Intervenciones quirúrgicas para el tratamiento temprano de la parálisis de Bell
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007468.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 26 enero 2021see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Neuromuscular
- Copyright:
-
- Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
IM assessed risk of bias, certainty of evidence, undertook data collection and analysis, and wrote the review.
KMcA devised the search strategy, designed the protocol, assessed risk of bias, undertook data collection and analysis, and wrote the review.
DW devised the search strategy, designed the protocol, assessed risk of bias, undertook data collection and analysis, and wrote the review.
PTD provided statistical knowledge and expertise required for the protocol and review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
None, Other
Declarations of interest
IM: none.
KMcA: none.
DW: none.
PTD has received grants from AbbVie, Gilead Sciences and SHIRE. Prof Donnan is a member of the New Drugs Committee of the Scottish Medicines Consortium.
Acknowledgements
Angela Gunn and Farhad Shokareh (FS), the Information Specialists of the Cochrane Neuromuscular Disease Group performed the searches. FS screened 2020 searches for RCTs for this update.
The Managing Editor of Cochrane Neuromuscular, Ruth Brassington, assisted with updating the methods section, based on a standard protocol developed by the group from an original created by Cochrane Airways.
Andrea Takeda acted as an independent systematic reviewer and extracted data for the newly included study and documented any differences between the extractions.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health. Cochrane Neuromuscular is also supported by the MRC Centre for Neuromuscular Disease.
With thanks to Jie Zhou, Department of Neurology, West China Hospital of Sichuan University, China and Ning Chen of West China Hospital of Sichuan University who kindly assisted with translations of Chinese studies.
Version history
| Published | Title | Stage | Authors | Version |
| 2021 Jan 26 | Surgical interventions for the early management of Bell's palsy | Review | Isabella Menchetti, Kerrie McAllister, David Walker, Peter T Donnan | |
| 2013 Oct 16 | Surgical interventions for the early management of Bell's palsy | Review | Kerrie McAllister, David Walker, Peter T Donnan, Iain Swan | |
| 2011 Feb 16 | Surgical interventions for the early management of Bell's palsy | Review | Kerrie McAllister, David Walker, Peter T Donnan, Iain Swan | |
| 2008 Oct 08 | Surgical interventions for the early management of Bell's palsy | Protocol | Iain Swan, Peter Donnan, Kerrie McAllister, David Walker | |
Differences between protocol and review
We have updated the 'Risk of bias' methodology since the protocol was published, in order to conform to the 2011 Cochrane methodology (Higgins 2011). We also added 'Summary of findings' tables and specified outcomes for inclusion in the table, in the Methods. We stated the comparisons that we would consider for inclusion and clarified this in the Methods.
We included searches of trials registries.
In this update, we also expanded most sections of the methods to fulfil MECIR reporting standards. We reported methods for use if meta‐analysis becomes possible, in Appendix 3. Changes to the protocol included the following.
-
Types of outcomes: clarified that the measurement of outcomes was not a selection criterion, but the outcomes were those of interest in eligible trials. We better defined 'psychosocial outcomes' by specifying the preferred measurement scales for both mood and appearance.
-
Primary outcome: expanded on to include justification for only reporting on and calculating a risk ratio for complete recovery data in both groups, for both comparisons.
-
Selection of studies: the Cochrane Neuromuscular Information Specialist performed an initial screen of titles and abstracts for randomised controlled trials.
-
Searching other resources: we did not contact the authors of all included trials for further additional information or for information on unpublished trials. We did not perform handsearching.
-
Data extraction and management: described an approach to papers requiring translation. An independent systematic reviewer performed the second data extraction for the newly included study and we resolved differences via discussion between lead author IM and review author KM.
-
Measures of treatment effect: describes use of standardised mean difference if studies report different scales for conceptually the same outcome.
-
Unit of analysis issues: described the approach to multi‐arm trials.
-
Dealing with missing data: clarified that no authors were contacted in relation to missing data.
-
Assessment of heterogeneity: included a comment on interpretation of Chi² statistic.
-
Assessment of reporting biases: described an approach to identification and reporting of unpublished studies, to analysis of reporting bias in the review. We also noted that funnel plots required 10 trials.
-
Data synthesis: specified comparisons more clearly and described an approach to combining data; described GRADE methodology in more detail. We stated that we would report random‐effects analyses, with sensitivity analysis using the fixed‐effect model.
-
Sensitivity analysis: stated that we would perform sensitivity analyses using risk of bias (previously 'quality') and specified a sensitivity analysis for fixed‐effect versus random‐effects models if heterogeneity was present.
Iain Swan withdrew from authorship. Isabella Menchetti joined the review as first author.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
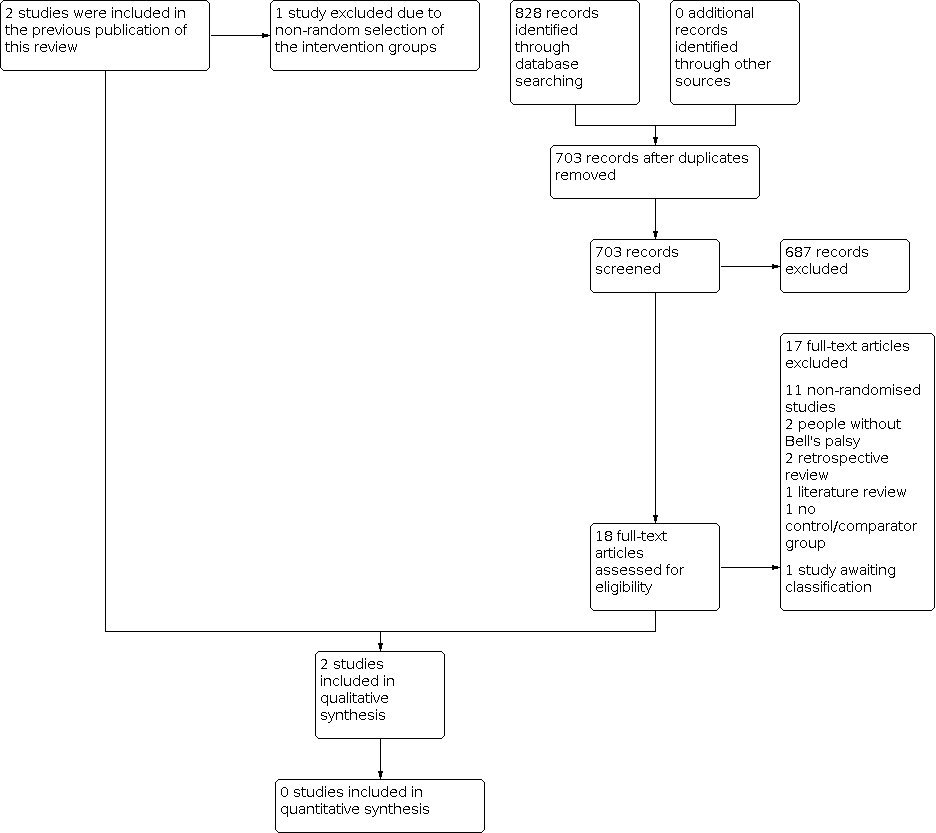
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Red = high risk of bias, yellow = unclear risk of bias and green = low risk of bias.

Comparison 1: Early surgery versus no treatment, Outcome 1: Complete recovery at 12 months

Comparison 1: Early surgery versus no treatment, Outcome 2: Complete recovery at 3 months

Comparison 1: Early surgery versus no treatment, Outcome 3: Complete recovery at 6 months

Comparison 1: Early surgery versus no treatment, Outcome 4: Synkinesis

Comparison 1: Early surgery versus no treatment, Outcome 5: Contractures

Comparison 2: Early surgery versus late surgery, Outcome 1: Complete recovery at 12 months

Comparison 2: Early surgery versus late surgery, Outcome 2: Complete recovery at 3 months

Comparison 2: Early surgery versus late surgery, Outcome 3: Complete recovery at 6 months
| Surgery compared with medical treatment for Bell's palsy | ||||||
| Patient or population: Bell's palsy Settings: hospital attendance with idiopathic facial paralysis Intervention: early surgery (within 3 months from denervation onset) Comparison: no surgical treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| No treatment | Early surgery | |||||
| Complete recovery of facial nerve function at 12 months Defined as: complete recovery HB grade I. Follow‐up: 12 months 1st study: scale 0–5 (0 = no function, 5 = complete function for 3 facial muscles) 2nd study: HBGS (complete = HB I, fair = HB II–III and poor recovery = HB IV–VI) | The 1st study (24 evaluable participants) compared surgery between the 2nd and 3rd week post onset to a no‐treatment control group. The 2nd study (33 evaluable participants) compared early surgery (within 2–3 months post paralysis onset) to no further treatment. Neither study reported a statistically significant difference in recovery of facial nerve function between the surgical and non‐surgical groups at 12 months (P > 0.05). We calculated the RR for complete recovery at 12 months for the 2nd study; 1/18 participants in the early surgery group achieved complete recovery at 12 months compared to 1/15 in the no‐treatment group (RR 0.76, 95% CI 0.05 to 11.11; P = 0.84). | 57 | ⊕⊝⊝⊝ | We are uncertain whether surgery affects recovery of facial nerve function at 1 year as the certainty of evidence was very low. 1 study did not perform statistical analysis, 1 did not state the method used. 1 study did not directly report 12‐month results. Different outcome measures in each study made combining results impractical. | ||
| Side effects and complications of treatment | The 1st study (24 evaluable participants) reported no complications of surgery. The 2nd study (41 evaluable participants) reported postoperative complications such as sensorineural hearing loss, tinnitus, vertigo and dizziness. There were no reported surgical complications such as wound dehiscence, infection, bleeding and numbness. 4 participants had sensorineural hearing loss at high frequencies, with bone conduction thresholds ranging from 35 dB to 50 dB at 4000 Hz. 3 participants reported tinnitus. There were no reported cases of postoperative vertigo or major labyrinthitis. | 57 | ⊕⊝⊝⊝ | The numbers involved in the included studies were small. Statistical analysis was not possible. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HB: House‐Brackmann; HBGS: House‐Brackmann grading system; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded twice because of very serious limitations in study design: 1 study did not report the method of randomisation. Both studies had unclear allocation concealment. Blinding of participants was not possible and neither study blinded outcome assessors. 1 study did not follow up large numbers of participants. We further downgraded the evidence for imprecision as there were small numbers of participants in both studies. 1 study also reported the primary and secondary facial nerve recovery outcomes of this review indirectly, which warranted further downgrading for indirectness. | ||||||
| Early surgery compared with late surgery for Bell's palsy | ||||||
| Patient or population: people with Bell's palsy Settings: hospital attendance with idiopathic facial paralysis Intervention: early surgery (within 3 months from denervation onset) Comparison: late surgery (later than 3 months from denervation onset) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Late surgery | Early surgery | |||||
| Complete recovery of facial nerve function at 12 months Defined as: complete recovery HB grade I. Follow‐up: 12 months 1st study: scale 0–5 (0 = no function, 5 = complete function for 3 facial muscles) 2nd study: HBGS (complete = HB I, fair = HB II–III, and poor recovery = HB IV–VI) | Within the 1 study (33 evaluable participants), 18 undergoing surgery within 2–3 months of denervation (early surgery) were compared with 8 participants undergoing surgery > 3 months from denervation onset (late surgery). There was no statistically significant difference in recovery of facial nerve function between the early surgical and late surgical groups at 12 months (P > 0.05). We calculated the RR for complete recovery at 12 months; 1/18 participants in the early surgery group achieved complete recovery at 12 months compared to 1/8 in the late surgery group. | RR 0.47 (0.03 to 6.60) | 26 (1 RCT) | ⊕⊝⊝⊝ | The evidence for recovery of facial nerve function with early vs late surgery was uncertain. | |
| Side effects and complications of treatment | The 1 study (41 evaluable participants) reported postoperative complications such as sensorineural hearing loss, tinnitus, vertigo and dizziness. There were no reported surgical complications such as wound dehiscence, infection, bleeding and numbness. 4 participants had sensorineural hearing loss at high frequencies, with bone conduction thresholds ranging from 35 dB to 50 dB at 4000 Hz. 3 participants reported tinnitus. There were no reported cases of postoperative vertigo or major labyrinthitis. | Not reported | 26 (1 RCT) | Unable to rate certainty of evidence | The trial that compared early and late surgery did not report adverse events separately by timing of surgery. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HB: House‐Brackmann; HBGS: House‐Brackmann grading system; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded three times: twice because of very serious limitations in study design (high risk of bias in most domains) and once for imprecision from small‐study size (26 participants). | ||||||
| Risk of bias | Interpretation | Within study | Across studies |
|---|---|---|---|
| Low risk of bias | Plausible bias unlikely to seriously alter the results. | Low risk of bias for all key domains. | Most information is from studies at low risk of bias. |
| Unclear risk of bias | Plausible bias that raises some doubt about the results. | Unclear risk of bias for ≥ 1 key domains. | Most information is from studies at low or unclear risk of bias. |
| High risk of bias | Plausible bias that seriously weakens the confidence in the results. | High risk of bias for ≥ 1 key domains. | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results. |
| Approach for summary assessments of the risk of bias for each important outcome (across domains) within and across studies (Higgins 2011). | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Complete recovery at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2 Complete recovery at 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 Complete recovery at 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4 Synkinesis Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5 Contractures Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Complete recovery at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 Complete recovery at 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3 Complete recovery at 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

