Aripiprazol para los trastornos del espectro autista (TEA)
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Phase 1: single‐blind, aripiprazole, flexibly dosed, to aripiprazole (2 to 15 mg/d) for 13 to 26 weeks. Chldren and adolescents with a stable response (> 25% decrease in ABC‐I subscale score and rating of "much improved" or "very much improved" on CGI‐I subscale score for 12 weeks randomised into phase 2) Phase 2: 1:1 randomisation to titrated dose of aripiprazole (2 to 15 mg/d) or placebo for 16 weeks in this double‐blind, randomised, placebo‐controlled, parallel‐group study | |
| Participants | Sample size: 85 children/adolescents entered phase 2 (double‐blind randomisation), from 157 in phase 1 (single‐blind stabilisation) Particpants randomised in phase 2 Sample size: 85 (intervention 41, placebo 44) Sex: 17 girls (intervention 11, placebo 6), 68 boys (intervention 30, placebo 38) Mean age: overall 10.4 (SD 2.8); intervention 10.1 (SD 2.80); placebo 10.8 (SD 2.77) Race: 59 white (intervention 31, placebo 28), 19 black/African American (intervention 8, placebo 11), 3 Asian (0 intervention, 3 placebo), 1 American Indian/Alaskan Native (0 intervention, 1 placebo), 3 other (2 intervention, 1 placebo) Inclusion criteria (both phases)
Inclusion criteria (phase 2)
| |
| Interventions | Aripiprazole 2, 5, 10 or 15 mg/d or placebo | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | Study dates: March 2011 to June 2012 Study location: United States Funding: Bristol‐Myers Squibb Conflicts of interest
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not described in the manuscript |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed via a centralised call‐in system |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded aripiprazole or matching placebo was taken once daily |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blinded aripiprazole or matching placebo was taken once daily |
| Incomplete outcome data (attrition bias) | High risk | Not all raw data were provided |
| Selective reporting (reporting bias) | Low risk | All outcomes in protocol were reported in the study |
| Other bias | Low risk | From the report, it does not appear that any other sources of bias are present |
| Methods | Randomised 1:1:1:1 to aripiprazole (5, 10 or 15 mg/d) or placebo in this 8‐week double‐blind, randomised, placebo‐controlled, parallel‐group study | |
| Participants | Sample size: 218 children and adolescents (intervention 166 (54 = 15 mg, 59 = 10 mg, 53 = 5 mg), placebo 52) Sex: 23 girls (intervention 19 (4 = 15 mg, 9 = 10 mg, 6 = 5 mg), placebo 4), 195 boys (intervention 147 (50 = 15 mg, 50 = 10 mg, 47 = 5 mg), placebo 48) Age: 166 between 6 and 12 years of age (intervention 131 (42 = 15 mg, 45 = 10 mg, 44 = 5 mg), placebo 35), 52 between 13 and 17 years of age (intervention 35 (12 = 15 mg, 14 = 10 mg, 9 = 5 mg), placebo 17) Race: 155 white (intervention = 120 (42 = 15 mg, 41 = 10 mg, 37 = 5 mg), placebo = 35), 50 black (intervention = 37 (9 = 15 mg, 13 = 10 mg, 15 = 5 mg), placebo = 13), 46 Asian (intervention = 43 (0 = 15 mg, 42 = 10 mg, 1 = 5 mg), placebo = 3), 7 other (intervention = 6 (3 = 15 mg, 1 = 10 mg, 2 = 5 mg), placebo = 1) Inclusion criteria
| |
| Interventions | Aripiprazole (5, 10 or 15 mg/day) versus placebo | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | Study dates: June 2006 to June 2008 Study location: United States Funding: Bristol‐Myers Squibb and Otsuka Pharmaceutical Conflicts of interest
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although a double‐blind placebo‐controlled trial was described, the study did not describe how randomisation occurred |
| Allocation concealment (selection bias) | Unclear risk | Although a double‐blind placebo‐controlled trial was described, the study did not describe the method of concealment of allocation of intervention type |
| Blinding of participants and personnel (performance bias) | Unclear risk | Although a double‐blind trial was described, the study did not describe assurance of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Although a double‐blind trial was described, the study did not describe assurance of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Sufficient information was provided to address incomplete outcome data and how LOCF analysis of such data was performed. Excluded children and adolescents and reasons for exclusion were reported fully |
| Selective reporting (reporting bias) | Low risk | Study protocol is available; its pre‐specified, primary outcomes have been reported online to reduce the likelihood of selective reporting |
| Other bias | Low risk | From the report, it seems clear that no other risks of bias are present |
| Methods | Randomised 1:1 to flexibly dosed aripiprazole (target dose 5, 10 or 15 mg/d) or placebo in this 8‐week, double‐blind, randomised, placebo‐controlled, parallel‐group study | |
| Participants | Sample size: 98 children/adolescents (intervention 47, placebo 51) Sex: 12 girls (intervention 7, placebo 5), 86 boys (intervention 40, placebo 46) Age: 83 between 6 and 12 years of age (intervention 46, placebo 37), 15 between 13 and 17 years of age (intervention 10, placebo 5) Race: 73 white (intervention 41, placebo 32), 18 black (intervention 11, placebo 7), 2 Asian (2 intervention, 0 placebo), 5 other (intervention 2, placebo 3) Inclusion criteria
| |
| Interventions | Aripiprazole (5, 10 or 15 mg/d) versus placebo | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | Study dates: June 2006 to April 2008 Study location: United States Funding: Bristol‐Myers Squibb and Otsuka Pharmaceutical Conflicts of interest
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomisation schedule using permuted block design was used for randomisation |
| Allocation concealment (selection bias) | Low risk | A call‐in interactive voice response system was readily available for participating intervention sites when children and adolescents were ready to be randomly assigned |
| Blinding of participants and personnel (performance bias) | Low risk | Medication bottle numbers were assigned to children and adolescents, thus reducing the risk that blinding could have been broken |
| Blinding of outcome assessment (detection bias) | Low risk | Investigators and caregivers were blinded to the intervention; dosage increases were made incrementally |
| Incomplete outcome data (attrition bias) | Low risk | Sufficient information was provided to address incomplete outcome data and the way LOCF analysis of such data was performed. Excluded children and adolescents and reasons for exclusion were reported fully. 3 randomised children were excluded from the analysis ‐ 1 child randomised to placebo was lost to follow‐up before entering the double‐blind intervention phase, 1 child randomised to placebo withdrew consent and 1 child randomised to aripiprazole discontinued on day 2 of the study before completing a post‐baseline efficacy evaluation. We do not think exclusion of these 3 children would alter study results, even if extreme results were obtained in all 3 cases |
| Selective reporting (reporting bias) | Low risk | Study protocol is available; its pre‐specified primary outcomes have been reported online to reduce the likelihood of selective reporting |
| Other bias | Low risk | From the report, it seems clear that no other risks of bias are present |
ABC: Aberrant Behavior Checklist.
ABC‐I: Aberrant Behavior Checklist ‐ Irritability.
ADI‐R: Autism Diagnostic Interview ‐ Revised.
ASD: autism spectrum disorders.
BMI: body mass index.
CGI: Clinical Global Impressions scale.
CGI‐I: Clinical Global Impressions ‐ Improvement scale.
CGI‐S: Clinical Global Impressions ‐ Severity scale.
CY‐BOCS: Children’s Yale‐Brown Obsessive Compulsive Scale.
DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision
LOCF: last observation carried forward.
SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Post hoc analysis of pooled results of Marcus 2009 and Owen 2009 | |
| Review article | |
| Review article | |
| Non‐randomised controlled trial | |
| Review article | |
| Review article | |
| Review article | |
| No placebo control group | |
| Case series | |
| Pooled analysis of results of Marcus 2009 and Owen 2009 | |
| Non‐randomised controlled trial | |
| Retrospective naturalistic study | |
| Open‐label study | |
| Pooled analysis of results of Marcus 2009 and Owen 2009 | |
| Open‐label study | |
| Open‐label study | |
| Post hoc HRQoL analysis of results of Marcus 2009 and Owen 2009 |
HRQoL: health‐related quality of life.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Aberrant Behavior Checklist (ABC) ‐ Irritability subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐6.17 [‐9.07, ‐3.26] |
| Analysis 1.1  Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 1 Aberrant Behavior Checklist (ABC) ‐ Irritability subscale: mean score changes. | ||||
| 2 Aberrant Behavior Checklist (ABC) ‐ Hyperactivity subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐7.93 [‐10.98, ‐4.88] |
| Analysis 1.2  Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 2 Aberrant Behavior Checklist (ABC) ‐ Hyperactivity subscale: mean score changes. | ||||
| 3 Aberrant Behavior Checklist (ABC) ‐ Stereotypy subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐2.66 [‐3.55, ‐1.77] |
| Analysis 1.3 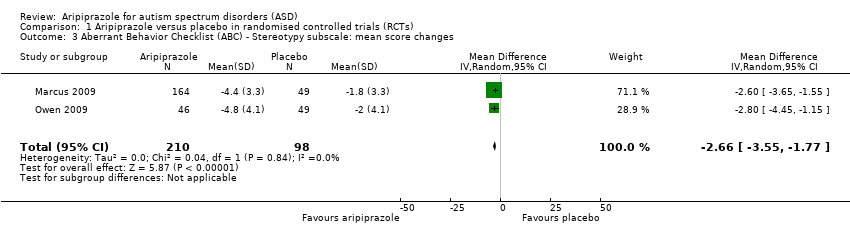 Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 3 Aberrant Behavior Checklist (ABC) ‐ Stereotypy subscale: mean score changes. | ||||
| 4 Aberrant Behavior Checklist (ABC) ‐ Inappropriate Speech subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.43 [‐2.60, ‐0.27] |
| Analysis 1.4  Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 4 Aberrant Behavior Checklist (ABC) ‐ Inappropriate Speech subscale: mean score changes. | ||||
| 5 Aberrant Behavior Checklist (ABC) ‐ Lethargy/Withdrawal subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐2.77, 0.40] |
| Analysis 1.5  Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 5 Aberrant Behavior Checklist (ABC) ‐ Lethargy/Withdrawal subscale: mean score changes. | ||||
| 6 Clinical Global Impression (CGI) ‐ Severity subscale: mean scores Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.96, ‐0.18] |
| Analysis 1.6  Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 6 Clinical Global Impression (CGI) ‐ Severity subscale: mean scores. | ||||
| 7 Clinical Global Impression (CGI) ‐ Improvement subscale: mean scores Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐1.75, ‐0.92] |
| Analysis 1.7  Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 7 Clinical Global Impression (CGI) ‐ Improvement subscale: mean scores. | ||||
| 8 Any extrapyramidal symptom event (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [0.98, 3.66] |
| Analysis 1.8 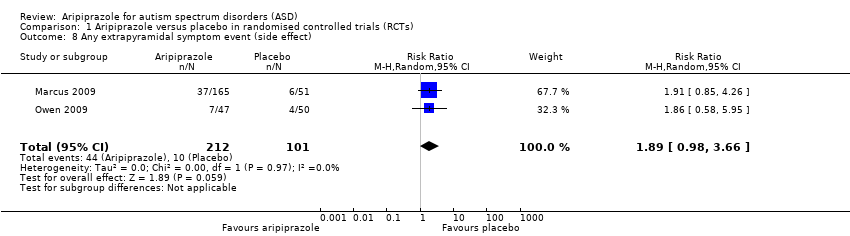 Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 8 Any extrapyramidal symptom event (side effect). | ||||
| 9 Children's Yale‐Brown Obsessive Compulsive Scale (CY‐BOCS): mean scores Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐3.86, 0.00] |
| Analysis 1.9 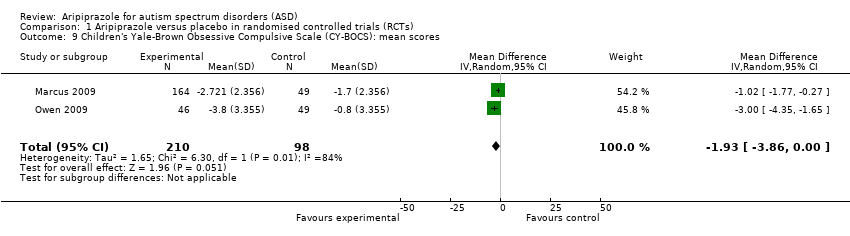 Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 9 Children's Yale‐Brown Obsessive Compulsive Scale (CY‐BOCS): mean scores. | ||||
| 10 Clinically relevant weight gain (side effect) Show forest plot | 2 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 3.78 [1.78, 8.02] |
| Analysis 1.10  Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 10 Clinically relevant weight gain (side effect). | ||||
| 11 Weight gain (side effect) Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | 1.13 [0.71, 1.54] |
| Analysis 1.11 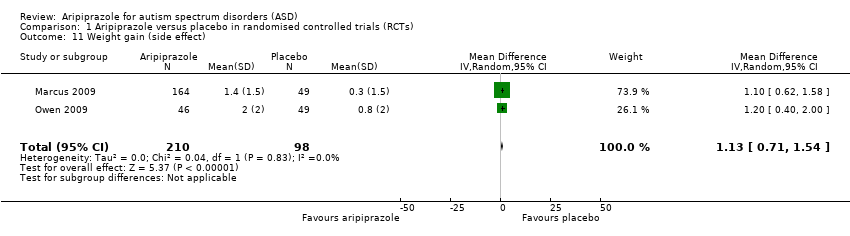 Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 11 Weight gain (side effect). | ||||
| 12 Body mass index (BMI) change from baseline (side effect) Show forest plot | 2 | 313 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.27, 1.16] |
| Analysis 1.12 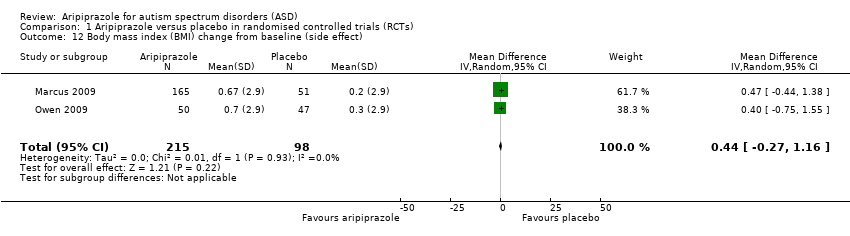 Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 12 Body mass index (BMI) change from baseline (side effect). | ||||
| 13 Elevated fasting triglycerides at endpoint (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.62, 3.67] |
| Analysis 1.13  Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 13 Elevated fasting triglycerides at endpoint (side effect). | ||||
| 14 Elevated low‐density lipoprotein at endpoint (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 3.19 [0.13, 76.36] |
| Analysis 1.14  Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 14 Elevated low‐density lipoprotein at endpoint (side effect). | ||||
| 15 Decreased high‐density lipoprotein at endpoint (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.11, 8.18] |
| Analysis 1.15 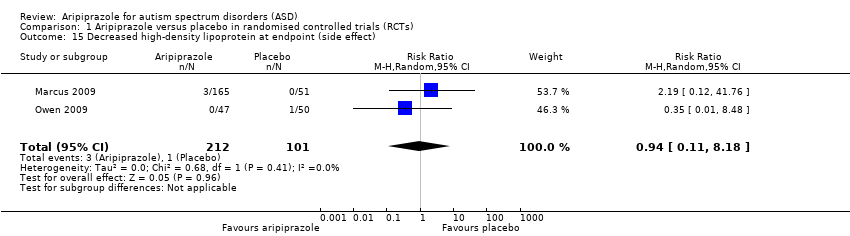 Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 15 Decreased high‐density lipoprotein at endpoint (side effect). | ||||
| 16 Elevated fasting blood glucose at endpoint (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.08, 32.11] |
| Analysis 1.16  Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 16 Elevated fasting blood glucose at endpoint (side effect). | ||||
| 17 Sedation (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 4.28 [1.58, 11.60] |
| Analysis 1.17  Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 17 Sedation (side effect). | ||||
| 18 Drooling (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 9.64 [1.29, 72.10] |
| Analysis 1.18  Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 18 Drooling (side effect). | ||||
| 19 Tremor (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 10.26 [1.37, 76.63] |
| Analysis 1.19  Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 19 Tremor (side effect). | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 1 Aberrant Behavior Checklist (ABC) ‐ Irritability subscale: mean score changes.

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 2 Aberrant Behavior Checklist (ABC) ‐ Hyperactivity subscale: mean score changes.
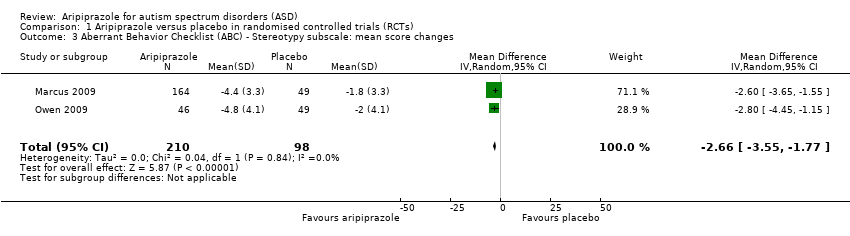
Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 3 Aberrant Behavior Checklist (ABC) ‐ Stereotypy subscale: mean score changes.

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 4 Aberrant Behavior Checklist (ABC) ‐ Inappropriate Speech subscale: mean score changes.
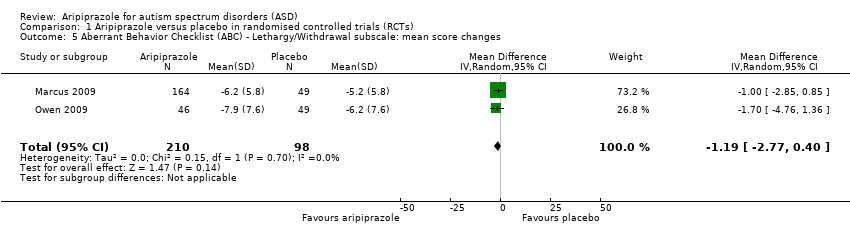
Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 5 Aberrant Behavior Checklist (ABC) ‐ Lethargy/Withdrawal subscale: mean score changes.

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 6 Clinical Global Impression (CGI) ‐ Severity subscale: mean scores.
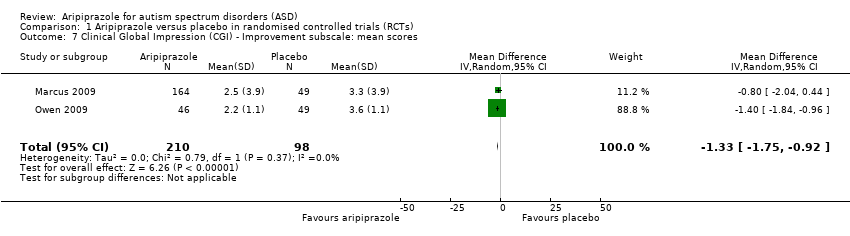
Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 7 Clinical Global Impression (CGI) ‐ Improvement subscale: mean scores.

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 8 Any extrapyramidal symptom event (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 9 Children's Yale‐Brown Obsessive Compulsive Scale (CY‐BOCS): mean scores.

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 10 Clinically relevant weight gain (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 11 Weight gain (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 12 Body mass index (BMI) change from baseline (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 13 Elevated fasting triglycerides at endpoint (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 14 Elevated low‐density lipoprotein at endpoint (side effect).
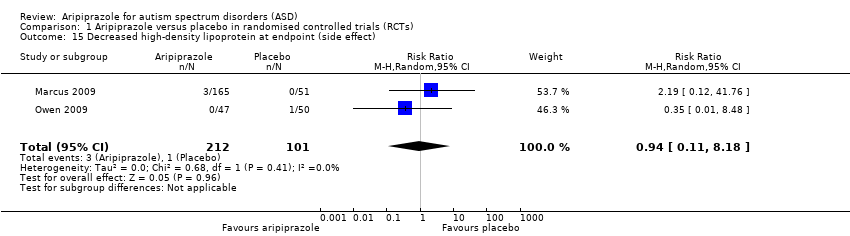
Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 15 Decreased high‐density lipoprotein at endpoint (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 16 Elevated fasting blood glucose at endpoint (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 17 Sedation (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 18 Drooling (side effect).
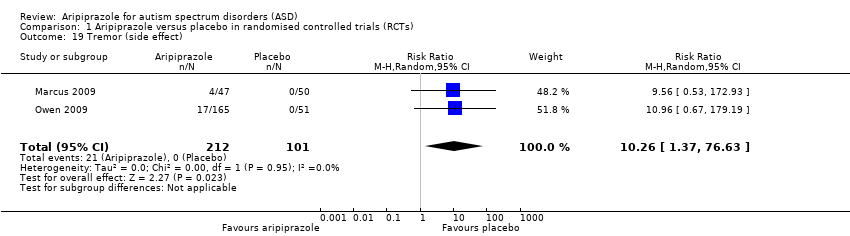
Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 19 Tremor (side effect).
| Aripiprazole compared with placebo for autism spectrum disorders (ASD)* | ||||
| Patient or population: children/youth with ASD Settings: ambulatory care Intervention: aripiprazole Comparison: placebo | ||||
| Outcomes | Effect (95% confidence interval) | Number of participants (studies) | Quality of the evidence | Comments |
| ABC ‐ Irritability subscale Mean score changes 8 weeks of treatment | MD ‐6.17 (‐9.07 to ‐3.26) points relative to placebo, in favour of aripiprazole | 308 (2) | ⊕⊕⊕⊝ | ‐ |
| ABC ‐ Hyperactivity subscale Mean score changes 8 weeks of treatment | MD ‐7.93 (‐10.98 to ‐4.88) points relative to placebo, in favour of aripiprazole | 308 (2) | ⊕⊕⊕⊝ | ‐ |
| ABC ‐ Stereotypy subscale Mean score changes 8 weeks of treatment | MD ‐2.66 (‐3.55 to ‐1.77) points relative to placebo, in favour of aripiprazole | 308 (2) | ⊕⊕⊕⊝ | ‐ |
| Weight gain 8 weeks of treatment | MD 1.13 (0.71 to 1.54) points relative to placebo | 308 (2) | ⊕⊕⊕⊝ | ‐ |
| Sedation 8 weeks of treatment | RR 4.28 (1.58 to 11.60) | 313 (2) | ⊕⊕⊕⊝ | ‐ |
| Tremor 8 weeks of treatment | RR 10.26 (1.37 to 76.63) | 313 (2) | ⊕⊕⊕⊝ | ‐ |
| Relapse rate 16 weeks of treatment | HR 0.57 (0.28 to 1.12) | 85 (1) | Lowb | ‐ |
| ABC: Aberrant Behavior Checklist; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; HR: hazard ratio;MD: mean difference; RR: risk ratio | ||||
| GRADE Working Group grades of evidence | ||||
| aQuality was rated as moderate because of unclear risk of bias for some domains in Marcus 2009 and Owen 2009, and because of the overall small number of studies conducted using aripiprazole in ASD. *A small number of studies have evaluated the use of aripiprazole for ASD. Only one study examined aripiprazole at a duration longer than eight weeks. Future trials of aripiprazole in children/adolescents with ASD are likely to impact the estimates found in this review. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Aberrant Behavior Checklist (ABC) ‐ Irritability subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐6.17 [‐9.07, ‐3.26] |
| 2 Aberrant Behavior Checklist (ABC) ‐ Hyperactivity subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐7.93 [‐10.98, ‐4.88] |
| 3 Aberrant Behavior Checklist (ABC) ‐ Stereotypy subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐2.66 [‐3.55, ‐1.77] |
| 4 Aberrant Behavior Checklist (ABC) ‐ Inappropriate Speech subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.43 [‐2.60, ‐0.27] |
| 5 Aberrant Behavior Checklist (ABC) ‐ Lethargy/Withdrawal subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐2.77, 0.40] |
| 6 Clinical Global Impression (CGI) ‐ Severity subscale: mean scores Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.96, ‐0.18] |
| 7 Clinical Global Impression (CGI) ‐ Improvement subscale: mean scores Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐1.75, ‐0.92] |
| 8 Any extrapyramidal symptom event (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [0.98, 3.66] |
| 9 Children's Yale‐Brown Obsessive Compulsive Scale (CY‐BOCS): mean scores Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐3.86, 0.00] |
| 10 Clinically relevant weight gain (side effect) Show forest plot | 2 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 3.78 [1.78, 8.02] |
| 11 Weight gain (side effect) Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | 1.13 [0.71, 1.54] |
| 12 Body mass index (BMI) change from baseline (side effect) Show forest plot | 2 | 313 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.27, 1.16] |
| 13 Elevated fasting triglycerides at endpoint (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.62, 3.67] |
| 14 Elevated low‐density lipoprotein at endpoint (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 3.19 [0.13, 76.36] |
| 15 Decreased high‐density lipoprotein at endpoint (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.11, 8.18] |
| 16 Elevated fasting blood glucose at endpoint (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.08, 32.11] |
| 17 Sedation (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 4.28 [1.58, 11.60] |
| 18 Drooling (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 9.64 [1.29, 72.10] |
| 19 Tremor (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 10.26 [1.37, 76.63] |

