Toxina botulínica para la hipertrofia del masetero
Resumen
Antecedentes
La hipertrofia benigna del músculo masetero es un fenómeno clínico poco común de etiología incierta que se caracteriza por un edema blando cerca del ángulo de la mandíbula. En ocasiones, el edema se puede asociar con dolor facial y puede ser lo suficientemente prominente como para considerarse cosméticamente desfigurante. Se han informado diferentes grados de éxito para algunas de las opciones terapéuticas para la hipertrofia del masetero, las cuales varían de un tratamiento sencillo con fármacos a la reducción quirúrgica más invasiva. La inyección de la toxina botulínica tipo A en el músculo masetero se considera en general una modalidad menos invasiva y se ha propuesto para esculpir cosméticamente la región inferior de la cara. La toxina botulínica tipo A es una potente neurotoxina producida por el organismo anaerobio Clostridium botulinum y cuando se inyecta en un músculo causa una interferencia en el mecanismo neurotransmisor produciendo una parálisis selectiva y la consiguiente atrofia del músculo. Esta revisión es una actualización de una revisión Cochrane publicada previamente.
Objetivos
Evaluar la eficacia y la seguridad de la toxina botulínica tipo A en comparación con el placebo o la ausencia de tratamiento, para el tratamiento de la hipertrofia benigna del masetero bilateral.
Métodos de búsqueda
Se hicieron búsquedas en las siguientes bases de datos desde su inicio hasta abril de 2013: Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL); MEDLINE (vía PubMed); EMBASE (vía embase.com); Web of Science; CINAHL; Academic Search Premier (vía EBSCOhost); ScienceDirect; LILACS (vía BIREME); PubMed Central y Google Scholar (desde 1700 hasta el 19 de abril de 2013). Se realizaron búsquedas en dos bases de datos bibliográficas de revistas regionales (IndMED e Iranmedex) que se esperaba que contuvieran ensayos pertinentes. También se realizaron búsquedas en las listas de referencias de los artículos pertinentes y se estableció contacto con los investigadores para identificar estudios adicionales publicados y no publicados.
Criterios de selección
Se consideraron para su inclusión los ensayos clínicos controlados aleatorizados (ECA) y ensayos clínicos controlados (ECC) que comparen las inyecciones intramusculares en el masetero de toxina botulínica versus placebo administradas para la escultura facial cosmética en individuos de cualquier edad con hipertrofia del masetero bilateral benigna, que se habían autoevaluado y cuyo diagnóstico se confirmó mediante examen clínico y radiológico. Se excluyó a los participantes con hipertrofia del masetero unilateral o contralateral compensatoria secundaria a radioterapia de cabeza y cuello.
Obtención y análisis de los datos
Dos autores de la revisión analizaron los resultados de la búsqueda de forma independiente. Para futuras actualizaciones, dos autores extraerán los datos de forma independiente y evaluarán la calidad de los ensayos mediante la herramienta Cochrane de riesgo de sesgo. Se calcularán los riesgos relativos (RR) y los correspondientes intervalos de confianza (IC) del 95% para todos los resultados dicotómicos y se calculará la diferencia de medias (DM) y el IC del 95% para los resultados continuos.
Resultados principales
Se recuperaron 683 referencias únicas de estudios. Después de examinar estas referencias, 660 fueron excluidas por no ser aplicables. Se evaluaron 23 artículos de texto completo para determinar su elegibilidad y todos estos estudios se excluyeron de la revisión.
Conclusiones de los autores
No fue posible identificar ningún ECA o ECC que evaluara la eficacia y la seguridad de las inyecciones intramasetarias de toxina botulínica para las personas con hipertrofia benigna bilateral del masetero. La ausencia de evidencia de alto nivel sobre la eficacia de esta intervención pone de relieve la necesidad de ECA bien diseñados y con un poder de decisión adecuado.
PICO
Resumen en términos sencillos
Toxina botulínica tipo A para la hipertrofia del masetero
La hipertrofia del músculo masetero ocurre como un aumento de volumen blando de los músculos de la mandíbula cerca del ángulo inferior de la mandíbula y rara vez presenta un importante problema de salud. Sin embargo, en algunos individuos el edema puede asociarse con dolor o puede ser tan grande que causa desfiguración facial. Aunque la causa de la afección es incierta, sí parece ser más común en ciertos grupos étnicos.
Los síntomas como el dolor pueden ser tratados con relajantes musculares y también se puede incluir ajustes de la mordedura o el uso de férulas dentales. La reducción quirúrgica del músculo de la mandíbula o las inyecciones de toxina botulínica tipo A directamente al músculo son otras opciones de tratamiento. La toxina botulínica tipo A (TbA) es una neurotoxina potente producida por el organismo anaerobio Clostridium botulinum. Cuando se inyecta la toxina botulínica tipo A en un músculo, ésta causa una interferencia en el mecanismo neurotransmisor que produce una pérdida selectiva de la función muscular y la consiguiente disminución de la masa muscular.
Aunque el uso de las inyecciones de toxina botulínica podría parecer que tiene ciertas ventajas sobre la cirugía, los autores de esta revisión no encontraron ningún estudio de alta calidad que evaluara la efectividad y los posibles efectos secundarios de la toxina botulínica tipo A para el tratamiento de la hipertrofia benigna del masetero. Se necesitan ensayos controlados aleatorizados bien diseñados para evaluar la efectividad y la seguridad (es decir, los efectos secundarios) de esta intervención.
Authors' conclusions
Background
Aetiology and prevalence
Benign masseter muscle hypertrophy is an uncommon clinical phenomenon of uncertain aetiology. It is characterised by a soft swelling, near the angle of the mandible, which can be associated with facial pain. The hypertrophy can be prominent enough to be considered cosmetically disfiguring.
More than 250 cases of benign bilateral masseter muscle hypertrophy have been reported since its first published description (Legg 1880). Prevalence data are scarce but in a recent study (Sannomya 2006) 90 (4%) of the patients with masseter hypertrophy were less than 10 years old and 3% were over 40 years of age (mean 30 years), with a male to female ratio of 1:1.
The aetiology of masseter muscle hypertrophy has been attributed to a number of factors including: emotional stress, chronic bruxism, masseteric hyper‐function and para‐function, and microtrauma (Harriman 1996; Serrat 1998; Wilson 1990). It reportedly occurs most frequently among pacific Asians and is associated with ethnic characteristics (e.g. prominence of the mandibular angle) and dietary habits (Jin Park 2007). The findings of several investigators suggest that the increase in muscle size is not caused by work hypertrophy but as a result of compensatory enlargement due to lack of a certain type of muscle fibre (Jin Park 2007; Satoh 2001). Tests have shown that the composition of muscle fibres in the enlarged masseter is very different from that in muscles with 'work hypertrophy' as well as that in normal masseter muscles (Satoh 2001), suggesting that the term 'hypertrophy' could be potentially misleading.
Other possible causes and associations have been suggested including: clenbuterol induced hypertrophy, overuse of anabolic steroids (Skoura 2001), localised scleroderma and facial hemi‐atrophy (Kim 2000), and a multifactorial origin in combination with a genetic basis (Giudice 1992). Benign masseter hypertrophy is also compatible with a rare genetic condition known as hypertrophic branchial myopathy (Kitagawa 2000).
Description of the condition
Signs and symptoms
Bilateral enlargement of the masseter muscles is often accompanied by pain, which may be intermittent and can be confused with pain arising from the parotid gland (Newton 1999; Nishida 1995). Clinical examination usually reveals a soft tissue mass near the angle of the mandible, which becomes more prominent on clenching of the teeth (Sannomya 2006).
Limitation of the mouth opening has been reported in some cases and particularly where the muscles are focally dystonic with tension in the region of the hypertrophied muscle (Papapetropoulos 2006). Midline deviation has also been observed in some cases, as well as masseteric (hemi‐masticatory) spasm (Kim 2000). It has also been suggested that the hypertrophied muscles of the jaw can lead to increased pressure in the temporo‐mandibular joints (TMJ), which can generate severe pain and mimic temporo‐mandibular dysfunction syndrome (TMD) (Chikani 2003).
Diagnosis
Diagnosis cannot solely be based on clinical findings and there are conflicting recommendations in the literature for investigating patients presenting with benign bilateral masseter hypertrophy. These recommendations include the following.
-
Standard radiographs (not essential but can sometimes assist in diagnosis).
-
Computed tomographic (CT) scan, magnetic resonance imaging (MRI) scan, or both (considered the gold standard in confirming a clinical suspicion).
-
Muscle biopsy.
-
Morphometric analysis.
-
Ultrasonographic measurement.
-
Electromyographic measurement.
Description of the intervention
Treatment options
A range of treatment modalities have been reported with variable degrees of success and failure. Treatment options include the following.
-
Pharmacotherapy: anxiolytics, muscle relaxants and antidepressants.
-
Dental restorations and occlusal adjustments to correct premature contacts and malocclusions, and prevention of para‐functional habits with orthotic appliances.
-
Botulinum toxin type A injections into the muscle.
-
Radiofrequency volumetric reduction.
-
Intra‐oral and extra‐oral surgical reduction of masseter size, removal of mandibular angle, neurectomy of the masseteric nerve, and resection of the buccal fat pad.
How the intervention might work
Botulinum toxin type A (BtA) is a powerful neurotoxin produced by the anaerobic organism Clostridium botulinum. When BtA is injected into a muscle it causes interference with the neurotransmitter mechanism producing selective paralysis and subsequent atrophy of the muscle.
Why it is important to do this review
Surgery has historically been the standard treatment for cosmetic reduction of masseter hypertrophy, but injection of BtA into the muscle, which is generally considered to be a less invasive modality, has more recently been advocated. This systematic review is an update of a previously published Cochrane review (Al‐Muharraqi 2009).
Objectives
To assess the efficacy and safety of botulinum toxin type A compared to placebo or no treatment, for the management of benign bilateral masseter hypertrophy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and controlled clinical trials (CCTs) were considered for inclusion.
Types of participants
Individuals of any age with bilateral benign masseter hypertrophy which has been self‐evaluated and confirmed by clinical and radiological examination were considered for inclusion. In view of the possible clinical diversity in presentation, we excluded studies involving participants with unilateral or compensatory contra lateral masseter hypertrophy resulting from head and neck radiotherapy from this review.
Types of interventions
Interventions included transcutaneous intra‐masseteric injections of botulinum toxin versus placebo or no treatment. We sought to include studies in which the intervention had been administered for cosmetic facial sculpting. We considered studies involving a single injection cycle in addition to studies in which all participants entered in to a trial had received repeat injections at similar time periods.
Types of outcome measures
Assessment was to include a follow‐up period of up to two years after the intervention.
Primary outcomes
-
Self‐assessed improvement in facial appearance and patient satisfaction using any validated scale or questionnaire.
-
Patient‐assessed improvement in pain or discomfort associated with the temporo‐mandibular joints or jaw muscles using any recognised validated pain scale.
Secondary outcomes
-
Objective evaluation of the change in facial contour, involving physical measurement. Change in facial contour could be measured by clinical photography or radiological measurement which could include three‐dimensional computed tomographic (CT) scans, magnetic resonance (MR) imaging, or ultrasonographic measurements of the thickness of the masseter muscle.
-
Adverse events including any specific adverse effects, systemic or local toxicity, any clinically diagnosed hypersensitivity and other unacceptable events associated with this treatment.
Search methods for identification of studies
Electronic searches
Databases searched
We extended and updated the searches for the following databases:
-
MEDLINE (via Pubmed) (1950 to 19 April 2013). See Appendix 1
-
EMBASE (via embase.com) (from 1980 to 19 April 2013). See Appendix 2
-
Web of Science (from 1945 to 19 April 2013). See Appendix 3
-
the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 4). See Appendix 4
-
CINAHL (from 1982 to 19 April 2013). See Appendix 5
-
Academic Search Premier (via EBSCOhost) (from 1897 to 19 April 2013). See Appendix 6
-
ScienceDirect (from 1823 to 19 April 2013). See Appendix 7
-
LILACS (via BIREME) (from 1982 to 19 April 2013). See Appendix 8
-
PubMed Central (from 1809 to 19 April 2013). See Appendix 9
-
Google Scholar (from 1700 to 19 April 2013). See Appendix 10
Although we did not search the Cochrane Movement Disorders Group Trials Register we clarified that it did not contain any relevant studies.
We updated our search of IndMED, a bibliographic database of Indian journals, available at (http://indmed.nic.in/) and a similar Iranian database, Iranmedex, available at (www.iranmedex.com), using free text terms appropriate for this review on 27 April 2013.
Searching other resources
We did not conduct any handsearching of journals but searched the reference lists of relevant articles in addition to the review authors' personal database of trial reports. We also contacted a number of investigators by electronic mail to ask for details of additional published and unpublished trials. There were no language restrictions on included studies and we arranged to translate any relevant non‐English papers.
Data collection and analysis
Selection of studies
Two review authors (ZF and EvZ) independently assessed the abstracts of studies resulting from the searches. We obtained full copies of all relevant and potentially relevant studies, those appearing to meet the inclusion criteria, and those for which there were insufficient data in the title and abstract to make a clear decision.The two review authors independently assessed the full text papers and resolved any disagreement on the eligibility of included studies through discussion and consensus. We excluded all irrelevant records and noted details of the studies and the reasons for their exclusion in the 'Characteristics of excluded studies' table in RevMan 5.2 (RevMan 2012).
Data extraction and management
The following methods of data extraction and management will apply for subsequent updates, and when future studies are identified. We will enter study details into the 'Characteristics of included studies' table in RevMan 5.2. The review authors will collect outcome data using a pre‐determined form designed for this purpose. Two authors (ZF and EvZ) will enter extracted data into RevMan 5.2., data will only be included if there is an independently reached consensus.
We will extract the following details.
-
Trial methods: (a) method of allocation, (b) masking of participants, personnel and outcome assessors, (c) exclusion of participants after randomisation and proportion and reasons for losses at follow‐up.
-
Participants: (a) country of origin and location (i.e. private clinic or academic institute), (b) sample size, (c) age, (d) sex, (e) inclusion and exclusion criteria.
-
Intervention: (a) type, dosage, route of administration, (b) length of follow‐up.
-
Control: (a) type, dosage, route of administration, (b) length of follow‐up.
-
Outcomes: (a) primary and secondary outcomes pre‐specified in the 'Types of outcome measures' section of this review.
If stated, we will record the sources of funding of any of the included studies.
Assessment of risk of bias in included studies
If studies are included in future updates each review author will grade the selected trials and assess every trial using a simple contingency form following the domain‐based evaluation described in the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011). We will compare the evaluations and discuss and resolve any inconsistencies and disagreements.
The following domains will be rated separately for each of the included studies as 'low risk of bias', 'high risk of bias', and 'unclear' if the risk of bias was uncertain or unknown:
-
sequence generation;
-
allocation concealment;
-
blinding (of participants, personnel and outcome assessors);
-
incomplete outcome data;
-
selective outcome reporting;
-
other bias.
These assessments will be reported in the 'Risk of bias' table for each individual study in the 'Characteristics of included studies' section of the review.
We will also categorise and report the overall risk of bias of each of the included studies according to the following:
-
low risk of bias (plausible bias unlikely to seriously alter the results) if all criteria were met;
-
unclear risk of bias (plausible bias that raises some doubt about the results) if one or more criteria were assessed as unclear; or
-
high risk of bias (plausible bias that seriously weakens confidence in the results) if one or more criteria were not met.
These assessments will be reported in the 'Risk of bias in included studies' section of this review.
Measures of treatment effect
If studies are included in future updates we will conduct analysis at the same level as the allocation. We will calculate risk ratios (RR) and corresponding 95% confidence interval (CI) for all dichotomous outcomes and the mean difference (MD) and 95% CI for continuous outcomes. We will use RevMan 5 for data analysis. Unless stated otherwise, we will use the Mantel‐Haenzel method to calculate the RR for dichotomous outcomes and MD for continuous outcomes. As it is likely that the timing of outcome assessment will vary between studies we will consider grouping the data according to the following time‐points: six months, one year and two years.
Unit of analysis issues
We expect to include trials of participants with bilateral hypertrophy in which the masseter muscles of an individual participant were the units of randomisation and subsequent analysis. We will analyse these data based on the advice provided in sections 9.3.8 in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
If studies are included in future updates we will make attempts to retrieve missing data from the investigators of the included trials, and if unsuccessful or the discrepancies are significant, we will provide a narrative synthesis of the data as reported.
Assessment of heterogeneity
If studies are included in future updates we will assess clinical heterogeneity by examining the characteristics of the studies and the similarity between types of participants, interventions and outcomes. In view of the expectation of a degree of clinical heterogeneity between the studies we will use a random‐effects model for statistical analyses. Statistical heterogeneity will be assessed using the Chi2 test and the I2 statistic, where I2 values over 50% indicate moderate to high heterogeneity (Higgins 2011).
Assessment of reporting biases
if future updates include studies we will follow the recommendations on testing for funnel plot asymmetry as described in section 10.4.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011) to assess publication bias.
Data synthesis
If future updates include studies two review authors (ZF and EvZ) will analyse the data and report the results as specified in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Higgins 2011). Where appropriate, we will calculate the pooled RR and 95% CI for dichotomous outcomes and the pooled MD and 95% CI for continuous outcomes. We will use the Mantel‐Haenzel method for combining results across studies using random‐effects models. In the event that there are insufficient clinically homogeneous trials for any specific intervention or insufficient study data that can be pooled, we will present a narrative synthesis.
Subgroup analysis and investigation of heterogeneity
If studies are included in future updates we will perform subgroup analysis by dose (i.e. low dose of BtA compared to medium or high doses). We define a low dose of BtA as ≤ 150 U per muscle and a medium to high dose as > 150 U per muscle.
Sensitivity analysis
If future updates contain a sufficient number of included studies we plan to conduct sensitivity analyses to assess the robustness of our review results. We will do this by repeating the analyses excluding studies with unclear or inadequate allocation concealment, blinding of outcomes assessment and completeness of follow‐up.
Results
Description of studies
Results of the search
A literature search conducted on April 19, 2013 identified 1147 records. After duplicates were removed, a total of 683 references remained for review of titles and abstracts (PubMed 258, EMBASE 361 (34 abstracts to conference proceedings), from which 181 unique, Web of Science: 181 (8 abstracts to conference proceedings), from which 41 unique, CENTRAL 105, from which 97 unique, CINAHL 23, from which 3 unique, Academic Search Premier 55, from which 11 unique, ScienceDirect: 47, from which 10 unique, LILACS: 5, from which 3 unique, PubMed Central/PMC: 54, from which 48 unique, Google Scholar: unknown set, 13 unique), citations to Cochrane Review: through Web of Science: 2, from which 1 unique, citations from Cochrane Review: through Web of Science: 30, from which 11 unique and citations to Cochrane Review: through Google Scholar: 13, from which 6 unique). After examination of the titles and abstracts of these references, we eliminated all but 23 and excluded them from further review (see Figure 1). We obtained full text copies of those remaining studies, translated them into the English language as required and subjected them to further evaluation. We examined the bibliographical references of these studies and, as with our searches of the IndMED and Iranmedex databases, they did not provide any further citations to potentially eligible studies. Two authors, Zbys Fedorowicz (ZF) and Esther van Zuuren (EvZ), independently assessed all of the full text papers, and resolved any disagreement on their eligibility for this review through discussion and consensus.
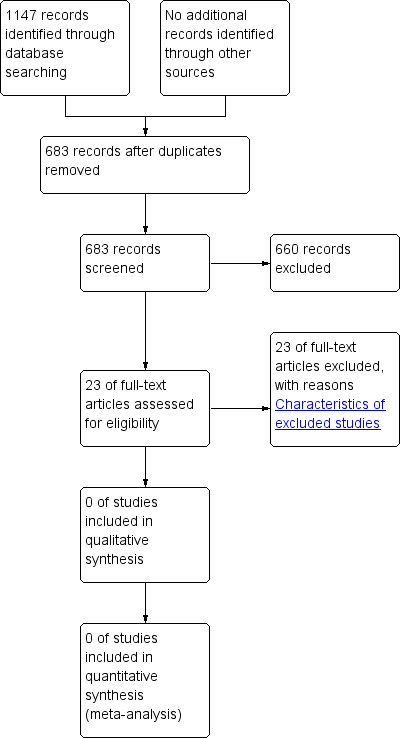
Study flow diagram.
Included studies
We retrieved a number of studies in our comprehensive search of the literature but none were eligible and therefore no trials were included in this review.
Excluded studies
We excluded all records which did not match our inclusion criteria and noted the reasons for their exclusion in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
A risk of bias analysis was not carried out because no trials were included in the review.
Effects of interventions
None of the studies retrieved in our searches met our inclusion criteria and therefore no data were available for analysis.
Discussion
The comprehensive search used in this review provided a large number of references to trials and thus the lack of relevant randomised controlled trials or controlled clinical trials as well as any robust evidence to support or refute the effectiveness of botulinum toxin for masseter hypertrophy, proved to be somewhat disappointing. Over the last 10 years a number of case reports and several recent cohort studies have sought to illustrate the effectiveness of botulinum toxin type A injections for benign masseter muscle hypertrophy, but questions remain unanswered as to whether management options based on this intervention can be considered both effective and safe.

Study flow diagram.

