Tratamiento tópico para las quemaduras faciales
Resumen
Antecedentes
Las lesiones por quemaduras son un problema de salud importante. Se producen con frecuencia en la región de la cabeza y el cuello. El rostro es el área central de la identidad de una persona que proporciona nuestro medio de comunicación más expresivo. Las intervenciones tópicas son actualmente la piedra angular del tratamiento de las quemaduras de la cara.
Objetivos
Evaluar los efectos de las intervenciones tópicas sobre la cicatrización de las heridas en personas con quemaduras faciales de cualquier profundidad.
Métodos de búsqueda
En diciembre de 2019, se hicieron búsquedas en el registro especializado del Grupo Cochrane de Heridas (Cochrane Wounds Specialised Register), en el Registro Cochrane Central de Ensayos Controlados (CENTRAL); Ovid MEDLINE (incluido In‐Process & Other Non‐Indexed Citations); Ovid Embase y EBSCO CINAHL Plus. También se buscaron en los registros de ensayos clínicos estudios en curso y no publicados, y se examinaron las listas de referencias de los estudios incluidos pertinentes, así como las revisiones, los metanálisis e informes de tecnología sanitaria para identificar estudios adicionales. No hubo restricciones en cuanto al idioma, la fecha de publicación ni el contexto de los estudios.
Criterios de selección
Fueron aptos para la inclusión en esta revisión los ensayos controlados aleatorizados (ECA) que evaluaran los efectos del tratamiento tópico para las quemaduras faciales.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, realizaron la selección de los estudios, la extracción de los datos, la evaluación del «riesgo de sesgo», y la evaluación de la certeza de la evidencia según los criterios GRADE.
Resultados principales
En esta primera actualización, se incluyeron 12 ECA con 507 participantes.
La mayoría de los ensayos incluyó adultos ingresados en centros especializados en quemaduras después de lesiones recientes por quemaduras.
Los agentes tópicos incluían agentes antimicrobianos (sulfadiazina de plata, Aquacel‐Ag, cerio‐sulfadiazina, crema de gentamicina, crema de acetato de mafenida, bacitracina), agentes no antimicrobianos (pomada húmeda para quemaduras expuestas (Moist Exposed Burn Ointment, MEBO)), apósitos salinos, sustitutos dérmicos (incluido el sustituto dérmico por bioingeniería (TransCyte)), el aloinjerto y el xenoinjerto (Porcine Xenoderm), y tratamientos varios (terapia con hormonas de crecimiento, hidrogel de factor estimulante de colonias de granulocitos‐macrófagos humanos recombinados (rhGMCS)), desbridamiento enzimático y crema con extracto de Helix Aspersa).
Casi toda la evidencia incluida en esta revisión se calificó como de certeza baja o muy baja, a menudo debido al riesgo de sesgo alto por procedimientos de aleatorización poco claros (es decir, generación de secuencias y ocultación de la asignación), a la falta de cegamiento de los participantes, proveedores y a veces, los evaluadores de los desenlaces; y a la imprecisión por el bajo número de participantes, tasas de eventos bajas o ambos, con frecuencia en estudios individuales.
Agentes antimicrobianos tópicos versus agentes no antimicrobianos tópicos
Hay evidencia de certeza moderada de que probablemente haya poca o ninguna diferencia entre los agentes antimicrobianos y los agentes no antimicrobianos (sulfadiazina de plata y MEBO) en cuanto al tiempo necesario para completar la cicatrización de las heridas (cociente de riesgos instantáneos (CRI) 0,84 (intervalo de confianza (IC) del 95%: 0,78 a 1,85; un estudio, 39 participantes). Los agentes antimicrobianos tópicos pueden suponer poca o ninguna diferencia en la proporción de heridas completamente curadas en comparación con los agentes no antimicrobianos tópicos (comparación sulfadiazina de plata y MEBO, riesgo relativo (RR) 0,94, IC del 95%: 0,68 a 1,29; un estudio, 39 participantes; evidencia de certeza baja). No se sabe con certeza si hay alguna diferencia en la infección de la herida (comparación entre el agente antimicrobiano tópico (Aquacel‐Ag) y el MEBO; RR 0,38, IC del 95%: 0,12 a 1,21; un estudio, 40 participantes; evidencia de certeza muy baja). Ningún ensayo informó de cambios en la superficie de la herida con el tiempo o de la curación parcial de la misma. Hay evidencia de certeza baja en cuanto a los desenlaces secundarios, la calidad de las cicatrices y la satisfacción del paciente. Dos estudios evaluaron el dolor, pero se informó sobre él de manera incompleta.
Agentes antimicrobianos tópicos versus otros agentes antimicrobianos tópicos
No es seguro que los agentes antimicrobianos tópicos marquen alguna diferencia en los efectos, ya que la evidencia es de certeza baja a muy baja. Para los desenlaces primarios, hay evidencia de certeza baja sobre el tiempo hasta la curación parcial (es decir, más del 90%) de las heridas (comparación sulfadiazina de plata versus sulfadiazina de plata de cerio: diferencia de medias (DM) ‐7,10 días, IC del 95%: ‐16,43 a 2,23; un estudio, 142 participantes). Hay evidencia de certeza muy baja con respecto a si los agentes antimicrobianos tópicos marcan una diferencia en la infección de las heridas (RR 0,73; IC del 95%: 0,46 a 1,17; un estudio, 15 participantes). Hay evidencia de certeza baja a muy baja sobre la proporción de quemaduras faciales que requieren cirugía, el dolor, la calidad de las cicatrices, los efectos adversos y la duración de la estancia en el hospital.
Sustitutos dérmicos versus agentes antimicrobianos tópicos
Hay evidencia de certeza baja de que un sustituto dérmico puede reducir ligeramente el tiempo de curación parcial (es decir, más del 90%) de las heridas en comparación con un agente antibacteriano no especificado (DM ‐6,00 días, IC del 95%: ‐8,69 a ‐3,31; un estudio, 34 participantes).
No está claro que exista alguna otra diferencia en los efectos con los sustitutos dérmicos, ya que la evidencia es de certeza muy baja. Los desenlaces incluían la infección de las heridas, el dolor, la calidad de las cicatrices, los efectos adversos del tratamiento y la duración de la estancia en el hospital.
Estudios individuales mostraron evidencia opuesta de certeza baja. Un sustituto dérmico diseñado mediante bioingeniería puede reducir ligeramente el dolor del procedimiento (DM ‐4,00; IC del 95%: ‐5,05 a ‐2,95; 34 participantes) y el dolor de fondo (DM ‐2,00; IC del 95%: ‐3,05 a ‐0,95; 34 participantes) en comparación con un agente antimicrobiano no especificado. Por el contrario, un apósito biológico (Xenoderm porcino) podría aumentar ligeramente el dolor en el caso de las quemaduras superficiales (DM 1,20, IC del 95%: 0,65 a 1,75; 15 participantes (30 heridas)), así como en las quemaduras profundas de espesor parcial (DM 3,00, IC del 95%: 2,34 a 3,66; 10 participantes (20 heridas)), en comparación con los agentes antimicrobianos (Physiotulle Ag (Coloplast)).
Tratamientos misceláneos versus tratamientos misceláneos
Los estudios individuales muestran efectos de las intervenciones de certeza baja a muy baja. La evidencia de certeza baja muestra que el MEBO puede reducir ligeramente el tiempo de curación completa de la herida en comparación con el apósito empapado en solución salina (DM ‐1,7 días, IC del 95%: ‐3,32 a ‐0,08; 40 participantes). Además, una crema que contenga Helix Aspersa puede aumentar ligeramente la proporción de heridas completamente curadas a los 14 días en comparación con el MEBO (RR 4,77; IC del 95%: 1,87 a 12,15; 43 participantes). No se sabe con certeza si algún tratamiento misceláneo en los estudios incluidos supone una diferencia en los efectos en el caso de los desenlaces de infección de la herida, la calidad de la cicatriz, el dolor y la satisfacción del paciente, ya que la evidencia es de certeza baja a muy baja.
Conclusiones de los autores
Principalmente hay evidencia de certeza baja a muy baja sobre los efectos de cualquier intervención tópica en la curación de heridas en personas con quemaduras faciales. El número de ECA sobre el cuidado de quemaduras está aumentando, pero el conjunto de evidencia sigue siendo obstaculizado debido al número insuficiente de estudios que siguen estándares basados en la evidencia adecuados para la realización y la notificación de ECA.
PICO
Resumen en términos sencillos
Tratamiento tópico para las quemaduras faciales
Pregunta de la revisión
Se revisó la evidencia sobre los efectos de los tratamientos tópicos (aplicados en la superficie de la piel) para la curación de las heridas por quemaduras en la cara o el cuello. Se quería saber qué tratamientos eran más efectivos para curar estas heridas y mejorar el aspecto de las cicatrices, lo cual es un tema particularmente importante en las lesiones por quemaduras faciales. También se quería conocer cómo los tratamientos tópicos afectaban al riesgo de complicaciones como la infección y el dolor, y cómo afectaban a la calidad de vida de las personas.
Antecedentes
Las lesiones por quemaduras son un importante problema de salud, y una importante causa mundial de discapacidad y desfiguración, tanto en adultos como en niños. Las mujeres y los niños de los países de bajos ingresos corren un riesgo especial. Las quemaduras plantean problemas particulares cuando se producen en la cabeza o el cuello. El rostro es fundamental para la identidad de una persona y desempeña un papel vital en la comunicación. Otras funciones básicas como la audición, el olfato y la respiración pueden verse afectadas como resultado directo de una quemadura facial. Los tratamientos tópicos como las cremas (no) antimicrobianas y los sustitutos dérmicos son los más frecuentemente utilizados para tratar las quemaduras faciales. Se quería comparar la efectividad de estos tratamientos para evaluar sus efectos beneficiosos y perjudiciales.
Características de los estudios
En diciembre de 2019, se buscaron ensayos controlados aleatorizados (ECA) que investigaran tratamientos tópicos para quemaduras faciales. Los ECA son estudios médicos en los que el tratamiento o la atención que reciben las personas se elige al azar. Este tipo de diseño de estudios proporciona la evidencia en salud más fiable acerca de si las diferentes formas de tratamiento o atención pueden marcar la diferencia. Se encontraron 12 estudios adecuados para su inclusión en esta actualización de la revisión, con 507 participantes con edades medias que oscilaban entre 5,3 y 41,9 años. Tres estudios compararon agentes antimicrobianos con no antimicrobianos, dos estudios compararon diferentes agentes antimicrobianos, cuatro estudios compararon los sustitutos dérmicos con agentes antimicrobianos, mientras que cuatro estudios compararon una variedad de tratamientos tópicos. Un estudio contribuyó a dos comparaciones. Ocho estudios fueron pequeños (menos de 40 participantes) y casi todos los estudios presentaban un riesgo de sesgo alto debido a la falta de cegamiento (los participantes y los evaluadores pueden haber sabido a qué grupo se asignaron los participantes e interpretar los efectos de manera diferente).
Resultados clave
En general, hay principalmente evidencia de certeza muy baja a baja sobre los efectos de cualquier intervención tópica en la curación de heridas o la infección en personas con quemaduras faciales. Además, hay evidencia de certeza baja a muy baja sobre los efectos de las intervenciones incluidas en la necesidad de cirugía, el dolor, la calidad de la cicatrización, la satisfacción del paciente, la duración de la estancia en el hospital y los efectos secundarios.
Todos los resultados fueron de alto riesgo de sesgo y variados, lo que puede haber exagerado los efectos.
Certeza de la evidencia
En general, la certeza de la evidencia sobre la efectividad de los tratamientos tópicos para las quemaduras faciales es baja o muy baja. No hay suficiente evidencia fiable sobre si los tratamientos tópicos mejoran los desenlaces en personas con quemaduras faciales, incluidas la mejora de la cicatrización de las heridas o las tasas de infección. Se requiere un mejor diseño de los ensayos y la presentación de informes de estos estudios para contribuir al cuidado de las quemaduras basado en la evidencia.
¿Qué grado de actualización tiene esta revisión?
Se hicieron búsquedas de estudios que se habían publicado hasta diciembre de 2019.
Authors' conclusions
Summary of findings
| Topical antimicrobial agent compared with topical non‐antimicrobial agent for facial burns | |||||||
| Patient or population: people with facial burns Setting: burn centres Intervention: topical antimicrobial agent Comparison: topical non‐antimicrobial agent | |||||||
| Outcomes (follow‐up) | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | ||
|---|---|---|---|---|---|---|---|
| Risk with topical non‐antimicrobial agent | Risk with topical antimicrobial agent | ||||||
| Time to complete wound healing (time to event data) (6 months) | Reported HR (adjusted for total body surface area burned) was 0.84 (0.78 to 1.85) (Ang 2000) | 39 (Ang 2000) | ⊕⊕⊕⊝ | Facial burns treated with SSD probably have a similar time to complete wound healing, compared with MEBO. | |||
| Time to complete wound healing (days) (until wound healing) | Mean time to wound healing 10.35 (SD 2.8) and 12.05 (SD 2.4) (Hindy 2009) | Mean time to wound healing 10.05 (SD 2.3) (Hindy 2009) | — | 60 | ⊕⊕⊝⊝ | There may be little or no difference in time to wound healing between topical anti‐microbial agents (Aquacel‐Ag) and non‐antimicrobial agents (MEBO) and saline‐soaked dressings) in facial burns. Data from Mabrouk 2012 not used, it was not stated or verifiable that all participants healed during the study. | |
| Proportion of wounds completely healed within 10 days (10 days) | Study population | RR 0.94 | 39 | ⊕⊕⊝⊝ | There may be little or no difference in the proportion of wounds completely healed within 10 days between SSD and MEBO. | ||
| 824 per 1000 | 774 per 1000 | ||||||
| Change in wound surface area over time or partial healing – not measured | No studies measured change in wound surface area over time or partial wound healing. | ||||||
| Infection (unclear follow‐up) | Study population | RR 0.38 | 40 | ⊕⊝⊝⊝ | It is uncertain whether Aquacel Ag increases or reduces the risk of infection compared with MEBO in facial burns as evidence is of very low certainty. | ||
| 400 per 1000 | 152 per 1000 | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; MEBO: Moist Exposed Burn Ointment; RR: risk ratio; SD: standard deviation; SSD: silver sulphadiazine. | |||||||
| GRADE Working Group grades of evidence | |||||||
| aDowngraded once for imprecision: limited number of participants (fewer than 100). bDowngraded once for unclear selection bias, performance and detection bias: unclear random sequence generation and allocation concealment, lack of blinding participants and providers, unclear blinding outcome assessment. Downgraded once for imprecision: effect estimates could not be calculated, limited number of participants (fewer than 100). | |||||||
| Topical antimicrobial compared with alternative antimicrobial agent for facial burns | ||||||
| Patient or population: people with facial burns Setting: burn centres Intervention: topical antimicrobial Comparison: alternative antimicrobial agent | ||||||
| Outcomes (follow‐up) | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with alternative antimicrobial agent | Risk with topical antimicrobial | |||||
| Time to complete wound healing | No studies measured time to complete wound healing. | |||||
| Proportion of wounds completely healed | No studies measured proportion of wounds completely healed. | |||||
| Change in wound surface area over time or partial healing (> 95%) (12 months) | The mean time to wound healing was 21.4 days | MD 7.10 days lower | — | 142 (Oen 2012) | ⊕⊕⊝⊝ Lowa | There may be little or no difference in time to wound healing between SSD and cerium‐SSD in facial burns. |
| Infection (until wound healing) | Study population | RR 0.73 | 15 | ⊕⊝⊝⊝ | It is uncertain whether mafenide acetate cream and gentamicin differs from mafenide acetate only in the risk of infection in facial burns as evidence is of very low certainty. | |
| 500 per 1000 | 365 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio; SSD: silver sulphadiazine. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once for imprecision: one study with 142 participants, optimal information size not reached. Downgraded once for high risk on performance bias and detection bias, and attrition bias not related to the outcome: lack of blinding participants, providers, and outcome assessor. | ||||||
| Skin substitute compared with topical antibiotic agent for facial burns | ||||||
| Patient or population: people with facial burns Setting: burn centres Intervention: skin substitute Comparison: topical antibiotic agent | ||||||
| Outcomes (follow‐up) | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with topical antibiotic agent | Risk with skin substitute | |||||
| Time to complete wound healing | No studies measured time to complete wound healing. | |||||
| Proportion of wounds completely healed | No studies measured proportion of wounds completely healed. | |||||
| Change in wound surface area over time or partial wound healing (> 90%) (until wound healing) | The mean time to wound healing was 15 days | MD 6 days lower | — | 34 | ⊕⊕⊝⊝ | A bioengineered skin substitute (TransCyte) might decrease time to 90% wound healing in facial burns, compared to a non‐specified antimicrobial agent. Data from Demling 1999, Horch 2005, and Wang 2015 not used because of concern of overlapping populations, lack of estimate of variance and comparison of wounds within participants. |
| Infection (until wound healing and unclear (2x)) | Study population | — | 56 | ⊕⊝⊝⊝ | It is uncertain whether skin substitutes increase or reduce the risk of infection compared with the use of topical antimicrobial agents in facial burns as evidence is of very low certainty. No events reported in 2 RCTs (Demling 1999; Horch 2005 (control group not reported)), third RCT reported 4 events in 50 wounds in 25 participants (Wang 2015). | |
| See comment | See comment | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once for risk of bias: unclear selection bias, high risk on performance bias and detection bias: unclear random sequence generation and allocation concealment, lack of blinding participants, providers and outcome assessor. Downgraded once for imprecision: one small‐sized study (34 participants). | ||||||
| Miscellaneous treatment compared with miscellaneous treatment for facial burns | ||||||
| Patient or population: people with facial burns Setting: burn centres Intervention: miscellaneous treatment (for details see comments) Comparison: miscellaneous treatment (for details see comments) | ||||||
| Outcomes (follow‐up) | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with miscellaneous treatment | Risk with miscellaneous treatment | |||||
| Time to complete wound healing (until wound healing) | The mean time to complete wound healing was 12.05 days | MD 1.7 days lower | — | 40 | ⊕⊕⊝⊝ | MEBO may slightly reduce mean time to wound healing in facial burns compared with saline‐soaked dressing. Data from Tsoutsos 2009 not used, it was not stated or verifiable that all participants healed during the study. |
| Proportion of wounds completely healed in 14 days (14 days) | Study population | RR 4.77 | 43 | ⊕⊕⊝⊝ | A cream containing Helix Aspersa (Elicina) may slightly increase the proportion completely healed at 14 days in facial burns, compared with MEBO. | |
| 188 per 1000 | 894 per 1000 | |||||
| Change in wound surface area over time or partial wound healing | Jiaao 2011 measured change in wound surface area over time, data not reported. | |||||
| Infection (follow‐up unclear) | Study population | Not estimable | 43 | ⊕⊝⊝⊝ | It is uncertain whether a cream containing Helix Aspersa (Elicina) increases or reduces the risk of infection compared with use of MEBO in facial burns as evidence is of very low certainty. | |
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; MEBO: Moist Exposed Burn Ointment; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once for risk of bias due to unclear selection bias, high risk on performance bias and detection bias: unclear random sequence generation and allocation concealment, lack of blinding participants and providers, and unclear blinding outcome assessor. Downgraded once for imprecision: one small‐sized study (40 participants). | ||||||
Background
(We have provided a glossary of some of the terms used in this review in Appendix 1.)
Burn injuries are an important health problem, resulting in 45,000 admissions annually in the US, of which more than 25,000 admissions to hospitals with specialised burn centres (American Burn Association 2016). In the UK, approximately 13,000 people a year are admitted to hospital for treatment of burns (Hettiaratchy 2004a), while in the Netherlands the annual figure is about 1300 people (Draisma 2017), 800 of whom are treated in one of the three Dutch burn centres (Dutch Burn Repository 2017 [pers comm]). Mortality rates from burn injuries have substantially decreased because of major improvements in burn care made in the 20th century. This has resulted in a shift in attention towards the functional outcome after a burn injury rather than mortality (Van Baar 2006). The head and neck region is estimated to be the site of burn injury in between 27% and 58% of burn cases (Hoogewerf 2013a). The face is central to our identity and also provides our most expressive means of communication. Appearance, communication, and other basic senses and abilities such as hearing, smelling and breathing may be affected as a direct result of a facial burn, or its sequelae (Serghiou 2004). Impaired function and distorted appearance may induce psychological problems, problems with social reintegration and affect quality of life (Van Loey 2003).
Description of the condition
A burn injury to the skin occurs when some, or all, the different layers of skin are destroyed by physical energy delivered via a hot liquid, flame, contact with a hot surface, ultraviolet/infrared radiation, radioactivity, electricity or chemicals (WHO 2014). The severity of burn wounds is characterised by their size and depth as well as their location and associated injuries. The size of a burn is measured by the percentage of total body surface area (% TBSA) affected, which is the percentage of the surface area of the skin burned, while the depth of a burn is determined by the layers of skin destroyed. So far, no consensus has been reached on the exact classifications of burns, especially not in relation to the classification of depth. Jaspers 2018 proposed a scheme based on a combination of currently used classifications. Burn wounds are classified as either superficial burns, partial‐thickness burns, deep partial‐thickness burns or subdermal burns. In superficial burns, only the epidermal layer is destroyed. Healing generally occurs within 5 to 10 days, resulting in a normal looking skin in one to three weeks with only a chance of pigment changes. In partial thickness burns, the upper part of the dermis is also comprised. However, regeneration of the epidermis is expected within two weeks, with very little, or no, scarring, due to the keratinocytes and epidermal stem cells in the appendages of the dermis (Jaspers 2018). In deep partial‐thickness burns, the epidermis and most of the dermis is destroyed, with damage to the skin appendages including hair follicles. Wound healing is delayed, depending on the surviving keratinocytes and epidermal stem cells in the remaining dermis. It is a general rule that if re‐epithelialisation does not occur within three weeks, then hypertrophic scarring may occur (Chipp 2017; Cubison 2006). Subdermal burns involve all the layers of skin and may involve structures underneath, such as muscle and bone, leaving little chance of healing from the epithelial elements at the bottom of the wound. In the case of a very small burn, healing might occur by contraction and growth of epithelial cell from the wound edges. Subdermal burns will nearly always result in hypertrophic (raised) scarring.
Subdermal facial burns are rare, since the face's high vascularity rapidly dissipates heat (Choi 2008). Facial burns are often caused by flash burns, which usually cause partial‐thickness burns. Nonetheless, subdermal facial burns do occur, especially in flame and contact burns, and in the event of prolonged exposure to a heating source, for example if the person was unconscious or paralysed at the time of accident. In addition, in some places (e.g. nose and ears) facial skin is very thin, and, therefore, more vulnerable to deep burns. When nose and ears are deeply burned, the anatomical structures can change or disappear. Full‐thickness burns often require surgical intervention. In the Netherlands, approximately 20% of the people with head and neck burns treated in a burn centre required primary facial surgery and 5% received facial reconstruction in a later phase (Hoogewerf 2013a).
Immediately after the thermal injury, the surfaces of burn wounds are thought to be sterile, but they are rapidly colonised by a variety of micro‐organisms (Erol 2004; Wysocki 2002). About 40% of the burn wound cultures on admission are colonised with one or more potentially pathogenic micro‐organisms (Dokter 2016). These micro‐organisms originate from the patient's own skin, respiratory and gastrointestinal flora, and from contact with contaminated surfaces in the external environment, hands of healthcare workers and even air (Erol 2004; Weber 2004; Wysocki 2002). Burn wounds provide a favourable niche for microbial colonisation and proliferation because of their protein‐rich environment and avascular necrotic tissue (Barret 2003; Erol 2004). This avascularity of eschar (necrotic tissue) results in impaired migration of host immune cells and restricts delivery of systemically administered antimicrobial agents to the area. The most common burn wound pathogens are Staphylococcus aureus and Pseudomonas aeruginosa (Nagoba 2010). Microbial colonisation of burn wounds has been associated with delayed wound healing, increased need for surgical interventions and prolonged length of stay (LOS) at burn centres (Vermeulen 2007).
Once 30% of the TBSA has been burned there may be systemic (whole body) responses in addition to local responses. This occurs because of the release of inflammatory mediators at the site of injury (Hettiaratchy 2004b). Besides generating excessive oedema in burns, these systemic reactions can further compromise the healing of a burn wound, and so it is important to consider adequate local treatment, as well as systemic management of a burn, as this may influence the final outcome of the injury.
Another possible outcome of a burn injury is hypertrophic scarring, which occurs when the balance between collagen synthesis and breakdown is disrupted (Herndon 2007). The postburn hypertrophic scar may present itself either as a pink to red in colour and slightly thickened, or as a red to purple inelastic mass of skin tissue. If a hypertrophic scar surrounds openings such as the eyes or mouth, functional impairment of the face can occur. The eyes for instance, may not close, due to the inelasticity and contraction of the hypertrophic skin, and the mouth may not open maximally. Furthermore, these scars can result in discomfort, because of itching, and sometimes cause neuropathic (nerve) pain (Van Loey 2008). The degree of hypertrophic scarring differs among individuals and depends on a variety of factors, one of which is time to wound healing, with hypertrophic scar formation being seen more often when wound healing takes more than 21 days (Chipp 2017; Cubison 2006). In general, a deeper burn wound results in the formation of more hypertrophic tissue. Other factors that have been found to be related to scar formation are female gender, young age, burn size, burns of the neck or upper limb, more than one surgical operation and meshed skin grafts (Gangemi 2008; Van der Wal 2012).
Description of the intervention
The focus of this review is topical treatment for facial burns. Topical treatment comprises any remedy, agent, substance, device or skin substitute that is placed on the face as a therapy for burn wounds. Interventions used in the topical treatment of facial burns can be divided into five main categories: topical antimicrobial agents; topical non‐antimicrobial agents; skin substitutes; wound preparation agents and antiseptics; and miscellaneous treatments, including alternative remedies. This definition excludes invasive surgical intervention, which is another important treatment in burn care. It also excludes negative pressure wound therapy. Numerous dressings and topical ointments are used to treat facial burns (De Haas 2005; Hansen 2004). Before applying topical or surgical treatment, a burn wound surface might need additional preparation in the form of debridement (removal of dead tissue). The debridement of burns is divided into two main approaches, namely:
-
superficial debridement: cleaning the wound surface using a brush, gauze or chemical, and removing the superficial loose wound surface;
-
surgical debridement: the excision of the burn wound, with removal of all non‐vital tissue.
This review considered only superficial debridement.
How the intervention might work
Topical antimicrobial agents
Topical antimicrobial agents are used to control and limit infection, and they are central to topical burn therapy. The ideal topical prophylactic antimicrobial agent would have a broad spectrum of activity with a long duration of action, low toxicity and the ability to penetrate eschar (necrotic tissue) without being absorbed by the body (Monafo 1990). Ideal topical antimicrobials do not hamper epithelial outgrowth and deliver a high concentration of active ingredients to devitalised, devascularised and potentially necrotic wounds, helping to provide a favourable wound healing environment. Use of topical antimicrobials may help to minimise wound deepening, and the need for extensive debridement and subsequent grafting. This is fundamentally important for facial wounds, where overzealous debridement may affect function and appearance (Leon‐Villapalos 2008).
The antimicrobial agents used in burn care include silver preparations. Silver sulphadiazine (SSD), in particular, is widely used and acts on burn eschar to limit the extent of non‐viable tissue in situations where surgery is either not possible, or would not be the immediate first option – as in facial burns (Leon‐Villapalos 2008). Cerium nitrate is another antimicrobial agent which penetrates burned tissue and has a broad spectrum of activity against Gram‐positive and Gram‐negative bacteria, and fungal species, especially in combination with SSD. Cerium nitrate also has a hardening effect on burn eschar, which is thought to prevent bacterial ingress and helps maintain a moist wound. Furthermore, cerium is supposed to bind and denature the lipid‐protein complex released from burned skin responsible for the profound immunosuppression associated with major cutaneous burns (Allgöwer 2008; Garner 2005). Despite their popularity and widespread use, silver‐based modalities are not without complications, including frequently observed delayed wound healing, which might be due to the retardation of sloughing in partial‐thickness burns. In addition, increased hypertrophic scarring has been described with SSD; while skin irritation, black staining of the skin and the possibility of systemic absorption of silver have also been reported (Atiyeh 2007; Pham 2007). Furthermore, Wasiak 2013 concluded in a Cochrane systematic review that "SSD was consistently associated with poorer healing outcomes than biosynthetic, silicon‐coated and silver dressings."
Other antimicrobial agents include natrium fusidate and nitrofuran. It has been reported that some antimicrobial medications might delay proper healing mechanisms of the wound (Le Duc 2007; Teepe 1993), and that improper use can contribute to the emergence of resistant microbes (Nagoba 2010).
Wound dressings are often used to create an optimal environment for epidermal wound healing. For a long time, a moist environment was regarded as optimal (Winter 1962), however, in the 1980s, Jonkman 1989 suggested that epidermal wound healing is best accelerated in an environment "between moist and dry," (i.e. a more jelly‐like wound exudate environment). Nowadays, several wound dressings have these moist or gel‐forming qualities. Occlusive dressings, such as hydrocolloids and hydrogel dressings, form a moist or jelly‐like environment by incorporating wound fluids into the dressing. Semi‐occlusive dressings (e.g. polyurethane film, foam or a hydrofibre) permit evaporation of excess water and prevent maceration, while maintaining a moist environment. Silicon‐coated nylon dressings function primarily as non‐adherent dressing layers, and, therefore, reduce potential damage during dressing changes (Walmsley 2002).
When these wound dressing include antimicrobial agents, these can be classified as topical antimicrobial agents.
Topical non‐antimicrobial agents
Topical non‐antimicrobial agents include all agents and wound dressings without any antimicrobial agent added. Multiple wound dressings do not include antimicrobial agents. Again, occlusive dressings, such as hydrocolloids and hydrogel dressings, semi‐occlusive dressings and silicon‐coated nylon dressings fall into this category.
Skin substitutes: biological and bioengineered dressings
Biological dressings (e.g. cadaver allografts (skin from corpses) and porcine (pig) skin xenografts) can be used to treat partial‐thickness burns. These provide temporary wound coverage until full healing can be achieved, or until autografting (skin graft(s) using the patient's own skin) can take place. Another biological dressing, amnion (derived from the membranous sac that surrounds the developing embryo), can be used as a wound dressing for burn treatment as well (Herndon 2017; Kesting 2008). In addition, bioengineered skin substitutes can be used as 'smart dressings' in topical therapy; these not only provide immediate wound cover, but are also available in large quantities, with a negligible possibility of disease transfer. These dressings have become part of standard care (Wurzer 2016). Costs of bioengineered skin substitutes are sometimes considered substantial (Pham 2007), but could be considered to be less substantial if total costs of burn care are taken into account (Hop 2014).
Wound preparation agents and antiseptics
Antiseptics are topical agents thought to prevent the growth of pathogenic micro‐organisms without damaging living tissue (Norman 2017). They can be used to cleanse facial burn surfaces after injury, or to prepare wounds for surgical debridement, or the application of a further topical agent. Examples of antiseptics include chlorhexidine digluconate and povidone iodine. Other wound‐preparation agents include enzymatic debriding agents. These agents prepare the wound by chemical debridement, but their use is controversial for facial burns (Leon‐Villapalos 2008).
Miscellaneous topical treatments, including alternative remedies
Several additional forms of topical therapy are available, including alternative remedies such as honey and Aloe vera. Honey is said to prevent bacterial growth, form a physical barrier, act as an enzymatic debrider, and promote epithelialisation and angiogenesis (formation of new blood vessels). Aloe vera could accelerate the wound healing process and rate of re‐epithelialisation in partial‐thickness burns (Maenthaisong 2007; Somboonwong 2000). Although honey and Aloe vera are sometimes considered to be antimicrobials, they are more often considered to be alternative treatments with possible antimicrobial/antiseptic properties and they are therefore included in the miscellaneous topical treatment category. Other alternative remedies, such as covering with banana or cabbage leaves, or potato skins, are sometimes used in places where treatment resources are limited (Bitter 2016). Any other topical treatment for facial burns which does not fall into one of the main groups above was included in this category of alternative remedies.
Why it is important to do this review
Treatment of facial burns is more demanding than treatment of burns on other parts of the body, not only because of the location of vital sensory and communication organs but also because the face is highly vascular. This high vascularisation increases the self‐healing potential of facial burns and, therefore, justifies a conservative approach to treatment, though this may require intensive daily care. There is uncertainty about which treatment is the most effective for facial burns, and, consequently, there are large variations in practice (De Haas 2005; Leon‐Villapalos 2008). Since treatment contributes to outcome – which is especially important for facial burns in terms of both physical and psychological functioning – it is important to consider the most effective treatment.
Existing guidelines to support clinical decision making in burn care are predominantly practice‐based or are concerned with the general treatment of burns (ISBI 2016). In addition, several systematic reviews have been published in the field of wound care; including reviews on dressings for superficial and partial‐thickness burns (Wasiak 2013), and the use of honey (Jull 2015), Aloe vera (Dat 2012), antiseptics (Norman 2017), and topical silver (Storm‐Versloot 2010; Vermeulen 2007); however, no review specifically considered facial burns. In conclusion, current published reviews do not address the effectiveness of topical treatment for facial burns.
Objectives
To assess the effects of topical interventions on wound healing in people with facial burns of any depth.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised controlled trials (RCTs) that evaluated the effects of topical treatments for facial burns. We decided to consider quasi‐RCTs only in the absence of RCTs.
Types of participants
We considered studies that included people of any age with a facial burn wound of any degree in any care setting. Any type of burn injury was eligible (flame, scald, chemical, etc). Ocular burn wounds were excluded.
Types of interventions
Studies were considered for inclusion if topical therapy was applied and compared with any comparator intervention. We defined topical therapy as any remedy, agent, substance, device or skin substitute (biological or bioengineered) that was applied to the surface of the facial wound in the acute phase with the aim of treating the burn. We defined the acute phase as the period of wound healing that occurred up to wound closure (epithelialisation). We divided the topical interventions considered for inclusion into the following five categories:
-
topical antimicrobial agents;
-
topical non‐antimicrobial agents;
-
skin substitutes: biological and bioengineered dressings;
-
wound preparation agents and antiseptics;
-
miscellaneous topical treatments, including alternative remedies.
The previously stated definition of topical therapy excluded surgical debridement and negative pressure wound therapy as index interventions in this review. Comparator interventions could include any other intervention, no intervention or a placebo intervention.
Types of outcome measures
Study outcomes did not form part of the selection process. We divided outcomes into primary and secondary outcomes.
Primary outcomes
-
Time to complete wound healing, or the proportion of the burn wounds completely healed in a specified time period (as defined by trial authors). Both time to wound healing reported as a time to event outcome and mean time to wound healing outcome were included.
-
Change in wound surface area over time, or the proportion of the burn wound completely healed (epithelialised) in a specific time period (as defined by the trial authors). In case of unclear definition of healing, data were included but this was mentioned.
-
Wound infection (as defined by the trial authors). We did not include data on wound colonisation unrelated to infection.
We accepted any definition of change in wound surface area over time, or proportion of wound surface area healed in a specified time period. In addition, we accepted any definition of wound infection. All primary outcomes were assessed as short‐term endpoints (i.e. three months).
Secondary outcomes
-
Proportion of facial burns requiring surgery (following treatment by topical agent).
-
Scar quality: observed and self‐reported (any definition of scar quality was accepted).
-
Pain (any assessment was accepted).
-
Patient satisfaction (any assessment was accepted).
-
Adverse effects: classified as: diagnosed by a clinician, diagnosed by laboratory results or patient‐reported symptoms.
-
Quality of life (any assessment was accepted).
-
Length of hospital stay (LOS).
Because we anticipated that primary studies would report and analyse secondary outcomes at different time points, we prespecified time points as either short‐term or long‐term. The short‐term endpoints (i.e. up to three months postburn) included the outcomes: pain, patient satisfaction, adverse effects and LOS; the long‐term endpoints (i.e. after three months and up to 12 months postburn) included the outcomes: proportion of facial burns requiring surgery, adverse effects, scar quality and quality of life.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant clinical trials:
-
the Cochrane Wounds Specialised Register (searched 18 December 2019);
-
the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 11) in the Cochrane Library (searched 18 December 2019);
-
Ovid MEDLINE including In‐Process & Other Non‐Indexed Citations (1946 to 18 December 2019);
-
Ovid Embase (1974 to 18 December 2019);
-
EBSCO CINAHL Plus (Cumulative Index to Nursing and Allied Health Literature; 1937 to 18 December 2019).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus can be found in Appendix 2. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2019). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2019). We combined the CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2019). There were no restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trials registries:
-
ClinicalTrials.gov (www.clinicaltrials.gov) (searched 18 December 2019);
-
World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/trialsearch) (searched 18 December 2019).
Search strategies for clinical trial registries can be found in Appendix 2.
Details of the search strategies used for the previous version of the review are given in Hoogewerf 2013b.
Searching other resources
We aimed to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, as well as relevant systematic reviews, meta‐analyses and health technology assessment reports. When necessary, we contacted authors of key papers and abstracts to request further information about their trials. We did not perform a separate search for adverse effects of interventions used, we considered adverse effects described in included studies only.
Data collection and analysis
Data collection and analysis were carried out according to methods stated in the published protocol (Van Baar 2009), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017).
Selection of studies
There were no restrictions on language of publication or publication status. For this update, two review authors (CH and MvB) independently assessed the titles and abstracts of studies identified from the search in terms of their relevance and design. We obtained full versions of articles if they matched the inclusion criteria from this initial assessment. The review authors independently assessed full‐text articles and determined a final selection of trials eligible for this review. A third review author (JH) evaluated any discrepancies and advised in case of disagreement.
Data extraction and management
Two review authors (CH and MvB) independently extracted and summarised details of trials using a data extraction sheet. To provide independent data extraction, a third person (Inge Spronk) not involved in one included study, performed data extraction and risk of bias assessment of the study from Oen 2012, together with CH. Data from a paper in the Chinese language was extracted by a member of Cochrane Wounds. We extracted data on the following items:
-
characteristics of the trial: method of randomisation, setting, location of care, country, source of funding;
-
participants: number, age, gender, type of burn, percentage TBSA burned, burn depth, concurrent illnesses;
-
intervention topical agents: type of dressing, dose used, frequency of dressing changes, time elapsed before treatment, concurrent interventions;
-
comparator intervention: type of dressing, dose used, frequency of dressing changes, time elapsed before treatment, concurrent interventions;
-
outcomes: types of outcomes measured, timing of outcomes;
-
results.
The review authors resolved any discrepancies by discussion with a third review author (MvB, update JH), and contacted the trial authors when information was missing from published reports or clarification was needed. Data from trials published in duplicate were included only once, but were maximally data extracted.
In cases where studies potentially contained the same participants, only the study with the largest sample size was included in data synthesis.
Assessment of risk of bias in included studies
Two review authors (CH and MvB) made systematic and independent assessments of the risk of bias of each trial, using the Cochrane 'Risk of bias' criteria (Higgins 2017). To provide independent assessment of risk of bias, a third person (Inge Spronk) not involved in one included study and CH performed data extraction and risk of bias assessment of the study from Oen 2012. A member of Cochrane Wounds (Zhenmi Liu) assessed a paper in the Chinese language. The criteria related to the following issues:
-
sequence generation;
-
allocation concealment;
-
blinding of participants, care providers and outcome assessors;
-
incomplete outcome data: assessment of dropout rate and intention‐to‐treat (ITT) analysis;
-
selective outcome reporting;
-
other sources of bias: baseline similarity, co‐interventions, compliance, similar timing of outcome assessment.
Risk of bias increases with each criterion that is judged to be negative. A detailed description of criteria for a judgement of 'low risk of bias', 'high risk of bias' or 'unclear risk of bias' is available in Appendix 3. Any discrepancies in judgement between the two review authors was resolved by discussion with a third review author (MvB, update JH). Final assessment of risk of bias was presented in a 'Risk of bias' graph (Figure 1), and a 'Risk of bias' summary (Figure 2).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
Data analysis was performed according to the Cochrane guidelines (Deeks 2019). One review author (MvB) entered quantitative data into Review Manager 5, this was checked by another review author (CH), and analysed using Review Manager 5 (Review Manager 2014). For each outcome, summary estimates of treatment effect (with 95% confidence intervals (CI)) were calculated for every comparison. Dichotomous outcomes were presented as risk ratios (RR) (see the Cochrane Handbook for Systematic Reviews of Interventions; Deeks 2019) with 95% CI, and continuous outcomes were presented as mean differences (MD) with 95% CI. We intended to use standardised mean differences (SMD) on occasions when studies assessed the same outcome (e.g. quality of life) but measured the outcome in different ways. Time to wound healing was analysed as a survival (time‐to‐event) outcome if possible, using an appropriate analytical method (i.e. hazard ratio; Cochrane Handbook for Systematic Reviews of Interventions; Deeks 2019). Where this was not possible, mean time to wound healing was presented.
Unit of analysis issues
We addressed the level at which randomisation occurred in our analysis. In general, the unit of randomisation and measurement was expected to be the patient. In case of a deviation, we contacted the original investigators whenever possible. In cases where no additional information was obtained, we only presented descriptive data in the review. In case of a within‐participant design, data were handled and presented separately.
Dealing with missing data
We contacted the original investigators to request missing data whenever possible. In cases where no information was obtained, we presented a narrative summary of available data.
If data were not provided in numerical format and only provided in graphs, we planned to estimate the mean scores and SDs from the graphs. If studies did not provide a mean (SD) for continuous data, we contacted the original investigators. If authors did not respond or were unable to provide the additional data, we included whatever data were available. If insufficient data were available for analyses, we only presented descriptive data in the review. If authors of trials provided both intention‐to‐treat and per protocol data, we would have used the intention‐to‐treat data rather than imputing missing data as originally stated in our protocol.
Assessment of heterogeneity
We planned to explore both clinical and statistical heterogeneity. Clinical heterogeneity was assessed using information on type of dressing, dose used and frequency of dressing changes. We planned to test statistical heterogeneity using the Chi2 test and estimate the amount of heterogeneity using the I2 statistic (with 95% CI) (Higgins 2003; Deeks 2019), which examines the percentage of total variation across studies due to heterogeneity rather than to chance.
The I2 statistic ranges from 0% to 100%, with higher values indicating greater heterogeneity. An I2 statistic of 55% to 100% can be interpreted as considerable heterogeneity.
Assessment of reporting biases
We planned to measure publication bias using the Begg funnel plot (Begg 1994), and the Egger test (Egger 1997), if the included studies were homogeneous and sufficient in number.
Data synthesis
We planned to perform a meta‐analysis for each primary outcome if clinical and statistical homogeneity indicated this would be appropriate (Higgins 2003), and calculate summary estimates of treatment effect for every comparison.
No totals were calculated if trial heterogeneity was considerable (I2 greater than 75%). If pooling was appropriate (I2 less than 75%), we used both a fixed‐effect and a random‐effects model. The fixed‐effect model ignores heterogeneity, and gives an estimate of the intervention effect, assuming a single intervention effect. The random‐effects model incorporates heterogeneity among studies (Deeks 2019; Ioannidis 2007).
In the study protocol, we planned to restrict the primary analyses to studies at low risk of bias. We defined studies with low risk of bias as RCTs which fulfilled the three criteria of adequate sequence generation, adequate allocation concealment and blinded outcome assessment. However, we conducted a mainly narrative overview, structured by the type of comparison, because statistical meta‐analyses were inappropriate in some cases. This mainly narrative overview was structured by the type of experimental interventions.
With the introduction of 'Summary of findings' tables in this update of the review, we decided to present data for all primary outcomes, structured by the type of experimental interventions.
Subgroup analysis and investigation of heterogeneity
We planned to investigate heterogeneity through subgroup and sensitivity analysis (Deeks 2019), when there was a sufficient number of studies in the meta‐analysis (i.e. more than 10). We planned to conduct subgroup analysis for:
-
partial‐thickness burns compared with full‐thickness burns, as the effects of topical interventions were expected to differ between patient groups with different burn depths;
-
adequate concealment of allocation (low risk of bias versus unclear or high risk of bias).
However, in the absence of sufficient studies in the meta‐analyses, we performed no subgroup analyses.
Sensitivity analysis
If there were a sufficient number of studies in the meta‐analysis, we planned to perform a sensitivity analysis showing how conclusions might be affected if studies at high risk of bias were excluded from the analyses. We planned to explore the effect of excluding studies with unclear and inadequate sequence generation and unclear and inadequate allocation concealment within the sensitivity analysis. However, in the absence of sufficient studies in meta‐analyses, we performed no sensitivity analysis.
'Summary of findings' tables and GRADE assessment of the certainty of evidence
We included 'Summary of findings' tables in this update, constructed using GRADE Pro/GDT software .
We limited the 'Summary of findings' tables to our primary outcomes: time to complete wound healing or the proportion of the burn wounds healed in a specified time period, change in wound surface area over time or the proportion of the burn wound completely healed (epithelialised) in a specific time period, and wound infection.
Two review authors assessed the certainty of the evidence (CH and MvB).
The certainty of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2019). We aimed to rate the overall certainty of evidence as high, moderate, low or very low using the GRADE approach (GRADE 2013; Ryan 2016).
Well‐designed and conducted RCTs are rated as high‐certainty evidence. We downgraded the evidence to moderate, low or very low depending on the presence of each of the following factors:
-
study limitations (risk of bias);
-
indirectness of evidence (directness of evidence);
-
imprecision (precision of results);
-
inconsistency (consistency of results) and
-
publication bias (existence of publication bias).
We downgraded the evidence for the risk of bias domain when studies had been classified at high risk of bias for one or more domains. We downgraded evidence for imprecision if the optimal information size was not met. We downgraded twice for imprecision when there were very few events or CIs around effects included both appreciable benefit and harm, or both (Guyatt 2011).
Results
Description of studies
See: Characteristics of included studies; Characteristics of ongoing studies; and Characteristics of excluded studies tables.
Results of the search
This is an update of a Cochrane Review first published in 2013 (Hoogewerf 2013b). The updated searches (18 December 2019), identified, after initial deduplication, 527 unique records.
We identified one additional study after reviewing the reference lists of the included papers (Tsoutsos 2009). The updated search resulted in 528 unique records in total.
Together with the four studies awaiting assessment in the previous version of the review (Hindy 2009; Jiaao 2011; Mabrouk 2012; Oen 2012), we screened 532 records.
Two review authors (CH and MvB) independently assessed the titles and abstracts of these records and judged 21 records from 18 studies to be potentially eligible for the review.
Seven new studies were included (Hindy 2009; Jiaao 2011; Lehna 2017; Mabrouk 2012; Oen 2012; Tsoutsos 2009; Wang 2015). With the five previously included studies there are now 12 included studies in this review.
Four studies were excluded in this update because they were not RCTs (Schulz 2016), or they did not involve facial burns (Aboelnaga 2018; Hundeshagen 2018; Moenadjat 2008). With the nine excluded studies in the previous version of the review, there are now 13 excluded studies in this review.
Three studies were duplicates and already included in the original review, as a study (Horch 2005), or in the section studies awaiting assessment (Mabrouk 2012; Oen 2012).
An overview of the inclusion of studies from the updated search is presented in the PRISMA study flow diagram (Figure 3).
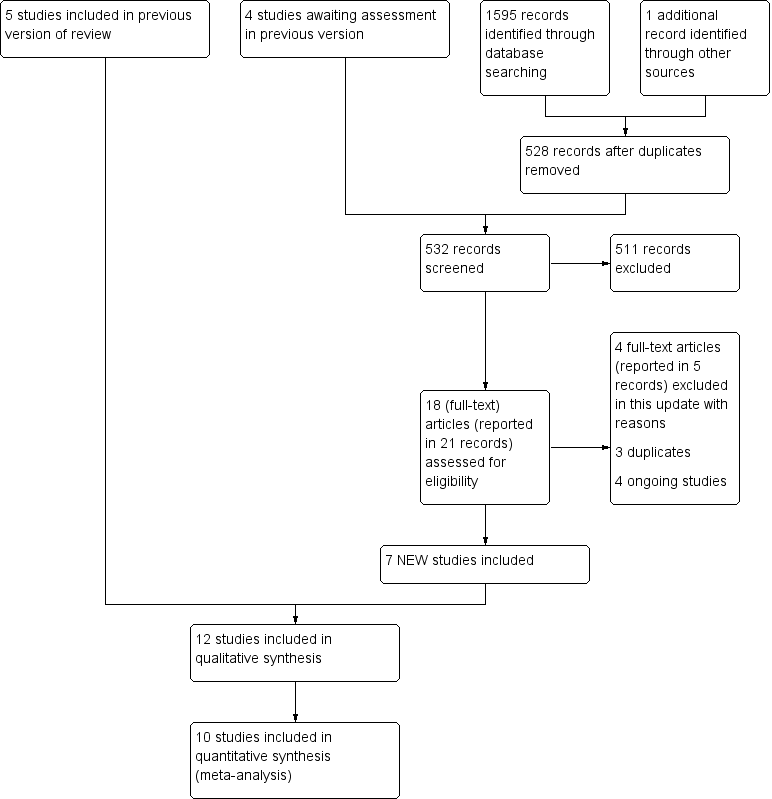
Study flow diagram.
Included studies
We included seven new studies (Hindy 2009; Jiaao 2011; Lehna 2017; Mabrouk 2012; Oen 2012; Tsoutsos 2009; Wang 2015), in addition to the five studies included in the previous review (Ang 2000; Demling 1999; Demling 2002; Desai 1991; Horch 2005). The characteristics of these studies are described in the Characteristics of included studies table and are summarised below.
Healthcare settings
Eleven RCTs took place in burn centres, the healthcare setting of the 12th study was unclear (Jiaao 2011). Four studies were conducted in North America (Demling 1999; Demling 2002; Desai 1991; Lehna 2017); three in Europe (Horch 2005; Oen 2012; Tsoutsos 2009), two in the Middle East (Hindy 2009; Mabrouk 2012), and three in Asia (Ang 2000; Jiaao 2011; Wang 2015).
Participants
The 12 included studies recruited 507 participants (range of sample size 10 to 180), although it is possible that this number might be lower due to a possible overlap of participants between two studies (Demling 1999; Demling 2002), which potentially would decrease the total to 486. Ten studies reported age of included participants and seven studies reported percentage TBSA burned. The mean age varied between 5.3 years (Jiaao 2011) up to 41.9 years (intervention group, Oen 2012). The mean percentage TBSA burned varied from 1.56% (control group, Ang 2000), to 50% (control group, Desai 1991). Two studies did not provide information on age or percentage TBSA burned (Hindy 2009; Tsoutsos 2009). For detailed information see Characteristics of included studies table.
Interventions
Three studies compared topical antimicrobial versus non‐antimicrobial agents. These studies included comparisons between SSD and the non‐antimicrobial Moist Exposed Burn Ointment (MEBO) (Ang 2000), and between a topical antimicrobial hydrocolloid dressing (Aquacel Ag) and MEBO (Hindy 2009; Mabrouk 2012).
Two studies compared topical antimicrobials with an alternative topical antimicrobial agent. One study compared cerium nitrate‐SSD (CN‐SSD) with SSD (Oen 2012), and one study compared an antimicrobial agent (i.e. 1% gentamicin cream) administered via iontophoresis in combination with mafenide acetate compared with mafenide acetate alone (Desai 1991).
Four studies compared skin substitutes with topical antimicrobial agents (Table 1). The skin substitutes included bioengineered skin substitutes (TransCyte), allograft (glycerolised cadaver skin), and a biological skin substitute (porcine Xenoderm) (Demling 1999; Demling 2002; Horch 2005; Wang 2015). These skin substitutes were compared with the antimicrobial agents Bacitracin, SSD and Physiotulle Ag.
| Comparison | Study | Follow‐up | Time to wound healing | Change in wound surface area, proportion of wound partly healed | Complete healing data reported? | Infection |
|---|---|---|---|---|---|---|
| Topical antimicrobial agent compared with topical non‐antimicrobial agent | ||||||
| SSD vs MEBO | 6 months | Time to event analysis, days to complete wound healing; number of participants healed at 10 days | – | Yes, stated | Not reported | |
| Silver hydrocolloid dressing (Aquacel Ag) vs MEBO vs saline‐soaked dressings | Until wound healing | Days to complete wound healing | – | Yes, stated | Not reported | |
| Silver hydrocolloid dressing (Aquacel Ag) vs MEBO | 6 months | Days to complete wound healing | – | Not stated nor verifiable | Reported, | |
| Topical antimicrobial agents compared with other topical antimicrobial agents | ||||||
| Mafenide acetate cream + gentamicin via iontophoresis (7 participants) vs usual care (mafenide acetate cream) (8 participants) | Until wound healing | Days to complete wound healing | – | Yes, stated | Clinical features | |
| Cerium‐SSD vs SSD | 12 months | Time to event anaysis, days to 90% wound healing | – | Yes, verifiable | Not reported | |
| Skin substitutes compared with topical antimicrobial agents | ||||||
| Biological skin substitute coated with fibronectin (TransCyte) vs Bacitracin | Until wound healing | Days to 95% wound healing | – | Yes, verifiable | Clinical features | |
| Bioactive skin substitute (a bilayered, biologically active, temporary skin substitute (TransCyte)) vs antibacterial ointment, not specified | Until wound healing | Days to 90% wound healing | – | Yes, verifiable | Not reported | |
| Glycerolised allograft cadaver (corpse) skin vs SSD | 6 months (maximum) | Days to complete wound healing | – | Yes, verifiable | Reported in 1 group, not prespecified, no definition | |
| Biological dressing (porcine Xenoderm) vs Physiotulle Ag (Coloplast) 50 wounds in 25 participants | 3 months (scar) | Days to 95% wound healing | – | Yes, verifiable | Clinical features | |
| Miscellaneous treatment compared with miscellaneous treatment | ||||||
| MEBO vs saline‐soaked dressing | Until wound healing | Days to complete wound healing | – | Yes, stated | Not reported | |
| rhGM‐CSF hydrogel (1 μg/cm2/day) vs placebo hydrogel (matrix hydrogel) | Until wound healing | Days to complete wound healing | % wound healing at 3, 5, 7 and 14 days after treatment | Yes, stated | Not reported | |
| Enzymatic debridement (collagenase) vs antimicrobial agent (Bacitracin) | 6 months | Wound epithelialisation: time to establish a wound bed | – | Yes, verifiable | Documented wound or blood infection confirmed by a positive laboratory result. | |
| Cream containing Helix Aspersa extract (terrestrial brown snail secretions extract (Elicina) vs MEBO | 2 years (scar) | Days to complete wound healing, number of participants healed at 14 days | – | Not stated or verifiable (days to complete wound healing) | Burn swab cultures; systemic infections, | |
MEBO: Moist Exposed Burn Ointment; rhGM‐CSF: recombinant human granulocyte‐macrophage colony‐stimulating factor; SSD: silver sulphadiazine.
Finally, four studies included comparisons of a variety of topical interventions, including the comparison of saline‐soaked dressings to sodium carboxymethyl‐cellulose silver (Aquacel Ag) and MEBO (Hindy 2009), the comparison of growth hormone therapy (recombinant human granulocyte‐macrophage colony‐stimulating factor hydrogel, rhGM CS) to a placebo hydrogel (Jiaao 2011), the comparison of enzymatic debridement to a topical antimicrobial agent (Lehna 2017), and a comparison of a cream containing Helix Aspersa extract (Elicina) to MEBO (Tsoutsos 2009).
Outcomes
Seven of 12 studies included 'time to complete wound healing' as an outcome of interest (Table 1). In addition, two studies reported the number of participants healed at 10 days (Ang 2000) and 14 days (Tsoutsos 2009). Five studies used 'change in wound surface area over time,' or 'the proportion of the burn wounds partly healed' as an outcome measure, by assessing the percentage wound healing at 3, 5, 7 and 14 days after treatment (Jiaao 2011), or the time to 90% or 95% wound healing (Demling 1999; Demling 2002; Oen 2012; Wang 2015).
Ideally 'time to wound healing' should be measured as a time‐to‐event outcome and reported in survival curves and expressed as hazard ratios. Only two studies (Ang 2000, Oen 2012) analysed time to wound healing properly, as a time‐to‐event outcome. For studies that reported on 'time to wound healing', if all the recruited participants did not heal within the study period it would have been wrong to report mean time to wound healing. Three out of 12 studies stated that all participants healed during the study, and in six studies this was verifiable, in the other three studies this was not stated or verifiable (Table 1).
Wound infection was a prespecified outcome in four studies (and another three studies reported this outcome although it had not been prespecified in the methods section (Table 1)). Two studies assessed bacterial wound colonisation only (Demling 2002; Oen 2012); these data were not included in the review.
Secondary outcomes reported in the included studies included the proportion of facial burns requiring surgery, pain, scar quality, patient satisfaction, LOS and adverse effects (Table 2).
| Comparison | Study | Follow‐up | Surgery | Scar quality | Pain | Patient satisfaction | Adverse effect | QoL | LOS |
|---|---|---|---|---|---|---|---|---|---|
| Topical antimicrobial agent compared with topical non‐antimicrobial agent | |||||||||
| SSD vs MEBO | 6 months | Reconstructive surgery, following wound closure | – | – | – | – | – | – | |
| Silver hydrocolloid dressing (Aquacel Ag) vs MEBO vs saline‐soaked dressings | Until wound healing | – | Quality of healing (4‐point scale) | VAS (0–10) | 4‐point scale (excellent‐ poor) | – | – | – | |
| Silver hydrocolloid dressing (Aquacel Ag) vs MEBO | 6 months | – | Vancouver Scar Scale, incidence hypertrophic scars | VAS (0–10) | 3‐point scale: (comfortable‐discomfortable) | – | – | – | |
| Topical antimicrobial agents compared with other topical antimicrobial agents | |||||||||
| Mafenide acetate cream and gentamicin via iontophoresis (7 participants) vs usual care (mafenide acetate cream) (8 participants) | Until wound healing | Surgery, following treatment by topical agent | – | – | – | – | – | LOS | |
| Cerium‐SSD vs SSD | 12 months | Surgery, following treatment by topical agent | POSAS, colour (dermaspectometer), elasticity (cutometer), functional and anatomical impairments | VAT (0–10) | – | – | – | – | |
| Skin substitutes compared with topical antimicrobial agents | |||||||||
| Biological skin substitute coated with fibronectin (TransCyte) vs Bacitracin | Until wound healing | – | – | VAS (0–10) | – | – | – | LOS | |
| Bioactive skin substitute (a bilayered, biologically active, temporary skin substitute (TransCyte)) vs antibacterial ointment, not specified | Until wound healing | – | – | VAS (0–10) | – | – | – | – | |
| Glycerolised allograft cadaver (corpse) skin vs SSD | 6 months (maximum) | – | Not specified | – | – | – | – | – | |
| Biological dressing (porcine Xenoderm) vs Physiotulle Ag (Coloplast) 50 wounds in 25 participants | 3 months (scar) | – | Vancouver Scar Scale | VAS (0–10) | – | – | – | – | |
| Miscellaneous treatment compared with miscellaneous treatment | |||||||||
| MEBO vs saline‐soaked dressing | Until wound healing | – | Quality of healing (4‐point scale) | VAS (0–10) | – | – | – | – | |
| Enzymatic debridement (Collagenase) vs antimicrobial agent (Bacitracin) | 6 months | – | POSAS | VAS (0–10) | – | All‐cause mortality, serious adverse effects | – | – | |
| Cream containing Helix Aspersa extract (Elicina) vs MEBO | 2 years (scar) | – | – | VAS (1–10) | – | – | – | – | |
LOS: length of hospital stay; MEBO: Moist Exposed Burn Ointment; POSAS: Patient and Observer Scar Assessment Scale; QoL: quality of life; SSD: silver sulphadiazine; VAS: visual analogue scale.
Sponsorship
Six of 12 studies explicitly stated that the authors had no conflict of interest (Ang 2000; Lehna 2017; Mabrouk 2012; Oen 2012; Tsoutsos 2009; Wang 2015), while the authors of other studies provided no information about sponsorship (Demling 1999; Demling 2002; Desai 1991; Hindy 2009; Horch 2005; Jiaao 2011). In three of these five studies, the intervention was an explicitly mentioned brand, but it was not stated whether this application was sponsored or purchased (Demling 1999; Demling 2002; Hindy 2009).
Excluded studies
Four additional potentially eligible studies were excluded in this update. Full‐text analysis resulted in four exclusions because the study was not an RCT (Schulz 2016), or did not involve facial burns (Aboelnaga 2018; Hundeshagen 2018; Moenadjat 2008).
In the original review, nine studies (10 records) were excluded because they were not RCTs (Branski 2008; Covey 1987; Hartmann 2007; Lansdown 2004; Li 2005; Liang 2007; Papp 1990), or because the focus of the study was not on facial burns (Ang 2001; Rege 1999).
The total number of excluded studies is now 13.
Studies awaiting assessment
No studies are awaiting classification.
Ongoing studies
Four studies are ongoing and have been detailed in the Characteristics of ongoing studies table (ACTRN12615001205527; ACTRN12618001631291; ACTRN12619001050145; TCTR20171004003).
Risk of bias in included studies
In most studies, there was a high risk of bias related to blinding (performance and detection bias), whereas the risk of bias related to random sequence and allocation concealment (selection bias) was often unclear. The risk of bias judgements are presented in the 'Risk of bias' summary (Figure 2) (part of Characteristics of included studies table), and the 'Risk of bias' graph (Figure 1), and are described below.
Allocation
Of the 12 included studies, only three studies adequately described the method of sequence generation (Ang 2000; Oen 2012; Tsoutsos 2009), and two studies adequately described the method of allocation concealment (Ang 2000; Oen 2012). Tsoutsos 2009 did not report on allocation concealment, but added in a personal communication, "the generator was used by the resident responsible for admitting the patient, who also checked for eligibility." This, in combination with the unequal number of randomised participants per group (27 versus 19) resulted in a judgement of high risk of bias. The other studies stated that participants were randomised, but did not describe the method of sequence generation or allocation concealment (Demling 1999; Demling 2002; Desai 1991; Hindy 2009; Horch 2005; Jiaao 2011Lehna 2017; Mabrouk 2012; Wang 2015).
Blinding
Review authors had to judge the blinding of participants, care providers and outcome assessors. One of 12 studies reported blinding of participants, care providers and assessors (Lehna 2017), one study reported blinding of participants and outcome assessors (personal communication, Tsoutsos 2009), and one study reported blinding of outcome assessors only (Ang 2000). Jiaao 2011 did not report blinding, but used recombinant human granulocyte‐macrophage stimulating factor (rhGMCSF) hydrogel and a placebo hydrogel (matrix hydrogel), suggesting that at least participants were blinded. In almost all studies, the nature of treatments made it impossible to blind participants and care providers and thus the review authors made a judgement of 'no' rather than one of 'unclear.' Three studies reported blinding of outcome assessors (Ang 2000; Lehna 2017; Tsoutsos 2009); four studies clearly did not undertake blinded outcome assessment (Demling 1999; Demling 2002; Oen 2012; Wang 2015), and in five studies it was unclear whether the outcome assessor was blinded (Desai 1991; Hindy 2009; Horch 2005; Jiaao 2011; Mabrouk 2012).
Incomplete outcome data
The item 'incomplete outcome data' includes a high dropout rate or an imbalance in dropout in combination with an absent ITT analysis. Seven studies showed no risk of attrition bias (Ang 2000; Demling 2002; Hindy 2009; Horch 2005; Lehna 2017; Tsoutsos 2009; Wang 2015), in four studies risk of bias was unclear (Demling 1999; Desai 1991; Jiaao 2011; Mabrouk 2012), and one study had a high risk of bias because of high dropout (Oen 2012).
The dropout rate was described and acceptable (i.e. did not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and did not lead to substantial bias) in seven studies (Ang 2000; Demling 2002; Hindy 2009; Horch 2005; Lehna 2017; Tsoutsos 2009; Wang 2015), while four studies did not report it or made it evident in the presentation of the outcome data (Demling 1999; Desai 1991; Jiaao 2011; Mabrouk 2012). In Oen 2012, loss to follow‐up was high (maximum 24.4% at 3 months, 34.2% at 6 months and 39.5% at 12 months); additional information on reasons for dropout were reported. Loss to follow‐up was related to shorter hospital stay and smaller burn size.
An imbalance in dropout in combination with a lack of ITT analysis was not observed. Two studies performed ITT analyses (Demling 2002; Oen 2012); another six studies did not report ITT analysis specifically, but it appeared likely when the studies were assessed (Hindy 2009; Horch 2005; Lehna 2017; Mabrouk 2012; Tsoutsos 2009; Wang 2015). One study stated that ITT analysis was performed, but assessment of the study showed clearly that it had not (Ang 2000). The final three studies were unclear on this topic (Demling 1999; Desai 1991; Jiaao 2011).
Selective reporting
Nine studies were free of the suggestion of selective outcome reporting (Ang 2000; Demling 1999; Demling 2002; Hindy 2009; Horch 2005; Mabrouk 2012; Oen 2012; Tsoutsos 2009; Wang 2015), in one study the reporting was unclear in the absence of sufficient information (no protocol or full‐text paper available) (Jiaao 2011), and in two studies risk of reporting bias seemed apparent (Desai 1991; Lehna 2017). Desai 1991 listed wound healing as an outcome of interest but did not report it in the results section. Lehna 2017 assessed wound infection, pain and scar quality but did not report the results.
Other potential sources of bias
Potential sources of bias existed in three studies, especially related to baseline characteristics. In two studies, the description of baseline characteristics was limited to only one important prognostic indicator (respectively %TBSA burned and age (Ang 2000; Tsoutsos 2009)), which was insufficient to make a judgement of low risk of bias for baseline similarity. In a third study, the %TBSA burned in both groups was not similar and no information was provided about aetiology (Desai 1991).
Bias related to co‐interventions was assessed and limited; three studies avoided (or similar) co‐interventions (Demling 1999; Mabrouk 2012; Oen 2012); four studies provided no information about co‐interventions (Desai 1991; Horch 2005; Jiaao 2011; Tsoutsos 2009), and another three studies presented small differences in treatment procedures in the methods sections (Ang 2000; Hindy 2009) or stated "subsequent care in the intervention group when needed" without reporting whether this care was applied (Demling 2002).
Due to the within‐participant design of two studies (i.e. two wounds in one participant), risks of above‐described sources of bias were small (Lehna 2017; Wang 2015). Baseline characteristics (i.e. age and %TBSA burned) did not differ and co‐interventions affected both wounds of the participant, except where localised co‐interventions were applied.
There were no specific unit of analysis issues. Two studies used a within‐participant design; one study did not conduct an appropriate analyses, reflecting the paired data (Lehna 2017).
Effects of interventions
See: Summary of findings 1 Topical antimicrobial agent compared with topical non‐antimicrobial agent for facial burns; Summary of findings 2 Topical antimicrobial compared with alternative antimicrobial agent for facial burns; Summary of findings 3 Skin substitute compared with topical antimicrobial agent for facial burns; Summary of findings 4 Miscellaneous treatment compared with miscellaneous treatment for facial burns
The effects of topical agents on facial burns are summarised in summary of findings Table 1: topical antimicrobial agent compared with topical non‐antimicrobial agent; summary of findings Table 2: topical antimicrobial agents compared with other topical antimicrobial agents; summary of findings Table 3: skin substitutes compared with topical antimicrobial agents; and summary of findings Table 4: miscellaneous treatment compared with miscellaneous treatment.
In addition, effects of interventions in individual studies are presented in Table 3.
| Study ID | Main baseline characteristics | Number of participants and dropout rate | Primary outcomes | Secondary outcomes |
|---|---|---|---|---|
| Number of participants: I: 22; C: 17. % TBSA burned (mean): I: 1.56 (SE 0.18; range 0.5–3.5). C: 2.16 (SE 0.38; range 0.13–6.0). | Initial number of participants: 115. After randomisation: I: 58; C: 57. Dropouts: I: 0; C:3. Participants with facial burns: I: 22; C:17. Participants in short‐term analysis: I: 22; C:17. Participants in long‐term analysis (6 months): I: 20; C:17. | Time to complete wound healing (number of days taken for face‐wound to heal): I: not reported; C: 2–35 days (range). Proportion completely healed in 10 days: I: 17/22; C: 14/17. | Need for surgery (need for reconstructive surgery 6 months PB): I: 0/20; C: 0/17. | |
| Number of participants: Minor burns: I: 5; C: 5. Mean age: Minor burns: I: 31 (SD 8) years; C: 29 (SD 7) years. Aetiology: flame. % TBSA burned (mean): Minor burns: I: 10 (SD 3); C: 7 (SD 2). % TBSA burned full‐thickness (mean): Minor burns: I: 0; C: 0. | Initial number of participants: 21. Number of participants with minor burns: 10. Number of participants with major burns: 11. After randomisation: Minor burns: I: 5; C: 5. Participants in short‐term analysis: Minor burns: I: 5; C: 5. | Time to partial wound healing (mean number of days to > 90% re‐epithelialisation): Minor burns: I: 8 (SD 1); C: 12 (SD 3); reported P < 0.05. Major burns: I: 8 (SD 2); C: 14 (SD 4); reported P < 0.05. Wound infection (signs of local wound infection): Minor burns: I: 0; C: 0. | Pain (procedural pain, during facial care (mean): Minor burns: I: 2 (SD 1); C: 5 (SD 1). Pain (background pain, between facial care) (mean): Minor burns: I: 1 (SD 0.5); C: 3 (SD 2). Length of stay (mean): Minor burns: I: 1 (SD 0.5); C: 3 (SD 1). | |
| Number of participants: I: 16; C: 18. Mean age: I: 39 (SD 9); C: 40 (SD 8). Aetiology: Flame: I: 11; C: 12. % TBSA burned (mean): I: 24 (SD 8); C: 21 (SD 9). % TBSA burned full‐thickness (mean): I: 12 (SD 7); C: 10 (SD 6). | Initial number of participants: 34. After randomisation: I: 16; C: 18. Participants in short‐term analysis: I: 16; C: 18. | Time to partial wound healing (mean number of days to > 95% re‐epithelialisation: I: 9 (SD 4); C: 15 (SD 4); reported P < 0.05. | Pain (procedural pain, during facial care) (mean): I: 3 (SD 1); C: 7 (SD 2). Pain (background pain, between facial care) (mean): I: 2 (SD 1); C: 4 (SD 2). | |
| Number of participants: I: 7; C: 8. Mean age: I: 11.4 (SE 1.2); C: 9.5 (SE 1.6). % TBSA burned (mean): I: 35 (SE 7); C: 50 (SE 6). % TBSA burned full‐thickness (mean): I: 20 (SE 9); C: 32 (SE 7). | Initial number of participants: 15. After randomisation: I: 7; C: 8. Participants in short‐term analysis: I: 7; C: 8. | Wound infection (occurrence of chondritis): I: 3; C: 4. | Need for surgery (mean number of surgical procedures): I: 1.2 (SE 0.1) C: 1.0 (SE 0); reported P < 0.05. Adverse effects (occurrence of gentamicin‐resistant micro‐organisms): I: 29%; C: 0%. Length of stay (mean): I: 26 (SE 1); C: 38 (SE 3). | |
| Number of participants: I: 20; C1; 20; C2: 20. | Initial number of participants: 60. After randomisation: I: 20; C1: 20; C2: 20. Participants in short‐term analysis: I: 20; C1: 20; C2: 20. | Time to complete wound healing (mean): I: 10.05 (SD 2.3); C1: 10.35 (SD 2.8); C2: 12.05 (SD 2.4), reported P < 0.05. | Pain: (mean) day 1 and 2: C1: 3.1 (SD 1.9); day 3 onwards: C1: 1.3 (SD 1.5), I and C2 not presented, least pain in C1, reported P < 0.05). Scar quality (quality of healing): excellent: I: 16; C1: 16; C2: 11, reported P > 0.05. Patient satisfaction: excellent: I: 11; C1: 12; C2: 2, reported P < 0.05. | |
| Number of participants: I: 5; C; 5. Median age I+C: 34.3 (range 24–67) years. | Initial number of participants: 10. After randomisation: I: 5; C: 5. Participants in short‐term analysis: I: 5; C:5. Participants in long‐term analysis: I: 5; C:5. | Time to complete wound healing (median) I: 10.5; C: 12.4, reported P < 0.05. Wound infection (signs of underlying infection) I: 0; C: not reported | Scar quality (scar formation, maximum 6 months): I: 0; C: 2. Adverse effects (localised partial integration of the biological dressing) I: 1; C: 0. | |
| Number of participants: I+C: 30 | Initial number of participants: 30. After randomisation: ? Participants in short‐term analysis: ? | Time to complete wound healing (mean): I: 13.47 (SD 1.08) days; C: 18.69 (SD 2.35) days, reported P < 0.01 Change in wound surface area over time: percentage of healing at 3, 5, 7 and 14 days after treatment, reported P < 0.01 at day 5, 7 and 14 days after treatment. | Not reported. | |
| Number of participants: 10 (within patient comparison). Age: 9 participants aged 18–64 years, 1 participant aged ≥ 65 years. | Initial number of participants: 10. After randomisation: 10. Participants in short‐term analysis: 10. Participants in long‐term analysis: no data provided. | Time to complete wound healing (days to complete epithelialisation, mean): I1: 37.6; I2: 37.4, reported P = 0.0853. Wound infection: not reported | Pain: not reported. Scar quality: not reported. Adverse effects: all‐cause mortality: 1. | |
| Number of participants: I1 (Aquacel Ag): 20; I2 (MEBO): 20. Mean age: I1: 41.4 years, I2: 37.5 years. Total TBSA range: I: 5–30, C: 7–30. | Initial number of participants: 40. After randomisation: I1: 20; I2: 20. Participants in short‐term analysis: I1: 20; I2: 20. Participants in long‐term analysis (3, 6 months), I1: 7–10; I2: 10–14 (table 3). | Time to complete wound healing (mean) I1: 10.5 days; I2: 12.4 days, reported P < 0.05. Wound infection (rate) I1: 3/20; I2: 8/20. | Pain (mean): I1: 4.1; I2: 4.6. Scar quality (Vancouver Scar Scale): I1: 4; I2: 7, reported P > 0.05. Patient satisfaction (comfortable, mild discomfort, discomfort): I1: 14, 2, 4; I2: 10, 4, 6. | |
| Number of participants: I1: 78; I2: 76. Mean age: I: 41.9 (SD 16.9) years; I2: 41.3 (SD 14.5) years. Aetiology: Flame: I1: 70; I2: 60. % TBSA burned (median (IQR): I1: 9.8 (5.0–19.4); I2: 9.3 (4.5–17.0). | Initial number of participants: 179. After randomisation: I1: 90; I2: 89. After postrandomisation exclusion: I1: 78; I2: 76. Participants in short‐term analysis (primary analysis): I1: 77; I2: 73. Participants in long‐term analysis (6 months) I1: 60; I2: 50) (12 months): I1: 50; I2: 46. | Time to wound partial healing: (survival analysis, all participants), reported P = 0.71. Median days (25–75th percentile) to healing (> 90%) in wounds without surgery: I1: 11.0 (7.0–15.0); I2: 9.0 (5.0–17.75), reported P = 0.17. Mean time to wound healing: I1: 14.3 (SD 14.5) days; I2: 21.4 (SD 37.6) days (data delivered upon request). | Need for surgery: I1: 13/77; I2: 15/73, reported P = 0.565, OR 0.8 (95% CI 0.3 to 1.8). Pain (background pain, before wound care, mean): I1: 0.6 (SEM 0.2); I2: 1.2 (SEM 0.4), reported P = 0.16. Pain (procedural pain, during wound care): I1: 1.3 (SEM 0.3); I2: 1.6 (SEM 0.5), reported P = 0.59. Scar quality at 12 months (Patient and Observer Scar Assessment Scale) (median): (patient report): I1: 1.2 (25–75th percentile 1.0–2.2); I2: 1.0 (25–75th percentile 1.0–1.8), reported P = 0.42; (observer report): I1: 1.4 (25–75th percentile 1.2–1.8); I2: 1.4 (25–75th percentile 1.2–1.6), reported P = 0.17. (mean): (patient report) I1: 2.0 (SD 1.5); I2: 1.8 (SD 1.5); (observer report) I1: 1.7 (SD 0.9); I2: 1.6 (SD 0.8) (delivered upon request). | |
| Number of participants I: 27; C: 16. | Initial number of participants: 46. After randomisation: I: 27; C: 19, after postrandomisation exclusion: I: 27; C: 16. Participants in short‐term analysis: not documented. | Time to complete wound healing (mean days: I: 11 (SD 2); C: 15 (SD 3), reported P < 0.0001. Number of participants with complete wound healing within 14 days: I: 27/27; C: 3/16, P value not reported. Wound infection: bacterial colonisation in all participants; absence of signs of systemic infection. | Pain (mean pain reduction scores): I: 2.7 (SD 1.35); C: 2.00 (SD 0.89), reported P interaction intervention by change pain = 0.072. | |
| Number of participants: 25 (within‐patient comparison): 15 superficial degree II burns; 10 deep degree II burns. % TBSA burned (mean): 45.1 (SD 25.2) (superficial degree II burns); 54.9 (SD 31.7) (deep degree II burns). | Initial number of participants: 25. After randomisation: 25. Participants in short‐term analysis: 25. Participants in long‐term analysis: no numbers provided. | Time to wound healing (mean days) Superficial degree II burns: I: 8.6 (SD 1.12); C: 8.67 (SD 1.18) (reported P = 0.855). Deep degree II burns: I: 15.80 (SD 1.55); C: 18.20 (SD 1.55), reported P = 0.005. Infection: Superficial degree II burns: I: 1/15; C: 1/15, P > 0.05 Deep degree II burns: I: 1/10; C: 1/10, reported P > 0.05. | Pain (mean VAS) Superficial degree II burns: I: 2.73 (SD 0.70); C: 1.53 (SD 0.83), reported P = 0.000. Deep degree II burns: I: 5.70 (SD 0.95); C: 2.70 (SD 0.48), reported P = 0.000. Scar quality at 3 months (mean Vancouver Scar Scale) Superficial degree II burns: I: 0.80 (SD 0.77); C: 0.80 (SD 0.77), reported P = 1.000. Deep degree II burns: I: 3.50 (SD 0.71); C: 5.50 (SD 1.08), reported P = 0.000. |
C: comparator; CI: confidence interval; I: intervention; IQR: interquartile range; MEBO: Moist Exposed Burn Ointment; OR: odds ratio; PB: postburn; SD: standard deviation; SE: standard error; SEM: standard error of the mean; TBSA: total body surface area.
Comparison 1: topical antimicrobial agent compared with topical non‐antimicrobial agent
Three studies including 139 people compared topical antimicrobial agents with topical non‐antimicrobial agents (Ang 2000; Hindy 2009; Mabrouk 2012). The antimicrobials agents included SSD and Aquacel‐Ag and were compared with MEBO or saline‐soaked dressings.
There is moderate to very low‐certainty evidence that antimicrobial agents and non‐antimicrobial agents are similar in time to wound healing and wound infection (summary of findings Table 1).
Primary outcomes
Time to complete wound healing
There is mixed‐certainty evidence that there is no clear difference in time to complete wound healing (no overall effect size available). One study revealed moderate
‐certainty evidence. suggesting that SSD and MEBO probably make little or no difference in time to complete wound healing (reported HR 0.84, 95% CI 0.78 to 1.85; 1 study, 39 participants; downgraded once for imprecision). Another study presented similar results on mean time to wound healing in a three‐group comparison of Aquacel‐Ag with MEBO or with saline‐soaked dressings, each applied in 20 patients (mean days to wound healing 10.05 (SD 2.3) versus 10.35 (SD 2.8) and 12.05 (SD 2.4), no overall effect estimate available, 1 study, 60 participants; downgraded once for risk of bias and once for imprecision (summary of findings Table 1); (Analysis 1.1).
Proportion of wounds completely healed
There is low‐certainty evidence that SSD and MEBO may make little or no difference to the proportion of wounds completely healed within 10 days (RR 0.94, 95% CI 0.68 to 1.29; 1 study, 39 participants; downgraded twice for imprecision; Analysis 1.2).
Change in wound surface area over time or partial healing
No studies measured change in wound surface area over time or partial healing.
Wound infection
We are uncertain whether there is a difference in wound infection between a topical antimicrobial (Aquacel‐Ag) and a non‐antimicrobial agent (MEBO) (RR 0.38, 95% CI 0.12 to 1.21; 1 study, 40 participants; very low‐certainty evidence, downgraded once for risk of bias and twice for imprecision; Analysis 1.3).
Secondary outcomes
Proportion of facial burns requiring surgery
One study assessed surgery. No participant in either group received surgery, thus no treatment effect was estimable (1 study, 37 participants; Analysis 1.4).
Scar quality
There is low‐certainty evidence that topical antimicrobial agents or topical non‐antimicrobial agents may make little or no difference to scar quality (RR 1.19, 95% CI 0.87 to 1.61; 1 study (2 comparisons), 60 participants; I2 = 29%; low‐certainty, evidence downgraded once for imprecision and once for risk of bias; Analysis 1.5).
Mabrouk 2012 addressed scar quality but provided incomplete results and could not be included in the analysis.
Pain
Two studies assessed and incompletely reported pain (Hindy 2009; Mabrouk 2012).
Patient satisfaction
Topical antimicrobial agents (i.e. hydrofibre Aquacel Ag) may result in slightly higher participant satisfaction, compared with non‐antimicrobial agents (including MEBO) and saline‐soaked dressings) (RR excellent/comfortable patient satisfaction 1.55, 95% CI 1.06 to 2.27; 2 studies (3 comparisons), 100 participants; I2 = 64%; low‐certainty evidence, downgraded once for imprecision and once for risk of bias; Analysis 1.6).
Adverse effects
None of the studies addressed adverse effects.
Quality of life
None of the studies addressed quality of life.
Length of hospital stay
None of the studies addressed LOS.
Comparison 2: topical antimicrobial agents compared with other topical antimicrobial agents
Two studies compared topical antimicrobial agents with another topical antimicrobial agent in 169 participants (Desai 1991; Oen 2012). The antimicrobial agents included CN‐SSD versus SSD and mafenide acetate cream and gentamicin via iontophoresis versus usual care (mafenide acetate cream).
There is low‐certainty evidence that antimicrobial agents are similar in time to wound healing and very low‐certainty evidence that they are similar in wound infection (summary of findings Table 2).
Primary outcomes
Time to complete wound healing
No studies measured time to complete wound healing.
Proportion of wounds completely healed.
No studies measured the proportion of wounds completely healed.
Change in wound surface area over time or partial healing
CN‐SSD and SSD may make little or no difference in time to complete wound healing (low‐certainty evidence, downgraded once for imprecision and once for risk of bias). Oen 2012 reported time to event analysis on time to more than 90% wound healing and found no differences between CN‐SSD (antimicrobial agent 1) versus SSD (antimicrobial agent 2) (survival analysis, Kaplan Meier; 129 participants; reported P = 0.71 (log‐rank test)), and provided mean time to wound healing (MD –7.10 days, 95% CI –16.43 to 2.23; 1 study, 142 participants; Analysis 2.1).
Wound infection
We are uncertain whether using several antimicrobial agents (mafenide acetate cream and gentamicin via iontophoresis versus usual care (mafenide acetate cream) reduces wound infection (infection, resulting in chondritis (inflammation of cartilage) (Desai 1991)), as the certainty of evidence is very low (RR 0.73, 95% CI 0.46 to 1.17; downgraded twice for risk of bias and twice for imprecision; Analysis 2.2).
Secondary outcomes
Proportion of facial burns requiring surgery
There is low‐certainty evidence that there is no clear difference between topical antimicrobial agents in the proportion of facial burns requiring surgery (RR 0.82, 95% CI 0.42 to 1.61; 1 study, 150 participants; downgraded once for risk of bias and once for imprecision; Analysis 2.3).
Scar quality
There is low‐certainty evidence that there is little or no difference between topical antimicrobial agents in scar quality as reported by the participant (MD 0.20 on a scale from 1‐10, 95% CI –0.40 to 0.80; 1 study, 85 participants; Analysis 2.5) and by the care provider/observer (MD 0.10, 95% CI –0.24 to 0.44; 1 study, 96 participants; Analysis 2.6). The evidence was downgraded once for risk of bias and once for imprecision.
Pain
There is low‐certainty evidence that there is little or no difference between topical antimicrobial agents in pain, as assessed on a 0‐10 scale with the visual analogue thermometer, in procedural pain (MD –0.30, 95% CI –1.44 to 0.84; 1 study, 150 participants; Analysis 2.7); or background pain (MD –0.60, 95% CI –1.48 to 0.28; 1 study, 150 participants; Analysis 2.8). The evidence was downgraded once for risk of bias and once for imprecision.
Patient satisfaction
Neither study reported patient satisfaction.
Adverse effects
It is uncertain whether gentamicin iontophoresis reduces adverse events (appearance of gentamicin‐resistant organisms) as the certainty of the evidence is very low (downgraded twice for risk of bias and twice for imprecision) (2/7 versus 0/8; RR 5.63, 95% CI 0.31 to 100.52; 1 study, 15 participants; Analysis 2.9).
Quality of life
Neither study reported quality of life.
Length of hospital stay
It is uncertain whether gentamicin iontophoresis reduces LOS as the certainty of the evidence is very low (downgraded twice for risk of bias and twice for imprecision) (MD –12.00 days, 95% CI –18.20 to –5.80; 1 study, 15 participants; Analysis 2.10); however, there are no differences if differences in burn size were taken into account.
Comparison 3: skin substitutes compared with topical antimicrobial agents
Four trials of 90 people, including 115 wounds, compared skin substitutes with topical antimicrobial agents. Three studies randomised and compared 65 participants, the fourth study randomised and compared two facial wounds within 25 participants (Wang 2015).
Four studies compared a skin substitute (a bioengineered skin substitute (Demling 1999; Demling 2002), an allograft (Horch 2005), or a xenograft (Wang 2015) with an topical antimicrobial agent (bacitracin (Demling 1999), SSD (Horch 2005), Physiotulle Ag (Wang 2015), or an unspecified antibacterial ointment (Demling 2002)).
There is low‐certainty evidence on the effects of skin substitutes versus topical antimicrobial agents in time to wound healing and very low‐certainty evidence on wound infection (summary of findings Table 3).
Primary outcome
Time to complete wound healing
One study reported time to complete wound healing (Horch 2005), but they did not use the appropriate statistical method (i.e. survival methods for time to event data). Data from Horch 2005 could not be used because of lack of estimate of variance. The authors reported the median time to re‐epithelialisation, which was significantly less in the allograft group (10.5 days; 5 participants) compared with the SSD group (12.4 days; 5 participants; reported P < 0.05).
Proportion of wounds completely healed
None of the studies reported on the proportion completely healed.
Change in wound surface area over time or partial healing
Three studies reported time to partial wound healing (time to 95% wound healing (Demling 1999; Wang 2015); time to 90% wound healing (Demling 2002)); but used no appropriate statistical method (i.e. survival methods for time to event data). Due to differences in study design (unit of analysis (participant versus wounds)) and data presentation (mean versus median), no overall effect estimate was calculated.
Because of concerns about possible patient overlap between two studies (Demling 1999; Demling 2002), only data from the most recent study with the largest sample size were included in the analysis.
There is low‐certainty evidence that a skin substitute may slightly reduce mean time to partial wound healing compared with the antibacterial ointment group (MD –6.00 days, 95% CI –8.69 to –3.31; 1 study, 34 participants; downgraded once for risk of bias and once for imprecision; Analysis 3.1).
Data from Wang 2015 could not be incorporated into the analysis because of a comparison of wounds within patients. Wang 2015 reported a mean time to wound healing in both superficial and deep partial thickness burns; mean time to wound healing was lower after skin substitute treatment in deep dermal burns (MD –2.40 days, 95% CI –3.76 to –1.04; 1 study, 10 participants (20 wounds); Analysis 3.2); but there were no differences in superficial burns (MD –0.07 days, 95% CI –0.89 to 0.75; 1 study, 15 participants (30 wounds); Analysis 3.2).
Wound infection
We are uncertain whether skin substitutes reduce wound infection as the certainty of the evidence is very low. Very low‐certainty evidence in combination with non‐estimable effects results in inconclusive effects of skin substitutes versus topical antimicrobial agents (Analysis 3.3, downgraded once for risk of bias and twice for imprecision). Three studies reported wound infection. In two studies, none of the participants showed signs of infection (Demling 1999; Horch 2005 (data on skin substitute only)). Wang 2015 reported "one infection in each group", probably one infected wound in each group.
Secondary outcomes
Proportion of facial burns requiring surgery
None of the studies reported proportion of facial burns requiring surgery.
Scar quality
It is uncertain whether skin substitutes improve scar quality as the certainty of the evidence is low to very low. In addition, incomparable data presentation resulted in an inconclusive outcome. Scar quality was a reported outcome in two studies and assessed as the proportion hypertrophic scar formation six months postburn (Horch 2005), or with the Vancouver Scar Scale (VSS) three months postburn in wounds within patients (Wang 2015). Very low‐certainty evidence from Horch 2005 showed that the skin substitute group may have little to no reduction in the prevalence of hypertrophic scars compared with the antimicrobial group (RR 0.20, 95% CI 0.01 to 3.35; 1 study, 10 participants; Analysis 3.4, downgraded once for risk of bias and twice for imprecision). It is uncertain whether xenografts reduce scar morbidity. Wang 2015 reported lower mean VSS scores after skin substitute treatment in deep dermal burns (MD –2.00 on a scale from 0‐15, 95% CI –2.80 to –1.20; 1 study, 10 participants (20 wounds); Analysis 3.5), but no differences in superficial burns (MD 0.00, 95% CI –0.55 to 0.55; 1 study, 15 participants (30 wounds); Analysis 3.5) (low‐certainty evidence, downgraded once for risk of bias and once for imprecision).
Pain
Three studies reported pain (Demling 1999; Demling 2002; Wang 2015); the studies of Demling assessed both procedural pain and background pain (Demling 1999; Demling 2002).
We are uncertain whether skin substitutes in general reduce pain as the certainty of the evidence is very low (downgraded once for risk of bias, once for inconsistency and once for imprecision).
Individual studies revealed inconclusive results based on low‐certainty evidence. In Demling 1999 and Demling 2002, pain scores were significantly lower in all comparisons between skin substitute groups (TransCyte) versus the topical treatment groups. Because of concerns about possible patient overlap between Demling 1999 and Demling 2002, the summary effect estimate was calculated based on data from the most recent Demling study only (Demling 2002). Low‐certainty evidence shows that skin substitutes may slightly reduce procedural pain (MD –4.00 on a 0‐10 scale, 95% CI –5.05 to –2.95; 1 study, 34 participants; Analysis 3.6) and background pain (MD –2.00, 95% CI –3.05 to –0.95; 1 study, 34 participants; Analysis 3.6). In contrast, low‐certainty evidence from Wang 2015 indicated that skin substitutes (biological dressing (porcine Xenoderm)), compared with antimicrobial agents (Physiotulle Ag (Coloplast)) might slightly increase pain in superficial burns (MD 1.20 on a 0‐10 scale, 95% CI 0.65 to 1.75; 1 study, 15 participants (30 wounds); Analysis 3.8), as well as deep partial thickness burns (MD 3.00, 95% CI 2.34 to 3.66; 1 study, 10 participants (20 wounds); Analysis 3.8).
Patient satisfaction
None of the studies reported patient satisfaction.
Adverse effects of treatment
It is uncertain whether skin substitutes reduce adverse effects of treatment as the certainty of the evidence is very low (downgraded once for risk of bias, and twice for imprecision). One study reported that one participant in the intervention group had a localised integration of the biological dressing, which was removed by two repeated dermabrasion manoeuvres (Horch 2005). After these manoeuvres, no more visible allograft remnants remained.
Quality of life
None of the studies reported quality of life.
Length of hospital stay
It is uncertain whether skin substitutes reduce LOS as the certainty of the evidence is very low (selective data reporting in Demling 1999) (MD –2.00 days, 95% CI –2.98 to –1.02; 1 study, 10 participants; downgraded once for risk of bias, and twice for imprecision; Analysis 3.9).
Comparison 4: miscellaneous treatment compared with miscellaneous treatment
Four studies compared a variety of miscellaneous topical treatments in 123 participants and 148 wounds including Jiaao 2011 (comparison growth hormone therapy (rhGM CS) versus a placebo hydrogel); Hindy 2009 (comparison MEBO versus saline); Lehna 2017 (comparison enzymatic debridement versus topical antimicrobial agent in wounds within participants); and Tsoutsos 2009 (comparison alternative topical agent versus MEBO).
Low to very low‐certainty evidence shows mainly inconclusive effectiveness of these treatments in time to wound healing and infection (summary of findings Table 4).
Primary outcomes
Time to complete wound healing and proportion of wounds completely healed
Four studies reported time to complete wound healing, one based on a comparison of wounds within patients (Lehna 2017). Tsoutsos 2009 also reported the proportion of participants who were completely healed at 14 days.
We did not aim to calculate overall effect estimates because of the heterogeneity of interventions. Individual studies showed low to very low‐certainty evidence for effects of interventions, downgraded once for risk of bias and once or twice for imprecision.
MEBO may slightly reduce time to wound healing compared with saline‐soaked dressing (MD –1.7 days, 95% CI –3.32 to –0.08; 1 study, 40 participants; Hindy 2009; Analysis 4.1). Lehna 2017 reported similar time to complete epithelialisation in 10 participants with two wounds each (mean: 37.6 days after enzymatic debridement versus 37.4 days after antimicrobial agent; reported p‐value not shown because of the use of inappropriate analyses not reflecting the paired character of the data). This is low‐certainty evidence downgraded once for risk of bias and once for imprecision in each case.
A cream containing Helix Aspersa may slightly increase the proportion of wounds completely healed at 14 days compared with MEBO (RR 4.77, 95% CI 1.87 to 12.15; 1 study, 43 participants; Analysis 4.2). This is low‐certainty evidence downgraded once for risk of bias and once for imprecision.
Change in wound surface area over time or partial healing
One study measured change in wound surface area over time or partial healing, by assessing percentage wound healing at 3, 5, 7 and 14 days after treatment (Jiaao 2011). Reporting of results was limited to the reported P < 0.01 difference at days 5, 7 and 14; no data were reported; no GRADE assessment was possible.
Wound infection
One study addressed wound infection. In the absence of clinical infections in both groups, no effect estimate was calculated (Tsoutsos 2009; Analysis 4.3). The certainty of evidence is very low (downgraded once for risk of bias and twice for serious imprecision). Lehna 2017 planned to assess wound infection but did not report outcomes addressing wound infections.
Secondary outcomes
Proportion of facial burns requiring surgery
None of the studies reported proportion of facial burns requiring surgery.
Scar quality
MEBO may make little or no difference to scar quality (quality of healing) compared with saline soaked dressings (RR excellent scar quality 1.45, 95% CI 0.92 to 2.29; 1 study, 40 participants; low‐certainty evidence downgraded once for risk of bias and once for imprecision; Analysis 4.4) (Hindy 2009). Lehna 2017 planned to assess scar quality but did not report outcomes addressing scar quality.
Pain
A cream containing Helix Aspersa may make little or no difference to pain, compared with MEBO (MD 0.70 points on a 0 to 10 scale, 95% CI 0.03 to 1.37; 1 study, 43 participants; low‐certainty evidence downgraded once for risk of bias and once for imprecision; Analysis 4.5) (Tsoutsos 2009). The data reported by Hindy 2009 were insufficient for analysis. They graphically presented pain scores per treatment group at days one or two postburn and from day three onwards. In addition, Hindy 2009 reported on participants treated with MEBO (comparator intervention 1) as "…patients felt the least pain on the first two days (mean 3.1 on a 0‐10 scale, SD [standard deviation] 1.9) and on day 3 onwards (mean 1.3, SD=1.5)", but data on other groups were not reported. Lehna 2017 planned to assess pain but did not report outcomes addressing pain.
Patient satisfaction
We are uncertain whether MEBO improves patient satisfaction compared with saline‐soaked dressings (excellent patient satisfaction; RR 6.00, 95% CI 1.54 to 23.44; 1 study, 40 participants; very low‐certainty evidence, downgraded once for risk of bias and twice for imprecision; Analysis 4.6) (Hindy 2009).
Adverse effects
None of the studies reported adverse effects.
Quality of life
None of the studies reported quality of life.
Length of hospital stay
None of the studies reported LOS.
Discussion
Summary of main results
We included 12 RCTs in this review that evaluated the effect of a variety of topical interventions for facial burns. These trials enrolled 507 participants, mainly adults, admitted in specialised burn centres after recent burn injuries.
Using GRADE, we assessed the certainty of the evidence, which is low to very low for almost all primary outcomes in the four comparisons. The reasons for these judgements are outlined in the 'Summary of findings' tables for the included comparisons.
The variety of topical interventions evaluated, the differences in unit of analyses and outcome measures between studies, and incomplete data presentation hampered pooling of data.
Wound healing
Wound healing was variously measured and reported including: time to complete wound healing time to 90% wound healing, or change in wounds and percentage of wound healed during follow‐up. Most studies reported time to wound healing, but only two studies used survival methods (the appropriate approach to statistical analysis of time to event data) (Ang 2000; Oen 2012).
The comparison between topical antimicrobial and non‐antimicrobial agents revealed moderate to low‐certainty evidence, indicating no or little difference in time to wound healing between topical agents.
Comparing between topical antimicrobial agents showed that topical antimicrobial agents may make no difference in time to wound healing as the evidence is low certainty.
The comparison between skin substitutes and topical antimicrobial agents showed low‐certainty evidence that a skin substitute (TransCyte) may slightly reduce time to wound healing.
Finally, when comparing miscellaneous treatments, single studies showed low or very low‐certainty effects of interventions. MEBO may slightly reduce time to wound healing, compared with saline‐soaked dressing. A cream containing Helix Aspersa (Elicina) may slightly increase the proportion completely healed at 14 days compared with MEBO.
Infection
There is no, or inconclusive, evidence that topical therapies may reduce infections in people with facial burns. The certainty of evidence is very low, derived from four comparisons including data from nine studies.
Seven studies addressed wound infection as a negative outcome. The lack of events in studies, in combination with incomplete reports hampered any conclusions on effectiveness of treatments regarding infections. In addition, two studies assessed wound colonization as a proxy for wound infection and thus did not contribute to the body of evidence. Overall, these data were insufficient to support any definite conclusions.
Secondary outcomes
Except for quality of life, at least one study addressed all other secondary outcomes (proportion of facial burns requiring surgery, scar quality, pain, adverse effects of treatment, patient satisfaction and LOS). Pain (eight studies) and scar quality (six studies) were the most frequently addressed outcomes.
We chose not to include the secondary outcomes in the 'Summary of findings' tables, because of the overall low to very low‐certainty of evidence in combination with the sparse and scattered information in the four comparisons.
Low‐certainty evidence from single studies on skin substitutes versus topical antimicrobial agents shows inconsistent results. Skin substitutes may slightly decrease pain (TransCyte) or slightly increase pain (xenograft). Pain was assessed using a visual analogue scale.
In addition, low‐certainty evidence indicates that MEBO may slightly improve patient satisfaction, compared with saline‐soaked dressings, as assessed on a 4‐point scale.
Other comparisons indicate no or little effects based on low‐certainty evidence or very low‐certainty evidence only. This impedes any conclusion on effects of topical agents in facial burns.
Because the certainty of evidence is low to very low, our findings barely contribute to the body of evidence that favours antimicrobial agents above non‐antimicrobial agents.
One study addressed the need for reconstructive surgery (Ang 2000); none of the participants needed surgery in the short follow‐up period (six months). Future studies addressing the need for reconstructive surgery should probably include a longer follow‐up to include all relevant events. Reconstructive surgery can be divided into urgent, essential and desirable procedures. The urgent and essential procedures might be performed within a six‐month follow‐up period, but the desirable procedure is usually postponed until scars have fully matured. This maturation can take one year or longer (Barret 2004). Therefore, differences in the need for reconstructive surgery between intervention and control group might appear in a later phase.
A few studies reported on adverse events, again not providing robust evidence.
Overall completeness and applicability of evidence
The objective of this review was to assess the effectiveness of topical interventions on wound healing in people with facial burns of any depth. All topical interventions were eligible for inclusion, and we identified multiple different interventions. Participants were recruited in burn centres from four continents. Studies included most outcome measures, except quality of life. The applicability of evidence might be restricted to specialised burn centres in high‐income countries, because of the relatively high costs of skin substitutes and wound dressings. In addition, part of the evidence stems from older studies (five RCTs were published before 2006), which could limit the applicability of the evidence because some topical interventions are probably less frequently applied nowadays. For instance, the application of iontophoresis in combination with an antimicrobial agent has not been described since Desai 1991, as far as we know. However, all topical interventions are still available on the market, and seem to be applied in global burn care.
Quality of the evidence
The certainty of evidence combined in this review is mainly low to very low and insufficient to allow definite conclusions to be drawn.
Limitations in study design
The substantial increase in the number of included RCTs did not result in high‐certainty evidence for the included comparisons. The RCTs added in this update had similar high risks of bias as the studies included in the original review. In addition, most newly included RCTs lacked precision, because of small sample sizes and absent sample size calculations. One RCT with a sample size calculation did not reach the necessary requirements (Oen 2012). As a result of this, the evidence in all comparisons but one was downgraded by two levels because of high risk of bias and imprecision.
The high risk of bias was mostly related to a high risk of performance and detection bias due to absent blinding of participants, providers and, to a lesser extent, outcome assessors. Blinding of participants and care providers is not easy in studies that compare topical interventions, but outcome assessors could have been blinded. Despite this possibility, only three out of twelve studies reported blinding of outcome assessors for all outcomes). Selection bias was a second source of bias.
Adequate sequence generation and allocation concealment were done in only a minority of studies (maximum three).
Imprecision of results
Imprecision remained a main issue in the update of the review, with seven small‐sized studies, of maximal 39 participants and low event rates. Thus, effect estimates could often not be calculated, or with wide CIs only. While pooling data from small trials could increase statistical power and give a more precise overall estimate of effect size, the studies in this review only partly compared similar interventions and outcome measures. In addition, the different unit of analysis in included studies further hampered data synthesis/the pooling of studies included in the update (Lehna 2017; Wang 2015), data from these studies could not be included in the calculation of pooled effect estimates.
Unexplained heterogeneity or inconsistency
Inconsistency could barely be addressed because of the limited evidence available. In our 'Summary of findings' tables, seven of the ten effect estimates were based on one study only.
Indirectness of evidence
Issues related to indirectness bias are probably limited; all studies included the target population and the primary outcome measures.
Publication bias
We could not assess publication bias with a Begg funnel plot or an Egger test, because the included studies were heterogeneous and sometimes poorly reported. However, included RCTs did not show clear indications of publication bias, for instance there was no sign of any selection of studies with large effects only.
Potential biases in the review process
Potential bias in the review process might have arisen as a result of the minimal response to our queries from authors of the eligible studies. The review authors tried to contact study authors by email in an attempt to retrieve all possible data to assess the studies thoroughly. Despite issuing a reminder, we received only three replies from Branski 2008, Tsoutsos 2009, and Lehna 2017, with answers to our questions. As a result of those answers, we excluded the Branski 2008 study as sequence generation and allocation concealment were inadequate and, therefore, we judged that it was not an RCT. Eight other studies scored unclear on these items, but as additional information was not provided, they were included. To prevent potential bias due to the inclusion of a study from our review group, a third person not involved in this study was added to provide independent assessment of risk of bias and data extraction. Another potential bias might have occurred due to a possible patient overlap between Demling 1999 and Demling 2002. Both studies were performed in the same hospital, and did not provide inclusion periods. Therefore, we were unable to confirm whether there was a patient overlap. To prevent any overlap in data, we only included data from the most recent study in the analyses and in the 'Summary of findings' tables (Demling 2002).
Agreements and disagreements with other studies or reviews
In general, the results of this updated review are in accordance with other systematic reviews on topical therapy in burns of any location (Jull 2015; Wasiak 2013). Wasiak 2013 is a Cochrane Review including 30 RCTs on wound dressing in partial and full thickness burns that found that both the quality of trial reporting and trial conduct were poor. In our review, only facial burns were eligible, resulting in 12 included studies. Several years ago, Danilla 2009 investigated the methodological quality of RCTs in burn care. They included 257 eligible studies and concluded that "the reporting standards of RCTs are highly variable and less than optimal in most cases." Furthermore, their results showed an increase in RCTs over time, of increasing size, without a significant improvement in methodological quality. Our review reflects the growing number of RCTs in burn care and also the increasing size of some of these RCTs. However, due to the limited certainty of the evidence, these RCTs contribute only to a limited extent to the knowledge in this field. Initiatives like Brölmann 2013 on guidelines for reporting RCTs will hopefully enhance the standard of reporting in RCTs in wound care.
Wasiak 2013 concluded: "It is impossible to draw firm and confident conclusions about the effectiveness of specific dressings, however SSD was consistently associated with poorer healing outcomes than biosynthetic, silicon‐coated and silver dressings whilst hydrogel‐treated burns had better healing outcomes than those treated with usual care." At the moment, there is no evidence regarding whether these results apply to facial burns as well.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Study flow diagram.

Comparison 1: Topical antimicrobial versus non‐antimicrobial agent, Outcome 1: Time to wound healing

Comparison 1: Topical antimicrobial versus non‐antimicrobial agent, Outcome 2: Time to wound healing: proportion completely healed within 10 days

Comparison 1: Topical antimicrobial versus non‐antimicrobial agent, Outcome 3: Infection

Comparison 1: Topical antimicrobial versus non‐antimicrobial agent, Outcome 4: Need for surgery

Comparison 1: Topical antimicrobial versus non‐antimicrobial agent, Outcome 5: Scar quality

Comparison 1: Topical antimicrobial versus non‐antimicrobial agent, Outcome 6: Patient satisfaction

Comparison 2: Topical antimicrobial versus alternative antimicrobial agent, Outcome 1: Time to wound healing

Comparison 2: Topical antimicrobial versus alternative antimicrobial agent, Outcome 2: Infection

Comparison 2: Topical antimicrobial versus alternative antimicrobial agent, Outcome 3: Need for surgery

Comparison 2: Topical antimicrobial versus alternative antimicrobial agent, Outcome 4: Need for surgery: mean number

Comparison 2: Topical antimicrobial versus alternative antimicrobial agent, Outcome 5: Scar quality, POSAS, patient reported

Comparison 2: Topical antimicrobial versus alternative antimicrobial agent, Outcome 6: Scar quality, POSAS, observer reported

Comparison 2: Topical antimicrobial versus alternative antimicrobial agent, Outcome 7: Pain: procedural pain

Comparison 2: Topical antimicrobial versus alternative antimicrobial agent, Outcome 8: Pain: background pain

Comparison 2: Topical antimicrobial versus alternative antimicrobial agent, Outcome 9: Adverse effects

Comparison 2: Topical antimicrobial versus alternative antimicrobial agent, Outcome 10: Length of hospital stay

Comparison 3: Skin substitute versus topical antibiotic agent, Outcome 1: Time to wound healing

Comparison 3: Skin substitute versus topical antibiotic agent, Outcome 2: Time to wound healing, within patient comparison

Comparison 3: Skin substitute versus topical antibiotic agent, Outcome 3: Infection

Comparison 3: Skin substitute versus topical antibiotic agent, Outcome 4: Scar quality

Comparison 3: Skin substitute versus topical antibiotic agent, Outcome 5: Scar quality, within‐patient comparison

Comparison 3: Skin substitute versus topical antibiotic agent, Outcome 6: Pain: procedural pain

Comparison 3: Skin substitute versus topical antibiotic agent, Outcome 7: Pain: background pain

Comparison 3: Skin substitute versus topical antibiotic agent, Outcome 8: Pain, within‐patient comparison

Comparison 3: Skin substitute versus topical antibiotic agent, Outcome 9: Length of hospital stay

Comparison 4: Miscellaneous treatment versus miscellaneous treatment, Outcome 1: Time to wound healing

Comparison 4: Miscellaneous treatment versus miscellaneous treatment, Outcome 2: Time to wound healing: number completely healed in 14 days

Comparison 4: Miscellaneous treatment versus miscellaneous treatment, Outcome 3: Infection

Comparison 4: Miscellaneous treatment versus miscellaneous treatment, Outcome 4: Scar quality

Comparison 4: Miscellaneous treatment versus miscellaneous treatment, Outcome 5: Pain

Comparison 4: Miscellaneous treatment versus miscellaneous treatment, Outcome 6: Patient satisfaction
| Topical antimicrobial agent compared with topical non‐antimicrobial agent for facial burns | |||||||
| Patient or population: people with facial burns Setting: burn centres Intervention: topical antimicrobial agent Comparison: topical non‐antimicrobial agent | |||||||
| Outcomes (follow‐up) | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | ||
|---|---|---|---|---|---|---|---|
| Risk with topical non‐antimicrobial agent | Risk with topical antimicrobial agent | ||||||
| Time to complete wound healing (time to event data) (6 months) | Reported HR (adjusted for total body surface area burned) was 0.84 (0.78 to 1.85) (Ang 2000) | 39 (Ang 2000) | ⊕⊕⊕⊝ | Facial burns treated with SSD probably have a similar time to complete wound healing, compared with MEBO. | |||
| Time to complete wound healing (days) (until wound healing) | Mean time to wound healing 10.35 (SD 2.8) and 12.05 (SD 2.4) (Hindy 2009) | Mean time to wound healing 10.05 (SD 2.3) (Hindy 2009) | — | 60 | ⊕⊕⊝⊝ | There may be little or no difference in time to wound healing between topical anti‐microbial agents (Aquacel‐Ag) and non‐antimicrobial agents (MEBO) and saline‐soaked dressings) in facial burns. Data from Mabrouk 2012 not used, it was not stated or verifiable that all participants healed during the study. | |
| Proportion of wounds completely healed within 10 days (10 days) | Study population | RR 0.94 | 39 | ⊕⊕⊝⊝ | There may be little or no difference in the proportion of wounds completely healed within 10 days between SSD and MEBO. | ||
| 824 per 1000 | 774 per 1000 | ||||||
| Change in wound surface area over time or partial healing – not measured | No studies measured change in wound surface area over time or partial wound healing. | ||||||
| Infection (unclear follow‐up) | Study population | RR 0.38 | 40 | ⊕⊝⊝⊝ | It is uncertain whether Aquacel Ag increases or reduces the risk of infection compared with MEBO in facial burns as evidence is of very low certainty. | ||
| 400 per 1000 | 152 per 1000 | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; MEBO: Moist Exposed Burn Ointment; RR: risk ratio; SD: standard deviation; SSD: silver sulphadiazine. | |||||||
| GRADE Working Group grades of evidence | |||||||
| aDowngraded once for imprecision: limited number of participants (fewer than 100). bDowngraded once for unclear selection bias, performance and detection bias: unclear random sequence generation and allocation concealment, lack of blinding participants and providers, unclear blinding outcome assessment. Downgraded once for imprecision: effect estimates could not be calculated, limited number of participants (fewer than 100). | |||||||
| Topical antimicrobial compared with alternative antimicrobial agent for facial burns | ||||||
| Patient or population: people with facial burns Setting: burn centres Intervention: topical antimicrobial Comparison: alternative antimicrobial agent | ||||||
| Outcomes (follow‐up) | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with alternative antimicrobial agent | Risk with topical antimicrobial | |||||
| Time to complete wound healing | No studies measured time to complete wound healing. | |||||
| Proportion of wounds completely healed | No studies measured proportion of wounds completely healed. | |||||
| Change in wound surface area over time or partial healing (> 95%) (12 months) | The mean time to wound healing was 21.4 days | MD 7.10 days lower | — | 142 (Oen 2012) | ⊕⊕⊝⊝ Lowa | There may be little or no difference in time to wound healing between SSD and cerium‐SSD in facial burns. |
| Infection (until wound healing) | Study population | RR 0.73 | 15 | ⊕⊝⊝⊝ | It is uncertain whether mafenide acetate cream and gentamicin differs from mafenide acetate only in the risk of infection in facial burns as evidence is of very low certainty. | |
| 500 per 1000 | 365 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio; SSD: silver sulphadiazine. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once for imprecision: one study with 142 participants, optimal information size not reached. Downgraded once for high risk on performance bias and detection bias, and attrition bias not related to the outcome: lack of blinding participants, providers, and outcome assessor. | ||||||
| Skin substitute compared with topical antibiotic agent for facial burns | ||||||
| Patient or population: people with facial burns Setting: burn centres Intervention: skin substitute Comparison: topical antibiotic agent | ||||||
| Outcomes (follow‐up) | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with topical antibiotic agent | Risk with skin substitute | |||||
| Time to complete wound healing | No studies measured time to complete wound healing. | |||||
| Proportion of wounds completely healed | No studies measured proportion of wounds completely healed. | |||||
| Change in wound surface area over time or partial wound healing (> 90%) (until wound healing) | The mean time to wound healing was 15 days | MD 6 days lower | — | 34 | ⊕⊕⊝⊝ | A bioengineered skin substitute (TransCyte) might decrease time to 90% wound healing in facial burns, compared to a non‐specified antimicrobial agent. Data from Demling 1999, Horch 2005, and Wang 2015 not used because of concern of overlapping populations, lack of estimate of variance and comparison of wounds within participants. |
| Infection (until wound healing and unclear (2x)) | Study population | — | 56 | ⊕⊝⊝⊝ | It is uncertain whether skin substitutes increase or reduce the risk of infection compared with the use of topical antimicrobial agents in facial burns as evidence is of very low certainty. No events reported in 2 RCTs (Demling 1999; Horch 2005 (control group not reported)), third RCT reported 4 events in 50 wounds in 25 participants (Wang 2015). | |
| See comment | See comment | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once for risk of bias: unclear selection bias, high risk on performance bias and detection bias: unclear random sequence generation and allocation concealment, lack of blinding participants, providers and outcome assessor. Downgraded once for imprecision: one small‐sized study (34 participants). | ||||||
| Miscellaneous treatment compared with miscellaneous treatment for facial burns | ||||||
| Patient or population: people with facial burns Setting: burn centres Intervention: miscellaneous treatment (for details see comments) Comparison: miscellaneous treatment (for details see comments) | ||||||
| Outcomes (follow‐up) | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with miscellaneous treatment | Risk with miscellaneous treatment | |||||
| Time to complete wound healing (until wound healing) | The mean time to complete wound healing was 12.05 days | MD 1.7 days lower | — | 40 | ⊕⊕⊝⊝ | MEBO may slightly reduce mean time to wound healing in facial burns compared with saline‐soaked dressing. Data from Tsoutsos 2009 not used, it was not stated or verifiable that all participants healed during the study. |
| Proportion of wounds completely healed in 14 days (14 days) | Study population | RR 4.77 | 43 | ⊕⊕⊝⊝ | A cream containing Helix Aspersa (Elicina) may slightly increase the proportion completely healed at 14 days in facial burns, compared with MEBO. | |
| 188 per 1000 | 894 per 1000 | |||||
| Change in wound surface area over time or partial wound healing | Jiaao 2011 measured change in wound surface area over time, data not reported. | |||||
| Infection (follow‐up unclear) | Study population | Not estimable | 43 | ⊕⊝⊝⊝ | It is uncertain whether a cream containing Helix Aspersa (Elicina) increases or reduces the risk of infection compared with use of MEBO in facial burns as evidence is of very low certainty. | |
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; MEBO: Moist Exposed Burn Ointment; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once for risk of bias due to unclear selection bias, high risk on performance bias and detection bias: unclear random sequence generation and allocation concealment, lack of blinding participants and providers, and unclear blinding outcome assessor. Downgraded once for imprecision: one small‐sized study (40 participants). | ||||||
| Comparison | Study | Follow‐up | Time to wound healing | Change in wound surface area, proportion of wound partly healed | Complete healing data reported? | Infection |
|---|---|---|---|---|---|---|
| Topical antimicrobial agent compared with topical non‐antimicrobial agent | ||||||
| SSD vs MEBO | 6 months | Time to event analysis, days to complete wound healing; number of participants healed at 10 days | – | Yes, stated | Not reported | |
| Silver hydrocolloid dressing (Aquacel Ag) vs MEBO vs saline‐soaked dressings | Until wound healing | Days to complete wound healing | – | Yes, stated | Not reported | |
| Silver hydrocolloid dressing (Aquacel Ag) vs MEBO | 6 months | Days to complete wound healing | – | Not stated nor verifiable | Reported, | |
| Topical antimicrobial agents compared with other topical antimicrobial agents | ||||||
| Mafenide acetate cream + gentamicin via iontophoresis (7 participants) vs usual care (mafenide acetate cream) (8 participants) | Until wound healing | Days to complete wound healing | – | Yes, stated | Clinical features | |
| Cerium‐SSD vs SSD | 12 months | Time to event anaysis, days to 90% wound healing | – | Yes, verifiable | Not reported | |
| Skin substitutes compared with topical antimicrobial agents | ||||||
| Biological skin substitute coated with fibronectin (TransCyte) vs Bacitracin | Until wound healing | Days to 95% wound healing | – | Yes, verifiable | Clinical features | |
| Bioactive skin substitute (a bilayered, biologically active, temporary skin substitute (TransCyte)) vs antibacterial ointment, not specified | Until wound healing | Days to 90% wound healing | – | Yes, verifiable | Not reported | |
| Glycerolised allograft cadaver (corpse) skin vs SSD | 6 months (maximum) | Days to complete wound healing | – | Yes, verifiable | Reported in 1 group, not prespecified, no definition | |
| Biological dressing (porcine Xenoderm) vs Physiotulle Ag (Coloplast) 50 wounds in 25 participants | 3 months (scar) | Days to 95% wound healing | – | Yes, verifiable | Clinical features | |
| Miscellaneous treatment compared with miscellaneous treatment | ||||||
| MEBO vs saline‐soaked dressing | Until wound healing | Days to complete wound healing | – | Yes, stated | Not reported | |
| rhGM‐CSF hydrogel (1 μg/cm2/day) vs placebo hydrogel (matrix hydrogel) | Until wound healing | Days to complete wound healing | % wound healing at 3, 5, 7 and 14 days after treatment | Yes, stated | Not reported | |
| Enzymatic debridement (collagenase) vs antimicrobial agent (Bacitracin) | 6 months | Wound epithelialisation: time to establish a wound bed | – | Yes, verifiable | Documented wound or blood infection confirmed by a positive laboratory result. | |
| Cream containing Helix Aspersa extract (terrestrial brown snail secretions extract (Elicina) vs MEBO | 2 years (scar) | Days to complete wound healing, number of participants healed at 14 days | – | Not stated or verifiable (days to complete wound healing) | Burn swab cultures; systemic infections, | |
| MEBO: Moist Exposed Burn Ointment; rhGM‐CSF: recombinant human granulocyte‐macrophage colony‐stimulating factor; SSD: silver sulphadiazine. | ||||||
| Comparison | Study | Follow‐up | Surgery | Scar quality | Pain | Patient satisfaction | Adverse effect | QoL | LOS |
|---|---|---|---|---|---|---|---|---|---|
| Topical antimicrobial agent compared with topical non‐antimicrobial agent | |||||||||
| SSD vs MEBO | 6 months | Reconstructive surgery, following wound closure | – | – | – | – | – | – | |
| Silver hydrocolloid dressing (Aquacel Ag) vs MEBO vs saline‐soaked dressings | Until wound healing | – | Quality of healing (4‐point scale) | VAS (0–10) | 4‐point scale (excellent‐ poor) | – | – | – | |
| Silver hydrocolloid dressing (Aquacel Ag) vs MEBO | 6 months | – | Vancouver Scar Scale, incidence hypertrophic scars | VAS (0–10) | 3‐point scale: (comfortable‐discomfortable) | – | – | – | |
| Topical antimicrobial agents compared with other topical antimicrobial agents | |||||||||
| Mafenide acetate cream and gentamicin via iontophoresis (7 participants) vs usual care (mafenide acetate cream) (8 participants) | Until wound healing | Surgery, following treatment by topical agent | – | – | – | – | – | LOS | |
| Cerium‐SSD vs SSD | 12 months | Surgery, following treatment by topical agent | POSAS, colour (dermaspectometer), elasticity (cutometer), functional and anatomical impairments | VAT (0–10) | – | – | – | – | |
| Skin substitutes compared with topical antimicrobial agents | |||||||||
| Biological skin substitute coated with fibronectin (TransCyte) vs Bacitracin | Until wound healing | – | – | VAS (0–10) | – | – | – | LOS | |
| Bioactive skin substitute (a bilayered, biologically active, temporary skin substitute (TransCyte)) vs antibacterial ointment, not specified | Until wound healing | – | – | VAS (0–10) | – | – | – | – | |
| Glycerolised allograft cadaver (corpse) skin vs SSD | 6 months (maximum) | – | Not specified | – | – | – | – | – | |
| Biological dressing (porcine Xenoderm) vs Physiotulle Ag (Coloplast) 50 wounds in 25 participants | 3 months (scar) | – | Vancouver Scar Scale | VAS (0–10) | – | – | – | – | |
| Miscellaneous treatment compared with miscellaneous treatment | |||||||||
| MEBO vs saline‐soaked dressing | Until wound healing | – | Quality of healing (4‐point scale) | VAS (0–10) | – | – | – | – | |
| Enzymatic debridement (Collagenase) vs antimicrobial agent (Bacitracin) | 6 months | – | POSAS | VAS (0–10) | – | All‐cause mortality, serious adverse effects | – | – | |
| Cream containing Helix Aspersa extract (Elicina) vs MEBO | 2 years (scar) | – | – | VAS (1–10) | – | – | – | – | |
| LOS: length of hospital stay; MEBO: Moist Exposed Burn Ointment; POSAS: Patient and Observer Scar Assessment Scale; QoL: quality of life; SSD: silver sulphadiazine; VAS: visual analogue scale. | |||||||||
| Study ID | Main baseline characteristics | Number of participants and dropout rate | Primary outcomes | Secondary outcomes |
|---|---|---|---|---|
| Number of participants: I: 22; C: 17. % TBSA burned (mean): I: 1.56 (SE 0.18; range 0.5–3.5). C: 2.16 (SE 0.38; range 0.13–6.0). | Initial number of participants: 115. After randomisation: I: 58; C: 57. Dropouts: I: 0; C:3. Participants with facial burns: I: 22; C:17. Participants in short‐term analysis: I: 22; C:17. Participants in long‐term analysis (6 months): I: 20; C:17. | Time to complete wound healing (number of days taken for face‐wound to heal): I: not reported; C: 2–35 days (range). Proportion completely healed in 10 days: I: 17/22; C: 14/17. | Need for surgery (need for reconstructive surgery 6 months PB): I: 0/20; C: 0/17. | |
| Number of participants: Minor burns: I: 5; C: 5. Mean age: Minor burns: I: 31 (SD 8) years; C: 29 (SD 7) years. Aetiology: flame. % TBSA burned (mean): Minor burns: I: 10 (SD 3); C: 7 (SD 2). % TBSA burned full‐thickness (mean): Minor burns: I: 0; C: 0. | Initial number of participants: 21. Number of participants with minor burns: 10. Number of participants with major burns: 11. After randomisation: Minor burns: I: 5; C: 5. Participants in short‐term analysis: Minor burns: I: 5; C: 5. | Time to partial wound healing (mean number of days to > 90% re‐epithelialisation): Minor burns: I: 8 (SD 1); C: 12 (SD 3); reported P < 0.05. Major burns: I: 8 (SD 2); C: 14 (SD 4); reported P < 0.05. Wound infection (signs of local wound infection): Minor burns: I: 0; C: 0. | Pain (procedural pain, during facial care (mean): Minor burns: I: 2 (SD 1); C: 5 (SD 1). Pain (background pain, between facial care) (mean): Minor burns: I: 1 (SD 0.5); C: 3 (SD 2). Length of stay (mean): Minor burns: I: 1 (SD 0.5); C: 3 (SD 1). | |
| Number of participants: I: 16; C: 18. Mean age: I: 39 (SD 9); C: 40 (SD 8). Aetiology: Flame: I: 11; C: 12. % TBSA burned (mean): I: 24 (SD 8); C: 21 (SD 9). % TBSA burned full‐thickness (mean): I: 12 (SD 7); C: 10 (SD 6). | Initial number of participants: 34. After randomisation: I: 16; C: 18. Participants in short‐term analysis: I: 16; C: 18. | Time to partial wound healing (mean number of days to > 95% re‐epithelialisation: I: 9 (SD 4); C: 15 (SD 4); reported P < 0.05. | Pain (procedural pain, during facial care) (mean): I: 3 (SD 1); C: 7 (SD 2). Pain (background pain, between facial care) (mean): I: 2 (SD 1); C: 4 (SD 2). | |
| Number of participants: I: 7; C: 8. Mean age: I: 11.4 (SE 1.2); C: 9.5 (SE 1.6). % TBSA burned (mean): I: 35 (SE 7); C: 50 (SE 6). % TBSA burned full‐thickness (mean): I: 20 (SE 9); C: 32 (SE 7). | Initial number of participants: 15. After randomisation: I: 7; C: 8. Participants in short‐term analysis: I: 7; C: 8. | Wound infection (occurrence of chondritis): I: 3; C: 4. | Need for surgery (mean number of surgical procedures): I: 1.2 (SE 0.1) C: 1.0 (SE 0); reported P < 0.05. Adverse effects (occurrence of gentamicin‐resistant micro‐organisms): I: 29%; C: 0%. Length of stay (mean): I: 26 (SE 1); C: 38 (SE 3). | |
| Number of participants: I: 20; C1; 20; C2: 20. | Initial number of participants: 60. After randomisation: I: 20; C1: 20; C2: 20. Participants in short‐term analysis: I: 20; C1: 20; C2: 20. | Time to complete wound healing (mean): I: 10.05 (SD 2.3); C1: 10.35 (SD 2.8); C2: 12.05 (SD 2.4), reported P < 0.05. | Pain: (mean) day 1 and 2: C1: 3.1 (SD 1.9); day 3 onwards: C1: 1.3 (SD 1.5), I and C2 not presented, least pain in C1, reported P < 0.05). Scar quality (quality of healing): excellent: I: 16; C1: 16; C2: 11, reported P > 0.05. Patient satisfaction: excellent: I: 11; C1: 12; C2: 2, reported P < 0.05. | |
| Number of participants: I: 5; C; 5. Median age I+C: 34.3 (range 24–67) years. | Initial number of participants: 10. After randomisation: I: 5; C: 5. Participants in short‐term analysis: I: 5; C:5. Participants in long‐term analysis: I: 5; C:5. | Time to complete wound healing (median) I: 10.5; C: 12.4, reported P < 0.05. Wound infection (signs of underlying infection) I: 0; C: not reported | Scar quality (scar formation, maximum 6 months): I: 0; C: 2. Adverse effects (localised partial integration of the biological dressing) I: 1; C: 0. | |
| Number of participants: I+C: 30 | Initial number of participants: 30. After randomisation: ? Participants in short‐term analysis: ? | Time to complete wound healing (mean): I: 13.47 (SD 1.08) days; C: 18.69 (SD 2.35) days, reported P < 0.01 Change in wound surface area over time: percentage of healing at 3, 5, 7 and 14 days after treatment, reported P < 0.01 at day 5, 7 and 14 days after treatment. | Not reported. | |
| Number of participants: 10 (within patient comparison). Age: 9 participants aged 18–64 years, 1 participant aged ≥ 65 years. | Initial number of participants: 10. After randomisation: 10. Participants in short‐term analysis: 10. Participants in long‐term analysis: no data provided. | Time to complete wound healing (days to complete epithelialisation, mean): I1: 37.6; I2: 37.4, reported P = 0.0853. Wound infection: not reported | Pain: not reported. Scar quality: not reported. Adverse effects: all‐cause mortality: 1. | |
| Number of participants: I1 (Aquacel Ag): 20; I2 (MEBO): 20. Mean age: I1: 41.4 years, I2: 37.5 years. Total TBSA range: I: 5–30, C: 7–30. | Initial number of participants: 40. After randomisation: I1: 20; I2: 20. Participants in short‐term analysis: I1: 20; I2: 20. Participants in long‐term analysis (3, 6 months), I1: 7–10; I2: 10–14 (table 3). | Time to complete wound healing (mean) I1: 10.5 days; I2: 12.4 days, reported P < 0.05. Wound infection (rate) I1: 3/20; I2: 8/20. | Pain (mean): I1: 4.1; I2: 4.6. Scar quality (Vancouver Scar Scale): I1: 4; I2: 7, reported P > 0.05. Patient satisfaction (comfortable, mild discomfort, discomfort): I1: 14, 2, 4; I2: 10, 4, 6. | |
| Number of participants: I1: 78; I2: 76. Mean age: I: 41.9 (SD 16.9) years; I2: 41.3 (SD 14.5) years. Aetiology: Flame: I1: 70; I2: 60. % TBSA burned (median (IQR): I1: 9.8 (5.0–19.4); I2: 9.3 (4.5–17.0). | Initial number of participants: 179. After randomisation: I1: 90; I2: 89. After postrandomisation exclusion: I1: 78; I2: 76. Participants in short‐term analysis (primary analysis): I1: 77; I2: 73. Participants in long‐term analysis (6 months) I1: 60; I2: 50) (12 months): I1: 50; I2: 46. | Time to wound partial healing: (survival analysis, all participants), reported P = 0.71. Median days (25–75th percentile) to healing (> 90%) in wounds without surgery: I1: 11.0 (7.0–15.0); I2: 9.0 (5.0–17.75), reported P = 0.17. Mean time to wound healing: I1: 14.3 (SD 14.5) days; I2: 21.4 (SD 37.6) days (data delivered upon request). | Need for surgery: I1: 13/77; I2: 15/73, reported P = 0.565, OR 0.8 (95% CI 0.3 to 1.8). Pain (background pain, before wound care, mean): I1: 0.6 (SEM 0.2); I2: 1.2 (SEM 0.4), reported P = 0.16. Pain (procedural pain, during wound care): I1: 1.3 (SEM 0.3); I2: 1.6 (SEM 0.5), reported P = 0.59. Scar quality at 12 months (Patient and Observer Scar Assessment Scale) (median): (patient report): I1: 1.2 (25–75th percentile 1.0–2.2); I2: 1.0 (25–75th percentile 1.0–1.8), reported P = 0.42; (observer report): I1: 1.4 (25–75th percentile 1.2–1.8); I2: 1.4 (25–75th percentile 1.2–1.6), reported P = 0.17. (mean): (patient report) I1: 2.0 (SD 1.5); I2: 1.8 (SD 1.5); (observer report) I1: 1.7 (SD 0.9); I2: 1.6 (SD 0.8) (delivered upon request). | |
| Number of participants I: 27; C: 16. | Initial number of participants: 46. After randomisation: I: 27; C: 19, after postrandomisation exclusion: I: 27; C: 16. Participants in short‐term analysis: not documented. | Time to complete wound healing (mean days: I: 11 (SD 2); C: 15 (SD 3), reported P < 0.0001. Number of participants with complete wound healing within 14 days: I: 27/27; C: 3/16, P value not reported. Wound infection: bacterial colonisation in all participants; absence of signs of systemic infection. | Pain (mean pain reduction scores): I: 2.7 (SD 1.35); C: 2.00 (SD 0.89), reported P interaction intervention by change pain = 0.072. | |
| Number of participants: 25 (within‐patient comparison): 15 superficial degree II burns; 10 deep degree II burns. % TBSA burned (mean): 45.1 (SD 25.2) (superficial degree II burns); 54.9 (SD 31.7) (deep degree II burns). | Initial number of participants: 25. After randomisation: 25. Participants in short‐term analysis: 25. Participants in long‐term analysis: no numbers provided. | Time to wound healing (mean days) Superficial degree II burns: I: 8.6 (SD 1.12); C: 8.67 (SD 1.18) (reported P = 0.855). Deep degree II burns: I: 15.80 (SD 1.55); C: 18.20 (SD 1.55), reported P = 0.005. Infection: Superficial degree II burns: I: 1/15; C: 1/15, P > 0.05 Deep degree II burns: I: 1/10; C: 1/10, reported P > 0.05. | Pain (mean VAS) Superficial degree II burns: I: 2.73 (SD 0.70); C: 1.53 (SD 0.83), reported P = 0.000. Deep degree II burns: I: 5.70 (SD 0.95); C: 2.70 (SD 0.48), reported P = 0.000. Scar quality at 3 months (mean Vancouver Scar Scale) Superficial degree II burns: I: 0.80 (SD 0.77); C: 0.80 (SD 0.77), reported P = 1.000. Deep degree II burns: I: 3.50 (SD 0.71); C: 5.50 (SD 1.08), reported P = 0.000. | |
| C: comparator; CI: confidence interval; I: intervention; IQR: interquartile range; MEBO: Moist Exposed Burn Ointment; OR: odds ratio; PB: postburn; SD: standard deviation; SE: standard error; SEM: standard error of the mean; TBSA: total body surface area. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Time to wound healing Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.2 Time to wound healing: proportion completely healed within 10 days Show forest plot | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.29] |
| 1.3 Infection Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.12, 1.21] |
| 1.4 Need for surgery Show forest plot | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5 Scar quality Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.87, 1.61] |
| 1.6 Patient satisfaction Show forest plot | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.06, 2.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Time to wound healing Show forest plot | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | ‐7.10 [‐16.43, 2.23] |
| 2.2 Infection Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.29, 2.58] |
| 2.3 Need for surgery Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.42, 1.61] |
| 2.4 Need for surgery: mean number Show forest plot | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 2.5 Scar quality, POSAS, patient reported Show forest plot | 1 | 95 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.40, 0.80] |
| 2.6 Scar quality, POSAS, observer reported Show forest plot | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.24, 0.44] |
| 2.7 Pain: procedural pain Show forest plot | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.44, 0.84] |
| 2.8 Pain: background pain Show forest plot | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.48, 0.28] |
| 2.9 Adverse effects Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.62 [0.31, 100.52] |
| 2.10 Length of hospital stay Show forest plot | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐12.00 [‐18.20, ‐5.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Time to wound healing Show forest plot | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐6.00 [‐8.69, ‐3.31] |
| 3.2 Time to wound healing, within patient comparison Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3 Infection Show forest plot | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.4 Scar quality Show forest plot | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 3.35] |
| 3.5 Scar quality, within‐patient comparison Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.6 Pain: procedural pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.7 Pain: background pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.8 Pain, within‐patient comparison Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.9 Length of hospital stay Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐2.98, ‐1.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Time to wound healing Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.2 Time to wound healing: number completely healed in 14 days Show forest plot | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.77 [1.87, 12.15] |
| 4.3 Infection Show forest plot | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.4 Scar quality Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.92, 2.29] |
| 4.5 Pain Show forest plot | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.03, 1.37] |
| 4.6 Patient satisfaction Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.00 [1.54, 23.44] |

