Uso de historias clínicas electrónicas para apoyar el abandono del hábito de fumar
Resumen
Antecedentes
Los sistemas de información sanitaria, como las historias clínicas electrónicas (HCE), los sistemas computarizados de apoyo a la toma de decisiones y la prescripción electrónica son componentes potencialmente valiosos para mejorar la calidad y la eficiencia de las intervenciones clínicas para el hábito de fumar.
Objetivos
Evaluar la eficacia de las intervenciones facilitadas por las historias clínicas electrónicas en las acciones de apoyo para el abandono del hábito de fumar realizadas por los médicos, los consultorios y los sistemas de prestación de asistencia sanitaria y en los resultados del abandono del hábito de fumar por parte de los pacientes.
Métodos de búsqueda
Se hicieron búsquedas en el Registro especializado del Grupo Cochrane de Adicción al Tabaco (Cochrane Tobacco Addiction Group), CENTRAL, MEDLINE, EMBASE, PsycINFO, CINAHL y en las listas de referencias y bibliografías de los estudios incluidos. Se hicieron búsquedas de estudios publicados entre enero 1990 y mayo 2014.
Criterios de selección
Se incluyeron los estudios aleatorizados y no aleatorizados que informaron sobre intervenciones orientadas al hábito de fumar a través de una HCE en contextos de atención sanitaria. La intervención podía incluir cualquier uso de una HCE para mejorar la documentación del estado del hábito de fumar o la ayuda para el abandono en pacientes que consumen tabaco, ya sea por acción directa o por retroalimentación de las medidas de resultado clínicas.
Obtención y análisis de los datos
Un autor de la revisión extrajo las características y el contenido de las intervenciones, los participantes, los resultados y los métodos de los estudios incluidos y un segundo autor de la revisión los verificó. Debido a la gran variación en la medición de los resultados, no fue posible realizar un metanálisis.
Resultados principales
Se incluyeron seis ensayos aleatorizados grupales, un estudio aleatorizado de pacientes y nueve estudios observacionales no aleatorizados de calidad aceptable a buena, que probaron el uso de una HCE existente para mejorar la documentación y el tratamiento del hábito de fumar. Ninguno de los estudios incluyó una evaluación directa de las tasas de abandono de los pacientes. En general, estos estudios encontraron sólo una mejoría moderada en algunas de las acciones recomendadas por el médico sobre el hábito de fumar.
Conclusiones de los autores
La documentación del estado del hábito de fumar y la remisión al asesoramiento para dejar de fumar parece aumentar después de las modificaciones de las HCE diseñadas para promover el registro y el tratamiento del hábito de fumar en las consultas. Es necesario realizar más estudios de investigación para mejorar el potencial de las HCE para promover un tratamiento adicional del hábito de fumar y los resultados del abandono en contextos de atención sanitaria.
PICOs
Resumen en términos sencillos
¿El uso de una historia clínica electrónica mejora la administración del tratamiento para dejar de fumar a los pacientes?
En muchos países se hacen grandes inversiones en tecnología para digitalizar las historias clínicas de los pacientes. Una de las posibilidades de las historias clínicas electrónicas (HCE) es que se podrían utilizar para recordar a los médicos y a otro personal médico que registren el hábito de fumar, proporcionen asesoramiento breve para dejar de fumar, prescriban fármacos y consulten el asesoramiento para dejar de fumar. También podrían ayudar a remitir a las personas a estos servicios y ser utilizados para medir el rendimiento de una clínica. Las HCE también podrían contribuir a que la administración de tratamientos contra el hábito de fumar se convierta en una práctica habitual al proporcionar remisiones electrónicas para servicios de tratamiento adicionales (p.ej., remisión a una línea telefónica para dejar de fumar). En esta revisión se incluyeron 16 estudios, nueve de los cuales fueron estudios observacionales, por lo que su calidad es inferior a la de los ensayos controlados aleatorizados. De las acciones recomendadas para los médicos que tratan a pacientes que consumen tabaco, sólo se encontraron modestas mejoras asociadas con los cambios en la HCE. Concretamente, la documentación sobre el hábito de fumar y la remisión a asesoramiento para dejar de fumar parecen aumentar tras los cambios en la HCE. Sin embargo, estos estudios no probaron ni demostraron un aumento en el número de personas que dejaron de fumar.
Authors' conclusions
Summary of findings
| Use of electronic health records to support smoking cessation | ||||
| Patient or population: People who smoke Settings: Healthcare clinics Intervention: Any use of an Electronic Health Record (EHR) to improve smoking status documentation or cessation assistance for patients who use tobacco, either by direct action or by feedback of clinical performance measures. Comparison: No EHR, or EHR without support for smoking cessation intervention | ||||
| Outcomes | Effect | No of Participants | Quality of the evidence | Comments |
| Smoking cessation | More intervention clinic than control clinic smokers quit (5.3% vs 1.9%, p < 0.001) | 1 cluster RCT, 26 clinics | ⊕⊝⊝⊝ very low1 | Indirect measurement based on EHR documentation of smoking status |
| Guideline recommended actions | Studies typically showed positive effects on outcomes including documenting smoking status, giving advice to quit, assessing interest in quitting, and providing assistance including referral. | 6 cluster RCTs, 98 clinics | ⊕⊕⊕⊝ Moderate2 | Studies did not all assess the same outcomes. Non randomized and uncontrolled studies also showed positive effects |
| GRADE Working Group grades of evidence | ||||
| 1 Only one study reported the outcome, and did not use direct patient report of cessation 2 Heterogeneity in the interventions and targeted behaviours | ||||
Background
Description of the condition
In 2012, an estimated 31.1% of men and 6.2% of women worldwide were daily smokers (Ng 2014). Although daily smoking has reduced among men and women, population growth has led to a significant increase in the number of smokers around the world (Ng 2014). Tobacco use currently kills more than five million people each year and this number is expected to increase substantially (WHO 2009). Even if prevalence rates remain unchanged, an estimated 500 million people will die as a direct result of tobacco usage over the next fifty years (WHO 2002).
The healthcare setting remains an underused venue to provide cessation assistance to tobacco users, particularly in developing countries. Recognizing this, Article 14 of the World Health Organization (WHO) Framework Convention on Tobacco Control emphasizes the necessity of promoting evidence‐based tobacco cessation and disseminating comprehensive guidelines and best practices. To achieve the goals of Article 14, such evidence‐based clinical practice guidelines exist, outlining strategies that healthcare settings can use to help smokers quit (Fiore 2008; NHS 2011).
Evidence‐based clinical practice guidelines for tobacco cessation support recommend systematic identification and intervention for tobacco use. Changes in health systems operations that institutionalise the identification and clinical treatment of patients using tobacco are a particularly promising way to take advantage of the primary care visit to help patients quit tobacco use (Fiore 2008).
A system level change that might increase the frequency of effective cessation delivery is to take advantage of the electronic medical record for clinician reminders, linking patients to cessation services, monitoring performance, and providing feedback.
Description of the intervention
We included both direct and indirect types of electronic health record (EHR)‐based interventions. EHRs could be used directly to remind clinicians to document tobacco use, to deliver brief advice, and to prescribe cessation medications, as well as to facilitate other cessation support such as referral to counselling.They also could be used indirectly to provide performance measures of cessation support by clinics or individual clinicians that are then publicly reported or fed back to those studied or to leaders for quality improvement.
How the intervention might work
Treatment for tobacco use in a healthcare setting first requires an assessment of tobacco use and patient willingness to stop using tobacco (Fiore 1991). Healthcare clinician advice has a small effect on cessation ‐ leading to approximately three to six per cent of patients stopping using tobacco (Stead 2013). However, higher rates of cessation are achieved when a coordinated system within the healthcare setting facilitates evidence‐based actions such as cessation counselling and use of cessation medications (Fiore 2008). In the absence of electronic records, a stamp or similar visual aid in a paper chart can serve as a clinician reminder to discuss tobacco use, to provide treatment, and to facilitate referrals. Chart audits by hand can also provide performance measure information needed for quality improvement. However, these paper‐based methods are time and resource expensive and unlikely to be performed consistently. EHRs provide a systematic mechanism to improve the fidelity of following clinical practice guidelines consistently (Hesse 2010).
Why it is important to do this review
Health information systems such as EHRs, computerized decision support systems, and electronic prescribing are increasingly identified as potentially valuable components to improve the quality and efficiency of patient care. EHRs are also very likely to disseminate rapidly, at least in developed countries, as healthcare systems modernize away from paper records.
Two occurrences ‐ inadequate tobacco cessation support during clinical encounters (Agaku 2014) and the rapid dissemination of EHRs ‐ create a need to evaluate the evidence for any beneficial connections between the two, and to identify any gaps in this evidence requiring additional research.
Objectives
To assess the effectiveness of electronic health record‐facilitated interventions on smoking cessation support actions by clinicians, clinics and healthcare delivery systems, and on patient smoking cessation outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials and observational studies (cohort, case‐control, cross sectional) were included. We included observational studies that included sufficient transparency in design, analysis, and reporting results.
Rationale for including non‐randomized studies: Our primary aim in this review is to determine the extent of evidence supporting EHRs as a means of enhancing the delivery of effective tobacco use cessation treatments in healthcare settings. Most clinical research in healthcare settings, including preventive measures such as smoking treatment, have involved observational rather than randomized studies. In part this reflects the challenges of conducting research in the healthcare setting. Therefore it is especially important to learn what we can from observational studies. Well‐done observational designs have the potential to fill the need for evidence when it is unavailable from randomized trials as well as to supplement those trials. Journal editors are now providing guidance for the publishing of observational studies, for example, the STROBE checklist (strobe‐statement.org) is now required for publication in some journals (The PLOS Medicine Editors 2014)
Types of participants
Participation can be considered at the individual patient level and at the clinic level where a group of clinics is the unit of randomization.
Types of interventions
We included any interventions that involved electronic health record systems in healthcare settings that were intended to improve documentation or assistance for patients who use tobacco, either by directly prompting clinician, clinic, or health system action or by measuring and reporting on clinical performance.
Types of outcome measures
Primary outcomes
Included studies measured abstinence from smoking at a minimum of six months from the date of the intervention. Smoking status might be measured directly from patient self‐reports or indirectly from patient medical records. We did not require biochemical validation of quit rates.
In addition to quit rates we included changes in smoking cessation support actions by clinicians, clinics, and health systems. These steps include: Ask ‐ systematically identify all tobacco users; Advise ‐ advise all users to quit; Assess ‐ determine willingness to make a quit attempt; Assist ‐ provide tobacco cessation counselling and medications; and Arrange ‐ ensure follow‐up contact. Changes in the rates of these action steps are important outcomes, since there is good evidence that they are associated with increased quit rates (Fiore 2008).
Search methods for identification of studies
Electronic searches
We searched the Specialised Register of the Cochrane Tobacco Addiction Group: this register includes controlled studies identified by systematic electronic searches of various databases including CENTRAL, MEDLINE, EMBASE, PsycINFO, hand searching of relevant specialty journals, conference proceedings and ’grey literature’ (e.g. unpublished reports, literature which is not covered by most electronic databases). We searched for the following keywords; 'Medical Records Systems*' OR 'Electronic Health Records*', or the following combinations of terms in title or abstract: '(electronic or automated or medical) AND record*'. See Appendix 1 for full strategy. The Register search was updated in July 2014. At the time of the search the Register included the results of searches of the Cochrane Central Register of Controlled trials (CENTRAL), issue 6, 2014; MEDLINE (via OVID) to update 20140627; EMBASE (via OVID) to week 201427; PsycINFO (via OVID) to update 20140630. See the Tobacco Addiction Group Module in the Cochrane Library for full search strategies and list of other resources searched.
In addition, we searched the following electronic databases without study design term limits in order to identify observational studies; The Cochrane Central Register of Controlled Trials (CENTRAL) via Cochrane Library, PUBMED (MEDLINE), OVID CINAHL, ISI Web of Science, Engineering Village, EMBASE, and Academic Search Premier. In each database we searched for the combination of the following key terms: (1) 'medical records' or 'health records'; (2) 'electronic' or 'automated'; (3) 'smoking or tobacco'; (4) 'cessation or quitting'; (5) 'feedback or reminders'. We limited these searches to records where at least the abstract was published in English from January 1990 through March 2014.
Searching other resources
In addition, we scanned the reference lists of retrieved studies for additional papers.
Data collection and analysis
Patient randomized or group randomized trials were analysed separately from non‐randomized studies.
Selection of studies
The title and abstract of records identified using the keyword searches were read independently by two of the authors. We looked for studies of interventions involving adult smokers and an electronic medical or health record that was used to directly or indirectly facilitate cessation support (e.g. by providing audit and feedback, by increasing rates of tobacco user identification and documentation).
Data extraction and management
The full text of each relevant article was read and study quality was assessed using a data abstraction form. Two authors independently extracted data about the research design, outcomes, and analysis, and all three authors adjudicated any significant differences between the two extracts.
Assessment of risk of bias in included studies
We estimated the risk of bias (ROB), including both the direction and magnitude. We independently assessed the ROB in randomized trials using the following ROB items:
(1) The presence of any sequence generation during randomizations
(2) Allocation sequence concealment
(3) Blinding of providers, participants and outcome assessors
(4) The completeness of outcome data
(5) Selective outcome reporting
We categorized each trial as being at low, unclear, or high risk of bias according to the standards described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We recognise that the potential biases are likely to be greater in observational studies. We used the ROB items as a starting point to assess included observational studies.
Measures of treatment effect
For the randomized trials we examined the treatment methods to determine if there was an acceptable level of inter‐study homogeneity to enable us to draw any inference.
Unit of analysis issues
For group randomized trials we determined if appropriate multilevel or other statistical methods were used to correct for non‐independence within groups.
Dealing with missing data
For a clinical trial that did not specify, we assumed an intention to treat analysis was followed ‐ this assumes missing participants have not quit smoking but are still included in the denominator.
Data synthesis
Rather than pool the included studies we reported descriptively the relationships between and within studies because there was no commonality among the outcomes reported.
Subgroup analysis and investigation of heterogeneity
We did not test for statistical heterogeneity or perform any subgroup analyses. The majority of studies involved patients seen in general medicine or primary care clinics. One study involved patients seen in primary dental care clinics.
Sensitivity analysis
We did not conduct a sensitivity analysis of included studies.
Results
Description of studies
We screened 69 records in the most recent update. A total of 16 studies met the eligibility criteria (Figure 1). Details of the design, intervention, and measures are presented in the Characteristics of included studies table. All of the studies except one (Frank 2004, Australia) were conducted in the United States. Fourteen of the studies were conducted in general practice/primary care medical clinics. One study (Rindal 2013) was conducted in dental clinics and another (Koplan 2008) was conducted in a single, large hospital. Overall six studies were group randomized trials (Bentz 2007; Linder 2009; Rindal 2013; Sherman 2008; Vidrine 2013; Vidrine 2013a), and one was a patient randomized study conducted in a single clinic (Frank 2004). A further two studies included a control or comparison group, and seven measured outcomes using a before and after design.
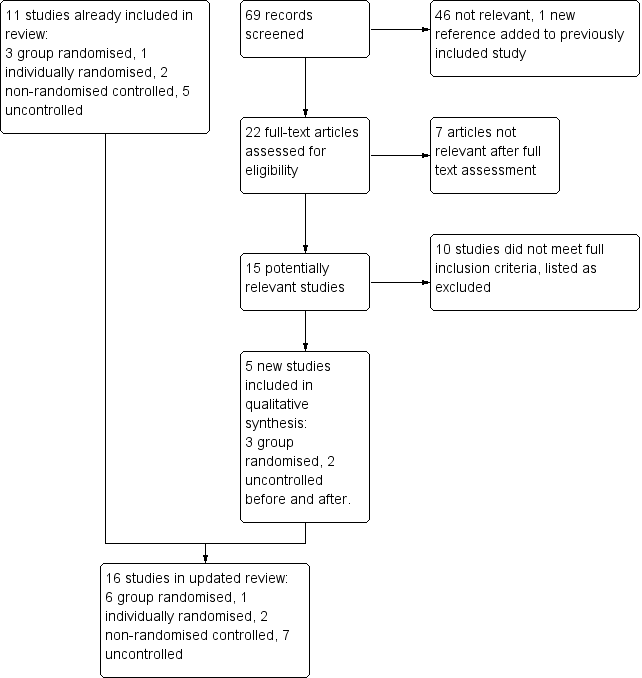
Study flow diagram for update 2014
Group Randomized Studies
We found six group randomized clinical trials (Bentz 2007; Linder 2009; Rindal 2013; Sherman 2008; Vidrine 2013; Vidrine 2013a) that assigned medical or dental clinics to either intervention or control conditions. One of the benefits of randomizing clinics rather than individual patients is the added protection against contamination of the control conditions when patients are seen in the same clinic (Campbell 2000). In each of these studies treatment conditions tested an electronic health record (EHR) with enhancements intended to facilitate the provider interaction with a smoker patient. Linder 2009 provided intervention clinics with additional tools within the EHR and clinical staff were reminded to use them. In Bentz 2007, the enhancement was based on information in an existing EHR. Clinical staff (physicians and medical assistants) in the intervention clinics received feedback reports on their use of the existing tools with smoking patients. Sherman 2008 also provided additional tools for clinical staff in the EHR system with some restrictions on use of the tools by the control clinics. The dental study (Rindal 2013) created text boxes or scripts within the intervention clinic dental record. The scripts served as language the dental providers could use based on patient‐specific information obtained during the dental encounter. In the other studies (Vidrine 2013; Vidrine 2013a) the intervention clinics were able to link patients through the EHR to a telephone quitline, and the quitline proactively called the patient.
Other studies
Of the other ten studies, three used a control condition or comparison clinic (Bentz 2002; Frank 2004; Szpunar 2006). In Bentz 2002, the comparison clinic was a paper records‐based clinic without an electronic health record. Szpunar 2006 used four control clinics, two were based on usual care and two had access to a new electronic health record vital sign screen but were provided no training or support on the use of the screen. Frank 2004 randomly assigned patients in one clinic to either intervention or usual care based on their family medical record number.
The seven additional studies (Adsit 2014; Koplan 2008; Lindholm 2010; Mathias 2012; McCullough 2009; Ragucci 2009; Spencer 1999) measured outcomes before and after the introduction of an enhancement to an existing electronic health record, without any comparison group. Adsit 2014 was conducted in a family practice clinic and a pulmonary specialty clinic within the same health system. Spencer 1999 was conducted in a single primary care clinic. Koplan 2008 studied the intervention in a single hospital. The McCullough 2009 and Ragucci 2009 studies each involved 3 clinics, and Lindholm 2010 studied one large health system with 18 primary care clinics. Ragucci 2009 and Mathias 2012 involved retrospective cohorts.
Length of follow‐up
There was wide variation in the type and length of follow‐up across the studies. For example, Rindal 2013 made telephone contact with dental patients a few days after a dental visit to measure intervention effects but no other follow‐up was conducted. Szpunar 2006 collected follow‐up data through a patient survey about two weeks after a medical care visit during an eight month study period. Bentz 2002 collected data during a three month period, and Frank 2004 collected 12 month outcome data. Spencer 1999 followed patients to 19 months. Lindholm 2010 provided data one year before and one year after the intervention. Koplan 2008 examined outcomes four months before and after implementation. McCullough 2009 followed a cohort for eight months. Mathias 2012 included patients with two or more visits during a six month post intervention period. Adsit 2014 collected outcome data from electronic records 6 months post intervention.
Excluded studies
We list seven excluded studies. See the Characteristics of excluded studies table for more detail.
Risk of bias in included studies
Allocation
All six group randomized studies described a method for matching or stratifying clinics prior to randomization, and patient populations were defined by clinic with no risk of differential patient recruitment, so all six were rated low risk for selection bias. Two studies (Bentz 2007; Linder 2009) were conducted in large health systems and, prior to randomizations, groups of clinics were created based on predetermined criteria such as the proportion of payment from government versus private insurance payers (Bentz 2007) or practice type (hospital based, community based or community health centre) (Linder 2009). In both of these studies all patients in a medical practice were included in the study. The Sherman 2008 study was conducted in a government funded health system, and clinics were randomly assigned, stratified by region (Northern vs Southern California) and size (large vs small). Vidrine 2013 and Vidrine 2013a pair matched community‐based primary care clinics based on patient demographics and clinic characteristics such as patient volume and smoking prevalence. Rindal 2013 stratified all dental clinics in one system by size and smoking volume then randomized clinics into intervention or control conditions.
In the other randomized trial, Frank 2004 randomly assigned patients within a single medical clinic. This was rated low risk for selection bias.
Among the controlled observational studies, there was no consistent method for choosing the control group. Bentz 2002 selected two clinics that were willing to participate, one used a paper chart and the other had recently switched to an electronic health record. Szpunar 2006 selected clinics based on a variety of criteria, including number of patients (population size), willingness to participate, and technical ability to complete the study. Control clinics were selected to match the intervention clinics based on a combination of number of patients and number of clinical providers. These studies were rated as high risk for selection bias.
Blinding
Since the intention of the intervention was to change provider performance, intervention providers could not be blind in any study design. Providers in control clinics may or may not have been aware of the study hypothesis but if they were and it acted as a prompt to change their behaviour the impact would be to reduce the intervention effect. Clinic patients (participants) would have been unlikely to be aware of any change in the procedure. For these reasons we rated most studies as low risk of bias. Frank 2004 was judged at high risk for performance bias since doctors saw patients allocated to both conditions. Sherman 2008 was judged at high risk because the change to the electronic record could not be restricted to intervention clinics so might have acted as a prompt in control clinics.
Outcomes were largely objective and obtained from medical records using similar procedures for intervention and control clinics so we judged most studies at low risk of detection bias. Ragucci 2009 was rated as high risk because patients self‐reported reported smoking cessation. Sherman 2008 was rated at high risk because providers self‐reported their referral patterns via surveys.
Incomplete outcome data
We considered both exclusions and attrition and found no concerns in most studies, Sherman 2008 surveyed clinicians about their activities and did not get 100% response.
Selective reporting
We examined studies for the completeness of their results. Sherman 2008 reported an increase within intervention clinics but failed to describe the comparable referral rate within control clinics.
Other potential sources of bias
We examined the group randomized trials for the potential of recruitment bias. Linder 2009 included all the medical clinics that belonged to a practice‐based research network. Rindal 2013 included all the clinics within a large system of dental clinics. Bentz 2007 reported the inclusion of 19 medical clinics that were part of a large health system. How clinics were selected to participate was not described but all patients in the selected clinics were included. The Sherman 2008 study involved 18 clinics but the criteria for study inclusion were not reported. Vidrine 2013 and Vidrine 2013a included 10 primary care clinics that were part of larger health systems but the selection criteria for the included clinics were not described.
Effects of interventions
See: Summary of findings for the main comparison
Outcomes are tabulated in Analysis 1.1
Evidence from group randomized studies
Smoking cessation
Only Linder 2009 reported a comparison of quit rates between control and intervention measured indirectly based on changes in the EHR documentation of smoking status. Significantly more smokers in the intervention clinics were subsequently documented as nonsmokers as compared to smokers in the control clinics (5.3% vs 1.9%, p < 0.001).
Clinical guideline recommended actions
Smoking status
Bentz 2007 and Linder 2009 measured documentation of smoking and found significantly higher rates after the intervention. Bentz 2007 reported no difference at baseline, but documentation increased among providers in the intervention clinics (94.5%) compared to control (88.1%) (p < 0.05). In Linder 2009 the comparable rates were 46% vs 54% (p < 0.001). However Rindal 2013 found no increase in already very high levels of dental care provider documentation of smoking status (97.5%).
Advise and assess interest in quitting
Bentz 2007 found higher rates of advice (71.6% vs 52.7%), and assessment (65.5% vs 40.1%), when comparing intervention and control clinics. Rindal 2013 reported only post intervention data and found an increase in dental provider actions. Compared to control clinics, providers in the intervention clinics were more likely to ask about interest in quitting (70% vs 87%) and to discuss strategies for quitting smoking (26% vs 47%).
Cessation assistance
A logical approach to increase the number of smokers who make attempts to quit smoking is to connect patients during a medical visit to the necessary resources to assist quitting. These resources might include physician assistance with a quitting plan or medications, or a referral to cessation counselling.
Linder 2009 found more smokers in the intervention clinics were referred to cessation counselling compared to the control clinics (4.5% vs 0.4% p<0.001) and making a contact with a cessation counsellor was more likely among intervention clinic smokers compared to control (3.9% vs 0.3%, p<0.001). However, they found smokers in the intervention clinics were no more likely to be prescribed a cessation medication. Based on similar clinic rates at baseline, Bentz 2007 reported an increase in documented assistance in the intervention clinics compared to the control clinics (20.1% vs 10.5%, p<0.001). However, referrals to the telephone‐based quitline did not increase. The researchers found variation across clinics and therefore adjusted their analysis for two factors: the presence of a "clinic champion" advocating for cessation support and the proportion of patients with more documented illnesses. This adjustment revealed an increase in referrals from intervention clinics (adjusted OR 1.5). Sherman 2008 found an increase in the last month estimated number of patients referred to telephone counselling from clinician self‐reports (15.6 vs 0.7), but no difference in the likelihood of patients from intervention clinics to receive a prescription for cessation medications. Using only post intervention data, Rindal 2013 reported significantly more dental patient smokers from intervention clinics reporting a quitline referral compared to control clinics (37% vs 17%). A similar intervention effect was found in Vidrine 2013 and Vidrine 2013a, although no baseline rates were reported. A significantly higher proportion of smokers enrolled in treatment with a quitline compared to control (Vidrine 2013; 15% vs 0.5%; Vidrine 2013a; 8% vs 0.6%).
Evidence from observational and patient randomized studies
Of the other studies, documentation of smoking status was the most commonly measured outcome. In the single patient randomized study (Frank 2004) that assessed smoking status, a preventive care reminder did not increase documentation of smoking status. However, many of the observational studies found that using an EHR system change to prompt the identification and documentation of smoking status boosted such interventions. These studies provided additional evidence of clinician assistance to smokers following an amended EHR. Four measured assistance with quitting at baseline and follow‐up (Koplan 2008; McCullough 2009; Spencer 1999; Szpunar 2006). All four found the intervention increased the rate of assistance provided to smokers. McCullough 2009 found an increase in documented assistance among smokers who were also asked about plans to quit smoking. After the EHR change, Koplan 2008 found an increase in both the proportion of admitted smokers referred to cessation counselling and an increase in physician orders for cessation medication. Adsit 2014 found more electronic referral to a quitline compared to a fax referral comparison (14% vs 0.3%). Mathias 2012 observed no change in prescriptions for cessation medications, but referrals for cessation counselling increased from 2% to 7%. They also reported a post intervention change in EHR documented 'not smoking' status from 17% to 20% (p < 0.06).
Discussion
Summary of main results
We included randomized and non‐randomized studies that tested the use of an electronic health record (EHR) to improve documentation and treatment of tobacco use among medical and dental patients. None of the studies included a direct assessment of patient quit rates. At least in the short term, documentation of tobacco status and quit assistance to smokers does appear to increase following the introduction of an electronic reminder to provide clinical support for patients who smoke (summary of findings Table for the main comparison).
Overall completeness and applicability of evidence
The goal of this review was to evaluate available evidence supporting computerized medical record systems as a method to enhance the delivery of effective tobacco use treatments.
The most common study design measured changes in clinician, clinic, or health system actions before and after the introduction of an enhancement to an electronic health record, but often without a control or comparison condition. Randomized controlled trials in real‐world settings such as medical or dental clinics are often designed as group randomized designs. Indeed this review included six studies with randomizations at the clinic level. Such designs provide an advantage for clinic settings but at the expense of complexity in design and sample size.
The most common enhancement of the EHR was the linking of smoking patients with a telephone based quitline (9/16 studies). The most recent studies had as their primary outcome the measurement of the rate of smokers receiving quitline treatment.
Quality of the evidence
Overall the studies included in this review were heterogeneous in design and intervention. For example, patient surveys, provider surveys, medical record reviews, and reports from quitline vendors were used to measure outcomes across the studies. Each of these methods introduces a different view of outcomes and each introduces a potential bias. Moreover, there was great variation in type of outcome. Therefore we were not able to perform statistical analysis or a meta‐analysis of the included studies.
Although 16 studies were included, we determined that five were high quality group randomized controlled trials. The sixth group randomized trial (Sherman 2008) demonstrated the difficulty of conducting such research within an existing healthcare system. In this study the researchers were unable to restrict the enhancement of the medical record system only to the intervention clinics. Instead, they relied on a visual request to maintain the delivery of the intervention.
There were various limitations to the non‐randomized studies. Most (7/9) lacked a control group and adopted a before and after design. Other limitations included small sample sizes and convenience sampling of included clinics which increases the potential risk of selection bias.

Study flow diagram for update 2014
| Study | Smoking cessation | Guideline recommended actions |
| Randomized controlled trials | ||
| Bentz 2007 | Guideline actions increased within the intervention clinics for smoking status (94.5% vs 88.1% p<0.05), advised to quit (71.6% vs 52.7%, p<0.001), assessed interest in quitting 65.5% vs 40.1% p<0.001), and provided assistance (20.1% vs 10.5%, p < 0.001). Quitline referral increased in the intervention clinics (adjusted OR 1.53) | |
| Linder 2009 | Significantly more smokers in the intervention clinics were subsequently documented as nonsmokers compared to smokers in the control clinics (5.3% vs 1.9%, p < 0.001) | Significantly more smokers were referred to cessation counselling in the intervention clinics (4.5% vs 0.4% in control clinics, p<0.001), and significantly more smokers from intervention clinics made contact with a cessation counsellor (3.9% vs 0.3% in control clinics, p<0.001). No difference in the proportion of documented smokers from control or intervention clinics prescribed any cessation medication (2.0% vs 2.0%). |
| Rindal 2013 | Significantly more smoking patients from intervention clinics versus control clinic patients reported dental provider actions: discussed interest in quitting (87% vs 70%); discussed quitting (47% vs 26%); and referral to quitline (37% vs 17%). | |
| Sherman 2008 | The average number of smokers per month referred to telephone counselling increased from 1.0 to 15.6 (p< 0.001) among intervention clinic providers, and from 0.2 to 0.7 (p<0.04) among control clinic providers. | |
| Vidrine 2013 | Patients from intervention clinics were more likely to enroll in quitline treatment compared to control clinics (15% vs 0.5%). | |
| Vidrine 2013a | Patients from intervention clinics were more likely to enroll in quitline treatment compared to control clinics (8% vs 0.6%). | |
| Controlled trials | ||
| Bentz 2002 | Documentation of tobacco use was unchanged in the paper chart clinic, but increased from 79% to 88% in the enhanced EHR clinic. | |
| Frank 2004 | Assessment of smoking status was unchanged between intervention and control patient visits (2.0% vs 1.8%, RR 1.12 , 95% CI 0.90 to 1.39). | |
| Szpunar 2006 | Asking about tobacco use increased in the intervention clinics from 88.4% to 92.8%. | |
| Uncontrolled trials | ||
| Adsit 2014 | The proportion of patients referred to the quitline increased from <1% to 14%. 5% enrolled in quitline treatment. | |
| Koplan 2008 | The proportion of smoking patients referred to cessation counselling increased from 0.8% to 2.1% (p < 0.001); medication ordered increased from 1.6% to 2.5% (p < 0.001). | |
| Lindholm 2010 | Tobacco use status in the EHR increased from 71.6% to 78.4% (p < 0.001). | |
| Mathias 2012 | The percentage of documented smokers with a change in smoking status from active to quit during the pre‐ or postintervention period increased from 17.1% in the preintervention cohort to 20.5% in the postintervention cohort (p = .06) | In the post enhancement period, cessation medication prescribing did not change (14.4% vs. 13.4%, p = .5), but quitline referral increased from 2% to 7% (p < 0.001). |
| McCullough 2009 | Tobacco use status increased from 71% to 84% (p < 0.001). Assessement of plan to quit increased from 25.% to 51% (p < 0.005), and smokers assessed for a plan to quit were more likely to receive cessation counselling (46% vs 14% among smokers not assessed, p < 0.001). | |
| Ragucci 2009 | Of 90 smokers in the study, 29 were quit at 6 months (32%) | |
| Spencer 1999 | Recording of tobacco use status increased from 18.4% to 80.3%. | |
Comparison 1 Study results, Outcome 1 All outcomes.
| Use of electronic health records to support smoking cessation | ||||
| Patient or population: People who smoke Settings: Healthcare clinics Intervention: Any use of an Electronic Health Record (EHR) to improve smoking status documentation or cessation assistance for patients who use tobacco, either by direct action or by feedback of clinical performance measures. Comparison: No EHR, or EHR without support for smoking cessation intervention | ||||
| Outcomes | Effect | No of Participants | Quality of the evidence | Comments |
| Smoking cessation | More intervention clinic than control clinic smokers quit (5.3% vs 1.9%, p < 0.001) | 1 cluster RCT, 26 clinics | ⊕⊝⊝⊝ very low1 | Indirect measurement based on EHR documentation of smoking status |
| Guideline recommended actions | Studies typically showed positive effects on outcomes including documenting smoking status, giving advice to quit, assessing interest in quitting, and providing assistance including referral. | 6 cluster RCTs, 98 clinics | ⊕⊕⊕⊝ Moderate2 | Studies did not all assess the same outcomes. Non randomized and uncontrolled studies also showed positive effects |
| GRADE Working Group grades of evidence | ||||
| 1 Only one study reported the outcome, and did not use direct patient report of cessation 2 Heterogeneity in the interventions and targeted behaviours | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All outcomes Show forest plot | Other data | No numeric data | ||
| 1.1 Randomized controlled trials | Other data | No numeric data | ||
| 1.2 Controlled trials | Other data | No numeric data | ||
| 1.3 Uncontrolled trials | Other data | No numeric data | ||

