One site versus two site phacotrabeculectomy for primary open angle glaucoma
Abstract
This is a protocol for a Cochrane Review (Intervention). The objectives are as follows:
To assess the effectiveness and safety of one site versus two site combined phacotrabeculectomy in people with cataract and POAG.
Background
Description of the condition
Glaucoma is generally defined as “a progressive optic neuropathy involving characteristic structural damage to the optic nerve and characteristic visual field defects” (Gupta 1997). Glaucoma can be classified into primary, secondary, and developmental glaucomas. Primary glaucomas are those in which there is no associated ocular disorder or associated diseases. Primary open angle glaucoma (POAG) is differentiated from primary angle‐closure glaucoma by the presence of a normal (open) anterior chamber angle (Gupta 1997).
Cataract is the partial or complete opacity on or in the lens or capsule impairing vision or causing blindness. Cataracts are classified by their morphology (size, shape, location) or etiology (cause and time of occurrence). When cataract is defined as a lens opacity combined with a decreased visual acuity, its prevalence ranges from 15% to 30% (Acosta 2006).
According to a World Health Organization report, although cataract is not a major cause of blindness in developed countries, globally it is still the leading cause; accounting for almost half of all cases despite improved delivery of cataract surgical services in many parts of the world (Resnikoff 2004). According to the same report, glaucoma is the second leading cause of blindness globally as well as in most regions.
The prevalence of both cataract and glaucoma increases with age. Leske 1983 and Tielsch 1996 have published detailed articles on risk factors for POAG. The Ocular Hypertension Treatment Study (OHTS) confirmed that age is a predictive factor for the development of POAG in individuals with ocular hypertension (Gordon 2002). Age is consistently associated with POAG. Both the incidence and the prevalence of POAG increase more than linearly with age (Boland 2007).
Additionally, there is an increased risk of cataract in some forms of glaucoma, such as pseudoexfoliative glaucoma. Antiglaucomatous medication may also have a role in the progression of nuclear cataracts as observed by the Early Management Glaucoma Trial (EMGT) group for POAG patients and the OHTS group for ocular hypertensive patients. Thus, not surprisingly, a considerable proportion of patients presenting for either cataract or glaucoma surgery suffer from a combination of both diseases (Heijl 2002; Kass 2002).
Presentation and diagnosis
Primary open angle glaucoma is a chronic, asymptomatic disease (Gupta 1997). Primary open angle glaucoma can be diagnosed by gonioscopy (an examination technique which allows the ophthalmologist to see if there is an open angle), typical optic disc changes (asymmetry loss of neuroretinal rim leading to increased cup/disc (C/D) ratio, localized loss, C/D asymmetry, disc haemorrhage), and classical glaucomatous visual field changes (nasal step, paracentral and arcuate scotoma). Intraocular pressure (IOP) is the main risk factor for glaucoma and the only parameter subject to treatment. Cataract is diagnosed with slit lamp examination, which allows the detection of lens opacity.
Description of the intervention
Visually significant cataract is treated by surgically removing the lens and replacing it with an intraocular lens (IOL). Riaz 2006 provides evidence from seven randomised controlled trials (RCTs) that phacoemulsification gives a better outcome than extracapsular cataract extraction (ECCE) with sutures. Phacoemulsification provides the best visual outcomes but will only be accessible to poorer countries if the cost of phacoemulsification and foldable IOLs decrease. Manual small incision cataract surgery provides early visual rehabilitation and comparable visual outcome to phacoemulsification. It has better visual outcomes than ECCE and can be used in any clinic that is currently carrying out ECCE with IOL. Further research from developing regions is needed to compare the cost and longer term outcomes of these procedures (e.g. posterior capsular opacification (PCO) and corneal endothelial cell damage).
Glaucoma treatment is based on reducing IOP. Glaucoma can be managed by medical treatment, laser therapy or surgical intervention. Below is a summary of Cochrane reviews evaluating these interventions.
Vass 2007 summarized the evidence for the effectiveness of different forms of topical medical treatment of POAG or OHT to prevent the progression or the onset of glaucomatous optic neuropathy. This review provided some evidence of a visual field protective effect by a particular type of beta‐blocker.
Burr 2008 observed that evidence from one trial suggests that for mild POAG, the risk of glaucoma progression up to five years is not significantly different whether treatment is initiated with medication or trabeculectomy. Reduced vision, cataract and eye discomfort are more likely with trabeculectomy. For more severe POAG, there is some evidence that initial medication (pilocarpine, now rarely used as first line treatment) is associated with a greater risk of glaucoma progression than surgery. Surgery lowers IOP more than medication.
Wilkins 2008 assessed the effects of intraoperative mitomycin C (MMC) compared to placebo in three groups. Intraoperative MMC reduces the risk of surgical failure in eyes that have undergone no previous surgery and in eyes at high risk of failure. No significant effect on failure was noted in the group undergoing trabeculectomy combined with cataract extraction. However, compared to placebo, intraoperative MMC reduced mean IOP at 12 months in all groups analysed in that review. Apart from an increase in cataract formation following MMC, there was insufficient power to detect any increase in other serious side effects such as endophthalmitis.
For a patient presenting with both cataract and POAG, there are three options available to the surgeon. Firstly, there is cataract surgery alone, secondly, glaucoma surgery alone or thirdly, combined cataract/glaucoma surgery. Cataract surgery alone would only be permitted in patients who have adequate target IOP. Trabeculectomy alone would be carried out on patients with inadequate target IOP. Combined surgery in patients who have both visual impairing cataract, advanced cupping and visual field loss with apparent good IOP control may be controversial and may be managed as cataract surgery alone. Patients with both diseases but poor IOP control would benefit from combined surgery and are the participants of interest in this review.
Combined cataract/glaucoma surgery can be performed with one site or two sites. Both procedures begin with a superior conjunctival incision followed by a scleral groove made approximately 2.5 mm posterior to the limbus. A 3.5 mm wide partial thickness scleral tunnel is dissected into the clear cornea using a crescent knife. Scissors are then used to make radial cuts at the sides of the tunnel to create a flap. A cellulose sponge is soaked in MMC and placed under the scleral flap and conjunctiva, for two to five minutes (on variable concentrations). Subsequently, in the two site combined surgery, a clear cornea temporal phacoemulsification and IOL implantation is performed. The trabeculectomy is then finalized (Figure 1). In one site combined surgery, the anterior chamber is entered under the scleral flap, and phacoemulsicification is performed. Subsequently, the sclerostomy is performed with a punch and the trabeculectomy is finalized (Figure 2).
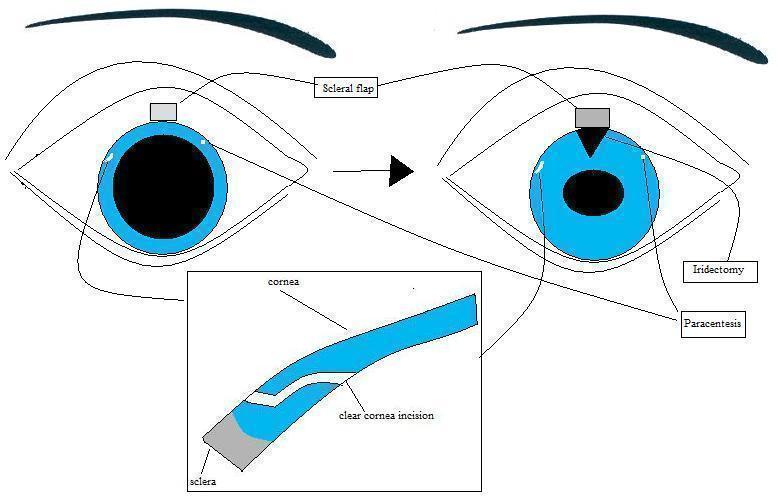
Two site phacotrabeculectomy. First illustration shows the flap configuration and the temporal corneal incision for phacoemulsification. Subsequently, the trabeculectomy is finalized.

One site phacotrabeculectomy. First illustration reveals the scleral flap configuration and phacoemulcification at the same site. Subsequently, the trabeculectomy is finalized.
How the intervention might work
The function of trabeculectomy is explained in Wilkins 2008 and the function of phacoemulsification in Riaz 2006.
There are some studies that have suggested that ECCE would result in IOP reduction (Calissendorff 1992; Cinotti 1988; Gunning 1991; Kooner 1988; McGuigan 1986; Onali 1991).
Unlike ECCE, phacoemulsification benefits the population with glaucoma as the cataract is removed without manipulating the conjunctiva. An unaltered conjunctiva is associated with a higher success rate for trabeculectomy.
Some reports have suggested that phacoemulsification alone would result in a long‐term reduction in IOP, with values between 1 and 5 mmHg (Hayashi 2000; Hayashi 2001; Perasalo 1997; Pohjalainen 2001; Shingleton 1999). Lens extraction for chronic angle‐closure glaucoma was studied in another Cochrane systematic review (Friedman 2006). The authors concluded that there is no evidence from good quality randomised trials or non‐randomised studies of the effectiveness of lens extraction for chronic primary angle‐closure glaucoma. However, a recent trial compared phacoemulsification alone versus combined phacotrabeculectomy in medically uncontrolled chronic angle‐closure glaucoma with coexisting cataract (Tham 2009). The trial investigators concluded that combined phacotrabeculectomy with adjunctive MMC is more effective than phacoemulsification alone in controlling IOP in medically uncontrolled chronic angle‐closure glaucoma eyes with coexisting cataract. Combined phacotrabeculectomy is associated with more postoperative complications. For acute primary angle‐closure glaucoma patients, a possible advantage of cataract extraction was observed in a RCT by Lam 2008. Early phacoemulsification appeared to be more effective in preventing IOP rise than laser peripheral iridotomy in eyes with post‐acute primary angle‐closure.
Why it is important to do this review
A considerable proportion of patients presenting with either cataract or glaucoma surgery suffer from a combination of both diseases and require a combined procedure. Although there is evidence to suggest that both procedures have similar IOP reduction and a similar improvement in visual acuity, controversy stills remains over the two procedures (Borggrefe 1999; Buys 2007; Cotran 2008; El Sayyad 1999; Rossetti 1997; Wyse 1998). One report suggested that more medications were required to maintain long‐term IOP control in eyes undergoing a single site combined procedure (Wyse 1998). Two reports observed that operating time was reduced in the one site surgery group (Buys 2007; Cotran 2008). Buys et al. also found that corneal endothelial cell counts were significantly lower in the two site group. A systematic review is needed to evaluate the effectiveness and safety of each technique.
Objectives
To assess the effectiveness and safety of one site versus two site combined phacotrabeculectomy in people with cataract and POAG.
Methods
Criteria for considering studies for this review
Types of studies
We will include RCTs only.
Types of participants
We will include trials in which the participants are diagnosed with uncontrolled POAG and visually significant cataract that could safely be extracted by phacoemulsification. There will be no restrictions with respect to gender, ethnicity and number of participants.
Types of interventions
We will include studies that compared one site versus two site combined trabeculectomy and phacoemulsification with IOL implantation. These procedures can be performed with or without adjunctives such as antimetabolites (MMC) or 5‐Fluorouracil (5‐FU).
Types of outcome measures
Primary outcomes
The primary outcomes for IOP will be:
1. The proportion of failed trabeculectomies at 12 months after surgery. Failure will be defined in this review as repeat surgery (trabeculectomy, glaucoma tube shunt or ciliary body ablation procedure) or uncontrolled IOP (usually more than 22 mmHg) despite additional topical or systemic medications (Wilkins 2008).
The primary outcomes for visual acuity will be:
a. The proportion of participants whose visual acuity did not improve following combined cataract/glaucoma surgery.
b. The proportion of participants not achieving good functional vision. Good functional vision is defined as vision better than or equal to 6/18 in the operated eye with usual spectacle correction.
Secondary outcomes
1. The proportion of complete success trabeculectomies at 12 months after surgery. Complete success is defined as patients that had no repeat surgery (trabeculectomy or glaucoma tube shunt or ciliary body ablation procedure) or no uncontrolled IOP (usually more than 22 mmHg) and no additional topical or systemic medications.
2. The mean IOP at 12 months after surgery.
3. Visual field as available throughout follow up and at last follow up as measured by any method. Progression of visual field damage is based on criteria defined in the methodology of each trial.
4. Progression of optic disc damage or nerve fibre layer loss according to the criteria defined in the methodology of the trial.
5. Quality of life measures as available in the trial reports.
6. Economic data as available in the trial reports.
Adverse outcomes
The adverse outcomes are adverse event rates in either group modified from Wilkins 2008:
1. Early complications:
1.1 Glaucoma
a. Wound leaks: the presence of a positive Seidel test (visible aqueous flow with the tear film stained with fluorescein).
b. Hypotony due to aqueous suppression or overdrainage (overfiltration): IOP is below 5 mmHg or is associated with complications such as macular oedema and sight loss or choroidal detachments.
c. Expulsive haemorrhage (suprachoroidal haemorrhage or choroidal effusion): choroidal haemorrhage usually at the time of surgery or during the early postoperative period while the eye is still soft leading to a marked rise in IOP.
d. Aqueous misdirection (malignant glaucoma, ciliary block glaucoma, ciliolenticular glaucoma and ciliovitreal block glaucoma) characterised by a shallow anterior chamber associated with a normal, or more commonly, raised IOP and in the presence of a patent iridotomy. High frequency ultrasound biomicroscopy may be used to visualize the configuration of anterior segment structures. This includes irido‐corneal touch, appositional angle‐closure, and anterior rotation of the ciliary body with apposition to the iris (Ruben 1997).
e. Intraocular pressure spikes.
1.2 Cataract
a. Complications during surgery, for example, capsular rupture with or without vitreous loss, iris prolapse and early postoperative complications such as postoperative inflammation.
2. Late complications
2.1 Glaucoma
a. Late endophthalmitis: an infection of the globe contents that even with prompt aggressive treatment often results in substantial loss of visual function. 'Late' here implies infection arising from organisms gaining access to the globe through thin‐walled drainage blebs or frank breaks in the conjunctival epithelium after the immediate postoperative period due to infectious agents which may have entered the eye during the surgical procedure.
2.2 Cataract
a. Complications at one year or more after surgery including the proportion of participants with retinal detachment, glaucoma, cystoid macular oedema, corneal endothelial cell loss, corneal decompensation or posterior capsule opacification.
Search methods for identification of studies
Electronic searches
We will search the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library), MEDLINE, EMBASE, Latin American and Caribbean Literature on Health Sciences (LILACS), the Australian New Zealand Clinical Trials Registry (ANZCTR), (www.actr.org.au) and the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com). There will be no date or language restrictions in the electronic search for trials.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), ANZCTR (Appendix 5) and mRCT(Appendix 6).
Searching other resources
We will search the reference lists of included studies to identify any additional trials. We will also use the Science Citation Index ‐ Expanded database to identify additional trials that may have cited any studies we include in the review.
Data collection and analysis
Selection of studies
Two review authors working independently will assess the titles and abstracts identified by the electronic and manual searches. We will obtain the full text copies of all relevant or potentially relevant trials and assess them against the 'Criteria for considering studies for this review'. For non‐English language trials identified as potentially relevant, we will get the methods and results sections translated and then assess them for quality.
Once we have obtained full text copies of potentially or definitely relevant trials, the two review authors will review and categorize each study as follows: 'exclude', 'unclear', or 'include'. We will document agreement in the assessment of trial quality between the review authors. We will settle any disagreement by discussion or, if necessary, by consultation with a third party. Papers identified by both review authors as 'exclude' will be excluded and documented in the review.
The review authors will be unmasked to the report authors, institutions and trial results during the assessment. We will attempt to obtain additional information when necessary.
Data extraction and management
Two review authors will independently extract the data for the primary and secondary outcomes onto paper data collection forms developed in collaboration with the Cochrane Eyes and Vision Group. We will resolve discrepancies by discussion. One review author will enter all data into RevMan (Higgins 2008a) following the guidelines set out in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions 5.0.1. A second author will then check the data entered.
Assessment of risk of bias in included studies
Two authors will independently evaluate included trials for methodological quality following guidelines provided in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 (Higgins 2008b). We will evaluate risk of bias in the included trials by assessing the following parameters: sequence generation, allocation concealment, masking (blinding) of patients, personnel and outcome assessors, incomplete outcomes, selective outcome reporting and other sources of bias. Each parameter will be judged as ‘Yes’ indicating low risk of bias, ‘No’ indicating high risk of bias, and ‘Unclear’ indicating unclear or unknown risk of bias.
We will request additional information from the authors of the trials if the risk of bias for any parameter is unclear.
Measures of treatment effect
Data analysis will follow guidelines set out in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 (Deeks 2008). We are planning to conduct intention‐to‐treat (ITT) analysis and per‐protocol analysis. We are planning to conduct meta‐analysis for the primary outcomes and visual field progression.
Dichotomous outcomes
For dichotomous outcomes we will calculate a summary risk ratio. We will also report the risk difference and number needed to treat. We will summarize data for the probability of failed trabeculectomy, people not achieving good functional vision and patients whose visual acuity did not improve at 12 months using relative risk. All adverse outcomes will be assessed as dichotomous variables by none/mild and moderate/severe.
Visual field progression and visual acuity will be analysed where possible as categorical variables. If reported as a score, the data will be analysed as continuous variables. If summary effect measures are reported, the logarithm of the odds ratio (Ln OR) and the standard error (SE) will be entered into Review Manager (RevMan) 5 as generic inverse variance measures. If other studies present similar outcomes using proportions, the OR and the 95% confidence interval (CI) will be calculated and the Ln OR and its SE will be calculated from the 95% CI and will be entered into RevMan 5 as a generic inverse variance effect measure (Burr 2008).
Continuous outcomes
We will calculate a mean difference for continuous outcomes and a standardized mean difference if different scales are used to measure continuous outcomes. Intraocular pressure, total number of medications, optic disc damage or nerve fibre layer loss will be evaluated as continuous data. Continuous data will be expressed as weighted mean differences (WMD) and an overall WMD will be calculated.
Time‐to‐event outcome
Time to glaucoma progression (failure of randomised treatment) will be assessed for one site versus two site surgery using the log hazard ratio and its standard error (variance) extracted directly or indirectly from the trial data (Parmar 1998).
Unit of analysis issues
We will include studies which have used one eye per participant and those which have used two eyes per participant but will take account of pairing of data in our analysis.
Dealing with missing data
We will attempt to contact the trial investigators for any missing data. If the investigators do not respond within four weeks, we will extract data as available from the published report. We will refer to guidelines in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 (Deeks 2008) for handling missing data. If patients with diseases other than POAG are included in the trials, we will ask the authors to send us data to separate analysis of POAG patients.
Assessment of heterogeneity
We will look for clinical heterogeneity by examining the study details then test for statistical heterogeneity between trial results using the Chi2 test and the I2 value. We will consider a P value of the Chi2 test less than 0.1, rather than the conventional level of 0.05, because a statistically significant result may indicate a problem with heterogeneity. A non‐significant result should not be taken as evidence of no heterogeneity. Care will be taken in the interpretation of the Chi2 test, since it has low power in the (common) situation of a meta‐analysis when studies have small sample size or are few in number.
Quantification of inconsistency across studies will be done with I2 values. A rough guide to interpretation is as follows:
-
0% to 40%: might not be important;
-
30% to 60%: may represent moderate heterogeneity*;
-
50% to 90%: may represent substantial heterogeneity*;
-
75% to 100%: considerable heterogeneity*.
*The importance of the observed value of I2 depends on (i) magnitude and direction of effects and (ii) strength of evidence for heterogeneity (e.g. P value from the Chi2 test, or a CI for I2).
I2 values of more than 50% will be seen as substantial statistical heterogeneity. We will also examine the funnel plot for statistical heterogeneity. When only one trial is included, we will not assess for heterogeneity.
Assessment of reporting biases
We will assess publication bias or a systematic difference between smaller and larger studies ('small study effects') by preparing a funnel plot (trial effect versus trial size) using RevMan 5.
Data synthesis
Data analysis will follow the guidelines set out in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 (Deeks 2008). We will calculate a summary risk ratio for dichotomous outcomes. If no significant statistical heterogeneity is detected either statistically or by review or there are a small number of trials in the analysis (three or fewer), a fixed‐effect model will be used. If heterogeneity has been detected, we will use a random‐effects model (DerSimonian 1986). The Peto odds ratio will be used to summarize data for rarer events such as adverse events.
Subgroup analysis and investigation of heterogeneity
We will not perform a subgroup analysis accounting for the inclusion or non‐inclusion of adjunctive antimetabolites (MMC or 5FU) since Wilkins 2008 found that there was no benefit with intraoperative MMC. We will probably not conduct a subgroup analysis of participants of black race either. Burr 2008 had planned a subgroup analysis for participants of black race but no trials were identified with participants solely of black race. In one trial 44% of the participants were non‐white (CIGTS 2001). The study did not analyse the results according to ethnic group apart from visual acuity loss.
Subgroup analysis may be used to investigate heterogeneity if there are sufficient trials identified within the review.
Sensitivity analysis
Sensitivity analysis will be performed, in order to evaluate how robust the results of the review are relative to decisions and assumptions made in the process of conducting the review (post hoc decisions). We will include all trials in our analysis initially, except those assessed as having a high risk of bias on 'allocation concealment and concealment approach'. We will then examine the effect of excluding trials assessed as having a high risk of bias on any of the parameters by repeating the analysis without these. We will conduct a sensitivity analysis excluding studies which have assumed that eyes within an individual are independent (cluster trials) to assess the influence of these studies on effect size.

Two site phacotrabeculectomy. First illustration shows the flap configuration and the temporal corneal incision for phacoemulsification. Subsequently, the trabeculectomy is finalized.

One site phacotrabeculectomy. First illustration reveals the scleral flap configuration and phacoemulcification at the same site. Subsequently, the trabeculectomy is finalized.

