Intervenciones quirúrgicas para el tratamiento temprano de la parálisis de Bell
Appendices
Appendix 1. ClinicalTrials.gov
Advanced search
Condition or disease: "Bell's palsy" OR "Bell palsy" OR "idiopathic facial paralysis" OR "facial paralysis" OR "facial palsy" OR "facial nerve"
Study type: Interventional Studies (Clinical Trials)
Intervention/treatment: "surgery" OR "surg*" OR "operative" OR "operat*" OR "decompression" OR "decompres"
17 studies
Appendix 2. World Health Organization International Clinical Trials Registry Platform (ICTRP)
Advanced search
(Bell's palsy OR Bell palsy OR idiopathic facial paralysis OR facial paralysis OR facial palsy OR facial nerve) in the Title
Recruitment status is ALL
107 records for 106 trials found
Appendix 3. Additional methods
Measures of treatment effect
If studies reported outcomes that were conceptually the same but were measured using different scales, we would have reported standardised mean differences with corresponding confidence intervals, ensuring that we entered data presented on a scale with a consistent direction of effect. We would have presented a rule‐of‐thumb interpretation alongside, whereby 0.2 represents a small effect, 0.5 a moderate effect and 0.8 a large effect (Cohen 1988), as described in Schünemann 2020b.
Unit of analysis issues
If in future updates two comparisons from the same trial (e.g. intervention A versus placebo and intervention B versus the same placebo group) are combined in the same meta‐analysis, we will follow guidance in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions to avoid double‐counting measurements. Our preferred approach will be to combine intervention groups if clinically appropriate, or if not, halve a control group (Higgins 2020).
Assessment of heterogeneity
There were insufficient studies to enable any statistical analysis. If we find sufficient studies at future updates, we will perform a Chi² test for homogeneity. If there is significant heterogeneity, we will attempt to identify the cause based on the characteristics of the studies included.
If we identify substantial unexplained heterogeneity, we will report it and explore possible causes by prespecified subgroup analysis. We will use the rough guide to interpretation as outlined in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions, as follows:
-
0% to 40%: might not be important;
-
30% to 60%: may represent moderate heterogeneity;
-
50% to 90%: may represent substantial heterogeneity;
-
75% to 100%: considerable heterogeneity.
We will avoid the use of absolute cut‐off values, but interpret the Chi² statistic in relation to the size and direction of effects and strength of evidence for heterogeneity (e.g. P value from the Chi² test, or CI for I² statistic) (Deeks 2020).
Assessment of reporting biases
If we can pool more than 10 trials, we will create and examine a funnel plot to explore possible small‐study biases.
Data synthesis
If meta‐analysis is possible in future updates, as a general rule, we will use a random‐effects model in Review Manager 5 (Review Manager 2014). Random‐effects models such as DerSimonian and Laird account for more uncertainty (DerSimonian 1986). We will perform a sensitivity analysis using the fixed‐effect model in the presence of heterogeneity, to determine whether results are systematically different.
Subgroup analysis and investigation of heterogeneity
We did not plan subgroup analyses.
Sensitivity analysis
If statistical analysis becomes possible in the future, we will perform a sensitivity analysis omitting studies of high risk of bias. In addition, risk of bias could be incorporated into mixed models simultaneously allowing for differences in bias using Bayesian methods, utilised in WinBUGS (Spiegelhalter 2000).
We will assess the effects of fixed‐effect versus random‐effects meta‐analysis if there is statistical heterogeneity.
Appendix 4. Embase (OvidSP) search strategy
Database: Embase <1974 to 2020 week 11>
Search strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 crossover‐procedure.sh. (62415)
2 double‐blind procedure.sh. (170250)
3 single‐blind procedure.sh. (38181)
4 randomized controlled trial.sh. (593541)
5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (1749674)
6 trial.ti. (292985)
7 clinical trial/ (966802)
8 or/1‐7 (2479571)
9 (animal/ or nonhuman/ or animal experiment/) and human/ (1928466)
10 animal/ or nonanimal/ or animal experiment/ (3929237)
11 10 not 9 (3270546)
12 8 not 11 (2315974)
13 limit 12 to (conference abstracts or embase) (1949659)
14 ((bell$ or facial or hemifacial$) adj3 (pals$ or paralys$ or paresi$ or spasm$)).mp. (29285)
15 (surg$ or operat$ or decompres$).mp. (4763691)
16 ((bell$ or facial or hemifacial$) adj3 (pals$ or paralys$ or paresi$ or spasm$) adj5 (surg$ or operat$ or decompres$)).mp. (2225)
17 Bell Palsy/su or Facial Nerve Paralysis/su or Hemifacial Spasm/su (2309)
18 16 or 17 (3745)
19 13 and 18 (151)
20 remove duplicates from 19 (150)
Appendix 5. Cochrane Neuromuscular Specialised Register (CRS Web) search strategy
#1 MESH DESCRIPTOR Bell Palsy WITH QUALIFIER SU AND INREGISTER 2
#2 MESH DESCRIPTOR facial paralysis WITH QUALIFIER SU AND INREGISTER 4
#3 MESH DESCRIPTOR Hemifacial Spasm WITH QUALIFIER SU AND INREGISTER 1
#4 (bell NEAR2 palsy) or (facial NEAR2 palsy) or (facial NEAR2 paralysis) or (facial NEAR2 paresis) AND INREGISTER 171
#5 (hemifacial NEAR2 palsy) OR (hemifacial NEAR2 paralysis) OR (hemifacial NEAR2 paresis) AND INREGISTER 2
#6 (facial NEAR2 palsy) or (facial NEAR2 paralysis) or (facial NEAR2 paresis) AND INREGISTER 137
#7 #4 OR #5 OR #6 171
#8 (surg* or operati* or decompressi*):AB,EH,EMT,KW,KY,MH,TI AND INREGISTER 956
#9 #8 AND #7 20
#10 #1 OR #2 OR #3 OR #9 21
Appendix 6. Cochrane Central Register of Controlled Trials (CENTRAL) (CRS Web) search strategy
#1 MESH DESCRIPTOR Bell Palsy WITH QUALIFIER SU AND CENTRAL:TARGET 2
#2 MESH DESCRIPTOR facial paralysis WITH QUALIFIER SU AND CENTRAL:TARGET 10
#3 MESH DESCRIPTOR Hemifacial Spasm WITH QUALIFIER SU AND CENTRAL:TARGET 4
#4 (bell NEAR2 palsy) or (facial NEAR2 palsy) or (facial NEAR2 paralysis) or (facial NEAR2 paresis) AND CENTRAL:TARGET 778
#5 (hemifacial NEAR2 palsy) OR (hemifacial NEAR2 paralysis) OR (hemifacial NEAR2 paresis) AND CENTRAL:TARGET 5
#6 (facial NEAR2 palsy) or (facial NEAR2 paralysis) or (facial NEAR2 paresis) AND CENTRAL:TARGET 692
#7 #4 OR #5 OR #6 AND CENTRAL:TARGET 779
#8 (surg* or operati* or decompressi*):AB,EH,EMT,KW,KY,MH,TI AND CENTRAL:TARGET 266584
#9 #8 AND #7 AND CENTRAL:TARGET 164
#10 #1 OR #2 OR #3 OR #9 AND CENTRAL:TARGET 168
Appendix 7. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) ALL <1946 to 19 March 2020>
Search strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 randomized controlled trial.pt. (502288)
2 controlled clinical trial.pt. (93580)
3 randomized.ab. (473646)
4 placebo.ab. (206169)
5 drug therapy.fs. (2188387)
6 randomly.ab. (329654)
7 trial.ab. (498947)
8 groups.ab. (2024489)
9 or/1‐8 (4661504)
10 exp animals/ not humans.sh. (4680615)
11 9 not 10 (4040593)
12 ((bell$ or facial or hemifacial$) adj3 (pals$ or paralys$ or paresi$ or spasm$)).mp. (20548)
13 (surg$ or operat$ or decompres$).mp. (3668969)
14 ((bell$ or facial or hemifacial$) adj3 (pals$ or paralys$ or paresi$ or spasm$) adj5 (surg$ or operat$ or decompres$)).mp. (1735)
15 bell palsy/su or facial paralysis/su or hemifacial spasm/su (2855)
16 14 or 15 (3781)
17 11 and 16 (367)
18 remove duplicates from 17 (366)
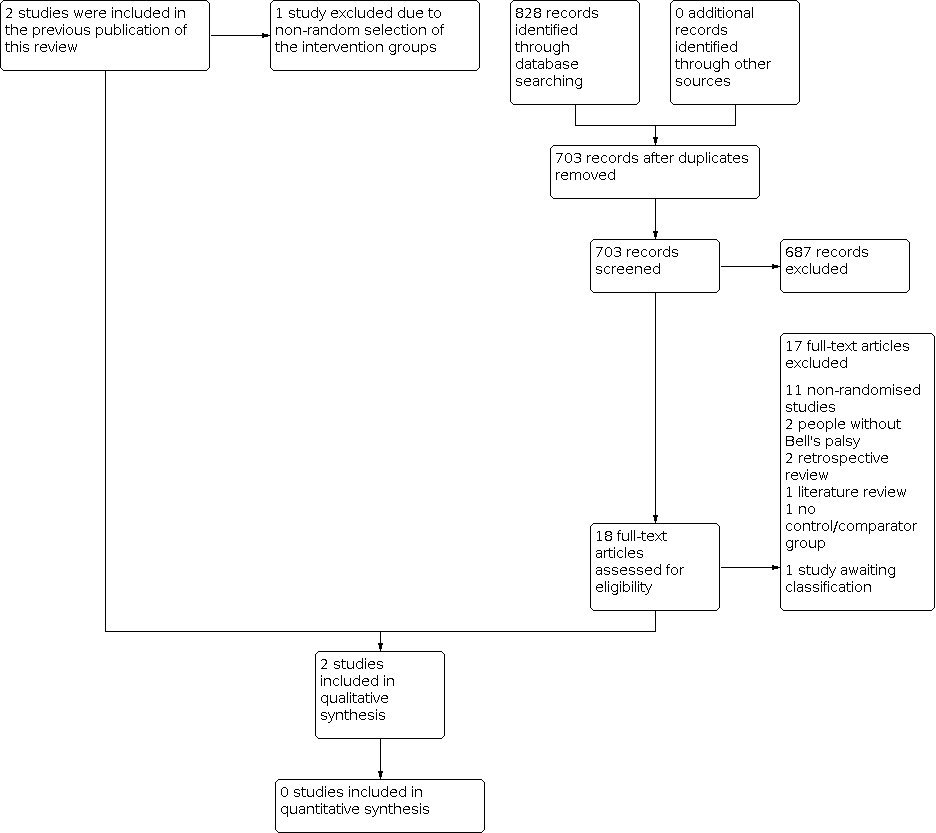
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Red = high risk of bias, yellow = unclear risk of bias and green = low risk of bias.

Comparison 1: Early surgery versus no treatment, Outcome 1: Complete recovery at 12 months

Comparison 1: Early surgery versus no treatment, Outcome 2: Complete recovery at 3 months

Comparison 1: Early surgery versus no treatment, Outcome 3: Complete recovery at 6 months

Comparison 1: Early surgery versus no treatment, Outcome 4: Synkinesis

Comparison 1: Early surgery versus no treatment, Outcome 5: Contractures

Comparison 2: Early surgery versus late surgery, Outcome 1: Complete recovery at 12 months

Comparison 2: Early surgery versus late surgery, Outcome 2: Complete recovery at 3 months

Comparison 2: Early surgery versus late surgery, Outcome 3: Complete recovery at 6 months
| Surgery compared with medical treatment for Bell's palsy | ||||||
| Patient or population: Bell's palsy Settings: hospital attendance with idiopathic facial paralysis Intervention: early surgery (within 3 months from denervation onset) Comparison: no surgical treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| No treatment | Early surgery | |||||
| Complete recovery of facial nerve function at 12 months Defined as: complete recovery HB grade I. Follow‐up: 12 months 1st study: scale 0–5 (0 = no function, 5 = complete function for 3 facial muscles) 2nd study: HBGS (complete = HB I, fair = HB II–III and poor recovery = HB IV–VI) | The 1st study (24 evaluable participants) compared surgery between the 2nd and 3rd week post onset to a no‐treatment control group. The 2nd study (33 evaluable participants) compared early surgery (within 2–3 months post paralysis onset) to no further treatment. Neither study reported a statistically significant difference in recovery of facial nerve function between the surgical and non‐surgical groups at 12 months (P > 0.05). We calculated the RR for complete recovery at 12 months for the 2nd study; 1/18 participants in the early surgery group achieved complete recovery at 12 months compared to 1/15 in the no‐treatment group (RR 0.76, 95% CI 0.05 to 11.11; P = 0.84). | 57 | ⊕⊝⊝⊝ | We are uncertain whether surgery affects recovery of facial nerve function at 1 year as the certainty of evidence was very low. 1 study did not perform statistical analysis, 1 did not state the method used. 1 study did not directly report 12‐month results. Different outcome measures in each study made combining results impractical. | ||
| Side effects and complications of treatment | The 1st study (24 evaluable participants) reported no complications of surgery. The 2nd study (41 evaluable participants) reported postoperative complications such as sensorineural hearing loss, tinnitus, vertigo and dizziness. There were no reported surgical complications such as wound dehiscence, infection, bleeding and numbness. 4 participants had sensorineural hearing loss at high frequencies, with bone conduction thresholds ranging from 35 dB to 50 dB at 4000 Hz. 3 participants reported tinnitus. There were no reported cases of postoperative vertigo or major labyrinthitis. | 57 | ⊕⊝⊝⊝ | The numbers involved in the included studies were small. Statistical analysis was not possible. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HB: House‐Brackmann; HBGS: House‐Brackmann grading system; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded twice because of very serious limitations in study design: 1 study did not report the method of randomisation. Both studies had unclear allocation concealment. Blinding of participants was not possible and neither study blinded outcome assessors. 1 study did not follow up large numbers of participants. We further downgraded the evidence for imprecision as there were small numbers of participants in both studies. 1 study also reported the primary and secondary facial nerve recovery outcomes of this review indirectly, which warranted further downgrading for indirectness. | ||||||
| Early surgery compared with late surgery for Bell's palsy | ||||||
| Patient or population: people with Bell's palsy Settings: hospital attendance with idiopathic facial paralysis Intervention: early surgery (within 3 months from denervation onset) Comparison: late surgery (later than 3 months from denervation onset) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Late surgery | Early surgery | |||||
| Complete recovery of facial nerve function at 12 months Defined as: complete recovery HB grade I. Follow‐up: 12 months 1st study: scale 0–5 (0 = no function, 5 = complete function for 3 facial muscles) 2nd study: HBGS (complete = HB I, fair = HB II–III, and poor recovery = HB IV–VI) | Within the 1 study (33 evaluable participants), 18 undergoing surgery within 2–3 months of denervation (early surgery) were compared with 8 participants undergoing surgery > 3 months from denervation onset (late surgery). There was no statistically significant difference in recovery of facial nerve function between the early surgical and late surgical groups at 12 months (P > 0.05). We calculated the RR for complete recovery at 12 months; 1/18 participants in the early surgery group achieved complete recovery at 12 months compared to 1/8 in the late surgery group. | RR 0.47 (0.03 to 6.60) | 26 (1 RCT) | ⊕⊝⊝⊝ | The evidence for recovery of facial nerve function with early vs late surgery was uncertain. | |
| Side effects and complications of treatment | The 1 study (41 evaluable participants) reported postoperative complications such as sensorineural hearing loss, tinnitus, vertigo and dizziness. There were no reported surgical complications such as wound dehiscence, infection, bleeding and numbness. 4 participants had sensorineural hearing loss at high frequencies, with bone conduction thresholds ranging from 35 dB to 50 dB at 4000 Hz. 3 participants reported tinnitus. There were no reported cases of postoperative vertigo or major labyrinthitis. | Not reported | 26 (1 RCT) | Unable to rate certainty of evidence | The trial that compared early and late surgery did not report adverse events separately by timing of surgery. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HB: House‐Brackmann; HBGS: House‐Brackmann grading system; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded three times: twice because of very serious limitations in study design (high risk of bias in most domains) and once for imprecision from small‐study size (26 participants). | ||||||
| Risk of bias | Interpretation | Within study | Across studies |
|---|---|---|---|
| Low risk of bias | Plausible bias unlikely to seriously alter the results. | Low risk of bias for all key domains. | Most information is from studies at low risk of bias. |
| Unclear risk of bias | Plausible bias that raises some doubt about the results. | Unclear risk of bias for ≥ 1 key domains. | Most information is from studies at low or unclear risk of bias. |
| High risk of bias | Plausible bias that seriously weakens the confidence in the results. | High risk of bias for ≥ 1 key domains. | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results. |
| Approach for summary assessments of the risk of bias for each important outcome (across domains) within and across studies (Higgins 2011). | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Complete recovery at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2 Complete recovery at 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 Complete recovery at 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4 Synkinesis Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5 Contractures Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Complete recovery at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 Complete recovery at 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3 Complete recovery at 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

