Topical NSAIDs for acute musculoskeletal pain in adults
Abstract
Background
Use of topical NSAIDs to treat acute musculoskeletal conditions has become widely accepted because they can provide pain relief without associated systemic adverse events. This review is an update of 'Topical NSAIDs for acute pain in adults' originally published in Issue 6, 2010.
Objectives
To determine the efficacy and safety of topically applied NSAIDs in acute musculoskeletal pain in adults.
Search methods
We searched the Cochrane Register of Studies Online, MEDLINE, and EMBASE to February 2015. We sought unpublished studies by asking personal contacts and searching online clinical trial registers and manufacturers websites. For the earlier review, we also searched our own in‐house database and contacted manufacturers.
Selection criteria
We included randomised, double‐blind, active or placebo (inert carrier)‐controlled trials in which treatments were administered to adults with acute pain resulting from strains, sprains or sports or overuse‐type injuries (twisted ankle, for instance). There had to be at least 10 participants in each treatment arm, with application of treatment at least once daily.
Data collection and analysis
Two review authors independently assessed studies for inclusion, and extracted data. We used numbers of participants achieving each outcome to calculate the risk ratio and numbers needed to treat for an additional beneficial outcome (NNT) or additional harmful outcome (NNH) compared with placebo or other active treatment. We reported 95% confidence intervals (CI). We were particularly interested to compare different formulations (gel, cream, plaster) of individual NSAIDs.
Main results
For this update we added 14 new included studies (3489 participants), and excluded four studies. We also identified 20 additional reports of completed or ongoing studies that have not been published in full. The earlier review included 47 studies.
This update included 61 studies. Most compared topical NSAIDs in the form of a gel, spray, or cream with a similar topical placebo; 5311 participants were treated with a topical NSAID, 3470 with placebo, and 220 with an oral NSAID. This was a 63% increase in the number of included participants over the previous version of this review. We also identified a number of studies in clinical trial registries with unavailable results amounting to about 5900 participants for efficacy and 5300 for adverse events.
Formulations of topical diclofenac, ibuprofen, ketoprofen, piroxicam, and indomethacin demonstrated significantly higher rates of clinical success (more participants with at least 50% pain relief) than matching topical placebo (moderate or high quality data). Benzydamine did not. Three drug and formulation combinations had NNTs for clinical success below 4. For diclofenac, the Emulgel® formulation had the lowest NNT of 1.8 (95% CI 1.5 to 2.1) in two studies using at least 50% pain intensity reduction as the outcome. Diclofenac plasters other than Flector® also had a low NNT of 3.2 (2.6 to 4.2) based on good or excellent responses in some studies. Ketoprofen gel had an NNT of 2.5 (2.0 to 3.4), from five studies in the 1980s, some with less well defined outcomes. Ibuprofen gel had an NNT of 3.9 (2.7 to 6.7) from two studies with outcomes of marked improvement or complete remission. All other drug and formulation combinations had NNT values above 4, indicating lesser efficacy.
There were insufficient data to compare reliably individual topical NSAIDs with each other or the same oral NSAID.
Local skin reactions were generally mild and transient, and did not differ from placebo (high quality data). There were very few systemic adverse events (high quality data) or withdrawals due to adverse events (low quality data).
Authors' conclusions
Topical NSAIDs provided good levels of pain relief in acute conditions such as sprains, strains and overuse injuries, probably similar to that provided by oral NSAIDs. Gel formulations of diclofenac (as Emugel®), ibuprofen, and ketoprofen, and some diclofenac patches, provided the best effects. Adverse events were usually minimal.
Since the last version of this review, the new included studies have provided additional information. In particular, information on topical diclofenac is greatly expanded. The present review supports the previous review in concluding that topical NSAIDs are effective in providing pain relief, and goes further to demonstrate that certain formulations, mainly gel formulations of diclofenac, ibuprofen, and ketoprofen, provide the best results. Large amounts of unpublished data have been identified, and this could influence results in updates of this review.
PICO
Plain language summary
Topical non‐steroidal anti‐inflammatory drugs for acute musculoskeletal pain in adults
Acute musculoskeletal pain describes conditions like a sprained ankle or a muscle pull. These usually get better over two or three weeks without treatment, but can be very painful while they last.
Topical non‐steroidal anti‐inflammatory drugs (NSAIDs) are applied to unbroken skin where it hurts as gels, creams, sprays, or plasters. Topical NSAIDs penetrate the skin, enter tissues or joints, and reduce processes causing pain in the tissue. Drug levels in the blood with topical NSAIDs are very much lower than with the same drug taken by mouth. This minimises the risk of harmful effects.
We searched medical databases for clinical trials comparing topical NSAIDs with placebo (creams or gels that do not contain a medicine) or other medicines in adults aged 16 years or older with musculoskeletal pain (typically sports injuries). The evidence is current to February 2015.
This review is an update of 'Topical NSAIDs for acute pain in adults' originally published in Issue 6, 2010. We identified 14 new studies to add to the 47 studies included in the earlier review. We also identified 14 studies in a clinical trial registry that are completed and three short reports from meetings, for which we could not find full details (about 4500 participants). Three more studies are ongoing (almost 900 participants).
The 61 included studies, involving 8386 participants, were generally of high‐quality. They tested a number of different topical drugs, mostly against a topical placebo (carrier without the NSAID), with application at least once a day. We were interested in participants having good pain reduction (by about half) around seven days after treatment started. At later times, most people are expected to get better even without treatment.
We looked at particular formulations of individual drugs. Gel formulations of diclofenac and ketoprofen were among the most effective, along with ibuprofen gel and diclofenac plaster. For diclofenac and ketoprofen gels, 7 or 8 people out of 10 with a painful strain, sprain, or muscle pull had much reduced pain after seven days, compared with only 2 or 3 out of 10 with placebo (high quality data). Other NSAIDs and formulations were better than placebo, but not by as much. Because both topical NSAIDs and topical placebo are rubbed into the skin in these studies, we know that any effect is not just from rubbing.
About 1 in 20 people experienced a mild and short‐lived side effect like redness at the application site. This was the same for topical NSAID and topical placebo (high quality data). Side effects like a stomach upset or feeling sick were uncommon, with no difference between topical NSAID and topical placebo (high quality data). There were no serious side effects.
Authors' conclusions
Summary of findings
| Topical NSAIDs compared with topical placebo for acute musculoskeletal pain in adults | ||||||
| Patient or population: adults with strains, sprains, or muscle pull Settings: community Intervention: topical NSAID (topical diclofenac, ibuprofen, and ketoprofen gels only shown here for efficacy) Comparison: topical placebo | ||||||
| Outcomes | Probable outcome with | Probable outcome with | RR, NNT, NNTp, or NNH | No of studies, participants | Quality of the evidence | Comments |
| Topical diclofenac gel (as Emulgel) Clinical success (eg 50% reduction in pain) | 780 in 1000 | 200 in 1000 | RR 3.4 (2.7 to 55) NNT 1.8 (1.5 to 2.1) | 2 studies 314 participants | High | Consistent results in 2 moderately sized recent studies of high quality |
| Topical ibuprofen gel Clinical success (eg 50% reduction in pain) | 420 in 1000 | 160 in 1000 | RR 2.7 (1.7 to 4.2) NNT 3.9 (2.7 to 6.7) | 2 studies 241 participants | Moderate | Modest effect size and numbers of participants |
| Topical ketoprofen gel Clinical success (eg 50% reduction in pain) | 720 in 1000 | 330 in 1000 | RR 2.2 (1.7 to 2.8) NNT 2.5 (2.0 to 3.4) | 5 studies 348 participants | Moderate | Modest effect size and numbers of participants, but studies small, with none recent |
| All topical NSAIDs Local adverse events | 46 in 1000 | 50 in 1000 | RR 1.0 (0.80 to 1.2) NNH not calculated | 42 studies 6125 participants | High | Large number of studies and participants with consistent results |
| All topical NSAIDs Systemic adverse events | 32 in 1000 | 35 in 1000 | RR 1.0 (0.7 to 1.3) NNH not calculated | 38 studies 5372 participants | High | Large number of studies and participants with consistent results |
| All topical NSAIDs Withdrawals ‐ adverse events | 11 in 1000 | 11 in 1000 | RR 1.0 (0.7 to 1.7) NNH not calculated | 42 studies 5790 participants | High | Large number of studies and participants with consistent results |
| Serious adverse events | 1 in total | 0 in total | Not calculated | All data | Low | Small numbers of events |
| GRADE Working Group grades of evidence | ||||||
| CI: confidence interval; RR: risk ratio; NNT: number needed to treat for an additional beneficial outcome; NNTp: number needed to treat to prevent an event happening; NNH: number needed to treat for an additional harmful outcome. | ||||||
Background
This review is an update of a review originally published in Issue 6 2010 on 'Topical NSAIDs for acute pain in adults' (Massey 2010). We have changed the title to specify musculoskeletal pain because topical NSAIDs are not normally used to treat visceral pain or headache. We felt that the new title better reflected the content of the review.
The use of topical NSAIDs for pain relief has been a controversial subject in analgesic practice. In some parts of the world (including much of Western Europe) they have been available for many years, are widely available without prescription, widely advertised, used extensively, and evidence for their use is considered adequate. In other parts of the world they were regarded as little more than placebo, with any apparent effect attributed to the process of rubbing at the site of the affected area. In some places (for example the US) their use was almost unknown until the mid‐2010s. In England, 5.2 million prescriptions for topical NSAIDs were dispensed in 2013 (PACT 2014), mainly for formulations of ibuprofen (2.45 million), piroxicam (1.18 million), and diclofenac (1.27 million).
There is good evidence for the efficacy of topical NSAIDs in acute and chronic musculoskeletal pain (Mason 2004a; Mason 2004b; Moore 1998a). In the US, the Food and Drug Administration licensed topical nonsteroidal products in 2007, and in England, the National Institute for Health and Care Excellence (NICE) recommended topical therapies as first line treatment in its guidelines for osteoarthritis in 2008 (NICE 2008). Earlier reviews of topical analgesics covered studies investigating the underlying science to explain biological plausibility in addition to clinical trials (Anon 2005; Moore 2008a).
This review is one of a series on topical analgesics, including topical capsaicin at low and high doses (Derry 2012a; Derry 2013), and topical NSAIDs in chronic pain conditions (Derry 2012b), and salicylate‐containing rubefacients (Derry 2014).
Description of the condition
Acute pain is usually defined as pain of less than three months' duration. It is often associated with injury, including trauma; surgery; musculoskeletal injuries such as strains, sprains, and over‐use injuries; or soft tissue injuries such as muscle soreness or cramps.
Description of the intervention
Clinicians prescribe NSAIDs on a routine basis for a range of mild to moderate pain. NSAIDs are the most commonly prescribed analgesic medications worldwide, and their efficacy for treating acute pain has been well demonstrated (Moore 2003). They reversibly inhibit cyclooxygenase (prostaglandin endoperoxide synthase), the enzyme mediating production of prostaglandins and thromboxane A2 (FitzGerald 2001). Prostaglandins mediate a variety of physiological functions such as maintenance of the gastric mucosal barrier, regulation of renal blood flow, and regulation of endothelial tone. They also play an important role in inflammatory and nociceptive processes. However, relatively little is known about the mechanism of action of this class of compounds aside from their ability to inhibit cyclooxygenase‐dependent prostanoid formation (Hawkey 1999).
NSAIDs taken orally or intravenously are transported to all parts of the body in the blood, and relatively high blood concentrations are needed to achieve effective tissue concentrations at the site of the pain and inflammation. These high concentrations throughout the body can give rise to a number of adverse events that can be unpleasant (for example, dyspepsia) or potentially serious (for example, gastrointestinal bleeding).
A topical medication is a one applied to body surfaces such as the skin or mucous membranes to treat ailments. A large range of types of topical formulation may be used, including but not limited to creams, foams, gels, lotions, ointments, and plasters. The exact formulation of a topical medication is often determined by the speed of drug absorption required. The need may be for slow absorption into the circulation to maintain low drug concentrations, and, perhaps, avoiding extensive first pass metabolism in the liver; plasters containing drug reservoirs may be used for this, as with transdermal opioids or contraceptive steroids. For rapid absorption, the formulation is enhanced by substances to improve or assist skin penetration, perhaps only to generate high concentrations in tissues rather than in the blood; gel formulations are useful for this purpose, which is why they are sometimes used for topical NSAIDs.
Topical NSAIDs
Topical NSAIDs are formulated for direct application to the painful site, and to produce a local pain‐relieving effect while avoiding body‐wide distribution of the drug at physiologically active levels (McPherson 2013). This method of application (dosing) necessarily limits their use to more superficial painful conditions such as sprains, strains, and muscle or tendon soreness. They would not, for example, be indicated for deep visceral pain or headaches. They are also not appropriate for use on broken skin, so would not be used on open wounds (accidental or surgical).
How the intervention might work
For a topical formulation to be effective, it must first penetrate the skin. Only when the drug has entered the lower layers of the skin can it be absorbed by the blood and transported to the site of action, or penetrate deeper into areas where inflammation occurs. Individual drugs have different degrees of penetration. A balance between lipid and aqueous solubility is needed to optimise penetration, and use of prodrug esters has been suggested as a way of enhancing permeability. Formulation is also crucial to good skin penetration. Experiments with artificial membranes or human epidermis suggest that creams are generally less effective than gels or sprays, but newer formulations such as microemulsions may have greater potential.
Once the drug has reached the site of action, it must be present at a sufficiently high concentration to inhibit cyclooxygenase enzymes, thereby reducing prostaglandin synthesis. This in turn reduces inflammation and relieves pain. It is probable that in acute conditions, topical NSAIDs exert their action primarily by local reduction of symptoms, independent of any systemic uptake and delivery. Tissue levels of NSAIDs applied topically certainly reach levels high enough to inhibit cyclooxygenase‐2 (Anon 2005; Haroutiunian 2010; Moore 2008a). However, plasma concentrations found after topical administration are only a fraction (usually much less than 5%) of the levels found in plasma following oral administration. Topical application can potentially limit systemic adverse events by increasing local effects, and minimising systemic concentrations of the drug. We know that the incidence of upper gastrointestinal bleeding is low with chronic use of topical NSAIDs (Evans 1995), although it has been reported, particularly in people with risk factors (Zimmerman 1995). We have no certain knowledge of lower effects on cardiovascular events, or renal failure, both of which have been associated with oral NSAID use.
Why it is important to do this review
Since the last review in 2010, a number of new studies have been published, nearly all of which investigated various formulations of diclofenac. These new studies are generally of higher quality than many of the earlier ones in this review, and have the potential to influence the strength of its conclusions substantially. Moreover, the additional information allows for analysis based not only on a particular drug, but also on the formulation of that drug. This can provide better insight into whether formulation affects efficacy of topical NSAIDs in acute musculoskeletal pain.
An updated review of evidence for topical NSAIDs was needed to inform choices made by consumers, prescribers, and commissioners (purchasers of healthcare).
Objectives
To determine the efficacy and safety of topically applied NSAIDs in acute musculoskeletal pain in adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled double‐blind studies comparing topical NSAIDs with placebo (inert carrier) or other active treatment for acute pain, with at least 10 participants per treatment arm and outcomes close to seven days (minimum three days). We excluded studies published only as short abstracts (which report insufficient data to assess methods) or studying experimentally induced pain (which does not correlate well with clinical pain). Because a cross‐over design is not appropriate for self limiting conditions such as sprains, strains, and contusions, we only considered parallel‐group designs.
Types of participants
Adults (aged 16 years or more) with acute musculoskeletal pain of at least moderate intensity resulting mainly from strains, sprains, or sports injuries. Typically for sports injuries, the injury would have occurred within 24 or 48 hours.
Types of interventions
Included studies had at least one treatment arm using a topical NSAID and a comparator arm using placebo (inert carrier without NSAID or other active treatment). The topical NSAID had to be applied at least once daily. We did not include salicylates in this review as they are no longer classified as topical NSAIDs and are covered in a separate review (Derry 2014).
Types of outcome measures
We sought information on participant characteristics including age, sex, and condition treated.
Primary outcomes
The primary outcome was 'clinical success', defined as at least a 50% reduction in pain or equivalent measure, such as a 'very good' or 'excellent' global assessment of treatment, or 'none' or 'slight' pain on rest or movement, measured on a categorical scale (Moore 1998a). We used the following hierarchy of outcomes to extract data for the primary outcome.
-
Participant reported reduction in pain of at least 50%.
-
Participant reported global assessment of treatment.
-
Pain on movement.
-
Pain at rest or spontaneous pain.
-
Undefined 'improvement'.
We used only participant reported outcomes of efficacy, and not physician or investigator reported outcomes.
Secondary outcomes
-
Numbers of participants with adverse events: local and systemic.
-
Numbers of withdrawals: all cause, lack of efficacy, and adverse events.
We anticipated that outcomes would be reported after different durations of treatment, and extracted data reported as close to seven days as possible, with a minimum of three days. We also extracted data for outcomes reported after longer durations of treatment. We anticipated that reporting of adverse events would vary between studies with regard to the terminology used, method of ascertainment, and categories reported (for example, occurring in at least 5% of participants or where there is a statistically significant difference between treatment groups). We took care to identify these details where relevant.
Search methods for identification of studies
Electronic searches
We searched the following databases without language restriction:
-
CENTRAL (The Cochrane Library), Issue 4, 2009 for the original review, and the Cochrane Register of Studies Online (CRSO) to 3 February 2015 for this update;
-
MEDLINE (via Ovid), from inception to December 2009 for the original review, and from 2008 to 3 February 2015 for this update;
-
EMBASE (via Ovid), from inception to December 2009 for the original review, and from 2008 to 3 February 2015 for this update;
-
Oxford Pain Relief Database for the original review (Jadad 1996a). This resource is no longer being updated.
See Appendix 1 for the CENTRAL search strategy, Appendix 2 for the MEDLINE search strategy, and Appendix 3 for the EMBASE search strategy.
Searching other resources
We searched the reference lists of review articles and included studies. We have previously asked manufacturers for details of unpublished studies, but did not make new requests.
We searched two clinical trial registries (clinicaltrials.gov (clinicaltrials.gov/) and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/)) and asked personal contacts about ongoing and unpublished studies.
Data collection and analysis
Selection of studies
We screened the titles and abstracts of studies identified by the searches to eliminate those that clearly did not satisfy the inclusion criteria, and obtained full reports of the remaining studies to determine inclusion in the review.
Data extraction and management
Review authors were not blinded to the authors' names and institutions, journal of publication, or study results at any stage of the review. Two review authors independently selected the studies for inclusion, assessed methodological quality and risk of bias, and extracted data. We resolved disagreements and uncertainties through discussion.
We abstracted information on participants, interventions, and outcomes from the original reports into a standard data extraction form. One review author entered data suitable for meta‐analysis into Review Manager 5 (RevMan 2014), and another review author checked it.
Assessment of risk of bias in included studies
We used the Oxford Quality Score as the basis for inclusion, limiting inclusion to studies that were randomised and double‐blind as a minimum (Jadad 1996b).
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions with any disagreements resolved by discussion (Higgins 2011). We assessed the following for each study.
-
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, for example, random number table; computer random number generator); unclear risk of bias (method used to generate sequence was not clearly stated). We excluded studies using a non‐random process that were therefore at high risk of bias (for example, odd or even date of birth; hospital or clinic record number).
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions before assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (for example, telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method was not clearly stated). We excluded studies that did not conceal allocation and were therefore at high risk of bias (for example, open list).
-
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, for example, identical tubes containing gel, or identical plasters; matched in appearance and smell); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how blinding was achieved). We excluded studies that were not double‐blind and were therefore at high risk of bias.
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk of bias (less than 10% of participants did not complete the study or used 'baseline observation carried forward' analysis, or both); unclear risk of bias (used 'last observation carried forward' analysis); or high risk of bias (used 'completer' analysis).
-
Size (checking for possible biases confounded by small size). Small studies have been shown to overestimate treatment effects, probably due to methodological weaknesses (Dechartres 2013; Nüesch 2010). We assessed studies as at low risk of bias if they had at least 200 participants, at unclear risk if they had 50 to 200 participants, and at high risk if they had fewer than 50 participants.
Measures of treatment effect
We used risk ratio (RR) to establish statistical difference and numbers needed to treat for an additional beneficial outcome (NNT) with 95% confidence intervals (CI). We pooled percentages as absolute measures of benefit or harm.
We used the following terms to describe adverse outcomes in terms of harm or prevention of harm.
-
When significantly fewer adverse outcomes occurred with treatment than with control (placebo or active), we used the term thenumber needed to treat to prevent one event (NNTp).
-
When significantly more adverse outcomes occurred with treatment compared with control (placebo or active), we used the term the number needed to treat for an additional harmful outcome or cause one event (NNH).
Unit of analysis issues
Randomisation was to the individual participant.
Dealing with missing data
Wherever possible we used intention‐to‐treat (ITT) analysis where the ITT population consists of participants who were randomised, applied at least one dose of the assigned study medication, and provided at least one post‐baseline assessment. We assigned missing participants zero improvement.
We also looked for information about methods of imputation for missing data.
Assessment of heterogeneity
We examined heterogeneity visually using L'Abbé plots (L'Abbé 1987), a visual method for assessing differences in results of individual studies, and with the I2 statistic.
Assessment of reporting biases
The aim of this review was to use dichotomous outcomes of known utility and of value to patients (Moore 2013). The review did not depend on what the authors of the original studies chose to report or not. Studies that did not report dichotomous results, but only average pain data, did not contribute to analyses.
We assessed publication bias using a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean an NNT of 10 or higher; Moore 2008b).
Data synthesis
We pooled data only for comparisons and outcomes where there were at least two studies and 200 participants (Moore 1998b). When two active treatment arms were compared with a placebo arm, we took care to avoid double counting of participants in the placebo arm: if both active groups contributed to an analysis, we split the placebo group between them.
We calculated RRs with 95% CIs using the fixed‐effect model (Morris 1995). A statistically significant benefit of topical NSAID over control was assumed when the lower limit of the 95% CI of the RR was greater than one. A statistically significant benefit of control over active treatment was assumed when the upper limit of the 95% CI was less than one. We calculated NNTs with 95% CIs using the pooled number of events by the method of Cook and Sackett (Cook 1995).
Statistically significant differences between NNTs for different topical NSAIDs were tested using the z test (Tramer 1997), where there were sufficient data to do so, and where the studies were sufficiently similar in types of participant, outcome, and duration to make such comparisons sensible.
Subgroup analysis and investigation of heterogeneity
We carried out separate analyses for individual NSAIDs, and, where the data permitted, for different formulations of individual NSAIDs.
Sensitivity analysis
The earlier review included sensitivity analyses for various factors that are now covered by the assessment of risk of bias.
Results
Description of studies
Results of the search
New searches for this update identified 20 publications that were examined in further detail to determine inclusion status. We also identified 20 additional studies in clinical trial registries. See Figure 1.

Study flow diagram.
We identified 14 new studies (11 publications, three unpublished reports) satisfying our inclusion criteria (Costantino 2011; Coudreuse 2010; González de Vega 2013; Hofman 2000; Klainguti 2010; Kuehl 2011; Li 2013; NCT01255423; NCT01272934; NCT01272947; Predel 2012; Predel 2013a; Predel 2013b; Saillant 1998), one post hoc analysis of a study that was included in the earlier review (Mueller 2010 in Predel 2004), and an additional publication and a pooled analysis that included new data for another study from the earlier review (Lionberger 2011 pooled analysis in Joussellin 2003).
Included studies
All except one of the new studies compared diclofenac with placebo. A number of different formulations were used, including diclofenac epolamine (DHEP) with or without heparin (Flectoparin Tissugel or Flector EP Tissugel) applied as a plaster (or patch), diclofenac diethylamine (DDEA) applied as a gel, and diclofenac with lethicin applied as a spray gel. The remaining study compared diclofenac gel with traumeel, a "fixed combination of plant and mineral extracts", applied as a gel or an ointment.
There were 47 studies in the original review; 14 new studies were included making a total of 61 studies in this updated review. All used a parallel group design. Forty‐four compared a topical NSAID with placebo, 13 a topical NSAID with an active comparator (a different topical NSAID, an oral NSAID, the same topical NSAID in a different formulation, or a compound of plant and mineral extracts), and four had both placebo and active comparators. In total, 5311 participants were treated with a topical NSAID, 3470 with placebo, and 220 with an oral NSAID. Topical NSAIDs used were benzydamine, diclofenac, etofenamate, felbinac, fentiazac, flunoxaprophen, flurbiprofen, ibuprofen, indomethacin, ketoprofen, ketorolac, lysine clonixinate, meclofenamic acid, naproxen, niflumic acid, and piroxicam. They were applied as creams, gels, sprays, foams, or plasters (patches). Topical placebos were the inert carriers, without the active NSAID. Oral NSAIDs used were ibuprofen (as tablets) and indomethacin (as capsules).
Most studies enrolled participants who had sprains, strains, and contusions, usually as a result of sports injuries, and treatment was started within a few hours or days. Other studies enrolled participants with overuse‐type injuries, such as tendinitis and acute low back pain, where pain had been present for days or weeks, but less than three months.
Participants were treated for at least five days, and up to three weeks, with most studies lasting seven to 14 days. Participants were usually assessed in clinic at intervals during treatment, and sometimes also at home using daily patient diaries. We used outcomes closest to seven days because many of these injuries are self limiting, with differences between active treatment and placebo being diminished or lost after longer intervals.
Most studies reported dichotomous outcomes suitable for a responder analysis, although group mean change (for pain or physical function, for example) was usually the primary outcomes. However, the definition of response varied both in the parameter measured (for example, pain, pain on movement, patient global evaluation of treatment), and in the scale used to measure it (for example, a 3‐, 4‐, or 5‐point scale for patient global evaluation).
Details of included studies are in the Characteristics of included studies table.
We identified 14 completed but apparently unpublished studies in a clinical trial registry for which no results have been posted (4403 participants, NCT00351104; NCT00352625; NCT00426985; NCT00640705; NCT00640939; NCT00680472; NCT00680784; NCT00765700; NCT00869063; NCT00869180; NCT00931866; NCT01874626; NCT01957215; NCT02324270). We have placed these under Characteristics of studies awaiting classification. We also identified three conference abstracts that relate to completed studies that do not appear to have been published, but that may satisfy our inclusion criteria. One is likely to be the same study as one of the included studies identified in a clinical trial registry (Pallay 2013 in NCT01272947), one relates to another study identified in the clinical trial registry that is awaiting classification (Ekman 2010 in NCT00765700), while we could find no published or unpublished reports of the other Sarzi‐Puttini 2014).
We also identified three ongoing studies with an estimated enrolment of 880 participants (NCT01945034; NCT02100670; NCT02290821). Details are in the Characteristics of ongoing studies table.
Excluded studies
For the original review, 25 studies were excluded after reading the full paper. For this update, we excluded four new studies (Cesarone 2008; Coulibaly 2009; Kuwabara 2013; Vinciguerra 2008). Details are in the Characteristics of excluded studies table.
Risk of bias in included studies
All studies were randomised and double‐blind. One study scored 2/5 (Sinniger 1981), 23 scored 3/5, 23 scored 4/5, and 14 scored 5/5 for methodological quality using the Oxford Quality Scale. A breakdown of the scores for individual studies is reported in the Characteristics of included studies table.
Comments on potential biases in individual studies are reported in the 'Risk of bias' section of the Characteristics of included studies table. The findings are displayed in Figure 2 and Figure 3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
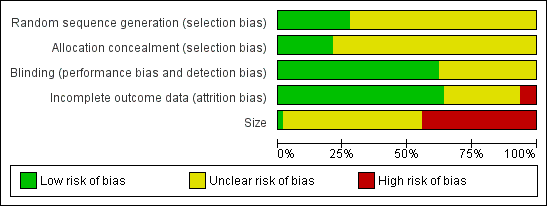
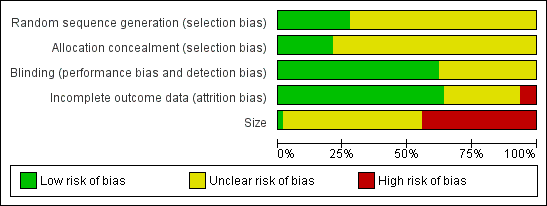
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Allocation
All the studies were randomised but only 17 adequately described the method used to generate the random sequence. Thirteen studies adequately described the method used to conceal allocation of the sequence. No studies were at high risk of bias for this item.
Blinding
All studies were double‐blind and 38 adequately described the method used to maintain the blinding. No studies were at high risk of bias for this item.
Incomplete outcome data
Thirty‐six studies included all participants in the primary analysis or provided sufficient data to allow missing participants to be included as non‐responders, and were judged at low risk of bias. We judged four studies to be at high risk of attrition bias (Campbell 1994; Kuehl 2011; Mazières 2005a; Russell 1991). Three unpublished studies contributed only to adverse event analyses and accounted for all participants for these outcomes (NCT01255423; NCT01272934; NCT01272947).
Other potential sources of bias
We judged one study that included more than 200 participants in each treatment arm to be at low risk of bias from size, but this study had a very high attrition rate (see 'Incomplete outcome data (attrition bias)') and did not report all the efficacy outcomes measured (Kuehl 2011). We judged 27 studies to be at high risk because they included fewer than 50 participants per treatment arm.
Effects of interventions
Three studies did not contribute data suitable for analysis of at least one outcome (González de Vega 2013; Gualdi 1987; Hoffmann 2012).
1. Topical NSAID versus placebo
Details of efficacy outcomes in individual studies are in Appendix 4, and of adverse events and withdrawals in Appendix 5. Appendix 6 has details of the concentration of topical products, the amount applied, the frequency of application, and an estimation of the daily dose of topical NSAID applied. Not all studies provided sufficient information to allow calculation of daily dose applied. For example, for topical diclofenac, the estimated doses applied varied between about 60 and 280 mg; for topical ketoprofen 100 to about 450 mg; for topical ibuprofen 300 to 800 mg.
Participants with clinical success
Topical diclofenac versus placebo
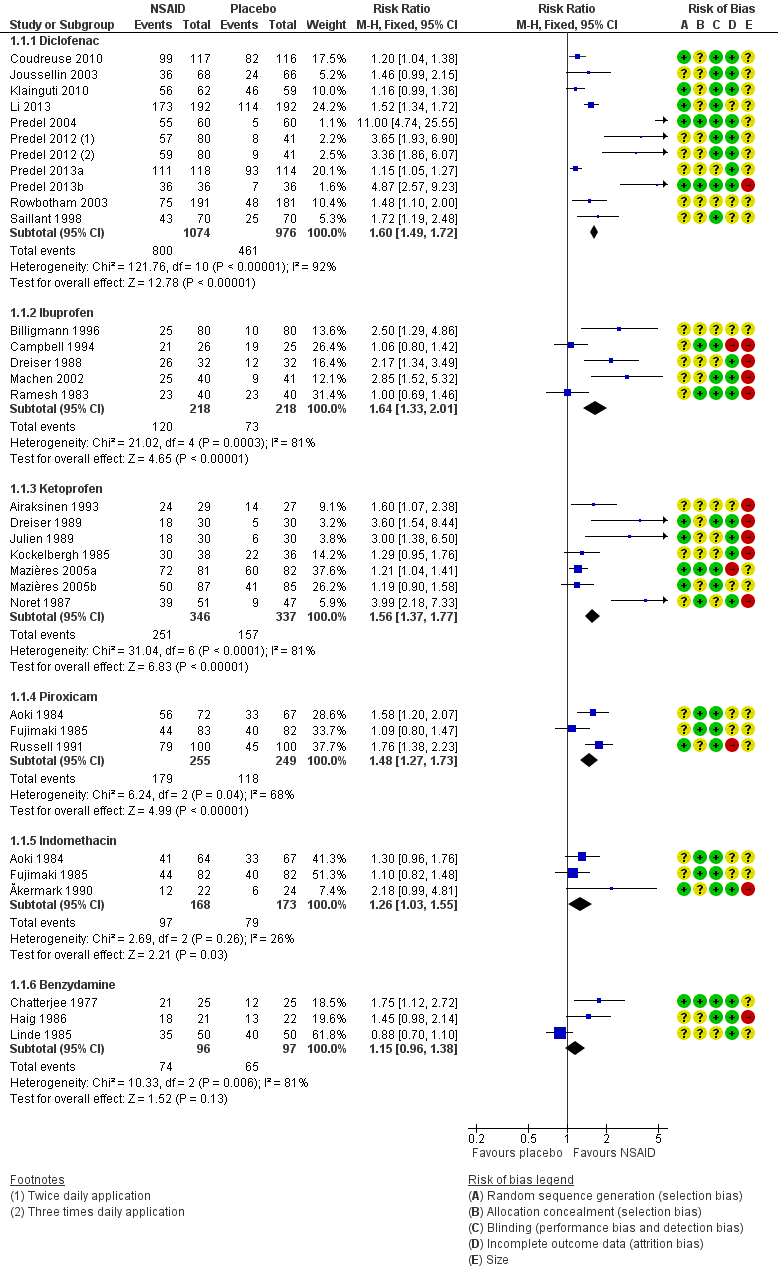
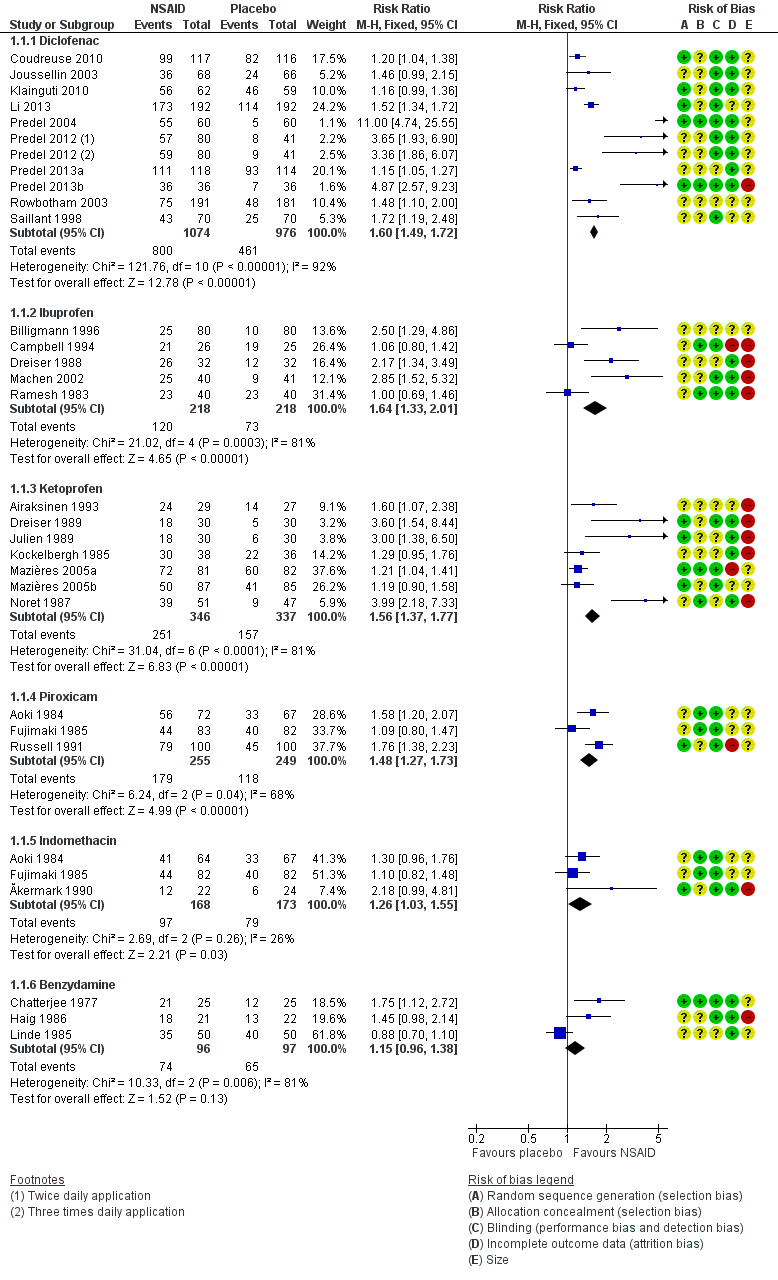
Ten studies contributed to this analysis (Coudreuse 2010; Joussellin 2003; Klainguti 2010; Li 2013; Predel 2004; Predel 2012; Predel 2013a; Predel 2013b; Rowbotham 2003), of which one (Predel 2012) had two active treatment arms. A total of 1074 participants were treated with topical diclofenac and 976 with placebo (Analysis 1.1; Figure 4).
Forest plot of comparison: 2 Individual NSAID versus placebo, outcome: 2.1 Clinical success.
-
The proportion of participants experiencing successful treatment with topical diclofenac was 74% (800/1074, range 39% to 100%).
-
The proportion of participants experiencing successful treatment with placebo was 47% (461/976, range 8% to 82%).
-
The RR for treatment compared with placebo was 1.6 (95% CI 1.5 to 1.7).
-
The NNT for successful treatment was 3.7 (3.2 to 4.3). For every four participants treated with topical diclofenac, one would experience successful treatment who would not have done so with placebo.
Effect of formulation
The effects of formulation are shown in Analysis 2.1 and Figure 5.

L'Abbé plot of clinical success in studies of topical diclofenac versus topical placebo. The size of the symbol is proportional to the size of the study (inset scale). Dark blue: Emulgel; light blue: spray/gel; red: Flector; pink: other patch or plaster.
-
Four studies used a Flector® plaster (1030 participants; Joussellin 2003; Li 2013; Rowbotham 2003; Saillant 1998). The RR for treatment compared with placebo was 1.5 (1.4 to 1.7), and the NNT was 4.7 (3.7 to 6.5).
-
Three studies used other makes of plaster (474 participants; Coudreuse 2010; Klainguti 2010; Predel 2004). The RR for treatment compared with placebo was 1.6 (1.4 to 1.8), and the NNT was 3.2 (2.6 to 4.2).
-
Two studies used Voltaren Emulgel (314 participants; Predel 2012; Predel 2013b). The RR for treatment compared with placebo was 3.8 (2.7 to 5.5), and the NNT was 1.8 (1.5 to 2.1).
-
One study used a spray gel (232 participants; Predel 2013a). The RR for treatment compared with placebo was 1.2 (1.05 to 1.3), and the NNT was 8.0 (4.8 to 24).
Diclofenac as the gel formulation Emulgel was statistically more efficacious than the plaster formulation as Flector plaster (z = 6.360; P value < 0.00001).
Topical ibuprofen versus placebo
Five studies contributed to this analysis (Billigmann 1996; Campbell 1994; Dreiser 1988; Machen 2002; Ramesh 1983). A total of 218 participants were treated with topical ibuprofen and 218 with placebo (Analysis 1.1).
-
The proportion of participants experiencing successful treatment with topical ibuprofen was 55% (120/218, range 31% to 81%).
-
The proportion of participants experiencing successful treatment with placebo was 33% (73/218, range 13% to 76%).
-
The RR of treatment compared with placebo was 1.6 (1.3 to 2.0).
-
The NNT for successful treatment was 4.6 (3.3 to 8.0). For every five participants treated with topical ibuprofen, one would experience successful treatment who would not have done so with placebo.
Effect of formulation
The effects of formulation are shown in Analysis 3.1.
-
Three studies used cream formulations (195 participants; Campbell 1994; Dreiser 1988; Ramesh 1983). Although this is just below our threshold for pooled analysis, we have included this analysis for completeness and the results should be interpreted with caution. The RR for treatment compared with placebo was 1.3 (1.03 to 1.6), and the NNT was 6.4 (3.4 to 41).
-
Two studies used gel formulations (241 participants; Billigmann 1996; Machen 2002). The RR for treatment compared with placebo was 2.7 (1.7 to 4.2), and the NNT was 3.9 (2.7 to 6.7).
There was no statistically significant difference between the gel and cream formulations (z = 1.160, P value = 0.246).
Topical ketoprofen versus placebo
Seven studies contributed to this analysis (Airaksinen 1993; Dreiser 1989; Julien 1989; Kockelbergh 1985; Mazières 2005b; Mazières 2005a; Noret 1987). A total of 346 participants were treated with topical ketoprofen, and 337 with placebo (Analysis 1.1).
-
The proportion of participants experiencing successful treatment with topical ketoprofen was 73% (251/346, range 57% to 89%).
-
The proportion of participants experiencing successful treatment with placebo was 47% (157/337, range 17% to 73%).
-
The RR of treatment compared with placebo was 1.6 (1.4 to 1.8).
-
The NNT for successful treatment was 3.9 (3.0 to 5.3). For every four participants treated with topical ketoprofen, one would experience successful treatment who would not have done so with placebo.
Effect of formulation
The effects of formulation are shown in Analysis 4.1 and Figure 6.

L'Abbé plot of clinical success in studies of topical ketoprofen versus topical placebo. The size of the symbol is proportional to the size of the study (inset scale). Light blue: ketoprofen gel; pink: ketoprofen plaster.
Two studies used a plaster formulation (335 participants; Mazières 2005b; Mazières 2005a). The RR for treatment compared with placebo was 1.2 (1.04 to 1.4), and the NNT was 8.2 (4.5 to 47).
Five studies used gel formulations (348 participants; Airaksinen 1993; Dreiser 1989; Julien 1989; Kockelbergh 1985; Noret 1987). The RR for treatment compared with placebo was 2.2 (1.7 to 2.8), and the NNT was 2.5 (2.0 to 3.4).
Ketoprofen as a gel formulation was statistically more efficacious than a plaster formulation (z = 3.860, P value = 0.00014).
Topical piroxicam versus placebo
Three studies contributed to this analysis (Aoki 1984; Fujimaki 1985; Russell 1991). A total of 255 participants were treated with topical piroxicam, and 249 with placebo (Analysis 1.1).
-
The proportion of participants experiencing successful treatment with topical piroxicam was 68% (179/255, range 53% to 79%).
-
The proportion of participants experiencing successful treatment with placebo was 47% (118/249, range 45% to 49%).
-
The RR of treatment compared with placebo was 1.5 (1.3 to 1.7).
-
The NNT for successful treatment was 4.4 (3.2 to 6.9). For every four participants treated with topical piroxicam, one would experience successful treatment who would not have done so with placebo.
Topical indomethacin versus placebo
Three studies contributed to this analysis (Ăkermark 1990; Aoki 1984; Fujimaki 1985). A total of 168 participants were treated with topical indomethacin, and 173 with placebo (Analysis 1.1).
-
The proportion of participants experiencing successful treatment with topical indomethacin was 58% (97/168, range 54% to 64%).
-
The proportion of participants experiencing successful treatment with placebo was 46% (79/173, range 25% to 49%).
-
The RR of treatment compared with placebo was 1.3 (1.03 to 1.6).
-
The NNT for successful treatment was 8.3 (4.4 to 65). For every eight participants treated with topical indomethacin, one would experience successful treatment who would not have done so with placebo.
Topical benzydamine versus placebo
Three studies contributed to this analysis (Chatterjee 1977; Haig 1986; Linde 1985). A total of 96 participants were treated with topical benzydamine, and 97 with placebo (Analysis 1.1). Although this is just below our threshold for pooled analysis, we have included this analysis for completeness and the results should be interpreted with caution.
-
The proportion of participants experiencing successful treatment with topical benzydamine was 77% (74/96, range 70% to 86%).
-
The proportion of participants experiencing successful treatment with placebo was 67% (65/97, range 48% to 80%).
-
The RR of treatment compared with placebo was 1.2 (0.96 to 1.4). There was no statistically significant difference between treatments (Figure 4).
Results for participants with clinical success with individual topical NSAIDs, where there were adequate data for analysis, are summarised below in 'Summary of results A' and Analysis 1.1, Analysis 2.1, Analysis 3.1, and Analysis 4.1.
| Summary of results A: Participants with clinical success | ||||||
| Comparison | Studies | Participants | NSAID (%) | Placebo (%) | Relative benefit (95% CI) | NNT (95% CI) |
| Diclofenac ‐ Flector plaster | 4 | 1030 | 63 | 41 | 1.5 (1.4 to 1.7) | 4.7 (3.7 to 6.5) |
| Diclofenac ‐ other plaster | 3 | 474 | 88 | 57 | 1.6 (1.4 to 1.8) | 3.2 (2.6 to 4.2) |
| Diclofenac ‐ Emulgel | 2 | 314 | 78 | 20 | 3.8 (2.7 to 5.5) | 1.8 (1.5 to 2.1) |
| Diclofenac ‐ other gel* | 1 | 232 | 94 | 82 | 1.2 (1.1 to 1.3) | 8.0 (4.8 to 24) |
| Ibuprofen ‐ cream* | 3 | 195 | 71 | 56 | 1.3 (1.03 to 1.6) | 6.4 (3.4 to 41) |
| Ibuprofen ‐ gel | 2 | 241 | 42 | 16 | 2.7 (1.7 to 4.2) | 3.9 (2.7 to 6.7) |
| Ketoprofen ‐ plaster | 2 | 335 | 73 | 60 | 1.2 (1.04 to 1.4) | 8.2 (4.5 to 47) |
| Ketoprofen ‐ gel | 5 | 348 | 72 | 33 | 2.2 (1.7 to 2.8) | 2.5 (2.0 to 3.4) |
| Piroxicam | 3 | 504 | 70 | 47 | 1.5 (1.3 to 1.7) | 4.4 (3.2 to 6.9) |
| Indomethacin | 3 | 341 | 58 | 46 | 1.3 (1.03 to 1.6) | 8.3 (4.4 to 65) |
| Benzydamine* | 3 | 193 | 77 | 67 | 1.2 (0.96 to 1.4) | not calculated |
* Results for these two comparisons are derived from very small amounts of data and are provided here for completeness. They should be interpreted with caution.
Local adverse events
Local adverse events were irritation of the area to which the topical NSAID was applied, including redness or erythema and itch or pruritus. Where reported, these were usually described as mild and transient.
All topical NSAIDs versus placebo
Forty‐two studies contributed to this analysis, of which three compared two different drugs with placebo (Aoki 1984; Diebshlag 1990; Fujimaki 1985). Three studies had two treatment arms comparing different formulations or application regimens for diclofenac with placebo, which have been combined for this analysis (Costantino 2011; Klainguti 2010; Predel 2012). In total, 3619 participants were treated with topical NSAIDs and 3121 with placebo (Analysis 5.1).
-
The proportion of participants experiencing a local adverse event with a topical NSAID was 4.3% (155/3619, range 0% to 33%).
-
The proportion of participants experiencing a local adverse event with placebo was 4.6% (145/3121, range 0% to 32%).
-
The RR of topical NSAID compared with placebo was 0.98 (0.80 to 1.2).
-
There was no significant difference between treatment groups so the NNH was not calculated.
Individual topical NSAIDs versus placebo
Results for local adverse events with individual topical NSAIDs, where there were adequate data for analysis, are in Summary of results B and Analysis 1.2.
| Summary of results B: Participants with local adverse events | ||||||
| Comparison | Studies | Participants | NSAID (%) | Placebo (%) | RR (95% CI) | NNH (95% CI) |
| All NSAIDs | 42 | 6740 | 4.3 | 4.6 | 0.98 (0.80 to 1.2) | Not calculated |
| Diclofenac | 15 | 3271 | 3.1 | 4.3 | 0.78 (0.56 to 1.1) | Not calculated |
| Ketoprofen | 8 | 852 | 11 | 9.5 | 1.2 (0.83 to 1.7) | Not calculated |
| Piroxicam | 3 | 522 | 2.3 | 5.4 | 0.42 (0.17 to 1.1) | Not calculated |
| Felbinac | 3 | 397 | 3.0 | 1.5 | 1.9 (0.49 to 7.5) | Not calculated |
| Indomethacin | 3 | 354 | 6.3 | 2.2 | 2.7 (0.91 to 7.7) | Not calculated |
| Ibuprofen | 3 | 321 | 10 | 4.3 | 2.3 (0.98 to 5.4) | Not calculated |
Systemic adverse events
All topical NSAIDs versus placebo
Thirty‐six studies contributed data on systemic adverse events, of which three compared two different drugs with placebo (Aoki 1984; Diebshlag 1990; Fujimaki 1985). Two studies had two treatment arms comparing different formulations or application regimens for diclofenac with placebo, which have been combined for this analysis (Klainguti 2010; Predel 2012). In total, 2956 participants were treated with a topical NSAID and 2620 with placebo (Analysis 5.2).
-
Twenty‐three studies reported no systemic adverse events in any arm of the study.
-
The proportion of participants experiencing a systemic adverse event with a topical NSAID was 3.1% (92/2956).
-
The proportion of participants experiencing a systemic adverse event with placebo was 3.5% (91/2620).
-
The RR of topical NSAID compared with placebo was 0.96 (0.73 to 1.3).
-
There was no significant difference between treatment groups so the NNH was not calculated.
A further six studies did not report the occurrence or otherwise of systemic adverse events (Billigmann 1996; Julien 1989; Kockelbergh 1985; Noret 1987; Ramesh 1983; Vecchiet 1991), while two studies did not report numbers of participants with systemic adverse events (Ăkermark 1990; Auclair 1989). Costantino 2011 reported that there were no systemic gastrointestinal adverse events. Two studies reported only on total adverse events, without distinguishing between local and systemic events (NCT01255423; NCT01272934).
Serious adverse events
Two studies reported serious adverse events. In Hoffmann 2012, one participant experienced three serious adverse events, none of which was judged to be related to the study medication (diclofenac plaster). In NCT01272934, one participant using diclofenac gel ruptured the ligaments of the wrist. There was no statement about likely relationship to the study medication, but this seems unlikely.
Adverse event withdrawals
Forty‐two studies reported data relating to adverse event withdrawals, of which three compared two different drugs with placebo (Aoki 1984; Diebshlag 1990; Fujimaki 1985). Two studies had two treatment arms comparing different formulations or application regimens for diclofenac with placebo, which have been combined for this analysis (Klainguti 2010; Predel 2012). In total, 3365 participants received a topical NSAID and 3040 placebo (Analysis 5.3).
-
Forty‐four comparisons reported no adverse event withdrawals.
-
The proportion of participants withdrawing from the study due to an adverse event after treatment with a topical NSAID was 0.98% (33/3365).
-
The proportion of participants withdrawing from the study due to an adverse event after treatment with placebo was 0.99% (30/3040).
-
The RR of topical NSAID compared to placebo was 1.0 (0.64 to 1.6).
-
There was no significant difference between treatment groups so the NNH was not calculated.
Four studies did not specifically mention adverse event withdrawals (Haig 1986; Hoffmann 2012; Klainguti 2010; Vecchiet 1991), while one reported that one participant withdrew with mild pruritus, but did not state the treatment arm (Joussellin 2003).
Ten studies specifically reported withdrawals due to lack of efficacy (Dreiser 1989; Dreiser 1994; Kuehl 2011; Machen 2002; Mazières 2005b; Mazières 2005a; Noret 1987; Predel 2013a; Russell 1991; Thorling 1990) (Appendix 5). Numbers of participants withdrawing were generally low, with rates of 6% or less, except in Kuehl 2011, where the rate was 10% with active treatment (diclofenac plaster) and 12% with placebo. We did not carry out any analysis because the outcome was inconsistently reported.
2. Topical NSAID versus active comparator
Details of efficacy outcomes in individual studies are in Appendix 4, and of adverse events and withdrawals in Appendix 5.
Participants with clinical success
Topical NSAID versus oral NSAID
-
Ăkermark 1990 compared indomethacin spray with indomethacin capsules, with response rates of 55% (12/22) with spray and 23% (5/22) with capsules.
-
Hosie 1993 compared felbinac foam with ibuprofen tablets, with response rates of 64% (81/127) with felbinac foam and 72% (96/133) with ibuprofen tablets.
-
Whitefield 2002 compared ibuprofen gel with ibuprofen tablets, with response rates of 60% (30/50) with gel and 54% (36/50) with tablets.
There were insufficient data for meta‐analysis for any one of these comparisons; felbinac is not known to be better than placebo.
Topical NSAID versus different formulation of the same topical NSAID
-
Fioravanti 1999 compared DHEP (diclofenac) gel formulated with and without lecithin, with response rates of 70% (35/50) in both treatment arms.
-
Mahler 2003 compared DHEP (diclofenac) gel formulated with and without lecithin, with response rates of 89% (82/92) with lecithin and 70% (62/88) without lecithin.
-
Gallacchi 1990 compared topical diclofenac formulated as Flector® gel and Emugel®, with response rates of 76% (19/25) in both treatment arms
-
Governali 1995 compared topical ketoprofen cream with gel, with response rates of 93% (14/15) with cream and 27% (4/15) with gel.
There were insufficient data for analysis.
Topical NSAID versus different topical NSAID
Eight studies compared one topical NSAID versus at least one other topical NSAID: piroxicam versus indomethacin (Aoki 1984; Fujimaki 1985; Sugioka 1984), ibuprofen versus ketoprofen (Curioni 1985; Picchio 1981), ketoprofen versus etofenamate (Curioni 1985; Tonutti 1994), ibuprofen versus etofenamate (Curioni 1985), ketorolac versus etofenamate (Diebshlag 1990), and diclofenac versus lysine clonixinate (Hofman 2000).
There were sufficient data to compare only piroxicam with indomethacin (Aoki 1984; Fujimaki 1985; Sugioka 1984; Analysis 6.1).
-
The proportion of participants experiencing clinical success with topical piroxicam was 56% (185/330, range 49% to 78%).
-
The proportion of participants experiencing clinical success with topical indomethacin was 45% (140/311, range 33% to 64%).
-
The RR of piroxicam compared with indomethacin was 1.2 (1.1 to 1.4).
-
The NNT for successful treatment was 9.1 (5.3 to 30). For every nine participants treated with topical piroxicam, one would experience a clinical success who would not have experienced one with topical indomethacin.
Topical NSAID versus different topical intervention
One study compared diclofenac gel with a herbal product called Traumeel gel under double‐blind conditions, with response rates for being pain‐free at seven days of 8/137 (5.8%) with diclofenac gel and 7/140 (5.0%) with Traumeel gel (González de Vega 2013).
Local adverse events
Topical NSAID versus oral NSAID
Two studies comparing a topical NSAID with an oral NSAID provided data on local adverse events (Ăkermark 1990; Hosie 1993). There were five events with topical NSAID and three with oral NSAID, which were too few for analysis.
Topical NSAID versus different topical NSAID
All nine studies comparing one topical NSAID with at least one other reported on local adverse events, with a total of 48 events in 1005 participants (4.8%). There were sufficient data to compare only piroxicam with indomethacin (Aoki 1984; Fujimaki 1985; Sugioka 1984; Analysis 6.2).
-
The proportion of participants experiencing local adverse events with topical piroxicam was 2.1% (7/340, range 1.2% to 2.8%).
-
The proportion of participants experiencing local adverse events with topical indomethacin was 10% (33/331, range 2.9% to 15%).
-
The RR of piroxicam compared with indomethacin was 0.21 (0.09 to 0.47).
-
The NNT to prevent a local adverse event was 13 (8.7 to 23). For every thirteen participants treated with topical piroxicam, one would not experience a local adverse event who would have experienced one with topical indomethacin.
Topical NSAID versus different topical intervention
González de Vega 2013 reported that adverse events were infrequent and mild to moderate in intensity, but did not distinguish between local and systemic events. Numbers of participants experiencing any adverse event were 8/147 with diclofenac gel and 14/148 with Traumeel gel.
Systemic adverse events
Ăkermark 1990 reported numbers of events, rather than numbers of participants with events, while Tonutti 1994 and Whitefield 2002 reported no adverse events attributable to the study medication, and Fioravanti 1999, Gallacchi 1990, Gualdi 1987, and Sugioka 1984 did not mention systemic adverse events. González de Vega 2013 did not distinguish between local and systemic events. In the remaining studies a total of 16 events were reported in topical NSAID treatment arms (797 participants, 2%) and 11 with ibuprofen tablets (134 participants, 8%).
Serious adverse events
No serious adverse events were reported in any treatment arm.
Withdrawals
The only withdrawals reported due to adverse events were in studies with placebo treatment arms (Ăkermark 1990; Fujimaki 1985), and have been reviewed.
Three studies reported withdrawals due to lack of efficacy (González de Vega 2013; Hofman 2000; Tonutti 1994) (Appendix 5). There were insufficient data for analysis.
Some studies reported exclusions from analysis (efficacy or safety, or both) following randomisation, mainly due to protocol violations or loss to follow‐up (Appendix 5). There is no reason to believe these exclusions would introduce systematic bias, and the numbers involved were not likely to influence results.
Discussion
This updated review of topical NSAIDs for acute musculoskeletal pain in adults differs from previous reviews. Previously, data allowed only for comparison of individual topical NSAIDs with placebo, irrespective of formulation. With a substantial amount of new data for diclofenac, it is possible to distinguish effects of formulation for individual NSAIDs. Because formulation chemistry can substantially affect the rate and total amount of drug accessing subcutaneous injured tissues, the effect of formulation may be as important as the individual NSAID used. Drug and formulation should thus be considered together when assessing efficacy, and this is now possible.
We have also included an assessment of the daily dose of NSAID applied to the skin. This involved having information on the concentration of NSAID in the preparation, the amount used, and the frequency of use. Not all studies reported all three, but an estimation of topical doses applied was possible. It varied by factors of three or four for each topical NSAID. However, for topical formulations, the key issue is less the dose applied but the amount that penetrates locally (producing analgesic effect) and the amount entering the systemic circulation (producing potential harms). Both will depend on the exact formulation of the topical agent, and whether there is occlusion (Moore 2008a).
Summary of main results
This review included 61 studies comparing a topical NSAID with placebo, another topical NSAID, or an oral NSAID. In total, 5311 participants were treated with a topical NSAID, 3470 with placebo, and 220 with an oral NSAID. There were 63% more participants than in the previous version of this review. Conditions treated were sprains, strains, and contusions, mainly resulting from sports injuries, and overuse injuries such as tendinitis.
Formulations of topical diclofenac, ibuprofen, ketoprofen, piroxicam, and indomethacin demonstrated significantly higher rates of clinical success than matching topical placebo lacking the NSAID; benzydamine did not. Three drug and formulation combinations had NNTs for clinical success below 4. For diclofenac, Emulgel® had the lowest NNT of 1.8 (1.5 to 2.1) in two studies using at least 50% pain intensity reduction as the outcome (high quality evidence). Diclofenac plasters other than Flector® also had a low NNT of 3.2 (2.6 to 4.2) based on good or excellent responses in relatively recent studies (high quality evidence). Ketoprofen gel had an NNT of 2.5 (2.0 to 3.4) from five studies in the 1980s, some with less well defined outcomes (moderate quality evidence). Ibuprofen gel had an NNT of 3.9 (2.7 to 6.7) from two studies with outcomes of marked improvement or complete remission (moderate quality evidence). All other drug and formulation combinations had NNT values above 4, indicating lesser efficacy.
These results are better than alternative topical products that might be used for acute musculoskeletal pain. There is no evidence to support the use of topical salicylate rubefacients (Derry 2014).
Treatment with a topical NSAID was not associated with an increase in local adverse events (skin reactions) compared with placebo (inert carrier), or in withdrawals due to adverse events (high quality evidence). The inert carrier was sometimes associated with mild skin irritation, but this rarely led to cessation of treatment, and quickly resolved. Systemic adverse events were uncommon and did not differ between topical NSAID and placebo (high quality evidence). Two participants experienced serious adverse events with diclofenac plaster and diclofenac gel, but it is unlikely that these were related to the study medications.
There were insufficient data directly comparing a topical NSAID with the same oral NSAID to draw conclusions about efficacy. Based on very limited data for oral NSAIDs, there were fewer systemic adverse events with topical than oral treatment. There were sufficient data only for topical piroxicam compared with topical indomethacin to compare one topical agent with another. These limited data suggested that piroxicam was more effective than indomethacin, and was less likely to cause local adverse events. It is worth noting here that topical indomethacin did not give significantly better pain relief than placebo in two of the three studies in this analysis.
Overall completeness and applicability of evidence
There is a tension between pooling studies to produce analyses with larger numbers and the subsequent large increases in clinical and statistical heterogeneity on the one hand, and using the approach of clinical homogeneity with subsequent smaller numbers of participants on the other hand. Previous reviews have taken the former approach; that is useful in demonstrating that topical NSAIDs 'work' by being significantly better than placebo. Because of the substantial increase in the amount of data available, in this review we have chosen to seek greater clinical homogeneity; this produces results that are more relevant to patient and prescriber choice.
There were too few studies comparing one topical NSAID versus another, or versus the same oral NSAID, to allow meaningful direct comparisons between individual drugs or routes of administration.
The conditions treated in these studies are representative of those likely to be suitable for acute treatment with topical NSAIDs. The mean age of participants in individual studies ranged from 25 to 57 years, and the nature of recruitment in many studies meant that participants were actively engaged in sporting activities. Nevertheless, older people in their 60s to 80s were also included in some studies, and the low levels of predominantly mild adverse events means that this route of administration of NSAIDs is suitable for all age groups able to manage the application process.
Information from other sources, mainly randomised studies lasting 12 weeks or more in older populations with arthritis, tend to confirm this. A systematic review of topical NSAIDs in older adults was difficult to interpret, but suggested that the range of withdrawal rates in these studies was similar with topical and oral NSAIDs (Makris 2010). It also claimed potentiation of warfarin effects, but that was with topical salicylate, not an NSAID. In contrast, a pooled safety analysis of topical diclofenac in people aged 75 years or older reported minimal changes, with a mean reduction in haemoglobin of less than 1 g/L with topical diclofenac, and a mean systolic blood pressure reduction of almost 4 mm Hg (Roth 2012). Two large randomised 12‐week studies comparing topical with oral diclofenac in arthritis reported lower rates of gastrointestinal adverse events with topical than oral, especially severe events, but larger reductions in haemoglobin with oral diclofenac (Simon 2009; Tugwell 2004).
The available evidence was limited by numbers to comment on rare but potentially serious adverse events. One example is the potential for photo‐sensitivity reactions with topical ketoprofen. Current advice from the Medicines and Healthcare products Regulatory Agency in the UK is to avoid direct sunlight, ultraviolet (UV) rays, sunlamps, and sunbeds while using topical ketoprofen, and to see a healthcare professional or go to hospital if they experience a skin reaction to sunlight, sunlamps, or sunbeds (MHRA 2009).
Quality of the evidence
All included studies were both randomised and double‐blind; none was considered at high risk of methodological bias. Many were carried out in the 1980s and 1990s when methodological rigor and detailed reporting were not given such high priority and studies did not always report details of the randomisation, treatment allocation, and blinding processes. More recent studies often did report methodological details, and tended to be larger (see Figure 4 for a comparison of quality of reporting for different dates and NSAIDs). Our primary outcome of clinical success was not always well‐defined, and was measured using different scales, but again more recent studies tended to report outcomes better.
The studies were conducted in different conditions, with somewhat different outcome definitions and duration, and with different topical NSAIDs and formulations. Moreover, the small size of many of the studies is likely to result in considerable chance variation (Counsell 1994; Moore 1998b). These factors would account for the high I2 values seen in several analyses. Despite these sources of potential clinical heterogeneity, most studies showed benefit of topical NSAID over placebo.
The design of studies to be able to demonstrate analgesic sensitivity is important in self limiting conditions such as strains and sprains. Too long a duration and the condition results in spontaneous resolution of painful symptoms, while too short a duration may be inadequate to show any effect. The decision by trialists to concentrate on outcomes closest to seven days of treatment appears to be prudent, and has been adopted in this and previous reviews. There are potential differences in response to treatment between strains and sprains and overuse‐type injuries such as tendinitis, and future reviews may examine this. At the present time, there are too few existing trials to explore any differences adequately.
Baseline pain may be a cause for concern. Seven studies did not report baseline pain levels (Billigmann 1996; Curioni 1985; Haig 1986; NCT01255423; NCT01272934; NCT01272947; Sinniger 1981), and a further 11 reported either mean levels of less than moderate pain or a significant proportion of individuals with less than moderate pain (Ăkermark 1990; Aoki 1984; Auclair 1989; Diebshlag 1990; Fujimaki 1985; Jenoure 1997; Linde 1985; Picchio 1981; Ramesh 1983; Sugioka 1984; Whitefield 2002), using recognised scales. Insufficient pain at baseline compromises the ability of a study to demonstrate any improvement. All the newly added studies reported baseline pain to be of at least moderate intensity.
Potential biases in the review process
There has been greater interest in topical NSAIDs in recent years, mainly because lower systemic drug levels reduce the risk of troublesome and severe adverse events, particularly in the gastrointestinal tract, and renal and cardiovascular systems. Most of the attention has been in chronic conditions such as osteoarthritis, with few studies in acute painful conditions. Low levels of serious adverse events with topical NSAIDs has been noted previously (Evans 1995), and the near absence of serious adverse events in this review is unlikely to be due to any biases in the review process.
One potential bias is that clinical trials for topical NSAIDs may not have been published. One previous review did find previously unpublished trials (Moore 1998a), but a subsequent attempt that included extensive contacts with pharmaceutical companies revealed no additional data (Mason 2004a). While some old unpublished studies of topical NSAIDs in acute painful conditions may exist, they constitute an unknown number of studies and participants whose results are unknown, and are likely to remain unknown. Furthermore, their relevance to current clinical practice may be limited as better formulations are developed. New systems of trial registration mean that we know what recent studies have been done or are ongoing; the number of studies and participants is known even if their results remain unknown. We identified in Clinicaltrials.gov three unpublished studies (612 participants) with adverse event data but no dichotomous efficacy data, 14 completed unpublished studies (4403 participants) with no results posted, and three ongoing studies (880 participants).
For the main topical NSAIDs of interest and where most information exists, about 4200 participants in this review provided data on efficacy for diclofenac, ibuprofen, ketoprofen, indomethacin, and benzydamine compared with placebo. For efficacy, there are unknown results from almost 5900 participants in studies known to have been done but essentially unpublished. Almost 6500 participants in this review provided information on local adverse events for topical NSAIDs compared with placebo. For local adverse events, the unknown results from known studies represents almost 5300 participants. It is clear that identified unpublished but unavailable study data amounts to a further potentially large increase in knowledge, over and above the 60% increase in numbers of participants already included in this updated review.
Based on efficacy data on known and available study results, unpublished trials showing no difference between any topical NSAID and topical placebo and involving 5500 participants would have to exist in order for the NNT to be as high as 9, at which point the effectiveness of topical NSAIDs would become clinically irrelevant (Moore 2006). This amount of unpublished negative data is obviously available, and while a negative result in all the identified studies is unlikely, knowledge would be greatly served by having these unpublished trial results available.
We have not yet attempted to obtain results from these clinical trials from the trial sponsors, because this takes a considerable amount of time and may not be successful. Moreover, the studies were spilt between nine different sponsors: Cerimon Pharmaceuticals (five studies), Novartis (four studies), Endo Pharmaceuticals (three studies), GlaxoSmithKline (two studies), Hisamatsu Pharmaceutical (two studies), and one each from Actavis, Pfizer, Imprimis Pharmaceutical, and Strategic Science & Technologies.
Agreements and disagreements with other studies or reviews
A review published in 2004 included some of the studies in this review and reported an NNT for all topical NSAIDs combined of 3.8 (3.4 to 4.4) for clinical success equivalent to half pain relief at seven days (Mason 2004a). That review found no difference between topical NSAID and placebo for local adverse events, as did this review. In turn, the Mason review was in broad agreement with the original systematic review on topical NSAIDs (Moore 1998a). To our knowledge, no previous review assembled sufficient trial data to analyse results by both drug and formulation, as was done here.

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Forest plot of comparison: 2 Individual NSAID versus placebo, outcome: 2.1 Clinical success.

L'Abbé plot of clinical success in studies of topical diclofenac versus topical placebo. The size of the symbol is proportional to the size of the study (inset scale). Dark blue: Emulgel; light blue: spray/gel; red: Flector; pink: other patch or plaster.

L'Abbé plot of clinical success in studies of topical ketoprofen versus topical placebo. The size of the symbol is proportional to the size of the study (inset scale). Light blue: ketoprofen gel; pink: ketoprofen plaster.

Comparison 1 Individual NSAID versus placebo, Outcome 1 Clinical success.

Comparison 1 Individual NSAID versus placebo, Outcome 2 Local adverse events.

Comparison 2 Diclofenac versus placebo (effect of formulation), Outcome 1 Clinical success.

Comparison 3 Ibuprofen versus placebo (effect of formulation), Outcome 1 Clinical success.
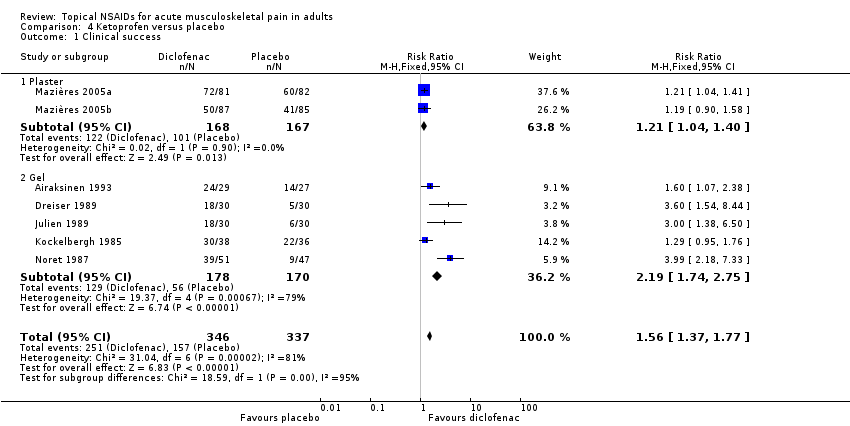
Comparison 4 Ketoprofen versus placebo, Outcome 1 Clinical success.
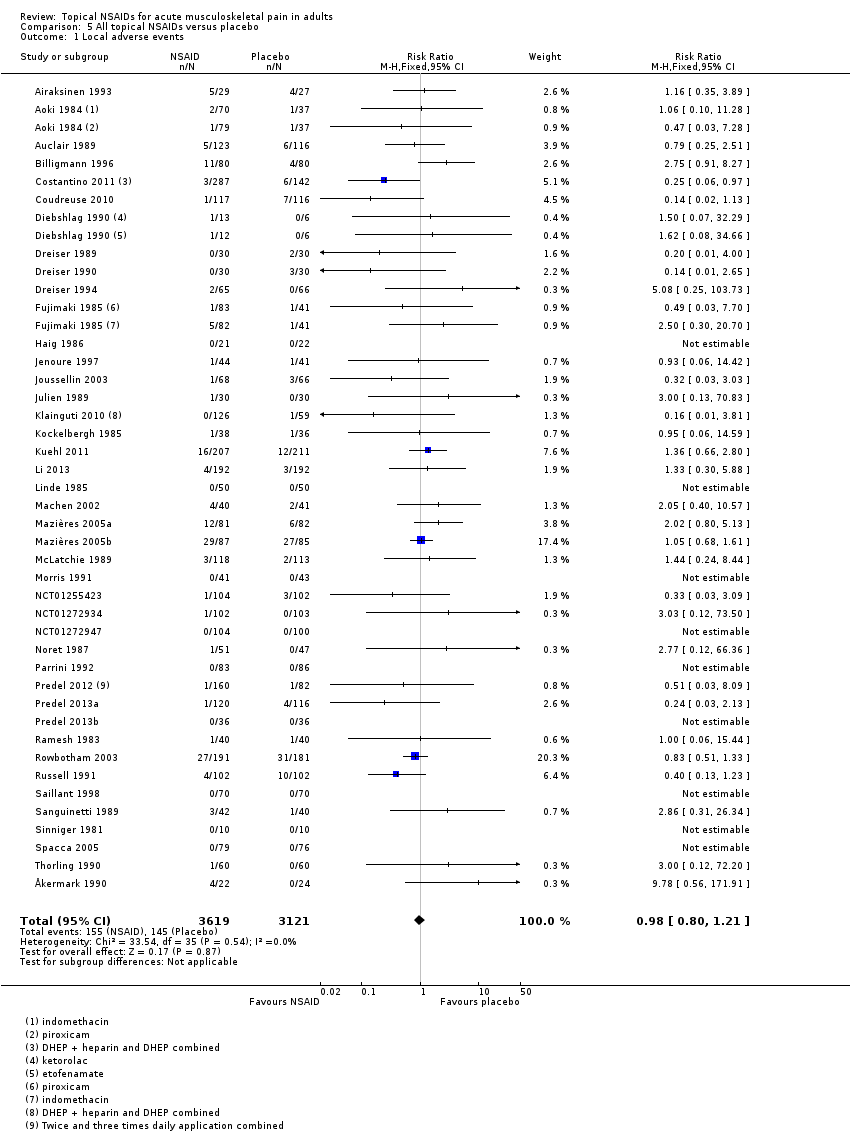
Comparison 5 All topical NSAIDs versus placebo, Outcome 1 Local adverse events.
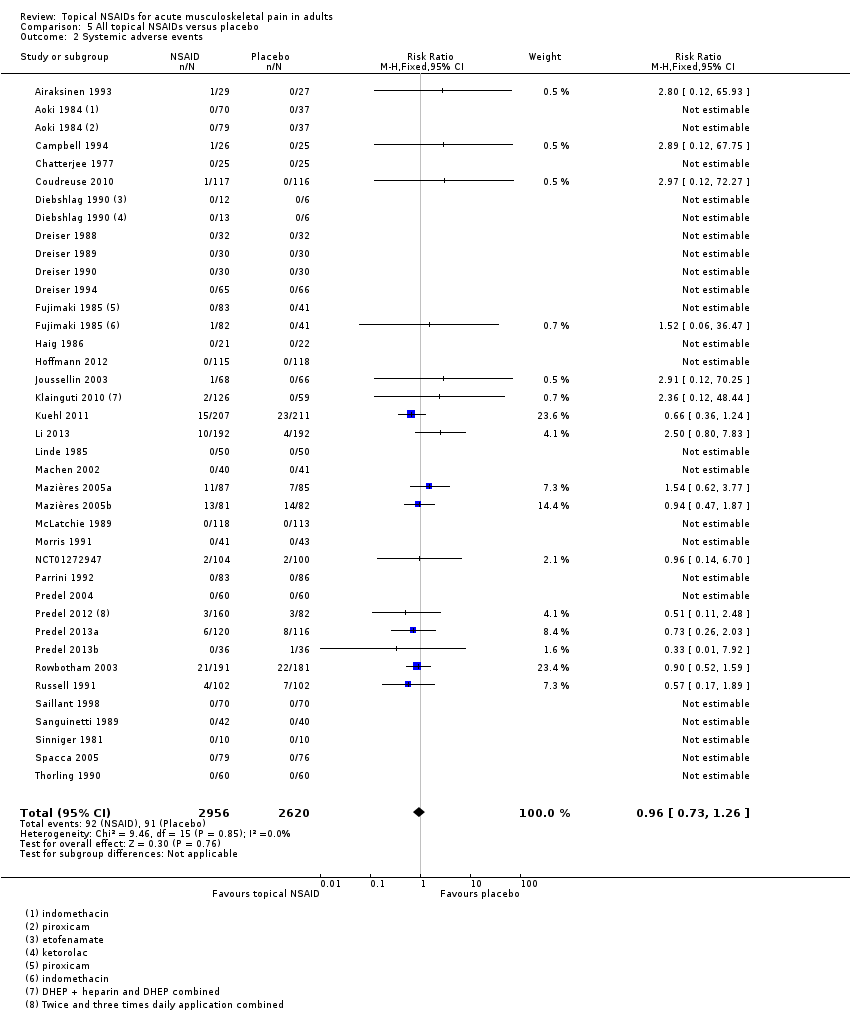
Comparison 5 All topical NSAIDs versus placebo, Outcome 2 Systemic adverse events.
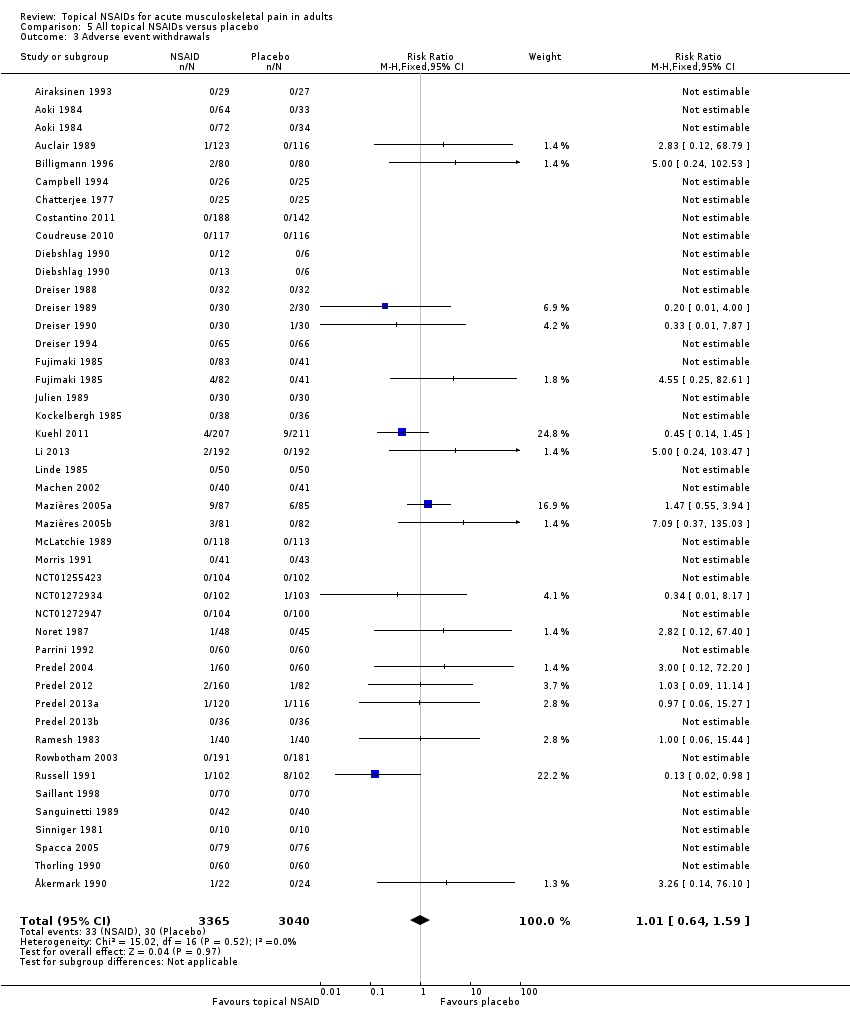
Comparison 5 All topical NSAIDs versus placebo, Outcome 3 Adverse event withdrawals.
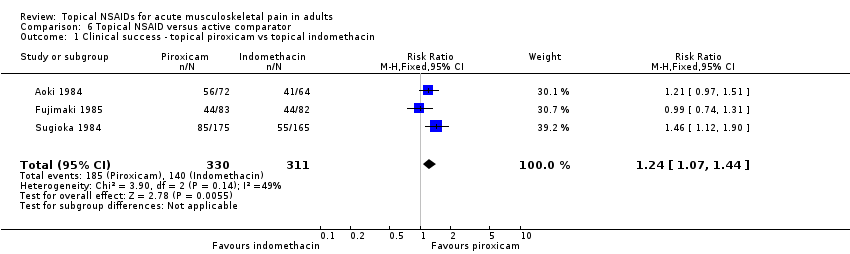
Comparison 6 Topical NSAID versus active comparator, Outcome 1 Clinical success ‐ topical piroxicam vs topical indomethacin.

Comparison 6 Topical NSAID versus active comparator, Outcome 2 Local adverse events ‐ topical piroxicam vs topical indomethacin.
| Topical NSAIDs compared with topical placebo for acute musculoskeletal pain in adults | ||||||
| Patient or population: adults with strains, sprains, or muscle pull Settings: community Intervention: topical NSAID (topical diclofenac, ibuprofen, and ketoprofen gels only shown here for efficacy) Comparison: topical placebo | ||||||
| Outcomes | Probable outcome with | Probable outcome with | RR, NNT, NNTp, or NNH | No of studies, participants | Quality of the evidence | Comments |
| Topical diclofenac gel (as Emulgel) Clinical success (eg 50% reduction in pain) | 780 in 1000 | 200 in 1000 | RR 3.4 (2.7 to 55) NNT 1.8 (1.5 to 2.1) | 2 studies 314 participants | High | Consistent results in 2 moderately sized recent studies of high quality |
| Topical ibuprofen gel Clinical success (eg 50% reduction in pain) | 420 in 1000 | 160 in 1000 | RR 2.7 (1.7 to 4.2) NNT 3.9 (2.7 to 6.7) | 2 studies 241 participants | Moderate | Modest effect size and numbers of participants |
| Topical ketoprofen gel Clinical success (eg 50% reduction in pain) | 720 in 1000 | 330 in 1000 | RR 2.2 (1.7 to 2.8) NNT 2.5 (2.0 to 3.4) | 5 studies 348 participants | Moderate | Modest effect size and numbers of participants, but studies small, with none recent |
| All topical NSAIDs Local adverse events | 46 in 1000 | 50 in 1000 | RR 1.0 (0.80 to 1.2) NNH not calculated | 42 studies 6125 participants | High | Large number of studies and participants with consistent results |
| All topical NSAIDs Systemic adverse events | 32 in 1000 | 35 in 1000 | RR 1.0 (0.7 to 1.3) NNH not calculated | 38 studies 5372 participants | High | Large number of studies and participants with consistent results |
| All topical NSAIDs Withdrawals ‐ adverse events | 11 in 1000 | 11 in 1000 | RR 1.0 (0.7 to 1.7) NNH not calculated | 42 studies 5790 participants | High | Large number of studies and participants with consistent results |
| Serious adverse events | 1 in total | 0 in total | Not calculated | All data | Low | Small numbers of events |
| GRADE Working Group grades of evidence | ||||||
| CI: confidence interval; RR: risk ratio; NNT: number needed to treat for an additional beneficial outcome; NNTp: number needed to treat to prevent an event happening; NNH: number needed to treat for an additional harmful outcome. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Diclofenac | 10 | 2050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.49, 1.72] |
| 1.2 Ibuprofen | 5 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.33, 2.01] |
| 1.3 Ketoprofen | 7 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.37, 1.77] |
| 1.4 Piroxicam | 3 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.27, 1.73] |
| 1.5 Indomethacin | 3 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.03, 1.55] |
| 1.6 Benzydamine | 3 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.96, 1.38] |
| 2 Local adverse events Show forest plot | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Diclofenac | 15 | 3271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.56, 1.10] |
| 2.2 Ibuprofen | 3 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.98, 5.43] |
| 2.3 Ketoprofen | 8 | 852 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.83, 1.70] |
| 2.4 Piroxicam | 3 | 522 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.08] |
| 2.5 Felbinac | 3 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.49, 7.50] |
| 2.6 Indomethacin | 3 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [0.91, 7.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 10 | 2050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.49, 1.72] |
| 1.1 Plaster ‐ Flector | 4 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.36, 1.71] |
| 1.2 Plaster ‐ other | 3 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.37, 1.75] |
| 1.3 Gel ‐ Emulgel | 2 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.84 [2.68, 5.50] |
| 1.4 Gel ‐ other | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.05, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 5 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.33, 2.01] |
| 1.1 Cream | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.03, 1.59] |
| 1.2 Gel | 2 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.69, 4.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 7 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.37, 1.77] |
| 1.1 Plaster | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.04, 1.40] |
| 1.2 Gel | 5 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [1.74, 2.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Local adverse events Show forest plot | 42 | 6740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.80, 1.21] |
| 2 Systemic adverse events Show forest plot | 36 | 5576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.26] |
| 3 Adverse event withdrawals Show forest plot | 42 | 6405 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.64, 1.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success ‐ topical piroxicam vs topical indomethacin Show forest plot | 3 | 641 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.07, 1.44] |
| 2 Local adverse events ‐ topical piroxicam vs topical indomethacin Show forest plot | 3 | 671 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.09, 0.47] |

