.1).
17. HALOPERIDOL versus COMBINATIONS: b. HALOPERIDOL + LORAZEPAM
We found two studies that compared haloperidol alone with a combination of haloperidol and lorazepam (n = 113, Battaglia 1997; Garza‐Trevino 1989).
17.1 Tranquillisation or asleep
Significantly more people in the combination group were asleep at three hours than those in the haloperidol alone group (1 RCT, n = 67, RR 1.83, 95% CI 1.11 to 3.02, Analysis 17.1).
17.2 Repeated need for rapid tranquillisation
Fewer people in the combination group required more than one (1 RCT, n = 67, RR 1.05, 95% CI 0.87 to 1.27) and more than three additional injections within 24 hours than in the haloperidol alone group (1 RCT, n = 67, RR 3.05, 95% CI 0.92 to 10.10, Analysis 17.2).
17.3 Global outcome ‐ no overall improvement
Significantly fewer people in the combination group showed no overall improvement by 30 minutes (1 RCT, n = 45, RR 2.67, 95% CI 1.25 to 5.68). By 60 minutes, although six people in the haloperidol alone group continued to show no overall improvement compared with none in the combination group; the difference between the groups was not statistically significant (1 RCT, n = 45, RR 14.77, 95% CI 0.88 to 247.54). No participants in either group showed ‘no overall improvement’ after 60 minutes. All participants in both groups demonstrated overall improvement (Analysis 17.3).
17.4 Adverse effects
17.4.1 General
One study (n = 67) found no difference for numbers experiencing one or more adverse effects (RR 1.16, 95% CI 0.62 to 2.18, Analysis 17.4).
17.4.2 Specific
In terms of a series of movement disorders, one study (n = 67) found no statistically significant differences between the groups (e.g. dystonia RR 8.25, 95% CI 0.46 to 147.45; required antiparkinson medication RR 2.74, 95% CI 0.81 to 9.25, Analysis 17.7).
The anticholinergic effect of dry mouth was not more common in the haloperidol alone group (1 RCT, n = 67, RR 0.91, 95% CI 0.20 to 4.21, Analysis 17.5). This also applies to cardiovascular effects such as hypertension (1 RCT, n = 67, RR 0.91, 95% CI 0.06 to 14.02) and dizziness (1 RCT, n = 67, RR 1.37, 95% CI 0.24 to 7.69, Analysis 17.6).
18. HALOPERIDOL versus COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM
We found one study (n = 60) comparing haloperidol versus haloperidol plus midazolam (Baldacara 2011).
18.1 Repeated need for tranquillisation
There were significant differences between groups in terms of need for additional drugs for tranquillisation at two hours in favour of haloperidol (RR 0.27, 95% CI 0.10 to 0.71); however, this was not confirmed at 12 hours (RR 0.88, 95% CI 0.69 to 1.13, Analysis 18.1).
18.2 Specific behaviour
18.2.1 Agitation
Average endpoint scores on OASS showed a significant difference in favour of haloperidol at one hour (MD ‐8.50, 95% CI ‐9.93 to ‐7.07), at two hours (MD ‐6.70, 95% CI ‐7.46 to ‐5.94, Analysis 18.2), at four hours (MD ‐5.50, 95% CI ‐6.48 to ‐4.52, Analysis 18.3), at six hours (MD ‐3.70, 95% CI ‐4.56 to ‐2.84), and at 12 hours (MD ‐11.20, 95% CI ‐12.24 to ‐10.16, Analysis 18.4).
18.2.2 Aggression
Continuous measurement on OAS at different rating time points showed a significant advantage of haloperidol over the combination group (Analysis 18.5), with the exception of the rating at four hours (MD ‐0.10, 95% CI ‐0.68 to 0.48, Analysis 18.6) and at six hours (MD 0.40, 95% CI 0.30 to 0.50, Analysis 18.7).
18.3 Global outcomes
Six people in the haloperidol group needed additional restraint, seclusion or special observation compared with 21 people in the combination group (1 RCT, n = 60, RR 0.29, 95% CI 0.13 to 0.61, Analysis 18.8).
18.4 Adverse effects
18.4.1 General
There were no significant differences between the compared groups in terms of number of people experiencing one or more adverse effect (RR 0.73, 95% CI 0.41 to 1.32, Analysis 18.9).
18.4.2 Specific
a. Arousal
More people in the combination group experienced excessive sedation by 12 hours (RR 0.25, 95% CI 0.08 to 0.80, Analysis 18.11). Continuous measurement on the Ramsay Sedation Scale (RSS) at various time points however failed to show differences between the compared groups (Analysis 18.12; Analysis 18.13, Analysis 18.14).
b. Cardiovascular
Five people in the combination group experienced hypotension episodes compared to no people in the haloperidol group (RR 0.09, 95% CI 0.01 to 1.57, Analysis 18.15).
c. Movement disorders
There were no significant differences in terms of EPS by 12 hours (RR 1.67, 95% CI 0.44 to 6.36, Analysis 18.16).
19. HALOPERIDOL versus COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE
We found two relevant studies (n = 376) (Baldacara 2011; Huf 2007).
19.1 Tranquillisation or asleep
More people in the haloperidol group compared with those in the combination group were not tranquil or asleep by 20 minutes (n = 316, RR 1.60, 95% CI 1.18 to 2.16), 40 minutes (n = 316, RR 1.26, 95% CI 0.83 to 1.91), 60 minutes (n = 316, RR 1.42, 95% CI 0.85 to 2.37) and 120 minutes (n = 316, RR 1.32, 95% CI 0.68 to 2.56). The difference between the groups was only statistically significant at 20 minutes (Analysis 19.1; Analysis 19.2).
19.2 Repeated need for tranquillisation
No more people in the haloperidol alone group required repeat rapid tranquillisation by two hours (2 RCTs, n = 376, RR 0.78, 95% CI 0.43 to 1.41) nor by 12 hours (1 RCT, n = 60, RR 1.10, 95% CI 0.81 to 1.49, Analysis 19.3).
19.3 Specific behaviours
19.3.1 Agitation
When rated on OASS, there was a significant advantage of haloperidol at one hour (1 RCT, n = 60, MD ‐24.50, 95% CI ‐27.32 to ‐21.68) and two hours (1 RCT, n = 60, MD ‐9.40, 95% CI ‐10.39 to ‐8.41, Analysis 19.4), and of combination at four hours (1 RCT, n = 60, MD 3.80, 95% CI 3.27 to 4.33, Analysis 19.5), at six hours (1 RCT, n = 60, MD 2.60, 95% CI 2.13 to 3.07) and at 12 hours (1 RCT, n = 60, MD 0.80, 95% CI 0.55 to 1.05, Analysis 19.6).
19.3.2 Aggression
While at continuous rating on OAS there was an significant advantage of haloperidol at one hour (1 RCT, n = 60, MD ‐4.50, 95% CI ‐6.28 to ‐2.72, Analysis 19.7), subsequent rating resulted significantly in favour of combination at four hours (1 RCT, n = 60, MD 0.60, 95% CI 0.49 to 0.71, Analysis 19.8), at six hours (1 RCT, n = 60, MD 1.10, 95% CI 0.91 to 1.29), and at 12 hours (1 RCT, n = 60, MD 1.80, 95% CI 1.67 to 1.93, Analysis 19.9).
No more people in the haloperidol alone group exhibited a further aggressive outburst within 24 hours (n = 316, RR 1.06. 95% CI 0.68 to 1.65, Analysis 19.10).
19.4 Global outcomes
More people in the haloperidol alone group had to be restrained by 120 minutes, however the difference between groups was not statistically significant (n = 316, RR 1.21, 95% CI 0.84 to 1.76, Analysis 19.11). The number of people who refused oral medication was roughly the same in both the haloperidol alone and combination group (n = 316, RR 0.99, 95% CI 0.61 to 1.60). Significantly more people in the haloperidol alone group required extra visits from the doctor (n = 316, RR 1.50, 95% CI 1.05 to 2.14, Analysis 19.12).
19.5 Adverse effects
19.5.1 General
Significantly more people in the haloperidol alone group than the combination group experienced one or more adverse effects (2 RCTs, n = 376, RR 2.01, 95% CI 1.07 to 3.80, Analysis 19.13). There were no differences between the compared group in terms of serious adverse events (Analysis 19.14).
19.5.2 Specific
a. Arousal
No significant differences were found between the compared groups (Analysis 19.15; Analysis 19.16; Analysis 19.17; Analysis 19.18).
b. Cardiovascular
Three people in the combination group experienced hypotension episodes compared to no people in the haloperidol group (1 RCT, n = 60, RR 0.14, 95% CI 0.01 to 2.65, Analysis 19.19).
c. Movement disorders
Nine people in the haloperidol alone group experienced acute dystonia compared with no one in the combination group (n = 316, RR 19.48, 95% CI 1.14 to 331.92); there were no differences in terms of number of EPS episodes by 12 hours between the compared groups (1 RCT, n = 60, RR 1.00, 95% CI 0.32 to 3.10, Analysis 19.20).
19.6 Service outcomes
Less people in the haloperidol alone group were not discharged by 14 days, but this was not a statistically significant difference between groups (n = 316, RR 0.87, 95% CI 0.72 to 1.05, Analysis 19.21).
19.7 Leaving the study early
Seventeen people from each group left the study early, therefore there were no statistical differences between groups (Analysis 19.22). When specific reasons were cited, again, no differences were clearly shown (e.g. withdrew consent n = 316, RR 1.03, 95% CI 0.06 to 16.25, Analysis 19.22).
20. HALOPERIDOL versus COMBINATIONS: e. HALOPERIDOL + RISPERIDONE
We found one study (n = 100) (Liu 2012).
20.1 Specific behaviour ‐ agitation
There was a statistically significant difference between the compared groups on the average scores of PANSS‐EC at 24 hours in favour of the comparison group (MD 3.82, 95% CI 1.35 to 6.29, Analysis 20.1); rating at three days, five days, one week and two weeks failed to show a significant difference (Analysis 20.2).
20.2 Global outcomes
More people in the combination group experienced no improvement at 72 hours (RR 0.38, 95% CI 0.11 to 1.33), at five days (RR 0.25, 95% CI 0.03 to 2.16), at one week (RR 0.67, 95% CI 0.12 to 3.82), at two weeks (RR 0.33, 95% CI 0.01 to 7.99) compared to people in the haloperidol group; however, differences were not significant (Analysis 20.3).
20.3 Adverse effects
a. Arousal
There were no significant differences in terms of insomnia (RR 1.20, 95% CI 0.39 to 3.68) and somnolence (RR 0.92, 95% CI 0.45 to 1.88, Analysis 20.4).
b. Movement disorders
There were no significant differences between the compared groups in terms of tremor (RR 0.94, 95% CI 0.54 to 1.65) and akathisia (RR 0.88, 95% CI 0.48 to 1.60) during 14 days (Analysis 20.5).
c. Miscellaneous
No significant differences were found when comparing the two groups concerning dry mouth (RR 0.89, 95% CI 0.37 to 2.12), constipation (RR 1.50, 95% CI 0.45 to 4.99), and increased salivation (RR 2.00, 95% CI 0.19 to 21.36) during 14 days (Analysis 20.6).
21. HALOPERIDOL versus COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE
We found one study (n = 64) that compared haloperidol versus olanzapine plus magnesium valproate (Shi 2010).
21.1 Specific behaviour ‐ agitation
There were no significant differences on average endpoint scores on PANSS‐EC at 72 hours (MD ‐0.04, 95% CI ‐1.33 to 1.25); however, rating on PANSS‐EC showed significant differences at seven days (MD 1.61, 95% CI 0.15 to 3.07) and 14 days (MD 1.82, 95% CI 0.61 to 3.03) in favour of combination group (Analysis 21.1).
21.2 Global outcomes ‐ no improvement
The same number of people experienced no improvement (RR 1.06, 95% CI 0.38 to 2.95, Analysis 21.2).
21.3 Mental state
There were no significant differences on average endpoint PANSS scores at 72 hours (MD ‐1.58, 95% CI ‐7.79 to 4.63), at seven days (MD 1.94, 95% CI ‐2.22 to 6.10), and at 14 days (MD 2.42, 95% CI ‐0.86 to 5.70, Analysis 21.3).
21.4 Adverse effects
Few people on the combination group experienced one or more adverse effects (RR 3.06, 95% CI 1.62 to 5.79) compared to haloperidol alone group (Analysis 21.4).
22. HALOPERIDOL versus COMBINATIONS: g. QUETIAPINE + MAGNESIUM VALPROATE
We found one study that compared haloperidol with a combination of quetiapine + magnesium valproate (n = 60, Guo 2007).
22.1 Specific behaviour ‐ agitation
Regarding the binary measure of agitation (less than 25% decrease in PANSS‐EC score) there was no difference between groups (n = 60, RR 1.17, 95% CI 0.44 to 3.06, Analysis 22.1).
When endpoint scores for agitation were recorded at three, seven and 14 days no differences were apparent (n = 60, MD 14 days 1.24, 95% CI ‐0.49 to 2.97, Analysis 22.2).
23. HALOPERIDOL versus COMBINATIONS: h. RISPERIDONE + CLONAZEPAM
We found two studies (n = 305) that compared haloperidol versus risperidone plus clonazepam (Fang 2012; Liu 2012).
23.1 Tranquillisation or asleep
There were no significant differences in the number of people not asleep at two hours (1 RCT, n = 205, RR 1.07, 95% CI 0.82 to 1.39) and at four hours (1 RCT, n = 205, RR 0.82, 95% CI 0.66 to 1.03, Analysis 23.1).
23.2 Specific behaviour ‐ agitation
Continuous measurement on average PANSS‐EC scores showed no significant differences between the compared groups at two hours (1 RCT, n = 108, MD ‐0.50, 95% CI ‐3.80 to 2.80, Analysis 23.2), at four hours (1 RCT, n = 126, MD ‐2.40, 95% CI ‐5.20 to 0.40, Analysis 23.3), at 24 hours (2 RCTs, n = 305, MD 0.71, 95% CI ‐0.56 to 1.98, Analysis 23.4), at three days (2 RCTs, n = 305, MD ‐0.41, 95% CI ‐1.66 to 0.83), at five days (2 RCTs, n = 305, MD 0.14, 95% CI ‐1.24 to 1.52), and at two weeks (1 RCT, n = 100, MD ‐0.88, 95% CI ‐2.34 to 0.58); the only exception was at week one in favour of haloperidol (1 RCT, n = 100, MD ‐2.56, 95% CI ‐4.57 to ‐0.55, Analysis 23.5).
23.3 Global outcomes
More people on the combination group experienced no improvement at 72 hours (RR 0.60, 95% CI 0.15 to 2.38), at five days (RR 0.25, 95% CI 0.03 to 2.16), at one week (RR 0.20, 95% CI 0.01 to 4.06), and at two weeks (RR 0.50, 95% CI 0.10 to 2.61) compared with haloperidol group (Analysis 23.6).
23.4 Mental state
There were no significant differences on average change score on PANSS at five days (1 RCT, n = 205, MD ‐3.50, 95% CI ‐7.07 to 0.07, Analysis 23.7).
23.5 Adverse effects
23.5.1 General
In the combination group there were fewer adverse events at a significant level (1 RCT, n = 205, RR 1.72, 95% CI 1.29 to 2.29, Analysis 23.8).
23.5.2 Specific
a. Arousal
There were no significant differences concerning arousal adverse events (e.g. insomnia during five days 1 RCT, n = 205, RR 1.54, 95% CI 0.45 to 5.31; insomnia during 14 days 1 RCT, n = 100, RR 0.50, 95% CI 0.20 to 1.23; somnolence during 14 days 1 RCT, n = 100, RR 2.75, 95% CI 0.94 to 8.06) between the compared groups (Analysis 23.9).
b. Cardiovascular
There were no significant differences between the groups concerning tachycardia during five days (1 RCT, n = 205, RR 1.29, 95% CI 0.36 to 4.66, Analysis 23.10).
c. Movement disorders
People in the haloperidol group experienced more movement disorder‐related adverse events (e.g. akathisia during five days 1 RCT, n = 205, RR 1.63, 95% CI 1.12 to 2.38; EPS during five days 1 RCT, n = 205, RR 2.22, 95% CI 1.52 to 3.23; tremor during 14 days 1 RCT, n = 100, RR 3.20, 95% CI 1.27 to 8.07, Analysis 23.11).
d. Miscellaneous
There were no significant differences in terms of anticholinergic adverse events (Analysis 23.12).
24. HALOPERIDOL versus COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM
We found 1 study (n = 76) that compared haloperidol versus ziprasidone plus clonazepam (Zhou 2014).
24.1 Specific behaviour ‐ agitation
There were no significant differences on the average endpoint PANSS‐EC scores at 12 hours (MD ‐0.28, 95% CI ‐0.80 to 1.24, Analysis 24.1), at 48 hours (MD ‐1.54, 95% CI ‐3.26 to 0.18), at three days (MD ‐0.42, 95% CI 1.63 to 0.79), at five days (MD ‐0.22, 95% CI ‐1.78 to 1.34), and at seven days (MD ‐0.19, 95% CI ‐1.57 to 1.19, Analysis 24.2).
24.2 Global outcomes
There were no significant differences on average endpoint CGI‐S scores at seven days (MD 0.00, 95% CI ‐0.26 to 0.26, Analysis 24.3).
24.3 Mental state
There were no significant differences on average endpoint PANSS total scores at seven days (MD 1.46, 95% CI ‐4.58 to 7.50) and sub‐scales (Analysis 24.4).
24.4 Leaving the study early
Three people in the haloperidol group left the study early compared to two people in the combination group (RR 1.50, 95% CI 0.27 to 8.48, Analysis 24.5).
Discussion
Summary of main results
1. COMPARISON 1. HALOPERIDOL versus PLACEBO
It can be seen from the summary of findings Table for the main comparison that, despite five trials providing data (total n = 700), the overall quality of evidence was not high. No study discussed economic outcomes.
1.1 Tranquillisation or asleep, repeated need for tranquillisation, agitation
When compared with placebo, significantly more people in the haloperidol group were asleep at two hours and demonstrated at least a 40% reduction in Positive and Negative Syndrome Scale Excited Component (PANSS‐EC) agitation score (Bristol Myers 2004a; Bristol‐Myers 2005b). With respect to evidence for rapid tranquillisation, the evidence was downgraded to ‘very low’ for ‘tranquil or asleep’ because there was no measure at 30 minutes, therefore, two hours was the closest time point. We think this is quite distant from the emergency situation and most data for this outcome came from Battaglia 2002 study, in which there is a serious risk of bias because the method of randomisation was not described, allocation concealment was not stated, there were incomplete outcome data and the trial was sponsored by the drug company, which could possibly impact on publication bias (please see summary of findings Table for the main comparison).
On the continuous measures of agitation, haloperidol seems to demonstrate a discernable effect. For example, people in the haloperidol group were reported to have experienced a significantly greater reduction in agitation than those in the placebo group at two hours (3 RCTs, n = 474, MD ‐4.48, 95% CI ‐5.78 to ‐3.18, Analysis 1.4). A four‐point reduction may be of clinical meaning, but we could not find this clearly highlighted.
1.2 Global outcome, mental state
Fewer people in the placebo group exhibited ‘marked improvement' than those who received haloperidol (Analysis 1.6), and four studies found that more people in the placebo group required benzodiazepines (Analysis 1.7). All data point to the real effect of use of haloperidol in these difficult and dangerous situations. Mental state data support this impression.
1.3 Adverse effects
More people in the haloperidol group experienced one or more adverse reactions than those in the placebo group (Analysis 1.12). Bristol‐Myers 2005b reported that of all adverse effects reported, only one was rated as 'serious' ‐ this was related to a participant in the placebo group.
More people in the haloperidol group than the placebo group were reported to be "over sedated". Haloperidol did seem to not cause more cardiac problems than placebo but, unsurprisingly, movement disorders were more prevalent. Often, however, and on a range of outcomes, the differences were not statistically significant. However, significantly more people in the haloperidol group did require antiparkinson drugs and experienced Extrapyramidal Side Effects (EPS) (Analysis 1.17).
1.4 Leaving the study early
It is problematic how to interpret data on leaving the study as, in some of these trials, people may not have been fully free to leave. Only 27 of 335 people left early ‐ with no differences between groups.
2. COMPARISON 2. HALOPERIDOL versus OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE
This comparison contains the larger trials (two RCTs, total n = 599), but, as can be seem from the summary of findings Table 2, the selected outcomes are absent or of low or very low quality.
2.1 Repeated need for rapid tranquillisation
Considering how difficult repeated injections are, that use of haloperidol necessitates less is important. The quality of these data is, however, low. We do not have any direct data on tranquillisation, but there is the implication from these data that haloperidol either sedates or tranquillises more effectively than aripiprazole.
2.2 Specific behaviours ‐ agitation
There was no difference between groups at about two hours with about half of each group having a considerable reduction in agitation scores. We graded these data as of very low quality (summary of findings Table 2). Two hours, is, however, a considerable time after an acute aggressive or agitated event and data at an earlier state would be helpful.
2.3 Global outcomes
All global outcomes – again all around two hours – show no difference between the two drugs (requiring additional benzodiazepines; average change scores on the Clinical Global Impression ‐ Severity (CGI‐S) rating scale; average endpoint scores on the Clinical Global Impression ‐ Improvement (CGI‐I) rating scale; average change scores on the CGI‐I rating scale in the schizophrenia subgroup). This is reassuring that aripiprazole does have some effect but its value in the very acute stage of management is not supported by these data. The few mental state data concur.
2.4 Adverse effects
For some adverse effects haloperidol seems to come off worse than aripiprazole although there was no difference between groups for 'adverse effects within 24 hours'. One person in the haloperidol group developed severe dystonia and one in the aripiprazole group had a tonic‐clonic seizure.
Evidence was not convincing that there were any differences when it came to cardiac or gastrointestinal effects. Movement disorders, however, all tended to be more common in the group allocated to haloperidol and risk of use of antiparkinson medication was increased if a person was allocated to haloperidol. Although this is not often the problem that is most paramount within an emergency, these effects result in an already difficult situation being even more unpleasant. Akathisia (RR nearly 3), for example, can be mistaken for the original agitation and result in administration of yet more haloperidol. These common effects (˜1/3 needed additional antiparkinson medication) are a considerable limitation for the value of haloperidol.
2.5 Leaving the study early
There were no significant differences in the number of people leaving early and, again, the meaning of these data is unclear as we are not sure how at liberty people were to leave the studies by the end of the trial.
Economic outcomes were not measured. Aripiprazole is considerably more expensive than haloperidol.
3. COMPARISON 3. HALOPERIDOL versus OTHER ANTIPSYCHOTICS: b. CHLORPROMAZINE
Although we found four relevant studies, all were small (total n = 153) and we could not rate overall findings more than of very low grade (summary of findings Table 3), and no important outcome had all trials contributing.
3.1 Tranquillisation or asleep, repeated need for rapid tranquillisation, not marked improvement
One small study (n = 39) of poor quality reported that more people allocated to chlorpromazine did experience sleep by two hours (Analysis 3.1). This does seem to fit with clinical experience.
Another study reported the number of people requiring more than one injection and, despite the suggestion of more sedation in the chlorpromazine group, there was no clear indication that either group avoided more or fewer additional injections – but, again the trial was small and of very limited quality (Analysis 3.2).
Although there was no clear difference for the outcome of "not marked improvement", more people in the chlorpromazine group were rated as showing "not any improvement" (Analysis 3.3). We are unclear if this has meaning or is a chance finding.
3.2 Adverse effects
Reporting of adverse effects was sporadic and therefore, not very helpful. From these data it is not clear that either drug has clear advantage over the other for allergic, cardiovascular, or movement disorders (Analysis 3.4).
3.3 Leaving the study early ‐ for any reason
This was the only outcome where all trials contributed. Few people left these studies early (5%). This could be a function of the coercive aspect of the treatment. Perhaps people were not fully free to leave; significantly fewer people left the haloperidol group (Analysis 3.5).
4. COMPARISON 4. HALOPERIDOL versus OTHER ANTIPSYCHOTICS: c. DROPERIDOL
Droperidol has been taken off the market because of “commercial reasons” (Khokhar 2016). However, there remains interest in its use (Isbister 2010). We found two relevant studies (n = 255) and the outcomes were of moderate grade (higher than others in this review) (summary of findings Table 4).
4.1 Tranquillisation or asleep, repeated need for rapid tranquillisation
The same amount of people in the haloperidol and droperidol groups did experience sleep by two hours (Analysis 4.1), with no significant differences concerning the time to get asleep (Analysis 4.2).
Droperidol did seem more effective ‐ more people in the haloperidol group required more than one injection, and this held true for the outcome of ‘required more than three injections’ (Analysis 4.3).
4.2 Global outcomes
To note, more people in the droperidol group required more benzodiazepines up to two hours (Analysis 4.4).
4.3 Adverse effects ‐ specific
Droperidol could be equally or more toxic than haloperidol in terms of total adverse events (Analysis 4.5) and specific adverse events (Analysis 4.6; Analysis 4.7; Analysis 4.8; Analysis 4.9).
Further studies are needed; much better evaluation is justified but in times of austerity in health care, it could be a clinically useful option.
5. COMPARISON 5. HALOPERIDOL versus OTHER ANTIPSYCHOTICS: d. LOXAPINE
We found three relevant studies (total n = 119), one of which offset adverse effects using biperiden, but that we could only rate the outcomes as being of low or very low grade (summary of findings Table 5). Loxapine is widely available in several European countries, Australasia and the USA. It is a shame that we do not have better data on this interesting compound that some consider to be inexpensive, but with 'atypical' properties (Glazer 1999).
5.1 Tranquillisation or asleep, agitation, aggression
Loxapine did seem more sedating than haloperidol although the one study lacked power (n = 54, Analysis 5.1). There were no differences between groups for improvement in levels of agitation or levels of aggression but, again, these outcomes were reported by only one very small study (n = 30, Analysis 5.2).
5.2 Global outcomes, mental state
There are several ways these trials reported global outcomes and measured mental state, but never any real suggestion of an effect favouring one or other drug.
5.3 Adverse effects
Even with the use of biperiden in one study, it might have been expected to see some differences between drugs. None were apparent in these trials.
6. COMPARISON 6. HALOPERIDOL versus OTHER ANTIPSYCHOTICS: e. OLANZAPINE
We found six studies (total n = 720) but no pre‐selected outcome of importance contained data from more than 400 people and all important outcomes we had to rate as being of very low grade (Summary of findings table 6).
6.1 Tranquillisation or asleep, need for more tranquillisation, specific behaviours
Olanzapine does seem more sedating than haloperidol (Analysis 6.1). In terms of requiring additional injections, a slight advantage in favours of olanzapine was demonstrated (Analysis 6.2).
When analysing agitation rating scales, a slight tendency in favour of olanzapine was demonstrated (Analysis 6.3; Analysis 6.4, Analysis 6.5; Analysis 6.6; Analysis 6.7).
In terms of both aggression and hostility, haloperidol and olanzapine showed no difference (Analysis 6.8; Analysis 6.9; Analysis 6.10; Analysis 6.11).
6.2 Global outcome, mental state
Most global outcomes demonstrated no clear difference between the compounds. For example, use of additional restraint, seclusion or special observation was comparable (Analysis 6.12). Olanzapine seems as effective as the older drug. This also seems to apply to the measures of mental state (Analysis 6.30).
6.3 Adverse effects
It was a concern that these industry‐sponsored trials were designed to under‐report adverse effects. It had been stipulated in the design of a trial that adverse effects would be reported only if, for example, more than 10% of people in a group experienced them (Kinon 2001d). Therefore, should 9% of people experienced worrying skin reactions in Kinon 2001d, this could have been omitted from the report.
Nevertheless, it does seem clear that haloperidol does cause more movement disorders than olanzapine. This must be seen as an advantage for the newer drug when being compared with haloperidol used alone.
6.4 Economic
There was no measure for economic outcomes. In older trials this is understandable, but not for comparisons of expensive drugs and the older compounds. It is likely that the expensive olanzapine could save money in the short term ‐ but, disappointingly, this is supposition.
7. COMPARISON 7. HALOPERDIOL versus OTHER ANTIPSYCHOTICS: f. PERPHENAZINE
Considering the recent resurgence of interest in perphenazine (Lieberman 2005), this is an important comparison. The one relevant study (n = 44) at least proves that comparative testing of these two treatments is possible, although outcomes are limited and the grade of evidence largely poor (summary of findings Table 7). Now that data suggest that perphenazine is a real option for people wanting a more gentle effective antipsychotic drug (Lieberman 2005), there is the possibility that it also would be of value in the emergency situation.
7.1 Global outcome ‐ no improvement
Limited data suggest global impression of improvement is not different between groups (Analysis 7.1).
7.2 Adverse effects
Considerably more people allocated to haloperidol experienced one or more adverse effects (Analysis 7.2) and required antiparkinson medication (Analysis 7.3), but these were not statistically significant in this small trial. Nevertheless, there is a suggestion that perphenazine may have advantages in terms of adverse effects.
This must be an area for future research. However, without the push of industry it is possible that perphenazine will continue to be under evaluated.
8. COMPARISON 8. HALOPERIDOL versus OTHER ANTIPSYCHOTICS: g. QUETIAPINE
We found only one study (n = 80) with the most of the outcomes rated at 28 days and of low grade (summary of findings Table 8).
8.1 Specific behaviour ‐ agitation
No differences were found between haloperidol and quetiapine across when using rating scales (Analysis 8.1; Analysis 8.2).
8.2 Mental state
Haloperidol and quetiapine showed similar results (Analysis 8.3); however, the Positive and Negative Syndrome Scale (PANSS) and sub‐scales were rated at 28 days, inappropriately with the aim of this review.
8.3 Adverse effects
More people allocated to haloperidol experienced one or more adverse effects (Analysis 8.4); this held true when taking into account movement disorders rating scales (Analysis 8.5).
9. COMPARISON 9. HALOPERIDOL versus OTHER ANTIPSYCHOTICS: h. RISPERIDONE
We found three relevant studies (n = 314), the outcomes of which we had to grade as of moderate, low or very low quality (summary of findings Table 9).
9.1 Tranquillisation or asleep, agitation, need for more benzodiazepine
We thought the best graded evidence was that, if given haloperidol as well as lorazepam, more people were asleep compared with risperidone – also plus lorazepam (Analysis 9.1). Tranquillisation is often now considered desirable rather than full sedation to sleep, but in certain circumstances the latter may be preferable. The key issue is whether agitation is decreased and, according to PANNS‐EC scores, both medications are effective (Analysis 9.3).
In the trial randomising lorazepam as part of the package of care, more subsequent benzodiazepines were used. There was no difference between groups (Analysis 9.7), just the indication that if benzodiazepines are part of the package of care they will be used again and again. This could be problematic for the cumulative adverse effects.
9.2 Adverse effects
The two drugs did not have many differences as regards adverse effects; one seems as good or as bad as the other (Analysis 9.9). For binary measures of movement disorder no differences were clearly apparent (Analysis 9.14). Continuous measures of akathisia and movement disorders did, however, favour risperidone (Analysis 9.15). These fine‐grain measures may pick up differences not clearly shown by the more blunt binary outcomes. Akathisia is unpleasant and can lead to clinicians misinterpreting that the person is agitated again. Even subtle decreases of akathisia could be valuable in management of agitation and aggression.
9.3 Economic
We found no data on economic outcomes of this comparison. These trials were undertaken at a time when risperidone was not an inexpensive option and omission of this type of data does not seem random.
10. COMPARISON 10. HALOPERIDOL versus OTHER ANTIPSYCHOTICS: i. THIOTHIXINE
The two small studies provided low level data (total n = 74, summary of findings Table 10).
10.1 Repeated need for rapid tranquillisation, agitation, global outcome
In the two studies that compared haloperidol with thiothixene, there was no outcome measure for tranquillisation or asleep. There were no significant differences between haloperidol and thiothixene with respect to the number of participants who required more than one injection (Analysis 10.1), agitation (Analysis 10.2), and ‘no response to the first injection’ (Analysis 10.3), but the study reporting these outcomes was very small indeed.
10.2 Adverse effects
There were no differences between the groups in terms of the number of participants who experienced one or more adverse effects (2 RCTs, n = 74, RR 1.47, 95% CI 0.97 to 2.22, Analysis 10.4).
The quality of these outcomes was, however, rated as ‘low’ or ‘very low’ because of risk of several biases (summary of findings Table 10).
11. COMPARISON 11. HALOPERIDOL versus OTHER ANTIPSYCHOTICS: j. ZIPRASIDONE
Ziprasidone is a relatively new drug when compared with others in this set of comparisons. It is clear that the pharmaceutical industry has recognised the paucity of data in this area and that profits are to be made if an expensive drug can be shown to be as effective as the older drug and have fewer adverse effects. We found five relevant studies (total n = 829) but, again we had to grade the quality of outcome evidence as low or very low (summary of findings Table 11). We have reconsidered as to whether we have been too harsh in judging the quality of evidence but, for the reasons outlined in the summary of findings Table 11, we feel we have to continue to grade these data as poor. For example, these large studies did not report the outcome of ‘Tranquillisation or asleep’ or even ‘Repeated need for tranquillisation’. In larger trials focused on the coercive treatment of aggressive people we do feel that this is an odd set of omissions.
11.1 Repeated need for tranquillisation, specific behaviours, global outcomes and mental state
All measures are not convincingly better for either drug. The CGI‐S measures are better for the ziprasidone group as are some sub‐scores of mental state measures.
11.2 Adverse effects
The technique of reporting adverse effects only if more than 2%/5% or even 10% of people suffered the effect results in under‐reporting. Haloperidol can be confidently expected to provide frequent adverse effects (Adams 2013), so this device may assist the new drug seem less toxic.
At 72 hours, significantly more people in the haloperidol group had experienced one or more adverse effects but, by seven days, the difference between the groups was not statistically significant (Analysis 11.15).
Ziprasidone does seem to cause fewer movement disorders than haloperidol (Analysis 11.26) and, perhaps less anticholinergic problems. However, even these trials, generated by the company with a pecuniary interest in the outcomes, did nevertheless suggest, that cardiac adverse effects seemed more common in the ziprasidone group (Analysis 11.28). This must be seen as an important concern.
11.3 Economic
In these trials, so obviously comparing an inexpensive with expensive drug we find no economic analysis. This bias by omission casts a further cloud on reporting of all other data.
12. COMPARISON 12. HALOPERIDOL versus OTHER ANTIPSYCHOTICS: k. ZUCLOPENTHIXOL ACETATE
Although we understand that zuclopenthixol acetate may be a choice when rather longer‐term sedation is required that would be expected with haloperidol (Jayakody 2012), we did expect to find more relevant trials. Whether to use haloperidol or zuclopenthixol acetate is a prevalent clinical dilemma in some countries. We found one small study (n = 70), the outcomes of which we had to grade as ‘very low’ (summary of findings Table 12).
This study reported few outcome with some expected results. For example, in this trial of a little longer duration, more people in the haloperidol group required more than three injections (Analysis 12.1) and there was the suggestion that, at seven days, haloperidol caused more tremor (Analysis 12.2).
This pioneering study is helpful in showing that fair testing could be undertaken for this important comparison. At present, however, best evidence from trials does not support or refute the choice one of these drugs over another in the emergency situation.
13. COMPARISON 13. HALOPERIDOL versus BENZODIAZEPINE: a. FLUNITRAZEPAM
We found one very small relevant study (n = 28) the outcomes of which were graded as being of very low quality (summary of findings Table 13).
Unsurprisingly with such a small study there were no differences for any outcomes. That even adverse effects were similar highlights how lacking in power this trial was to show real differences. Flunitrazepam may be effective and a real alternative, but this trial does not show this. It does, however, illustrate that trials with this comparison are possible.
14. COMPARISON 14. HALOPERIDOL versus BENZODIAZEPINE: b. LORAZEPAM
Although we found four relevant studies with more than the average number of total participants (n = 207), few outcomes contain data from more than 70 people. The use of haloperidol alone in preference to lorazepam or vice versa is based on such limited evidence of poor quality that this could be considered an embarrassment to a subspecialty that recommends coercive use of these interventions (NICE 2005; NICE 2015).
14.1 Tranquillisation or asleep
Evidence is limited and we had to grade these data as of ‘very poor’ quality (summary of findings Table 14). There was a suggestion that lorazepam is significantly more sedating after a few hours (Analysis 14.1). This may not be desirable as tranquillisation is frequently the aim without necessarily sedating.
14.2 Other global outcome
There were no differences in numbers requiring more than one and more than three injections and no difference between groups for the outcome of ‘showing no improvement’ at 30 and 60 minutes (Analysis 14.3). Mental state scores on 37 people were the same.
14.3 Adverse effects
These underpowered studies did not highlight many expected differences. However, significantly more participants experienced EPS in the haloperidol group compared with the lorazepam group (Analysis 14.11). Apnoea was not reported.
15. COMPARISON 15. HALOPERIDOL versus BENZODIAZEPINE: c. MIDAZOLAM
We found one small relevant study with data we could use (n = 84), but the limited outcomes we could use for the summary of findings Table 15 we had to grade as being of low quality.
15.1 Tranquillisation or asleep: average time
Midazolam is a highly effective hypnotic (Olkkola 2008) and the skewed data on tranquillisation did seem to support that it was significantly faster than haloperidol in this clinical situation.
15.2 Adverse effects
Midazolam is known to cause apnoea but in this trial it was a person in the haloperidol group that experienced this (Analysis 15.3). Nevertheless, data from this small study should not be considered conclusive. Other data from better trials (TREC CG; TREC 2003) and other sources suggest that use of midazolam could be accompanied with full resource for control of airways and intravenous reversal of the apneic effects with flumazenil (Olkkola 2008).
16. COMPARISON 16. HALOPERIDOL versus COMBINATIONS: a. HALOPERIDOL + LEVOMEPROMAZINE
We found one very small study (n = 19), the outcomes of which we had to grade as being of very low quality (summary of findings Table 16).
Clinicians find themselves in an unusual position because of the prevalence of haloperidol and its use for psychosis‐induced aggression but in the recognition of its toxicity. We think it understandable that researchers investigate whether combinations could offset some adverse effects and levomepromazine may do this, but the included trial is too small to illustrate anything except that such a study is possible.
17. COMPARISON 17. HALOPERIDOL versus COMBINATIONS: b. HALOPERIDOL + LORAZEPAM
We were rather surprised to find that there are only two trials of this comparison. We think that this combination is in common use but the two trials have a total of only just over 100 people and we had to grade outcomes all of very low quality (summary of findings Table 17).
17.1 Tranquillisation or asleep
More people in the combination group were asleep at three hours than those in the haloperidol alone group but these were limited very low‐grade data. Also, three hours is a very long time when a person is acutely disturbed and not an outcome of great clinical utility in regard to the focus of this review.
17.2 Repeated need for rapid tranquillisation
There was some suggestion that the addition of lorazepam did assist the tranquillisation process but no data were conclusive and all were of very poor grade. Significantly fewer people in the combination group exhibited “no overall improvement by 30 minutes”.
17.3 Adverse effects
Overall, there was no difference between the groups. Best evidence suggests that addition of lorazepam does not offset adverse effects. In these small poorly reported and probably poorly conducted trials dystonia was common and could be considered to be at unacceptable levels. People undergoing coercive rapid tranquillisation have enough difficult experiences without the addition of needless distressing adverse effects. We have no data that this will cause further mistrust of healthcare professionals, but it does seem a reasonable assumption.
18. COMPARISON 18. HALOPERIDOL versus COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM
We found one relevant study (n = 60) with outcomes of very low grade only (summary of findings Table 18).
18.1 Repeated need for tranquillisation, specific behaviours, global outcomes
An overall advantage in favour of haloperidol was shown in terms of agitation and aggression rating scales, and need for seclusion or restraint (Analysis 18.1; Analysis 18.2; Analysis 18.3; Analysis 18.4; Analysis 18.5; Analysis 18.6; Analysis 18.7).
18.2 Adverse effects
Haloperidol and combination data resulted in similar but, again, data from this small study should not be considered conclusive.
19. HALOPERIDOL versus COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE
We found two relevant study (n = 376) and outcomes were graded of moderate quality (higher than others in this review) or low quality (summary of findings Table 19).
19.1 Tranquillisation or asleep, repeated need for tranquillisation
Haloperidol used on its own clearly is quickly tranquillising, but the combination with promethazine makes is significantly more swift. The difference between being tranquil at 20 and 40 minutes can be of great importance in the clinical front line (Analysis 19.1; Analysis 19.2).
There were no significant differences in terms of requiring additional drugs for tranquillisation (Analysis 19.3).
19.2 Specific behaviours ‐ agitation, aggression
When using agitation and aggression rating scales, an initial advantage for haloperidol was demonstrated (Analysis 19.4; Analysis 19.7); however, this should be replicated by other studies as it appears to come from one trial only of low‐moderate quality (Baldacara 2011).
On other measures of aggression there was not much difference (requiring repeat rapid tranquillisation, exhibiting further aggressive outburst within 24 hours. More people in the haloperidol alone group had to be restrained by 120 minutes, but the difference between groups was not statistically significant. However, significantly more people in the haloperidol alone group required extra visits from the doctor, which is an indication of the clinical concern of the healthcare professionals. Such visits can be for further management of aggression or disturbance, or to address adverse effects.
19.3 Adverse effects
Haloperidol is a potent cause of adverse effects. Significantly more people in the haloperidol alone group than the combination group experienced one or more adverse effects. Acute dystonia – a most distressing effect – was common (n = 316, RR 19.48, 95% CI 1.14 to 331.92, Analysis 19.20), and frequency of occurrence resulted in this trial being terminated prematurely. Use of promethazine offsets the dystonia. The report specifically states that the Steering Group of this trial felt that the guidance from APA in the USA and NICE in the UK was unethical to continue.
20. COMPARISON 20. HALOPERIDOL versus COMBINATIONS: e. HALOPERIDOL + RISPERIDONE
We found one study (n = 100) with outcomes of low‐grade quality (summary of findings Table 20).
20.1 Specific behaviour, global outcomes
An initial advantage ‐ at 24 hours ‐ for the combination group was demonstrated (Analysis 20.1), even though it was not confirmed by subsequent time points (Analysis 20.2); further studies should be carried out, in particularly focused on the first hours in order to better understand the effect of the combination versus haloperidol.
More people allocated to the haloperidol group showed improvement when compared to the haloperidol + risperidone group, even if this difference was not significant (Analysis 20.3).
20.2 Adverse effects
The two compared groups showed comparable results (Analysis 20.4, Analysis 20.5, Analysis 20.6).
21. COMPARISON 21. HALOPERIDOL versus COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE
We found one small study (n = 64) with outcomes of very low‐grade quality (summary of findings Table 21).
21.1 Specific behaviour ‐ agitation
People allocated in the combination group showed a lower level of agitation, rated using the PANSS‐EC, at seven and 14 days; unfortunately, the first time point available in this trial was at 72 hours and showed no differences between the compared groups (Analysis 21.1). These timings are not adequate in order to fulfil the aim of this study, and even more highlight the need for further studies focused on the early effects.
21.2 Global outcomes, mental state
No significant differences were found as an equal number of people showed no improvement in both groups (Analysis 21.2); the PANSS total scores showed a trend over the weeks in advantage for the combination group, even if not significant (Analysis 21.3).
21.3 Adverse effects
The number of people experiencing one or more adverse effects was almost three times in the haloperidol group (Analysis 21.4).
22. COMPARISON 22. HALOPERIDOL versus COMBINATIONS: g. QUETIAPINE + MAGNESIUM VALPROATE
The one study that compared haloperidol with a combination of quetiapine + magnesium valproate was small and outcomes were graded as very low quality (n = 60, summary of findings Table 22).
Although this one trial was probably underpowered, there did not seem to be a suggestion that the quetiapine‐magnesium valproate combination had any affect over and above that seen with haloperidol alone.
Global effect had to be indirectly inferred from no effect on the PANSS‐EC, and further aggressive behaviour had to be surmised from scale‐derived data for agitation at 72 hours. Risk of bias was rated as serious because the method of randomisation was not reported, allocation concealment was not stated and it was an open‐label trial. There was no measure for economic outcomes. Any use of this quetiapine‐valproate combination must be considered experimental.
23. COMPARISON 23. HALOPERIDOL versus COMBINATIONS: h. RISPERIDONE + CLONAZEPAM
We found two studies (n = 305) and outcomes were graded as of low and very low grade (summary of findings Table 23).
23.1 Tranquillisation or asleep, specific behaviour
There were no differences when comparing the number of people not asleep in the first four hours (Analysis 23.1) and the PANSS‐EC rating scores (Analysis 23.2; Analysis 23.3; Analysis 23.4; Analysis 23.5).
23.2 Global outcomes, mental state
More people allocated to the haloperidol group experienced an improvement across the explored time points, even though the difference was not significant (Analysis 23.6). At five days when looking at the PANSS total scores, the two groups showed similar results (Analysis 23.7).
23.3 Adverse effects
More adverse events were recorded in the haloperidol group (Analysis 23.8), with a significant predominance of movement disorders‐related adverse effects (Analysis 23.11).
24. COMPARISON 24. HALOPERIDOL versus COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM
We found one study (n = 76) and outcomes were graded as of very low quality (summary of findings Table 24).
There did not seem to be a suggestion of superiority of ziprasidone + clonazepam than haloperidol alone; no significant differences were found between the compared groups.
Overall completeness and applicability of evidence
1. Completeness
Despite the great number of comparisons in this review, there are no data on several important questions. For example, although guidelines recommend use of haloperidol intravenously in exceptional circumstances, not one randomised controlled trial was found that investigated the efficacy and safety of haloperidol via this route of administration. For other routes of administration, even where some data do exist, evidence is graded as being of largely poor quality. For example, there is no strong evidence of the clear advantage of olanzapine or risperidone over the older haloperidol. The evidence for perphenazine is not good ether, but it could be seem as an inexpensive option to haloperidol. In addition, not one of the studies evaluated satisfaction with care, acceptance of treatment, quality of life or economic costs. Unfortunately, a large proportion of the efficacy and safety data produced by the trials was left redundant due to poor and/or selective reporting. This is clearly an area of evaluation in which researchers have invested heavily and this leaves those compiling guidance having to use judgements that are based less on good evidence than they should be.
2. Applicability
Many of the included trials were more of the explanatory type of study rather than practical/pragmatic and easily applicable to real‐world practice (Thorpe 2009). Few seem to have been designed with clear clinical needs as a priority. This does not mean that applicability is impossible. It just necessitates a level of conjecture that could have been lessened should more pragmatic trials have been available. Two examples of how trials are near or far from real‐world practice and needs are outlined below.
2.1 Participants seen in real‐world practice
Many of these trials seem to have been undertaken in circumstances very far from real clinical practice. For example, the UK's NICE guidelines recommend that rapid tranquillisation should only be considered "once de‐escalation and other strategies have failed to calm the service user" (NICE 2005, p14) and that “p.r.n. medication can be used as part of a de‐escalation strategy but p.r.n. medication used alone is not de‐escalation.” (NICE 2015, p15). However, in a number of studies, participants (in some cases their legal representatives) were required to provide written informed consent (Battaglia 2002; Breier 2002; Bristol Myers 2004a; Bristol‐Myers 2005b; Brook 2000; Eli Lilly 2004; Kinon 2001d; Currier 2004; Higashima 2004; Lim 2010), Further, participants in many studies all had to undergo a screening period ranging from two to 96 hours (Battaglia 2002; Breier 2002; Bristol Myers 2004a; Bristol‐Myers 2005b; Brook 2000; Currier 2004; Eli Lilly 2004; Fruensgaard 1977; Kinon 2001d; Lim 2010, Shu 2010). Participants in these studies have intervention delayed for screening and are deemed competent to give informed consent ‐ a situation that is not common in the heat of an emergency and makes applicability problematic ‐ a matter of conjecture.
Other studies, however, recognised that conduct of the study had to involve deferred or waived consent ‐ and in this way making it more like clinical practice. In Battaglia 1997, Huf 2007, Nobay 2004 and Walther 2014, informed consent was obtained wherever possible. It was waived in Bailine 1987, Dorevitch 1999 and Foster 1997 because it was acknowledged that if people were that violent or psychologically disorganised to require rapid tranquillisation they would not be likely to be able to give informed consent. In Tuason 1986, informed consent was obtained as soon as participants condition permitted and in Resnick 1984 it was deemed that participants were unable to give informed consent because they were involuntary patients. This last group of trials is likely to be involving people closer to real‐world circumstances.
2.2 Outcomes of value in real‐world practice
Where people are a risk to themselves or others, rapid tranquillisation is often used to prevent the prolonged physical restraint of people, which can result in injury and death. It was therefore surprising that few studies measured tranquillisation, asleep, or global functioning outcomes at time points that one would associate with a rapid intervention. There were only three studies that measured tranquil or asleep on or around 30 minutes (Huf 2007; Currier 2004; Fruensgaard 1977), with a further two measuring at 60 minutes (Kinon 2001d; Salzman 1991), with global improvement being measured at 30 minutes in the Garza‐Trevino 1989 and at 60 minutes in the Kewala 1984 study. Also, not all studies documented simple and important outcomes such as repeated need for rapid tranquillisation. We are therefore left inferring the clinical implications of outcomes that are not recorded in routine care. This is an entirely avoidable situation if those designing trials listened to the needs of clinicians and people who are subject to this coercive care.
Quality of the evidence
1. General
Twenty‐nine of the 41 included studies were published after the first CONSORT guidance (1996). Few studies complied with this internationally agreed standard. Disappointingly, a large proportion of data was rendered unusable, which could have been avoided had the trials been better reported.
2. Specific
Although this review includes 41 studies and approximately 3500 participants, there were 24 different comparisons. Trials were largely very small and meta‐analysis could be undertaken for a minority of comparisons and, even then, for only some outcomes. Overall, the majority of the evidence had to be graded as 'very low', usually because of considerable ‐ and often avoidable ‐ limits in research design, selective reporting and imprecision.
The best available evidence came from the Huf 2007 trial which compared haloperidol with haloperidol plus promethazine, from the Calver 2015 trial which compared haloperidol with droperidol, and from the Nobay 2004 trial that compared haloperidol with midazolam. Nevertheless, these studies also had limitations to their research design which led to the evidence being graded as 'moderate'.
Potential biases in the review process
There are a number of ways in which bias could have been introduced into this review.
i. Double‐data extraction
In some systematic reviews, two people independently inspect all citations obtained from the search and extracted the data to minimise bias. However, this was not the case with this review, with only 20% of the screening of citations and only 10% of the data extraction being performed by two review authors.
ii. Potential conflict of interest
Another source of potential bias is that one of the authors of this review (CEA) was an investigator in the Huf 2007 trial.
Agreements and disagreements with other studies or reviews
The review authors are not aware of any other studies or reviews that have focused solely on use of haloperidol alone for people who are aggressive secondary to serious mental illnesses. Pratt 2008 conducted a systematic review (not meta‐analysis), focusing on the practice of rapid tranquillisation in the UK and concluded that there was a lack of high‐quality evidence.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 1 Tranquillisation or asleep ‐ asleep.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 2 Repeated need for tranquillisation.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 3 Specific behaviour: 1a. Agitation ‐ various measures.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 4 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 2 hours.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 5 Specific behaviour: 1c. Agitation ‐ average scores ‐ ii. up to 24 hours.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 6 Global outcome: 1. Not improved.
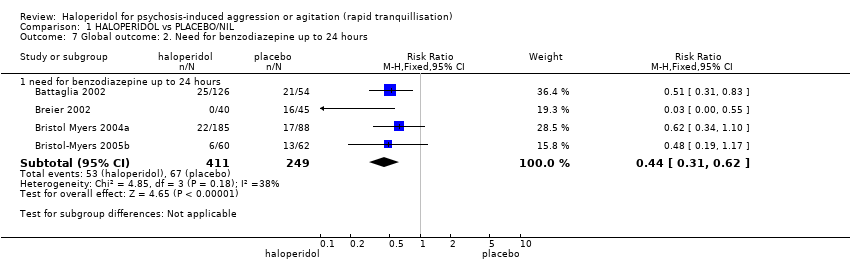
Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 7 Global outcome: 2. Need for benzodiazepine up to 24 hours.
Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 8 Global outcome: 3a. Average scores ‐ i. up to 2 hours.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 9 Global outcome: 3b. Average scores ‐ ii. up to 24 hours.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 10 Mental state: 1a. Average scores ‐ i. up to 2 hours.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 11 Mental state: 1b. Average scores ‐ ii. up to 24 hours.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 12 Adverse effects: 1a. General.
Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 13 Adverse effects: 1b. General ‐ serious.
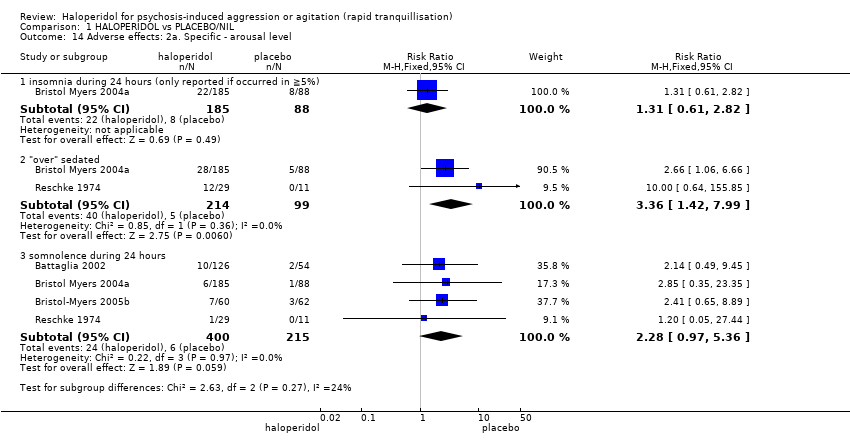
Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 14 Adverse effects: 2a. Specific ‐ arousal level.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 15 Adverse effects: 2b. Specific ‐ cardiovascular ‐ miscellaneous outcomes.
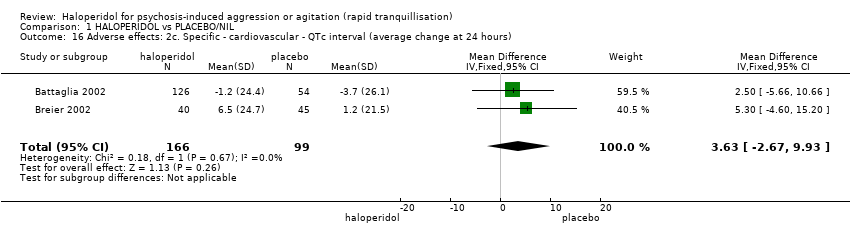
Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 16 Adverse effects: 2c. Specific ‐ cardiovascular ‐ QTc interval (average change at 24 hours).

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 17 Adverse effects: 2d. Specific ‐ movement disorders.
Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 18 Adverse effects: 2e. Specific ‐ movement disorders: i. average scores ‐ i. up to 24 hours.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 19 Adverse effects: 2f. Specific ‐ miscellaneous.

Comparison 1 HALOPERIDOL vs PLACEBO/NIL, Outcome 20 Leaving the study early.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 1 Repeated need for rapid tranquillisation: needing additional injection.
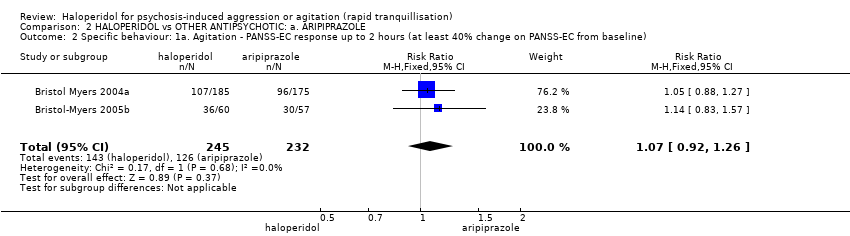
Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 2 Specific behaviour: 1a. Agitation ‐ PANSS‐EC response up to 2 hours (at least 40% change on PANSS‐EC from baseline).

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ up to 2 hours.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 4 Global outcome: 1. Need for benzodiazepine.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 5 Global outcome: 2. Average scores ‐ up to 2 hours.
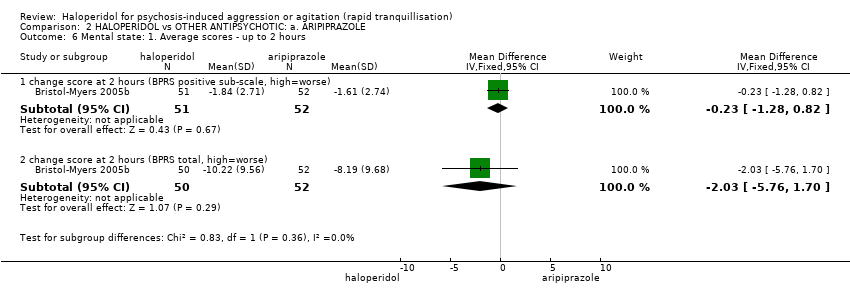
Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 6 Mental state: 1. Average scores ‐ up to 2 hours.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 7 Adverse effects: 1a. General.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 8 Adverse effects: 1b. General ‐ serious.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 9 Adverse effects: 2a. Specific ‐ arousal level.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 10 Adverse effects: 2b. Specific ‐ cardiac.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 11 Adverse effects: 2c. Specific ‐ gastrointestinal.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 12 Adverse effects: 2d. Specific ‐ movement disorder.

Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 13 Adverse effects: 2e. Specific ‐ miscellaneous.
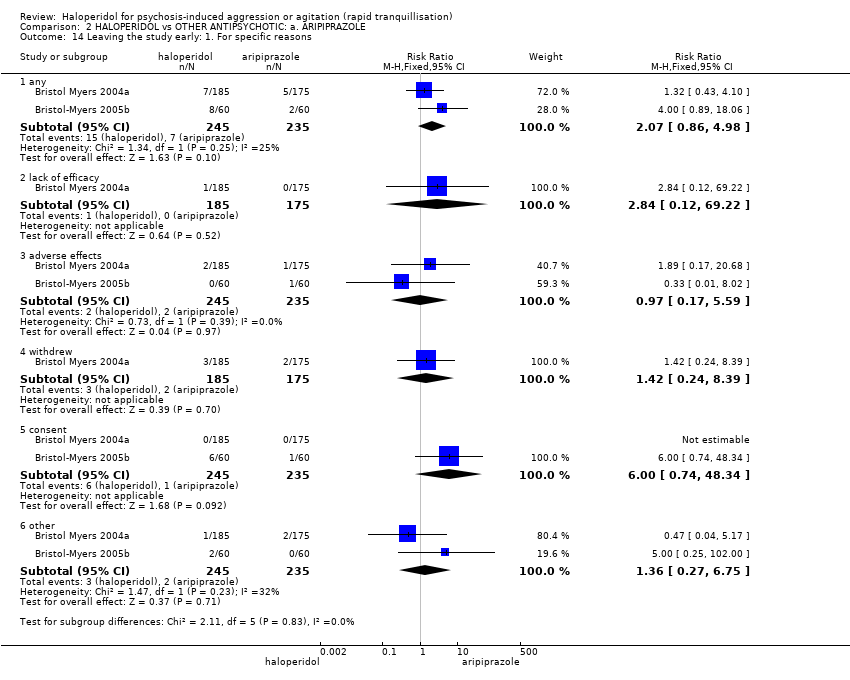
Comparison 2 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: a. ARIPIPRAZOLE, Outcome 14 Leaving the study early: 1. For specific reasons.

Comparison 3 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE, Outcome 1 Tranquillisation or asleep ‐ asleep.
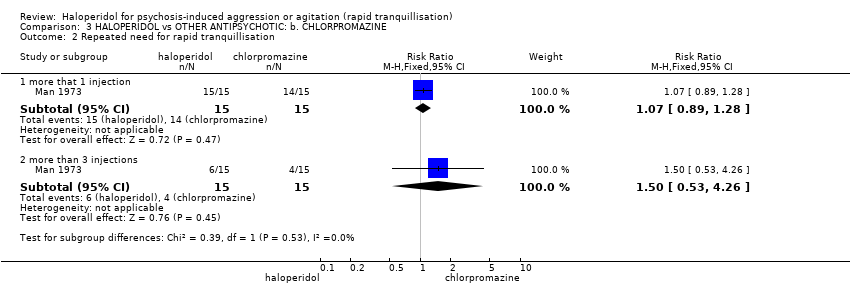
Comparison 3 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE, Outcome 2 Repeated need for rapid tranquillisation.

Comparison 3 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE, Outcome 3 Global outcome: 1. Not improved.
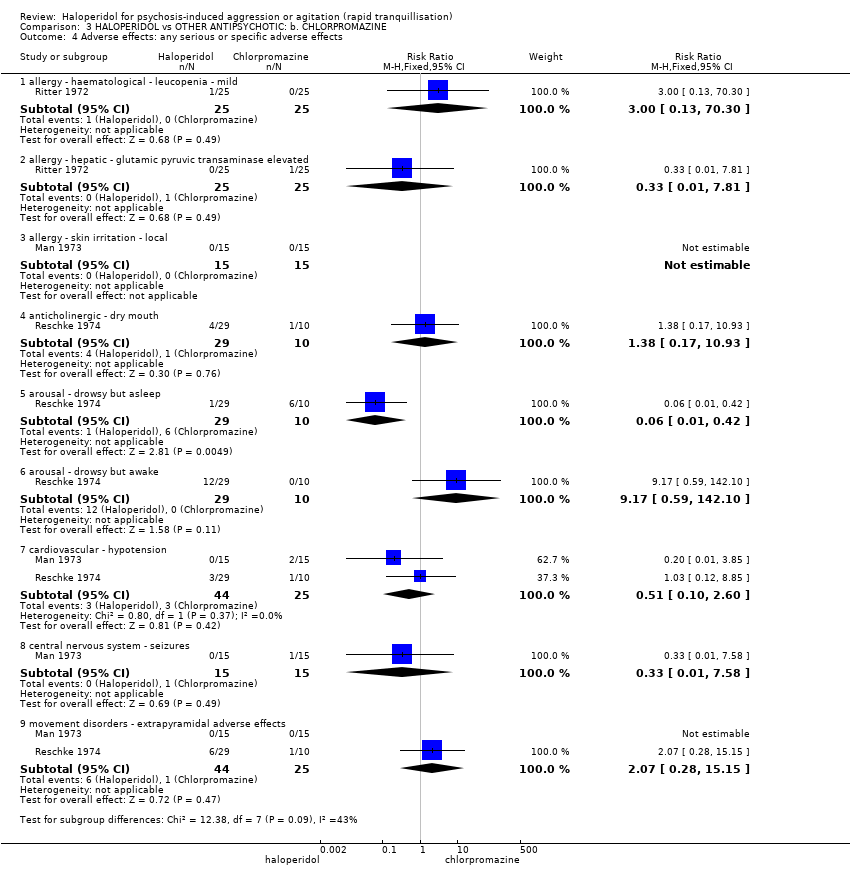
Comparison 3 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE, Outcome 4 Adverse effects: any serious or specific adverse effects.

Comparison 3 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE, Outcome 5 Leaving the study early.
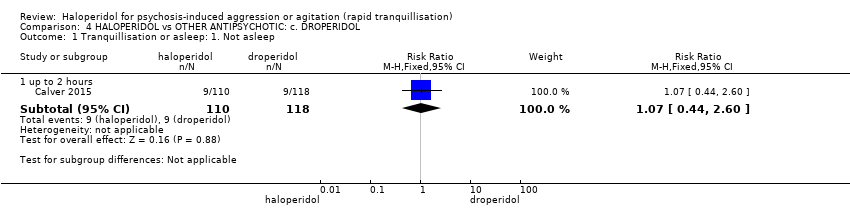
Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 1 Tranquillisation or asleep: 1. Not asleep.

Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 2 Tranquillisation or asleep: 2. Time to sleep.

Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 3 Repeated need for rapid tranquillisation.
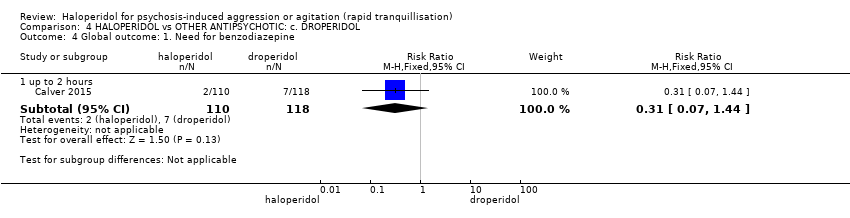
Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 4 Global outcome: 1. Need for benzodiazepine.

Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 5 Adverse effects: 1. General.
Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 6 Adverse effects: 2a. Specific ‐ arousal level.

Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 7 Adverse effects: 2b. Specific ‐ cardiovascular.
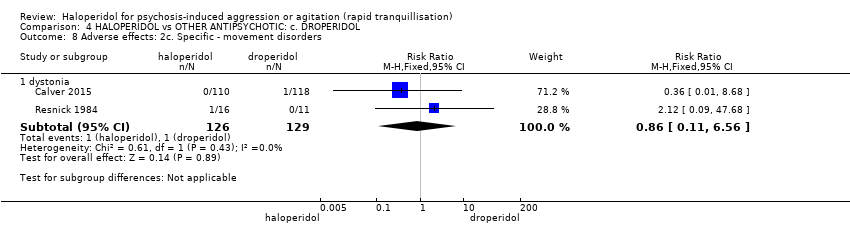
Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 8 Adverse effects: 2c. Specific ‐ movement disorders.
Comparison 4 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: c. DROPERIDOL, Outcome 9 Adverse effects: 2d. Specific ‐ miscellaneous.

Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 1 Tranquillisation or asleep ‐ not asleep up to 24 hours.
Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 2 Specific behaviour: 2. Aggression ‐ various measures.

Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 3 Global outcome: 1. General ‐ no change ‐ i. over 24 hours.

Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 4 Global outcome: 2a. Specific ‐ not sedated ‐ i. by 30 minutes.
Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 5 Global outcome: 2b. Specific ‐ not sedated ‐ ii. up to 2 hours.

Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 6 Mental state: 1. Average scores.
Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 7 Adverse effects: 1. General.
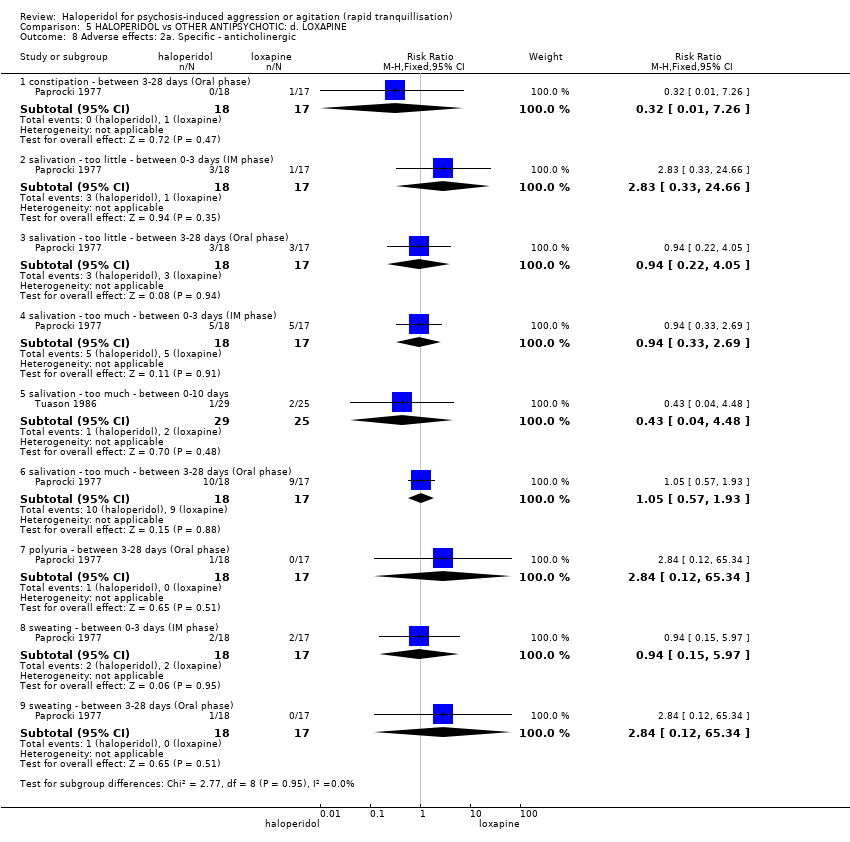
Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 8 Adverse effects: 2a. Specific ‐ anticholinergic.

Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 9 Adverse effects: 2b. Specific ‐ arousal level.

Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 10 Adverse effects: 2c. Specific ‐ cardiovascular.

Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 11 Adverse effects: 2d. Specific ‐ movement disorders.

Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 12 Adverse effects: 2e. Specific ‐ miscellaneous.

Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 13 Leaving the study early: 1. For general reasons.

Comparison 5 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: d. LOXAPINE, Outcome 14 Leaving the study early: 2. Specific reasons.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 1 Tranquillisation or asleep ‐ asleep.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 2 Repeated need for tranquillisation ‐ needing additional injection.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 3 Specific behaviour: 1a. Agitation ‐ various measures.
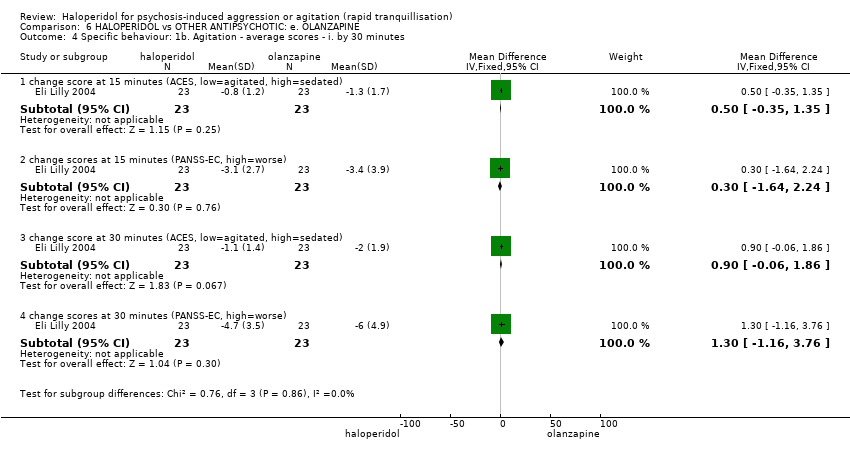
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 4 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. by 30 minutes.
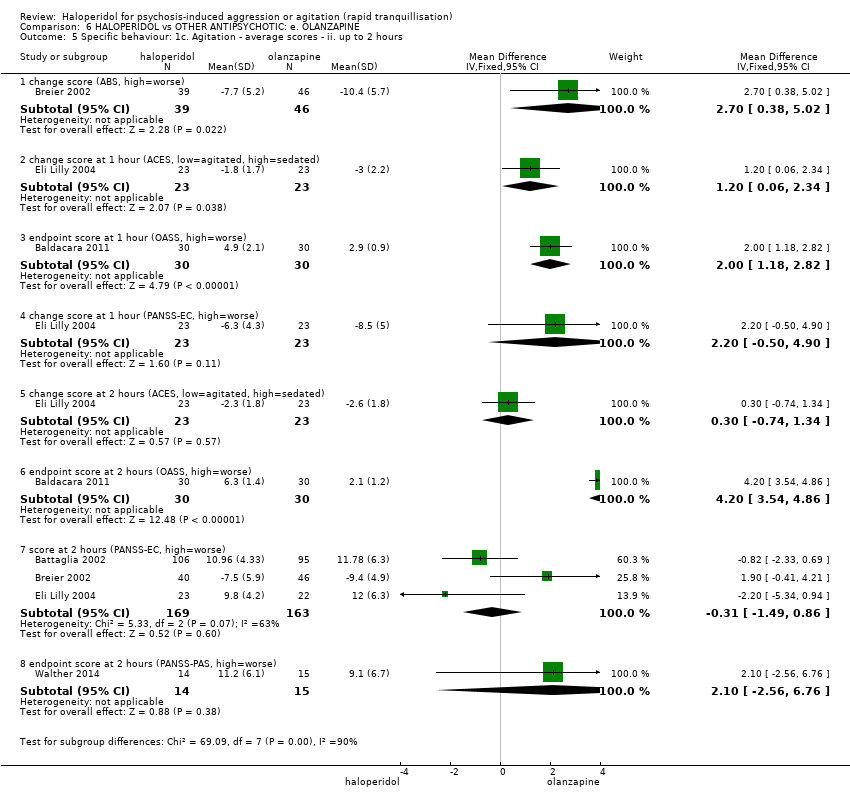
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 5 Specific behaviour: 1c. Agitation ‐ average scores ‐ ii. up to 2 hours.
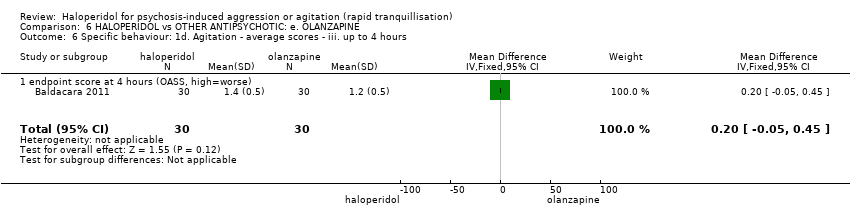
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 6 Specific behaviour: 1d. Agitation ‐ average scores ‐ iii. up to 4 hours.
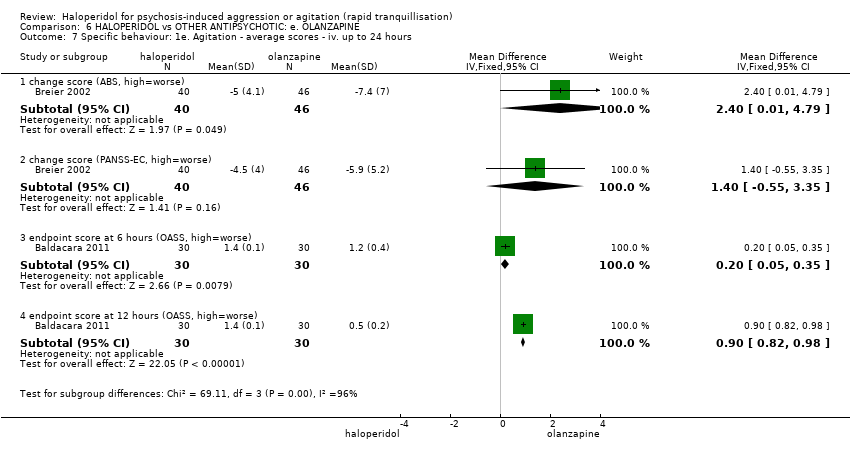
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 7 Specific behaviour: 1e. Agitation ‐ average scores ‐ iv. up to 24 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 8 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours.
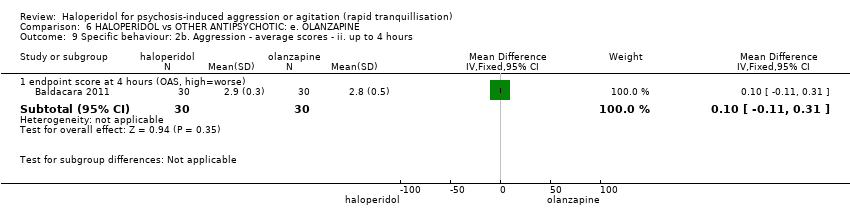
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 9 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours.
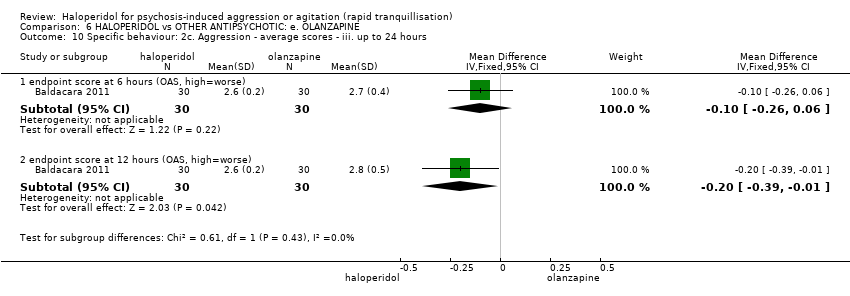
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 10 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 11 Specific behaviour: 3. Hostility.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 12 Global outcome: 1a. General ‐ need for additional measures.
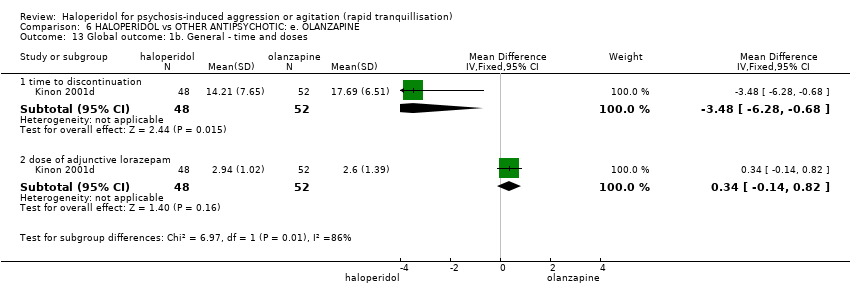
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 13 Global outcome: 1b. General ‐ time and doses.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 14 Global outcome: 1c. General ‐ average scores ‐ up to 2 hours.
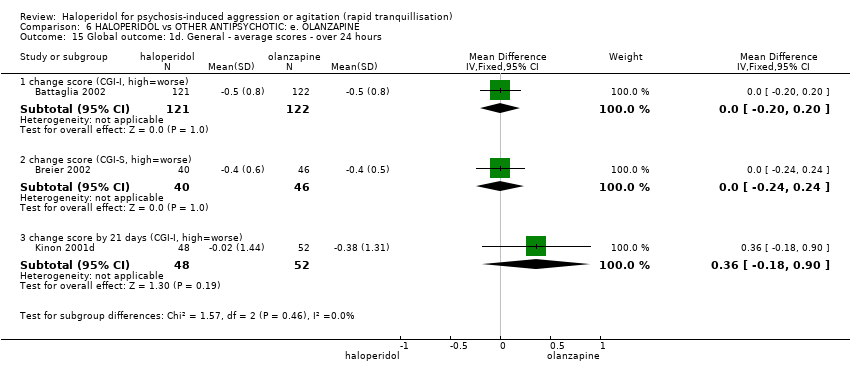
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 15 Global outcome: 1d. General ‐ average scores ‐ over 24 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 16 Global outcome: 2a. Specific ‐ alert ‐ i. up to 2 hours.
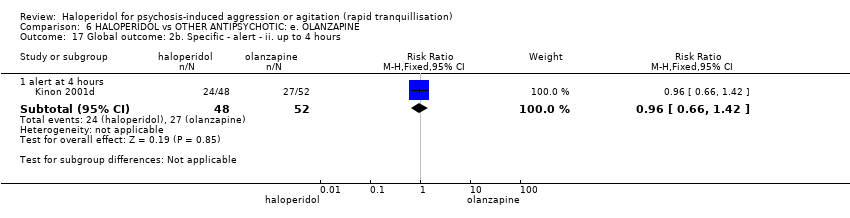
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 17 Global outcome: 2b. Specific ‐ alert ‐ ii. up to 4 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 18 Global outcome: 2c. Specific ‐ alert ‐ iii. up to 24 hours.
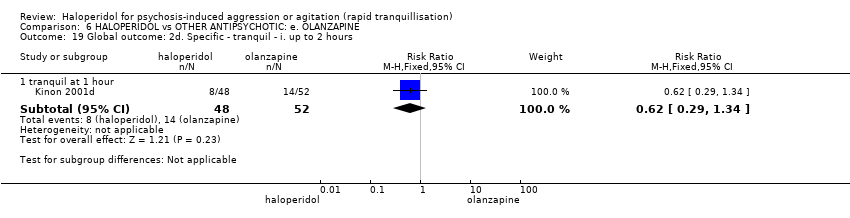
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 19 Global outcome: 2d. Specific ‐ tranquil ‐ i. up to 2 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 20 Global outcome: 2e. Specific ‐ tranquil ‐ ii. up to 4 hours.
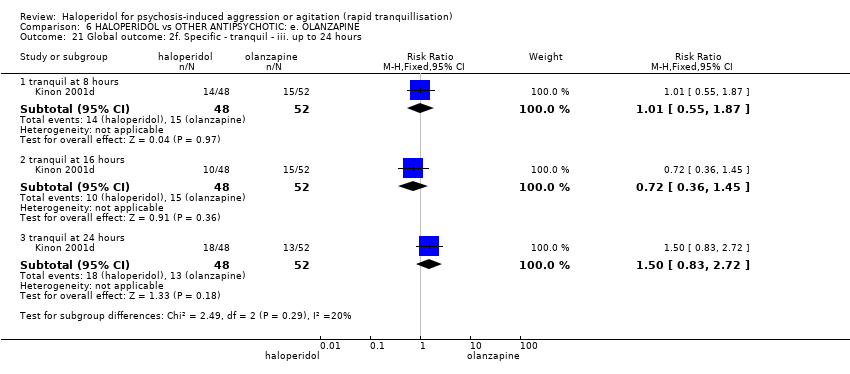
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 21 Global outcome: 2f. Specific ‐ tranquil ‐ iii. up to 24 hours.
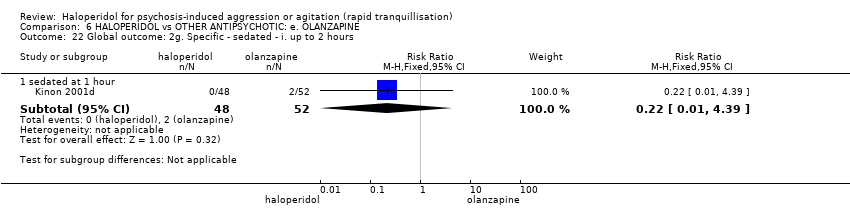
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 22 Global outcome: 2g. Specific ‐ sedated ‐ i. up to 2 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 23 Global outcome: 2h. Specific ‐ sedated ‐ ii. up to 4 hours.
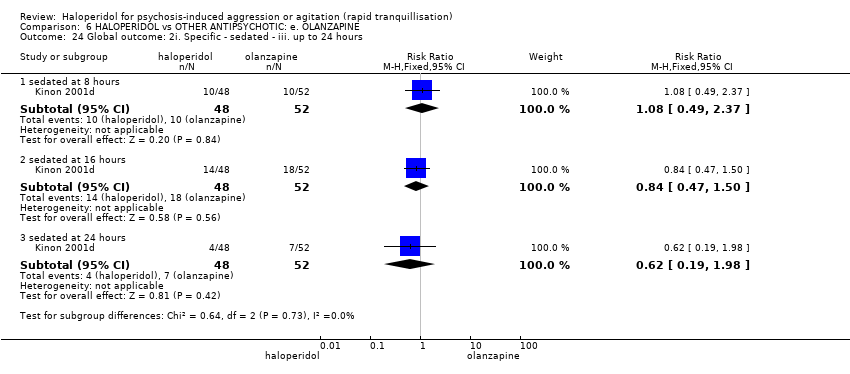
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 24 Global outcome: 2i. Specific ‐ sedated ‐ iii. up to 24 hours.
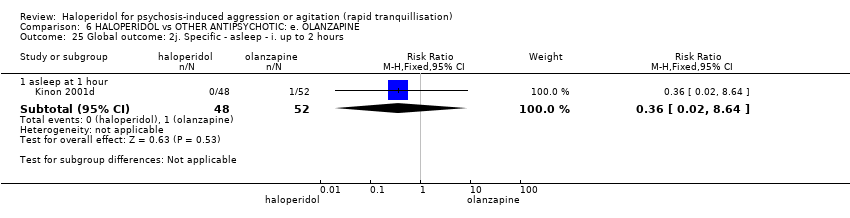
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 25 Global outcome: 2j. Specific ‐ asleep ‐ i. up to 2 hours.
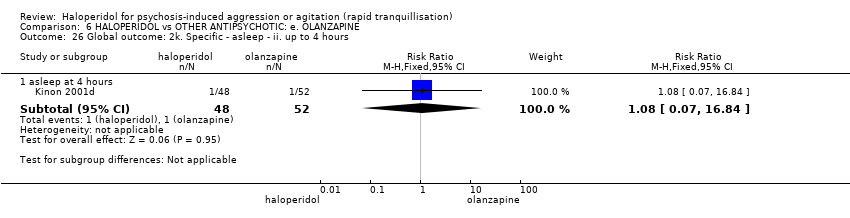
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 26 Global outcome: 2k. Specific ‐ asleep ‐ ii. up to 4 hours.
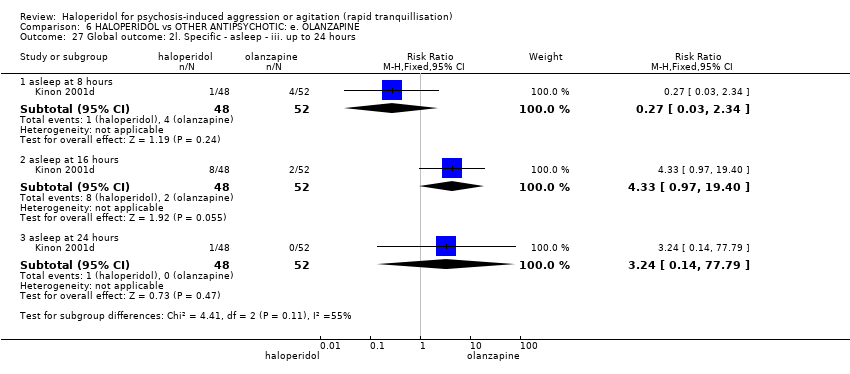
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 27 Global outcome: 2l. Specific ‐ asleep ‐ iii. up to 24 hours.
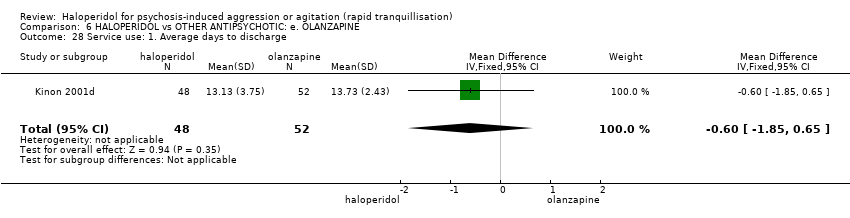
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 28 Service use: 1. Average days to discharge.
| Study | Intervention | Mean | SD | N |
| days 1‐7 | ||||
| Kinon 2001d | Haloperidol | 2.59 | 6.79 | 48 |
| Kinon 2001d | Olanzapine | 1.57 | 5.52 | 52 |
| days 8‐14 | ||||
| Kinon 2001d | Haloperidol | 0.92 | 4.05 | 48 |
| Kinon 2001d | Olanzapine | 0.33 | 2.23 | 52 |
| days 15‐21 | ||||
| Kinon 2001d | Haloperidol | 0.55 | 2.74 | 48 |
| Kinon 2001d | Olanzapine | 0 | 0 | 52 |
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 29 Service use: 2. Average patient‐hours used during 24 hours (skewed data).
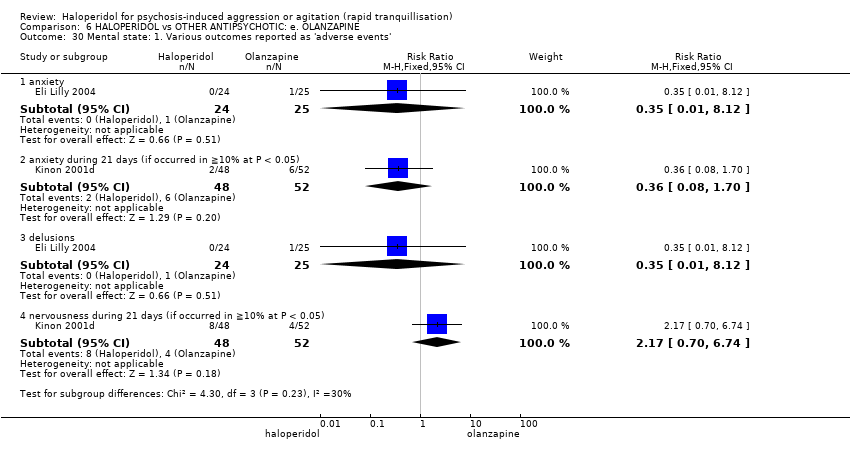
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 30 Mental state: 1. Various outcomes reported as 'adverse events'.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 31 Mental state: 2a. Average scores ‐ i. by 30 minutes.
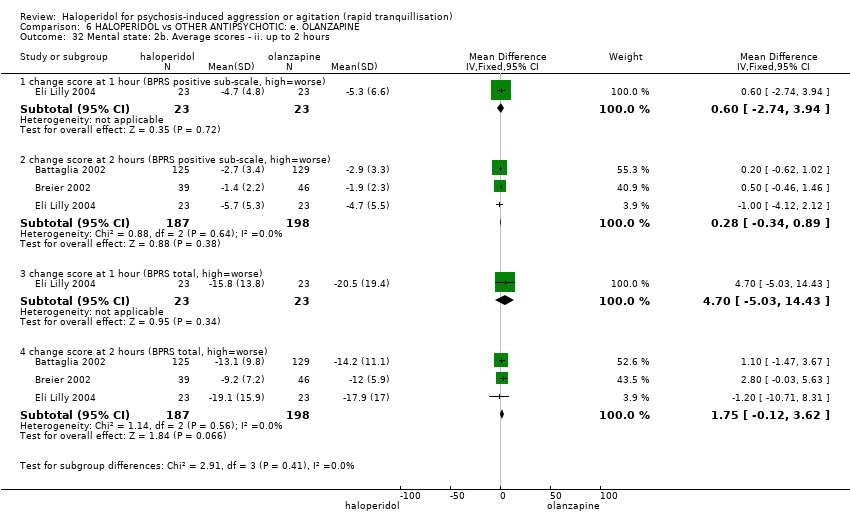
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 32 Mental state: 2b. Average scores ‐ ii. up to 2 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 33 Mental state: 2c. Average scores ‐ iii. up to 24 hours.
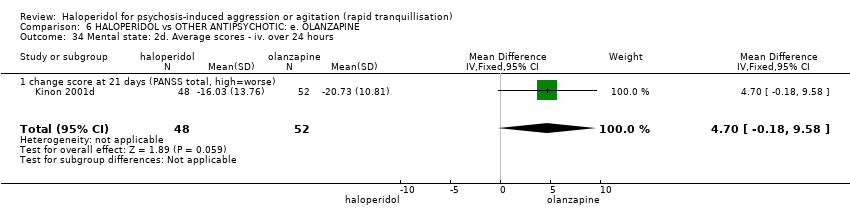
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 34 Mental state: 2d. Average scores ‐ iv. over 24 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 35 Adverse effects: 1a. General.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 36 Adverse effects: 1b. General ‐ serious.
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 37 Adverse effects: 2a. Specific ‐ anticholinergic.
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 38 Adverse effects: 2b. Specific ‐ arousal level ‐ i. various.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 39 Adverse effects: 2c. Specific ‐ arousal level ‐ ii. average scores ‐ i. up to 2 hours.
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 40 Adverse effects: 2d. Specific ‐ arousal level ‐ ii. average scores ‐ ii. up to 4 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 41 Adverse effects: 2e. Specific ‐ arousal level ‐ ii. average scores ‐ iii. up to 24 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 42 Adverse effects: 2f. Specific ‐ cardiovascular i. binary.
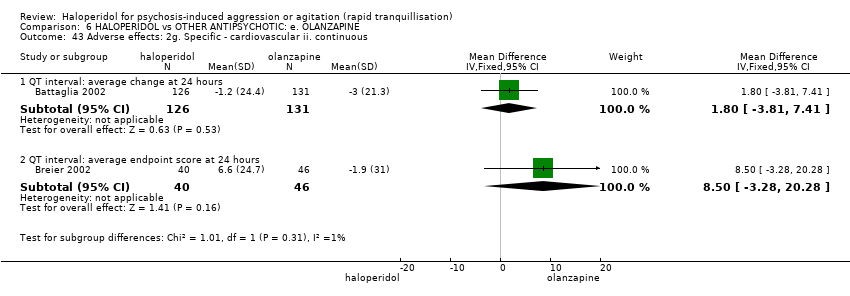
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 43 Adverse effects: 2g. Specific ‐ cardiovascular ii. continuous.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 44 Adverse effects: 2h. Specific ‐ gastrointestinal.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 45 Adverse effects: 2i. Specific ‐ movement disorder ‐ i. various.
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 46 Adverse effects: 2j. Specific ‐ movement disorder ‐ ii. average scores ‐ i. up to 24 hours.
| Study | Intervention | Mean | SD | N | Notes |
| endpoint score at 24 hours (SAS, high=worse) ‐ (skewed data) | |||||
| Eli Lilly 2004 | Haloperidol | 1.4 | 2.8 | 22 | |
| Eli Lilly 2004 | Olanzapine | 2.2 | 3.5 | 20 | |
| endpoint score at 24 hours (BAS, high=worse) ‐ (skewed data) | |||||
| Eli Lilly 2004 | Haloperidol | 1.5 | 1.7 | 22 | |
| Eli Lilly 2004 | Olanzapine | 1.7 | 2.6 | 20 | |
Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 47 Adverse effects: 2k. Specific ‐ movement disorder ‐ iii. average scores ‐ i. up to 24 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 48 Adverse effects: 2l. Specific ‐ movement disorder ‐ vi. average scores ‐ i. over 24 hours.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 49 Adverse effects: 2m. Specific ‐ miscellaneous.

Comparison 6 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: e. OLANZAPINE, Outcome 50 Leaving the study early.
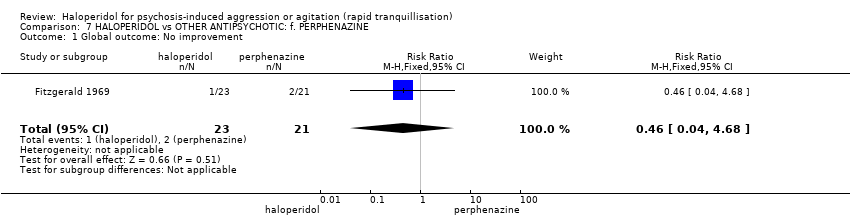
Comparison 7 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: f. PERPHENAZINE, Outcome 1 Global outcome: No improvement.

Comparison 7 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: f. PERPHENAZINE, Outcome 2 Adverse effects: 1. General.
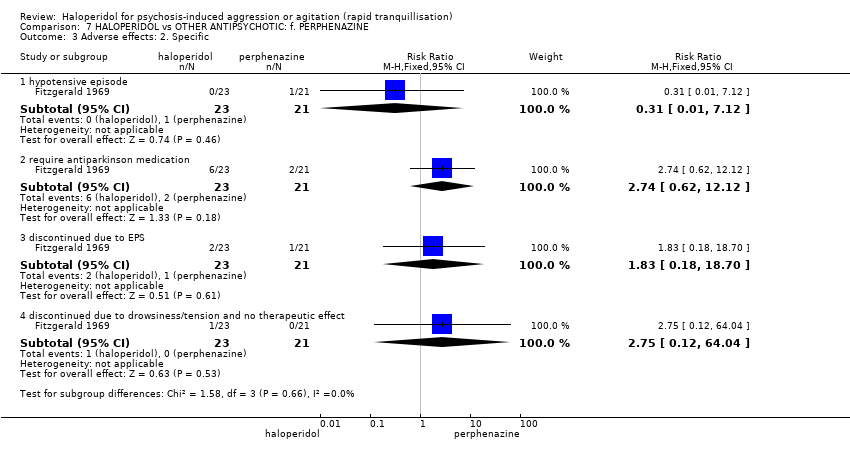
Comparison 7 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: f. PERPHENAZINE, Outcome 3 Adverse effects: 2. Specific.

Comparison 7 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: f. PERPHENAZINE, Outcome 4 Leaving the study early: 1. Specific reasons.
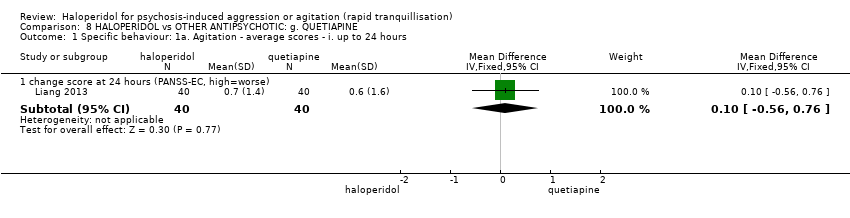
Comparison 8 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: g. QUETIAPINE, Outcome 1 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 24 hours.

Comparison 8 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: g. QUETIAPINE, Outcome 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. over 24 hours.

Comparison 8 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: g. QUETIAPINE, Outcome 3 Mental state: Average scores ‐ i. over 24 hours.
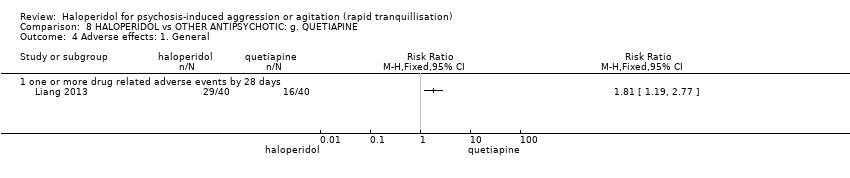
Comparison 8 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: g. QUETIAPINE, Outcome 4 Adverse effects: 1. General.
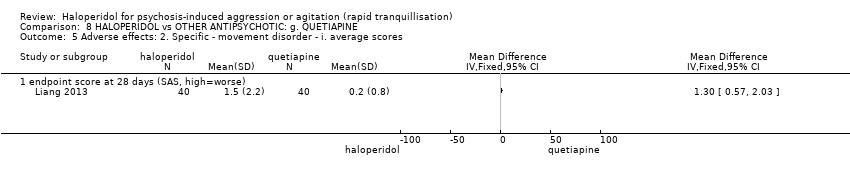
Comparison 8 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: g. QUETIAPINE, Outcome 5 Adverse effects: 2. Specific ‐ movement disorder ‐ i. average scores.

Comparison 8 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: g. QUETIAPINE, Outcome 6 Leaving the study early.
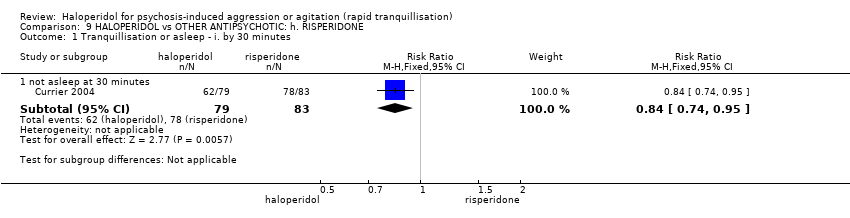
Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 1 Tranquillisation or asleep ‐ i. by 30 minutes.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 2 Tranquillisation or asleep ‐ ii. up to 2 hours.
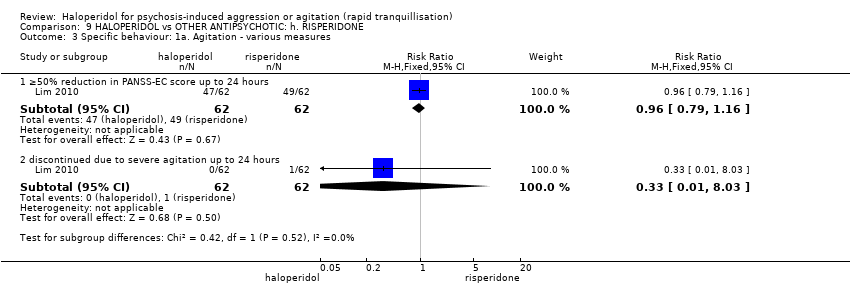
Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 3 Specific behaviour: 1a. Agitation ‐ various measures.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 4 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 2 hours.
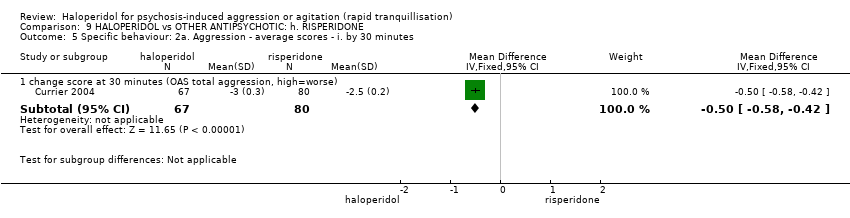
Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 5 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. by 30 minutes.
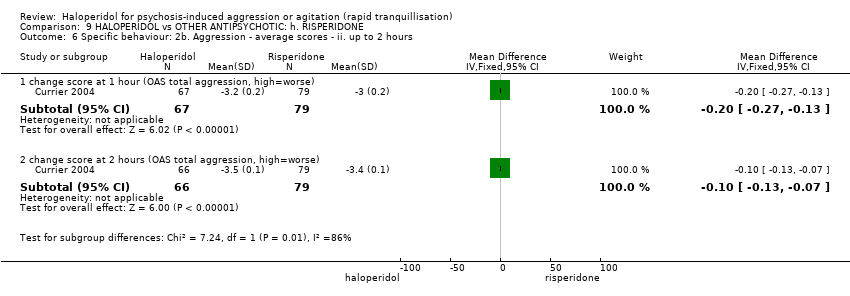
Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 6 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 2 hours.
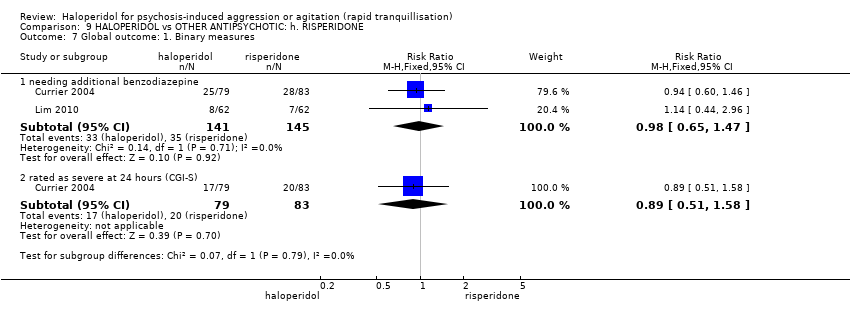
Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 7 Global outcome: 1. Binary measures.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 8 Global outcome: 2. Continuous measures.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 9 Adverse effects: 1a. General ‐ one or more adverse effects.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 10 Adverse effects: 2a. Specific ‐ arousal.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 11 Adverse effects: 2b. Specific ‐ cardiovascular ‐ i. binary outcomes.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 12 Adverse effects: 2c. Specific ‐ cardiovascular ‐ ii. pulse rate ‐ i. up to 2 hours.
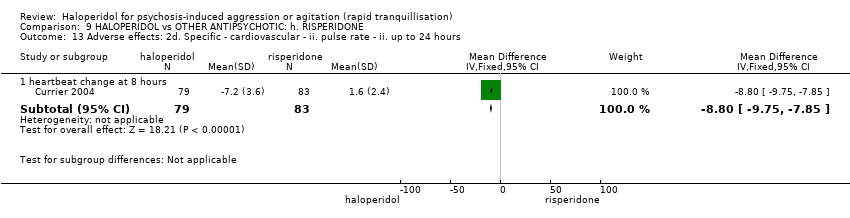
Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 13 Adverse effects: 2d. Specific ‐ cardiovascular ‐ ii. pulse rate ‐ ii. up to 24 hours.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 14 Adverse effects: 2e. Specific ‐ movement disorder ‐ i. binary outcomes.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 15 Adverse effects: 2f. Specific ‐ movement disorders ‐ ii. average scores ‐ i. up to 24 hours.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 16 Adverse effects: 2g. Specific ‐ movement disorder ‐ ii. average scores ‐ ii. over 24 hours.

Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 17 Adverse effects: 2h. Specific ‐ miscellaneous.
Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 18 Leaving the study early ‐ i. up to 24 hours.
Comparison 9 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: h. RISPERIDONE, Outcome 19 Leaving the study early ‐ ii. over 24 hours.

Comparison 10 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: i. THIOTHIXENE, Outcome 1 Repeated need for rapid tranquillisation.

Comparison 10 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: i. THIOTHIXENE, Outcome 2 Specific behaviour: 1. Agitation ‐ rated as 'adverse effect'.

Comparison 10 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: i. THIOTHIXENE, Outcome 3 Global outcome.
Comparison 10 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: i. THIOTHIXENE, Outcome 4 Adverse effects: 1. General ‐ one or more adverse effects.

Comparison 10 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: i. THIOTHIXENE, Outcome 5 Adverse effects: 2a. Specific ‐ movement disorders.

Comparison 10 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: i. THIOTHIXENE, Outcome 6 Adverse effects: 2b. Specific ‐ others.
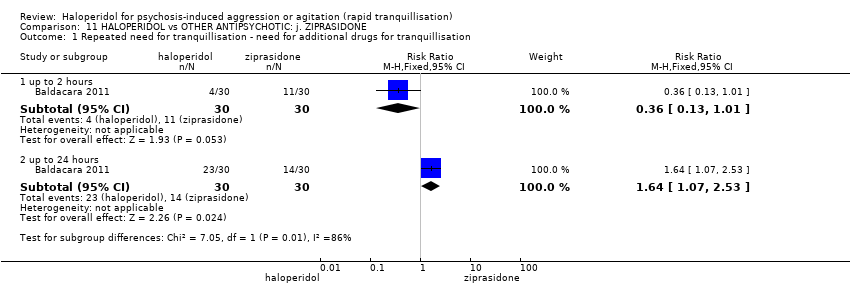
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 1 Repeated need for tranquillisation ‐ need for additional drugs for tranquillisation.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 2 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours.
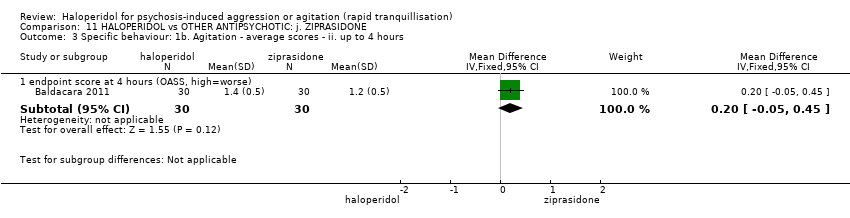
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. up to 4 hours.
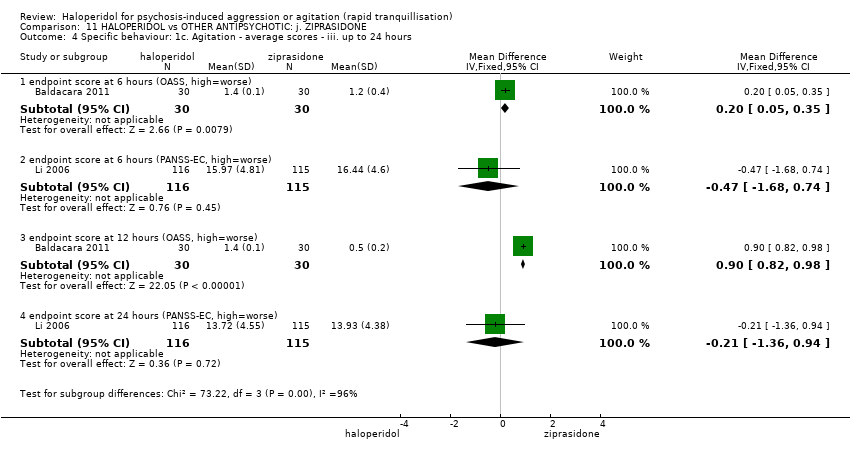
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 4 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 5 Specific behaviour: 1d. Agitation ‐ average scores ‐ iv. over 24 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 6 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 7 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours.
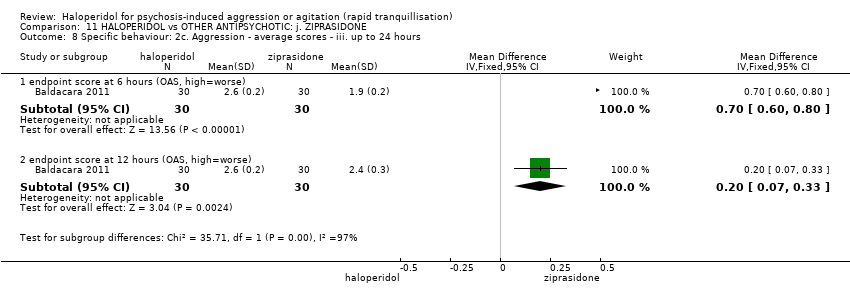
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 8 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours.
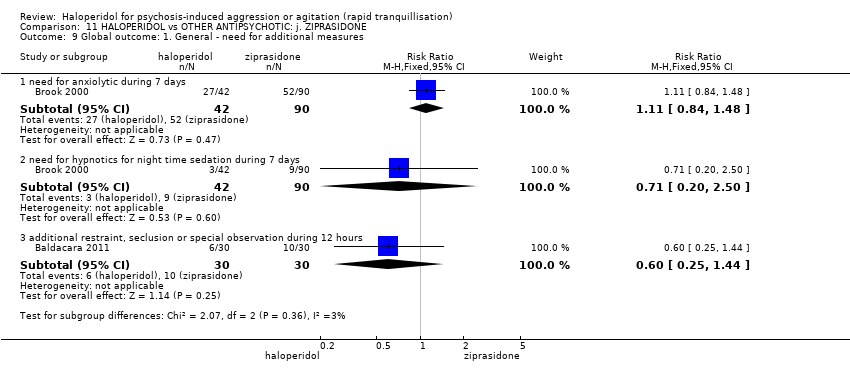
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 9 Global outcome: 1. General ‐ need for additional measures.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 10 Global outcome: 2. Average scores ‐ i. over 24 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 11 Mental state: 1a. Average scores ‐ i. up to 2 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 12 Mental state: 1b. Average scores ‐ ii. up to 4 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 13 Mental state: 1c. Average scores ‐ iii. up to 24 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 14 Mental state: 1d. Average scores ‐ iv. over 24 hours.
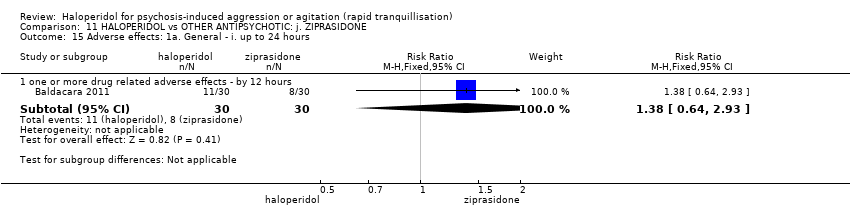
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 15 Adverse effects: 1a. General ‐ i. up to 24 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 16 Adverse effects: 1b. General ‐ ii. over 24 hours.
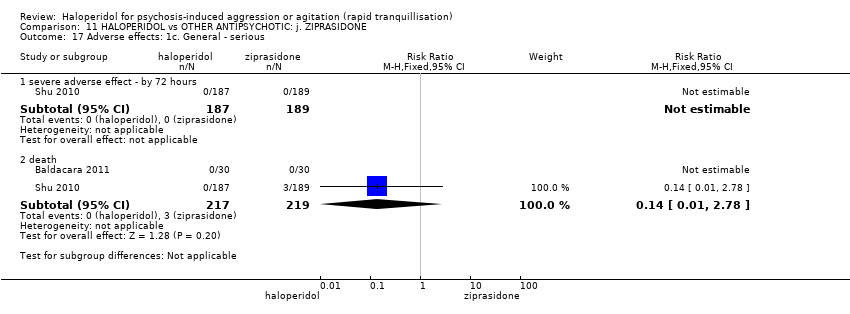
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 17 Adverse effects: 1c. General ‐ serious.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 18 Adverse effects: 2a. Specific ‐ anticholinergic.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 19 Adverse effects: 2b. Specific ‐ arousal level ‐ i. various.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 20 Adverse effects: 2c. Specific ‐ arousal level ‐ ii. average scores ‐ i. up to 2 hours.
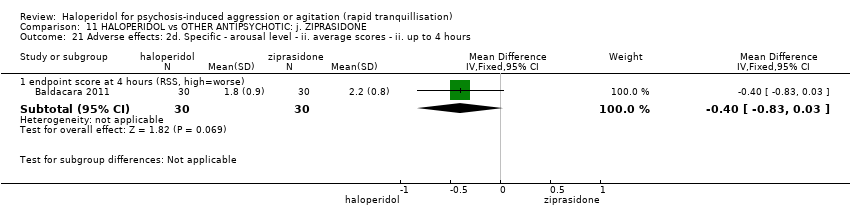
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 21 Adverse effects: 2d. Specific ‐ arousal level ‐ ii. average scores ‐ ii. up to 4 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 22 Adverse effects: 2e. Specific ‐ arousal level ‐ ii. average scores ‐ iii. up to 24 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 23 Adverse effects: 2f. Specific ‐ cardiovascular.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 24 Adverse effects: 2g. Specific ‐ gastrointestinal.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 25 Adverse effects: 2h. Specific ‐ hematological tests.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 26 Adverse effects: 2i. Specific ‐ movement disorders ‐ i. binary measures.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 27 Adverse effects: 2j. Specific ‐ movement disorders ‐ ii. average scores ‐ i. over 24 hours.
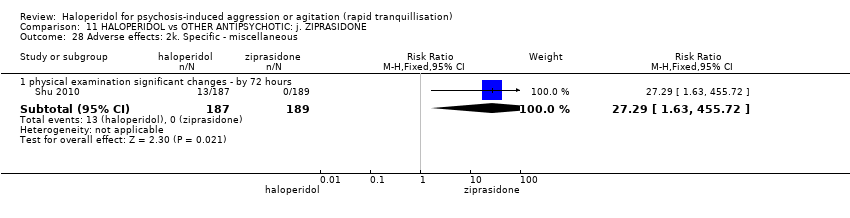
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 28 Adverse effects: 2k. Specific ‐ miscellaneous.
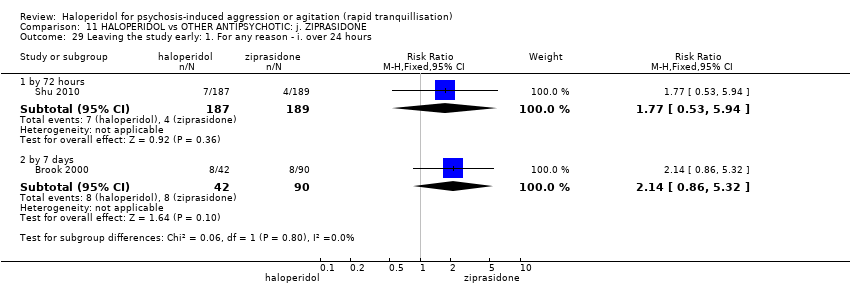
Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 29 Leaving the study early: 1. For any reason ‐ i. over 24 hours.

Comparison 11 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: j. ZIPRASIDONE, Outcome 30 Leaving the study early: 2. For specific reasons ‐ i. over 24 hours.

Comparison 12 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: k. ZUCLOPENTHIXOL ACETATE, Outcome 1 Repeated need for tranquillisation: more than 3 injections.

Comparison 12 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: k. ZUCLOPENTHIXOL ACETATE, Outcome 2 Adverse effects: 1a. Specific ‐ movement disorders.

Comparison 12 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: k. ZUCLOPENTHIXOL ACETATE, Outcome 3 Adverse effects: 1b. Specific ‐ miscellaneous.

Comparison 12 HALOPERIDOL vs OTHER ANTIPSYCHOTIC: k. ZUCLOPENTHIXOL ACETATE, Outcome 4 Leaving the study early.

Comparison 13 HALOPERIDOL vs BENZODIAZEPINE: a. FLUNITRAZEPAM, Outcome 1 Specific behaviour: 1. Aggression ‐ binary measures ‐ i. up to 2 hours.
Comparison 13 HALOPERIDOL vs BENZODIAZEPINE: a. FLUNITRAZEPAM, Outcome 2 Global outcome: 1. Need for seclusion or restraint.

Comparison 13 HALOPERIDOL vs BENZODIAZEPINE: a. FLUNITRAZEPAM, Outcome 3 Adverse effects: 1. Specific ‐ movement disorders ‐ i. binary measures ‐ i. by 30 minutes.

Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 1 Tranquillisation or asleep ‐ not asleep.

Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 2 Repeated need for rapid tranquillisation.
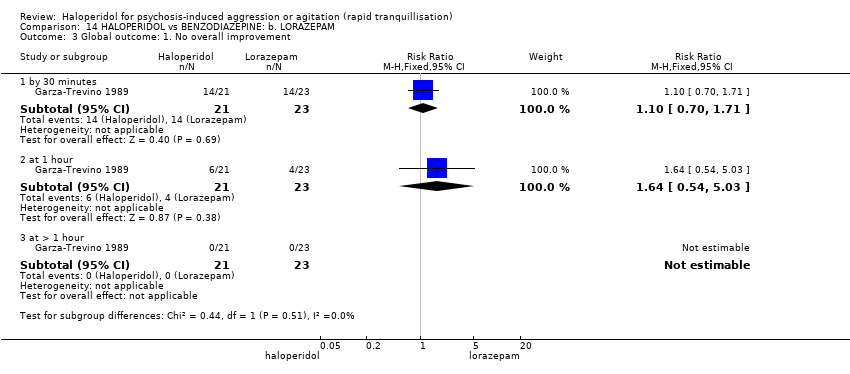
Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 3 Global outcome: 1. No overall improvement.

Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 4 Mental state: 1a. Average scores ‐ i. up to 2 hours.
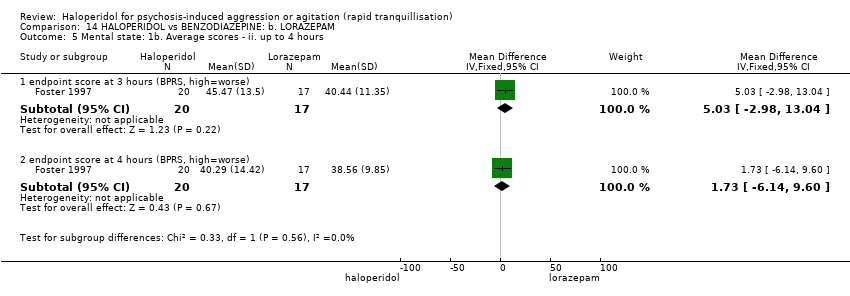
Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 5 Mental state: 1b. Average scores ‐ ii. up to 4 hours.

Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 6 Adverse effects: 1a. General.
Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 7 Adverse effects: 1b. General ‐ serious.

Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 8 Adverse effects: 2a. Specific ‐ anticholinergic.

Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 9 Adverse effects: 2b. Specific ‐ arousal.
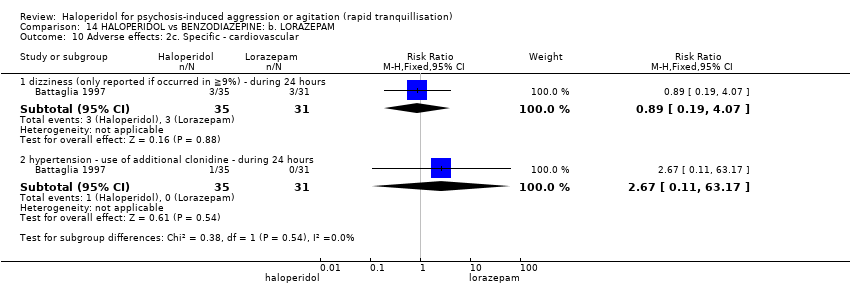
Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 10 Adverse effects: 2c. Specific ‐ cardiovascular.

Comparison 14 HALOPERIDOL vs BENZODIAZEPINE: b. LORAZEPAM, Outcome 11 Adverse effects: 2d. Specific ‐ movement disorder.
| Study | Intervention | Average | SD | N | Notes |
| average time to sedation | |||||
| Nobay 2004 | Haloperidol | 28.3 | 25 | 42 | |
| Nobay 2004 | Midazolam | 18.3 | 14 | 42 | |
| average time to arousal | |||||
| Nobay 2004 | Haloperidol | 126.6 | 85 | 42 | |
| Nobay 2004 | Midazolam | 81.9 | 66 | 42 | |
Comparison 15 HALOPERIDOL vs BENZODIAZEPINE: c. MIDAZOLAM, Outcome 1 Tranquillisation or asleep: average times in minutes ‐ (skewed data).

Comparison 15 HALOPERIDOL vs BENZODIAZEPINE: c. MIDAZOLAM, Outcome 2 Global outcome: 1. Need for rescue drug.

Comparison 15 HALOPERIDOL vs BENZODIAZEPINE: c. MIDAZOLAM, Outcome 3 Adverse effects: 1. General ‐ one or more drug related adverse effect.

Comparison 15 HALOPERIDOL vs BENZODIAZEPINE: c. MIDAZOLAM, Outcome 4 Adverse effects: 2. Specific.

Comparison 16 HALOPERIDOL vs COMBINATIONS: a. HALOPERIDOL + LEVOMEPROMAZINE, Outcome 1 Global outcome: 1. No overall improvement.

Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 1 Tranquillisation or asleep ‐ asleep.
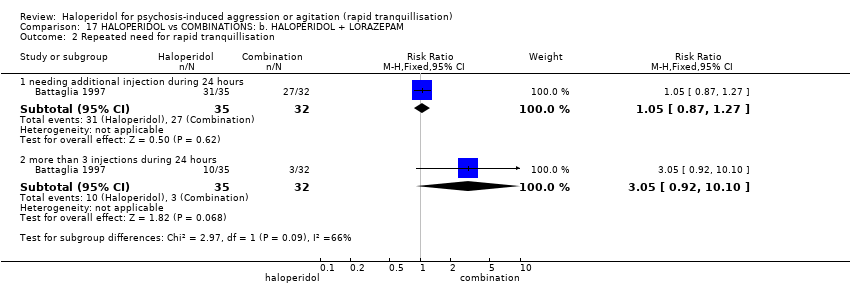
Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 2 Repeated need for rapid tranquillisation.

Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 3 Global outcome: 1. No overall improvement.

Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 4 Adverse effects: 1. General.

Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 5 Adverse effects: 2a. Specific ‐ anticholinergic.
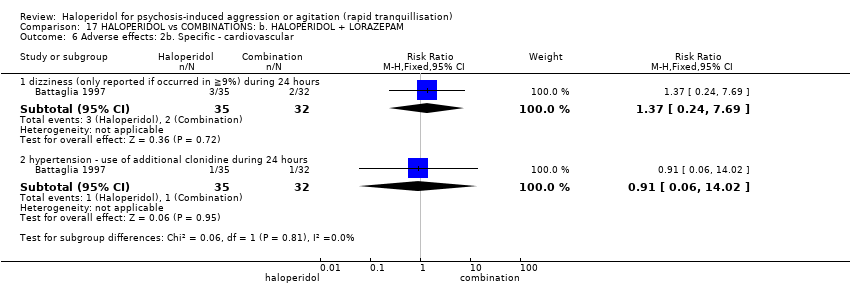
Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 6 Adverse effects: 2b. Specific ‐ cardiovascular.

Comparison 17 HALOPERIDOL vs COMBINATIONS: b. HALOPERIDOL + LORAZEPAM, Outcome 7 Adverse effects: 2c. Specific ‐ movement disorders.
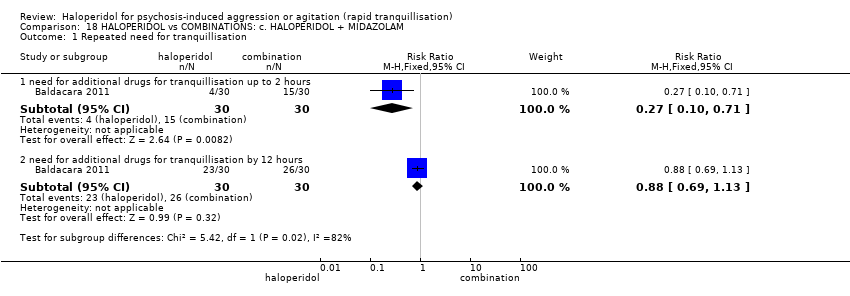
Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 1 Repeated need for tranquillisation.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 2 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours.
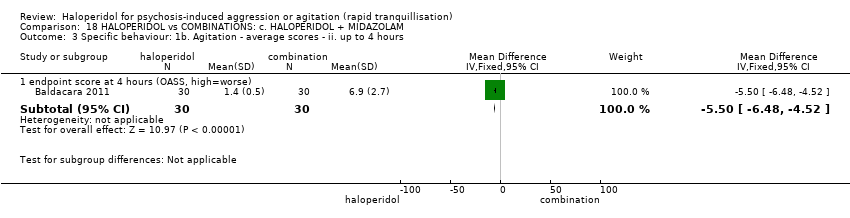
Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. up to 4 hours.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 4 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 5 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 6 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 7 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours.
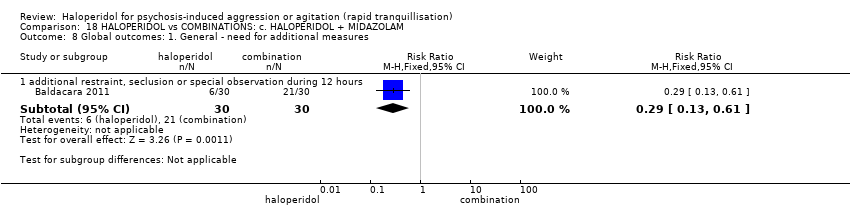
Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 8 Global outcomes: 1. General ‐ need for additional measures.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 9 Adverse effects: 1b. General ‐ one or more adverse events.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 10 Adverse effects: 1a. General ‐ serious.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 11 Adverse effects: 2a. Specific ‐ arousal ‐ i. various.
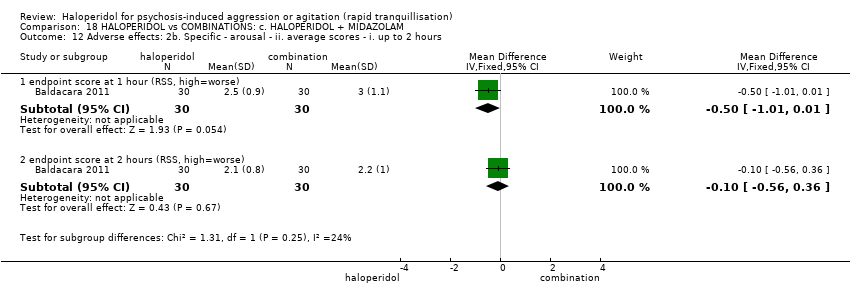
Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 12 Adverse effects: 2b. Specific ‐ arousal ‐ ii. average scores ‐ i. up to 2 hours.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 13 Adverse effects: 2c. Specific ‐ arousal ‐ ii. average scores ‐ ii. up to 4 hours.
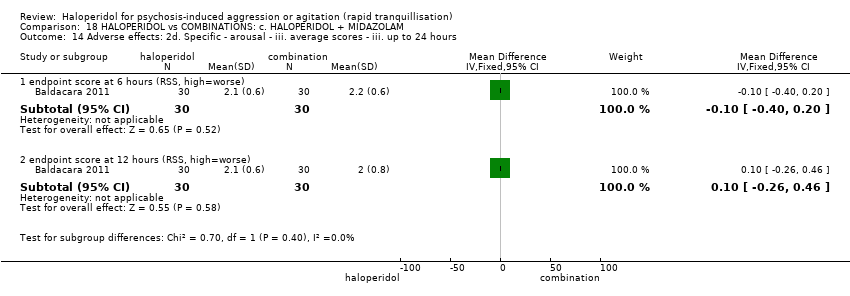
Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 14 Adverse effects: 2d. Specific ‐ arousal ‐ iii. average scores ‐ iii. up to 24 hours.
Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 15 Adverse effects: 2e. Specific ‐ cardiovascular.

Comparison 18 HALOPERIDOL vs COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM, Outcome 16 Adverse effects: 2f. Specific ‐ movement disorders.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 1 Tranquillisation or asleep ‐ not tranquil or asleep ‐ i. by 30 minutes.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 2 Tranquillisation or asleep ‐ not tranquil or asleep ‐ ii. up to 2 hours.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 3 Repeated need for tranquillisation ‐ need for additional drugs for tranquillisation.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 4 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 5 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. up to 4 hours.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 6 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours.
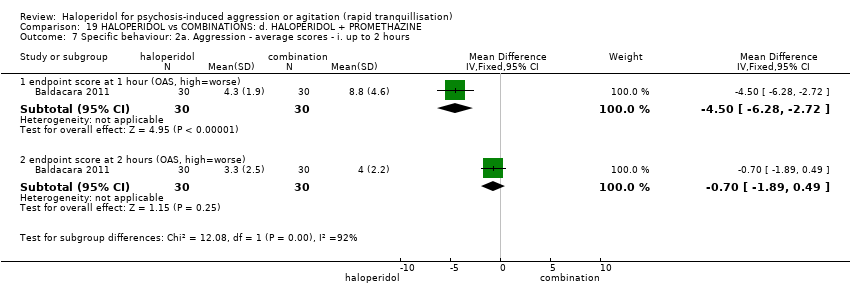
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 7 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 8 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 9 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours.
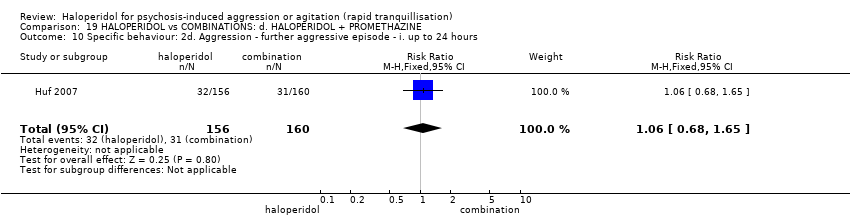
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 10 Specific behaviour: 2d. Aggression ‐ further aggressive episode ‐ i. up to 24 hours.
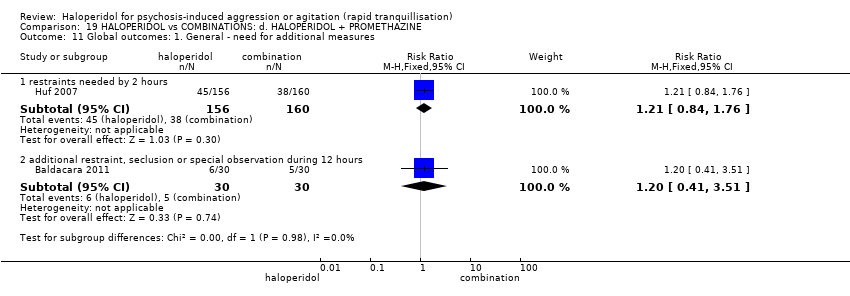
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 11 Global outcomes: 1. General ‐ need for additional measures.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 12 Global outcomes: 2. Specific.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 13 Adverse effects: 1a. General.
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 14 Adverse effects: 1b. General ‐ serious.
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 15 Adverse effects: 2a. Specific ‐ arousal ‐ i. various.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 16 Adverse effects: 2b. Specific ‐ arousal ‐ ii. average scores ‐ i. up to 2 hours.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 17 Adverse effects: 2c. Specific ‐ arousal ‐ ii. average scores ‐ ii. up to 4 hours.
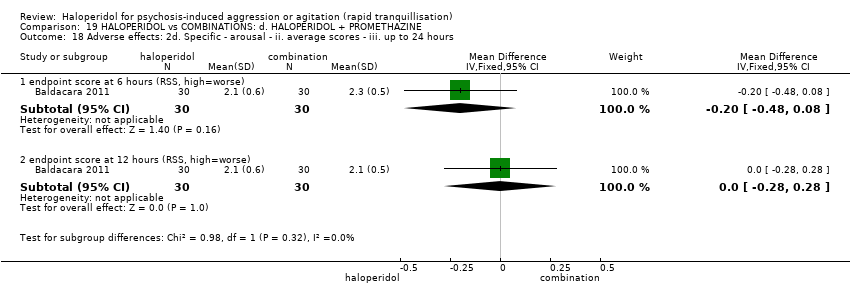
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 18 Adverse effects: 2d. Specific ‐ arousal ‐ ii. average scores ‐ iii. up to 24 hours.
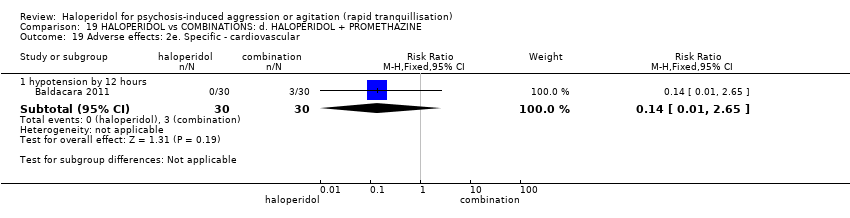
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 19 Adverse effects: 2e. Specific ‐ cardiovascular.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 20 Adverse effects: 2f. Specific ‐ movement disorders.

Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 21 Service outcomes: 1. Not discharged by 14 days.
Comparison 19 HALOPERIDOL vs COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE, Outcome 22 Leaving the study early.
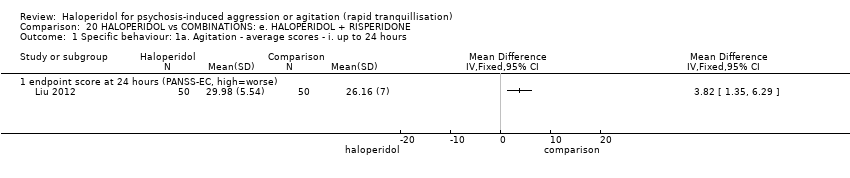
Comparison 20 HALOPERIDOL vs COMBINATIONS: e. HALOPERIDOL + RISPERIDONE, Outcome 1 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 24 hours.

Comparison 20 HALOPERIDOL vs COMBINATIONS: e. HALOPERIDOL + RISPERIDONE, Outcome 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. over 24 hours.

Comparison 20 HALOPERIDOL vs COMBINATIONS: e. HALOPERIDOL + RISPERIDONE, Outcome 3 Global outcome: No improvement.
Comparison 20 HALOPERIDOL vs COMBINATIONS: e. HALOPERIDOL + RISPERIDONE, Outcome 4 Adverse effects: 1a. Specific ‐ arousal level ‐ i. various.

Comparison 20 HALOPERIDOL vs COMBINATIONS: e. HALOPERIDOL + RISPERIDONE, Outcome 5 Adverse effects: 1b. Specific ‐ movement disorders ‐ i. various.

Comparison 20 HALOPERIDOL vs COMBINATIONS: e. HALOPERIDOL + RISPERIDONE, Outcome 6 Adverse effects: 1c. Specific ‐ miscellaneous.

Comparison 21 HALOPERIDOL vs COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE, Outcome 1 Specific behaviour: 1. Agitation ‐ average scores ‐ over 24 hours.

Comparison 21 HALOPERIDOL vs COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE, Outcome 2 Global outcome: No improvement.

Comparison 21 HALOPERIDOL vs COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE, Outcome 3 Mental state: Average scores ‐ i. over 24 hours.
Comparison 21 HALOPERIDOL vs COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE, Outcome 4 Adverse effects: General ‐ over 24 hours.

Comparison 22 HALOPERIDOL vs COMBINATIONS: g. QUETIAPINE + MAGNESIUM VALPROATE, Outcome 1 Specific behaviour: 1a. Agitation ‐ binary measures.

Comparison 22 HALOPERIDOL vs COMBINATIONS: g. QUETIAPINE + MAGNESIUM VALPROATE, Outcome 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. over 24 hours.
Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 1 Tranquillisation or asleep.

Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 2 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours.

Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 4 hours.
Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 4 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours.
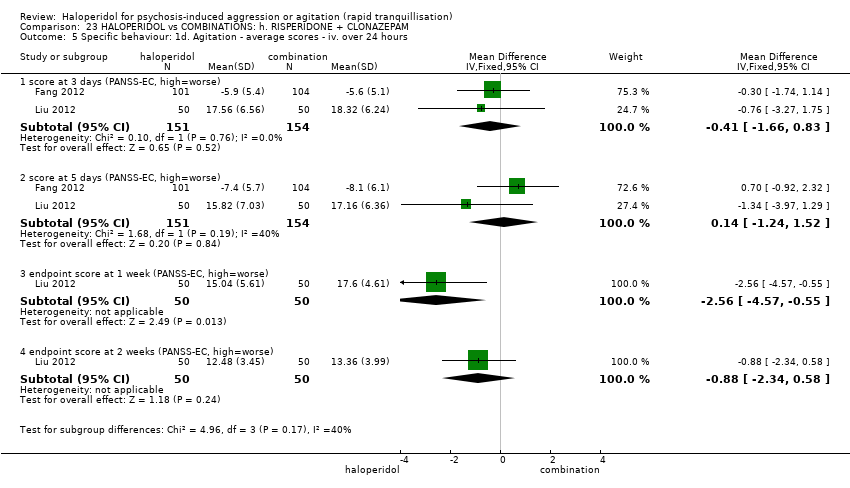
Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 5 Specific behaviour: 1d. Agitation ‐ average scores ‐ iv. over 24 hours.

Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 6 Global outcome: No improvement.
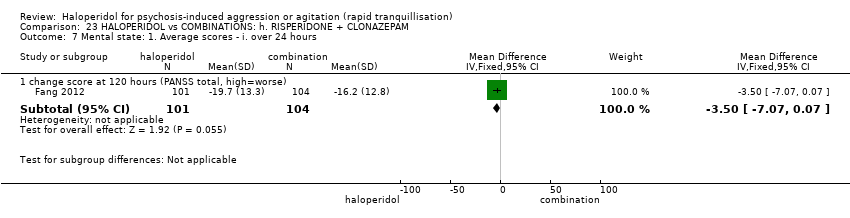
Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 7 Mental state: 1. Average scores ‐ i. over 24 hours.
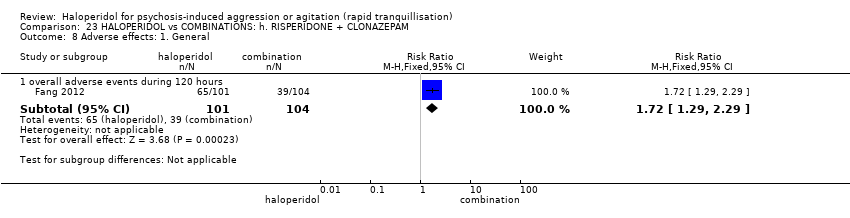
Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 8 Adverse effects: 1. General.

Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 9 Adverse effects: 2a. Specific ‐ arousal ‐ i. various.

Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 10 Adverse effects: 2b. Specific ‐ cardiovascular ‐ i. various.

Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 11 Adverse effects: 2c. Specific ‐ movement disorders ‐ i. various.
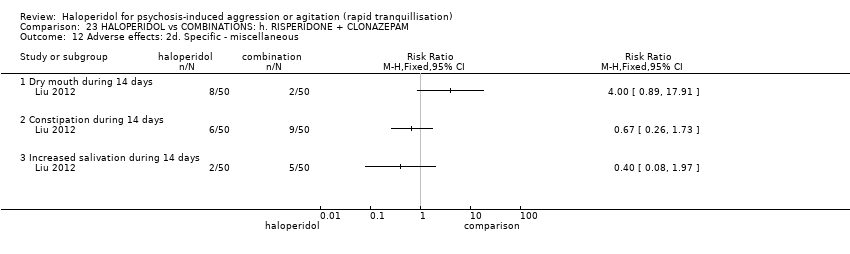
Comparison 23 HALOPERIDOL vs COMBINATIONS: h. RISPERIDONE + CLONAZEPAM, Outcome 12 Adverse effects: 2d. Specific ‐ miscellaneous.

Comparison 24 HALOPERIDOL vs COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM, Outcome 1 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 24 hours.

Comparison 24 HALOPERIDOL vs COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM, Outcome 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. over 24 hours.

Comparison 24 HALOPERIDOL vs COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM, Outcome 3 Global outcome: 1. Average scores ‐ i. over 24 hours.

Comparison 24 HALOPERIDOL vs COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM, Outcome 4 Mental state: 1. Average scores ‐ i. over 24 hours.

Comparison 24 HALOPERIDOL vs COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM, Outcome 5 Leaving the study early.
| Methods | Allocation: randomised, clearly described, concealed. |
| Participants | Diagnosis: thought to have psychoses. |
| Interventions | 1. Haloperidol IM: dose flexible within recommended limits. N = 150. |
| Outcomes | All outcomes are grouped by time: by 30 minutes, up to two hours, up to four hours, up to 24 hours and finally over 24 hours. Tranquillisation or asleep (measured at 30 minutes, 2 hours, 4 hours and 24 hours). |
| Notes | * Powered to be able to identify a difference of ˜20% between groups for primary outcome with adequate degree of certainty. |
| IM: intramuscular | |
| HALOPERIDOL compared with PLACEBO/NIL for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PLACEBO/NIL | HALOPERIDOL | |||||
| Tranquillisation or asleep | Low1 | RR 0.88 | 220 | ⊕⊝⊝⊝ | ||
| 965 per 1000 | 849 per 1000 | |||||
| Moderate1 | ||||||
| 980 per 1000 | 862 per 1000 | |||||
| High1 | ||||||
| 995 per 1000 | 876 per 1000 | |||||
| Repeated need for tranquillisation | Low1 | RR 0.51 | 660 | ⊕⊕⊝⊝ | ||
| 400 per 1000 | 204 per 1000 | |||||
| Moderate1 | ||||||
| 600 per 1000 | 306 per 1000 | |||||
| High1 | ||||||
| 800 per 1000 | 408 per 1000 | |||||
| Specific behaviour ‐ threat or injury to self or others | The mean specific behaviour ‐ threat or injury to self or others in the intervention groups was | 474 | ⊕⊝⊝⊝ | |||
| Global outcome ‐ overall improvement (not any improvement) | Low1 | RR 0.28 | 40 | ⊕⊕⊝⊝ | ||
| 200 per 1000 | 56 per 1000 | |||||
| Moderate1 | ||||||
| 350 per 1000 | 98 per 1000 | |||||
| High1 | ||||||
| 500 per 1000 | 140 per 1000 | |||||
| Adverse effects: Specific ‐ dystonia within 24 hours | Moderate1 | RR 7.49 | 207 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| High1 | ||||||
| 10 per 1000 | 75 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control risk ‐ moderate risk is roughly equal to that of the control group. | ||||||
| HALOPERIDOL compared with OTHER ANTIPSYCHOTICS: a. ARIPIPRAZOLE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTICS: a. ARIPIPRAZOLE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for rapid tranquillisation ‐ needing additional injection | Low1 | RR 0.78 | 473 | ⊕⊕⊝⊝ | ||
| 200 per 1000 | 156 per 1000 | |||||
| Moderate1 | ||||||
| 400 per 1000 | 312 per 1000 | |||||
| High1 | ||||||
| 650 per 1000 | 507 per 1000 | |||||
| Specific behaviours ‐ agitation: CABS average change score at 2 hours | The mean specific behaviours ‐ agitation: average change score at 2 hours in the intervention groups was | 470 | ⊕⊝⊝⊝ | |||
| Global outcomes: need for benzodiazepine 2 | Low1 | RR 1.26 | 477 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 63 per 1000 | |||||
| Moderate1 | ||||||
| 100 per 1000 | 126 per 1000 | |||||
| High1 | ||||||
| 200 per 1000 | 252 per 1000 | |||||
| Adverse effects: any serious, specific ‐ dystonia during 24 hours | Low6 | RR 6.63 | 477 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate6 | ||||||
| 50 per 1000 | 332 per 1000 | |||||
| High6 | ||||||
| 100 per 1000 | 663 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk roughly equates with that of the trial control group. | ||||||
| HALOPERIDOL compared with OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTIC: b. CHLORPROMAZINE | HALOPERIDOL | |||||
| Tranquillisation or asleep | Low1 | RR 1.93 | 39 | ⊕⊝⊝⊝ | ||
| 200 per 1000 | 386 per 1000 | |||||
| Moderate1 | ||||||
| 500 per 1000 | 965 per 1000 | |||||
| High1 | ||||||
| 700 per 1000 | 1000 per 1000 | |||||
| Repeated need for rapid tranquillisation ‐ more that 1 injection | Low1 | RR 1.07 | 30 | ⊕⊝⊝⊝ | ||
| 800 per 1000 | 856 per 1000 | |||||
| Moderate1 | ||||||
| 900 per 1000 | 963 per 1000 | |||||
| High1 | ||||||
| 990 per 1000 | 1000 per 1000 | |||||
| Specific behaviour ‐ threat or injury of harm to self or others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome ‐ not any improvement | Low1 | RR 0.15 | 89 | ⊕⊝⊝⊝ | ||
| 1000 per 1000 | 150 per 1000 | |||||
| Moderate1 | ||||||
| 300 per 1000 | 45 per 1000 | |||||
| High1 | ||||||
| 500 per 1000 | 75 per 1000 | |||||
| Adverse effects: specific ‐ cardiovascular ‐ hypotension | Low1 | RR 0.51 | 30 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 10 per 1000 | |||||
| Moderate1 | ||||||
| 150 per 1000 | 30 per 1000 | |||||
| High1 | ||||||
| 250 per 1000 | 50 per 1000 | |||||
| Adverse effects: specific ‐ central nervous system ‐ seizures | Low1 | RR 0.33 | 30 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 3 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 17 per 1000 | |||||
| High1 | ||||||
| 150 per 1000 | 50 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control risk ‐ moderate risk roughly equates to that of the control group | ||||||
| HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: c. DROPERIDOL for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTIC: c. DROPERIDOL | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not asleep up to 2 hours | Study population | RR 1.07 | 228 | ⊕⊕⊕⊝ | ||
| 82 per 1.000 | 88 per 1.000 | |||||
| Repeated need for rapid tranquillisation ‐ more than 1 injection | Low | RR 2.38 | 255 | ⊕⊕⊕⊝ | ||
| 50 per 1.000 | 119 per 1.000 | |||||
| Moderate | ||||||
| 70 per 1.000 | 167 per 1.000 | |||||
| High | ||||||
| 360 per 1.000 | 857 per 1.000 | |||||
| Specific behaviour ‐ threat or injury to self or others ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Global outcome ‐ need for benzodiazepine | Study population | RR 0.31 | 228 | ⊕⊕⊕⊝ | ||
| 59 per 1.000 | 18 per 1.000 | |||||
| Adverse effects: specific ‐ dystonia | Study population | RR 0.86 | 255 | ⊕⊕⊕⊝ | ||
| 8 per 1.000 | 7 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 CI include no effect 2 Risk of bias: rated 'serious' ‐ method of randomisation not reported, described as double blind but no further information given regarding rater blinding, small short study. 3 Publication bias: rated 'strongly suspected' ‐ small study. 4 Moderate risk roughly equates with that of the trial control group, 5 Adverse effects ‐ imprecision ‐ 95% confidence interval. 6 Adverse effects ‐ publication bias ‐ small study. | ||||||
| HALOPERIDOL compared with OTHER ANTIPSYCHOTICS: d. LOXAPINE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients to psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTICS: d. LOXAPINE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not asleep by 12 hours | Low1 | RR 4.31 | 54 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 43 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 215 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 431 per 1000 | |||||
| Global outcome: specific | Low1 | RR 3.50 | 30 | ⊕⊝⊝⊝ | * data for prespecified outcome Repeated need for rapid tranquillisation were not reported | |
| 10 per 1000 | 57 per 1000 | |||||
| Moderate1 | ||||||
| 100 per 1000 | 569 per 1000 | |||||
| High1 | ||||||
| 200 per 1000 | 1000 per 1000 | |||||
| Specific behaviour ‐ aggression ‐ no overall improvement | Low1 | RR 1.10 | 30 | ⊕⊝⊝⊝ | ||
| 500 per 1000 | 550 per 1000 | |||||
| Moderate1 | ||||||
| 650 per 1000 | 715 per 1000 | |||||
| High1 | ||||||
| 800 per 1000 | 880 per 1000 | |||||
| Global outcome: no change at 48 hours | Low1 | RR 0.93 | 56 | ⊕⊕⊝⊝ | ||
| 10 per 1000 | 9 per 1000 | |||||
| Moderate1 | ||||||
| 70 per 1000 | 65 per 1000 | |||||
| High1 | ||||||
| 140 per 1000 | 130 per 1000 | |||||
| Adverse effects: Specific ‐ dystonia between 0‐3 days (IM phase) | Low1 | RR 0.94 | 35 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 9 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 47 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 94 per 1000 | |||||
| Adverse effects: Specific ‐ rigidity between 0‐3 days (IM phase) | Low1 | RR 0.88 | 35 | ⊕⊝⊝⊝ | ||
| 750 per 1000 | 660 per 1000 | |||||
| Moderate1 | ||||||
| 850 per 1000 | 748 per 1000 | |||||
| High1 | ||||||
| 950 per 1000 | 836 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the control group. | ||||||
| HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: e. OLANZAPINE for psychosis induced aggression or agitation | ||||||
| Patient or population: psychosis induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTIC: e. OLANZAPINE | Risk with HALOPERIDOL | |||||
| Tranquilisation or asleep | Low | RR 1.16 | 257 | ⊕⊝⊝⊝ | ||
| 200 per 1.000 | 232 per 1.000 | |||||
| Moderate | ||||||
| 700 per 1.000 | 812 per 1.000 | |||||
| High | ||||||
| 900 per 1.000 | 1000 per 1.000 | |||||
| Repeated need for tranquillisation | Low | RR 1.06 | 392 | ⊕⊝⊝⊝ | ||
| 50 per 1.000 | 53 per 1.000 | |||||
| Moderate | ||||||
| 200 per 1.000 | 212 per 1.000 | |||||
| High | ||||||
| 350 per 1.000 | 371 per 1.000 | |||||
| Specific behaviour ‐ threat or injury to self or others | Low | RR 0.96 | 45 | ⊕⊝⊝⊝ | ||
| 200 per 1.000 | 192 per 1.000 | |||||
| Moderate | ||||||
| 400 per 1.000 | 384 per 1.000 | |||||
| High | ||||||
| 600 per 1.000 | 576 per 1.000 | |||||
| Global outcome ‐ need for benzodiazepine during 24 hours | Low | RR 1.05 | 343 | ⊕⊝⊝⊝ | ||
| 50 per 1.000 | 53 per 1.000 | |||||
| Moderate | ||||||
| 150 per 1.000 | 158 per 1.000 | |||||
| High | ||||||
| 250 per 1.000 | 263 per 1.000 | |||||
| Adverse effects: Specific ‐ dystonia during 24 hours | Low | RR 12.92 | 343 | ⊕⊝⊝⊝ | ||
| 0 per 1.000 | 0 per 1.000 | |||||
| Moderate | ||||||
| 0 per 1.000 | 0 per 1.000 | |||||
| High | ||||||
| 5 per 1.000 | 65 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the control group. 2 Risk of bias: rated 'very serious' ‐ method of randomisation not reported, allocation concealment not stated, described as double blind but no further details are given regarding blinding, selective reporting, sponsored by drug company. 3 Indirectness: rated 'serious' ‐ tranquil at 30 minutes is not measured, therefore used not asleep at 2 hour. 4 Publication bias: rated 'strongly suspected' ‐ sponsored by drug company. 5 Indirectness: rated 'serious' ‐ not threat or injury to self or others as stated in protocol, therefore inferred further aggressive behaviour by less than a 40% reduction in PANSS‐EC score. 6 Risk of bias: rated 'serious' ‐ method of randomisation is not reported, allocation concealment not stated, described as double blind but no further information given, incomplete outcome data, selective reporting, sponsored by drug company. 7 Imprecision: rated 'serious' ‐ 95% confidence interval is wide. | ||||||
| HALOPERIDOL compared with OTHER ANTIPSYCHOTIC: f. PERPHENAZINE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTIC: f. PERPHENAZINE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for rapid tranquillisation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Specific behaviour ‐ injury or threat of self to others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome: general ‐ no improvement | Low1 | RR 0.46 | 44 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 5 per 1000 | |||||
| Moderate1 | ||||||
| 100 per 1000 | 46 per 1000 | |||||
| High1 | ||||||
| 200 per 1000 | 92 per 1000 | |||||
| Adverse effect: Specific ‐ hypotensive episode | Low1 | RR 0.31 | 44 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 3 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 15 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 31 per 1000 | |||||
| Adverse effect ‐ discontinued due to EPS | Low1 | RR 1.83 | 44 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 18 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 92 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 183 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the trial control group. | ||||||
| HALOPERIDOL compared to OTHER ANTIPSYCHOTICS: g. QUETIAPINE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTICS: g. QUETIAPINE | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Repeated need for tranquillisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Specific behaviour ‐ agitation | MD 0.10 higher | ‐ | 80 | ⊕⊕⊝⊝ | ||
| Global outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Adverse effects | Low | RR 1.81 | 80 | ⊕⊕⊝⊝ | ||
| 200 per 1.000 | 362 per 1.000 | |||||
| Moderate | ||||||
| 400 per 1.000 | 724 per 1.000 | |||||
| High | ||||||
| 600 per 1.000 | 1000 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Imprecision: rated 'very serious' ‐ small study, 95% CI are wide. | ||||||
| HALOPERIDOL compared with OTHER ANTIPSYCHOTIC: g. RISPERIDONE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTIC: g. RISPERIDONE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not asleep | Low1 | RR 0.84 | 162 | ⊕⊕⊕⊝ | ||
| 700 per 1000 | 588 per 1000 | |||||
| Moderate1 | ||||||
| 900 per 1000 | 756 per 1000 | |||||
| High1 | ||||||
| 1000 per 1000 | 840 per 1000 | |||||
| Repeated need for rapid tranquillisation ‐ needing additional benzodiazepine | Low | RR 0.98 | 286 | ⊕⊕⊝⊝ | ||
| 100 per 1000 | 98 per 1000 | |||||
| Moderate | ||||||
| 200 per 1000 | 196 per 1000 | |||||
| High | ||||||
| 300 per 1000 | 294 per 1000 | |||||
| Specific behaviours ‐ agitation | Low1 | RR 1.15 | 124 | ⊕⊕⊝⊝ | ||
| 50 per 1000 | 58 per 1000 | |||||
| Moderate1 | ||||||
| 200 per 1000 | 230 per 1000 | |||||
| High1 | ||||||
| 350 per 1000 | 402 per 1000 | |||||
| Global outcome ‐ rated as severe ‐ CGI‐S Follow‐up: 1 days | Low1 | RR 0.89 | 162 | ⊕⊝⊝⊝ | ||
| 100 per 1000 | 89 per 1000 | |||||
| Moderate1 | ||||||
| 200 per 1000 | 178 per 1000 | |||||
| High1 | ||||||
| 300 per 1000 | 267 per 1000 | |||||
| Adverse effects: Specific ‐ EPS during 24 hours | Low1 | RR 1.6 | 124 | ⊕⊕⊝⊝ | ||
| 10 per 1000 | 16 per 1000 | |||||
| Moderate1 | ||||||
| 100 per 1000 | 160 per 1000 | |||||
| High1 | ||||||
| 200 per 1000 | 320 per 1000 | |||||
| Adverse effects: Specific ‐ acute dystonia during 24 hours | Low1 | RR 5 | 286 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 50 per 1000 | |||||
| Moderate1 | ||||||
| 20 per 1000 | 100 per 1000 | |||||
| High1 | ||||||
| 30 per 1000 | 150 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the trial control group. | ||||||
| HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: h. THIOTHIXENE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTIC: h. THIOTHIXENE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for rapid tranquillisation | Low1 | RR 1.07 | 30 | ⊕⊝⊝⊝ | ||
| 850 per 1000 | 910 per 1000 | |||||
| Moderate1 | ||||||
| 900 per 1000 | 963 per 1000 | |||||
| High1 | ||||||
| 950 per 1000 | 1000 per 1000 | |||||
| Specific behaviour ‐ threat or injury to self or others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome | Low1 | RR 0.50 | 44 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 25 per 1000 | |||||
| Moderate1 | ||||||
| 250 per 1000 | 125 per 1000 | |||||
| High1 | ||||||
| 450 per 1000 | 225 per 1000 | |||||
| Adverse effects: specific ‐ hypotensive episode | Low | Not estimable | 30 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Adverse effects: specific ‐ tachycardia | Low1 | RR 0.28 | 44 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 3 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 14 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 28 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control risk ‐ moderate risk roughly equates to that of the trial control group. | ||||||
| HALOPERIDOL compared to OTHER ANTIPSYCHOTIC: i. ZIPRASIDONE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTIC: i. ZIPRASIDONE | Risk with HALOPERIDOL | |||||
| Tranquilisation or asleep ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No trial reported this outcome. |
| Repeated need for tranquilisation | Study population | RR 1.64 | 60 | ⊕⊝⊝⊝ | ||
| 467 per 1.000 | 765 per 1.000 | |||||
| Specific behaviour ‐ agitation | MD 0.06 higher | ‐ | 231 | ⊕⊕⊝⊝ | ||
| Global outcome | MD 0.34 higher | ‐ | 132 | ⊕⊝⊝⊝ | ||
| Adverse effects: Specific ‐ dystonia during 72 hours | Low | RR 10.26 | 508 | ⊕⊝⊝⊝ | ||
| 2 per 1.000 | 21 per 1.000 | |||||
| Moderate | ||||||
| 4 per 1.000 | 41 per 1.000 | |||||
| High | ||||||
| 10 per 1.000 | 103 per 1.000 | |||||
| Adverse effects: Specific ‐ clinically significant abnormal ECG during 72 hours | Study population | RR 1.01 | 376 | ⊕⊝⊝⊝ | ||
| 127 per 1.000 | 128 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No trial reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'serious' ‐ method of allocation concealment not stated, blinding of participants uneffective 2 Imprecision: rated 'very serious' ‐ less than 100 patients, 95% CI extends beyond the no effect point 3 Risk of bias: rated 'serious' ‐ method of randomisation is not described, allocation concealment is not stated, single‐blind. 4 Indirectness: rated 'serious' ‐ not threat or injury to self or others, therefore had to use scale derived data for agitation. 5 Risk of bias: rated 'very serious' ‐ allocation of concealment not stated, open trial, adverse effects only reported where they occurred in ≥10% of people, sponsored by drug company. 6 Publication bias: rated 'strongly suspected' ‐ sponsored by drug company. 7 Imprecision: rated 'serious' ‐ 95% confidence intervals are wide. 8 Moderate risk roughly is roughly equal to that of the control group. 9 Indirectness: rated 'serious' ‐ abnormal ECG ‐ not necessarily a serious adverse effect. | ||||||
| HALOPERIDOL compared with OTHER ANTIPSYCHOTICS: j. ZUCLOPENTHIXOL ACETATE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| OTHER ANTIPSYCHOTICS: j. ZUCLOPENTHIXOL ACETATE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for rapid tranquillisation ‐ more than 3 injections | Low1 | RR 2.54 | 70 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 127 per 1000 | |||||
| Moderate1 | ||||||
| 200 per 1000 | 508 per 1000 | |||||
| High1 | ||||||
| 350 per 1000 | 889 per 1000 | |||||
| Specific behaviour ‐ threat or injury to self or others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Adverse effects: specific ‐ tremor by 7 days | Low1 | RR 4.16 | 70 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 42 per 1000 | |||||
| Moderate1 | ||||||
| 50 per 1000 | 208 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 416 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the control group. | ||||||
| HALOPERIDOL compared with BENZODIAZEPINE: a. FLUNITRAZEPAM for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| BENZODIAZEPINE: a. FLUNITRAZEPAM | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for tranquillisation within 24 hours ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Specific behaviours ‐ aggression | Low1 | RR 1.15 | 28 | ⊕⊝⊝⊝ | ||
| 650 per 1000 | 747 per 1000 | |||||
| Moderate1 | ||||||
| 800 per 1000 | 920 per 1000 | |||||
| High1 | ||||||
| 950 per 1000 | 1000 per 1000 | |||||
| Global outcome ‐ need for restraint or seclusion | See comment | See comment | Not estimable | 28 | ⊕⊝⊝⊝ | No events in either group. |
| Adverse effects: specific ‐ EPS | See comment | See comment | Not estimable | 28 | ⊕⊝⊝⊝ | No events in either group. |
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the control group. | ||||||
| HALOPERIDOL compared with BENZODIAZEPINE: b. LORAZEPAM for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| BENZODIAZEPINE: b. LORAZEPAM | HALOPERIDOL | |||||
| Tranquillisation or asleep | Low1 | RR 1.05 | 60 | ⊕⊝⊝⊝ | ||
| 500 per 1000 | 525 per 1000 | |||||
| Moderate1 | ||||||
| 700 per 1000 | 735 per 1000 | |||||
| High1 | ||||||
| 900 per 1000 | 945 per 1000 | |||||
| Repeated need for rapid tranquillisation | Low1 | RR 1.14 | 66 | ⊕⊝⊝⊝ | ||
| 500 per 1000 | 570 per 1000 | |||||
| Moderate1 | ||||||
| 750 per 1000 | 855 per 1000 | |||||
| High1 | ||||||
| 900 per 1000 | 1000 per 1000 | |||||
| Specific behaviour ‐ threat or injury to self or others within 24 hours ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome: no overall improvement ‐ at 60 minutes | Low1 | RR 1.64 | 44 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 82 per 1000 | |||||
| Moderate1 | ||||||
| 150 per 1000 | 246 per 1000 | |||||
| High1 | ||||||
| 100 per 1000 | 164 per 1000 | |||||
| Adverse effects: specific ‐ dystonia (only reported if occurred in ≥9% during 24 hours) | Low1 | RR 3.54 | 66 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 35 per 1000 | |||||
| Moderate1 | ||||||
| 30 per 1000 | 106 per 1000 | |||||
| High1 | ||||||
| 50 per 1000 | 177 per 1000 | |||||
| Adverse effects: specific ‐ hypertonia/rigidity (only reported if occurred in ≥9%) during 24 hours | Low7 | RR 6.22 | 66 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate7 | ||||||
| 50 per 1000 | 311 per 1000 | |||||
| High7 | ||||||
| 100 per 1000 | 622 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the trial control group. | ||||||
| HALOPERIDOL compared with BENZODIAZEPINE: c. MIDAZOLAM for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| BENZODIAZEPINE: c. MIDAZOLAM | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for rapid tranquillisation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Specific behaviour ‐ threat or injury to self or others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome | Low1 | RR 1.14 | 84 | ⊕⊕⊝⊝ | ||
| 50 per 1000 | 57 per 1000 | |||||
| Moderate1 | ||||||
| 150 per 1000 | 171 per 1000 | |||||
| High1 | ||||||
| 250 per 1000 | 285 per 1000 | |||||
| Adverse effects ‐ general ‐ one or more drug‐related adverse effect | Low4 | RR 5.00 | 84 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate4 | ||||||
| 50 per 1000 | 250 per 1000 | |||||
| High4 | ||||||
| 100 per 1000 | 500 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Medium risk is roughly equal to that of the trial control group. | ||||||
| HALOPERIDOL compared with 1. COMBINATIONS ‐ HALOPERIDOL + LEVOMEPROMAZINE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| COMBINATIONS: a. HALOPERIDOL + LEVOMEPROMAZINE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for tranquillisation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Specific behaviours ‐ threat or injury to self or others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Global outcome: general ‐ no overall improvement | Low1 | RR 8.18 | 19 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate1 | ||||||
| 200 per 1000 | 1000 per 1000 | |||||
| High1 | ||||||
| 400 per 1000 | 1000 per 1000 | |||||
| Adverse effect: any serious, specific ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Economic outcome ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Low risk is roughly equal to that of the trial control group. | ||||||
| HALOPERIDOL compared with COMBINATIONS: b. HALOPERIDOL + LORAZEPAM for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| COMBINATIONS: b. HALOPERIDOL + LORAZEPAM | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not asleep by 3 hours | Low1 | RR 1.83 | 67 | ⊕⊝⊝⊝ | ||
| 200 per 1000 | 366 per 1000 | |||||
| Moderate1 | ||||||
| 400 per 1000 | 732 per 1000 | |||||
| High1 | ||||||
| 600 per 1000 | 1000 per 1000 | |||||
| Repeated need for rapid tranquillisation ‐ needing additional injection during 24 hours | Low1 | RR 1.05 | 67 | ⊕⊝⊝⊝ | ||
| 750 per 1000 | 788 per 1000 | |||||
| Moderate1 | ||||||
| 850 per 1000 | 892 per 1000 | |||||
| High1 | ||||||
| 950 per 1000 | 997 per 1000 | |||||
| Specific behaviour ‐ threat or injury to self or others ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Global outcome: no overall improvement ‐ at 30 minutes | Low1 | RR 2.67 | 45 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 134 per 1000 | |||||
| Moderate1 | ||||||
| 250 per 1000 | 668 per 1000 | |||||
| High1 | ||||||
| 450 per 1000 | 1000 per 1000 | |||||
| Adverse effects: Specific ‐ dystonia (only reported if occurred in ≥9%) during 24 hours | Low7 | RR 8.25 | 67 | ⊕⊝⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate7 | ||||||
| 50 per 1000 | 412 per 1000 | |||||
| High7 | ||||||
| 100 per 1000 | 825 per 1000 | |||||
| Adverse effects: Specific ‐ hypertonia/rigidity (only reported if occurred in ≥9%) during 24 hours | Low1 | RR 2.74 | 67 | ⊕⊝⊝⊝ | ||
| 10 per 1000 | 27 per 1000 | |||||
| Moderate1 | ||||||
| 30 per 1000 | 82 per 1000 | |||||
| High1 | ||||||
| 50 per 1000 | 137 per 1000 | |||||
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Moderate risk is roughly equal to that of the control group. | ||||||
| HALOPERIDOL compared to COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATIONS: c. HALOPERIDOL + MIDAZOLAM | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Repeated need for tranquillisation | Study population | RR 0.88 | 60 | ⊕⊝⊝⊝ | ||
| 867 per 1.000 | 763 per 1.000 | |||||
| Specific behaviour ‐ agitation | MD 11.20 lower | ‐ | 60 | ⊕⊝⊝⊝ | ||
| Global outcome | Low | RR 0.29 | 60 | ⊕⊝⊝⊝ | ||
| 350 per 1.000 | 102 per 1.000 | |||||
| Moderate | ||||||
| 700 per 1.000 | 203 per 1.000 | |||||
| High | ||||||
| 950 per 1.000 | 275 per 1.000 | |||||
| Adverse effects | Study population | RR 1.67 | 60 | ⊕⊝⊝⊝ | ||
| 100 per 1.000 | 167 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'very serious' ‐ method of allocation not stated, patients instructed to not reveal their allocation although the trial is described as double blind, selective reporting, small study. 2 Imprecision: rated 'very serious' ‐ small study, 95% CI cross the no effect line. | ||||||
| HALOPERIDOL compared to COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATIONS: d. HALOPERIDOL + PROMETHAZINE | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep | Study population | RR 1.60 | 316 | ⊕⊕⊕⊝ | ||
| 281 per 1.000 | 450 per 1.000 | |||||
| Repeated need for tranquillisation | Low | RR 0.78 | 376 | ⊕⊝⊝⊝ | ||
| 50 per 1.000 | 39 per 1.000 | |||||
| Moderate | ||||||
| 120 per 1.000 | 94 per 1.000 | |||||
| High | ||||||
| 450 per 1.000 | 351 per 1.000 | |||||
| Specific behaviours ‐ aggression | Study population | RR 1.06 | 316 | ⊕⊕⊕⊝ | ||
| 194 per 1.000 | 205 per 1.000 | |||||
| Global outcomes | Study population | RR 1.21 | 316 | ⊕⊕⊕⊝ | ||
| 238 per 1.000 | 287 per 1.000 | |||||
| Adverse effects: specific ‐ acute dystonia | Low | RR 19.48 | 316 | ⊕⊕⊝⊝ | ||
| 0 per 1.000 | 0 per 1.000 | |||||
| Moderate | ||||||
| 50 per 1.000 | 974 per 1.000 | |||||
| High | ||||||
| 100 per 1.000 | 1000 per 1.000 | |||||
| Adverse effects: specific ‐ seizure | Study population | RR 2.05 | 316 | ⊕⊕⊝⊝ | ||
| 6 per 1.000 | 13 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No trial reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'serious' ‐ open trial. 2 Moderate risk is roughly equal to that of the trial control group. 3 Inconsistency: rated 'very serious' ‐ I2 86%. 4 Publication bias: rated 'strongly suspected' ‐ visual inspection of the funnel plot. 5 Indirectness: overall improvement not reported, therefore global improvement inferred from need for restraints at by 120 minutes. 6 Imprecision: rated 'serious' ‐ 95% confidence intervals are wide. 7 Low risk is roughly equal to that of the trial control group. | ||||||
| HALOPERIDOL compared to COMBINATIONS: e. HALOPERIDOL + RISPERIDONE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATIONS: e. HALOPERIDOL + RISPERIDONE | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Repeated need for tranquillisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Specific behaviour ‐ agitation | MD 3.82 higher | ‐ | 100 | ⊕⊕⊝⊝ | ||
| Global outcome assessed with: no improvement at 72 hours | Study population | RR 0.38 | 100 | ⊕⊕⊝⊝ | ||
| 160 per 1.000 | 61 per 1.000 | |||||
| Adverse effects: specific assessed with akathisia during 14 days | Study population | RR 0.88 | 100 | ⊕⊕⊝⊝ | ||
| 320 per 1.000 | 282 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'serious' ‐ allocation concealment procedures not stated, blinding procedures not stated. 2 Imprecision: rated 'serious' ‐ 95% CIs are wide. | ||||||
| HALOPERIDOL compared to COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATIONS: f. OLANZAPINE + MAGNESIUM VALPROATE | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Repeated need for tranquillisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Specific behaviour ‐ agitation | MD 0.04 lower | ‐ | 64 | ⊕⊝⊝⊝ | ||
| Global outcome | Study population | RR 1.06 | 64 | ⊕⊝⊝⊝ | ||
| 182 per 1.000 | 193 per 1.000 | |||||
| Adverse effects | Low | RR 3.06 | 64 | ⊕⊝⊝⊝ | ||
| 100 per 1.000 | 306 per 1.000 | |||||
| Moderate | ||||||
| 240 per 1.000 | 734 per 1.000 | |||||
| High | ||||||
| 500 per 1.000 | 1000 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'serious' ‐ allocation concealment procedures not stated, blinding procedures not stated, small study. 2 Imprecision: rated 'very serious' ‐ small study, 95% CI cross the no effect area. | ||||||
| HALOPERIDOL compared with COMBINATIONS: d. QUETIAPINE + MAGNESIUM VALPROATE for psychosis‐induced aggression or agitation | ||||||
| Patient or population: patients with psychosis‐induced aggression or agitation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| COMBINATIONS: d. QUETIAPINE + MAGNESIUM VALPROATE | HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Repeated need for tranquillisation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Specific behaviours ‐ agitation at 3 days | The mean specific behaviours ‐ agitation at 3 days in the intervention groups was | 60 | ⊕⊝⊝⊝ | |||
| Global effect | Low3 | RR 1.17 | 60 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 58 per 1000 | |||||
| Moderate3 | ||||||
| 200 per 1000 | 234 per 1000 | |||||
| High3 | ||||||
| 350 per 1000 | 409 per 1000 | |||||
| Adverse effects ‐ any specific, serious ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No study reported this outcome. |
| Economic outcome ‐ not reported | See comment | See comment | Not estimable | 0 | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'very serious' ‐ method of randomisation is not reported, allocation concealment not stated, open‐label, selective reporting. | ||||||
| HALOPERIDOL compared to COMBINATIONS: h. RISPERIDONE + CLONAZEPAM for psychosis induced aggression or agitation | ||||||
| Patient or population: psychosis induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATIONS: h. RISPERIDONE + CLONAZEPAM | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep | Study population | RR 0.82 | 205 | ⊕⊝⊝⊝ | ||
| 673 per 1.000 | 552 per 1.000 | |||||
| Repeated need for tranquillisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Specific behaviour ‐ agitation | MD 0.50 lower | ‐ | 108 | ⊕⊝⊝⊝ | ||
| Global outcome | Study population | RR 0.60 | 100 | ⊕⊕⊝⊝ | ||
| 100 per 1.000 | 60 per 1.000 | |||||
| Adverse effects | Low | RR 1.63 | 205 | ⊕⊝⊝⊝ | ||
| 150 per 1.000 | 244 per 1.000 | |||||
| Moderate | ||||||
| 300 per 1.000 | 489 per 1.000 | |||||
| High | ||||||
| 500 per 1.000 | 815 per 1.000 | |||||
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'very serious' ‐ open study, evidence of reporting bias, sponsored trial. 2 Imprecision: rated 'serious' ‐ 95% CI cross the no effect area. 3 Risk of bias: rated 'serious' ‐ allocation concealment and blinding procedures not stated. 4 Imprecision: rated 'serious' ‐ 95% CI are wide. 5 Imprecision: rated 'serious' ‐ 95% CI are wide. | ||||||
| HALOPERIDOL compared to COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM for psychosis‐induced aggression or agitation | ||||||
| Patient or population: psychosis‐induced aggression or agitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with COMBINATIONS: i. ZIPRASIDONE + CLONAZEPAM | Risk with HALOPERIDOL | |||||
| Tranquillisation or asleep ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Repeated need for tranquillisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Specific behaviour ‐ agitation | MD 0.28 lower | ‐ | 71 | ⊕⊝⊝⊝ | ||
| Global outcome ‐ no improvement ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Adverse effects ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Economic outcome ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'very serious' ‐ randomisation, allocation and blinding procedures not stated, evidence of attrition bias, small study. 2 Imprecision: rated 'very serious' ‐ small study, 95% CI are wide. | ||||||
| Focus of review | Reference |
| Completed and maintained reviews | |
| 'As required' medication regimens for seriously mentally ill people in hospital | |
| Benzodiazepines for psychosis‐induced aggression or agitation | |
| Chlorpromazine for psychosis induced aggression or agitation | |
| Clotiapine for acute psychotic illnesses | |
| Containment strategies for people with serious mental illness | |
| Droperidol for acute psychosis | |
| Haloperidol plus promethazine for psychosis‐induced aggression | |
| Olanzapine IM or velotab for acutely disturbed/agitated people with suspected serious mental illnesses | |
| Seclusion and restraint for serious mental illnesses | |
| Zuclopenthixol acetate for acute schizophrenia and similar serious mental illnesses | |
| Reviews in the process of being completed | |
| Risperidone for psychosis induced aggression or agitation | |
| Haloperidol for long term aggression in psychosis | |
| Loxapine inhaler for psychosis‐induced aggression | |
| Quetiapine for psychosis‐induced aggression | |
| De‐escalation techniques for psychosis‐induced aggression | |
| Description | Study |
| Explicitly referred to how participants, study personnel and raters were blinded + tested blinding. | |
| Explicitly referred to how participants, study personnel and raters were blinded. | |
| Report that drugs had identical appearance ‐ no further details regarding rater blinding. | Fitzgerald 1969, Fruensgaard 1977, Paprocki 1977, Resnick 1984, Ritter 1972 |
| Explicitly state that rater was blinded ‐ no reference to participant blinding. Not all study personnel could have been blinded because person preparing drug (not blinded), often administered drug. | |
| Study personnel and raters blinded, not clear if attempts made to blind participants. | |
| Raters blinded ‐ not clear if study personnel and participants were blinded. | |
| "Double blind" ‐ no further information regarding blinding of participants, study personnel and raters. | |
| Described as double‐blind, but during oral phase haloperidol described as being in capsule form, and aripiprazole in tablet form, therefore unclear whether drugs had identical appearance. Not explicit if raters were blinded. | |
| Described as double‐blind during IM phase ‐ open during oral phase ‐ no further information given regarding blinding. | |
| Described as "modified double", whereby staff administering drugs were not blinded, but raters were ‐ not clear if participants were blinded. | |
| Single‐blind studies ‐ "open label, rater blind". | |
| Reported to be single‐blind, but unclear who was blind. | |
| Open‐label and rater blinding for some outcomes (BPRS). | |
| Rater blinding for assessment of BPRS, but unclear regarding rater blinding for other outcomes and whether participants and study personnel were blind. | |
| Open studies | Brook 2000, Fang 2012, Garza‐Trevino 1989, Guo 2007, Higashima 2004, Huf 2007 |
| Not stated | |
| BPRS ‐ Brief Psychiatric Rating Scale | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ asleep Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 not asleep up to 2 hours | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.82, 0.95] |
| 2 Repeated need for tranquillisation Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 needing additional injection during 24 hours (agitation only) | 4 | 660 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.42, 0.62] |
| 3 Specific behaviour: 1a. Agitation ‐ various measures Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 PANSS‐EC response at 2 hours (at least 40% change on PANSS‐EC from baseline) | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.28, 2.07] |
| 4 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 change score (ABS, high=worse) | 3 | 474 | Mean Difference (IV, Fixed, 95% CI) | ‐4.48 [‐5.78, ‐3.18] |
| 4.2 change score (ACES, low=agitated, high=sedated) | 2 | 390 | Mean Difference (IV, Fixed, 95% CI) | 0.83 [0.39, 1.26] |
| 4.3 score (PANSS‐EC, high=worse) | 3 | 514 | Mean Difference (IV, Fixed, 95% CI) | ‐3.67 [‐4.75, ‐2.59] |
| 4.4 change score (PANSS‐EC, high=worse, schizophrenia subgroup) | 1 | 199 | Mean Difference (IV, Fixed, 95% CI) | ‐2.57 [‐4.05, ‐1.09] |
| 4.5 change score (PANSS‐EC, high=worse, non‐sedated patients subgroup based on ACES) | 1 | 240 | Mean Difference (IV, Fixed, 95% CI) | ‐2.64 [‐3.95, ‐1.33] |
| 4.6 change score (PANSS‐EC, high=worse, non‐sedated patients subgroup based on ACES) | 1 | 263 | Mean Difference (IV, Fixed, 95% CI) | ‐2.97 [‐4.27, ‐1.67] |
| 5 Specific behaviour: 1c. Agitation ‐ average scores ‐ ii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 change score (ABS, high=worse) | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐2.4 [‐4.13, ‐0.67] |
| 5.2 change score (PANSS‐EC, high=worse) | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐1.4 [‐2.97, 0.17] |
| 6 Global outcome: 1. Not improved Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 not marked improvement | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.44, 0.84] |
| 6.2 not any improvement | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.08, 1.07] |
| 7 Global outcome: 2. Need for benzodiazepine up to 24 hours Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 need for benzodiazepine up to 24 hours | 4 | 660 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.31, 0.62] |
| 8 Global outcome: 3a. Average scores ‐ i. up to 2 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 endpoint score (CGI‐I, high=worse) | 2 | 390 | Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐0.96, ‐0.51] |
| 8.2 change score (CGI‐I, high=worse, schizophrenia subgroup) | 1 | 199 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐0.98, ‐0.30] |
| 8.3 change score (CGI‐S, high=worse) | 2 | 390 | Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.78, ‐0.18] |
| 9 Global outcome: 3b. Average scores ‐ ii. up to 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 change score (CGI‐I, high=worse) | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | ‐0.4 [‐0.62, ‐0.18] |
| 9.2 change score (CGI‐S, high=worse) | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐0.2 [‐0.46, 0.06] |
| 10 Mental state: 1a. Average scores ‐ i. up to 2 hours Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 change score up to 2 hours (BPRS positive sub‐scale, high=worse) | 3 | 372 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐1.54, ‐0.54] |
| 10.2 change score up to 2 hours (BPRS total, high=worse) | 3 | 371 | Mean Difference (IV, Fixed, 95% CI) | ‐5.69 [‐7.38, ‐4.00] |
| 11 Mental state: 1b. Average scores ‐ ii. up to 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 change score up to 24 hours (BPRS positive sub‐scale, high=worse) | 2 | 264 | Mean Difference (IV, Fixed, 95% CI) | ‐1.61 [‐2.34, ‐0.89] |
| 11.2 change score up to 24 hours (BPRS total, high=worse) | 2 | 264 | Mean Difference (IV, Fixed, 95% CI) | ‐4.81 [‐6.82, ‐2.81] |
| 12 Adverse effects: 1a. General Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 one or more drug related adverse events during 24 hours | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.22, 2.20] |
| 12.2 increased severity of adverse effects after 2nd injection | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [1.88, 5.63] |
| 12.3 overall adverse events during 72 hours | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.23, 2.59] |
| 13 Adverse effects: 1b. General ‐ serious Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 death | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 rated as serious | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.29] |
| 13.3 tonic clonic seizure | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Adverse effects: 2a. Specific ‐ arousal level Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 insomnia during 24 hours (only reported if occurred in ≧5%) | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.61, 2.82] |
| 14.2 "over" sedated | 2 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.36 [1.42, 7.99] |
| 14.3 somnolence during 24 hours | 4 | 615 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [0.97, 5.36] |
| 15 Adverse effects: 2b. Specific ‐ cardiovascular ‐ miscellaneous outcomes Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 dizziness during 24 hours (only reported if occurred in ≧5%) | 2 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.48, 3.65] |
| 15.2 hypotension during 24 hours | 2 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.05, 27.44] |
| 15.3 QTc abnormality | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.1 [0.33, 28.98] |
| 15.4 sinus tachycardia during 24 hours (only reported if occurred in ≥5%) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.1 [0.33, 28.98] |
| 15.5 tachycardia during 24 hours (only reported if occurred in ≥5%) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 16.15] |
| 16 Adverse effects: 2c. Specific ‐ cardiovascular ‐ QTc interval (average change at 24 hours) Show forest plot | 2 | 265 | Mean Difference (IV, Fixed, 95% CI) | 3.63 [‐2.67, 9.93] |
| 17 Adverse effects: 2d. Specific ‐ movement disorders Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 akathisia during 24 hours (only reported if occurred in ≥5%) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.43 [0.77, 233.23] |
| 17.2 dystonia during 24 hours | 2 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.49 [0.93, 60.21] |
| 17.3 extrapyramidal effects ‐ use of antiparkinson drugs during 24 hours | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.57 [1.37, 22.65] |
| 17.4 EPS during 24 hours | 3 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.79 [2.19, 21.07] |
| 18 Adverse effects: 2e. Specific ‐ movement disorders: i. average scores ‐ i. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.1 change score at 24 hours (BAS, high=worse) | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.17, 0.35] |
| 18.2 change score at 24 hours (SAS, high=worse) | 1 | 167 | Mean Difference (IV, Fixed, 95% CI) | 1.89 [0.77, 3.01] |
| 19 Adverse effects: 2f. Specific ‐ miscellaneous Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 agitation during 24 hours (only reported if occurred in ≧5%) | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.29, 2.19] |
| 19.2 dry mouth | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.6 [0.21, 61.86] |
| 19.3 headache during 24 hours (only reported if occurred in ≧5%) | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.55, 3.00] |
| 19.4 pain at injection site | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.22] |
| 19.5 pain (1st injection) | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.00, 1.97] |
| 19.6 pain (2nd injection) | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [1.88, 5.63] |
| 19.7 nausea during 24 hours (only reported if occurred in ≧5%) | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.13, 3.67] |
| 19.8 vomiting during 24 hours (only reported if occurred in ≧5%) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 16.15] |
| 20 Leaving the study early Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 any reason | 3 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.53, 2.11] |
| 20.2 lack of efficacy | 3 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.03, 0.49] |
| 20.3 withdrew | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.35 [0.17, 64.15] |
| 20.4 consent | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.2 [0.77, 49.98] |
| 20.5 adverse effects | 3 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.21, 15.39] |
| 20.6 could not be evaluated due to being asleep | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.05, 27.44] |
| 20.7 other | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.27, 12.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Repeated need for rapid tranquillisation: needing additional injection Show forest plot | 2 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.62, 0.99] |
| 2 Specific behaviour: 1a. Agitation ‐ PANSS‐EC response up to 2 hours (at least 40% change on PANSS‐EC from baseline) Show forest plot | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.92, 1.26] |
| 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ up to 2 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 change score (ACES, low=agitated, high=sedated) | 2 | 470 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.30, 0.55] |
| 3.2 change score (CABS, high=worse) | 2 | 470 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐2.10, 1.01] |
| 3.3 change score (PANSS‐EC, high=worse) | 1 | 357 | Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐2.12, 1.16] |
| 3.4 change score (PANSS‐EC, high=worse, schizophrenia subgroup) | 1 | 257 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐1.48, 0.96] |
| 3.5 change score (PANSS‐EC, high=worse, non‐sedated patients subgroup based on ACES) | 1 | 316 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐1.42, 0.76] |
| 3.6 change score (PANSS‐EC, high=worse, non‐sedated patients subgroup based on AEs) | 1 | 346 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐1.40, 0.72] |
| 4 Global outcome: 1. Need for benzodiazepine Show forest plot | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.74, 2.16] |
| 5 Global outcome: 2. Average scores ‐ up to 2 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 change score (CGI‐S, high=worse) | 2 | 470 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.22, 0.36] |
| 5.2 endpoint score (CGI‐I, high=worse) | 2 | 470 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.23, 0.19] |
| 5.3 change score (CGI‐I, high=worse, schizophrenia subgroup) | 1 | 257 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.30, 0.26] |
| 6 Mental state: 1. Average scores ‐ up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 change score at 2 hours (BPRS positive sub‐scale, high=worse) | 1 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐1.28, 0.82] |
| 6.2 change score at 2 hours (BPRS total, high=worse) | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐2.03 [‐5.76, 1.70] |
| 7 Adverse effects: 1a. General Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 one or more drug related adverse effects during 24 hours | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.95, 1.46] |
| 7.2 increased severity of adverse effects after 2nd injection | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.03, 1.74] |
| 7.3 overall adverse events during 72 hours | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.04, 1.70] |
| 8 Adverse effects: 1b. General ‐ serious Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 any | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.18, 1.81] |
| 8.2 tonic clonic seizure | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.62] |
| 8.3 death | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Adverse effects: 2a. Specific ‐ arousal level Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 insomnia ‐ between 0‐1 days (IM phase) (only reported if occurred in ≥5%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.01, 4.27] |
| 9.2 insomnia ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.47, 1.44] |
| 9.3 "over" sedated | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.93, 2.95] |
| 9.4 somnolence ‐ between 0‐1 days | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.25, 2.00] |
| 9.5 somnolence ‐ between 0‐1 days (IM phase) (only reported if occurred in ≥5%) | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [0.60, 8.16] |
| 9.6 somnolence ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.30, 27.02] |
| 10 Adverse effects: 2b. Specific ‐ cardiac Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 dizziness ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.33, 1.48] |
| 10.2 sinus tachycardia ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.66 [0.35, 126.06] |
| 10.3 tachycardia ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.06] |
| 11 Adverse effects: 2c. Specific ‐ gastrointestinal Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 dyspepsia ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.41 [1.36, 79.76] |
| 11.2 diarrhoea ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.28, 3.21] |
| 11.3 nausea ‐ between 0‐1 days (IM phase) (only reported if occurred in ≥5%) | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.05, 0.60] |
| 11.4 nausea ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.09] |
| 11.5 vomiting ‐ between 0‐1 days (IM phase) (only reported if occurred in ≥5%) | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.10] |
| 11.6 vomiting ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.07, 1.92] |
| 12 Adverse effects: 2d. Specific ‐ movement disorder Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 akathisia ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.94, 8.61] |
| 12.2 akathisia ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.58, 8.40] |
| 12.3 dystonia ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.63 [1.52, 28.86] |
| 12.4 EPS ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.46 [1.22, 73.13] |
| 12.5 EPS ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.09 [1.65, 30.58] |
| 12.6 Use of antiparkinson drugs ‐ between 3 ‐ 7 days (oral phase) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.94 [2.50, 9.78] |
| 12.7 dystonia | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 69.22] |
| 13 Adverse effects: 2e. Specific ‐ miscellaneous Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 agitation ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.46, 3.22] |
| 13.2 agitation ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.68, 1.74] |
| 13.3 anxiety ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.54, 3.16] |
| 13.4 headache ‐ between 0 ‐1 day (IM phase) (only reported if occurred in ≥5%) | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.45, 1.59] |
| 13.5 headache ‐ between 3 ‐ 7 days (oral phase) (only reported if occurred in ≥ 2%) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.57, 1.97] |
| 13.6 pain at injection site | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.62] |
| 13.7 pain (1st injection) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.06, 1.54] |
| 13.8 pain (2nd injection) | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.03, 1.74] |
| 14 Leaving the study early: 1. For specific reasons Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 any | 2 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.86, 4.98] |
| 14.2 lack of efficacy | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 69.22] |
| 14.3 adverse effects | 2 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.17, 5.59] |
| 14.4 withdrew | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.24, 8.39] |
| 14.5 consent | 2 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.74, 48.34] |
| 14.6 other | 2 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.27, 6.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ asleep Show forest plot | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.04, 3.60] |
| 1.1 not asleep up to 2 hours | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.04, 3.60] |
| 2 Repeated need for rapid tranquillisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 more that 1 injection | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.89, 1.28] |
| 2.2 more than 3 injections | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.53, 4.26] |
| 3 Global outcome: 1. Not improved Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 not marked improvement | 2 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.61, 1.02] |
| 3.2 not any improvement | 2 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.05, 0.49] |
| 4 Adverse effects: any serious or specific adverse effects Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 allergy ‐ haematological ‐ leucopenia ‐ mild | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.30] |
| 4.2 allergy ‐ hepatic ‐ glutamic pyruvic transaminase elevated | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.81] |
| 4.3 allergy ‐ skin irritation ‐ local | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 anticholinergic ‐ dry mouth | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.17, 10.93] |
| 4.5 arousal ‐ drowsy but asleep | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.42] |
| 4.6 arousal ‐ drowsy but awake | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.17 [0.59, 142.10] |
| 4.7 cardiovascular ‐ hypotension | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.60] |
| 4.8 central nervous system ‐ seizures | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 4.9 movement disorders ‐ extrapyramidal adverse effects | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.28, 15.15] |
| 5 Leaving the study early Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 any reason/general reasons | 4 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.71] |
| 5.2 could not be evaluated due to being asleep | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.01, 0.52] |
| 5.3 severe hypotensive reaction | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.85] |
| 5.4 deviation from protocol | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep: 1. Not asleep Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 up to 2 hours | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.44, 2.60] |
| 2 Tranquillisation or asleep: 2. Time to sleep Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 up to 2 hours | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 5.20 [‐6.05, 16.45] |
| 3 Repeated need for rapid tranquillisation Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 more than 1 injection | 2 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [1.27, 4.47] |
| 3.2 more than 3 injections | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.09, 47.68] |
| 4 Global outcome: 1. Need for benzodiazepine Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 up to 2 hours | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.07, 1.44] |
| 5 Adverse effects: 1. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 overall adverse events up to 2 hours | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.23] |
| 5.2 patients with one or more AEs | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.02, 1.46] |
| 6 Adverse effects: 2a. Specific ‐ arousal level Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 "over" sedated | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.68] |
| 7 Adverse effects: 2b. Specific ‐ cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 hypotension up to 2 hours | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.36] |
| 8 Adverse effects: 2c. Specific ‐ movement disorders Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 dystonia | 2 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.11, 6.56] |
| 9 Adverse effects: 2d. Specific ‐ miscellaneous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 desaturation | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ not asleep up to 24 hours Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.31 [0.54, 34.48] |
| 2 Specific behaviour: 2. Aggression ‐ various measures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 no overall improvement | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.69, 1.76] |
| 3 Global outcome: 1. General ‐ no change ‐ i. over 24 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 up to 48 hours (CGI‐I, high=worse) | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.14, 6.15] |
| 3.2 up to 72 hours (CGI‐I, high=worse) | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.04, 3.49] |
| 3.3 at endpoint (CGI‐I, high=worse) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.12, 1.32] |
| 4 Global outcome: 2a. Specific ‐ not sedated ‐ i. by 30 minutes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 at 30 minutes | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.65, 2.54] |
| 5 Global outcome: 2b. Specific ‐ not sedated ‐ ii. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 at 1 hour | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.5 [0.86, 14.18] |
| 5.2 at 2 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.98, 50.16] |
| 6 Mental state: 1. Average scores Show forest plot | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 6.10 [4.48, 7.72] |
| 6.1 endpoint score (BPRS, high=worse) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 6.10 [4.48, 7.72] |
| 7 Adverse effects: 1. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 one or more drug related adverse effect | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.44, 1.45] |
| 8 Adverse effects: 2a. Specific ‐ anticholinergic Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 constipation ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.26] |
| 8.2 salivation ‐ too little ‐ between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.33, 24.66] |
| 8.3 salivation ‐ too little ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.22, 4.05] |
| 8.4 salivation ‐ too much ‐ between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.33, 2.69] |
| 8.5 salivation ‐ too much ‐ between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.04, 4.48] |
| 8.6 salivation ‐ too much ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.57, 1.93] |
| 8.7 polyuria ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 65.34] |
| 8.8 sweating ‐ between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.15, 5.97] |
| 8.9 sweating ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 65.34] |
| 9 Adverse effects: 2b. Specific ‐ arousal level Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 asleep ‐ by 12 hours | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.72, 1.04] |
| 9.2 drowsiness ‐ between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.70, 1.12] |
| 9.3 drowsiness ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 33.16 [2.15, 511.57] |
| 9.4 drowsiness during 72 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.20, 2.79] |
| 9.5 insomnia ‐ between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.26] |
| 9.6 insomnia ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.63 [0.37, 119.59] |
| 10 Adverse effects: 2c. Specific ‐ cardiovascular Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 blood pressure ‐ increased ‐ between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.45] |
| 10.2 dizziness ‐ between 3‐28 days | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.09, 1.52] |
| 10.3 dyspnoea ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 65.34] |
| 10.4 palpitations during 72 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 10.5 pulse rate ‐ increased ‐ between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.79] |
| 11 Adverse effects: 2d. Specific ‐ movement disorders Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 akathisia between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.16, 2.02] |
| 11.2 akathisia between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.26, 1.61] |
| 11.3 akathisia between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.62, 2.27] |
| 11.4 dysarthria between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.44] |
| 11.5 dyskinesia between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.79] |
| 11.6 dystonia between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.06, 13.93] |
| 11.7 dystonia between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.26, 1.61] |
| 11.8 involuntary movement between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.26] |
| 11.9 motor retardation between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.06, 13.93] |
| 11.10 muscle spasms between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.33, 4.82] |
| 11.11 oculogyric crisis between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.6 [0.11, 61.11] |
| 11.12 rigidity between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.65, 1.19] |
| 11.13 rigidity between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.13, 5.68] |
| 11.14 rigidity during between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.93, 1.38] |
| 11.15 tremor between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.42, 2.13] |
| 11.16 tremor during between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.71, 1.76] |
| 11.17 tremor between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.6 [0.11, 61.11] |
| 11.18 use of antiparkinson medication | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.66, 12.16] |
| 11.19 dystonia during 72 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 96.13] |
| 11.20 extrapyramidal effects ‐ use of antiparkinson drugs during 72 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.17, 2.07] |
| 11.21 EPS during 72 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.98, 50.16] |
| 11.22 thick tongue ‐ between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.79] |
| 12 Adverse effects: 2e. Specific ‐ miscellaneous Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 allergy ‐ itch ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 65.34] |
| 12.2 allergy ‐ tissue reaction at injection site ‐ between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 nervousness ‐ between 0‐10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.79] |
| 12.4 pain ‐ during 24 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.13, 3.44] |
| 12.5 pain ‐ headache ‐ between 0‐3 days (IM phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.26] |
| 12.6 pain ‐ myalgia ‐ between 3‐28 days (Oral phase) | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 65.34] |
| 13 Leaving the study early: 1. For general reasons Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 90% CI) | Subtotals only | |
| 13.1 by 72 hours | 1 | 35 | Risk Ratio (M‐H, Fixed, 90% CI) | 0.63 [0.21, 1.85] |
| 13.2 by 10 days | 1 | 54 | Risk Ratio (M‐H, Fixed, 90% CI) | 1.18 [0.67, 2.07] |
| 13.3 by endpoint | 1 | 35 | Risk Ratio (M‐H, Fixed, 90% CI) | 0.94 [0.42, 2.13] |
| 14 Leaving the study early: 2. Specific reasons Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 adverse effects by 72 hours | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 65.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ asleep Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 not asleep up to 2 hours | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.02, 1.32] |
| 2 Repeated need for tranquillisation ‐ needing additional injection Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 by 2 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.47, 33.73] |
| 2.2 by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.29 [1.67, 6.47] |
| 2.3 by 24 hours | 3 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.75, 1.51] |
| 3 Specific behaviour: 1a. Agitation ‐ various measures Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 response ‐ up to 2 hours (≥40% reduction in PANSS‐EC) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.58, 1.58] |
| 3.2 agitation ‐ reported as 'adverse effect' | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 15.73] |
| 3.3 agitation during 21 days (if occurred in ≧10% at P < 0.05) ‐ reported as 'adverse effect' | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.33, 3.51] |
| 4 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. by 30 minutes Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 change score at 15 minutes (ACES, low=agitated, high=sedated) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.35, 1.35] |
| 4.2 change scores at 15 minutes (PANSS‐EC, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.64, 2.24] |
| 4.3 change score at 30 minutes (ACES, low=agitated, high=sedated) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.06, 1.86] |
| 4.4 change scores at 30 minutes (PANSS‐EC, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐1.16, 3.76] |
| 5 Specific behaviour: 1c. Agitation ‐ average scores ‐ ii. up to 2 hours Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 change score (ABS, high=worse) | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | 2.7 [0.38, 5.02] |
| 5.2 change score at 1 hour (ACES, low=agitated, high=sedated) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 1.2 [0.06, 2.34] |
| 5.3 endpoint score at 1 hour (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [1.18, 2.82] |
| 5.4 change score at 1 hour (PANSS‐EC, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 2.2 [‐0.50, 4.90] |
| 5.5 change score at 2 hours (ACES, low=agitated, high=sedated) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.74, 1.34] |
| 5.6 endpoint score at 2 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [3.54, 4.86] |
| 5.7 score at 2 hours (PANSS‐EC, high=worse) | 3 | 332 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐1.49, 0.86] |
| 5.8 endpoint score at 2 hours (PANSS‐PAS, high=worse) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [‐2.56, 6.76] |
| 6 Specific behaviour: 1d. Agitation ‐ average scores ‐ iii. up to 4 hours Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.05, 0.45] |
| 6.1 endpoint score at 4 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.05, 0.45] |
| 7 Specific behaviour: 1e. Agitation ‐ average scores ‐ iv. up to 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 change score (ABS, high=worse) | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [0.01, 4.79] |
| 7.2 change score (PANSS‐EC, high=worse) | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐0.55, 3.35] |
| 7.3 endpoint score at 6 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.05, 0.35] |
| 7.4 endpoint score at 12 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.9 [0.82, 0.98] |
| 8 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 endpoint score at 1 hour (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.13, 1.67] |
| 8.2 endpoint score at 2 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.41, 1.41] |
| 9 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 endpoint score at 4 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.11, 0.31] |
| 10 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 endpoint score at 6 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.26, 0.06] |
| 10.2 endpoint score at 12 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.39, ‐0.01] |
| 11 Specific behaviour: 3. Hostility Show forest plot | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.12] |
| 12 Global outcome: 1a. General ‐ need for additional measures Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 need for benzodiazepine during 24 hours | 2 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.63, 1.74] |
| 12.2 need for benzodiazepine during 7 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.79, 1.27] |
| 12.3 additional restraint, seclusion or special observation | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.70, 3.17] |
| 13 Global outcome: 1b. General ‐ time and doses Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 time to discontinuation | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐3.48 [‐6.28, ‐0.68] |
| 13.2 dose of adjunctive lorazepam | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [‐0.14, 0.82] |
| 14 Global outcome: 1c. General ‐ average scores ‐ up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 endpoint score (CGI‐I, high=worse) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.65, 0.45] |
| 15 Global outcome: 1d. General ‐ average scores ‐ over 24 hours Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1 change score (CGI‐I, high=worse) | 1 | 243 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.20, 0.20] |
| 15.2 change score (CGI‐S, high=worse) | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.24, 0.24] |
| 15.3 change score by 21 days (CGI‐I, high=worse) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [‐0.18, 0.90] |
| 16 Global outcome: 2a. Specific ‐ alert ‐ i. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 alert at 1 hour | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.99, 1.55] |
| 17 Global outcome: 2b. Specific ‐ alert ‐ ii. up to 4 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 alert at 4 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.66, 1.42] |
| 18 Global outcome: 2c. Specific ‐ alert ‐ iii. up to 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 alert at 8 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.67, 1.60] |
| 18.2 alert at 16 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.58, 1.78] |
| 18.3 alert at 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.20] |
| 19 Global outcome: 2d. Specific ‐ tranquil ‐ i. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 tranquil at 1 hour | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.29, 1.34] |
| 20 Global outcome: 2e. Specific ‐ tranquil ‐ ii. up to 4 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 tranquil at 4 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.94, 3.62] |
| 21 Global outcome: 2f. Specific ‐ tranquil ‐ iii. up to 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 tranquil at 8 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.55, 1.87] |
| 21.2 tranquil at 16 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.36, 1.45] |
| 21.3 tranquil at 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.83, 2.72] |
| 22 Global outcome: 2g. Specific ‐ sedated ‐ i. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 sedated at 1 hour | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.39] |
| 23 Global outcome: 2h. Specific ‐ sedated ‐ ii. up to 4 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 sedated at 4 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.18, 1.03] |
| 24 Global outcome: 2i. Specific ‐ sedated ‐ iii. up to 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 24.1 sedated at 8 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.49, 2.37] |
| 24.2 sedated at 16 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.47, 1.50] |
| 24.3 sedated at 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.19, 1.98] |
| 25 Global outcome: 2j. Specific ‐ asleep ‐ i. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.1 asleep at 1 hour | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.64] |
| 26 Global outcome: 2k. Specific ‐ asleep ‐ ii. up to 4 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26.1 asleep at 4 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.07, 16.84] |
| 27 Global outcome: 2l. Specific ‐ asleep ‐ iii. up to 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 27.1 asleep at 8 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.34] |
| 27.2 asleep at 16 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.33 [0.97, 19.40] |
| 27.3 asleep at 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.14, 77.79] |
| 28 Service use: 1. Average days to discharge Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.85, 0.65] |
| 29 Service use: 2. Average patient‐hours used during 24 hours (skewed data) Show forest plot | Other data | No numeric data | ||
| 29.1 days 1‐7 | Other data | No numeric data | ||
| 29.2 days 8‐14 | Other data | No numeric data | ||
| 29.3 days 15‐21 | Other data | No numeric data | ||
| 30 Mental state: 1. Various outcomes reported as 'adverse events' Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30.1 anxiety | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.12] |
| 30.2 anxiety during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.08, 1.70] |
| 30.3 delusions | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.12] |
| 30.4 nervousness during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.70, 6.74] |
| 31 Mental state: 2a. Average scores ‐ i. by 30 minutes Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 31.1 change score at 15 minutes (BPRS positive sub‐scale, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐2.11, 1.51] |
| 31.2 change score at 30 minutes (BPRS positive sub‐scale, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐2.70, 1.90] |
| 31.3 change score at 15 minutes (BPRS total, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐5.42, 4.02] |
| 31.4 change score at 30 minutes (BPRS total, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐7.35, 6.75] |
| 32 Mental state: 2b. Average scores ‐ ii. up to 2 hours Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 32.1 change score at 1 hour (BPRS positive sub‐scale, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐2.74, 3.94] |
| 32.2 change score at 2 hours (BPRS positive sub‐scale, high=worse) | 3 | 385 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐0.34, 0.89] |
| 32.3 change score at 1 hour (BPRS total, high=worse) | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 4.70 [‐5.03, 14.43] |
| 32.4 change score at 2 hours (BPRS total, high=worse) | 3 | 385 | Mean Difference (IV, Fixed, 95% CI) | 1.75 [‐0.12, 3.62] |
| 33 Mental state: 2c. Average scores ‐ iii. up to 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 33.1 change score at 24 hours (BPRS positive sub‐scale, high=worse) | 2 | 340 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.97, 0.37] |
| 33.2 change score at 24 hours (BPRS total, high=worse) | 2 | 340 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [‐1.34, 2.29] |
| 34 Mental state: 2d. Average scores ‐ iv. over 24 hours Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 4.70 [‐0.18, 9.58] |
| 34.1 change score at 21 days (PANSS total, high=worse) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 4.70 [‐0.18, 9.58] |
| 35 Adverse effects: 1a. General Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 35.1 one or more drug related adverse events | 3 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.09, 1.76] |
| 36 Adverse effects: 1b. General ‐ serious Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 36.1 one or more treatment emergent adverse events | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.36, 1.83] |
| 36.2 treatment emergent adverse events ‐ all | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.69, 2.19] |
| 36.3 overall serious adverse effects | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 36.4 death | 3 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Adverse effects: 2a. Specific ‐ anticholinergic Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 37.1 increased salivation during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.73 [0.54, 176.18] |
| 38 Adverse effects: 2b. Specific ‐ arousal level ‐ i. various Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 38.1 insomnia | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.39, 2.78] |
| 38.2 insomnia during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.57, 8.19] |
| 38.3 somnolence at 24 hours | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.45, 2.41] |
| 38.4 somnolence during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.67, 3.12] |
| 38.5 excessive sedation during 24 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.23] |
| 39 Adverse effects: 2c. Specific ‐ arousal level ‐ ii. average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 39.1 endpoint score at 1 hour (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.13, 0.73] |
| 39.2 endpoint score at 2 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.36, 0.36] |
| 40 Adverse effects: 2d. Specific ‐ arousal level ‐ ii. average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 40.1 endpoint score at 4 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.61, 0.21] |
| 41 Adverse effects: 2e. Specific ‐ arousal level ‐ ii. average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 41.1 endpoint score at 6 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.38, 0.18] |
| 41.2 endpoint score at 12 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.26, 0.26] |
| 42 Adverse effects: 2f. Specific ‐ cardiovascular i. binary Show forest plot | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.38] |
| 42.1 clinically significant ECG change | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 42.2 hypotension during 24 hours | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.38] |
| 43 Adverse effects: 2g. Specific ‐ cardiovascular ii. continuous Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 43.1 QT interval: average change at 24 hours | 1 | 257 | Mean Difference (IV, Fixed, 95% CI) | 1.8 [‐3.81, 7.41] |
| 43.2 QT interval: average endpoint score at 24 hours | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 8.5 [‐3.28, 20.28] |
| 44 Adverse effects: 2h. Specific ‐ gastrointestinal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 44.1 abdominal pain | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.13, 73.04] |
| 44.2 vomiting | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.2 [0.26, 103.03] |
| 45 Adverse effects: 2i. Specific ‐ movement disorder ‐ i. various Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 45.1 dystonia during 24 hours | 2 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.92 [1.67, 99.78] |
| 45.2 dystonia during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.73 [0.54, 176.18] |
| 45.3 hypertonia during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.73 [0.54, 176.18] |
| 45.4 EPS during 24 hours | 3 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.35 [2.27, 30.63] |
| 45.5 EPS during study | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 15.73] |
| 45.6 extrapyramidal effects ‐ use of antiparkinson drugs during 24 hours | 1 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.51 [1.92, 10.58] |
| 45.7 extrapyramidal effects ‐ use of antiparkinson drugs during 21 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.73 [0.54, 176.18] |
| 45.8 extrapyramidal effects ‐ use of antiparkinson drugs during study | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 15.73] |
| 46 Adverse effects: 2j. Specific ‐ movement disorder ‐ ii. average scores ‐ i. up to 24 hours Show forest plot | 1 | 483 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.16, 0.53] |
| 46.1 change score at 24 hours (SAS, high=worse) | 1 | 242 | Mean Difference (IV, Fixed, 95% CI) | 1.31 [0.56, 2.06] |
| 46.2 change score at 24 hours (BAS, high=worse) | 1 | 241 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [0.09, 0.47] |
| 47 Adverse effects: 2k. Specific ‐ movement disorder ‐ iii. average scores ‐ i. up to 24 hours Show forest plot | Other data | No numeric data | ||
| 47.1 endpoint score at 24 hours (SAS, high=worse) ‐ (skewed data) | Other data | No numeric data | ||
| 47.2 endpoint score at 24 hours (BAS, high=worse) ‐ (skewed data) | Other data | No numeric data | ||
| 48 Adverse effects: 2l. Specific ‐ movement disorder ‐ vi. average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 48.1 endpoint score at 96 hours (AIMS, high=worse) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.87, 1.27] |
| 48.2 endpoint score at 96 hours (BARS, high=worse) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.8 [‐0.01, 1.61] |
| 48.3 endpoint score at 96 hours (SAS, high=worse) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 2.2 [‐0.07, 4.47] |
| 49 Adverse effects: 2m. Specific ‐ miscellaneous Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 49.1 pain | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.13, 73.04] |
| 49.2 pain during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.33, 3.51] |
| 49.3 pain ‐ headache during 21 days (if occurred in ≧10% at P < 0.05) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.88, 5.32] |
| 49.4 other ‐ nose bleed | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.13, 73.04] |
| 49.5 other ‐ clinically relevant laboratory changes | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 50 Leaving the study early Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 50.1 any reason | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.04, 2.65] |
| 50.2 adverse effects | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.67 [1.13, 66.75] |
| 50.3 lack of efficacy | 2 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.09, 1.44] |
| 50.4 lost at follow‐up | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.10, 2.82] |
| 50.5 participants decision | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.57, 8.19] |
| 50.6 non compliance | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.42, 11.30] |
| 50.7 physician decision | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.33 [0.50, 37.42] |
| 50.8 sponsor decision | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global outcome: No improvement Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.04, 4.68] |
| 2 Adverse effects: 1. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 one or more adverse effect | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.61, 2.80] |
| 2.2 clinically significant laboratory changes | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Adverse effects: 2. Specific Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 hypotensive episode | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.12] |
| 3.2 require antiparkinson medication | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.62, 12.12] |
| 3.3 discontinued due to EPS | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.18, 18.70] |
| 3.4 discontinued due to drowsiness/tension and no therapeutic effect | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.12, 64.04] |
| 4 Leaving the study early: 1. Specific reasons Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 discontinued due to EPS | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.18, 18.70] |
| 4.2 discontinued due to drowsiness/tension and no therapeutic effect | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.12, 64.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 change score at 24 hours (PANSS‐EC, high=worse) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.56, 0.76] |
| 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 change score at 72 hours (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 change score at 7 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 change score at 10 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 change score at 14 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 change score at 28 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mental state: Average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 change score at 28 days (PANSS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 change score at 28 days (PANSS positive sub‐scale, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 change score at 28 days (PANSS negative sub‐scale, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects: 1. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 one or more drug related adverse events by 28 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse effects: 2. Specific ‐ movement disorder ‐ i. average scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 endpoint score at 28 days (SAS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Leaving the study early Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 any reason | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 lost at follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ i. by 30 minutes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 not asleep at 30 minutes | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.74, 0.95] |
| 2 Tranquillisation or asleep ‐ ii. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 not asleep at 1 hour | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.59, 0.92] |
| 2.2 not asleep at 2 hours | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.51, 0.99] |
| 3 Specific behaviour: 1a. Agitation ‐ various measures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 ≥50% reduction in PANSS‐EC score up to 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.79, 1.16] |
| 3.2 discontinued due to severe agitation up to 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.03] |
| 4 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 endpoint score at 2 hours (PANSS‐PAS, high=worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐5.22, 4.42] |
| 5 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. by 30 minutes Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 change score at 30 minutes (OAS total aggression, high=worse) | 1 | 147 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐0.58, ‐0.42] |
| 6 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 change score at 1 hour (OAS total aggression, high=worse) | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.27, ‐0.13] |
| 6.2 change score at 2 hours (OAS total aggression, high=worse) | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.13, ‐0.07] |
| 7 Global outcome: 1. Binary measures Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 needing additional benzodiazepine | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.65, 1.47] |
| 7.2 rated as severe at 24 hours (CGI‐S) | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.51, 1.58] |
| 8 Global outcome: 2. Continuous measures Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 dose of lorazapam | 1 | 147 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.49, 0.29] |
| 8.2 time to additional dose | 1 | 147 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐2.17, 2.57] |
| 9 Adverse effects: 1a. General ‐ one or more adverse effects Show forest plot | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.84, 1.23] |
| 9.1 up to 24 hours | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.84, 1.23] |
| 10 Adverse effects: 2a. Specific ‐ arousal Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 sedated at 30 minutes | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.39, 2.59] |
| 10.2 sedated at 60 minutes | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.15, 1.72] |
| 10.3 sedated at 120 minutes | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.98, 1.23] |
| 10.4 somnolence during 24 hours (only reported if occurred in ≥5%) | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.43, 2.12] |
| 10.5 somnolence during 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.31, 1.96] |
| 10.6 insomnia ‐ severe | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.47] |
| 10.7 somnolence ‐ severe | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.13, 76.20] |
| 11 Adverse effects: 2b. Specific ‐ cardiovascular ‐ i. binary outcomes Show forest plot | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.45, 4.70] |
| 11.1 dizziness during 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.26, 3.82] |
| 11.2 orthostatic hypertension | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.25 [0.26, 107.67] |
| 12 Adverse effects: 2c. Specific ‐ cardiovascular ‐ ii. pulse rate ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 heartbeat change at 60 minutes | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐9.4 [‐9.99, ‐8.81] |
| 13 Adverse effects: 2d. Specific ‐ cardiovascular ‐ ii. pulse rate ‐ ii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 heartbeat change at 8 hours | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐8.8 [‐9.75, ‐7.85] |
| 14 Adverse effects: 2e. Specific ‐ movement disorder ‐ i. binary outcomes Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 dystonia during 24 hours | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.28, 7.28] |
| 14.2 dyskinesia during 24 hours | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.13, 76.20] |
| 14.3 hyperkinesia during 24 hours (only reported if occurred in ≥5%) | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.20 [0.48, 36.79] |
| 14.4 hypertonia/rigidity during 24 hours | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.47] |
| 14.5 tremor during 24 hours | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.25 [0.26, 107.67] |
| 14.6 movement disorder | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.6 [0.55, 4.62] |
| 15 Adverse effects: 2f. Specific ‐ movement disorders ‐ ii. average scores ‐ i. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1 change score at 24 hours (BAS akathisia, high=worse) | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | 0.3 [0.24, 0.36] |
| 15.2 change score at 24 hours (SAS movement disorders, high=worse) | 1 | 162 | Mean Difference (IV, Fixed, 95% CI) | 0.4 [0.34, 0.46] |
| 16 Adverse effects: 2g. Specific ‐ movement disorder ‐ ii. average scores ‐ ii. over 24 hours Show forest plot | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | 0.58 [‐0.32, 1.47] |
| 16.1 endpoint score at 96 hours (AIMS, high=worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 endpoint score at 96 hours (BARS, high=worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.36, 1.56] |
| 16.3 endpoint score at 96 hours (SAS, high=worse) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐2.20, 3.00] |
| 17 Adverse effects: 2h. Specific ‐ miscellaneous Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 agitation during 24 hours (only reported if occurred in ≥5%) | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.27, 4.06] |
| 17.2 agitation ‐ severe | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.19, 22.72] |
| 17.3 anxiety ‐ severe | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.13, 76.20] |
| 17.4 headache during 24 hours (only reported if occurred in ≥5%) | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.37, 4.71] |
| 17.5 headache during 24 hours | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.71] |
| 18 Leaving the study early ‐ i. up to 24 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 due to severe agitation | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.03] |
| 18.2 due to acute dystonia | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 102.07] |
| 18.3 due to adverse effects | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.07, 16.51] |
| 18.4 unspecified general reasons | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.42, 1.44] |
| 19 Leaving the study early ‐ ii. over 24 hours Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.89] |
| 19.1 Lack of efficacy at 96 hours | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Repeated need for rapid tranquillisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 more than 1 injection | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.89, 1.28] |
| 1.2 more than 3 injections | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.57, 10.93] |
| 2 Specific behaviour: 1. Agitation ‐ rated as 'adverse effect' Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 6.52] |
| 3 Global outcome Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 no response to 1st injection | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.57, 11.05] |
| 3.2 no improvement at 1 hour | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.14, 1.84] |
| 3.3 no improvement at 2 hours | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.31] |
| 3.4 no improvement at 3 hours | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.06, 12.49] |
| 3.5 no improvement at 4 hours | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.06, 12.49] |
| 3.6 no improvement at 5 hours | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.2 [0.21, 82.72] |
| 4 Adverse effects: 1. General ‐ one or more adverse effects Show forest plot | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.97, 2.22] |
| 5 Adverse effects: 2a. Specific ‐ movement disorders Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 ataxia | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.06, 12.49] |
| 5.2 thick tongue | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [0.11, 58.67] |
| 5.3 use of antiparkinson drugs | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Adverse effects: 2b. Specific ‐ others Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 anticholinergic ‐ blurred vision | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.2 [0.21, 82.72] |
| 6.2 anticholinergic ‐ dry mouth | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.31] |
| 6.3 arousal ‐ drowsiness | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.02, 2.90] |
| 6.4 arousal ‐ lightheadedness | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.2 [0.21, 82.72] |
| 6.5 cardiac ‐ chest pain | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 6.52] |
| 6.6 cardiac ‐ dizziness | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.28, 22.20] |
| 6.7 cardiac ‐ hypotensive crisis | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.8 cardiac ‐ orthostatic hypotension | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.06, 12.49] |
| 6.9 cardiac ‐ tachycardia | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 6.52] |
| 6.10 other ‐ depressed mood | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [0.11, 58.67] |
| 6.11 other ‐ diaphoresis | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 6.52] |
| 6.12 other ‐ weakness | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.30, 25.21] |
| 6.13 other ‐ unsteadiness | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Repeated need for tranquillisation ‐ need for additional drugs for tranquillisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 up to 2 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.13, 1.01] |
| 1.2 up to 24 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.07, 2.53] |
| 2 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 endpoint score at 1 hour (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐7.70 [‐9.41, ‐5.99] |
| 2.2 endpoint score at 2 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐3.90 [‐6.38, ‐1.42] |
| 2.3 endpoint score at 2 hours (PANSS‐EC, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐1.13, 1.25] |
| 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 endpoint score at 4 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.05, 0.45] |
| 4 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 endpoint score at 6 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.05, 0.35] |
| 4.2 endpoint score at 6 hours (PANSS‐EC, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐1.68, 0.74] |
| 4.3 endpoint score at 12 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.9 [0.82, 0.98] |
| 4.4 endpoint score at 24 hours (PANSS‐EC, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐1.36, 0.94] |
| 5 Specific behaviour: 1d. Agitation ‐ average scores ‐ iv. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 endpoint scores at 48 hours (PANSS‐EC, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐1.03, 1.15] |
| 5.2 endpoint scores at 72 hours (PANSS‐EC, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 0.62 [‐0.45, 1.69] |
| 6 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 endpoint score at 1 hour (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.77, 0.77] |
| 6.2 endpoint score at 2 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.25, 1.65] |
| 7 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 endpoint score at 4 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.04, 0.64] |
| 8 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 endpoint score at 6 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.60, 0.80] |
| 8.2 endpoint score at 12 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.07, 0.33] |
| 9 Global outcome: 1. General ‐ need for additional measures Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 need for anxiolytic during 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.84, 1.48] |
| 9.2 need for hypnotics for night time sedation during 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.20, 2.50] |
| 9.3 additional restraint, seclusion or special observation during 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.25, 1.44] |
| 10 Global outcome: 2. Average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 change score at 72 hours (CGI‐S, high=worse) | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.13, 0.55] |
| 10.2 change score at 7 days (CGI‐S, high=worse) | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | 0.51 [0.07, 0.95] |
| 11 Mental state: 1a. Average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 endpoint score at 2 hours (BPRS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Mental state: 1b. Average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 endpoint score at 4 hours (BPRS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Mental state: 1c. Average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 endpoint score at 24 hours (BPRS, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Mental state: 1d. Average scores ‐ iv. over 24 hours Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 endpoint score at 48 hours (BPRS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.05 [‐6.45, 2.35] |
| 14.2 endpoint score at 72 hours (PANSS total, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 2.45 [‐2.19, 7.09] |
| 14.3 endpoint score at 72 hours (PANSS positive sub‐scale, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 1.11 [‐0.45, 2.67] |
| 14.4 endpoint score at 72 hours (PANSS negative sub‐scale, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐4.19 [‐5.71, ‐2.67] |
| 14.5 endpoint score at 72 hours (PANSS general pathology score, high=worse) | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.27, 2.27] |
| 14.6 score at 72 hours (BPRS, high=worse) | 3 | 511 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐1.93, 1.07] |
| 14.7 change score at 7 days (BPRS, high=worse) | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | 2.93 [‐0.81, 6.67] |
| 15 Adverse effects: 1a. General ‐ i. up to 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 one or more drug related adverse effects ‐ by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.64, 2.93] |
| 16 Adverse effects: 1b. General ‐ ii. over 24 hours Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 one or more drug related adverse effects ‐ by 72 hours | 4 | 799 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.47, 2.06] |
| 16.2 one or more drug related adverse effects ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.93, 1.83] |
| 17 Adverse effects: 1c. General ‐ serious Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 severe adverse effect ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 death | 2 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.78] |
| 18 Adverse effects: 2a. Specific ‐ anticholinergic Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 blurred vision by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.97 [1.15, 13.68] |
| 18.2 constipation by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.06] |
| 18.3 dry mouth by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [1.22, 7.22] |
| 18.4 hypersalivation by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [1.11, 5.87] |
| 19 Adverse effects: 2b. Specific ‐ arousal level ‐ i. various Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 excessive sedation at 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.22, 4.56] |
| 19.2 insomnia ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.53] |
| 19.3 lethargy ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.67, 2.35] |
| 19.4 somnolence ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.43, 3.12] |
| 20 Adverse effects: 2c. Specific ‐ arousal level ‐ ii. average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 20.1 endpoint score at 1 hour (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.46, 0.46] |
| 20.2 endpoint score at 2 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.46, 0.46] |
| 21 Adverse effects: 2d. Specific ‐ arousal level ‐ ii. average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.1 endpoint score at 4 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.83, 0.03] |
| 22 Adverse effects: 2e. Specific ‐ arousal level ‐ ii. average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 22.1 endpoint score at 6 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.40, 0.20] |
| 22.2 endpoint score at 12 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.40, 0.20] |
| 23 Adverse effects: 2f. Specific ‐ cardiovascular Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 hypotension at 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.31] |
| 23.2 dizziness ‐ by 72 hours | 2 | 607 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.28, 1.19] |
| 23.3 ECG ‐ abnormal ‐ by 72 hours | 2 | 607 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.17, 0.84] |
| 23.4 ECG change ‐ clinically significant ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.60, 1.71] |
| 23.5 tachycardia ‐ by 72 hours | 3 | 739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.23, 1.04] |
| 24 Adverse effects: 2g. Specific ‐ gastrointestinal Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 24.1 nausea by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.06] |
| 24.2 vomiting by 72 hours | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.02, 5.72] |
| 24.3 vomiting by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 2.82] |
| 25 Adverse effects: 2h. Specific ‐ hematological tests Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.1 abnormal lab results ‐ by 72 hours | 2 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.69, 1.17] |
| 25.2 abnormal lab results ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.47, 2.15] |
| 25.3 aspartate aminotransference ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.12 [0.62, 199.64] |
| 25.4 glucose increased ‐ by 24 hours | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.31, 2.92] |
| 25.5 haemogram abnormal ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.35] |
| 25.6 lactate dehydrogenase increase ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.39] |
| 25.7 liver function abnormal ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.39] |
| 25.8 triglyceride increase ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.96 [0.24, 102.14] |
| 26 Adverse effects: 2i. Specific ‐ movement disorders ‐ i. binary measures Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26.1 akathisia ‐ by 72 hours | 3 | 739 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [1.34, 4.01] |
| 26.2 akathisia ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 [1.13, 16.31] |
| 26.3 dystonia ‐ by 72 hours | 2 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.26 [1.67, 63.17] |
| 26.4 dystonia ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.68 [0.76, 9.47] |
| 26.5 EPS ‐ by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.64, 190.53] |
| 26.6 EPS ‐ by 72 hours | 2 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.13 [7.59, 48.21] |
| 26.7 EPS ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 34.29 [4.70, 250.02] |
| 26.8 extrapyramidal effects ‐ use of antiparkinson drugs ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.30 [1.82, 5.97] |
| 26.9 hypertonia/rigidity ‐ by 72 hours | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.81 [0.78, 280.47] |
| 26.10 hypertonia ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [0.90, 14.25] |
| 26.11 myotonia ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.84 [1.85, 8.00] |
| 26.12 pyramidal tract syndrome ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.18 [1.00, 295.55] |
| 26.13 reduced movement ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.17, 3.91] |
| 26.14 torsional spasm ‐ by 72 hours | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.30 [0.93, 11.70] |
| 26.15 tremor ‐ by 72 hours | 2 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [1.37, 5.11] |
| 26.16 tremor ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 [0.82, 22.48] |
| 27 Adverse effects: 2j. Specific ‐ movement disorders ‐ ii. average scores ‐ i. over 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 27.1 change score at 72 hours (BAS, high=worse) | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [0.18, 0.76] |
| 27.2 score at 72 hours (SAS, high=worse) | 2 | 191 | Mean Difference (IV, Fixed, 95% CI) | 3.59 [2.15, 5.03] |
| 27.3 change score at 7 days (BAS, high=worse) | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | 0.9 [0.51, 1.29] |
| 27.4 change score at 7 days (SAS, high=worse) | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | 6.1 [3.91, 8.29] |
| 28 Adverse effects: 2k. Specific ‐ miscellaneous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 28.1 physical examination significant changes ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 27.29 [1.63, 455.72] |
| 29 Leaving the study early: 1. For any reason ‐ i. over 24 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 29.1 by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.53, 5.94] |
| 29.2 by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.86, 5.32] |
| 30 Leaving the study early: 2. For specific reasons ‐ i. over 24 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30.1 adverse events ‐ by 72 hours | 2 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [0.45, 12.75] |
| 30.2 adverse events ‐ by 7 days | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.06, 4.65] |
| 30.3 withdrew consent ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.05 [0.24, 104.55] |
| 30.4 other ‐ by 72 hours | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Repeated need for tranquillisation: more than 3 injections Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [1.19, 5.46] |
| 2 Adverse effects: 1a. Specific ‐ movement disorders Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 movement disorder ‐ tremor by 7 days | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.16 [0.93, 18.62] |
| 3 Adverse effects: 1b. Specific ‐ miscellaneous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 pain/allergy ‐ reaction at injection site | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.55 [0.15, 84.14] |
| 4 Leaving the study early Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1. Aggression ‐ binary measures ‐ i. up to 2 hours Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.86, 1.55] |
| 1.1 ≥50% reduction at 90 minutes (OAS) | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.86, 1.55] |
| 2 Global outcome: 1. Need for seclusion or restraint Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Adverse effects: 1. Specific ‐ movement disorders ‐ i. binary measures ‐ i. by 30 minutes Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 EPS by 30 minutes | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ not asleep Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 at 1 hour | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.76, 1.44] |
| 1.2 by 3 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.14, 3.27] |
| 2 Repeated need for rapid tranquillisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 more than 1 injection | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.91, 1.43] |
| 2.2 more than 3 injections | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.50, 2.45] |
| 3 Global outcome: 1. No overall improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 by 30 minutes | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.70, 1.71] |
| 3.2 at 1 hour | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.54, 5.03] |
| 3.3 at > 1 hour | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Mental state: 1a. Average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 endpoint score at 1 hour (BPRS, high=worse) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 3.26 [‐4.13, 10.65] |
| 4.2 endpoint score at 2 hours (BPRS, high=worse) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 4.07 [‐2.62, 10.76] |
| 5 Mental state: 1b. Average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 endpoint score at 3 hours (BPRS, high=worse) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 5.03 [‐2.98, 13.04] |
| 5.2 endpoint score at 4 hours (BPRS, high=worse) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 1.73 [‐6.14, 9.60] |
| 6 Adverse effects: 1a. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 one or more drug‐related adverse effect up to 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.60, 2.10] |
| 7 Adverse effects: 1b. General ‐ serious Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8 Adverse effects: 2a. Specific ‐ anticholinergic Show forest plot | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.14, 2.04] |
| 8.1 dry mouth up to 24 hours (only reported if occurred ≥9%) | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.14, 2.04] |
| 9 Adverse effects: 2b. Specific ‐ arousal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 asleep ‐ at 1 hour | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.40, 1.99] |
| 10 Adverse effects: 2c. Specific ‐ cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 dizziness (only reported if occurred in ≧9%) ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.19, 4.07] |
| 10.2 hypertension ‐ use of additional clonidine ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.11, 63.17] |
| 11 Adverse effects: 2d. Specific ‐ movement disorder Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 ataxia (only reported if occurred in ≧9%) ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.04, 4.65] |
| 11.2 dystonia (only reported if occurred in ≧9%) ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.54 [0.42, 30.03] |
| 11.3 EPS | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.0 [2.11, 106.49] |
| 11.4 hypertonia/rigidity (only reported if occurred in ≧9%) ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.22 [0.33, 115.91] |
| 11.5 speech disorder (only reported if occurred in ≧9%) ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.35, 9.01] |
| 11.6 tremor (only reported if occurred in ≧9%) ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.17, 18.60] |
| 11.7 use of additional benztropine ‐ during 24 hours | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.68, 5.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep: average times in minutes ‐ (skewed data) Show forest plot | Other data | No numeric data | ||
| 1.1 average time to sedation | Other data | No numeric data | ||
| 1.2 average time to arousal | Other data | No numeric data | ||
| 2 Global outcome: 1. Need for rescue drug Show forest plot | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.46, 2.87] |
| 3 Adverse effects: 1. General ‐ one or more drug related adverse effect Show forest plot | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 101.11] |
| 4 Adverse effects: 2. Specific Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 hypotensive | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.61] |
| 4.2 apnoea | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global outcome: 1. No overall improvement Show forest plot | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.18 [0.50, 133.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ asleep Show forest plot | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.11, 3.02] |
| 1.1 not asleep by 3 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.11, 3.02] |
| 2 Repeated need for rapid tranquillisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 needing additional injection during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.87, 1.27] |
| 2.2 more than 3 injections during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.92, 10.10] |
| 3 Global outcome: 1. No overall improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 by 30 minutes | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [1.25, 5.68] |
| 3.2 at 1 hour | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.77 [0.88, 247.54] |
| 3.3 at > 1 hour | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Adverse effects: 1. General Show forest plot | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.62, 2.18] |
| 4.1 one or more drug‐related adverse effect during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.62, 2.18] |
| 5 Adverse effects: 2a. Specific ‐ anticholinergic Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 dry mouth (only reported if occurred in ≧9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.20, 4.21] |
| 6 Adverse effects: 2b. Specific ‐ cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 dizziness (only reported if occurred in ≧9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.24, 7.69] |
| 6.2 hypertension ‐ use of additional clonidine during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.06, 14.02] |
| 7 Adverse effects: 2c. Specific ‐ movement disorders Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 ataxia (only reported if occurred in >9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.03, 2.78] |
| 7.2 dystonia (only reported if occurred in >9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.25 [0.46, 147.45] |
| 7.3 hypertonia/rigidity (only reported if occurred in >9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.30, 25.05] |
| 7.4 tremor (only reported if occurred in >9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.17, 19.21] |
| 7.5 speech disorder (only reported if occurred in >9%) during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.30, 5.03] |
| 7.6 use of additional benztropine during 24 hours | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.81, 9.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Repeated need for tranquillisation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 need for additional drugs for tranquillisation up to 2 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.10, 0.71] |
| 1.2 need for additional drugs for tranquillisation by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.13] |
| 2 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 endpoint score at 1 hour (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐8.5 [‐9.93, ‐7.07] |
| 2.2 endpoint score at 2 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐6.7 [‐7.46, ‐5.94] |
| 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 endpoint score at 4 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐5.5 [‐6.48, ‐4.52] |
| 4 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 endpoint score at 6 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐4.56, ‐2.84] |
| 4.2 endpoint score at 12 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐11.2 [‐12.24, ‐10.16] |
| 5 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 endpoint score at 1 hour (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.44, 0.04] |
| 5.2 endpoint score at 2 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐4.21, ‐0.59] |
| 6 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 endpoint score at 4 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.68, 0.48] |
| 7 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 endpoint score at 6 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.30, 0.50] |
| 7.2 endpoint score at 12 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.9 [‐2.58, ‐1.22] |
| 8 Global outcomes: 1. General ‐ need for additional measures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 additional restraint, seclusion or special observation during 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.13, 0.61] |
| 9 Adverse effects: 1b. General ‐ one or more adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 during 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.41, 1.32] |
| 10 Adverse effects: 1a. General ‐ serious Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 death | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Adverse effects: 2a. Specific ‐ arousal ‐ i. various Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 excessive sedation by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.08, 0.80] |
| 12 Adverse effects: 2b. Specific ‐ arousal ‐ ii. average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 endpoint score at 1 hour (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.01, 0.01] |
| 12.2 endpoint score at 2 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.56, 0.36] |
| 13 Adverse effects: 2c. Specific ‐ arousal ‐ ii. average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 endpoint score at 4 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.78, 0.18] |
| 14 Adverse effects: 2d. Specific ‐ arousal ‐ iii. average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 endpoint score at 6 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.40, 0.20] |
| 14.2 endpoint score at 12 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.26, 0.46] |
| 15 Adverse effects: 2e. Specific ‐ cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 hypotension by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.57] |
| 16 Adverse effects: 2f. Specific ‐ movement disorders Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 EPS by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.44, 6.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep ‐ not tranquil or asleep ‐ i. by 30 minutes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 at 20 minutes | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.18, 2.16] |
| 2 Tranquillisation or asleep ‐ not tranquil or asleep ‐ ii. up to 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 at 40 minutes | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.83, 1.91] |
| 2.2 at 1 hour | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.85, 2.37] |
| 2.3 at 2 hours | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.68, 2.56] |
| 3 Repeated need for tranquillisation ‐ need for additional drugs for tranquillisation Show forest plot | 2 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.29] |
| 3.1 by 2 hours | 2 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.43, 1.41] |
| 3.2 by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.81, 1.49] |
| 4 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 endpoint score at 1 hour (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐24.5 [‐27.32, ‐21.68] |
| 4.2 endpoint score at 2 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐9.40 [‐10.39, ‐8.41] |
| 5 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 endpoint score at 4 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [3.27, 4.33] |
| 6 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 endpoint score at 6 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.6 [2.13, 3.07] |
| 6.2 endpoint score at 12 hours (OASS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [0.55, 1.05] |
| 7 Specific behaviour: 2a. Aggression ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 endpoint score at 1 hour (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐4.50 [‐6.28, ‐2.72] |
| 7.2 endpoint score at 2 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.89, 0.49] |
| 8 Specific behaviour: 2b. Aggression ‐ average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 endpoint score at 4 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.49, 0.71] |
| 9 Specific behaviour: 2c. Aggression ‐ average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 endpoint score at 6 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [0.91, 1.29] |
| 9.2 endpoint score at 12 hours (OAS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.8 [1.67, 1.93] |
| 10 Specific behaviour: 2d. Aggression ‐ further aggressive episode ‐ i. up to 24 hours Show forest plot | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.65] |
| 11 Global outcomes: 1. General ‐ need for additional measures Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 restraints needed by 2 hours | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.84, 1.76] |
| 11.2 additional restraint, seclusion or special observation during 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.41, 3.51] |
| 12 Global outcomes: 2. Specific Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 doctor called to see patient | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.05, 2.14] |
| 12.2 refuse oral drugs | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.61, 1.60] |
| 13 Adverse effects: 1a. General Show forest plot | 2 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.07, 3.80] |
| 13.1 one or more adverse effects up to 24 hours | 2 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.07, 3.80] |
| 14 Adverse effects: 1b. General ‐ serious Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 seizure | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.19, 22.39] |
| 14.2 death | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Adverse effects: 2a. Specific ‐ arousal ‐ i. various Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 excessive sedation by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.23] |
| 16 Adverse effects: 2b. Specific ‐ arousal ‐ ii. average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.1 endpoint score at 1 hour (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.38, 0.58] |
| 16.2 endpoint score at 2 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.50, 0.30] |
| 17 Adverse effects: 2c. Specific ‐ arousal ‐ ii. average scores ‐ ii. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 17.1 endpoint score at 4 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.78, 0.18] |
| 18 Adverse effects: 2d. Specific ‐ arousal ‐ ii. average scores ‐ iii. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.1 endpoint score at 6 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.48, 0.08] |
| 18.2 endpoint score at 12 hours (RSS, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.28, 0.28] |
| 19 Adverse effects: 2e. Specific ‐ cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 hypotension by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.65] |
| 20 Adverse effects: 2f. Specific ‐ movement disorders Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 acute dystonia | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.48 [1.14, 331.92] |
| 20.2 EPS by 12 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.32, 3.10] |
| 21 Service outcomes: 1. Not discharged by 14 days Show forest plot | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.72, 1.05] |
| 22 Leaving the study early Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 any reason | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.54, 1.94] |
| 22.2 absconded before receiving drug | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.24] |
| 22.3 incomplete information in notes | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.51, 2.46] |
| 22.4 seizure before drug was given | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.13, 74.95] |
| 22.5 transfer to another hospital/notes lost | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.21, 5.00] |
| 22.6 withdrew consent | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.06, 16.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 endpoint score at 24 hours (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 endpoint score at 3 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 endpoint score at 5 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 endpoint score at 1 week (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 endpoint score at 2 weeks (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Global outcome: No improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 at 72 hours | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 at 5 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 at 1 week | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 at 2 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects: 1a. Specific ‐ arousal level ‐ i. various Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 insomnia during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 somnolence during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse effects: 1b. Specific ‐ movement disorders ‐ i. various Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 tremor during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 akathisia during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Adverse effects: 1c. Specific ‐ miscellaneous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Dry mouth during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Constipation during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Increased salivation during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1. Agitation ‐ average scores ‐ over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 endpoint score at 72 hours (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 endpoint score at 7 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 endpoint score at 14 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Global outcome: No improvement Show forest plot | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.38, 2.95] |
| 3 Mental state: Average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 endpoint score at 72 hours (PANSS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 endpoint score at 7 days (PANSS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 endpoint score at 14 days (PANSS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects: General ‐ over 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 one or more drug related adverse effects ‐ by 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1a. Agitation ‐ binary measures Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.44, 3.06] |
| 1.1 <25% reduction, no effect (PANSS‐EC) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.44, 3.06] |
| 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 endpoint score at 3 days (PANSS‐EC, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐2.31, 2.35] |
| 2.2 endpoint score at 7 days (PANSS‐EC, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.16 [‐0.98, 3.30] |
| 2.3 endpoint score at 14 days (PANSS‐EC, high=worse) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.24 [‐0.49, 2.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tranquillisation or asleep Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 not asleep at 2 hours | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.82, 1.39] |
| 1.2 not asleep at 4 hours | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.66, 1.03] |
| 2 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 change score at 2 hours (PANSS‐EC, high=worse) | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐3.80, 2.80] |
| 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 4 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 change score at 4 hours (PANSS‐EC, high=worse) | 1 | 126 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐5.20, 0.40] |
| 4 Specific behaviour: 1c. Agitation ‐ average scores ‐ iii. up to 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 score at 24 hours (PANSS‐EC, high=worse) | 2 | 305 | Mean Difference (IV, Fixed, 95% CI) | 0.71 [‐0.56, 1.98] |
| 5 Specific behaviour: 1d. Agitation ‐ average scores ‐ iv. over 24 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 score at 3 days (PANSS‐EC, high=worse) | 2 | 305 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐1.66, 0.83] |
| 5.2 score at 5 days (PANSS‐EC, high=worse) | 2 | 305 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐1.24, 1.52] |
| 5.3 endpoint score at 1 week (PANSS‐EC, high=worse) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐2.56 [‐4.57, ‐0.55] |
| 5.4 endpoint score at 2 weeks (PANSS‐EC, high=worse) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐2.34, 0.58] |
| 6 Global outcome: No improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 at 72 hours | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 at 5 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 at 1 week | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 at 2 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Mental state: 1. Average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 change score at 120 hours (PANSS total, high=worse) | 1 | 205 | Mean Difference (IV, Fixed, 95% CI) | ‐3.5 [‐7.07, 0.07] |
| 8 Adverse effects: 1. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 overall adverse events during 120 hours | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.29, 2.29] |
| 9 Adverse effects: 2a. Specific ‐ arousal ‐ i. various Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 insomnia during 5 days (only reported if occurred in ≥5%) | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.45, 5.31] |
| 9.2 insomnia during 14 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.20, 1.23] |
| 9.3 somnolence during 14 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.94, 8.06] |
| 10 Adverse effects: 2b. Specific ‐ cardiovascular ‐ i. various Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 tachycardia during 120 hours (only reported if occurred in ≥5%) | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.36, 4.66] |
| 11 Adverse effects: 2c. Specific ‐ movement disorders ‐ i. various Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 akathisia during 5 days (only reported if occurred in ≥5%) | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.12, 2.38] |
| 11.2 akathisia during 14 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.98, 5.58] |
| 11.3 EPS during 5 days (only reported if occurred in ≥5%) | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [1.52, 3.23] |
| 11.4 tremor during 14 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.2 [1.27, 8.07] |
| 12 Adverse effects: 2d. Specific ‐ miscellaneous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.1 Dry mouth during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Constipation during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Increased salivation during 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1a. Agitation ‐ average scores ‐ i. up to 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 endpoint score at 12 hours (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ ii. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 endpoint score at 48 hours (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 endpoint score at 3 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 endpoint score at 5 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 endpoint score at 7 days (PANSS‐EC, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Global outcome: 1. Average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 endpoint score at 7 days (CGI‐S, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Mental state: 1. Average scores ‐ i. over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 endpoint score at 7 days (PANSS total, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 endpoint score at 7 days (PANSS positive sub‐scale, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 endpoint score at 7 days (PANSS negative sub‐scale, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 endpoint score at 7 days (PANSS general psychopathology sub‐scale, high=worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Leaving the study early Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 any reason | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 lost at follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 non compliance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| AE | Dose | 1 mg (N = 57) | 5 mg (N = 63) | 15 mg (N = 58) |
| At least 1 AE | 28 | 30 | 27 | |
| AE rated as serious | 1 | 2 | 0 | |
| Tachycardia | 3 | 2 | 0 | |
| Sinus tachycardia | 1 | 0 | 0 | |
| Vomiting | 1 | 0 | 3 | |
| Nausea | 0 | 6 | 2 | |
| Dizziness | 4 | 7 | 7 | |
| Headache | 4 | 11 | 8 | |
| Somnolence | 3 | 5 | 6 | |
| Akathisia | 0 | 2 | 0 | |
| Dystonia | 0 | 0 | 1 | |
| Agitation | 0 | 0 | 3 | |
| Pain at injection site | Not reported | 1 | 1 | |
| QTC abnormality | 4 | 4 | 3 | |
| AE: adverse event | ||||
| Outcome | Dose | Mean | SD | N |
| Mean dose of study drug | 2.5 mg | 4 | 1.5 | 48 |
| 5 mg | 6.9 | 2.7 | 45 | |
| 7.5 mg | 9.8 | 3.8 | 46 | |
| Mean dose of benzodiazepines | 2.5 mg | 3.2 | 1.1 | 48 |
| 5 mg | 2.0 | 0 | 45 | |
| 7.5 mg | 3.0 | 1.4 | 46 |
| Rating scale (Mean change at 2 hr) | Dose | Mean | SD | N |
| Specific behaviour: ACES | 2.5 mg | 1.3 | 1.5 | 48 |
| 5 mg | 2.3 | 1.9 | 45 | |
| 7.5 mg | 2.4 | 1.7 | 46 | |
| Specific behaviour: ABS | 2.5 mg | ‐5.8 | 5.5 | 48 |
| 5 mg | ‐9.0 | 5.5 | 45 | |
| 7.5 mg | ‐10.5 | 5.6 | 46 | |
| Specific behaviour: PANSS‐EC | 2.5 mg | ‐5.5 | 4.6 | 48 |
| 5 mg | ‐8.1 | 5.3 | 45 | |
| 7.5 mg | ‐8.7 | 5 | 46 | |
| Mental state: BPRS Total | 2.5 mg | ‐8.2 | 9.1 | 48 |
| 5 mg | ‐10.4 | 7.5 | 45 | |
| 7.5 mg | ‐12.0 | 7 | 46 | |
| Mental state: BPRS Positive | 2.5 mg | ‐1.5 | 3.1 | 48 |
| 5 mg | ‐1.7 | 2.8 | 45 | |
| 7.5 mg | ‐2.1 | 2.9 | 46 | |
| ABS: Agitated Behavior Scale | ||||
| Rating scale (mean change at 24 hr) | Dose | Mean | SD | N |
| Specific behaviour: ACES | 2.5 mg | 0.9 | 0.8 | 48 |
| 5 mg | 1.1 | 1.1 | 45 | |
| 7.5 mg | 1 | 1 | 46 | |
| Specific behaviour: ABS | 2.5 mg | ‐5.7 | 4.2 | 48 |
| 5 mg | ‐6.7 | 5.9 | 45 | |
| 7.5 mg | ‐7.7 | 5.8 | 46 | |
| Specific behaviour: PANSS‐EC | 2.5 mg | ‐4.9 | 4.3 | 48 |
| 5 mg | ‐5.5 | 4.9 | 45 | |
| 7.5 mg | ‐5.5 | 4.1 | 46 | |
| Global outcomes: CGI‐S (24 hr) | 2.5 mg | ‐0.3 | 0.5 | 48 |
| 5 mg | ‐0.5 | 0.8 | 45 | |
| 7.5 mg | ‐0.6 | 0.7 | 46 | |
| Mental state: BPRS Total | 2.5 mg | ‐8.4 | 7.4 | 48 |
| 5 mg | ‐9.2 | 7.8 | 45 | |
| 7.5 mg | ‐9.6 | 7.5 | 46 | |
| Mental state: BPRS Positive | 2.5 mg | ‐1.5 | 2.3 | 48 |
| 5 mg | ‐2.0 | 2.6 | 45 | |
| 7.5 mg | ‐1.9 | 2.7 | 46 | |
| ABS: Agitated Behavior Scale | ||||
|
| Dose | 2.5 mg | 5 mg | 7.5 mg |
| Outcome |
| N = 48 | N = 45 | N = 46 |
| Global outcome: > 1 injection | 25 | 17 | 14 | |
| Leaving the study early: lack of efficacy | 0 | 2 | 0 | |
| Adverse effects: EPS | 0 | 0 | 0 | |
| Adverse effects: akathisia | 0 | 2 | 0 | |
| Adverse effects: QT abnormality | 0 | 4 | 2 | |
| EPS: Extrapyramidal Side Effects | ||||
| Rating scale (Mean change 2 hr after 1st injection) | Dose | Mean | SD | N |
| Specific behaviour: ACES | 1 mg | 0.65 | 1.64 | 56 |
| 5 mg | 1.01 | 1.65 | 62 | |
| 15 mg | 0.99 | 1.73 | 58 | |
| Specific behaviour: CABS | 1 mg | ‐5.15 | 6.8 | 56 |
| 5 mg | ‐5.97 | 6.81 | 62 | |
| 15 mg | ‐7.04 | 7.01 | 58 | |
| Global outcomes: CGI‐I | 1 mg | 3.07 | 0.98 | 56 |
| 5 mg | 2.82 | 1.10 | 62 | |
| 15 mg | 2.66 | 1.07 | 58 | |
| Global outcomes: CGI‐S | 1 mg | ‐0.63 | 1.15 | 56 |
| 5 mg | ‐0.82 | 1.12 | 62 | |
| 15 mg | ‐0.99 | 1.17 | 58 | |
| Mental state: BPRS Total | 1 mg | ‐6.53 | 9.61 | 55 |
| 5 mg | ‐8.16 | 9.66 | 61 | |
| 15 mg | ‐8.88 | 9.89 | 56 | |
| Mental state: BPRS Positive | 1 mg | ‐1.20 | 2.72 | 55 |
| 5 mg | ‐1.47 | 2.71 | 61 | |
| 15 mg | ‐1.86 | 2.82 | 57 | |
| ACES: Agitation‐Calmness Evaluation Scale | ||||
|
| Dose | 1 mg | 5 mg | 15 mg |
| Outcome | N | N = 57 | N = 63 | N = 58 |
| PEC response ≥ 40% | 21 | 31 | 32 | |
| Need for rescue medication | 11 | 5 | 12 | |
| Discontinued for any reason | 2 | 3 | 1 | |
| Discontinued due to adverse event | 0 | 0 | 1 | |
| Withdrew consent | 2 | 2 | 0 | |
| Discontinued due to other known cause | 0 | 1 | 0 | |
| PEC: Psychiatric Emergency Centre | ||||
| AE | Dose | 1 mg (N = 57) | 5 mg (N = 63) | 15 mg (N = 58) |
| At least 1 AE | 28 | 30 | 27 | |
| AE rated as serious | 1 | 2 | 0 | |
| Tachycardia | 3 | 2 | 0 | |
| Sinus tachycardia | 1 | 0 | 0 | |
| Vomiting | 1 | 0 | 3 | |
| Nausea | 0 | 6 | 2 | |
| Dizziness | 4 | 7 | 7 | |
| Headache | 4 | 11 | 8 | |
| Somnolence | 3 | 5 | 6 | |
| Akathisia | 0 | 2 | 0 | |
| Dystonia | 0 | 0 | 1 | |
| Agitation | 0 | 0 | 3 | |
| Pain at injection site | Not reported | 1 | 1 | |
| QTC abnormality | 4 | 4 | 3 | |
| AE: adverse event | ||||
| Outcome | Dose | Mean | SD | N |
| Mean dose of study drug | 2.5 mg | 4 | 1.5 | 48 |
| 5 mg | 6.9 | 2.7 | 45 | |
| 7.5 mg | 9.8 | 3.8 | 46 | |
| Mean dose of benzodiazepines | 2.5 mg | 3.2 | 1.1 | 48 |
| 5 mg | 2.0 | 0 | 45 | |
| 7.5 mg | 3.0 | 1.4 | 46 |
| Rating scale (Mean change at 2 hr) | Dose | Mean | SD | N |
| Specific behaviour: ACES | 2.5 mg | 1.3 | 1.5 | 48 |
| 5 mg | 2.3 | 1.9 | 45 | |
| 7.5 mg | 2.4 | 1.7 | 46 | |
| Specific behaviour: ABS | 2.5 mg | ‐5.8 | 5.5 | 48 |
| 5 mg | ‐9.0 | 5.5 | 45 | |
| 7.5 mg | ‐10.5 | 5.6 | 46 | |
| Specific behaviour: PANSS‐EC | 2.5 mg | ‐5.5 | 4.6 | 48 |
| 5 mg | ‐8.1 | 5.3 | 45 | |
| 7.5 mg | ‐8.7 | 5 | 46 | |
| Mental state: BPRS Total | 2.5 mg | ‐8.2 | 9.1 | 48 |
| 5 mg | ‐10.4 | 7.5 | 45 | |
| 7.5 mg | ‐12.0 | 7 | 46 | |
| Mental state: BPRS Positive | 2.5 mg | ‐1.5 | 3.1 | 48 |
| 5 mg | ‐1.7 | 2.8 | 45 | |
| 7.5 mg | ‐2.1 | 2.9 | 46 | |
| ABS: Agitated Behavior Scale | ||||
| Rating scale (mean change at 24 hr) | Dose | Mean | SD | N |
| Specific behaviour: ACES | 2.5 mg | 0.9 | 0.8 | 48 |
| 5 mg | 1.1 | 1.1 | 45 | |
| 7.5 mg | 1 | 1 | 46 | |
| Specific behaviour: ABS | 2.5 mg | ‐5.7 | 4.2 | 48 |
| 5 mg | ‐6.7 | 5.9 | 45 | |
| 7.5 mg | ‐7.7 | 5.8 | 46 | |
| Specific behaviour: PANSS‐EC | 2.5 mg | ‐4.9 | 4.3 | 48 |
| 5 mg | ‐5.5 | 4.9 | 45 | |
| 7.5 mg | ‐5.5 | 4.1 | 46 | |
| Global outcomes: CGI‐S (24 hr) | 2.5 mg | ‐0.3 | 0.5 | 48 |
| 5 mg | ‐0.5 | 0.8 | 45 | |
| 7.5 mg | ‐0.6 | 0.7 | 46 | |
| Mental state: BPRS Total | 2.5 mg | ‐8.4 | 7.4 | 48 |
| 5 mg | ‐9.2 | 7.8 | 45 | |
| 7.5 mg | ‐9.6 | 7.5 | 46 | |
| Mental state: BPRS Positive | 2.5 mg | ‐1.5 | 2.3 | 48 |
| 5 mg | ‐2.0 | 2.6 | 45 | |
| 7.5 mg | ‐1.9 | 2.7 | 46 | |
| ABS: Agitated Behavior Scale | ||||
|
| Dose | 2.5 mg | 5 mg | 7.5 mg |
| Outcome |
| N = 48 | N = 45 | N = 46 |
| Global outcome: > 1 injection | 25 | 17 | 14 | |
| Leaving the study early: lack of efficacy | 0 | 2 | 0 | |
| Adverse effects: EPS | 0 | 0 | 0 | |
| Adverse effects: akathisia | 0 | 2 | 0 | |
| Adverse effects: QT abnormality | 0 | 4 | 2 | |
| EPS: Extrapyramidal Side Effects | ||||
| Rating scale (Mean change 2 hr after 1st injection) | Dose | Mean | SD | N |
| Specific behaviour: ACES | 1 mg | 0.65 | 1.64 | 56 |
| 5 mg | 1.01 | 1.65 | 62 | |
| 15 mg | 0.99 | 1.73 | 58 | |
| Specific behaviour: CABS | 1 mg | ‐5.15 | 6.8 | 56 |
| 5 mg | ‐5.97 | 6.81 | 62 | |
| 15 mg | ‐7.04 | 7.01 | 58 | |
| Global outcomes: CGI‐I | 1 mg | 3.07 | 0.98 | 56 |
| 5 mg | 2.82 | 1.10 | 62 | |
| 15 mg | 2.66 | 1.07 | 58 | |
| Global outcomes: CGI‐S | 1 mg | ‐0.63 | 1.15 | 56 |
| 5 mg | ‐0.82 | 1.12 | 62 | |
| 15 mg | ‐0.99 | 1.17 | 58 | |
| Mental state: BPRS Total | 1 mg | ‐6.53 | 9.61 | 55 |
| 5 mg | ‐8.16 | 9.66 | 61 | |
| 15 mg | ‐8.88 | 9.89 | 56 | |
| Mental state: BPRS Positive | 1 mg | ‐1.20 | 2.72 | 55 |
| 5 mg | ‐1.47 | 2.71 | 61 | |
| 15 mg | ‐1.86 | 2.82 | 57 | |
| ACES: Agitation‐Calmness Evaluation Scale | ||||
|
| Dose | 1 mg | 5 mg | 15 mg |
| Outcome | N | N = 57 | N = 63 | N = 58 |
| PEC response ≥ 40% | 21 | 31 | 32 | |
| Need for rescue medication | 11 | 5 | 12 | |
| Discontinued for any reason | 2 | 3 | 1 | |
| Discontinued due to adverse event | 0 | 0 | 1 | |
| Withdrew consent | 2 | 2 | 0 | |
| Discontinued due to other known cause | 0 | 1 | 0 | |
| PEC: Psychiatric Emergency Centre | ||||

