Aripiprazol para los trastornos del espectro autista (TEA)
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL; part of The Cochrane Library)
#1MeSH descriptor Antipsychotic Agents, this term only
#2aripiprazol* or abilify or abilitat
#3atypical Near/3 antipsychotic*
#4(#1 OR #2 OR #3)
#5MeSH descriptor Child Development Disorders, Pervasive explode all trees
#6asperger*
#7autis* or ASD or ASDs
#8kanner*
#9rett*
#10childhood near/3 schizophren*
#11(pervasiv* NEXT development* NEXT disorder*) OR PDD or PDDs
#12MeSH descriptor Social Behavior Disorders, this term only
#13MeSH descriptor Child Behavior Disorders, this term only
#14MeSH descriptor Speech Disorders, this term only
#15MeSH descriptor Language Development Disorders, this term only
#16(speech or language) near/3 (disorder* or delay*)
#17MeSH descriptor Communication Disorders, this term only
#18(behav* or communicat*) NEXT (disorder* or impair*)
#19(#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18)
#20(#4 AND #19)
Ovid MEDLINE
1 ( aripiprazol$ or abilify or abilitat).mp.
2 (atypical adj3 antipsychotic$).mp.
3 antipsychotic agents/
4 or/1‐3
5 exp Child Development Disorders, Pervasive/
6 asperger$.tw.
7 (autis$ or ASD or ASDs).tw.
8 kanner$.tw.
9 rett$.tw.
10 (childhood adj3 schizophren$).tw.
11 (pervasive development$ disorder$ or PDD or PDDs).tw.
12 Social Behavior Disorders/
13 Child Behavior Disorders/
14 speech disorders/
15 language development disorders/
16 ((speech or language) adj3 (delay$ or disorder$)).tw.
17 Communication Disorders/
18 ((behav$ or communicat$) adj (disorder$ or impair$)).tw.
19 or/5‐18
20 randomised controlled trial.pt.
21 controlled clinical trial.pt.
22 randomi#ed.ab.
23 placebo$.ab.
24 drug therapy.fs.
25 randomly.ab.
26 trial.ab.
27 groups.ab.
28 or/20‐27
29 exp animals/ not humans.sh.
30 28 not 29
31 4 and 19 and 30
Embase (Ovid)
1 aripiprazole/
2 (aripiprazol$ or abilify or abilitat).mp.
3 atypical antipsychotic agent/
4 (atypical adj3 antipsychotic$).mp.
5 or/1‐4
6 exp autism/
7 asperger$.tw.
8 (autis$ or ASD or ASDs).tw.
9 kanner$.tw.
10 rett$.tw.
11 (childhood adj3 schizophren$).tw.
12 (pervasive development$ disorder$ or PDD or PDDs).tw.
13 sociopathy/
14 behavior disorder/
15 speech disorder/
16 language disability/
17 ((speech or language) adj3 (delay$ or disorder$)).tw.
18 communication disorder/
19 ((behav$ or communicat$) adj (disorder$ or impair$)).tw.
20 or/6‐19
21 exp Clinical trial/
22 Randomization/
23 Single blind procedure/
24 Double blind procedure/
25 Crossover procedure/
26 Placebo/
27 Randomi#ed.tw.
28 RCT.tw.
29 (random$ adj3 (allocat$ or assign$)).tw.
30 randomly.ab.
31 groups.ab.
32 trial.ab.
33 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
34 Placebo$.tw.
35 Prospective study/
36 (crossover or cross‐over).tw.
37 prospective.tw.
38 or/21‐37
39 5 and 20 and 38
CINAHL Plus: Cumulative Index to Nursing and Allied Health Literature (EBSCOhost)
S39 S18 and S23 and S38
S38 S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37
S37 TI (evaluat* study or evaluat* research) or AB (evaluate* study or evaluat* research) or TI (effectiv* study or effectiv* research) or AB (effectiv* study or effectiv* research) OR TI (prospectiv* study or prospectiv* research) or AB(prospectiv* study or prospectiv* research) or TI (follow‐up study or follow‐up research) or AB (follow‐up study or follow‐up research)
S36 "cross over*"
S35 crossover*
S34 (MH "Crossover Design")
S33 (tripl* N3 mask*) or (tripl* N3 blind*)
S32 (trebl* N3 mask*) or (trebl* N3 blind*)
S31 (doubl* N3 mask*) or (doubl* N3 blind*)
S30 (singl* N3 mask*) or (singl* N3 blind*)
S29 (clinic* N3 trial*) or (control* N3 trial*)
S28 (random* N3 allocat* ) or (random* N3 assign*)
S27 randomis* or randomiz*
S26 (MH "Meta Analysis")
S25 (MH "Clinical Trials+")
S24 MH random assignment
S23 S19 or S20 or S21 or S22
S22 atypical N3 antipsychotic*
S21 (MH "Antipsychotic Agents")
S20 Aripiprazol* or abilify or abilitat
S19 (MH "Aripiprazole")
S18 (S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17)
S17 TI (speech N3 delay*) or TI (speech N3 disorder*) or AB (speech N3 delay*) or AB (speech N3 disorder*)
S16 TI (language N3 delay*) or TI (language N3 disorder*) or AB (language N3 delay*) or AB (language N3 disorder*)
S15 TI (communicat* disorder*) or TI (communicat* impair*)
S14 AB (communicat* disorder*) or AB (communicat* impair*)
S13 AB (behav* disorder*) or AB (behav* impair*)
S12 TI (behav* disorder*) or TI (behav* impair*)
S11 (MH "Speech Disorders")
S10 (MH "Language Disorders")
S9 (MH "Communicative Disorders")
S8 TI(childhood schizophrenia) or AB(childhood schizophrenia)
S7 TI(Kanner*) or AB (Kanner*)
S6 TI (Rett* ) or AB (Rett*)
S5 TI (Asperger* ) or AB (Asperger*)
S4 TI (autis* or ASD or ASDs) or AB (autis* or ASD or ASDs)
S3 AB (pervasive development* disorder* or PDD or PDDs)
S2 TI (pervasive development* disorder* or PDD or PDDs)
S1 (MH "Child Development Disorders, Pervasive+")
PsycINFO
PsycINFO (Ovid) was searched from 2014 onwards for the review update
1 exp pervasive developmental disorders/
2 Developmental disabilities/
3 pervasive development$ disorder$.tw.
4 (pervasive adj3 child$).tw.
5 autis$.tw.
6 asperger$.tw.
7 (autis$ or ASD or ASDs).tw.
8 (ASD or ASDs or PDD or PDDs).tw.
9 Rett$.tw.
10 Kanner$.tw.
11 or/1‐10
12 aripiprazole/
13 Neuroleptic Drugs/
14 (atypical adj3 (antipsychotic$ or anti‐psychotic$)).tw.
15 aripiprazol$.tw.
16 Abilify.tw.
17 Abilitat.tw.
18 (second generation adj (antipsychotic$ or anti‐psychotic$)).tw.
19 or/12‐18
20 clinical trials/
21 random$.tw.
22 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).tw.
23 (crossover$ or "cross over$").tw.
24 trial$.tw.
25 group$.ab.
26 control.ab.
27 exp program evaluation/
28 treatment effectiveness evaluation/
29 treatment outcome clinical trial.md.
30 ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw.
31 (allocat$ or assign$).tw.
32 placebo.ab.
33 or/20‐32
34 11 and 19 and 33
PsycINFO (EBSCOhost) was searched in 2011 for the original review
S41 S21 and S26 and S40
S40 S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39
S39 (evaluation N3 stud* or evaluation N3 research*)
S38 (effectiveness N3 stud* or effectiveness N3 research*)
S37 DE "Placebo" or DE "Evaluation" or DE "Program Evaluation" OR DE "Educational Program Evaluation" OR DE "Mental Health Program Evaluation"
S36 (DE "Random Sampling" or DE "Clinical Trials") or (DE "Experiment Controls")
S35 "cross over*"
S34 crossover*
S33 (tripl* N3 mask*) or (tripl* N3 blind*)
S32 (trebl* N3 mask*) or (trebl* N3 blind*)
S31 (doubl* N3 mask*) or (doubl* N3 blind*)
S30 (singl* N3 mask*) or (singl* N3 blind*)
S29 (clinic* N3 trial*) or (control* N3 trial*)
S28 (random* N3 allocat* ) or (random* N3 assign*)
S27 randomis* or randomiz*
S26 S22 or S23 or S24 or S25
S25 (atypical N3 antipsychotic*)
S24 DE "Neuroleptic Drugs"
S23 (aripiprazol* or abilify or abilitat
S22 DE "Aripiprazole"
S21 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20
S20 TI (speech N3 disorder*) Or TI (speech N3 delay*)
S19 AB (speech N3 disorder*) Or Ab (speech N3 delay*)
S18 AB (language N3 disorder*) Or Ab (language N3 delay*)
S17 TI (language N3 disorder*) Or TI (language N3 delay*)
S16 TI(communicat* disorder*) or TI (communicat* impair*)
S15 AB (communicat* disorder*) or AB (communicat* impair*)
S14 AB (behav* disorder*) or AB (behav* impair*)
S13 TI (behav* disorder*) or TI (behav* impair*)
S12 DE "Behavior Disorders"
S11 DE "Language Disorders" OR DE "Language Delay"
S10 DE "Speech Disorders"
S9 DE "Communication Disorders"
S8 TI(childhood schizophrenia) or AB(childhood schizophrenia)
S7 TI(Kanner*) or AB (Kanner*)
S6 TI (Rett* ) or AB (Rett*)
S5 TI (Asperger* ) or AB (Asperger*)
S4 TI (autis* ) or AB (autis*)
S3 TI (pervasive development* disorder* or PDD or PDDs)
S2 AB (pervasive development* disorder* or PDD or PDDs)
S1 DE "Pervasive Developmental Disorders" OR DE "Pervasive Developmental Disorders" OR DE "Aspergers Syndrome" OR DE "Autism" OR DE "Rett Syndrome"
Cochrane Database of Systematic Reviews (CDSR; part of The Cochrane Library)
#1MeSH descriptor: [Antipsychotic Agents] this term only
#2(aripiprazol* or abilify or abilitat):ti,ab
#3(atypical near/3 (antipsychotic* or anti‐psychotic*)):ti,ab
#4#1 or #2 or #3
#5MeSH descriptor: [Child Development Disorders, Pervasive] explode all trees
#6MeSH descriptor: [Developmental Disabilities] this term only
#7asperger*:ti,ab
#8(autis* or ASD or ASDs):ti,ab
#9kanner*:ti,ab
#10rett*:ti,ab
#11(childhood near/3 schizophren*):ti,ab
#12(pervasiv* next development* next disorder* or PDD or PDDs):ti,ab
#13#5 or #6 or #7 or #8 or #9 or #10 or #11 or #12
#14#4 and #13
Database of Abstracts of Reviews of Effects (DARE; part of The Cochrane Library)
#1MeSH descriptor: [Antipsychotic Agents] this term only
#2(aripiprazol* or abilify or abilitat):ti,ab
#3(atypical near/3 (antipsychotic* or anti‐psychotic*)):ti,ab
#4#1 or #2 or #3
#5MeSH descriptor: [Child Development Disorders, Pervasive] explode all trees
#6MeSH descriptor: [Developmental Disabilities] this term only
#7asperger*:ti,ab
#8(autis* or ASD or ASDs):ti,ab
#9kanner*:ti,ab
#10rett*:ti,ab
#11(childhood near/3 schizophren*):ti,ab
#12(pervasiv* next development* next disorder* or PDD or PDDs):ti,ab
#13#5 or #6 or #7 or #8 or #9 or #10 or #11 or #12
#14#4 and #13
#15#1
Conference Proceedings Citation Index ‐ Science (CPCI‐S; Web of Science)
#3 2 AND #1
#2 TS=(aripiprazol* or abilify or abilitat or "atypical antipsychotic*" or "atypical anti‐psychotic*" )
#1 TS=(autis* or asperger* or kanner* or childhood schizophren* or ASD or ASDs or "childhood pervasive disorder* " or "pervasive disorder* of childhood" or PDD or PDDs)
Autism Data
aripiprazole or abilify or abilitat
ZETOC (limited to conferences)
conference: aripiprazole autis*
WorldCat (limited to theses and dissertations)
(kw: autis* or kw: asperger* or kw: pervasiv*) AND (kw: aripiprazole OR kw: abilify OR kw: abilitat or kw: atypical) and yr: 1990‐2011 and mt: deg.
ClinicalTrials.gov
aripiprazole OR abilify OR abilitat OR atypical antipsychotics AND autis* OR pervasive OR asperge*
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP)
Condition : autism OR autistic or asperger or aspergers or pervasive or ASD or ASDs or PDD or PDDS
AND
Intervention: aripiprazole OR abilify OR abilitat OR atypical antipsychotic*
Appendix 2. Search summary
| Database | Search | Database date range/issue/volume | Number of | Limits |
| Cochrane Central Register of Controlled Trials (CENTRAL) | 04/05/2011 | 2011 Issue 11 | 84 | limit to 1990‐ |
| 20/11/2014 | 2014 Issue 10 | 26 | limit to 2011‐current | |
| 14/10/2015 | 2015 Issue 9 | 8 | limit to 2014‐2015 | |
| Ovid MEDLINE | 04/05/2011 | 1948 to April Week 3 2011 | 567 | limit to 1990‐ |
| 20/11/2014 | 1946 to November Week 2 2014 | 150 | ed=20110401‐20141120 | |
| 14/10/2015 | 1946 to October Week 1 2015 | 23 | ed=20141101‐20151001 | |
| Embase (Ovid) | 04/05/2011 | 1980 to 2011 Week 17 | 710 | limit to 1990‐ |
| 20/11/2014 | 1980 to 2014 Week 46 | 144 | limit to 2011‐current | |
| 14/10/2015 | 1980 to 2015 Week 41 | 27 | em=201446‐201541 | |
| CINAHL Plus (EBSCOhost) | 04/05/2011 | 1937 to current | 45 | limit to 1990‐ |
| 20/11/2014 | 1937 to current | 31 | EM 20110430‐ | |
| 14/10/2015 | 1937 to current | 5 | EM 20141101‐ | |
| PsycINFO (EBSCOost) | 04/05/2011 | 1887 to current | 83 | limit to 1990‐ |
| PsycINFO (Ovid) | 20/11/2014 | 1887 to current | 59 | up=20110502‐20141117 |
| 14/10/2015 | 1806 to October Week 1 2015 | 10 | up=20141117‐20151005 | |
| Cochrane Database of Systematic Reviews (CDSR) | 20/11/2014 | 11 of 12, November 2014 | 4 | no limits as not searched previously |
| 14/10.2015 | 10 of 12 October 2015 | 0 | 2014‐2015 | |
| Database of Abstracts of Reviews of Effects (DARE) | 20/11/2014 | Issue 4 of 4, October 2014 | 8 | no limits as not searched previously |
| 14/10/2015 | Issue 2 of 4, April 2015 | 1 | 2014‐2015 | |
| Conference Proceedings Citation Index ‐ Science (CPCI‐S, Web of Science) | 04/05/2011 | 1990 to current | 22 | limit to 1990‐ |
| 20/11/2014 | 1990 to current | 2 | limit to 2011‐2014 | |
| 14/10/2015 | 1990 to current | 1 | limit to 2014‐2015 | |
| 04/05/2011 | all available years | 20 | no limits | |
| 20/11/2014 | all available years | 21 | 2011‐2014 | |
| 14/10/2015 | all available years | 0 | 2014‐2015 | |
| ZETOC (conference proceedings) | 04/05/2011 | all available years | 0 | no limits |
| 20/11/2014 | all available years | 0 | no limits | |
| 14/10/2015 | all available years | 0 | no limits | |
| WorldCat.org (limited to theses and dissertations) | 04/05/2011 | all available years | 11 | 1990‐ |
| 20/11/2014 | all available years | 1 | 2011‐2014 | |
| 14/10/2015 | all available years | 0 | 2014‐2015 | |
| 04/05/2011 | all available years | 20 | no limits | |
| 24/11/2014 | all available years | 16 | no limits | |
| 14/10/2015 | all available years | 16 | no limits | |
| World Health Organisation International Clinical Trials Registry Platform (WHO ICTRP) | 04/05/2011 | all available years | 7 | no limits |
| 24/11/2014 | all available years | 6 | no limits | |
| 14/10/2015 | all available years | 20 | no limits | |
| Total records up to 2011 | 1569 | |||
| Total records 2011 to 2015 | 579 |
Appendix 3. Planned methods archived for future updates of this review
| Measures of treatment effect | Continuous outcomes We will use standardised mean differences (SMDs) for continuous outcomes that are measured with similar, but not identical, instruments. |
| Unit of analysis issues
| Cluster‐randomised trials We do not anticipate finding cluster‐randomised trials. Cross‐over trials If we find any cross‐over trials, we will conduct a meta‐analysis to combine results using the inverse variance method as recommended by Elbourne 2002. In the case that data from a cross‐over trial are limited and restricted, we will use available data within the first phase, only up to the point of cross‐over. Repeated observations on participants If studies report repeated observations on participants, we will extract time points closer to 3 and 6 months and at the end of the study. |
| Assessment of reporting bias
| If we find enough studies (n = 10), we will draw funnel plots (effect size versus standard error) to assess the presence of small‐study effects. Although asymmetry may represent the relationship between trial size and effect size, it can also demonstrate publication bias. If we find funnel plot asymmetry, we will investigate clinical diversity as a possible explanation for asymmetry. |
| Subgroup analysis and investigation of heterogeneity
| Assuming a sufficient number of included studies, we will undertake a subgroup analysis to explore the possibility of differential responses to aripiprazole based on:
If data are presented in unuseable forms, we will attempt to contact study authors for raw data. If we cannot obtain these data, we will not include the study in the meta‐analysis but instead will describe the results individually. |
| Sensitivity analysis | We will conduct sensitivity analyses to assess the impact of risk of bias on results in the event that we find a sufficient number of studies. For example, we will exclude studies rated as having high risk of bias for sequence generation or allocation concealment, or both, to find any impact on results of the meta‐analysis. |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 1 Aberrant Behavior Checklist (ABC) ‐ Irritability subscale: mean score changes.

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 2 Aberrant Behavior Checklist (ABC) ‐ Hyperactivity subscale: mean score changes.
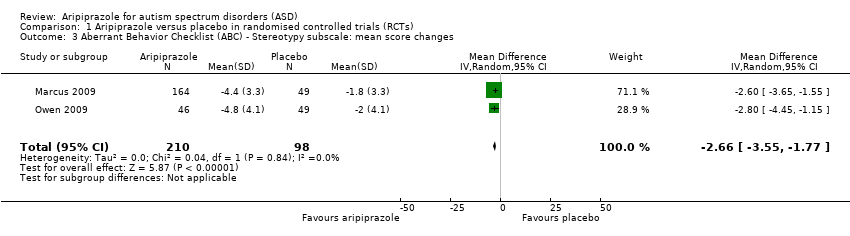
Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 3 Aberrant Behavior Checklist (ABC) ‐ Stereotypy subscale: mean score changes.

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 4 Aberrant Behavior Checklist (ABC) ‐ Inappropriate Speech subscale: mean score changes.
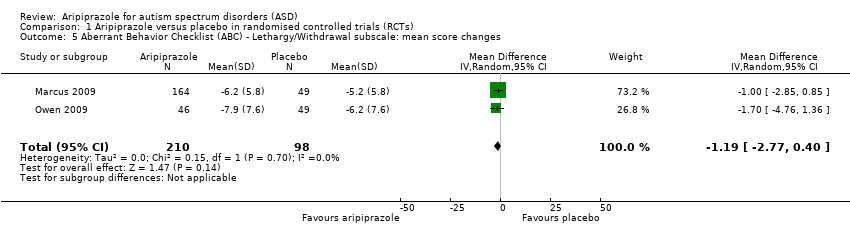
Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 5 Aberrant Behavior Checklist (ABC) ‐ Lethargy/Withdrawal subscale: mean score changes.

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 6 Clinical Global Impression (CGI) ‐ Severity subscale: mean scores.
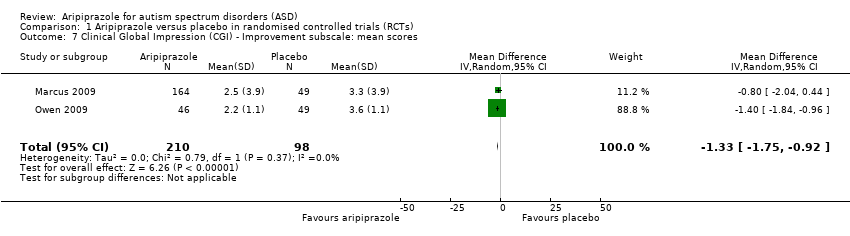
Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 7 Clinical Global Impression (CGI) ‐ Improvement subscale: mean scores.

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 8 Any extrapyramidal symptom event (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 9 Children's Yale‐Brown Obsessive Compulsive Scale (CY‐BOCS): mean scores.

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 10 Clinically relevant weight gain (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 11 Weight gain (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 12 Body mass index (BMI) change from baseline (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 13 Elevated fasting triglycerides at endpoint (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 14 Elevated low‐density lipoprotein at endpoint (side effect).
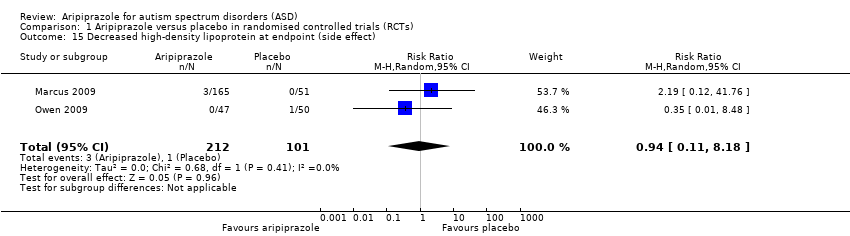
Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 15 Decreased high‐density lipoprotein at endpoint (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 16 Elevated fasting blood glucose at endpoint (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 17 Sedation (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 18 Drooling (side effect).
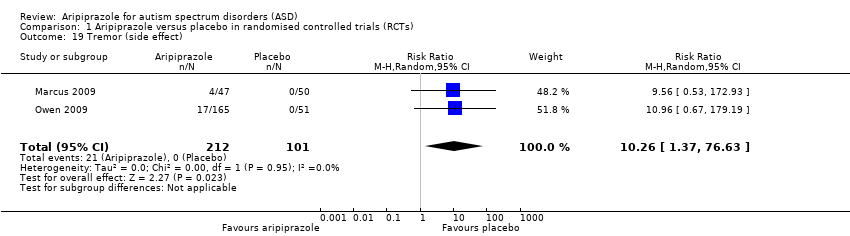
Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 19 Tremor (side effect).
| Aripiprazole compared with placebo for autism spectrum disorders (ASD)* | ||||
| Patient or population: children/youth with ASD Settings: ambulatory care Intervention: aripiprazole Comparison: placebo | ||||
| Outcomes | Effect (95% confidence interval) | Number of participants (studies) | Quality of the evidence | Comments |
| ABC ‐ Irritability subscale Mean score changes 8 weeks of treatment | MD ‐6.17 (‐9.07 to ‐3.26) points relative to placebo, in favour of aripiprazole | 308 (2) | ⊕⊕⊕⊝ | ‐ |
| ABC ‐ Hyperactivity subscale Mean score changes 8 weeks of treatment | MD ‐7.93 (‐10.98 to ‐4.88) points relative to placebo, in favour of aripiprazole | 308 (2) | ⊕⊕⊕⊝ | ‐ |
| ABC ‐ Stereotypy subscale Mean score changes 8 weeks of treatment | MD ‐2.66 (‐3.55 to ‐1.77) points relative to placebo, in favour of aripiprazole | 308 (2) | ⊕⊕⊕⊝ | ‐ |
| Weight gain 8 weeks of treatment | MD 1.13 (0.71 to 1.54) points relative to placebo | 308 (2) | ⊕⊕⊕⊝ | ‐ |
| Sedation 8 weeks of treatment | RR 4.28 (1.58 to 11.60) | 313 (2) | ⊕⊕⊕⊝ | ‐ |
| Tremor 8 weeks of treatment | RR 10.26 (1.37 to 76.63) | 313 (2) | ⊕⊕⊕⊝ | ‐ |
| Relapse rate 16 weeks of treatment | HR 0.57 (0.28 to 1.12) | 85 (1) | Lowb | ‐ |
| ABC: Aberrant Behavior Checklist; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; HR: hazard ratio;MD: mean difference; RR: risk ratio | ||||
| GRADE Working Group grades of evidence | ||||
| aQuality was rated as moderate because of unclear risk of bias for some domains in Marcus 2009 and Owen 2009, and because of the overall small number of studies conducted using aripiprazole in ASD. *A small number of studies have evaluated the use of aripiprazole for ASD. Only one study examined aripiprazole at a duration longer than eight weeks. Future trials of aripiprazole in children/adolescents with ASD are likely to impact the estimates found in this review. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Aberrant Behavior Checklist (ABC) ‐ Irritability subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐6.17 [‐9.07, ‐3.26] |
| 2 Aberrant Behavior Checklist (ABC) ‐ Hyperactivity subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐7.93 [‐10.98, ‐4.88] |
| 3 Aberrant Behavior Checklist (ABC) ‐ Stereotypy subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐2.66 [‐3.55, ‐1.77] |
| 4 Aberrant Behavior Checklist (ABC) ‐ Inappropriate Speech subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.43 [‐2.60, ‐0.27] |
| 5 Aberrant Behavior Checklist (ABC) ‐ Lethargy/Withdrawal subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐2.77, 0.40] |
| 6 Clinical Global Impression (CGI) ‐ Severity subscale: mean scores Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.96, ‐0.18] |
| 7 Clinical Global Impression (CGI) ‐ Improvement subscale: mean scores Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐1.75, ‐0.92] |
| 8 Any extrapyramidal symptom event (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [0.98, 3.66] |
| 9 Children's Yale‐Brown Obsessive Compulsive Scale (CY‐BOCS): mean scores Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐3.86, 0.00] |
| 10 Clinically relevant weight gain (side effect) Show forest plot | 2 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 3.78 [1.78, 8.02] |
| 11 Weight gain (side effect) Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | 1.13 [0.71, 1.54] |
| 12 Body mass index (BMI) change from baseline (side effect) Show forest plot | 2 | 313 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.27, 1.16] |
| 13 Elevated fasting triglycerides at endpoint (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.62, 3.67] |
| 14 Elevated low‐density lipoprotein at endpoint (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 3.19 [0.13, 76.36] |
| 15 Decreased high‐density lipoprotein at endpoint (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.11, 8.18] |
| 16 Elevated fasting blood glucose at endpoint (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.08, 32.11] |
| 17 Sedation (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 4.28 [1.58, 11.60] |
| 18 Drooling (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 9.64 [1.29, 72.10] |
| 19 Tremor (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 10.26 [1.37, 76.63] |

