Фенитоин для наружного применения в лечении пролежней
Abstract
Background
Pressure ulcers are common in clinical practice and pose a significant health problem worldwide. Apart from causing suffering to patients, they also result in longer hospital stays and increase the cost of health care. A variety of methods are used for treating pressure ulcers, including pressure relief, patient repositioning, biophysical strategies, nutritional supplementation, debridement, topical negative pressure, and local treatments including dressings, ointments and creams such as bacitracin, silver sulphadiazine, neomycin, and phenytoin. Phenytoin is a drug more commonly used in the treatment of epilepsy, but may play an important role in accelerating ulcer healing.
Objectives
To assess the effects of topical phenytoin on the rate of healing of pressure ulcers of any grade, in any care setting.
Search methods
In September 2016, we searched the following electronic databases to identify relevant randomized clinical trials: the Cochrane Wounds Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library); Ovid MEDLINE; Ovid Embase; and EBSCO CINAHL Plus. We handsearched conference proceedings from the European Pressure Ulcer Advisory Panel, European Wound Management Association and the Tissue Viability Society for all available years. We searched the references of the retrieved trials to identify further relevant trials. We also searched clinical trials registries to identify ongoing and unpublished studies. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria
We included all randomized controlled trials (RCTs) addressing the effects (both benefits and harms) of topical phenytoin on the healing of pressure ulcers of any grade compared with placebo or alternative treatments or no therapy, irrespective of blinding, language, and publication status.
Data collection and analysis
Two review authors independently selected studies, extracted information on participants, interventions, methods and results and assessed risk of bias using Cochrane methodological procedures. For dichotomous variables, we calculated the risk ratio (RR) with 95% confidence interval (CI). For continuous variables, we calculated the mean difference with 95% CI. We rated the quality of the evidence by using Grading of Recommendations, Assessment, Development and Evaluation approach (GRADE).
Main results
Three small RCTs met our inclusion criteria and included a total of 148 participants. These compared three treatments with topical phenytoin: hydrocolloid dressings, triple antibiotic ointment and simple dressings. In the three RCTs, 79% of participants had grade II ulcers, and 21% of participants had grade I ulcers; no participants had grade III or IV ulcers. Two RCTs had a high risk of bias overall and the other RCT was at unclear risk of bias due to poor reporting. Two RCTs had three intervention arms and the other had two intervention arms.
Two studies compared topical phenytoin with hydrocolloid dressing (84 participants analysed). The available data suggest that hydrocolloid dressings may improve ulcer healing compared to topical phenytoin (39.3% ulcers healed for phenytoin versus 71.4% ulcers healed for hydrocolloid dressings (RR 0.55, 95% CI 0.33 to 0.92; 56 participants, 1 study; low quality evidence). We downgraded the evidence twice: once due to serious limitations (high risk of bias) and once due to the small sample size and small number of events. Two studies compared topical phenytoin with simple dressings (81 participants analysed). From the available data, we are uncertain whether topical phenytoin improves ulcer healing compared to simple dressings (39.3% ulcers healed for phenytoin versus 29.6% ulcers healed for the simple dressing (RR 1.33, 95% CI 0.63 to 2.78; 55 participants, 1 study; very low quality evidence). This evidence was downgraded once due to serious limitations (high risk of bias) and twice due to the low number of outcome events and resulting wide CI which included the possibility of both increased healing and reduced healing. We therefore considered it to be insufficient to determine the effect of topical phenytoin on ulcer healing. One study compared topical phenytoin with triple antibiotic ointment, however, none of the outcomes of interest to this review were reported. No adverse drug reactions or interactions were detected in any of the three RCTs. Minimal pain was reported in all groups in one trial that compared topical phenytoin with hydrocolloid dressings and triple antibiotic ointment.
Authors' conclusions
This review has considered the available evidence and the result shows that it is uncertain whether topical phenytoin improves ulcer healing for patients with grade I and II pressure ulcers. No adverse events were reported from three small trials and minimal pain was reported in one trial. Therefore, further rigorous, adequately powered RCTs examining the effects of topical phenytoin for treating pressure ulcers, and to report on adverse events, quality of life and costs are necessary.
PICOs
Резюме на простом языке
Фенитоин для наружного (накожного) применения в лечении пролежней
Вопрос обзора
Мы рассмотрели доказательства влияния фенитоина (лекарство, применяемое внутрь для лечения эпилепсии) при использовании в качестве крема или в повязке, накладываемых непосредственно на пролежни. Мы хотели выяснить влияние фенитоина на заживление язв и наличие у него вредоносного побочного эффекта.
Актуальность
Пролежни ‐ это участки кожи и подкожной клетчатки, которые были повреждены в результате длительного давления и/или воздействия поперечных сил (например, силы, действующие на кожу при длительном нахождении в относительно вертикальном положении в постели или кресле). В группу людей, подверженных риску развития пролежней, входят те, кто имеют травмы спинного мозга, и те, кто неподвижны или чья подвижность ограничена, например, пожилые люди и люди, находящиеся на постельном режиме в течение длительного времени из‐за кратковременных или долгосрочных заболеваний. Требуется много времени, чтобы вылечить пролежни. Люди с пролежнями на протяжении длительного времени часто страдают от боли и лечения, которое затрудняет осуществление повседневной деятельности; это оказывает влияние на их качество жизни и может привести к дополнительным затратам на их медицинское обслуживание.
Фенитоин для внутреннего применения ‐ это лекарство, которое используется для контроля эпилептических припадков. Было высказано предположение, что фенитоин для наружного применения (наносится непосредственно на кожу) может помочь в заживлении пролежней, так как он был использован для наружного заживления и снижения боли и отека (воспаления) некоторых ран, в том числе появившиеся в результате травматического повреждения, других типов язв и ожогов. Препарат наружного применения может использоваться в виде крема, лосьона или пропитанной медикаментом повязки.
Характеристика исследований
В сентябре 2016 года мы провели поиск рандомизированных контролируемых клинических исследований (РКИ), которые сравнивали фенитоин наружного применения с другими вариантами лечения пролежней. Мы нашли три небольших РКИ, которые включали в общей сложности 148 человека с пролежнями. Средний возраст участников в двух исследованиях был 45 лет и 75 лет в одном исследовании. Двадцать один процент участников имел язвы I степени (менее тяжелый тип, с опухшей, но неповрежденной кожей) и 79% имели язвы II степени (несколько более серьезные). Ни у кого не было язв III или IV степени (наиболее тяжелые типы). Клинические испытания сравнивали фенитоин наружного применения с тремя другими видами лечения пролежней: гидроколлоидными перевязочными материалами, мазью с тремя антибиотиками и простыми повязками. Результаты исследования дают основания считать, что гидроколлоидные перевязочные материалы могут немного улучшить заживление язв по сравнению с фенитоином для наружного применения. Однако мы не уверены, улучшает ли фенитоин наружного применения заживление язв по сравнению с простыми повязками. Исследование, в котором сравнивали фенитоин наружного применения с мазью с тремя антибиотиками, не представило информации по исходам, которые являлись исходами, изучавшимися в этом обзоре.
Качество доказательств
Неясно, улучшает ли фенитоин для наружного применения заживление язв у пациентов с I и II степенью пролежней. Не сообщалось о каких‐либо нежелательных явлениях в трех небольших клинических испытаниях, и только в одном испытании сообщалось о минимальной боли. Отчеты о клинических испытаниях не содержали информации о некоторых показателях, которые мы изучали, таких, как стоимость лечения и качество жизни. Два РКИ имели высокий риск смещения (систематической ошибки) в целом, что могло повлиять на результаты, а отчет о другом РКИ не содержал достаточно данных о том, как оно было проведено. Необходимы более тщательные, надлежащим образом разработанные РКИ, чтобы установить пользу фенитоина для наружного применения в лечении пролежней.
Это резюме на простом языке актуально по состоянию на сентябрь 2016 года.
Authors' conclusions
Summary of findings
| Topical phenytoin compared with hydrocolloid dressing for pressure ulcers | ||||||
| Patient or population: people with a pressure ulcer Settings: family homes or nursing homes Intervention: topical phenytoin Comparison: hydrocolloid dressing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with hydrocolloid dressing | Risk with topical phenytoin | |||||
| Time to complete healing | See comments | See comments | The study data reported did not provide a suitable representation of the outcome of interest | |||
| Proportion of ulcers healed within trial period (eight weeks) | 714 per 1000 | 393 per 1000 (236 to 657) | RR 0.55 (0.33 to 0.92) | 56 (1 study) | ⊕⊕⊝⊝ | |
| Adverse events | See comments | See comments | Not estimable | 84 (2 studies) | No adverse drug reactions or interactions were detected in the included RCTs. | |
| Pain | See comments | See comments | Not estimable | 28 (1 study) | Minimal pain was reported in all groups in one study. Study authors made this statement without any data to support it. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded two levels: serious limitation (risk of bias), serious imprecision (small number of events ) | ||||||
| Topical phenytoin compared with triple antibiotic ointment for pressure ulcers | ||||||
| Patient or population: people with a pressure ulcer Settings: nursing homes Intervention: topical phenytoin Comparison: triple antibiotic ointment | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with triple antibiotic ointment | Risk with topical phenytoin | |||||
| Time to complete healing | See comments | See comments | The study data reported did not provide a suitable representation of the outcome of interest | |||
| Proportion of ulcers healed within trial period (eight weeks) | See comments | See comments | This outcome was not reported for this comparison | |||
| Adverse events | See comments | See comments | Not estimable | 28 (1 study) | No adverse drug reactions or interactions were detected in the included RCT. | |
| Pain | See comments | See comments | Not estimable | 28 (1 study) | Minimal pain was reported in all groups in one study. Study authors made this statement without any data to support it. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Topical phenytoin compared with a simple dressing for pressure ulcers | ||||||
| Patient or population: people with a pressure ulcer Settings: family homes or nursing homes, and a hospital Intervention: topical phenytoin Comparison: a simple dressing | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with a simple dressing | Risk with topical phenytoin | |||||
| Time to complete healing | See comments | See comments | This outcome was not reported for this comparison | |||
| Proportion of ulcers healed within trial period (eight weeks) | 296 per 1000 | 394 per 1000 (187 to 824) | RR 1.33 (0.63 to 2.78) | 55 (1 study) | ⊕⊝⊝⊝ very low1 | |
| Adverse events | See comments | See comments | Not estimable | 81 (2 studies) | No adverse drug reactions or interactions were detected in the included RCTs. | |
| Pain | See comments | See comments | This outcome was not reported for this comparison. | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded three levels: once for serious limitation (risk of bias), and twice for very serious imprecision (small number of events and a wide confidence interval which includes the possibility of both increased healing and reduced healing) | ||||||
Background
Description of the condition
A pressure ulcer can be defined as localised injury to the skin or underlying tissue, or both, usually over a bony prominence, as a result of pressure, or pressure in combination with shear. A number of contributing or confounding factors are also associated with pressure ulcers; the significance of these factors has yet to be elucidated (EPUAP/NPUAP/PPPIA 2014). Pressure ulcers are a widespread, expensive and painful healthcare problem, with prevalence rates that range from 8.8% to 53.2% (Gallagher 2008; Moore 2012), and incidence rates that vary from 7% to 71.6% (Scott 2006; Moore 2011). Mean prevalence has been reported as 21% in acute care settings and as 12% in long‐stay settings (Moore 2013a). Prevalence among hospice patients has been reported as 35.7%, and in community care patients as 0.04% and 4% (Moore 2013a). Similarly, mean incidence has been reported as 17.6% in an acute care setting, 6.63% in a long‐stay setting and 20.4% in a hospice setting (Moore 2013a). Pressure ulcers represent a major concern as they can take a long time to heal, reduce quality of life for many people, and are a burden on healthcare systems and society.
The cost of pressure ulcer treatment is substantial. Pressure ulcers represent a significant cost burden in the UK, both to patients and to healthcare providers. The cost of treating a pressure ulcer varies from GBP 1214 (grade I ‐ least severe type of pressure ulcer) to GBP 14,108 (grade IV ‐ most severe) (Dealey 2012). Costs will increase with the severity of the ulcer, and the incidence of complications is higher in more severe cases (Dealey 2012). In the USA, it has been reported that the average hospital treatment cost associated with grade IV pressure ulcers and related complications was USD 129,248 for hospital‐acquired ulcers during one admission, and USD 124,327 for community‐acquired ulcers over an average of four admissions; this is much more than previously estimated (Brem 2010). In the Netherlands, pressure ulcers are thought to be the third most expensive health problem (Haalboom 2000). In Flanders, the mean cost of local treatment per patient per day varied between EUR 2.34 and EUR 77.36 in hospitals, and between EUR 2.42 and EUR 16.18 in nursing homes (Demarré 2015). Pressure ulcers impose a heavy burden on healthcare systems in terms of cost, patient suffering, and the need for skilled medical intervention.
Pressure ulcers can be graded according to criteria established by the European Pressure Advisory Panel and National Pressure Ulcer Advisory Panel as follows (EPUAP/NPUAP/PPPIA 2014):
-
Grade I: non‐blanchable erythema (redness) of intact skin. Discolouration of the skin, warmth, oedema (swelling), induration (hardness) may also be used as indicators, particularly on individuals with darker skin.
-
Grade II: partial‐thickness skin loss involving epidermis, dermis, or both. The ulcer is superficial and presents clinically as an abrasion or blister.
-
Grade III: full‐thickness skin loss involving damage necrosis of subcutaneous tissue that may extend down to, but not through, underlying fascia (layers).
-
Grade IV: extensive destruction, tissue necrosis (death), or damage to muscle, bone, or supporting structures with or without full‐thickness skin loss.
A variety of risk factors for pressure ulcer formation have been identified in different populations. Besides unrelieved pressure, shearing forces and friction, endogenous (personal) conditions that predispose people to pressure ulcers include old age, diabetes, terminal illness, sepsis, neurological and vascular diseases (Bliss 1999). Other risk factors include incontinence, immobility, altered mental status, lower blood pressure and higher body temperature (Allman 1986; Bergstrom 1992; Armstrong 2001; Schouchoff 2002; Benoit 2012).
Description of the intervention
A variety of methods have been used for treating pressure ulcers, such as pressure relief using beds, mattresses or cushions and patient repositioning (NICE 2014; Gillespie 2014; McGinnis 2014; McInnes 2015; Moore 2015); biophysical strategies such as electrical stimulation, ultrasound, ultraviolet irradiation, massage therapy, and laser treatment (Nussbaum 1994; Akbari Sari 2006; Aziz 2012; Chen 2014; Zhang 2015); nutritional supplementation (Hartgrink 1998; Langer 2014); debridement (removal of dead tissue) (Witkowski 1991; Moore 2013b); topical (skin surface) negative pressure (Philbeck 1999; Dumville 2015a); and local treatments including hydrocolloid dressings and phenytoin (Hollisaz 2004; Dumville 2015b). Hydrocolloid dressings are advanced wound dressings designed to control the environment with the aim of promoting wound healing; examples include Granuflex and Duoderm.
Phenytoin is an effective anti‐epileptic medication. The possibility of using phenytoin for wound healing was first recognised in 1939 when it was observed that patients receiving oral phenytoin had a side‐effect of gingival hyperplasia (increase in the size of the gums) (Kimball 1939). In 1958, Shapiro carried out the first controlled clinical trial examining the effects of oral phenytoin on periodontal (mouth) wounds and reported that phenytoin accelerated wound healing and reduced pain and inflammation (Shapiro 1958). The first double‐blind, placebo‐controlled clinical study involving topical phenytoin treatment in venous stasis ulcers demonstrated that, when compared with controls, the use of phenytoin promoted wound healing (Simpson 1965). Since this study, a number of other studies have suggested the effectiveness of topical phenytoin in the treatment of a variety of wounds including diabetic ulcers (Pai 2001), trophic ulcers in leprosy (Bhatia 2004), chronic leg ulcers (Carneiro 2003), pressure ulcers (Rhodes 2001), and superficial burn wounds (Carneiro 2002). The topical application of phenytoin can be usually administered as either powder, via an impregnated dressing, or as cream (Fonseka 2010; Pereira 2010; Hokkam 2011; Shaw 2011).
How the intervention might work
It has been reported that phenytoin may promote ulcer healing by stimulating the following processes: fibroblast proliferation (cells that play a central part in wound healing) (Shapiro 1958; Cloyd 1994; Margolis 1995; Eser 2012); collagen deposition (Margolis 1995; Habibipour 2003; Beigom Taheri 2015); vessel ingrowth (Dill 1997; Simsek 2014; Sayar 2014); glucocorticoid antagonism (reduces inflammation) (Margolis 1995; Baharvand 2014; Simsek 2014), and antibacterial activity (Dill 1997; Patil 2013); as well as enhancing the number of macrophages in the wound (cells that 'eat' cellular debris) (Song 1997; Sehgal 2014).
Why it is important to do this review
Previous systematic reviews reported evidence about topical phenytoin for treating pressure ulcers (Reddy 2006; Reddy 2008; Reddy 2011; Smith 2013), but the data were inconsistent and insufficient to enable conclusions to be drawn. In addition, they did not report the outcomes in detail. The effects of topical phenytoin on the healing of pressure ulcers is not known, therefore it is important to undertake a comprehensive assessment of all the available evidence.
Objectives
To assess the effects of topical phenytoin on the rate of healing of pressure ulcers of any grade, in any care setting.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomized controlled trials (RCTs), including cluster trials, that addressed the effects (both benefits and harms) of topical phenytoin on the healing of pressure ulcers of any grade, irrespective of blinding status, language, and publication status.
Types of participants
We included studies involving people of any age, of any gender and race/ethnicity, and in any care setting, who were described as having a pressure ulcer (pressure sore, bed sore or decubitus ulcer) of any grade.
Types of interventions
The topical application of phenytoin (administered as either powder, impregnated dressing, cream and/or other preparation) to treat pressure ulcers compared with placebo or alternative treatments or no therapy.
Types of outcome measures
Primary outcomes
-
Time to complete healing (we defined complete healing as intact skin)
-
Proportion of ulcers healed within trial period.
Secondary outcomes
-
Adverse events, such as any drug allergies and adverse drug reactions
-
Pain, measured using tools such as a visual analogue scale
-
The overall cost of healing pressure ulcers in different grades of pressure ulcers
-
Quality of life, using tools such as the Nottingham Health Profile.
Search methods for identification of studies
Electronic searches
We searched the following databases with the assistance of Cochrane Wound's Information Specialist:
-
the Cochrane Wounds Specialised Register (searched 10 September 2016);
-
the Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library 2016, Issue 9);
-
Ovid MEDLINE (1946 to 27 September 2016);
-
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (searched 27 September 2016);
-
Ovid Embase (1974 to 27 September 2016);
-
EBSCO CINAHL Plus (1937 to 27 September 2016).
CENTRAL was searched using the strategy in Appendix 1.This strategy was adapted where appropriate for the Cochrane Wounds Specialised Register. The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity and precision‐maximizing version (2011 revision) (Lefebvre 2011). The Embase and CINAHL Plus searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2015). There was no restriction on the basis of date or language of publication.
The search strategies for Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus are listed in Appendix 1.
Searching other resources
We handsearched conference proceedings from the European Pressure Ulcer Advisory Panel, the European Wound Management Association and the Tissue Viability Society from inception to 27 September 2016. We searched the reference sections of the retrieved trial reports to identify further relevant trials.
We also searched the following clinical trials registries for any ongoing and unpublished studies (searched 27 September 2016):
-
Clinical Trials.gov (clinicaltrials.gov);
-
WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en);
-
Chinese Clinical Trial Registry (ChiCTR) (www.chictr.org.cn/searchprojen.aspx).
Data collection and analysis
We carried out data collection and analysis according to methods stated in the published protocol (Hao 2010), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Selection of studies
Two review authors (XYH and YFS) identified the trials for inclusion according to the selection criteria independently of each other. When it was unclear from the title or abstract whether a paper fulfilled the criteria, we obtained a copy of the full text and the two review authors jointly decided whether the study met the inclusion criteria. We listed the excluded studies with the reasons for their exclusion. We resolved disagreement by discussion with a third review author (TKG). We set out a study flow diagram as recommended by the PRISMA statement to illustrate the process of screening and selecting studies for inclusion in the review (Figure 1) (Liberati 2009).
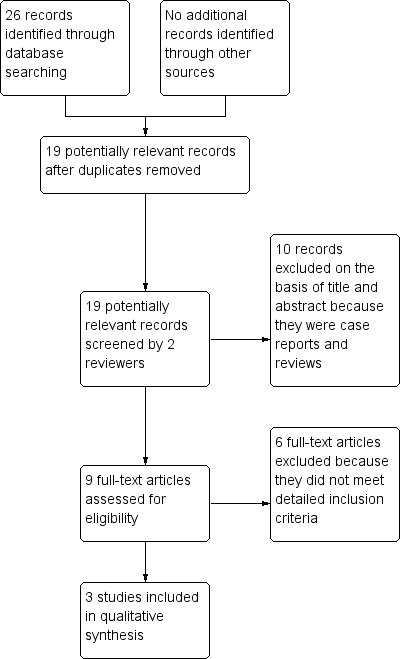
Study flow diagram.
Data extraction and management
Two review authors (XYH and YFS) independently extracted the following data from each study:
-
first author
-
year the trial was conducted
-
year of publication
-
country
-
inclusion and exclusion criteria
-
baseline characteristics such as age, sex ratio, concurrent disease, and presence of existing pressure ulcers on entering the study (site, number, grade and size of ulcer(s)), by treatment group
-
description of interventions and co interventions and number of participants in each group
-
data for the primary and the secondary outcomes
-
risk of bias (described below)
-
duration of follow‐up
-
source of funding.
We sought any unclear or missing information by contacting the authors of the individual trials. If there was any doubt about whether the trials shared the same participants, completely or partially (by identifying common authors and centres), we contacted the authors of the trials to clarify whether the trial had been published in duplicate. We resolved any differences in opinion through discussion.
Assessment of risk of bias in included studies
Two review authors (XYH and YFS) independently assessed each included study using the Cochrane tool for assessment of risk of bias (Higgins 2011a). This tool addresses six specific domains, namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues (e.g. extreme baseline imbalance; see Appendix 2 for details of criteria on which the judgements were based). Blinding and completeness of outcome data were assessed for each outcome separately. We completed a 'Risk of bias' table for each eligible study. We discussed any disagreement amongst all review authors to achieve a consensus.
We have presented an assessment of risk of bias using a 'Risk of bias' summary figure, which presents all of the judgments in a cross‐tabulation of study by entry. This display of internal validity indicates the weight the reader may give the results of each study.
Measures of treatment effect
We carried out this systematic review according to Cochrane methods (Higgins 2011a), and used the Cochrane software package Review Manager 5 (RevMan 2014). For dichotomous variables, we calculated the risk ratio (RR) with 95% confidence interval (CI). For continuous variables, we calculated the mean difference (MD) with 95% CI. We carried out the analysis according to the number of pressure ulcers in each group. For time to complete healing, we checked the time to complete healing of participants who reached complete healing in each intervention group, and these original data were then presented and analysed in this review.
Unit of analysis issues
We considered individual participants as the unit of analysis. We checked how participants, or ulcers, had been randomized and whether there was evidence that more than one pressure ulcer on a single person had been analysed by examining the number of participants and the number of pressure ulcers in each trial. We did not include any cluster‐randomized studies and cross‐over trials in this review.
Dealing with missing data
We attempted to contact the original investigators of trials to request information re missing data. As we received no response, we assessed the risk of attrition bias according to our risk of bias criteria and based on available data (Higgins 2011b). We reported the number and percentage of dropouts/withdrawals. Studies that had missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups, we assessed as being at a low risk of bias for attrition. If the percentage of dropouts/withdrawals was less than 10%, we classed the trial as being at a low risk of bias, but if dropouts/withdrawals exceeded 10%, we classed the trial as being at high risk of bias.
Where we considered data to be missing at random, we analysed the available information. Where outcome data were missing, we used an available‐case analysis, based on the numbers of participants for whom outcome data were known. We also addressed the potential impact of the missing data on the findings of the review in the discussion section (Higgins 2011c).
Assessment of heterogeneity
We intended to explore heterogeneity using the Chi² test, with P values of less than 0.1 considered to be statistically significant, and to measure heterogeneity using the I² statistic (Higgins 2003). We considered that an I² value greater than 50% represented substantial heterogeneity. We intended to explore clinical heterogeneity by examining potentially influential factors, for example, care setting or participant characteristics. No studies were pooled in this review, but this was largely due to a lack of suitable data for pooling rather than because of heterogeneity.
Assessment of reporting biases
In future, if 10 or more studies are included for meta‐analysis, we will assess visual asymmetry of funnel plots to identify any potential reporting or publication bias (Sterne 2011).
Data synthesis
We had decided to use a fixed‐effect model to pool all results when there was no evidence of heterogeneity between studies (DeMets 1987). If there was evidence of heterogeneity, we would have used a random‐effects model (DerSimonian 1986). Where pooling was not appropriate, because there was substantial heterogeneity or lack of suitable data for pooling, we presented the outcomes in a descriptive way. No pooling of results occurred in this review due to a lack of suitable data.
'Summary of findings' tables
We present the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE approach. The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We presented the following outcomes in the 'Summary of findings' tables:
-
time to complete healing;
-
proportion of ulcers healed within trial period;
-
adverse events;
-
pain.
Results
Description of studies
Results of the search
See: Characteristics of included studies; Characteristics of excluded studies.
We identified 26 relevant citations through database searches. After we had removed duplicates, 19 citations remained. We excluded ten of these on the basis of their title and abstract. We assessed 9 full‐text articles for eligibility and excluded six because they did not meet our detailed inclusion criteria. This left us with three studies that we included in this review (see Figure 1 for Study flow diagram).
Included studies
Three RCTs met the inclusion criteria. These had recruited a total of 148 participants (Subbanna 2007, 26 participants; Rhodes 2001, 39 participants; Hollisaz 2004, 83 participants). Only Hollisaz 2004 reported an a priori sample size estimation and showed the full details of the sample size estimation. The trials were published between 2001 and 2007. The studies were conducted in America (Rhodes 2001), Iran (Hollisaz 2004), and India (Subbanna 2007), and they were all single‐centre studies. The studies were carried out in nursing homes (Rhodes 2001), family homes or nursing homes (Hollisaz 2004), and a hospital (Subbanna 2007).
Hollisaz 2004 and Subbanna 2007 restricted recruitment to people with spinal cord disorders, while Rhodes 2001 excluded people with spinal cord disorders and did not report the cause of the pressure ulcers. All trials provided detailed information on patient exclusion criteria. Subbanna 2007 and Hollisaz 2004 included relatively young participants with a mean age of 45 years. Rhodes 2001 included participants with a mean age of 75 years. The mean duration of ulcers before treatment in Hollisaz 2004 and Subbanna 2007 was six and 10 weeks, respectively; mean duration was not reported in Rhodes 2001. Subbanna 2007 and Rhodes 2001 included only participants with grade II pressure ulcers; Hollisaz 2004 included participants with grade I and grade II ulcers. Pressure ulcers were all classified according guidance provided by the National Pressure Ulcer Advisory Panel (NPUAP 1995). The trials considered three comparators to topical phenytoin: triple antibiotic ointment (Rhodes 2001); hydrocolloid dressing (Rhodes 2001; Hollisaz 2004); and simple dressings (Hollisaz 2004; Subbanna 2007). The triple antibiotic ointment contained three antibiotics ‐ bacitracin, neomycin, and polymyxin B sulphates. The simple dressings intervention involved covering the ulcer with a wet saline gauze dressing after it was cleaned. In the three RCTs, two RCTs have three intervention arms respectively and another RCT has two intervention arms. The participants of every group in each comparison were comparable. The concentration of phenytoin solution was 5 mg/mL in Subbanna 2007 and 20 mg/mL in Rhodes 2001; the concentration used was not reported in Hollisaz 2004. Rhodes 2001 reported on time to complete healing and pain, while Hollisaz 2004 reported on proportion of ulcers healed within trial period. All three RCTs reported adverse events. None of the included studies reported costs or quality of life. The Rhodes 2001 study did not report the sources of funding, but the Hollisaz 2004 study received financial support from the Jaonbazan Medical and Engineering Research Center, and the Subbanna 2007 study received financial support from the intramural research funds of the Christian Medical College, Vellore, India.
Excluded studies
We excluded six studies and provided details of the reasons for their exclusion in the Characteristics of excluded studies table. These six studies were excluded because they did not meet detailed inclusion criteria after we had retrieved them in full text for more detailed evaluation (el Zayat 1989; Lodha 1991; Agarwal 1998; Chauhan 2003; Shaw 2010; Panahi 2015). Two of the excluded trials, Agarwal 1998 and el Zayat 1989 were not randomized trials. Chauhan 2003 included only participants with venous ulcers, diabetic ulcers and mixed wounds. Lodha 1991 included only participants with abscess wounds. Shaw 2010 included only participants with diabetic foot ulcers. Panahi 2015 included 60 participants (41 with pressure ulcers, 13 with diabetic wounds and 6 with venous ulcers). The included 60 participants were divided into two groups (Aloe vera‐olive oil combination cream group and phenytoin cream) by simple randomization rather than stratified randomization based on the type of the three diseases (pressure ulcers, diabetic wounds and venous ulcers). Therefore, participants with pressure ulcers in Panahi 2015 failed to meet the inclusion criteria for this review.
Risk of bias in included studies
An overall summary of the methodological quality of the included trials can be found in Figure 2 and Figure 3. The studies were either at high or unclear risk of bias.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
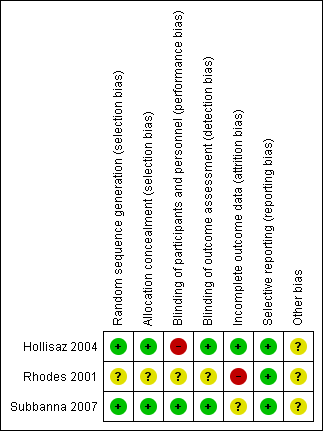
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
The participant was the unit of randomization in the included trials. Subbanna 2007 used a computer‐generated randomization list and Hollisaz 2004 used a random‐number table to ensure random assignment to the groups. Hollisaz 2004 and Subbanna 2007 used appropriate methods of concealment of allocation sequence that prevented foreknowledge of intervention assignments. Hollisaz 2004 reported that the treatment category for each participant was prepared by a statistician and contained within an opaque sealed envelope that was then opened by the general practitioner to determine which treatment would be used at the start of the study. Subbanna 2007 reported that the treatments were prepared and labelled using the randomization number provided by pharmacists from the institution's pharmacy. The nursing staff who administered pressure ulcer dressings were blinded to treatment assignment. Rhodes 2001 did not report sufficient details about sequence generation or allocation concealment to assess whether the study used appropriate methods. Therefore, the methods used for generating the allocation sequence and concealing the group allocation were of low risk of bias in Subbanna 2007 and Hollisaz 2004 but were unclear in Rhodes 2001.
Blinding
Subbanna 2007 reported successful blinding at all levels; the phenytoin solution was indistinguishable from the normal saline in presentation, colour, density, and odour, therefore, blinding of participants was achieved. So we judged Subbanna 2007 to be at a low risk of performance bias. The nursing staff were blinded to treatment assignment and undertook changes of the pressure ulcer dressings. Outcome assessors were also reported as blinded to treatment allocation. Hollisaz 2004 reported blinding of personnel and outcome assessors, but due to significant differences across the three treatment methods, blinding of participants was considered impossible and so we judged the study to be at a high risk of performance bias. Rhodes 2001 did not report whether participants, personnel and/or outcome assessors were blinded and so we judged this trial to be at an unclear risk of bias for these domains.
Incomplete outcome data
Two participants (7% of the included participants) in Subbanna 2007 were lost to follow‐up. Eight participants (17% of the included participants; three in the phenytoin group, three in the Duoderm group, and two in the triple antibiotic ointment group) did not complete the study in Rhodes 2001. We contacted the original investigators and there were no response. The final analysis in both trials did not include these dropouts/withdrawals; there was therefore a high risk of attrition bias in the estimates, especially in Rhodes 2001. Hollisaz 2004 reported that all participants completed the study, with no losses to follow‐up or treatment withdrawals. So we judged Hollisaz 2004 to be at a low risk of attrition bias.
Selective reporting
The included studies did not provide enough information to permit us to make a judgement regarding selective reporting, or to permit identification of any problem that might introduce bias. All of the trials provided information on the outcomes identified in their trial methods, so we considered them to be at a low risk of reporting bias.
Other potential sources of bias
We identified no other potential sources of bias in the included studies.
Effects of interventions
See: Summary of findings for the main comparison Topical phenytoin compared with hydrocolloid dressing for pressure ulcers; Summary of findings 2 Topical phenytoin compared with triple antibiotic ointment for pressure ulcers; Summary of findings 3 Topical phenytoin group compared with a simple dressing for pressure ulcers
Comparison 1: Topical phenytoin compared with hydrocolloid dressing (two trials)
summary of findings Table for the main comparison
Two trials with 28 participants (Rhodes 2001), and 56 participants (Hollisaz 2004) respectively, compared phenytoin with a hydrocolloid dressing.
Time to complete healing
In Rhodes 2001, the mean time to complete healing in the phenytoin group was 35.3 ±14.3 (mean ± standard deviation (SD)) days compared with 51.8 ± 19.6 (mean ± SD) days for the hydrocolloid dressing (Duoderm) group. However, time to complete healing is a type of time‐to‐event data and the study data reported do not provide a suitable representation of the outcome of interest. Due to uncertainty regarding the number of participants covered by the reported data, this result is not appropriate for use. Hollisaz 2004 did not report data on the time to complete healing.
Proportion of ulcers healed within trial period
The Hollisaz 2004 trial reported that 20 out of 28 (71.4%) participants' ulcers had completely healed at eight weeks in the hydrocolloid dressing group compared with 11 out of 28 (39.3%) participants in the topical phenytoin group. The results suggest that hydrocolloid dressings may improve ulcer healing at eight weeks compared with topical phenytoin (RR 0.55, 95% CI 0.33 to 0.92; 56 participants, 1 study; Analysis 1.1). Rhodes 2001 did not report data on the ulcers healed at eight weeks. This evidence is low quality; we downgraded it once for risk of bias due to lack of blinded participants and once for imprecision due to the small trial size and low number of events.
Adverse events
No adverse drug reactions or interactions were detected in the included RCTs.
Pain
Minimal pain was reported in all groups in Rhodes 2001. This statement was made by study authors. No data were available to support it.
Costs and quality of life
None of the included RCTs in this comparison reported on costs or quality of life.
Comparison 2: Topical phenytoin compared with triple antibiotic ointment (one trial)
One trial with 28 participants compared phenytoin with triple antibiotic ointment (Rhodes 2001).
Time to complete healing
The mean time to healing in the topical phenytoin group was 35.3 ± 14.3 (mean ± SD) days compared with 53.8 ± 8.5 (mean ± SD) days for the triple antibiotic ointment group. However, time to complete healing is a type of time‐to‐event data and the study data reported do not provide a suitable representation of the outcome of interest. Due to uncertainty regarding the number of participants covered by the reported data, this result is not appropriate for use.
Proportion of ulcers healed within trial period
This outcome was not reported for this comparison.
Adverse events
No adverse drug reactions or interactions were detected in the included RCT.
Pain
Minimal pain was reported in all groups in Rhodes 2001. This statement was made by study authors. No data were available to support it.
Costs and quality of life
The included RCT in this comparison did not report on costs or quality of life.
Comparison 3: Topical phenytoin compared with a simple dressing (two trials)
Two trials compared phenytoin with a simple dressing (Hollisaz 2004, with 55 participants; Subbanna 2007, with 26 participants).
Time to complete healing
This outcome was not reported for this comparison.
Proportion of ulcers healed within trial period
The Hollisaz 2004 trial reported that 11 out of 28 (39.3%) participants had completely ulcers healed at eight weeks in the topical phenytoin group compared with eight out of 27 (29.6%) participants in the simple dressing group. We are uncertain whether topical phenytoin improves ulcer healing compared with simple dressings (RR 1.33, 95% CI 0.63 to 2.78; 55 participants, 1 study; Analysis 2.1). This evidence is very low quality, we downgraded once for risk of bias due to lack of blinded participants and twice for imprecision due to the small number of events and wide confidence intervals that include the possibility of both increased healing and reduced healing. Subbanna 2007 did not report data on the proportion of ulcers healed within the trial period.
Adverse events
No adverse drug reactions or interactions were detected in the included RCTs.
Pain
Pain was not reported for this comparison.
Costs and quality of life
None of the included RCTs in this comparison reported on costs or quality of life.
Discussion
Summary of main results
The objective of our systematic review was to assess the effects of topical phenytoin on the rate of healing of pressure ulcers. However, only one study reported quantitative data suitable for a meta‐analysis (Hollisaz 2004). We have presented 'Summary of findings' tables with GRADE ratings for the relevant outcomes (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3). The conclusions are based on the results of three small RCTs that compared topical phenytoin to either hydrocolloid dressings, triple antibiotic ointment, or simple dressings. The results of one study suggest that hydrocolloid dressings may slightly improve ulcer healing compared to topical phenytoin (low quality evidence). We are uncertain whether topical phenytoin improves ulcer healing compared to simple dressings (very low quality evidence). One study compared topical phenytoin with triple antibiotic ointment, however, none of the outcomes of interest to this review was reported suitably to allow data to be considered further. The evidence for both comparisons for which there are data is of low to very low quality due to the paucity of data and risk of bias, therefore, we considered the available evidence to be insufficient to determine the effect of topical phenytoin for treating pressure ulcers. Adverse drug reactions or interactions were negligible in all three RCTs. Minimal pain was only reported in all groups in Rhodes 2001 . Study authors made this statement. There were no data to support it. For time to complete healing, the study data reported did not provide a suitable representation of the outcome of interest. It was not possible to address the other outcomes of this review, for example, costs and quality of life, because data on these were not reported.
Overall completeness and applicability of evidence
As the main results were from one study of 56 participants, it is highly unlikely that the results are generalisable. Pressure ulcers that penetrate only into the epidermis or dermis can heal through tissue regeneration, whereas deeper wounds that extend through the dermis, heal by the formation of scar tissue, since subcutaneous tissues, glands, and hair follicles cannot regenerate (Sieggreen 1987). In the three included RCTs, 79% of participants had grade II ulcers, and 21% participants had grade I ulcers; no participants had grade III or IV ulcers. Therefore, we are uncertain if effectiveness would be similar in participants with grade III or IV ulcers, and further research in this area needs to be conducted. Time to complete healing was not reported as time‐to‐event data, therefore, the effect of topical phenytoin on this outcome, together with costs and quality of life, are unknown.
Quality of the evidence
The quality of the evidence for each outcome was low to very low. While this analysis provided an estimate of the best available evidence, this review was based on only three RCTs with small sample sizes that were underpowered to detect significant differences. Also, only Hollisaz 2004 reported an a priori sample size estimation and showed the full details of the estimation. In addition, some methodological limitations may have affected the validity of this review. An overall summary of the methodological quality of the included trials can be found in Figure 2 and Figure 3. The main reasons for downgrading our estimation of the quality of the evidence were risk of bias, and imprecision. The results available came from one study that analyzed a small number of participants (56 participants). This resulted in the study recording a small number of events, which can give rise to imprecision. We downgraded a second time for imprecision where there was a small number of events and wide confidence intervals that included the possibility of both increased and reduced healing.
Potential biases in the review process
We searched databases and trial registries comprehensively, together with reference lists of relevant studies for published RCTs, with no restrictions on language. However, the possibility of publication bias still cannot be excluded. For time to complete healing, as we were unable to verify whether all the participants' ulcers completely healed during the study, we presented the original data as a narrative summary.
Agreements and disagreements with other studies or reviews
Our results concur with Mao 2010 and Shaw 2011. Mao 2010 reported that, from the available published data, routine use of topical phenytoin in pressure ulcer treatment cannot be recommended until more data from rigorously‐designed studies become available. Shaw 2011 conducted a randomized, controlled, double‐blind, clinical trial to evaluate the effect of topical phenytoin on healing in diabetic foot ulcers. This study did not support the topical application of the phenytoin‐containing alginate‐based, hydrogel dressing in the treatment of diabetic foot ulcers compared with alginate‐based dressing. However, Rhodes 2001 and Anstead 1996 were in disagreement with our result. Rhodes 2001 reported that topical application of phenytoin demonstrated more rapid results in all aspects of ulcer healing. Anstead 1996 described that people with large Grade IV pressure ulcers responded rapidly to treatment with topical phenytoin after conventional treatments were unsuccessful, and suggested that topical phenytoin was effective in wound healing and deserved further investigation. Additionally, Pitiakoudis 2004 reported that topical phenytoin enhanced wound healing by stimulating lymphocytic chemotaxis and up‐regulation of angiogenesis.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
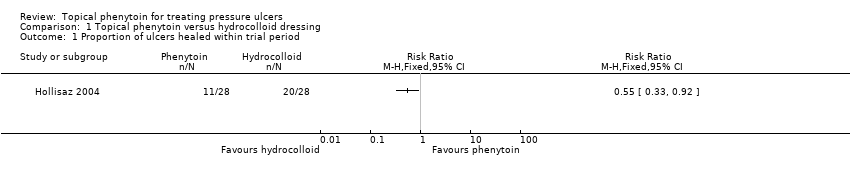
Comparison 1 Topical phenytoin versus hydrocolloid dressing, Outcome 1 Proportion of ulcers healed within trial period.

Comparison 2 Topical phenytoin versus simple dressing, Outcome 1 Proportion of ulcers healed within trial period.
| Topical phenytoin compared with hydrocolloid dressing for pressure ulcers | ||||||
| Patient or population: people with a pressure ulcer Settings: family homes or nursing homes Intervention: topical phenytoin Comparison: hydrocolloid dressing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with hydrocolloid dressing | Risk with topical phenytoin | |||||
| Time to complete healing | See comments | See comments | The study data reported did not provide a suitable representation of the outcome of interest | |||
| Proportion of ulcers healed within trial period (eight weeks) | 714 per 1000 | 393 per 1000 (236 to 657) | RR 0.55 (0.33 to 0.92) | 56 (1 study) | ⊕⊕⊝⊝ | |
| Adverse events | See comments | See comments | Not estimable | 84 (2 studies) | No adverse drug reactions or interactions were detected in the included RCTs. | |
| Pain | See comments | See comments | Not estimable | 28 (1 study) | Minimal pain was reported in all groups in one study. Study authors made this statement without any data to support it. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded two levels: serious limitation (risk of bias), serious imprecision (small number of events ) | ||||||
| Topical phenytoin compared with triple antibiotic ointment for pressure ulcers | ||||||
| Patient or population: people with a pressure ulcer Settings: nursing homes Intervention: topical phenytoin Comparison: triple antibiotic ointment | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with triple antibiotic ointment | Risk with topical phenytoin | |||||
| Time to complete healing | See comments | See comments | The study data reported did not provide a suitable representation of the outcome of interest | |||
| Proportion of ulcers healed within trial period (eight weeks) | See comments | See comments | This outcome was not reported for this comparison | |||
| Adverse events | See comments | See comments | Not estimable | 28 (1 study) | No adverse drug reactions or interactions were detected in the included RCT. | |
| Pain | See comments | See comments | Not estimable | 28 (1 study) | Minimal pain was reported in all groups in one study. Study authors made this statement without any data to support it. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Topical phenytoin compared with a simple dressing for pressure ulcers | ||||||
| Patient or population: people with a pressure ulcer Settings: family homes or nursing homes, and a hospital Intervention: topical phenytoin Comparison: a simple dressing | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with a simple dressing | Risk with topical phenytoin | |||||
| Time to complete healing | See comments | See comments | This outcome was not reported for this comparison | |||
| Proportion of ulcers healed within trial period (eight weeks) | 296 per 1000 | 394 per 1000 (187 to 824) | RR 1.33 (0.63 to 2.78) | 55 (1 study) | ⊕⊝⊝⊝ very low1 | |
| Adverse events | See comments | See comments | Not estimable | 81 (2 studies) | No adverse drug reactions or interactions were detected in the included RCTs. | |
| Pain | See comments | See comments | This outcome was not reported for this comparison. | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded three levels: once for serious limitation (risk of bias), and twice for very serious imprecision (small number of events and a wide confidence interval which includes the possibility of both increased healing and reduced healing) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed within trial period Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed within trial period Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

