نقش دوز کلورپرومازین برای درمان افراد مبتلا به اسکیزوفرنی
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Allocation: randomised ‐ block randomised into 11 groups. | |
| Participants | Diagnosis: schizophrenia. | |
| Interventions | 1. Chlorpromazine: dose 600 mg/day. N = 11*. *interventions started after 2 weeks of fixed doses of 300 mg/day CPZ | |
| Outcomes | Leaving the study early. Adverse effects. Mental state: BPRS. Unable to use ‐ Laboratory tests (physiological). Blood pressure (physiological). Pulse rate (physiological). | |
| Notes | Lost to follow‐up: 1 patient lost during 5th week, assumed not treatment related. Reason given: he had a habit of leaving the hospital without authorisation. Unclear which treatment group he belonged to. Plan to assume that from one of the α‐methyldopa groups (3/5 chance of this). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "within a block: patients were then randomly assigned" pp119, p3. Comment: Block randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "double‐blind conditions", "identical‐appearing tablets...administered three times a day" pp119, p6. Comment: probably done (double, tablets of identical appearance, same amount of tablets and treatment scheme to all patients). |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "one male patient left the hospital without authorization during the 5th week of treatment" pp120, p4. Comment: 1 person lost to follow‐up, data at the time of loss (5th week) reported as end data. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | Quote: "This study was supported in part by the Medical Research Council of Canada and by an FCAC grant (Financial Consumer Agency of Canada, Quebec)" pp125,p3. |
| Methods | Allocation: randomised ‐ block randomised but not specified how. | |
| Participants | Diagnosis: schizophrenia. | |
| Interventions | 1. Chlorpromazine: dose 150 mg/day**. N = 16. | |
| Outcomes | Adverse effects. Unable to use ‐ Mental state: IMPS, BPRS (N and mean, no SD). Blood pressure, body temperature, pulse, EEG, serum cholesterol (physiological). Body weight (N and mean, no SD). Service use: duration of hospitalisation (N and mean, no SD). | |
| Notes | * Initially 71 people randomised but people lost to follow‐up and unclear from which group, ‐ so outcomes reported on only those who completed. ** Initial low doses increased over 10 days to final fixed dose level. Lost to follow‐up: The number of participants requiring additional sedation with chloral hydrate or a barbiturate on more than 5 occasions during the 24 weeks of treatment were 5 in the PL group, 4 in the CPZ‐150 group, 2 in the CPZ‐300 group and 1 in the CPZ‐600 group. Study #4 of 6 separate studies reported in paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "each treatment group was first subdivided by age at the time of beginning the study" pp479, p6 Comment: Block randomisation, no details. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "double‐blind study" pp479, p5; "all patients received the same volume of medication (10 mL twice daily) but in different concentration" pp262, p1 Comment: probably done. |
| Incomplete outcome data (attrition bias) | High risk | Quote: no quote. Comment: 8% loss to follow‐up ignored in analyses. |
| Selective reporting (reporting bias) | High risk | Quote: "one subject dropped out because of rash and anaemia, one because of agranulocytosis, two because of elopement from the hospital and two because of requisite surgery" pp263, p2. Comment: clinical outcomes reported by group of allocation, leaving the study not reported by group of allocation. |
| Other bias | Unclear risk | Quote: "The authors are grateful to Mr. Robert C. Zwahlen of Smith, Kline and French Laboratories for generous supplies of medication for this study" pp269, p5. Comment: we are unclear if this support went beyond supply of medication. |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: schizophrenia. | |
| Interventions | 1. Chlorpromazine: dose 300 mg/day. N = 208. * In gradual increasing doses, reaching the maximal doses after 45 days. | |
| Outcomes | Global state: GIS, GSIS, GAS, relapse. Leaving the study early. Unable to use ‐ Ocular changes (lens change, corneal change, skin changes, photosensitivity, visual acuity). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned to four treatment groups" pp483, p5. Comment: no details provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The low dose and placebo group also received liquid concentrate, on a double‐blind basis" pp483, p6. Comment: identical liquid administration. Blinding probably done. |
| Incomplete outcome data (attrition bias) | Low risk | Lost to follow‐up: 51 of high dose group (41 disabling adverse effects, 10 other), 31 of low‐dose group (4 disabling adverse effects, 27 other). |
| Selective reporting (reporting bias) | High risk | No SD for several outcomes, several outcomes not reported (see above). |
| Other bias | Low risk | Quote: "This investigation was supported by Public Health Service grants...from the National Institute of Mental Health, and Public Health Service" pp494 ,p6. |
| Methods | Allocation: randomised. | |
| Participants | Diagnosis: psychosis of schizophrenic type. History: emergency ward patients. Presence of thought disorder, delusions or auditory hallucinations. | |
| Interventions | 1. Chlorpromazine: dose 200 mg/day. N = 15. Additional medication: Nitrazepam 5‐10 mg (N = 30) for sleep, diazepam 5 mg (N = 18) for daytime sedation ‐ not reported by group. Commented as: results not deviating significantly from those of the remaining patients, therefore additional medication was disregarded in final evaluation. | |
| Outcomes | Leaving the study early. Adverse effects: modified Simpson and Angus. Unable to use ‐ Prolactin levels in CSF (physiological). Prolactin levels in plasma (physiological). Total prolactin, FSH, LH, TSH and oestradiol in CSF (physiological). Total prolactin, FSH, LH, TSH and oestradiol in plasma (physiological). Monoamine metabolite (HVA, MOPEG and 5‐HIAA) levels in CSF (physiological). | |
| Notes | One man had diabetes mellitus and was treated with insulin (64 IU daily). Commented that the results obtained with this patient were similar to those seen in the rest of the group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A research assistant not involved in the project attempted to assign an equal number of men and women to each dose using the technique of restricted randomisation (Armitage (1971))” pp152, p1. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) | Low risk | Quote: “For drug administration the “double‐dummy technique” was used, i.e. every patient received the same total number of tablets containing CPZ or placebo. The appearance of the placebo tablets was identical to those containing CPZ” pp152, p1. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Four patients dropped out before the 2‐week evaluation since dose had to be adjusted for ethical reasons. Thus a man and a woman on 600mg showed extreme somnolence, requiring dose reduction. In a man on 400mg and a woman on 200mg the doses were increased because of insufficient effect on the severe psychosis. Another six patients dropped out after the 2‐week, but the 4‐week, evaluation. In a woman and a man on 400mg, the dose was increased due to insufficient antipsychotic effect. A man on 400mg who was markedly improved clinically was omitted because of severe elevation of transaminase levels. Another man on 200mg and a woman on 400mg were also both quite well and insisted on being discharged from the hospital. A woman on 600mg refused further medication" pp154,p5. Comment: loss to follow‐up described from each group in detail. |
| Selective reporting (reporting bias) | High risk | For some scales only graphs without numeric data. |
| Other bias | Low risk | Quote: “This investigation was supported by grants from the Swedish Medical Research Council (No. 21X‐02291, 14X‐03560), National Institutes of Health, Bethesda, Maryland, and USA (MH 27254‐01), F.Hoffmann‐La Roche, Basle, Switzerland, ‘Forenade Liv’ Mutual Group Life Insurance Company, Stockholm, Sweden, Svenska Lakaresallskapet: Anton och Dorothea Bexelius Minnesfond, Bror Gadelius Minnesfond, Karolinska Institutets Fonder, AB Leo, Sweden, and Magnus Bergvalls Stiftelse” pp171, p2. Comment: publication bias unlikely. |
| Methods | Allocation: randomised (divided into large‐dose group and small‐dose group according to the odd‐even number). Blindness: Not mentioned. Duration: 8 weeks. | |
| Participants | Diagnosis: CCMD ‐3. N = 120. (60 in CPZ group, 60 in clozapine group). Age: 18‐60 years, mean ˜ 33 years. Sex: 31 male, 29 female. History: inpatients. Excluded: severe physical diseases, alcohol and substance dependence; pregnancy and lactation. | |
| Interventions | 1. Medium dose CPZ: dose titrated between 425 mg ‐ 600 mg/day (mean 494.1 mg/day)*. 2. Low dose CPZ: dose titrated between 250 mg ‐ 400 mg/day (mean 338.3 mg/day)*. 3. Medium dose clozapine*. 4. Low dose clozapine*. *Other antipsychotics, antidepressants, Antimanic Drugs and MECT were not allowed during the treatment. Benzhexol, sedatives and tranquillizers and β blocker were used if necessary. | |
| Outcomes | Global state: CGI‐SI. Mental state: PANSS. Adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. No detailed information was described in the study. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. No detailed information was described in the study. |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned. No detail information was described in the study. |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes were reported. |
| Selective reporting (reporting bias) | Low risk | No selective reporting was found. |
| Other bias | Low risk | Not obvious. |
BPRS ‐ Brief Psychiatric Rating Scale
CCMD‐3 ‐ Chinese Classification of Mental Disorders Version 3
CGI‐I ‐ Clinical Global Impression ‐ Improvement scale
CGI‐SI ‐ Clinical Global Impression ‐ Severity of Illness scale
CPRS ‐ Comprehensive Psychopathological Rating Scale
CPZ ‐ Chlorpromazine
CSF ‐ cerebrospinal fluid
DRI ‐ Discharge Readiness Inventory
EEG ‐ electroencephalogram
FSH ‐ follicle‐stimulating hormone
GIS ‐ Global Improvement Scale (aka CGI‐I)
GRS ‐ Global Rating Scale
GSIS ‐ Global Severity of Illness Scale (aka CGI‐SI)
GAS ‐ Global Assessment Scale
IMPS ‐ Inpatient Multidimensional Psychiatric Scale
IU ‐ international unit
LH ‐ luteinizing hormone
NOSIE ‐ Nurse's Observation Scale for Inpatient Evaluation
OBRS ‐ Oklahoma Behaviour Rating Scale
PANSS‐ Positive And Negative Syndrome Scale
TSH ‐ thyroid‐stimulating hormone
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Allocation: randomised (times 3 factorial design). Participants: psychogeriatric inpatients (total = 151, 81 completed with schizophrenia). Interventions: CPZ vs thioridazine and bed time dose vs day time fractional doses and 30 mg vs 60 mg vs 90 mg vs 120 mg. All CPZ doses ‘low’ according to definitions in this review. Outcomes: leaving early, 'response', behaviour (needing chlordiazepoxide, MIBS), side effects ‐ no usable data, all reported for total group and not for people with schizophrenia, all regression scores rather than averages or binary outcomes. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised, "double‐blind". Participants: people with schizophrenia. Interventions: rimcazole 20 mg ‐ 80 mg/day vs rimcazole 100 mg ‐ 400 mg/day vs CPZ 400 mg ‐1600 mg/day vs placebo. | |
| Allocation: "double‐blind", randomisation not stated. Participants: people with schizophrenia. Interventions: study 1‐ continuous use of antipsychotics vs antipsychotics 5 days/week vs antipsychotics 4 days/week. Study 2 ‐ antiparkinsonian treatment vs placebo as an adjunct to antipsychotics. | |
| Allocation: randomised, "double‐blind". Participants: men with schizophrenia who are physically healthy but apathetic and withdrawn. Interventions: Combination of CPZ with: dextroamphetamine vs isocarboxazid vs trifluoperazine vs imipramine vs placebo, not dose. | |
| Allocation: not stated (possibly CCT). Participants: people with schizophrenia. Interventions: risperidone vs chlorpromazine vs clozapine. | |
| Allocation: not randomised, case series. | |
| Allocation: randomised (table of random numbers). | |
| Allocation: randomised, table of random numbers (Study #1 and #2 of Clark 1970). | |
| Allocation: randomised, "double‐blind" (Study #3 of Clark 1970). | |
| Allocation: randomised, "double‐blind" (Study #6 of Clark 1970). | |
| Allocation: randomised. | |
| Allocation: not randomised, cross‐over investigating plasma levels. | |
| Allocation: randomised, cross‐over. | |
| Allocation: not randomised, case series investigating plasma levels. | |
| Allocation: randomised. Participants: healthy people, not people with schizophrenia. | |
| Allocation: "assigned", "double‐blind", cross‐over, implied randomisation. Participants: people with schizophrenia. Interventions: chlorpromazine, thioridazine, trifluoperazine, haloperidol, placebo (sodium bicarbonate) administered at 90‐min intervals ‐ order of administration randomised, not dose. | |
| Allocation: randomised. | |
| Allocation: randomised, cross‐over. | |
| Allocation: all people received the 3 drug treatments, "order determined by Latin Square". Participants: healthy men, not people with schizophrenia. | |
| Allocation: randomised. | |
| Allocation: randomised, crossover. Participants: outpatients with schizophrenia diagnosed with persistent dyskinesia (total = 3). Interventions: schedule A (weeks 1 & 2(0), 3 & 4(four times a day), 5(0), 6 & 7(once daily), 8(0), 9 &10 (four times a day), 11(0), 12 & 13(once daily), 14(0).) vs schedule B (weeks 1& 2(0), 3 & 4(once daily), 5(0), 6 & 7(once daily), 8(0), 9 & 10(once daily), 11(0), 12 &13 (four times a day).), not dose. | |
| Allocation: randomised, "double‐blind". Participants: people with schizophrenia (untreated Acute Schizophrenic Psychosis). Interventions: 4 groups of varying CPZ doses with daily injection of vitamin preparation (containing B1, B6 and B12) vs placebo. | |
| Allocation: randomised, "double‐blind". Participants: acutely psychotic people. Interventions: bed time dose of CPZ vs day time fractional doses of CPZ. | |
| Allocation: randomised, but the procedure was not entirely successful (top p295). Participants: hospitalised chronic psychotic people. Interventions: high vs low chlorpromazine equivalent dosages ‐ unclear what antipsychotic drugs were used. | |
| Allocation: randomised, "double‐blind", cross‐over. Participants: hospitalised chronic psychotic people. Interventions: CPZ preparation A vs CPZ preparation B. Comparison of different brands, not different doses. | |
| Allocation: "blindness" achieved by randomisation of duration of treatment time periods, not dose. | |
| Allocation: "carefully matched". | |
| Allocation: randomised. Participants: inpatients with schizophrenia. Interventions: all participants given 3 or more antipsychotics with total dosage more than 1500 mg CPZ equivalence (mg CP), RAS group vs control group with continuation of polypharmacy, multiple antipsychotics. | |
| Allocation: randomised. Participants: people with schizophrenia. Interventions: all participants undergo dose reduction of antipsychotic drugs (for low potency drugs: dose decreased with 25 mg CP or lower per week. For high potency drugs: dose decreased with 50 mg CP or lower per week), multiple antipsychotics. | |
| Allocation: randomised. Participants: people with schizophrenia, aged 20 or over. Interventions: control group vs dose reduction (dose decreased to 80% or less than the initial dose over 3‐6 months according to SCAP method), multiple antipsychotics. | |
| Allocation: randomised. Participants: inpatients with schizophrenia. Interventions: CPZ 100 mg (three time a day) vs CPZ 100 mg (three time a day) + trihexyphenidyl 2 mg (three time a day) vs CPZ 200 mg (three time a day) + trihexyphenidyl 2 mg (three time a day), both CPZ doses ‘low’ according to definitions in this review. |
CPZ ‐ Chlorpromzine
MIBS ‐ Missouri Inpatient Behaviour Scale
CCT ‐ Controlled clinical trial
BPRS ‐ Brief Psychiatric Rating Scale
RAS ‐ Reduction and Simplification of Antipsychotics
mg CP ‐ chlorpromazine equivalence
SCAP ‐ Safety Correction of High Dose Antipsychotic Polypharmacy
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: 1. Not improved ‐ short term (CGI‐SI) Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.28, 2.44] |
| Analysis 1.1  Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 1 Global state: 1. Not improved ‐ short term (CGI‐SI). | ||||
| 2 Global state: 2. Requiring additional medication (sedation with chloral hydrate or a barbiturate > 5 times) ‐ medium term Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.40, 23.64] |
| Analysis 1.2  Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 2 Global state: 2. Requiring additional medication (sedation with chloral hydrate or a barbiturate > 5 times) ‐ medium term. | ||||
| 3 Leaving the study early Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 3 Leaving the study early. | ||||
| 3.1 any reason | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.32, 3.55] |
| 3.2 adverse effects | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.02, 2.32] |
| 3.3 inefficacy of treatment | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.24 [0.24, 74.01] |
| 3.4 other reason | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.09, 9.27] |
| 4 Mental state: 1a. Average endpoint score (BPRS total, high = poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 4 Mental state: 1a. Average endpoint score (BPRS total, high = poor). | ||||
| 4.1 anxious depression | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.35, 0.15] |
| 4.2 thinking disturbance | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐0.24, 5.44] |
| 4.3 withdrawal retardation | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐3.76, ‐0.24] |
| 5 Mental state: 1b. Average endpoint score (PANSS total and subscores, high = poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 5 Mental state: 1b. Average endpoint score (PANSS total and subscores, high = poor). | ||||
| 5.1 negative | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐2.34, 2.00] |
| 5.2 positive | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐1.98, 1.50] |
| 5.3 total | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [‐5.39, 6.11] |
| 6 Adverse effects: 1. Central nervous system ‐ extrapyramidal symptoms Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 6 Adverse effects: 1. Central nervous system ‐ extrapyramidal symptoms. | ||||
| 6.1 akathisia ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.28, 6.68] |
| 6.2 akinesia ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 4.75] |
| 6.3 dystonia ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.05, 1.29] |
| 6.4 gait disturbance ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.11, 1.17] |
| 6.5 muscle tension ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.03, 0.67] |
| 6.6 rigidity ‐ elbow ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.21, 1.31] |
| 6.7 rigidity ‐ unspecified ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.05] |
| 6.8 tremor ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.17, 1.10] |
| 6.9 unspecified extrapyramidal symptoms ‐ short term | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.30, 0.74] |
| 6.10 unspecified extrapyramidal symptoms ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.05, 1.27] |
| 7 Adverse effects: 2. Anticholinergic Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 7 Adverse effects: 2. Anticholinergic. | ||||
| 7.1 blurred vision ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.06, 1.63] |
| 7.2 ejaculation disturbance ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 erectile disturbance ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 constipation ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.42, 7.99] |
| 7.5 constipation ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.09] |
| 7.6 salivation ‐ dry mouth ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.58, 2.34] |
| 7.7 salivation ‐ too much ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.85] |
| 7.8 tachycardia ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 4.75] |
| 7.9 urinary disturbance/trouble starting urination ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.37, 24.58] |
| 8 Adverse effects: 3. Cardiovascular Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 8 Adverse effects: 3. Cardiovascular. | ||||
| 8.1 electrocardiogram abnormal ‐ short term | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 0.94] |
| 8.2 orthostatic symptoms (faint, dizzy, weak) ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.21, 18.98] |
| 8.3 orthostatic symptoms ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.09] |
| 8.4 tachycardia ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 4.75] |
| 8.5 vertigo ‐ short term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.21, 5.07] |
| 9 Adverse effects: 4. Central nervous system ‐ other than extrapyramidal symptoms Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 9 Adverse effects: 4. Central nervous system ‐ other than extrapyramidal symptoms. | ||||
| 9.1 depression ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 drowsiness ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.47, 2.14] |
| 9.3 EEG abnormal ‐ short term | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.33, 1.82] |
| 9.4 head feels heavy ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.34] |
| 9.5 headache ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [0.12, 46.22] |
| 9.6 sedation ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.05, 0.55] |
| 9.7 somnolence ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.59, 2.08] |
| 9.8 vertigo ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.19, 4.43] |
| 10 Adverse effects: 5. Dermatological Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 10 Adverse effects: 5. Dermatological. | ||||
| 10.1 dermatitis ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.07, 37.03] |
| 10.2 rash ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.39, 4.62] |
| 10.3 rash ‐ 'photosensitivity: erythema' ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.03, 7.74] |
| 11 Adverse effects: 6. Endocrine and metabolic Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 11 Adverse effects: 6. Endocrine and metabolic. | ||||
| 11.1 amenorrhoea ‐ short term | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.03, 6.74] |
| 11.2 lactation ‐ short term | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.73] |
| 11.3 metrorrhagia ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 4.11] |
| 12 Adverse effects: 7. Gastrointestinal Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 12 Adverse effects: 7. Gastrointestinal. | ||||
| 12.1 constipation ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.42, 7.99] |
| 12.2 constipation ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.09] |
| 12.3 unspecified ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.27, 93.55] |
| 13 Adverse effects: 8. Genitourinary Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.13  Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 13 Adverse effects: 8. Genitourinary. | ||||
| 13.1 urinary disturbance/trouble starting urination ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.37, 24.58] |
| 14 Adverse effects: 9. Haematological Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.14  Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 14 Adverse effects: 9. Haematological. | ||||
| 14.1 agranulocytosis ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.07, 37.03] |
| 14.2 anaemia ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.07, 37.03] |
| 15 Adverse effects: 10. Hepatological Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.15  Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 15 Adverse effects: 10. Hepatological. | ||||
| 15.1 abnormal liver function ‐ short term | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.33, 5.45] |
| 16 Adverse effects: 11. Others Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.16  Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 16 Adverse effects: 11. Others. | ||||
| 16.1 agitation and restlessness ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.07, 37.03] |
| 16.2 excitement ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.05] |
| 16.3 restlessness, insomnia ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.22, 2.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Not improved, severely ill, relapse (GAS) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 1 Global state: Not improved, severely ill, relapse (GAS). | ||||
| 1.1 no clinically important improvement | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [1.01, 1.25] |
| 1.2 severely ill | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.96, 1.24] |
| 1.3 relapse | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.17, 4.32] |
| 2 Leaving the study early Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 2 Leaving the study early. | ||||
| 2.1 any reason | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.40, 0.89] |
| 2.2 adverse effects | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.26] |
| 2.3 death | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.14] |
| 2.4 deterioration of behaviour | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.7 [1.34, 5.44] |
| 3 Adverse effects: 1. Central nervous system ‐ extrapyramidal symptoms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 3 Adverse effects: 1. Central nervous system ‐ extrapyramidal symptoms. | ||||
| 3.1 akathisia | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.55, 1.83] |
| 3.2 dystonia | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.02, 0.45] |
| 3.3 parkinsonian reaction | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.30, 0.90] |
| 3.4 required antiparkinsonian medication | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.26, 0.59] |
| 3.5 unspecified | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.32, 0.59] |
| 4 Adverse effects: 2. Anticholinergic symptoms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 4 Adverse effects: 2. Anticholinergic symptoms. | ||||
| 4.1 blurred vision | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.14] |
| 4.2 constipation | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.09, 0.79] |
| 4.3 salivation ‐ too little (dry mouth) | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.61] |
| 4.4 salivation ‐ too much | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.04, 0.90] |
| 4.5 urinary disturbance | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.09, 2.70] |
| 5 Adverse effects: 3. Central nervous system ‐ other than extrapyramidal symptoms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 5 Adverse effects: 3. Central nervous system ‐ other than extrapyramidal symptoms. | ||||
| 5.1 drowsiness | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.27, 0.57] |
| 5.2 seizures | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.04, 0.74] |
| 6 Adverse effects: 4. Cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.6  Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 6 Adverse effects: 4. Cardiovascular. | ||||
| 6.1 dizziness, faintness | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.16, 0.56] |
| 6.2 peripheral oedema | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.63] |
| 6.3 syncope | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.09, 2.70] |
| 7 Adverse effects: 5. Dermatological Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.7  Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 7 Adverse effects: 5. Dermatological. | ||||
| 7.1 photosensitivity | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.03, 0.24] |
| 7.2 rashes, itching | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.58] |
| 8 Adverse effects: 6. Gastrointestinal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.8  Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 8 Adverse effects: 6. Gastrointestinal. | ||||
| 8.1 constipation | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.09, 0.79] |
| 8.2 diarrhoea | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.09, 2.70] |
| 8.3 nausea, vomiting | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.19, 2.33] |
| 9 Adverse effects: 7. Genitourinary Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.9  Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 9 Adverse effects: 7. Genitourinary. | ||||
| 9.1 urinary disturbance | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.09, 2.70] |
| 10 Adverse effects: 8. Others Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.10  Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 10 Adverse effects: 8. Others. | ||||
| 10.1 occular ‐ any lens/corneal opacities | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.23, 0.52] |
| 10.2 occular ‐ corneal changes only | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.30] |
| 10.3 occular ‐ lens changes only | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.88, 3.46] |
| 10.4 occular ‐ lens and corneal changes | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.02, 0.26] |
| 10.5 nasal congestion | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 7.03] |
Chlorpromazine structure
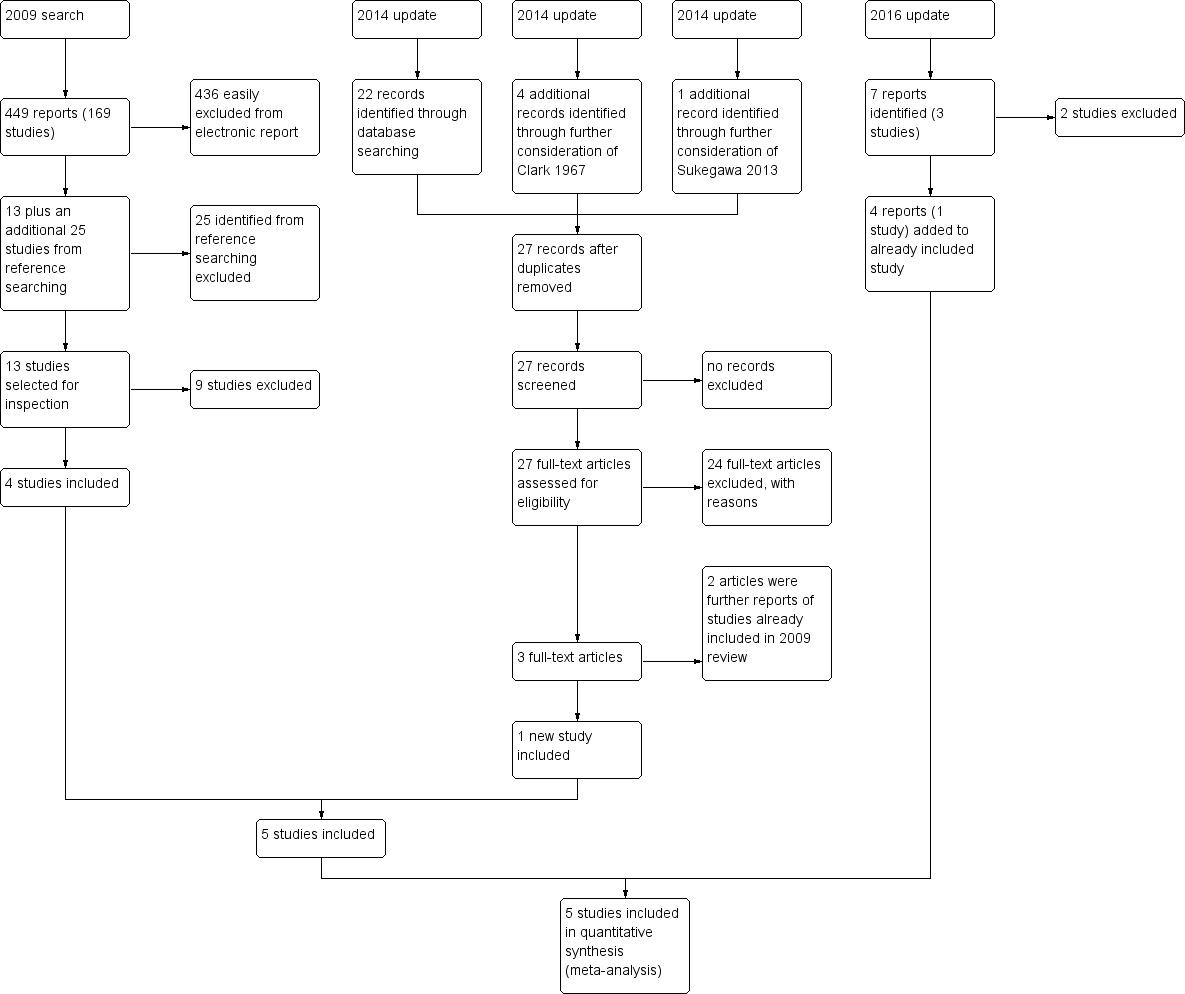
Study flow diagram
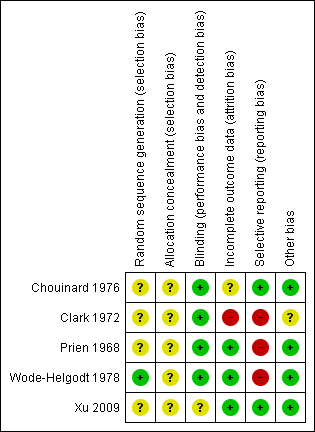
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
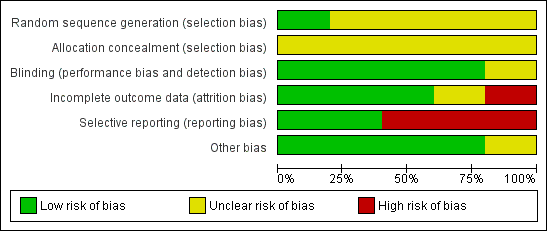
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
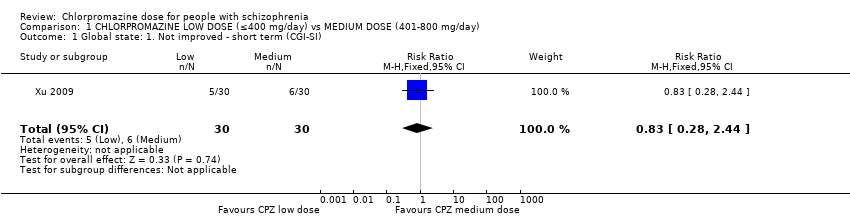
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 1 Global state: 1. Not improved ‐ short term (CGI‐SI).
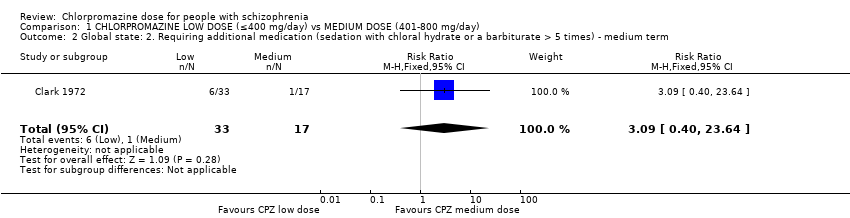
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 2 Global state: 2. Requiring additional medication (sedation with chloral hydrate or a barbiturate > 5 times) ‐ medium term.
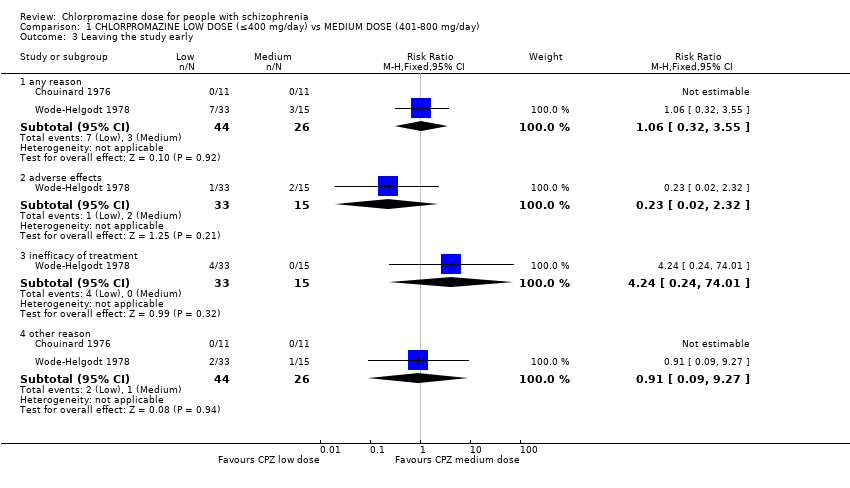
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 3 Leaving the study early.
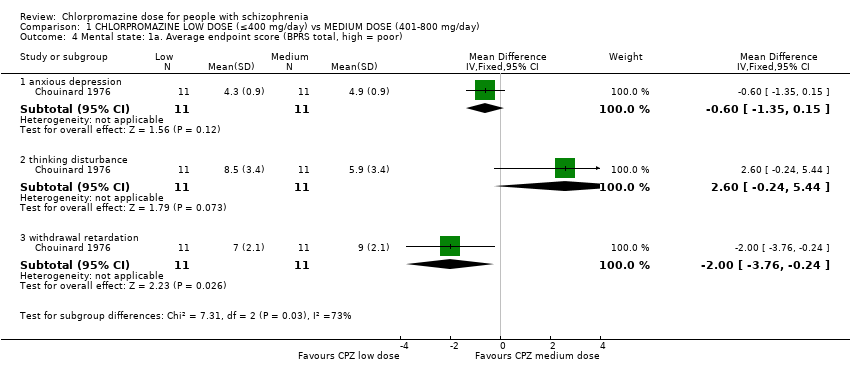
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 4 Mental state: 1a. Average endpoint score (BPRS total, high = poor).
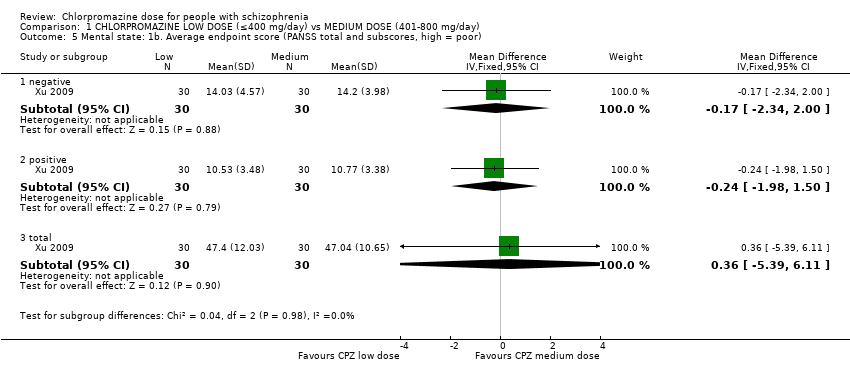
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 5 Mental state: 1b. Average endpoint score (PANSS total and subscores, high = poor).
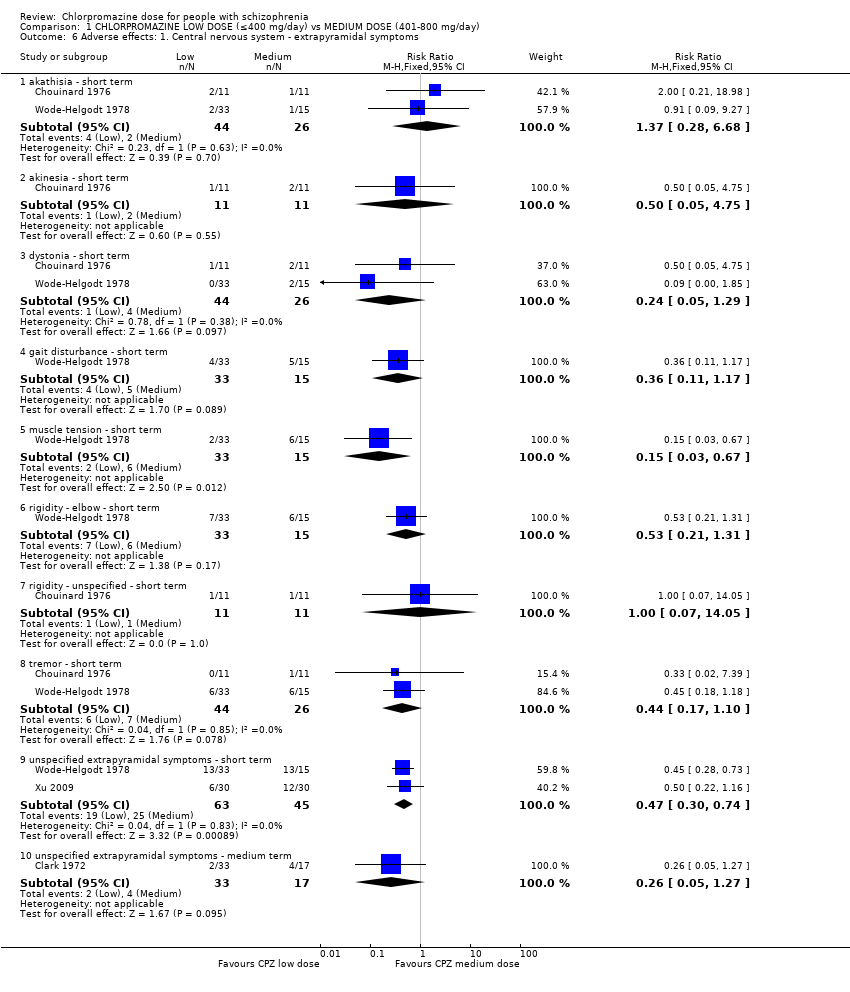
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 6 Adverse effects: 1. Central nervous system ‐ extrapyramidal symptoms.
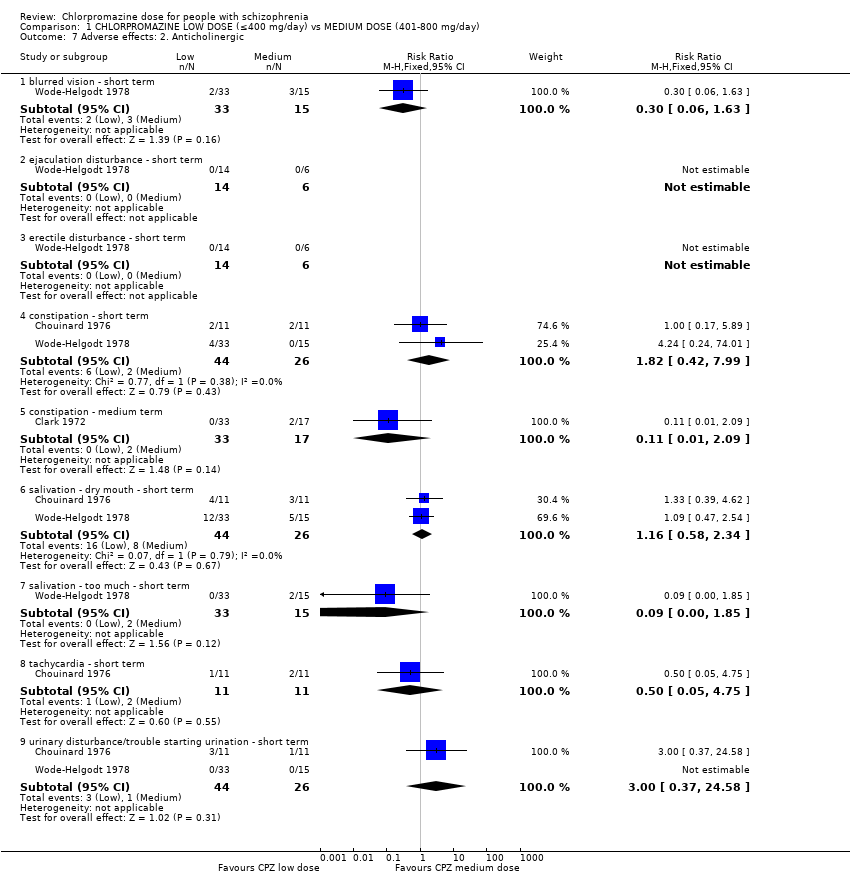
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 7 Adverse effects: 2. Anticholinergic.
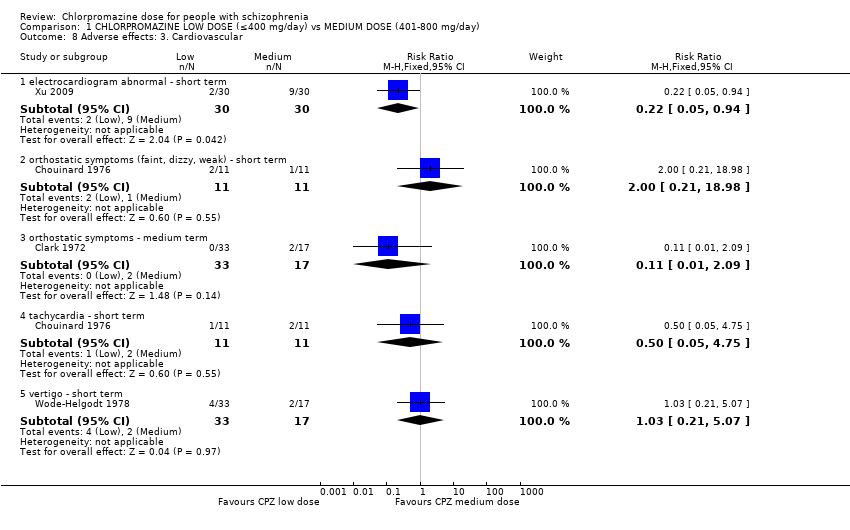
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 8 Adverse effects: 3. Cardiovascular.
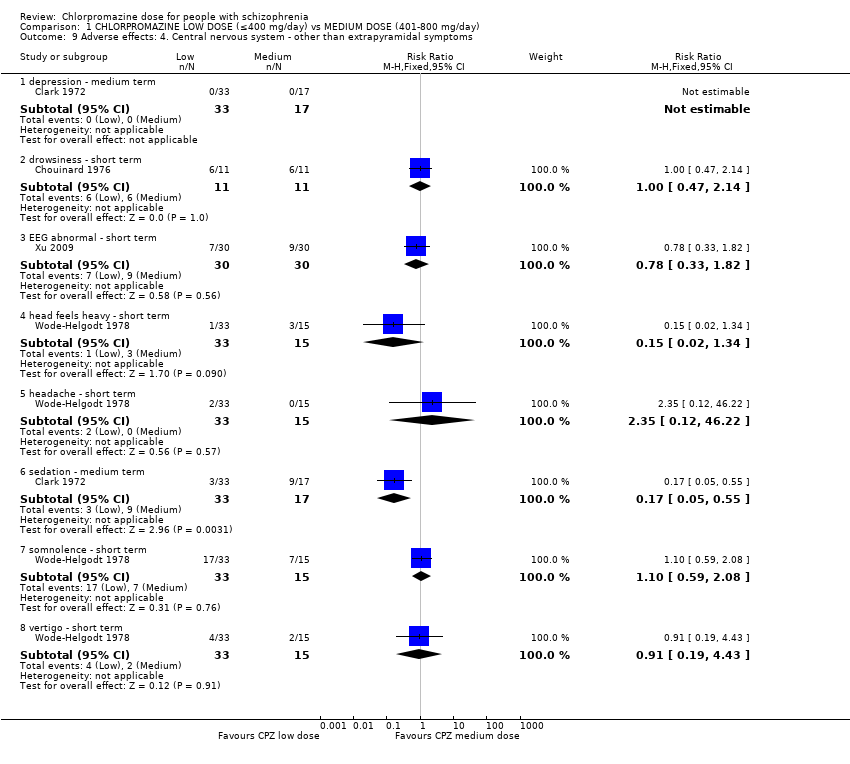
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 9 Adverse effects: 4. Central nervous system ‐ other than extrapyramidal symptoms.
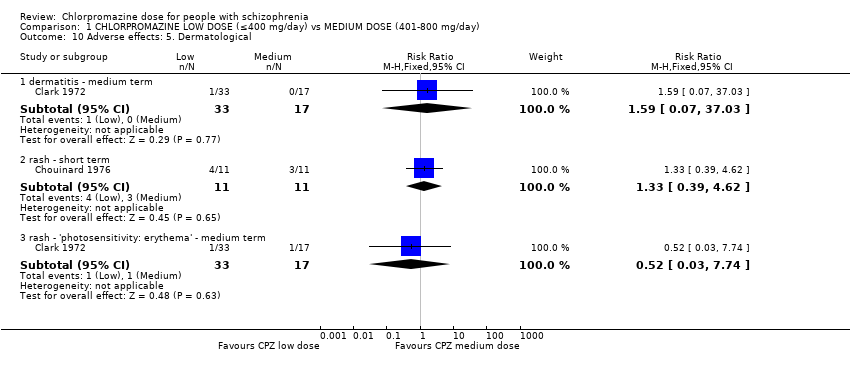
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 10 Adverse effects: 5. Dermatological.
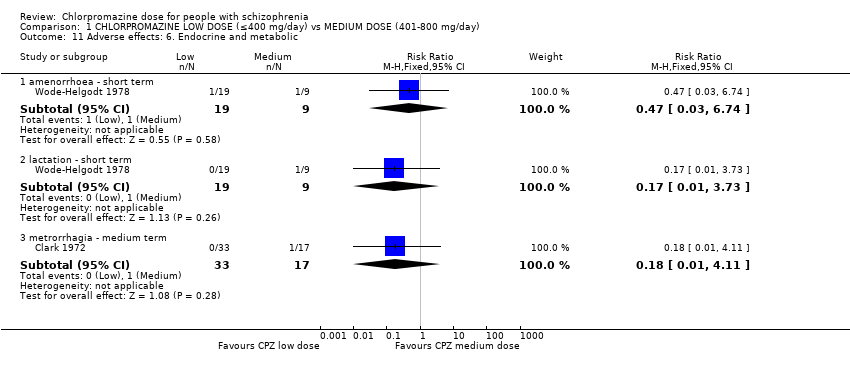
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 11 Adverse effects: 6. Endocrine and metabolic.
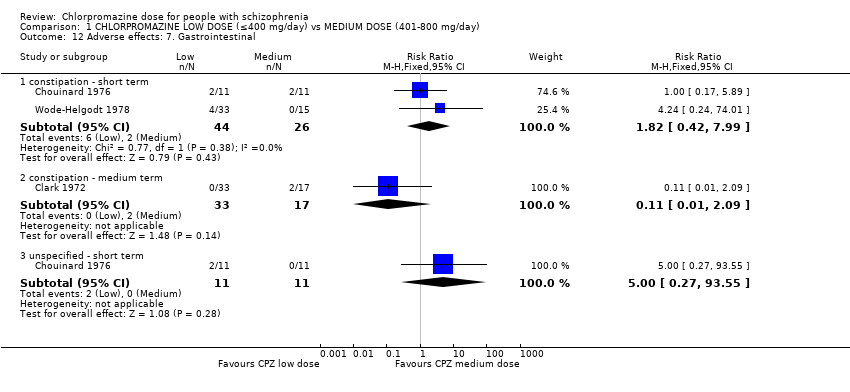
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 12 Adverse effects: 7. Gastrointestinal.
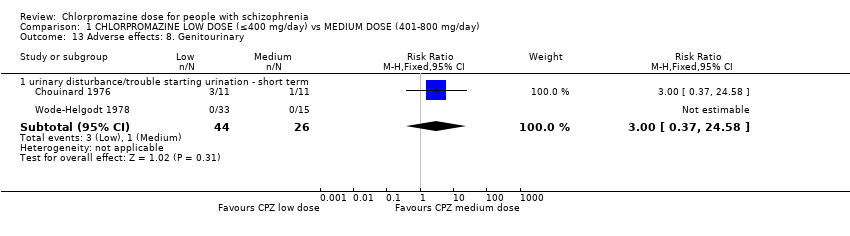
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 13 Adverse effects: 8. Genitourinary.
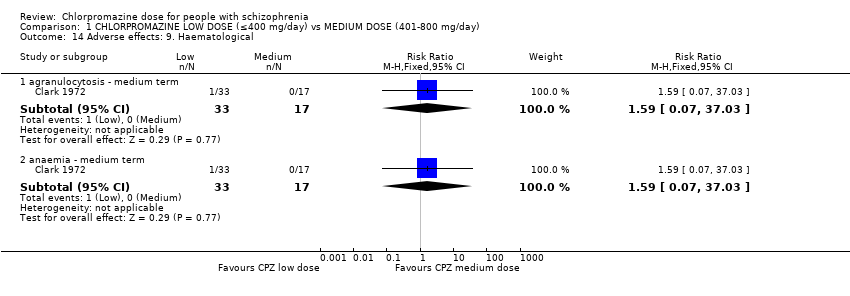
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 14 Adverse effects: 9. Haematological.
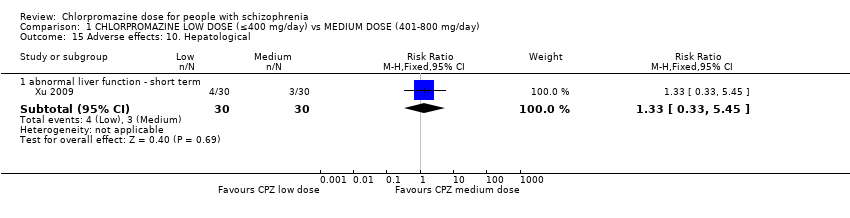
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 15 Adverse effects: 10. Hepatological.
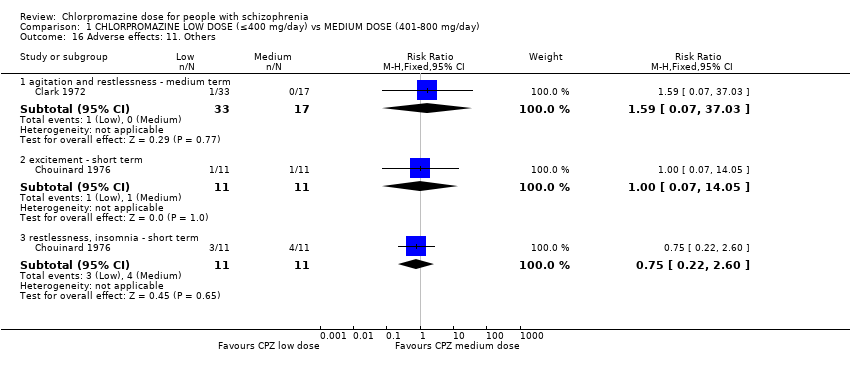
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 16 Adverse effects: 11. Others.
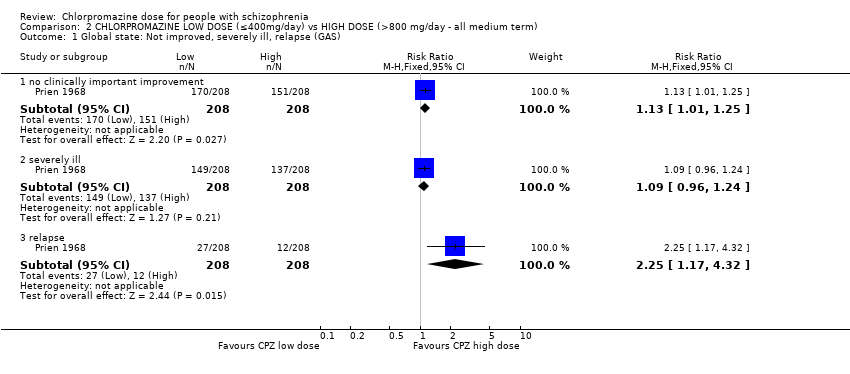
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 1 Global state: Not improved, severely ill, relapse (GAS).
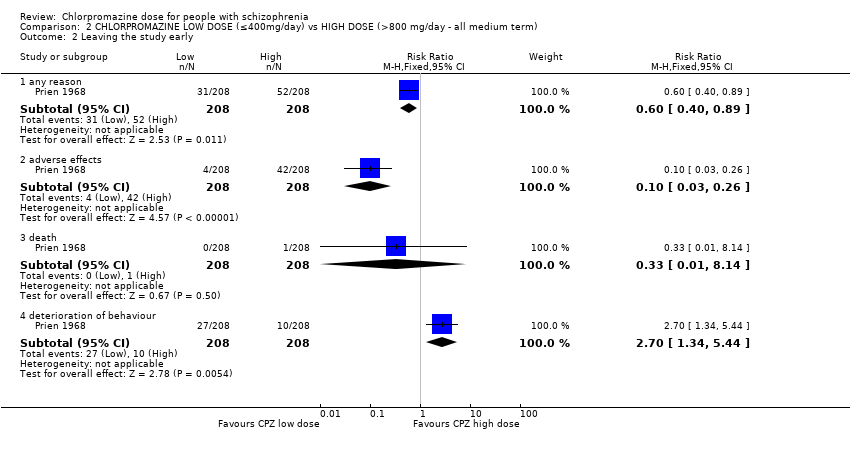
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 2 Leaving the study early.
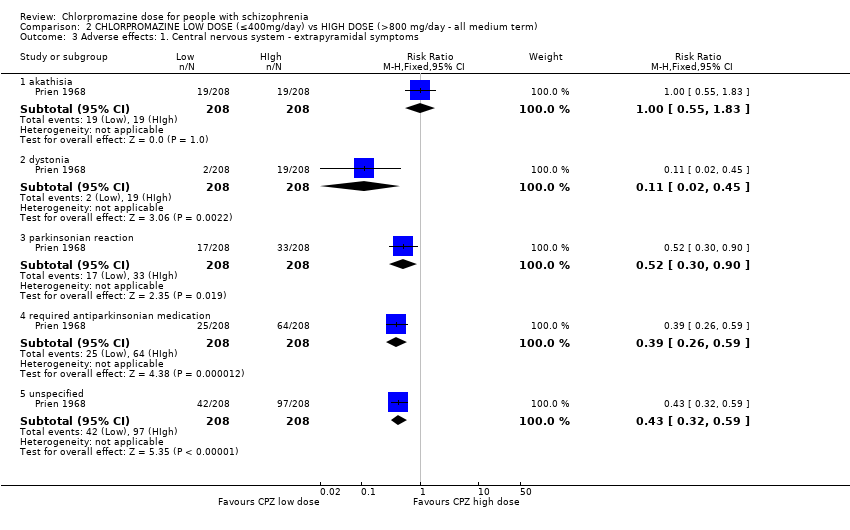
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 3 Adverse effects: 1. Central nervous system ‐ extrapyramidal symptoms.
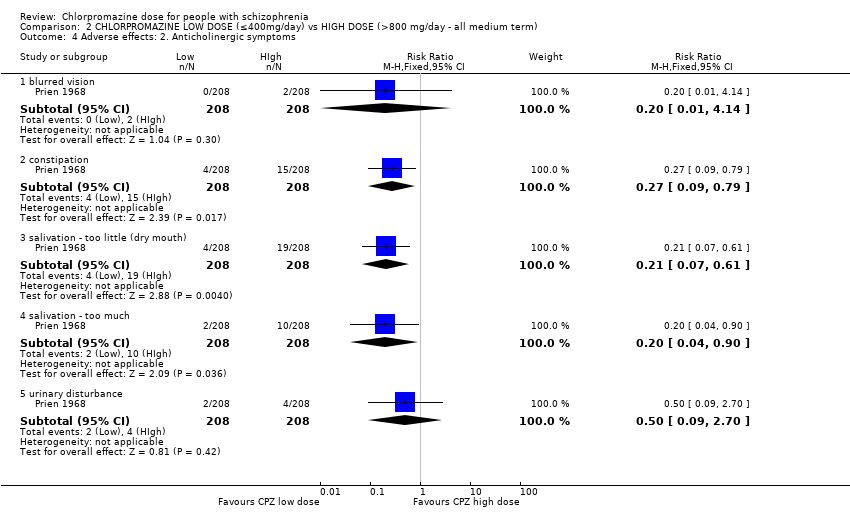
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 4 Adverse effects: 2. Anticholinergic symptoms.
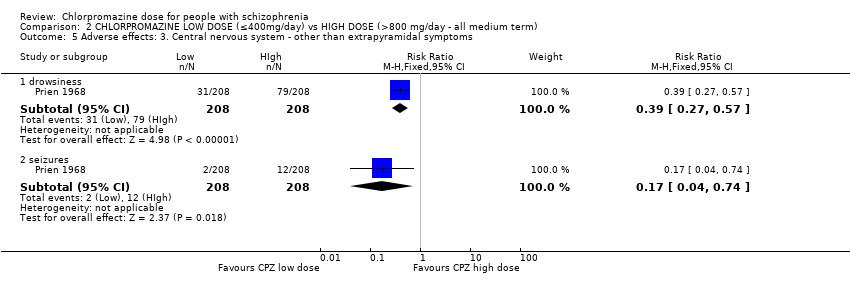
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 5 Adverse effects: 3. Central nervous system ‐ other than extrapyramidal symptoms.
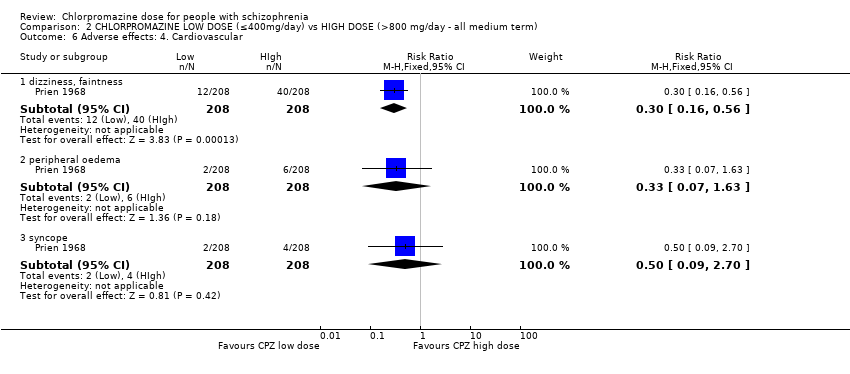
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 6 Adverse effects: 4. Cardiovascular.
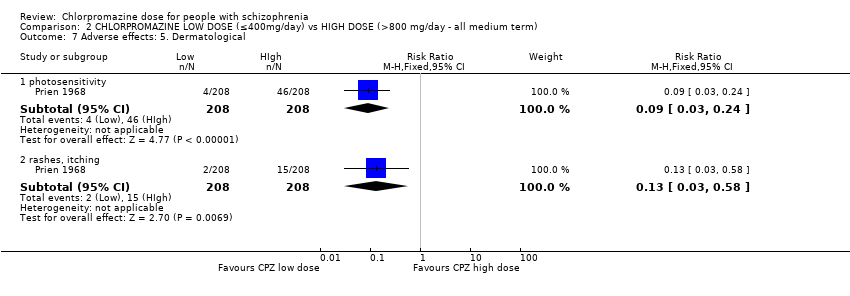
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 7 Adverse effects: 5. Dermatological.
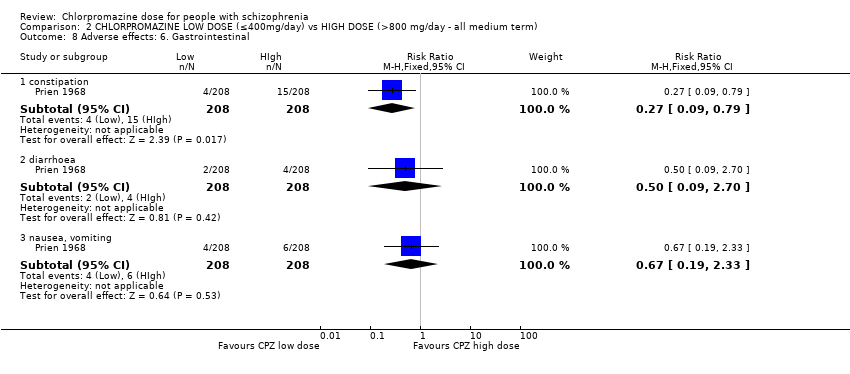
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 8 Adverse effects: 6. Gastrointestinal.
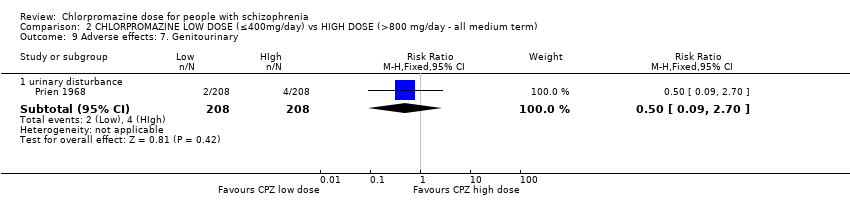
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 9 Adverse effects: 7. Genitourinary.
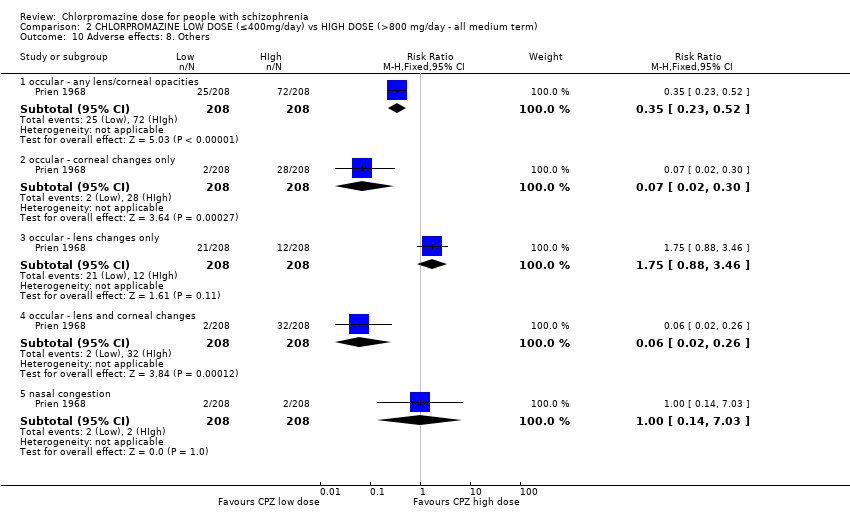
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 10 Adverse effects: 8. Others.
| Medication | Comparison | For people with | Relevant excluded study | Relevant existing Cochrane review | |
| Antiparkinsonian drug ‐ unspecified | Antiparkinsonian treatment versus placebo | Movement disorders | ‐ | ||
| Antipsychotic drugs ‐ unspecified | Doses | Any antipsychotic at high chlorpromazine equivalent dosages vs lower chlorpromazine equivalent dosages | Schizophrenia | ‐ | |
| Dose reduction of multiple antipsychotics versus control group | ‐ | ||||
| Timing of treatment | Treatment days per week of antipsychotics | ‐ | |||
| Chlorpromazine | Mostly versus other antipsychotic drug | Chlorpromazine brand comparisons | ‐ | ||
| Chlorpromazine versus clopenthixol | |||||
| Chlorpromazine versus clozapine | |||||
| Chlorpromazine versus haloperidol | |||||
| Chlorpromazine versus placebo | Borison 1991; Clark 1967; Clark 1970a; Clark 1970b; Eitan 1992 | ||||
| Chlorpromazine versus rimcazole | ‐ | ||||
| Chlorpromazine versus risperidone | |||||
| Chlorpromazine versus thioridazine | |||||
| Chlorpromazine versus trifluoperazine | |||||
| Doses | Ultra‐low doses of chlorpromazine | ‐ | |||
| Ultra‐low doses of thioridazine | ‐ | ||||
| Timing of treatment | Night doses versus daytime doses of chlorpromazine | ‐ | |||
| Single doses versus divided doses of chlorpromazine | ‐ | ||||
| Q.I.D versus O.D of chlorpromazine | ‐ | ||||
| Clopenthixol | Clopenthixol versus placebo | ||||
| Clozapine | Clozapine versus risperidone | ||||
| Dextroamphetamine | Dextroamphetamine versus imipramine | ||||
| Dextroamphetamine versus isocarboxazid | |||||
| Dextroamphetamine versus placebo | ‐ | ||||
| Dextroamphetamine versus trifluoperazine | ‐ | ||||
| Haloperidol | Haloperidol versus placebo | ||||
| Haloperidol versus trifluoperazine | |||||
| Haloperidol versus thioridazine | |||||
| Imipramine | ‐ | ‐ | |||
| Isocarboxazid | Isocarboxazid versus placebo | ||||
| Isocarboxazid versus trifluoperazine | |||||
| Rimcazole | Rimcazole versus placebo | ‐ | |||
| Doses | Rimcazole dose | ‐ | |||
| Risperidone | ‐ | ‐ | |||
| Thioridazine | Thioridazine versus placebo | ||||
| Timing of treatment | Night doses versus daytime doses of thioridazine | ‐ | |||
| Timing of treatment | Thioridazine ‐ single doses versus divided doses | ‐ | |||
| Trifluoperazine | Trifluoperazine versus placebo | ||||
| Trifluoperazine versus thioridazine | |||||
| Trihexyphenidyl | Trihexyphenidyl versus placebo | Movement disorders | ‐ | ||
| Vitamin preparation | Vitamin preparation versus placebo | Schizophrenia | |||
| Methods | Allocation: clearly randomised, well‐described concealment. Blindness: triple‐blinding clearly described including information on method administration, volume and concentration of chlorpromazine. Duration: 28 weeks (2 weeks of no antipsychotic medication). |
| Participants | Diagnosis: people with schizophrenia ‐ diagnosed by any criteria. Age: any. Sex: both. N = 450.* History: acute or long‐term illness. Excluded: borderline cases, mental deficiency (IQ < 70), bad physical health with complicating organic illness or known brain damage, medical conditions constraining use of high doses, alcoholism or drug use, showing severe suicidal or aggressive impulses. |
| Interventions | 1. Chlorpromazine: dose 300 mg/day. N = 150. |
| Outcomes | Leaving the study early (reasons, timing, gender and treatment group provided). Deaths. Adverse effects (clinically important and specific). Needing additional medication (information about doses, timings and reasons for drug administration provided). Effectiveness:
|
| Notes | * 150 in each group allows for good statistical power. |
| CHLORPROMAZINE LOW DOSE (≤ 400 mg/day) compared to MEDIUM DOSE (401 mg/day to 800 mg/day) for people with schizophrenia | |||||
| Patient or population: people with schizophrenia | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| MEDIUM DOSE (401‐800 mg/day) | CHLORPROMAZINE LOW DOSE (≤ 400 mg/day) | ||||
| Global state: no improvement ‐ short term | Low1 | RR 0.83 | 60 | ⊕⊝⊝⊝ | |
| 100 per 1000 | 83 per 1000 | ||||
| Moderate1 | |||||
| 200 per 1000 | 166 per 1000 | ||||
| High1 | |||||
| 300 per 1000 | 249 per 1000 | ||||
| Global state: needing additional medication (sedation with chloral hydrate or a barbiturate > 5 times) ‐ medium term | Low1 | RR 3.09 | 50 | ⊕⊕⊝⊝ | |
| 20 per 1000 | 62 per 1000 | ||||
| Moderate1 | |||||
| 60 per 1000 | 185 per 1000 | ||||
| High1 | |||||
| 100 per 1000 | 309 per 1000 | ||||
| Mental state: average endpoint score (PANSS total, high=poor) | The mean mental state: average endpoint score (PANSS total, high = poor) in the intervention groups was | 60 | ⊕⊝⊝⊝ | ||
| Leaving the study early ‐ any reason | Low1 | RR 1.06 | 70 | ⊕⊕⊕⊝ | |
| 50 per 1000 | 53 per 1000 | ||||
| Moderate1 | |||||
| 100 per 1000 | 106 per 1000 | ||||
| High1 | |||||
| 150 per 1000 | 159 per 1000 | ||||
| Behaviour: agitation and restlessness ‐ medium term (categorised as adverse event) | Study population | RR 1.59 | 50 | ⊕⊝⊝⊝ | |
| 0 per 1000 | 0 per 1000 | ||||
| Moderate | |||||
| 0 per 1000 | 0 per 1000 | ||||
| Adverse effects: extrapyramidal symptoms (unspecified extrapyramidal symptoms ‐ short term) | Low | RR 0.47 | 108 | ⊕⊕⊕⊝ | |
| 200 per 1000 | 94 per 1000 | ||||
| Moderate | |||||
| 500 per 1000 | 235 per 1000 | ||||
| High | |||||
| 800 per 1000 | 376 per 1000 | ||||
| Adverse event: death | No trial reported this outcome | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Indirectness: rated serious: downgraded by 1 ‐ unclear if clinically important improvement, no prespecified medium‐term data available. | |||||
| CHLORPROMAZINE LOW DOSE (≤ 400 mg/day) compared to HIGH DOSE (> 800 mg/day‐ all medium term) for people with schizophrenia | ||||||
| Patient or population: patients with people with schizophrenia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comment | |
| Assumed risk | Corresponding risk | |||||
| HIGH DOSE (>800 mg/day‐ all medium term) | CHLORPROMAZINE LOW DOSE (≤ 400 mg/day) | |||||
| Global state: no clinically important improvement ‐ medium term | Low1 | RR 1.13 | 416 | ⊕⊕⊕⊝ | ||
| 300 per 1000 | 339 per 1000 | |||||
| Moderate1 | ||||||
| 700 per 1000 | 791 per 1000 | |||||
| High1 | ||||||
| 900 per 1000 | 1000 per 1000 | |||||
| Global state: requiring additional medication (sedation with chloral hydrate or a barbiturate > 5 times) | See comment | See comment | Not estimable | 0 | See comment | No data available |
| Mental state: no clinically important change in mental state | See comment | See comment | Not estimable | 0 | See comment | No data available |
| Leaving the study early ‐ any reason | Low1 | RR 0.60 | 416 | ⊕⊕⊕⊝ | ||
| 100 per 1000 | 60 per 1000 | |||||
| Moderate1 | ||||||
| 250 per 1000 | 150 per 1000 | |||||
| High1 | ||||||
| 500 per 1000 | 300 per 1000 | |||||
| Behaviour: deterioration of behaviour (categorised as reason to leave early) | Low | RR 2.70 | 416 | ⊕⊕⊝⊝ | ||
| 20 per 1000 | 54 per 1000 | |||||
| Moderate | ||||||
| 50 per 1000 | 135 per 1000 | |||||
| High | ||||||
| 100 per 1000 | 270 per 1000 | |||||
| Adverse effects: unspecified extrapyramidal symptoms | Low | RR 0.43 | 416 | ⊕⊕⊕⊝ | ||
| 200 per 1000 | 86 per 1000 | |||||
| Moderate | ||||||
| 500 per 1000 | 215 per 1000 | |||||
| High | ||||||
| 700 per 1000 | 301 per 1000 | |||||
| Adverse event: death | Low | RR 0.33 | 416 | ⊕⊕⊕⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 5 per 1000 | 2 per 1000 | |||||
| High | ||||||
| 50 per 1000 | 17 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated serious: downgraded by 1 ‐ randomisation not described well, no mention of allocation concealment, concern regarding selective reporting. 2Indirectness: rated serious: downgraded by 1 ‐ unclear if clinically important change in behaviour, rated within trial as leaving the study early outcome. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: 1. Not improved ‐ short term (CGI‐SI) Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.28, 2.44] |
| 2 Global state: 2. Requiring additional medication (sedation with chloral hydrate or a barbiturate > 5 times) ‐ medium term Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.40, 23.64] |
| 3 Leaving the study early Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 any reason | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.32, 3.55] |
| 3.2 adverse effects | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.02, 2.32] |
| 3.3 inefficacy of treatment | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.24 [0.24, 74.01] |
| 3.4 other reason | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.09, 9.27] |
| 4 Mental state: 1a. Average endpoint score (BPRS total, high = poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 anxious depression | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.35, 0.15] |
| 4.2 thinking disturbance | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐0.24, 5.44] |
| 4.3 withdrawal retardation | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐3.76, ‐0.24] |
| 5 Mental state: 1b. Average endpoint score (PANSS total and subscores, high = poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 negative | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐2.34, 2.00] |
| 5.2 positive | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐1.98, 1.50] |
| 5.3 total | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [‐5.39, 6.11] |
| 6 Adverse effects: 1. Central nervous system ‐ extrapyramidal symptoms Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 akathisia ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.28, 6.68] |
| 6.2 akinesia ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 4.75] |
| 6.3 dystonia ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.05, 1.29] |
| 6.4 gait disturbance ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.11, 1.17] |
| 6.5 muscle tension ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.03, 0.67] |
| 6.6 rigidity ‐ elbow ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.21, 1.31] |
| 6.7 rigidity ‐ unspecified ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.05] |
| 6.8 tremor ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.17, 1.10] |
| 6.9 unspecified extrapyramidal symptoms ‐ short term | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.30, 0.74] |
| 6.10 unspecified extrapyramidal symptoms ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.05, 1.27] |
| 7 Adverse effects: 2. Anticholinergic Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 blurred vision ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.06, 1.63] |
| 7.2 ejaculation disturbance ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 erectile disturbance ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 constipation ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.42, 7.99] |
| 7.5 constipation ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.09] |
| 7.6 salivation ‐ dry mouth ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.58, 2.34] |
| 7.7 salivation ‐ too much ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.85] |
| 7.8 tachycardia ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 4.75] |
| 7.9 urinary disturbance/trouble starting urination ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.37, 24.58] |
| 8 Adverse effects: 3. Cardiovascular Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 electrocardiogram abnormal ‐ short term | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 0.94] |
| 8.2 orthostatic symptoms (faint, dizzy, weak) ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.21, 18.98] |
| 8.3 orthostatic symptoms ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.09] |
| 8.4 tachycardia ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 4.75] |
| 8.5 vertigo ‐ short term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.21, 5.07] |
| 9 Adverse effects: 4. Central nervous system ‐ other than extrapyramidal symptoms Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 depression ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 drowsiness ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.47, 2.14] |
| 9.3 EEG abnormal ‐ short term | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.33, 1.82] |
| 9.4 head feels heavy ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.34] |
| 9.5 headache ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [0.12, 46.22] |
| 9.6 sedation ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.05, 0.55] |
| 9.7 somnolence ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.59, 2.08] |
| 9.8 vertigo ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.19, 4.43] |
| 10 Adverse effects: 5. Dermatological Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 dermatitis ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.07, 37.03] |
| 10.2 rash ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.39, 4.62] |
| 10.3 rash ‐ 'photosensitivity: erythema' ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.03, 7.74] |
| 11 Adverse effects: 6. Endocrine and metabolic Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 amenorrhoea ‐ short term | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.03, 6.74] |
| 11.2 lactation ‐ short term | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.73] |
| 11.3 metrorrhagia ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 4.11] |
| 12 Adverse effects: 7. Gastrointestinal Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 constipation ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.42, 7.99] |
| 12.2 constipation ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.09] |
| 12.3 unspecified ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.27, 93.55] |
| 13 Adverse effects: 8. Genitourinary Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 urinary disturbance/trouble starting urination ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.37, 24.58] |
| 14 Adverse effects: 9. Haematological Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 agranulocytosis ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.07, 37.03] |
| 14.2 anaemia ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.07, 37.03] |
| 15 Adverse effects: 10. Hepatological Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 abnormal liver function ‐ short term | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.33, 5.45] |
| 16 Adverse effects: 11. Others Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 agitation and restlessness ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.07, 37.03] |
| 16.2 excitement ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.05] |
| 16.3 restlessness, insomnia ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.22, 2.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Not improved, severely ill, relapse (GAS) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 no clinically important improvement | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [1.01, 1.25] |
| 1.2 severely ill | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.96, 1.24] |
| 1.3 relapse | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.17, 4.32] |
| 2 Leaving the study early Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 any reason | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.40, 0.89] |
| 2.2 adverse effects | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.26] |
| 2.3 death | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.14] |
| 2.4 deterioration of behaviour | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.7 [1.34, 5.44] |
| 3 Adverse effects: 1. Central nervous system ‐ extrapyramidal symptoms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 akathisia | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.55, 1.83] |
| 3.2 dystonia | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.02, 0.45] |
| 3.3 parkinsonian reaction | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.30, 0.90] |
| 3.4 required antiparkinsonian medication | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.26, 0.59] |
| 3.5 unspecified | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.32, 0.59] |
| 4 Adverse effects: 2. Anticholinergic symptoms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 blurred vision | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.14] |
| 4.2 constipation | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.09, 0.79] |
| 4.3 salivation ‐ too little (dry mouth) | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.61] |
| 4.4 salivation ‐ too much | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.04, 0.90] |
| 4.5 urinary disturbance | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.09, 2.70] |
| 5 Adverse effects: 3. Central nervous system ‐ other than extrapyramidal symptoms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 drowsiness | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.27, 0.57] |
| 5.2 seizures | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.04, 0.74] |
| 6 Adverse effects: 4. Cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 dizziness, faintness | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.16, 0.56] |
| 6.2 peripheral oedema | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.63] |
| 6.3 syncope | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.09, 2.70] |
| 7 Adverse effects: 5. Dermatological Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 photosensitivity | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.03, 0.24] |
| 7.2 rashes, itching | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.58] |
| 8 Adverse effects: 6. Gastrointestinal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 constipation | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.09, 0.79] |
| 8.2 diarrhoea | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.09, 2.70] |
| 8.3 nausea, vomiting | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.19, 2.33] |
| 9 Adverse effects: 7. Genitourinary Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 urinary disturbance | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.09, 2.70] |
| 10 Adverse effects: 8. Others Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 occular ‐ any lens/corneal opacities | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.23, 0.52] |
| 10.2 occular ‐ corneal changes only | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.30] |
| 10.3 occular ‐ lens changes only | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.88, 3.46] |
| 10.4 occular ‐ lens and corneal changes | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.02, 0.26] |
| 10.5 nasal congestion | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 7.03] |

