Neuraminidase inhibitors for preventing and treating influenza in adults and children
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008965.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 10 abril 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Infecciones respiratorias agudas
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
TJ, PD, CDM, MT, MJ and CH were authors of the separate relevant Cochrane reviews. The protocol for the 2012 review was written by TJ, PD and CDM. All authors contributed to the writing of this protocol and devised the approach strategies to the data sources. CH provided logistical support. For the 2012 review, all authors reconstructed clinical trials using the CONSORT statement‐based extraction template, TJ reviewed regulatory material and TJ, MJ, CH, RH and CDM applied the inclusion criteria. CDM supervised the process and arbitrated when necessary. MJ carried out the statistical analyses. RH reviewed the Japanese data together with MJ and PD. TJ reviewed the FDA files. CDM and MT screened the electronic searches. TJ prepared the final text and all authors contributed to the final draft. Toby Lasserson contributed editorial support.
For the 2014 review TJ, PD, CDM, MT, RH, MJ and CH amended the protocol. TJ and PD applied the inclusion criteria to the oseltamivir clinical study reports. CH and IO applied the inclusion criteria to the zanamivir clinical study reports. MJ supervised the process and arbitrated when necessary. MJ carried out the statistical analyses. RH reviewed the Japanese data together with MJ and PD. TJ, PD, CH, IO, ES, DN and JH extracted the clinical study reports. CDM and MT screened the electronic search updates. TJ prepared the final text and all authors contributed to the final draft.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
The review has been prepared with support from a NIHR (UK) grant 10/80/01
Declarations of interest
All review authors have applied for and received competitive research grants. TJ, PD, CDM, MT, RH, MJ and CH are co‐recipients of the NIHR grant to carry out this review ( http://www.nets.nihr.ac.uk/projects/hta/108001 ). In addition:
Prof Jefferson receives royalties from his books published by Blackwell and Il Pensiero Scientifico Editore, Rome. Dr Jefferson is occasionally interviewed by market research companies for anonymous interviews about Phase 1 or 2 pharmaceutical products. In 2011‐2013 Dr Jefferson acted as an expert witness in a litigation case related to oseltamivir phosphate; Tamiflu [Roche] and in a labour case on influenza vaccines in healthcare workers in Canada. In 1997‐99 Dr Jefferson acted as consultant for Roche, in 2001‐2 for GSK and in 2003 for Sanofi‐Synthelabo for pleconaril (an anti‐rhinoviral which did not get approval from FDA). Dr Jefferson is a consultant for IMS Health.
Dr Doshi received EUR 1500 from the European Respiratory Society in support of his travel to the society's September 2012 annual congress in Vienna, where he gave an invited talk on oseltamivir.
Prof Del Mar was a Board member of two companies to commercialise research at Bond University, part of his responsibilities as Pro‐Vice Chancellor (Research) until 2010, receives fees for editorial and guideline developmental work and royalties from books, and is in receipt of institutional grants from NHMRC (Aus), NIHR (UK) and HTA (UK) and from a private donor (for support of the editorial base of the Cochrane ARI Group).
Dr Hama receives royalties from two books published in 2008 titled "Tamiflu: harmful as was afraid" and "In order to escape from drug‐induced encephalopathy". Dr Hama provided scientific opinions and expert testimony on 11 adverse reaction cases related to oseltamivir and gefitinib.
Dr Howick has received expenses and payments from Johns Hopkins and the American Society for Neurophysiological Monitoring as an EBM consultant. Dr Howick has received funding from the Wellcome Trust, the Medical Research Council of the UK, the Economics and Social Science Research Council of the UK and he is currently a National Institute for Health Research non‐clinical research fellow. He has received payment from the Canadian Medical Association Journal for writing a book review and receives royalties from the publication of his book from Blackwell/Wiley.
Dr Heneghan receives payment for running educational courses at the University of Oxford and University of Oxford ISIS consulting services for external teaching and training. He also receives royalties for books (Evidence Based Toolkit series by Blackwell BMJ Books).
Dr Onakpoya has no additional interests to disclose.
Dr Thompson has no additional interests to disclose.
Dr Jones has no additional interests to disclose.
Dr Spencer has no additional interests to disclose.
Dr Nunan has no additional interests to disclose.
Dr Mahtani has no additional interests to disclose.
Acknowledgements
Thanks to Jon Deeks, Timothy Aoki, Carlo Di Pietrantonj, Vittorio Demicheli, Janet Wale, John Bartlett, Sree Nair, Tom Fahey, Matthew Shun‐Shin, Anthony Harnden, Nigel Matheson, M Symmonds‐Abrahams and Aziz Sheikh, for input and advice on earlier versions of related reviews. Thanks to Ruth Foxlee, Alex Rivetti and Nia Roberts for helping out with the searches. Peter Collignon and Marcus Muellner helped us with aspects of the review. Thanks to Nicola Ring and Ruth Jepson for advice on the inclusion of qualitative data. We thank Toby Lasserson for providing advice and an independent check of our 'Risk of bias' judgements. The European Medicines Agency (EMA) (formerly EMEA) provided all clinical study reports and reviewers' comments in their archive. Hoffman‐La Roche SA and GlaxoSmithKline provided us with full clinical study reports and answered our queries. Thanks also to the Australian National Health and Medical Research Council (NHMRC) and the UK National Health Service (NHS) Research and Development fund for grants to enable the 2009 healthy adults review update. Philip Carter and Deborah Cohen shared some of their Freedom of Information material; Eliana Ferroni helped develop and cross‐check the TOC. Finally, we wish to thank the following people for commenting on the draft protocol: Maryann Napoli, Janet Wale, Paul Glasziou, David Boltz, Elaine Beller and Anca Zalmanovici Trestioreanu and Marcus Muellner. Thanks to the following people for commenting on the draft 2012 review: Chris Cates, Janet Wale, Paul Glasziou, David Boltz and Robert Ware and the following people for commenting on the draft 2014 review: Chris Cates, Elizabeth Dooley, Janet Wale, David Boltz and Robert Ware.
This project was funded by the NIHR Health Technology Assessment programme and will be published in full in the Health Technology Assessment journal series. Visit the HTA programme website for more details: http://www.nets.nihr.ac.uk/projects/hta/108001. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health. The National Institute of Health Research (NIHR) School of Primary Care Research (SPCR) provides financial support for Dr Carl Heneghan and funding for an investigators' meeting in Oxford (UK).
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Apr 10 | Neuraminidase inhibitors for preventing and treating influenza in adults and children | Review | Tom Jefferson, Mark A Jones, Peter Doshi, Chris B Del Mar, Rokuro Hama, Matthew J Thompson, Elizabeth A Spencer, Igho J Onakpoya, Kamal R Mahtani, David Nunan, Jeremy Howick, Carl J Heneghan | |
| 2012 Jan 18 | Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children | Review | Tom Jefferson, Mark A Jones, Peter Doshi, Chris B Del Mar, Carl J Heneghan, Rokuro Hama, Matthew J Thompson | |
| 2011 Dec 07 | Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children ‐ a review of clinical study reports | Protocol | Tom Jefferson, Mark A Jones, Peter Doshi, Chris B Del Mar, Carl J Heneghan, Rokuro Hama, Matthew J Thompson | |
| 2011 Jan 19 | Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children ‐ a review of clinical study reports | Protocol | Tom Jefferson, Mark A Jones, Peter Doshi, Chris B Del Mar, Carl J Heneghan, Rokuro Hama, Matthew J Thompson | |
Differences between protocol and review
We have made a number of changes to the text of A159 during the process of turning the protocol into the review. This reflects our evolving understanding of the issues, during the relatively long period when work on the review was underway.
We have changed the review title to reflect the nature of the evidence. The old title was: Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children ‐ a review of clinical study reports.
We have also re‐written the objective twice, tightening up the text to bring it in line with our initial intentions and clarifying its meaning. The old objectives were: "To review clinical study reports (CSRs) identified from published and unpublished randomised controlled trials (RCTs) and relevant regulatory data on effectiveness and harms of NIs for influenza in all age groups" and "To review published and unpublished clinical study reports and other relevant regulatory data on effectiveness and harms of NIs for influenza in all age groups (and compare them with our published review)."
We changed the emphasis of the objectives on unpublished study reports as we had decided from the start to concentrate on regulatory information. Similarly, comparison of published versus unpublished data is an important and worthwhile effort, but the original objective possibly misled readers as to its importance in our work. We had always conceptualised it as a low‐priority task we could carry out only if we had time following our review of unpublished data. We have also avoided using acronyms, which we thought cumbersome and confusing to the reader.
Our initial intention was to review clinical study reports and regulatory comments making up what we have subsequently called 'regulatory information'. The edits do not reflect a change in intent but our slowly evolving understanding of the problems we faced and our solutions to address these problems. As one of many examples, the transition from a world in which studies were identified by names and years (Nicholson 2000), to one in which the same trial is identified by a series of letters and numbers (WV15670), was not easy.
While the review was underway, we identified several unforeseen issues, such as placebo content and the effect of oseltamivir on antibodies. To test the relevant hypotheses we carried out post‐protocol analyses, which had not been present in the original protocol but were derived from our protocol‐stated intention to assess programmes and not single trials. These are now reported in their entirety in Appendix 10.
In May 2013, we added amendments to the review for: data analyses from oseltamivir trials Module 2s, clinical outcomes and adverse events added in the Feedback section. In the text we explain the rationale and methods applying to regulatory information received after our 2011 time lock, which could not be implemented in time for the current review (see also Appendix 2). For the 16 May 2013 amendments see Feedback.
Notes
Since the January 2012 version of A159, we have now completed the review of regulatory information which became available after our original time lock. We have assessed additional evidence from oseltamivir Module 2s, evidence on adverse events following exposure to NIs and clinically relevant outcomes, and cross‐referenced this with individual listings contained in Modules 3 to 5. We now hold all the relevant full clinical study reports, which we are making publicly accessible with this review.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Antiviral Agents [adverse effects, *therapeutic use];
- Drug Evaluation;
- Enzyme Inhibitors [adverse effects, *therapeutic use];
- Europe;
- Health Status;
- Influenza, Human [*drug therapy, *prevention & control];
- Japan;
- Legislation, Drug;
- Neuraminidase [*antagonists & inhibitors];
- Oseltamivir [adverse effects, *therapeutic use];
- Pneumonia [prevention & control];
- Publication Bias;
- Randomized Controlled Trials as Topic;
- United Kingdom;
- United States;
- Zanamivir [adverse effects, *therapeutic use];
Medical Subject Headings Check Words
Adult; Child; Humans;
PICO

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
'Other bias' includes potentially active placebos.
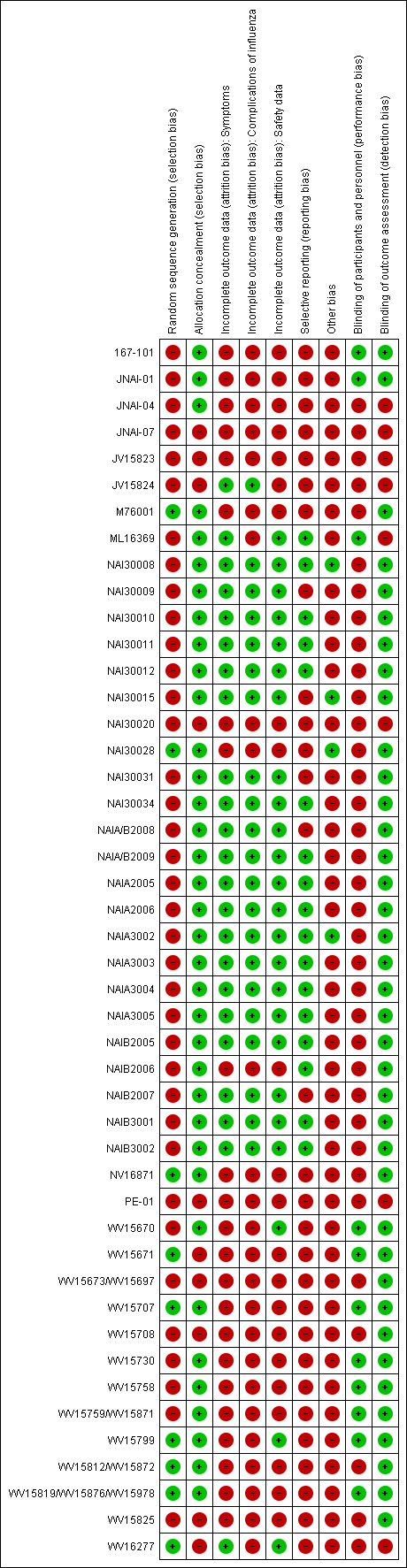
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
'Other bias' includes potentially active placebos.
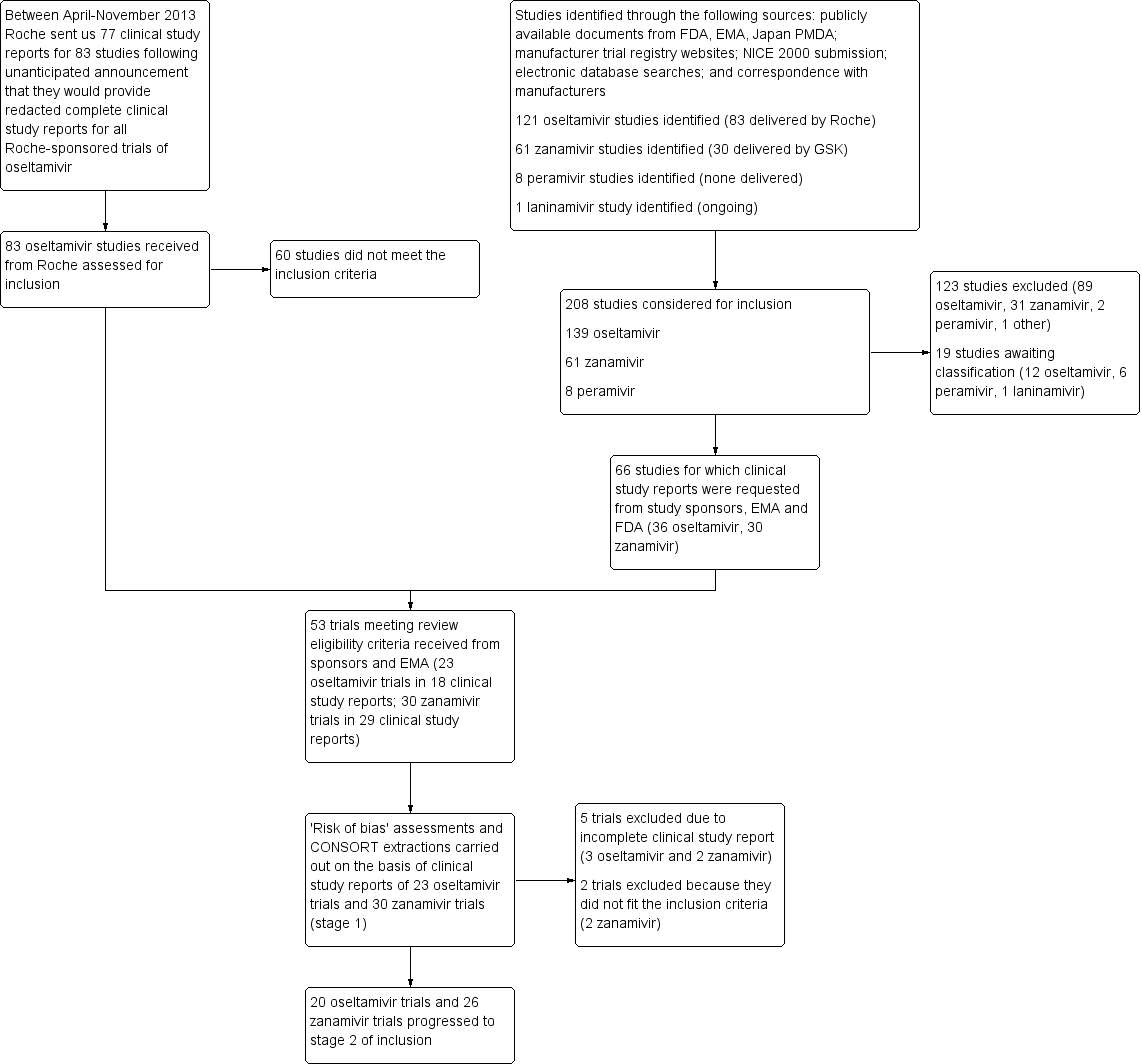
Flow diagram describing the number of studies identified, inclusion, exclusion and progression from identification to stage 1 to stage 2 of the review.
NB Because of the absence of trial programmes for both drugs listing all sponsored trials completed or underway, we had to rely on a variety of sources for the reconstruction of the trial programmes and retrieval of relevant clinical study reports. This complexity is reflected in the flowchart, illustrating the study selection process for this review. The two main pathways were the spontaneous release of 77 clinical full clinical study reports by Roche and the requests to regulatory authorities and GSK for all the relevant reports. There was overlap in trial reports retrieved following the different pathways
![Forest plot of comparison: 1 Oseltamivir versus placebo for treatment, outcome: 1.1 Time to first alleviation of symptoms in adult treatment (ITT population) [hours].](/es/cdsr/doi/10.1002/14651858.CD008965.pub4/media/CDSR/CD008965/image_n/nCD008965-AFig-FIG04.png)
Forest plot of comparison: 1 Oseltamivir versus placebo for treatment, outcome: 1.1 Time to first alleviation of symptoms in adult treatment (ITT population) [hours].

Forest plot of comparison: 3 Zanamivir versus placebo for treatment, outcome: 3.1 Time to first alleviation of symptoms in adult treatment (days).
![Forest plot of comparison: 3 Zanamivir versus placebo for treatment, outcome: 3.68 Time to first alleviation of symptoms in adults with/without relief medication [days].](/es/cdsr/doi/10.1002/14651858.CD008965.pub4/media/CDSR/CD008965/image_n/nCD008965-AFig-FIG06.png)
Forest plot of comparison: 3 Zanamivir versus placebo for treatment, outcome: 3.68 Time to first alleviation of symptoms in adults with/without relief medication [days].

Forest plot of comparison: 1 Oseltamivir versus placebo for treatment, outcome: 1.17 Complications: pneumonia in adult treatment.

Example Diary card from case‐report form for Zanamivir trial

Example Diary card from case‐report form for Zanamivir trial (cont)

Forest plot of comparison: 2 Oseltamivir versus placebo for prophylaxis, outcome: 2.19 Adverse events: headache in adult prophylaxis (on‐treatment).
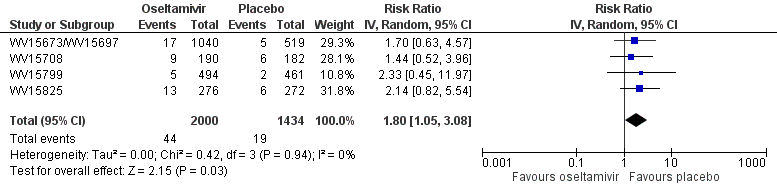
Forest plot of comparison: 2 Oseltamivir versus placebo for prophylaxis, outcome: 2.54 Adverse events: psychiatric body system in adult prophylaxis (on‐ and off‐treatment).

Sample "Adverse event or intercurrent illness" form (oseltamivir study M76001)

Sample "Secondary illness" form (oseltamivir study WV15670)

Sample "Diagnosis of secondary illness" form, page 1/2 (oseltamivir study WV15978)
Sample "Diagnosis of secondary illness" form, page 2/2 (oseltamivir study WV15978)

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 1 Time to first alleviation of symptoms in adult treatment (ITT population).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 2 Hospital admission in adult treatment (safety population).
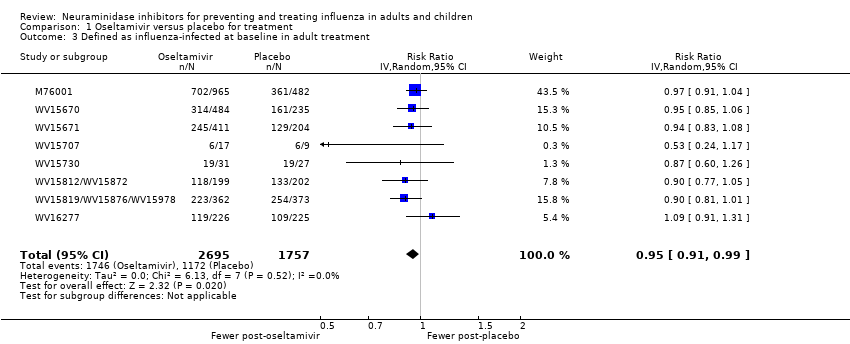
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 3 Defined as influenza‐infected at baseline in adult treatment.
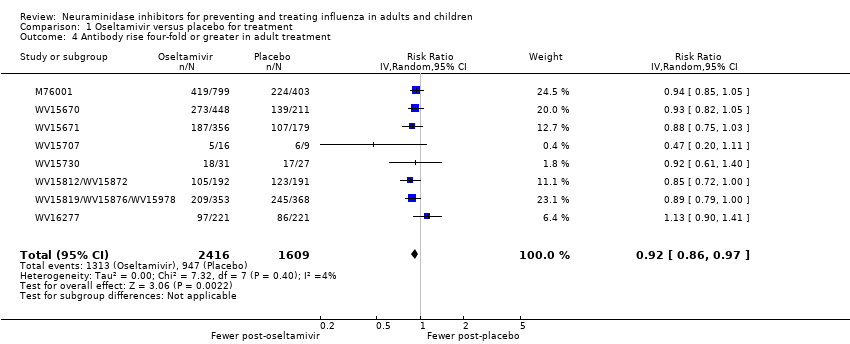
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 4 Antibody rise four‐fold or greater in adult treatment.
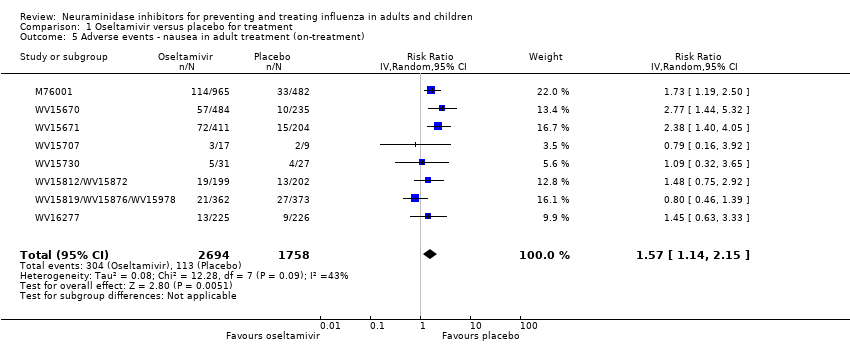
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 5 Adverse events ‐ nausea in adult treatment (on‐treatment).
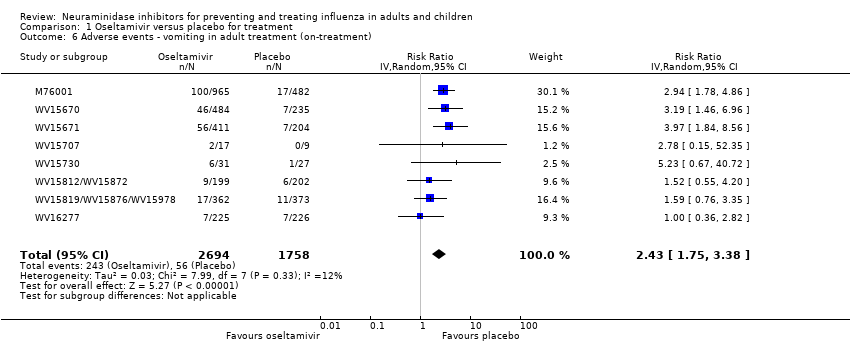
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 6 Adverse events ‐ vomiting in adult treatment (on‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 7 Adverse events ‐ diarrhoea in adult treatment (on‐treatment).
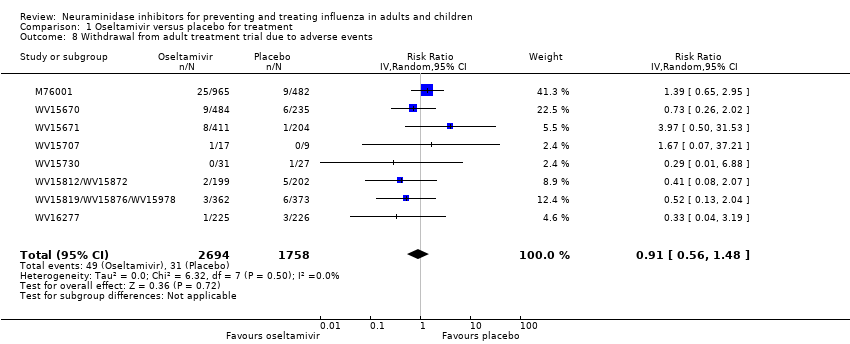
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 8 Withdrawal from adult treatment trial due to adverse events.
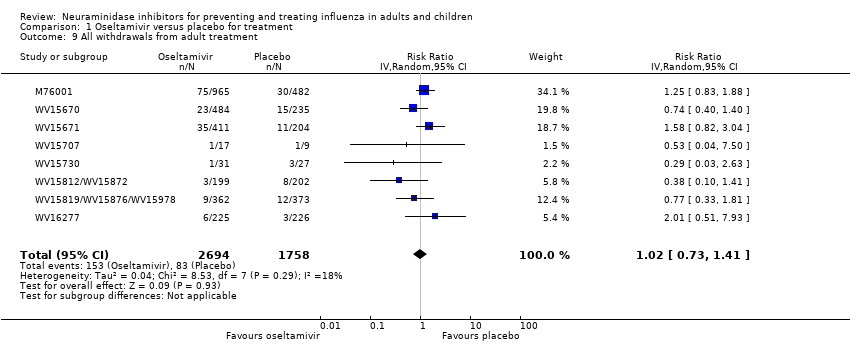
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 9 All withdrawals from adult treatment.

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 10 Adverse events ‐ cough in adult treatment (on‐treatment).
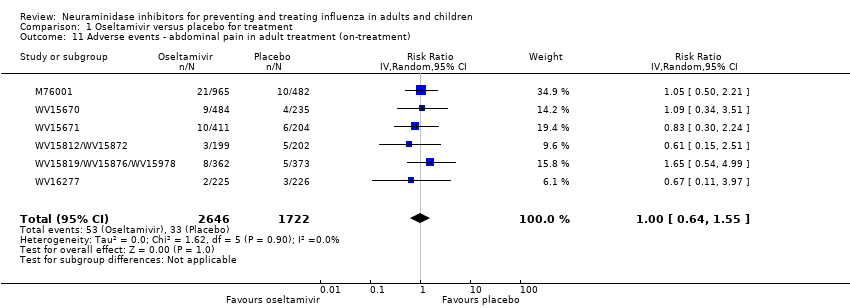
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 11 Adverse events ‐ abdominal pain in adult treatment (on‐treatment).
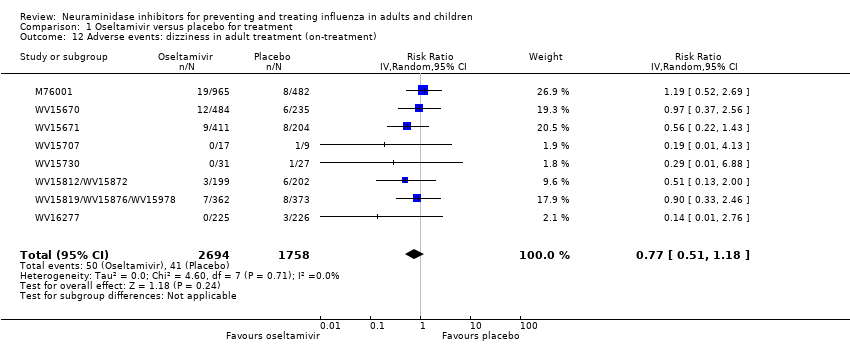
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 12 Adverse events: dizziness in adult treatment (on‐treatment).
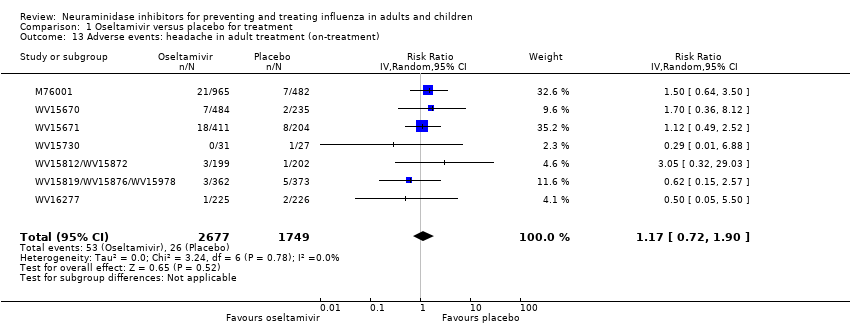
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 13 Adverse events: headache in adult treatment (on‐treatment).
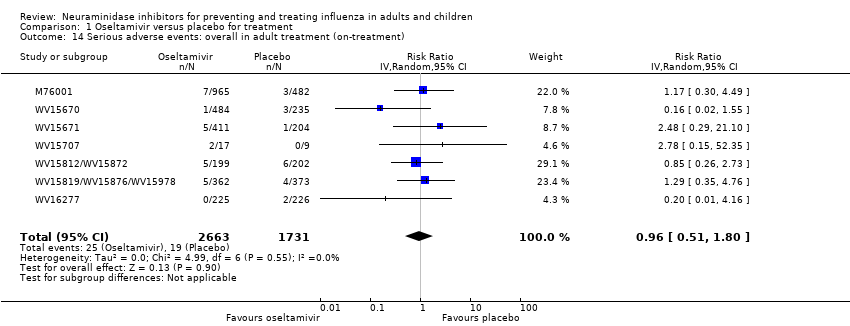
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 14 Serious adverse events: overall in adult treatment (on‐treatment).
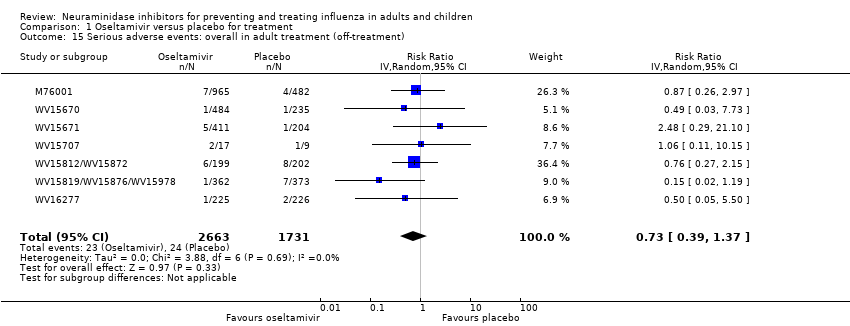
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 15 Serious adverse events: overall in adult treatment (off‐treatment).
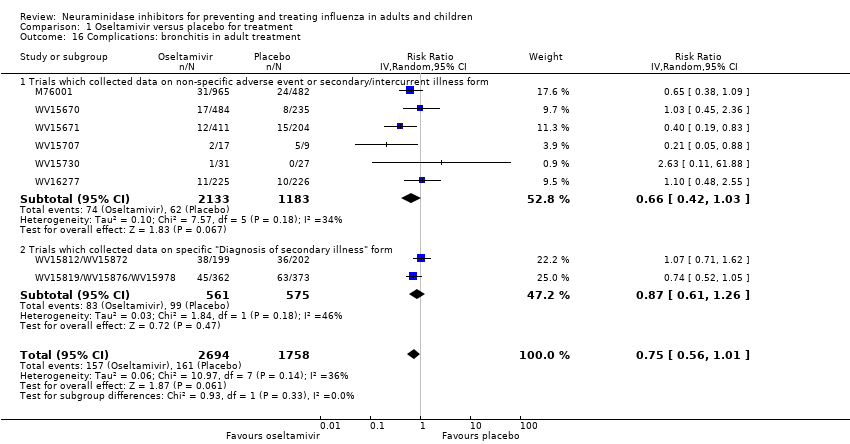
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 16 Complications: bronchitis in adult treatment.

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 17 Complications: pneumonia in adult treatment.

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 18 Complications: sinusitis in adult treatment.

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 19 Complications: otitis media in adult treatment.
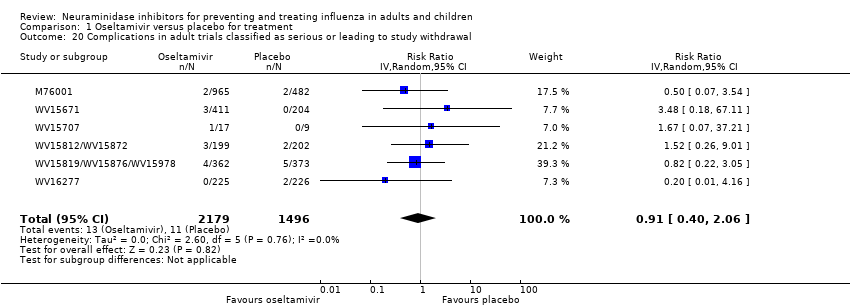
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 20 Complications in adult trials classified as serious or leading to study withdrawal.

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 21 Culture‐positive at baseline in adult treatment.
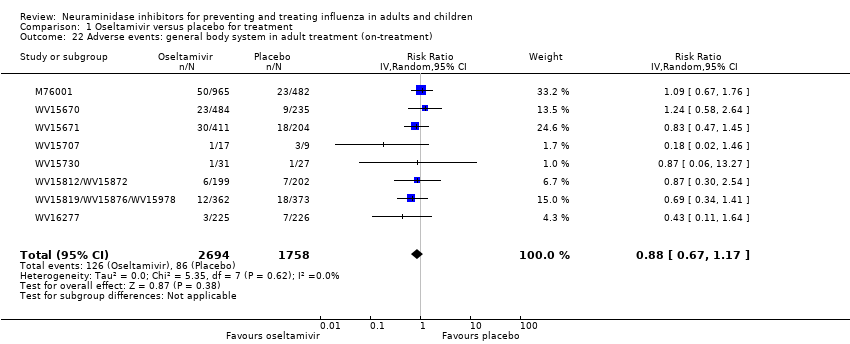
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 22 Adverse events: general body system in adult treatment (on‐treatment).
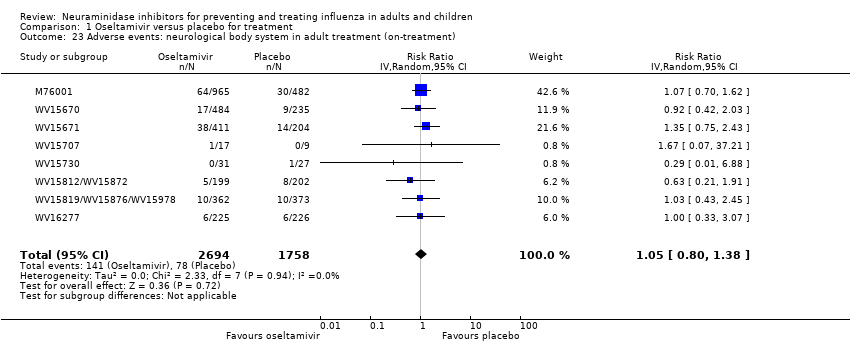
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 23 Adverse events: neurological body system in adult treatment (on‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 24 Adverse events: respiratory body system in adult treatment (on‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 25 Adverse events: infection body system in adult treatment (on‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 26 Adverse events: gastrointestinal body system in adult treatment (on‐treatment).
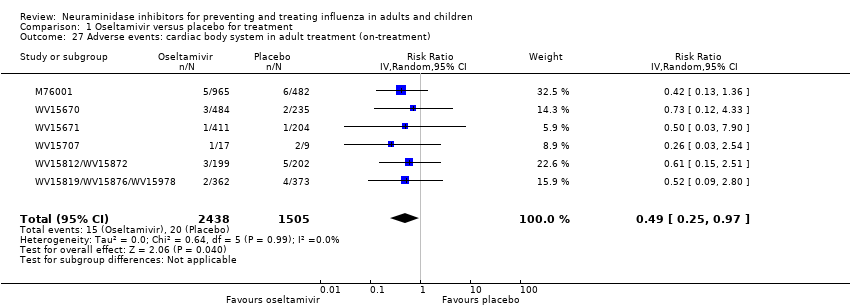
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 27 Adverse events: cardiac body system in adult treatment (on‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 28 Adverse events: ear body system in adult treatment (on‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 29 Adverse events: eye body system in adult treatment (on‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 30 Adverse events: metabolism body system in adult treatment (on‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 31 Adverse events: musculoskeletal body system in adult treatment (on‐treatment).
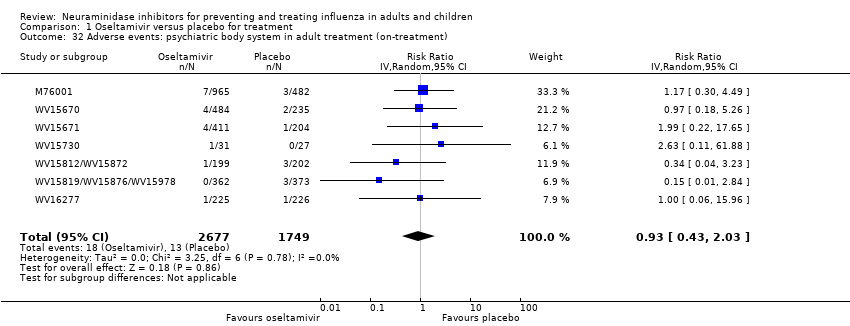
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 32 Adverse events: psychiatric body system in adult treatment (on‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 33 Adverse events: skin body system in adult treatment (on‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 34 Adverse events: cardiac body system in adult treatment (off‐treatment).
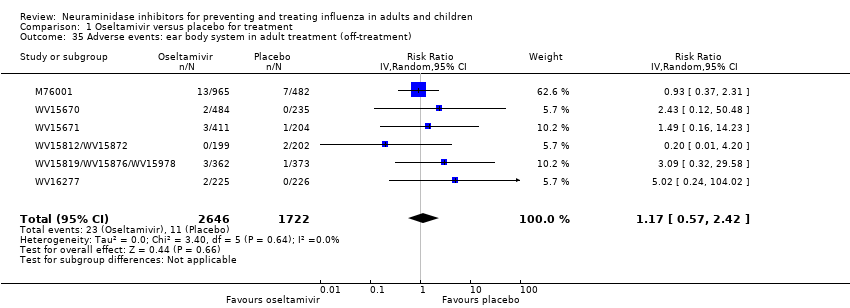
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 35 Adverse events: ear body system in adult treatment (off‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 36 Adverse events: gastrointestinal body system in adult treatment (off‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 37 Adverse events: general body system in adult treatment (off‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 38 Adverse events: infection body system in adult treatment (off‐treatment).
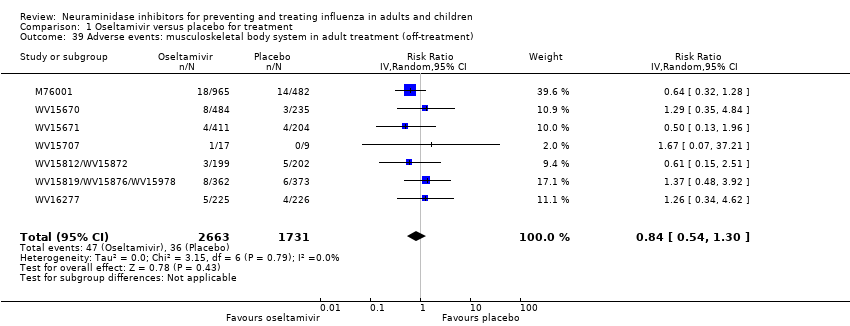
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 39 Adverse events: musculoskeletal body system in adult treatment (off‐treatment).
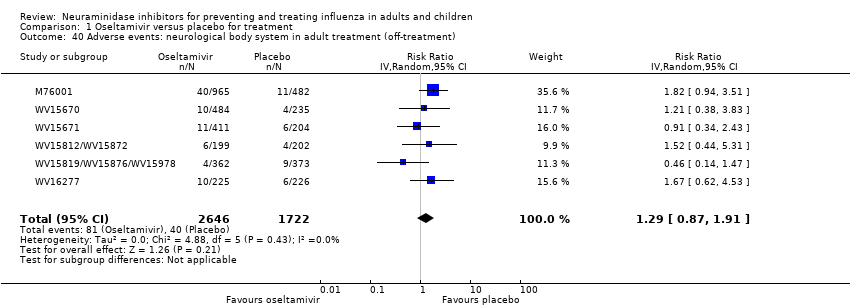
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 40 Adverse events: neurological body system in adult treatment (off‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 41 Adverse events: respiratory body system in adult treatment (off‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 42 Adverse events: skin body system in adult treatment (off‐treatment).
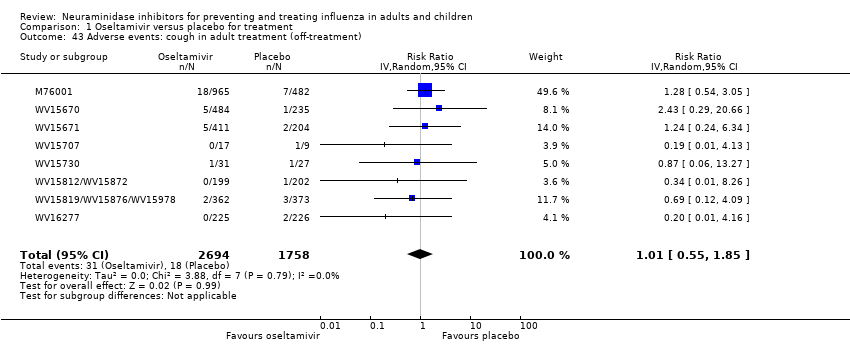
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 43 Adverse events: cough in adult treatment (off‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 44 Adverse events: headache in adult treatment (off‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 45 Adverse events: nausea in adult treatment (off‐treatment).
![Comparison 1 Oseltamivir versus placebo for treatment, Outcome 46 Time to first alleviation of symptoms in child treatment [hours].](/es/cdsr/doi/10.1002/14651858.CD008965.pub4/media/CDSR/CD008965/image_n/nCD008965-CMP-001-46.png)
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 46 Time to first alleviation of symptoms in child treatment [hours].

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 47 Hospital admission in child treatment (safety population).
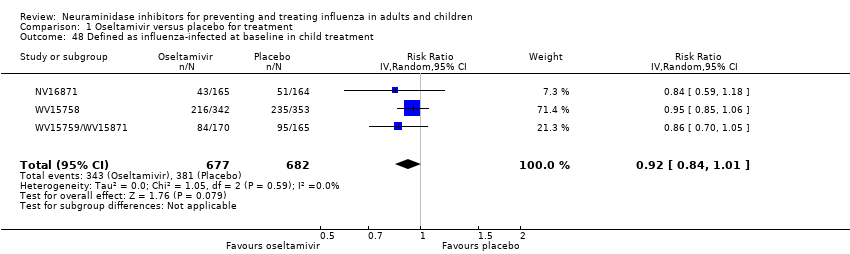
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 48 Defined as influenza‐infected at baseline in child treatment.
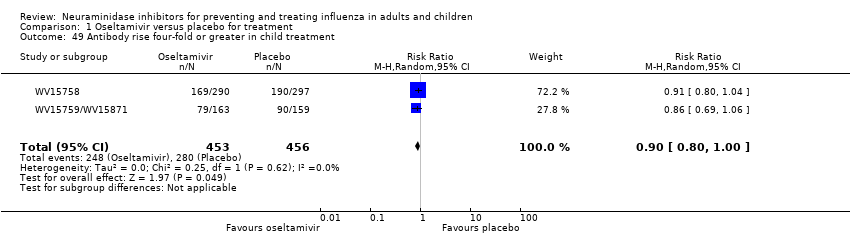
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 49 Antibody rise four‐fold or greater in child treatment.

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 50 Complications: bronchitis in child treatment.

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 51 Complications: otitis media in child treatment.

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 52 Complications: pneumonia in child treatment.

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 53 Complications: sinusitis in child treatment.

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 54 Complications: pneumonia in child treatment by on‐ and off‐treatment.
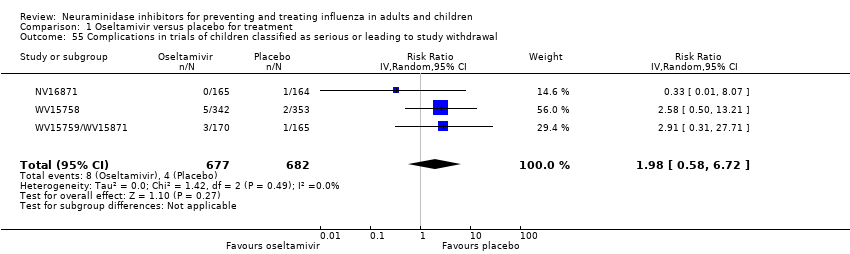
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 55 Complications in trials of children classified as serious or leading to study withdrawal.

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 56 Withdrawal from child treatment trial due to adverse events.

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 57 All withdrawals from child treatment.

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 58 Serious adverse events: overall in child treatment (on‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 59 Serious adverse events: overall in child treatment (off‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 60 Adverse events: abdominal pain in child treatment (on‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 61 Adverse events: diarrhoea in child treatment (on‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 62 Adverse events: nausea in child treatment (on‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 63 Adverse events: vomiting in child treatment (on‐treatment).
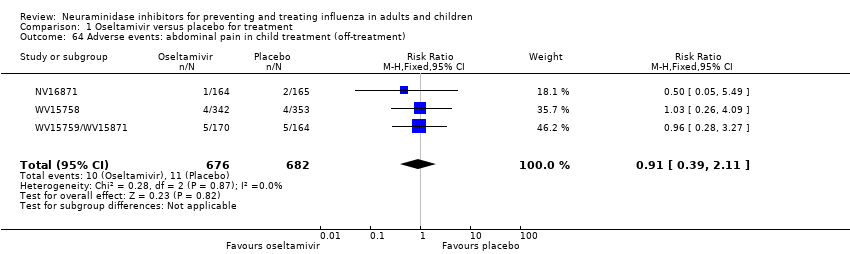
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 64 Adverse events: abdominal pain in child treatment (off‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 65 Adverse events: cough in child treatment (off‐treatment).
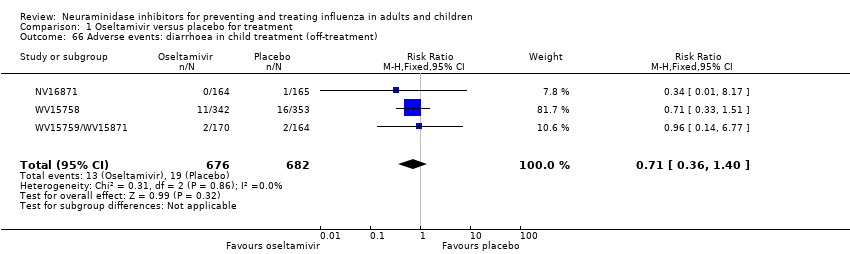
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 66 Adverse events: diarrhoea in child treatment (off‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 67 Adverse events: headache in child treatment (off‐treatment).
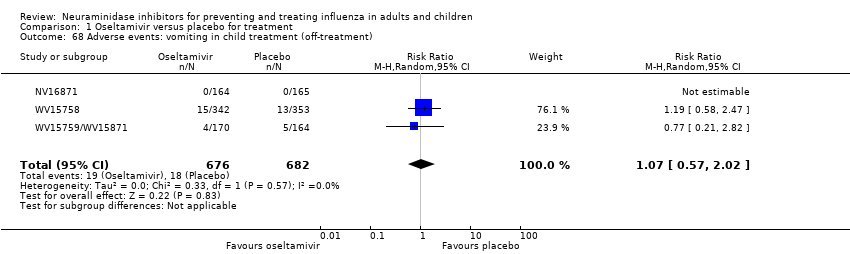
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 68 Adverse events: vomiting in child treatment (off‐treatment).
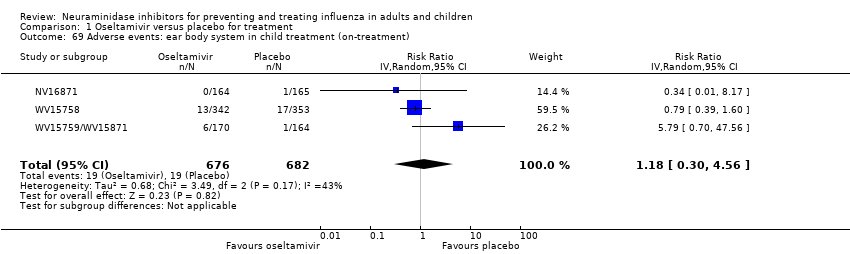
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 69 Adverse events: ear body system in child treatment (on‐treatment).
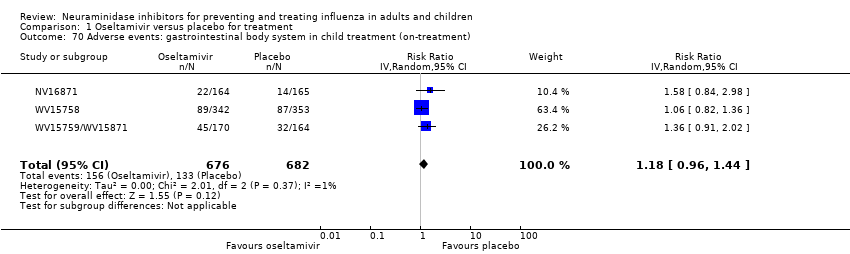
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 70 Adverse events: gastrointestinal body system in child treatment (on‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 71 Adverse events: general body system in child treatment (on‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 72 Adverse events: infection body system in child treatment (on‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 73 Adverse events: neurological body system in child treatment (on‐treatment).
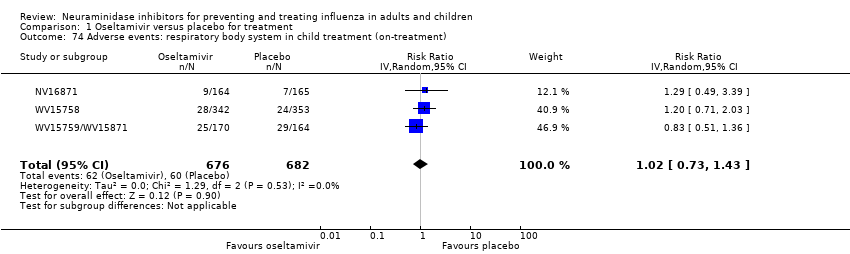
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 74 Adverse events: respiratory body system in child treatment (on‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 75 Adverse events: skin body system in child treatment (on‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 76 Adverse events: ear body system in child treatment (off‐treatment).
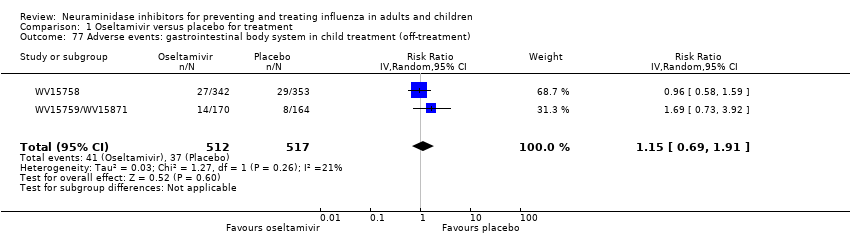
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 77 Adverse events: gastrointestinal body system in child treatment (off‐treatment).
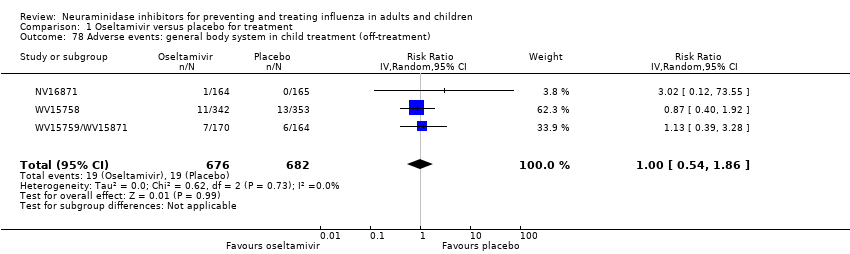
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 78 Adverse events: general body system in child treatment (off‐treatment).
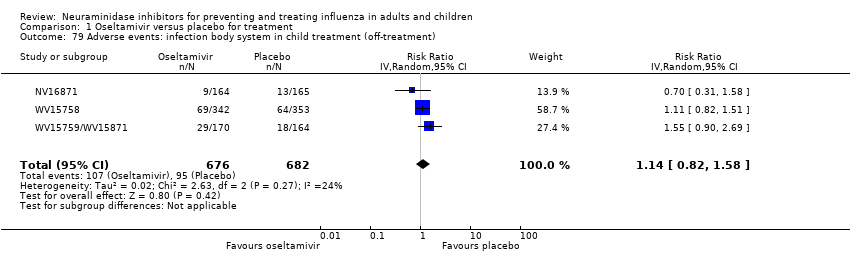
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 79 Adverse events: infection body system in child treatment (off‐treatment).
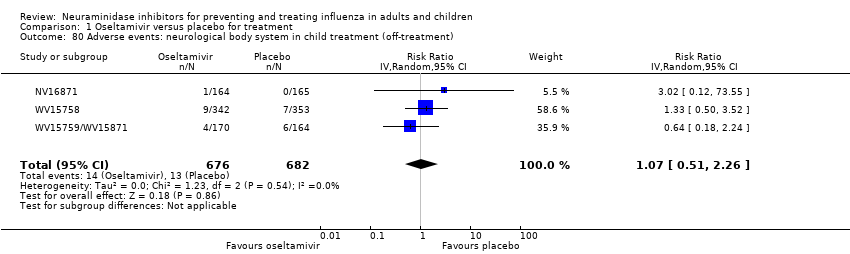
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 80 Adverse events: neurological body system in child treatment (off‐treatment).

Comparison 1 Oseltamivir versus placebo for treatment, Outcome 81 Adverse events: respiratory body system in child treatment (off‐treatment).
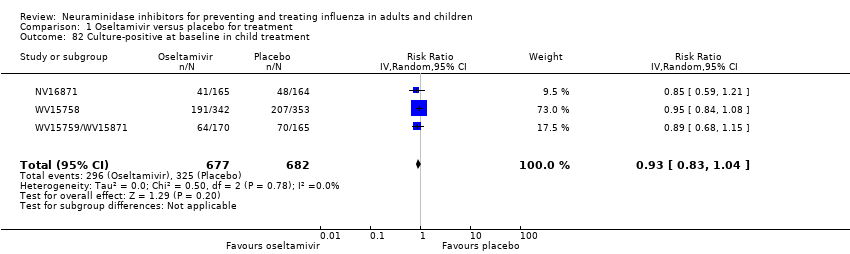
Comparison 1 Oseltamivir versus placebo for treatment, Outcome 82 Culture‐positive at baseline in child treatment.

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 1 Symptomatic influenza in adult prophylaxis of individuals.
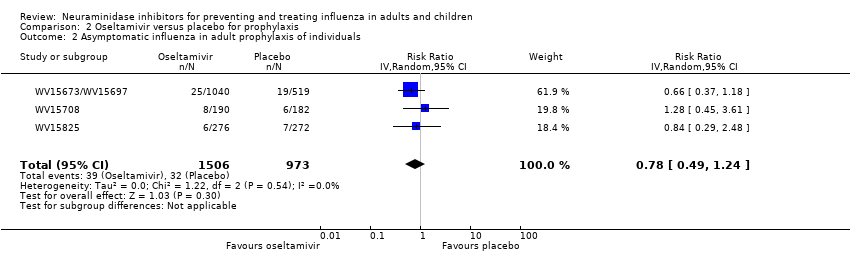
Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 2 Asymptomatic influenza in adult prophylaxis of individuals.

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 3 Symptomatic influenza in household prophylaxis.

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 4 Asymptomatic influenza in household prophylaxis.

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 5 Influenza‐like illness reported as adverse event (on‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 6 Influenza‐like illness reported as adverse event (off‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 7 Hospitalisation in adult prophylaxis (safety population).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 8 Complications: bronchitis in adult prophylaxis.

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 9 Complications: sinusitis in adult prophylaxis.

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 10 Adverse events leading to study withdrawal in adult prophylaxis.
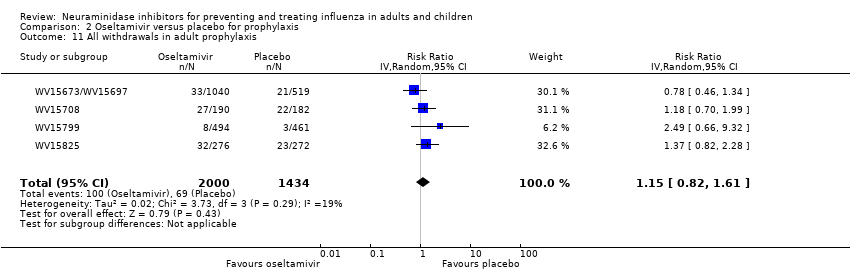
Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 11 All withdrawals in adult prophylaxis.

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 12 Serious adverse events in adult prophylaxis (on‐treatment).
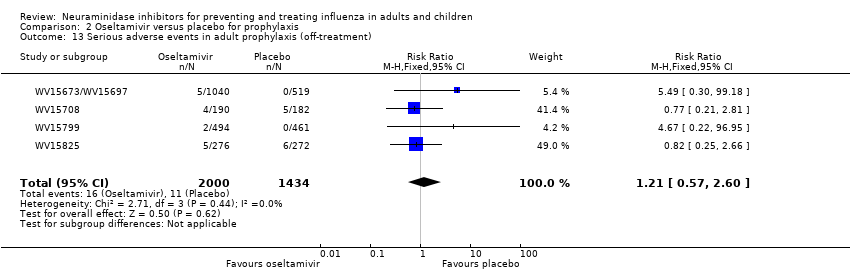
Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 13 Serious adverse events in adult prophylaxis (off‐treatment).
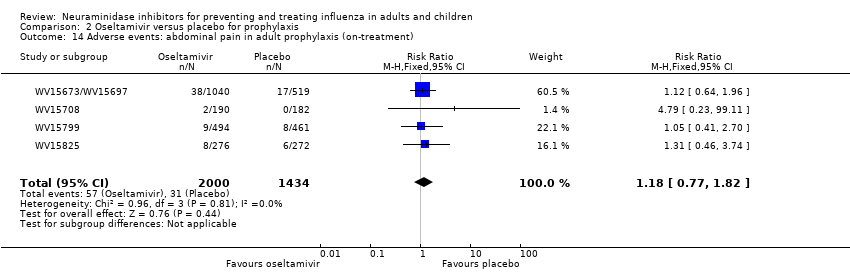
Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 14 Adverse events: abdominal pain in adult prophylaxis (on‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 15 Adverse events: cough in adult prophylaxis (on‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 16 Adverse events: diarrhoea in adult prophylaxis (on‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 17 Adverse events: dizziness in adult prophylaxis (on‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 18 Adverse events: fatigue in adult prophylaxis (on‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 19 Adverse events: headache in adult prophylaxis (on‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 20 Adverse events: nausea in adult prophylaxis (on‐treatment).
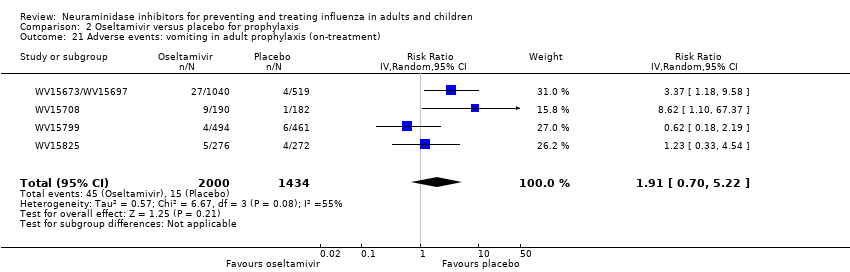
Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 21 Adverse events: vomiting in adult prophylaxis (on‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 22 Adverse events: cough in adult prophylaxis (off‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 23 Adverse events: fatigue in adult prophylaxis (off‐treatment).
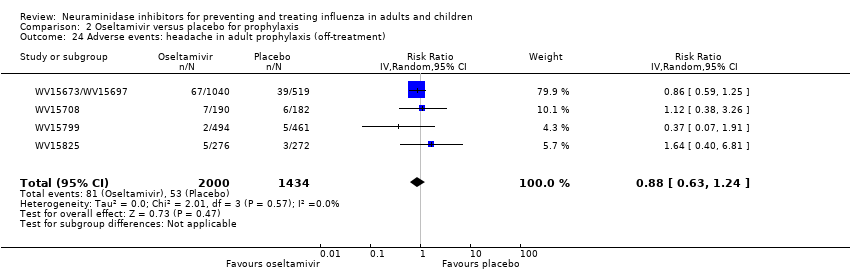
Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 24 Adverse events: headache in adult prophylaxis (off‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 25 Adverse events: blood body system in adult prophylaxis (on‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 26 Adverse events: cardiac body system in adult prophylaxis (on‐treatment).
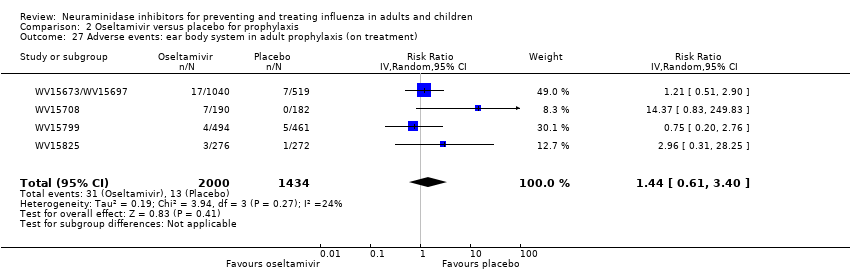
Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 27 Adverse events: ear body system in adult prophylaxis (on treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 28 Adverse events: eye body system in adult prophylaxis (on‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 29 Adverse events: gastrointestinal body system in adult prophylaxis (on‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 30 Adverse events: general body system in adult prophylaxis (on‐treatment).
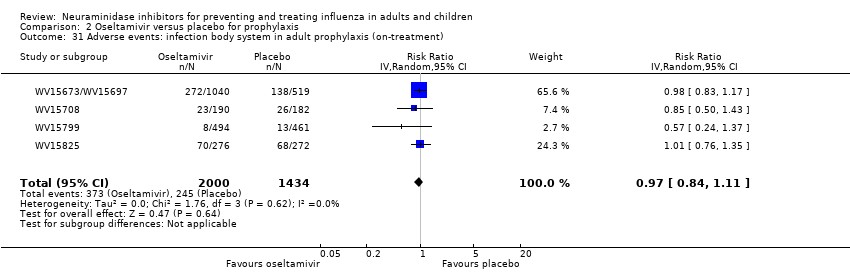
Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 31 Adverse events: infection body system in adult prophylaxis (on‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 32 Adverse events: immune body system in adult prophylaxis (on‐treatment).
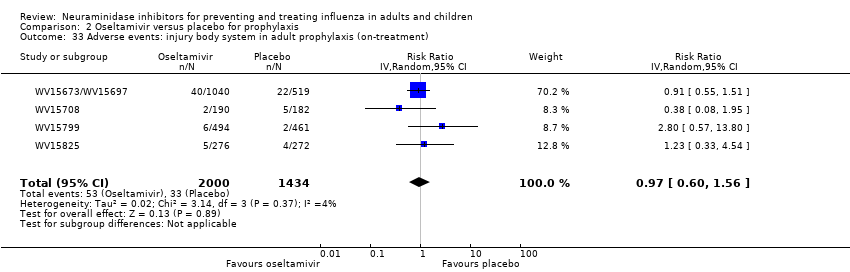
Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 33 Adverse events: injury body system in adult prophylaxis (on‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 34 Adverse events: metabolism body system in adult prophylaxis (on‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 35 Adverse events: musculoskeletal body system in adult prophylaxis (on‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 36 Adverse events: neurological body system in adult prophylaxis (on‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 37 Adverse events: psychiatric body system in adult prophylaxis (on‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 38 Adverse events: renal body system in adult prophylaxis (on‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 39 Adverse events: reproductive body system in adult prophylaxis (on treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 40 Adverse events: respiratory body system in adult prophylaxis (on‐treatment).
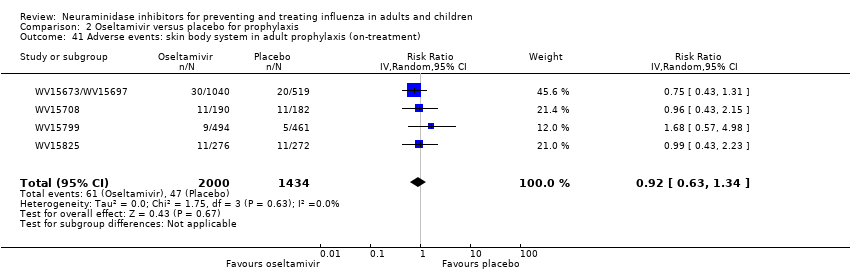
Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 41 Adverse events: skin body system in adult prophylaxis (on‐treatment).
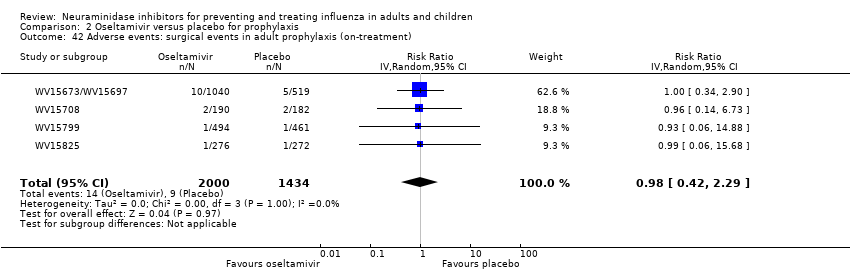
Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 42 Adverse events: surgical events in adult prophylaxis (on‐treatment).
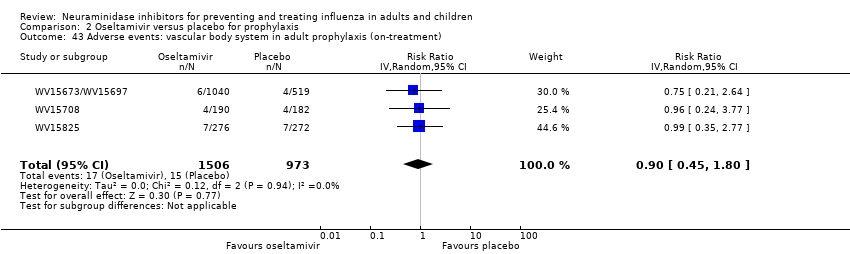
Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 43 Adverse events: vascular body system in adult prophylaxis (on‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 44 Adverse events: cardiac body system in adult prophylaxis (off‐treatment).
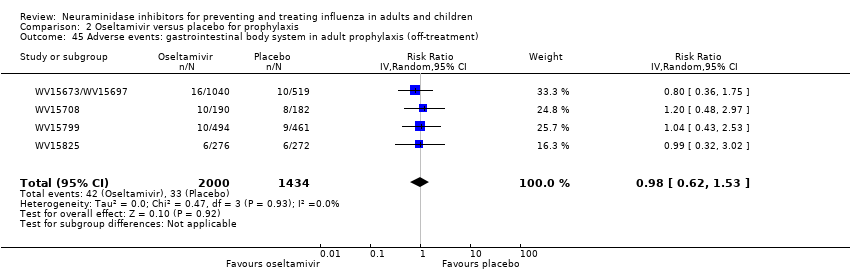
Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 45 Adverse events: gastrointestinal body system in adult prophylaxis (off‐treatment).
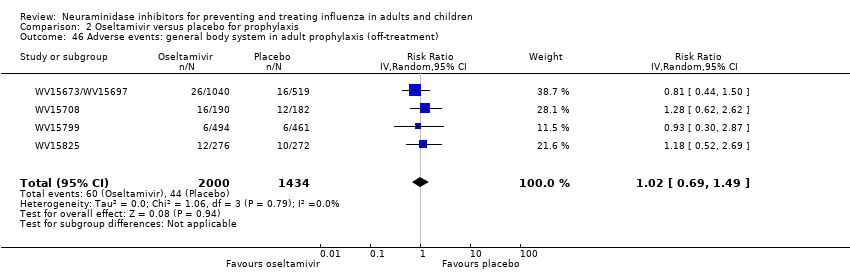
Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 46 Adverse events: general body system in adult prophylaxis (off‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 47 Adverse events: infection body system in adult prophylaxis (off‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 48 Adverse events: injury body system in adult prophylaxis (off‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 49 Adverse events: musculoskeletal body system in adult prophylaxis (off‐treatment).
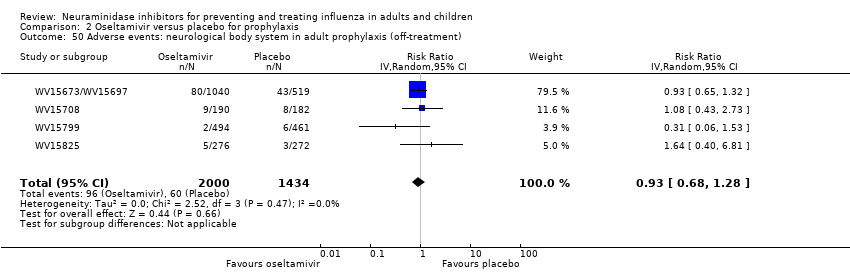
Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 50 Adverse events: neurological body system in adult prophylaxis (off‐treatment).
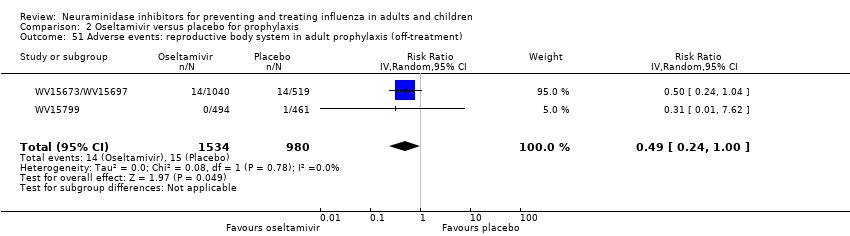
Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 51 Adverse events: reproductive body system in adult prophylaxis (off‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 52 Adverse events: respiratory body system in adult prophylaxis (off‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 53 Adverse events: skin body system in adult prophylaxis (off‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 54 Adverse events: psychiatric body system in adult prophylaxis (on and off‐treatment).

Comparison 2 Oseltamivir versus placebo for prophylaxis, Outcome 55 Adverse events: renal body system in adult prophylaxis (on and off‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 1 Time to first alleviation of symptoms in adult treatment (days).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 2 Complications: pneumonia in adult treatment.

Comparison 3 Zanamivir versus placebo for treatment, Outcome 3 Complications: pneumonia confirmed with X‐ray in adult treatment.
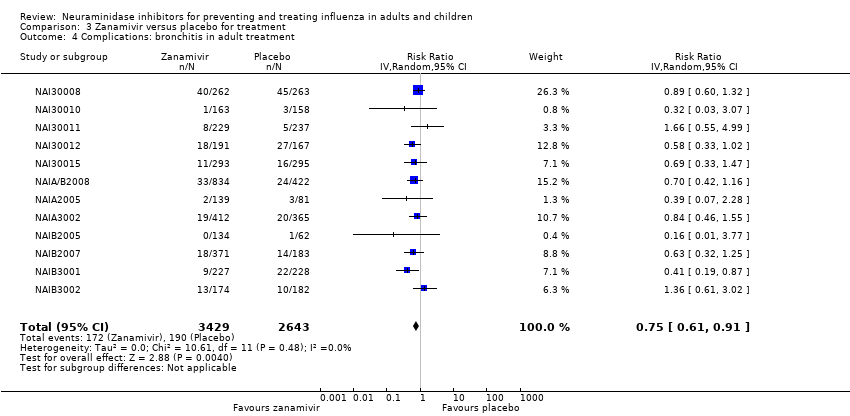
Comparison 3 Zanamivir versus placebo for treatment, Outcome 4 Complications: bronchitis in adult treatment.

Comparison 3 Zanamivir versus placebo for treatment, Outcome 5 Complications: sinusitis in adult treatment.

Comparison 3 Zanamivir versus placebo for treatment, Outcome 6 Complications: otitis media in adult treatment.
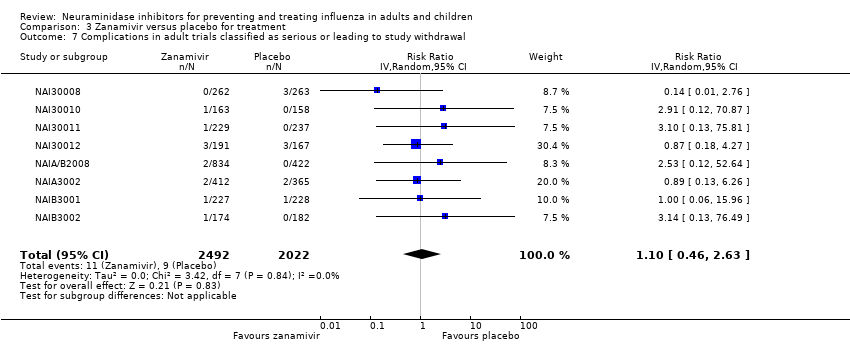
Comparison 3 Zanamivir versus placebo for treatment, Outcome 7 Complications in adult trials classified as serious or leading to study withdrawal.

Comparison 3 Zanamivir versus placebo for treatment, Outcome 8 Proportion diagnosed as influenza‐infected in adult treatment.

Comparison 3 Zanamivir versus placebo for treatment, Outcome 9 Proportion with four‐fold rise in antibody titre in adult treatment.
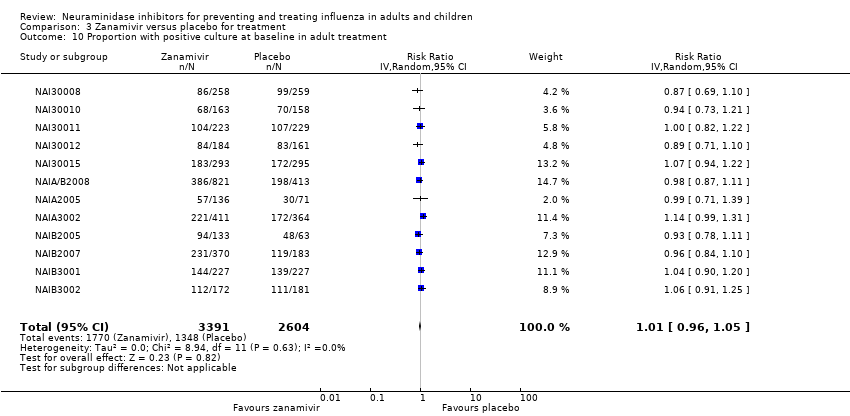
Comparison 3 Zanamivir versus placebo for treatment, Outcome 10 Proportion with positive culture at baseline in adult treatment.

Comparison 3 Zanamivir versus placebo for treatment, Outcome 11 Serious adverse events in adult treatment.

Comparison 3 Zanamivir versus placebo for treatment, Outcome 12 Adverse events leading to study withdrawal in adult treatment.

Comparison 3 Zanamivir versus placebo for treatment, Outcome 13 All withdrawals in adult treatment.

Comparison 3 Zanamivir versus placebo for treatment, Outcome 14 Time to first alleviation of symptoms in children (days).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 15 Complications: pneumonia in child treatment.
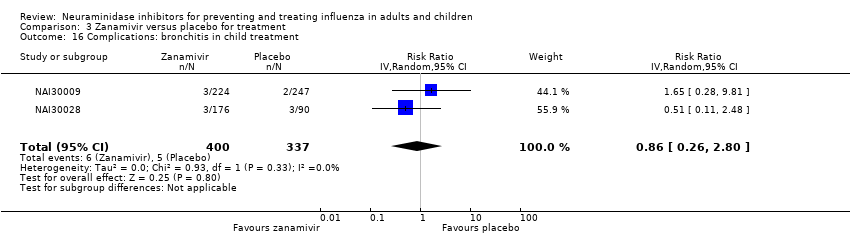
Comparison 3 Zanamivir versus placebo for treatment, Outcome 16 Complications: bronchitis in child treatment.
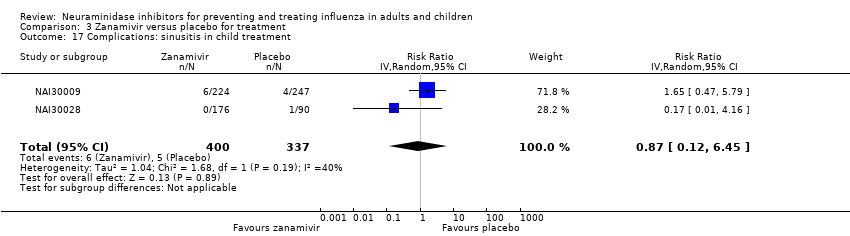
Comparison 3 Zanamivir versus placebo for treatment, Outcome 17 Complications: sinusitis in child treatment.

Comparison 3 Zanamivir versus placebo for treatment, Outcome 18 Complications: otitis media in child treatment.
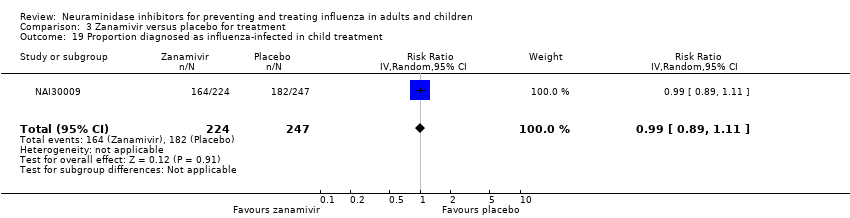
Comparison 3 Zanamivir versus placebo for treatment, Outcome 19 Proportion diagnosed as influenza‐infected in child treatment.
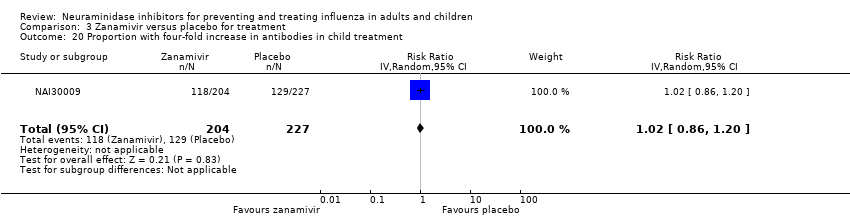
Comparison 3 Zanamivir versus placebo for treatment, Outcome 20 Proportion with four‐fold increase in antibodies in child treatment.

Comparison 3 Zanamivir versus placebo for treatment, Outcome 21 Proportion with positive culture at baseline in child treatment.
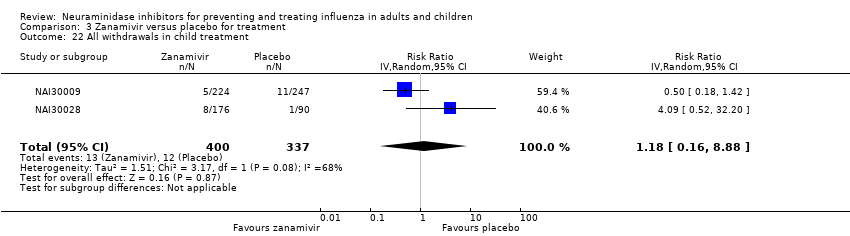
Comparison 3 Zanamivir versus placebo for treatment, Outcome 22 All withdrawals in child treatment.
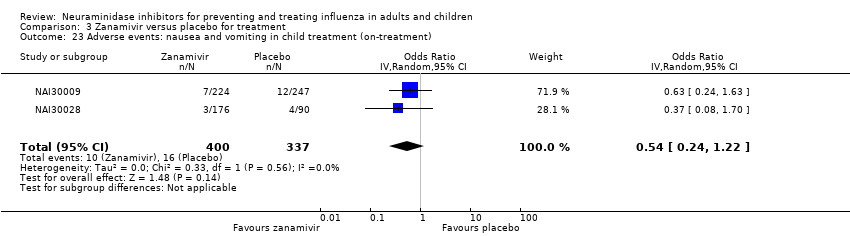
Comparison 3 Zanamivir versus placebo for treatment, Outcome 23 Adverse events: nausea and vomiting in child treatment (on‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 24 Adverse events: diarrhoea in child treatment (on‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 25 Adverse events: gastrointestinal body system in child treatment (on‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 26 Adverse events: respiratory body system in child treatment (on‐treatment).
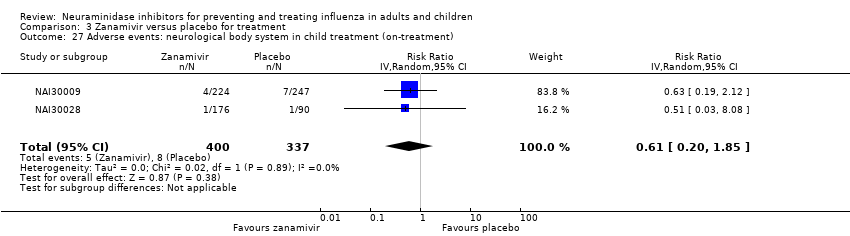
Comparison 3 Zanamivir versus placebo for treatment, Outcome 27 Adverse events: neurological body system in child treatment (on‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 28 Adverse events: ear, nose and throat body system in child treatment (on‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 29 Adverse events: skin body system in child treatment (on‐treatment).
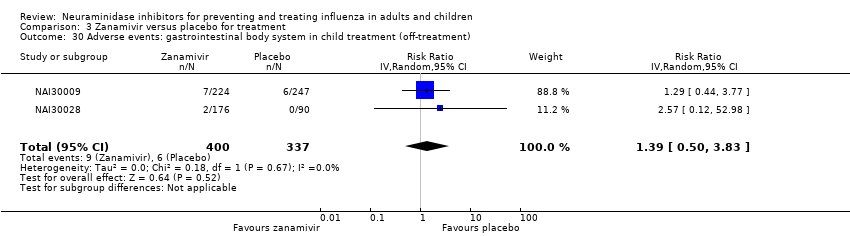
Comparison 3 Zanamivir versus placebo for treatment, Outcome 30 Adverse events: gastrointestinal body system in child treatment (off‐treatment).
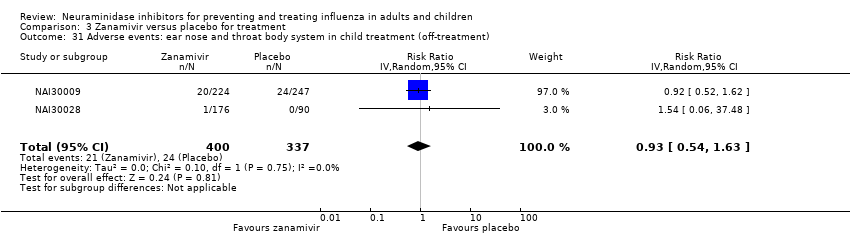
Comparison 3 Zanamivir versus placebo for treatment, Outcome 31 Adverse events: ear nose and throat body system in child treatment (off‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 32 Adverse events: nausea/vomiting in adult treatment (on‐treatment).
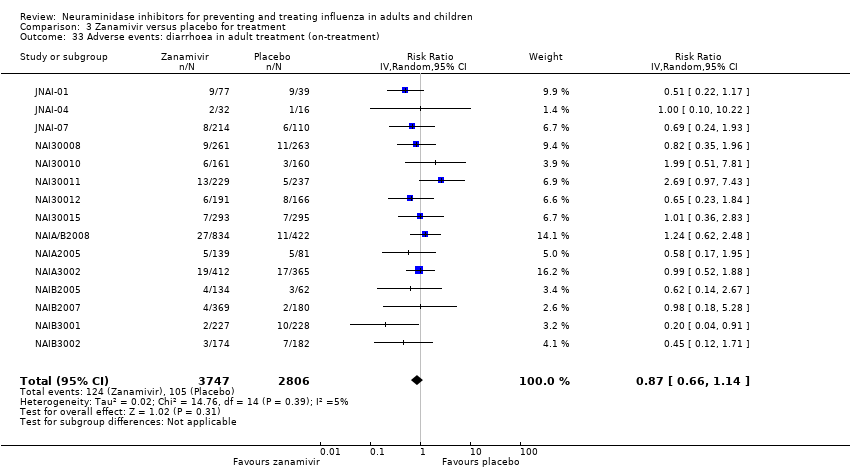
Comparison 3 Zanamivir versus placebo for treatment, Outcome 33 Adverse events: diarrhoea in adult treatment (on‐treatment).
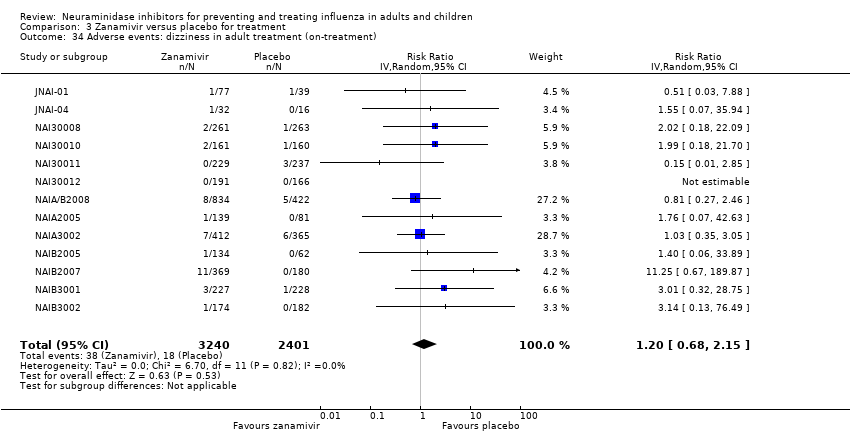
Comparison 3 Zanamivir versus placebo for treatment, Outcome 34 Adverse events: dizziness in adult treatment (on‐treatment).
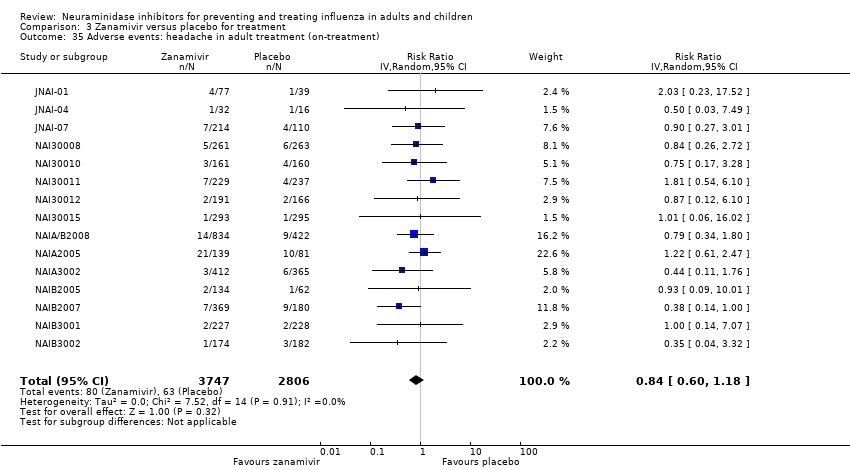
Comparison 3 Zanamivir versus placebo for treatment, Outcome 35 Adverse events: headache in adult treatment (on‐treatment).
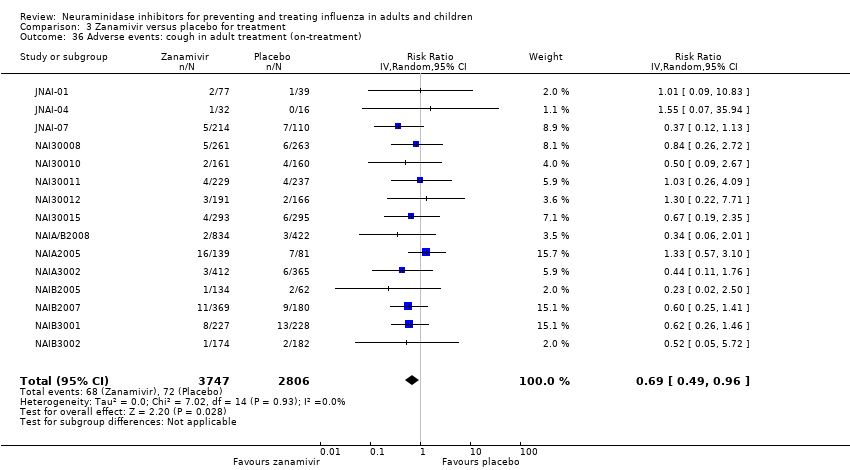
Comparison 3 Zanamivir versus placebo for treatment, Outcome 36 Adverse events: cough in adult treatment (on‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 37 Adverse events: gastrointestinal body system in adult treatment (on‐treatment).
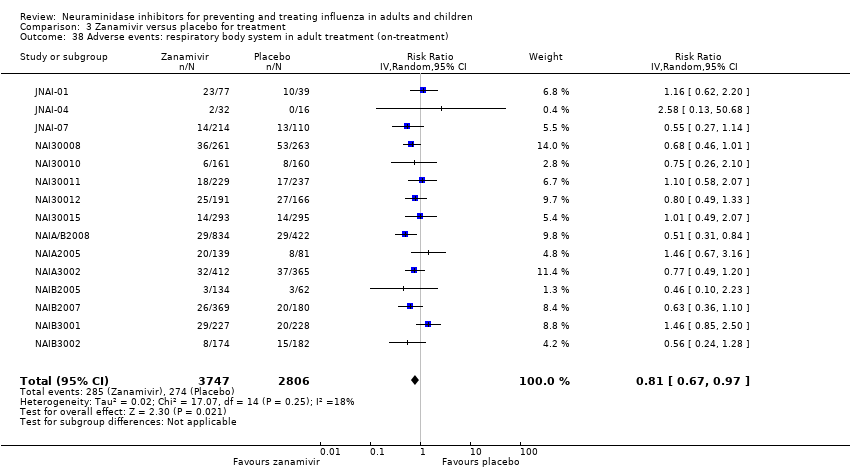
Comparison 3 Zanamivir versus placebo for treatment, Outcome 38 Adverse events: respiratory body system in adult treatment (on‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 39 Adverse events: neurological body system in adult treatment (on‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 40 Adverse events: ear, nose and throat body system in adult treatment (on‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 41 Adverse events: skin body system in adult treatment (on‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 42 Adverse events: musculoskeletal body system in adult treatment (on‐treatment).
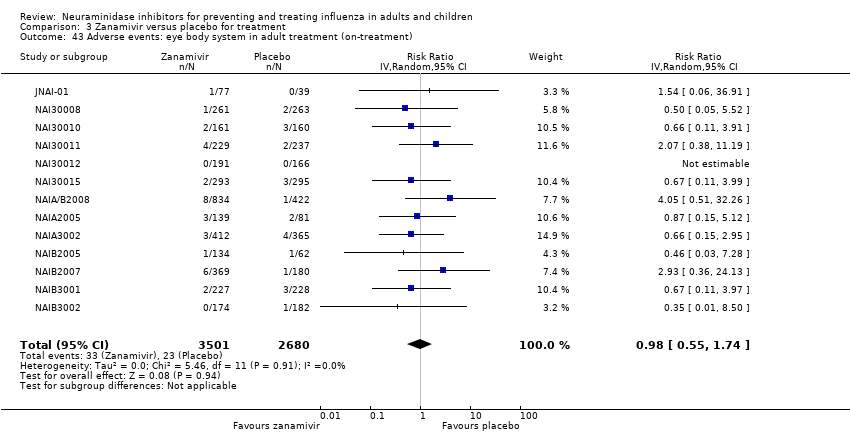
Comparison 3 Zanamivir versus placebo for treatment, Outcome 43 Adverse events: eye body system in adult treatment (on‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 44 Adverse events: hepato body system in adult treatment (on‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 45 Adverse events: renal body system in adult treatment (on‐treatment).
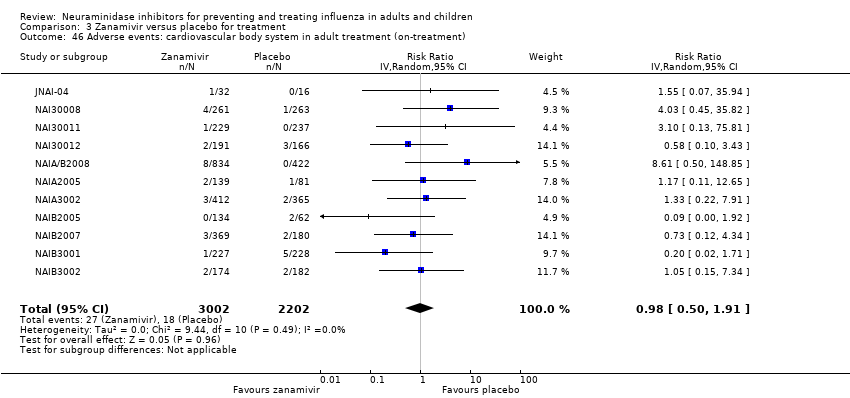
Comparison 3 Zanamivir versus placebo for treatment, Outcome 46 Adverse events: cardiovascular body system in adult treatment (on‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 47 Adverse events: blood body system in adult treatment (on‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 48 Adverse events: psychiatric body system in adult treatment (on‐treatment).
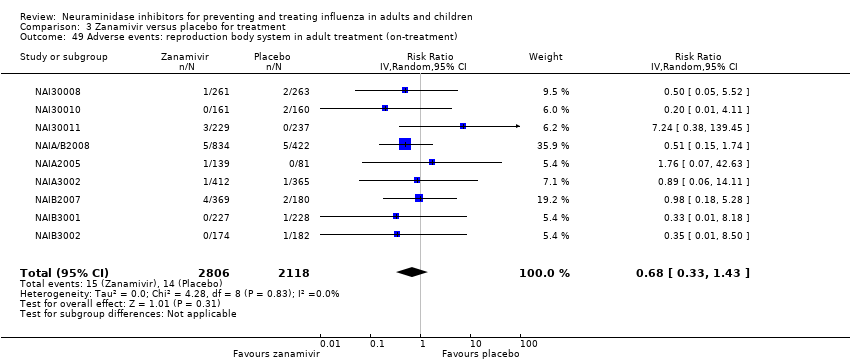
Comparison 3 Zanamivir versus placebo for treatment, Outcome 49 Adverse events: reproduction body system in adult treatment (on‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 50 Adverse events: endocrine and metabolic body system in adult treatment (on‐treatment).
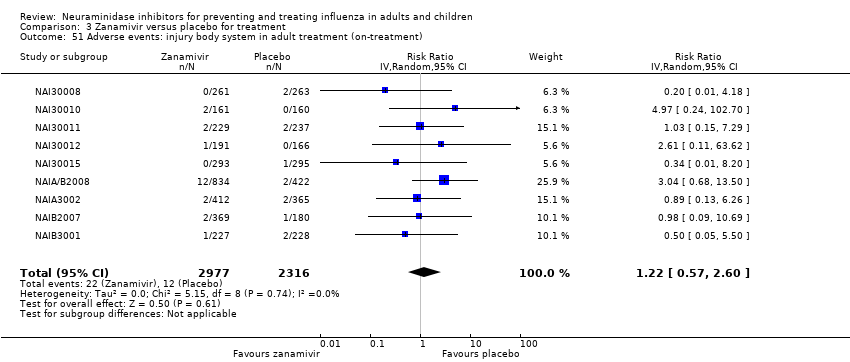
Comparison 3 Zanamivir versus placebo for treatment, Outcome 51 Adverse events: injury body system in adult treatment (on‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 52 Adverse events: non‐site specific events in adult treatment (on‐treatment).
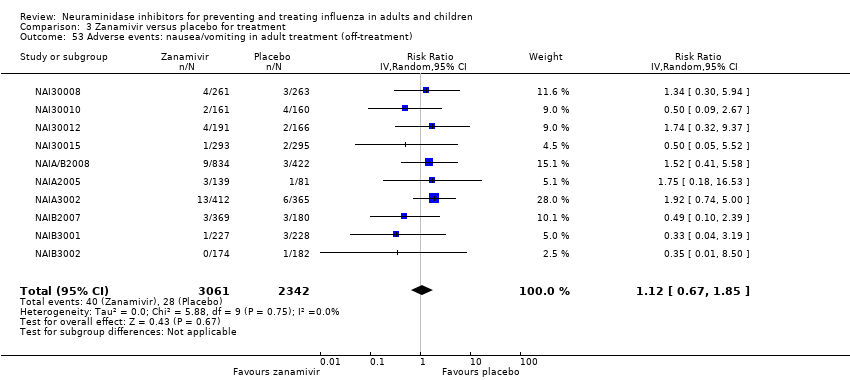
Comparison 3 Zanamivir versus placebo for treatment, Outcome 53 Adverse events: nausea/vomiting in adult treatment (off‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 54 Adverse events: cough in adult treatment (off‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 55 Adverse events: respiratory body system in adult treatment (off‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 56 Adverse events: headache in adult treatment (off‐treatment).
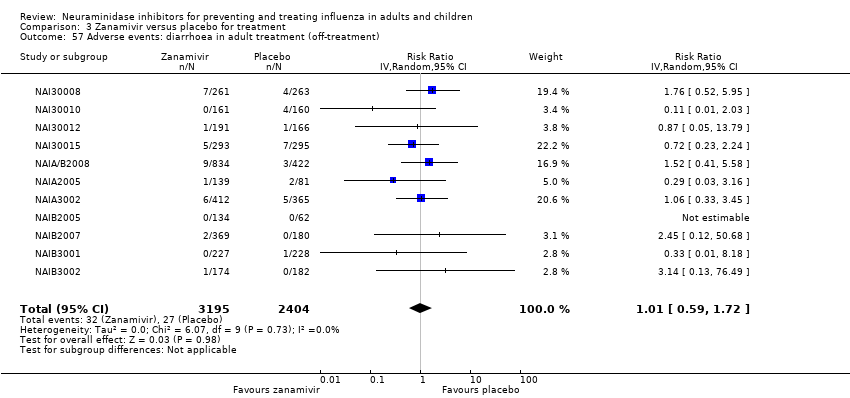
Comparison 3 Zanamivir versus placebo for treatment, Outcome 57 Adverse events: diarrhoea in adult treatment (off‐treatment).
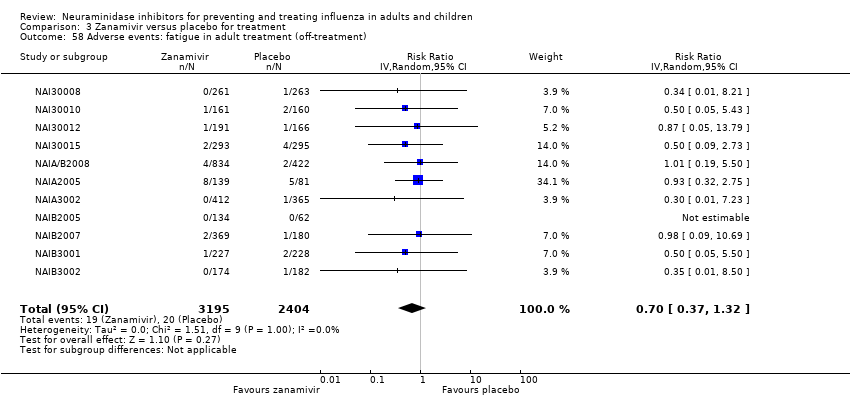
Comparison 3 Zanamivir versus placebo for treatment, Outcome 58 Adverse events: fatigue in adult treatment (off‐treatment).
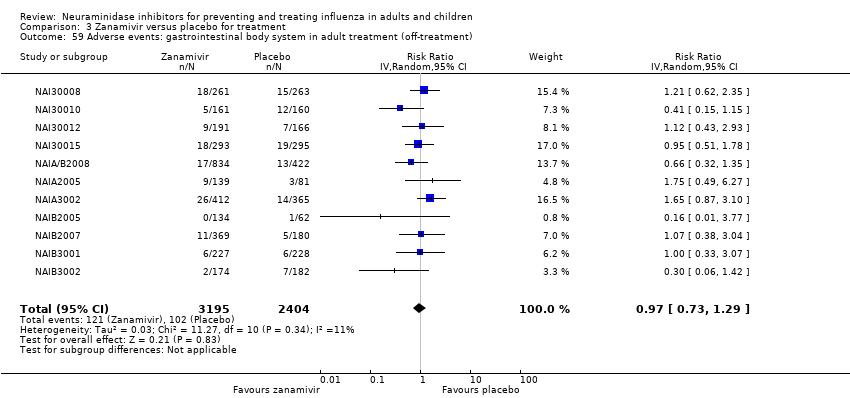
Comparison 3 Zanamivir versus placebo for treatment, Outcome 59 Adverse events: gastrointestinal body system in adult treatment (off‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 60 Adverse events: neurological body system in adult treatment (off‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 61 Adverse events: ear, nose and throat in adult treatment (off‐treatment).

Comparison 3 Zanamivir versus placebo for treatment, Outcome 62 Adverse events: skin body system in adult treatment (off‐treatment).
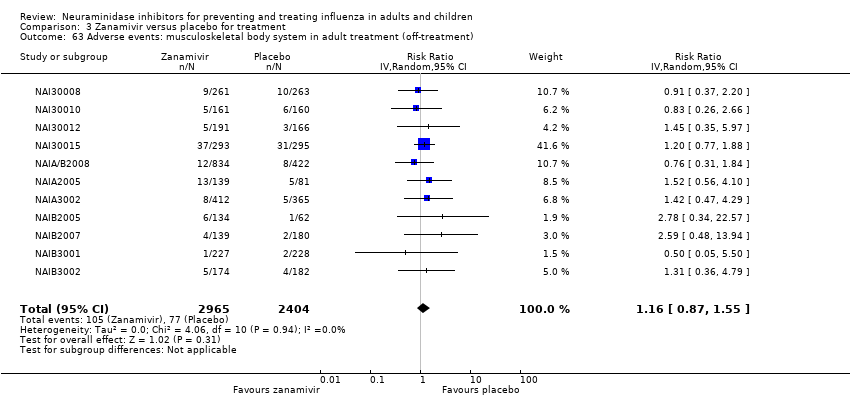
Comparison 3 Zanamivir versus placebo for treatment, Outcome 63 Adverse events: musculoskeletal body system in adult treatment (off‐treatment).
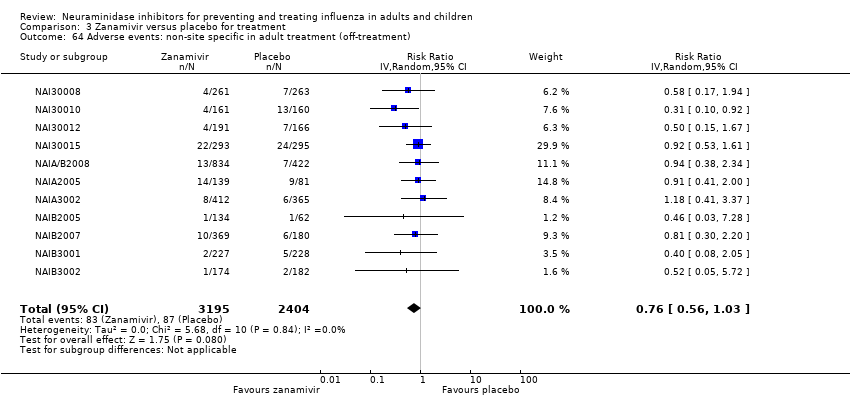
Comparison 3 Zanamivir versus placebo for treatment, Outcome 64 Adverse events: non‐site specific in adult treatment (off‐treatment).
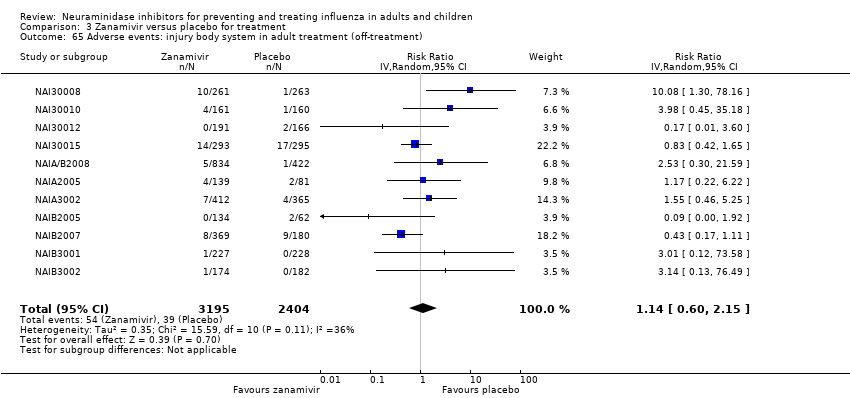
Comparison 3 Zanamivir versus placebo for treatment, Outcome 65 Adverse events: injury body system in adult treatment (off‐treatment).
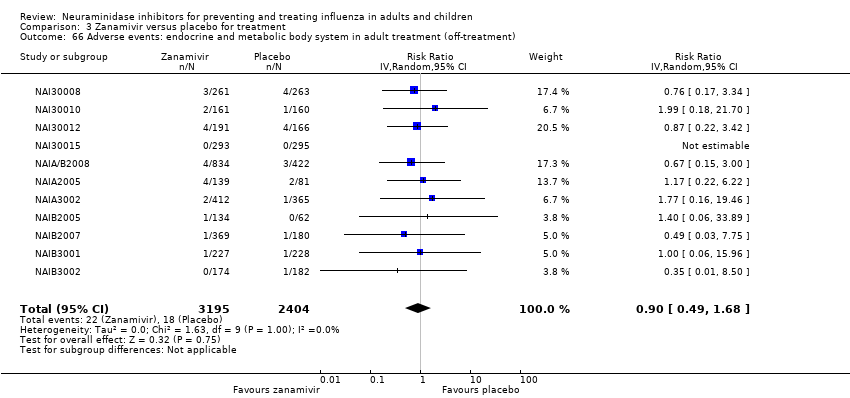
Comparison 3 Zanamivir versus placebo for treatment, Outcome 66 Adverse events: endocrine and metabolic body system in adult treatment (off‐treatment).
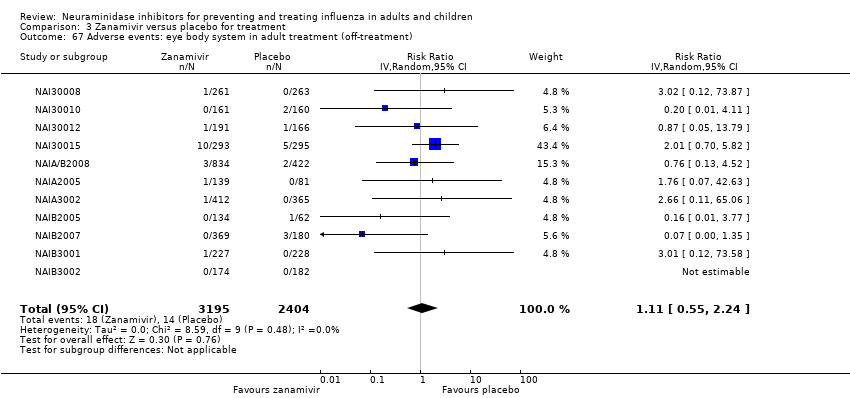
Comparison 3 Zanamivir versus placebo for treatment, Outcome 67 Adverse events: eye body system in adult treatment (off‐treatment).
![Comparison 3 Zanamivir versus placebo for treatment, Outcome 68 Time to first alleviation of symptoms in adults with/without relief medication [days].](/es/cdsr/doi/10.1002/14651858.CD008965.pub4/media/CDSR/CD008965/image_n/nCD008965-CMP-003-68.png)
Comparison 3 Zanamivir versus placebo for treatment, Outcome 68 Time to first alleviation of symptoms in adults with/without relief medication [days].
![Comparison 3 Zanamivir versus placebo for treatment, Outcome 69 Time to first alleviation of symptoms in adults by infection status [days].](/es/cdsr/doi/10.1002/14651858.CD008965.pub4/media/CDSR/CD008965/image_n/nCD008965-CMP-003-69.png)
Comparison 3 Zanamivir versus placebo for treatment, Outcome 69 Time to first alleviation of symptoms in adults by infection status [days].

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 1 Symptomatic influenza in prophylaxis of individuals.

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 2 Asymptomatic influenza in prophylaxis of individuals.
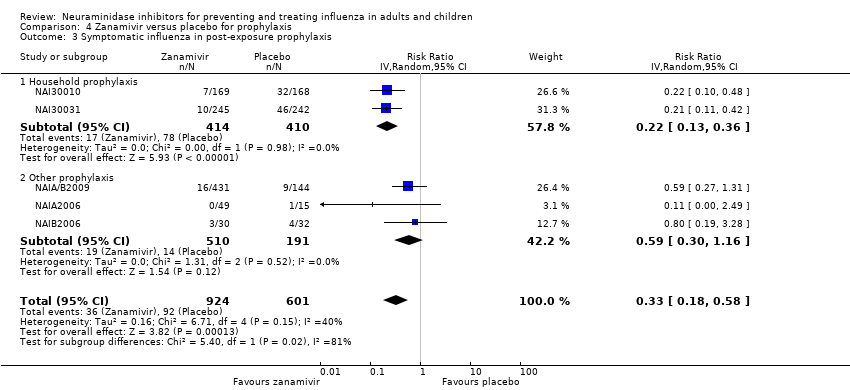
Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 3 Symptomatic influenza in post‐exposure prophylaxis.

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 4 Asymptomatic influenza in post‐exposure prophylaxis.

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 5 Complications: pneumonia in adult prophylaxis.

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 6 Complications: bronchitis in adult prophylaxis.

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 7 Complications: sinusitis in adult prophylaxis.

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 8 Complications classified as serious or leading to study withdrawal.
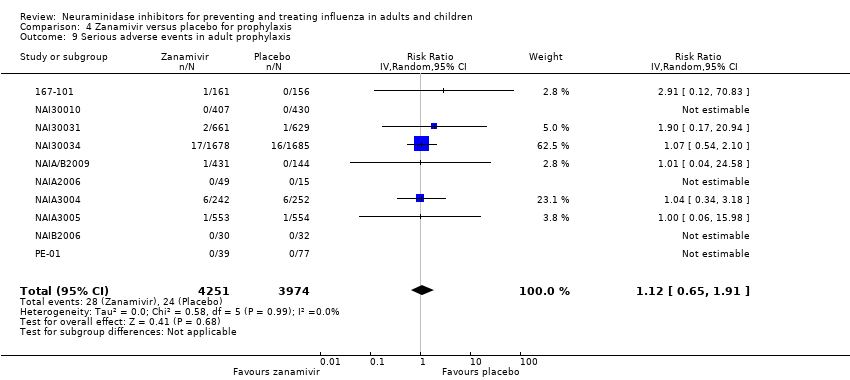
Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 9 Serious adverse events in adult prophylaxis.
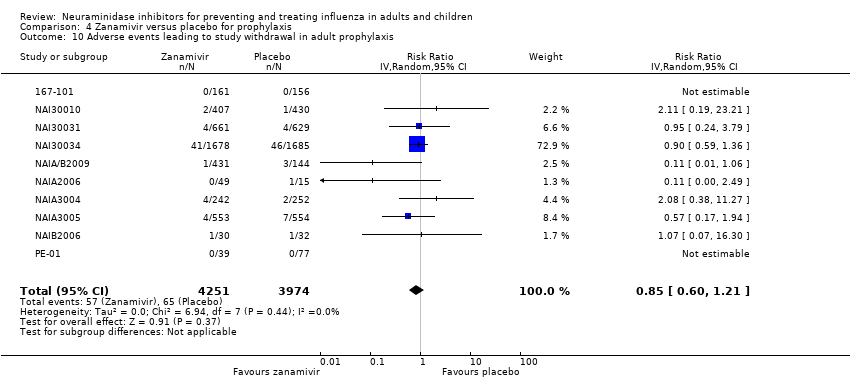
Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 10 Adverse events leading to study withdrawal in adult prophylaxis.

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 11 All withdrawals in adult prophylaxis.
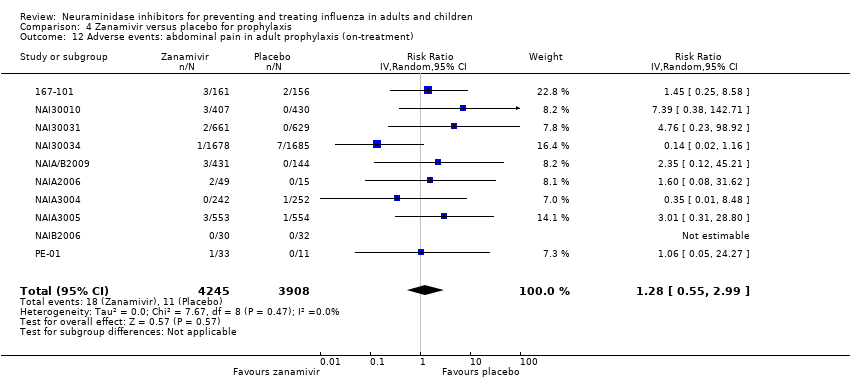
Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 12 Adverse events: abdominal pain in adult prophylaxis (on‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 13 Adverse events: cough in adult prophylaxis (on‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 14 Adverse events: diarrhoea in adult prophylaxis (on‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 15 Adverse events: dizziness in adult prophylaxis (on‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 16 Adverse events: fatigue in adult prophylaxis (on‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 17 Adverse events: headache in adult prophylaxis (on‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 18 Adverse events: blood body system in adult prophylaxis (on‐treatment).
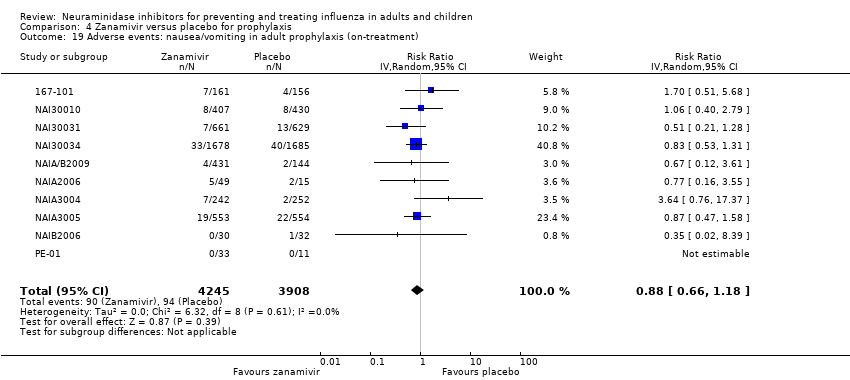
Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 19 Adverse events: nausea/vomiting in adult prophylaxis (on‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 20 Adverse events: cardiovascular body system in adult prophylaxis (on‐treatment).
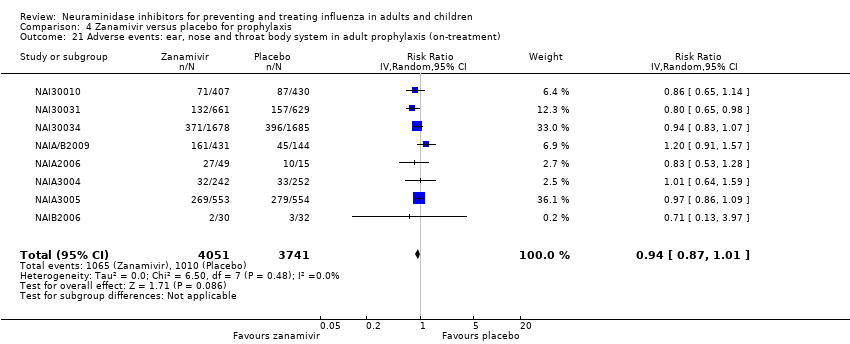
Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 21 Adverse events: ear, nose and throat body system in adult prophylaxis (on‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 22 Adverse events: endocrine and metabolic body system in adult prophylaxis (on‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 23 Adverse events: eye body system in adult prophylaxis (on‐treatment).
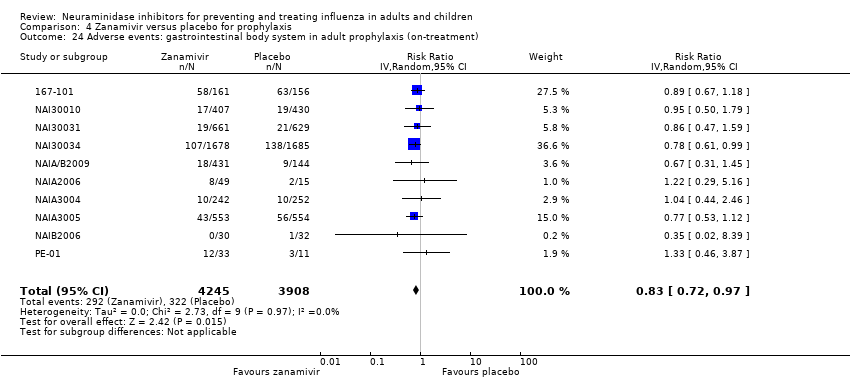
Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 24 Adverse events: gastrointestinal body system in adult prophylaxis (on‐treatment).
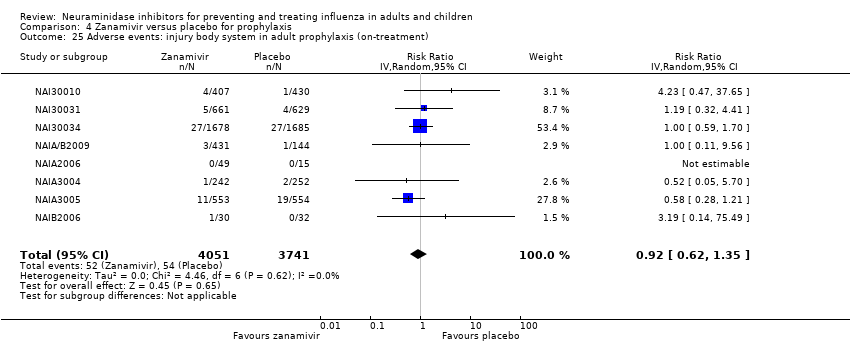
Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 25 Adverse events: injury body system in adult prophylaxis (on‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 26 Adverse events: musculoskeletal body system in adult prophylaxis (on‐treatment).
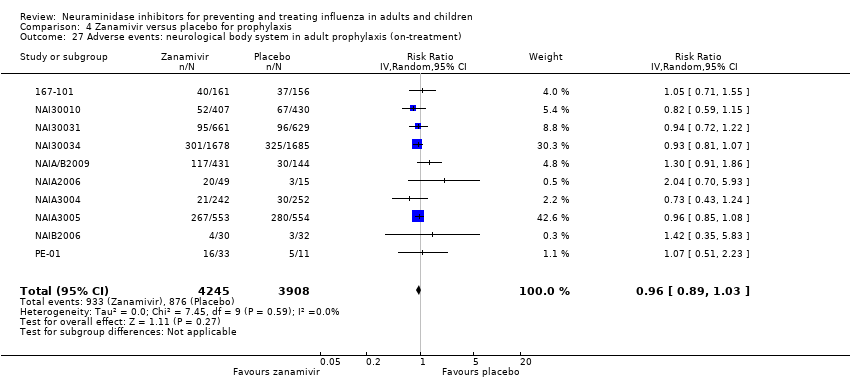
Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 27 Adverse events: neurological body system in adult prophylaxis (on‐treatment).
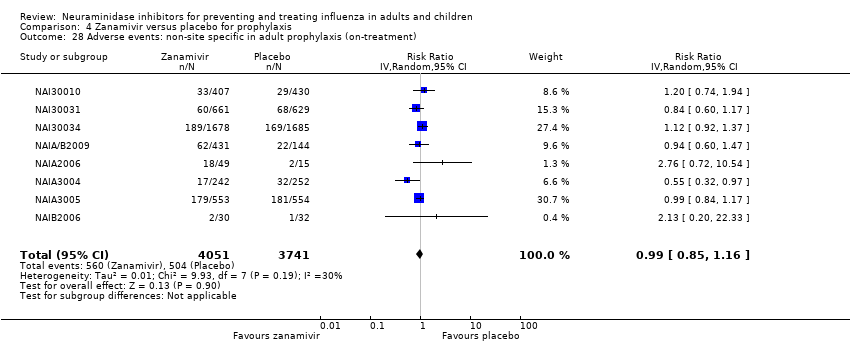
Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 28 Adverse events: non‐site specific in adult prophylaxis (on‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 29 Adverse events: psychiatric body system in adult prophylaxis (on‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 30 Adverse events: renal body system in adult prophylaxis (on‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 31 Adverse events: reproductive body system in adult prophylaxis (on‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 32 Adverse events: respiratory body system in adult prophylaxis (on‐treatment).
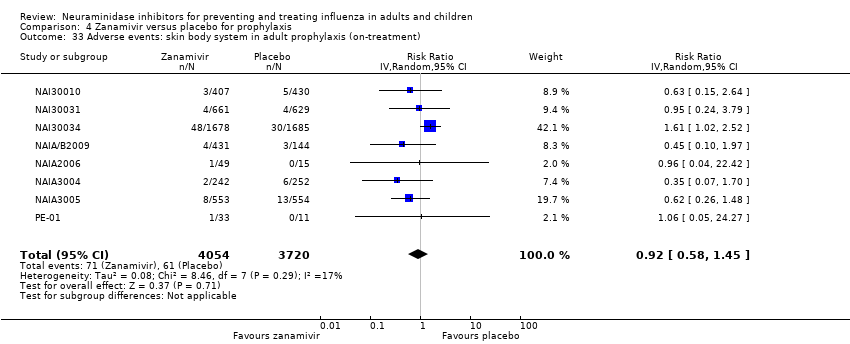
Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 33 Adverse events: skin body system in adult prophylaxis (on‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 34 Adverse events: gastrointestinal body system in adult prophylaxis (off‐treatment).
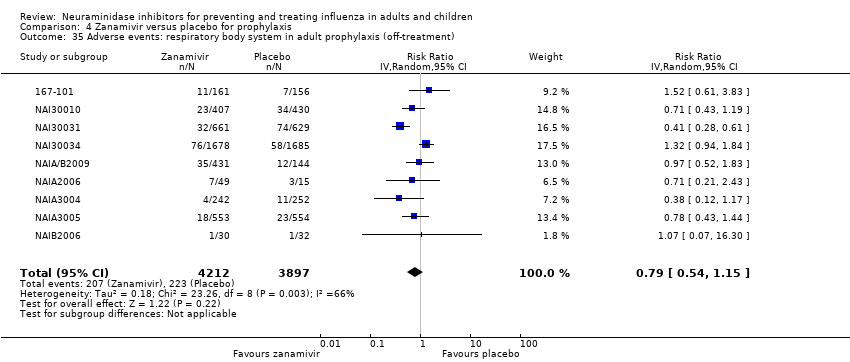
Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 35 Adverse events: respiratory body system in adult prophylaxis (off‐treatment).
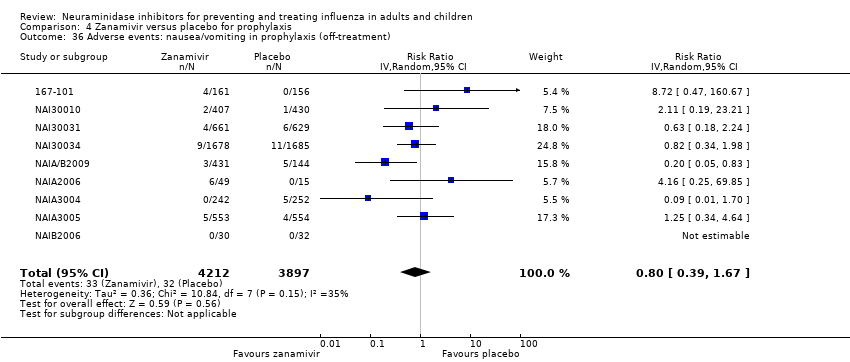
Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 36 Adverse events: nausea/vomiting in prophylaxis (off‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 37 Adverse events: diarrhoea in prophylaxis (off‐treatment).
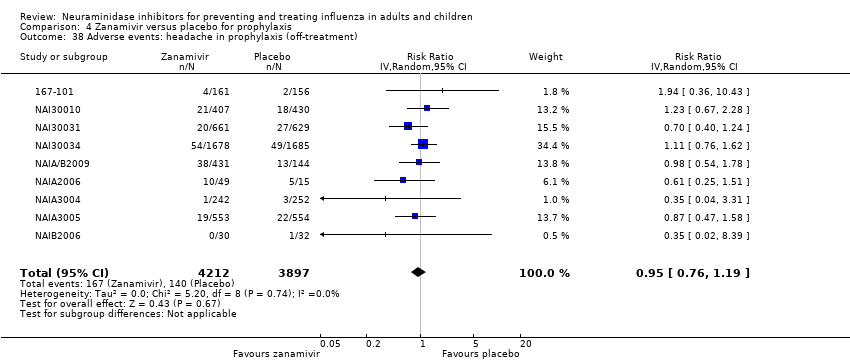
Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 38 Adverse events: headache in prophylaxis (off‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 39 Adverse events: cough in prophylaxis (off‐treatment).
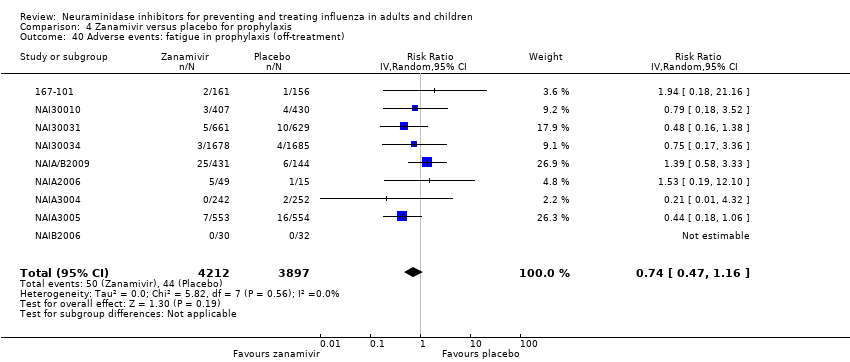
Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 40 Adverse events: fatigue in prophylaxis (off‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 41 Adverse events: neurological body system in prophylaxis (off‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 42 Adverse events: ear, nose and throat in prophylaxis (off‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 43 Adverse events: musculoskeletal body system in prophylaxis (off‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 44 Adverse events: non‐site specific in prophylaxis (off‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 45 Adverse events: injury in prophylaxis (off‐treatment).

Comparison 4 Zanamivir versus placebo for prophylaxis, Outcome 46 Adverse events: endocrine and metabolic in prophylaxis (off‐treatment).
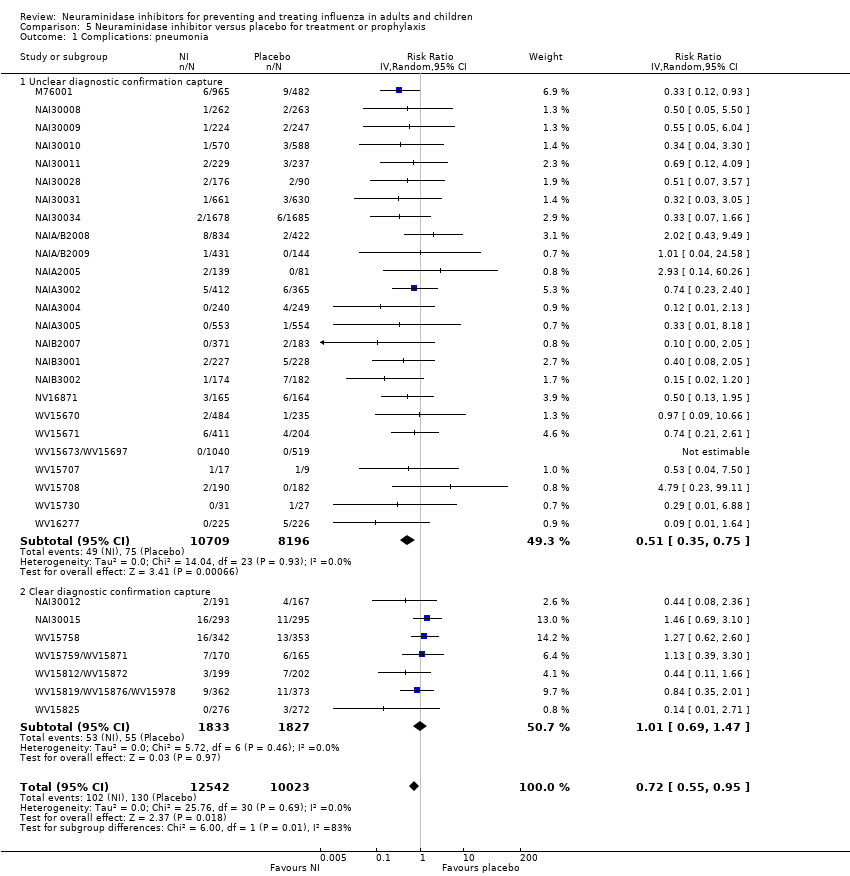
Comparison 5 Neuraminidase inhibitor versus placebo for treatment or prophylaxis, Outcome 1 Complications: pneumonia.
| Study | Where in CRF (PDF pg #) | Data captured | Person reporting (participant/investigator) | Where reported | Specific field for recording confirmatory assessment (e.g. CXR) | Confirmation (including px) |
| M76001 | 1167 | Yes/no answer to question: "Is this event a secondary illness related to influenza?" Secondary illness is defined: sinusitis, otitis, bronchitis, pneumonia + other chest infections that are not diagnosed as bronchitis and/or pneumonia | Investigator | In form for "Adverse events or intercurrent illness" | No | Px |
| NV16871 | 361, 389 | Form states: Have there been any changes in the patient’s health including any new conditions or worsening of existing conditions since day 1 (please include secondary illnesses)? Yes/No. If "Yes", please record the details on the "Adverse events or secondary illness" form in the Additional Forms section of the CRF on pg 30.0. All serious adverse events must be reported within 1 working day of occurrence to Roche Pg 30.0 of CRF (PDF pg 389) defines secondary illnesses as sinusitis, otitis media, bronchitis and pneumonia, and asks additional questions such as relationship to test drug and outcome, and leaves space for investigator's comments on the adverse event | Investigator | Secondary illness not listed as efficacy outcomes Recording of secondary illnesses was to occur in a form titled "Adverse event or secondary illness" | No | Px |
| WV15670 | 732, 754, 791, 832 | CRF form (PDF pg 732) states: Secondary illness reminder Has the patient reported any sinusitis, otitis, bronchitis, other chest infection or pneumonia since baseline? yes [ ] Complete secondary illness page (not the adverse event page) no [ ] Secondary illness page CRF (PDF pg 754) requests information on date of onset, date resolved, whether treatment was given and, if so, what treatment or medical procedures, total daily dose, and start/end date of treatment or medical procedure In addition, participants could fill in information related to a secondary illness in their diary card in the free‐text box called "Notes" which prompts participants: "Please can you record below any extra information about your flu which may be of interest to us, (for example: did your flu symptoms re‐occur, and if so when?), and have you taken any other treatments. If so please record the treatment name and the dates you took it." (PDF pg 791) | Participant mediated through Investigator | For investigators, on "Secondary illness" form For participants, on "Notes" section of diary card | No | |
| WV15671 | 740, 889, 1018 | CRF form (PDF pg 740) states: Secondary illness reminder Has the patient reported any sinusitis, otitis, bronchitis, other chest infection or pneumonia since baseline? yes [ ] Complete secondary illness page (not the adverse event page) no [ ] Secondary illness page CRF (PDF pg 889) requests information on date of onset, date resolved, whether treatment was given and, if so, what treatment or medical procedures, total daily dose and start/end date of treatment or medical procedure Secondary illnesses are listed as sinusitis, otitis, bronchitis, pneumonia and other chest infections that are not diagnosed as bronchitis and/or pneumonia In addition, participants could fill in information related to a secondary illness in their diary card in the free‐text box called "Notes" which prompts participants: "Please can you record below any extra information about your flu which may be of interest to us, (for example: did your flu symptoms re‐occur, and if so when?), and have you taken any other treatments. If so please record the treatment name and the dates you took it." (PDF pg 1018) | Participant mediated through investigator | Mentioned in M1 and RAP as tertiary outcomes not mentioned in protocol | No | Px |
| WV15673/WV15697 | From 483 | No mention of pneumonia, secondary illness, complications in the CRFs | Unclear | Secondary illnesses not listed in protocol as endpoints. They are listed as safety endpoints in the RAP which states that "pre‐defined" secondary illnesses were "sinusitis, otitis, bronchitis, pneumonia, and other chest infections that are not diagnosed as bronchitis and/or pneumonia, plus recurrence of symptoms from the diary card once alleviation had occurred." (PDF pg 479) | ||
| WV15707 | From 98 | Pg 117 Secondary illness reminder: Has the patient reported any sinusitis, otitis, bronchitis, other chest infection or pneumonia since baseline? yes [] ‐ Complete secondary illness page (not the adverse event page) no [] Pg 131: Diagnostic procedures 1) Were there any diagnostic procedures or tests carried out since day 1 as a result of influenza or secondary illness that were not scheduled as part of protocol? Type of diagnostic procedure or test 1 Chest X‐rays, 2 ECG, 3 Bacterial culture, 4 Bronchoscopy, 5 Pulmonary function test, 6 Viral culture (other than influenza), 7 Blood tests (other than antibody sample), 8 Other specify No Secondary illness page CRF (PDF pg 158) requests information on date of onset, date resolved, whether treatment was given and, if so, what treatment or medical procedures, total daily dose and start/end date of treatment or medical procedure | Participant mediated through investigator | Mentioned in RAP as tertiary endpoints pg 57‐8 | Yes | Px |
| WV15708 | From 460 | Secondary illness reminder at pg 474: Has the patient reported any new episodes of sinusitis, otitis, bronchitis, other chest infection or pneumonia since screening? yes [] ... Complete adverse event page no [] "Adverse events" CRF collected data on date of onset, initial intensity, test drug adjustment, whether treatment was given (if so, what), most extreme intensity, relationship to test drug, outcome, whether it led to hospitalisation and a free‐text line for recording "Comments on AE" (e.g. PDF pg 479) | Participant mediated through investigator | Secondary illness not mentioned in protocol | No | Px |
| WV15730 | From 340 | Secondary illness reminder: Has the patient reported any sinusitis, otitis, bronchitis, other chest infection or pneumonia since baseline? yes [] ... Complete secondary illness page (not the adverse event page) no [] The secondary illness page is descriptive of dates and px | Participant mediated through investigator | Listed as tertiary endpoints in RAP at pg 297 | No | Px |
| WV15758 | From 637 | Has the patient reported any new adverse events or symptoms (including intercurrent illnesses and secondary illnesses)? yes [] record in the adverse events/intercurrent illness section of the case no [] report form Diagnostic confirmation of otitis media from pg 648 onwards | Participant mediated through investigator | Listed as secondary illnesses in core report Module 1‐2 (pg 36) | Yes | Px |
| WV15759/871 | From 665 | Has the subject reported any adverse events including secondary and intercurrent illnesses? | Participant mediated through investigator | Secondary illnesses not mentioned in protocol, but secondary outcome in core report Note: worth looking at comparisons 1.49 to 1.51 in RM5. No effect but in bronchitis this study has a more conservative effect than NV 16871 which has no definitions and no diagnostics | Yes | Px |
| WV15799 | From 642 | Secondary illness defined as in M76001. There is a generic physical examination form at pg 704 including "lungs" normal/abnormal specify_______ At pg 709 has the patient reported any new AE including intercurrent or secondary illnesses yes/no. If y record the adverse events/intercurrent illness section of the CRF (noted at pf 746 on to be at the back of the CRF) with FULL HISTORY, PHYSICAL EXAMINATION AND DIAGNOSTIC WORK UP QUESTIONS FOR BRON+PNUM+LRTI+SIN+OM including questions about CXR, MRI, sputum etc. | Investigator | Proportion of contacts who are classified as having a secondary illness subsequent to a confirmed episode of influenza listed as tertiary endpoints | Yes | Px |
| WV15812/872 | From 285 | Has the patient reported any new adverse events or symptoms (including intercurrent illnesses and secondary illnesses)? yes [] record in the adverse events/intercurrent illness section of the case no [] report form At pg 450‐74 is DIAGNOSIS OF SECONDARY ILLNESS page which is very similar to the one at serial 10 EXHAUSTIVE list of diagnostic procedures | Participant mediated through investigator | Listed as secondary tertiary in protocol at pg 252 | Yes | Px |
| WV15819/978/876 | From 412 | Pg 437 (adverse event reminder): Has the patient reported any new adverse events or symptoms (including intercurrent illnesses)? yes [] record in the adverse events/intercurrent illness section of the case no [] report form In CRF pg 447 and 443 usual secondary illness reminder From pg 471 DIAGNOSTIC OF SECONDARY ILLNESS. This is a one page list of diagnostics similar to that at serial 10. The question is: "Were there any diagnostic procedures or tests carried out since day 1 as a result of influenza or secondary illness that were not scheduled as part of protocol?" If yes list per serial 10 From pg 486 is a list of diagnostic tests | Participant mediated through investigator | Secondary illness listed as secondary (required antibiotics) and tertiary outcomes in core report and as an addition in protocol amendment at pg 21 | Yes | Px |
| WV15825 | From 389 | There is a usual note: please go to diagnosis of secondary illness at back of CRF. Pg 487: Is this event a secondary illness related to influenza? DIAGNOSTIC OF SECONDARY ILLNESS From pg 510‐40 with exhaustive list of diagnostics as per serial 10 | Participant mediated through investigator | Secondary illness listed as secondary outcomes in protocol pg 346 Secondary illnesses recorded on "Adverse events" CRF | Yes | Px |
| WV16277 | From 415 | Not found | Not found | Secondary illness not listed as efficacy outcomes | ||
| #Events within the first 2 days of the study were excluded | ||||||
| Mentioned study | File name | Pages where study is mentioned (separated by commas) | Note |
| 113502 |
|
|
|
| 113625 |
|
|
|
| 113678 |
|
|
|
| 114045 |
|
|
|
| NAI108166 |
|
|
|
| 105934 |
|
|
|
| NAI106784 |
|
|
|
| 107485 |
|
|
|
| 108127 |
|
|
|
| 112311 |
|
|
|
| 112312 |
|
|
|
| 113268 |
|
|
|
| GCP/95/045 |
|
|
|
| NAI10901 | Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐admin2.pdf | 15.15 |
|
| NAI10902 |
|
|
|
| NAI30008 | Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐admin2.pdf | 15 | 7 documents with 14 instances |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐admin3.pdf | 13 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview7.pdf | 19, 19, 20 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview8.pdf | 1, 1, 3, 4, 4 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview9.pdf | 7.7 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/21036ltr.pdf | 2 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_MEDR.pdf | 33 |
| |
| NAI30009 | Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview8.pdf | 1.2 | 7 documents with 110 instances |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_ADMINCORRES_P1.pdf | 10, 10, 12, 13, 13, 14, 14, 17, 29, 42, 61, 62, 64, 64, 65, 65, 68 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_ADMINCORRES_P2.pdf | 33, 34, 36, 43, 43, 43, 43, 52, 52, 52, 53, 53, 56, 57 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_BIOPHARMR.pdf | 5, 5, 5, 6, 6, 8, 8 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_MEDR.pdf | 3, 3, 3, 3, 3, 3, 3, 4, 4, 5, 8, 9, 9, 10, 10, 11, 11, 11, 14, 14, 15, 16, 17, 19, 19, 19, 20, 20, 22, 23, 23, 23, 24, 24, 24, 25, 25, 25, 25, 25, 25, 26, 26, 26, 27, 27, 28, 28, 28, 29, 29, 31, 31, 31, 31 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_MICROBR.pdf | 3, 3, 4, 4, 4 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_STATR.pdf | 2, 2, 2, 4, 7, 12, 18, 18, 18, 19 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_ADMINCORRES_P1.pdf | 31.56 | 1 document with 2 instances | |
| NAI30010 | Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview8.pdf | 1.2 | 6 documents with 65 instances |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_ADMINCORRES_P1.pdf | 10, 12, 13, 14, 14, 15, 17, 62, 62, 62, 64 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_ADMINCORRES_P2.pdf | 34, 34, 36, 43, 53 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_BIOPHARMR.pdf | 5, 5, 6, 6 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_MEDR.pdf | 3, 3, 3, 3, 3, 4, 5, 18, 19, 21, 21, 22, 23, 23, 23, 23, 24, 25, 25, 25, 26, 26, 26, 26, 27, 27, 27, 28, 28, 29, 29, 29, 30, 31, 31, 31, 32 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_STATR.pdf | 2, 2, 13, 13, 13, 19 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_BIOPHARMR.pdf | 6 | 1 document with 1 instance | |
| NAI30012 | Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview7.pdf | 1 | 1 document with 1 instance |
| NAI30015 |
|
|
|
| NAI30020 |
|
|
|
| NAI30028 |
|
|
|
| NAI30034 |
|
|
|
| NAI40012 |
|
|
|
| NAIA1009 | Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_ADMINCORRES_P1.pdf | 56 | 4 documents with 17 instances |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_ADMINCORRES_P2.pdf | 1, 1, 1, 48, 49, 52 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_BIOPHARMR.pdf | 5, 5, 6 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_MEDR.pdf | 3, 3, 6, 7, 20, 31, 31 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview5.pdf | 18 | 5 documents with 5 instances | |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview6.pdf | 9 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_ADMINCORRES_P2.pdf | 52 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_BIOPHARMR.pdf | 11 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_STATR.pdf | 2 |
| |
| NAIA3002 | Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐admin1.pdf | 15 | 13 documents with 122 instances |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐admin2.pdf | 6, 6, 7, 7, 14, 15, 22, 22, 23 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐admin3.pdf | 1, 4, 4, 12, 12, 12, 12, 17 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview1.pdf | 4, 14, 14, 14, 14, 14, 15, 15, 15, 15, 16 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview2.pdf | 1, 2, 3, 4, 4, 5, 6, 6, 6, 8, 8, 9, 9, 9, 12, 12, 15, 16, 16, 16, 17 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview3.pdf | 5, 5, 6, 6, 6, 7, 7, 7, 8, 8, 9, 9, 9, 10, 11, 12, 13, 13, 14, 15, 15, 17, 18, 18, 19, 20, 21 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview4.pdf | 1, 1, 1, 1, 2, 6 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview6.pdf | 4, 5, 10, 12 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview7.pdf | 1, 1, 2, 2, 2, 2, 3, 3, 4, 4, 5, 5, 7, 8, 10, 11, 12, 14, 16, 16, 16, 16, 16, 17 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview8.pdf | 2, 2, 6, 6, 8, 8, 9, 10 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview9.pdf | 10 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐stats.pdf | 7 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_BIOPHARMR.pdf | 5 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview1.pdf | 15 | 1 document with 1 instance | |
| NAIA3003
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview7.pdf | 17, 17, 18 | 3 documents with 6 instances |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview8.pdf | 4.4 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview9.pdf | 22 |
| |
| NAIA3004 | Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐admin3.pdf | 14 | 4 documents with 8 instances |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview6.pdf | 7 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview7.pdf | 18, 18, 19 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview8.pdf | 4, 4, 4 |
| |
| NAIA3005 | Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐admin3.pdf | 14 | 5 documents with 12 instances |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview1.pdf | 5 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview5.pdf | 12, 12, 12, 13, 14, 15, 15 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview7.pdf | 14.15 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_ADMINCORRES_P2.pdf | 38 |
| |
| NAIB1002 |
|
|
|
| NAIB3002 | Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐admin1.pdf | 15 | 14 documents with 99 instances |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐admin2.pdf | 14, 15, 15, 15 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐admin3.pdf | 11.12 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview1.pdf | 4, 14, 14, 14, 14, 14, 14, 14, 14 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview2.pdf | 9, 9, 9, 9, 9, 9, 10, 11, 12, 12, 12, 12, 13, 13, 13, 14, 14, 14, 15, 15, 16, 16, 16, 17 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview3.pdf | 4, 5, 6, 6, 6, 7, 7, 7, 8, 8, 8, 9, 9, 11, 12, 12, 13, 13, 14, 15, 17, 18, 18, 19, 20, 21 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview4.pdf | 1, 1, 1, 1, 2 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview5.pdf | 4 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview6.pdf | 4, 5, 10, 12 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview7.pdf | 2, 3, 3, 7, 8, 10, 11, 14, 15, 16, 16, 16 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview8.pdf | 7, 8, 8, 8, 9, 9 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview9.pdf | 10.2 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐stats.pdf | 7 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_BIOPHARMR.pdf | 5.5 |
| |
| NAI30011 |
|
|
|
| NAIB2007 | Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐admin1.pdf | 15 | 7 documents with 18 instances |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐admin2.pdf | 15 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview1.pdf | 5 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview4.pdf | 14, 15, 15, 16, 16, 17, 17, 17, 18 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview6.pdf | 3 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview7.pdf | 8, 10, 10, 15 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview8.pdf | 2 |
| |
| NAIA2006 |
|
|
|
| NAIB2006 |
|
|
|
| NAIB1007 |
|
|
|
| C94‐009 | Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview5.pdf | 17 | 1 document with 1 instance |
| C94‐085
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview5.pdf | 17 | 2 documents with 2 instances |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview9.pdf | 22 |
| |
| NAIB1001 | Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_BIOPHARMR.pdf | 17 | 1 document with 1 instance |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/20000426_001/21‐036‐S001_RELENZA_BIOPHARMR.pdf | 6 | 1 document with 1 instance | |
| NAIA2005 | Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐admin1.pdf | 15 | 10 documents with 44 instances |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐admin2.pdf | 7, 17, 10 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐admin3.pdf | 2.4 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview1.pdf | 4.5 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview4.pdf | 2, 2, 3, 3, 3, 3, 5, 6, 6, 6, 6, 8, 8, 8, 9, 11, 12, 12, 13, 14, 14, 14, 14, 14, 15, 18 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview5.pdf | 7.7 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview6.pdf | 3.4 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview7.pdf | 2, 5, 9, 15 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview8.pdf | 10 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐microbiology.pdf | 21 |
| |
| NAIB2005 | Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐admin1.pdf | 15 | 9 documents with 43 instances |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐admin2.pdf | 17, 20, 20, 22, 23 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview1.pdf | 5.5 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview4.pdf | 3, 3, 3, 7, 8, 8, 8, 9, 10, 11, 11, 11, 11, 11, 12, 12, 12, 13, 14, 14, 14, 14, 14, 14, 14, 15 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview5.pdf | 7.7 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview6.pdf | 3.4 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview7.pdf | 2, 9, 15 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview8.pdf | 2 |
| |
| Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐microbiology.pdf | 21 |
| |
| NAIA/B2008 | Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview6.pdf | 4 | 1 document with 1 instance |
| NAIA2010 | Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036‐medreview5.pdf | 16 | 1 document with 1 instance |
| NAIA/B2009 |
|
|
|
| 167‐02 |
|
|
|
| 167‐03 |
|
|
|
| 167‐05 |
|
|
|
| 167‐04 |
|
|
|
| JNAI‐03 |
|
|
|
| JNAI‐02 |
|
|
|
| JNAI‐01 |
|
|
|
| JNAI‐07 |
|
|
|
| JNAI‐04 |
|
|
|
| PE‐01 |
|
|
|
| 167‐101 |
|
|
|
| 167T3‐11 |
|
|
|
| Zanamivir trials citation by trial ID and source FDA file. Page numbers separated by commas (where applicable) indicate which trial is cited where in which regulatory file. Blank spaces indicate no citation for known trials. All the studies have been searched in the folder "Tamiflu and Relenza/Relenza/Relenza ‐ NDA 021036/19990726_000/021036". File name is left blank when the study was not present in that folder. | |||
| Referenced study | File name | Pages where study is mentioned (separated by commas) | Note |
| NP15717 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_bior.pdf | 46.46 | 6 documents with 13 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P2.pdf | 14, 15, 15 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20040624_010/021087_S016_TAMIFLU CAPSULES ‐ DRY POWDER_BIOPHARMR.pdf | 3 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Admindocs_P2.pdf | 2 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_BioPharmr.pdf | 5, 8, 10, 13, 31 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20040624_010/021087_S016_TAMIFLU CAPSULES ‐ DRY POWDER_BIOPHARMR.pdf | 3 |
| |
| NP15718 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_bior.pdf | 17 | 1 document with 1 instance |
| NP15728 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_bior.pdf | 16.35 | 3 documents with 6 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P1.pdf | 11 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P2.pdf | 45, 46, 47 |
| |
| NP15757 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/20001117_002/21‐087SE1‐002_review.pdf | 92, 93, 104, 122, 126, 131, 144, 144, 145 | 1 document with 9 instances |
| NP15826 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P2.pdf | 47 | 9 documents with 26 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/20040624_016/021087_S016_TAMIFLU CAPSULES ‐ DRY POWDER_ADMINCORRES.pdf | 6 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20040624_010/021087_S016_TAMIFLU CAPSULES ‐ DRY POWDER_BIOPHARMR.pdf | 3 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Admindocs_P2.pdf | 2 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_BioPharmr.pdf | 4, 5, 5, 8, 8, 8, 10, 17, 29, 30, 30, 30, 30, 30, 31, 31 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Medr.pdf | 9.1 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Statr.pdf | 9.1 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/20040624_016/021087_S016_TAMIFLU CAPSULES ‐ DRY POWDER_ADMINCORRES.pdf | 6 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20040624_010/021087_S016_TAMIFLU CAPSULES ‐ DRY POWDER_BIOPHARMR.pdf | 3 |
| |
| NP15827 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P1.pdf | 10.12 | 2 documents with 7 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P2.pdf | 16, 16, 17, 17, 17 |
| |
| WP15525 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_bior.pdf | 21, 25, 26, 27, 27, 27, 27, 42, 42, 44 | 3 document with 13 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Admindocs_P2.pdf | 2.2 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_BioPharmr.pdf | 29 |
| |
| WP15647 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_bior.pdf | 24, 27, 27 | 2 documents with 4 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P2.pdf | 44 |
| |
| WP15648 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_bior.pdf | 39 | 3 documents with 8 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P2.pdf | 44.44 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/20001117_002/21‐087SE1‐002_review.pdf | 94, 128, 153, 153, 154 |
| |
| WP15676 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_bior.pdf | 28.33 | 3 documents with 4 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P1.pdf | 11 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P2.pdf | 45 |
| |
| WV15670 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_bior.pdf | 2, 44, 44 | 6 documents with 45 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P1.pdf | 6, 19, 37, 38, 39, 39, 39, 39, 40, 41, 41, 42, 43, 44, 48, 48, 49, 49 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P2.pdf | 1, 25, 25, 35, 35, 39, 39, 47 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Statr.pdf | 3, 3, 4, 4, 5, 5, 5, 8, 9, 10, 17, 17, 21, 22, |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/20001117_002/21‐087SE1‐002_review.pdf | 189 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20040624_010/021087_S016_TAMIFLU CAPSULES ‐ DRY POWDER_BIOPHARMR.pdf | 3 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20040624_010/021087_S016_TAMIFLU CAPSULES ‐ DRY POWDER_BIOPHARMR.pdf | 3 |
| |
| WV15671 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_bior.pdf | 2, 44, 44 | 7 documents with 50 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P1.pdf | 6, 16, 19, 24, 24, 25, 25, 26, 27, 27, 28, 32, 34, 35, 36, 37, 38, 39, 39, 39, 40, 41, 46, 49, 49 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P2.pdf | 1, 25, 25, 35, 38, 47 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Statr.pdf | 3, 4, 4, 5, 5, 5, 5, 9, 10, 10, 15, 17, 21 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/20001117_002/21‐087SE1‐002_review.pdf | 189 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20040624_010/021087_S016_TAMIFLU CAPSULES ‐ DRY POWDER_BIOPHARMR.pdf | 3 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20040624_010/021087_S016_TAMIFLU CAPSULES ‐ DRY POWDER_BIOPHARMR.pdf | 3 |
| |
| WV15673 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P1.pdf | 3 | 3 documents with 50 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P2.pdf | 18, 18, 18, 20, 21, 21, 21, 22, 39 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/20001117_002/21‐087SE1‐002_review.pdf | 58, 59, 71, 71, 71, 71, 71, 72, 72, 73, 73, 76, 76, 76, 76, 76, 77, 77, 79, 82, 83, 83, 84, 122, 124, 125, 126, 128, 131, 131, 132, 133, 134, 134, 145, 145, 156, 169, 177, 189 |
| |
| WV15697 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P2.pdf | 39 | 2 documents with 40 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/20001117_002/21‐087SE1‐002_review.pdf | 58, 59, 71, 71, 71, 71, 71, 72, 72, 73, 73, 76, 76, 76, 76, 76, 77, 77, 79, 82, 83, 83, 84, 122, 126, 128, 131, 131, 131, 132, 133, 134, 145, 145, 152, 153, 156, 162, 189 |
| |
| WV15708 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P1.pdf | 3 | 3 documents with 39 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P2.pdf | 23, 35, 39, 41 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/20001117_002/21‐087SE1‐002_review.pdf | 71, 71, 71, 71, 71, 72, 72, 72, 72, 75, 75, 75, 75, 77, 77, 78, 79, 79, 82, 82, 122, 125, 125, 126, 131, 134, 134, 135, 135, 149, 151, 152, 152, 153 |
| |
| WV15708D | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P1.pdf | 3 | 2 documents with 3 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P2.pdf | 23.35 |
| |
| WV15730 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_bior.pdf | 44.44 | 5 documents with 15 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P1.pdf | 6, 9, 19, 49, 50, 50 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P2.pdf | 1, 1, 25, 25, 27 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/20001117_002/21‐087SE1‐002_review.pdf | 189 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20040624_010/021087_S016_TAMIFLU CAPSULES ‐ DRY POWDER_BIOPHARMR.pdf | 3 |
| |
| WV15731 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Admindocs_P2.pdf | 17 | 4 documents with 9 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Medr.pdf | 5, 30, 37 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Microbr.pdf | 5.6 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Statr.pdf | 5, 30, 37 |
| |
| WV15758 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Admindocs_P1.pdf | 12, 19, 19, 36 | 9 documents with 92 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Admindocs_P2.pdf | 2, 8, 17, 39, 39, 57, 57 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_BioPharmr.pdf | 3, 4, 5, 5, 5, 8, 10, 17, 27, 30 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Corres.pdf | 6.9 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Medr.pdf | 5, 5, 9, 9, 10, 11, 12, 12, 16, 18, 18, 18, 19, 19, 31, 31, 31, 33, 33, 35, 36, 37, 37, 37, 37, 37, 37, 37, 40, 43 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Microbr.pdf | 2, 4, 5, 6 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Statr.pdf | 5, 5, 9, 9, 10, 11, 12, 12, 16, 18, 18, 18, 19, 19, 31, 31, 31, 33, 33, 35, 36, 37, 37, 37, 37, 37, 37, 37, 40, 43 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/20040624_016/021087_S016_TAMIFLU CAPSULES ‐ DRY POWDER_ADMINCORRES.pdf | 6, 6, 8 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20040624_010/021087_S016_TAMIFLU CAPSULES ‐ DRY POWDER_BIOPHARMR.pdf | 2.3 |
| |
| WV15759 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Admindocs_P1.pdf | 12.13 | 7 documents with 44 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Admindocs_P2.pdf | 39 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Medr.pdf | 5, 10, 30, 30, 30, 30, 31, 32, 32, 33, 34, 37, 37, 37, 40, 44 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Microbr.pdf | 2, 4, 4, 5, 6 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Statr.pdf | 5, 10, 30, 30, 30, 30, 31, 32, 32, 33, 34, 37, 37, 37, 40, 44 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/20040624_016/021087_S016_TAMIFLU CAPSULES ‐ DRY POWDER_ADMINCORRES.pdf | 6, 6, 9 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20040624_010/021087_S016_TAMIFLU CAPSULES ‐ DRY POWDER_BIOPHARMR.pdf | 2 |
| |
| WV15799 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/20001117_002/21‐087SE1‐002_review.pdf | 28, 28, 28, 28, 28, 29, 29, 30, 30, 30, 30, 30, 31, 31, 31, 31, 32, 32, 32, 32, 32, 33, 33, 34, 34, 35, 35, 35, 36, 37, 37, 37, 37, 37, 38, 38, 38, 39, 39, 40, 40, 40, 40, 40, 58, 60, 71, 71, 71, 71, 71, 72, 72, 73, 76, 76, 76, 77, 78, 79, 84, 85, 122, 125, 125, 126, 126, 128, 131, 140, 140, 140, 143, 147, 149, 156, 162, 169, 175, 187, 203, 208, 208 | 4 documents with 89 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Medr.pdf | 10.11 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Statr.pdf | 10.11 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/20040624_016/021087_S016_TAMIFLU CAPSULES ‐ DRY POWDER_ADMINCORRES.pdf | 6.7 |
| |
| WV15812 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P1.pdf | 3, 6, 10, 12 | 2 documents with 9 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P2.pdf | 6, 8, 10, 25, 35 |
| |
| WV15819 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P1.pdf | 6, 10, 12, 15 | 2 documents with 8 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/19991027_000/21087_Tamiflu_medr_P2.pdf | 2, 6, 6, 39 |
| |
| WV15825 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/20001117_002/21‐087SE1‐002_review.pdf | 41, 41, 41, 41, 42, 42, 42, 42, 42, 42, 43, 44, 58, 59, 71, 71, 71, 71, 71, 72, 72, 72, 72, 73, 73, 75, 75, 77, 77, 78, 79, 79, 79, 80, 80, 80, 81, 82, 85, 125, 125, 126, 126, 128, 131, 134, 134, 135, 135, 137, 137, 138, 145, 150, 151, 152, 152, 155, 156, 162, 169, 180, 204, 211 | 1 document with 64 instances |
| WV15871 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Admindocs_P1.pdf | 12.13 | 7 documents with 42 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Admindocs_P2.pdf | 39 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Medr.pdf | 5, 11, 30, 31, 31, 32, 32, 32, 33, 34, 37, 37, 37, 37, 37, 37, 40 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Microbr.pdf | 2, 5, 6 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Statr.pdf | 5, 11, 30, 31, 31, 32, 32, 32, 33, 34, 37, 37, 37, 37, 37, 37, 40 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021087/20040624_016/021087_S016_TAMIFLU CAPSULES ‐ DRY POWDER_ADMINCORRES.pdf | 6 |
| |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20040624_010/021087_S016_TAMIFLU CAPSULES ‐ DRY POWDER_BIOPHARMR.pdf | 2 |
| |
| WV15872 | Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Medr.pdf | 11.33 | 2 documents with 4 instances |
| Tamiflu and Relenza/Tamiflu/Tamiflu ‐ NDA 021246/20001214_000/21‐246_Tamiflu_Statr.pdf | 11.33 |
| |
| Oseltamivir trials citation by trial ID and source FDA file. Page numbers separated by commas (where applicable) indicate which trial is cited where in which regulatory file. Blank spaces indicate no citation for known trials. Search strategy: | |||
| Mentioned study | File name | Pages where study is mentioned (separated by commas) | Note |
| NAI106784 |
|
|
|
| 107485 |
|
|
|
| 108127 |
|
|
|
| 112311 |
|
|
|
| 112312 |
|
|
|
| 113268 |
|
|
|
| GCP/95/045 |
|
|
|
| NAI10901 |
|
|
|
| NAI10902 |
|
|
|
| NAI30008 | Relenza treatment submission executive summary.pdf | 4 | 3 documents with 10 instances |
| Relenza treatment submission full document.pdf | 5, 26, 26, 26, 146 |
| |
| Relenza treatment submission main text.pdf | 5, 26, 26, 26 |
| |
| NAI30009 | NAI30010 study report pdf\FINAL NAI30010 for sign‐off.pdf | 102 | 7 documents with 461 instances |
| NAI30009 study report pdf\CSR30009.pdf |
|
| |
| NAI30009 study report pdf\NAI 30009 HO final FSR.pdf |
|
| |
| NAI30009 study report pdf\suptables.pdf |
|
| |
| NAI30009 study report pdf\tables.pdf |
|
| |
| Relenza treatment submission full document.pdf | 16, 16, 17, 18, 18, 18, 18, 19, 27, 30, 31, |
| |
| Relenza treatment submission main text.pdf | 16, 16, 17, 18, 18, 18, 18, 19, 27, 30, 31, 76, 128, 130, 132, 134, 144 |
| |
| NAI30010 | NAI30010 study report\Final NAI30010 for sign‐off.pdf |
| 7 documents with 399 instances |
| NAI30010 study report pdf\NAI30010 HO final FSR.pdf |
|
| |
| NAI30010 study report pdf\suptables.pdf |
|
| |
| NAI30010 study report pdf\tables.pdf |
|
| |
| Relenza prophylaxis submission.pdf | 2, 5, 8, 11, 12, 19, 20, 21, 23, 24 |
| |
| Relenza treatment submission full document.pdf | 16, 16, 17, 18, 18, 18, 27, 30, 31, 76, 135, 137, 139, 141, 143, 144 |
| |
| Relenza treatment submission main text.pdf | 16, 16, 17, 18, 18, 18, 27, 30, 31 |
| |
| NAI30012 | Relenza treatment submission executive summary.pdf | 4 | 3 documents with 8 instances |
| NAI30012 | Relenza treatment submission full document.pdf | 5, 26, 26, 146 |
|
| NAI30012 | Relenza treatment submission main text.pdf | 5, 26, 26 |
|
| NAI30015 | Relenza treatment submission full document.pdf | 146 | 1 document with 1 instance |
| NAI30020 |
|
|
|
| NAI30028 |
|
|
|
| NAI30031 |
|
|
|
| NAI30034 |
|
|
|
| NAI40012 |
|
|
|
| NAIA1009
| NAI30010 study report pdf\FINAL NAI30010 for sign‐off.pdf | 101 | 2 documents with 3 instances |
| NAI30009 study report pdf\CSR30009.pdf | 28.34 |
| |
| NAIA3002
| NAI30010 study report pdf\FINAL NAI30010 for sign‐off.pdf | 102 | 9 documents with 513 instances |
| NAI30009 study report pdf\CSR30009.pdf | 34.95 |
| |
| NAI30009 study report pdf\NAI30009 HO final FSR.pdf | 22 |
| |
| NAIA3002 study report pdf\NAIA3002 full study report.pdf |
|
| |
| NAIA3002 study report pdf\NAIA3002 supporting tables 2.pdf |
|
| |
| NAIA3005 study report pdf\A3005cr01.pdf | 25 |
| |
| NAIB3002 study report pdf\NAIB3002 full study report.pdf | 28, 47, 49 |
| |
| Relenza treatment submission full document.pdf | 16, 16, 17, 17, 18, 19, 27, 30, 31, 63, 63, 63, 76, 106, 106, 107, 107, 109, 109, 112, 112, 114, 114, 115, 115, 144 |
| |
| Relenza treatment submission main text.pdf | 16, 16, 17, 17, 18, 19, 27, 30, 31 |
| |
| NAIA3003 | Relenza prophylaxis submission.pdf | 10 | 1 document with 1 instance |
| NAIA3004 | Relenza prophylaxis submission.pdf | 10 | 1 document with 1 instance |
| NAIA3005 | NAI30010 study report pdf\FINAL NAI30010 for sign‐off.pdf | 36, 94, 94, 94, 95, 96, 96, 101 | 5 documents with 310 instances |
| NAI30010 study report pdf\NAI30010 HO FSR.pdf | 6.18 |
| |
| NAIA3005 study report pdf\A3005cr01.pdf |
|
| |
| NAIA3005 study report pdf\TABS.pdf |
|
| |
| Relenza prophylaxis submission.pdf | 2, 5, 6, 12, 13, 13, 15, 15, 16, 16, 17, 17, 18, 18 |
| |
| NAIB1002 |
|
|
|
| NAIB3001 | NAI30009 study report pdf\CSR30009.pdf | 34, 50, 95 | 11 documents with 374 instances |
| NAI30009 study report pdf\NAI 30009 HO final FSR.pdf | 10.22 |
| |
| NAI30010 study report pdf\FINAL NAI30010 for sign‐off.pdf | 102 |
| |
| NAI30010 study report pdf \NAI30010 HO FSR.pdf | 17.17 |
| |
| NAIA3002 study report pdf\NAIA3002 full study report.pdf | 28 |
| |
| NAIA3005 study report pdf\A3005cr01.pdf | 25 |
| |
| NAIB3001 study report pdf\NAIB3001 full study report.pdf |
|
| |
| NAIB3001 study report pdf\NAIB3001 supporting tables 1.pdf |
|
| |
| NAIB3002 study report pdf\NAIB3002 full study report.pdf | 28 |
| |
| Relenza treatment submission full document.pdf | 16, 16, 17, 18, 18, 18, 18, 27, 30, 31, 32, 63, 63, 63, 76, 99, 99, 101, 101, 103, 103, 105, 105, 144, 162 |
| |
| Relenza treatment submission main text.pdf | 16, 16, 17, 18, 18, 18, 18, 27, 30, 31, 32 |
| |
| NAIB3002 | NAI30009 study report pdf\CSR30009.pdf | 34.95 | 10 documents with 579 instances |
| NAI30009 study report pdf\NAI 30009 HO final FSR.pdf | 22 |
| |
| NAI30010 study report pdf\FINAL NAI30010 for sign‐off.pdf | 102 |
| |
| NAIA3002 study report pdf\NAIA3002 full study report.pdf | 28, 48, 50 |
| |
| NAIA3005 study report pdf\A3005cr01.pdf | 25 |
| |
| NAIB3002 study report pdf\NAIB3002 full study report.pdf |
|
| |
| NAIB3002 study report pdf\NAIB3002supporting tables 1.pdf |
|
| |
| NAIB3002 study report pdf\NAIB3002supporting tables 2.pdf |
|
| |
| Relenza treatment submission full document.pdf | 16, 16, 17, 17, 18, 19, 27, 30, 31, 63, 63, 63, 76, 117, 117, 117, 118, 118, 120, 120, 122, 122, 124, 124, 125, 125, 127, 127, 144 |
| |
| Relenza treatment submission main text.pdf | 16, 16, 17, 17, 18, 19, 27, 30, 31 |
| |
| NAI30011 | Relenza treatment submission full document.pdf | 146 | 1 document with 1 instance |
| NAIB2007 | NAI30009 study report pdf\CSR30009.pdf | 95 | 10 documents with 379 instances |
| NAI30009 study report pdf\NAI 30009 HO final FSR.pdf | 10 |
| |
| NAIA3002 study report pdf\NAIA3002 full study report.pdf | 28, 28, 29 |
| |
| NAIA3005 study report pdf\A3005cr01.pdf | 25 |
| |
| NAIB2007 study report pdf\b2007cr.pdf |
|
| |
| NAIB2007 study report pdf\TABLES.pdf |
|
| |
| NAIB3001 study report pdf\NAIB3001 full study report.pdf | 25.26 |
| |
| NAIB3002 study report pdf\NAIB3002 full study report.pdf | 28, 28, 29 |
| |
| Relenza treatment submission full document.pdf | 16, 16, 17, 18, 18, 19, 27, 30, 31, 76, 91, 91, 92, 92, 94, 94, 96, 96, 98, 98, 144 |
| |
| Relenza treatment submission main text.pdf | 16, 16, 17, 18, 18, 19, 27, 30, 31 |
| |
| NAIA2006 | NAIA2005 study report pdf\a2005cr.pdf | 38, 73, 74 | 4 documents with 6 instances |
| NAIA3002 study report pdf\NAIA3002 full study report.pdf | 28 |
| |
| NAIA3005 study report pdf\A3005cr01.pdf | 25 |
| |
| NAIB3002 study report pdf\NAIB3002 full study report.pdf | 28 |
| |
| NAIB2006 | NAIA3002 study report pdf\NAIA3002 full study report.pdf | 28 | 3 documents with 3 instances |
| NAIA3005 study report pdf\A3005cr01.pdf | 25 |
| |
| NAIB3002 study report pdf\NAIB3002 full study report.pdf | 28 |
| |
| NAIB1007 |
|
|
|
| C94‐009 |
|
|
|
| C94‐085 |
|
|
|
| NAIB1001 |
|
|
|
| NAIB_1001 |
|
|
|
| NAIA2005
| NAI30009 study report pdf\CSR30009.pdf | 95 | 12 documents with 895 instances |
| NAIA2005 study report pdf\a2005cr.pdf |
|
| |
| NAIA2005 study report pdf\APPS_ALL.pdf |
|
| |
| NAIA2005 study report pdf\TBS_ALL.pdf |
|
| |
| NAIA3002 study report pdf\NAIA3002 full study report.pdf | 28, 28, 48, 48 |
| |
| NAIA3005 study report pdf\A3005cr01.pdf | 25 |
| |
| NAIB2005 study report pdf\b2005cr.pdf | 7, 7, 22, 25, 26, 34, 34, 42, 71, 72, 72 |
| |
| NAIB2007 study report pdf\b2007cr.pdf | 76 |
| |
| NAIB3001 study report pdf\NAIB3001 full study report.pdf | 25 |
| |
| NAIB3002 study report pdf\NAIB3002 full study report.pdf | 28, 28, 47, 47 |
| |
| Relenza treatment submission full document.pdf | 16, 16, 16, 16, 17, 18, 27, 30, 76, 77, 77, 77, 79, 79, 79, 80, 80, 82, 82, 84, 84, 85, 144, 144 |
| |
| Relenza treatment submission main text.pdf | 16, 16, 16, 16, 17, 18, 27, 30 |
| |
| NAIB2005
| NAI30009 study report pdf\CSR30009.pdf | 95 | 12 documents with 838 instances |
| NAIA2005 study report pdf\a2005cr.pdf | 7, 8, 8, 24, 24, 25, 43, 70, 74, 74 |
| |
| NAIA3002 study report pdf\NAIA3002 full study report.pdf | 28, 28, 48, 48 |
| |
| NAIA3005 study report pdf\A3005cr01.pdf | 25 |
| |
| NAIB2005 study report pdf\APPSNEW.pdf |
|
| |
| NAIB2005 study report pdf\b2005cr.pdf |
|
| |
| NAIB2005 study report pdf\TBS_ALL.pdf |
|
| |
| NAIB2007 study report pdf\b2007cr.pdf | 76 |
| |
| NAIB3001 study report pdf\NAIB3001 full study report.pdf | 25 |
| |
| NAIB3002 study report pdf\NAIB3002 full study report.pdf | 28, 28, 47, 47 |
| |
| Relenza treatment submission full document.pdf | 16, 16, 16, 16, 17, 18, 27, 30, 76, 77, 79, 79, 85, 85, 85, 86, 86, 88, 88, 90, 90, 144, 144 |
| |
| Relenza treatment submission main text.pdf | 16, 16, 16, 16, 17, 18, 27, 30 |
| |
| NAIA/B2008
| NAI30009 study report pdf\CSR30009.pdf | 95 | 6 documents with 16 instances |
| NAI30009 study report pdf\NAI 30009 HO final FSR.pdf | 10 |
| |
| NAIA3002 study report pdf\NAIA3002 full study report.pdf | 28, 28, 29, 29 |
| |
| NAIA3005 study report pdf\A3005cr01.pdf | 25 |
| |
| NAIB3001 study report pdf\NAIB3001 full study report.pdf | 25, 26, 26, 26, 77 |
| |
| NAIB3002 study report pdf\NAIB3002 full study report.pdf | 28, 28, 29, 29 |
| |
| NAIA2010 | NAIA3005 study report pdf\A3005cr01.pdf | 25 | 1 document with 1 instance |
| NAIA/B2009 | NAIA3002 study report pdf\NAIA3002 full study report.pdf | 28 | 3 documents with 3 instances |
| NAIA3005 study report pdf\A3005cr01.pdf | 25 |
| |
| NAIB3002 study report pdf\NAIB3002 full study report.pdf | 28 |
| |
| 167‐02 |
|
|
|
| 167‐03 |
|
|
|
| 167‐05 |
|
|
|
| 167‐04 |
|
|
|
| JNAI‐03 |
|
|
|
| JNAI‐02 |
|
|
|
| JNAI‐01 |
|
|
|
| JNAI‐07 |
|
|
|
| JNAI‐04 |
|
|
|
| PE‐01 |
|
|
|
| 167‐101 |
|
|
|
| 167T3‐11 |
|
|
|
| Zanamivir trials citation by trial ID and source NICE file. Page numbers separated by commas (where applicable) indicate which trial is cited where in which file. Blank spaces indicate no citation for known trials | |||
| Referenced study | File name volume* | Pages where study is mentioned (separated by commas) | Note |
| 133312 |
|
|
|
| GS97‐802 |
|
|
|
| 133312 |
|
|
|
| GS‐97‐801 |
|
|
|
| JP15734 |
|
|
|
| JP15735 |
|
|
|
| JV15823 |
|
|
|
| JV15824 |
|
|
|
| JV16284 |
|
|
|
| M76001 | 1 | 33, 36, 37, 37, 38, 38, 39, 67, 68, 94, 95, 224 | 1 document with 12 instances |
| M76006 |
|
|
|
| ML20910 |
|
|
|
| ML22789 |
|
|
|
| ML22879 |
|
|
|
| MV21118 |
|
|
|
| MV22841 |
|
|
|
| NCT00298233 |
|
|
|
| NCT00555893 |
|
|
|
| NCT00707941 |
|
|
|
| NCT00799760 |
|
|
|
| NCT00830323 |
|
|
|
| ML25018 |
|
|
|
| NCT00867139 |
|
|
|
| NCT00873886 |
|
|
|
| NCT01002729 |
|
|
|
| NP15717 | 6 | 32, 75, 76, 77 | 2 documents with 5 instances |
| 8 | 68 |
| |
| 6 | 73.98 | 1 document with 2 instances | |
| NP15718 |
|
|
|
| NP15728 |
|
|
|
| NP15757 | 8 | 68 | 1 document with 1 instance |
| NP15826 | 6 | 32, 75, 75, 75, 76, 76, 77, 78, 79, 80, 98 | 1 document with 11 instances |
| NP15827 | 8 | 68 | 1 document with 1 instance |
| NP22770 |
|
|
|
| NP25138 |
|
|
|
| NP25139 |
|
|
|
| NV16871 |
|
|
|
| NV20234 |
|
|
|
| NV20235 |
|
|
|
| NV20236 |
|
|
|
| NV20237 |
|
|
|
| NV22155 |
|
|
|
| NV25118 |
|
|
|
| NV25182 |
|
|
|
| PP16351 |
|
|
|
| WP15517 | 1 | 185.245 | 1 document with 2 instances |
| WP15525 | 1 | 185.245 | 1 document with 2 instances |
| WP15647 |
|
|
|
| WP15648 |
|
|
|
| WP15676 |
|
|
|
| WP15901 |
|
|
|
| WP22849 |
|
|
|
| WV144181 |
|
|
|
| WV15670 | 1 | 33, 36, 37, 37, 38, 38, 39, 47, 48, 48, 49, 49, 50, 53, 54, 54, 55, 163, 171, 188, 207, 209, 224, 245, 245, 252, 253, 253 | 7 documents with 1193 instances |
| 10 | 7, 36, 37, 37 |
| |
| 2 |
|
| |
| 3 |
|
| |
| 4 | 90 |
| |
| 6 | 35.98 |
| |
| 8 | 65 |
| |
| 2 | 20, 20, 20, 20, 20 | 1 document with 5 instances | |
| WV15671
| 1 | 33, 36, 37, 37, 38, 38, 39, 47, 48, 49, 49, 50, 53, 54, 54, 55, 163, 171, 188, 207, 209, 224, 245, 245 | 7 documents with 1222 instances |
| 10 | 7, 36, 37, 37 |
| |
| 2 | 82 |
| |
| 4 |
|
| |
| 5 |
|
| |
| 6 | 35.98 |
| |
| 8 | 66 |
| |
| WV15673 | 8 | 66 | 1 document with 1 instance |
| WV15673D | 8 | 66 | 1 document with 1 instance |
| WV15697 | 8 |
| 1 document with 1 instance |
| WV15697D | 8 |
| 1 document with 1 instance |
| WV15707 | 1 | 33, 36, 37, 37, 38, 67, 68, 224, 245, 245, 245, 246 | 1 document with 12 instances |
| WV15708 |
|
|
|
| WV15708D |
|
|
|
| WV15730 | 1 | 33, 36, 37, 37, 38, 38, 39, 47, 53, 54, 55, 186, 207, 224, 245, 245, 246 | 4 documents with 22 instances |
| 10 | 7, 36, 37 |
| |
| 2 | 82 |
| |
| 4 | 90 |
| |
| WV15731 | 6 | 98 | 1 document with 1 instance |
| WV15758 | 1 | 36, 37, 82, 83, 84, 85, 86, 92, 94, 95, 97, 106, 224, 246 | 4 documents with 424 instances |
| 6 |
|
| |
| 7 |
|
| |
| 8 | 68 |
| |
| WV15759 | 1 | 36, 37, 94, 95, 95, 109, 113, 114, 121, 122, 224, 246 | 1 document with 12 instances |
| WV15799 | 1 | 137, 139, 139, 232, 233 | 3 documents with 499 instances |
| 8 |
|
| |
| 9 |
|
| |
| WV15812 | 1 | 36, 37, 37, 38, 38, 39, 67, 68, 68, 107, 107, 107, 108, 108, 121, 121, 122, 123, 224, 246 | 2 documents with 197 instances |
| 10 |
|
| |
| WV15819 | 1 | 33, 36, 37, 37, 38, 58, 58, 59, 59, 60, 61, 62, 62, 65, 65, 67, 68, 224, 246 | 2 documents with 173 instances |
| 10 |
|
| |
| WV15825 | 8 | 66, 66 | 1 document with 2 instances |
| WV15871 | 1 | 109, 246 | 1 document with 2 instances |
| WV15872 | 1 | 36, 37, 37, 38, 38, 39, 67, 68, 68, 107, 107, 108, 108, 121, 121, 122, 123, 224 | 1 document with 18 instances |
| WV15876 | 1 | 246, 246 | 1 document with 2 instances |
| WV15978 | 1 | 67, 70, 175, 246, 246 | 1 document with 5 instances |
| WV16193 |
|
|
|
| ML16369 |
|
|
|
| Oseltamivir trials citation by trial ID and source NICE file. Page numbers separated by commas (where applicable) indicate which trial is cited where in which file. Blank spaces indicate no citation for known trials. All the studies have been searched in the folder "Roche submission". When there is the number of the volume but no pages are mentioned, it means that the code of the study is cited more than 100 times. *Number of the volume of the Tamiflu NICE submission. | |||
| Study | Sample size | Median days to alleviation for all participants | Difference in days (P value) | Median days to alleviation and no use of relief medication | Difference in days (P value) | |||
| Zanamivir (n) | Placebo (n) | Zanamivir | Placebo | Zanamivir | Placebo | |||
| NAI30008 | 262 | 263 | 6.0 | 7.0 | 1.0 (0.123) | 8.0 | 10.0 | 2.0 (0.037) |
| NAI30009 | 224 | 247 | 4.5 | 5.0 | 0.5 (0.011) | 5.0 | 6.0 | 1.0 (0.002) |
| NAI30010 | 76 | 81 | 4.5 | 5.5 | 1.0 (0.033) | 5.5 | 6.75 | 1.25 (0.150) |
| NAI30011 | 237 | 229 | 4.50 | 5.00 | 0.50 (0.495) | 7.0 | 7.0 | 0.0 (0.623) |
| NAI30012 | 191 | 167 | 6.5 | 7.5 | 1.0 (0.159) | 9.0 | 10.0 | 1.0 (0.131) |
| NAI30015 | 293 | 295 | 2.17 | 2.67 | 0.5 (0.166) | 3.17 | 3.83 | 0.66 (0.058) |
| NAIA3002 | 412 | 365 | 5.5 | 6.0 | 0.5 (0.228) | 7.0 | 8.0 | 1.0 (0.054) |
| NAIB3002 | 174 | 182 | 5.0 | 7.5 | 2.5 (< 0.001) | 5.5 | 8.25 | 2.75 (< 0.001) |
| *Alleviation defined as no fever (temperature < 37.8 °C), cough recorded as none or mild and muscle/joint aches and pains, sore throat, feverishness/chills and headache recorded as absent/minimal | ||||||||
| Oseltamivir versus placebo for treating influenza in healthy adults | ||||||
| Patient or population: healthy adults with influenza | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Risk difference (95% CI) | NNTB or NNTH (95% CI) | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Oseltamivir versus placebo for treatment | |||||
| Time to first alleviation of symptoms in adult treatment (ITT population) (hours) | The mean time (hours) to first alleviation of symptoms adults in the intervention groups was | 16.8 hours (8.4 to 25.1) | 3954 | N/A | N/A | |
| Adverse events: nausea in adult treatment (on‐treatment) | Study population | RR 1.57 | 4452 | ‐3.66% (‐7.39 to ‐0.9) | NNTH = 28 (14 to 112) | |
| 64 per 1000 | 101 per 1000 | |||||
| Adverse events: vomiting in adult treatment (on‐treatment) | Study population | RR 2.43 | 4452 | ‐4.56% (‐7.58 to ‐2.39) | NNTH = 22 (14 to 42) | |
| 32 per 1000 | 77 per 1000 | |||||
| Adverse events: diarrhoea in adult treatment (on‐treatment) | Study population | RR 0.67 | 4452 | 2.33% (0.14 to 3.81) | NNTB = 43 (27 to 709) | |
| 71 per 1000 | 47 per 1000 | |||||
| Complications: self reported, investigator‐mediated, unverified pneumonia in adult treatment | Study population | RR 0.55 | 4452 | 1.00% (0.22 to 1.49) | NNTB = 100 (67 to 451) | |
| 22 per 1000 | 12 per 1000 (7 to 20) | |||||
| Adverse events: cardiac body system in adult treatment (on‐treatment) | Study population | RR 0.49 | 3943 | 0.68% (0.04 to 1.00) | NNTB = 148 (101 to 2509) | |
| 13 per 1000 | 7 per 1000 | |||||
| Adverse events: hospital admission in adult treatment | Study population | RR 0.92 (0.57 to 1.50) | 4394 (7) | 0.15% (‐0.78 to 0.91) | NNTB = 687 (NNTB 110 to ∞ to NNTH 128) | |
| 18 per 1000 | 17 per 1000 (11 to 28) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Negative risk differences indicate harms; positive risk differences indicate benefits. | ||||||
| Oseltamivir versus placebo for treating influenza in healthy children | ||||||
| Patient or population: healthy children with influenza | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Risk difference (95% CI) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Oseltamivir versus placebo for treatment | |||||
| Time to first alleviation of symptoms in child treatment (hours) | The mean time (hours) to first alleviation of symptoms in children in the intervention groups was | 1329 | Not significant | |||
| Hospital admission in child treatment (safety population) | Study population | RR 1.92 | 1359 | ‐0.81% (‐3.72 to 0.26) | NNTH = 124 (NNTB 379 to ∞ to NNTH 27) | |
| 9 per 1000 | 17 per 1000 | |||||
| Complications: bronchitis in child treatment | Study population | RR 0.65 | 1359 | 1.08% (‐1.69 to 2.25) | NNTB = 93 (NNTB 45 to ∞ to NNTH 59) | |
| 31 per 1000 | 20 per 1000 | |||||
| Complications: otitis media in child treatment | Study population | RR 0.8 | 1359 | 3.26% (‐0.33 to 6.18) | NNTB = 31 (NNTB 17 to ∞ to NNTH 308) | |
| 163 per 1000 | 130 per 1000 | |||||
| Complications: pneumonia in child treatment | Study population | RR 1.06 | 1359 | ‐0.22% (‐3.07 to 1.41) | NNTH = 450 (NNTB 71 to ∞ to NNTH 33) | |
| 37 per 1000 | 39 per 1000 | |||||
| Adverse events: diarrhoea in child treatment (on‐treatment) | Study population | RR 0.87 | 1358 | 0.93% (‐2.01 to 3.02) | NNTB 108 (NNTB 34 to ∞ to NNTH 50) | |
| 72 per 1000 | 63 per 1000 | |||||
| Adverse events: vomiting in child treatment (on‐treatment) | Study population | RR 1.7 | 1358 | 5.34% (1.75 to 10.29) | NNTH = 19 (10 to 57) | |
| 76 per 1000 | 130 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Negative risk differences indicate harm; positive risk differences indicate benefits. | ||||||
| Zanamivir versus placebo for treating influenza in healthy adults | ||||||
| Patient or population: healthy adults with influenza | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Risk difference (95% CI) | NNTB or NNTH (95% CI) | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Zanamivir versus placebo for treatment | |||||
| Time to first alleviation of symptoms in adult treatment (days) | The mean time (days) to first alleviation of symptoms in adults in the intervention groups was | 0.60 days (0.39 to 0.81) | 5411 | N/A | N/A | |
| Complications: pneumonia confirmed with X‐ray in adult treatment | Study population | RR 1.02 | 946 | ‐0.06% (‐6.56 to 2.11) | NNTH = 1540 (NNTB 48 to ∞ to NNTH 16) | |
| 32 per 1000 | 33 per 1000 | |||||
| Adverse events: nausea/vomiting in adult treatment (on‐treatment) | Study population | RR 0.6 | 6553 | 1.63% (0.24 to 2.48) | NNTB = 62 (41 to 411) | |
| 41 per 1000 | 24 per 1000 | |||||
| Adverse events: psychiatric body system in adult treatment (on‐treatment) | Study population | RR 1.16 | 4732 | ‐0.09% (‐0.76 to 0.24) | NNTH 1132 (NNTB 421 to ∞ to NNTH 132) | |
| 6 per 1000 | 6 per 1000 | |||||
| Complications: bronchitis in adult treatment | Study population | RR 0.75 (0.61 to 0.91) | 6072 (12) | 1.80% (0.65 to 2.80) | NNTB 56 (36 to 155) | |
| 72 per 1000 | 54 per 1000 (44 to 65) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Negative risk differences indicate harms; positive risk differences indicate benefits. | ||||||
| Zanamivir versus placebo for treating influenza in healthy children | ||||||
| Patient or population: healthy children with influenza | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Risk difference (95% CI) | NNTB or NNTH (95% CI) | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Zanamivir versus placebo for treatment | |||||
| Time to first alleviation of symptoms in children (days) | The mean time (days) to first alleviation of symptoms in children in the intervention groups was | 723 | NA | NA | ||
| Complications: sinusitis in child treatment | Study population | RR 0.87 | 737 | 0.19% (‐8.09 to 1.31) | NNTB = 519 (NNTB 13 to ∞ to NNTH 77) | |
| 15 per 1000 | 13 per 1000 | |||||
| Complications: otitis media in child treatment | Study population | RR 1.0 | 737 | 0.00% (‐5.13 to 2.92) | NNTB = > 1000 (NNTB 35 to ∞ to NNTH 20) | |
| 71 per 1000 | 71 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Negative risk differences indicate harms; positive risk differences indicate benefits. | ||||||
| Oseltamivir versus placebo for preventing influenza in healthy children | |||||
| Patient or population: healthy children without influenza | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Comments | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Oseltamivir versus placebo for treatment | ||||
| No data | — | — | — | — | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| Zanamivir versus placebo for preventing influenza in healthy adults | ||||||
| Patient or population: healthy adults without influenza | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Risk difference (95% CI) | NNTB or NNTH (95% CI) | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Zanamivir versus placebo for prophylaxis | |||||
| Symptomatic influenza in prophylaxis of individuals | Study population | RR 0.39 (0.22 to 0.70) | 5275 (4) | 1.98% (0.98 to 2.54) | NNTB = 51 (40 to 103) | |
| 33 per 1000 | 13 per 1000 | |||||
| Asymptomatic influenza in prophylaxis of individuals | Study population | RR 0.97 | 5275 | 0.14% (‐1.1 to 1.1) | NNTB = 729 (NNTB 91 to ∞ to NNTH 91) | |
| 50 per 1000 | 48 per 1000 | |||||
| Symptomatic influenza in household prophylaxis | Study population | RR 0.22 | 824 | 14.84% (12.18 to 16.55) | NNTB = 7 (6 to 9) | |
| 190 per 1000 | 42 per 1000 | |||||
| Asymptomatic influenza in household prophylaxis | Study population | RR 0.90 | 824 | 1.32% (‐2.2 to 3.84) | NNTB = 76 (NNTB 26 to ∞ to NNTH 46) | |
| 107 per 1000 | 97 per 1000 | |||||
| Complications: pneumonia in adult prophylaxis | Study population | RR 0.30 | 7662 | 0.32% (0.09 to 0.41) | NNTB = 311 (244 to 1086) | |
| 5 per 1000 | 1.5 per 1000 | |||||
| Complications: bronchitis in adult prophylaxis | Study population | RR 0.49 (0.02 to 1.19) | 7662 (6) | 0.79% (‐0.29 to 1.24) | NNTB = 127 (to NNTB 81 to ∞ to NNTH 341) | |
| 15 per 1000 | 8 per 1000 (3 to 18) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Negative risk differences indicate harms; positive risk differences indicate benefits. | ||||||
| Oseltamivir versus placebo for preventing influenza in healthy adults | ||||||
| Patient or population: healthy adults without influenza | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Risk difference (95% CI) | NNTB or NNTH (95% CI) | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Oseltamivir versus placebo for prophylaxis | |||||
| Symptomatic influenza in adult prophylaxis of individuals | Study population | RR 0.45 | 2479 | 3.05% (1.83 to 3.88) | NNTB = 33 (26 to 55) | |
| 55 per 1000 | 25 per 1000 | |||||
| Symptomatic influenza in household prophylaxis | Study population | RR 0.2 | 405 | 13.6% (9.52 to 15.47) | NNTB = 7 (6 to 11) | |
| 170 per 1000 | 34 per 1000 | |||||
| Adverse events: psychiatric body systems in adult prophylaxis (all events on‐ and off‐treatment) | Study population | RR 1.80 | 3434 | ‐1.06% (‐2.76 to ‐0.07) | NNTH = 94 (36 to 1538) | |
| 13 per 1000 | 23 per 1000 | |||||
| Adverse events: headache in adult prophylaxis (on‐treatment) | Study population | RR 1.18 | 3434 | ‐3.15% (‐5.78 to ‐0.88) | NNTH = 32 (18 to 115) | |
| 175 per 1000 | 207 per 1000 | |||||
| Adverse events: nausea in adult prophylaxis (on‐treatment) | Study population | RR 1.96 | 3434 | ‐4.15% (‐9.51 to ‐0.86) | NNTH = 25 (11 to 116) | |
| 43 per 1000 | 85 per 1000 | |||||
| Adverse events: vomiting in adult prophylaxis (on‐treatment) | Study population | RR 1.91 | 3434 | ‐0.95% (‐4.41 to 0.31) | NNTH = 106 (NNTB 319 to ∞ to NNTH 23) | |
| 10 per 1000 | 20 per 1000 | |||||
| Adverse events: headache in adult prophylaxis (off‐treatment) | Study population | RR 0.88 | 3434 | 0.44% (‐0.89 to 1.37) | NNTB = 226 (NNTB 74 to ∞ to NNTH 113) | |
| 37 per 1000 | 33 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Negative risk differences indicate harms; positive risk differences indicate benefits. | ||||||
| Zanamivir versus placebo for preventing influenza in healthy children | |||||
| Patient or population: healthy children without influenza | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Comments | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Zanamivir versus placebo for treatment | ||||
| No data | — | — | — | — | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| Oseltamivir | Placebo | Total | ||||
| Event type | # Events | % | # Events | % | # Events | % |
| Confusion | 5 | 0.25 | 1 | 0.07 | 6 | 0.17 |
| Depression | 14 | 0.7 | 6 | 0.42 | 20 | 0.58 |
| Hallucinations | 2 | 0.1 | 0 | 0.00 | 2 | 0.06 |
| Anxiety | 7 | 0.35 | 8 | 0.56 | 15 | 0.44 |
| Psychosis | 2 | 0.1 | 1 | 0.07 | 3 | 0.09 |
| Schizophrenia | 1 | 0.05 | 0 | 0.00 | 1 | 0.03 |
| Bipolar disorder | 0 | 0 | 1 | 0.07 | 1 | 0.03 |
| Sleeping disorder | 2 | 0.1 | 0 | 0.00 | 2 | 0.06 |
| Aggression | 1 | 0.05 | 0 | 0.00 | 1 | 0.03 |
| Stress symptoms | 3 | 0.15 | 0 | 0.00 | 3 | 0.09 |
| Restlessness | 1 | 0.05 | 0 | 0.00 | 1 | 0.03 |
| Nervousness | 1 | 0.05 | 0 | 0.00 | 1 | 0.03 |
| Suicide ideation | 1 | 0.05 | 0 | 0.00 | 1 | 0.03 |
| Paranoia | 1 | 0.05 | 0 | 0.00 | 1 | 0.03 |
| Alcohol related | 6 | 0.3 | 2 | 0.14 | 8 | 0.23 |
| Total | 47 | 2.35 | 19 | 1.32 | 66 | 1.92 |
| Of the 66 events, 12 were classified as severe intensity (10 oseltamivir, 2 placebo) | ||||||
| Positive serology | Group | Total | |
| Placebo N % | Tamiflu N % | ||
| No | 166 | 192 | 358 |
| Yes | 34 | 13 | 47 |
| Total | 200 | 205 | 405 |
| ITTIINAB population: ITT influenza‐infected index cases who had negative virology at baseline Chi² P = 0.001 | |||
| Trial ID | Description oseltamivir/batch no. | Description placebo/batch no. | Certified content (oseltamivir) | Certified content (placebo) | Ref (PDF page number) |
| M76001 | Size 2 capsules containing 75 mg oseltamivir/V01‐00 (GS 4104), batch number GMZ 0082 | Size 2 placebo capsules for oseltamivir/V02‐00 (GS 4104), batch number GMZ 0083 | Unknown (certificate of analysis not provided) | Unknown (certificate of analysis not provided) | 20 |
| WP16263 | Grey opaque body, light yellow opaque cap/PT2247C01 | Grey opaque body, ivory opaque cap/GMZ 0163
| Oseltamivir 97.5 mg | Dehydrocholic acid | 19 and 422 |
| WV15670 | Size 2 capsules containing 75 mg Ro 64‐0796/V01‐00 (GS 4104), batch number GMZ 0067; caramel opaque body, caramel opaque cap | Size 2 placebo capsules for Ro 64‐0796/V02‐00 (GS 4104), batch number GMZ 0066; caramel opaque body, caramel opaque cap | Oseltamivir 94.3 mg | Dehydrocholic acid 6.13 mg | 13, 834‐5 |
| WV15671 | Capsules (size 2) containing 75 mg Ro 64‐0796 (GS 4104)/V01; batch number GMZ 0067/GMZ 0065. Capsules are caramel opaque body, caramel opaque cap. Also used batch GMZ 0067 capsules caramel opaque body, caramel opaque cap (for oseltamivir 94.3 mg) | Matching placebo capsules (size 2) for Ro 64‐0796 (GS 4104)/V02; batch number GMZ 0066. Capsules are caramel opaque body, caramel opaque cap | Oseltamivir 93.1 mg and oseltamivir 94.3 mg | Dehydrocholic acid 6.13 mg | 13, 764‐7 |
| WV15673/WV15697 | GS 4104 (Ro 64‐0796) provided as size 2 caramel‐coloured capsules containing 75 mg of active drug and packaging material consisting of dehydrocholic acid, dibasic calcium phosphate dihydrate, pregelatinised starch, povidone, talc and sodium stearyl fumarate." Ro 64‐0796/V01‐00 batch GMZ 0067; caramel opaque body, caramel opaque cap | Placebo provided as size 2 caramel‐coloured capsules, containing dehydrocholic acid, dibasic calcium phosphate dihydrate, pregelatinised starch, povidone, talc and sodium stearyl fumarate Ro64‐0796/V02‐00 batch GMZ 0066; caramel opaque body, caramel opaque cap | 94.3 mg | Dehydrocholic acid 6.13 mg | 385, 540‐2 |
| WV15707 | Ro 64‐0796 was provided as a size 2 capsule containing 75 mg of active drug and packaging material consisting of pregelatinised starch, povidone, talc and sodium stearyl fumarate. Ro 64‐0796 (GS4104)/V01‐00 batch number GMZ 0082 | Placebo was provided as a size 2 capsule containing dehydrocholic acid, dibasic calcium phosphate dihydrate and packaging material consisting of pregelatinised starch, povidone, talc and sodium stearyl fumarate. Placebo Ro 64‐0796/V02‐00 batch number GMZ 0066 | Ro 64‐0796/002 100.5 mg | Dehydrocholic acid | 3, 517‐9 |
| WV15708 | Size 2 capsules of 75 mg; Ro 64‐0796/V01‐00 batch no. GMZ 0082; caramel opaque body, caramel opaque cap | Matching size 2 placebo capsules Ro 64‐0796/V02‐00 batch no. GMZ 0083; caramel opaque body, caramel opaque cap | Oseltamivir 100.5 mg | Dehydrocholic acid | 21‐2, 517‐9 |
| WV15730 | Ro 64‐0796 was provided as a caramel, opaque, size 2 capsule containing 75 mg of active drug and packaging material consisting of pregelatinised starch, povidone, talc and sodium stearyl fumarate. Ro 64‐0796 (GS4104)/V01‐00 batch number GMZ 0082 | Placebo was provided as a caramel, opaque, size 2 capsule containing dehydrocholic acid, dibasic (calcium phosphate dihydrate and packaging material consisting of pregelatinised starch, povidone, talc and sodium stearyl fumarate. Placebo Ro 64‐0796/V02‐00 batch number GMZ 0083 | Oseltamivir 100.5 mg | Dehydrocholic acid | 24, 504‐5 |
| WV15758 | 2 batches of the paediatric formulation were used in the present study: 1. Ro 64‐0796/V20‐01 (0.6% syrup); batch no. G HK 0180/05 2. Ro 64‐0796/V20‐01 (0.6% syrup); batch no. G HK 0180/06 | 2 batches of the corresponding placebo formulation were used: 1. Ro 64‐0796/V19‐01; batch no. G HK 0179/04 2. Ro 64‐0796/V19‐01; batch no. G HK 0179/05 | Oseltamivir 0.768 g (G HK 0180/05), 0.763 g (G HK 0180/06) | Dehydrocholic acid | 27, 1043‐5 |
| WV15759/15871 | Ro 64‐0796 was to be provided as a dry powder for reconstitution with water. The powdered formulation contains the active ingredient, sorbitol and saccharin sodium (sweeteners), betacarotene (colouring agent), permageal 31 tutti frutti (flavour), cellulose, xanthan gum and methylhydroxy/propylhydroxybenzoate | Unknown (certificates of analysis not in our possession) | Unknown (certificates of analysis not in our possession) | 36 | |
| WV15799 | Ro 64‐0796 was provided as ivory, opaque, size 2 capsule containing 75 mg of active drug and packaging material consisting of pregelatinised starch, povidone, talc and sodium stearyl fumarate. Ro 64‐0796 (GS4104)/V14‐00 batch numbers GMZ 0124/03 and GMZ 0129/03 | Placebo was provided as an ivory, opaque, size 2 capsule containing dehydrocholic acid, dibasic calcium phosphate dihydrate and packaging material consisting of pregelatinised starch, povidone, talc and sodium stearyl fumarate. Placebo Ro 64‐0796/V16‐00 batch number GMZ 0136 | Unknown (certificates of analysis not in our possession) | Unknown (certificates of analysis not in our possession) | 24 |
| WV15812/WV15872 | Ro 64‐0976 was provided as size 2 capsules containing 75 mg of active drug and packaging material consisting of pregelatinised starch, povidone, talc and sodium stearyl fumarate | Matching placebo was provided as size 2 capsules containing dehydrocholic acid, dibasic calcium phosphate dihydrate, pregelatinised starch, povidone, talc, sodium stearyl fumarate | Unknown (certificate of analysis not provided) | Unknown (certificate of analysis not provided) | 18 |
| WV15876/WV15819/WV15978 | Capsules (size 2) containing 95.8 mg oseltamivir phosphate, equivalent to 75 mg oseltamivir: formulation V14; batch numbers GMZ 0124/03, GMZ 0129/03. Both batches: grey opaque body, ivory opaque cap The following statement appears after the description of the placebo; whether it applies to oseltamivir capsules is unclear: "Excipients for each capsule consisted of dehydrocholic acid, dibasic calcium diphosphate dihydrate, pregelatinized starch, povidone, talc, sodium stearyl fumarate." | Matching placebo capsules (size 2) for oseltamivir: formulation V16; batch numbers GMZ 0136, GMZ 0163. Both batches: grey opaque body, ivory opaque cap The following statement appears after the description of the placebo; whether it applies to oseltamivir capsules is unclear: "Excipients for each capsule consisted of dehydrocholic acid, dibasic calcium diphosphate dihydrate, pregelatinized starch, povidone, talc, sodium stearyl fumarate." | Oseltamivir 95.7 mg (GMZ 0124/03); 97.9 mg (GMZ 0129/03) | Dehydrocholic acid | 21, 944‐9 |
| WV16277 | Oseltamivir was provided as size 2 capsules containing 75 mg of active drug and packaging material consisting of pregelatinised starch, povidone, talc and sodium stearyl fumarate.. Oseltamivir: 75 mg capsules, Ro 64‐0796/V14, batch number PT2247C01 Capsules are caramel opaque body, caramel opaque cap | Matching placebo was provided as size 2 capsules, containing dehydrocholic acid, dibasic calcium phosphate dihydrate, pregelatinised starch, povidone, talc, sodium stearyl fumarate Matching placebo capsules: Ro 64‐0796/V16, batch number GMZ 0163. Capsules are caramel opaque body, caramel opaque cap | Unknown (certificate of analysis not provided) | Unknown (certificate of analysis not provided) | 20 |
| NV16871 | Capsules containing 75 mg of active drug and packaging material consisting of pregelatinised starch, povidone, talc and sodium stearyl fumarate. All participants over the age of 13 or who weighed > 40 kg received this dosage form. 2. A paediatric suspension containing 12 mg oseltamivir per ml of reconstituted solution and the following excipients: sorbitol, titanium dioxide, sodium benzoate, xanthan gum, monosodium citrate, saccharin sodium and Permaseal 11900‐31 tutti frutti (flavour). All participants of 12 years and under or who weighed ≤ 40 kg received this dosage form or 10 doses Ro 64‐0796/V14 (oseltamivir 75 mg capsules), batch PT2247C01 Ro 64‐0796/V37 (oseltamivir powder for oral suspension), batch B1023 | Matching placebo was to be provided as capsules and as suspension Ro 64‐0796/V16 (placebo capsules), batch GMZ 0163 Ro 64‐0796/VF01 (placebo powder for oral suspension), batch C0318A001 | Unknown (certificate of analysis not provided) | Unknown (certificate of analysis not provided) | 13‐14, 24 |
| NB Most content dosage unavailable at review time‐lock. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to first alleviation of symptoms in adult treatment (ITT population) Show forest plot | 8 | 3954 | Mean Difference (IV, Random, 95% CI) | ‐16.76 [‐25.10, ‐8.42] |
| 2 Hospital admission in adult treatment (safety population) Show forest plot | 7 | 4394 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.57, 1.50] |
| 3 Defined as influenza‐infected at baseline in adult treatment Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.91, 0.99] |
| 4 Antibody rise four‐fold or greater in adult treatment Show forest plot | 8 | 4025 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.86, 0.97] |
| 5 Adverse events ‐ nausea in adult treatment (on‐treatment) Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 1.57 [1.14, 2.15] |
| 6 Adverse events ‐ vomiting in adult treatment (on‐treatment) Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 2.43 [1.75, 3.38] |
| 7 Adverse events ‐ diarrhoea in adult treatment (on‐treatment) Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.46, 0.98] |
| 8 Withdrawal from adult treatment trial due to adverse events Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.56, 1.48] |
| 9 All withdrawals from adult treatment Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.73, 1.41] |
| 10 Adverse events ‐ cough in adult treatment (on‐treatment) Show forest plot | 6 | 3943 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.41, 0.96] |
| 11 Adverse events ‐ abdominal pain in adult treatment (on‐treatment) Show forest plot | 6 | 4368 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.64, 1.55] |
| 12 Adverse events: dizziness in adult treatment (on‐treatment) Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.51, 1.18] |
| 13 Adverse events: headache in adult treatment (on‐treatment) Show forest plot | 7 | 4426 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.72, 1.90] |
| 14 Serious adverse events: overall in adult treatment (on‐treatment) Show forest plot | 7 | 4394 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.51, 1.80] |
| 15 Serious adverse events: overall in adult treatment (off‐treatment) Show forest plot | 7 | 4394 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.39, 1.37] |
| 16 Complications: bronchitis in adult treatment Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.56, 1.01] |
| 16.1 Trials which collected data on non‐specific adverse event or secondary/intercurrent illness form | 6 | 3316 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.42, 1.03] |
| 16.2 Trials which collected data on specific "Diagnosis of secondary illness" form | 2 | 1136 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.61, 1.26] |
| 17 Complications: pneumonia in adult treatment Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.33, 0.90] |
| 17.1 Trials which collected data on non‐specific adverse event or secondary/intercurrent illness form | 6 | 3316 | Risk Ratio (IV, Random, 95% CI) | 0.44 [0.22, 0.88] |
| 17.2 Trials which collected data on specific "Diagnosis of secondary illness" form | 2 | 1136 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.33, 1.44] |
| 18 Complications: sinusitis in adult treatment Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.76, 1.40] |
| 18.1 Trials which collected data on non‐specific adverse event or secondary/intercurrent illness form | 6 | 3316 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.74, 1.50] |
| 18.2 Trials which collected data on specific "Diagnosis of secondary illness" form | 2 | 1136 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.52, 1.80] |
| 19 Complications: otitis media in adult treatment Show forest plot | 6 | 4368 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.57, 2.15] |
| 19.1 Trials which collected data on non‐specific adverse event or secondary/intercurrent illness form | 4 | 3232 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.46, 2.12] |
| 19.2 Trials which collected data on specific "Diagnosis of secondary illness" form | 2 | 1136 | Risk Ratio (IV, Random, 95% CI) | 1.57 [0.41, 6.02] |
| 20 Complications in adult trials classified as serious or leading to study withdrawal Show forest plot | 6 | 3675 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.40, 2.06] |
| 21 Culture‐positive at baseline in adult treatment Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.95, 1.07] |
| 22 Adverse events: general body system in adult treatment (on‐treatment) Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.67, 1.17] |
| 23 Adverse events: neurological body system in adult treatment (on‐treatment) Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.80, 1.38] |
| 24 Adverse events: respiratory body system in adult treatment (on‐treatment) Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.65, 1.00] |
| 25 Adverse events: infection body system in adult treatment (on‐treatment) Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.71, 1.01] |
| 26 Adverse events: gastrointestinal body system in adult treatment (on‐treatment) Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 1.25 [1.08, 1.45] |
| 27 Adverse events: cardiac body system in adult treatment (on‐treatment) Show forest plot | 6 | 3943 | Risk Ratio (IV, Random, 95% CI) | 0.49 [0.25, 0.97] |
| 28 Adverse events: ear body system in adult treatment (on‐treatment) Show forest plot | 7 | 4426 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.61, 1.60] |
| 29 Adverse events: eye body system in adult treatment (on‐treatment) Show forest plot | 7 | 4426 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.52, 1.92] |
| 30 Adverse events: metabolism body system in adult treatment (on‐treatment) Show forest plot | 7 | 4394 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.46, 1.43] |
| 31 Adverse events: musculoskeletal body system in adult treatment (on‐treatment) Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.59, 1.73] |
| 32 Adverse events: psychiatric body system in adult treatment (on‐treatment) Show forest plot | 7 | 4426 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.43, 2.03] |
| 33 Adverse events: skin body system in adult treatment (on‐treatment) Show forest plot | 7 | 4426 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.63, 2.06] |
| 34 Adverse events: cardiac body system in adult treatment (off‐treatment) Show forest plot | 7 | 4394 | Risk Ratio (IV, Random, 95% CI) | 1.21 [0.55, 2.64] |
| 35 Adverse events: ear body system in adult treatment (off‐treatment) Show forest plot | 6 | 4368 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.57, 2.42] |
| 36 Adverse events: gastrointestinal body system in adult treatment (off‐treatment) Show forest plot | 7 | 4394 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.74, 1.58] |
| 37 Adverse events: general body system in adult treatment (off‐treatment) Show forest plot | 7 | 4394 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.50, 1.62] |
| 38 Adverse events: infection body system in adult treatment (off‐treatment) Show forest plot | 7 | 4426 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.61, 1.03] |
| 39 Adverse events: musculoskeletal body system in adult treatment (off‐treatment) Show forest plot | 7 | 4394 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.54, 1.30] |
| 40 Adverse events: neurological body system in adult treatment (off‐treatment) Show forest plot | 6 | 4368 | Risk Ratio (IV, Random, 95% CI) | 1.29 [0.87, 1.91] |
| 41 Adverse events: respiratory body system in adult treatment (off‐treatment) Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.71, 1.24] |
| 42 Adverse events: skin body system in adult treatment (off‐treatment) Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.42, 1.56] |
| 43 Adverse events: cough in adult treatment (off‐treatment) Show forest plot | 8 | 4452 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.55, 1.85] |
| 44 Adverse events: headache in adult treatment (off‐treatment) Show forest plot | 6 | 4368 | Risk Ratio (IV, Random, 95% CI) | 1.34 [0.83, 2.15] |
| 45 Adverse events: nausea in adult treatment (off‐treatment) Show forest plot | 6 | 4368 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.50, 2.23] |
| 46 Time to first alleviation of symptoms in child treatment [hours] Show forest plot | 3 | 1329 | Mean Difference (IV, Random, 95% CI) | ‐8.04 [‐33.34, 17.26] |
| 46.1 Otherwise healthy children | 1 | 669 | Mean Difference (IV, Random, 95% CI) | ‐29.40 [‐47.04, ‐11.76] |
| 46.2 Children with chronic asthma | 2 | 660 | Mean Difference (IV, Random, 95% CI) | 5.18 [‐11.06, 21.41] |
| 47 Hospital admission in child treatment (safety population) Show forest plot | 3 | 1359 | Risk Ratio (IV, Random, 95% CI) | 1.92 [0.70, 5.23] |
| 48 Defined as influenza‐infected at baseline in child treatment Show forest plot | 3 | 1359 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.84, 1.01] |
| 49 Antibody rise four‐fold or greater in child treatment Show forest plot | 2 | 909 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.80, 1.00] |
| 50 Complications: bronchitis in child treatment Show forest plot | 3 | 1359 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.27, 1.55] |
| 51 Complications: otitis media in child treatment Show forest plot | 3 | 1359 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.62, 1.02] |
| 52 Complications: pneumonia in child treatment Show forest plot | 3 | 1359 | Risk Ratio (IV, Random, 95% CI) | 1.06 [0.62, 1.83] |
| 53 Complications: sinusitis in child treatment Show forest plot | 3 | 1359 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.58, 1.72] |
| 54 Complications: pneumonia in child treatment by on‐ and off‐treatment Show forest plot | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 54.1 On‐treatment | 3 | 1359 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.48, 1.60] |
| 54.2 Off‐treatment | 3 | 1359 | Risk Ratio (IV, Random, 95% CI) | 2.83 [0.52, 15.31] |
| 55 Complications in trials of children classified as serious or leading to study withdrawal Show forest plot | 3 | 1359 | Risk Ratio (IV, Random, 95% CI) | 1.98 [0.58, 6.72] |
| 56 Withdrawal from child treatment trial due to adverse events Show forest plot | 2 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.33, 3.01] |
| 57 All withdrawals from child treatment Show forest plot | 2 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.56, 1.60] |
| 58 Serious adverse events: overall in child treatment (on‐treatment) Show forest plot | 2 | 1029 | Risk Ratio (IV, Random, 95% CI) | 1.97 [0.59, 6.56] |
| 59 Serious adverse events: overall in child treatment (off‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.38, 8.46] |
| 60 Adverse events: abdominal pain in child treatment (on‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.62, 1.95] |
| 61 Adverse events: diarrhoea in child treatment (on‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.58, 1.28] |
| 62 Adverse events: nausea in child treatment (on‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.50, 1.51] |
| 63 Adverse events: vomiting in child treatment (on‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (IV, Random, 95% CI) | 1.70 [1.23, 2.35] |
| 64 Adverse events: abdominal pain in child treatment (off‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.39, 2.11] |
| 65 Adverse events: cough in child treatment (off‐treatment) Show forest plot | 2 | 1029 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.27, 1.85] |
| 66 Adverse events: diarrhoea in child treatment (off‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.36, 1.40] |
| 67 Adverse events: headache in child treatment (off‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.55, 2.34] |
| 68 Adverse events: vomiting in child treatment (off‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.57, 2.02] |
| 69 Adverse events: ear body system in child treatment (on‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (IV, Random, 95% CI) | 1.18 [0.30, 4.56] |
| 70 Adverse events: gastrointestinal body system in child treatment (on‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (IV, Random, 95% CI) | 1.18 [0.96, 1.44] |
| 71 Adverse events: general body system in child treatment (on‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.47, 1.92] |
| 72 Adverse events: infection body system in child treatment (on‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.59, 0.95] |
| 73 Adverse events: neurological body system in child treatment (on‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.17, 2.62] |
| 74 Adverse events: respiratory body system in child treatment (on‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.73, 1.43] |
| 75 Adverse events: skin body system in child treatment (on‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.71, 2.22] |
| 76 Adverse events: ear body system in child treatment (off‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.52, 2.32] |
| 77 Adverse events: gastrointestinal body system in child treatment (off‐treatment) Show forest plot | 2 | 1029 | Risk Ratio (IV, Random, 95% CI) | 1.15 [0.69, 1.91] |
| 78 Adverse events: general body system in child treatment (off‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.54, 1.86] |
| 79 Adverse events: infection body system in child treatment (off‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.82, 1.58] |
| 80 Adverse events: neurological body system in child treatment (off‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.51, 2.26] |
| 81 Adverse events: respiratory body system in child treatment (off‐treatment) Show forest plot | 3 | 1358 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.65, 1.35] |
| 82 Culture‐positive at baseline in child treatment Show forest plot | 3 | 1359 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.83, 1.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic influenza in adult prophylaxis of individuals Show forest plot | 3 | 2479 | Risk Ratio (IV, Random, 95% CI) | 0.45 [0.30, 0.67] |
| 2 Asymptomatic influenza in adult prophylaxis of individuals Show forest plot | 3 | 2479 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.49, 1.24] |
| 3 Symptomatic influenza in household prophylaxis Show forest plot | 1 | 405 | Risk Ratio (IV, Random, 95% CI) | 0.20 [0.09, 0.44] |
| 4 Asymptomatic influenza in household prophylaxis Show forest plot | 1 | 405 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.39, 3.33] |
| 5 Influenza‐like illness reported as adverse event (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.73, 1.35] |
| 6 Influenza‐like illness reported as adverse event (off‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 0.62 [0.34, 1.16] |
| 7 Hospitalisation in adult prophylaxis (safety population) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.66, 1.94] |
| 8 Complications: bronchitis in adult prophylaxis Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.41, 1.35] |
| 9 Complications: sinusitis in adult prophylaxis Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.75, 2.62] |
| 10 Adverse events leading to study withdrawal in adult prophylaxis Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.57, 2.18] |
| 11 All withdrawals in adult prophylaxis Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.15 [0.82, 1.61] |
| 12 Serious adverse events in adult prophylaxis (on‐treatment) Show forest plot | 3 | 2479 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.53, 1.66] |
| 13 Serious adverse events in adult prophylaxis (off‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.57, 2.60] |
| 14 Adverse events: abdominal pain in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.77, 1.82] |
| 15 Adverse events: cough in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.68, 1.36] |
| 16 Adverse events: diarrhoea in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.09 [0.64, 1.86] |
| 17 Adverse events: dizziness in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.15 [0.66, 2.01] |
| 18 Adverse events: fatigue in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.89, 1.40] |
| 19 Adverse events: headache in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.18 [1.05, 1.33] |
| 20 Adverse events: nausea in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.96 [1.20, 3.20] |
| 21 Adverse events: vomiting in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.91 [0.70, 5.22] |
| 22 Adverse events: cough in adult prophylaxis (off‐treatment) Show forest plot | 3 | 2479 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.36, 1.45] |
| 23 Adverse events: fatigue in adult prophylaxis (off‐treatment) Show forest plot | 3 | 2479 | Risk Ratio (IV, Random, 95% CI) | 1.33 [0.57, 3.13] |
| 24 Adverse events: headache in adult prophylaxis (off‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.63, 1.24] |
| 25 Adverse events: blood body system in adult prophylaxis (on‐treatment) Show forest plot | 3 | 2479 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.30, 3.25] |
| 26 Adverse events: cardiac body system in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.36, 1.58] |
| 27 Adverse events: ear body system in adult prophylaxis (on treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.44 [0.61, 3.40] |
| 28 Adverse events: eye body system in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.56, 1.81] |
| 29 Adverse events: gastrointestinal body system in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.38 [1.17, 1.63] |
| 30 Adverse events: general body system in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.88, 1.20] |
| 31 Adverse events: infection body system in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.84, 1.11] |
| 32 Adverse events: immune body system in adult prophylaxis (on‐treatment) Show forest plot | 1 | 1559 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.45, 1.64] |
| 33 Adverse events: injury body system in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.60, 1.56] |
| 34 Adverse events: metabolism body system in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.36 [0.73, 2.54] |
| 35 Adverse events: musculoskeletal body system in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.79, 1.22] |
| 36 Adverse events: neurological body system in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.21 [1.03, 1.42] |
| 37 Adverse events: psychiatric body system in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.81 [0.97, 3.37] |
| 38 Adverse events: renal body system in adult prophylaxis (on‐treatment) Show forest plot | 3 | 2479 | Risk Ratio (IV, Random, 95% CI) | 3.17 [0.96, 10.49] |
| 39 Adverse events: reproductive body system in adult prophylaxis (on treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.77, 1.42] |
| 40 Adverse events: respiratory body system in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.90, 1.20] |
| 41 Adverse events: skin body system in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.63, 1.34] |
| 42 Adverse events: surgical events in adult prophylaxis (on‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.42, 2.29] |
| 43 Adverse events: vascular body system in adult prophylaxis (on‐treatment) Show forest plot | 3 | 2479 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.45, 1.80] |
| 44 Adverse events: cardiac body system in adult prophylaxis (off‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.50 [0.67, 3.35] |
| 45 Adverse events: gastrointestinal body system in adult prophylaxis (off‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.62, 1.53] |
| 46 Adverse events: general body system in adult prophylaxis (off‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.69, 1.49] |
| 47 Adverse events: infection body system in adult prophylaxis (off‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.68, 1.17] |
| 48 Adverse events: injury body system in adult prophylaxis (off‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.49, 2.09] |
| 49 Adverse events: musculoskeletal body system in adult prophylaxis (off‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.63, 1.72] |
| 50 Adverse events: neurological body system in adult prophylaxis (off‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.68, 1.28] |
| 51 Adverse events: reproductive body system in adult prophylaxis (off‐treatment) Show forest plot | 2 | 2514 | Risk Ratio (IV, Random, 95% CI) | 0.49 [0.24, 1.00] |
| 52 Adverse events: respiratory body system in adult prophylaxis (off‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.69, 1.32] |
| 53 Adverse events: skin body system in adult prophylaxis (off‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.32, 1.69] |
| 54 Adverse events: psychiatric body system in adult prophylaxis (on and off‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 1.80 [1.05, 3.08] |
| 55 Adverse events: renal body system in adult prophylaxis (on and off‐treatment) Show forest plot | 4 | 3434 | Risk Ratio (IV, Random, 95% CI) | 2.01 [0.74, 5.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to first alleviation of symptoms in adult treatment (days) Show forest plot | 13 | 5411 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.81, ‐0.39] |
| 2 Complications: pneumonia in adult treatment Show forest plot | 11 | 5876 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.58, 1.40] |
| 3 Complications: pneumonia confirmed with X‐ray in adult treatment Show forest plot | 2 | 946 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.35, 3.02] |
| 4 Complications: bronchitis in adult treatment Show forest plot | 12 | 6072 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.61, 0.91] |
| 5 Complications: sinusitis in adult treatment Show forest plot | 12 | 6072 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.84, 1.48] |
| 6 Complications: otitis media in adult treatment Show forest plot | 10 | 5494 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.54, 1.20] |
| 7 Complications in adult trials classified as serious or leading to study withdrawal Show forest plot | 8 | 4514 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.46, 2.63] |
| 8 Proportion diagnosed as influenza‐infected in adult treatment Show forest plot | 15 | 6569 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.98, 1.06] |
| 9 Proportion with four‐fold rise in antibody titre in adult treatment Show forest plot | 13 | 5113 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.96, 1.06] |
| 10 Proportion with positive culture at baseline in adult treatment Show forest plot | 12 | 5995 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.96, 1.05] |
| 11 Serious adverse events in adult treatment Show forest plot | 10 | 4388 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.49, 1.50] |
| 12 Adverse events leading to study withdrawal in adult treatment Show forest plot | 13 | 6116 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.66, 1.39] |
| 13 All withdrawals in adult treatment Show forest plot | 12 | 6065 | Risk Difference (IV, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 14 Time to first alleviation of symptoms in children (days) Show forest plot | 2 | 723 | Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐2.32, 0.15] |
| 15 Complications: pneumonia in child treatment Show forest plot | 2 | 737 | Risk Ratio (IV, Random, 95% CI) | 0.53 [0.12, 2.38] |
| 16 Complications: bronchitis in child treatment Show forest plot | 2 | 737 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.26, 2.80] |
| 17 Complications: sinusitis in child treatment Show forest plot | 2 | 737 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.12, 6.45] |
| 18 Complications: otitis media in child treatment Show forest plot | 2 | 737 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.59, 1.72] |
| 19 Proportion diagnosed as influenza‐infected in child treatment Show forest plot | 1 | 471 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.89, 1.11] |
| 20 Proportion with four‐fold increase in antibodies in child treatment Show forest plot | 1 | 431 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.86, 1.20] |
| 21 Proportion with positive culture at baseline in child treatment Show forest plot | 1 | 469 | Risk Ratio (IV, Random, 95% CI) | 1.06 [0.87, 1.27] |
| 22 All withdrawals in child treatment Show forest plot | 2 | 737 | Risk Ratio (IV, Random, 95% CI) | 1.18 [0.16, 8.88] |
| 23 Adverse events: nausea and vomiting in child treatment (on‐treatment) Show forest plot | 2 | 737 | Odds Ratio (IV, Random, 95% CI) | 0.54 [0.24, 1.22] |
| 24 Adverse events: diarrhoea in child treatment (on‐treatment) Show forest plot | 2 | 737 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.15, 1.75] |
| 25 Adverse events: gastrointestinal body system in child treatment (on‐treatment) Show forest plot | 2 | 737 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.52, 1.73] |
| 26 Adverse events: respiratory body system in child treatment (on‐treatment) Show forest plot | 2 | 737 | Risk Ratio (IV, Random, 95% CI) | 0.56 [0.29, 1.10] |
| 27 Adverse events: neurological body system in child treatment (on‐treatment) Show forest plot | 2 | 737 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.20, 1.85] |
| 28 Adverse events: ear, nose and throat body system in child treatment (on‐treatment) Show forest plot | 2 | 737 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.63, 1.67] |
| 29 Adverse events: skin body system in child treatment (on‐treatment) Show forest plot | 2 | 737 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.34, 2.98] |
| 30 Adverse events: gastrointestinal body system in child treatment (off‐treatment) Show forest plot | 2 | 737 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.50, 3.83] |
| 31 Adverse events: ear nose and throat body system in child treatment (off‐treatment) Show forest plot | 2 | 737 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.54, 1.63] |
| 32 Adverse events: nausea/vomiting in adult treatment (on‐treatment) Show forest plot | 15 | 6553 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.39, 0.94] |
| 33 Adverse events: diarrhoea in adult treatment (on‐treatment) Show forest plot | 15 | 6553 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.66, 1.14] |
| 34 Adverse events: dizziness in adult treatment (on‐treatment) Show forest plot | 13 | 5641 | Risk Ratio (IV, Random, 95% CI) | 1.20 [0.68, 2.15] |
| 35 Adverse events: headache in adult treatment (on‐treatment) Show forest plot | 15 | 6553 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.60, 1.18] |
| 36 Adverse events: cough in adult treatment (on‐treatment) Show forest plot | 15 | 6553 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.49, 0.96] |
| 37 Adverse events: gastrointestinal body system in adult treatment (on‐treatment) Show forest plot | 15 | 6453 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.72, 1.09] |
| 38 Adverse events: respiratory body system in adult treatment (on‐treatment) Show forest plot | 15 | 6553 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.67, 0.97] |
| 39 Adverse events: neurological body system in adult treatment (on‐treatment) Show forest plot | 15 | 6553 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.82, 1.29] |
| 40 Adverse events: ear, nose and throat body system in adult treatment (on‐treatment) Show forest plot | 14 | 6229 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.76, 1.05] |
| 41 Adverse events: skin body system in adult treatment (on‐treatment) Show forest plot | 13 | 6181 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.60, 1.18] |
| 42 Adverse events: musculoskeletal body system in adult treatment (on‐treatment) Show forest plot | 15 | 6553 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.49, 1.04] |
| 43 Adverse events: eye body system in adult treatment (on‐treatment) Show forest plot | 13 | 6181 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.55, 1.74] |
| 44 Adverse events: hepato body system in adult treatment (on‐treatment) Show forest plot | 9 | 4788 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.39, 1.76] |
| 45 Adverse events: renal body system in adult treatment (on‐treatment) Show forest plot | 11 | 5205 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.41, 1.72] |
| 46 Adverse events: cardiovascular body system in adult treatment (on‐treatment) Show forest plot | 11 | 5204 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.50, 1.91] |
| 47 Adverse events: blood body system in adult treatment (on‐treatment) Show forest plot | 11 | 5272 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.43, 1.49] |
| 48 Adverse events: psychiatric body system in adult treatment (on‐treatment) Show forest plot | 10 | 4732 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.57, 2.38] |
| 49 Adverse events: reproduction body system in adult treatment (on‐treatment) Show forest plot | 9 | 4924 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.33, 1.43] |
| 50 Adverse events: endocrine and metabolic body system in adult treatment (on‐treatment) Show forest plot | 11 | 5477 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.58, 1.53] |
| 51 Adverse events: injury body system in adult treatment (on‐treatment) Show forest plot | 9 | 5293 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.57, 2.60] |
| 52 Adverse events: non‐site specific events in adult treatment (on‐treatment) Show forest plot | 12 | 6065 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.78, 1.39] |
| 53 Adverse events: nausea/vomiting in adult treatment (off‐treatment) Show forest plot | 10 | 5403 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.67, 1.85] |
| 54 Adverse events: cough in adult treatment (off‐treatment) Show forest plot | 11 | 5599 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.71, 1.29] |
| 55 Adverse events: respiratory body system in adult treatment (off‐treatment) Show forest plot | 11 | 5599 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.71, 1.14] |
| 56 Adverse events: headache in adult treatment (off‐treatment) Show forest plot | 11 | 5599 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.69, 1.20] |
| 57 Adverse events: diarrhoea in adult treatment (off‐treatment) Show forest plot | 11 | 5599 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.59, 1.72] |
| 58 Adverse events: fatigue in adult treatment (off‐treatment) Show forest plot | 11 | 5599 | Risk Ratio (IV, Random, 95% CI) | 0.70 [0.37, 1.32] |
| 59 Adverse events: gastrointestinal body system in adult treatment (off‐treatment) Show forest plot | 11 | 5599 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.73, 1.29] |
| 60 Adverse events: neurological body system in adult treatment (off‐treatment) Show forest plot | 11 | 5599 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.72, 1.16] |
| 61 Adverse events: ear, nose and throat in adult treatment (off‐treatment) Show forest plot | 11 | 5599 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.91, 1.19] |
| 62 Adverse events: skin body system in adult treatment (off‐treatment) Show forest plot | 11 | 5599 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.68, 1.78] |
| 63 Adverse events: musculoskeletal body system in adult treatment (off‐treatment) Show forest plot | 11 | 5369 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.87, 1.55] |
| 64 Adverse events: non‐site specific in adult treatment (off‐treatment) Show forest plot | 11 | 5599 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.56, 1.03] |
| 65 Adverse events: injury body system in adult treatment (off‐treatment) Show forest plot | 11 | 5599 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.60, 2.15] |
| 66 Adverse events: endocrine and metabolic body system in adult treatment (off‐treatment) Show forest plot | 11 | 5599 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.49, 1.68] |
| 67 Adverse events: eye body system in adult treatment (off‐treatment) Show forest plot | 11 | 5599 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.55, 2.24] |
| 68 Time to first alleviation of symptoms in adults with/without relief medication [days] Show forest plot | 7 | 3396 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.47, 1.29] |
| 69 Time to first alleviation of symptoms in adults by infection status [days] Show forest plot | 12 | 4873 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.78, ‐0.37] |
| 69.1 Influenza‐infected | 12 | 3233 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐0.99, ‐0.35] |
| 69.2 Not influenza‐infected | 12 | 1640 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.86, ‐0.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic influenza in prophylaxis of individuals Show forest plot | 4 | 5275 | Risk Ratio (IV, Random, 95% CI) | 0.39 [0.22, 0.70] |
| 2 Asymptomatic influenza in prophylaxis of individuals Show forest plot | 4 | 5275 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.76, 1.24] |
| 3 Symptomatic influenza in post‐exposure prophylaxis Show forest plot | 5 | 1525 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.18, 0.58] |
| 3.1 Household prophylaxis | 2 | 824 | Risk Ratio (IV, Random, 95% CI) | 0.22 [0.13, 0.36] |
| 3.2 Other prophylaxis | 3 | 701 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.30, 1.16] |
| 4 Asymptomatic influenza in post‐exposure prophylaxis Show forest plot | 5 | 1525 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.65, 1.20] |
| 5 Complications: pneumonia in adult prophylaxis Show forest plot | 6 | 7662 | Risk Ratio (IV, Random, 95% CI) | 0.30 [0.11, 0.80] |
| 6 Complications: bronchitis in adult prophylaxis Show forest plot | 6 | 7662 | Risk Ratio (IV, Random, 95% CI) | 0.49 [0.20, 1.19] |
| 7 Complications: sinusitis in adult prophylaxis Show forest plot | 6 | 7662 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.64, 1.36] |
| 8 Complications classified as serious or leading to study withdrawal Show forest plot | 5 | 6825 | Risk Ratio (IV, Random, 95% CI) | 1.09 [0.36, 3.26] |
| 9 Serious adverse events in adult prophylaxis Show forest plot | 10 | 8225 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.65, 1.91] |
| 10 Adverse events leading to study withdrawal in adult prophylaxis Show forest plot | 10 | 8225 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.60, 1.21] |
| 11 All withdrawals in adult prophylaxis Show forest plot | 8 | 7792 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.64, 1.03] |
| 12 Adverse events: abdominal pain in adult prophylaxis (on‐treatment) Show forest plot | 10 | 8153 | Risk Ratio (IV, Random, 95% CI) | 1.28 [0.55, 2.99] |
| 13 Adverse events: cough in adult prophylaxis (on‐treatment) Show forest plot | 10 | 8153 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.82, 1.01] |
| 14 Adverse events: diarrhoea in adult prophylaxis (on‐treatment) Show forest plot | 10 | 8153 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.73, 1.40] |
| 15 Adverse events: dizziness in adult prophylaxis (on‐treatment) Show forest plot | 10 | 8153 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.59, 1.96] |
| 16 Adverse events: fatigue in adult prophylaxis (on‐treatment) Show forest plot | 10 | 8153 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.88, 1.16] |
| 17 Adverse events: headache in adult prophylaxis (on‐treatment) Show forest plot | 10 | 8153 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.89, 1.04] |
| 18 Adverse events: blood body system in adult prophylaxis (on‐treatment) Show forest plot | 8 | 7792 | Risk Ratio (IV, Random, 95% CI) | 1.18 [0.62, 2.25] |
| 19 Adverse events: nausea/vomiting in adult prophylaxis (on‐treatment) Show forest plot | 10 | 8153 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.66, 1.18] |
| 20 Adverse events: cardiovascular body system in adult prophylaxis (on‐treatment) Show forest plot | 8 | 7792 | Risk Ratio (IV, Random, 95% CI) | 1.15 [0.77, 1.71] |
| 21 Adverse events: ear, nose and throat body system in adult prophylaxis (on‐treatment) Show forest plot | 8 | 7792 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.87, 1.01] |
| 22 Adverse events: endocrine and metabolic body system in adult prophylaxis (on‐treatment) Show forest plot | 7 | 7730 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.69, 1.08] |
| 23 Adverse events: eye body system in adult prophylaxis (on‐treatment) Show forest plot | 8 | 7792 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.51, 1.21] |
| 24 Adverse events: gastrointestinal body system in adult prophylaxis (on‐treatment) Show forest plot | 10 | 8153 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.72, 0.97] |
| 25 Adverse events: injury body system in adult prophylaxis (on‐treatment) Show forest plot | 8 | 7792 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.62, 1.35] |
| 26 Adverse events: musculoskeletal body system in adult prophylaxis (on‐treatment) Show forest plot | 10 | 8153 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.93, 1.19] |
| 27 Adverse events: neurological body system in adult prophylaxis (on‐treatment) Show forest plot | 10 | 8153 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.89, 1.03] |
| 28 Adverse events: non‐site specific in adult prophylaxis (on‐treatment) Show forest plot | 8 | 7792 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.85, 1.16] |
| 29 Adverse events: psychiatric body system in adult prophylaxis (on‐treatment) Show forest plot | 7 | 7730 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.48, 2.29] |
| 30 Adverse events: renal body system in adult prophylaxis (on‐treatment) Show forest plot | 7 | 7730 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.35, 1.26] |
| 31 Adverse events: reproductive body system in adult prophylaxis (on‐treatment) Show forest plot | 10 | 8153 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.55, 1.09] |
| 32 Adverse events: respiratory body system in adult prophylaxis (on‐treatment) Show forest plot | 9 | 8109 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.80, 0.94] |
| 33 Adverse events: skin body system in adult prophylaxis (on‐treatment) Show forest plot | 8 | 7774 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.58, 1.45] |
| 34 Adverse events: gastrointestinal body system in adult prophylaxis (off‐treatment) Show forest plot | 9 | 8109 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.63, 1.13] |
| 35 Adverse events: respiratory body system in adult prophylaxis (off‐treatment) Show forest plot | 9 | 8109 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.54, 1.15] |
| 36 Adverse events: nausea/vomiting in prophylaxis (off‐treatment) Show forest plot | 9 | 8109 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.39, 1.67] |
| 37 Adverse events: diarrhoea in prophylaxis (off‐treatment) Show forest plot | 9 | 8109 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.54, 1.57] |
| 38 Adverse events: headache in prophylaxis (off‐treatment) Show forest plot | 9 | 8109 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.76, 1.19] |
| 39 Adverse events: cough in prophylaxis (off‐treatment) Show forest plot | 9 | 8109 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.99, 1.73] |
| 40 Adverse events: fatigue in prophylaxis (off‐treatment) Show forest plot | 9 | 8109 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.47, 1.16] |
| 41 Adverse events: neurological body system in prophylaxis (off‐treatment) Show forest plot | 9 | 8109 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.82, 1.24] |
| 42 Adverse events: ear, nose and throat in prophylaxis (off‐treatment) Show forest plot | 8 | 7792 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.84, 1.17] |
| 43 Adverse events: musculoskeletal body system in prophylaxis (off‐treatment) Show forest plot | 9 | 8109 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.77, 1.39] |
| 44 Adverse events: non‐site specific in prophylaxis (off‐treatment) Show forest plot | 8 | 7792 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.74, 1.32] |
| 45 Adverse events: injury in prophylaxis (off‐treatment) Show forest plot | 8 | 7792 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.34, 1.10] |
| 46 Adverse events: endocrine and metabolic in prophylaxis (off‐treatment) Show forest plot | 8 | 7792 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.60, 1.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complications: pneumonia Show forest plot | 32 | 22565 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.55, 0.95] |
| 1.1 Unclear diagnostic confirmation capture | 25 | 18905 | Risk Ratio (IV, Random, 95% CI) | 0.51 [0.35, 0.75] |
| 1.2 Clear diagnostic confirmation capture | 7 | 3660 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.69, 1.47] |

