مهارکنندههای انتخابی بازجذب سروتونین (SSRI) در بهبود سکته مغزی
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
[1] The CENTRAL search strategy looks different to the one I have stored. Please see below the correct strategy and add it into your appendices.
#1 MeSH descriptor: [Cerebrovascular Disorders] this term only
#2 MeSH descriptor: [Basal Ganglia Cerebrovascular Disease] explode all trees
#3 MeSH descriptor: [Brain Ischemia] explode all trees
#4 MeSH descriptor: [Carotid Artery Diseases] explode all trees
#5 MeSH descriptor: [Intracranial Arterial Diseases] explode all trees
#6 MeSH descriptor: [Intracranial Embolism and Thrombosis] explode all trees
#7 MeSH descriptor: [Intracranial Hemorrhages] explode all trees
#8 MeSH descriptor: [Stroke] explode all trees
#9 MeSH descriptor: [Brain Infarction] explode all trees
#10 MeSH descriptor: [Vertebral Artery Dissection] this term only
#11 (stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva* or apoplex* or SAH):ti,ab,kw (Word variations have been searched)
#12 ((brain* or cerebr* or cerebell* or intracran* or intracerebral) near/5 (isch?emi* or infarct* or thrombo* or emboli* or occlus*)):ti,ab,kw (Word variations have been searched)
#13 ((brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)):ti,ab,kw (Word variations have been searched)
#14 MeSH descriptor: [Hemiplegia] this term only
#15 MeSH descriptor: [Paresis] explode all trees
#16 (hemipleg* or hemipar* or paresis or paretic):ti,ab,kw (Word variations have been searched)
#17 MeSH descriptor: [Gait Disorders, Neurologic] explode all trees
#18 {or #1‐#17}
#19 MeSH descriptor: [Serotonin Uptake Inhibitors] explode all trees
#20 ((serotonin or 5‐HT or 5 HT or 5‐hydroxytryptamine or 5 hydroxytryptamine) near/5 (uptake or reuptake or re‐uptake) near/5 inhib*):ti,ab,kw (Word variations have been searched)
#21 SSRI*:ti,ab,kw (Word variations have been searched)
#22 (alaproclat* or cericlamin* or citalopram or clomipramin* or dapoxetin* or etoperidon* or escitalopram or femoxetin* or fenfluramin* or fluoxetin* or fluvoxamin* or nonfenfluramin* or paroxetin* or sertralin$ or trazodone or vilazodone or zimelidine):ti,ab,kw (Word variations have been searched)
#23 (Celexa or Cipramil or Cipram or Recital or Emocal or Dalsan or Sepram or Seropram or Citox or Priligy or Lexapro or Cipralex or Seroplex or Esertia or Prozac or Fontex or Seromex or Seronil or Sarafem or Ladose or Motivest or Fluctin or fluox or Lovan or Luvox or Fevarin or Faverin or Favoxil or Movox or Paxil or Seroxat or Sereupin or Aropax or Deroxat or Divarius or Rexetin or Xetanor or Paroxat or Loxamine or Zoloft or Lustral or Serlain or Asentra):ti,ab,kw (Word variations have been searched)
#24 {or #19‐#23}
Appendix 2. MEDLINE (Ovid) search strategy
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vertebral artery dissection/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. hemiplegia/ or exp paresis/
6. (hemipleg$ or hemipar$ or paresis or paretic).tw.
7. exp Gait Disorders, Neurologic/
8. or/1‐7
9. exp Serotonin Uptake Inhibitors/
10. ((serotonin or 5‐HT or 5 HT or 5‐hydroxytryptamine or 5 hydroxytryptamine) adj5 (uptake or reuptake or re‐uptake) adj5 inhib$).tw.
11. SSRI$1.tw.
12. (alaproclat$ or cericlamin$ or citalopram or dapoxetin$ or escitalopram or femoxetin$ or fluoxetin$ or fluvoxamin$ or paroxetin$ or sertralin$ or trazodone or vilazodone or zimelidine).tw,nm.
13. (Celexa or Cipramil or Cipram or Recital or Emocal or Dalsan or Sepram or Seropram or Citox or Priligy or Lexapro or Cipralex or Seroplex or Esertia or Prozac or Fontex or Seromex or Seronil or Sarafem or Ladose or Motivest or Fluctin or fluox or Lovan or Luvox or Fevarin or Faverin or Favoxil or Movox or Paxil or Seroxat or Sereupin or Aropax or Deroxat or Divarius or Rexetin or Xetanor or Paroxat or Loxamine or Zoloft or Lustral or Serlain or Asentra).tw,nm.
14. 9 or 10 or 11 or 12 or 13
15. 8 and 14
16. exp animals/ not humans.sh.
17. 15 not 16
18. Randomized Controlled Trials as Topic/
19. random allocation/
20. Controlled Clinical Trials as Topic/
21. control groups/
22. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/
23. Clinical Trials Data Monitoring Committees/
24. double‐blind method/
25. single‐blind method/
26. Placebos/
27. placebo effect/
28. cross‐over studies/
29. Multicenter Studies as Topic/
30. Therapies, Investigational/
31. Drug Evaluation/
32. Research Design/
33. Program Evaluation/
34. evaluation studies as topic/
35. randomized controlled trial.pt.
36. controlled clinical trial.pt.
37. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt.
38. multicenter study.pt.
39. (evaluation studies or comparative study).pt.
40. meta analysis.pt.
41. meta‐analysis as topic/
42. random$.tw.
43. (controlled adj5 (trial$ or stud$)).tw.
44. (clinical$ adj5 trial$).tw.
45. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
46. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
47. ((multicenter or multicentre or therapeutic) adj5 (trial$ or stud$)).tw.
48. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
49. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
50. (coin adj5 (flip or flipped or toss$)).tw.
51. latin square.tw.
52. versus.tw.
53. (cross‐over or cross over or crossover).tw.
54. placebo$.tw.
55. sham.tw.
56. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw.
57. controls.tw.
58. (treatment$ adj6 order).tw.
59. (meta‐analy$ or metaanaly$ or meta analy$ or systematic review or systematic overview).tw.
60. or/18‐59
61. 17 and 60
Appendix 3. Embase (Ovid) search strategy
1. cerebrovascular disease/ or basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or cerebral artery disease/ or cerebrovascular accident/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or stroke/
2. stroke unit/ or stroke patient/
3. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
5. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
6. hemiparesis/ or hemiplegia/ or paresis/
7. (hemipleg$ or hemipar$ or paresis or paretic).tw.
8. or/1‐7
9. exp serotonin uptake inhibitor/
10. ((serotonin or 5‐HT or 5 HT or 5‐hydroxytryptamine or 5 hydroxytryptamine) adj5 (uptake or reuptake or re‐uptake) adj5 inhib$).tw.
11. SSRI$1.tw.
12. (alaproclat$ or cericlamin$ or citalopram or dapoxetin$ or escitalopram or femoxetin$ or fluoxetin$ or fluvoxamin$ or paroxetin$ or sertralin$ or trazodone or vilazodone or zimelidine).tw.
13. (Celexa or Cipramil or Cipram or Recital or Emocal or Dalsan or Sepram or Seropram or Citox or Priligy or Lexapro or Cipralex or Seroplex or Esertia or Prozac or Fontex or Seromex or Seronil or Sarafem or Ladose or Motivest or Fluctin or fluox or Lovan or Luvox or Fevarin or Faverin or Favoxil or Movox or Paxil or Seroxat or Sereupin or Aropax or Deroxat or Divarius or Rexetin or Xetanor or Paroxat or Loxamine or Zoloft or Lustral or Serlain or Asentra).tw,tn.
14. 9 or 10 or 11 or 12 or 13
15. 8 and 14
16. limit 15 to human
17. Randomized Controlled Trial/
18. Randomization/
19. Controlled Study/
20. control group/
21. clinical trial/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or controlled clinical trial/
22. Double Blind Procedure/
23. Single Blind Procedure/ or triple blind procedure/
24. placebo/
25. "types of study"/
26. research subject/
27. random$.tw.
28. (controlled adj5 (trial$ or stud$)).tw.
29. (clinical$ adj5 trial$).tw.
30. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
31. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
32. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
33. (coin adj5 (flip or flipped or toss$)).tw.
34. versus.tw.
35. placebo$.tw.
36. controls.tw.
37. or/17‐36
38. 16 and 37
Appendix 4. CINAHL (Ebsco) search strategy
S23. S12 and S22
S22. S13 or S17 or S18 or S19 or S20 or S21
S21. AB Celexa or Cipramil or Cipram or Recital or Emocal or Dalsan or Sepram or Seropram or Citox or Priligy or Lexapro or Cipralex or Seroplex or Esertia or Prozac or Fontex or Seromex or Seronil or Sarafem or Ladose or Motivest or Fluctin or fluox or Lovan or Luvox or Fevarin or Faverin or Favoxil or Movox or Paxil or Seroxat or Sereupin or Aropax or Deroxat or Divarius or Rexetin or Xetanor or Paroxat or Loxamine or Zoloft or Lustral or Serlain or Asentra
S20. TI Celexa or Cipramil or Cipram or Recital or Emocal or Dalsan or Sepram or Seropram or Citox or Priligy or Lexapro or Cipralex or Seroplex or Esertia or Prozac or Fontex or Seromex or Seronil or Sarafem or Ladose or Motivest or Fluctin or fluox or Lovan or Luvox or Fevarin or Faverin or Favoxil or Movox or Paxil or Seroxat or Sereupin or Aropax or Deroxat or Divarius or Rexetin or Xetanor or Paroxat or Loxamine or Zoloft or Lustral or Serlain or Asentra
S19. TI ( alaproclat* or cericlamin* or citalopram or dapoxetin* or escitalopram or femoxetin* or fluoxetin* or fluvoxamin* or paroxetin* or sertralin* or trazodone or vilazodone or zimelidine ) OR AB ( alaproclat* or cericlamin* or citalopram or dapoxetin* or escitalopram or femoxetin* or fluoxetin* or fluvoxamin* or paroxetin* or sertralin* or trazodone or vilazodone or zimelidine )
S18. TI SSRI* OR AB SSRI*
S17. S14 and S15 and S16
S16. TI inhib* OR AB inhib*
S15. TI ( uptake or reuptake or re‐uptake ) OR AB ( uptake or reuptake or re‐uptake )
S14. TI ( serotonin or 5‐HT or 5 HT or 5‐hydroxytryptamine or 5 hydroxytryptamine ) OR AB ( serotonin or 5‐HT or 5 HT or 5‐hydroxytryptamine or 5 hydroxytryptamine )
S13. (MH "Serotonin Uptake Inhibitors+")
S12. S1 or S2 or S3 or S6 or S9 or S10 or S11
S11. TI ( hemipleg* or hemipar* or paresis or paretic ) or AB ( hemipleg* or hemipar* or paresis or paretic )
S10. (MH "Hemiplegia")
S9. S7 and S8
S8. TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )
S7. TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid )
S6. S4 and S5
S5. TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* )
S4. TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral )
S3. TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH )
S2. (MH "Stroke Patients") OR (MH "Stroke Units")
S1. (MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR (MH "Intracranial Embolism and Thrombosis") OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections")
Appendix 5. AMED (Ovid) search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. hemiplegia/
6. (hemipleg$ or hemipar$ or paresis or paretic).tw.
7. or/1‐6
8. antidepressive agents/
9. ((serotonin or 5‐HT or 5 HT or 5‐hydroxytryptamine or 5 hydroxytryptamine) adj5 (uptake or reuptake or re‐uptake) adj5 inhib$).tw.
10. SSRI$1.tw.
11. (alaproclat$ or cericlamin$ or citalopram or dapoxetin$ or escitalopram or femoxetin$ or fluoxetin$ or fluvoxamin$ or paroxetin$ or sertralin$ or trazodone or vilazodone or zimelidine).tw.
12. (Celexa or Cipramil or Cipram or Recital or Emocal or Dalsan or Sepram or Seropram or Citox or Priligy or Lexapro or Cipralex or Seroplex or Esertia or Prozac or Fontex or Seromex or Seronil or Sarafem or Ladose or Motivest or Fluctin or fluox or Lovan or Luvox or Fevarin or Faverin or Favoxil or Movox or Paxil or Seroxat or Sereupin or Aropax or Deroxat or Divarius or Rexetin or Xetanor or Paroxat or Loxamine or Zoloft or Lustral or Serlain or Asentra).tw.
13. 8 or 9 or 10 or 11 or 12
14. 7 and 13
Appendix 6. PsycINFO (Ovid) search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or exp cerebral ischemia/ or cerebral small vessel disease/ or cerebrovascular accidents/ or subarachnoid hemorrhage/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. (hemipleg$ or hemipar$ or paresis or paretic).tw.
6. hemiparesis/ or hemiplegia/
7. or/1‐6
8. exp serotonin reuptake inhibitors/
9. ((serotonin or 5‐HT or 5 HT or 5‐hydroxytryptamine or 5 hydroxytryptamine) adj5 (uptake or reuptake or re‐uptake) adj5 inhib$).tw.
10. SSRI$1.tw.
11. (alaproclat$ or cericlamin$ or citalopram or dapoxetin$ or escitalopram or femoxetin$ or fluoxetin$ or fluvoxamin$ or paroxetin$ or sertralin$ or trazodone or vilazodone or zimelidine).tw.
12. (Celexa or Cipramil or Cipram or Recital or Emocal or Dalsan or Sepram or Seropram or Citox or Priligy or Lexapro or Cipralex or Seroplex or Esertia or Prozac or Fontex or Seromex or Seronil or Sarafem or Ladose or Motivest or Fluctin or fluox or Lovan or Luvox or Fevarin or Faverin or Favoxil or Movox or Paxil or Seroxat or Sereupin or Aropax or Deroxat or Divarius or Rexetin or Xetanor or Paroxat or Loxamine or Zoloft or Lustral or Serlain or Asentra).tw.
13. 8 or 9 or 10 or 11 or 12
14. 7 and 13
Appendix 7. Search strategy for the trial registers
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch;search strategy)
1. Basic search: CEREBR* AND selective serotonin OR CEREBR* AND alaproclate OR CEREBR* AND cericlamine OR CEREBR* AND citalopram OR CEREBR* AND clomipramine OR CEREBR* AND dapoxetine OR CEREBR* AND etoperidone OR CEREBR* AND escitalopram OR CEREBR* AND femoxetine OR CEREBR* AND fenfluramine OR CEREBR* AND fluoxetine OR CEREBR* AND fluvoxamine OR CEREBR* AND norfenfluramine OR CEREBR* AND paroxetine OR CEREBR* AND sertraline OR CEREBR* AND trazodone OR CEREBR* AND vilazodone OR CEREBR* AND zimelidine
2. Basic search: STROKE AND selective serotonin OR STROKE AND alaproclate OR STROKE AND cericlamine OR STROKE AND citalopram OR STROKE AND clomipramine OR STROKE AND dapoxetine OR STROKE AND etoperidone OR STROKE AND escitalopram OR STROKE AND femoxetine OR STROKE AND fenfluramine OR STROKE AND fluoxetine OR STROKE AND fluvoxamine OR STROKE AND norfenfluramine OR STROKE AND paroxetine OR STROKE AND sertraline OR STROKE AND trazodone OR STROKE AND vilazodone OR STROKE AND zimelidine
US National Institutes of Health Trials Register (ClinicalTrials.gov) search strategy
( “selective serotonin” OR alaproclate OR cericlamine OR citalopram OR clomipramine OR dapoxetine OR etoperidone OR escitalopram OR femoxetine OR fenfluramine OR fluoxetine OR fluvoxamine OR norfenfluramine OR paroxetine OR sertraline OR trazodone OR vilazodone OR zimelidine ) AND EXACT "Interventional" [STUDY‐TYPES] AND Stroke [DISEASE]
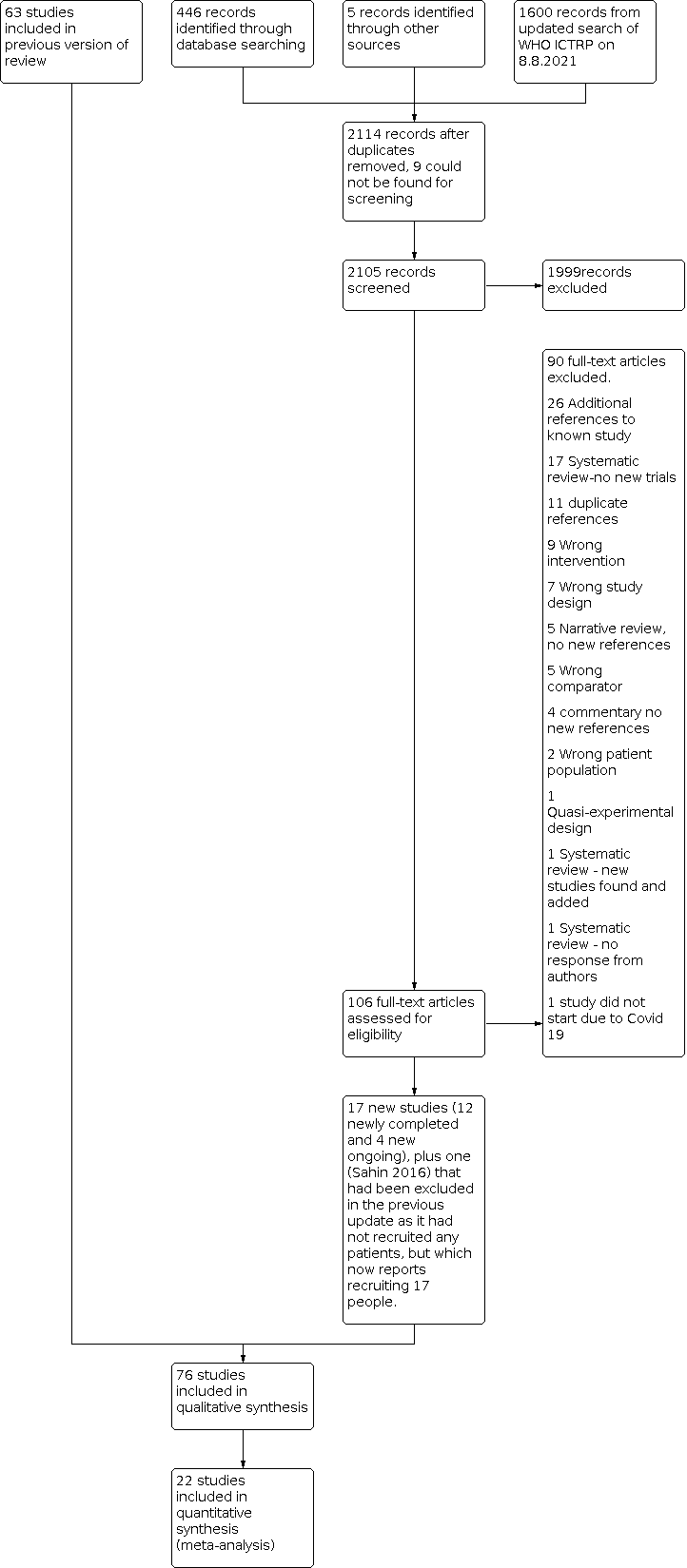
PRISMA flow diagram for this update

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each 'risk of bias' item presented as percentages across all included studies.

Funnel plot, all studies irrespective or risk of bias, for disability at end of treatment.

Comparison 1: SSRI versus control at end of treatment, by SSRI, Outcome 1: Disability (primary outcome). Studies at low risk of bias

Comparison 1: SSRI versus control at end of treatment, by SSRI, Outcome 2: Independent on modified Rankin score (mRS 0 to 2) (primary outcome). Studies at low risk of bias

Comparison 1: SSRI versus control at end of treatment, by SSRI, Outcome 3: Neurological deficit score (studies at low risk of bias)

Comparison 1: SSRI versus control at end of treatment, by SSRI, Outcome 4: Motor deficits (studies at low risk of bias)

Comparison 1: SSRI versus control at end of treatment, by SSRI, Outcome 5: Depression, continuous data (studies at low risk of bias)

Comparison 1: SSRI versus control at end of treatment, by SSRI, Outcome 6: Depression, dichotomous data (studies at low risk of bias)

Comparison 1: SSRI versus control at end of treatment, by SSRI, Outcome 7: Death (trials at low risk of bias)

Comparison 1: SSRI versus control at end of treatment, by SSRI, Outcome 8: Seizures (studies at low risk of bias)

Comparison 1: SSRI versus control at end of treatment, by SSRI, Outcome 9: Gastrointestinal side effects (studies at low risk of bias)

Comparison 1: SSRI versus control at end of treatment, by SSRI, Outcome 10: Bleeding (studies at low risk of bias)

Comparison 1: SSRI versus control at end of treatment, by SSRI, Outcome 11: Fractures (studies at low risk of only)

Comparison 1: SSRI versus control at end of treatment, by SSRI, Outcome 12: Cognition (trials at low risk of bias)

Comparison 1: SSRI versus control at end of treatment, by SSRI, Outcome 13: Leaving the study before the end of scheduled follow‐up for reasons other than death (trials at low risk of bias)

Comparison 1: SSRI versus control at end of treatment, by SSRI, Outcome 14: Fatigue at end of treatment (studies at low risk of bias only)

Comparison 1: SSRI versus control at end of treatment, by SSRI, Outcome 15: Quality of life at end of treatment (studies at low risk of bias)

Comparison 1: SSRI versus control at end of treatment, by SSRI, Outcome 16: Disability (all studies regardless of risk of bias)

Comparison 1: SSRI versus control at end of treatment, by SSRI, Outcome 17: Independent on modified Rankin score (mRS 0 to 2) (all studies regardless of risk of bias)

Comparison 2: SSRI versus control at end of follow up, by SSRI, Outcome 1: Disability (studies at low risk of bias only)

Comparison 2: SSRI versus control at end of follow up, by SSRI, Outcome 2: Independent on modified rankin score (0‐2) (studies at low risk of bias only)

Comparison 2: SSRI versus control at end of follow up, by SSRI, Outcome 3: Depression, continuous data (studies at low risk of bias only)

Comparison 2: SSRI versus control at end of follow up, by SSRI, Outcome 4: Depression, dichotomous (studies at low risk of bias only)

Comparison 2: SSRI versus control at end of follow up, by SSRI, Outcome 5: Motor deficits (studies at low risk of bias only)

Comparison 2: SSRI versus control at end of follow up, by SSRI, Outcome 6: Cognition (studies at low risk of bias only)

Comparison 2: SSRI versus control at end of follow up, by SSRI, Outcome 7: Death (studies at low risk of bias only)

Comparison 2: SSRI versus control at end of follow up, by SSRI, Outcome 8: Leaving the trial before the end of follow‐up, for reasons other than death ( studies at low risk of bias)

Comparison 2: SSRI versus control at end of follow up, by SSRI, Outcome 9: Disability, all studies irrespective of risk of bias

Comparison 2: SSRI versus control at end of follow up, by SSRI, Outcome 10: Independent on mRS (0‐2) all studies irrespective of risk of bias
| Fluoxetine versus control at end of treatment, by SSRI, for stroke recovery* | ||||||
| Patient or population: people with stroke recovery *Summary of findings table based on studies with low risk of bias. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Control | Fluoxetine versus control at end of treatment | |||||
| Disability (primary analysis) |
| SMD 0.0 (‐0.05, 0.05) |
| 5436 | ⊕⊕⊕⊕ | ‐ |
| Independent on modified Rankin score (mRS 0 to 2) (primary analysis) | Study population | RR 0.98
| 5926 | ⊕⊕⊕⊕ | ‐ | |
| 1541/2971 (i.e. 52 per hundred) | 1498/2955 (i.e. 51 per hundred) | |||||
| Neurological deficit score |
| SMD ‐0.39 (95% CI (‐1.12 to 0.33) |
| 30 participants, one study | ⊕⊕⊝⊝ Lowa | This is a small effect (based on the 'rule‐of‐thumb' method for interpreting SMD) |
| Depression (continuous data) |
| SMD ‐0.14 (‐0.19 to ‐0.08) |
| 5356 | ⊕⊕⊕⊕ | This is a small effect (based on the 'rule‐of‐thumb' method for interpreting SMD) |
| Death | Study population | RR 1.01 | 6090 | ⊕⊕⊕⊝ | ‐ | |
| 168/3029 (i.e. 55 per thousand) | 170/3061 (i.e. 56 per thousand) | |||||
| Number of seizures | Study population | RR 1.40 | 6080 | ⊕⊕⊕⊝ Moderatec | ‐ | |
| 54/3024 (i.e. 18 per thousand) | 76/3056 (i.e. 25 per thousand) | |||||
| Bone fractures | Study population | RR 2.35 | 6080 | ⊕⊕⊕ ⊕ | ‐ | |
| 40/3024 (i.e. 13 per thousand) | 93/3056 (i.e. 30 per thousand) | |||||
| *The basis for the assumed risk (e.g. the mean control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aNeurological deficit from only one trial of 30 people so we have downgraded for imprecision (GRADE 2013). bDeath downgraded for imprecision. cSeizures downgraded for imprecision. Note that because we included only the low risk of bias studies in our review, none of the evidence was downgraded because of study quality. A range of different outcome scales were used for disability (including Barthel Index and daily activities subscale of the Stroke Impact Scale), and depression (including emotional role function of the Stroke Impact Scale). | ||||||
| mRS (RR and 95% CI) | Disability (SMD and 95% CI) | |
|---|---|---|
| Fixed‐effect | 0.98 (0.93, 1.03) | ‐0.0 (‐0.05, 0.05) |
| Random‐effects | 0.98 (0.92, 1.04) | ‐0.00 (‐0.05, 0.05) |
| CI: confidence interval | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Disability (primary outcome). Studies at low risk of bias Show forest plot | 5 | 5436 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.05, 0.05] |
| 1.1.1 Fluoxetine | 5 | 5436 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.05, 0.05] |
| 1.2 Independent on modified Rankin score (mRS 0 to 2) (primary outcome). Studies at low risk of bias Show forest plot | 5 | 5926 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.93, 1.03] |
| 1.3 Neurological deficit score (studies at low risk of bias) Show forest plot | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.12, 0.33] |
| 1.3.1 Fluoxetine | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.12, 0.33] |
| 1.4 Motor deficits (studies at low risk of bias) Show forest plot | 6 | 5518 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.02, 0.08] |
| 1.4.1 Fluoxetine | 6 | 5518 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.02, 0.08] |
| 1.5 Depression, continuous data (studies at low risk of bias) Show forest plot | 4 | 5356 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.19, ‐0.08] |
| 1.5.1 Fluoxetine | 4 | 5356 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.19, ‐0.08] |
| 1.6 Depression, dichotomous data (studies at low risk of bias) Show forest plot | 3 | 5907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.65, 0.86] |
| 1.6.1 Fluoxetine | 3 | 5907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.65, 0.86] |
| 1.7 Death (trials at low risk of bias) Show forest plot | 6 | 6090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.82, 1.24] |
| 1.7.1 Fluoxetine | 6 | 6090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.82, 1.24] |
| 1.8 Seizures (studies at low risk of bias) Show forest plot | 6 | 6080 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.00, 1.98] |
| 1.8.1 Fluoxetine | 6 | 6080 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.00, 1.98] |
| 1.9 Gastrointestinal side effects (studies at low risk of bias) Show forest plot | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.33, 8.83] |
| 1.9.1 Fluoxetine | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.33, 8.83] |
| 1.10 Bleeding (studies at low risk of bias) Show forest plot | 6 | 6088 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.69, 1.70] |
| 1.10.1 Fluoxetine (except for Asadollahi 2018) | 6 | 6088 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.69, 1.70] |
| 1.11 Fractures (studies at low risk of only) Show forest plot | 6 | 6080 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.62, 3.41] |
| 1.12 Cognition (trials at low risk of bias) Show forest plot | 4 | 5373 | Mean Difference (IV, Fixed, 95% CI) | ‐1.22 [‐2.37, ‐0.07] |
| 1.13 Leaving the study before the end of scheduled follow‐up for reasons other than death (trials at low risk of bias) Show forest plot | 6 | 6090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.03, 2.40] |
| 1.13.1 Fluoxetine | 6 | 6090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.03, 2.40] |
| 1.14 Fatigue at end of treatment (studies at low risk of bias only) Show forest plot | 4 | 5524 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐1.24, 1.11] |
| 1.15 Quality of life at end of treatment (studies at low risk of bias) Show forest plot | 3 | 5482 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.02, 0.02] |
| 1.16 Disability (all studies regardless of risk of bias) Show forest plot | 32 | 7667 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.23, ‐0.14] |
| 1.16.1 Fluoxetine | 19 | 6590 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.13, ‐0.04] |
| 1.16.2 Sertraline | 1 | 130 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.38 [‐1.76, ‐0.99] |
| 1.16.3 Paroxetine | 5 | 293 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.29 [‐1.55, ‐1.03] |
| 1.16.4 Citalopram | 5 | 446 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐0.88, ‐0.48] |
| 1.16.5 Escitalopram | 2 | 208 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.00, ‐0.34] |
| 1.17 Independent on modified Rankin score (mRS 0 to 2) (all studies regardless of risk of bias) Show forest plot | 8 | 6792 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.93, 1.01] |
| 1.17.1 Fluoxetine | 6 | 6039 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.94, 1.03] |
| 1.17.2 Sertraline | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.97, 1.04] |
| 1.17.3 Citalopram | 1 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.82, 0.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Disability (studies at low risk of bias only) Show forest plot | 2 | 2591 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐2.59, 2.11] |
| 2.2 Independent on modified rankin score (0‐2) (studies at low risk of bias only) Show forest plot | 2 | 3137 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.89, 1.19] |
| 2.3 Depression, continuous data (studies at low risk of bias only) Show forest plot | 2 | 2684 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.36, 0.44] |
| 2.4 Depression, dichotomous (studies at low risk of bias only) Show forest plot | 1 | 3083 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.73, 1.04] |
| 2.5 Motor deficits (studies at low risk of bias only) Show forest plot | 2 | 2688 | Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐3.00, 1.46] |
| 2.6 Cognition (studies at low risk of bias only) Show forest plot | 2 | 2689 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐2.32, 1.62] |
| 2.7 Death (studies at low risk of bias only) Show forest plot | 2 | 3144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.77, 1.12] |
| 2.8 Leaving the trial before the end of follow‐up, for reasons other than death ( studies at low risk of bias) Show forest plot | 2 | 3188 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.54, 1.51] |
| 2.9 Disability, all studies irrespective of risk of bias Show forest plot | 2 | 2691 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐2.56, 2.06] |
| 2.10 Independent on mRS (0‐2) all studies irrespective of risk of bias Show forest plot | 2 | 3134 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.89, 1.19] |

