Antidepresivos para el tratamiento de los pacientes con depresión y dependencia del alcoholismo concomitantes
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 138 depressed people with alcohol dependence (56 men and 82 women; mean (± SD) age 43.6 ± 9.1 years). Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. | |
| Interventions | Drugs:
Psychotherapy: manualized clinical case management was delivered by experienced addiction clinicians. Scheduled duration of treatment: 12 weeks Sites: 7 addiction clinics spanning urban, provincial, and rural catchments in Australia. Setting: outpatients Route of administration: orally Starting dose:
Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence:
Dropouts Adverse effects | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: 47.1% of participants had current anxiety disorder. Other substance‐use disorders: 14.5% of participants had current substance dependence. Other characteristics of study Other pharmacological treatment offered: all participants received naltrexone. Funding sources: study funded by Health Research Council of New Zealand grant HRC 07/138. Declaration of interest: authors declared no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed using a computer‐generated random number table. |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was conducted by an administrative staff member independent of study investigators or research clinicians, and the allocation sequence record was stored securely. |
| Blinding of participants and personnel (performance bias) | Low risk | The investigators, doctors, participants, and any other staff members taking part in the experiment were unaware which of the groups any particular participant belonged to. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were imputed using appropriate methods. |
| Selective reporting (reporting bias) | Unclear risk | Numbers of dropouts per group were missing. |
| Methods | Randomized, placebo‐controlled, double‐blind trial | |
| Participants | 30 people with alcohol dependence with dysthymia (24 men and 6 women; mean (± SD) age: 44.5 ± 2.6 years). Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder: information not available. | |
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 12 weeks Site: 1 centre, Department of Clinical Psychiatry, Policlinico, Milan, Italy Setting: inpatient setting for first 4 weeks, then outpatients for following 8 weeks. Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence: information not available Dropouts Adverse effects: information not available | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: information not available Other substance‐use disorders: participants with other substance‐use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: information not available Funding sources: information not available Declaration of interest: information not available Other information Standard errors were converted into SDs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation stated but no further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information insufficient to permit judgement. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Unclear risk | Method to account for missing data not described. Intention‐to‐treat approach not reported. No high numbers of dropouts or unbalanced between groups. |
| Selective reporting (reporting bias) | High risk | DBI and DOTES scores are reported in 2 figures (figures 3 and 4 of the publication) in which the titles of the y axis do not correspond to those reported in the legends and in the text, and the positions of the points do not correspond to the values indicated in the y axes. Accordingly, these results were not included in the present meta‐analysis. |
| Methods | Double‐blind, randomized, comparative trial | |
| Participants | 44 depressed people with alcohol dependence (number of men and women: information not available; mean age: information not available). Sociodemographic characteristics available only for 36 participants (20 mirtazapine, 16 amitriptyline). Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. | |
| Interventions | Drugs:
Psychotherapy: information not available. Scheduled duration of treatment: 8 weeks. Site: Ege University School of Medicine Hospital, Specialized Addiction Unit, Izmir, Turkey Setting: inpatient Route of administration: orally Starting dose:
Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence: data not available Alcohol craving:
Dropouts: data not available Adverse effects:
Bodyweight:
Anxiety:
| |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Anxiety:
Global assessment: information not available. Weight (mean ± SD):
Other psychiatric comorbidity: participants with other psychiatric disorders were excluded. Other substance use disorders: participants with other substance use disorders were excluded. Other characteristics of study Other pharmacological treatment: no other pharmacological treatment was allowed. Funding sources: not available. Declaration of interest: not available. Other information After their inclusion in study, participants were admitted at a specialized department for alcohol detoxification on an inpatient basis. Alcohol consumption was prohibited during hospitalization and people who consumed alcohol during study were excluded from study. At end of alcohol detoxification treatment (approximately 10‐14 days), people were included in study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation stated. No further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind stated. Medication and placebo prepared to appear identical ("Both the clinicians and patients were blind to the treatment. Drugs were given in identical‐looking opaque capsules"). |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | High risk | Intention‐to‐treat approach not used ("Dropouts were not included in the analysis due to missing data"). People who consumed alcohol during study were excluded by study. |
| Selective reporting (reporting bias) | High risk | People who consumed alcohol during study were excluded by study. |
| Methods | Randomized, double‐blind, comparative trial | |
| Participants | 39 people with alcohol dependence (all men; mean age: information not available) with a significant degree of anxious‐depressive symptomatology. Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder: information not available | |
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 3 weeks Site: Alcoholism Treatment Service of East Louisiana State Hospital, Mandeville, LA, USA Setting: inpatients Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence: data not available Dropouts Adverse effects | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: information not available Other substance‐use disorders: information not available Other characteristics of study Other pharmacological treatment: other concomitant therapy not allowed Funding source: medications were supplied by Laboratories of Pfizer Inc. Declaration of interest: information not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation stated. No further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind stated. Medications prepared to appear identical. Evaluations conducted by 2 independent investigators and the results pooled. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | High risk | No information on dropouts provided. Methods applied to account for missing data not described. Intention‐to‐treat approach not reported. |
| Selective reporting (reporting bias) | High risk | No information on dropouts provided. Methods applied to account for missing data not described. Intention‐to‐treat approach not reported. |
| Methods | Randomized, placebo‐controlled, double‐blind trial | |
| Participants | 40 depressed people with alcohol dependence (all men; mean age: majority aged 31‐50 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder: information not available | |
| Interventions | Drugs:
Psychotherapy: none Scheduled duration of treatment: 3 weeks Site: Alcoholic Treatment Service, East Louisiana State Hospital, Jackson, LA, USA Setting: inpatients for first 3‐4 days for treatment of alcohol withdrawal, then 3 weeks for trial Route of administration: orally Starting dose:
Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence: data not available Dropouts Adverse effects | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: information not available. Other substance‐use disorders: information not available. Other characteristics of study Other pharmacological treatment offered: participants received pharmacological treatment to control the acute symptoms of alcohol withdrawal for 3‐4 days. Funding sources: information not available Declaration of interest: information not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation stated. No further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind stated. Medication and placebo prepared to appear identical. No specific reference made to blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | High risk | Methods applied to account for missing data not described. Intention‐to‐treat approach not reported. People who left study were replaced by other people ("One patients taking imipramine left the hospital ... and global evaluation were omitted. Two additional patients left without permission just after entering the trial, and were therefore replaced in the study. One had received six doses of imipramine and the other one placebo"). |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement. |
| Methods | Randomized comparative trial | |
| Participants | 122 depressed people with alcohol dependence (95 men and 27 women; mean age: 42 years) Inclusion criteria:
Exclusion criteria: information not available Participants with bipolar disorder: information not available | |
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 3‐4 weeks Site: Alcohol Unit, casa di Cura Villa Silvia per malattie nervose e mentali, Senigallia, Italy Setting: inpatients Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available | |
| Outcomes | Depression
Alcohol dependence: data not available Dropouts: data not available Adverse effects: data not available | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence: data not available Other psychiatric comorbidity: information not available Other substance use disorders: information not available Other characteristics of study Other pharmacological treatment: information not available Funding sources: information not available Declaration of interest: information not available Other information Data on response and remission were excluded because evaluated using a self‐administered scale. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation stated. No further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | High risk | No information on the design (double‐blind or open trial), on the preparation and appearance of medications, and on blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on study design (double‐blind or open trial). |
| Blinding of outcome assessment (detection bias) subjective | High risk | No information on study design (double‐blind or open trial). |
| Incomplete outcome data (attrition bias) | High risk | Methods applied to account for missing data. Intention‐to‐treat approach not reported. No high number of dropouts or unbalanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement. |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 51 depressed people with alcohol dependence (26 men and 25 women; age (mean ± SD) = 34.8 ± 10.2 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. | |
| Interventions | Drugs:
Psychotherapy:
Scheduled duration of treatment: 12 weeks Site: Western Psychiatric Institute and Clinic of the University of Pittsburgh, Pittsburgh, USA Setting: inpatients for first 2 weeks of abstinence, then outpatients Route of administration: orally Starting dose:
Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence:
Global assessment:
Dropouts: information not available Adverse effects: information not available | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance‐use disorders: participants with substance‐use disorders were excluded. Abuse of other substances was not an exclusionary criterion, provided that alcohol was the main substance of abuse. Other characteristics of study Other pharmacological treatment: other pharmacological treatments were not allowed. Funding sources: work was supported by the National Institute on Alcohol Abuse and Alcoholism (grants AA09127 and AA10523), and by the Mental Health Clinical Research Center, Rockville, MD (grant MH30915). Declarations of interest: information not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization balanced for gender and race. It was not reported whether a computer‐generated list was used. |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to permit judgement. Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Medications were administered in identical opaque capsules. Substantial blood levels of fluoxetine were observed in more than 99% of participants assigned to fluoxetine. Not reported if blood analyses were made also to participants who received placebo. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis and last point carried forward analysis applied. |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement. |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 14 depressed people with alcohol dependence (10 men and 4 women; mean age = 41.3 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder: information not available | |
| Interventions | Drugs:
Psychotherapy:
Scheduled duration of treatment: 12 weeks Site: University of Pittsburgh, Western Psychiatric Institute and Clinic, Pittsburgh, USA Setting: outpatients Route of administration: orally Starting dose:
Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence:
Craving for alcohol:
Dropouts Adverse effects | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment: participants did not receive other pharmacological treatments. Funding sources: study received grants from the National Institute on Alcohol Abuse and Alcoholism (R21 AA022123, R21 AA022863, R01 AA013370, R01 AA015173, K24 AA15320) and from the National Institute on Drug Abuse (R01 DA019142, P50 DA05605, K02 DA017822). Declarations of interest: information not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process to permit judgement of low or high risk. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Medications were identical in appearance (identical‐looking opaque capsules). |
| Blinding of outcome assessment (detection bias) objective | Low risk | Medications were identical in appearance (identical‐looking opaque capsules). |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat approach reported. |
| Selective reporting (reporting bias) | Low risk | No dropouts |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 76 people with alcohol dependence (all men; mean age = 42 years) in association with a predominant chronic anxiety or depressive reaction. Inclusion criteria: information not available Exclusion criteria:
Participants with bipolar disorder: information not available | |
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 3 weeks Site: Alcoholism Treatment Service of Southeast Louisiana Hospital, Mandeville, LA, USA Setting: inpatients Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence: data not available Dropouts Adverse effects | |
| Notes | Baseline characteristics of participants included inGallant 1969 arm a; Gallant 1969 arm b Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were included. Other substance use disorders: information not available Other characteristics of study Other pharmacological treatment offered: information not available Funding source: the project was partially supported by PHS Grant MH‐03701‐08, Psychopharmacology Research Branch, NIMH. Declarations of interest: information not available Other information In the original study, 100 participants were divided into 4 groups:
In the present meta‐analysis, participants were divided into 2 substudies:
The first arm (Gallant 1969 arm a) was included in the 'Effects of interventions: Antidepressants versus placebo' comparison and the second arm (Gallant 1969 arm b) in the 'Effects of interventions: Antidepressants versus other medications' comparison. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process to permit judgement of low or high risk. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Medications were identical in appearance and were coded in accordance with double‐blind procedure to ensure that all personal involved in project remained blind as to which group any given participant belonged. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Unclear risk | Methods applied to account for missing data not described. Intention‐to‐treat approach not reported. However, there were no dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. However, there were no dropouts. |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 71 people with alcohol dependence (all men; mean age: 42 years) in association with a predominant chronic anxiety or depressive reaction (diagnosis of depression was uncertain). Inclusion criteria: information not available Exclusion criteria:
Participants with bipolar disorder: information not available | |
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 3 weeks Site: Alcoholism Treatment Service of Southeast Louisiana Hospital, Mandeville, LA, USA Setting: inpatients Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence: data not available (probably because of inpatient setting). Dropouts Adverse effects | |
| Notes | Baseline characteristics of participants included inGallant 1969 arm a; Gallant 1969 arm b Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were included. Other substance use disorders: information not available Other characteristics of study Other pharmacological treatment offered: information not available Funding source: the project was partially supported by PHS Grant MH‐03701‐08, Psychopharmacology Research Branch, NIMH. Declarations of interest: information not available Other information In the original study, 100 patients were divided into 4 groups:
In the present meta‐analysis, participants were divided into 2 substudies:
The first arm (Gallant 1969 arm a) was included in the 'Effects of interventions: Antidepressants versus placebo' comparison and the second arm (Gallant 1969 arm b) in the 'Effects of interventions: Antidepressants versus other medications' comparison. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding of participants and personnel (performance bias) | Low risk | Medications were identical in appearance and were coded in accordance with double‐blind procedure to ensure that all personal involved in project remained blind as to which group any given participant belonged. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Unclear risk | Methods applied to account for missing data not described. Intention‐to‐treat approach not reported. However, there were no dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 83 depressed people with alcohol dependence (44 men and 39 women; mean age = 47 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. | |
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 24 weeks Site: Alcohol Unit of the Hospital ‘Clínico y Provincial' in Barcelona, Spain Setting: outpatients Route of administration: orally Starting dose: 50 mg/day Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence:
Dropouts Adverse effects | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: people with other mental disorders were excluded. Other substance‐use disorders: people with substance‐use disorders were excluded. Other characteristics of study Other pharmacological treatment: other pharmacological treatments were not allowed. Funding source: information not available Declarations of interest: information not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation stated. The investigator did not have access to the randomization code. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Matching packets containing placebo were provided for all possible sertraline dose progressions, so that titration could be performed double blind. Medication was dispensed in bottles with MEMS caps, which contain an electronic monitoring device that records the date and time of bottle cap openings (Aprex Corp, San Diego, CA). |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat method utilized in all statistical analyses. |
| Selective reporting (reporting bias) | Low risk | For alcohol consumption data, participants with missing assessments at last observation were treated as non‐abstinent. For depression scale scores, missing data were handled on the principle of last observation carried forward. |
| Methods | Randomized, double‐blind, comparative trial | |
| Participants | 286 depressed people with alcohol dependence (222 men and 64 women; mean age: information not available). Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were included. | |
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 6 weeks (responders were proposed to continue the same treatment up to 12 weeks). Site: Department of Substance Use Prevention and Treatment, Institute of Psychiatry and Neurology, Warsaw, Poland Setting: outpatients Starting dose: information not available Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence: data not available Craving for alcohol:
Anxiety:
Dropout Adverse effects | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Craving for alcohol:
Anxiety:
Other psychiatric comorbidity: information not available Other substance use disorders: information not available Other characteristics of study Other pharmacological treatment: other pharmacological treatments were not allowed. Funding source: information not available Declarations of interest: information not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and key study personnel ensured. Both drugs were blinded to participants. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | Only full analysis set were evaluated by the present meta‐analysis. |
| Selective reporting (reporting bias) | Low risk | Only full analysis set were evaluated by the present meta‐analysis. |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 41 depressed people with alcohol dependence (20 men and 21 women; age (mean ± SD): 42.9 ± 8.6 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. | |
| Interventions | Drugs:
Psychotherapy:
Scheduled duration of treatment: 10 weeks Site: Alcohol Research Center, Department of Psychiatry, University of Connecticut School of Medicine, Farmington, CT, USA Setting: outpatients Route of administration: orally Starting dose:
Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence:
Anxiety:
Sleep quality:
Dropouts Adverse effects | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Anxiety:
Quality of sleep
Other psychiatric comorbidity: participants with other mental disorders were included. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding sources: study supported by NIH Grants P50‐AA03510, K24‐AA13736, and M01‐RR06192 and the Bristol‐Myers Squibb Co. Declarations of interest: information not available Other information After a baseline assessment, participants entered a 1‐week single‐blind placebo treatment, followed by random assignment to nefazodone or placebo groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment to treatment groups used an urn randomization procedure, which balanced group assignment on sex, age, educational level, percentage of heavy drinking days, and severity of depressive symptoms at the time of the initial assessment. |
| Allocation concealment (selection bias) | Low risk | Method of concealment not reported but unlikely that selection bias was introduced. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and key study personnel ensured. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information provided on blinding of assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information provided on blinding of assessors. |
| Incomplete outcome data (attrition bias) | Low risk | Mixed model analysis used to examine variables measured at each visit during study. This procedure allows the inclusion of all cases (41 participants) by estimating individual trajectories even when other data points are missing because of participant attrition. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 189 depressed people with alcohol dependence (123 men; 66 women; age (mean ± SD): placebo = 44.0 ± 8.0 years; sertraline = 41.7 ± 9.4 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. | |
| Interventions | Drugs:
Psychotherapy: at each study visit, participants received supportive therapy delivered according to a manual developed specifically for study consisting in:
Scheduled duration of treatment: for up to 10 weeks Sites: 13 investigative sites in USA Setting: outpatients Route of administration: orally Starting dose: 50 mg/day Pattern of dose reduction:
| |
| Outcomes | Depression:
Alcohol dependence:
Dropout Adverse effects | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: supported by Pfizer Pharmaceuticals. Manuscript preparation supported by NIH grant K24 AA13736. Declarations of interest: information not available. Other information In the original study, 328 participants were divided into the 2 groups on whether initially elevated HRSD score declined with cessation of heavy drinking:
In the present meta‐analysis, participants were divided into 2 substudies:
Both the substudies (Kranzler 2006 arm A; Kranzler 2006 arm B) were included in the 'Effects of interventions: Antidepressants versus placebo' comparison. Unfortunately, the original study did not report the adverse events for the single substudies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization schedule. |
| Allocation concealment (selection bias) | Unclear risk | Assignment to medication group was done according to a computer‐generated randomization schedule for groups A and B, with the medication groups within each stratum balanced for recent outpatient/inpatient status. Despite random assignment, participants who received placebo were older, reported more drinks per week during the pretreatment period, and had higher CGI depression scores at baseline. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | All analyses used an intention‐to‐treat approach. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. However, some of them were reported only as a % reduction (e.g. BDI score). |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 139 depressed people with alcohol dependence (86 men and 53 women; age (mean ± SD): placebo = 42.9 ± 9.2 years; sertraline = 41.8 ± 9.4 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. | |
| Interventions | Drugs:
Psychotherapy: At each study visit, participants received supportive therapy delivered according to a manual developed specifically for study consisting of:
Scheduled duration of treatment: up to 10 weeks Sites: 13 investigative sites in the USA Setting: outpatients Route of administration: orally Starting dose: 50 mg/day Pattern of dose reduction:
| |
| Outcomes | Depression:
Alcohol dependence:
Dropout Adverse effects | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: study supported by Pfizer Pharmaceuticals. Manuscript preparation supported by NIH grant K24 AA13736. Declarations of interest: information not available Other information In the original study, 328 participants were divided into the 2 groups on whether initially elevated HRSD score declined with cessation of heavy drinking:
In the present meta‐analysis, participants were divided into 2 substudies:
Both the substudies (Kranzler 2006 arm A; Kranzler 2006 arm B) were included in the 'Effects of interventions: Antidepressants versus placebo' comparison. Unfortunately, the original study did not report the adverse events for the single substudies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization schedule reported. |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment was not described. Despite random assignment, participants who received placebo were older, reported more drinks per week during the pretreatment period, and had higher CGI depression scores at baseline. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | All analyses used an intention‐to‐treat approach. Weekly comparisons including only participants for whom data were available from that visit, whereas end of study analyses used last observation carried forward analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. However, some of them were reported only as a % reduction (e.g. BDI score). |
| Methods | Randomized, placebo‐controlled trial | |
| Participants | 41 people with alcohol dependence (number of men and women not available; mean age = 36‐37 years) with non‐severe affective disorders Inclusion criteria: information not available Exclusion criteria: information not available Participants with bipolar disorder: information not available | |
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 3 weeks Site: Russia Setting: inpatients Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence: no information provided Anxiety:
Dropouts: data not available Adverse effects: information not provided | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Anxiety:
Other psychiatric comorbidity: information not available Other substance use disorders: information not available Other characteristics of study Other pharmacological treatment offered: other treatments were not administrated during study Funding sources: information not available. Other information In the original study, 90 people with alcohol dependence were randomly divided into 4 groups:
In the present meta‐analysis, study was divided into 2 substudies:
The first substudy (Krupitsky 1993 arm A) was included in the 'Effects of interventions: Antidepressants versus placebo' comparison and the second substudy (Krupitsky 1993 arm B) in the 'Antidepressants versus other medications' comparison | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | High risk | Not reported if it was a double‐ or single‐blind study. |
| Blinding of outcome assessment (detection bias) objective | Low risk | Not reported if it was a double‐ or single‐blind study. |
| Blinding of outcome assessment (detection bias) subjective | High risk | Not reported if it was a double‐ or single‐blind study. |
| Incomplete outcome data (attrition bias) | High risk | Number of dropouts not reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Methods | Randomized, controlled trial | |
| Participants | 38 people with alcohol dependence (number of men and women not available; mean age = 36‐37 years) with affective disorders not severe Inclusion criteria: information not available Exclusion criteria: information not available Participants with bipolar disorder: information not available | |
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 3 weeks Site: Russia Setting: inpatients Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence: no information provided Anxiety:
Dropouts: data not available Adverse effects: data not available | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Anxiety:
Other psychiatric comorbidity: information not available Other substance use disorders: information not available Other characteristics of study Other pharmacological treatment offered: other treatments were not administrated during study Funding sources: information not available Other information In the original study, 90 people with alcohol dependence were randomly divided into 4 groups:
In the present meta‐analysis, study was divided into 2 substudies:
The first substudy (Krupitsky 1993 arm A) was included in the 'Effects of interventions: Antidepressants versus placebo' comparison and the second substudy (Krupitsky 1993 arm B) in the 'Antidepressants versus other medications' comparison. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | High risk | Not reported if it was a double‐ or single‐blind study. |
| Blinding of outcome assessment (detection bias) objective | Low risk | Not reported if it was a double‐ or single‐blind study. |
| Blinding of outcome assessment (detection bias) subjective | High risk | Not reported if it was a double‐ or single‐blind study. |
| Incomplete outcome data (attrition bias) | High risk | Number of dropouts not reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 60 depressed people with alcohol dependence (47 men and 13 women; age (mean ± SD): escitalopram = 43.9 ± 1.1 years; placebo = 40.9 ± 1.3 years). Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. | |
| Interventions | Drugs:
Psychotherapy:
Scheduled duration of treatment: 13 weeks Site: a single‐site at the Department of Narcology (Addiction Psychiatry) of the Bekhterev PsychoNeurological Research Institute, St Petersburg, Russia Setting: outpatients Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence:
Craving for alcohol:
Global response:
Anxiety:
Dropouts Adverse effects | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: information not available. Declarations of interest: information not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed by means of generation random numbers in Excel. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered drug containers of identical appearance. |
| Blinding of participants and personnel (performance bias) | Low risk | The investigators, doctors, participants, and any other staff members taking part in the experiment were unaware which of the groups any particular person belonged to. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | High risk | Methods: participants who relapsed to heavy drinking were excluded from study. Results: the relatively small number of alcohol consumption days in the groups was due to participants who relapsed being excluded from trial. |
| Selective reporting (reporting bias) | High risk | Methods: participants who relapsed to heavy drinking were excluded from study. Results: the relatively small number of alcohol consumption days in the groups was due to participants who relapsed being excluded from trial. |
| Methods | Randomized, single‐blind, comparative trial | |
| Participants | 30 people with alcohol dependence of an original group constituted by 60 participants (41 men and 19 women; mean age: 47 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. | |
| Interventions | Treatment:
Psychotherapy: cognitive behavioural psychotherapy administered in individual sessions and family interventions, twice a week Scheduled duration of treatment: 3 weeks Site: Drug and Alcohol Addiction Clinic, Athens University Psychiatric Clinic, Eginition Hospital, Athens, Greece Setting: inpatients for 1 week, then residential treatment Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence: no information available Anxiety:
Global assessment:
Dropouts Adverse effects: data not available | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Anxiety:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance‐use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: information not available Declarations of interest: information not available Other information In the original study, 60 participants were included into 4 groups:
Participants but not clinicians were blind to group status In the present meta‐analysis, we divided the control group (only psychotherapy) into 2 smaller groups, and compared these 2 smaller groups to the 2 antidepressants, using 3 subgroups:
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random sequence generation was used ("At the end of the first week, individuals were randomly/electronically allocated to one of the three groups"). |
| Allocation concealment (selection bias) | Low risk | Computer sequence of allocation used. |
| Blinding of participants and personnel (performance bias) | High risk | Single‐blind study ("Patients but not clinicians were blind to group status"). |
| Blinding of outcome assessment (detection bias) objective | Low risk | Single‐blind study ("Patients but not clinicians were blind to group status"). |
| Blinding of outcome assessment (detection bias) subjective | High risk | Single‐blind study ("Patients but not clinicians were blind to group status"). |
| Incomplete outcome data (attrition bias) | High risk | Dropouts were not included in the analysis due to missing data. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Methods | Randomized, single‐blind, comparative trial | |
| Participants | 30 people with alcohol dependence of an original group constituted by 60 participants (41 men and 19 women; mean age: 47 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. | |
| Interventions | Treatment:
Psychotherapy: cognitive behavioural psychotherapy was administered in individual sessions and family interventions, twice a week Scheduled duration of treatment: 3 weeks Site: Drug and Alcohol Addiction Clinic, Athens University Psychiatric Clinic, Eginition Hospital, Athens, Greece Setting: inpatients for 1 week, then residential treatment Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence: no information available Anxiety:
Global assessment:
Dropouts Adverse effects: data not available | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Anxiety:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance‐use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: information not available Declarations of interest: information not available Other information In the original study, 60 participants were included into 4 groups:
Participants but not clinicians were blind to group status In the present meta‐analysis, we divided the control group (only psychotherapy) into 2 smaller groups, and compared these 2 smaller groups to the 2 antidepressants, using 3 subgroups:
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random sequence generation reported ("At the end of the first week, individuals were randomly/electronically allocated to one of the three groups") |
| Allocation concealment (selection bias) | Low risk | Computer sequence of allocation reported. |
| Blinding of participants and personnel (performance bias) | High risk | Single‐blind study ("Patients but not clinicians were blind to group status"). |
| Blinding of outcome assessment (detection bias) objective | Low risk | Single‐blind study ("Patients but not clinicians were blind to group status"). |
| Blinding of outcome assessment (detection bias) subjective | High risk | Single‐blind study ("Patients but not clinicians were blind to group status"). |
| Incomplete outcome data (attrition bias) | High risk | Dropouts were not included in the analysis due to missing data. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Methods | Randomized, single‐blind, comparative trial | |
| Participants | 40 people with alcohol dependence of an original group constituted by 60 participants (41 men and 19 women; mean age: 47 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. | |
| Interventions | Drugs:
Psychotherapy: cognitive behavioural psychotherapy was administered in individual sessions and family interventions, twice a week. Scheduled duration of treatment: 3 weeks Site: Drug and Alcohol Addiction Clinic, Athens University Psychiatric Clinic, Eginition Hospital, Athens, Greece Setting: inpatients for 1 week, then residential treatment Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence: no information available Anxiety:
Global assessment:
Dropouts Adverse effects: data not available | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Anxiety:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: information not available Declarations of interest: information not available Other information In the original study, 60 participants were included into 4 groups:
Participants but not clinicians were blind to group status. In the present meta‐analysis, we divided the control group (only psychotherapy) into 2 smaller groups, and compared these 2 smaller groups to the 2 antidepressants, using 3 subgroups:
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random sequence generation reported ("At the end of the first week, individuals were randomly/electronically allocated to one of the three groups") |
| Allocation concealment (selection bias) | Low risk | Computer sequence of allocation reported. |
| Blinding of participants and personnel (performance bias) | High risk | Single‐blind study, and the outcomes are likely to be influenced by lack of blinding ("Patients but not clinicians were blind to group status"). |
| Blinding of outcome assessment (detection bias) objective | Low risk | Single‐blind study, and the outcomes are likely to be influenced by lack of blinding ("Patients but not clinicians were blind to group status"). |
| Blinding of outcome assessment (detection bias) subjective | High risk | Single‐blind study, and the outcomes are likely to be influenced by lack of blinding ("Patients but not clinicians were blind to group status"). |
| Incomplete outcome data (attrition bias) | High risk | Dropouts were not included in the analysis due to missing data. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Methods | Randomized, double‐blind, comparative trial | |
| Participants | 129 people with alcohol dependence with depression or dysthymic disorder (111 men and 18 women; mean age: approximately 38 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder: information not available | |
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 4‐8 weeks (depending on the centre concerned) Sites: 7 centres, in France Setting: unclear Route of administration: orally Starting dose: tianeptine = 37.5 mg/day; amitriptyline = 75.0 mg/day Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence: data not available Anxiety:
Dropouts Adverse effects | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: information not available Other substance use disorders: information not available Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were allowed. Funding source: information not available Declarations of interest: information not available Other information In the original study, the duration of the trial was 4‐8 weeks depending on the centre concerned. In the present meta‐analysis, only data of 4 weeks were analyzed. Before the onset of the trial, participants received a pretreatment with a placebo for 3‐10 days to screen out placebo‐responder participants. After this period, participants received tianeptine or amitriptyline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind stated. Medication and placebo prepared to appear identical. No specific reference made to blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | High risk | Intention‐to‐treat analysis not used. However, the numbers of dropouts were low (10/64, 12/65) and were not unbalanced between groups. |
| Selective reporting (reporting bias) | High risk | Several data were missing (final MADRS score for amitriptyline, adverse effects, and alcohol consumption). |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 28 depressed people with alcohol dependence (24 men and 4 women; mean age = desimipramine: 36.0 ± 22 years; placebo = 41.0 ± 15.5 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder: information not available | |
| Interventions | Drugs:
Psychotherapy: participants were encouraged to participate in Alcoholics Anonymous and any other psychosocial treatments. Scheduled duration of treatment: 6 months Sites: Department of the New York (NY) Hospital‐Cornell Medical Center, and the University of Miami (FL), School of Medicine, USA Setting: outpatients Route of administration: orally Starting dose: medication was prescribed in divided doses for the first week, then changed to bedtime dosing Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence:
Dropouts Adverse effects | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: grants from the National Institute on Alcohol Abuse and Alcoholism (AA06866 and AA08111) Declarations of interest: information not available Other information Final HRSD score and difference between baseline and final HRSD score were excluded because they were expressed as medians and interquartile ranges. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind stated and blinding of key study personnel ensured by sentences of the evaluation of plasma desipramine concentration. "Plasma desipramine concentration was assessed and results were reviewed by a physician not involved in patient ratings to verify compliance and make dose recommendations. Equivalent dosing instructions were given by the nonblinded physician to blinded therapists for placebo‐treated patients to preserve the double‐blind study design." |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | High risk | Intention to treat was not used. Participants who relapsed, who demonstrated non‐compliance, or who did not improve were removed from study. |
| Selective reporting (reporting bias) | High risk | Participants who relapsed, who demonstrated non‐compliance, or who did not improve were removed from study. |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 69 depressed people with alcohol dependence (34 men and 35 women; age (mean ± SD): imipramine = 37.4 ± 6.7 years; placebo = information not available (report stated "10.6 ± 9.1")). Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. | |
| Interventions | Drugs:
Psychotherapy:
Scheduled duration of treatment: 12 weeks Site: Depression Evaluation Service, New York State Psychiatric Institute, New York, NY, USA Setting: outpatients Route of administration: orally Starting dose:
Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence:
Global response Dropouts Adverse effects: data not available | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: history of hypomania was not exclusionary. Other substance‐use disorders: history of current abuse of other substances was not exclusionary, provided that alcohol was clearly the main substance of abuse. Other characteristics of study Other pharmacological treatment offered: information not available Funding source: grants from the National Institute on Alcohol Abuse and Alcoholism (AA9539), the state of New York, and the Mental Health Clinical Research Center (NIMH 30906). Medications were supplied by Ciba‐Geigy Corp. Declarations of interest: information not available Other information Eligible participants were given single‐blind placebo for 1 week. Participants whose depression was not rated 'much improved' or 'very much improved' on the improvement item of the CGI scale for depression were randomized to receive placebo or imipramine. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | High risk | Double‐blind stated and medications prepared to appear identical. No specific reference made to blinding of participants and personnel. Plasma dosage of imipramine performed but no information on blinding of personnel provided. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analyses used. "Ratings from the last observation were carried forward for those subjects who did not complete all 12 weeks of treatment. Intention‐to‐treat analyses included all randomized patients and carried last observation forward for dropouts and those who completed less than 12 weeks." |
| Selective reporting (reporting bias) | High risk | Treatment outcomes were reported only for completers. |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 35 people with alcohol dependence (number of men and women: not available; mean age: information not available) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder: information not available | |
| Interventions | Drugs:
Psychotherapy:
Scheduled duration of treatment: 4 weeks Site: Alcoholism Treatment Unit, Mapperley Hospital, Nottingham, UK Setting: inpatients Route of administration: orally Starting dose:
Pattern of dose reduction:
| |
| Outcomes | Depression:
Alcohol dependence: data not available Dropouts Adverse effects | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: sociopathic personality disorder was present in 6 participants; 3 were considered to have significant anxiety; there were no diagnoses of schizophrenia or psychotic illness. Other substance‐use disorders: information not available Other characteristics of study Other pharmacological treatment offered:
Funding source: Bencard. Declarations of interest: information not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation stated. No further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind stated. No information on blinding of participants and personnel and on the appearance of medications. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | High risk | Intention‐to‐treat analysis not used. However, the number of dropouts was low and balanced between groups. |
| Selective reporting (reporting bias) | High risk | Data of participants who dropped out of study were not included in results. |
| Methods | Randomized, placebo‐controlled trial | |
| Participants | 82 depressed people with alcohol dependence (50 men and 32 women; age (mean ± SD): sertraline = 41 ± 11 years; placebo = 42 ± 10 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. | |
| Interventions | Drugs:
Psychotherapy:
Scheduled duration of treatment: 12 weeks Site: Alcohol Research Center, Center for Drug and Alcohol Programs, Charleston, SC, USA Setting: outpatients Route of administration: orally Starting dose: 50 mg/day Pattern of dose reduction: titrated back down 50 mg over 7‐day period | |
| Outcomes | Depression:
Alcohol dependence:
Dropout Adverse effects | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: National Institute on Alcohol Abuse and Alcoholism (grant AA10476). Pfizer supplied study drug and matched placebo. Declarations of interest: information not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomization used. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind, placebo‐controlled medication design applied. Medications dispensed in identically tablets. No further details provided on blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information provided on blinding of assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information provided on blinding of assessors. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis used. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Methods | Randomized, double‐blind, comparative trial | |
| Participants | 80 depressed people with alcohol dependence (44 men and 36 women; age (mean ± SD): memantine = 47.5 ± 8.3 years; escitalopram = 47.9 ± 8.3 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. | |
| Interventions | Drugs:
Psychotherapy: no psychosocial intervention was offered. Scheduled duration of treatment: 26 weeks (6 months) Sites: 3 centres, Helsinki, Finland, and Europe. Setting: outpatients Route of administration: orally Starting dose:
Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence:
Anxiety:
Cognitive functioning:
Quality of life:
Dropouts Adverse effects | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other medications prescribed by the patient's physician were allowed, except other antidepressants. Funding source: National Public Health Institute, the Finnish Foundation for Alcohol Research and Helsinki Health Center Research. Study medication provided by Lundbeck Oy Ab, Turku, Finland Declaration of interest: no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All participants meeting the inclusion criteria were randomly assigned by an independent person to escitalopram or memantine groups using a 1:1 ratio and random permuted blocks (Vassar Statistics randomizing algorithm). |
| Allocation concealment (selection bias) | Low risk | Randomization was concealed until study database was locked on by an independent clinical study monitor. |
| Blinding of participants and personnel (performance bias) | Low risk | Study medication was double‐dummy packed: participant took 2 tablets every time, 1 of which was the active medicine and 1 was an identical placebo for the second medication. The medication was labelled and controlled by an independent supplier. |
| Blinding of outcome assessment (detection bias) objective | Low risk | Outcome analysis was performed by an independent source. |
| Blinding of outcome assessment (detection bias) subjective | Low risk | Outcome analysis was performed by an independent source. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis used. |
| Selective reporting (reporting bias) | Low risk | Data of all randomized participants were reported except for 1 participant due to an interrupted interview. |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 26 depressed people with alcohol dependence (number of men and women: data not available; mean age: data not available) Inclusion criteria:
Exclusion criteria: information not available Participants with bipolar disorder: information not available | |
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 6 months Site: Depression Evaluation Service, New York State Psychiatric Institute, New York, NY, USA Setting: outpatients Route of administration: orally Starting dose:
Pattern of dose reduction: information not available | |
| Outcomes | Depression: data not available Alcohol dependence: data not available Global response Dropouts: data not available Adverse effects: data not available | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: information not available. Other substance use disorders: information not available. Other characteristics of study Other pharmacological treatment offered: information not available. Funding sources: supported in part by training grant MH‐15144 from NIMH, grants AA‐07688 and AA‐08030 from the National Institute on Alcohol Abuse and Alcoholism, and Scientist Development Award for Clinicians DA‐00154 from the National Institute on Drug Abuse. CIBA/Geigy provided imipramine and matching placebo. Declarations of interest: information not available Other information Only data of the double‐blind trial were included in the present meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information insufficient to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to permit judgement. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information insufficient to permit judgement. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Unclear risk | Information insufficient to permit judgement. |
| Selective reporting (reporting bias) | High risk | Not all of study's prespecified outcomes were reported. |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 29 depressed people with alcohol dependence (number of men and women: data not available; mean age: data not available) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. | |
| Interventions | Drugs:
Psychotherapy: weekly individual cognitive‐behavioural therapy Scheduled duration of treatment: 14 weeks Sites: University of Pennsylvania and the Carrier Foundation, USA Setting: outpatients Starting dose: 50 mg/day Pattern of dose reduction: tapering during the last 2 weeks of treatment | |
| Outcomes | Depression:
Alcohol dependence:
Dropouts: information not available Adverse effects: information not available | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: National Institute on Alcohol Abuse and Alcoholism (R01‐AA09544 and KO2‐AA00239) and by Veteran Affairs Medical Center. Pfizer Inc. provided sertraline and matching placebo. Declarations of interest: information not available Other information In the original study participants were divided into 2 groups:
Participants with lifetime depression were then divided into 2 subgroups:
In the meta‐analysis, we included only participants with current depression. Unfortunately, dropouts and adverse events were not provided for each subgroup. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation referred to 1 randomization schedule for both sites. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind stated and medications prepared to appear identical. No specific reference made to blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis used. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 79 depressed people with alcohol dependence (49 men and 30 women; age (mean ± SD): sertraline = 43.9 ± 11.5 years; placebo = 43.4 ± 8.9 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. | |
| Interventions | Drugs:
Psychotherapy: weekly individual CBT Scheduled duration of treatment: 14 weeks Site: University of Pennsylvania Treatment Research Center, USA Setting: outpatients Route of administration: orally Starting dose: 50 mg/day Pattern of dose reduction:
| |
| Outcomes | Depression:
Alcohol dependence:
Dropouts Adverse effects | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: National Institute on Alcohol Abuse and Alcoholism (grant R01‐AA09544‐10). Pfizer Inc., USA, Pharmaceutical Group provided sertraline and matching placebo. Declaration of interest: the authors declared the grants received. Other information In the original study participants were divided into 4 groups:
In the meta‐analysis, data were analyzed and included in 2 substudies:
Both the substudies (Pettinati 2010 arm A; Pettinati 2010 arm B) were included in the 'Effects of interventions: Antidepressants versus placebo' comparison. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomization reported. |
| Allocation concealment (selection bias) | Low risk | Urn randomization used to evenly distribute participants across groups using 4 pretreatment variables: gender, regular smoking, HRSD scores at time of randomization, and drinking frequency. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were imputed using appropriate methods. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 91 depressed people with alcohol dependence (57 men and 34 women; age (mean ± SD): sertraline plus naltrexone = 43.4 ± 10.2 years; naltrexone = 42.9 ± 8.1) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. | |
| Interventions | Drugs:
Psychotherapy: weekly individual CBT Scheduled duration of treatment: 14 weeks Site: University of Pennsylvania Treatment Research Center, USA Setting: outpatients Route of administration: orally Starting doses:
Pattern of dose reduction:
| |
| Outcomes | Depression:
Alcohol dependence:
Dropouts Adverse effects | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: National Institute on Alcohol Abuse and Alcoholism (grant R01‐AA09544‐10). Pfizer Inc., USA, Pharmaceutical Group provided sertraline and matching placebo. Declaration of interest: the authors declared the grants received. Other information In the original study participants were divided into 4 groups:
In the meta‐analysis, data were analyzed and included in 2 substudies:
Both the substudies (Pettinati 2010 arm A; Pettinati 2010 arm B) were included in the 'Effects of interventions: Antidepressants versus placebo' comparison. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomization. |
| Allocation concealment (selection bias) | Low risk | Urn randomization used to evenly distribute participants across groups using 4 pretreatment variables: gender, regular smoking, HRSD scores at the time of randomization, and drinking frequency. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were imputed using appropriate methods. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 36 depressed people with alcohol dependence (33 men and 3 women; age (mean ± SD): sertraline = 40.5 ± 7.7 years; placebo = 41.2 ± 5.6 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. | |
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 6 weeks Site: Alcoholic Rehabilitation Unit of the Veterans Administration Medical Center, East Orange, New Jersey, USA Setting: inpatients for the first 2 weeks then outpatients for 6 weeks Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence: information not available Dropouts Adverse effects: information not available. | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Abuse of other substances was not an exclusion. Other characteristics of study Other pharmacological treatment offered: participants who had received antidepressant medication in the previous month were excluded. Funding source: information not available Declarations of interest: information not available Other information Final HRSD and BDI scores and number of withdrawal for medical reasons were provided separately for participants with primary depression (arm A; 15 participants) and participants with secondary depression (arm B; 21 participants). For the other outcomes, data were provided for the entire sample of participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding of participants and key study personnel Information ensured but information insufficient to permit judgement. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Unclear risk | Intention‐to‐treat analysis referred to, but for participants who did not complete the study (dropouts), the last observation was not carried forward. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 64 depressed people with alcohol dependence (29 men and 35 women; age (mean ± SD): 40.2 ± 8.2 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. | |
| Interventions | Drugs:
Psychotherapy: cognitive‐behavioural skills training and psychoeducational group for alcohol‐dependence and depression led by an experienced therapist; group sessions lasted 1 hour once per week. Scheduled duration of treatment: 12 weeks Site: Harborview Medical Center Outpatient Psychiatry Clinic, University of Washington, Seattle, USA Setting: outpatients Route of administration: orally Starting dose:
Pattern of dose reduction: information not available | |
| Outcomes | Depression:
Alcohol dependence:
Dropouts Adverse effects | |
| Notes | Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were included. Other substance use disorders: participants with substance use disorders were excluded. Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: supported in part by Bristol‐Meyers Squibb. Declarations of interest: information not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not provided. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and key study personnel ensured. |
| Blinding of outcome assessment (detection bias) objective | Low risk | Blinding of outcome assessment ensured. |
| Blinding of outcome assessment (detection bias) subjective | Low risk | Blinding of outcome assessment ensured. |
| Incomplete outcome data (attrition bias) | Low risk | Missing data were imputed using appropriate methods. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
ADS: Alcohol Dependence Scale; ALT: alanine aminotransferase; AST: aspartate aminotransferase; AUDIT: Alcohol Use Disorders Identification Test; BAI: Beck Anxiety Inventory; BDI: Beck Depression Inventory; BPRS: Brief Psychiatric Rating Scale; CBT: cognitive behavioural therapy; CGI: Clinical Global Impression scale; DBI: Drinking Behaviour Interview; DrInC: Drinker Inventory of Consequences scale; DOTES: Dosage Record and Treatment Emergent Symptoms Scale; DSM: Diagnostic and Statistic Manual of Mental Disorders; DSM‐III‐R: Diagnostic and Statistic Manual of Mental Disorders III ‐ Revised; DSM‐IV: Diagnostic and Statistic Manual of Mental Disorders ‐ IV; ECT: electroconvulsive therapy; GAF: General Assessment of Functioning scale; GAS: Global Assessment Scale; GGT: γ‐glutamyltransferase; HRSA: Hamilton Rating Scale for Anxiety; HRSD: Hamilton Rating Scale for Depression; ICD: International Classification of Diseases; LDQ: Leeds Dependence Questionnaire; LDRS: Lehmann Depression Rating Scale; MADRS: Montgomery and Åsberg Depression Rating Scale; MAST: Michigan Alcoholism Screening Test; MET: Motivational Enhancement Therapy; MMPI: Minnesota Multiphasic Personality Inventory; MMSE: Mini‐Mental State Examination; OCDS: Obsessive‐Compulsive Drinking Scale; PACS: Penn Alcohol Craving scale; PSQI: Pittsburgh Sleep Quality Index; SCL‐90: Symptom Check List‐90; SD: standard deviation; SOFAS: The Social and Occupational Functioning Assessment Scale; STAI: State Trait Anxiety Inventory; UKU: Udvalg for Kliniske Undersogelser Side Effect Rating Scale; VAS: Visual Analogue Scale; ZUNG: Zung Self‐Assessment Depression Scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Type of participants not in the inclusion criteria: no depression | |
| Type of participants not in the inclusion criteria: no alcohol dependence | |
| Data of single group not available | |
| Study design not in the inclusion criteria: no control group | |
| Type of participants and type of intervention not in the inclusion criteria: no depression, no use of antidepressant medications | |
| Type of intervention not in the inclusion criteria: no antidepressant medications used | |
| Type of participants and type of intervention not in the inclusion criteria: no depression, no alcohol dependence; no antidepressant medications used | |
| Study design not in the inclusion criteria: no control group | |
| Type of participants and type of intervention not in the inclusion criteria: no alcohol dependence; no antidepressant medications used | |
| Type of participants not in the inclusion criteria: only 22% of participants had depression (single data of these participants not available) | |
| Type of participants not in the inclusion criteria: no depression | |
| Type of participants not in the inclusion criteria: no depression | |
| Study population not in the inclusion criteria: people aged < 18 years | |
| Study design not in the inclusion criteria: no control group | |
| Type of participants not in the inclusion criteria: people aged < 18 years | |
| Study design not in the inclusion criteria: no control group | |
| Study design not in the inclusion criteria: no control group | |
| Study design not in the inclusion criteria: no randomised trial | |
| Study design not in the inclusion criteria: commentary on Mason 1996 | |
| Type of participants not in the inclusion criteria: no alcohol dependence. Only 73% of recruited participants with alcohol dependence; data of these participants not reported. | |
| Study design not in the inclusion criteria: case report | |
| Commentary | |
| Study design not in the inclusion criteria: no control group | |
| Type of intervention not in the inclusion criteria: the name of the antidepressant medication used was not indicated | |
| Type of participants not in the inclusion criteria: no alcohol dependence | |
| Type of participants not in the inclusion criteria: no alcohol dependence | |
| Study design not in the inclusion criteria: no control group for the antidepressant. Study compared the efficacy of aripripazole plus escitalopram to escitalopram in reducing the severity of depression and craving for alcohol in people with alcohol dependence and depression. | |
| Type of participants not in the inclusion criteria: no depression | |
| Lack of information: number of patients for each group; time of collected data (1 month, 3 months, or 6 months?) | |
| Type of participants not in the inclusion criteria: no depression | |
| Type of participants not in the inclusion criteria: no depression | |
| Type of participants not in the inclusion criteria: no depression | |
| Study design not in the inclusion criteria: no control group | |
| Type of participants not in the inclusion criteria: no alcohol dependence. Only 14% of recruited participants with alcohol dependence; data of these participants not reported. | |
| Type of participants not in the inclusion criteria: no depression | |
| Data on severity of depression, craving for alcohol, and consumption of alcohol were reported to be improved but not provided as score achieved in the different scales used | |
| Type of intervention not in the inclusion criteria: the name of antidepressant medications was not indicated. | |
| Study design not in the inclusion criteria. No control group for the antidepressant. Study compared the efficacy of sertraline in people with post‐traumatic stress disorder and alcohol dependence with comorbid anxiety or depression to that in people with post‐traumatic stress disorder and alcohol dependence but without comorbid anxiety or depression. | |
| Study design not in the inclusion criteria: no control group for the antidepressant. Study compared the efficacy of aripripazole plus escitalopram to escitalopram in reducing the severity of depression and craving for alcohol in people with alcohol dependence and depression. | |
| Type of participants not in the inclusion criteria: no depression | |
| Study design not in the inclusion criteria: no control group | |
| Study design not in the inclusion criteria: no control group | |
| Study design not in the inclusion criteria: review | |
| Study design not in the inclusion criteria: no control group for the antidepressant. Study compared the efficacy of naltrexone plus sertraline to sertraline in reducing the severity of depression and alcohol consumption in people with alcohol dependence and depression. | |
| Study design not in the inclusion criteria: statistical elaboration of previous studies | |
| Study design not in the inclusion criteria: no control group for the antidepressant. Study compared the efficacy of naltrexone plus sertraline to sertraline in reducing the severity of depression and alcohol consumption in people with alcohol dependence and depression. | |
| Type of participants not in the inclusion criteria: no depression | |
| Type of participants not in the inclusion criteria: no depression | |
| Study design not in the inclusion criteria: no control group | |
| Study design not in the inclusion criteria: no control group | |
| Study design not in the inclusion criteria: no control group | |
| Study design not in the inclusion criteria: no control group | |
| Study design not in the inclusion criteria: no control group | |
| Study design not in the inclusion criteria: no control group for the antidepressant. Study compared the efficacy of chlordiazepoxide plus imipramine to placebo. | |
| Study design not in the inclusion criteria: no control group for the antidepressant. Study compared the efficacy of acamprosate plus escitalopram to escitalopram in reducing the severity of depression and alcohol consumption in people with alcohol dependence and depression. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Requested from the authors |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Requested from the authors |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depression severity: final score (interviewer‐rated scales) Show forest plot | 14 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Antidepressants versus placebo: all studies, Outcome 1 Depression severity: final score (interviewer‐rated scales). | ||||
| 1.1 All studies | 14 | 1074 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.49, ‐0.04] |
| 1.2 Selective serotonin reuptake inhibitors (SSRIs) | 10 | 881 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.40, 0.07] |
| 1.3 5‐HT2 antagonists | 2 | 97 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.69, 0.11] |
| 2 Depression severity: final score (self‐administered scales) Show forest plot | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Antidepressants versus placebo: all studies, Outcome 2 Depression severity: final score (self‐administered scales). | ||||
| 2.1 All studies | 8 | 373 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.64, 0.07] |
| 2.2 SSRIs | 5 | 300 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.69, 0.18] |
| 2.3 5‐HT2 antagonists | 2 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.59, 0.64] |
| 3 Depression severity: difference between basal and final score (interviewer‐rated scales) Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Antidepressants versus placebo: all studies, Outcome 3 Depression severity: difference between basal and final score (interviewer‐rated scales). | ||||
| 3.1 All studies | 5 | 447 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.12, 0.42] |
| 3.2 SSRIs | 4 | 408 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.18, 0.32] |
| 4 Depression severity: difference between basal and final score (self‐administered scales) Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Antidepressants versus placebo: all studies, Outcome 4 Depression severity: difference between basal and final score (self‐administered scales). | ||||
| 4.1 All studies | 4 | 121 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.16, 0.56] |
| 4.2 SSRIs | 2 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.16, 0.73] |
| 4.3 5‐HT2 antagonists | 2 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.59, 0.64] |
| 5 Response to antidepressive treatment Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Antidepressants versus placebo: all studies, Outcome 5 Response to antidepressive treatment. | ||||
| 5.1 All studies | 10 | 805 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.08, 1.82] |
| 5.2 Tricyclic antidepressants (TCAs) | 4 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [1.09, 2.34] |
| 5.3 SSRIs | 5 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.87, 1.63] |
| 6 Full remission of depression Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Antidepressants versus placebo: all studies, Outcome 6 Full remission of depression. | ||||
| 6.1 All studies | 4 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.77, 1.83] |
| 6.2 SSRIs | 3 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.74, 1.36] |
| 7 Consumption of alcohol: abstinent days (%) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Antidepressants versus placebo: all studies, Outcome 7 Consumption of alcohol: abstinent days (%). | ||||
| 7.1 All studies | 9 | 821 | Mean Difference (IV, Random, 95% CI) | 1.34 [‐1.66, 4.34] |
| 7.2 SSRIs | 7 | 711 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐3.20, 2.26] |
| 8 Consumption of alcohol: abstinent participants (number) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Antidepressants versus placebo: all studies, Outcome 8 Consumption of alcohol: abstinent participants (number). | ||||
| 8.1 All studies | 7 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [1.22, 2.39] |
| 8.2 SSRIs | 4 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.02, 2.68] |
| 8.3 5‐HT2 antagonists | 2 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.77, 3.39] |
| 9 Consumption of alcohol: drinking days (per week) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 Antidepressants versus placebo: all studies, Outcome 9 Consumption of alcohol: drinking days (per week). | ||||
| 9.1 All studies | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐1.15 [‐2.35, 0.05] |
| 9.2 5‐HT2 antagonists | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐1.15 [‐2.35, 0.05] |
| 10 Consumption of alcohol: drinks (per drinking days) Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1 Antidepressants versus placebo: all studies, Outcome 10 Consumption of alcohol: drinks (per drinking days). | ||||
| 10.1 All studies | 7 | 451 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐1.79, ‐0.46] |
| 10.2 SSRIs | 3 | 271 | Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐2.58, ‐0.26] |
| 10.3 5‐HT2 antagonists | 3 | 111 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐2.00, ‐0.11] |
| 11 Consumption of alcohol: drinks (per week) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 Antidepressants versus placebo: all studies, Outcome 11 Consumption of alcohol: drinks (per week). | ||||
| 11.1 All studies | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐5.06 [‐12.30, 2.18] |
| 11.2 5‐HT2 antagonists | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐5.06 [‐12.30, 2.18] |
| 12 Consumption of alcohol: heavy drinking days (per week) Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1 Antidepressants versus placebo: all studies, Outcome 12 Consumption of alcohol: heavy drinking days (per week). | ||||
| 12.1 All studies | 5 | 313 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.85, 0.20] |
| 12.2 SSRIs | 2 | 189 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.09, 0.27] |
| 12.3 5‐HT2 antagonists | 2 | 55 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐2.09, 1.22] |
| 13 Consumption of alcohol: heavy drinkers (number) Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.13  Comparison 1 Antidepressants versus placebo: all studies, Outcome 13 Consumption of alcohol: heavy drinkers (number). | ||||
| 13.1 All studies | 7 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.57, 1.07] |
| 13.2 SSRIs | 6 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.69, 1.11] |
| 13.3 5‐HT2 antagonists | 2 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.68, 4.67] |
| 14 Consumption of alcohol: time to first relapse (days) Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.14  Comparison 1 Antidepressants versus placebo: all studies, Outcome 14 Consumption of alcohol: time to first relapse (days). | ||||
| 14.1 All studies | 6 | 348 | Mean Difference (IV, Random, 95% CI) | 2.54 [‐8.79, 13.87] |
| 14.2 SSRIs | 6 | 348 | Mean Difference (IV, Random, 95% CI) | 2.54 [‐8.79, 13.87] |
| 15 Liver enzyme levels: γ‐glutamyltransferase (U/L) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.15  Comparison 1 Antidepressants versus placebo: all studies, Outcome 15 Liver enzyme levels: γ‐glutamyltransferase (U/L). | ||||
| 15.1 All studies | 2 | 56 | Mean Difference (IV, Random, 95% CI) | ‐8.39 [‐26.47, 9.68] |
| 16 Depression and alcohol: global response Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.16  Comparison 1 Antidepressants versus placebo: all studies, Outcome 16 Depression and alcohol: global response. | ||||
| 16.1 All studies | 3 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 2.37 [1.34, 4.19] |
| 16.2 TCAs | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.09, 4.02] |
| 17 Acceptability: dropouts Show forest plot | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.17  Comparison 1 Antidepressants versus placebo: all studies, Outcome 17 Acceptability: dropouts. | ||||
| 17.1 All studies | 17 | 1159 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.79, 1.22] |
| 17.2 TCAs | 4 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.48, 3.06] |
| 17.3 SSRIs | 8 | 759 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.79, 1.36] |
| 17.4 5‐HT2 antagonists | 4 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.38, 1.64] |
| 18 Tolerability of treatment: adverse events Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.18  Comparison 1 Antidepressants versus placebo: all studies, Outcome 18 Tolerability of treatment: adverse events. | ||||
| 18.1 Withdrawal for medical reasons: all studies | 10 | 947 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.65, 2.04] |
| 18.2 Withdrawal for medical reasons: SSRIs | 7 | 786 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.52, 2.32] |
| 18.3 Withdrawal for medical reasons: TCAs | 2 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.10, 8.41] |
| 18.4 Total adverse events: all studies | 5 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.97, 1.44] |
| 18.5 Total adverse events: TCAs | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.13, 2.42] |
| 18.6 Total adverse events: SSRIs | 3 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.92, 1.23] |
| 18.7 Dry mouth: all studies | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.96, 3.81] |
| 18.8 Insomnia: all studies | 4 | 564 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.02, 2.77] |
| 18.9 Insomnia: SSRIs | 2 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [1.04, 2.96] |
| 18.10 Headache: all studies | 3 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.89, 1.64] |
| 18.11 Headache: SSRIs | 2 | 414 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.87, 1.61] |
| 18.12 Dizziness: all studies | 2 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.42, 6.73] |
| 18.13 Diarrhoea: all studies | 2 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.37, 10.22] |
| 18.14 Nausea: all studies | 3 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.66, 3.23] |
| 18.15 Nausea: SSRIs | 2 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.62, 3.35] |
| 18.16 Constipation: all studies | 2 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.19, 15.64] |
| 18.17 Total serious adverse events: all studies | 7 | 774 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.80, 1.86] |
| 18.18 Total serious adverse events: SSRIs | 5 | 721 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.80, 1.86] |
| 18.19 Worsening of clinical condition because of relapse: all studies | 2 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.73, 10.87] |
| 18.20 Worsening of clinical condition because of relapse: SSRIs | 2 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.73, 10.87] |
| 18.21 Depression: all studies | 2 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [0.30, 17.69] |
| 18.22 Depression: SSRIs | 2 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [0.30, 17.69] |
| 19 Suicide attempts Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.19  Comparison 1 Antidepressants versus placebo: all studies, Outcome 19 Suicide attempts. | ||||
| 19.1 All studies | 4 | 602 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.23, 7.61] |
| 19.2 SSRIs | 4 | 602 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.23, 7.61] |
| 20 Secondary outcomes: craving Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.20  Comparison 1 Antidepressants versus placebo: all studies, Outcome 20 Secondary outcomes: craving. | ||||
| 20.1 All studies | 2 | 29 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐3.27, 5.27] |
| 21 Secondary outcomes: severity of dependence Show forest plot | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.21  Comparison 1 Antidepressants versus placebo: all studies, Outcome 21 Secondary outcomes: severity of dependence. | ||||
| 21.1 All studies | 2 | 168 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.44, 0.17] |
| 22 Secondary outcomes: severity of anxiety Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.22  Comparison 1 Antidepressants versus placebo: all studies, Outcome 22 Secondary outcomes: severity of anxiety. | ||||
| 22.1 All studies | 3 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐6.31 [‐10.33, ‐2.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depression severity: final score Show forest plot | 2 | 60 | Mean Difference (IV, Random, 95% CI) | ‐2.61 [‐6.92, 1.70] |
| Analysis 2.1  Comparison 2 Antidepressants versus psychotherapy, Outcome 1 Depression severity: final score. | ||||
| 2 Global assessment: final score Show forest plot | 2 | 60 | Mean Difference (IV, Random, 95% CI) | 5.92 [1.30, 10.54] |
| Analysis 2.2  Comparison 2 Antidepressants versus psychotherapy, Outcome 2 Global assessment: final score. | ||||
| 3 Acceptability: dropouts Show forest plot | 2 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.31, 6.54] |
| Analysis 2.3  Comparison 2 Antidepressants versus psychotherapy, Outcome 3 Acceptability: dropouts. | ||||
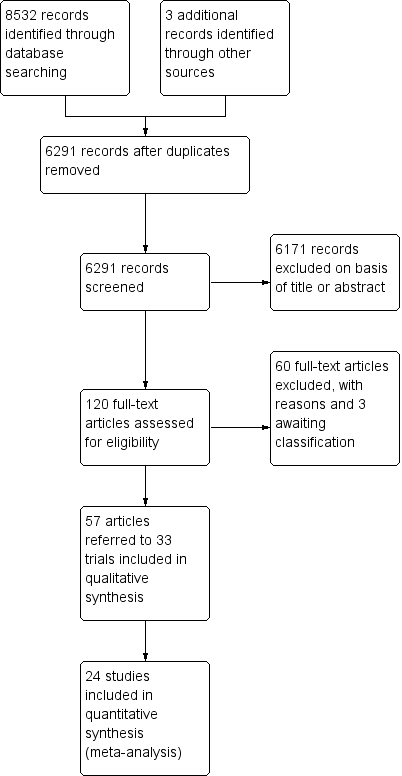
Study flow diagram.
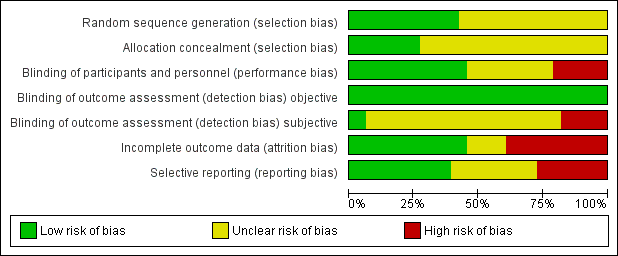
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
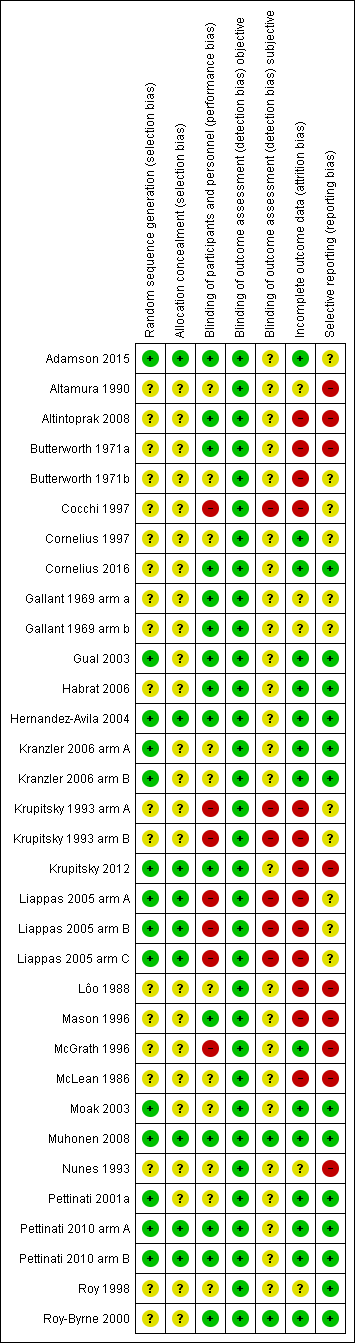
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
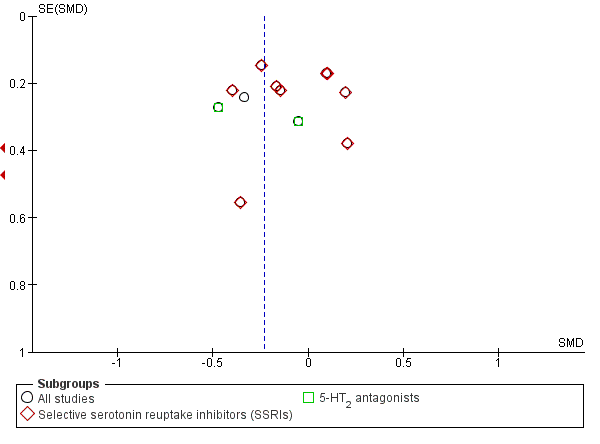
Funnel plot of comparison: 1 Antidepressants versus placebo: all studies, outcome: 1.1 Depression severity: final score (interviewer‐rated scales).
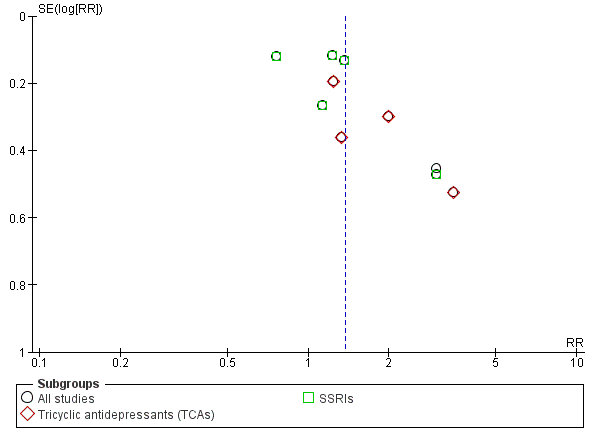
Funnel plot of comparison: 1 Antidepressants versus placebo: all studies, outcome: 1.5 Response to antidepressive treatment.
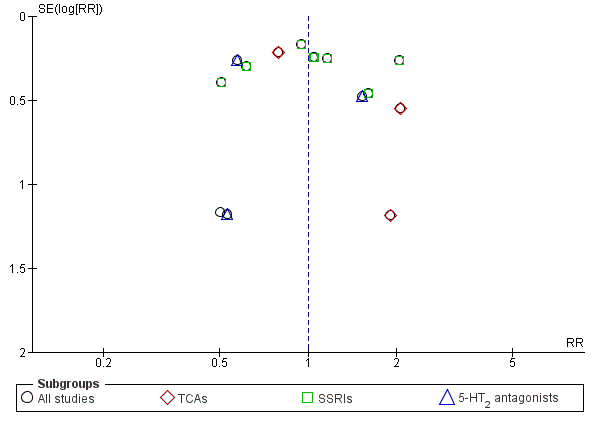
Funnel plot of comparison: 1 Antidepressants versus placebo: all studies, outcome: 1.17 Acceptability: dropouts.
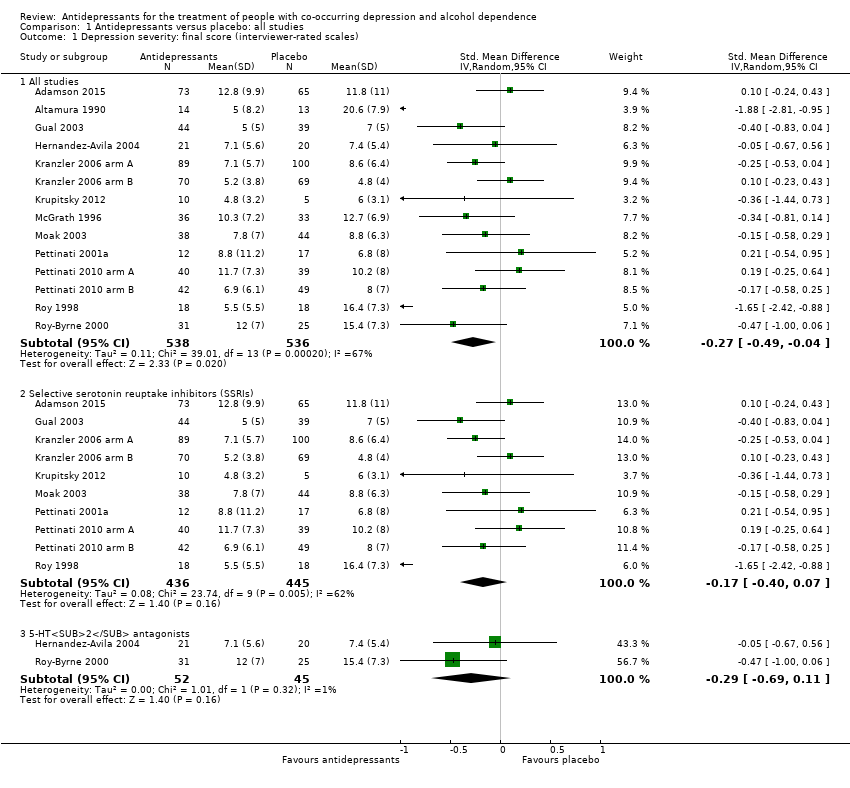
Comparison 1 Antidepressants versus placebo: all studies, Outcome 1 Depression severity: final score (interviewer‐rated scales).
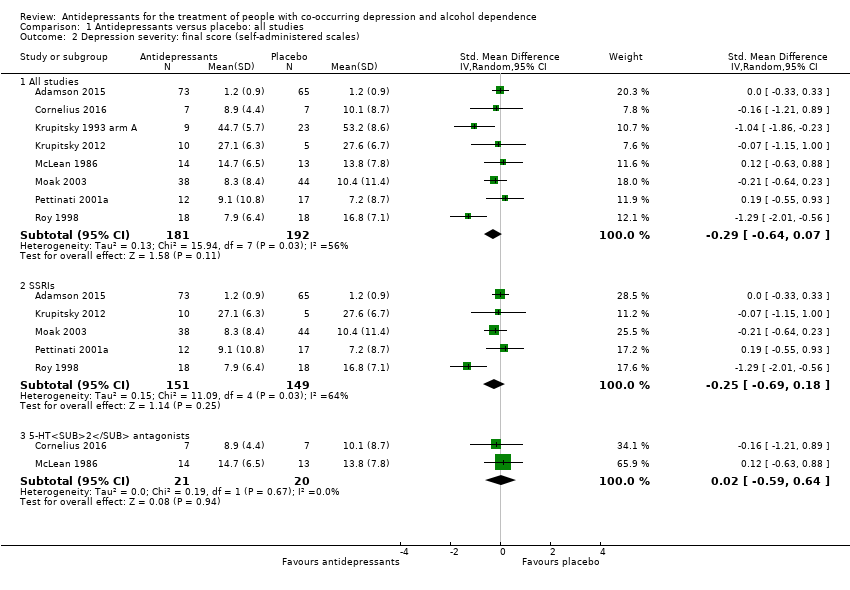
Comparison 1 Antidepressants versus placebo: all studies, Outcome 2 Depression severity: final score (self‐administered scales).
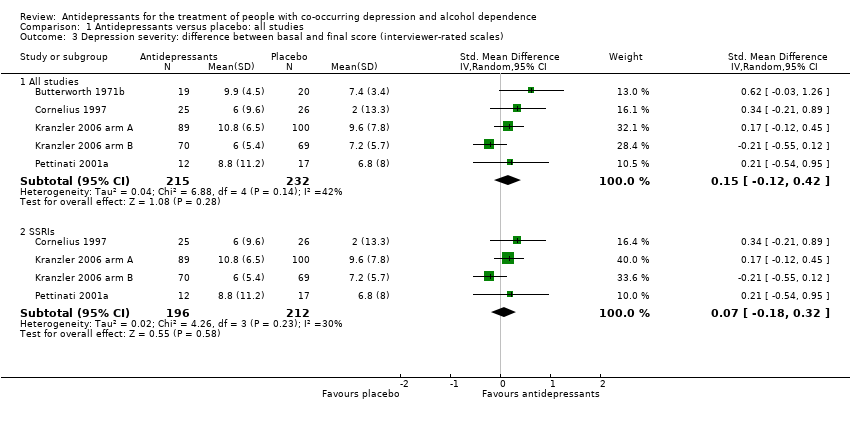
Comparison 1 Antidepressants versus placebo: all studies, Outcome 3 Depression severity: difference between basal and final score (interviewer‐rated scales).
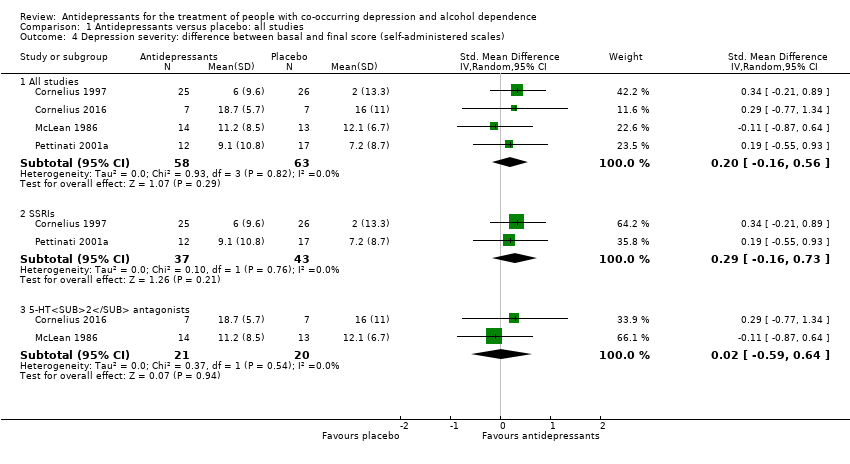
Comparison 1 Antidepressants versus placebo: all studies, Outcome 4 Depression severity: difference between basal and final score (self‐administered scales).
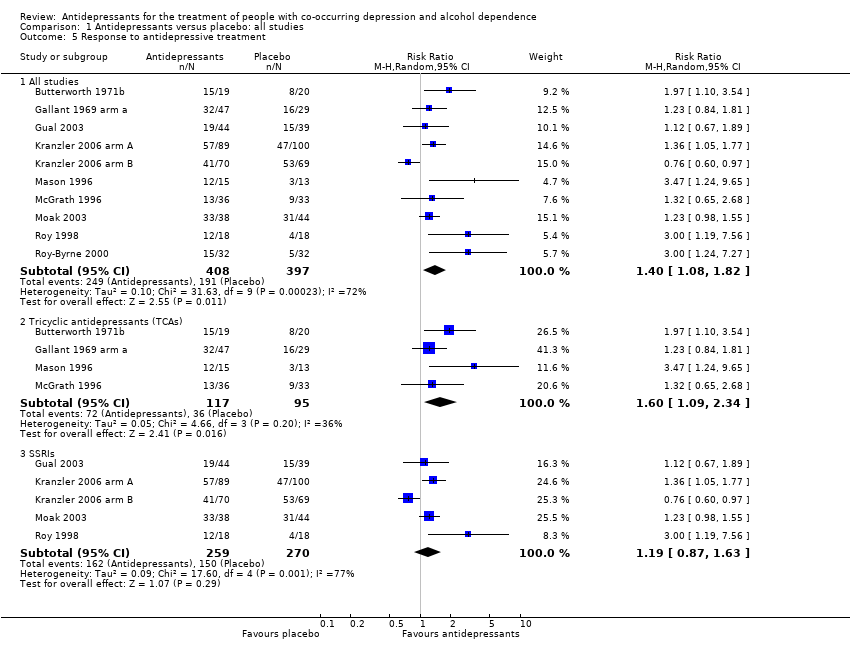
Comparison 1 Antidepressants versus placebo: all studies, Outcome 5 Response to antidepressive treatment.
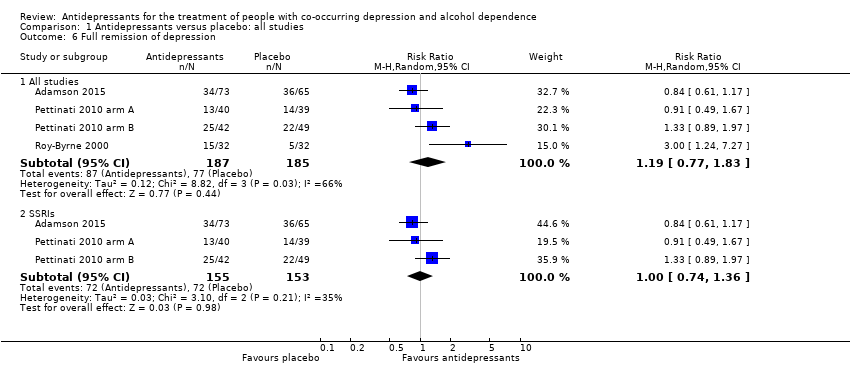
Comparison 1 Antidepressants versus placebo: all studies, Outcome 6 Full remission of depression.
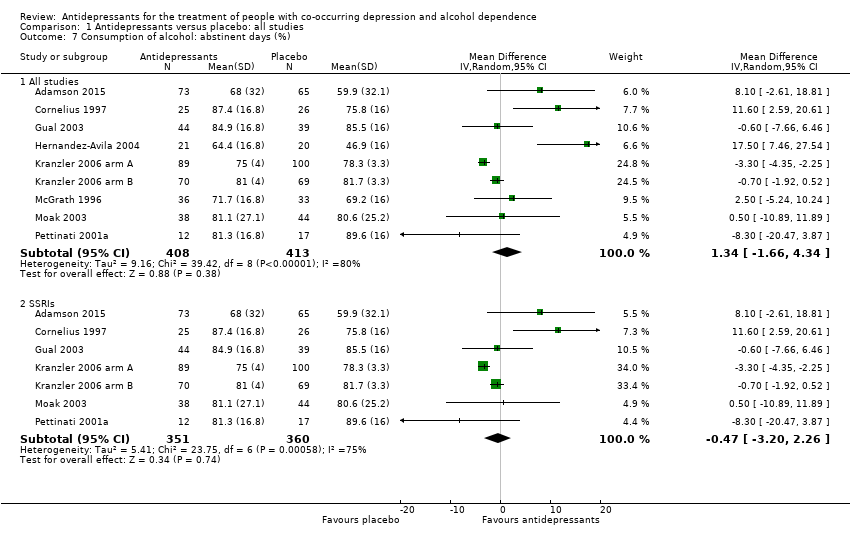
Comparison 1 Antidepressants versus placebo: all studies, Outcome 7 Consumption of alcohol: abstinent days (%).
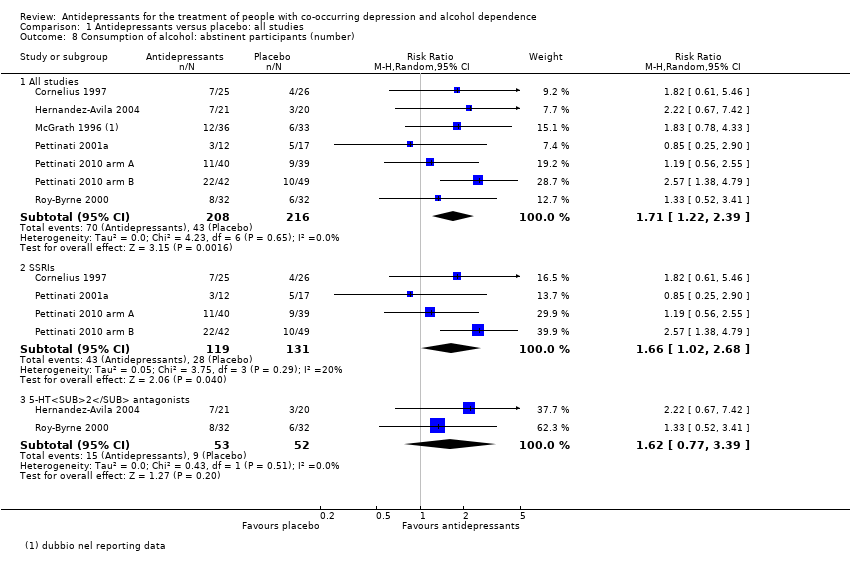
Comparison 1 Antidepressants versus placebo: all studies, Outcome 8 Consumption of alcohol: abstinent participants (number).
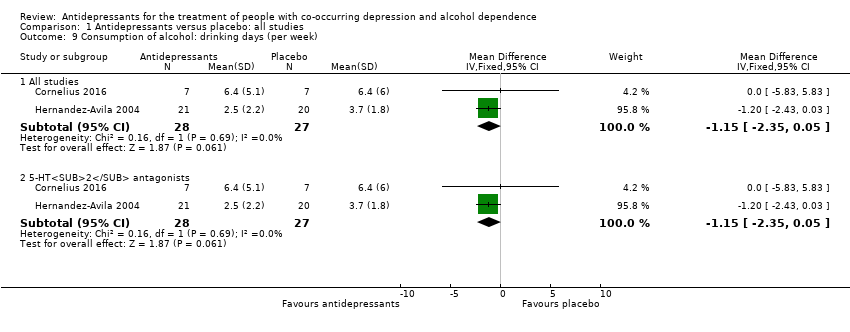
Comparison 1 Antidepressants versus placebo: all studies, Outcome 9 Consumption of alcohol: drinking days (per week).
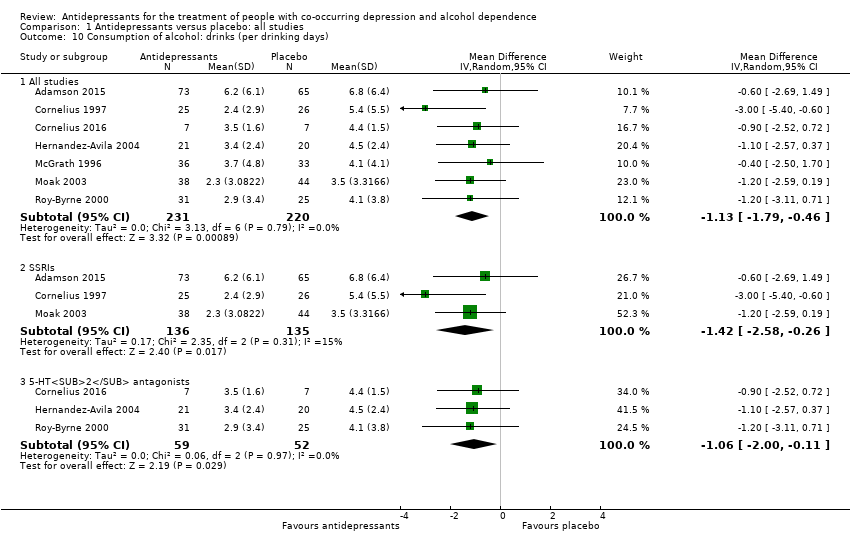
Comparison 1 Antidepressants versus placebo: all studies, Outcome 10 Consumption of alcohol: drinks (per drinking days).
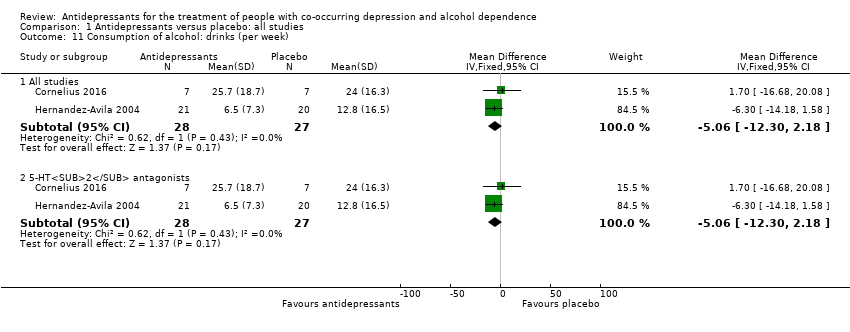
Comparison 1 Antidepressants versus placebo: all studies, Outcome 11 Consumption of alcohol: drinks (per week).
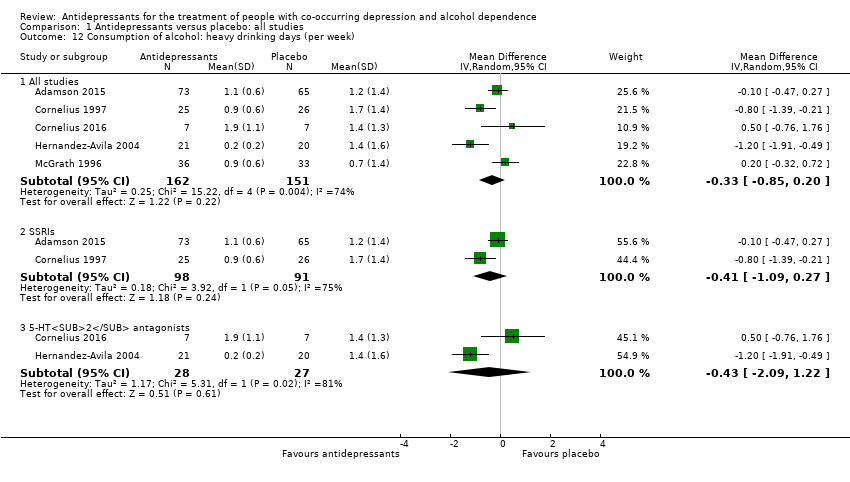
Comparison 1 Antidepressants versus placebo: all studies, Outcome 12 Consumption of alcohol: heavy drinking days (per week).
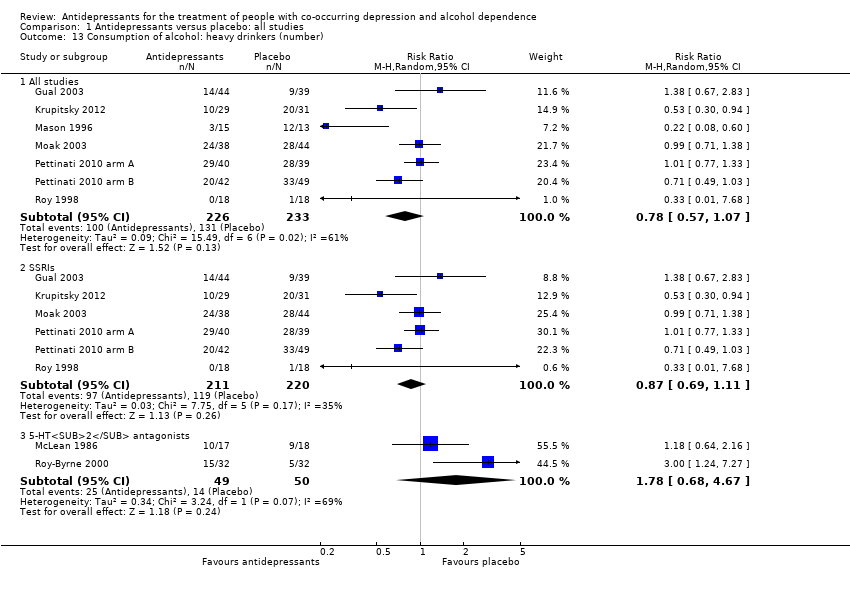
Comparison 1 Antidepressants versus placebo: all studies, Outcome 13 Consumption of alcohol: heavy drinkers (number).
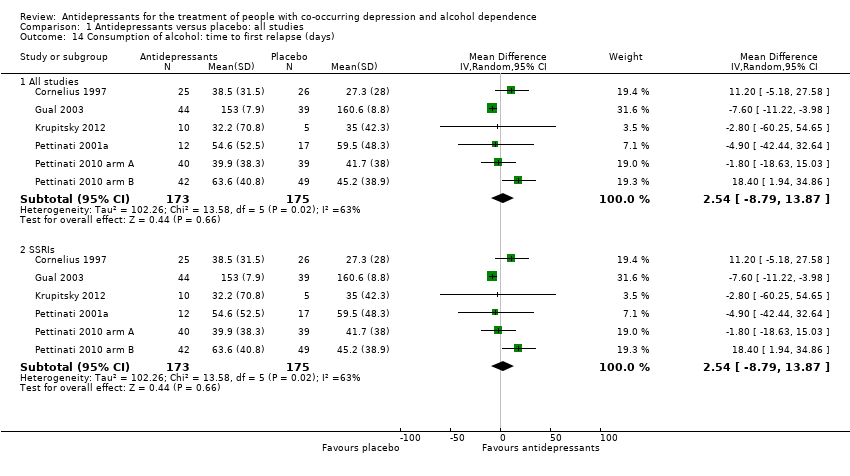
Comparison 1 Antidepressants versus placebo: all studies, Outcome 14 Consumption of alcohol: time to first relapse (days).
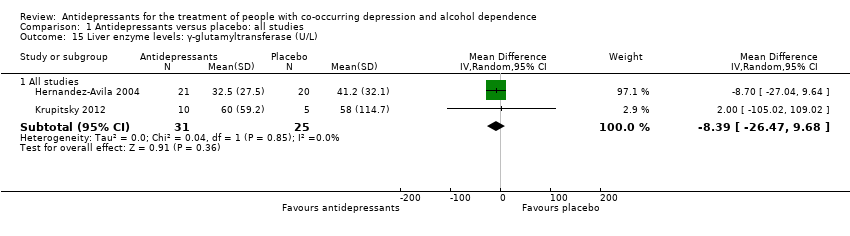
Comparison 1 Antidepressants versus placebo: all studies, Outcome 15 Liver enzyme levels: γ‐glutamyltransferase (U/L).
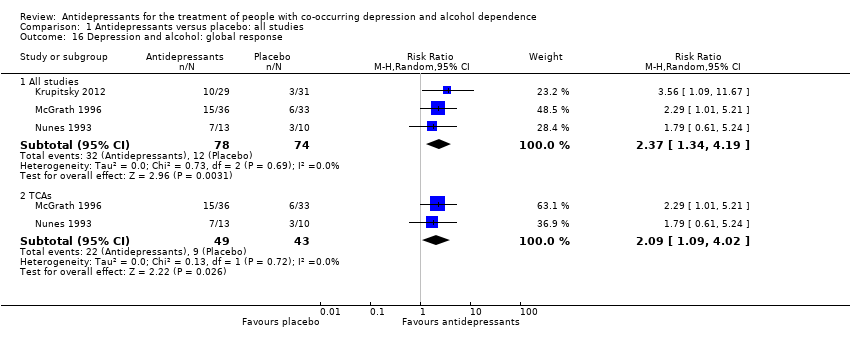
Comparison 1 Antidepressants versus placebo: all studies, Outcome 16 Depression and alcohol: global response.
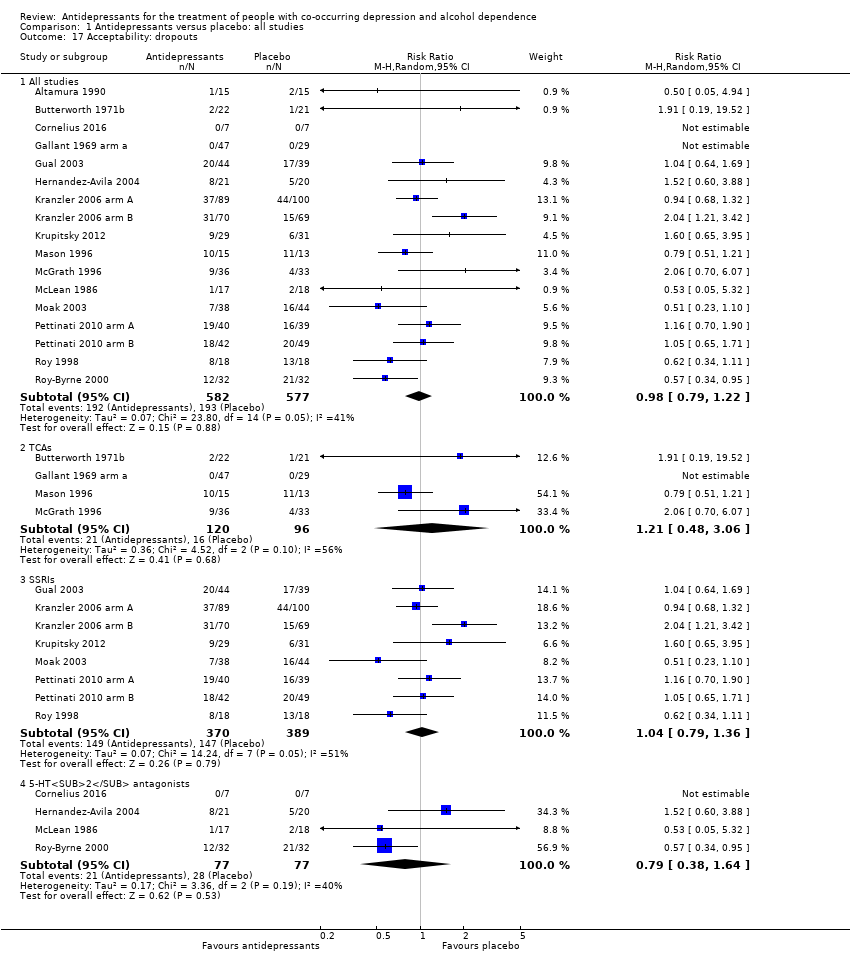
Comparison 1 Antidepressants versus placebo: all studies, Outcome 17 Acceptability: dropouts.
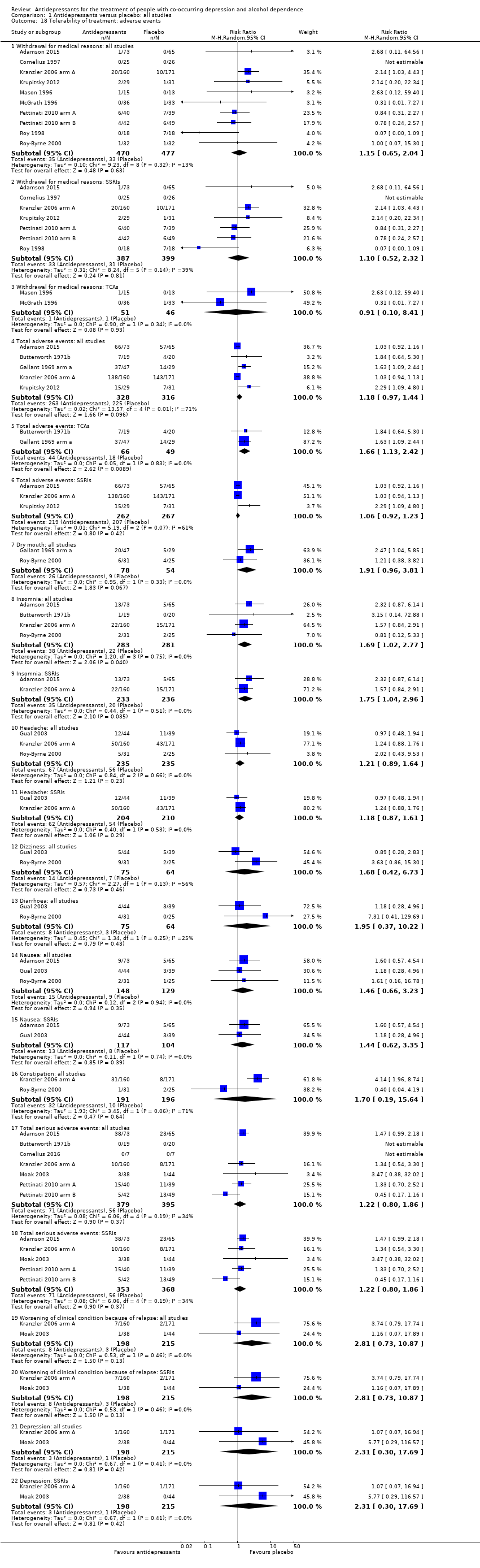
Comparison 1 Antidepressants versus placebo: all studies, Outcome 18 Tolerability of treatment: adverse events.
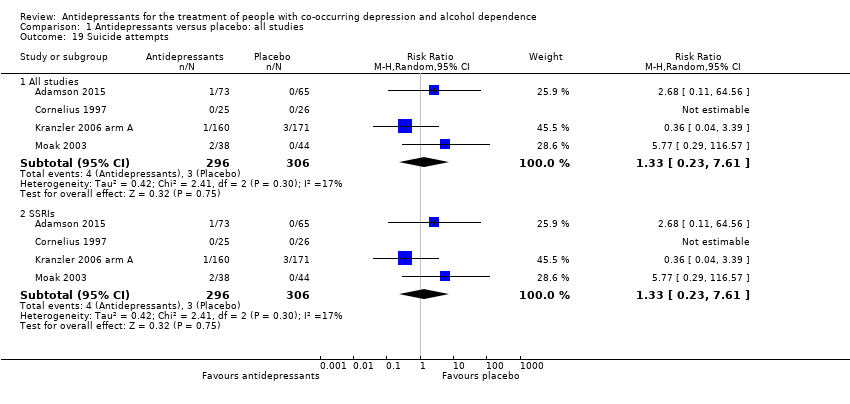
Comparison 1 Antidepressants versus placebo: all studies, Outcome 19 Suicide attempts.
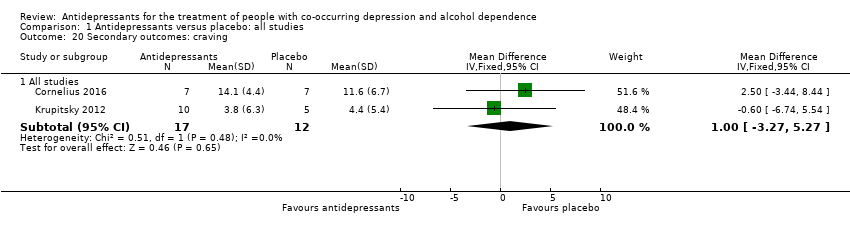
Comparison 1 Antidepressants versus placebo: all studies, Outcome 20 Secondary outcomes: craving.
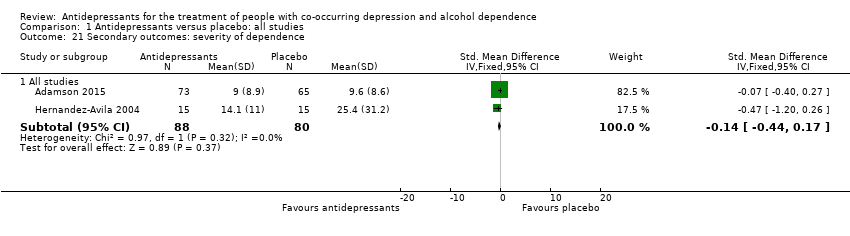
Comparison 1 Antidepressants versus placebo: all studies, Outcome 21 Secondary outcomes: severity of dependence.
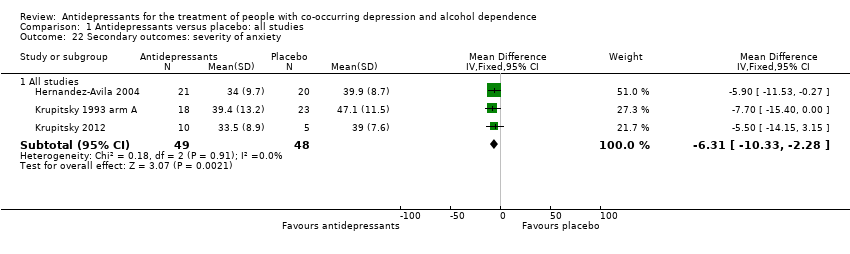
Comparison 1 Antidepressants versus placebo: all studies, Outcome 22 Secondary outcomes: severity of anxiety.
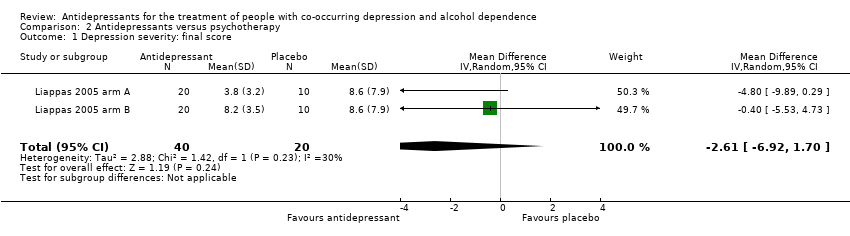
Comparison 2 Antidepressants versus psychotherapy, Outcome 1 Depression severity: final score.
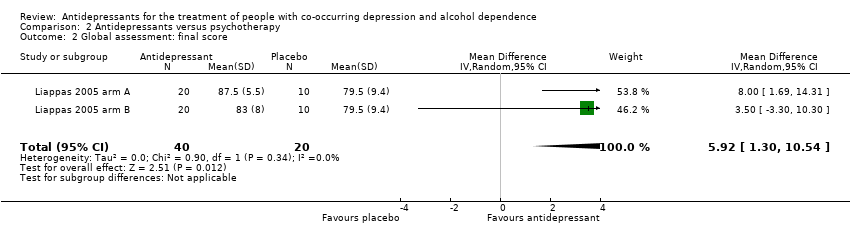
Comparison 2 Antidepressants versus psychotherapy, Outcome 2 Global assessment: final score.
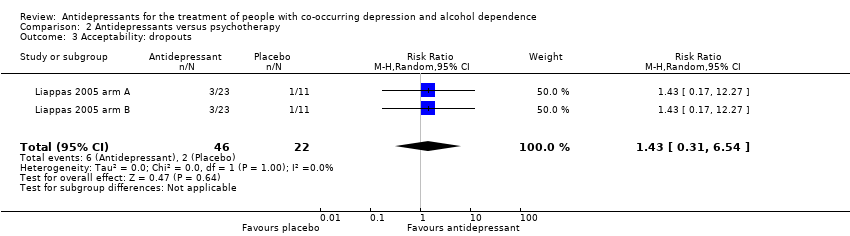
Comparison 2 Antidepressants versus psychotherapy, Outcome 3 Acceptability: dropouts.
| Antidepressants compared to placebo: all studies | ||||||
| Patient or population: people with co‐occurring depression and alcohol dependence Settings: unknown | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Antidepressants | |||||
| Depression severity: final score (interviewer‐rated scales) | ‐ | The mean depression: final score (interviewer‐rated scales) ‐ all studies in the intervention groups was 0.27 standard deviations lower (0.49 lower to 0.04 lower) | ‐ | 1074 | ⊕⊕⊝⊝ | ‐ |
| Response to antidepressive treatment | Study population | RR 1.40 | 805 | ⊕⊝⊝⊝ | ‐ | |
| 481 per 1000 | 674 per 1000 | |||||
| 392 per 1000 | 521 per 1000 | |||||
| Consumption of alcohol: abstinent days (%) | ‐ | The mean alcohol: abstinent days (%) ‐ all studies in the intervention groups was 1.34 higher (1.66 lower to 4.34 higher) | ‐ | 821 | ⊕⊕⊝⊝ | ‐ |
| Consumption of alcohol: abstinent participants (number) | Study population | RR 1.71 | 424 | ⊕⊕⊕⊝ | ‐ | |
| 199 per 1000 | 340 per 1000 | |||||
| 188 per 1000 | 321 per 1000 | |||||
| Consumption of alcohol: drinks (per drinking days) | ‐ | The mean alcohol: drinks (per drinking days) ‐ all studies in the intervention groups was | ‐ | 451 | ⊕⊕⊕⊝ | ‐ |
| Acceptability: dropouts | Study population | RR 0.98 | 1159 | ⊕⊕⊝⊝ | ‐ | |
| 334 per 1000 | 328 per 1000 | |||||
| 307 per 1000 | 301 per 1000 | |||||
| Tolerability of treatment: withdrawal for medical reasons | Study population | RR 1.15 | 947 | ⊕⊕⊝⊝ | ‐ | |
| 69 per 1000 | 80 per 1000 | |||||
| 32 per 1000 | 37 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Nine studies at unclear risk for selection bias; two studies at high risk and six at unclear risk for performance bias; 13 studies at unclear risk for detection bias (subjective); one study at high risk and two at unclear risk for attrition bias; three studies at high risk and one at unclear risk for reporting bias. 6Seven studies with unclear risk of selection bias; one study at high risk and five studies at unclear risk for performance bias; all studies at unclear risk for detection bias (subjective); one study at high risk and two at unclear risk for reporting bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depression severity: final score (interviewer‐rated scales) Show forest plot | 14 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All studies | 14 | 1074 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.49, ‐0.04] |
| 1.2 Selective serotonin reuptake inhibitors (SSRIs) | 10 | 881 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.40, 0.07] |
| 1.3 5‐HT2 antagonists | 2 | 97 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.69, 0.11] |
| 2 Depression severity: final score (self‐administered scales) Show forest plot | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 All studies | 8 | 373 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.64, 0.07] |
| 2.2 SSRIs | 5 | 300 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.69, 0.18] |
| 2.3 5‐HT2 antagonists | 2 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.59, 0.64] |
| 3 Depression severity: difference between basal and final score (interviewer‐rated scales) Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 All studies | 5 | 447 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.12, 0.42] |
| 3.2 SSRIs | 4 | 408 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.18, 0.32] |
| 4 Depression severity: difference between basal and final score (self‐administered scales) Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 All studies | 4 | 121 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.16, 0.56] |
| 4.2 SSRIs | 2 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.16, 0.73] |
| 4.3 5‐HT2 antagonists | 2 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.59, 0.64] |
| 5 Response to antidepressive treatment Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 All studies | 10 | 805 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.08, 1.82] |
| 5.2 Tricyclic antidepressants (TCAs) | 4 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [1.09, 2.34] |
| 5.3 SSRIs | 5 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.87, 1.63] |
| 6 Full remission of depression Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 All studies | 4 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.77, 1.83] |
| 6.2 SSRIs | 3 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.74, 1.36] |
| 7 Consumption of alcohol: abstinent days (%) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 All studies | 9 | 821 | Mean Difference (IV, Random, 95% CI) | 1.34 [‐1.66, 4.34] |
| 7.2 SSRIs | 7 | 711 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐3.20, 2.26] |
| 8 Consumption of alcohol: abstinent participants (number) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 All studies | 7 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [1.22, 2.39] |
| 8.2 SSRIs | 4 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.02, 2.68] |
| 8.3 5‐HT2 antagonists | 2 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.77, 3.39] |
| 9 Consumption of alcohol: drinking days (per week) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 All studies | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐1.15 [‐2.35, 0.05] |
| 9.2 5‐HT2 antagonists | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐1.15 [‐2.35, 0.05] |
| 10 Consumption of alcohol: drinks (per drinking days) Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 All studies | 7 | 451 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐1.79, ‐0.46] |
| 10.2 SSRIs | 3 | 271 | Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐2.58, ‐0.26] |
| 10.3 5‐HT2 antagonists | 3 | 111 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐2.00, ‐0.11] |
| 11 Consumption of alcohol: drinks (per week) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 All studies | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐5.06 [‐12.30, 2.18] |
| 11.2 5‐HT2 antagonists | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐5.06 [‐12.30, 2.18] |
| 12 Consumption of alcohol: heavy drinking days (per week) Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 All studies | 5 | 313 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.85, 0.20] |
| 12.2 SSRIs | 2 | 189 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.09, 0.27] |
| 12.3 5‐HT2 antagonists | 2 | 55 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐2.09, 1.22] |
| 13 Consumption of alcohol: heavy drinkers (number) Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 All studies | 7 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.57, 1.07] |
| 13.2 SSRIs | 6 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.69, 1.11] |
| 13.3 5‐HT2 antagonists | 2 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.68, 4.67] |
| 14 Consumption of alcohol: time to first relapse (days) Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 All studies | 6 | 348 | Mean Difference (IV, Random, 95% CI) | 2.54 [‐8.79, 13.87] |
| 14.2 SSRIs | 6 | 348 | Mean Difference (IV, Random, 95% CI) | 2.54 [‐8.79, 13.87] |
| 15 Liver enzyme levels: γ‐glutamyltransferase (U/L) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 All studies | 2 | 56 | Mean Difference (IV, Random, 95% CI) | ‐8.39 [‐26.47, 9.68] |
| 16 Depression and alcohol: global response Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 All studies | 3 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 2.37 [1.34, 4.19] |
| 16.2 TCAs | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.09, 4.02] |
| 17 Acceptability: dropouts Show forest plot | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 All studies | 17 | 1159 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.79, 1.22] |
| 17.2 TCAs | 4 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.48, 3.06] |
| 17.3 SSRIs | 8 | 759 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.79, 1.36] |
| 17.4 5‐HT2 antagonists | 4 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.38, 1.64] |
| 18 Tolerability of treatment: adverse events Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 Withdrawal for medical reasons: all studies | 10 | 947 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.65, 2.04] |
| 18.2 Withdrawal for medical reasons: SSRIs | 7 | 786 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.52, 2.32] |
| 18.3 Withdrawal for medical reasons: TCAs | 2 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.10, 8.41] |
| 18.4 Total adverse events: all studies | 5 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.97, 1.44] |
| 18.5 Total adverse events: TCAs | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.13, 2.42] |
| 18.6 Total adverse events: SSRIs | 3 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.92, 1.23] |
| 18.7 Dry mouth: all studies | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.96, 3.81] |
| 18.8 Insomnia: all studies | 4 | 564 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.02, 2.77] |
| 18.9 Insomnia: SSRIs | 2 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [1.04, 2.96] |
| 18.10 Headache: all studies | 3 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.89, 1.64] |
| 18.11 Headache: SSRIs | 2 | 414 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.87, 1.61] |
| 18.12 Dizziness: all studies | 2 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.42, 6.73] |
| 18.13 Diarrhoea: all studies | 2 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.37, 10.22] |
| 18.14 Nausea: all studies | 3 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.66, 3.23] |
| 18.15 Nausea: SSRIs | 2 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.62, 3.35] |
| 18.16 Constipation: all studies | 2 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.19, 15.64] |
| 18.17 Total serious adverse events: all studies | 7 | 774 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.80, 1.86] |
| 18.18 Total serious adverse events: SSRIs | 5 | 721 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.80, 1.86] |
| 18.19 Worsening of clinical condition because of relapse: all studies | 2 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.73, 10.87] |
| 18.20 Worsening of clinical condition because of relapse: SSRIs | 2 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.73, 10.87] |
| 18.21 Depression: all studies | 2 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [0.30, 17.69] |
| 18.22 Depression: SSRIs | 2 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [0.30, 17.69] |
| 19 Suicide attempts Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.1 All studies | 4 | 602 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.23, 7.61] |
| 19.2 SSRIs | 4 | 602 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.23, 7.61] |
| 20 Secondary outcomes: craving Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 20.1 All studies | 2 | 29 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐3.27, 5.27] |
| 21 Secondary outcomes: severity of dependence Show forest plot | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.1 All studies | 2 | 168 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.44, 0.17] |
| 22 Secondary outcomes: severity of anxiety Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 22.1 All studies | 3 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐6.31 [‐10.33, ‐2.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Depression severity: final score Show forest plot | 2 | 60 | Mean Difference (IV, Random, 95% CI) | ‐2.61 [‐6.92, 1.70] |
| 2 Global assessment: final score Show forest plot | 2 | 60 | Mean Difference (IV, Random, 95% CI) | 5.92 [1.30, 10.54] |
| 3 Acceptability: dropouts Show forest plot | 2 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.31, 6.54] |

