Trasplante de células madre alogénicas versus tratamiento convencional para el linfoma de linfocitos T cutáneo primario avanzado
Resumen
Antecedentes
Los linfomas de linfocitos T cutáneos (LLTC) primarios pertenecen al grupo de linfomas no Hodgkin y generalmente presentan un curso indolente. Sin embargo, algunos pacientes progresan a estadios tumorales avanzados o leucémicos. Hasta la fecha, no hay cura para esos casos. En los últimos años varias publicaciones informaron respuestas duraderas en algunos pacientes después del trasplante de células madre alogénicas (TCMA). Esta es una actualización de una revisión Cochrane publicada por primera vez en 2011 y actualizada en 2013.
Objetivos
Comparar la eficacia y la seguridad de los tratamientos convencionales con el trasplante de células madre alogénicas en pacientes con linfomas de linfocitos T cutáneos primarios avanzados.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL, Número 1), MEDLINE (1950 a enero de 2013), en bases de datos de Internet de ensayos en curso, en actas de congresos de la Sociedad Americana de Oncología Clínica (ASCO, 2009 a julio de 2013) y en la Sociedad Americana de Hematología (ASH, 2009 a julio de 2013). También se estableció contacto con los miembros de la European Organisation for Research and Treatment of Cancer (EORTC) Cutaneous Lymphoma Task Force para verificar las actividades de los estudios en curso. Se buscaron manualmente citas bibliográficas obtenidas de los ensayos identificados y artículos de revisión pertinentes. Además, se buscaron manualmente ensayos controlados aleatorizados del European Group for Blood and Marrow Transplantation (EBMT) y la International Conference on Cutaneous T‐cell Lymphoma, ASCO y ASH hasta julio de 2013.
Criterios de selección
Los ensayos elegibles para inclusión fueron ensayos controlados aleatorizados (ECA) genéticamente que compararon el TCMA más tratamiento de acondicionamiento (independientemente de los agentes) versus el tratamiento convencional para el LLTC avanzado.
Obtención y análisis de los datos
Dos autores de la revisión habrían extraído los datos de los estudios elegibles y evaluado su calidad. La medida de resultado primaria fue la supervivencia general y los resultados secundarios fueron el tiempo de progresión, la tasa de respuesta, la mortalidad relacionada con el tratamiento, los eventos adversos y la calidad de vida.
Resultados principales
No se identificaron ensayos controlados aleatorizados en la búsqueda actualizada de enero de 2013. En 2011, se encontraron 2077 citas, pero ninguna relacionada con ensayos controlados relevantes asignados al azar genéticamente o no. Los 41 estudios que se consideraron potencialmente adecuados se excluyeron después de revisar los textos completos debido a que eran no aleatorios, no incluían LLTC o eran artículos de revisión.
Conclusiones de los autores
Se planificó informar sobre evidencia de ensayos controlados asignados al azar genéticamente o no que compararan el tratamiento convencional y el trasplante de células madre alogénicas. Sin embargo, no se ha identificado ningún ensayo controlado aleatorizado que aborde esta cuestión. No obstante, se deben iniciar ensayos controlados prospectivos aleatorizados genéticamente para evaluar la función precisa del TCMA para el LLTC avanzado.
Resumen en términos sencillos
Función del trasplante de células madre alogénicas en pacientes con linfoma de linfocitos T cutáneo primario en estadio avanzado
Los linfomas de linfocitos T cutáneos (LLTC) primarios son un tipo de neoplasia hematológica denominado linfomas no Hodgkin. Los LLCT se ven generalmente primero en la piel. Se caracteriza por un aumento no controlado de linfocitos T, que son un tipo especial de leucocitos. La mayoría de las personas que contraen la enfermedad son mayores de 60 años. En comparación con otras enfermedades de los linfocitos T, este tipo suele progresar lentamente. El curso y el resultado probable de la enfermedad también es mejor para este tipo de cáncer. Sin embargo, todavía no hay cura. El subtipo más frecuente de LLTC es la micosis fungoide (MF), que habitualmente crece despacio en estadios tempranos. Sin embargo, aproximadamente el 20% de las personas en estadios iniciales de la enfermedad empeorarán y progresarán a la fase tumoral o desarrollarán un tipo de leucemia llamado síndrome de Sézary. La mayoría de estas personas son sometidas a quimioterapias para matar las células cancerosas. La quimioterapia puede implicar uno o más medicamentos (conocidos como monoquimioterapia o poliquimioterapia). Aunque algunos pacientes responden bien al principio del tratamiento, la enfermedad suele reaparecer y la esperanza de vida es incierta. Además, muchos pacientes pueden experimentar graves efectos secundarios del tratamiento. En los últimos años varias publicaciones informaron respuestas duraderas después de una intervención llamada trasplante de células madre alogénicas (TCMA). Esto es cuando el paciente recibe un trasplante de células madre de otro donante. Antes de comenzar el trasplante, el paciente se somete a un tratamiento para reducir el tamaño del tumor. Esto se llama acondicionamiento de intensidad completa o reducida (RIC). El acondicionamiento de intensidad reducida permite al paciente evitar los regímenes estándar de la terapia de alta dosis. Parece ser igualmente eficaz pero con una toxicidad significativamente menor. El uso de acondicionamiento de intensidad reducida significa que las personas mayores, que son la mayoría de los pacientes con esta enfermedad, pueden ser tratadas con células madre.
Se planeó llevar a cabo una revisión del efecto del trasplante de células madre alogénicas comparado con el tratamiento estándar en pacientes con linfomas de linfocitos T cutáneos. Sin embargo, después de búsquedas exhaustivas, no se encontraron estudios relevantes.
En su lugar, sólo se ha podido presentar un resumen de algunas series de casos y evaluaciones clínicas. Esto no permite evaluar claramente las posibilidades y limitaciones de este tratamiento. Sin embargo, sí muestran que el trasplante de células madre alogénicas puede ser de algún beneficio, con efectos secundarios aceptables. Por lo tanto, puede considerarse como una opción de tratamiento prometedora para los pacientes con LLTC avanzado, pero se necesita más investigación.
Authors' conclusions
Background
Description of the condition
Primary cutaneous T‐cell lymphomas (CTCLs) are a clinically and histologically distinct group of T‐lymphocyte malignancies that manifest primarily in the skin. CTCLs are still incurable by conventional therapies. The most common subtype of CTCL is Mycosis fungoides (MF) which typically runs an indolent course in early stages (Whittaker 2007) but is not yet curable. A representative retrospective analysis of 525 patients with MF shows that approximately 30% of patients have advanced staged disease (Kim 2003) and 20% of those with early‐stage (IB) disease will develop disease progression (Whittaker 2007). Patients with advanced stages of CTCL (World Health Organisation (WHO)/European Organisation for Research and Treatment of Cancer (EORTC) stage > IIB) have a poor prognosis. The optimal therapy in this situation is still undefined, despite the use of various therapies.
Incidence rates in Germany differ between 3 to 4 per million inhabitants for CTCL overall (Stang 2006). In the United States of America, incidence rates for advanced CTCL including erythrodermic MF and Sézary syndrome (SS, leukaemic variant of MF) were estimated to account for approximately 10% of all patients with cutaneous lymphoma (Vidulich 2009). In Norway, patients with tumour stage and erythrodermic MF or Sézary syndrome represent approximately 30% of all patients with CTCL (Saunes 2009). Therefore, the incidence for advanced CTCL (tumour and erythrodermic stage, WHO/EORTC stage > IIB) in Germany can be estimated at approximately one case per million inhabitants.
Standard conventional therapy for CTCL is stage dependent and includes a variety of topical and systemic treatments recommended by consensus guidelines (Stadler 2008; Whittaker 2003; Whittaker 2007): radiation, photopheresis, UV‐irradiation, monoclonal antibodies (e.g. alemtuzumab), interferon‐alpha, cytostatic monotherapy (e.g. gemcitabine, liposomal doxorubicin) or even polychemotherapy (CHOP = cyclophosphamide/doxorubicin/vincristine/prednisone). Following these consensus guidelines, only short‐term clinical responses can be achieved in advanced stage CTCL in most cases and median survival is around 2.9 years. In patients with SS, median survival is even worse at approximately 13 months (Stadler 2008; Whittaker 2003; Whittaker 2007).
As conventional treatments usually do not lead to long‐term disease control in advanced stage CTCL, high‐intensity regimens have been investigated as an alternative option for treatment. Disappointingly, high‐dose chemotherapy with autologous stem cell transplantation showed only short‐lived responses as well (Duarte 2008).
Description of the intervention
Allogeneic blood stem cell transplantation has been shown to provide a substantial graft‐versus‐lymphoma (GvL) effect in primary CTCL (Duarte 2008). In several retrospective analyses, alloSCT led to long‐term remissions in many patients with advanced stage CTCL (WHO/EORTC > IIB) (Dearden 2007; Duarte 2008; Duarte 2010; Duvic 2010; Introcaso 2008; Molina 2005). Nevertheless, alloSCT has only been explored in single cases, case series or retrospectively (Wu 2009). Approximately 80% of 20 patients with advanced stage CTCL summarised by Duarte et al. had achieved remission at the 3 year follow up after alloSCT. Conditioning regimens were mixed in these case series and consisted of myeloablative (full‐intensity) and also non‐myeloablative (reduced‐intensity) conditioning (RIC). RIC appeared to be equally effective as conventional myeloablative regimens in terms of stable haematopoietic engraftment and remission induction but with significantly less toxicity (Duarte 2008). This is particularly interesting for the broader use of RIC alloSCT in older patients who represent the majority of CTCL individuals.
How the intervention might work
The use of allogeneic stem cells offers the advantage of a tumour cell free graft. More importantly, alloSCT provides the basis of adoptive immunotherapy, leading to the so‐called "graft‐versus‐tumour‐effect" (GVT) (Foss 2004). Patients who relapsed from CTCL after alloSCT have been shown to develop complete remissions again after donor lymphocyte infusions (DLI) which strongly supports the existence of a GVT effect (Duarte 2008). Severe graft‐versus‐host disease (GvHD) is one of the major complications after alloSCT. Nevertheless, limited GvHD is associated with prolonged disease‐free survival. As GvHD frequently occurs in the skin, the major manifestation site of MF or CTCL, this phenomenon may also contribute to the efficacy of alloSCT in this disease entity (Giralt 2001).
In 2009, Wu et al. published a meta‐analysis including 39 cases of advanced CTCL treated with stem cell transplantation and previously reported in the literature. Twenty patients received an allogeneic transplantation and 19 patients received an autologous transplantation. Analysis of the overall survival (OS) showed a more favourable outcome for patients who received alloSCT. Event‐free survival showed a more durable response in patients who received alloSCT. In the allogeneic group, the majority (70%) of patients experienced persistent GvHD with mild to moderate severity (Wu 2009).
Up to date, there are several clinical phase II/III trials investigating the role of alloSCT in lymphomas, but the majority of these trials do not focus on primary CTCL. Instead, most of these trials are open to a wide range of haematological malignancies. Among them primary CTCL might be present as well. In the ongoing clinical trial NCT00506129 at M.D. Anderson Cancer Center, 25 patients with advanced CTCL are due to receive alloSCT in a non‐randomised, open label, uncontrolled, single group assignment.
Multicentre, genetically randomised, double blind clinical trials are still lacking.
Why it is important to do this review
The results of this systematic review and meta‐analysis will provide evidence on the role of alloSCT versus standard conventional therapy in the treatment of advanced primary CTCL. By systematically identifying all studies conducted to date and subsequent meta‐analysis, we aim to overcome the statistical limitations of individual trials, which are mostly underpowered. In addition, a systematic identification of all genetically randomised controlled trials conducted to date and a critical review of their reliability and validity is required. In case of missing prospective and genetically randomised controlled trials we will show the need for further investigations. Non‐randomised trials are expected to suffer from a pronounced selection bias. It should be kept in mind that most patients eligible for the discussed treatment options have advanced stage disease with usually several prior therapies. We expect our timely meta‐analysis to strongly influence future treatment recommendations.
Objectives
To compare the efficacy and safety of conventional therapies with allogeneic stem cell transplantation in patients with advanced primary cutaneous T‐cell lymphomas.
Methods
Criteria for considering studies for this review
Types of studies
Apart from the intervention or control, participants in the study groups were intended to receive identical care. Only studies on the principle of genetic assignment were considered for this review. This is named after Gregor Mendel (1822–1884), a Moravian and an Augustinian monk. In the haematological context, for a patient’s sibling to be a suitable donor, he/she must have inherited the same tissue type as the patient from their mother and father. Since the chances of existence for a match depend on the random assortment of genes at fertilisation, only one in four siblings will be expected to have the same tissue type as the patient. Thus, whether or not a patient has a matched sibling donor available is essentially a random process and the presence or absence of a donor can be used as a surrogate for randomisation. Genetic assignment is a method to overcome the lack of randomised evidence in standard controlled trials (Wheatley 2004).
Any published or unpublished genetically randomised trials were eligible for inclusion in the review. We intended to include both full text publications and abstract publications. Due to the need of genetic assignment in an allogeneic stem cell transplantation trial only sibling donor versus no donor trials would be included. The design of quasi‐randomised trials is considered to be of low quality leading to unreliable study results. Cross‐over trials are generally considered to be inappropriate if the primary outcome is irreversible (e.g. mortality) (Higgins 2011b). In the absence of genetically randomised controlled clinical trials, we have sought to summarise and discuss applicable published results, keeping in mind that all conclusions made are not associated with a high level of evidence and need to be interpreted carefully.
Types of participants
Adult patients (age ≥ 18 years) with a confirmed diagnosis of advanced primary CTCL, without gender or ethnicity restriction, were included. We considered advanced stages of CTCL (tumour stage, WHO/EORTC stage > IIB), including newly diagnosed patients, and those with relapsed or resistant disease.
Types of interventions
The main intervention had to be alloSCT compared to conventional chemotherapy or immunotherapy. We considered any chemotherapeutic and immuno‐chemotherapeutic regimen for comparison.
The intervention was defined as follows:
-
alloSCT plus conditioning therapy regardless of agents, with or without radiotherapy, RIC or non‐RIC as treatment for advanced CTCL.
The control intervention was defined as follows:
-
conventional therapy for advanced CTCL such as radiation, photopheresis, UV‐irradiation, monoclonal antibodies (e.g. alemtuzumab), interferon‐alpha, cytostatic monotherapy (e.g. gemcitabine or liposomal doxorubicin) or polychemotherapies (e.g. CHOP).
Types of outcome measures
Primary outcomes
-
Overall survival (OS) was evaluated as the primary efficacy endpoint.
Secondary outcomes
-
Time to progression.
-
Response rate (e.g. mSWAT, tumour burden index, RECIST).
-
Treatment‐related mortality.
-
Adverse events (e.g. GvHD, treatment side effects).
-
Quality of life.
Search methods for identification of studies
We adapted search strategies from those suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). No language restriction was applied during the search strategy, in order to reduce language bias. Due to the specificity of alloSCT trials the search strategies had to consider genetic assignment, making the finding and assessment of these studies much more difficult than for standard randomised trials.
Electronic searches
We searched for relevant trials in the following databases:
-
MEDLINE (see Appendix 1 for search strategy),1950 to May 2011.
-
the Cochrane Central Register of Controlled Trials (CENTRAL), up to May 2011 (see Appendix 2 for search strategy).
We searched on 19th July 2010 and 5th May 2011. For details of the search strategies see Appendix 1; Appendix 2.
We updated the search on 28th January 2013 in MEDLINE (203 hits) and CENTRAL (no hits) see Appendix 3; Appendix 4.
We also searched databases of ongoing trials:
-
Current Controlled Trials: www controlled‐trials.com
-
Clinical Trials: www.clinicaltrials.gov
We performed the searches on 20th October 2010, 14th April 2011 and 2nd July 2013.
Searching other resources
We handsearched:
-
citations from identified trials and relevant review articles;
-
the following conference proceedings:
-
-
ASH (American Society of Hematology) 2009 to July 2013;
-
ASCO (American Society of Clinical Oncology) 2009 to July 2013.
-
Randomised controlled trials from ASH and ASCO were handsearched by the Cochrane Haematological Malignancies Group (CHMG) and are included in CENTRAL.
In addition, randomised controlled trials from the European Group for Blood and Marrow Transplantation (EBMT), International Conference on Cutaneous T‐cell Lymphoma, ASCO and ASH up to 2010 were handsearched by the authors.
We also contacted members of the European Organisation for Research and Treatment of Cancer (EORTC) cutaneous lymphoma task force to check for ongoing study activities.
Data collection and analysis
Selection of studies
Two review authors independently screened titles and abstracts of studies identified from the above sources (Figure 1). The first screening discarded studies that were clearly ineligible. If this could not be done satisfactorily based on the title and abstract, we obtained a full text version and discussed eligibility. The aim was to be overly inclusive rather than to risk losing relevant trials. We assessed selected studies with an eligibility form to determine whether they met the inclusion criteria. We resolved any disagreement by discussion. If necessary, we would have sought further information from the authors where articles contained insufficient data to make a decision about eligibility. The eligibility form contained the following questions/criteria:
Update 2013
-
Is the study described as genetically randomised in order to avoid bias?
-
Is the diagnosis of CTCL histologically confirmed?
-
Were the participants in the experimental group treated by allogeneic stem cell transplantation?
-
Were the participants in the control group treated by chemotherapy or immuno‐chemotherapy or immunotherapy?
-
Due to the need of genetic assignment in an allogeneic stem cell transplantation trial only sibling donor versus no donor trials will be included.
Data extraction and management
Two authors would have undertaken data extraction, independently, and obtained data concerning details of study population, intervention and outcomes using a standardised data extraction form. This form would have included at least the following terms:
-
General information: author, title, source, publication date, country, language, duplicate publications;
-
Study characteristics: trial design, aims, setting and dates, source of participants, examination of proportion of the donor group (genetic assignment), inclusion/exclusion criteria, treatment allocation, comparability of groups, subgroup analysis, statistical methods, power calculations, compliance with assigned treatment, length of follow‐up, time‐point of randomisation (genetic assignment);
-
Participant characteristics: age, gender, ethnicity, number of participants recruited/allocated/evaluated, participants lost to follow up, additional diagnoses, percentage actually receiving transplant;
-
Interventions: setting, type of (multi‐agent) chemotherapy (intensity of induction and conditioning regimen, number of cycles, with or without radiation), type and dosage of immunotherapy, type and dosage of antibodies, transplantation with or without growth factor support, transplant details, infection prophylaxis, type of maintenance treatment, type of salvage treatment, duration of follow‐up;
-
Outcomes: overall survival, progression‐free survival, response rate, treatment‐related mortality, adverse events, quality of life.
The criteria were according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Where possible, we would have sought missing data from the authors.
Assessment of risk of bias in included studies
Two review authors would have independently evaluated all included trials using a list of selected quality criteria. Whether a criterion would have been fulfilled or not would have been evaluated on a three‐point scale: agree, uncertain, disagree. The criteria were according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
-
Prospective donor availability reporting.
-
Blinding of outcome assessors.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other sources of bias.
We would have resolved disagreement between the rating review authors by consensus. The review authors were not blinded to the names of authors, institutions, journals or the outcomes of the trials.
Measures of treatment effect
For binary outcomes, we planned to calculate risk ratios (RR) with 95% confidence intervals (CI) for each trial. We would have calculated time‐to‐event data as Hazard Ratios (HR) according to Parmar (Parmar 1998; Tierney 2007).
We planned to assess heterogeneity of treatment effects between trials using a Chi2 test with a significance level at P < 0.1. The I² statistic would have been used to quantify possible heterogeneity (I² > 30% moderate heterogeneity, I² > 75% considerable heterogeneity) (Deeks 2011).
In meta‐analyses with at least 10 trials, we would have explored potential publication bias by generating a funnel plot and tested this statistically by using a linear regression test (at least 10 trials) (Egger 1997; Lau 2006; Sterne 2008). We considered a P value of less than 0.1 significant for this test.
Dealing with missing data
We planned to impute missing continuous data where necessary (e.g. calculating standard deviations (SDs) from standard errors and P values) and clearly document this in the review. Where it would not have been possible to calculate missing SDs, we would have carried out available case analysis based on participants who completed a trial (Higgins 2011a).
Assessment of heterogeneity
We would have inspected heterogeneity (the degree of difference between the results of different trials) graphically in the results of the trials and assessed this by calculating a test of heterogeneity (Chi2 and I2). We planned to explore potential causes of heterogeneity by sensitivity and subgroup analysis using meta‐regression (Deeks 2011).
Assessment of reporting biases
In meta‐analyses with at least ten trials we planned to examine a funnel plot estimating the treatment effect against the precision of trials (plots of the log of the relative risk for efficacy against the standard error) in order to estimate potential asymmetry that may indicate selection bias (the selective publication of trials with positive findings) or methodological flaws in the small studies. We would also have estimated publication bias by the formal linear regression test (Egger 1997; Sterne 2008).
Data synthesis
With included studies we planned to perform analyses according to the recommendations of The Cochrane Collaboration. We would have used the Cochrane statistical package Review Manager 5 (RevMan) for analysis.
We would have pooled hazard ratios (HRs) and their confidence intervals for time‐to‐event outcomes using an inverse variance method, and risk ratios (RRs) and their confidence intervals for dichotomous data using the Mantel‐Haenszel method. We planned to use a fixed‐effect model. We would have repeated primary analyses using a random‐effects model (DerSimonian and Laird method) for sensitivity analyses.
Subgroup analysis and investigation of heterogeneity
We would have considered the following subgroups:
-
age (adults < 65 years, adults ≥ 65 years);
-
stage;
-
type and intensity of preparative regimen;
-
antibody usage;
-
immunotherapy (interferon) usage;
-
patients newly diagnosed or with relapse;
-
duration of follow‐up;
-
RIC versus non‐RIC (conditioning therapy).
We planned to assess heterogeneity of treatment effects between trials by using a Chi2 test with a significance level at P < 0.1. We would have used the I² statistic to quantify possible heterogeneity.
Sensitivity analysis
If studies had been included we would have done sensitivity analyses based on:
-
quality components, including full text publications/abstracts, preliminary results versus mature results;
-
fixed‐effect versus random‐effects model.
Results
Description of studies
We did not find any randomised controlled trials that were eligible for inclusion in this review.
Results of the search
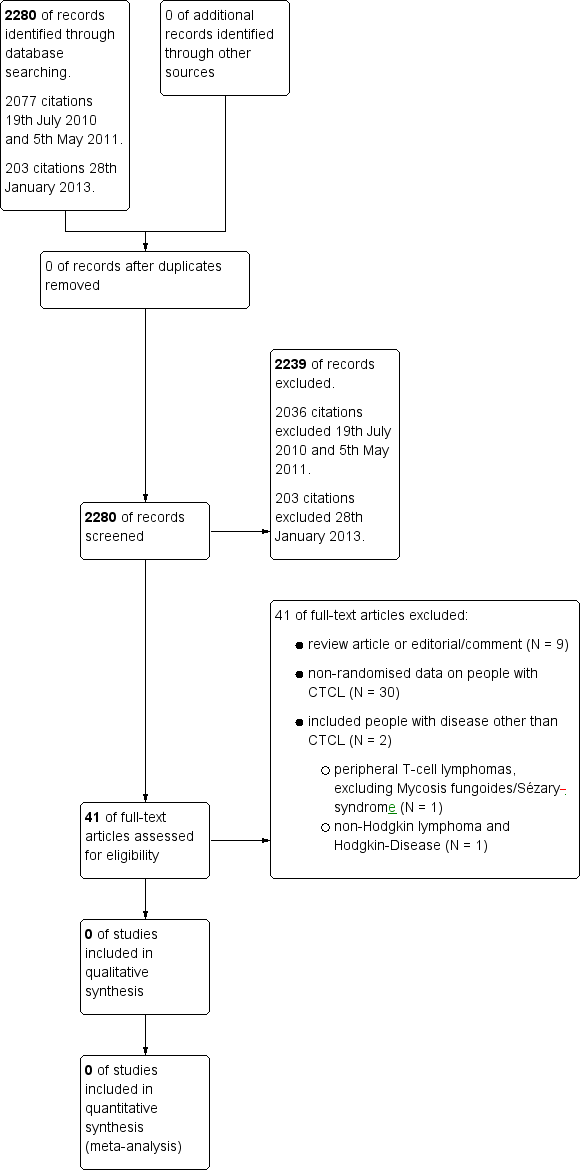
We updated the search on 28th January 2013 and found 203 citations. Altogether with the first search, we identified 2280 citations. Overall, we excluded 2239 citations (Figure 1).
We searched the first time on 19th July 2010 and then again on 5th May 2011. Altogether, we identified 2077 citations from these searches. After title and abstract screening 2036 citations were excluded. A total of 41 full texts were screened independently by two authors but no genetically or non‐genetically randomised trials could be found for inclusion in this review.
Reasons for exclusion were as follows (Figure 1):
-
review article or editorial/comment (N = 9);
-
non‐randomised data on people with CTCL (N = 30);
-
included people with disease other than CTCL (N = 2);
-
-
peripheral T‐cell lymphomas, excluding Mycosis fungoides/Sézary syndrome or cutaneous lymphomas (N = 1);
-
non‐Hodgkin lymphoma and Hodgkin disease (N = 1).
-
The search of two trial registers (run on 20th October 2010, 14th April 2011 and 2nd July 2013) identified 122 unique references to trials. Ongoing genetically or non‐genetically randomised controlled trials including CTCL could not be found. Only three ongoing non‐randomised trials including CTCL patients could be identified.
Included studies
We did not find any studies that were eligible for inclusion in this review.
Excluded studies
Forty‐one studies that were initially thought to be potentially suitable for this review by both authors were finally excluded after full text screening for being non‐randomised, not including CTCL or being review articles. For details see Characteristics of excluded studies.
Risk of bias in included studies
We did not find any studies that were eligible for inclusion in this review.
Effects of interventions
We did not find any studies that were eligible for inclusion in this review.
Discussion
No data of genetically or non‐genetically randomised controlled trials were available to show a superiority either of conventional therapy or of alloSCT for the treatment of advanced primary CTCL. This may be due to the rarity especially of advanced stage CTCL and the lack of development of consistent treatment protocols. Therefore, no validated conclusions or recommendations for clinical practice can be provided at this point.
We were unable to extract any data from genetically randomised controlled trials. However, several case series, retrospective comparisons and randomised trials for conventional therapies are available. Although this information lacks reliability, we have summarised these publications in order to demonstrate the available information until today. It should be kept in mind that all conclusions made need to be interpreted cautiously.
Since there is currently no internationally accepted standard of care for advanced forms of CTCL and no definitive treatment available which produces durable responses in these stages of disease in most patients, several case reports, case series and retrospective analyses have been done to explore if alloSCT in advanced stage CTCL can be a potential curative treatment. Retrospective analyses showed that alloSCT can lead to long‐term remissions in patients with advanced stage CTCL (Dearden 2007; Duarte 2008; Duarte 2010; Duvic 2010; Introcaso 2008; Molina 2005). Over two‐thirds of the reported patients remained alive and with no evidence of disease after a median follow‐up of more than three years (Duarte 2008). Wu et al. reported overall survival rates of 85% at one year and 80% at five years. Event‐free survival was 65% at one year and 60% at five years (Wu 2009).
This appears superior to the expected median disease‐free survival and even overall survival for patients with advanced stage CTCL in case series. Previously reported life expectancies based on overall survival at five years are 40% to 65% in stage IIB, 45% to 57% for erythrodermic stage III disease, 15% to 40% for patients with stage IVA and 0% to 15% with IVB disease (Whittaker 2003). Patients with Sézary syndrome have an even worse prognosis with an overall median survival of 32 months from diagnosis (Whittaker 2003).
With regard to the duration of response or median event‐free survival, published data on the outcome of alloSCT also show a superiority if compared to the published data on various conventional therapies in advanced CTCL. Standard conventional therapy for CTCL is stage dependent and includes a variety of topical and systemic treatments recommended by consensus guidelines (Stadler 2008; Whittaker 2003; Whittaker 2007). Patients with advanced stage MF or SS are usually treated with interferon alpha, oral bexarotene and extracorporeal photopheresis, before therapy is escalated to novel biologic agents like antibodies or cytotoxic chemotherapy (Hoff 2010). Conventional therapies in advanced stage disease can achieve high overall response rates, but most of them are short lived. This leads to many different consecutive therapies during the course of disease. Often the initial beneficial effects are lost during disease progression and patients require a more aggressive therapy in the next cycle of treatment.
Oral bexarotene, which is one of the standard therapies in early and advanced CTCL, shows an overall response rate of 45% and a median duration of response of 299 days (Apisarnthanarax 2002; Knobler 2004; Lansigan 2010; Whittaker 2003). There seems to be a dose‐dependent response rate (Hoff 2010), with 300 mg/day being the most effective dose which is tolerated well (Whittaker 2003). According to Whittaker et al., bexarotene has shown significant clinical efficacy in patients with relapsed or refractory CTCL; responses are even seen in patients with large cell transformation and with erythrodermic SS, who were refractory to other systemic therapies (Whittaker 2007). Therefore, retinoids like oral bexarotene are an important treatment option for patients with resistant and late stage disease but in most cases they do not produce durable remissions (Whittaker 2007).
Interferon alpha, another standard drug in early CTCL and generally used as first line therapy for stage IIB, III and SS (Hoff 2010), shows overall response rates ranging between 40% and 80% (Apisarnthanarax 2002; Hoff 2010; Lansigan 2010; Whittaker 2003; Whittaker 2007), with complete remissions in about 17% of the cases (Apisarnthanarax 2002; Hoff 2010; Knobler 2004; Whittaker 2003). Median duration of response is short (4 to 28 months) (Apisarnthanarax 2002), but it is also stated that several responses were durable (Lansigan 2010) and that the length of a complete response was not correlated to the stage of disease (Knobler 2004). Larger doses of interferon alpha increased the response rate (Whittaker 2003), but a poor efficacy has been shown in stage III and IV disease, in long‐lasting disease and in heavily pretreated patients (Apisarnthanarax 2002).
Histone deacetylase inhibitors, like vorinostat or romidepsin, are approved for relapsed or treatment‐refractory MF in the United States. The overall response rates in stages IIB to IV are 25% to 30% (Whittaker 2007), with an overall response rate of 24% in highly refractory patients for vorinostat and a response rate of 42% in stages IB to IV for romidepsin (Gardner 2009). Lansigan et al. reported a response rate of 30% for vorinostat as well as for romidepsin, with a response duration of 9.8 months for vorinostat and 8 to 14 months in refractory, heavily pretreated patients for romidepsin (Lansigan 2010).
In the United States, denileukin diftitox has been recently approved for treatment of patients with relapsed MF if tumours express a special subunit of the interleukin‐2 receptor (CD25). At least 20% of lymphocytes in skin biopsies have to express CD25 to justify the use of denileukin diftitox (Lansigan 2010). Overall response rates in stages IB to IV were 30% with complete remission in 10% of the cases. Median duration of response was only 6.9 months from time of first dose (Apisarnthanarax 2002; Gardner 2009; Knobler 2004; Lansigan 2010; Whittaker 2003; Whittaker 2007). Patients experienced an improved quality of life (Knobler 2004) and there are also single reports of durable responses which lasted between five and nine years (Gardner 2009).
Another treatment, psoralen+UV‐A (PUVA), shows an overall response rate of 95% in all stages but seems to be mainly effective in stage I disease. However, patients with more extensive disease, erythroderma or tumours do not have good response rates or relapse quickly (Knobler 2004). In stage IIB no significant response has been seen (Whittaker 2003; Whittaker 2007). One study showed a complete response rate of 46% in stage III with a maximum duration response of 5.7 years. Regarding disease‐specific survival at 5 and 10 years there was no difference between patients who relapsed when compared to those who did not (Whittaker 2007). Although some patients with late stage disease responded to PUVA, most of the responses were non‐durable at the end of the study periods (Apisarnthanarax 2002). As the response to PUVA in late stage disease is usually poor, it is only recommended as palliative therapy (Apisarnthanarax 2002).
Extracorporeal photopheresis (ECP) is generally used as a first line systemic therapy for erythrodermic disease (stage III) and Sézary syndrome. Compared to historical control groups, patients treated with ECP show a prolonged survival (median survival of 30 months, as compared to 60 months), and durable responses are not uncommon (Apisarnthanarax 2002; Hoff 2010). Overall response rates in stages III to IV differ between 50% and 88% with a complete response rate of 14% to 25% (Apisarnthanarax 2002; Knobler 2004; Whittaker 2003). Other studies show an overall response rate in stage III of 73% in relapsed MF or SS with a median survival of 5.2 years in erythrodermic patients and 3.3 years in SS patients (Whittaker 2003). To achieve a good response it seems important to have a CD4:CD8 ratio < 10 (Knobler 2004) and the best responders are those patients with a short duration of disease, an absence of lymphadenopathy or visceral disease, low Sézary cell counts (10% to 20%) and a lack of prior immunosuppressive chemotherapy (Apisarnthanarax 2002; Lansigan 2010).
Monoclonal antibodies have been developed since the 1980s to specifically target malignant cells and spare normal tissue in order to minimise adverse side effects. One monoclonal antibody that has shown efficacy in the treatment of MF or SS is alemtuzumab, which targets the CD52‐antigen on malignant and normal T‐cells. Alemtuzumab has shown high overall responses rates in MF and SS patients, with an overall response rate of 50% and a complete response rate of 25% (Apisarnthanarax 2002; Whittaker 2007). However, because the duration of response is short and severe side effects like opportunistic infections, viral reactivation (cytomegalovirus), prolonged lymphopenia and cardiac toxicity exist, the use of alemtuzumab has a limited role in the treatment of CTCL (Apisarnthanarax 2002; Hoff 2010). Low‐dose subcutaneous alemtuzumab seems to be well tolerated and effective in palliative treatment for MF or SS patients, with a low risk for opportunistic infections (Gardner 2009). Zanolimumab, a monoclonal antibody which targets the CD4 receptor on T‐lymphocytes, has also shown high response rates with an overall response rate of 56% in refractory CTCL patients and a median duration of response of 81 weeks (Gardner 2009; Lansigan 2010). Adverse side effects are comparably limited, with low‐grade infections and eczematous dermatitis being the most frequently reported (Gardner 2009; Lansigan 2010).
Total skin electron beam therapy (TSEB) is used for CTCL patients with infiltrative plaques, tumours or erythrodermic disease. The rate of complete remission after TSEB is dependent on the stage of disease and the treatment seems to be mainly effective in stage III disease (erythrodermic CTCL). So far, there are no consistent data on remission rates, disease‐free survival or overall survival after TSEB therapy. Complete response rates of 60%, a five‐year disease‐free survival of 26% and a median overall survival of 3.4 years have been reported (Whittaker 2003; Whittaker 2007). It has been shown that higher rates of complete responses and disease‐free progression can be achieved with a more intense regimen (Whittaker 2003; Whittaker 2007). Other studies report a complete remission rate of 24% in stage IIB, 26% in stage III and 8% in stage IV with a five‐year relapse‐free survival of 2% in stage IIB, 10% in stage III and 0% in stage IV (Knobler 2004). Gardner et al. observed complete response rates of 50% in advanced refractory CTCL, with an overall one‐year survival of 48% and most of the patients relapsing within one year after completing treatment (Gardner 2009). Although data are not consistent, it is undisputed that TSEB comprises one of the most effective skin‐directed therapies in tumour stage and erythrodermic patients, even though responses may be short lived in most cases. Especially in combination therapy TSEB can achieve good results: TSEB plus adjuvant ECP has shown an improvement of overall survival in stages T3/T4. TSEB combined with ECP even improved disease‐free survival, cause‐specific survival and progression‐free survival in stage T4 (Apisarnthanarax 2002). TSEB with prior chemotherapy showed longer disease‐free survival in stages IIB‐IV but overall survival was not affected significantly (Knobler 2004). Other studies combining TSEB and chemotherapy showed a higher response rate when compared to topical sequential therapy (PUVA, TSEB or oral methotrexate), but disease‐free survival and overall survival were not affected in late stage disease (Apisarnthanarax 2002).
Although responses to systemic chemotherapies are usually short lived, single‐agent and multi‐agent chemotherapies are frequently used in patients with late stage disease and achieve high overall response rates. Gemcitabine, a pyrimidine antimetabolite, has demonstrated impressive clinical activity with a response rate of 70% and a median duration of response of eight months in chemotherapy‐naive patients with advanced and refractory CTCL (Lansigan 2010; Whittaker 2007). Another study also showed a response rate of 70% with complete responses in 11% of the cases and a 15‐month median duration of response with a dose of 1200 mg/m² (Whittaker 2007). Data published by Apisarnthanarax et al. shows similar response rates and also reports on observed tumour reduction and flattening in five of six patients with stage IIB. One patient with stage IVB even achieved complete visceral response of hepatic lesions (Apisarnthanarax 2002). These observations indicate that gemcitabine may be an effective therapy for the treatment of MF, especially in the tumour stages of disease (Apisarnthanarax 2002). Doxorubicin, used as a pegylated liposomal form, shows overall response rates between 80% and 88% with complete responses of 42% up to 60% and a median disease‐free survival of 12 to 13.3 months (Apisarnthanarax 2002; Lansigan 2010; Whittaker 2007). Methotrexate (MTX), in late stage disease mostly used orally, achieves overall response rates of 58% with complete response of 41% in stage III disease, median survival is 8.4 years (Apisarnthanarax 2002; Whittaker 2003). Low to medium doses of 25 mg to 75 mg weekly are sufficient to achieve sustainable remissions and seem to be well tolerated with only minimal side effects (Hoff 2010). Combined chemotherapy (e.g. CHOP) achieves an overall response of 80% with a complete response of 27%, but a median duration of response of only eight months and a median survival of 13.5 months in heavily pretreated patients (Lansigan 2010; Whittaker 2007). Other trials showed overall response rates of 81% in late stage disease with a complete response of 38% and a median duration of response ranging from 5 to 41 months (Apisarnthanarax 2002; Whittaker 2003). Owing to significant treatment‐related toxicity, such as myelosuppression and high risk of infection, and the often low durations of response with multi‐agent chemotherapy, it should be used only in patients who are refractory to other treatments or who present with extensive adenopathy and visceral involvement and require immediate palliation (Whittaker 2007). Despite a high number of chemotherapeutic agents, no single‐ or multi‐agent regimen seems to be significantly superior to other cytotoxic substances and no specific regimen has been found to increase survival (Apisarnthanarax 2002; Horwitz 2008; Knobler 2004). All authors recommend that various combined therapies should be considered before moving on to systemic cytotoxic chemotherapy, since even single‐agent chemotherapy can lead to problematic short‐ and long‐term haematologic toxicity (Hoff 2010).
Since no conventional therapy is capable of repeatedly delivering durable remissions until now, autologous and allogeneic stem cell transplantation have been investigated for their use in advanced CTCL. Autologous stem cell transplantation (autoSCT) achieved promising complete responses of 83% but median progression‐free survival ranged from less than 100 days to about seven months (Apisarnthanarax 2002; Duarte 2008). Overall survival rates after autoSCT were 68% at one year and 23% at five years, event‐free survival rates were 20% at one year and 0% at five years (Wu 2009).
Due to the poor results of autoSCT, alloSCT remained to be examined as treatment for advanced CTCL. Although only a small number of patients have been treated with alloSCT until now, the results are consistent and promising. All cases of alloSCT in advanced stage CTCL, myeloablative and non‐myeloablative, published to date, show a decreased relapse rate and increased overall‐ and event‐free survival when compared to published data about conventional therapies as described above. The latest report by Duarte et al., being the first large retrospective multicentre analysis of alloSCT for MF and SS, showed an incidence of relapse of 38% at one year and 47% at three years after transplantation. Progression‐free survival was 42% at one year and 34% at three years. The current progression‐free survival at the last published follow‐up was 52% in patients who received non‐myeloablative alloSCT and 29% in patients who received myeloablative alloSCT. Estimated overall survival rate was 66% at one year and 53% at three years (Duarte 2010).
The use of allogeneic stem cells offers the advantage of a tumour cell free graft and provides the basis of adoptive immunotherapy (Foss 2004). The increased overall survival may be consistent with the potential graft‐versus‐lymphoma (GVL)/tumour effect, mediated by donor T‐cells. Unfortunately, the GVL effect lacks specificity, resulting in additional targeting of normal tissue which leads to one of the major complications after alloSCT: a high incidence of severe graft‐versus‐host‐disease mediated by allo reactive T‐lymphocytes (Ringdén 2009). Separation of the GVL effect from GvHD is difficult but needs further investigation, as 30% to 50% of patients clinically develop GvHD and 30% to 50% of the patients with severe acute GvHD die as a consequence of GvHD (Hoff 2010). Chronic GvHD requires continued immunosuppression, which can cause various infections and is the most significant determinant of long‐term morbidity in patients undergoing alloSCT (Hoff 2010). In the reported 20 cases by Wu et al., 90% of the alloSCT patients experienced acute or chronic GvHD. In the final follow‐up (median of 45 months) 70% of patients presented with a GvHD and two patients died because of GvHD complications (Wu 2009). In the latest case series reported by Duvic et al., TSEB was used as preparative regimen in 15 patients for debulking the skin before transplantation with the intention of reducing the severity of cutaneous GvHD. However, there is no evidence that this had an effect on the severity of acute or chronic GvHD. Four of 15 patients receiving TSEB did not show any signs of GvHD compared to two of four patients not receiving TSEB (Duvic 2010). Whether the combination with TSEB is an effective treatment to prevent or to reduce the GvHD merits further investigation, since TSEB is a conventional therapy that by itself can achieve a median overall survival of 3.4 years (Whittaker 2007) and because of the negative impact of GvHD on morbidity and mortality. Nevertheless, limited GvHD (especially mild chronic GvHD) is associated with prolonged disease‐free survival, providing evidence for a simultaneously present GVL effect (Ringdén 2009). As GvHD frequently occurs in the skin, the major manifestation site of MF or CTCL, this phenomenon may also contribute to the efficacy of alloSCT in this disease entity (Giralt 2001).
Beginning with the early 1970s and during the first decades of clinical alloSCT or bone marrow transplantation, myeloablative conditioning regimens were regularly used as standard treatments. These regimens were accompanied by a high rate of treatment‐related mortality. Therefore, alloSCT represented a treatment option only for younger patients (< 55 years of age) in good general condition. In parallel with the increasing optimisation of supportive therapies during the last 20 years, specifically with anti‐infectious medications, treatment‐related mortality of the myeloablative conditionings could be lowered significantly but still represents a clinical problem (Aoudjhane 2005; Karanes 2008). Pioneered by the transplantation group of the Fred Hutchinson Cancer Center in Seattle, USA, RIC regimens were developed in order to decrease treatment‐related toxicity and mortality during the early phase of alloSCT (Feinstein 2001). The underlying principle of the RIC regimens is to provide sufficient immunosuppression to facilitate engraftment without the high toxicity of conventional myeloablative regimens. This approach successfully opened the way to alloSCT also for older (> 55 years and even > 65 years of age) or multi‐morbid patients (Ljungman 2010; Rocha 2009; Storb 2009). Today, RIC regimens mostly consist of fludarabine in combination with alkylating agents or low dose total body irradiation (2 Gray) in combination with immunosuppression by cyclosporine A plus mycophenolate mofetil or short course methotrexate.
In contrast to the classic myeloablative regimens, there is a generally increased risk of relapses after RIC regimens because of the lowered cytotoxic efficacy and the not fully established donor‐derived immunosystem during the early post‐transplant period (Aoudjhane 2005). Kolb and colleagues have successfully introduced donor lymphocyte infusions in patients suffering from relapsed chronic myelogenous leukaemia (CML) after alloSCT as an effective method to enhance the graft‐versus‐leukaemia effect (Kolb 1998). This approach was extended to other malignancies and a number of RIC‐based treatment protocols nowadays include the prophylactic use of donor lymphocyte infusions (DLI) beginning at a certain point of time after alloSCT. Donor lymphocyte infusions usually contain a mixture of all circulating white blood cells including the lymphocytic part which consists mostly of CD3+ T‐cells. The administration of prophylactic or therapeutic DLI has become an important component of the treatment after alloSCT (Roddie 2011). The anti‐leukaemic activity of DLI is dependent on the immunogenicity of the underlying disease. The most susceptible disease towards DLI is CML followed by acute myelogenous leukaemia and lymphatic malignancies (Kolb 2008). Therefore, the results following administration of DLI are heterogeneous. It has been shown as a proof of principle that relapsed CTCL or SS patients after alloSCT are susceptible to DLI; therefore, justifying the use in these patients (Duarte 2010).
The first reports about CTCL or SS patients who were transplanted with cells from an allogeneic donor were published during the mid 1990s. These selected patients received myeloablative conditioning regimens and were, therefore, young (< 55 years of age) and clinically in good general condition (Koeppel 1994; Molina 1999). As the median age of onset of CTCL or SS is between 60 and 65 years, those cases did not reflect the typical clinical situation. For these patients, RIC‐based transplant regimens seem to represent a promising treatment approach, especially when combined with post‐transplant therapies with DLI.
The oldest patient reported until now is a 66‐year old patient included in the retrospective analysis of allogeneic haematopoetic cell transplantation for patients with advanced stage MF and SS, undertaken by the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation, published by Duarte et al. (Duarte 2010). Unfortunately, sex, stage of disease, treatment regimen (myeloablative or non‐myeloablative) and outcome are not mentioned individually,.Therefore, it is not possible to assess how a patient at this age and with advanced CTCL responds to alloSCT. It is also not mentioned how many patients were older than 60 years, only a median age of 46.5 years with a range from 22 to 66 years is stated (Duarte 2010). Before this study was published, the oldest patient who received non‐myeloablative alloSCT reported in the literature was a 63‐year old female who died one year after transplantation as a result of early sepsis and in progressive disease. In the same study was a 60‐year old male in complete remission with no signs of GvHD 20 months after transplantation, and a 61‐year old male in stable disease with acute and chronic GvHD 19 months after transplantation (Duvic 2010). Prior to these reports, only one patient aged 60 was described receiving non‐myeloablative alloSCT and being alive with no evidence of disease 24 months after transplantation (Soligo 2003). Although advanced CTCL is a disease of the older population, until now there are only four cases of patients aged 60 or older among the total of about 40 reported cases (Duarte 2008; Duvic 2010; Wu 2009). In addition to that, an unknown number of patients aged 60 years or older is included in the latest retrospective analysis by Duarte et al. including 55 previously unreported cases (Duarte 2010). Authors of case reports, case series and retrospective analyses agree that alloSCT appears to be useful in younger patients with advanced CTCL. With respect to the average age of disease, non‐myeloablative alloSCT in particular needs to be investigated intensively in older patients and also patients with comorbidity to evaluate if this treatment is an option for the majority of CTCL patients. Against the background of growing experience with alloSCT, the aging population and the better management of side‐effects, it is expected that more and more elderly patients will be treated. An ongoing study about non‐myeloablative alloSCT in CTCL at Stanford University includes patients up to the age of 75 years. Another clinical trial about peripheral alloSCT with non‐myeloablative conditioning at the National Heart, Lung and Blood Institute (NHLBI) at the National Institutes of Health (NIH) in Maryland includes patients up to the age of 70 years. Although these studies also are non‐randomised, uncontrolled, single‐armed clinical trials it is likely that more data about patients between 60 and 75 years of age will be available in the future.
Until now, the optimal conditioning regimen and the best timing of alloSCT in the course of disease remains unknown. All twenty‐one patients who underwent alloSCT reviewed by Duarte et al. were pretreated, with a reported median of seven prior treatments (Duarte 2008). The allogeneic group reviewed by Wu et al. received a median number of 6.5 therapies prior to SCT (Wu 2009). Patients in the latest reports by Duvic et al. and Duarte et al. had a median number of four prior therapies (Duarte 2010; Duvic 2010). As these reviews and reports include all case reports and case series conducted to the date of their publication, it seems that mainly patients were included who had a long history of disease or an aggressive course that resulted in high numbers of prior treatment. Contrary to this is the suggestion by Duarte et al. that alloSCT can show improved results if the procedure is performed early during the course of the disease and before patients get heavily pretreated, particularly for patients receiving non‐myeloablative alloSCT (Duarte 2008). In this publication it remains unclear what exactly early and heavy pretreatment means, as only three of 21 patients reported in the review had less than four prior therapies and it is not mentioned which specific kind of therapy they received before inclusion into the studies. In the report published in 2010, Duarte et al. at least clarified the term 'early' as "patients with advanced stage MF/SS in first or second complete response, partial response or relapse/progression having received three or fewer prior lines of systemic therapy" (Duarte 2010). Patients in this defined early phase of disease showed decreased non‐relapse mortality and relapse/progression but increased progression‐free survival, overall survival and current progression‐free survival at last follow‐up (Duarte 2010). These results support the assumption expressed two years before. Likewise, Ringdén et al. imply that an early transplant (earlier stage of disease with less tumour burden) can increase effectiveness of GVL activity and decrease the risk of cancer recurrence after alloSCT (Ringdén 2009). These assumptions demonstrate the importance of controlled trials for alloSCT, especially with non‐myeloablative conditioning, to clarify whether this treatment can induce long‐term complete remissions in the early phase of advanced disease. This could reduce the use of systemic therapies which often show significant treatment‐related toxicity (especially multi‐agent cytotoxic chemotherapy) and induce only short‐lived responses in the majority of cases. On the other hand, the recommendation of early alloSCT does not change the fact that alloSCT should be considered in patients with refractory disease or short‐lived responses with standard therapies (Wu 2009), uninfluenced by the number of prior therapies or the state of disease. Until now, alloSCT seems to be a treatment which is able to induce long‐lasting complete and durable remissions, at least in some patients.
The latest publication by Duvic et al. is the largest single centre study conducted so far which uses non‐myeloablative alloSCT. It includes 19 patients with a median age of 50 years and a median of four prior therapies (Duvic 2010). This selection appears to be superior to earlier studies which had a median age of 42 years and an average of 6.5 prior therapies (Wu 2009), considering the suggestion that results of RIC alloSCT improve when used after less heavy pretreatment. Although the median age implies that some older patients were included, even here only patients younger than 65 years of age were eligible for inclusion. Ten out of 19 patients were in complete clinical remission immediately before transplantation, seven patients were in partial response and none had progressive disease at the time of transplantation (Duvic 2010). Therefore, it is not clear whether the therapeutical success of complete remission is based on prior therapies and whether these patients would have achieved the same results without alloSCT. Duvic et al. reported 58% complete clinical and molecular remissions with a median follow‐up of 1.7 years (Duvic 2010). This validates the results of other reports with 65% event‐free survival at one year (Wu 2009) or 42% progression‐free survival at one year (Duarte 2010) after alloSCT.
Summary of main results
According to the search and quality criteria, we found no studies to be eligible for inclusion in this review. All 41 studies that were thought to be potentially suitable by both screening authors were finally excluded after full text screening for being non‐randomised, not including CTCL or being review articles. Nevertheless, several retrospective analyses and case series addressed the question of alloSCT for patients with advanced CTCL or Sézary syndrome. According to these publications with limited evidence, alloSCT has the potential to achieve long term remissions. The use of reduced intensity conditioning offers the possibility to treat older patients. Until now, genetically randomised controlled trials are lacking to precisely assess the role of alloSCT in advanced CTCL. Therefore, it should be kept in mind that all conclusions made are not associated with a high level of evidence and need to be interpreted carefully.
Overall completeness and applicability of evidence
We did not find any studies that were eligible for inclusion in this review.
Quality of the evidence
Due to the fact that we found no eligible studies, Grading of Recommendations Assessment, Development and Evaluation (GRADE) could not be performed.
Potential biases in the review process
No biases in the review process are known.
Agreements and disagreements with other studies or reviews
We agree with findings of Duarte et al. 2010 (Duarte 2010) supporting allogeneic stem cell transplantation as a treatment option for advanced cutaneous T‐cell lymphoma.