دیلاتاسیون مکانیکال دهانه رحم در طول زایمان سزارین انتخابی پیش از آغاز زایمان برای کاهش موربیدیتی پس از جراحی
چکیده
پیشینه
حین زایمان سزارین انتخابی (برنامهریزی شده)، برخی از متخصصان زایمانی به طور معمول دهانه رحم را با استفاده از فورسپس اسفنجی، یک انگشت یا دیگر ابزار، حین جراحی دیلاته میکنند، چون دهانه رحم زنی که وارد فاز زایمان نشده، ممکن است دیلاته نشود و این امر ممکن است باعث انسداد خون یا درناژ lochia شود. با این حال، دیلاتاسیون مکانیکال دهانه رحم طی زایمان سزارین ممکن است منجر به آلوده شدن با میکروارگانیسمهای واژینال در طول دیلاتاسیون شود و خطر عفونت یا ترومای دهانه رحم را افزایش دهد.
اهداف
تعیین تاثیرات دیلاتاسیون مکانیکی دهانه رحم در طول زایمان سزارین انتخابی روی موربیدیتی پس از جراحی.
روشهای جستوجو
ما پایگاه ثبت کارآزماییهای گروه بارداری و زایمان در کاکرین؛ ClinicalTrials.gov؛ پلتفرم بینالمللی پایگاه ثبت کارآزماییهای بالینی (ICTRP) سازمان جهانی بهداشت و فهرستهای منابع مطالعات بازیابی شده را در 20 سپتامبر 2017، جستوجو کردیم.
معیارهای انتخاب
کارآزماییهای تصادفیسازی شده، شبه‐تصادفیسازی شده و خوشهای‐تصافیسازی و کنترل شدهای را وارد کردیم که دیلاتاسیون دهانه رحم را حین جراحی با استفاده از یک انگشت، فورسپس اسفنجی، یا سایر ابزارها در طول زایمان سزارین انتخابی در برابر عدم دیلاتاسیون مکانیکال مقایسه کرده بودند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم کارآزماییها را برای ورود و خطر سوگیری (bias) ارزیابی کردند، دادهها را استخراج و دقت آنها را ارزیابی کردند. کیفیت شواهد را با استفاده از رویکرد درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE) ارزیابی کردیم.
نتایج اصلی
هشت مطالعه را با مجموع 2227 زن که تحت زایمان سزارین انتخابی قرار گرفته بودند، وارد کردیم. از این تعداد، 1097 زن تحت دیلاتاسیون دهانه رحم حین جراحی با کمک انگشت اشاره که دو دستکش پوشیده بود یا دیلاتور Hegar که وارد کانال دهانه رحم قرار داده شد تا دیلاته شود، قرار گرفتند و 1130 زن نیز تحت دیلاتاسیون دهانه رحم حین جراحی قرار نگرفتند. شش مورد از هشت مطالعه وارد شده در معرض خطر سوگیری (bias) بالا برای برخی از دامنههای خطر سوگیری بودند.
شواهدی با کیفیت بسیار پائین نشان میدهند که مشخص نیست دیلاتاسیون دهانه رحم تاثیری بر خونریزی پس از زایمان داشته باشد یا خیر (تخمین مقدار خون از دست رفته بیش از 1000 میلیلیتر؛ خطر نسبی (RR): 1.97؛ 95% فاصله اطمینان (CI): 0.48 تا 8.13؛ 5/205 در برابر 3/242؛ یک مطالعه؛ 447 زن).
شواهد با کیفیت پائین یا بسیار پائین نشان دادند که هیچ تفاوت روشنی در نیاز به ترانسفیوژن خون (RR: 3.54؛ 95% CI؛ 0.37 تا 33.79؛ دو مطالعه؛ 847 زن)، هموگلوبین پس از جراحی (تفاوت میانگین (MD): 0.05‐؛ 95% CI؛ 0.15‐ تا 0.06؛ سه مطالعه؛ 749 زن) یا هماتوکریت (MD: %0.01؛ 95% CI؛ 0.18‐ تا 0.20؛ یک مطالعه؛ 400 زن)، بروز افت هموگلوبین بیش از 0.5 گرم/دسیلیتر نسبت به پایه (RR: 0.92؛ 95% CI؛ 0.64 تا 1.31؛ دو مطالعه؛ 722 زن) یا مقدار افت هموگلوبین (MD: ‐0.01 گرم/دسیلیتر؛ 95% CI؛ 0.14‐ تا 0.13؛ سه مطالعه؛ 796 زن)، بروز خونریزی ثانویه پس از زایمان درون شش هفته (RR: 1.18؛ 95% CI؛ 0.07 تا 18.76؛ یک مطالعه؛ 447 زن)، موربیدیتی تبدار (RR: 1.18؛ 95% CI؛ 0.76 تا 1.85؛ هفت مطالعه؛ 2126 زن)، اندومتریت (RR: 0.94؛ 95% CI؛ 0.35 تا 2.52؛ چهار مطالعه؛ 1536 زن) یا توقف یا کندی بازگشت رحم به وضعیت طبیعی (subinvolution) (RR: 0.34؛ 95% CI؛ 0.08 تا 1.36؛ دو مطالعه؛ 654 زن) وجود ندارد؛ نتایج، خطر عدم تاثیر را برای همه پیامدها قطع کردند. هیچ دادهای در مورد ترومای دهانه رحم وجود نداشت.
یک بهبود جزئی را با دیلاتاسیون مکانیکی برای این پیامدهای ثانویه، که در پروتکل از پیش مشخص نشده بودند یافتیم؛ متوسط مقدار خون از دست رفته، ضخامت حفره اندومتر، محصولات باقیمانده لقاح، پیچ خوردن برش رحمی و نسبت بهبود. شواهد برای این پیامدها بر پایه یک یا دو مطالعه استوار بود. دیلاتاسیون دهانه رحم تاثیر روشنی بر این پیامدهای ثانویه، که در پروتکل از پیش مشخص نشده بود، دارد: عفونت زخم، عفونت مجاری ادراری (UTI)، زمان صرف شده برای جراحی، موربیدیتی عفونی و بینقصی اسکار رحمی.
نتیجهگیریهای نویسندگان
در این زمان، شواهد استفاده از دیلاتاسیونهای مکانیکی دهانه رحم را طی زایمان سزارین انتخابی برای کاهش موربیدیتی پس از جراحی تایید یا رد نمیکند.
مطالعات بزرگ و با طراحی خوب برای مقایسه تاثیر دیلاتاسیون مکانیکی دهانه رحم حین جراحی با عدم دیلاتاسیون مکانیکی دهانه رحم حین جراحی برای کاهش موربیدیتی پس از جراحی مورد نیاز است.
PICO
خلاصه به زبان ساده
باز کردن ساختگی دهانه رحم حین یک زایمان سزارین برنامهریزی شده پیش از شروع زایمان برای کاهش مشکلات پس از جراحی
موضوع چیست؟
دهانه رحم، کانال عبوری شبیه گردن باریک است که در انتهای پائینی رحم قرار دارد که وارد واژن میشود. دهانه رحم زن در آغاز بارداری محکم و دیلاته نشده است، اما به تدریج و در تمام طول مدت بارداری نرم میشود. باز شدن پیشرونده، یا دیلاتاسیون، دهانه رحم با انقباضات رحمی در طول زایمان رخ میدهد. دیلاتاسیون مکانیکی دهانه رحم در زایمان سزارین، پیش از شروع زایمان، باز کردن مصنوعی و ساختگی دهانه رحم است. این کار توسط جراح، با استفاده از یک انگشت پوشیده شده با دستکش، فورسپس اسفنجی یا دیگر ابزارهای جراحی انجام میشود.
چرا این موضوع مهم است؟
برخی از متخصصان زایمانی معتقد هستند که باز کردن دهانه رحم به درناژ خون از رحم، به دنبال تولد با زایمان سزارین برنامهریزی شده و پیش از آغاز زایمان، کمک میکند. افزایش درناژ ممکن است خطر عفونت داخل رحمی و خونریزی پس از زایمان (postpartum haemorrhage) را کاهش دهد. از طرف دیگر، باز کردن مکانیکال دهانه رحم میتواند منجر به آلوده شدن رحم با میکروارگانیسمهای واژینال و افزایش خطر عفونت یا ترومای دهانه رحم شود. برای تعیین تاثیرات دیلاتاسیون مکانیکی دهانه رحم طی یک زایمان سزارین برنامهریزی شده پیش از شروع زایمان، روی مقدار خون از دست رفته پس از جراحی یا عفونت رحمی، در مقایسه با عدم دیلاتاسیون مکانیکی، دست به کار میشویم.
ما چه شواهدی را پیدا کردیم؟
ما به جستوجوی شواهدی از کارآزماییهای تصادفیسازی و کنترل شده در سپتامبر 2017 پرداختیم. هشت مطالعه را با مجموع 2227 زن شناسایی کردیم که پیش از شروع زایمان، تحت زایمان سزارین انتخابی (برنامهریزی شده) قرار گرفتند. از بین این زنان، 1097 زن در طول جراحی تحت دیلاتاسیون دهانه رحم با انگشت اشاره، یا در یک مطالعه با دیلاتور Hegar قرار گرفتند، در حالی که 1130 زن در طول جراحی تحت دیلاتاسیون دهانه رحم قرار نگرفتند.
شواهد با کیفیت پائین یا بسیار پائین نشان میدهند که مشخص نیست دیلاتاسیون دهانه رحم تاثیری بر خونریزی پس از جراحی (مقدار خون از دست رفته تخمین زده شده بیش از 1000 میلیلیتر)، نیاز به ترانسفیوژن خون، و دیگر معیارهای خون از دست رفته، خونریزی بعد از زایمان درون شش هفته، موربیدیتی تبدار (عفونت رحم)، یا توقف یا کندی بازگشت رحم به وضعیت طبیعی (subinvolution) (رحم که پس از زایمان به اندازه عادی خود باز نمیگردد) داشته باشد. هیچ دادهای در مورد ترومای دهانه رحم وجود نداشت.
یک بهبود جزئی را با دیلاتاسیون مکانیکی برای برخی از پیامدها که در پروتکل اصلی ما مشخص نشده بودند، پیدا کردیم، اما شواهد این پیامدها بر اساس یک یا دو مطالعه (میانگین اتلاف خون، ضخامت حفره اندومتر، محصولات باقیمانده لقاح، پیچیده شدن برش رحمی و نرخ بهبود) بنا شده بود. دیلاتاسیون دهانه رحم تاثیر روشنی بر سایر پیامدهای ثانویه ندارد (دوباره در پروتکل اصلی ما مشخص نشده): عفونت زخم، عفونت مجاری ادراری (UTI)، زمان صرف شده برای جراحی، موربیدیتی عفونی و یکپارچگی اسکار رحمی.
این یافتهها چه معنایی دارد؟
این موضوع که دیلاتاسیون دهانه رحم تاثیری بر کاهش مشکلات پس از جراحی پس از زایمان سزارین دارد یا خیر، نامطمئن است. این بدان معنی است که شواهد کافی برای تشویق یا دلسرد کردن استفاده از دیلاتاسیونهای مکانیکی دهانه رحم در زایمان سزارین انتخابی برای کاهش سلامت‐بیماری پس از جراحی وجود ندارد.
Authors' conclusions
Summary of findings
| Manual cervical dilatation versus no dilatation for reducing postoperative morbidity | ||||||
| Patient or population: women with non‐labour caesarean section Comparison: no dilatation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Cervical dilatation versus no dilatation | |||||
| Postpartum haemorrhage (blood loss at least 1000 mL; | Study population | RR 1.97 | 447 | ⊕⊝⊝⊝ | ||
| 12 per 1000 | 24 per 1000 | |||||
| Medium risk population | ||||||
| 12 per 1000 | 24 per 1000 | |||||
| Need for blood transfusion (within 24 hours post caesarean section) | Study population | RR 3.54 | 847 | ⊕⊝⊝⊝ | ||
| 2 per 1000 | 7 per 1000 | |||||
| Medium risk population | ||||||
| 2 per 1000 | 7 per 1000 | |||||
| Drop from baseline haemoglobin > 0.5 g/dL | Study population | RR 0.92 | 722 | ⊕⊕⊝⊝ | ||
| 163 per 1000 | 150 per 1000 | |||||
| Medium risk population | ||||||
| 223 per 1000 | 205 per 1000 | |||||
| Secondary postpartum haemorrhage (within 6 weeks postpartum) | Study population | RR 1.18 | 447 | ⊕⊝⊝⊝ | ||
| 4 per 1000 | 5 per 1000 | |||||
| Medium risk population | ||||||
| 4 per 1000 | 5 per 1000 | |||||
| Febrile morbidity (at least 38° Celsius, measured on 2/10 days postpartum) | Study population | RR 1.18 | 2126 | ⊕⊕⊝⊝ | ||
| 31 per 1000 | 37 per 1000 | |||||
| Medium risk population | ||||||
| 21 per 1000 | 25 per 1000 | |||||
| Endometritis | Study population | RR 0.94 | 1536 | ⊕⊝⊝⊝ | ||
| 11 per 1000 | 10 per 1000 | |||||
| Medium risk population | ||||||
| 6 per 1000 | 6 per 1000 | |||||
| Uterine subinvolution | Study population | RR 0.34 | 654 | ⊕⊝⊝⊝ | ||
| 23 per 1000 | 8 per 1000 | |||||
| Medium risk population | ||||||
| 19 per 1000 | 6 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aHigh risk of bias for some of domains (‐1) bOne study with small number of events (‐1) cWide confidence interval crossing the line of no effect (‐1) dSmall number of events and wide cross interval crossing the line of no effect (‐2) | ||||||
Background
Description of the condition
Non‐labour elective caesarean section has increased in low‐, middle‐, and high‐income countries due to the indication of repeated caesarean or breech presentation (Chanthasenanont 2007; Lydon‐Rochelle 2006; Swende 2007; Van Roosmalen 1995). Vaginal birth after caesarean section is still a less common practice in low‐ and middle‐income countries (Chanrachakul 2000; Van der Walt 1994).
In one study on caesarean delivery for term breech, the puerperal morbidity following elective caesarean section (caesarean section before labour starts) were lower than for emergency caesarean section (after labour starts) in terms of puerperal fever or pelvic infection (1.5% versus 2.3%), and anaemia and/or haemorrhage (5.7% versus 7.0%) (Krebs 2003). Similarly, in another study, postpartum haemorrhage was reported at 4.84% in elective and 6.75% in non‐elective caesarean sections (Magann 2005). Another study indicated that women undergoing caesarean section without labour were less likely to have early postpartum haemorrhage compared with women undergoing induction of labour, but they found no difference in wound infection, puerperal febrile morbidity, blood transfusion, or intraoperative trauma (Allen 2006). Chongsuvivatwong 2010 found that when they evaluated complications after deliveries between vaginal delivery and caesarean section, fever, minor wound infection, peritonitis, urinary problems, and haemorrhage were more common in elective caesarean section than in vaginal delivery. In addition, Liu 2007 found elective caesarean section increased the overall risk of severe morbidity, major puerperal infection, wound disruption, wound hematoma, haemorrhage requiring hysterectomy, any hysterectomy, or other complications, such as anaesthetic complications, cardiac arrest, or thromboembolism.
Description of the intervention
A woman's cervix is firm and undilated at the beginning of pregnancy, but progressive remodeling occurs during gestation, until the cervix is soft at term, especially the nulliparous cervix (Myers 2008). The progressive dilatation of the cervix needs uterine contraction during labour. A mechanical dilatation of the cervix during non‐labour elective caesarean section is defined as an artificial dilatation of the cervix performed by finger, sponge forceps, or other instruments.
How the intervention might work
Some obstetricians believe that the cervix of women at non‐labour caesarean section is undilated and might cause obstruction of blood or lochia drainage, leading to postpartum haemorrhage and endometritis from collection of lochia or debris. Dilatation of the cervix helps with the drainage of blood during postpartum, reducing intrauterine infection or the risk of postpartum haemorrhage. To avoid this problem, some obstetricians routinely dilate the cervix from above during an elective, non‐labour caesarean section using finger, sponge forceps, or other instruments. A comparative study at Mir Hosseini Hospital in Iran, found lower incidence of postoperative endometritis in women who underwent elective caesarean section with cervical dilatation using a ring forcep (5.7%) compared with women who did not receive cervical dilatation (16.8% (Malkamy 1995). Collection of blood in the intrauterine cavity and distended uterus in women after elective caesarean section due to repeated sections was reported in two cases, and dilatation and evacuation were needed for treatment (Bollapragada 2002). However, this has not been proven in clinical trials. In contrast, mechanical cervical dilatation using sponge forceps or a finger during caesarean section may result in contamination by vaginal micro‐organisms during dilatation, and increase the risk of infection or cervical trauma. A study found that a positive culture at the lower uterine segment predicted postpartum endometritis (Sherman 1999).
Why it is important to do this review
The information currently available about the advantages of cervical dilatation at caesarean section is inconclusive. Therefore, evidence to support the effectiveness or safety of cervical dilatation at caesarean section is needed. This is an update a review, first published in Liabsuetrakul 2011.
Objectives
To determine the effects of mechanical dilatation of the cervix during elective caesarean section on postoperative morbidity.
Methods
Criteria for considering studies for this review
Types of studies
All randomised, quasi‐randomised, or cluster‐randomised controlled trials comparing cervical dilatation during caesarean section to no intervention.
Types of participants
All women underwent non‐labour elective caesarean section for any indications, regardless of degree of cervical dilatation.
Types of interventions
Mechanical dilatation of the cervix using a finger, sponge forceps, or other instrument during non‐labour caesarean section versus no mechanical dilatation.
Types of outcome measures
Primary outcomes
-
Postpartum haemorrhage: estimated blood loss greater than 1000 mL immediately or delayed after caesarean section
Secondary outcomes
-
Need for blood transfusion: blood transfusion given during or within 24 hours after caesarean section
-
Hematocrit or haemoglobin concentration: concentration of hematocrit or haemoglobin at postoperative period, or the change in concentration compared to preoperative or baseline level
-
Secondary postpartum haemorrhage: late vaginal bleeding, within six weeks postpartum
-
Febrile morbidity: temperature of 38 degrees Celsius or higher on any two of the first 10 days postpartum, exclusive of the first 24 hours, of unknown causes
-
Endometritis: febrile morbidity due to intrauterine infection, clinically diagnosed by fever, uterine tenderness, foul‐smelling discharge, or a combination
-
Cervical trauma: signs of vaginal bleeding due to the tear of cervical tissue from mechanical dilatation
-
Uterine subinvolution: delayed or absent involution of the uterus during the postpartum period
Based on the included studies in the first version of this review, we added these secondary outcomes.
-
Blood loss: estimated blood loss recorded during and immediately after caesarean section
-
Wound infection: presence of purulent or serous wound discharge with induration, warmth, and tenderness
-
Urinary tract infection: clinical signs plus identification of micro‐organisms of 105 on culture
-
Operative time: duration of operative time
Based on the ongoing studies identified after searching the trial registers for this update of the review, we added these secondary outcomes.
-
Infectious morbidity: rate of endometritis, wound infection, febrile morbidity, and urinary tract infection.
-
Endometrial cavity thickness: the decidual thickness and the intracavitary fluid collection measured by transabdominal ultrasound in mm
-
Retained products of conception: necessity of oxytocin administration, cervical dilatation, or curettage within six weeks
-
Integrity of uterine scar: scar thickness less than 2.3 mm
-
Distortion of uterine incision: any deviation from the full thickness apposition of the anterior uterine wall
-
Healing ratio: anterior wall thickness/anterior wall thickness + height of the wedge‐shaped defect
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (20 September 2017).
The Register is a database containing over 24,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register, including the detailed search strategies for CENTRAL, MEDLINE, Embase, and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library, and select the ‘Specialized Register’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE Ovid;
-
weekly searches of Embase Ovid;
-
monthly searches of CINAHL EBSCO;
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals, plus monthly BioMed Central email alerts.
Search results are screened by two people, and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Studies awaiting classification).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned, and ongoing trial reports on 20 September 2017, using the terms given in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies. We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeLiabsuetrakul 2011.
For this update, the following methods were used to assess eight reports that were identified as a result of the updated search, and searching other sources.
The following methods section of this review was based on a standard template used by Cochrane Pregnancy and Childbirth. The process of independent study selection, data extraction, and assessment of risk of bias was completed by two review authors, who used the data extraction form provided by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion, all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion, or if required, we consulted a third person, a researcher from Cochrane Pregnancy and Childbirth editorial team.
Data extraction and management
Two review authors extracted the data. We resolved discrepancies through discussion or, if required, we consulted the third person. We entered the data into Review Manager 5 software, and checked for accuracy (RevMan 2014).
When information regarding any of the above was unclear, we had planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
For each included study, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study, we described the method used to conceal allocation to interventions prior to assignment, and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study, we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study, we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high, or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described the completeness of data, including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups, or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses that we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation) or greater than 20% of missing outcome;
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes in the methods section and all expected outcomes of interest to the review were reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes were reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For each included study, we described any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high, unclear, or low risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias, and whether it was likely to impact on the findings. We explored the impact of the level of bias through, undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this update, we assessed the quality of the evidence using the GRADE approach, as outlined in the GRADE handbook, in order to assess the quality of the body of evidence relating to the following outcomes, for the main comparison of cervical dilatation versus no dilatation (Schünemann 2013).
-
Postpartum haemorrhage: estimated blood loss greater than 1000 mL immediately or delayed after caesarean section
-
Need for blood transfusion: blood transfusion given during or within 24 hours after caesarean section
-
Drop in baseline haemoglobin (g/dL)
-
Secondary postpartum haemorrhage: late vaginal bleeding, within six weeks postpartum
-
Febrile morbidity: temperature of 38 degrees Celsius or higher on any two of the first 10 days postpartum, exclusive of the first 24 hours, of unknown causes.
-
Endometritis: febrile morbidity due to intrauterine infection, clinically diagnosed by fever, uterine tenderness, foul‐smelling discharge, or a combination
-
Uterine subinvolution: delayed or absent involution of the uterus during the postpartum period
We used GRADEpro GDT software to import data from Review Manager 5.3, to create the ’Summary of findings’ table (GRADEpro GDT). A summary of the intervention effect and a measure of quality for each of the above outcomes were produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from high quality by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference, with 95% confidence intervals, if outcomes were measured in the same way between trials. In future updates, if applicable, we will use the standardised mean difference, with 95% confidence intervals, to combine trials that measure the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
Cluster‐randomised trials were eligible, but none were identified in this update. In future updates, if we identify any, we will include cluster‐randomised trials in the analyses along with individually randomised trials if there is little heterogeneity between the study designs, and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We will adjust the sample sizes of the cluster‐randomised trials using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions, using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. We will also acknowledged heterogeneity in the randomisation unit, and perform a subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials
This study design is not eligible for inclusion.
Dealing with missing data
We noted levels of attrition for the included studies. In future updates, if we include more eligible studies, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect through sensitivity analyses.
For all outcomes, we conducted the analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I², and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30%, and either Tau² was greater than zero, or the P value was less than 0.10 in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we explored it through prespecified subgroup analyses.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using Review Manager 5 software (RevMan 2014). If we did not find heterogeneity, we used fixed‐effect meta‐analysis to combine data, where it was reasonable to assume that studies were estimating the same underlying treatment effect. We had planned to use subgroup or sensitivity analyses to explore the clinical or methodological heterogeneity, respectively. If we had still detected substantial heterogeneity, we had planned to use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. We treated the random‐effects summary as the average range of possible treatment effects, and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. If we used random‐effects analyses, we presented the results as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we had planned to investigate it using subgroup analyses and sensitivity analyses, and reconsider whether an overall summary was meaningful. If it was, we planned to use random‐effects analysis to produce it.
We had planned to carry out the following prespecified subgroup analyses, if available:
-
nulliparous woman versus multiparous woman;
-
no prior caesarean section versus repeat caesarean section;
-
no premature rupture of membrane versus premature rupture of membrane;
-
dilatation with finger versus Hegar dilator.
We had planned to assess subgroup differences by interaction tests available within RevMan 5 (RevMan 2014). We would have reported the results of subgroup analyses, quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We had planned to perform the following sensitivity analysis.
Sensitivity analyses would have included:
-
omission of studies at high risk of bias;
-
repeating analyses using fixed‐ or random‐effects;
-
omission of studies published as abstracts or non‐peer reviewed publications
Results
Description of studies
Results of the search
See: Figure 1.

Study flow diagram
We included three studies previously in Liabsuetrakul 2011 (Ahmed 2005; Güngördük 2009; Tosun 2011).
An updated search in September 2017 retrieved a further eight relevant reports. One trial registry record was an earlier report of Tosun 2011. We attempted to contact the trialists involved in the Cengiz 2013 study, but have received no reply to date. This study remains in Studies awaiting classification. We included five new studies in this updated review (Abdelaziz 2014; Ezegwui 2015; Kirscht 2017; Sakinci 2015; Yazicioglu 2012) .
Included studies
A total of 2227 women participated in the eight included studies comparing intraoperative cervical dilatation with no cervical dilatation. All studies included both nulliparous and multiparous women, as well as no prior and repeat caesarean section, except one study, which included only nulliparous with no prior caesarean section (Yazicioglu 2012). Four studies did not specify whether they included rupture of membranes (Abdelaziz 2014; Ahmed 2005; Ezegwui 2015; Kirscht 2017), and only one study used Hegar dilatation (Kirscht 2017). Please see the 'Characteristics of included studies' table for further details.
(1) Study location and settings
The studies were conducted in Egypt (Abdelaziz 2014), Qatar (Ahmed 2005), Turkey (Güngördük 2009; Sakinci 2015; Tosun 2011; Yazicioglu 2012), Nigeria (Ezegwui 2015), and Germany (Kirscht 2017).
(2) Participants
All participants in the included studies were undergoing non‐labour elective caesarean section. Seven studies included a mixture of nulliparous and multiparous women (Abdelaziz 2014; Ahmed 2005; Ezegwui 2015; Güngördük 2009; Kirscht 2017; Sakinci 2015; Tosun 2011). All eight studies included women with term pregnancies; two studies also included those with gestational ages less than 37 weeks (Kirscht 2017; Tosun 2011).
The included studies excluded the following categories of women: women undergoing caesarean section due to multiple pregnancies (Abdelaziz 2014; Ahmed 2005; Sakinci 2015; Tosun 2011; Yazicioglu 2012); preterm births (Abdelaziz 2014; Ahmed 2005; Güngördük 2009; Sakinci 2015; Tosun 2011; Yazicioglu 2012); rupture of membranes (Abdelaziz 2014; Ezegwui 2015; Güngördük 2009; Sakinci 2015; Tosun 2011; Yazicioglu 2012); evidence of infection (Ezegwui 2015; Güngördük 2009; Kirscht 2017; Sakinci 2015; Tosun 2011); placenta previa (Abdelaziz 2014; Ezegwui 2015; Sakinci 2015), maternal diseases such as anaemia or diabetes mellitus (Abdelaziz 2014; Ezegwui 2015; Güngördük 2009; Sakinci 2015), and need for blood transfusion before or during caesarean section (Güngördük 2009; Sakinci 2015; Tosun 2011).
(3) Interventions
All included studies compared intraoperative cervical dilatation by the insertion of a double‐gloved index finger (Abdelaziz 2014; Ahmed 2005; Ezegwui 2015; Güngördük 2009; Sakinci 2015; Tosun 2011; Yazicioglu 2012), or Hegar dilator (Kirscht 2017), into the cervical canal to dilate it (the outer glove was removed after digital dilatation), with no intraoperative cervical dilatation.
(4) Outcomes
One study reported the primary outcome of postpartum haemorrhage of at least of 1000 mL (Kirscht 2017).
Two studies measured the need for blood transfusion (Güngördük 2009; Kirscht 2017). Six studies reported haematocrit or haemoglobin levels, but in various terms, as levels of haematocrit or haemoglobin at postoperative period, or the change of level compared to preoperative or baseline level (Abdelaziz 2014; Ahmed 2005; Güngördük 2009; Kirscht 2017; Sakinci 2015; Tosun 2011). One study reported on secondary postpartum haemorrhage (Kirscht 2017). Seven studies measured febrile morbidity (Abdelaziz 2014; Ahmed 2005; Ezegwui 2015; Güngördük 2009; Kirscht 2017; Sakinci 2015; Tosun 2011). Four studies identified endometritis (Abdelaziz 2014; Güngördük 2009; Kirscht 2017; Tosun 2011). Two studies reported uterine subinvolution (Ezegwui 2015; Kirscht 2017), and no study reported on cervical trauma.
We also reported on the following outcomes that were not prespecified in our protocol: blood loss (Güngördük 2009), wound infection (Abdelaziz 2014; Ahmed 2005; Güngördük 2009; Kirscht 2017; Tosun 2011), urinary tract infection (Güngördük 2009; Kirscht 2017), infectious morbidity (Güngördük 2009), operative time (Abdelaziz 2014; Güngördük 2009; Kirscht 2017; Sakinci 2015), endometrial cavity thickness (Sakinci 2015; Tosun 2011), retained products of conception (Kirscht 2017), integrity of uterine scar (Abdelaziz 2014), and distortion of uterine incision and healing ratio (Yazicioglu 2012).
(5) Dates of study, funding sources and declarations of interest
Dates when the studies were conducted were reported as: 16 November 2002 to 25 February 2003 (Ahmed 2005); December 2002 to March 2005 (Yazicioglu 2012); June 2007 to December 2007 (Güngördük 2009); 22 July 2008 to 15 September 2009 (Tosun 2011); November 2011 to July 2013 (Kirscht 2017); February 2013 to September 2014 (Abdelaziz 2014); and March 2013 to February 2014 (Sakinci 2015). Study dates were not reported in one study (Ezegwui 2015).
One study declared there was no funding (Kirscht 2017). Funding sources were not reported for the remaining seven studies.
Declarations of interest of trial authors were reported that 'all authors declare no conflict of interest' in four studies (Ezegwui 2015; Kirscht 2017; Sakinci 2015; Tosun 2011). There was no information on declarations of interest in four studies (Abdelaziz 2014; Ahmed 2005; Güngördük 2009; Yazicioglu 2012).
Please see the 'Characteristics of included studies' table for further details.
Excluded studies
There were no excluded studies.
Risk of bias in included studies
A summary of the risk of bias judgements that we made for each included study is presented in Figure 2 and Figure 3. In conclusion, one study had a high overall risk of bias (Ahmed 2005), two studies we judged to be unclear risk of bias (Abdelaziz 2014; Ezegwui 2015), and five studies at low risk of bias (Güngördük 2009; Kirscht 2017; Sakinci 2015; Tosun 2011; Yazicioglu 2012).
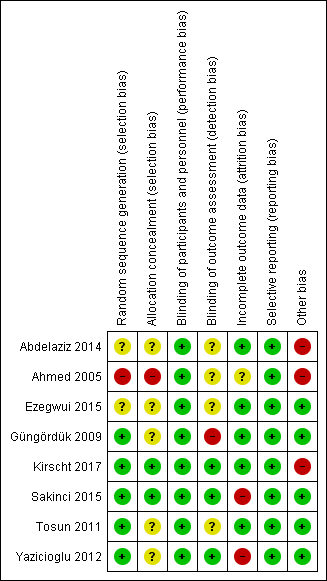
Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Five of eight studies had adequate random sequence generation, and we rated them as having a low risk of bias for random sequence (Güngördük 2009; Kirscht 2017; Sakinci 2015; Tosun 2011; Yazicioglu 2012). Random sequence was unclear in two studies (Abdelaziz 2014; Ezegwui 2015). Ahmed 2005 used alternation, in which the sequence was predictable and unconcealed, leading to a high risk of bias for selection bias.
We rated allocation concealment as being of unclear risk of bias for five studies (Abdelaziz 2014; Ezegwui 2015; Güngördük 2009; Tosun 2011; Yazicioglu 2012). Güngördük 2009 used sealed envelopes, but did not state whether they were opaque or consecutively numbered. Two studies had adequate allocation concealment, and we rated them as a low risk of bias (Kirscht 2017; Sakinci 2015).
Blinding
All studies compared the intervention to no intervention. It was not feasible to blind the intervention, but the outcome was not likely to be influenced by lack of blinding; thus we rated all studies as low risk of performance bias.
We rated three of the eight studies as a low risk of detection bias, because the outcomes were assessed in a blinded manner (Kirscht 2017; Sakinci 2015; Yazicioglu 2012). We rated one study as high risk, as procedure allocation was recorded in the women's charts (Güngördük 2009). The other four studies had insufficient information to permit judgement (Abdelaziz 2014; Ahmed 2005; Ezegwui 2015; Tosun 2011).
Incomplete outcome data
Five of the eight studies reported no or low losses to follow‐up (Abdelaziz 2014; Ezegwui 2015; Güngördük 2009; Kirscht 2017; Tosun 2011), but Ahmed 2005 did not mention whether any data were missing. Two studies had a high risk of bias due to losses to follow‐up greater than 20%, or reasons for missing an outcome was likely to be related to the true outcome (Sakinci 2015; Yazicioglu 2012).
Selective reporting
All included studies were judged to be at low risk. For a study, all outcomes were reported as in the study protocol published (Kirscht 2017). For the remaining seven studies, the study protocols were not found, but the prespecified outcomes mentioned in the methods section were reported thus they were also judged as low risk (Abdelaziz 2014; Ahmed 2005; Ezegwui 2015; Güngördük 2009; Sakinci 2015; Tosun 2011; Yazicioglu 2012).
Other potential sources of bias
Five studies did not appear to be at risk of other sources of bias (Ezegwui 2015; Güngördük 2009; Sakinci 2015; Tosun 2011; Yazicioglu 2012), but three studies had substantial discrepancies in numbers of participants reported (Abdelaziz 2014; Ahmed 2005; Kirscht 2017). Abdelaziz 2014 presented unbalanced samples without details of allocation ratio, including 299 cases with cervix opened, and other 240 cases with unopened cervix. The number of samples in the abstract and methods showing 67 and 64 in cervical dilatation and no dilatation, but in the results, they presented 62 and 121 in cervical dilatation and no dilatation for the outcome of febrile morbidity (Ahmed 2005). Kirscht 2017 described the randomisation of 1:1 allocation but the results showed 205 women with cervical dilatation and 242 women without dilatation.
Effects of interventions
Cervical dilatation versus no dilatation
Primary outcomes
One study reported on postpartum haemorrhage (estimated blood loss greater than 1000 mL (Kirscht 2017)). It was uncertain whether mechanical dilatation during caesarean section had any impact on the risk of postpartum haemorrhage (risk ratio (RR) 1.97, (95% confidence interval (CI) 0.48 to 8.13; 5/205 versus 3/242; one study, 447 women; very low‐quality evidence; Analysis 1.1). The quality of the evidence was downgraded due to limitations in study design and imprecision.
Secondary outcomes
Note that infectious morbidity was reported differently in seven included studies (febrile morbidity, endometritis, wound infection, urinary tract infection, infectious morbidity; see Characteristics of included studies); we included these outcomes at different stages of the review process, as explained below.
-
It was uncertain whether cervical dilatation increased the need for blood transfusions during or within 24 hours following caesarean section (RR 3.54, 95% CI 0.37 to 33.79; two studies, 847 women; very low‐quality evidence; Analysis 1.2 (Güngördük 2009; Kirscht 2017). The quality of the evidence was downgraded for limitations in study design and imprecision (small number of events and wide CIs crossing the line of no effect).
-
There was no clear effect seen in postoperative haemoglobin concentrations (MD ‐0.05 g/dL, 95% CI ‐0.15 to 0.06; three trials, 749 women; Analysis 1.3 (Güngördük 2009; Sakinci 2015; Tosun 2011); postoperative hematocrit concentrations (MD 0.01%, 95% CI ‐0.18 to 0.20; one trial, 400 women; Analysis 1.4 (Güngördük 2009); incidence of postoperative drop from baseline in haemoglobin > 0.5 g/dL (RR 0.92, 95% CI 0.64 to 1.31; two studies, 722 women; low‐quality evidence; Analysis 1.5 (Abdelaziz 2014; Ahmed 2005); or postoperative drop from baseline in mean haemoglobin (MD ‐0.01, 95% CI ‐0.14 to 0.13; three studies, 796 women; Analysis 1.6 (Kirscht 2017; Sakinci 2015; Tosun 2011).
-
In one study of 447 women, cervical dilatation did not have a clear effect on secondary postpartum haemorrhage, measured within six weeks postpartum (RR 1.18, 95% CI 0.07 to 18.76; very low‐quality evidence; Analysis 1.7 (Kirscht 2017).
-
There was no clear effect of dilatation on febrile morbidity: RR 1.18, 95% CI 0.76 to 1.85; seven studies, 2126 women; low‐quality evidence; Analysis 1.8 (Abdelaziz 2014; Ahmed 2005; Ezegwui 2015; Güngördük 2009; Kirscht 2017; Sakinci 2015; Tosun 2011);
-
There was no clear effect of dilatation on endometritis: RR 0.94, 95% CI 0.35 to 2.52; four studies, 1536 women; very low‐quality evidence; Analysis 1.9 (Abdelaziz 2014; Güngördük 2009; Kirscht 2017; Tosun 2011);
-
No study reported the outcome of cervical trauma.
-
Cervical dilatation did not have a clear impact on uterine subinvolution (RR 0.34, 95% CI 0.08 to 1.36; two studies, 654 women; very low‐quality evidence; Analysis 1.10 (Ezegwui 2015; Kirscht 2017).
Based on the included studies in the first version of this review, we added these secondary outcomes.
-
In one study of 400 women, blood loss was reduced in the dilatation group compared with the no dilatation group (mean difference (MD) ‐48.49 mL, 95% CI ‐88.75 to ‐8.23; Analysis 1.11 (Güngördük 2009).
-
There was no clear difference in wound infection between groups: RR 1.13, 95% CI 0.44 to 2.90; random‐effects, Tau² = 0.49, I² = 71%; 37/841 versus 33/878; Analysis 1.12 (Abdelaziz 2014; Ahmed 2005; Güngördük 2009; Kirscht 2017; Tosun 2011).
-
There was no clear difference in urinary tract infection between groups: RR 0.92, 95% CI 0.34 to 2.53; two studies, 847 women; Analysis 1.13 (Güngördük 2009; Kirscht 2017);
-
Cervical dilatation did not have a clear effect on the operative time of 1585 women in four studies (MD ‐0.05 minutes, 95% CI ‐2.62 to 2.53; random‐effects, Tau² = 6.23, I² = 95%; Analysis 1.14 (Abdelaziz 2014; Güngördük 2009; Kirscht 2017; Sakinci 2015).
Based on the new and ongoing studies identified during the searches for this update of the review, we added these secondary outcomes.
-
There was no clear difference in infectious morbidity between groups: RR 0.91, 95% CI 0.51 to 1.61; one study, 400 women; Analysis 1.15 (Güngördük 2009).
-
Endometrial cavity thickness was slightly reduced in the dilatation group compared to the no dilatation group (MD ‐1.89, 95% CI ‐3.30 to ‐0.48; random‐effects, Tau² = 0,84, I² = 81%; two studies, 349 women; Analysis 1.16 (Sakinci 2015; Tosun 2011).
-
There was lower rate of retained product of conception in the dilatation group than in the no dilatation group (RR 0.04, 95% CI 0.00 to 0.63; 0/205 versus 15/242; one study, 447 women; Analysis 1.17 (Kirscht 2017).
-
There was no clear difference seen in the integrity (or thickness) of the uterine scar (RR 2.68, 95% CI 0.74 to 9.61; one trial, 539 women; Analysis 1.18 (Abdelaziz 2014).
-
In the dilatation group, there was lower rate of distortion of the uterine incision (RR 0.35, 95% CI 0.14 to 0.88; one study, 67 women; Analysis 1.19 (Yazicioglu 2012).
-
In the dilatation group, there was a higher healing ratio (MD 0.35, 95% CI 0.14 to 0.88; one study, 67 women; Analysis 1.20 (Yazicioglu 2012).
We could not perform subgroup analysis in this review, since nulliparous and multiparous women who underwent primary and repeat caesarean section were included as a total population in all seven studies, except the study by Yazicioglu 2012.
Discussion
Summary of main results
We included eight studies, with a total of 2227 women undergoing elective caesarean section; 1097 with intraoperative cervical dilatation using finger or Hegar dilator, and 1130 without cervical dilatation. Low‐ or very low‐quality evidence suggested it was unclear whether cervical dilatation had any impact on postpartum haemorrhage (estimated blood loss greater than 1000 mL), the need for blood transfusion, postoperative haemoglobin or haematocrit, the incidence or amount of drop from baseline haemoglobin above 0.5 g/dL, secondary postpartum haemorrhage (within six weeks), febrile morbidity, endometritis, or uterine subinvolution; the results crossed the line of no effect for all of the outcomes. There were no data for cervical trauma.
We found a slight improvement with mechanical dilatation for these secondary outcomes, not prespecified in the protocol: mean blood loss, endometrial cavity thickness, retained products of conception, distortion of uterine incision, and healing ratio. The evidence for these outcomes was based on one or two studies. Cervical dilatation did not have a clear effect on these secondary outcomes, not prespecified in the protocol: wound infection, urinary tract infection, operative time, infectious morbidity, and integrity of uterine scar.
Overall completeness and applicability of evidence
Although there were five studies added in this update to the three already included studies, only one included study reported the primary outcome of postpartum haemorrhage with blood loss at least 1000 mL (Kirscht 2017). Secondary outcomes not pre‐specified in the protocol were reported in this review. Due to substantial heterogeneity, random‐effects model on the outcomes of wound infection, operative time and endometrial thickness was performed, but the finding of wound infection from a sensitivity analyses by omission of a study at high risk of bias (Ahmed 2005) was not changed.
One study showed that a positive culture at the lower uterine segment increased an incidence of endometritis (Sherman 1999). However, we could not determine whether cervical dilatation was associated with febrile morbidity and endometritis. Further well‐designed randomised controlled studies with sufficient sample sizes and adequate methodological quality are needed.
Quality of the evidence
A summary of the 'Risk of bias' judgements that we made for each included study is presented in Figure 2 and Figure 3. In this update, we assessed the quality of evidence considering the study limitations, consistency of effect, imprecision, indirectness, and publication bias for the primary and main secondary outcomes. The quality of the evidence for the seven outcomes presented in summary of findings Table for the main comparison varied from low to very low. We based our downgrading decisions on limitations in study design, small numbers of events, and wide confidence intervals that crossed the line of no effect.
Potential biases in the review process
Two authors independently selected studies, reviewed risks of bias, and extracted data. TL conducted the GRADE quality assessments, and KP reviewed them; there were no discrepancies. None of the authors of this review have any conflict of interest on this issue. We were unable to formally assess publication bias because the number of studies for each outcome was less than 10 studies. We performed a comprehensive search of the literature in an attempt to identify all relevant published and unpublished randomised trials.
Agreements and disagreements with other studies or reviews
Two descriptive studies reported a lower risk of postoperative endometritis and collection of blood in the intrauterine cavity among women undergoing cervical dilatation with elective caesarean section (Bollapragada 2002; Malkamy 1995).
A retrospective study showed that cervical dilatation was performed in 34% of women undergoing elective caesarean section, during 2008 to 2011, in a university hospital in Germany (Berlit 2013). However, strong evidence of the benefits of this procedure is needed before it can be recommended for routine use.
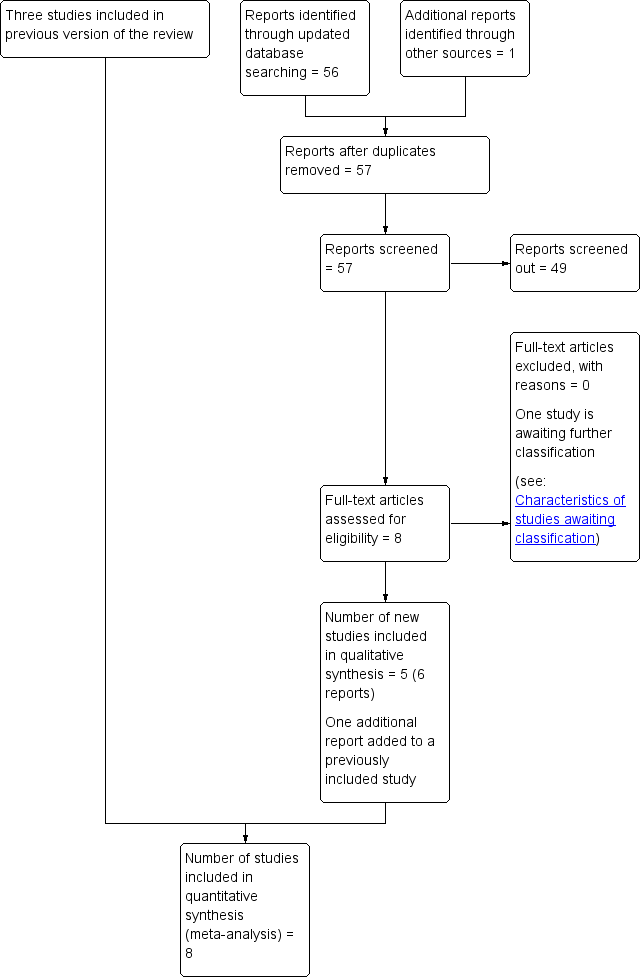
Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
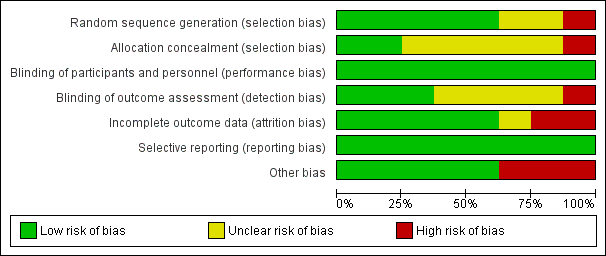
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 1 Postpartum haemorrhage (blood loss at least 1000 mL).

Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 2 Need for blood transfusion.
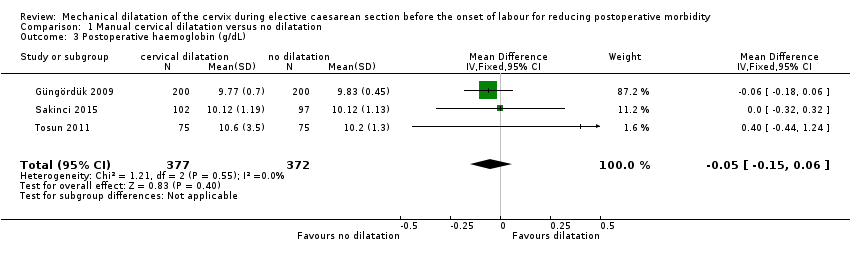
Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 3 Postoperative haemoglobin (g/dL).

Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 4 Postoperative haematocrit (%).

Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 5 Drop from baseline haemoglobin > 0.5 g/dL.
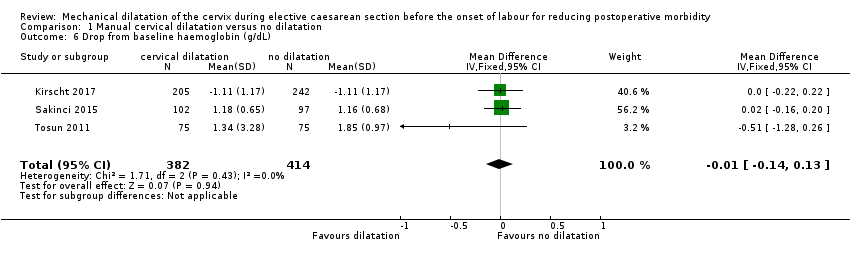
Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 6 Drop from baseline haemoglobin (g/dL).

Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 7 Secondary postpartum haemorrhage (within 6 weeks).

Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 8 Febrile morbidity.

Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 9 Endometritis.

Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 10 Uterine subinvolution.

Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 11 Blood loss (mL).
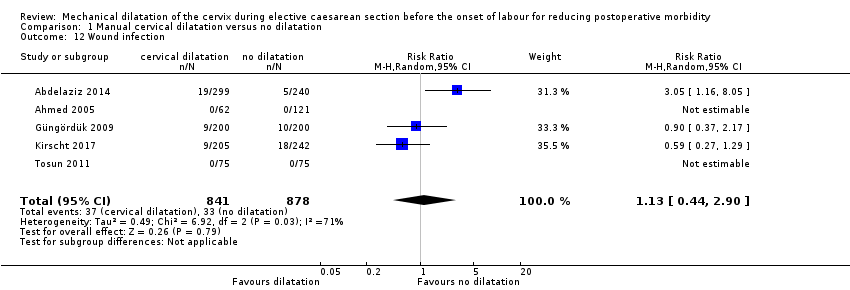
Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 12 Wound infection.

Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 13 Urinary tract infection.

Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 14 Operative time (min).

Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 15 Infectious morbidity.

Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 16 Endometrial cavity thickness (mm).
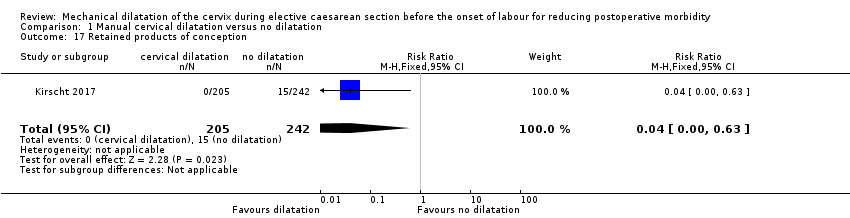
Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 17 Retained products of conception.
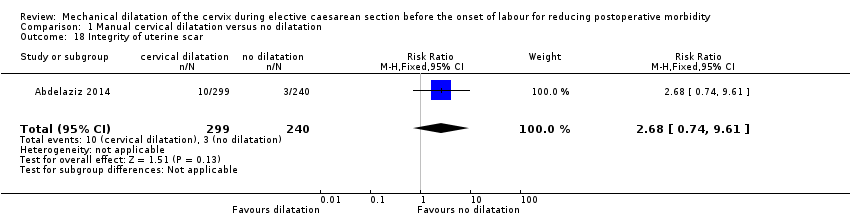
Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 18 Integrity of uterine scar.

Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 19 Distortion of uterine incision.

Comparison 1 Manual cervical dilatation versus no dilatation, Outcome 20 Healing ratio.
| Manual cervical dilatation versus no dilatation for reducing postoperative morbidity | ||||||
| Patient or population: women with non‐labour caesarean section Comparison: no dilatation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Cervical dilatation versus no dilatation | |||||
| Postpartum haemorrhage (blood loss at least 1000 mL; | Study population | RR 1.97 | 447 | ⊕⊝⊝⊝ | ||
| 12 per 1000 | 24 per 1000 | |||||
| Medium risk population | ||||||
| 12 per 1000 | 24 per 1000 | |||||
| Need for blood transfusion (within 24 hours post caesarean section) | Study population | RR 3.54 | 847 | ⊕⊝⊝⊝ | ||
| 2 per 1000 | 7 per 1000 | |||||
| Medium risk population | ||||||
| 2 per 1000 | 7 per 1000 | |||||
| Drop from baseline haemoglobin > 0.5 g/dL | Study population | RR 0.92 | 722 | ⊕⊕⊝⊝ | ||
| 163 per 1000 | 150 per 1000 | |||||
| Medium risk population | ||||||
| 223 per 1000 | 205 per 1000 | |||||
| Secondary postpartum haemorrhage (within 6 weeks postpartum) | Study population | RR 1.18 | 447 | ⊕⊝⊝⊝ | ||
| 4 per 1000 | 5 per 1000 | |||||
| Medium risk population | ||||||
| 4 per 1000 | 5 per 1000 | |||||
| Febrile morbidity (at least 38° Celsius, measured on 2/10 days postpartum) | Study population | RR 1.18 | 2126 | ⊕⊕⊝⊝ | ||
| 31 per 1000 | 37 per 1000 | |||||
| Medium risk population | ||||||
| 21 per 1000 | 25 per 1000 | |||||
| Endometritis | Study population | RR 0.94 | 1536 | ⊕⊝⊝⊝ | ||
| 11 per 1000 | 10 per 1000 | |||||
| Medium risk population | ||||||
| 6 per 1000 | 6 per 1000 | |||||
| Uterine subinvolution | Study population | RR 0.34 | 654 | ⊕⊝⊝⊝ | ||
| 23 per 1000 | 8 per 1000 | |||||
| Medium risk population | ||||||
| 19 per 1000 | 6 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aHigh risk of bias for some of domains (‐1) bOne study with small number of events (‐1) cWide confidence interval crossing the line of no effect (‐1) dSmall number of events and wide cross interval crossing the line of no effect (‐2) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postpartum haemorrhage (blood loss at least 1000 mL) Show forest plot | 1 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.48, 8.13] |
| 2 Need for blood transfusion Show forest plot | 2 | 847 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.54 [0.37, 33.79] |
| 3 Postoperative haemoglobin (g/dL) Show forest plot | 3 | 749 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.15, 0.06] |
| 4 Postoperative haematocrit (%) Show forest plot | 1 | 400 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.18, 0.20] |
| 5 Drop from baseline haemoglobin > 0.5 g/dL Show forest plot | 2 | 722 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.64, 1.31] |
| 6 Drop from baseline haemoglobin (g/dL) Show forest plot | 3 | 796 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.14, 0.13] |
| 7 Secondary postpartum haemorrhage (within 6 weeks) Show forest plot | 1 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.07, 18.76] |
| 8 Febrile morbidity Show forest plot | 7 | 2126 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.76, 1.85] |
| 9 Endometritis Show forest plot | 4 | 1536 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.35, 2.52] |
| 10 Uterine subinvolution Show forest plot | 2 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.08, 1.36] |
| 11 Blood loss (mL) Show forest plot | 1 | 400 | Mean Difference (IV, Fixed, 95% CI) | ‐48.49 [‐88.75, ‐8.23] |
| 12 Wound infection Show forest plot | 5 | 1719 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.44, 2.90] |
| 13 Urinary tract infection Show forest plot | 2 | 847 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.34, 2.53] |
| 14 Operative time (min) Show forest plot | 4 | 1585 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐2.62, 2.53] |
| 15 Infectious morbidity Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.51, 1.61] |
| 16 Endometrial cavity thickness (mm) Show forest plot | 2 | 349 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐3.30, ‐0.48] |
| 17 Retained products of conception Show forest plot | 1 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.63] |
| 18 Integrity of uterine scar Show forest plot | 1 | 539 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.68 [0.74, 9.61] |
| 19 Distortion of uterine incision Show forest plot | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.14, 0.88] |
| 20 Healing ratio Show forest plot | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [0.03, 0.13] |

