Abordajes quirúrgicos para las luxaciones de la faceta articular de la columna cervical en adultos
Resumen
Antecedentes
La elección del abordaje quirúrgico para el tratamiento de las luxaciones subaxiales de la faceta articular de la columna cervical es un tema polémico entre los cirujanos de columna. Las razones de lo anterior incluyen diferencias en la familiaridad con la técnica y la experiencia de los cirujanos con los diferentes abordajes quirúrgicos, y la interpretación variable de los estudios de imágenes con respecto a la existencia de una herniación traumática del disco intervertebral y del estado neurológico del paciente. Además, debido a que los abordajes son disímiles, es probable encontrar variaciones importantes en los resultados neurológicos, radiográficos y clínicos.
Objetivos
Comparar los efectos (beneficiosos y perjudiciales) de los diferentes abordajes quirúrgicos utilizados para tratar a adultos con luxaciones agudas de la faceta articular de la columna cervical.
Métodos de búsqueda
Se hicieron búsquedas en el registro especializado del Grupo Cochrane de Lesiones Óseas, Articulares y Musculares (Cochrane Bone, Joint and Muscle Trauma Group) (9 mayo 2014), Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (The Cochrane Library, 2014 número 4), MEDLINE (1946 hasta abril, semana 5, 2014), MEDLINE In‐Process & Other Non‐Indexed Citations (8 mayo 2013), EMBASE (1980 hasta 2014, semana 18), Latin American and Caribbean Health Sciences (9 mayo 2014), registros de ensayos, actas de congresos y listas de referencias de artículos hasta mayo 2014.
Criterios de selección
Se incluyeron ensayos controlados aleatorios y cuasialeatorios que compararon abordajes quirúrgicos para el tratamiento de las luxaciones agudas de la faceta articular de la columna cervical en adultos con y sin lesión de la médula espinal.
Obtención y análisis de los datos
Dos autores de la revisión seleccionaron los estudios de forma independiente, evaluaron el riesgo de sesgo y extrajeron los datos.
Resultados principales
Se incluyó un ensayo controlado aleatorio y un ensayo controlado cuasialeatorio con un total de 94 participantes que informaban los resultados para un máximo de 84 participantes. Un ensayo incluyó a pacientes con lesiones de la médula espinal y los otros incluyeron a pacientes sin lesiones de la médula espinal. Ambos ensayos compararon abordajes quirúrgicos anteriores versus posteriores. Ambos ensayos estuvieron en riesgo alto de sesgo, que incluyó sesgo de selección (un ensayo), sesgo de realización (ambos ensayos) y sesgo de deserción (un ensayo). Los datos sólo se combinaron para uno de los resultados: consolidación viciosa. Se consideró que las pruebas fueron de muy baja calidad para todos los resultados, lo cual también refleja la imprecisión de los resultados; este hecho significa que el nivel de incertidumbre acerca de los cálculos es alto.
Ningún ensayo encontró diferencias entre los dos abordajes en la recuperación o el estado neurológico, según se observó en un estudio de acuerdo a las pequeñas diferencias clínicamente insignificantes en las puntuaciones neurológicas de la NASS (Northern American Spine Society) (0 a 100: puntuación óptima) al año de seguimiento: puntuación media del abordaje anterior: 85,23 versus puntuación media del abordaje posterior: 83,86; diferencia de medias (DM) 1,37 a favor del abordaje anterior, intervalo de confianza (IC) del 95%: ‐9,76 a 12,50; 33 participantes; un estudio). El mismo ensayo no encontró diferencias relevantes entre los abordajes un año más tarde en la calidad de vida informada por el paciente medida con las puntuaciones de la 36‐item Short Form Survey del componente físico (DM ‐0,08; IC del 95%: ‐7,26 a 7,10) y mental (DM 2,88; IC del 95%: ‐3,32 a 9,08). Ningún ensayo halló pruebas de diferencias significativas en el dolor a largo plazo, o la consolidación viciosa (2/38 versus 2/46; cociente de riesgos [CR] 1,18; IC del 95%: 0,04 a 34,91). Un ensayo encontró una mejor alineación sagital y más “normal” después del abordaje anterior (DM ‐10,31 grados a favor del abordaje anterior, IC del 95%: ‐14,95 grados a ‐5,67 grados), mientras que el otro ensayo no informó diferencias significativas en la alineación cervical. No hubo pruebas suficientes para indicar diferencias entre los grupos en los eventos adversos médicos, las tasas de fracaso del instrumental y la infección. Un ensayo halló que los diversos participantes presentaron trastornos de la voz y la deglución después del abordaje anterior en la cirugía (11/20) versus ninguno (0/22) en el grupo de abordaje posterior: CR 25,19; IC del 95%: 1,58 a 401,58); todos se recuperaron alrededor de los tres meses.
Conclusiones de los autores
Las pruebas de calidad muy baja de dos ensayos indicaron pocas diferencias en el estado neurológico a largo plazo, el dolor o la calidad de vida informada por el paciente entre los abordajes quirúrgicos anteriores y posteriores al tratamiento de los pacientes con luxaciones subaxiales de la faceta articular de la columna cervical. La alineación sagital puede lograrse mejor con el abordaje anterior. No hubo pruebas suficientes disponibles para indicar diferencias entre los grupos en los eventos adversos médicos, las tasas de fracaso del instrumental y la infección. Todos los trastornos de la voz y de la deglución que ocurrieron de forma exclusiva en el grupo de abordaje anterior se resolvieron alrededor de los tres meses. No existe seguridad en cuanto a estas pruebas y, por lo tanto, no es posible decir si un abordaje es mejor que el otro. No hubo pruebas disponibles para otros enfoques. Se justifica la realización de ensayos aleatorios multicéntricos adicionales de calidad más alta.
PICO
Resumen en términos sencillos
Abordajes quirúrgicos para las luxaciones de los huesos del cuello
La parte ósea de la espalda que se encuentra en el cuello es denominada columna cervical. Consta de siete huesos (o vértebras). El movimiento relativo de estas vértebras es realizado principalmente por articulaciones pequeñas (llamadas facetas articulares) ubicadas entre cada una de las vértebras. Las facetas articulares de la columna cervical facilitan el buen movimiento del cuello, aunque son vulnerables a la luxación. Habitualmente, las luxaciones de la faceta articular de la columna cervical son causadas por traumatismos de alto impacto como accidentes de tránsito o ataques violentos. Aproximadamente la mitad de los pacientes con dichas luxaciones sufre una lesión en la médula espinal que se encuentra dentro de la columna. Este trastorno puede dar lugar a deficiencias significativas en la función (p.ej., parálisis). Generalmente, se necesita cirugía para estas lesiones graves con objeto de mantener los huesos del cuello en su lugar.
¿Cuáles son los diferentes abordajes quirúrgicos?
Existen dos etapas principales en la cirugía: la reducción y la fijación. La reducción es la restauración de la articulación o el hueso lesionado o dislocado a la posición anatómica normal, y puede lograrse con cirugía o mediante la reducción cerrada, que se realiza con tracción o manipulación. La fijación es el procedimiento médico utilizado para estabilizar una o más articulaciones, o un hueso fracturado, generalmente al insertar quirúrgicamente dispositivos como clavos, tornillos, placas y varillas. La fijación de la lesión en general es realizada con abordaje quirúrgico anterior o posterior. En el abordaje cervical anterior, la cirugía se realiza a través de una incisión sobre la superficie frontal del cuello, mientras que el abordaje cervical posterior consta de una incisión a lo largo de la línea media sobre la parte posterior del cuello y la disección a través del músculo de las vértebras cervicales. Este abordaje proporciona un acceso directo a las facetas articulares dislocadas.
Descripción de los estudios incluidos en la revisión
Se realizaron búsquedas en la bibliografía médica hasta mayo de 2014 y se encontraron dos estudios relevantes que incluían a un total de 94 adultos con luxaciones de la faceta articular de la columna cervical. Un ensayo incluyó a individuos con lesiones de la médula espinal y los otros incluyeron a individuos sin lesiones de la médula espinal. Ambos estudios compararon el abordaje quirúrgico anterior versus posterior.
Calidad de la evidencia
Los dos estudios fueron pequeños y ambos se encontraban en alto riesgo de sesgo. Por lo tanto, se consideró que la calidad de las pruebas era muy baja.
Resumen de las pruebas
Ningún estudio encontró diferencias entre los dos abordajes en el estado neurológico y el dolor un año más tarde. Un estudio tampoco encontró diferencias entre los dos abordajes en la calidad de vida informada por el paciente. Aunque un estudio halló que el abordaje anterior dio lugar a una curvatura más normal del cuello, el otro estudio informó que no hubo diferencias entre los dos abordajes con respecto a la alineación de las vértebras del cuello. Las pruebas fueron insuficientes para indicar diferencias entre los dos abordajes en cuanto a los eventos adversos médicos, las tasas de fracaso del instrumental y la infección. Aunque más de la mitad (11) de los 20 pacientes del grupo de abordaje anterior de un estudio presentaron trastornos de la voz y la deglución, todos se resolvieron alrededor de los tres meses.
Conclusión
La calidad de las pruebas fue muy baja, lo que indica que no existe seguridad en cuanto a la dirección y el tamaño del efecto. Por lo tanto, no es posible establecer si es mejor el abordaje anterior o el posterior para el tratamiento quirúrgico de los individuos con luxaciones de las facetas articulares de la columna cervical. Se sugiere que existe la necesidad de investigación adicional para informar la elección del abordaje quirúrgico.
Authors' conclusions
Summary of findings
| Anterior versus posterior approach for cervical facet dislocations in adults | ||||||
| Population: adults with cervical spine facet dislocations | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| Posterior approach | Anterior approach | |||||
| Final post‐surgical neurological status | Mean 83.86 | The mean final post‐surgical neurological status in the anterior group was | 33 | ⊕⊝⊝⊝ | Another study (47 participants), which included patients with spinal cord injury, reported that there was no significant differences in neurological recovery between the two groups (very low quality evidence)4 | |
| Functional aspects and quality of life ‐ SF‐36 physical scores | Mean 46.91 (range 35.2 to 58.9) | The mean SF‐36 physical score in the anterior group was | 33 | ⊕⊝⊝⊝ | ||
| Emotional aspects and quality of life ‐ SF‐36 mental scores | Mean 49.3 (range 29.9 to 62.1) | The mean SF‐36 mental score in the anterior group was | 33 | ⊕⊝⊝⊝ | ||
| Pain | Mean 81.67 (range 58 to 100) | The mean NASS pain score was | 33 | ⊕⊝⊝⊝ | Another study (47 participants), which included patients with spinal cord injury, reported that seven participants in each group had neck pain (7/20 anterior group versus 7/27 posterior group; RR 1.35, 95% CI 0.56 to 3.23) (very low quality evidence)4 | |
| Non‐fusion at follow‐up | Study population | RR 1.18 (0.04 to 34.91) | 84 (2 studies) | ⊕⊝⊝⊝ | ||
| 43 per 1000 | 51 per 1000 | |||||
| Sagittal alignment | Mean | The mean sagittal alignment was | 36 | ⊕⊝⊝⊝ | Lower (negative) degrees indicate physiological cervical alignment. Another study (47 participants), which included patients with spinal cord injury, reported no differences in changes in alignment between the two approaches (very low quality evidence)4 | |
| Complications | Swallowing/voice disorders (short‐term) | RR 25.19 | 42 | ⊕⊝⊝⊝ | Very low quality evidence3 did not confirm between‐group differences in medical adverse events, rates of instrumentation failure and infection. The only significant difference found related to voice and swallowing disorders after anterior approach surgery; this was reported only in one study. All had recovered by three months | |
| 0 per 1000 | 550 per 1000 (actual results) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. The assumed risk is based on that provided in the included studies. 2. This study excluded patients with spinal cord injury. 3. The evidence was downgraded two levels for limitations in study design and implementation (primarily performance bias from lack of clinician and participant blinding, and attrition bias) and one level for imprecision (small sample size and wide CI). 4. The evidence was downgraded two levels for limitations in study design and implementation (primarily selection bias from inadequate randomisation method and lack of allocation concealment, performance bias from lack of clinician and participant blinding, detection bias from lack of outcome assessment blinding and other bias, especially imbalances in baseline characteristics) and one level for imprecision (small sample size and either wide CI or a clearly large range in values). | ||||||
Background
Description of the condition
Fractures and dislocations of the spine are among the most challenging in trauma clinical practice. Vertebral column injuries occur in approximately 6% of trauma patients, half of which sustain spinal cord or nerve root neurological deficits (Burney 1993). The main causes of traumatic spinal cord injury (SCI) are motor vehicle‐related accidents (39.5% to 55%), violence (14.2% to 29.5%), falls (18.8% to 23%) and sports injuries (7.3% to 11.1%) (Burke 2001; DeVivo 1997; Nobunaga 1999). For definitions of some of the terms used in this review, see the Glossary in Table 1.
| Term | Definition |
| Discectomy | Excision (cutting out), in part or whole, of an intervertebral disc. The most common indication is disc displacement or herniation (see 'Hernia'). In addition to standard surgical removal, it can be performed by percutaneous discectomy or by laparoscopic discectomy, the former being the more common |
| Fracture | A break in a bone |
| Fracture fixation | The use of usually metallic devices inserted into or through bone to hold a fracture in a set position and alignment while it heals |
| Facet dislocation | Complete displacement that occurs between facets of the interior (located below) and superior (located above) articular processes of adjacent vertebrae |
| Hernia | Protrusion (pushing out) of tissue, structure or part of an organ through the muscular tissue or the membrane by which it is normally contained |
| Reduction | The restoration, by surgical or manipulative procedures, of a part to its normal anatomical relation |
| Surgical decompression | A surgical operation for the relief of pressure in a body compartment or on a body part |
| Pseudarthrosis | A pathological entity characterised by persistent non‐union of bone fragments, leading to formation of a false joint |
| Non‐union | Failure of healing at the ends of a fracture |
| Fusion | Formation of an ankylosis by surgical means |
The incidence of SCIs in North America has remained stable over the past 30 years, and ranges between 27 and 47 cases per million population (Fisher 2006). In addition, it has been estimated that the annual incidence of SCIs requiring hospitalisation in developed countries is approximately 11.5 to 53.4 per million population (Kraus 1975; Surkin 2000). The most common site of SCI is the cervical region, accounting for 50% to 64% of traumatic SCIs (Tator 1995). Approximately 40% of these injuries are associated with a neurological deficit (Lasfargues 1995). Moreover, the incidence of traumatic disc herniations at the site of injury in this population may be about 54% (Robertson 1992).
The cervical or neck region of the spine consists of seven vertebrae. The first and second cervical vertebrae (C1 and C2), respectively, the atlas and axis, form the upper cervical spine. The lower cervical spine comprises the third to the seventh vertebrae (C3 to C7). Below this is the thoracic region of the spine, starting with the T1 vertebra. Relative movement of the vertebrae is primarily via the facet joints. Starting from below C2, intervertebral discs lie between the cylindrical parts (centrum) of adjacent vertebrae. These discs act as shock absorbers, as well as allowing movement.
A traditionally used classification system for subaxial cervical spine injuries is that described by Allen 1982. This is based on the mechanism of injury, and is divided into six categories: compression‐flexion, vertical compression, distraction‐flexion, compression‐extension, distraction‐extension and lateral flexion. Facet dislocations are classified as distraction‐flexion injuries and account for approximately 10% of all subaxial cervical spine fractures. They may be unilateral or bilateral. Although pure ligamentous facet injuries, by definition, are classified as facet dislocations, it is important to note that facet fractures are part of the same spectrum of injury. Both types are probably the result of subtle differences in injury mechanism, in which pure ligamentous injuries occur when distractive forces across the posterior elements outweigh shear forces, whereas facet fractures take place when the facet is subjected to a relatively greater shear force. Both have similar associated instability patterns and diagnostic, therapeutic and prognostic factors (Bellabarba 2006).
Description of the intervention
Several aspects in the management of facet dislocations are controversial. Intervention may be considered in two stages: a) reduction, which can be performed closed with skull traction or surgically (by an anterior or posterior approach), and b) internal fixation, which can be performed by an anterior or posterior approach.
It should be clearly established whether it is safe to undertake closed manipulation of the neck either awake or under anaesthesia. The timing of such closed reduction is important. Some authors believe that an early, successful, closed reduction protects neurological elements during the mobilisation of the patient and may potentially improve neurorecovery in compromised patients compared with delayed reduction (Kahn 1998; Lee 1994). Occasionally, gentle manipulation by an experienced surgeon can be necessary to reduce a perched facet during the traction procedure (Star 1990). Any treatment will be complicated by the presence of an intervertebral disc prolapse. Whether magnetic resonance imaging (MRI) is required before intervention is uncertain, with some authors arguing that, due to the high incidence of herniated discs at the time of cervical dislocations, an MRI scan should be obtained prior to reduction (Eismont 1991; Robertson 1992) and others considering that imaging is unnecessary during immediate, awake, closed traction in an alert and cooperative patient (Hart 2002).
Generally, facet dislocations are stabilised posteriorly, but there has been a trend towards anterior surgery, due to concern regarding the potential for any disc herniation to lead to spinal cord compression (De Lure 2003; Doran 1993; Eismont 1991; Harrington 1991; Harrop 2001; Maiman 1986).
Open posterior reduction and stabilisation is accomplished by a distraction manoeuvre and the placing of a small instrument between the dislocated facets or, if necessary, by removal of the superior part of the caudal facet, allowing the dislocated facet to fall back to its original position. The subsequent fixation may be performed with sublaminar or spinous process wires, or both, or lateral mass or pedicle screws.
The anterior approach is initiated with decompression and discectomy at the level (of the spine) compromised by the injury, followed by a reduction manoeuvre (if necessary) that can be performed in many ways, including distraction with sequential application of weight, direct manipulation or reduction through Caspar vertebral pins. Thereafter, fixation may be achieved with iliac crest graft or cage placement at the intervertebral space associated with anterior cervical plating.
Some studies justify anterior procedures on the grounds of safety (Ordonez 2000; Reindl 2006), whereas others promote posterior cervical reduction and fusion even in the presence of a herniated disc (Abumi 2000), or anterior cervical discectomy and grafting followed by a posterior reduction and fusion (Allred 2001).
How the intervention might work
All surgical approaches (including anterior‐ and posterior‐only approaches) for cervical facet dislocation may be used for reduction and fixation in an attempt to acquire anatomical realignment and bony fusion, and maximise neurological recovery, long‐term relief of pain, functional recuperation and early return to activities of daily living.
Why it is important to do this review
The seriousness of cervical spine facet dislocations, including the risk of major complications such as paralysis, points to the need for evidence‐based practice. Although there appears to be a trend towards the use of the anterior approach for some types of these injuries, a recent survey found poor overall agreement between surgeons on the choice of surgical approach (Nassr 2008). This underlying variation in treatment indicates the uncertainty on the best approach and the need to conduct a systematic review of the best evidence to inform practice.
Objectives
To compare the effects (benefits and harms) of the different surgical approaches used for treating adults with acute cervical spine facet dislocation.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐randomised (a method of allocating participants to a treatment which is not strictly random, e.g. by date of birth, hospital record number, alternation) controlled trials.
Types of participants
We included all studies relating to adults with an acute (less than three weeks) and radiologically confirmed distraction‐flexion dislocation or fracture‐dislocation of the lower cervical spine with or without neurological deficit. Trials including participants with unilateral or bilateral facet dislocation injuries were included. Trials including adolescents or people with other cervical spine injuries, or both, were included, provided separate data could be obtained or the proportion included was small.
Types of interventions
Included were trials comparing different surgical approaches for these injuries. The main comparison was the anterior versus the posterior approach for either open reduction and fixation or fixation after initial closed reduction.
1. Anterior surgical approaches, including:
-
anterior cervical surgical reduction and fixation;
-
closed reduction followed by anterior cervical surgical fixation.
2. Posterior surgical approaches, including:
-
posterior cervical surgical reduction and fixation;
-
closed reduction followed by posterior cervical surgical fixation.
3. Combined approaches following closed reduction:
-
anterior‐posterior;
-
anterior‐posterior‐anterior;
-
posterior‐anterior.
4. Combined approaches (with open reduction):
-
anterior‐posterior;
-
anterior‐posterior‐anterior;
-
posterior‐anterior.
Types of outcome measures
Primary outcomes
-
Final post‐surgical neurological status (recovery or deterioration)
Secondary outcomes
-
Functional aspects and quality of life
-
Pain
-
Radiographical outcomes
-
Bone fusion
-
Dislocation reduction or sagittal realignment
-
-
Complications
-
Economic data
We considered only validated measure instruments for analysis. We accepted outcomes measured at any time of follow‐up.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (9 May 2014), The Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library, 2014 Issue 4), MEDLINE (1946 to April Week 5 2014), MEDLINE In‐Process & Other Non‐Indexed Citations (8 May 2013), EMBASE (1980 to 2014 Week 18) and LILACS (Latin American and Caribbean Health Sciences) database (9 May 2014). We also searched Current Controlled Trials (May 2014) and the World Health Organization (WHO) International Clinical Trials Registry Platform (May 2014). No language restrictions were applied.
We present search strategies composed of descriptors for the clinical condition, intervention of interest, as well as an RCT filter for each database in Appendix 1. We combined the MEDLINE search with the sensitivity‐ and precision‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying RCTs (Lefebvre 2011) and the LILACS search with the optimal search strategy for clinical trials in LILACS (Castro 1999).
Searching other resources
We also searched the reference lists of relevant articles, including narrative reviews, and book chapters. We screened conference proceedings from Orthopaedic Trauma Association Annual Meetings (1996 to 2013) and EuroSpine Meetings (2007 to 2013). We also contacted other researchers and experts in the field.
Data collection and analysis
The intended methodology for data collection and analysis was described in our published protocol (Del Curto 2009).
Selection of studies
Two review authors (DDC and DEM) independently assessed the titles and abstracts of the identified articles to determine potential relevance. The same authors analysed the full text of potentially relevant articles to assess eligibility. All disagreements were resolved by discussion or by a third opinion (JCB).
Data extraction and management
Two review authors (DDC and DEM) independently extracted data from each study using a pre‐piloted data extraction form. All unresolved disagreements were resolved by discussion with another author (JCB).
Assessment of risk of bias in included studies
Two review authors (DDC and DEM) independently assessed the risk of bias of the included studies using The Cochrane Collaboration's 'Risk of bias' tool (Higgins 2008). This tool incorporates the assessment of randomisation (sequence generation and allocation concealment), blinding of participants and personnel (for performance bias), blinding of outcome assessment (for detection bias), completeness of outcome data, selection of outcomes reported and other sources of bias. Other considered sources of bias included bias resulting from major imbalances in baseline characteristics (e.g. the presence of SCI) and performance bias, where we assessed the risk of bias from systematic differences in the experience of the operating surgeon(s) and subsequent rehabilitation. All unresolved disagreements were resolved by discussion with a third author (JCB). Titles of journals, names of authors or supporting institutions were not masked at any stage.
Measures of treatment effect
Where appropriate, we calculated risk ratios (RR) with 95% confidence intervals (CIs) for dichotomous outcomes and mean differences (MD) with 95% CIs for continuous outcomes. When data from primary studies were not parametric (e.g. effects reported as medians and quartiles, etc) or were not adequately reported (e.g. without standard deviations), we presented these in text or tables, or both.
Unit of analysis issues
The individual participant was both the unit of randomisation and the unit of analysis in included trials. We avoided other unit of analysis issues such as presenting total numbers of complications rather than numbers of participants with complications, where participants have more than one complication.
Dealing with missing data
For dichotomous data, we attempted to perform intention‐to‐treat analyses including all participants randomised to the intervention groups. For continuous data, we intended, if necessary by contacting trial investigators, to favour 'last observation carried forward' analyses for accounting for missing data. When our efforts to acquire missing data from authors were unsuccessful, we performed available case analyses and thus just used the data that were available. We stipulated beforehand that we would assume there were no dropouts in any study that did not report dropouts.
Assessment of heterogeneity
We assessed heterogeneity by visual inspection of the forest plot (analysis) together with consideration of the Chi² test for heterogeneity and the I² statistic (Higgins 2003). We based our quantitative assessment of heterogeneity mainly on the I² statistic, and interpreted it according to the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011) where 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity and 75% to 100% may represent very substantial (considerable) heterogeneity.
Assessment of reporting biases
In future, should a sufficient number of studies be available (10 studies), we will attempt to assess publication bias by preparing a funnel plot. However, we remain aware that asymmetry in the funnel plot can be associated with other reasons than publication bias (e.g. chance, real heterogeneity and clinical particularities inherent to each of the included studies, such as participants at high risk for the outcome).
Data synthesis
When appropriate, we pooled the results of comparable groups and calculated the 95% CI. In future updates, we plan to present MDs for continuous outcomes unless data are derived from disparate outcome measures, in which case standardised mean differences (SMDs) will be presented. We used the random‐effects model because, as expected, there was substantial clinical and methodological heterogeneity between studies, which could generate substantial statistical heterogeneity.
Subgroup analysis and investigation of heterogeneity
We planned but could not carry out subgroup analyses by age and gender, fracture type (unilateral versus bilateral facet dislocation), neurological status (neurologically intact, incomplete and complete SCI) and use/non‐use of MRI before reduction.
Sensitivity analysis
Where possible, we planned sensitivity analyses to examine various aspects of trial and review methodology, including the effects of intention‐to‐treat and available data analyses, and the inclusion of trials without concealment of allocation or those reported only in abstract form.
Quality assessment and 'Summary of findings' table
We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach to assess the quality of evidence relating to the listed outcomes (Higgins 2011, Section 12.2) and generated a 'Summary of findings' table for the sole comparison tested in the review.
Results
Description of studies
Results of the search
We completed the search in May 2014. We screened a total of 949 records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (122 records); CENTRAL (169), MEDLINE (75), EMBASE (578), LILACS (5), Current Controlled Trials (0) and the WHO International Clinical Trials Registry Platform (0). We did not identify any potentially eligible studies from other sources such as the manual search of references.
We identified three potentially eligible studies, for which full reports were obtained. Upon study selection, we included two studies (Brodke 2003; Kwon 2007) and excluded one (Kandziora 2005). We did not identify any ongoing studies.
A flow diagram summarising the study selection process is presented in Figure 1.
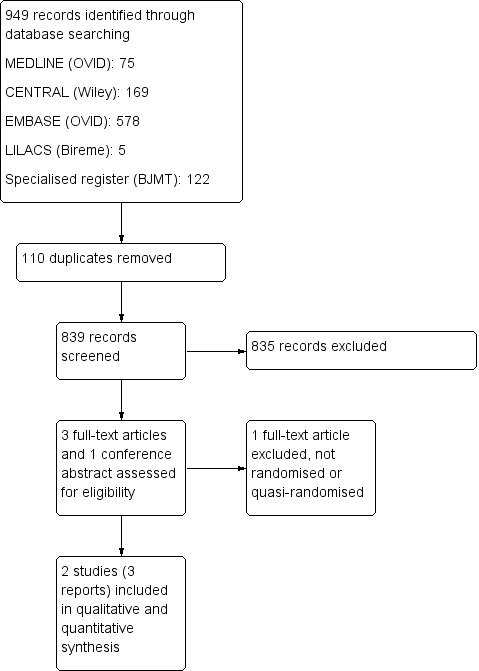
Study flow diagram.
Included studies
We included two studies in this review (Brodke 2003; Kwon 2007). Brodke 2003 was also reported in abstract form at the Orthopaedic Trauma Association Annual Meeting in September 1996. For further details, please see the Characteristics of included studies.
Design
Brodke 2003 was a quasi‐randomised trial, where allocation was according to the day of admission. In Kwon 2007, participants were randomised "according to a block randomisation procedure".
Sample sizes
The two studies included a total of 94 participants. Brodke 2003 included 52 participants, and presented results for at least six months of follow‐up for 47 participants. Kwon 2007 included 42 participants, and presented results for 12 months of follow‐up for 33 participants.
Setting
There was no information available as to whether studies were conducted in single or multiple centres. Brodke 2003 was conducted in the USA and Kwon 2007 in Canada.
Participants
Most of the participants in each trial were male (79% of the trial population in Brodke 2003 and 74% in Kwon 2007). The mean age was 35 years in both studies. An important difference between participants in the studies was that Brodke 2003 specifically included individuals with spinal cord injuries (SCIs), whereas Kwon 2007 included individuals with unilateral facet fractures or dislocations without SCIs. Brodke 2003 included individuals with different types of spine injuries, most of them including facet joint dislocations. However, Brodke 2003 also included seven people with burst type fractures only.
Interventions
Both trials compared the anterior surgical approach versus the posterior surgical approach in the treatment of cervical spine injuries.
Outcomes
Primary outcomes
Final post‐surgical neurological status was evaluated in Brodke 2003 on the last day of follow‐up (minimum of six months) using the Frankel classification (Frankel 1969) and the American Spinal Injury Association (ASIA) motor index score (ASIA 1992).
Kwon 2007 reported on neurological function using NASS (Northern American Spine Society) neurological scores after 12 months of follow‐up.
Secondary outcomes
Clinical outcomes were assessed in both studies. Kwon 2007 reported on functional aspects and quality of life via the 35‐item Short‐Form Survey (SF‐36). Both studies reported pain as an outcome. However, Brodke 2003 reported only the numbers of participants reporting neck pain at final follow‐up and Kwon 2007 reported postoperative pain on day one and day two, assessed via a visual analogue scale (VAS), and at one year, assessed via the NASS cervical spine pain score.
Radiographical outcomes were reported in both studies. Brodke 2003 and Kwon 2007 both evaluated fusion rates and sagittal alignment at the level of injury. Brodke 2003 also assessed kyphotic angulation across the injury site and anterior‐posterior displacement of the vertebral body.
Both studies reported complications as an outcome.
Kwon 2007 reported on the duration of time required for a participant to achieve a standard set of discharge criteria. The relation between these criteria and actual discharge criteria was not discussed in the trial report.
Excluded studies
We excluded one study because it was not randomised (Kandziora 2005) (see the Characteristics of excluded studies).
Risk of bias in included studies
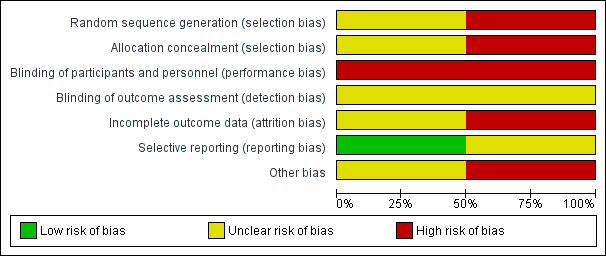
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
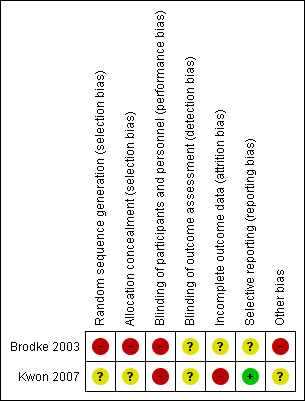
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
Since Brodke 2003 was quasi‐randomised with allocation based on the day of admission, we judged this trial to be at high risk of selection bias relating to inadequate sequence generation and lack of allocation concealment. Kwon 2007 reported only that it used a block randomisation process. Given the lack of details on which to make a judgement, we rated Kwon 2007 as being at unclear risk of selection bias relating to sequence generation and allocation concealment.
Blinding
Due to the nature of the interventions, blinding was not feasible either for participants or treatment providers. Hence, we judged both trials to be at high risk of performance bias relating to lack of blinding. Both trials referred to independent but not treatment‐blinded outcome assessment. We judged detection bias to be unclear for both trials.
Incomplete outcome data
Both studies described the participants who were lost to follow‐up as well as those who were excluded. However, outcome data for those participants were not available, even after our attempts to obtain these data from the trial authors.
We judged Brodke 2003 to be at unclear risk of attrition bias, as there were similar losses to follow‐up from the final analysis in both groups. Of the five excluded participants (9.6%), two had died of other injuries in the early postoperative period in the anterior group (9%) and three had less than six months of follow‐up in the posterior group (10%).
We considered Kwon 2007 to be at high risk of bias as losses to follow‐up were not evenly distributed between allocation groups. For patient‐reported outcomes of functional aspects and quality of life, 6 of 20 participants from the anterior group (30%) and 3 of 22 (14%) from the posterior group were lost to follow‐up or did not send back outcome questionnaires at the one year follow‐up. For radiographical outcomes, 2 of 20 (10%) participants randomised to anterior surgery and 3 (14%) of those randomised to posterior surgery did not complete follow‐up at the one‐year postoperative time point.
Selective reporting
All the outcomes described in the methods of both studies were presented in the results. However, we judged Brodke 2003 to be at unclear risk of selective reporting bias because the scoring system for the Frankel classification of neurological status was incompletely described, as well as being inappropriate. We judged Kwon 2007 to be at low risk for reporting bias.
Other potential sources of bias
In Brodke 2003, there were important between‐group baseline differences in type of injury and neurological status; additionally, the mean time to surgery was twice as long in the anterior group as in the posterior group (10 versus 5 days). We thus judged this trial to be at high risk of other bias. We judged Kwon 2007 to be at unclear risk of other bias because of the variety of instrumentation devices used in both participant groups, especially in the posterior fixation group, in which lateral mass screws with plates, or posterior wiring, or both, were used.
Effects of interventions
The only surgical approaches compared in these trials included in this review were the anterior and posterior approaches.
Primary outcomes
Final post‐surgical neurological status
Brodke 2003 presented preoperative and final follow‐up data on neurological outcomes based on Frankel grades (see below) and the ASIA motor score (ASIA 1992).
The Frankel classification grading system is shown below (Frankel 1969).
-
Grade A: complete neurological injury ‐ no motor and sensory function below the injured level
-
Grade B: some sensation present below the lesion (injured level) but the motor paralysis is complete below that level
-
Grade C: some motor power present below the lesion but it is of no practical use to the patient
-
Grade D: useful motor power below the level of lesion
-
Grade E: normal motor and sensory functions (some abnormalities may still be present)
The baseline distribution of Frankel grades differed between the groups; for example, 10 (50% of 20) participants in the anterior group had grade A injuries, compared with 16 (59% of 27) in the posterior group. Whereas 14 of 20 (70%) participants in the anterior group and 15 of 27 (56%) participants in the posterior group improved one grade or more, this outcome measure is unsatisfactory given the different extents of recovery between the different levels and the differences in the baseline distribution.
The mean ASIA motor index scores (0 to 100, where 100 is the optimal score) increased from 43 to 64 in the anterior group and from 40 to 54 in the posterior group. This difference was reported to be not statistically significant but the study authors did not provide standard deviations or respond to our request for these data.
Neurological status at one year of follow‐up was evaluated in Kwon 2007 using NASS neurological scores (0 to 100, where 100 is the optimal score) in 33 participants (14 in the anterior group and 19 in the posterior group) and found no difference between groups (anterior mean: 85.23 versus posterior mean: 83.86; MD 1.37, 95% CI ‐9.76 to 12.50) (Analysis 1.1).
Secondary outcomes
Functional aspects and quality of life
Brodke 2003 did not report these outcomes.
In Kwon 2007, 12 months after surgery, 33 participants (14 versus 19) completed the SF‐36 and the NASS cervical spine questionnaire. There were no statistically significant differences between participants for the SF‐36 physical component score (anterior mean: 46.83 versus posterior mean: 46.91; MD ‐0.08, 95% CI ‐7.26 to 7.10) or mental component scores (anterior mean: 52.31 versus posterior mean: 49.43; MD 2.88, 95% CI ‐3.32 to 9.08) (Analysis 1.2). These are both scored on a 0 to 100 scale, with 100 being the best score and a score of 50 representing the mean for a normal population; neither of the mean differences amounted to a clinically important difference.
Pain
Brodke 2003 reported that seven participants in each group had neck pain at final follow‐up (7/20 anterior approach versus 7/27 posterior approach; RR 1.35, 95% CI 0.56 to 3.23; Analysis 1.3).
In Kwon 2007, pain was assessed using a VAS on the first and second postoperative days. On this scale, 0 means no pain and 10 represents the worst pain. Pain scores on both days favoured the anterior group, but the MD was less than the minimum clinically important difference and the results on the second postoperative day only were of borderline statistical significance (anterior mean: 2.10 versus posterior mean: 2.98; MD ‐0.88, 95% CI ‐1.76 to 0.00) (Analysis 1.4). At one year of follow‐up, pain was evaluated using NASS cervical spine pain scores and no difference was apparent between the two groups (anterior mean: 85.81 versus posterior mean: 81.67; MD 4.14, 95% CI ‐5.54 to 13.82) (Analysis 1.5).
Radiographical outcomes
Two participants in the anterior group of Brodke 2003 and two in the posterior group of Kwon 2007 were found to have non‐fusion or union at final follow‐up. Hence, only two participants had non‐bony fusion in each group in the pooled results (2/38 versus 2/46; RR 1.18, 95% CI 0.04 to 34.91; Analysis 1.6).
Brodke 2003 reported, without providing usable data, that there were “no significant differences in improved alignment or loss of correction between groups”. In Kwon 2007, however, the average sagittal alignment at the level of injury of participants treated by the anterior approach was 8.76 degrees (SD 5.96 degrees) of lordosis; whereas in those treated by posterior approach, the mean sagittal alignment was 1.55 degrees (SD 8.17 degrees) of kyphosis, which represents a statistically significant difference favouring the anterior group (MD ‐10.31 degrees, 95% CI ‐14.95 degrees to ‐5.67 degrees) (Analysis 1.7). The normal curvature at the cervical spine is lordotic: Kwon 2007 reported that 3 of 18 participants in the anterior group versus 11 of 19 in the posterior group were kyphotic across the injured segment.
Complications
These data are presented in Analysis 1.8. Brodke 2003 reported one case of instrumentation failure in each group (RR 1.35, 95% CI 0.09 to 20.31) and that four participants (two in each group) developed medical complications (three pneumonia and one acute respiratory distress syndrome) (RR 1.35, 95% CI 0.21 to 8.78). There were also two deaths that were unrelated to the surgery in the anterior group in Brodke 2003.
In Kwon 2007, 11 of 20 participants treated by the anterior approach had difficulties with swallowing or voice disorders at the time of discharge; no such complications were reported in those treated by the posterior approach (RR 25.19, 95% CI 1.58 to 401.58). However, 10 of these participants had recovered by six weeks of follow‐up and the remaining participant had recovered at three months. Four of 22 participants randomised to the posterior group had neck wound infection during their hospitalisation, whereas one participant in the anterior group had infection at the site of the iliac graft harvesting (RR 0.28, 95% CI 0.03 to 2.26). An elderly participant in the anterior group presented with a variety of medical complications in the acute postoperative period (Analysis 1.8).
Economic data
Neither trial provided cost data. Kwon 2007 reported a significantly longer mean operating time for the anterior group (134 versus 103 minutes; reported P value = 0.0002) but warned that as these times include patient positioning and setup, they "may not accurately reflect skin‐to‐skin time". No participant required blood transfusion in Kwon 2007, who reported that mean blood loss was minimal. Actual length of stay data were not provided by Kwon 2007. Instead, Kwon 2007 reported a proxy, which was the time required for participants to meet the criteria for discharge from the hospital after surgery. Individual participant data and notes on complications (not swallowing/speech difficulties) from Kwon 2007 are presented in Table 2. In the 20 participants in the anterior group, the median time to achieve discharge criteria was 2.75 days; in the 22 participants in the posterior group, the time was 3.5 days (reported p value = 0.096, Mann‐Whitney U‐test).
| Anterior approach | Posterior approach | ||||
| Case | Days | Complications | Case | Days | Complications |
| 1 | 1 | 1 | 1.5 | ||
| 2 | 1.5 | 2 | 1.5 | ||
| 3 | 1.5 | 3 | 2 | ||
| 4 | 1.5 | 4 | 2 | ||
| 5 | 1.5 | 5 | 2 | ||
| 6 | 1.5 | 6 | 2.5 | ||
| 7 | 2 | 7 | 2.5 | ||
| 8 | 2 | 8 | 2.5 | ||
| 9 | 2.5 | 9 | 3 | ||
| 10 | 2.5 | 10 | 3 | Pseudarthrosis requiring revision | |
| 11 | 3 | 11 | 3.5 | ||
| 12 | 3 | 12 | 3.5 | ||
| 13 | 3 | Infection at bone graft site at 3 weeks | 13 | 4 | |
| 14 | 3.5 | 14 | 4 | ||
| 15 | 4 | 15 | 4 | ||
| 16 | 4 | 16 | 5 | ||
| 17 | 4 | 17 | 5 | ||
| 18 | 4.5 | 18 | 6 | (non‐union but not listed as a complication) | |
| 19 | 4.5 | 19 | 17 | Wound infection, 2 weeks of oral antibiotics | |
| 20 | 24 | Severe medical complications postoperatively | 20 | 18 | Wound infection, 2 weeks of oral antibiotics |
| 21 | 28 | Wound infection, 3 weeks of oral antibiotics | |||
| 22 | 42 | MSRA wound infection, 6 weeks of intravenous antibiotics + surgical debridement | |||
MSRA = methicillin‐resistant Staphylococcus aureus
Discussion
Summary of main results
We included two RCTs in this review, involving a total of 94 participants but reporting on a maximum of 84 participants. Follow‐up was approximately one year. Both studies compared anterior and posterior surgical approaches for the stabilisation of a cervical spine facet dislocation. The trials included different and heterogeneous populations: Brodke 2003 included only people with spinal cord injury whereas Kwon 2007 excluded people with spinal cord injury (Kwon 2007). We were able to pool data for one outcome only: non‐union. We judged the quality of the evidence to be very low for all outcomes.
There were no significant differences between groups regarding final post‐surgical neurological status in either trial. Brodke 2003 used non‐validated outcome measure instruments, whereas Kwon 2007 used one validated score. Even though participants with SCIs were excluded in Kwon 2007, the study included 10 participants with single‐level radiculopathy associated with partial motor deficit who fully recovered at follow‐up. Considering that reduction was performed during surgery, prior to stabilisation, these participants were at risk of spinal cord or nerve root injury that could lead to neurological deterioration. However, none occurred, as was demonstrated by the lack of neurological deterioration at one year of follow‐up.
In Kwon 2007, only 33 participants completed functional aspects and quality of life questionnaires at final follow‐up, and again there were no between‐group differences between the anterior and posterior groups. No differences regarding long‐term pain and number of days to achieve specific discharge criteria were found in this study. Pain was evaluated on the first and second postoperative days, and results demonstrated a borderline statistical significance favouring the anterior approach only on day two. Lower postoperative pain with the anterior approach than with the posterior approach is plausible since the stripping of the superficial and deep muscles, necessary for exposure of the spine in the posterior approach, is likely to increase pain at the access site after surgery.
Although heterogeneous in nature, pooled data from the two studies showed no significant differences related to non‐fusion (two participants in each group) between the anterior and posterior approaches. Brodke 2003 reported no differences in changes in alignment between the two approaches. Conversely, Kwon 2007 found better sagittal alignment after one year of follow‐up in the group treated using the anterior approach.
There was insufficient evidence from the two studies to indicate between‐group differences in medical adverse events, rates of instrumentation failure and infection. In Kwon 2007, there were minor complications related to the surgical access in the anterior approach group amounting to voice and swallowing disorders in 11 participants; however, all had recovered by three months.
Overall completeness and applicability of evidence
Outcome data were available for a maximum of 84 participants with cervical spine facet dislocations. Moreover, the populations of the two trials differed in clinically important ways, the main differences being that Brodke 2003 allowed the inclusion of participants with SCIs and a variety of skeletal injuries (including those with burst fractures) as well as facet dislocations, whereas Kwon 2007 included only participants with unilateral facet fractures or dislocations but without SCIs. (Ideally, trials comparing approaches for the surgical management of facet dislocations should analyse outcomes for homogeneous populations that can be divided into different subgroups: unilateral and bilateral dislocations, and total and partial neurological deficits.)
The measurement of outcomes differed in the two trials. Final post‐surgical neurological status was evaluated through ad‐hoc conversion of Frankel scores into a non‐validated numerical scale in Brodke 2003. In contrast, Kwon 2007 assessed neurological function using the validated NASS neurologic score.
Notably, various types of instrumentation with different biomechanical qualities were used in the posterior group in Kwon 2007. In current practice, lateral mass and pedicular screws have generally replaced interspinous and oblique wiring techniques for posterior fixation of the cervical spine; a small number of articles have been published reporting such fixation methods in recent years.
Quality of the evidence
We downgraded the evidence for all outcomes two levels due to major limitations in study design and implementation. This reflects our finding that both studies were at very high risk of bias, including risk of performance bias from lack of blinding. Additionally, we considered Brodke 2003 to be at high risk from: selection bias, because it used an inappropriate method of random sequence generation that in turn precluded allocation concealment; detection bias from lack of blinding of outcome assessments; and other bias, in particular between‐group imbalances in fracture type and extent of cord injury. We judged Kwon 2007 to be at high risk of attrition bias and at unclear risk of selection and detection biases.
We further downgraded the evidence for imprecision, given that both trials had small sample sizes. Thus, overall, we judged the evidence to be of very low quality, which means that we are very uncertain about the estimate.
Potential biases in the review process
In this systematic review we tried to follow the pre‐established criteria and methods included in the published protocol (Del Curto 2009). However, findings in one included study led us to change our primary outcome, as surgery took place several days after reduction (Brodke 2003). Hence, the immediate change in neurological status would be the result of anatomical changes in the spinal canal caused by reduction rather than stabilisation.
We developed a search strategy designed to capture the largest possible number of relevant studies and performed the search in May 2014. We also searched the reference lists of relevant articles, conference proceedings and ongoing clinical trials. Despite efforts to make our strategy more sensitive, the possibility that we have missed potentially eligible studies should not be ruled out. We were unsuccessful in our attempts to obtain further data and information from the authors of both studies.
Agreements and disagreements with other studies or reviews
In a narrative review that drew on evidence from experimental biomechanical studies in cadavers and retrospective studies as well as RCTs, Dvorak 2007 proposed an algorithm to guide the choice of surgical approach for subaxial cervical spine injuries, which included those covered in our review. Dvorak 2007 considered that anterior and posterior approaches to fixation were both viable treatment options for the majority of unilateral or bilateral facet fracture dislocations and pointed out, as we have, the advantages and disadvantages of the two approaches. While noting the difficulties with swallowing and voice disorders associated with the anterior approach, Dvorak 2007 observed that the long‐term clinical significance of segmental kyphosis found more often with the posterior approach "remains to be seen".

Study flow diagram.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
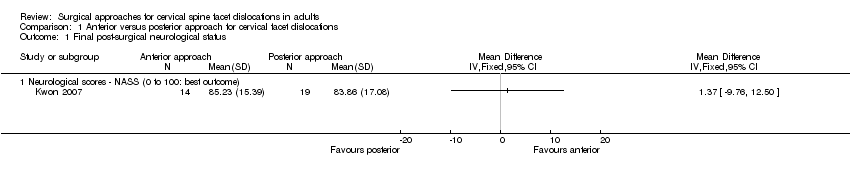
Comparison 1 Anterior versus posterior approach for cervical facet dislocations, Outcome 1 Final post‐surgical neurological status.

Comparison 1 Anterior versus posterior approach for cervical facet dislocations, Outcome 2 Functional aspects and quality of life ‐ SF‐36.

Comparison 1 Anterior versus posterior approach for cervical facet dislocations, Outcome 3 Pain at final follow‐up (6+ months).

Comparison 1 Anterior versus posterior approach for cervical facet dislocations, Outcome 4 Pain postoperative.

Comparison 1 Anterior versus posterior approach for cervical facet dislocations, Outcome 5 Pain scores ‐ NASS (0 to 100: no pain).

Comparison 1 Anterior versus posterior approach for cervical facet dislocations, Outcome 6 Non fusion at follow‐up.

Comparison 1 Anterior versus posterior approach for cervical facet dislocations, Outcome 7 Sagittal alignment (degrees).

Comparison 1 Anterior versus posterior approach for cervical facet dislocations, Outcome 8 Complications.
| Anterior versus posterior approach for cervical facet dislocations in adults | ||||||
| Population: adults with cervical spine facet dislocations | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| Posterior approach | Anterior approach | |||||
| Final post‐surgical neurological status | Mean 83.86 | The mean final post‐surgical neurological status in the anterior group was | 33 | ⊕⊝⊝⊝ | Another study (47 participants), which included patients with spinal cord injury, reported that there was no significant differences in neurological recovery between the two groups (very low quality evidence)4 | |
| Functional aspects and quality of life ‐ SF‐36 physical scores | Mean 46.91 (range 35.2 to 58.9) | The mean SF‐36 physical score in the anterior group was | 33 | ⊕⊝⊝⊝ | ||
| Emotional aspects and quality of life ‐ SF‐36 mental scores | Mean 49.3 (range 29.9 to 62.1) | The mean SF‐36 mental score in the anterior group was | 33 | ⊕⊝⊝⊝ | ||
| Pain | Mean 81.67 (range 58 to 100) | The mean NASS pain score was | 33 | ⊕⊝⊝⊝ | Another study (47 participants), which included patients with spinal cord injury, reported that seven participants in each group had neck pain (7/20 anterior group versus 7/27 posterior group; RR 1.35, 95% CI 0.56 to 3.23) (very low quality evidence)4 | |
| Non‐fusion at follow‐up | Study population | RR 1.18 (0.04 to 34.91) | 84 (2 studies) | ⊕⊝⊝⊝ | ||
| 43 per 1000 | 51 per 1000 | |||||
| Sagittal alignment | Mean | The mean sagittal alignment was | 36 | ⊕⊝⊝⊝ | Lower (negative) degrees indicate physiological cervical alignment. Another study (47 participants), which included patients with spinal cord injury, reported no differences in changes in alignment between the two approaches (very low quality evidence)4 | |
| Complications | Swallowing/voice disorders (short‐term) | RR 25.19 | 42 | ⊕⊝⊝⊝ | Very low quality evidence3 did not confirm between‐group differences in medical adverse events, rates of instrumentation failure and infection. The only significant difference found related to voice and swallowing disorders after anterior approach surgery; this was reported only in one study. All had recovered by three months | |
| 0 per 1000 | 550 per 1000 (actual results) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. The assumed risk is based on that provided in the included studies. 2. This study excluded patients with spinal cord injury. 3. The evidence was downgraded two levels for limitations in study design and implementation (primarily performance bias from lack of clinician and participant blinding, and attrition bias) and one level for imprecision (small sample size and wide CI). 4. The evidence was downgraded two levels for limitations in study design and implementation (primarily selection bias from inadequate randomisation method and lack of allocation concealment, performance bias from lack of clinician and participant blinding, detection bias from lack of outcome assessment blinding and other bias, especially imbalances in baseline characteristics) and one level for imprecision (small sample size and either wide CI or a clearly large range in values). | ||||||
| Term | Definition |
| Discectomy | Excision (cutting out), in part or whole, of an intervertebral disc. The most common indication is disc displacement or herniation (see 'Hernia'). In addition to standard surgical removal, it can be performed by percutaneous discectomy or by laparoscopic discectomy, the former being the more common |
| Fracture | A break in a bone |
| Fracture fixation | The use of usually metallic devices inserted into or through bone to hold a fracture in a set position and alignment while it heals |
| Facet dislocation | Complete displacement that occurs between facets of the interior (located below) and superior (located above) articular processes of adjacent vertebrae |
| Hernia | Protrusion (pushing out) of tissue, structure or part of an organ through the muscular tissue or the membrane by which it is normally contained |
| Reduction | The restoration, by surgical or manipulative procedures, of a part to its normal anatomical relation |
| Surgical decompression | A surgical operation for the relief of pressure in a body compartment or on a body part |
| Pseudarthrosis | A pathological entity characterised by persistent non‐union of bone fragments, leading to formation of a false joint |
| Non‐union | Failure of healing at the ends of a fracture |
| Fusion | Formation of an ankylosis by surgical means |
| Anterior approach | Posterior approach | ||||
| Case | Days | Complications | Case | Days | Complications |
| 1 | 1 | 1 | 1.5 | ||
| 2 | 1.5 | 2 | 1.5 | ||
| 3 | 1.5 | 3 | 2 | ||
| 4 | 1.5 | 4 | 2 | ||
| 5 | 1.5 | 5 | 2 | ||
| 6 | 1.5 | 6 | 2.5 | ||
| 7 | 2 | 7 | 2.5 | ||
| 8 | 2 | 8 | 2.5 | ||
| 9 | 2.5 | 9 | 3 | ||
| 10 | 2.5 | 10 | 3 | Pseudarthrosis requiring revision | |
| 11 | 3 | 11 | 3.5 | ||
| 12 | 3 | 12 | 3.5 | ||
| 13 | 3 | Infection at bone graft site at 3 weeks | 13 | 4 | |
| 14 | 3.5 | 14 | 4 | ||
| 15 | 4 | 15 | 4 | ||
| 16 | 4 | 16 | 5 | ||
| 17 | 4 | 17 | 5 | ||
| 18 | 4.5 | 18 | 6 | (non‐union but not listed as a complication) | |
| 19 | 4.5 | 19 | 17 | Wound infection, 2 weeks of oral antibiotics | |
| 20 | 24 | Severe medical complications postoperatively | 20 | 18 | Wound infection, 2 weeks of oral antibiotics |
| 21 | 28 | Wound infection, 3 weeks of oral antibiotics | |||
| 22 | 42 | MSRA wound infection, 6 weeks of intravenous antibiotics + surgical debridement | |||
| MSRA = methicillin‐resistant Staphylococcus aureus | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final post‐surgical neurological status Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Neurological scores ‐ NASS (0 to 100: best outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Functional aspects and quality of life ‐ SF‐36 Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Physical scores (0 to 100: best outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Mental scores (0 to 100: best outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Pain at final follow‐up (6+ months) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Pain postoperative Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Pain day 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Pain day 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Pain scores ‐ NASS (0 to 100: no pain) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Non fusion at follow‐up Show forest plot | 2 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.04, 34.91] |
| 7 Sagittal alignment (degrees) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Complications Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Instrumentation failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Medical complications | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Swallowing/voice disorders | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

