Interventions visant à améliorer l'observance des traitements médicamenteux de la dépendance tabagique
Résumé scientifique
Contexte
Les traitements pharmacologiques de la dépendance tabagique, comme le traitement nicotinique de substitution (TNS), se sont avérés des interventions sûres et efficaces pour le sevrage tabagique. Des niveaux plus élevés d'observance de ces traitements médicamenteux augmentent la probabilité d'un arrêt du tabac prolongé, mais de nombreux fumeurs les utilisent à une dose plus faible et pendant moins de temps que ce qui serait optimal. Il est donc important de déterminer l'efficacité des interventions conçues spécifiquement pour augmenter l'observance thérapeutique. De telles interventions peuvent inclure l'apport d'informations complémentaires sur l'importance de la prise de médicaments et d'un soutien supplémentaire pour surmonter les problèmes de maintien de l'observance.
Objectifs
L'objectif principal de cette revue était d'évaluer l'efficacité des interventions pour augmenter l'observance des traitements médicamenteux de sevrage tabagique, comme le TNS, le bupropion, la nortriptyline et la varénicline (et schémas combinés). Leur efficacité était évaluée par rapport à un groupe témoin, généralement les soins standard. Les objectifs secondaires étaient i) d'évaluer les approches d'intervention les plus efficaces ; ii) de déterminer l'impact des interventions sur les précurseurs potentiels de l'observance, comme la compréhension du traitement et les perceptions sur son efficacité ; et iii) d'évaluer les résultats clés influencés par l'observance préalable, principalement l'arrêt du tabac.
Stratégie de recherche documentaire
Nous avons effectué des recherches dans les bases de données suivantes à l'aide de mots‐clés et de vedettes de la nomenclature médicale : registre Cochrane des essais contrôlés (CENTRAL, dans la Bibliothèque Cochrane), MEDLINE (OVID SP) (de 1946 à la 3ème semaine de juillet 2014), EMBASE (OVID SP) (de 1980 à la semaine 29 de 2014) et PsycINFO (OVID SP) (de 1806 à la 4ème semaine de juillet 2014). Nous avons consulté le registre spécialisé du groupe Cochrane sur le tabagisme le 9 juillet 2014. Des recherches de références ascendantes et descendantes ont été entreprises.
Critères de sélection
Études randomisées, randomisées par grappes ou quasi randomisées dans lesquelles les participants utilisant un traitement pharmacologique actif de sevrage tabagique étaient attribués à un bras d'intervention ou témoin. Les participants admissibles étaient des fumeurs adultes (18+). Les interventions admissibles comprenaient toute intervention différente des soins standard et dont le contenu avait clairement pour objectif premier d'augmenter l'observance du traitement médicamenteux de la dépendance tabagique. Les groupes de comparaison acceptables étaient ceux ayant fourni des soins standard, ce qui selon le contexte pouvait comporter un soutien minimal ou un soutien comportemental à divers degrés. Les études incluses devaient utiliser une mesure du comportement d'observance permettant d'évaluer d'une façon ou d'une autre le degré d'observance.
Recueil et analyse des données
Deux auteurs de la revue ont recherché des études et extrait des données des études incluses de manière indépendante. Le risque de biais a été évalué selon les recommandations du manuel Cochrane. Pour les mesures de résultats continues, nous avons rapporté les quantités d'effet sous forme de différences moyennes standardisées (DMS). Pour les mesures de résultat dichotomiques, nous avons rendu compte de l'ampleur de l'effet sous la forme de risques relatifs (RR). Nous avons calculé les tailles d'effet combinées avec des intervalles de confiance (IC) à 95 % à l'aide du modèle à effets fixes.
Résultats principaux
Notre stratégie de recherche a permis de récupérer 3 165 références uniques parmi lesquelles 31 études étaient potentiellement admissibles pour inclusion. Parmi celles‐ci, 23 ont été exclues à l'étape d'évaluation du texte intégral ou ont été identifiées comme des études en attente de classement sous réserve de plus amples informations. Nous avons inclus au final huit études portant sur 3 336 participants randomisés. Les interventions, qui étaient toutes complémentaires à un soutien comportemental standard, consistaient typiquement à fournir de plus amples informations sur les raisons de l'observance thérapeutique et à souligner l'importance de celle‐ci, ainsi qu'à soutenir l'élaboration de stratégies pour surmonter les problèmes de maintien de l'observance.
Cinq études signalent si oui ou non les participants sont parvenus à un niveau satisfaisant pré‐spécifié d'observance du traitement médicamenteux. Des preuves indiquent que les interventions d'observance ont conduit à des améliorations modestes dans l'observance thérapeutique, avec un risque relatif (RR) de 1,14 (IC à 95 % de 1,02 à 1,28 ; P = 0,02 ; n = 1 630). Quatre études rapportent des mesures continues de l'observance du traitement. Bien que la différence moyenne standardisée (DMS) favorise les interventions d'observance, l'effet est petit et pas statistiquement significatif (DMS 0,07 ; IC à 95 % de ‐0,03 à 0,17 ; n = 1 529). En appliquant le système GRADE, le niveau de preuve de ces résultats a été évalué, respectivement, comme étant modéré et faible.
Des preuves indiquent que les interventions d'observance ont conduit à des améliorations modestes des taux de sevrage. Le risque relatif pour parvenir au sevrage était similaire à celui pour une meilleure observance. Il n'était pas significatif dans la méta‐analyse de quatre études fournissant des données sur le sevrage à court terme : RR = 1,07 (IC à 95 % de 0,95 à 1,21 ; n = 1 755), mais des preuves statistiquement significatives d'un meilleur sevrage à six mois ou plus ont été observées dans un autre ensemble de quatre études : RR = 1,16 (IC à 95 % de 1,01 à 1,34 ; P = 0,03 ; n = 3 049). En appliquant le système GRADE, le niveau de preuve pour ces deux résultats a été évalué comme étant faible.
Les interventions étant de nature semblable et le nombre d'études faible, il n'a pas été possible d'étudier si différents types d'approches d'intervention ont été plus efficaces que d'autres. Outre l'observance ou le sevrage, d'autres critères pertinents n'étaient pas rapportés.
Aucune preuve ne suggère que les interventions visant à augmenter l'observance du traitement médicamenteux aient entraîné des effets indésirables. Toutes les études incluses ont été évaluées comme étant à risque de biais élevé ou imprécis. Celui‐ci était souvent dû à un manque de clarté du compte‐rendu, qui rendait les évaluations peu claires, plutôt qu'à des preuves nettes de l'échec d'une protection adéquate contre les risques de biais.
Conclusions des auteurs
Il existe des éléments de preuve indiquant que les interventions qui consacrent une attention particulière à l'amélioration de l'observance du traitement médicamenteux de sevrage tabagique, en fournissant des informations et en facilitant la résolution de problèmes, peuvent augmenter l'observance, bien que ces données ne soient pas solides mais limitées à la fois en qualité et en quantité. Certaines preuves indiquent également que de telles interventions améliorent les chances de sevrage mais, là aussi, les données sont relativement faibles.
PICOs
Résumé simplifié
Est‐il possible d'améliorer l'observance des traitements médicamenteux qui aident les fumeurs à arrêter ?
Les médicaments qui aident les gens à arrêter de fumer comme le traitement nicotinique de substitution (TNS) sont des traitements sûrs et efficaces pour le sevrage tabagique. Cependant, beaucoup de personnes ne prennent pas les médicaments prescrits conformément à l'ordonnance. Dans la présente revue, nous avons cherché à savoir s'il existait des approches efficaces pour augmenter l'observance de ces traitements, ce qui devrait améliorer les chances des fumeurs d'arrêter. De telles approches ou interventions impliquent généralement de fournir des informations supplémentaires sur les médicaments et d'aider les gens à surmonter les problèmes qu'ils éprouvent à suivre la prescription.
Une recherche systématique a permis d'identifier huit études sur des interventions pour améliorer l'observance médicamenteuse, portant sur un total de 3 336 participants. Cinq études ont évalué si les participants parvenaient à un niveau satisfaisant pré‐spécifié pour la prise de médicaments, et la combinaison statistique des résultats suggère que les interventions ont conduit à des améliorations modestes. Quatre études ont évalué la quantité de médicaments prise, et ont mis en évidence un petit effet qui peut être dû au hasard. Des preuves indiquent également que les interventions pour augmenter l'observance du traitement médicamenteux ont conduit à des améliorations modestes sur le sevrage tabagique. Les preuves incluses dans cette revue sont considérées comme étant de qualité faible à modérée, ce qui suggère que de plus amples recherches sont nécessaires si nous voulons augmenter notre confiance dans ces résultats.
En résumé, il existe des éléments de preuve indiquant que les interventions qui consacrent une attention particulière à l'amélioration de l'observance du traitement médicamenteux de sevrage tabagique peuvent augmenter l'observance, bien que les données ne soient pas solides mais limitées à la fois en termes de qualité et de quantité. Certaines preuves indiquent aussi que ces approches améliorent les chances d'arrêter de fumer mais, encore une fois, les données sont relativement faibles.
Authors' conclusions
Summary of findings
| Interventions to increase adherence compared to standard care for improving adherence to medications for tobacco dependence and abstinence from smoking | |||||
| Patient or population: Adult smokers | |||||
| Outcomes | Relative effect (95% CI) | Illustrative comparative risks (95% CI) | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Standard care | Interventions to increase adherence | ||||
| Adherence to medications for tobacco dependence (dichotomous outcomes) | RR 1.14 | Study population | 1630 | ⊕⊕⊕⊝ | |
| 368 per 1000 achieve a specified satisfactory level of adherence | 419 per 1000 (375 to 471) achieve a specified satisfactory level of adherence | ||||
| Adherence to medications for tobacco dependence (continuous outcomes) | SMD 0.07 (‐0.03 to 0.17) | The mean level of adherence is 0 | The mean level of adherence is 0.07 standard deviations higher (0.03 lower to 0.17 higher) | 1529 | ⊕⊕⊝⊝ |
| Short‐term abstinence from smoking (<6 months) | RR 1.07 | Study population | 1755 | ⊕⊕⊝⊝ | |
| 363 per 1000 achieve abstinence | 389 per 1000 (345 to 439) achieve abstinence | ||||
| Long‐term abstinence from smoking (≥6 months) | RR 1.16 | Study population | 3049 | ⊕⊕⊝⊝ | |
| 171 per 1000 achieve abstinence | 198 per 1000 (173 to 229) achieve abstinence | ||||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | |||||
| GRADE Working Group grades of evidence | |||||
| 1All studies are judged to be at high or unclear risk of bias which lowers confidence in estimate of effect 2Includes sufficient sample size for single adequately powered trial but 95% CI overlaps no effect and ranges from very small harm to small benefit 3Includes sufficient sample size for single adequately powered trial but 95% CI overlaps no effect and ranges from small harm to substantial benefit 4Substantial heterogeneity with inconsistency in point estimates and limited overlap of confidence intervals | |||||
Background
Description of the condition
Smoking is the single largest preventable cause of disease and premature death worldwide, being a key causal factor in heart disease, stroke, chronic lung disease and cancers. Pharmacological treatments for tobacco dependence such as nicotine replacement therapy (NRT) are widely considered to be safe and effective interventions for smoking cessation. A systematic review found that participants using NRT were over 1.5 times more likely to achieve abstinence (Stead 2012a). Participants using bupropion, nortriptyline and varenicline are also more likely to stop smoking than those using placebo (Hughes 2014; Cahill 2012).
There is observational evidence that people who adhere to medication to a greater extent are more likely to achieve abstinence. One problem with interpreting such evidence is that people whose quit attempt is faltering may also choose not to adhere to their medication. However, even studies that control for this reverse causation still suggest that prior adherence promotes later abstinence (Shiffman 2007; Shiffman 2008; Hollands 2013). A recent review of this relationship, although highlighting the lack of high quality studies, suggests that the degree of adherence predicts subsequent abstinence (Raupach 2014). Observational evidence can, however, never prove causality. Trials that show that interventions to improve adherence also improve the rate of abstinence are stronger evidence for causality in this respect.
Studies show that many smokers who use medications for tobacco dependence do so at a lower dose and for less time than the evidence suggests is optimal (Shiffman 2008; Cheong 2010; Hays 2010; Swan 2010). For example, Burns and Levinson (Burns 2008) report that users of NRT, on average, continue medication for less than half the time for which it is prescribed. These findings provide another reason why assessing the effectiveness of interventions to improve adherence is important.
Description of the intervention
Interventions that specifically aim to increase adherence to prescribed medications vary widely in their content and characteristics (Haynes 2008). Examples may include, but are not limited to, improved or increased information provision, monitoring and feedback concerning performance, reminders, and psychological therapy or counselling (see Appendix 1 for more details). In the specific context of medications for tobacco dependence, general behavioural support for smoking cessation may include components that target increasing medication adherence. Interventions that are additional to standard behavioural support and that devote special attention to improving adherence may also be delivered. Such interventions may include further educating individuals about the value of taking medications and providing additional support to overcome problems with maintaining adherence.
Why it is important to do this review
As far as we are aware, no published systematic reviews address this question. Reviews of studies of behavioural support interventions (such as Lancaster 2008; Stead 2005; Stead 2012b), which may include elements that target medication adherence, are not designed to disentangle the specific effects of those components that focus on increasing adherence. Previous reviews of interventions designed to increase adherence have focused on specific patient groups or treatment contexts, or have not covered smoking cessation treatments (Nieuwlaat 2014). A specific review of the topic is valuable because we cannot be certain that findings relating to adherence to other medications are generalisable to smoking cessation medications, as these provide a unique treatment context with specific compliance issues. For example, smoking cessation treatment is relatively short term and its use dictated by a specific behaviour (if an individual resumes smoking, medication use typically ends) rather than an illness. Additionally, the drawbacks of failing to adhere are less significant than they may be in the treatment of illness. For example, individuals may successfully quit smoking without adhering to therapy, or if they fail to adhere and continue to smoke, they may not feel that they have lost anything or experienced any adverse effects. Finally, there is evidence to suggest that it may be more difficult to persuade individuals of the benefits of using smoking cessation medications compared with other health conditions. Hammond and colleagues (Hammond 2004) found that over a third of smokers reported that use of pharmacotherapies (NRT or bupropion) would either make no difference or actually reduce the likelihood of quitting smoking. Smokers who perceived cessation assistance methods to be beneficial were more likely to use medication in the future. Some users may perceive risks of harm to their health from the medication that outweigh the potential benefits.
Objectives
The primary objective of the review was to assess the effectiveness of interventions aiming to increase adherence to medications for smoking cessation, such as NRT, bupropion, nortriptyline and varenicline (and combination regimens). This was considered in comparison to a control group, typically representing standard care. Secondary objectives were to i) assess which intervention approaches are most effective; ii) determine the impact of interventions on potential precursors of adherence, such as understanding of the treatment and efficacy perceptions; and iii) evaluate key outcomes influenced by prior adherence, principally smoking cessation.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, cluster‐randomised or quasi‐randomised studies in which participants using active pharmacological treatment for smoking cessation were allocated to an intervention arm or a control arm. Pharmacological treatments comprised those that are prescribed to increase cessation rates (e.g. NRT, bupropion, nortriptyline, varenicline and combination regimens).
Types of participants
Adult individuals (18 years and over) defined as smokers at point of entry into the trial.
Types of interventions
Interventions to increase adherence may vary significantly in their nature, with a workable taxonomy provided in a previous Cochrane review (Haynes 2008). This taxonomy is provided in Appendix 1. The nature of the interventions considered in the current review was not specified beyond reference to exclusion criteria.
Eligible interventions comprised any intervention that differed from standard care administered to smokers, and where the differing intervention content had a clear principal focus on increasing adherence to medications for tobacco dependence, reflected in described content and stated aims. We did not include interventions that systematically alter the active pharmacological characteristics of a given medication, such as dose strength, length of treatment or means of delivery. Interventions that include the use of financial incentives were not eligible. Acceptable comparison groups were those that provided standard or usual care. Depending on setting, this can comprise minimal support or varying degrees of behavioural support.
Types of outcome measures
To be considered for inclusion, studies must have used a measure of adherence behaviour allowing some assessment of the degree of adherence. This was defined as a continuous measure ‐ such as the amount of medication consumed over a given treatment period ‐ or as a dichotomous outcome, indicating whether the treatment is being used to a specified degree (e.g. adherence for x number of days, or x amount of medication consumed). This is in contrast to a single binary measure without nuance (i.e. any amount of medication at any time vs. non‐use), which was not considered an appropriate measure.
Adherence could be measured by means of a behavioural endpoint using an electronic measure, pill counts by a third party, or through a self‐report or questionnaire measure (or combinations thereof).
Primary outcomes
Primary outcome:
-
Adherence to medication for tobacco dependence
Where treatment periods were assessed at multiple timepoints, the longest timepoint reported was used. Where multiple measures of adherence were reported, we have used the most stringent measure that is available.
Secondary outcomes
-
Abstinence from smoking measured near or at a time point relevant to the measure of adherence
Where multiple measures of abstinence were reported, we used the most stringent. If there were data from multiple timepoints, we report data measured near or at a timepoint relevant to the measure of adherence. In addition, we also report abstinence at the longest available timepoint should that differ, in order to assess the long‐term benefit of the intervention on cessation rates.
-
Factors plausibly associated with increases in adherence such as, but not limited to:
‐ intention or motivation to change health behaviour
‐ attitudes towards treatment, or understanding of the treatment
-
Adverse events
Any adverse events or harms reported in included trials were noted, including clinical levels of depression or anxiety.
Search methods for identification of studies
Electronic searches
We searched the following databases on 25th July 2014:
-
Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library),
-
MEDLINE (OVID SP) (1946 to July Week 3 2014),
-
EMBASE (OVID SP) (1980 to Week 29 2014),
-
PsycINFO (OVID SP) (1806 to July Week 4 2014).
In addition, the Cochrane Tobacco Addiction Group Specialized Register was searched on 9th July 2014. The search strategies were developed to comprise searches both for keywords and medical subject headings under existing database organisational schemes. Those used are presented in Appendix 2.
We searched databases in the metaRegister of Controlled Trials to identify ongoing studies. Ongoing studies are presented in 'Characteristics of ongoing studies'. We also searched published Cochrane reviews of behavioural support for smoking cessation (Lancaster 2008, Stead 2005, Stead 2012b) for relevant studies.
Searching other resources
We conducted forwards and backwards citation searches from included studies. We did not handsearch journals.
Data collection and analysis
Selection of studies
Two review authors independently screened all search results (titles and abstracts) for possible inclusion, and those selected by either or both authors were subject to full‐text assessment. Two review authors independently assessed the selected articles for inclusion. Any discrepancies were resolved by consensus, overseen by a third review author acting as arbiter as necessary. We list excluded studies after full‐text assessment in the table 'Characteristics of Excluded Studies', giving reasons for exclusion.
Data extraction and management
We developed a data extraction form, which was piloted and amended as necessary. We extracted the following main sets of data from each included study:
-
lead author;
-
date;
-
study participant inclusion criteria;
-
participants (participant condition(s) and demographics: race/ethnicity, gender, religion/culture, socioeconomic status, age);
-
study design and timetable; randomisation; allocation concealment;
-
interventions (content and format of interventions, including details of information provided; intervention setting and delivery provider; delivery of any co‐interventions, theoretical basis of intervention if stated);
-
numbers of participants in each trial arm;
-
outcome measures; time(s) at which outcomes assessed;
-
results;
-
balance of baseline characteristics;
-
analysis;
-
additional comments.
Two review authors independently extracted data. Data extraction was checked by a third review author and any errors or inconsistencies resolved. The first review author entered the data into RevMan, with another review author checking the accuracy of the data entry.
Assessment of risk of bias in included studies
We assessed and report on the risk of bias of included studies by outcome, in accordance with the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We report on the following individual domains:
-
random sequence generation (selection bias);
-
allocation concealment (selection bias);
-
blinding of participants and personnel (performance bias);
-
blinding of outcome assessment (detection bias) (assessed for each main outcome or class of outcome);
-
incomplete outcome data (attrition bias) (assessed for each main outcome or class of outcome);
-
selective reporting (reporting bias);
-
other sources of bias (validity and reliability of outcome measures; comparability of baseline characteristics; consistency in intervention delivery (i.e. was the information standardised/scripted; was fidelity to protocol monitored)).
Two review authors independently assessed the risk of bias in included studies, with any disagreements resolved by discussion and consensus, and with a third review author acting as arbiter as necessary. We present our assessment in Risk of Bias tables for each included study.
A summary risk of bias judgement was derived for each study from those domains judged to be most critical in this intervention context, informed by criteria outlined in another recent Cochrane review of interventions to increase adherence to medications (Nieuwlaat 2014) and additionally including assessment of incomplete outcome data. These key domains were as follows: random sequence generation; allocation concealment; blinding of outcome assessment; incomplete outcome data and validity and reliability of outcome measures. We applied an algorithm suggested in Section 8.7 (Table 8.7a) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Specifically, if the judgement in at least one of these domains was 'high risk of bias' then summary risk of bias was determined to be high. If no judgements of 'high' risk were made, but the judgement in at least one domain was 'unclear risk of bias' then the summary risk of bias was determined to be unclear. Summary risk of bias was only judged 'low' if judgements in all domains were 'low risk of bias'.
The GRADE system was used to assess the quality of the evidence for the primary and secondary outcomes across studies and a Summary of Findings table produced (Higgins 2011).
Measures of treatment effect
For continuous outcomes where the precise nature of the measures used differ but the outcomes were regarded as comparable, they were integrated and standardised to have common effect sizes, defined as the standardised mean difference (SMD). The effect measure for comparable dichotomous outcomes is the risk ratio (RR). We obtained a pooled effect size with 95% confidence interval (CI) using the fixed‐effects model, in line with our protocol. We do, however, also report effects using the random‐effects model due to observed clinical heterogeneity in study characteristics.
Unit of analysis issues
There were no cluster‐randomised trials included and no unit of analysis errors were observed.
Dealing with missing data
We suggest elsewhere (Hollands 2013) that, in the context of smoking cessation medications, it would be informative for measures of adherence to include only those participants who continue a quit attempt and not all those allocated to receive a given intervention. Including those people who abandon a quit attempt is less appropriate because first, treatment such as NRT is not indicated when a person has ceased trying to quit smoking, and second, it potentially confounds adherence with initial uptake (which may be influenced by different factors). As such, we are most interested in adherence to medication in those individuals who continue to engage with a treatment programme and do not drop out from the intervention and hence invariably remain in the study. We therefore intended to analyse data for our primary outcome in this way where available. In practice, primary outcomes for included studies were more often presented as intention‐to‐treat, with three exceptions where it was clear that adherence was assessed for only those who remained engaged with treatment or at least with the study (Mooney 2005; Nollen 2011; Smith 2013). We conducted a sensitivity analysis to examine if this affected the results for the primary adherence outcome, providing this was not prevented by missing data or continuous outcome data (for which imputation is problematic). Results were not found to be affected by these three studies not using ITT data. For smoking cessation outcomes, ITT data were provided in all cases, with a conservative approach being taken that assumed that drop‐out implies abstinence was not achieved.
Assessment of heterogeneity
We tested for heterogeneity by inspecting overlapping confidence intervals and further quantified this using the I² statistic (which describes the percentage of the variability in effect estimates due to heterogeneity rather than sampling error). A value greater than 50% was considered to represent substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
We did not assess likelihood of publication bias using funnel plots (Sutton 2000) as there were insufficient studies to do so.
Data synthesis
We conducted a narrative synthesis of the included studies, presenting studies’ major characteristics and results. If studies were sufficiently similar in terms of setting, population, interventions and outcomes (including the time(s) at which these are assessed), we pooled the data statistically. In line with our protocol, a fixed‐effects model for meta‐analysis was selected to obtain a pooled effect size with 95% CIs, as we grouped substantially similar studies. We do, however, also report effects using the random‐effects model due to observed clinical heterogeneity in study characteristics, such as differences in the outcome measures used.
Mantel‐Haenszel meta‐analytic methods (Mantel 1959) were used for analysis of dichotomous outcomes. These are the default methods in the Review Manager software, and are considered the most appropriate when data are sparse, either in terms of event rates being low or study size being small (Higgins 2011). In such cases, the estimates of the standard errors of the effect that are used in inverse variance methods may be poor. Inverse variance methods were used for analysis of continuous outcomes and as the measures varied between studies, we used standardised mean differences.
Subgroup analysis and investigation of heterogeneity
In all trials, smokers were motivated to quit or reduce smoking and in both arms received counselling or support to help them do so, whilst the intervention groups were given special interventions to enhance adherence. The interventions used to enhance adherence comprised a combination of two intervention strategies outlined within the taxonomy of interventions to increase adherence that is included in Appendix 1 (Haynes 2008). These were either: a) instruction for patients or b) counselling about the patients’ target condition, the importance of therapy and compliance with therapy. Given this, we did not feel there were sufficient differences between intervention approaches to justify subgroup analysis. We also proposed to conduct a subgroup analysis looking at differential effects on adherence by the type of prescribed medication but there were insufficient studies to meaningfully examine this.
Sensitivity analysis
We assessed the impact of missing ITT data in a sensitivity analysis as previously described. We also proposed to remove studies at higher risk of bias (those not considered to be at low risk of bias) from the analysis to check the robustness of the results. However, no studies were assessed as being at low risk of bias.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies for additional details of studies. An additional Table 1 provides a brief overview of the nature of adherence interventions used in the included studies.
| Study | Brief description of specific intervention components intended to increase adherence* | Additional contact time relative to standard care? | Medication for which adherence was targeted |
| Chan 2010 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT |
| Chan 2011 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT |
| Marteau 2012 | Tailored and communicated about NRT dosage using a more potent rationale (genotype versus phenotype) | No | NRT |
| Mooney 2005 | Personalised feedback of questionnaire responses regarding medication | No | NRT |
| Mooney 2007 | Personalised feedback of externally validated medication adherence | Yes | Bupropion |
| Nollen 2011 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | Varenicline |
| Schmitz 2005 | Personalised feedback of externally validated medication adherence | Yes | Bupropion |
| Smith 2013 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT |
* For further details see Characteristics of Included Studies
Results of the search
Our search strategy retrieved 3165 unique references. 31 studies were identified as potentially eligible for inclusion. Of these, 23 studies were excluded at full‐text screening stage or identified as studies awaiting classification subject to further information. The flow of studies through the systematic review process is shown in Figure 1.
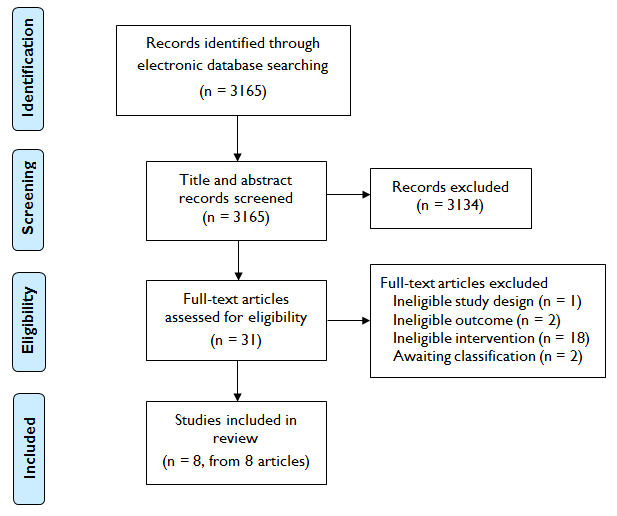
Study flow diagram
Included studies
We included eight studies involving 3336 randomised participants (Chan 2010; Chan 2011; Marteau 2012; Mooney 2005; Mooney 2007; Schmitz 2005; Nollen 2011; Smith 2013).
Types of studies
All trials were randomised controlled trials with parallel groups. Four trials involved randomisation into two groups which were both included in our analysis (Marteau 2012;Mooney 2007; Schmitz 2005; Nollen 2011) and three trials involved randomisation into three groups, but where only two of these groups were eligible for this review (Chan 2010; Chan 2011; Mooney 2005). One trial involved a 2 x 2 x 2 factorial design with eight randomised groups, but these groups were collapsed into a two‐group comparison relevant to this review by the study authors (Smith 2013).
Types of participants and settings
Participants were typically healthy general population samples of smokers. Only one study included participants with a specific clinical condition which was erectile dysfunction (Chan 2010). The mean ages of participants in trials ranged from 34.6 (Mooney 2005) to 49 (Schmitz 2005). In two trials, all participants were female (Mooney 2007; Schmitz 2005). In one trial, all participants were male (Chan 2010). In the remaining trials, % female ranged from 19.4 (Chan 2011) to 62.5 (Nollen 2011). Five trials took place in the USA (Mooney 2005; Mooney 2007; Schmitz 2005; Nollen 2011; Smith 2013), two in Hong Kong, China (Chan 2010; Chan 2011) and one was conducted in the UK (Marteau 2012). Regarding setting, all but one of the included studies featured interventions that were delivered in‐person, with the other delivering the intervention by phone (Smith 2013). The interventions were delivered in clinic settings apart from one that was delivered by phone (Smith 2013) and two where the setting was unclear (Chan 2010; Chan 2011). Those delivering the intervention were trained counsellors (Chan 2010; Chan 2011; Mooney 2005; Nollen 2011; Smith 2013), nurses (Marteau 2012; Schmitz 2005) or CBT therapists (Mooney 2007).
Types of interventions
The trials all provided some behavioural support to participants in the control arm ‐ its form at minimum comprising dosing instructions and weekly checks of side effects (Schmitz 2005). Support for the control arm varied from a single session of twenty minutes (Mooney 2005) to seven weekly sessions (Mooney 2007; Schmitz 2005; Marteau 2012). In the main, the intervention consisted of an additional component to the standard behavioural support, with additional contact time for those in the intervention arm being provided in six studies (Chan 2010; Chan 2011; Mooney 2007; Schmitz 2005; Nollen 2011; Smith 2013). In the other two studies (Marteau 2012; Mooney 2005), the nature of the contact changed but its duration did not significantly differ. The interventions typically provided information on the rationale for, and emphasised the importance of, adherence to medication, and aided participants in developing strategies to overcome problems and barriers to maintaining adherence. As such, they included a combination of two intervention strategies outlined within the taxonomy of interventions to increase adherence (Haynes 2008) that is included in Appendix 1. These are: a) instruction for patients on medication use or b) counselling about smoking, and the value of medication in overcoming addiction. In terms of specific components, two interventions included personalised feedback of externally validated medication adherence (Schmitz 2005; Mooney 2007), one study included an additional component of personalised feedback of questionnaire responses regarding medication (Mooney 2005); one study tailored and communicated about NRT dosage using a different rationale (genotype versus phenotype) (Marteau 2012) and four studies added additional counselling contact time to standard behavioural support, focusing specifically on medication adherence (Chan 2010; Chan 2011; Nollen 2011; Smith 2013). Medications that were being used by participants in the trials were NRT in five studies (Chan 2010; Chan 2011; Marteau 2012; Mooney 2005; Smith 2013), bupropion in two studies (Mooney 2007; Schmitz 2005) and varenicline in one study (Nollen 2011).
Types of outcome measures
Measures of adherence varied but four studies used a dichotomous outcome, meaning people were either classified as achieving or not achieving a level of adherence that represented multiple weeks of what was deemed adequate adherence (Chan 2010; Chan 2011; Mooney 2007; Schmitz 2005). Three studies used a continuous outcome, measured as the percentage of prescribed medication that was consumed (Marteau 2012; Nollen 2011) or number of days on which it was used (Smith 2013). One study presented both a dichotomous and a continuous outcome (Mooney 2005). The definitions of adequate adherence naturally varied by medication type and because there may not be agreed standards for what constitutes desirable levels of adherence. Furthermore, the operationalisation of this was not always clear. In assessing adherence, five studies used pill counts (Marteau 2012; Mooney 2005; Nollen 2011) or electronic monitoring systems (Schmitz 2005; Mooney 2007). One study clearly used self‐report (Smith 2013), whilst this was probable but not clearly the case in two others (Chan 2010; Chan 2011). The period for which the primary adherence outcome was being assessed ranged from approximately two weeks (Mooney 2005; Smith 2013) to three months (Nollen 2011). Six studies reported biochemically validated abstinence outcomes, although only five provide useable data in study reports (Chan 2010; Chan 2011; Marteau 2012; Mooney 2005; Nollen 2011; but not Mooney 2007). One study provided self‐reported abstinence data (Smith 2013) and Schmitz 2005 did not report abstinence. Time of abstinence outcome measurement ranged from two weeks (Mooney 2005) to six months (Chan 2010; Chan 2011; Marteau 2012; Smith 2013).
Excluded studies
We excluded 20 studies (see Characteristics of excluded studies). Two were excluded because they did not include an eligible adherence outcome (Shaughnessy 1987; Willemsen 2006), one was not an eligible study design (Raupach 2010), whilst the other 17 studies did not include an eligible intervention.
Risk of bias in included studies
It is clear from the risk of bias summary (Figure 2) that the included studies were often difficult to assess for bias on our criteria because insufficient information was given in published reports. For two studies, we were able to judge that at least five of nine assessment domains were at low risk of bias (Chan 2011; Marteau 2012). Very few judgements were made suggesting a high risk of bias for any domain, with the only two examples being risk of bias due to validity and reliability of outcome measures for Smith 2013 and due to incomplete outcome data for Mooney 2005. For summary risk of bias judgements, as described in Assessment of risk of bias in included studies, two studies were assessed as at high risk of bias (Mooney 2005; Smith 2013) with all others were assessed as at unclear risk of bias.
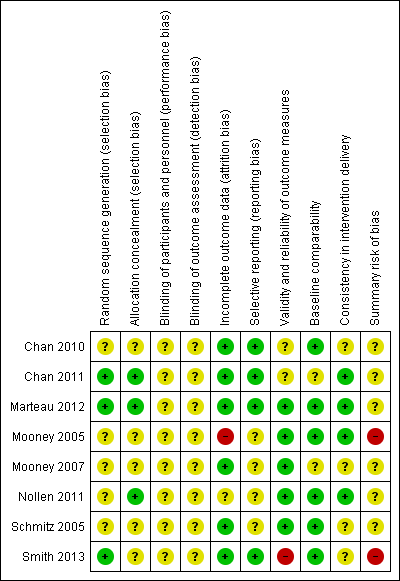
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two studies (Chan 2011; Marteau 2012) were judged to be at low risk of selection bias with details being provided of an adequate sequence generation process and steps to ensure allocation concealment. One study (Nollen 2011) provided details of adequate allocation concealment but not sequence generation. In the other studies, insufficient detail was provided to permit a judgement of high or low risk of selection bias (Chan 2010; Mooney 2005; Mooney 2007; Schmitz 2005; Smith 2013).
Blinding
None of the included studies were regarded as being at low risk of bias in relation to blinding. In all cases, those delivering the intervention were not blind to it. However, the nature of the intervention means that it would be impractical to blind those delivering the intervention and attempts to do so may introduce additional limitations (such as reducing potency of the intervention by impairing its delivery and introducing further systematic differences between the intervention exposures by group). Furthermore, it is unclear as to the degree of risk of bias this places on the outcome data, particularly given the typical use of objective measures of adherence in the included studies. Efforts were made to blind outcome assessors to the secondary abstinence outcome in some cases (Chan 2010; Marteau 2012), although only clearly in one example to the primary adherence outcome (Chan 2011). Where attempts to blind outcome assessors were not apparent (Mooney 2005; Mooney 2007; Schmitz 2005; Nollen 2011; Smith 2013), the use of objective outcome measures, for all other than Smith 2013, may mitigate the risk of bias impacting on the primary adherence outcome.
Incomplete outcome data
We deemed six studies to have been sufficiently explicit in using intention to treat analysis or addressing substantial and/or differential attrition to be considered as at low risk of bias (Chan 2010; Chan 2011; Marteau 2012; Mooney 2007; Schmitz 2005; Smith 2013). One study was determined to be at unclear risk of bias (Nollen 2011) with one judged to be at high risk of bias (Mooney 2005).
Selective reporting
Four trials were pre‐registered on a clinical trials register enabling us to corroborate that specified outcomes remained consistent (Chan 2010; Chan 2011; Marteau 2012; Nollen 2011). One of these also published a protocol (Marteau 2012). The other four studies were to our knowledge not registered and so selective reporting within the final report could not reasonably be ruled out (Mooney 2005; Mooney 2007; Schmitz 2005; Nollen 2011).
Other potential sources of bias
We regarded other potential sources of bias that were highly relevant to this review to be validity and reliability of outcome measures, comparability of baseline characteristics, and consistency in intervention delivery. Three studies were assessed as at low risk of bias for all of these criteria (Marteau 2012; Mooney 2005; Nollen 2011). Regarding validity and reliability of outcome measures, one study was assessed to be at high risk of bias, clearly using self‐report to assess the primary adherence outcome (Smith 2013), two were assessed as at unclear risk of bias (Chan 2010; Chan 2011) and the remaining five studies were judged to be at low risk of bias. For comparability of baseline characteristics, two studies were determined to be at unclear risk of bias (Chan 2011; Mooney 2007) with the remainder at low risk of bias. Regarding consistency in intervention delivery, four studies were assessed as at unclear risk of bias (Chan 2010; Mooney 2007; Schmitz 2005; Smith 2013) and the remaining four at low risk of bias (Chan 2011; Marteau 2012; Mooney 2005; Nollen 2011).
Effects of interventions
Primary adherence outcomes
Analysis 1.1 Adherence ‐ dichotomous outcomes
For dichotomous data, analysis comprises data from five studies (Chan 2010; Chan 2011; Mooney 2005; Mooney 2007; Schmitz 2005). Chan 2010 and Chan 2011 assessed whether or not there had been continuous use of NRT, for 4 weeks and 8 weeks respectively. Mooney 2005 assessed whether or not people were using 12 pieces of nicotine gum per day for every day for the first 15 days of a quit attempt. Mooney 2007 and Schmitz 2005 both assessed whether or not participants had taken two daily doses in an optimal schedule over the 7‐week treatment period. A pooled analysis of these data show that these interventions increased the proportion of participants achieving a specified satisfactory level of adherence, with a Relative Risk (RR) of 1.14 (95% confidence interval (CI) 1.02 to 1.28, n = 1630, I² = 46% Analysis 1.1, that is statistically significant (P = 0.02). In other words, adherence was 14% higher in the intervention group than in the control group. Whilst we specified use of a fixed‐effect model in our protocol, we also conducted a pooled analysis using the random‐effects model due to clinical heterogeneity in study characteristics, such as differences in the outcome measures used. This analysis resulted in an RR of 1.30 (95% CI 0.99 to 1.72, I² = 46%), indicating a larger magnitude of effect but one that is no longer statistically significant (P = 0.06).
Analysis 1.2 Adherence ‐ continuous outcomes
Data were available in four studies that expressed adherence as a continuous outcome (Marteau 2012; Mooney 2005; Nollen 2011; Smith 2013). Marteau 2012 assessed the proportion of prescribed NRT consumed over the four week treatment period and gave a group mean, whilst Nollen 2011 assessed the proportion of prescribed varenicline doses taken over three months, for those remaining engaged to provide data. Mooney 2005 reported the mean number of nicotine gums used during the first 15 days of a quit attempt in those who completed the treatment period only. Smith 2013 assessed self‐reported number of days of nicotine patch use in the first 2 weeks, for those remaining engaged to provide data.
Pooled analysis of these data showed a small and not statistically significant improvement in adherence with a standardised mean difference (SMD) of 0.07 (95% CI ‐0.03 to 0.17, n = 1529, I² = 0%, Analysis 1.2). No significant statistical heterogeneity was observed.
Secondary abstinence outcomes
We report assessments at the timepoint that most closely concords with the assessment of adherence, plus the longest timepoint available should there be additional timepoints reported.
Analysis 2.1 Short‐term abstinence (< 6 months)
This analysis comprised data from four studies (Marteau 2012; Mooney 2005; Nollen 2011; Smith 2013). Additional data have been requested for Mooney 2007 which will be added to this analysis should we receive them.
Marteau 2012 assessed biochemically validated prolonged abstinence at 28 days, Mooney 2005 assessed biochemically validated point‐prevalent abstinence at 2 weeks and Nollen 2011 assessed biochemically validated point‐prevalent abstinence at 3 months. Smith 2013 measured self‐reported 30‐day point‐prevalent abstinence at 6 weeks.
A pooled analysis of these data gave an RR of 1.07 (95% CI 0.95 to 1.21, n = 1755, I² = 0%, Analysis 2.1). This shows a small and not statistically significant effect of adherence interventions on short‐term abstinence from smoking.
Analysis 2.2 Long‐term abstinence (≥ 6 months)
We extracted data on abstinence at the longest follow‐up reported. This analysis comprised data from four studies (Chan 2010; Chan 2011; Marteau 2012; Smith 2013). All four studies assessed abstinence at 6 months, this being biochemically validated in all cases apart from Smith 2013. A pooled analysis of these studies gave a statistically significant effect of adherence interventions on longer‐term abstinence (RR = 1.16, 95% CI 1.01 to 1.34, n = 3049, P = 0.03, I² = 72%). Participants given information to improve adherence were 16% more likely to be abstinent at 6 months than those given standard behavioural support for smoking cessation.
The substantial heterogeneity observed is attributed primarily to Smith 2013, being the only study where the direction of effect slightly favours the control. To illustrate its impact on statistical heterogeneity, its removal from this analysis resulted in no observed heterogeneity (I² = 0%) and a stronger effect of the intervention (RR = 1.63, 95% CI 1.24 to 2.14, n = 2062). This post‐hoc exploratory finding is, however, presented as a sensitivity analysis, as we did not pre‐specify such an exclusion on this basis and we cannot explain the contrary effect in Smith 2013 by reference to differences between the clinical characteristics of this and other studies. Whilst there were such differences, in that the intervention is delivered by telephone and because abstinence is assessed only by self‐report, cessation support delivered by telephone has been shown to increase quitting (Stead 2013) and the use of a self‐report measure is more likely to bias the results towards favouring the intervention arm as participants may feel more pressure to falsely report abstinence. Whilst we specified use of a fixed‐effects model in our protocol, we also conducted a pooled analysis using the random‐effects model due to observed heterogeneity in study characteristics, such as differences in the outcome measures used. This analysis resulted in a RR of 1.36 (95% CI 0.96 to 1.94, I² = 72%), indicating a larger magnitude of effect but one that is no longer statistically significant.
Other secondary outcomes
No studies reported any further relevant secondary outcomes that did not relate to adherence or cessation, other than adverse events.
Adverse events
Adverse events are reported by trial arm in Marteau 2012; Mooney 2005; and Smith 2013. In Marteau 2012, no adverse events occurred that were plausibly related to the intervention or its effect on participants' exposure to medication. There were also no differences between groups in levels of anxiety at either one week or six month assessment times. In Mooney 2005, there was no difference in adverse events between groups and in Smith 2013 there were no serious adverse events during the study.
Discussion
Summary of main results
There was some evidence that interventions that devote special attention to improving adherence through providing information and facilitating problem‐solving can lead to modest increases in adherence, when added to behavioural support for smoking cessation. In turn there was some evidence that such interventions may lead to modest increases in abstinence. However, the limited nature of the available evidence ‐ as a result of the small number of studies, clinical heterogeneity, and impaired study quality ‐ precludes strong statements about the effects of interventions. Lack of data produced imprecise estimates of effect and overall the results are suggestive but inconclusive that adherence interventions may enhance adherence and abstinence.
Primary outcome
The extant evidence suggests that adherence interventions may lead to a modest increase in the proportion of participants achieving a specified satisfactory level of adherence (as reflected in dichotomous outcomes), but at best a small effect on aggregate levels of adherence (as reflected in continuous outcomes). Both outcomes are important, with evidence, at least as far as NRT is concerned, suggesting that the more medication that is consumed the better, and that high levels are inevitably better than low levels. But because there is unlikely to be clear guidance as to what should be regarded as an adequate or effective level of adherence to a given medication, dichotomous measures may be subject to greater variation, arbitrariness and be less directly comparable and interpretable. Applying the GRADE system, the quality of evidence for the effect estimates for dichotomous and continuous adherence outcomes was assessed as moderate and low, respectively (see summary of findings Table for the main comparison). This suggests that further research is at the least likely to have an important impact on the confidence we can have in the estimates, and may change the estimates.
Should this pattern of results ‐ with a larger effect on levels of adequate rather than aggregate adherence, but drawn from few studies ‐ represent a true effect, it may reflect the potential of adherence interventions to work mainly by changing the distribution of adherence, i.e. by shifting those who would always be relatively adherent over a threshold, rather than systematically increasing use in all exposed to it. This may mean that such interventions would most efficiently be targeted at those with a realistic chance of attaining adequate levels of adherence, possibly determined by assessing factors shown to predict adherence. Characteristics of the treatment could also be shaped to attempt to increase the overall background levels of adherence ‐ in essence meaning that there would be less work for the intervention to do to facilitate adequate levels to be reached. For example, characteristics of the medication (Hollands 2013) and its delivery (Hajek 1999) have been shown to impact on adherence.
Secondary outcomes
There is some evidence that adherence interventions lead to improved rates of cessation, with the estimated effect being more convincing for the effect on long‐term abstinence at six months or more. This is consistent with evidence that suggests that increasing adherence will benefit subsequent cessation and that a wide variety of behavioural support interventions have a small effect on long‐term smoking cessation, although it may also be due to effects on other potential precursors. Applying the GRADE system, the quality of evidence for the effect estimates for short‐term and long‐term abstinence outcomes was assessed as low for both (see summary of findings Table for the main comparison). This suggests that further research is very likely to have an important impact on the confidence we can have in the estimates, and is likely to change the results. There was also no evidence of adverse unintended effects on behaviour (with all pooled estimates being in the direction of effect of improving health outcomes) and no evidence of adverse clinical or psychological consequences.
There were not enough studies to examine whether specific types of intervention were more effective than others. Furthermore, much of the content of the included interventions appeared relatively homogenous. It is of course possible that this may in part be a function of either the lack of detail in reporting or the lack of consistency in terminology used to describe interventions, rather than reflecting true homogeneity. Ongoing initiatives to improve the reporting of behavioural interventions (e.g. Michie 2013), in combination with a larger and more varied set of included studies and intervention components therein, may allow a meaningful and nuanced analysis of the effects of specific components to be conducted in future.
Although there is a perception that adherence is often suboptimal, the studies in this review that have quantified it show high adherence on the whole. One problem assessing the degree of adherence is that almost all studies used different measures of adherence. Two studies (Marteau 2012; Nollen 2011) reported the mean percentage of prescribed doses taken, and used objective rather than self‐report measures. Both of these studies demonstrated high adherence, being over 82% in both arms for Nollen 2011 study and over 63% in both arms for Marteau 2012 (despite reflecting an ITT analysis in which no response at follow‐up was taken as zero adherence). Dichotomous measures of adequate adherence were less obviously comparable because their criteria varied more, but in three studies over 50% of participants achieved adequate levels of adherence (Chan 2011; Mooney 2007; Schmitz 2005). Levels of adherence were much lower in one study (Chan 2010), with only 14% of the intervention arm being classified as having adhered adequately. The authors attribute this in part to cost, as only one week of free medication was provided to participants, with cost being given as the main reason for not continuing with medication.
Overall completeness and applicability of evidence
Whilst the included studies do encompass a reasonable range of participants and intervention and treatment characteristics, the small number of studies mean that the depth of evidence relating to any given characteristic and thus completeness is lacking. In terms of the applicability of the evidence, all trials in this review featured participants who were motivated to quit or reduce smoking and who had agreed to receive medication and behavioural support to assist them in doing so. Furthermore, none of the included studies reported specifically targeting participants who were more likely to be non‐adherent, such as those who had previously been unable to adhere to medication regimens. In these contexts, there is evidence that medication use is quite high but also that special interventions to enhance adherence may increase this further. However, improving on levels of adherence that are already high may be challenging and this may help explain why the interventions in this review achieved only modest effects. These characteristics are shared by, for example, smoking cessation services delivered in primary care, suggesting that the findings should be generalisable to these settings. Most people who stop smoking, however, either do not use medication or use it without behavioural support, and typically any medication must be purchased at considerable cost. It is likely that adherence is much lower in this context and that interventions to improve adherence may be particularly helpful. These will have to be delivered by media other than the in‐person consultation ‐ typically by trained counsellors or clinical staff ‐ that was examined in all but one of these trials (Smith 2013, which featured an intervention delivered over the phone). At the moment, we know little about what to do to increase adherence outside of in‐person, clinic settings, though it is plausible that the same kinds of approaches that look like they may be effective in clinical contexts may also prove to be outside these. In order to address applicability to settings and populations in which there is low adherence to medications, researchers will be required to specifically target contexts in which low adherence is known to be prevalent or where participants are specifically identified as having previously been non‐adherent to medication regimens.
Quality of the evidence
At the level of individual studies included in this review, most gave inadequate information to allow us to evaluate whether or not they were at risk of bias. This is reflected in the majority of summary risk of bias assessments being judged as unclear. Despite being published in the era of the CONSORT statement, descriptions of attempts to address selection bias through adequate randomisation and allocation concealment were often inadequate. Only two of the included studies clearly did so (Chan 2011; Marteau 2012). It is possible that this led to bias, but unlikely. In the context of smoking cessation clinics, trial participants are usually unknown to the therapists and there is therefore no clear incentive or basis for therapists to assign particular participants to particular arms and subvert the randomisation. That said, inadequate procedures or description of these should be easy to address and future trials should do so clearly. One potential source of bias that is common throughout the studies is the involvement of the practitioners providing the adherence intervention in collecting data on the degree to which people were adhering and the related issue of the lack of blinding of separate outcome assessors. This may provide an incentive to falsely inflate adherence for people who have received an adherence intervention. This concern is mitigated somewhat by the use of ‘pill counts’, common to all but one of these trials (Smith 2013). It is encouraging that the use of such more objective measures appears commonplace in this intervention context, meaning that measurement issues were not considered to confer particular risk of bias. This is contrary to what was found in a recent Cochrane review focusing on adherence to prescription medications, where most studies used self report measures (Nieuwlaat 2014). Furthermore, whilst in the past, electronic monitoring approaches have been applied primarily to the opening and closing of pill bottles, making them suitable for certain types of medications only, technology has been developed that will enable this to be used for other types of medication storage. A final key risk of bias domain concerned incomplete outcome data, as, in adherence studies, participants lost to follow‐up are likely to be non‐adherent, thus inflating adherence levels when this is not adequately accounted for. This was not determined to be a major issue for the included studies as either analysis was conducted as randomised, or dropout was neither differential by arm or considered substantial in proportion. A global assessment of the evidence for each outcome in the review, through applying the GRADE system, incorporates concerns about the limitations of the included studies and finds that the evidence for our results ranges from low to moderate. This suggests that further research will be valuable in increasing the reliability and precision of effect estimates and the confidence we can place in them.
Potential biases in the review process
Key possible limitations of the review are that first, we failed to identify all relevant research for inclusion in the review. We did take steps to minimise this possibility such as backward and forward citation searching and searching the Tobacco Addiction Group's specialised register in addition to electronic database searches, but this remains possible and will be addressed if necessary when the review is updated. Second, there is the possibility of publication bias, particularly given that all included studies were journal publications. Although this is difficult to examine with small numbers of included studies, we did ultimately include trials that showed effects that did not favour the adherence intervention.
Agreements and disagreements with other studies or reviews
We are not aware of other reviews of this specific literature. Cochrane reviews show that behavioural support increases smoking cessation and, typically, such studies include people using medication, and adherence advice is included in standard smoking cessation support (Lancaster 2008; Stead 2012b). However, it is not possible within these reviews to identify the specific impact of intervention components focused on increasing adherence, as these were combined with other types of behavioural support for participants. In terms of reviews of interventions to increase adherence, Haynes 2008 (recently updated in Nieuwlaat 2014) produced a Cochrane review but excluded tobacco dependence medications. Consistent with what we found, they reported that information and counselling approaches improved adherence and patient outcomes.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Primary outcome (adherence), Outcome 1 Adherence ‐ Dichotomous outcomes.
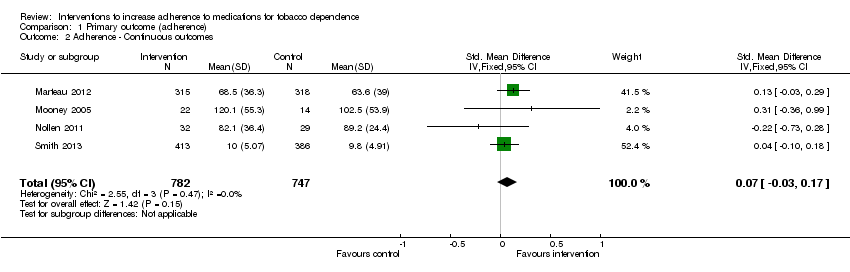
Comparison 1 Primary outcome (adherence), Outcome 2 Adherence ‐ Continuous outcomes.

Comparison 2 Secondary outcomes, Outcome 1 Short‐term abstinence < 6 months.

Comparison 2 Secondary outcomes, Outcome 2 Long‐term abstinence ≥ 6 months.
| Interventions to increase adherence compared to standard care for improving adherence to medications for tobacco dependence and abstinence from smoking | |||||
| Patient or population: Adult smokers | |||||
| Outcomes | Relative effect (95% CI) | Illustrative comparative risks (95% CI) | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Standard care | Interventions to increase adherence | ||||
| Adherence to medications for tobacco dependence (dichotomous outcomes) | RR 1.14 | Study population | 1630 | ⊕⊕⊕⊝ | |
| 368 per 1000 achieve a specified satisfactory level of adherence | 419 per 1000 (375 to 471) achieve a specified satisfactory level of adherence | ||||
| Adherence to medications for tobacco dependence (continuous outcomes) | SMD 0.07 (‐0.03 to 0.17) | The mean level of adherence is 0 | The mean level of adherence is 0.07 standard deviations higher (0.03 lower to 0.17 higher) | 1529 | ⊕⊕⊝⊝ |
| Short‐term abstinence from smoking (<6 months) | RR 1.07 | Study population | 1755 | ⊕⊕⊝⊝ | |
| 363 per 1000 achieve abstinence | 389 per 1000 (345 to 439) achieve abstinence | ||||
| Long‐term abstinence from smoking (≥6 months) | RR 1.16 | Study population | 3049 | ⊕⊕⊝⊝ | |
| 171 per 1000 achieve abstinence | 198 per 1000 (173 to 229) achieve abstinence | ||||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | |||||
| GRADE Working Group grades of evidence | |||||
| 1All studies are judged to be at high or unclear risk of bias which lowers confidence in estimate of effect 2Includes sufficient sample size for single adequately powered trial but 95% CI overlaps no effect and ranges from very small harm to small benefit 3Includes sufficient sample size for single adequately powered trial but 95% CI overlaps no effect and ranges from small harm to substantial benefit 4Substantial heterogeneity with inconsistency in point estimates and limited overlap of confidence intervals | |||||
| Study | Brief description of specific intervention components intended to increase adherence* | Additional contact time relative to standard care? | Medication for which adherence was targeted |
| Chan 2010 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT |
| Chan 2011 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT |
| Marteau 2012 | Tailored and communicated about NRT dosage using a more potent rationale (genotype versus phenotype) | No | NRT |
| Mooney 2005 | Personalised feedback of questionnaire responses regarding medication | No | NRT |
| Mooney 2007 | Personalised feedback of externally validated medication adherence | Yes | Bupropion |
| Nollen 2011 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | Varenicline |
| Schmitz 2005 | Personalised feedback of externally validated medication adherence | Yes | Bupropion |
| Smith 2013 | Added counselling contact time to standard behavioural support, focusing specifically on medication adherence | Yes | NRT |
| * For further details see Characteristics of Included Studies | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adherence ‐ Dichotomous outcomes Show forest plot | 5 | 1630 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.02, 1.28] |
| 2 Adherence ‐ Continuous outcomes Show forest plot | 4 | 1529 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.03, 0.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term abstinence < 6 months Show forest plot | 4 | 1755 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.95, 1.21] |
| 2 Long‐term abstinence ≥ 6 months Show forest plot | 4 | 3049 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.01, 1.34] |

