Aripiprazol (intramuscular) para la agresión o agitación inducida por la psicosis (tranquilización rápida)
Resumen
Antecedentes
Los pacientes que presentan psicosis pueden volverse agresivos. Los antipsicóticos, como el aripiprazol en forma intramuscular, pueden usarse en dichas situaciones.
Objetivos
Evaluar los efectos del aripiprazol intramuscular en el tratamiento de la agresión o agitación inducida por la psicosis (tranquilización rápida).
Métodos de búsqueda
El 11 diciembre 2014 y el 11 abril 2017, se realizó una búsqueda en el registro de ensayos basado en estudios del Grupo Cochrane de Esquizofrenia (Cochrane Schizophrenia Group’s Study‐based Register of Trials), basado en búsquedas regulares en CINAHL, BIOSIS, AMED, Embase, PubMed, MEDLINE, PsycINFO y en registros de ensayos clínicos.
Criterios de selección
Todos los ensayos controlados aleatorios (ECA) que asignaron al azar a los pacientes con agresión o agitación inducida por la psicosis para recibir aripiprazol intramuscular u otra intervención intramuscular.
Obtención y análisis de los datos
Se analizaron de forma independiente las citas y, cuando fue posible, los resúmenes, se ordenaron los documentos, se los volvió a analizar y se evaluó la calidad de los mismos. Se incluyeron los estudios que cumplieron con los criterios de selección. Al menos dos autores de la revisión extrajeron de forma independiente los datos de los estudios incluidos. Se optó por un modelo de efectos fijos. Los datos dicotómicos se analizaron mediante el cociente de riesgos (CR) y los intervalos de confianza (IC) del 95%. Se analizaron los datos continuos mediante las diferencias de medias (DM) y sus IC. Se evaluó el riesgo de sesgo de los estudios incluidos y se utilizó el enfoque GRADE para crear las tablas de "Resumen de los hallazgos".
Resultados principales
La búsqueda encontró 63 registros que se referían a 21 ensayos posibles. Sólo fue posible incluir tres estudios, todos completados durante el último decenio, con 885 participantes, de los cuales 707 fueron incluidos para los análisis cuantitativos en esta revisión sistemática. Debido a las comparaciones limitadas, el tamaño pequeño de los ensayos y la escasez de resultados "pragmáticos"? investigados e informados, la evidencia se calificó principalmente como de calidad baja o muy baja. Ningún ensayo informó datos útiles para uno de los resultados primarios de "calmo" o "dormido" a los 30 minutos. Los resultados económicos tampoco fueron informados en los ensayos.
En comparación con placebo, menos pacientes del grupo de aripiprazol necesitaron inyecciones adicionales en comparación con el grupo de placebo (2 ECA, n = 382; CR 0,69; IC del 95%: 0,56 a 0,85; evidencia de muy baja calidad). La mejoría clínicamente importante en la agitación a las dos horas estuvo a favor del grupo de aripiprazol (2 ECA, n = 382; CR 1,50; IC del 95%: 1,17 a 1,92; evidencia de muy baja calidad). Los números de pacientes que no responden después de la primera inyección también favorecieron al aripiprazol (1 ECA, n = 263; CR 0,49; IC del 95%: 0,34 a 0,71; evidencia de baja calidad). Aunque no se encontró ningún efecto, más pacientes del grupo de aripiprazol comparado con el de placebo experimentaron efectos adversos (1 ECA, n = 117; CR 1,51; IC del 95%: 0,93 a 2,46; evidencia de muy baja calidad).
El aripiprazol requirió más inyecciones en comparación con el haloperidol (2 ECA, n = 477; CR 1,28; IC del 95%: 1,00 a 1,63; evidencia de muy baja calidad), sin diferencias significativas en la agitación (2 ECA, n = 477; CR 0,94; IC del 95%: 0,80 a 1,11; evidencia de muy baja calidad) y un número similar de pacientes que no respondieron después de la primera inyección (1 ECA, n = 360; CR 1,18; IC del 95%: 0,78 a 1,79; evidencia de baja calidad). El aripiprazol y el haloperidol no difirieron cuando se tuvo en cuenta el número general de pacientes que experimentaron al menos un efecto adverso (1 ECA, n = 113; CR 0,91; IC del 95%: 0,61 a 1,35; evidencia de muy baja calidad).
En comparación con el aripiprazol, la olanzapina fue mejor para reducir la agitación (1 ECA, n = 80; CR 0,77; IC del 95%: 0,60 a 0,99; evidencia de baja calidad) y tuvo un efecto más favorable sobre las puntuaciones del cambio en el estado global (1 ECA, n = 80; DM 0,58; IC del 95%: 0,01 a 1,15; evidencia de baja calidad ), ambos a las dos horas. No se encontró ninguna diferencia en cuanto a la posibilidad de experimentar al menos un efecto adverso durante las 24 horas después del tratamiento (1 ECA, n = 80; CR 0,75; IC del 95%: 0,45 a 1,24; evidencia de muy baja calidad). Sin embargo, los participantes asignados al aripiprazol experimentaron menos somnolencia (1 ECA, n = 80; CR 0,25; IC del 95%: 0,08 a 0,82; evidencia de baja calidad).
Conclusiones de los autores
La evidencia disponible es de calidad deficiente aunque hay alguna evidencia de que el aripiprazol es efectivo en comparación con el placebo y el haloperidol, pero no comparado con la olanzapina. Sin embargo, al considerar que la evidencia proviene sólo de tres estudios, se debe proceder con cuidado al generalizar estos resultados a la práctica real. Esta revisión destaca firmemente la necesidad de más ensayos de alta calidad sobre el aripiprazol intramuscular en el tratamiento de los pacientes con agresión o agitación aguda.
PICOs
Resumen en términos sencillos
¿Cuán efectivo es el aripiprazol para calmar a los pacientes que presentan agresión o agitación debido a la psicosis?
Antecedentes
Los pacientes con psicosis pueden escuchar voces (alucinaciones) o tener pensamientos anormales (delirios) que pueden hacer que la persona sienta miedo, angustia y agitación (intranquilidad, excitación o irritabilidad). Dichas emociones pueden dar lugar, a veces, a un comportamiento agresivo o violento. Este hecho plantea un reto y un dilema para los profesionales de la salud mental que tienen que diagnosticar y administrar, a menudo rápidamente, el mejor tratamiento disponible para prevenir que los pacientes agresivos se dañen a ellos mismos o a otras personas.
El aripiprazol es una medicación que puede usarse para tratar la psicosis y también calmar a los pacientes que presentan agresión o agitación debido a la psicosis. Puede administrarse por vía oral o mediante inyección (intramuscular). Sin embargo, el aripiprazol también puede causar efectos secundarios desagradables como cefaleas, trastornos gástricos y sueño o somnolencia excesiva.
Esta revisión busca la evidencia de los ensayos controlados aleatorios que evalúan la efectividad del aripiprazol intramuscular para los pacientes que presentan agitación y agresión como resultado de la psicosis.
Búsqueda
El Especialista en Información del Grupo Cochrane de Esquizofrenia (Cochrane Schizophrenia Group) realizó una búsqueda electrónica en 2014 y en 2017 para obtener estudios que habían asignado al azar a los adultos con agresión debido a la psicosis para recibir inyecciones de aripiprazol o inyecciones de placebo (tratamiento de simulacro) o inyecciones de otro antipsicótico. La búsqueda encontró 63 registros relevantes, con referencia a 21 ensayos. Los autores de la revisión examinaron estos registros para su inclusión o exclusión.
Resultados principales
Solo tres estudios pudieron ser incluidos. La evidencia es limitada debido al pequeño número de ensayos y a la mala calidad de los datos informados. Menos personas que recibieron aripiprazol requirieron más inyecciones para lograr la calma que los que recibieron placebo o haloperidol. En términos generales, el aripiprazol causó un número similar de efectos adversos que el placebo o el haloperidol. Comparado con la olanzapina, el aripiprazol fue menos efectivo para calmar a los pacientes aunque causó menos sueño o somnolencia
Conclusiones
Hay alguna evidencia disponible, aunque es de calidad deficiente, y es difícil establecer conclusiones acerca de la efectividad del aripiprazol a partir de dichos datos. Por lo tanto, no es posible ofrecer orientación clara para los profesionales de la salud y los pacientes con problemas de salud mental en cuanto al uso del aripiprazol como un tranquilizante rápido. Se necesita más investigación para ayudar a los pacientes a considerar qué medicación es mejor para calmar a los pacientes, tiene menos efectos adversos y funciona rápidamente.
Authors' conclusions
Summary of findings
| ARIPIPRAZOLE compared to PLACEBO/NIL for psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Patient or population: psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with PLACEBO/NIL | Risk with ARIPIPRAZOLE | |||||
| Tranquillisation or asleep | Not reported | |||||
| Repeated need for tranquillisation ‐ needing additional injections during 24 hours | Low | RR 0.69 | 382 | ⊕⊝⊝⊝ | ||
| 250 per 1.000 | 173 per 1.000 | |||||
| Moderate | ||||||
| 600 per 1.000 | 414 per 1.000 | |||||
| High | ||||||
| 750 per 1.000 | 518 per 1.000 | |||||
| Specific behaviour: Agitation ‐ clinically important change (PANSS ‐EC reduction ≥ 40% from baseline) ‐ up to 2 hours | Low | RR 1.50 | 382 | ⊕⊝⊝⊝ | ||
| 100 per 1.000 | 150 per 1.000 | |||||
| Moderate | ||||||
| 350 per 1.000 | 525 per 1.000 | |||||
| High | ||||||
| 700 per 1.000 | 1000 per 1.000 | |||||
| Global state: non‐responders to the first injection | Low | RR 0.49 | 263 | ⊕⊕⊝⊝ | ||
| 200 per 1.000 | 98 per 1.000 | |||||
| Moderate | ||||||
| 450 per 1.000 | 221 per 1.000 | |||||
| High | ||||||
| 700 per 1.000 | 343 per 1.000 | |||||
| Adverse effects: one or more adverse events during 24 hours (only reported if occurred in ≥ 5% of people) | Study population | RR 1.51 | 117 | ⊕⊝⊝⊝ | ||
| 295 per 1.000 | 446 per 1.000 | |||||
| Economic outcomes | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'serious' (downgraded by 1) ‐ randomisation procedure is not reported for both the included studies, and allocation concealment procedure is not consistently reported in Bristol‐Myers 2005. While for the 'randomisation' bias, studies are at least reported as 'randomised', as for the latter that allocation concealment was correctly handled could not be implied. 2 Indirectness: rated 'very serious' (downgraded by 2) ‐ participants included in the studies had levels of agitation not so pronounced by inclusion criteria, potentially under‐estimating or more likely over‐estimating true effectiveness. | ||||||
| ARIPIPRAZOLE compared to OTHER ANTIPSYCHOTIC: a. HALOPERIDOL for psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Patient or population: psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTIC: a. HALOPERIDOL | Risk with ARIPIPRAZOLE | |||||
| Tranquillisation or asleep | Not reported | |||||
| Repeated need for tranquillisation ‐ needing additional injections during 24 hours | Low | RR 1.28 | 477 | ⊕⊝⊝⊝ | , | |
| 100 per 1.000 | 128 per 1.000 | |||||
| Moderate | ||||||
| 300 per 1.000 | 384 per 1.000 | |||||
| High | ||||||
| 500 per 1.000 | 640 per 1.000 | |||||
| Specific behaviour: Agitation ‐ clinically important change (PANSS ‐EC reduction ≥ 40% from baseline) ‐ up to 2 hours | Study population | RR 0.94 | 477 | ⊕⊝⊝⊝ | ||
| 576 per 1.000 | 541 per 1.000 | |||||
| Global state: non‐responders to the first injection | Study population | RR 1.18 | 360 | ⊕⊕⊝⊝ | ||
| 184 per 1.000 | 217 per 1.000 | |||||
| Adverse effects: one or more adverse events during 24 hours (only reported if occurred in ≥ 5% of people) | Study population | RR 0.91 | 113 | ⊕⊝⊝⊝ | ||
| 491 per 1.000 | 447 per 1.000 | |||||
| Adverse effects: movement disorders ‐ EPS during 24 hours (only reported if occurred in ≥ 5% of people) | Low | RR 0.29 | 471 | ⊕⊝⊝⊝ | ||
| 50 per 1.000 | 14 per 1.000 | |||||
| Moderate | ||||||
| 100 per 1.000 | 29 per 1.000 | |||||
| High | ||||||
| 300 per 1.000 | 87 per 1.000 | |||||
| Economic outcomes | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'serious' (downgraded by 1) ‐ randomisation procedure is not reported for both the included studies, and allocation concealment procedure is not consistently reported in Bristol‐Myers 2005. While for the 'randomisation' bias studies are at least reported as 'randomised', as for the latter that allocation concealment was correctly handled could not be implied. 2 Indirectness: rated 'very serious' (downgraded by 2) ‐ participants included in the studies had levels of agitation not so pronounced by inclusion criteria, potentially under‐estimating or more likely over‐estimating true effectiveness. | ||||||
| ARIPIPRAZOLE compared to OTHER ANTIPSYCHOTIC: b. OLANZAPINE for psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Patient or population: psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTIC: b. OLANZAPINE | Risk with ARIPIPRAZOLE | |||||
| Tranquillisation or asleep | Not reported | |||||
| Repeated need for tranquillisation | Not reported | |||||
| Specific behaviour: Agitation ‐ clinically important change (PANSS ‐EC reduction ≥ 40% from baseline) ‐ up to 2 hours | Low | RR 0.77 | 80 | ⊕⊕⊝⊝ | ||
| 500 per 1.000 | 385 per 1.000 | |||||
| Moderate | ||||||
| 800 per 1.000 | 616 per 1.000 | |||||
| High | ||||||
| 900 per 1.000 | 693 per 1.000 | |||||
| Global state: CGI‐S change score up to 2 hours | MD 0.58 | ‐ | 80 | ⊕⊕⊝⊝ | ||
| Adverse effects: one or more adverse effects during 24 hours | Study population | RR 0.75 | 80 | ⊕⊝⊝⊝ | ||
| 500 per 1.000 | 375 per 1.000 | |||||
| Adverse effects: somnolence during 24 hours | Low | RR 0.25 | 80 | ⊕⊕⊝⊝ | ||
| 100 per 1.000 | 25 per 1.000 | |||||
| Moderate | ||||||
| 300 per 1.000 | 75 per 1.000 | |||||
| High | ||||||
| 700 per 1.000 | 175 per 1.000 | |||||
| Economic outcomes | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Imprecision ‐ rated 'serious' (downgraded by 1): Optimal Information Size (OIS) criterion is not met meaning that imprecision of effect estimates could not be properly handled. 2 Imprecision ‐ rated 'very serious' (downgraded by 2): Optimal Information Size (OIS) criterion is not met and CI overlaps no effect. High imprecision of effect estimates could not be properly excluded. 3 Indirectness ‐ rated 'serious' (downgraded by 1): attribution to the intervention drugs is suspected but can not be confirmed since in the study results it is showed that almost all the participants were administered with 'treatment as usual' drugs. | ||||||
Background
Description of the condition
Aggression and agitation are psychiatric emergencies that can often be associated with psychosis, substance misuse or epilepsy (Tardiff 1982). Aggression is described as hostile, injurious, or destructive behaviour, and can occur in about 3% of psychiatric outpatients (Tardiff 1985) and in up to 7% to 10% of inpatient settings. Agitation is described as a state of troubled mind or feelings (Makins 1994) and is characterised by restlessness, excitability and irritability, and for some people, this can result in verbal and physical aggressive behaviour (Mohr 2005). As is often the case, patients experiencing these episodes find new environments stressful and become aggressive (Ferracuti 1993). Aggression and agitation within the psychiatric setting imposes a significant challenge to clinicians who, while attempting to make an accurate diagnosis and formulation (Schleifer 2011), have to intervene quickly in order to manage the risk that the service user may present to themselves, other service users and staff (NICE 2015).
There have been numerous national and international guidelines that created protocols for managing people with aggression and violence (APA 2006; CPA 2005; NICE 2015). Some evidence from these guidelines may be conflicting in nature. For example, the use of zuclopenthixol acetate is not recommended by NICE, but recommended by Australian and Canadian guidelines. This is not surprising as there has only been one trial of zuclopenthixol acetate in this area and the small sample size from this trial could lead to varied interpretations. An individual requiring rapid tranquillisation is frequently unable or unwilling to consent and the treatment is being provided in his or her best interest.
In the UK, NICE guidelines recommend that a comprehensive risk assessment is the first step in management of aggression and non‐pharmacological methods recommended for use include de‐escalation, physical restraints and seclusion. Where rapid tranquillisation through oral therapy is refused, is not indicated by previous clinical response, is not a proportionate response, or is ineffective, administration of an intramuscular combination of haloperidol and promethazine or intramuscular lorazepam is recommended (NICE 2015).
In the USA, the first intervention involves staff members talking to the patient in an attempt to calm him or her. Attempts to restrain the patient should be done only by a team trained in safe restraint procedures to minimise risk of harm to patients or staff. Antipsychotics and benzodiazepines are often helpful in reducing the patient’s level of agitation. If the patient will take oral medication, rapidly dissolving forms of olanzapine and risperidone can be used for quicker effect and to reduce non adherence. If a patient refuses oral medication, most states allow for emergency administration despite the patient’s objection. Short‐acting parenteral formulations of first‐ and second‐generation antipsychotic agents (e.g. haloperidol, ziprasidone, and olanzapine), with or without a parenteral benzodiazepine (e.g. lorazepam), are available for emergency administration in acutely agitated patients (APA 2006).
There have been only a handful of surveys looking at the prevalence and practices in managing acute agitation. Cunane 1994 found that in the UK, clinicians preferred using chlorpromazine, whereas Binder 1999 found that in the USA, clinicians preferred a combination of haloperidol and benzodiazepines. In a more recent survey, Huf 2002a found that Brazilian psychiatrists preferred a combination of haloperidol and promethazine. Surveys of this nature are notoriously difficult to conduct specially in resource‐poor settings, which partly explains the paucity of such surveys from low‐income countries. There have been a few good quality pragmatic trials comparing various interventions such as haloperidol, olanzapine, promethazine, benzodiazepines including lorazepam and midazolam, and other medications for managing acute agitation (Alexander 2004; Huf 2002b; Raveendran 2007).
Description of the intervention
Aripiprazole is a quinolinone derivative, 7‐[4‐[4‐(2,3‐dichlorophenyl)‐1‐piperazinyl]butoxy]‐3,4‐dihydrocarbostyril (Kikuchi 1995; Figure 1). It is available as oral tablets, orally disintegrating tablets, oral solution and intramuscular injections. The intramuscular preparations are available in single‐dose vials as a ready‐to‐use, 9.75 mg/1.3 mL (7.5 mg/mL) clear, colourless, sterile, aqueous solution. Inactive ingredients for this solution include 150 mg/mL of sulfobutylethercyclodextrin (SBECD), tartaric acid, sodium hydroxide and water for injection (Abilify 2017). Intramuscular aripiprazole seems to have two medians for peak plasma concentrations, one at one hour and the second at three hours. It has 100% bioavailability and the pharmacokinetics is linear over a dose range of 1 mg to 45 mg. It is likely to be eliminated by the liver using the P450 system of isoenzymes (CYP2D6 and CYP3A4, Abilify 2017).

Aripiprazole structure
Aripiprazole solution, for injection, is licensed for rapid control of agitation and disturbed behaviours in adults when oral therapy is not appropriate. The dose for adults is 9.75 mg (1.3 mL) as a single intramuscular injection initially with an effective dose range of 5.25 mg to 15 mg as a single injection. Second injections can be administered two hours after the first, No more than three injections should be given in any 24‐hour period.
How the intervention might work
According to treatment guidelines, aripiprazole is classified amongst the second‐generation antipsychotics with amisulpride, clozapine, olanzapine, quetiapine, risperidone, sertindole, ziprasidone and zotepine. However, aripiprazole has been described as the prototype of a new and third generation of antipsychotics, the so called 'dopamine‐serotonin system stabilisers' (McGavin 2002; Swainston Harrison 2004).
It is reported to exert its antipsychotic effects by acting as a partial agonist at dopamine D2 and 5‐HT1A receptors and antagonist activity at 5‐HT2A receptors (Shapiro 2003). It has been postulated that through dopamine and serotonin system stabilisation, a partial D2 agonist would be able to act as an antagonist in pathways where an abundance of dopamine was leading to 'psychosis', yet it would stimulate receptors as an agonist at sites in which low‐dopaminergic tone would produce adverse effects.
Aripiprazole, however, also has an affinity to other receptors including D3, D4, 5‐HT2c, 5HT7, alpha‐1 adrenergic and histamine receptors, which could be related to some adverse effects (such as headache, gastrointestinal upset, headedness, somnolence; FDA 2004). The recommended target dose for aripiprazole is 10 mg to 15 mg per day (dose range 10 mg to 30 mg/day). Phase III trials were initially conducted in Japan in 1995 and the drug was granted Approved Status by the FDA (USA) on the 15 November 2002 for the treatment of schizophrenia. Aripiprazole has since been licensed in most countries worldwide.
Why it is important to do this review
Aripiprazole has been around since 2002 and its use has been gradually increasing in the UK. Recent data state that for aripiprazole the spending on NHS prescriptions in England was about £6m in 2008, about £10m in 2010, rising to £15m in 2013 (NHS 2014). An intramuscular formulation of aripiprazole has recently been licensed for use in treatment of acute agitation. The increase in prescribing costs of oral aripiprazole seems to be in keeping with the general increase in atypical antipsychotic prescribing in the UK. If previous trends are to go by, we could expect a general increase in cost of intramuscular aripiprazole use to the UK's NHS over the next few years. In terms of raw cost data, there are considerable differences between the older generation and newer generation of antipsychotics. For example, in the UK a vial of haloperidol 5 mg/mL costs 30 pence, a vial of promethazine 25 mg/mL 70 pence, whereas, a vial of olanzapine 5 mg/mL about £3.48, and finally a vial of aripiprazole 9.75 mg costs £3.43 in the UK (BNF 2014).
With the increasing number of the antipsychotic medications that are available for intramuscular use and treatment of acute agitation, there is an urgent need to systematically review and evaluate the effects of intramuscular aripiprazole. This is one of a series of similar reviews (Table 1).
| Focus of review | Reference |
| Completed and maintained reviews | |
| 'As required' medication regimens for seriously mentally ill people in hospital | |
| Benzodiazepines for psychosis‐induced aggression or agitation | |
| Chlorpromazine for psychosis‐induced aggression or agitation | |
| Clotiapine for acute psychotic illnesses | |
| Containment strategies for people with serious mental illness | |
| De‐escalation techniques for psychosis‐induced aggression | |
| Droperidol for acute psychosis | |
| Haloperidol for long‐term aggression in psychosis | |
| Haloperidol for psychosis‐induced aggression or agitation (rapid tranquillisation) | |
| Haloperidol plus promethazine for psychosis‐induced aggression | |
| Olanzapine IM or velotab for acutely disturbed/agitated people with suspected serious mental illnesses | |
| Seclusion and restraint for serious mental illnesses | |
| Zuclopenthixol acetate for acute schizophrenia and similar serious mental illnesses | |
| Reviews in the process of being completed | |
| Risperidone for psychosis‐induced aggression or agitation | |
| Loxapine inhaler for psychosis‐induced aggression | |
| Clozapine for people with psychosis‐induced aggression or agitation | |
| Quetiapine for psychosis‐induced aggression or agitation | |
Objectives
To evaluate the effects of intramuscular aripiprazole in the treatment of psychosis‐induced aggression or agitation (rapid tranquillisation).
Methods
Criteria for considering studies for this review
Types of studies
All relevant randomised control trials. If a trial was described as ’double‐blind’ but implied randomisation, we included such trials in a sensitivity analysis (see Sensitivity analysis). If their inclusion did not result in a substantive difference, they remained in the analyses. If their inclusion resulted in statistically significant differences, we did not add the data from these lower‐quality studies to the results of the better trials, but presented such data within a subcategory. We planned to exclude quasi‐randomised studies, such as those allocating by alternate days of the week or where allocation was undertaken on surname.
Types of participants
People exhibiting aggression or agitation (or both) thought to be due to psychosis, regardless of age and sex. Should studies have involved people with other diagnoses, such as drug or alcohol intoxication, organic problems including dementia, non‐psychotic mental illnesses or learning disabilities, we would have included these as long as the majority of participants (> 50%) were experiencing psychosis.
Types of interventions
1. Aripiprazole
Given alone, intramuscularly in any dose.
versus:
A. Other antipsychotic medications
Given alone, intramuscularly in any dose.
B. Placebo
Active or non‐active, intramuscular, in any dose.
Types of outcome measures
Outcomes grouped by time: by 30 minutes, up to two hours, up to four hours, up to 24 hours, and over 24 hours.
We endeavoured to report binary outcomes recording clear and clinically meaningful degrees of change (e.g. global impression of much improved, or more than 50% improvement on a rating scale ‐ as defined within the trials) before any others. Thereafter, we listed other binary outcomes and then those that were continuous.
Primary outcomes
1. Tranquil or asleep
1.1 Tranquil or asleep ‐ by up to 30 minutes *
2. Repeated need for rapid tranquillisation
Secondary outcomes
1. Tranquillisation or asleep
1.1 Tranquil or asleep ‐ over 30 minutes *
1.2 Time to tranquillisation/sleep
1.3 Time to tranquillisation
1.4 Time to sleep
2. Specific behaviours
2.1 Self‐harm, including suicide
2.2 Injury to others
2.3 Agitation
2.3.1 Another episode of agitation by 24 hours
2.3.2 Clinically important change in agitation *
2.3.3 Any change in agitation
2.3.4 Average endpoint score ‐ agitation scales
2.3.5 Average change score ‐ agitation scales
2.4 Aggression
2.4.1 Another episode of aggression by 24 hours
2.4.2 Clinically important change in aggression *
2.4.3 Any change in aggression
2.4.4 Average endpoint score ‐ aggression scales
2.4.5 Average change score ‐ aggression scales
3. Global state
3.1 Overall improvement
3.2 Use of additional medication
3.3 Use of restraints/seclusion
3.4 Relapse ‐ as defined by each study
3.5 Recurrence of violent incidents
3.6 Needing extra visits from the doctor
3.7 Refusing oral medication
4. Service use
4.1 Duration of hospital stay
4.2 Re‐admission
4.3 Clinically important engagement with services
4.4 Any engagement with services
4.5 Average endpoint engagement score
4.6 Average change in engagement scores
5. Mental state
5.1 Clinically important change in general mental state
5.2 Any change in general mental state
5.3 Average endpoint general mental state score
5.4 Average change in general mental state scores
6. Adverse effects
6.1 Death
6.2 Any general adverse effects
6.3 Any serious specific adverse effects
6.4 Average endpoint general adverse effect score
6.5 Average change in general adverse effect scores
6.6 Clinically important change in specific adverse effects
6.7 Any change in specific adverse effects
6.8 Average endpoint specific adverse effects
6.9 Average change in specific adverse effects
7. Leaving the study early
7.1 For specific reasons
7.2 For general reasons
8. Satisfaction with treatment
8.1 Recipient of treatment satisfied with treatment
8.2 Recipient of treatment average satisfaction score
8.3 Recipient of treatment average change in satisfaction scores
8.4 Informal treatment provider satisfied with treatment
8.5 Informal treatment providers' average satisfaction score
8.6 Informal treatment providers' average change in satisfaction scores
8.7 Professional providers satisfied with treatment
8.8 Professional providers' average satisfaction score
8.9 Professional providers' average change in satisfaction scores
9. Acceptance of treatment
9.1 Accepting treatment
9.2 Average endpoint acceptance score
9.3 Average change in acceptance scores
10. Quality of life
10.1 Clinically important change in quality of life *
10.2 Any change in quality of life *
10.3 Average endpoint quality of life score
10.4 Average change in quality of life score
10.5 Clinically important change in specific aspects of quality of life *
10.6 Any change in specific aspects of quality of life *
10.7 Average endpoint specific aspects of quality of life
10.8 Average change in specific aspects of quality of life
10. Economic outcomes
10.1 Direct costs
10.2 Indirect costs
Outcomes used for 'Summary of findings' tables
We used the GRADE approach to interpret findings (Schünemann 2011) and used the GRADEpro GDT web application to export data from this review to create 'Summary of findings' tables. These tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient‐care and decision‐making. We selected the following main outcomes for inclusion in the 'Summary of findings' tables.
1. Tranquillisation or asleep ‐ by 30 minutes.
2. Repeated need for rapid tranquillisation ‐ within 24 hours.
3. Specific behaviours ‐ agitation or aggression.
4. Global state ‐ clinically important overall improvement *.
5. Adverse effects ‐ any serious, specific adverse effects.
6. Economic outcomes
Search methods for identification of studies
No language restriction was applied within the limitations of the search tools.
Electronic searches
Cochrane Schizophrenia Group’s Study‐Based Register of Trials
On 11 December 2014 and 11 April 2017, the Information Specialist searched the register using the following search strategy:
(*Aripiprazole*) in Intervention AND (*Aggress* or *Violent* or *Agitation* or *Tranq*) in Healthcare Condition Fields of Study
In such study‐based register, searching the major concept retrieves all the synonyms and relevant studies because all the studies have already been organised based on their interventions and linked to the relevant topics.
This register is compiled by systematic searches of major resources (including AMED, BIOSIS, CINAHL, Embase, MEDLINE, PsycINFO, PubMed, and registries of clinical trials) and their monthly updates, handsearches, grey literature, and conference proceedings (see Group’s Module). There is no language, date, document type, or publication status limitations for inclusion of records into the register.
Searching other resources
1. Reference searching
We inspected references of all included studies for further relevant studies.
2. Personal contact
Where necessary, we attempted to contact the first author of each included study for information regarding unpublished trials.
Data collection and analysis
Selection of studies
For the 2014 search, review authors SJ, and KS independently inspected citations from the searches and identified relevant abstracts. A random 20% sample was independently re‐inspected by SS to ensure reliability. Where disputes arose, the full report was acquired for more detailed scrutiny. SJ and KS obtained and inspected full reports of the abstracts meeting the review criteria. If it had not been possible to resolve disagreement by discussion, we would have attempted to contact the authors of the study for clarification.
For the 2017 search, review author EGO independently inspected citations and identified relevant abstracts. EGO also re‐inspected the results of the 2014 search.
Data extraction and management
1. Extraction
For the 2014 search, review author JS extracted data from all included studies. In addition, to ensure reliability, review authors KS and SS independently extracted data from a random sample of these studies, comprising 10% of the total. We would have discussed any disagreement, documented decisions and, if necessary, contacted authors of studies. We extracted data presented only in graphs and figures whenever possible, but included only if two review authors independently had the same result. We attempted to contact authors through an open‐ended request in order to obtain missing information or for clarification whenever necessary.
For the 2017 search, review author EGO extracted data from the new included study and re‐inspected the data extraction from previously included studies.
2. Management
2.1 Forms
We extracted data onto standard, simple forms.
2.2 Scale‐derived data
We included continuous data from rating scales only if:
a) the psychometric properties of the measuring instrument had been described in a peer‐reviewed journal (Marshall 2000); and
b) the measuring instrument had not been written or modified by one of the trialists for that particular trial;
c) the instrument should be a global assessment of an area of functioning and not sub‐scores which are not, in themselves, validated or shown to be reliable, however there are exceptions; we included sub‐scores from mental state scales measuring positive and negative symptoms of schizophrenia.
Ideally, the measuring instrument should either be i. self‐report or ii. completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly, therefore, in Description of studies we noted if this was the case or not.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint), which can be difficult in unstable and difficult to measure conditions such as schizophrenia. We decided primarily to use endpoint data, and only use change data if the former were not available. We combined endpoint and change data in the analysis and we used mean differences (MD) rather than standardised mean differences (SMD) throughout (Deeks 2011).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we aimed to apply the following standards to all data before inclusion:
a) standard deviations (SDs) and means are reported in the paper or obtainable from the authors;
b) when a scale starts from the finite number zero, the SD, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, (Altman 1996; Higgins 2011);
c) if a scale started from a positive value (such as the Positive and Negative Syndrome Scale (PANSS), (Kay 1986)), which can have values from 30 to 210), the calculation described above was modified to take the scale starting point into account. In these cases skew is present if 2 SD > (S‐ S min), where S is the mean score and ’S min’ is the minimum score.
Endpoint scores on scales often have a finite start and end point and these rules can be applied. Skewed data pose less of a problem when looking at means if the sample size is large (> 200) and we entered these into the syntheses. We planned to present skewed data from studies of less than 200 participants in ‘Additional tables’ rather than in analyses.
When continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. These data were entered into analyses.
2.5 Common measure
To facilitate comparison between trials, we intended to convert variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, we made efforts to convert outcome measures to dichotomous data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the PANSS (Kay 1986), this could be considered as a clinically significant response (Leucht 2005; Leucht 2005a). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicates a favourable outcome for aripiprazole is effective for psychosis‐induced aggression or agitation. Where keeping to this makes it impossible to avoid outcome titles with clumsy double‐negatives (e.g. 'Not un‐improved'), we reported data where the left of the line indicates an unfavourable outcome. This is noted in the relevant graphs.
Assessment of risk of bias in included studies
Review authors EGO and JS assessed risk of bias by using the criteria described in the Cochrane Handbook for Systematic reviews of Interventions (Higgins 2011a) to assess trial quality. This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
We noted the level of risk of bias in both the text of the review and in the 'Summary of findings' tables.
Measures of treatment effect
1. Binary data
For binary outcomes, we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). The number needed to treat for an additional beneficial outcome/number needed to treat for an additional harmful outcome(NNTB/NNTH) statistic with its confidence intervals is intuitively attractive to clinicians but is problematic both in its accurate calculation in meta‐analyses and interpretation (Hutton 2009). For binary data presented in the 'Summary of findings' tables, where possible, we calculated illustrative comparative risks.
2. Continuous data
For continuous outcomes we estimated mean difference (MD) between groups. We preferred not to calculate effect size measures (SMD). However, if scales of very considerable similarity had been used, we would have presumed there was a small difference in measurement, and calculated the effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
If clustering was not been accounted for in primary studies, we would have presented data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. We would have attempted to contact the first authors of studies to obtain intra‐class correlation coefficients (ICCs) for their clustered data and to adjust for this by using accepted methods (Gulliford 1999).
If clustering had been incorporated into the analysis of primary studies, we would have reported these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
Cochrane Schizophrenia have sought statistical advice and have been advised that the binary data presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC is not reported, it is assumed to be 0.1 (Ukoumunne 1999).
Where cluster studies are appropriately analysed taking into account ICCs, and relevant data documented in the report, synthesis with other studies would be possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason, cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we would have only used data from the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, where relevant, we presented the additional treatment arms in separate comparisons. If data were binary, we simply added and combined within the two‐by‐two table. If data were continuous, we combined data following the formula in section the Cochrane Handbook for Systematic reviews of Interventions (Higgins 2011). If the additional treatment arms had not been relevant, we would not have reproduced such data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for, we would not reproduce these data or use them within analyses (except for the outcome 'leaving the study early'). When more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we addressed this within the 'Summary of findings' tables by down‐grading quality.
2. Binary
In the case where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat analysis, ITT). Those leaving the study early were all assumed to have the same rates of negative outcome as those who completed, with the exception of the outcome of death and adverse effects. For these outcomes, we used the rate of those who stayed in the study ‐ in that particular arm of the trial ‐ for those who did not. We undertook a sensitivity analysis to test how prone the primary outcomes were to change when 'completer' data only were compared to the ITT analysis using the above assumptions.
3. Continuous
3.1 Attrition
In the case where attrition for a continuous outcome was between 0% and 50% and 'completer'‐data were reported, we reproduced these.
3.2 Standard deviations (SDs)
If SDs were not reported, we first tried to obtain the missing values from the authors. If not available, where there were missing measures of variance for continuous data, but an exact standard error (SE) and confidence intervals were available for group means, and either 'P' value or 't' values were available for differences in mean, we would have calculated them according to the rules described in the Cochrane Handbook for Systematic reviews of Interventions (Higgins 2011). When only the SE are reported, SDs can be calculated by the formula SD = SE * square root (n). Chapters 7.7.3 and 16.1.3 of the Cochrane Handbook for Systematic reviews of Interventions (Higgins 2011) present detailed formulae for estimating SDs from P values, t or F values, confidence intervals, ranges or other statistics. If these formulae do not apply, we could calculate the SDs according to a validated imputation method which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. We would have examined the validity of the imputations in a sensitivity analysis excluding imputed values.
3.3 Assumptions about participants who left the trials early or were lost to follow‐up
Various methods are available to account for participants who left the trials early or were lost to follow‐up. Some trials just present the results of study completers, others use the method of last observation carried forward (LOCF), while more recently methods such as multiple imputation or mixed‐effects models for repeated measurements (MMRM) have become more of a standard. While the latter methods seem to somewhat better than LOCF (Leon 2006), we feel that the high percentage of participants leaving the studies early and differences in the reasons for leaving the studies early between groups could be often the core problem in randomised schizophrenia trials. We therefore did not exclude studies based on the statistical approach used. However, preferably we used the more sophisticated approaches, e.g. we preferred MMRM or multiple‐imputation to LOCF and only presented completer analyses if some kind of ITT data were not available at all; this issue was addressed in the item 'incomplete outcome data' of the 'Risk of bias' tool.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We inspected all studies for clearly outlying people or situations which we had not predicted would arise. If such situations or participant groups had arisen, we would have discussed them.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We inspected all studies for clearly outlying methods which we had not predicted would arise. If such methodological outliers had arisen, we would have discussed them.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
We investigated heterogeneity between studies by considering the I2 statistic alongside the Chi2 'P' value. The I2 statistic provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on i. magnitude and direction of effects and ii. strength of evidence for heterogeneity (e.g. 'P' value from Chi2 test, or a CI for I2). We interpreted an I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistic, as evidence of substantial levels of heterogeneity (Chapter 9. Cochrane Handbook for Systematic Reviews of Interventions) (Deeks 2011). If substantial levels of heterogeneity had been found in the primary outcome, we planned to explore reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in Chapter 10. of the Cochrane Handbook for Systematic reviews of Interventions (Sterne 2011).
1. Protocol versus full study
We tried to locate protocols of included randomised trials. If the protocol was available, we compared outcomes in the protocol and in the published report . If the protocol was not available, we compared outcomes listed in the methods section of the trial report with actually reported results.
2. Funnel plot
We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We decided that we would not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar size. In future, where funnel plots are possible, we will seek statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model: it puts added weight onto small studies which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We chose fixed‐effect model for all analyses.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
1.1 Primary outcomes
We did not anticipate any subgroup analyses.
2. Investigation of heterogeneity
If inconsistency was high, we reported it. First, we investigated whether data were entered correctly. Second, if data were correct, we visually inspected the graph and successfully removed outlying studies to see if homogeneity was restored. For this review, we decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, we would present such data. If not, we would not pool data, but would discuss this issue. We know of no supporting research for this 10% cut‐off but are investigating use of prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity were obvious, we stated hypotheses regarding these for future reviews or versions of this review. We did not anticipate undertaking analyses relating to these.
Sensitivity analysis
1. Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in some way as to imply randomisation. For the main outcomes of interest, we we would have included these studies and if there was no substantive difference when the implied randomised studies were added to those with better description of randomisation, then we would have used all relevant from data these studies.
2. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data), we compared the findings of the main outcomes of interest when we used our assumption compared with 'completer' data only. Where there was a substantial difference, we reported results and discussed them but continued to employ our assumption.
Where assumptions had to be made regarding missing SDs data (see Dealing with missing data), we compared the findings of the main outcomes of interest when we used our assumption compared with 'completer' data only. We undertook a sensitivity analysis to test how prone results were to change when 'completer' data only were compared to the imputed data using the above assumption. If there was a substantial difference, we reported results and discussed them but continued to employ our assumption.
3. Risk of bias
We analysed the effects of excluding trials that were judged to be at high risk of bias across one or more of the domains of randomisation (see Assessment of reporting biases). If the exclusion of trials at high risk of bias did not substantially alter the direction of effect or the precision of the effect estimates, then we included data from these trials in the analyses.
4. Imputed values
We also would have undertaken a sensitivity analysis to assess the effects of including data from trials if we had used imputed values for ICC in calculating the design effect in cluster‐randomised trials.
If substantial differences were noted in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we did not pool data from the excluded trials with the other trials contributing to the outcome, but presented them separately.
5. Fixed and random effects
All data were synthesised using a fixed‐effect model; however, we also synthesised data for the main outcomes using a random‐effects model to evaluate whether this altered the significance of the results.
Results
Description of studies
For substantive descriptions of studies please see Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification and Characteristics of ongoing studies.
Results of the search
The electronic search (December 2014) yielded 51 citations of potentially eligible studies. We obtained 51 full‐text papers, related to 17 different studies (two included studies, 13 excluded, one awaiting classification, one ongoing study, Figure 2).
The last search (April 2017) yielded 25 citations of potentially eligible studies, with 13 of those already cited in the previous search; therefore, we obtained and closely inspected 12 new full‐text papers related to four studies (one study included, three studies excluded, Figure 3).

Study flow diagram (2014 search).

Study flow diagram (2017 search).
Included studies
Details of the three studies included in the review are provided in the Characteristics of included studies table.
1. Length of studies
The duration of the studies, limited to intramuscular (IM) intervention phase, was up to 24 hours (Andrezina 2006, Bristol‐Myers 2005; Kittipeerachon 2016).
2. Participants
A total number of 707 patients were considered for this review; please note that for Bristol‐Myers 2005, the participants reported below refer to those allocated to selected comparisons only (placebo, aripiprazole 9.75 mg, haloperidol).
2.1 Clinical state
Participants in the trials presented with acute aggression or agitation due to psychosis and therefore were deemed by the treating clinician to be appropriate candidates for IM rapid tranquillisation therapy.
2.2 Diagnosis
Overall, 526 (74.4%) of participants had a diagnosis of schizophrenia, 176 (24.9%) of schizoaffective disorder, and 5 (0.7%) of schizophreniform disorder.
2.3 Exclusion
Exclusion criteria in Andrezina 2006 and Bristol‐Myers 2005 studies were similar: psychoactive substance dependence within two months of the study start, significant risk of committing suicide, neurologic condition, psychiatric condition requiring pharmacotherapy other than those included, significant medical condition, known non‐responders to antipsychotic medication; PANSS (Excited Component (PEC) score ≥ 32; in Andrezina 2006, schizophreniform disorder was considered an exclusion criteria, whilst in Bristol‐Myers 2005 it was not.Kittipeerachon 2016, a more pragmatic randomised controlled trial (RCT), had less restrictive exclusion criteria: known allergy to aripiprazole or olanzapine, pregnancy or breastfeeding.
2.4 Age
Age range was of 18‐69 years in Andrezina 2006, 18‐66 years in Bristol‐Myers 2005, and 18‐65 years in Kittipeerachon 2016.
2.5 Sex
Participants of the studies involved in this review were 435 men (61.5%) and 272 women (38.5%).
3. Study size
The total number of participants randomised was 805 (Andrezina 2006, n = 448; Bristol‐Myers 2005, n = 357, of which 179 were considered for comparisons; Kittipeerachon 2016, n = 80).
4. Setting
All the included studies were located in emergency room departments and subsequently in hospital, involving newly admitted patients.
5. Interventions
5.1 Aripiprazole IM
Data in this systematic review relate to the 9.75 mg dose of aripiprazole for all the studies (Andrezina 2006; Bristol‐Myers 2005; Kittipeerachon 2016).
5.2 Placebo IM
Both Andrezina 2006 and Bristol‐Myers 2005 used placebo given intramuscularly.
5.3 Haloperidol IM
Dosage varied from 6.5 mg (Bristol‐Myers 2005) to 7.5 mg (Andrezina 2006).
5.4 Olanzapine IM
Dosage was 10 mg (Kittipeerachon 2016).
6. Outcomes
Binary data concerning the repeated need for tranquillisation were available for Andrezina 2006 and Bristol‐Myers 2005 only, since in Kittipeerachon 2016 repeated administration of intervention drugs was prohibited by the methodological design; with respect to adverse effects, binary data were available for all the included trials. All the trials employed continuous scales to measure other clinical outcomes; in Andrezina 2006 and Bristol‐Myers 2005 continuous measurements for adverse effects were presented as means without standard deviations (SDs), standard error (SE) or confidence intervals (CIs), and therefore were unusable for quantitative analyses. The various rating scales, from which we were able to obtain usable data, are listed below:
6.1 Specific behaviour ‐ Agitation
a. Agitated Behavior Scale (ABS/CABS)
The ABS (Corrigan 1989), is a 14‐item scale used for measuring agitation levels. The ABS was originally designed to monitor agitated behaviour in the recovery period after stroke and there are three factor‐based sub scores: I. aggression; II. disinhibition; III. lability. High scores indicated higher levels of aggression.
b. Agitation calmness Evaluation scale (ACES)
The ACES (Breier 2001), is a single‐item rating scale developed by Eli Lilly and company. On this scale, 1 = 'marked agitation', 4 = 'normal', 9 = 'unable to be aroused'.
c. Positive and Negative Syndrome Scale ‐ Excited Component (PANSS‐EC)
The PANSS‐EC (Montoya 2011) is a five‐item subscale of the PANSS scale (excitement, tension, hostility, uncooperativeness, and poor impulse control). As in the original scale, items are rated from one ('not present') to seven ('extremely severe'). Scores range from five to 35, with mean scores ≥ 20 indicating agitation. A high score indicates high levels of agitation.
6.2 Global state
a. Clinical Global Impression (CGI)
The CGl (Guy 1976) is not a diagnostic tool but rather, enables clinicians to quantify the severity of symptoms of any mental health problem at one point in time. Clinicians are then able to use this to track whether there has been any improvement or worsening of symptoms over time. A seven‐point rating scale is used with high scores indicating increased severity or less recovery.
b. Clinical Global Impression ‐ Severity (CGI‐S)
The CGI‐S (Guy 1976) requires clinicians to consider the severity of a person’s symptoms in relation to the clinicians past experience of people with the same diagnosis. Clinicians then have to give a rating from one (= 'normal') to seven (= 'extremely ill'). High scores indicate increased severity.
c. Clinical Global Impression ‐ Improvement (CGI‐I)
The CGI‐I (Guy 1976) enables clinicians to assess whether a person’s symptoms have improved or worsened following an intervention. Based on the clinicians judgement, a rating on a seven‐point scale is given from one (= 'very much improved') to seven (= 'very much worse'). Therefore, low scores indicate greater improvement.
6.3 Mental state
a. Brief Psychiatric Rating Scale (BPRS)
The Brief Psychiatric Rating Scale was originally developed by Overall and Gorham (Overall 1962) as a 14‐item scale to measure the severity of a range of psychiatric symptoms, including psychosis. This rating scale items evolved over time and now consists of 24 items which can be rated on a seven‐point scale from ‘not present’ to ‘extremely severe’. A high score would suggest poor mental health. It is not clear for the majority of the studies included in this review, which version of the BPRS was used.
b. Positive and Negative Syndrome Scale (PANSS)
The PANSS was developed and published by Kay, Flszbein and Opler (Kay 1987). The PANSS is designed as a brief interview, whereby the severity of 30 symptoms of schizophrenia can be assessed on a scale of one ('not present') to seven ('extremely severe'). A high score would indicate more severe symptoms. The PANSS can be divided into separate subscales by focusing on the statements relating to positive symptoms (e.g. hallucinations), negative symptoms (e.g. social withdrawal) or general psychopathology (e.g. anxiety and uncooperativeness).
6.4 Adverse effects
a. Udvalg for Kliniske Undersogelser (UKU) side effects rating scale
The UKU (Lingjaerde 1987) is a questionnaire that divides adverse effects into four general categories of symptoms: psychic, neurologic, autonomic, and miscellaneous; for each adverse effects, the interviewer is asked to assess the probability of causal relationship with psychotropic drugs.
b. Simpson‐Angus Scale (SAS)
The SAS (Simpson 1970) is a 10‐item scale which measures drug‐induced parkinsonism (extrapyramidal side effects). Each item is scored from zero to four. A high score would indicate increased levels of parkinsonism.
7. Missing outcomes
No studies evaluated satisfaction with care, acceptance of treatment, quality of life, or economic outcomes.
8. Funders
Andrezina 2006 and Bristol‐Myers 2005 studies received sponsorship from pharmaceutical companies.
Excluded studies
In total we excluded 16 studies. Of these, seven had to be excluded based on the characteristics of participants. These studies (Kane 2002; Cutler 2006; Fleischhacker 2009; Liang 2005; McEvoy 2007; Potkin 2003; Tandon 2006) were excluded because although participants were suspected to have psychosis, these people were not displaying an aggressive or agitated behaviour. Five studies had to be excluded because they involved oral administration of aripiprazole (Chen 2009; Kinon 2008; Li 2009; Liu 2012; Xie 2011) or the intramuscular administration of aripiprazole long acting (NCT01469039). Three studies (Bao 2007; Simpson 2010; Wang 2014) had to be excluded because the intervention medication was not aripiprazole alone.
Risk of bias in included studies
Although each ’Summary of findings’ table describes bias by outcome, an overview is reported here and a graphical impression is seen in Figure 4 and in Figure 5.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All included studies were described as randomised. In Andrezina 2006, the randomisation process is not stated (unclear sequence generation risk); however, allocation concealment was preserved by the utilisation of a centralised call‐in system (low allocation concealment risk). In Bristol‐Myers 2005 patients were randomly assigned in 1:1:1:1:1:1 ratio to the six arms of the study but no further information was provided regarding the process of randomisation; no details were provided regarding allocation concealment (overall unclear risk of selection bias). In Kittipeerachon 2016, the randomisation process is low risk as it is described as blindly picking the name of the assigned medication from a box with equal numbers of folded papers printed with each medication; allocation concealment management is not stated (unclear risk ).
Blinding
Andrezina 2006 is described as a double‐blinded study but no further details are provided (unclear risk of performance bias; unclear risk of detection bias). In the Bristol‐Myers 2005 study, authors stated double‐blinding but different dilution instructions for drug injections were provided, so no blinding was possible during the preparation of injections; in most cases the same person prepared and administered the drug (high risk of performance bias). To limit the magnitude of this bias, in the efficacy evaluations study investigators were blinded to treatment (low risk of detection bias), giving an overall unclear risk for blinding. In one case of medical emergency, the treating physician broke the blind design as knowledge of investigational product was considered to be critical to the patient management; however, investigators remained blinded. In Kittipeerachon 2016, patients and the physician investigator were blinded to treatment assignment.
Incomplete outcome data
Two included studies used a 'Last Observation Carried Forward' (LOCF) approach to manage missing data. In Andrezina 2006, there was no evidence of attrition bias (low risk of attrition bias). Concerning Bristol‐Myers 2005, reasons for leaving the study early were reported; however, number of participants completing different assessments at the same time point vary and reasons are not given (unclear risk of attrition bias). In Kittipeerachon 2016, procedures to manage missing data are not reported in the method section but no attritions were recorded throughout the trial (low risk of attrition bias).
Selective reporting
In both Andrezina 2006 and Bristol‐Myers 2005, adverse effects were reported only if they occurred in ≥ 5% of participants (high risk of reporting bias). In Kittipeerachon 2016, there was no evidence of reporting bias.
Other potential sources of bias
Both Andrezina 2006 and Bristol‐Myers 2005 were supported by manufacturers of one of the compared drugs (high risk of other bias). Kittipeerachon 2016 had no obvious risk of other bias.
Effects of interventions
See: Summary of findings for the main comparison ARIPIPRAZOLE (IM) compared to PLACEBO/NIL for psychosis‐induced aggression or agitation (rapid tranquillisation); Summary of findings 2 ARIPIPRAZOLE (IM) compared to OTHER ANTIPSYCHOTIC: a. HALOPERIDOL for psychosis‐induced aggression or agitation (rapid tranquillisation); Summary of findings 3 ARIPIPRAZOLE (IM) compared to OTHER ANTIPSYCHOTIC: b. OLANZAPINE for psychosis‐induced aggression or agitation (rapid tranquillisation)
See summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3.
In the following section, the summary statistic for binary outcome results is reported as risk ratio (RR), and for continuous outcomes we used mean difference (MD). In all instances 95% confidence intervals (CIs) are reported.
1. COMPARISON 1. ARIPIPRAZOLE (intramuscular) versus PLACEBO/NIL
1.1 Repeated need for tranquillisation
Overall, significantly fewer participants allocated to aripiprazole needed additional injections during 24 hours, when compared to placebo (2 RCTs, n = 382, RR 0.69, 95% CI 0.56 to 0.85, very low‐quality evidence). Analyses for numbers needing one additional injection showed no differences between groups (1 RCT, n = 119, RR 1.38, 95% CI 0.69 to 2.80) but significantly more people receiving placebo required two additional injections (1 RCT, n = 119, RR 0.36, 95% CI 0.19 to 0.70, Analysis 1.1).
1.2 Specific behaviour ‐ agitation
1.2.1 Clinically important change
Aripiprazole had an effect on agitation. At two hours more participants in the aripiprazole group showed a positive response for agitation (defined as a PANSS ‐ EC reduction of ≥ 40% from baseline, 2 RCTs, n = 382, RR 1.50. 95% CI 1.17 to 1.92, very low‐quality evidenceAnalysis 1.2).
1.2.2 Average scores
Continuous measures of agitation (up to two hours) also favoured aripiprazole: ABS change score (2 RCTs, n = 380, MD ‐3.77, 95% CI ‐5.39 to ‐2.16), ACES change score (2 RCTs, n = 380, MD ‐0.71, 95% CI ‐1.15 to ‐0.28) and PANSS‐EC change score (1 RCT, n = 261, MD ‐2.49, 95% CI ‐4.28 to ‐0.70).
These findings were still favourable for aripiprazole for both a schizophrenia subgroup (2 RCTs, n = 263, MD ‐2.55, 95% CI ‐3.78 to ‐1.32), and a non‐sedated participants subgroup ‐ based on ACES score (2 RCTs, n = 355, MD ‐2.68, 95% CI ‐3.75 to ‐1.62) and on AEs (2 RCTs, n = 365, MD ‐2.83, 95% CI ‐3.92 to ‐1.75, Analysis 1.3).
1.3 Global state
Various
There were fewer non‐responders at first injection in aripiprazole group (1 RCT, n = 263, RR 0.49, 95% CI 0.34 to 0.71,low‐quality evidence) and participants allocated to aripiprazole had a significantly lower need for additional intervention with benzodiazepines (2 RCTs, n = 382, RR 0.48, 95% CI 0.28 to 0.80, Analysis 1.4).
1.3.2 Average scores ‐ up to two hours
Rating scales showed a significant advantage for participants allocated to aripiprazole: CGI‐I endpoint score (2 RCTs, n = 380, MD ‐0.73, 95% CI ‐0.97 to ‐0.49), CGI‐S change score (2 RCTs, n = 380, MD ‐0.56, 95% CI ‐0.86 to ‐0.26). CGI‐I endpoint score was also lower in the aripiprazole group for participants with a schizophrenia diagnosis (1 RCT, n = 75, MD ‐0.71, 95% CI ‐1.17 to ‐0.25, Analysis 1.5).
1.4 Mental state ‐ average scores ‐ up to two hours
Change score of PANSS‐derived BPRS total (1 RCT, n = 110, MD ‐3.39, 95% CI ‐7.03 to 0.25) and positive subscale (1 RCT, n = 110, MD ‐0.62, 95% CI ‐1.65 to 0.41) were similar between aripiprazole and placebo groups (Analysis 1.6).
1.5 Adverse effects
1.5.1 General
More participants allocated to aripiprazole experienced one or more drug‐related adverse effects by two hours (1 RCT, n = 117, RR 1.51, 95% CI 0.93 to 2.46, very low‐quality evidence), and onset or worsening of adverse effects following the second injection (1 RCT, n = 262, RR 2.40, 95% CI 1.36 to 4.23, Analysis 1.7).
Similar findings were found for serious adverse effects, which were higher in the aripiprazole group when compared to placebo group (2 RCTs, n = 379, RR 3.77, 95% CI 0.63 to 22.60); in the aripiprazole group a tonic clonic seizure event occurred (1 RCT, n = 117, RR 3.26, 95% CI 0.14 to 78.49, Analysis 1.8). No deaths were reported.
1.5.2 Specific ‐ arousal
No clear effect was found for other effects such as 'insomnia during 24 hours' (1 RCT, n = 262, RR 0.62, 95% CI 0.25 to 1.52), 'over'‐sedated (1 RCT, n = 262, RR 1.59, 95% CI 0.60 to 4.20) somnolence (2 RCTs, n = 379, RR 1.52, 95% CI 0.57 to 4.00, Analysis 1.9).
1.5.3 Specific ‐ cardiovascular
Again, similar numbers in each treatment group experienced dizziness (2 RCTs, n = 379, RR 1.77, 95% CI 0.66 to 4.70), tachycardia (1 RCT, n = 117, RR 4.36, 95% CI 0.50 to 37.82) during 24 hours and sinus tachycardia (1 RCT, n = 117, RR 0.36, 95% CI 0.02 to 8.72, Analysis 1.10).
1.5.4 Specific ‐ movement disorders
Drug‐related adverse effects were experienced only by participants allocated to aripiprazole during 24 hours but no effect between treatment groups was observed for akathisia (1 RCT, n = 117, RR 7.61, 95% CI 0.40 to 144.21), dystonia (1 RCT, n = 117, RR 3.26, 95% CI 0.14 to 78.49) and extrapyramidal symptoms (1 RCT, n = 262, RR 1.50 95% CI 0.06 to 36.45, Analysis 1.11).
1.5.5 Miscellaneous
More participants allocated to the aripiprazole group experienced nausea during 24 hours (2 RCTs, n = 379, RR 3.97 95% CI 1.13 to 13.92).
Concerning other miscellaneous adverse effects, no observable differences were found: agitation (2 RCTs, n = 379, RR 0.88 95% CI 0.33 to 2.37), headache (2 RCTs, n = 379, RR 1.66 95% CI 0.74 to 3.71), pain at injection site (2 RCTs, n = 379, RR 1.10 95% CI 0.30 to 3.94), and vomiting (1 RCT, n = 117, RR 2.18 95% CI 0.20 to 23.37, Analysis 1.12).
1.6 Leaving the study early
We found no clear difference for numbers of participants leaving the study early for 'any reason' (2 RCTs, n = 382, RR 1.29, 95% CI 0.35 to 4.74); 'lack of efficacy' (2 RCTs, n = 382, RR 0.47, 95% CI 0.07 to 3.06), 'consent withdrawal' (2 RCTs, n = 382, RR 1.68, 95% CI 0.23 to 12.46), 'adverse effects' (2 RCTs, n = 382, RR 2.25, 95% CI 0.25 to 20.54), and 'other' reasons (2 RCTs, n = 382, RR 1.05, 95% CI 0.15 to 7.44, Analysis 1.13).
2. COMPARISON 2. ARIPIPRAZOLE (intramuscular) versus OTHER ANTIPSYCHOTIC: a. HALOPERIDOL (intramuscular)
2.1 Repeated need for tranquillisation
Compared to haloperidol, more participants allocated to aripiprazole needed additional injections during 24 hours (2 RCTs, n = 477, RR 1.28, 95% CI 1.00 to 1.63, very low‐quality evidence). A comparable pattern was found in terms of need of 1 additional injection (1 RCT, n = 117, RR 1.34, 95% CI 0.66 to 2.70) and 2 additional injections (1 RCT, n = 117, RR 2.37, 95% CI 0.77 to 7.26) during 24 hours (Analysis 2.1). Even though not being statistically significant, leading to an equal to higher number of additional injections needed must be taken into account when considering clinical significance.
2.2 Specific behaviour ‐ agitation
2.2.1 Clinically important change
Clinically important change in agitation by two hours (defined as a PANSS‐EC reduction of ≥ 40% from baseline) was similar between groups (2 RCTs, n = 477, RR 0.94, 95% CI 0.80 to 1.11, very low‐quality evidence;Analysis 2.2).
2.2.2 Average scores ‐ up to two hours
Continuous measures of agitation showed similar findings, with no observable differences in ABS change score (2 RCTs, n = 473, MD 0.55, 95% CI ‐1.00 to 2.10), ACES change score (2 RCTs, n = 473, MD 0.12, 95% CI ‐0.30 to 0.55) and PANSS‐EC change score in the total sample (1 RCT, n = 357, MD 0.48, 95% CI ‐1.16 to 2.12), nor in schizophrenia subgroup (2 RCTs, n = 336, MD 0.07, 95% CI ‐0.99 to 1.13), or non‐sedated patients (based on ACES score: 2 RCTs, n = 422, MD 0.12, 95% CI ‐0.82 to 1.06). Based on AEs: 2 RCTs, n = 448, MD 0.26, 95% CI ‐0.68 to 1.19, Analysis 2.3).
2.3 Global state
Similar numbers of participants were 'non‐responders to the first injection (1 RCT, n = 360, RR 1.18, 95% CI 0.78 to 1.79, low‐quality evidence) and similar numbers needed benzodiazepine up to 24 hours (2 RCTs, n = 477, RR 0.79, 95% CI 0.46 to 1.35, Analysis 2.4).
No differences between aripiprazole and haloperidol were observed for global state (as measured by CGI scale): CGI‐I endpoint score for total sample (2 RCTs, n = 473, MD 0.02, 95% CI ‐0.19 to 0.23) and in the schizophrenia subgroup (1 RCT, n = 79, MD 0.02, 95% CI ‐0.44 to 0.48), and CGI‐S change score (2 RCTs, n = 473, MD ‐0.07, 95% CI ‐0.36 to 0.22, Analysis 2.5).
2.4 Mental state
Participants in aripiprazole and haloperidol groups also had similar scores on mental state scales: PANSS‐derived BPRS change score (1 RCT, n = 102, MD 2.03, 95% CI ‐1.70 to 5.76) and in the positive subscale (1 RCT, n = 103, MD 0.23, 95% CI ‐0.82 to 1.28) by two hours (Analysis 2.6).
2.5 Adverse effects
2.5.1 General
The same proportion of participants of aripiprazole and haloperidol group experienced one or more drug‐related adverse effects during 24 hours (1 RCT, n = 113, RR 0.91, 95% CI 0.61 to 1.35, very low‐quality evidence); however, more people allocated to haloperidol experienced onset or worsening of adverse effects after the second injection (1 RCT, n = 358, RR 0.74, 95% CI 0.57 to 0.96, Analysis 2.7).
More adverse effects rated as serious were recorded in the aripiprazole group but this was not an observable difference between the treatments (2 RCTs, n = 471, RR 1.73, 95% CI 0.54 to 5.52), as was 'event of tonic clonic seizure' (1 RCT, n = 113, RR 3.05, 95% CI 0.13 to 73.38). No death events were recorded (Analysis 2.8).
2.5.2 Specific ‐ arousal
More people allocated to haloperidol experienced insomnia during 24 hours (1 RCT, n = 358, RR 0.48, 95% CI 0.23 to 0.97) and, were 'over'‐sedated (1 RCT, n = 358, RR 0.60, 95% CI 0.34 to 1.07); no differences in terms of somnolence were found between aripiprazole and haloperidol groups (2 RCTs, n = 471, RR 0.81, 95% CI 0.41 to 1.58, Analysis 2.9).
2.5.3 Specific ‐ cardiovascular
No real differences were found as regards the number of participants experiencing dizziness (2 RCTs, n = 471, RR 1.41, 95% CI 0.66 to 3.01) and tachycardia (1 RCT, n = 113, RR 4.07, 95% CI 0.47 to 35.31); three events of sinus tachycardia were recorded in the haloperidol group but again, no effect found (1 RCT, n = 113, RR 0.15, 95% CI 0.01 to 2.75, Analysis 2.10).
2.5.4 Specific ‐ movement disorders
Significantly more participants allocated to haloperidol experienced extrapyramidal symptoms (2 RCTs, n = 471, RR 0.29, 95% CI 0.12 to 0.70, very low‐quality evidence); but this effect was not observed for akathisia (1 RCT, n = 113, RR 0.51, 95% CI 0.13 to 1.94) and dystonia (1 RCT, n = 113, RR 0.25, 95% CI 0.03 to 2.21), although more events were recorded in the haloperidol group (Analysis 2.11).
2.5.5 Miscellaneous
A favourable effect for haloperidol was found in terms of number of participants experiencing nausea (2 RCTs, n = 471, RR 5.52, 95% CI 1.63 to 18.70). No effect was found for 'pain at injection site' (2 RCTs, n = 471, RR 3.12, 95% CI 0.75 to 12.91), agitation (2 RCTs, n = 471, RR 1.04 95% CI 0.42 to 2.58), headache (2 RCTs, n = 471, RR 1.16 95% CI 0.62 to 2.18), or vomiting during 24 hours (1 RCT, n = 113, RR 2.04 95% CI 0.19 to 21.82, Analysis 2.12).
2.6 Leaving the study early
No differences in numbers leaving the study early were observed between aripiprazole and haloperidol groups for either any reason (2 RCTs, n = 477, RR 0.49 95% CI 0.20 to 1.18), consent withdrawal (2 RCTs, n = 477, RR 0.35 95% CI 0.10 to 1.28), lack of efficacy (2 RCTs, n = 477, RR 1.05, 95% CI 0.15 to 7.43), adverse effects: 2 RCTs, n = 477, RR 1.06, 95% CI 0.18 to 6.04 or other reason (2 RCTs, n = 477, RR 0.75, 95% CI 0.15 to 3.78, Analysis 2.13).
3. COMPARISON 3. ARIPIPRAZOLE (intramuscular) versus OTHER ANTIPSYCHOTIC: b. OLANZAPINE (intramuscular)
3.1 Specific behaviour ‐ agitation
More participants allocated to olanzapine had an improvement in agitation at two hours (1 RCT, n = 80, RR 0.77, 95% CI 0.60 to 0.99, low‐quality evidence;Analysis 3.1). Continuous measurements showed similar findings, results showing a favourable effect for olanzapine at two hours using ACES change scores (1 RCT, n = 80, MD 0.58, 95% CI 0.10 to 1.06) and PANSS‐EC change scores (1 RCT, n = 80, MD 3.30, 95% CI 1.25 to 5.35, Analysis 3.2).
3.2 Global state
Olanzapine had a favourable effect compared to aripiprazole on global state when measured using CGI‐S change scores (1 RCT, n = 80, MD 0.58, 95% CI 0.01 to 1.15, low‐quality evidence;Analysis 3.3).
3.3 Adverse effects
3.3.1 General
A comparable amount of participants in both groups experienced at least one adverse effect (1 RCT, n = 80, RR 0.75, 95% CI 0.45 to 1.24, very low‐quality evidence;Analysis 3.4). No serious adverse events occurred (Analysis 3.5).
3.3.2 Specific ‐ arousal
Significantly more participants allocated to olanzapine experienced somnolence during 24 hours (1 RCT, n = 80, RR 0.25, 95% CI 0.08 to 0.82, low‐quality evidence; Analysis 3.6).
3.3.3 Specific ‐ cardiovascular
No significant differences were observed for systolic and diastolic pressure change from baseline, and for pulse rate change from baseline, both at two and 24 hours (Analysis 3.7).
3.3.4 Specific ‐ movement disorders
Aripiprazole and olanzapine results were also similar for rigidity (1 RCT, n = 80, RR 1.33, 95% CI 0.32 to 5.58) and tremor (1 RCT, n = 80, RR 1.50, 95% CI 0.26 to 8.50) during 24 hours. None of the participants experienced akathisia or dystonia (Analysis 3.8), with similar continuous ratings for the former both at two hours (1 RCT, n = 80, MD ‐0.05, 95% CI ‐0.26 to 0.16) and at 24 hours (1 RCT, n = 80, MD ‐0.03, 95% CI ‐0.29 to 0.23, Analysis 3.9).
3.3.5 Specific ‐ miscellaneous
Again, no effect was observed when aripiprazole and olanzapine were compared for headache (1 RCT, n = 80, RR 2.00, 95% CI 0.19 to 21.18), nausea (1 RCT, n = 80, RR 3.00, 95% CI 0.13 to 71.51), and pain at injection site (1 RCT, n = 80, RR 2.67, 95% CI 0.76 to 9.33), even though as regards the latter more events occurred in the aripiprazole group (Analysis 3.10).
3.4 Leaving the study early
None of the involved participants left this study early (Analysis 3.11).
Discussion
Summary of main results
1. COMPARISON 1. ARIPIPRAZOLE (intramuscular) versus PLACEBO/NIL
Two studies were included (Andrezina 2006; Bristol‐Myers 2005), with a total number for this comparison of 382 participants (aripiprazole, n = 232; placebo, n = 150); available outcomes were rated of very low‐low quality evidence (summary of findings Table for the main comparison). No data concerning 'tranquillisation or asleep' and economic outcomes were available.
1.1 Repeated need for rapid tranquillisation, agitation
When compared to placebo, fewer people in the aripiprazole group required additional injections (Analysis 1.1). Significantly more participants in the aripiprazole group showed an at least 40% reduction in PANSS‐EC agitation score (Analysis 1.2) and Analysis 1.3 showed higher improvement in agitation over continuous measurements, supporting potential benefits of this drug. However, outcomes for agitation were at two hours, a time point that meets only partially the issue of rapid tranquillisation.
1.2 Global state
More people allocated to placebo failed to respond to the first injection and required additional benzodiazepines over 24 hours (Analysis 1.4); whilst the former statement is based on data from Andrezina 2006 only, the latter comes from the both included studies (Andrezina 2006; Bristol‐Myers 2005). Continuous measurement scales (CGI‐I, CGI‐S) emphasise this outcome, with people in placebo group having worse rating scores (Analysis 1.5).
1.3 Adverse effects ‐ any serious, specific adverse effects
Even though not formerly meeting statistical significance, a higher proportion of participants allocated to aripiprazole experienced one or more drug‐related adverse effects, and after the second injection more problems with adverse effects were recorded compared to people in the placebo group (Analysis 1.7). Adverse effects rated as serious were more common for participants allocated to aripiprazole, despite not meeting statistical significance (Analysis 1.8).
When specific adverse effects were taken into account, a significant difference was observed only for the 'nausea during the 24 hours' outcome, more common in the aripiprazole group (Analysis 1.12); concerning the other adverse effects (insomnia, 'over'‐sedation, somnolence, dizziness, tachycardia, akathisia, dystonia, extrapyramidal symptoms , agitation, headache, pain at injection site, vomiting), recorded events were higher in the aripiprazole group, but statistical significance was not met (Analysis 1.9; Analysis 1.10; Analysis 1.11). However, it is important to consider that in both included studies (Andrezina 2006; Bristol‐Myers 2005), data refer only to adverse effects that occurred in ≥ 5% of people.
2. COMPARISON 2. ARIPIPRAZOLE (intramuscular) versus OTHER ANTIPSYCHOTIC: a. HALOPERIDOL (intramuscular)
Data from a total number of 477 participants were extracted from included studies (Andrezina 2006; Bristol‐Myers 2005, aripiprazole, n = 232; haloperidol, n = 245); the overall quality of the evidence was very low (summary of findings Table 2), suggesting caution in the interpretation of quantitative synthesis data.
2.1 Repeated need for rapid tranquillisation, agitation
Despite having comparable response rates and agitation rating scales at two hours (Analysis 2.2; Analysis 2.3), more participants allocated to aripiprazole required at least one additional injection during 24 hours (Analysis 2.1) when compared to haloperidol. Considering how difficult the management of repeated injections could be in an agitation setting and the potential distress of this for the user in need of tranquillisation, that use of haloperidol necessitates less injections is important. Direct data on tranquillisation were not available.
Despite in both Andrezina 2006 and Bristol‐Myers 2005 it is stated that primary outcomes were recorded at multiple time points before two hours (0.5, 0.75, 1, 1.5 hours for Andrezina 2006, 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75 hours for Bristol‐Myers 2005), no numeric data were available on related publications; two hours is a considerable amount of time after an acute aggressive or agitated event.
2.2 Global state ‐ overall improvement
Proportions of non‐responders to the first injection between aripiprazole and haloperidol groups were comparable (Analysis 2.4), and so were the global outcome measurement scales (Analysis 2.6).
2.3 Adverse effects ‐ any serious, specific adverse effects
Compared groups did not differ in terms of number of people that experienced at least one drug‐related adverse effect (Analysis 2.7); one person in the aripiprazole group had a tonic‐clonic seizure and one in the haloperidol group developed severe dystonia (Analysis 2.8). More participants allocated to haloperidol developed insomnia or, by contrast, were 'over'‐sedated during the 24 hours (Analysis 2.9), whilst nausea was significantly more common in the aripiprazole group (Analysis 2.12); with the exception of drug‐related movement disorders, that tended to be more common with haloperidol (Analysis 2.11), other adverse effects showed no differences between the compared groups (Analysis 2.10).
2.4 Economic outcomes
No data concerning economic outcomes were available in the two included studies.
3. COMPARISON 3: ARIPIPRAZOLE (intramuscular) versus OTHER ANTIPSYCHOTIC: b. OLANZAPINE (intramuscular)
One study was included (Kittipeerachon 2016) with a total number of 80 participants (aripiprazole, n = 40; olanzapine, n = 40); outcomes were rated being of very low to low quality (summary of findings Table 3). No data concerning 'tranquillisation or asleep' and economic outcomes were available.
3.1 Agitation
As for participants allocated to aripiprazole, a significantly minor PANSS (Excited Component (PEC) response rate by two hours was observed (Analysis 3.1). Nevertheless, there are no outcomes available before two hours.
3.2 Global state ‐ overall improvement
A significantly minor CGI‐S change score was observed for aripiprazole when compared to olanzapine, at two hours (Analysis 3.3).
3.3 Adverse effects ‐ any serious, specific adverse effects
Compared groups did not differ in terms of number of people who experienced one or more drug‐related adverse effects (Analysis 3.4); no adverse events were rated as serious in both groups (Analysis 3.5). Aripiprazole and olanzapine results were comparable for all the investigated adverse events (Analysis 3.7; Analysis 3.8; Analysis 3.9; Analysis 3.10), with the exception of somnolence, which was more common in participants allocated to olanzapine (Analysis 3.6). Somnolence data at two hours could help to clarify if this issue could be a major PEC response rate of olanzapine.
2.4 Economic outcomes
No data concerning economic outcomes were available.
Overall completeness and applicability of evidence
1. Completeness
Data on several important questions are missing: satisfaction with care, acceptance of treatment, quality of life and economic outcomes. Current evidence‐based knowledge on aripiprazole in agitation is limited by the paucity of randomised controlled trials (RCTs) and low quality of available data, thus suggesting strong caution in the interpretation of results.
2. Applicability
Two out of three of the currently available studies were undertaken in circumstances quite different from real clinical practice: involved participants constituted a specific population of voluntarily hospitalised patients that, while meeting specific agitation criteria for enrolment, "their level of agitation was not so pronounced that they could not give informed consent or participate in the study" (Andrezina 2006): this appears to be in line with the inclusion criteria applied in the two trials (Andrezina 2006; Bristol‐Myers 2005), in which a PEC score ≥ 15 but ≤ 32 was requested. Participants in the included studies had their intervention delayed for screening and until deemed competent to give informed consent, a situation that is not common in the heat of an emergency and makes applicability problematic even if it is not impossible; it just necessitates a level of conjecture that could have been lessened had more pragmatic trials been available.
While in Kittipeerachon 2016, a more pragmatic methodological design can be observed, generalisability of the outcomes is partly limited by the contrast between the lack of outcomes prior to two hours and the administration of a 'treatment as usual' during the 24 hours.
Quality of the evidence
This review includes three trials completed over the last decade with a total sample size of 885 participants with schizophrenia, schizoaffective or schizophreniform disorder, of which 707 were included for quantitative analyses in this systematic review. Disappointingly, a proportion of interesting data were unusable, which could have been avoided had the trials been better reported. Overall, the majority of the evidence had to be graded as very low, usually because of considerable ‐ and often avoidable ‐ limits in research design, selective reporting and imprecision.
Potential biases in the review process
There are a number of ways in which bias could have been introduced into this review.
1. Trial selection and data extraction
For Cochrane reviews, at least two review authors independently inspect all citations obtained from the search and extract the data to minimise bias with a third completing a random sample check. This was achieved in the 2014 search but for the 2017 search only one review author inspected the search results and extracted data. However, the number of citations found in this search was low and in addition, to limit the possibility of this bias, screening obtained from the search was performed on two different occasions and compared.
2. Review author conflict of interest
No conflict of interest is declared by the review authors.
Agreements and disagreements with other studies or reviews
The authors are not aware of any other studies or reviews that have focused solely on use of aripiprazole IM alone for people who are aggressive secondary to serious mental illnesses.

Study flow diagram (2014 search).

Study flow diagram (2017 search).

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 ARIPIPRAZOLE (intramuscular) vs PLACEBO/NIL, Outcome 1 Repeated need for tranquillisation.

Comparison 1 ARIPIPRAZOLE (intramuscular) vs PLACEBO/NIL, Outcome 2 Specific behaviour: 1a. Agitation ‐ clinically important change (PANSS‐EC reduction ≥ 40% from baseline).

Comparison 1 ARIPIPRAZOLE (intramuscular) vs PLACEBO/NIL, Outcome 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 2 hours.

Comparison 1 ARIPIPRAZOLE (intramuscular) vs PLACEBO/NIL, Outcome 4 Global state: 1. Various.

Comparison 1 ARIPIPRAZOLE (intramuscular) vs PLACEBO/NIL, Outcome 5 Global state: 2. Average scores ‐ i. up to 2 hours.

Comparison 1 ARIPIPRAZOLE (intramuscular) vs PLACEBO/NIL, Outcome 6 Mental state: 1. Average scores ‐ i. up to 2 hours.

Comparison 1 ARIPIPRAZOLE (intramuscular) vs PLACEBO/NIL, Outcome 7 Adverse effects: 1a. General.

Comparison 1 ARIPIPRAZOLE (intramuscular) vs PLACEBO/NIL, Outcome 8 Adverse effects: 1b. General ‐ serious.

Comparison 1 ARIPIPRAZOLE (intramuscular) vs PLACEBO/NIL, Outcome 9 Adverse effects: 2a. Specific ‐ arousal.

Comparison 1 ARIPIPRAZOLE (intramuscular) vs PLACEBO/NIL, Outcome 10 Adverse effects: 2b. Specific ‐ cardiovascular.

Comparison 1 ARIPIPRAZOLE (intramuscular) vs PLACEBO/NIL, Outcome 11 Adverse effects: 2c. Specific ‐ movement disorders.

Comparison 1 ARIPIPRAZOLE (intramuscular) vs PLACEBO/NIL, Outcome 12 Adverse effects: 2d. Specific ‐ miscellaneous.

Comparison 1 ARIPIPRAZOLE (intramuscular) vs PLACEBO/NIL, Outcome 13 Leaving the study early.

Comparison 2 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL (intramuscular), Outcome 1 Repeated need for tranquillisation.

Comparison 2 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL (intramuscular), Outcome 2 Specific behaviour: 1a. Agitation ‐ clinically important change (PANSS‐ EC reduction ≥ 40% from baseline).

Comparison 2 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL (intramuscular), Outcome 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 2 hours.

Comparison 2 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL (intramuscular), Outcome 4 Global state: 1. Various.

Comparison 2 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL (intramuscular), Outcome 5 Global outcome: 2. Average scores ‐ i. up to 2 hours.

Comparison 2 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL (intramuscular), Outcome 6 Mental state: 1. Average scores ‐ i. up to 2 hours.

Comparison 2 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL (intramuscular), Outcome 7 Adverse effects: 1a. General.

Comparison 2 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL (intramuscular), Outcome 8 Adverse effects: 1b. General ‐ serious.

Comparison 2 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL (intramuscular), Outcome 9 Adverse effects: 2a. Specific ‐ arousal.

Comparison 2 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL (intramuscular), Outcome 10 Adverse effects: 2b. Specific ‐ cardiovascular.

Comparison 2 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL (intramuscular), Outcome 11 Adverse effects: 2c. Specific ‐ movement disorders.

Comparison 2 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL (intramuscular), Outcome 12 Adverse effects: 2d. Specific ‐ miscellaneous.

Comparison 2 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: a. HALOPERIDOL (intramuscular), Outcome 13 Leaving the study early.

Comparison 3 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: b. OLANZAPINE (intramuscular), Outcome 1 Specific behaviour: 1a. Agitation ‐ clinically important change (PANSS‐ EC reduction ≥ 40% from baseline).

Comparison 3 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: b. OLANZAPINE (intramuscular), Outcome 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 2 hours.

Comparison 3 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: b. OLANZAPINE (intramuscular), Outcome 3 Global state: 1. Average scores ‐ i. up to 2 hours.

Comparison 3 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: b. OLANZAPINE (intramuscular), Outcome 4 Adverse effects: 1a. General.
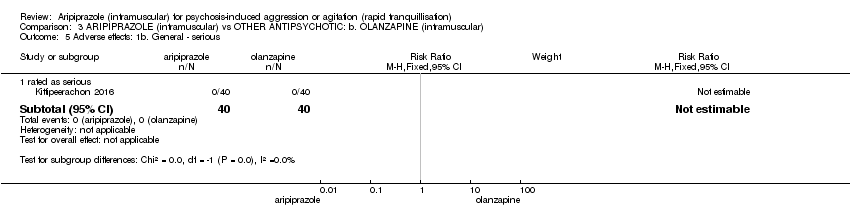
Comparison 3 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: b. OLANZAPINE (intramuscular), Outcome 5 Adverse effects: 1b. General ‐ serious.

Comparison 3 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: b. OLANZAPINE (intramuscular), Outcome 6 Adverse effects: 2a. Specific ‐ arousal.

Comparison 3 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: b. OLANZAPINE (intramuscular), Outcome 7 Adverse effects: 2b. Specific ‐ cardiovascular.

Comparison 3 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: b. OLANZAPINE (intramuscular), Outcome 8 Adverse effects: 2c. Specific ‐ movement disorders ‐ i. dichotomous.

Comparison 3 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: b. OLANZAPINE (intramuscular), Outcome 9 Adverse effects: 2d. Specific ‐ movement disorders ‐ ii. average scores.

Comparison 3 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: b. OLANZAPINE (intramuscular), Outcome 10 Adverse effects: 2e. Specific ‐ miscellaneous.

Comparison 3 ARIPIPRAZOLE (intramuscular) vs OTHER ANTIPSYCHOTIC: b. OLANZAPINE (intramuscular), Outcome 11 Leaving the study early.
| Methods | Allocation: randomised, clearly described, concealed. |
| Participants | Diagnosis: thought to be psychoses. History: acutely ill, aggressive and/or agitated. |
| Interventions | 1. Aripiprazole IM: dose flexible within recommended limits. N = 150. |
| Outcomes | All outcomes are grouped by time: by 30 minutes, up to two hours, up to four hours, up to 24 hours, and over 24 hours. Tranquillisation or asleep ‐ tranquil; asleep. Repeated need for tranquillisation ‐ needing additional injections. Quality of life outcomes. |
| Notes | * Powered to be able to identify a difference of ˜20% between groups for primary outcome with adequate degree of certainty |
| IM: intramuscular | |
| Aripiprazole | Combination | Comparison #1 | Comparison #2 | Excluded study | Suggested title of review | Cochrane review | |
| IM | Short acting | + quetiapine | aripiprazole + olanzapine | quetiapine + olanzapine | Antipsychotic combinations for psychosis‐induced aggression or agitation (rapid tranquillisation) | None | |
| Long acting (441mg) | Long acting (882 mg) | placebo | Aripiprazole for long‐term aggression in psychosis; Placebo for long‐term aggression in psychosis | None | |||
| Oral | aripiprazole + magnesium valproate | Magnesium valproate (+/‐aripiprazole) for psychosis‐induced aggression | None | ||||
| oral olanzapine | Aripiprazole (oral) for psychosis‐induced aggression or agitation (rapid tranquillisation); Olanzapine (oral) for psychosis‐induced aggression or agitation (rapid tranquillisation); | ? expansion of this review | |||||
| + clonazepam | oral clozapine | Aripiprazole plus benzodiazepines for psychosis‐induced aggression or agitation (rapid tranquillisation); Clozapine for psychosis‐induced aggression or agitation (rapid tranquillisation); | ? expansion of this review; Toal 2012 | ||||
| Non‐aripiprazole | |||||||
| IM haloperidol + lorazepam + benztropine | oral quetiapine | Benzodiazepines for psychosis‐induced aggression or agitation (rapid tranquillisation); Haloperidol for psychosis‐induced aggression or agitation (rapid tranquillisation); Quetiapine for psychosis‐induced aggression or agitation (rapid tranquillisation) | |||||
| * One sub‐study with people with aggression/agitation. | |||||||
| ARIPIPRAZOLE compared to PLACEBO/NIL for psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Patient or population: psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with PLACEBO/NIL | Risk with ARIPIPRAZOLE | |||||
| Tranquillisation or asleep | Not reported | |||||
| Repeated need for tranquillisation ‐ needing additional injections during 24 hours | Low | RR 0.69 | 382 | ⊕⊝⊝⊝ | ||
| 250 per 1.000 | 173 per 1.000 | |||||
| Moderate | ||||||
| 600 per 1.000 | 414 per 1.000 | |||||
| High | ||||||
| 750 per 1.000 | 518 per 1.000 | |||||
| Specific behaviour: Agitation ‐ clinically important change (PANSS ‐EC reduction ≥ 40% from baseline) ‐ up to 2 hours | Low | RR 1.50 | 382 | ⊕⊝⊝⊝ | ||
| 100 per 1.000 | 150 per 1.000 | |||||
| Moderate | ||||||
| 350 per 1.000 | 525 per 1.000 | |||||
| High | ||||||
| 700 per 1.000 | 1000 per 1.000 | |||||
| Global state: non‐responders to the first injection | Low | RR 0.49 | 263 | ⊕⊕⊝⊝ | ||
| 200 per 1.000 | 98 per 1.000 | |||||
| Moderate | ||||||
| 450 per 1.000 | 221 per 1.000 | |||||
| High | ||||||
| 700 per 1.000 | 343 per 1.000 | |||||
| Adverse effects: one or more adverse events during 24 hours (only reported if occurred in ≥ 5% of people) | Study population | RR 1.51 | 117 | ⊕⊝⊝⊝ | ||
| 295 per 1.000 | 446 per 1.000 | |||||
| Economic outcomes | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'serious' (downgraded by 1) ‐ randomisation procedure is not reported for both the included studies, and allocation concealment procedure is not consistently reported in Bristol‐Myers 2005. While for the 'randomisation' bias, studies are at least reported as 'randomised', as for the latter that allocation concealment was correctly handled could not be implied. 2 Indirectness: rated 'very serious' (downgraded by 2) ‐ participants included in the studies had levels of agitation not so pronounced by inclusion criteria, potentially under‐estimating or more likely over‐estimating true effectiveness. | ||||||
| ARIPIPRAZOLE compared to OTHER ANTIPSYCHOTIC: a. HALOPERIDOL for psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Patient or population: psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTIC: a. HALOPERIDOL | Risk with ARIPIPRAZOLE | |||||
| Tranquillisation or asleep | Not reported | |||||
| Repeated need for tranquillisation ‐ needing additional injections during 24 hours | Low | RR 1.28 | 477 | ⊕⊝⊝⊝ | , | |
| 100 per 1.000 | 128 per 1.000 | |||||
| Moderate | ||||||
| 300 per 1.000 | 384 per 1.000 | |||||
| High | ||||||
| 500 per 1.000 | 640 per 1.000 | |||||
| Specific behaviour: Agitation ‐ clinically important change (PANSS ‐EC reduction ≥ 40% from baseline) ‐ up to 2 hours | Study population | RR 0.94 | 477 | ⊕⊝⊝⊝ | ||
| 576 per 1.000 | 541 per 1.000 | |||||
| Global state: non‐responders to the first injection | Study population | RR 1.18 | 360 | ⊕⊕⊝⊝ | ||
| 184 per 1.000 | 217 per 1.000 | |||||
| Adverse effects: one or more adverse events during 24 hours (only reported if occurred in ≥ 5% of people) | Study population | RR 0.91 | 113 | ⊕⊝⊝⊝ | ||
| 491 per 1.000 | 447 per 1.000 | |||||
| Adverse effects: movement disorders ‐ EPS during 24 hours (only reported if occurred in ≥ 5% of people) | Low | RR 0.29 | 471 | ⊕⊝⊝⊝ | ||
| 50 per 1.000 | 14 per 1.000 | |||||
| Moderate | ||||||
| 100 per 1.000 | 29 per 1.000 | |||||
| High | ||||||
| 300 per 1.000 | 87 per 1.000 | |||||
| Economic outcomes | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'serious' (downgraded by 1) ‐ randomisation procedure is not reported for both the included studies, and allocation concealment procedure is not consistently reported in Bristol‐Myers 2005. While for the 'randomisation' bias studies are at least reported as 'randomised', as for the latter that allocation concealment was correctly handled could not be implied. 2 Indirectness: rated 'very serious' (downgraded by 2) ‐ participants included in the studies had levels of agitation not so pronounced by inclusion criteria, potentially under‐estimating or more likely over‐estimating true effectiveness. | ||||||
| ARIPIPRAZOLE compared to OTHER ANTIPSYCHOTIC: b. OLANZAPINE for psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Patient or population: psychosis‐induced aggression or agitation (rapid tranquillisation) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER ANTIPSYCHOTIC: b. OLANZAPINE | Risk with ARIPIPRAZOLE | |||||
| Tranquillisation or asleep | Not reported | |||||
| Repeated need for tranquillisation | Not reported | |||||
| Specific behaviour: Agitation ‐ clinically important change (PANSS ‐EC reduction ≥ 40% from baseline) ‐ up to 2 hours | Low | RR 0.77 | 80 | ⊕⊕⊝⊝ | ||
| 500 per 1.000 | 385 per 1.000 | |||||
| Moderate | ||||||
| 800 per 1.000 | 616 per 1.000 | |||||
| High | ||||||
| 900 per 1.000 | 693 per 1.000 | |||||
| Global state: CGI‐S change score up to 2 hours | MD 0.58 | ‐ | 80 | ⊕⊕⊝⊝ | ||
| Adverse effects: one or more adverse effects during 24 hours | Study population | RR 0.75 | 80 | ⊕⊝⊝⊝ | ||
| 500 per 1.000 | 375 per 1.000 | |||||
| Adverse effects: somnolence during 24 hours | Low | RR 0.25 | 80 | ⊕⊕⊝⊝ | ||
| 100 per 1.000 | 25 per 1.000 | |||||
| Moderate | ||||||
| 300 per 1.000 | 75 per 1.000 | |||||
| High | ||||||
| 700 per 1.000 | 175 per 1.000 | |||||
| Economic outcomes | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Imprecision ‐ rated 'serious' (downgraded by 1): Optimal Information Size (OIS) criterion is not met meaning that imprecision of effect estimates could not be properly handled. 2 Imprecision ‐ rated 'very serious' (downgraded by 2): Optimal Information Size (OIS) criterion is not met and CI overlaps no effect. High imprecision of effect estimates could not be properly excluded. 3 Indirectness ‐ rated 'serious' (downgraded by 1): attribution to the intervention drugs is suspected but can not be confirmed since in the study results it is showed that almost all the participants were administered with 'treatment as usual' drugs. | ||||||
| Focus of review | Reference |
| Completed and maintained reviews | |
| 'As required' medication regimens for seriously mentally ill people in hospital | |
| Benzodiazepines for psychosis‐induced aggression or agitation | |
| Chlorpromazine for psychosis‐induced aggression or agitation | |
| Clotiapine for acute psychotic illnesses | |
| Containment strategies for people with serious mental illness | |
| De‐escalation techniques for psychosis‐induced aggression | |
| Droperidol for acute psychosis | |
| Haloperidol for long‐term aggression in psychosis | |
| Haloperidol for psychosis‐induced aggression or agitation (rapid tranquillisation) | |
| Haloperidol plus promethazine for psychosis‐induced aggression | |
| Olanzapine IM or velotab for acutely disturbed/agitated people with suspected serious mental illnesses | |
| Seclusion and restraint for serious mental illnesses | |
| Zuclopenthixol acetate for acute schizophrenia and similar serious mental illnesses | |
| Reviews in the process of being completed | |
| Risperidone for psychosis‐induced aggression or agitation | |
| Loxapine inhaler for psychosis‐induced aggression | |
| Clozapine for people with psychosis‐induced aggression or agitation | |
| Quetiapine for psychosis‐induced aggression or agitation | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Repeated need for tranquillisation Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 needing additional injections during 24 hours | 2 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.56, 0.85] |
| 1.2 needing 1 additional injection during 24 hours | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.69, 2.80] |
| 1.3 needing 2 additional injections during 24 hours | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.19, 0.70] |
| 2 Specific behaviour: 1a. Agitation ‐ clinically important change (PANSS‐EC reduction ≥ 40% from baseline) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 up to 2 hours | 2 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.17, 1.92] |
| 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 change score (ABS, high=worse) | 2 | 380 | Mean Difference (IV, Fixed, 95% CI) | ‐3.77 [‐5.39, ‐2.16] |
| 3.2 change score (ACES, low=agitated, high=sedated) | 2 | 380 | Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.15, ‐0.28] |
| 3.3 change score (PANSS‐EC, high=worse) | 1 | 261 | Mean Difference (IV, Fixed, 95% CI) | ‐2.49 [‐4.28, ‐0.70] |
| 3.4 change score (PANSS‐EC, high=worse, schizophrenia subgroup) | 2 | 263 | Mean Difference (IV, Fixed, 95% CI) | ‐2.55 [‐3.78, ‐1.32] |
| 3.5 change score (PANSS‐EC, high=worse, non‐sedated patients subgroup based on ACES) | 2 | 355 | Mean Difference (IV, Fixed, 95% CI) | ‐2.68 [‐3.75, ‐1.62] |
| 3.6 change score (PANSS‐EC, high=worse, non‐sedated patients subgroup based on AEs) | 2 | 365 | Mean Difference (IV, Fixed, 95% CI) | ‐2.83 [‐3.92, ‐1.75] |
| 4 Global state: 1. Various Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 non‐responders to the first injection | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.34, 0.71] |
| 4.2 need for benzodiazepine up to 24 hours | 2 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.28, 0.80] |
| 5 Global state: 2. Average scores ‐ i. up to 2 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 endpoint score (CGI‐I, high=worse) | 2 | 380 | Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐0.97, ‐0.49] |
| 5.2 endpoint score (CGI‐I, high=worse, schizophrenia subgroup | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.17, ‐0.25] |
| 5.3 change score (CGI‐S, high=worse) | 2 | 380 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐0.86, ‐0.26] |
| 6 Mental state: 1. Average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 change score up to 2 hours (BPRS total, high=worse) | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐3.39 [‐7.03, 0.25] |
| 6.2 change score up to 2 hours (BPRS positive subscale, high=worse) | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐1.65, 0.41] |
| 7 Adverse effects: 1a. General Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 one or more drug related adverse events during 24 hours | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.93, 2.46] |
| 7.2 onset or increased severity of adverse effects after 2nd injection | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [1.36, 4.23] |
| 8 Adverse effects: 1b. General ‐ serious Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 death | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 rated as serious | 2 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.77 [0.63, 22.60] |
| 8.3 tonic clonic seizure | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.14, 78.49] |
| 9 Adverse effects: 2a. Specific ‐ arousal Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 insomnia during 24 hours | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.25, 1.52] |
| 9.2 "over" sedated during 24 hours | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.60, 4.20] |
| 9.3 somnolence during 24 hours | 2 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.57, 4.00] |
| 10 Adverse effects: 2b. Specific ‐ cardiovascular Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 dizziness during 24 hours | 2 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.66, 4.70] |
| 10.2 sinus tachycardia during 24 hours | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.72] |
| 10.3 tachycardia during 24 hours | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.36 [0.50, 37.82] |
| 11 Adverse effects: 2c. Specific ‐ movement disorders Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 akathisia during 24 hours | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.61 [0.40, 144.21] |
| 11.2 dystonia during 24 hours | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.14, 78.49] |
| 11.3 EPS during 24 hours | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.06, 36.45] |
| 12 Adverse effects: 2d. Specific ‐ miscellaneous Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 agitation during 24 hours | 2 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.33, 2.37] |
| 12.2 headache during 24 hours | 2 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.74, 3.71] |
| 12.3 pain at injection site | 2 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.30, 3.94] |
| 12.4 nausea during 24 hours | 2 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.97 [1.13, 13.92] |
| 12.5 vomiting during 24 hours | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.20, 23.37] |
| 13 Leaving the study early Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 any reason | 2 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.35, 4.74] |
| 13.2 lack of efficacy | 2 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.07, 3.06] |
| 13.3 withdrew consent | 2 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.23, 12.46] |
| 13.4 adverse effects | 2 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.25, 20.54] |
| 13.5 other | 2 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.15, 7.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Repeated need for tranquillisation Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 needing additional injections during 24 hours | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.00, 1.63] |
| 1.2 needing 1 additional injection during 24 hours | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.66, 2.70] |
| 1.3 needing 2 additional injections during 24 hours | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.77, 7.26] |
| 2 Specific behaviour: 1a. Agitation ‐ clinically important change (PANSS‐ EC reduction ≥ 40% from baseline) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 up to 2 hours | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.11] |
| 3 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 change score (ABS, high=worse) | 2 | 473 | Mean Difference (IV, Fixed, 95% CI) | 0.55 [‐1.00, 2.10] |
| 3.2 change score (ACES, low=agitated, high=sedated) | 2 | 473 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.30, 0.55] |
| 3.3 change score (PANSS‐EC, high=worse) | 1 | 357 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [‐1.16, 2.12] |
| 3.4 change score (PANSS‐EC, high=worse, schizophrenia subgroup) | 2 | 336 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.99, 1.13] |
| 3.5 change score (PANSS‐EC, high=worse, non‐sedated patients subgroup based on ACES) | 2 | 422 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.82, 1.06] |
| 3.6 change score (PANSS‐EC, high=worse, non‐sedated patients subgroup based on AEs) | 2 | 448 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.68, 1.19] |
| 4 Global state: 1. Various Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 non‐responders to the first injection | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.78, 1.79] |
| 4.2 need for benzodiazepine up to 24 hours | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.46, 1.35] |
| 5 Global outcome: 2. Average scores ‐ i. up to 2 hours Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 endpoint score (CGI‐I, high=worse) | 2 | 473 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.19, 0.23] |
| 5.2 endpoint score (CGI‐I, high=worse, schizophrenia subgroup | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.44, 0.48] |
| 5.3 change score (CGI‐S, high=worse) | 2 | 473 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.36, 0.22] |
| 6 Mental state: 1. Average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 change score up to 2 hours (BPRS total, high=worse) | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | 2.03 [‐1.70, 5.76] |
| 6.2 change score up to 2 hours (BPRS positive subscale, high=worse) | 1 | 103 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.82, 1.28] |
| 7 Adverse effects: 1a. General Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 one or more drug related adverse events during 24 hours | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.61, 1.35] |
| 7.2 onset or increased severity of adverse effects after 2nd injection | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.57, 0.96] |
| 8 Adverse effects: 1b. General ‐ serious Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 death | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 rated as serious | 2 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.54, 5.52] |
| 8.3 tonic clonic seizure | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.13, 73.38] |
| 9 Adverse effects: 2a. Specific ‐ arousal Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 insomnia during 24 hours | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.23, 0.97] |
| 9.2 "over" sedated during 24 hours | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.34, 1.07] |
| 9.3 somnolence during 24 hours | 2 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.41, 1.58] |
| 10 Adverse effects: 2b. Specific ‐ cardiovascular Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 dizziness during 24 hours | 2 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.66, 3.01] |
| 10.2 sinus tachycardia during 24 hours | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.75] |
| 10.3 tachycardia during 24 hours | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.07 [0.47, 35.31] |
| 11 Adverse effects: 2c. Specific ‐ movement disorders Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 akathisia during 24 hours | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.13, 1.94] |
| 11.2 dystonia during 24 hours | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.21] |
| 11.3 EPS during 24 hours | 2 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.12, 0.70] |
| 12 Adverse effects: 2d. Specific ‐ miscellaneous Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 agitation during 24 hours | 2 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.42, 2.58] |
| 12.2 headache during 24 hours | 2 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.62, 2.18] |
| 12.3 pain at injection site | 2 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.75, 12.91] |
| 12.4 nausea during 24 hours | 2 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.52 [1.63, 18.70] |
| 12.5 vomiting during 24 hours | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.19, 21.82] |
| 13 Leaving the study early Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 any reason | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.20, 1.18] |
| 13.2 lack of efficacy | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.15, 7.43] |
| 13.3 withdrew consent | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.10, 1.28] |
| 13.4 adverse effects | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.18, 6.04] |
| 13.5 other | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.15, 3.78] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Specific behaviour: 1a. Agitation ‐ clinically important change (PANSS‐ EC reduction ≥ 40% from baseline) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 up to 2 hours | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.60, 0.99] |
| 2 Specific behaviour: 1b. Agitation ‐ average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 change score (ACES, low=agitated, high=sedated) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.58 [0.10, 1.06] |
| 2.2 change score (PANSS‐EC, high=worse) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 3.30 [1.25, 5.35] |
| 3 Global state: 1. Average scores ‐ i. up to 2 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 change score (CGI‐S, high=worse) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.58 [0.01, 1.15] |
| 4 Adverse effects: 1a. General Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 one or more drug related adverse events during 24 hours | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.45, 1.24] |
| 5 Adverse effects: 1b. General ‐ serious Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 rated as serious | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Adverse effects: 2a. Specific ‐ arousal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 somnolence during 24 hours | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.08, 0.82] |
| 7 Adverse effects: 2b. Specific ‐ cardiovascular Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 systolic blood pressure change up to 2 hours | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 2.85 [‐6.77, 12.47] |
| 7.2 diastolic blood pressure change up to 2 hours | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.05 [‐8.09, 5.99] |
| 7.3 pulse rate change up to 2 hours | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐6.80, 5.46] |
| 7.4 systolic blood pressure change up to 24 hours | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 3.37 [‐5.43, 12.17] |
| 7.5 diastolic blood pressure change up to 24 hours | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐8.55, 5.95] |
| 7.6 pulse rate change up to 24 hours | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐6.05 [‐13.43, 1.33] |
| 8 Adverse effects: 2c. Specific ‐ movement disorders ‐ i. dichotomous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 akathisia up to 24 hours | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 dystonia up to 24 hours | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 rigidity up to 24 hours | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.32, 5.58] |
| 8.4 tremor up to 24 hours | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.26, 8.50] |
| 9 Adverse effects: 2d. Specific ‐ movement disorders ‐ ii. average scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 SAS change score up to 2 hours (high=worse) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.26, 0.16] |
| 9.2 SAS change score up to 24 hours (high=worse) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.29, 0.23] |
| 10 Adverse effects: 2e. Specific ‐ miscellaneous Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 headache during 24 hours | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| 10.2 pain at injection site during 24 hours | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.76, 9.33] |
| 10.3 nausea during 24 hours | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.51] |
| 11 Leaving the study early Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |

