Probióticos para el tratamiento de la diarrea persistente en niños
Resumen
Antecedentes
Según algunos estudios, la diarrea persistente (diarrea de más de 14 días de duración) representa un tercio de todos los casos de muertes relacionadas con la diarrea en países en desarrollo. Los probióticos pueden ayudar en el tratamiento.
Objetivos
Evaluar los probióticos para el tratamiento de la diarrea persistente en niños.
Métodos de búsqueda
Se hicieron búsquedas en el Registro Especializado de Ensayos Controlados del Grupo Cochrane de Enfermedades Infecciosas (Cochrane Infectious Diseases Group), CENTRAL, MEDLINE, EMBASE y LILACS. También se contactó con los autores de los ensayos incluidos y las organizaciones que trabajan en el campo, y se verificaron las listas de referencias. La fecha de la búsqueda más reciente fue el 13 de diciembre 2012
Criterios de selección
Ensayos controlados aleatorizados que comparan un agente probiótico específico con placebo o ningún probiótico en niños con diarrea persistente.
Obtención y análisis de los datos
Dos revisores evaluaron la elegibilidad, el riesgo de sesgo, y extrajeron y analizaron los datos. Las diferencias se resolvieron mediante discusión. Se realizaron los análisis estadísticos con un modelo de efectos fijos, y los resultados se expresaron como diferencia de medias (DM) para los resultados continuos, con intervalos de confianza (IC) del 95%.
Resultados principales
Se incluyeron cuatro ensayos, con un número total de 464 participantes; un ensayo tenía bajo riesgo de sesgo. El metanálisis indicó que los probióticos redujeron la duración de la diarrea persistente (diferencia de medias 4,02 días, IC del 95%: 4,61 a 3,43 días; n = 324, dos ensayos). La frecuencia de las deposiciones se redujo con los probióticos en dos ensayos. Un ensayo informó una estancia hospitalaria más corta, lo que fue significativo, pero los casos fueron pocos. No se informó sobre eventos adversos.
Conclusiones de los autores
Hay pruebas limitadas que sugieren que los probióticos pueden ser eficaces para el tratamiento de la diarrea persistente en los niños.
Resumen en términos sencillos
Probióticos para la diarrea persistente en niños
La diarrea persistente se define como un episodio diarreico de inicio agudo pero que luego se extiende por 14 días o más. Es una causa importante de morbilidad y mortalidad en niños menores de cinco años en los países en desarrollo. No se sabe del todo la causa de la diarrea persistente pero probablemente es de naturaleza compleja; esto a su vez dificulta el manejo de la afección. Los probióticos son bacterias y levaduras con características similares a las bacterias que habitualmente se encuentran en el intestino sano. Estos microorganismos, llamados "bacterias amistosas", se han usado en varios estudios para tratar la diarrea infecciosa aguda con resultados alentadores. Esta revisión encontró cuatro ensayos que incluyeron niños con diarrea persistente. Dos estudios, con un total combinado de 324, mostraron que los probióticos acortan la duración de la diarrea y reducen la frecuencia de las heces en el día 5. Un estudio (235 niños) sugirió que los probióticos reducen la estancia hospitalaria. Tres de los cuatro ensayos informaron que no hubo eventos adversos. Sin embargo, esta revisión se ve limitada por el reducido número de ensayos con pocos participantes, y por lo tanto, puede no representar una estimación fiable del efecto de los probióticos.
Authors' conclusions
Summary of findings
| Probiotic compared to placebo for treating children with persistent diarrhoea | ||||||
| Patient or population: Children with persistent diarrhoea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Probiotic | |||||
| Duration of diarrhoea | The mean duration of diarrhoea ranged across control groups from | The mean Duration of diarrhoea in the intervention groups was | 324 | ⊕⊕⊕⊝ | ||
| Stool frequency on day 5 | See comment | See comment | Not estimable | 327 | ⊕⊕⊝⊝ | Both studies showed a benefit with probiotics, however the size of the benefit was very different in the two trials so the data were not pooled |
| Hospital stay | The mean hospital stay in the control groups was | The mean Hospital stay in the intervention groups was | 235 | ⊕⊕⊕⊝ | ||
| Death from any cause ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not estimable |
| Weight‐for‐age z score ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not estimable |
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 No serious study limitations: Basu 2007 adequately concealed allocation and blinded both participants and study staff to be considered at low risk of bias. As this was the larger study contributing 86% of the data to the meta‐analysis we did not downgrade for study limitations. Gaon 2003 did not adequately describe the study methodology. | ||||||
Background
The World Health Organization (WHO) defines persistent diarrhoea as an illness of proven or presumed infectious aetiology that lasts 14 days or more (Anonymous 1988). The definition excludes causes of chronic diarrhoea that may appear as persistent diarrhoea; for example, celiac disease, food‐related enteropathies, and congenital enteropathies. Persistent diarrhoea accounts for 3% to 20% of all diarrhoeal episodes in children aged less than five years (IWGPD 1996). It is also directly responsible for between 36% and 54% of all diarrhoea‐related deaths according to two large, community‐based studies (Schorling 1990; Fauveau 1992). Thus, the main consequences of persistent diarrhoea are morbidity (with an increased risk of hospital admission), death, and malnutrition.
The cause of persistent diarrhoea is not known and the pathogenic mechanisms are not well understood; most of the viruses, parasites, and bacterial pathogens that cause acute diarrhoea have also been associated with persistent diarrhoea (Ochoa 2004). The management of persistent diarrhoea is complex because the etiology and pathogenesis are complex. It includes adequate dietary management, micronutrient supplementation, adequate rehydration, and antimicrobials (Ochoa 2004). In developing countries, where persistent diarrhoea is a problem, it is recognized that frequent recurrence of acute diarrhoeal episodes (less than 14 days' duration) result in nutritional compromise, which is in turn the most important epidemiological risk factor for persistent diarrhoea (Bhandari 1989). Other risk factors for persistent diarrhoea include lack of breastfeeding and immune deficiencies (Bhutta 2004).
Probiotics are defined as living organisms that when administered in adequate amounts confer a health benefit on the host (Pineiro 2007). They are used widely for various indications because of their widespread acceptance and general lack of adverse effects. Probiotics most commonly used include Bifidobacterium and two genera of lactic acid bacteria, namely, Lactobacillus and Streptococcus. Acute infectious diarrhoea is the most investigated field in the area of probiotic use in children; five recent systematic reviews have described the role of probiotics in acute infectious diarrhoea (Szajewska 2001; Huang 2002; Van Niel 2002; Allen 2003; McFarland 2006). Each review demonstrated that probiotics had a good safety profile, significantly reduced the duration of diarrhoea by 13.4 to 30.5 hours (range), reduced stool frequency, and reduced the duration of hospital stays. However, the effects of probiotics in acute diarrhoea are not generalizable to persistent diarrhoea, since most trials of probiotics in acute diarrhoea have been done in otherwise healthy, well nourished children from developed countries.
The rationale for using probiotics to treat infectious diarrhoea is based on the assumption that they modify the composition of the intestinal microflora and act against enteric pathogens. It has recently been reported that probiotics have multiple properties that attenuate inflammation in cases of inflammatory bowel disease. The main mechanisms of action include: induction of regulatory T cells that suppress inflammation‐inducing effector cells, maintenance of the gastrointestinal barrier function, and the interference with the ability of pathogens to colonize and infect the mucosa (Boirivant 2007). Probably two or more of these mechanisms operate simultaneously, and these mechanisms may also be beneficial in persistent diarrhoea. Moreover, the beneficial effects of probiotics in acute diarrhoea in children seem to be strain‐dependent, dose‐dependent (greater for doses of > 1010 colony forming units), significant in people with viral gastroenteritis, and more evident when treatment with probiotics is initiated early in the course of disease (Szajewska 2005).
Objectives
To evaluate the efficacy and adverse effects of probiotics for the treatment of persistent diarrhoea in children.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
Children (0 to 18 years of age) with persistent diarrhoea (duration ≥ 14 days) that is proven (pathogens isolated from stools) or presumed to be caused by an infectious agent.
Types of interventions
Intervention
Specific, identified probiotic.
Trials investigating yogurt or other fermented foods in which a specific probiotic agent is not identified are not eligible.
Control
Placebo or no treatment.
Intervention and control arms to be otherwise treated identically in relation to other treatments and drugs.
Types of outcome measures
Primary
-
Duration of diarrhoea.
Secondary
-
Stool frequency.
-
Stool volume.
-
Weight‐for‐age z score.
-
Hospital stay.
-
Death from any cause.
Adverse events
-
Serious (leads to death, hospitalisation, or disability, is life‐threatening, or requires intervention to prevent permanent impairment).
-
Requiring discontinuation of treatment.
-
Other.
Search methods for identification of studies
We identified all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Databases
We searched the following databases using the search terms and strategy as described in Table 1: Cochrane Infectious Disease Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library; MEDLINE; EMBASE; and LILACS. We also searched the metaRegister of Controlled Trials (mRCT) using 'diarrhoea', ' probiotic*', 'lactobacill*' and 'bifidobacter*' as search terms.
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | diarrhea | diarrhea | diarrhea | diarrhea | diarrhea |
| 2 | diarrhoea | diarrhoea | diarrhoea | diarrhoea | diarrhoea |
| 3 | 1 or 2 | 1 or 2 | 1 or 2 | 1 or 2 | 1 or 2 |
| 4 | probiotic* | lactobacill* | lactobacill* | lactobacill$ | probiotic$ |
| 5 | lactobacill* | lactococc* | lactococc* | lactococc$ | lactobacill$ |
| 6 | Bifidobacter* | Bifidobacter* | Bifidobacter* | Bifidobacter$ | Bifidobacter$ |
| 7 | 4 or 5 or 6 | Enterococc* | Enterococc* | Enterococc$ | 4 or 5 or 6 |
| 8 | 3 and 7 | Streptococc* | Streptococc* | Streptococc$ | 3 and 7 |
| 9 | child* | Saccharomyces | Saccharomyces | Saccharomyces | child$ |
| 10 | infant* | 4‐9/OR | 4‐9/OR | 4‐9/OR | Infant$ |
| 11 | pediatr* | 3 and 10 | 3 and 10 | 3 and 10 | pediatr$ |
| 12 | 9 or 10 or 11 | child* | child* | child$ | 9 or 10 or 11 |
| 13 | 8 and 12 | Infant* | Infant* | Infant$ | 8 and 12 |
| 14 | — | pediatr* | pediatr* | pediatr$ | — |
| 15 | — | 12 or 13 or 14 | 12 or 13 or 14 | 12 or 13 or 14 | — |
| 16 | — | 11 and 15 | 11 and 15 | 11 and 15 | — |
aCochrane Infectious Diseases Group Specialized Register.
bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Lefebvre 2008); upper case: MeSH or EMTREE heading; lower case: free text term.
Organizations
To help identify unpublished and ongoing trials, we contacted researchers at organizations including the International Scientific Association for Probiotics and Prebiotics.
Reference lists
We checked the reference lists of all studies identified by the above methods.
Data collection and analysis
Selection of studies
Two authors (CABM and NYCP) independently screened the search results using article titles and abstracts (where available). The full text of the selected articles was retrieved and scrutinized to ensure that multiple publications from the same trial were included only once. The same authors (CABM and NYCP) then independently selected articles for inclusion according to a standardized form to assess the eligibility of trials. Disagreements were resolved through discussion with a third author (GBA). The trial authors were contacted for clarification if it was unclear whether a trial is eligible for inclusion. Excluded trials along with the reason for exclusion are listed in the section, 'Characteristics of excluded studies '.
Data extraction and management
Two authors (GBA and RARG) independently extracted the data using standard forms. Any differences were resolved through discussion with a third author (CABM). Attempts were made to obtain any missing data from the trial authors. We aimed to extract the following data: hazard ratios and standard deviations for duration of diarrhoea if trials reported them, otherwise we extracted mean and standard deviations; the number of stools and the number of person days; the mean stool output from the start of the intervention; the mean weight for age z score; the mean duration of hospital stay and its standard deviations; and the number of deaths in each group. The authors carried out an intention‐to‐treat analysis, and extracted the number of participants randomized and analysed in each group for all outcomes.
Assessment of risk of bias in included studies
Two authors (GBA and NYCP) independently assessed the risk of bias of each trial using The Cochrane Collaboration's risk of bias tool (Higgins 2008). We followed the guidance to make judgements on the risk of bias in six domains: sequence generation; allocation concealment; blinding (of participants, personnel, and outcome assessors); incomplete outcome data; selective outcome reporting; and other sources of bias. We categorized these judgements as 'yes' (low risk of bias), 'no' (high risk of bias), or 'unclear'. Where our judgment was unclear we attempted to contact the trial authors for clarification.
Assessment of reporting biases
We assessed publication bias using the funnel plot if there were about 10 or more trials included in a meta‐analysis.
Data synthesis
We analysed the data using Review Manager 5. Results were combined unless diversity (clinical and methodological heterogeneity) or statistical heterogeneity (non‐overlapping confidence intervals) made this unreasonable. We pooled dichotomous data using the risk ratio and calculated the number needed to treat when appropriate. If continuous data were summarized by arithmetic means and standard deviation data, then we combined them using the mean differences; where continuous data were summarized using geometric means, we combined them on the log scale using the generic inverse variance method and reported them on the natural scale. The hazard ratio was combined on the log scale using the generic inverse variance method for time to event data. We presented all results with 95% confidence intervals.
The intention‐to‐treat principle was applied; however, if there was a discrepancy in the number randomized and the number analysed in each group, we calculated the percentage loss to follow up in each group. We assessed pooled data using available case analysis rather than intention‐to‐treat analysis with imputation. We used a fixed‐effect model unless there was statistically significant heterogeneity between trials, in which case used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
Heterogeneity amongst trials was investigated by looking at whether a graphical plot of the confidence intervals for the results of each study overlapped, using a standard Chi2 test with significance set at P < 0.10, and using the I2 test statistic (> 50% will be considered as substantial heterogeneity). Subgroup analyses were subdivided by: identified diarrhoeal pathogens, trial low‐ to middle‐income/high‐income setting, probiotic strain and dosage of probiotic.
Sensitivity analysis
We performed sensitivity analysis in order to explore whether effect size was different in adequately concealed trials compared with the rest.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Our search (in August 2010) identified 198 potentially relevant studies. Independent review (CABM, NYCP) of titles and/or abstracts identified 23 potentially relevant studies for review (21 full‐text and 2 abstracts). An additional study was found through the reference list of included studies, and one through a different search undertaken for another related publication. Of these studies, two were duplicated publications of prior reports, and one was a preliminary report of a potentially relevant study. Finally, four trials met inclusion criteria and were included in the review. In one trial, which referred to chronic diarrhoea, participants actually met the criteria for persistent diarrhoea (Castañeda 1995). One unpublished trial is awaiting further evaluation. The International Scientific Association for Probiotics and Prebiotics was contacted in October 2008 but no additional references were found. A search update in December 2012 revealed one additional trial which was excluded.
Risk of bias in included studies
See: Characteristics of included studies.
Generation of allocation sequence
Three trials used adequate methods to generate the allocation sequence.The method used in the other trial was unclear (Gaón 2003).
Allocation concealment
Allocation concealment was mixed. One study demonstrated adequate allocation concealment (Basu 2007), two studies were unclear as to whether allocation concealment was performed (Castañeda 1995; Gaón 2003), and in the remaining study allocation concealment was not performed.
Blinding
Blinding of the participants, providers, and assessors was only done in one trial (Basu 2007). Two trials described double blinding in broad terms, which made it impossible to know exactly who was blinded (Castañeda 1995; Gaón 2003). The remaining study was open‐labelled.
Follow up and exclusions
Reported outcome data was considered satisfactory with a low risk of bias in three studies. One trial (Touhami 1992) were considered to be at high risk of bias due to number of exclusions (>10%).
Selective reporting
Reporting appeared to include all important outcomes in one study (Basu 2007). In two studies (Touhami 1992; Castañeda 1995) it was unclear whether important persistent diarrhoea outcomes had not occurred or had not been reported. In Gaón 2003, the treatment failure outcome was not reported.
Other potential sources of bias
One trial was apparently free of other problems that could put it at a risk of bias (Basu 2007). It was unclear whether the remaining trials were free from potential sources of bias. Small number of patients were recruited in the three intervention groups in Gaón 2003, there was insufficient data to assess the baseline balance in Castañeda 1995, and the incorrect enrolment of 14 children could have introduced baseline imbalance in Touhami 1992.
Effects of interventions
The primary outcome was reported in two studies (Gaón 2003; Basu 2007), and after combining the results of the trials, diarrhoea duration was reduced with probiotics by ‐4.02 days (MD, 95% CI ‐4.61 to ‐3.43; 324 participants, two trials, Figure 1).
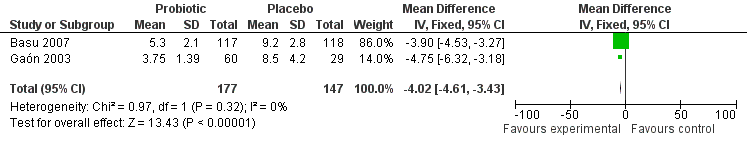
Forest plot of comparison: 1 Probiotic versus placebo, outcome: 1.1 duration of diarrhoea.
For the secondary efficacy endpoint of stool frequency on day 5, data were available from two trials (Gaón 2003; Basu 2007). The data from the three‐arm study by Gaón 2003 showed that on day 5, the stool frequency decreased to 2.0 ± 2.0 in the saccharomyces group and 1.5 ± 0.9 in the lactobacilli group, respectively, in comparison with placebo (5.2 ± 3.0), (P < 0.001). The data from the study by Basu 2007 showed a significant difference in stool frequency on day 5 during treatment between probiotic and placebo groups (5.2 ± 2.1 versus 10.2 ± 3.2).
For the secondary efficacy endpoint of stool volume, data from one trial (Touhami 1992) suggested no difference in total stool volume between probiotic group and placebo group (1130 ± 250 versus 830 ± 180, P = 0.89, authors' calculation). For the secondary endpoint of hospital stay, one trial (Basu 2007) found a reduction in hospital stay (7.3 ± 1.6 versus 15.5 ± 1.5, P < 0.05). No studies reported weight‐for‐age Z score or death from any cause. Three out of four trials reported that no adverse events occurred.
Despite we planned an intention‐to‐treat analysis, it was not feasible, so we performed a complete‐case analysis.
Discussion
Summary of main results
See: Summary of findings table 1.
Despite the comprehensive search strategy used, only four relevant trials were identified. There were variability between studies in probiotic tested, treatment regimens, and definitions of outcomes measures; and the small number of studies limited the ability to perform a subgroup analysis, especially with regards to the probiotic strains and identified diarrhoeal pathogens.
Concerning the primary outcome, two trials (Gaón 2003; Basu 2007) examined whether probiotics reduced the duration of diarrhoea. The results of the pooled analysis were significant in favour of the intervention group. Because there were only two trials and the number of participants in the studies were small, it is hard to draw a definitive conclusion about the effects of probiotics on the duration of persistent diarrhoea. One problem with the analysis presented in these trials was that the primary outcome was not treated as a time‐to‐event outcome.
Concerning secondary outcomes, there was evidence from two studies (Gaón 2003; Basu 2007) that probiotic treatment leads to a reduction in stool frequency on day 5. One study (Touhami 1992) reported that stool volume showed no significant difference between the two groups, and other study (Basu 2007) showed a significant reduction in hospital stay. No conclusion regarding probiotic's impact on weight‐for‐age Z score or death can be drawn from this review as trials included were not designed to look at these outcomes.
One trial did not report adverse events (Touhami 1992), and three trials reported that no events occurred.
Completeness and applicability of evidence
All trials were conducted in hospitals and involved children up to the age of six years from middle‐income countries. Studies including children from low‐income countries, where persistent diarrhoea is more common and interventions most likely to be beneficial, were not found. Moreover, the number of studies and the number of participants included in review analysis were small, and review outcomes were poorly reported.
Although two of the included trials (Gaón 2003; Basu 2007) showed a significant reduction of diarrhoea duration and stool frequency, the available data does not support the use of probiotics as a standard treatment for persistent diarrhoea.
One trial, Castañeda 1995, classified participants as having chronic diarrhoea; however, they met criteria for persistent diarrhoea (which was confirmed by the author). In addition, the endpoints of this trial were not the same as the intended outcome measures of the review.
Quality of the evidence
All four included trials were randomized, controlled studies. However, only one trial (Basu 2007) was at a low risk of bias because it had adequate concealment of randomisation, good follow up, and blinded outcome assessment. Two trials (Castañeda 1995; Gaón 2003) were at a unclear risk of bias, since they had an unclear risk of bias for one or more key domains, specifically for allocation concealment and blinding. The remaining trial (Touhami 1992) was at a high risk of bias with no allocation concealment, no blinding, and excessive loss to follow‐up (more than 10%). Hence, the identified evidence did not permit a robust conclusion to be reached on the efficacy of probiotics for treating persistent diarrhoea.
Potential biases in the review process
The literature search was conducted by the Cochrane Infectious Diseases Group Information Specialist, making it unlikely that any relevant trial have been missed; however, it is possible that we have overlooked small unpublished trials.
Although we tried to collect all relevant data, the possibility of missing data remained. We have contacted the authors for data but have not yet received a reply or have not obtained useful information.
Another limitation of this review is the fact that the meta‐analysis for duration of diarrhoea was performed with only two trials, especially when one of them is a small three‐arm trial. The two intervention groups of the three‐arm trial were combined into one before performing the meta‐analysis.
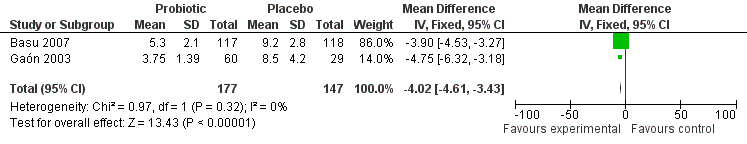
Forest plot of comparison: 1 Probiotic versus placebo, outcome: 1.1 duration of diarrhoea.
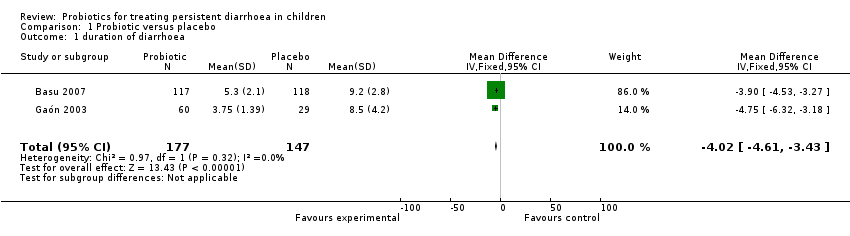
Comparison 1 Probiotic versus placebo, Outcome 1 duration of diarrhoea.
| Probiotic compared to placebo for treating children with persistent diarrhoea | ||||||
| Patient or population: Children with persistent diarrhoea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Probiotic | |||||
| Duration of diarrhoea | The mean duration of diarrhoea ranged across control groups from | The mean Duration of diarrhoea in the intervention groups was | 324 | ⊕⊕⊕⊝ | ||
| Stool frequency on day 5 | See comment | See comment | Not estimable | 327 | ⊕⊕⊝⊝ | Both studies showed a benefit with probiotics, however the size of the benefit was very different in the two trials so the data were not pooled |
| Hospital stay | The mean hospital stay in the control groups was | The mean Hospital stay in the intervention groups was | 235 | ⊕⊕⊕⊝ | ||
| Death from any cause ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not estimable |
| Weight‐for‐age z score ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not estimable |
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 No serious study limitations: Basu 2007 adequately concealed allocation and blinded both participants and study staff to be considered at low risk of bias. As this was the larger study contributing 86% of the data to the meta‐analysis we did not downgrade for study limitations. Gaon 2003 did not adequately describe the study methodology. | ||||||
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | diarrhea | diarrhea | diarrhea | diarrhea | diarrhea |
| 2 | diarrhoea | diarrhoea | diarrhoea | diarrhoea | diarrhoea |
| 3 | 1 or 2 | 1 or 2 | 1 or 2 | 1 or 2 | 1 or 2 |
| 4 | probiotic* | lactobacill* | lactobacill* | lactobacill$ | probiotic$ |
| 5 | lactobacill* | lactococc* | lactococc* | lactococc$ | lactobacill$ |
| 6 | Bifidobacter* | Bifidobacter* | Bifidobacter* | Bifidobacter$ | Bifidobacter$ |
| 7 | 4 or 5 or 6 | Enterococc* | Enterococc* | Enterococc$ | 4 or 5 or 6 |
| 8 | 3 and 7 | Streptococc* | Streptococc* | Streptococc$ | 3 and 7 |
| 9 | child* | Saccharomyces | Saccharomyces | Saccharomyces | child$ |
| 10 | infant* | 4‐9/OR | 4‐9/OR | 4‐9/OR | Infant$ |
| 11 | pediatr* | 3 and 10 | 3 and 10 | 3 and 10 | pediatr$ |
| 12 | 9 or 10 or 11 | child* | child* | child$ | 9 or 10 or 11 |
| 13 | 8 and 12 | Infant* | Infant* | Infant$ | 8 and 12 |
| 14 | — | pediatr* | pediatr* | pediatr$ | — |
| 15 | — | 12 or 13 or 14 | 12 or 13 or 14 | 12 or 13 or 14 | — |
| 16 | — | 11 and 15 | 11 and 15 | 11 and 15 | — |
| aCochrane Infectious Diseases Group Specialized Register. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 duration of diarrhoea Show forest plot | 2 | 324 | Mean Difference (IV, Fixed, 95% CI) | ‐4.02 [‐4.61, ‐3.43] |

