Agonista de la hormona liberadora de gonadotropina versus HCG para la activación de ovocitos en los ciclos de tecnología de reproducción asistida con antagonistas hormonales
Resumen
Antecedentes
La gonadotropina coriónica humana (HCG) se utiliza rutinariamente para la maduración final de los ovocitos, desencadenando ciclos de fecundación in vitro (FIV)/inyección intracitoplasmática de espermatozoides (ICSI), pero el uso de la HCG para este fin puede tener inconvenientes. Los agonistas de la hormona liberadora de gonadotropina (GnRH) presentan una alternativa a la HCG en los regímenes de tratamiento de hiperestimulación ovárica controlada (COH) en los que el ciclo se ha regulado a la baja con un antagonista de la GnRH. Ésta es una actualización de una revisión publicada por primera vez en 2010.
Objetivos
Evaluar la efectividad y la seguridad de los agonistas de la GnRH en comparación con la HCG para la activación de la maduración final de ovocitos en la FIV y la ICSI para las pacientes que reciben hiperestimulación ovárica controlada en un protocolo de antagonistas de la GnRH.
Métodos de búsqueda
Se realizaron búsquedas en bases de datos que incluyen el Registro Especializado de Ensayos Controlados del Grupo de Trastornos Menstruales y Subfertilidad (MDSG), el Registro Cochrane Central de Ensayos Controlados (CENTRAL), MEDLINE, EMBASE, PsycINFO, el Cumulative Index to Nursing and Allied Health Literature (CINAHL) y los registros de ensayos de artículos publicados y no publicados (en cualquier idioma) sobre ensayos controlados aleatorizados (ECA) de agonistas de la hormona liberadora de gonadotropina versus GCH para la activación de ovocitos en ciclos de tratamiento de FIV/ICSI con antagonistas de la GnRH. La búsqueda está actualizada hasta el 8 de septiembre de 2014.
Criterios de selección
Se incluyeron ECA que compararon los resultados clínicos de los desencadenantes de los agonistas de la GnRH frente a la GCH para el desencadenante de la maduración final de los ovocitos en mujeres que se sometían a ciclos de tratamiento de FIV/ICSI con antagonistas de la GnRH.
Obtención y análisis de los datos
Dos o más autores de la revisión seleccionaron los estudios de forma independiente, extrajeron los datos y evaluaron el riesgo de sesgo del estudio. Los efectos del tratamiento se resumieron utilizando un modelo de efectos fijos, y se realizaron análisis de subgrupos para explorar las posibles fuentes de heterogeneidad. Los efectos del tratamiento se expresaron como diferencias de medias (DM) para los resultados continuos y como odds ratios (OR) para los resultados dicotómicos, junto con intervalos de confianza (IC) del 95%. Los resultados principales fueron nacidos vivos y la tasa de síndrome de hiperestimulación ovárica (SHEO) por mujeres asignadas al azar. Se utilizaron los métodos de los Grados de Recomendación, Valoración, Desarrollo y Evaluación (GRADE) para evaluar la calidad de la evidencia de cada comparación.
Resultados principales
Se incluyeron 17 ECA (n = 1847), de los cuales 13 estudios evaluaron ciclos autólogos frescos y cuatro estudios evaluaron ciclos donante‐receptor. En los ciclos autólogos frescos, los agonistas de la GnRH se asociaron con una tasa de nacidos vivos más baja que la observada con la GCH (OR 0,47, IC del 95%: 0,31 a 0,70; cinco ECA, 532 mujeres, I2 = 56%, evidencia de calidad moderada). Esto sugiere que para una mujer con una probabilidad del 31% de lograr un nacimiento vivo con el uso de la HCG, la probabilidad de un nacimiento vivo con el uso de un agonista de la GnRH sería entre el 12% y el 24%.
En las mujeres que se sometían a ciclos autólogos frescos, los agonistas de la GnRH se asociaban a una menor incidencia de SHEO leve, moderado o grave que la GCH (OR 0,15, IC del 95%: 0,05 a 0,47; ocho ECA, 989 mujeres, I² = 42%, evidencia de calidad moderada). Esto sugiere que para una mujer con un 5% de riesgo de SHEO leve, moderado o grave con el uso de GCH, el riesgo de SHEO con el uso de un agonista de GnRH sería entre cero y 2%.
En las mujeres que se someten a ciclos autólogos frescos, los agonistas de la GnRH se asociaron a una tasa de embarazo en curso más baja que la observada con la GCH (OR 0,70; IC del 95%: 0,54 a 0,91; 11 estudios, 1198 mujeres, I2 = 59%, evidencia de baja calidad) y a una tasa de aborto temprano más alta (OR 1,74; IC del 95%: 1,10 a 2,75; 11 ECA, 1198 mujeres, I² = 1%, evidencia de calidad moderada). Sin embargo, el efecto dependía del tipo de apoyo a la fase luteínica proporcionado (con o sin actividad de la hormona luteinizante (HL)); la tasa más alta de embarazos en el grupo con HL se aplicó sólo al grupo que recibió apoyo a la fase luteínica sin actividad de la HL (OR 0,36, IC del 95%: 0,21 a 0,62; I2 = 73%, cinco ECA, 370 mujeres). No se encontró evidencia de una diferencia entre los grupos en cuanto al riesgo de embarazo múltiple (OR 3,00; IC del 95%: 0,30 a 30,47; dos ECA, 62 mujeres, I2 = 0%, evidencia de baja calidad).
En las mujeres con ciclos donante‐receptor, no hay evidencia que sugieran una diferencia entre los grupos en la tasa de nacidos vivos (OR 0,92, IC del 95%: 0,53 a 1,61; un ECA, 212 mujeres) o en la tasa de embarazos en curso (OR 0,88, IC del 95%: 0,58 a 1,32; tres ECA, 372 mujeres, I² = 0%). Se encontró evidencia de una menor incidencia de SHEO en el grupo de agonistas de GnRH que en el grupo de GCH (OR 0,05, IC del 95%: 0,01 a 0,28; tres ECA, 374 mujeres, I² = 0%).
La principal limitación en la calidad de la evidencia fue el riesgo de sesgo asociado a la mala información sobre los métodos de los estudios incluidos.
Conclusiones de los autores
El desencadenamiento de la maduración final de los ovocitos con un agonista de la GnRH en lugar de la GCH en los ciclos de tratamiento de FIV/ICSI con antagonistas de la GnRH autólogos frescos evita el SHEO en detrimento de la tasa de nacidos vivos. En los ciclos donante‐receptor, el uso de agonistas de la GnRH en lugar de la GCH dio lugar a una menor incidencia del SHEO, sin que hubiera evidencia de una diferencia en la tasa de nacidos vivos.
La evidencia sugiere que el agonista de la GnRH como desencadenante final de la maduración de los ovocitos en los ciclos autólogos frescos se asocia con una menor tasa de nacimientos vivos, una menor tasa de embarazos en curso (embarazos de más de 12 semanas) y una mayor tasa de abortos espontáneos tempranos (menos de 12 semanas). El agonista de la GnRH como desencadenante de la maduración de los ovocitos podría ser útil para las mujeres que deciden evitar las transferencias en fresco (por cualquier motivo), las mujeres que donan ovocitos a las receptoras o las que desean congelar sus óvulos para utilizarlos posteriormente en el contexto de la preservación de la fertilidad.
PICO
Resumen en términos sencillos
Agonista de la hormona liberadora de gonadotropina versus HCG para la activación de ovocitos en los ciclos de tecnología de reproducción asistida con antagonistas hormonales
Pregunta de la revisión
Se revisó la evidencia sobre los efectos de los agonistas de la GnRH en la maduración final de los ovocitos que se desencadenan en los ciclos de tratamiento de FIV/ICSI con antagonistas de la GnRH.
Antecedentes
El desencadenamiento de la maduración de los ovocitos es el proceso de diferenciación final de un ovocito inmaduro antes de la fecundación en ciclos no estimulados o estimulados con técnicas de reproducción asistida. Dos hormonas pueden utilizarse para desencadenar la maduración de los ovocitos: la gonadotropina coriónica humana (HCG), que es el tratamiento estándar, y el agonista de la hormona liberadora de gonadotropina (agonista de la GnRH). En esta revisión, se evaluaron los beneficios y daños de los agonistas de la GnRH como desencadenantes de la maduración de los ovocitos. La evidencia está actualizada hasta septiembre de 2014.
Características de los estudios
Se incluyeron 17 estudios con 1817 mujeres. Los investigadores evaluaron los ciclos frescos o de donantes en mujeres con riesgo variable de síndrome de hiperestimulación ovárica (SHEO). Los autores de cuatro estudios declararon que los estudios fueron financiados comercialmente. La mayoría de los estudios no revelaron su fuente de financiación.
Resultados clave
Los agonistas de la GnRH desencadenan una reducción significativa del riesgo de hiperestimulación ovárica, pero también disminuyen las posibilidades de embarazo en los ciclos de tratamiento de FIV/ICSI autólogo fresco en comparación con la GCH. El uso de un agonista de la GnRH como desencadenante de la maduración de los ovocitos podría ser útil para las mujeres que deciden evitar las transferencias en fresco (por cualquier motivo), las mujeres que donan ovocitos a las receptoras o las que desean congelar sus óvulos para utilizarlos posteriormente en el contexto de la preservación de la fertilidad.
Calidad de la evidencia
La calidad general de la evidencia fue moderada en la mayoría de las comparaciones. La principal limitación en la calidad de la evidencia fue el riesgo de sesgo asociado a la mala información sobre los métodos de estudio.
Authors' conclusions
Summary of findings
| GnRH agonist compared with HCG for oocyte maturation triggering in antagonist‐assisted reproductive technology | ||||||
| Population: subfertile women | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| HCG for oocyte maturation triggering | GnRH agonist | |||||
| Live birth | 313 per 1000 | 176 per 1000 | OR 0.47 | 532 | ⊕⊕⊕⊝ | |
| OHSS (mild, moderate or severe): overall risk | 5 per 1000 | 1 per 1000 | OR 0.15 | 989 | ⊕⊕⊕⊝ | |
| OHSS (moderate or severe): overall risk | 5 per 1000 | 1 per 1000 | OR 0.21 | 989 | ⊕⊕⊕⊝ | Low event rate: 4 of 9 RCTs reported no events in either arm |
| OHSS (mild, moderate or severe) in women at high risk of OHSS | 308 per 1000 | 26 per 1000 (4 to 131) | OR 0.06 | 212 women (3 studies) | ⊕⊕⊕⊝ | |
| Ongoing pregnancy | 256 per 1000 | 194 per 1000 | OR 0.7 | 1198 | ⊕⊕⊝⊝ | |
| Miscarriage | 67 per 1000 | 111 per 1000 | OR 1.74 | 1198 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aOne of the studies at high risk of bias because of premature termination. bAll studies at high risk of bias in 1 or more domains. None clearly reported blinded outcome assessment. cMost studies at high risk of bias in 1 or more domains. None clearly reported blinded outcome assessment. dSubstantial heterogeneity: I2 = 59% to 66%. e5/11 studies at high risk of bias because of early termination and/or inadequate allocation concealment. None clearly reported blinded outcome assessment. | ||||||
Background
Description of the condition
After oocyte growth is stimulated by gonadotropins, the next step in in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI) treatment consists of triggering the oocytes to go through the last stage of maturation, so that they can be retrieved and fertilised. This final oocyte maturation is usually triggered by human chorionic gonadotropin (HCG), but use of HCG for this purpose may have drawbacks. Some studies have suggested a negative impact of HCG on endometrial receptivity (Simon 1995; Forman 1998; Simon 1998) and embryo quality (Valbuena 2001; Tavaniotou 2002). In addition, the sustained luteotrophic effect of HCG is associated with increased chances of ovarian hyperstimulation syndrome (OHSS), which is an iatrogenic complication of assisted reproductive technology (ART).
OHSS may be associated with massive ovarian enlargement, ascites, hydrothorax, liver dysfunction and renal failure. It can lead to cancellation of an IVF cycle and the need for prolonged bed rest or hospitalisation, which may have a significant emotional, social and economic impact or—in its most severe form—may even result in mortality (Delvigne 2003).
Gonadotropin‐releasing hormone (GnRH) agonists present an alternative to HCG for triggering endogenous luteinising hormone (LH) release (Gonen 1990; Olivennes 1996; Olivennes 2001; Tay 2002). Use of GnRH agonist triggering is applicable only in IVF with controlled ovarian hyperstimulation (COH) treatment regimens in which the cycle has been down‐regulated by a GnRH antagonist. Because of the specific mode of action of the antagonist, the pituitary remains responsive to a GnRH agonist, provided that the GnRH antagonist treatment utilised standard doses (Felberbaum 1995; Orvieto 2006).
Description of the intervention
A midcycle single bolus of GnRH agonist may be injected subcutaneously (0.2 to 0.5 mg of triptorelin, leuprorelin or buserelin) (Itskovitz‐Eldor 2000; Humaidan 2005) or administered intranasally (200 µg buserelin) (Pirard 2006).
How the intervention might work
A single injection of a GnRH agonist results in an acute release of LH and follicle‐stimulating hormone (FSH)—the so‐called flare‐up. Serum LH and FSH levels rise after four hours and 12 hours, respectively, and are elevated for 24 to 36 hours. The amplitude of the surges is similar to that seen in the normal menstrual cycle, but, in contrast to the natural cycle, the LH surge consists of two phases: a short ascending limb (> 4 hours) and a long descending limb (> 20 hours). This has no bearing on luteal phase steroid levels, which are qualitatively similar to those observed in the natural cycle (Segal 1992; Itskovitz‐Eldor 2000; Fauser 2002; Nevo 2003; Kol 2004).
Consequently, oocyte maturation triggering with GnRH agonists may provide several advantages over that achieved with HCG. First, GnRH agonists reduce the risk of OHSS due to quick and irreversible luteolysis (Kol 2004). Second, a more physiological LH and FSH surge is induced by the agonists, which may result in better oocyte and embryo quality (Humaidan 2005). Third, GnRH agonists may improve endometrial quality as a result of the lower luteal phase steroid levels (Forman 1998; Simon 1998).
Why it is important to do this review
This is an update of a review first published in 2010 (Youssef 2010). HCG is the standard medication for final oocyte maturation triggering. More recently, GnRH agonists have been proposed, especially as they may prevent OHSS to a large extent. Summarising the available evidence shows what is known about the effectiveness and safety of GnRH agonists in comparison with HCG and hence will help fertility experts and women to make informed decisions on final oocyte maturation triggering by GnRH antagonists in IVF/ICSI treatment cycles.
Objectives
To evaluate the effectiveness and safety of GnRH agonists in comparison with HCG for triggering final oocyte maturation in IVF and ICSI for women undergoing COH in a GnRH antagonist protocol.
Methods
Criteria for considering studies for this review
Types of studies
-
Only published and unpublished randomised controlled trials (RCTs) were included in the review.
-
Non‐randomised studies (e.g. studies with evidence of inadequate sequence generation such as alternate days and participant numbers), as they are associated with high risk of bias, were excluded from the review.
-
Cross‐over trials were excluded, as the design is not valid in this context.
Types of participants
Inclusion criteria
-
Subfertile couples undergoing IVF or ICSI for therapeutic reasons or for oocyte donation and randomly assigned to receive a GnRH agonist or HCG for final oocyte maturation triggering.
Exclusion criteria
-
Women who were not undergoing IVF or ICSI (i.e. those undergoing intrauterine insemination (IUI)).
Types of interventions
-
GnRH agonists in comparison with HCG for final oocyte maturation triggering in GnRH antagonist–controlled hyperstimulation cycles, IVF or ICSI followed by embryo transfer (ET) with or without luteal phase support, in autologous or donor cycles.
Types of outcome measures
Primary outcomes
-
Live birth rate (LBR) per woman randomised: live birth defined as delivery of a live fetus after 20 completed weeks of gestation.
-
Incidence of OHSS per woman randomised (mild, moderate or severe): detected by clinical, laboratory or imaging grading of OHSS.
Secondary outcomes
-
Ongoing pregnancy rate (OPR) per woman randomised: ongoing pregnancy defined as pregnancy beyond 12 weeks.
-
Clinical pregnancy rate (CPR) per woman randomised: clinical pregnancy defined as presence of a fetal heart rate with transvaginal ultrasound.
-
Early miscarriage rate per woman randomised.
-
Multiple pregnancy rate per woman randomised.
Search methods for identification of studies
All published and unpublished RCTs of GnRH agonists versus HCG for final oocyte maturation triggering were sought, without language restriction and in consultation with the Menstrual Disorders and Subfertility Group (MDSG) Trials Search Co‐ordinator, using the following search strategy.
Electronic searches
2014 update
We searched the following electronic databases, trial registers and websites to 8 September 2014: the MDSG Specialised Register of Controlled Trials (Appendix 1), the Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 2), MEDLINE (Appendix 3), EMBASE (Appendix 4), PsycINFO (Appendix 5) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL)(Appendix 6). Other electronic sources of trials included the following.
-
Trial registers for ongoing and registered trials: http://www.controlled‐trials.com, http://clinicaltrials.gov/ct2/home, http://www.who.int/trialsearch/Default.aspx.
-
Citation indexes: http://scientific.thomson.com/products/sci/ Conference abstracts.
-
Conference abstracts in the Web of Knowledge: http://www.wokinfo.com
-
Latin American and Caribbean Health Science Information Database (LILACS) database, for trials from the Portuguese and Spanish‐speaking world: http://bases.bireme.br/cgi ‐bin/ wxislind.exe/iah/online/?IsisScript=iah/i ah.xis&base=LILACS&lang=i&form=F.
-
PubMed: www.ncbi.nlm.nih.gov/pubmed/.
-
Open System for Information on Grey Literature in Europe (OpenSIGLE) database (http://opensigle.inist.fr/) and Google for grey literature.
MEDLINE and EMBASE search strategies use different filters for identifying randomised trials. The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying randomised trials, which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.0.1, Chapter 6, 6.4.11). EMBASE and CINAHL searches were combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/methodology/filters.html#random).
Searching other resources
-
Reference lists of relevant clinical practice guidelines, review articles and studies.
-
Letters seeking information about unpublished or incomplete RCTs sent to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
-
After an initial screen of titles and abstracts retrieved by the search, conducted by MAFMY and MVW, the full texts of all potentially eligible studies were retrieved. These full‐text articles were examined for compliance with the inclusion criteria, and review authors selected studies eligible for inclusion in the review. We corresponded with study investigators as required to clarify study eligibility. The selection process was documented on a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart (Figure 1).
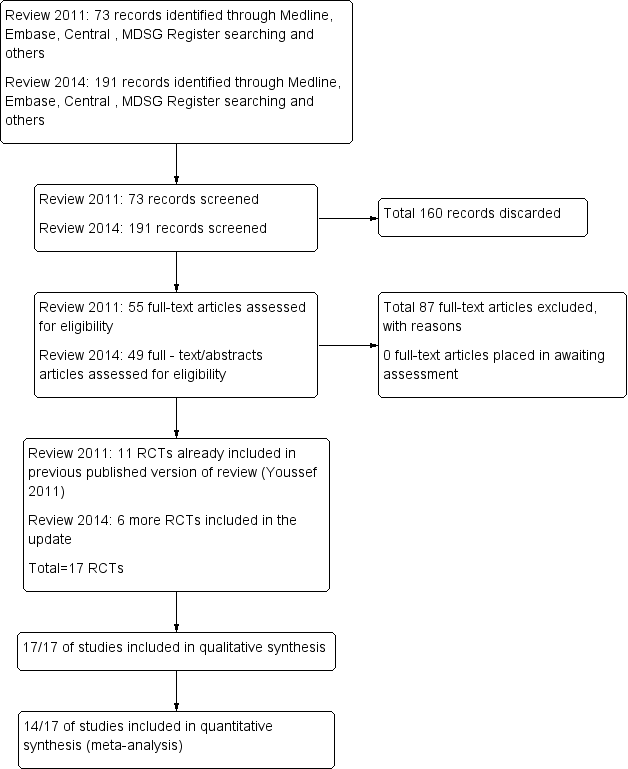
Study flow diagram.
Data extraction and management
-
Two review authors independently extracted data from eligible studies using a standard data extraction form that they designed and pilot‐tested. Disagreements were resolved by discussion or by consultation with a third review author. Extracted data included study characteristics and outcome data (see data extraction table for details, Characteristics of included studies).
-
Data entry was carried out by the same two review authors.
Studies were analysed for the following quality criteria and methodological details.
Trial characteristics
Study design
-
Method of randomisation.
-
Multi‐centre or single‐centre design.
-
Presence or absence of blinding to treatment allocation.
-
Number of participants randomised, excluded or lost to follow‐up.
-
Presence of intention‐to‐treat (ITT) analysis.
-
Presence of a power calculation.
Characteristics of study participants
-
Subfertile women undergoing IVF/ICSI treatment cycles.
-
At high or low risk to develop OHSS.
Interventions used
-
Types of ovarian hyperstimulation protocols used.
-
Types of final oocyte maturation triggering used: route of administration, duration and dose.
-
Types of luteal phase support provided: dose, duration and route of administration.
Outcomes
-
LBR.
-
Incidence of OHSS.
-
Ongoing pregnancy rate.
-
Clinical pregnancy rate.
-
Miscarriage rate.
-
Multiple pregnancy rate.
Assessment of risk of bias in included studies
Two review authors independently assessed the included studies for risk of bias using the risk of bias assessment tool of The Cochrane Collaboration (Higgins 2011) to assess allocation (random sequence generation and allocation concealment); blinding of participants, personnel and outcome assessors; incomplete outcome data; selective reporting; and other bias. Disagreements were resolved by discussion or by consultation with a third review author.
Randomisation
Randomisation was considered adequate if any random method of allocation was described and was verifiable, that is,
-
using a computerised random number generator; or
-
referring to a number table.
Concealment of allocation (selection bias)
-
This was considered adequate if a third‐party system; serially numbered sealed, opaque envelopes; or a similar system was described. Concealment was stated as 'unclear ' if no information was available pertaining to allocation concealment.
Blinding of participants and personnel (performance bias)
-
This was examined with regard to likelihood of bias influencing primary outcomes. We considered it unlikely that blinding would influence findings for live birth, but likely that blinding could influence findings for OHSS, so unblinded studies were rated as having high risk of bias for this outcome.
Blinding of outcome assessors (detection bias)
-
This was examined with regard to likelihood of bias influencing primary outcomes. We considered it unlikely that blinding would influence findings for live birth, but likely that blinding could influence findings for OHSS, so unblinded studies were rated as having high risk of bias for this outcome.
Incomplete outcome data
-
Low risk of bias was allocated if no outcome data were missing, or if missing outcome data were balanced in numbers across intervention groups with similar reasons provided for missing data across groups.
Selective outcome reporting
-
Low risk of bias was allocated if all of a study's primary, secondary and additional outcomes of interest in the review were reported in a prespecified way; when fewer outcome measures were reported than planned, this was considered to be a source of bias.
Other potential sources of bias
We considered other potential forms of bias (e.g. baseline imbalance of groups, premature discontinuation of study).
Measures of treatment effect
For dichotomous data (e.g. live birth rates), the numbers of events in control and intervention groups of each study were used to calculate odds ratios (ORs) with 95% confidence intervals (CIs) for each individual trial.
Unit of analysis issues
The primary analysis was per woman randomised (e.g. live birth rate or miscarriage rate per woman randomised, defined as the number of women achieving a live birth divided by the number of women treated). Data per cycle were not included in the analysis.
Dealing with missing data
When possible, data were extracted to allow for an ITT analysis, defined as including all randomised participants in the denominator. When appropriate, study authors were contacted to provide further information or missing data. Data obtained in this manner were included in our analyses. Women lost to follow‐up were assumed to be not pregnant.
Assessment of heterogeneity
We considered whether clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by the measure of the I2 statistic. An I2 measurement greater than 50% was taken to indicate substantial heterogeneity (Higgins 2011). We tested the effect of using a random‐effects model when heterogeneity was substantial.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If 10 or more studies were included in an analysis, we planned to use a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
Data from primary studies were combined using the fixed‐effect model in the following comparisons.
-
GnRH agonist versus HCG in fresh autologous cycles.
-
GnRH agonist versus HCG in donor‐recipient cycles.
-
-
An increase in the odds of a particular outcome, which may be beneficial (e.g. live birth) or detrimental (e.g. OHSS, miscarriage), was displayed graphically in the meta‐analyses to the right of the centre‐line (i.e. in favour of GnRH agonist), and a decrease in the odds of an outcome to the left of the centre‐line (i.e. in favour of HCG).
-
-
-
For the meta‐analysis, the number of women experiencing the event in each group of the trial was recorded. Meta‐analysis of binary data was performed using the Mantel‐Haenszel method with a fixed‐effect model, and the OR and the 95% CI were calculated using RevMan 5 software.
-
-
-
We performed a separate analysis for oocyte donor‐recipient cycles.
-
Subgroup analysis and investigation of heterogeneity
We considered clinical and methodological differences between studies that might account for any heterogeneity.
When data were available, we conducted subgroup analyses to determine separate evidence within the following subgroups in studies of autologous cycles.
Type of luteal phase support (for the outcomes of live birth, OHSS and ongoing pregnancy)
-
Luteal phase support with LH activity (single or two doses of HCG, recLH and repeated GnRH doses)
-
Luteal phase support without LH activity (progesterone only or progesterone plus oestradiol).
Risk of OHSS (for the outcome of OHSS)
-
Studies of women with low OHSS risk: Low risk was defined as studies excluding women with polycystic ovary syndrome (PCOS) or women with high numbers of ovarian follicles (≥ 14 follicles) ≥ 11 mm in diameter.
-
Studies of women with high OHSS risk: High risk was defined as studies including women with PCOS or women with high numbers of ovarian follicles (≥ 14 follicles) ≥ 11 mm in diameter.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding study eligibility and analysis. These analyses included consideration of whether the review conclusions would have differed if:
-
we had used a random‐effects model for the primary outcomes;
-
we had reported risk ratios rather than odds ratios; or
-
we had included only moderate or severe OHSS as an outcome (not including mild OHSS).
Results
Description of studies
For details about the studies, please see: Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification.
Results of the search
In searches to date (2011 and 2014), a total of 264 references were identified. Most references identified by the search were excluded at the first screening step, as they were clearly irrelevant (n = 160). The most frequent reasons for exclusion were the following: The article was a review or a commentary/editorial, or the study was clearly of a non‐randomised design or reported comparisons of no interest (n = 87); 17 RCTs fulfilled the inclusion criteria (Figure 1).
Included studies
Design
-
Seventeen RCTs, 13 in fresh autologous cycles and four in donor‐recipient cycles, including 1847 randomly assigned women, met the inclusion criteria and were fully reviewed. Randomisation was done as soon as oocyte maturation triggering was planned in all except one trial. In this trial, randomisation was timed to occur at the beginning of stimulation (Kolibianakis 2005). Three abstracts (Segal 1992; Ossina 2004; Peňa 2007) were published in conference proceedings. As it was not possible to obtain further information from the authors of these abstracts, they were excluded from the quantitative analysis. See Characteristics of included studies.
-
Ten studies were randomised controlled single‐centre studies (Segal 1992; Acevedo 2006; Babayof 2006; Humaidan 2006; Pirard 2006; Peňa 2007; Engmann 2008; Galindo 2009; Melo 2009; Papanikolaou 2010). Three studies were two‐centre studies (Beckers 2003; Humaidan 2005; Kolibianakis 2005), one was a three‐centre study (Humaidan 2010), one was a four‐centre study (Humaidan 2013) and two studies were six‐centre studies (Fauser 2002; Ossina 2004).
-
Nine studies reported sample size calculations for the primary outcome (Beckers 2003; Humaidan 2005; Kolibianakis 2005; Babayof 2006; Engmann 2008; Galindo 2009; Melo 2009; Humaidan 2010; Humaidan 2013). No sample size calculation was performed in three studies (Fauser 2002; Acevedo 2006; Pirard 2006); in five studies, this information was not provided (Segal 1992; Ossina 2004; Humaidan 2006; Peňa 2007; Papanikolaou 2010).
-
Three studies failed to achieve the intended sample size (Humaidan 2005; Kolibianakis 2005; Humaidan 2013). Nine studies recruited only a small number of women (Fauser 2002, n = 57; Beckers 2003, n = 40; Humaidan 2005, n = 45; Acevedo 2006, n = 60; Babayof 2006, n = 28; Pirard 2006, n = 30; Engmann 2008, n= 66; Melo 2009, n = 100; Papanikolaou 2010; n = 39).
-
Fourteen RCTs were published as full‐text articles (Fauser 2002; Beckers 2003; Humaidan 2005; Kolibianakis 2005; Acevedo 2006; Babayof 2006; Humaidan 2006; Pirard 2006; Engmann 2008; Galindo 2009; Melo 2009; Humaidan 2010; Papanikolaou 2010; Humaidan 2013) and three as abstracts (Segal 1992; Ossina 2004; Peňa 2007) in conference proceedings.
-
For details of study risk of bias, see the Characteristics of included studies table.
-
Source of funding (Lundh 2012): Four studies (28%) reported that they received industry funding (Beckers 2003; Engmann 2008; Papanikolaou 2010; Humaidan 2013).
Participants
-
Analysed studies (14/17) included 791 women in the intervention groups and 779 in the control groups. All were women with subfertility from 18 to 40 years of age. All participants were undergoing IVF/ICSI treatment cycles followed by fresh ET in autologous or donor cycles.
-
The number of randomly assigned participants ranged from 23 (Pirard 2006) to 302 (Humaidan 2010), including both GnRH agonist and HCG groups.
-
Baseline characteristics were comparable between groups (Characteristics of included studies).
-
Ten studies included women at low risk of developing OHSS (Fauser 2002; Beckers 2003; Humaidan 2005; Kolibianakis 2005; Acevedo 2006; Humaidan 2006; Galindo 2009; Melo 2009; Humaidan 2010; Papanikolaou 2010), and only three studies randomised women with PCOS or with retrieved oocytes with more than 14 follicles (Babayof 2006; Engmann 2008; Humaidan 2013). Risk of OHSS was reported unclearly in four studies (Segal 1992; Ossina 2004; Pirard 2006; Peňa 2007).
Intervention
-
All included studies compared GnRH agonist versus HCG for final oocyte maturation triggering in GnRH antagonist down‐regulated IVF and ICSI cycles.
-
Five studies used 250 μg of recombinant HCG (rHCG) for final oocyte maturation triggering in the control group (Acevedo 2006; Babayof 2006; Galindo 2009; Melo 2009; Papanikolaou 2010). A three‐arm study compared LH versus rHCG versus GnRH (Beckers 2003). Other included studies used 10,000 IU of urinary HCG for final oocyte maturation triggering, except one (Engmann 2008), which used a dose ranging from 3300 to 10,000 IU, depending on follicular response.
-
Luteal phase support: Five studies used progesterone (P) plus oestradiol (E2) in fresh autologous cycles (Kolibianakis 2005; Humaidan 2005; Babayof 2006; Humaidan 2006; Engmann 2008) and one study in donor‐recipient cycles (Acevedo 2006). Two studies used the combination of P + E2 + single dose of 1500 IU hCG (Humaidan 2010) or two doses of 1500 IU HCG (Humaidan 2013); one study used P only in fresh autologous cycles (Fauser 2002) and two studies in donor‐recipient cycles (Galindo 2009; Melo 2009); one study used the combination of P + six doses of recLH (Papanikolaou 2010); one study used repeated administration of GnRH agonist (Pirard 2006); and one study provided no luteal phase support (Beckers 2003).
Outcomes
-
Five studies reported live birth rate in fresh autologous cycles (Humaidan 2005; Babayof 2006; Humaidan 2006; Humaidan 2010; Papanikolaou 2010) and one study in donor‐recipient cycles (Galindo 2009).
-
Eight studies reported OHSS incidence in fresh autologous cycles (Kolibianakis 2005; Babayof 2006; Humaidan 2006; Pirard 2006; Engmann 2008; Humaidan 2010; Papanikolaou 2010; Humaidan 2013) and three studies in donor‐recipient cycles (Acevedo 2006; Galindo 2009; Melo 2009).
-
All included studies reported ongoing pregnancy rate, clinical pregnancy rate and early miscarriage rate in both groups.
-
Multiple pregnancy rate was reported in all donor‐recipient cycles and in two studies in fresh autologous cycles (Babayof 2006; Papanikolaou 2010).
-
Three studies were published as abstracts with no details on outcome measures (Segal 1992; Ossina 2004; Peňa 2007); therefore they were included only in the qualitative research—not in the meta‐analysis.
Excluded studies
In searches to date (2011 and 2014), a total of 87 studies were excluded. Reasons for exclusion are explained in the Characteristics of excluded studies table.
Risk of bias in included studies
Risk of bias in the included studies is summarised in Figure 2 and Figure 3.
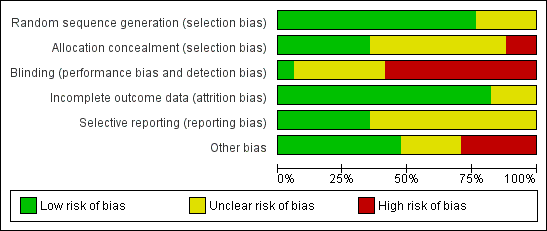
Risk of bias graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological risk of bias summary: review authors' judgements about each methodological quality item for each included study.
Allocation
-
Thirteen studies were rated as having low risk of bias related to sequence generation, and four were rated as having unclear risk of bias in this domain.
-
Six studies were rated as having low risk of bias related to allocation concealment, and nine were rated as having unclear risk of this bias. In two trials, the allocation was not adequately concealed; these studies were rated as having high risk of bias (Kolibianakis 2005; Acevedo 2006).
Blinding
-
One study clearly reported blinding of assessors (Melo 2009) and was deemed to be at low risk of bias related to blinding. Six studies did not clearly report on blinding and were rated as having unclear risk of bias related to assessment of OHSS. Ten reported lack of blinding and were rated as having high risk of bias related to assessment of OHSS.
Incomplete outcome data
Fourteen studies were rated as having low risk of attrition bias. Three were rated as having unclear risk of bias in this domain.
Intention‐to‐treat analysis
-
We contacted the following investigators of individual studies via email to ask for additional information, so we could perform analyses on an ITT basis (Fauser 2002; Humaidan 2005; Acevedo 2006; Humaidan 2006; Humaidan 2010). We could not identify contact details for the authors of two abstracts (Ossina 2004; Peňa 2007); therefore we excluded these studies from analysis on the basis of missing relevant data.
-
Only five studies performed an ITT analysis (Humaidan 2006; Galindo 2009; Humaidan 2010; Papanikolaou 2010; Humaidan 2013).
-
In seven studies, no ITT analysis was performed (Fauser 2002; Beckers 2003; Humaidan 2005; Kolibianakis 2005; Acevedo 2006; Pirard 2006; Engmann 2008), and it was unclear whether ITT was used in two studies (Babayof 2006; Melo 2009). However, for all of these studies, the number of women randomised was known; therefore the ITT data could be imputed.
Selective reporting
Six studies were rated as having low risk of selective reporting bias; 11 were rated as having unclear risk of bias in this domain, in most cases because live birth and/or OHSS was not reported.
Other potential sources of bias
For eight studies, no additional potential sources of bias were noted. Four studies were rated as having unclear risk of other bias because they were reported only as abstracts and provided insufficient details on methods.
Five studies were deemed at high risk of other potential bias. All of these studies were prematurely discontinued. In one case (Kolibianakis 2005), study discontinuation was triggered by preplanned stopping rules. In other cases (Beckers 2003; Humaidan 2005; Pirard 2006), the interim analysis was unplanned and/or stopping rules were unclear. Three of these studies were stopped prematurely as the result of a significantly lower pregnancy rate in the GnRH agonist triggering group, and in one trial with six arms, two arms were stopped prematurely for the same reason (Pirard 2006). One study was stopped prematurely before the estimated sample size had been obtained as a result of the death of one of the local principal investigators and job rotations among other investigators (Humaidan 2013).
Effects of interventions
Primary outcomes
1.1 Live birth rate per woman randomised
1.1.1 Fresh autologous cycles
GnRH agonist trigger was associated with a lower live birth rate than was seen with HCG (OR 0.47, 95% CI 0.31 to 0.70; five RCTs, 532 women, I² = 56%, moderate‐quality evidence). This means that for a woman with a 31% chance of achieving live birth with the use of HCG, the chance of a live birth with the use of a GnRh agonist will be between 12% and 24%. Use of a random‐effects model did not substantially affect the results (OR 0.38, 95% CI 0.17 to 0.89), nor did use of risk ratios have a substantial effect. Statistical heterogeneity for this outcome was moderate. The live birth rate varied from 15% to 53% in the HCG group and from 5% to 24% in the agonist group (Analysis 1.1; Figure 4).
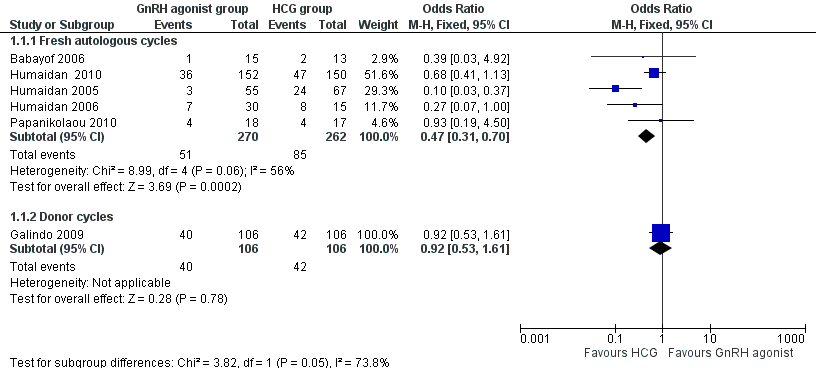
GnRH agonist versus HCG for oocyte maturation triggering, outcome: 1.1 Live birth rate per women randomly assigned.
1.1.2 Donor‐recipient cycles
No evidence of a difference in live birth rate was noted between GnRH agonist and HCG groups in donor‐recipient cycles (OR 0.92, 95% CI 0.53 to 1.61; one RCT, 212 women) (Analysis 1.1; Figure 4).
1.2 Live birth rate in autologous cycles: subgroup analysis on luteal support approach
The subgroup analysis based on luteal phase support methods used in the included studies revealed differences in live birth rates between trials that used luteal phase support with LH activity and trials that used luteal phase support without LH activity. Both groups showed evidence of differences in live birth rate in favour of HCG, but this difference was significantly greater in studies that used luteal support without LH activity (studies with luteal phase support with LH activity: OR 0.63, 95% CI 0.40 to 0.98; three RCTs, 382 women, I2 = 0%; studies with luteal phase support without LH activity: OR 0.13, 95% CI 0.04 to 0.39; two RCTS, 150 women, I2 = 73%; test for subgroup differences: Chi² = 6.65, df = 1 (P value 0.010), I² = 85.0%) (Analysis 1.2).
1.3 Ovarian hyperstimulation syndrome (OHSS)
1.3.1 Fresh autologous cycles
GnRH agonist was associated with lower risk of OHSS (mild, moderate or severe) than was seen with HCG (OR 0.15, 95% CI 0.05 to 0.47; eight RCTs, 989 women, I² = 42%, moderate‐quality evidence; Analysis 1.3). This suggests that for a woman with a 5% risk of OHSS using HCG, the rate would be between nil and 2% with use of a GnRH agonist. Use of a random‐effects model did not substantially affect the results (OR 0.17, 95% CI 0.03 to 0.98; I2 = 42%) (Analysis 1.3; Figure 5).

GnRH agonist versus HCG for oocyte maturation triggering, outcome: 1.2 OHSS incidence per women randomly assigned.
1.3.2 Donor‐recipient cycles
We found evidence of a lower incidence of OHSS in the GnRH agonist group than in the HCG group (OR 0.05, 95% CI 0.01 to 0.28; three RCTs, 374 women, I² = 0%) (Analysis 1.3; Figure 5).
1.4 Incidence of OHSS in autologous cycles: subgroup analysis on luteal support approach
The subgroup analysis based on luteal phase support methods used in the included studies found no evidence of a difference in OHSS rates between trials that used luteal phase support with LH activity and trials that used luteal phase support without LH activity (test for subgroup differences: Chi² = 3.39, df = 1 (P value 0.07), I² = 71%). No evidence was found of a difference between GnRH agonist and HCG groups among women who had luteal phase support with LH activity (OR 0.47, 95% CI 0.11 to 2.09; I2 = 25%, five RCTs), but the OHSS rate was lower in the GnRH agonist group among women who had luteal phase support without LH activity (OR 0.04, 95% CI 0.01 to 0.34; I2 = 0%) (Analysis 1.4).
Secondary outcomes
1.5 Ongoing pregnancy rate per woman randomised
1.5.1 Fresh autologous cycles
GnRH agonist trigger was associated with a lower ongoing pregnancy rate when compared with HCG (OR 0.70, 95% CI 0.54 to 0.91; 11 RCTs, 1198 women, I² = 54%, moderate‐quality evidence) (Analysis 1.5; Figure 6).

GnRH agonist versus HCG for oocyte maturation triggering, outcome: 1.3 Ongoing pregnancy rate per women randomly assigned.
1.5.2 Donor‐recipient cycles
We observed no evidence of differences between groups in ongoing pregnancy rate (OR 0.88, 95% CI 0.58 to 1.32; three RCTs, 374 women, I² = 0%) (Figure 5).
1.6 Ongoing pregnancy rate in autologous cycles: subgroup analysis on luteal support approach
The subgroup analysis based on luteal phase support methods used in the included studies indicated differences in ongoing pregnancy rate between trials that used luteal phase support with LH activity and those that used luteal phase support without LH activity (test for subgroup differences: Chi² = 8.1, df = 1 (P value 0.004), I² = 88%). No evidence was found of differences between groups among women who had luteal phase support with LH activity (OR 0.89, 95% CI 0.65 to 1.21; I2 = 27%, five RCTs), but the ongoing pregnancy rate in the HCG group was higher among women who had luteal phase support without LH activity (OR 0.36, 95% CI 0.21 to 0.62; I2 = 73%, five RCTs, 370 women) (Analysis 1.6).
1.7 Clinical pregnancy rate per woman randomised
1.7.1 Fresh autologous cycles
We found no evidence of a difference between groups in clinical pregnancy rate (OR 0.81, 95% CI 0.61 to 1.04; 11 RCTs, 1198 women, I² = 49%) (Analysis 1.7).
1.7.2 Donor‐recipient cycles
We found no evidence of a difference between groups in clinical pregnancy rate (OR 0.87, 95% CI 0.57 to 1.33; three RCTs, 372 women, I² = 0%) (Analysis 1.7).
1.8 Miscarriage rate per woman randomised
1.8.1 Fresh autologous cycles
GnRH agonist trigger was associated with a higher early miscarriage rate when compared with HCG (OR 1.74, 95% CI 1.10 to 2.75; 11 RCTs, 1198 women, I² = 1%) (Analysis 1.8).
1.8.2 Donor‐recipient cycles
We found no evidence of differences between groups in miscarriage rate (OR 1.14, 95% CI 0.56 to 2.32; three RCTs, 372 women, I² = 0%) (Analysis 1.8).
1.9 Multiple pregnancy per woman randomised
1.9.1 Fresh autologous cycles
We found no evidence of differences between groups in multiple pregnancy rate (OR 3.00, 95% CI 0.30 to 30.47; two RCTs, 62 women, I² = 0%) (Analysis 1.9).
1.9.2 Donor‐recipient cycles
We found no evidence of differences between groups in multiple pregnancy rate (OR 1.73, 95% CI 0.86 to 3.48; three RCTs, 374 women, I² = 0%) (Analysis 1.9).
Additional analyses
Subgroup and sensitivity analyses
10.1 OHSS incidence: effect of risk
OHSS in women at low risk of OHSS
No evidence of a difference between GnRh agonist and HCG was noted in the rate of OHSS among women at low risk of OHSS (OR 0.79, 95% CI 0.18 to 3.47; six RCTs, 777 women, I2 = 66%; Analysis 1.10). Heterogeneity for this analysis was substantial, probably as a result of the low event rate, with four of the six RCTs reporting no events in either arm.
OHSS in women at high risk of OHSS
GnRH agonist was associated with a significantly lower risk of OHSS when compared with HCG among women at high risk of OHSS (OR 0.06, 95% CI 0.01 to 0.34; three RCTs, 212 women, I2 = 0%; Analysis 1.10).
10.2 Effect of including only moderate or severe OHSS as an outcome
After cases with mild OHSS were excluded, GnRH agonist was associated with lower risk of moderate or severe OHSS when compared with HCG (OR 0.21, 95% CI 0.07 to 0.66; four RCTs, 112 women, I2 = 20%; Analysis 1.2). The analysis included only 16 events reported by four RCTs. A further five RCTs reported no events in either arm.
Results were similar among women at high risk of OHSS: GnRH agonist was associated with significantly lower risk of moderate or severe OHSS when compared with HCG (OR 0.09, 95% CI 0.02 to 0.52; four RCTs, 112 women, I2 = 0%; Analysis 1.10).
10.3 Use of risk ratios rather than odds ratios
Use of risk ratios rather than odds ratios did not materially affect our findings.
Findings of other subgroup and sensitivity analyses are described above, under the section on relevant comparisons.
Assessment of publication bias
A funnel plot was constructed for the outcome of ongoing pregnancy (Figure 7). This plot was not symmetrical, as a greater number of effect estimates were placed on the left side of the graph. This could imply publication bias, but in this case it seems more likely that the effect was due to the fact that the more extreme effect estimates were derived from studies that did not use luteal support with LH.

Funnel plot of comparison: 1 GnRH agonist versus HCG for oocyte maturation triggering, outcome: 1.5 Ongoing pregnancy rate per woman randomised.
Discussion
Summary of main results
This review update on the benefits and harms of GnRH agonist trigger in subfertile women treated with GnRH antagonist in IVF/ICSI treatment cycles found that use of GnRH agonist trigger compared with HCG triggering was associated with a markedly reduced live birth rate and an increased early miscarriage rate but was beneficial in preventing OHSS in fresh autologous cycles among women at high risk of OHSS. No differences between interventions in OHSS incidence were noted among women at low risk of OHSS. Overall (regardless of underlying risk) for a woman with a 5% risk of mild, moderate or severe OHSS with use of HCG, the risk of OHSS with use of a GnRh agonist was between nil and 2%, and for women with a 5% risk of developing moderate or severe OHSS with use of HCG, the risk with use of a GnRH antagonist was between nil and 3% (summary of findings Table for the main comparison).
In donor‐recipient cycles, use of GnRH agonist instead of HCG also resulted in a lower incidence of OHSS. No evidence was found of a difference in live birth or ongoing pregnancy rate, although the results were consistent with those for fresh autologous cycles.
Overall completeness and applicability of evidence
Quality of the evidence
GRADE assessment found that evidence for most review outcomes was of moderate quality. Exceptions included ongoing pregnancy and multiple pregnancy, which were rated as having low‐quality evidence. Reasons for downgrading evidence quality included poor reporting of study methods, premature study termination, failure to blind outcome assessment and statistical heterogeneity. For some outcomes, confidence intervals were wide as the result of low event rates (summary of findings Table for the main comparison).
The authors of four studies stated that the studies were commercially funded. The authors of most studies failed to disclose their funding source.
Potential biases in the review process
Strengths of this review include comprehensive systematic searching for eligible studies, rigid inclusion criteria for RCTs and data extraction and analysis by two independent review authors. Furthermore, the possibility of publication bias was minimised by inclusion of both published and unpublished studies (such as abstracts from meetings). However, as with any review, we cannot guarantee that we found all eligible studies.
Agreements and disagreements with other studies or reviews
Our results are in agreement with those of a previous review (Griesinger 2006). However, that review included only three small randomised controlled studies (Fauser 2002; Humaidan 2005; Kolibianakis 2005) involving 275 randomised women.
How can poor reproductive outcomes following oocyte triggering with GnRH agonist be explained? In previous studies, oocyte maturity, fertilisation rate and embryo development were comparable between GnRH agonist and HCG‐induced final oocyte maturation. This was found both in fresh autologous cycles (Griesinger 2006) and in donor cycles (Bodri 2009; Erb 2009). Furthermore, frozen‐thawed cycles with embryos obtained after oocyte triggering with GnRH agonist resulted in high pregnancy rates (Griesinger 2007a; Griesinger 2007b). Hence, oocyte triggering with GnRH agonist appears to have no major impact on oocyte and embryo quality.
It seems more likely that GnRH agonist induces a luteal phase defect. This luteal phase defect may result from the short half‐life of the induced LH surge, leading to premature luteolysis of corpus luteum and significantly lower steroidal and non‐steroidal hormones, thus affecting endometrial receptivity (Lanzone 1994; Peñarrubia 1998; Nevo 2003; Emperaire 2004; Humaidan 2005). Consequently, further studies have been conducted to evaluate different modified luteal phase strategies with LH activity supplementation in terms of administration of small dosages of HCG around the time of oocyte maturation trigger (Humaidan 2010; Humaidan 2013) or with repeated administration of recLH (Papanikolaou 2010), or without LH supplementation but with the help of progesterone and oestradiol administration (Engmann 2008). Our subgroup analysis shows that, although modified luteal phase support with LH was associated with pregnancy rates almost comparable with those of HCG, the difference in OHSS risk was no longer present. Apparently, available regimens could not compensate for the induced luteal phase defect in GnRH agonist–triggered cycles.
Our meta‐analysis of fresh autologous cycles and donor‐recipient cycles found that use of a GnRH agonist trigger is associated with a significantly reduced incidence of OHSS when compared with HCG, as none of the women in the pooled studies developed any form of OHSS when in the GnRH agonist group. The shorter half‐life of the endogenous LH surge and subsequent pituitary suppression and withdrawal of LH support for the corpora luteum may lead to early luteolysis (Kol 2004; Kol 2008). Moreover, significantly lower luteal levels of inhibins and steroid hormones suggest that the corpora luteum may secrete lower levels of other non‐steroidal substances, and the vasoactive properties of vascular endothelial growth factor (VEGF) may be involved in OHSS. This may explain the mechanism of OHSS prevention with the use of GnRH agonists (Nevo 2003; Cerrillo 2011).

Study flow diagram.

Risk of bias graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological risk of bias summary: review authors' judgements about each methodological quality item for each included study.
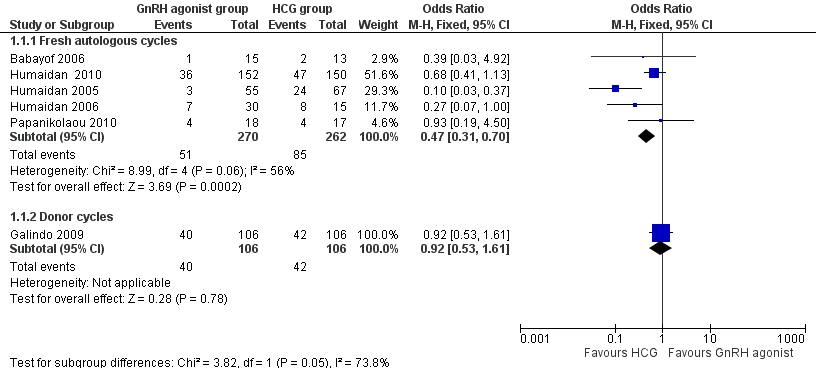
GnRH agonist versus HCG for oocyte maturation triggering, outcome: 1.1 Live birth rate per women randomly assigned.

GnRH agonist versus HCG for oocyte maturation triggering, outcome: 1.2 OHSS incidence per women randomly assigned.

GnRH agonist versus HCG for oocyte maturation triggering, outcome: 1.3 Ongoing pregnancy rate per women randomly assigned.

Funnel plot of comparison: 1 GnRH agonist versus HCG for oocyte maturation triggering, outcome: 1.5 Ongoing pregnancy rate per woman randomised.

Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 1 Live birth rate per woman randomised.

Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 2 Live birth rate in autologous cycles: luteal phase support approach.

Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 3 OHSS incidence per woman randomised.

Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 4 OHSS rate in autologous cycles: luteal support approach.

Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 5 Ongoing pregnancy rate per woman randomised.

Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 6 Ongoing pregnancy rate in autologous cycles: luteal phase support approach.

Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 7 Clinical pregnancy per woman randomised.

Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 8 Miscarriage rate per woman randomised.

Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 9 Multiple pregnancy rate per woman randomised.

Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 10 Subgroup and sensitivity analyses—OHSS incidence in autologous cycles: risk and severity.
| GnRH agonist compared with HCG for oocyte maturation triggering in antagonist‐assisted reproductive technology | ||||||
| Population: subfertile women | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| HCG for oocyte maturation triggering | GnRH agonist | |||||
| Live birth | 313 per 1000 | 176 per 1000 | OR 0.47 | 532 | ⊕⊕⊕⊝ | |
| OHSS (mild, moderate or severe): overall risk | 5 per 1000 | 1 per 1000 | OR 0.15 | 989 | ⊕⊕⊕⊝ | |
| OHSS (moderate or severe): overall risk | 5 per 1000 | 1 per 1000 | OR 0.21 | 989 | ⊕⊕⊕⊝ | Low event rate: 4 of 9 RCTs reported no events in either arm |
| OHSS (mild, moderate or severe) in women at high risk of OHSS | 308 per 1000 | 26 per 1000 (4 to 131) | OR 0.06 | 212 women (3 studies) | ⊕⊕⊕⊝ | |
| Ongoing pregnancy | 256 per 1000 | 194 per 1000 | OR 0.7 | 1198 | ⊕⊕⊝⊝ | |
| Miscarriage | 67 per 1000 | 111 per 1000 | OR 1.74 | 1198 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aOne of the studies at high risk of bias because of premature termination. bAll studies at high risk of bias in 1 or more domains. None clearly reported blinded outcome assessment. cMost studies at high risk of bias in 1 or more domains. None clearly reported blinded outcome assessment. dSubstantial heterogeneity: I2 = 59% to 66%. e5/11 studies at high risk of bias because of early termination and/or inadequate allocation concealment. None clearly reported blinded outcome assessment. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate per woman randomised Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Fresh autologous cycles | 5 | 532 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.31, 0.70] |
| 1.2 Donor cycles | 1 | 212 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.53, 1.61] |
| 2 Live birth rate in autologous cycles: luteal phase support approach Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Live birth in studies using modified luteal phase support with LH activity | 3 | 382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.40, 0.98] |
| 2.2 Live birth in studies using modified luteal phase support without LH activity (P ± E2) | 2 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.04, 0.39] |
| 3 OHSS incidence per woman randomised Show forest plot | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Fresh autologous cycles | 8 | 989 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.05, 0.47] |
| 3.2 Donor cycles: mild, moderate or severe OHSS | 3 | 372 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.28] |
| 4 OHSS rate in autologous cycles: luteal support approach Show forest plot | 8 | 989 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.05, 0.47] |
| 4.1 OHSS in studies using modified luteal phase support with LH activity | 5 | 789 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.11, 2.09] |
| 4.2 OHSS in studies using modified luteal phase support without LH activity | 3 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.34] |
| 5 Ongoing pregnancy rate per woman randomised Show forest plot | 14 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Autologous cycles | 11 | 1198 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.54, 0.91] |
| 5.2 Donor cycles | 3 | 372 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.58, 1.32] |
| 6 Ongoing pregnancy rate in autologous cycles: luteal phase support approach Show forest plot | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Ongoing pregnancy in studies using modified luteal phase support with LH activity | 5 | 789 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.65, 1.21] |
| 6.2 Ongoing pregnancy in studies using modified luteal phase support without LH activity (P ± E2) | 5 | 370 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.21, 0.62] |
| 7 Clinical pregnancy per woman randomised Show forest plot | 14 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Autologous cycles | 11 | 1198 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.63, 1.04] |
| 7.2 Donor cycles | 3 | 372 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.57, 1.33] |
| 8 Miscarriage rate per woman randomised Show forest plot | 14 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Autologous cycles | 11 | 1198 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.10, 2.75] |
| 8.2 Donor cycles | 3 | 372 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.56, 2.32] |
| 9 Multiple pregnancy rate per woman randomised Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Autologous cycles | 2 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.30, 30.47] |
| 9.2 Donor cycles | 3 | 372 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.86, 3.48] |
| 10 Subgroup and sensitivity analyses—OHSS incidence in autologous cycles: risk and severity Show forest plot | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Autologous cycles: studies of women at low OHSS risk reporting mild, moderate or severe OHSS | 6 | 777 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.18, 3.47] |
| 10.2 Autologous cycles: studies of women at high OHSS risk reporting mild, moderate or severe OHSS | 3 | 212 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.34] |
| 10.3 Autologous cycles: all studies (women at high or low OHSS risk) reporting moderate or severe OHSS | 8 | 989 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.66] |
| 10.4 Autologous cycles: studies of women at high OHSS risk reporting moderate or severe OHSS | 3 | 212 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.02, 0.52] |

