Capsaïcine topique pour la douleur neuropathique chronique chez l'adulte
Résumé scientifique
Contexte
Les crèmes topiques contenant de la capsaïcine sont utilisées pour traiter la douleur associée à un large éventail de troubles chroniques, notamment la douleur neuropathique. Une fois appliquée sur la peau, la capsaïcine entraîne une augmentation de la sensibilité aux stimuli nocifs, suivie d'une période de sensibilité réduite et d'une désensibilisation durable après des applications répétées. L'efficacité et la tolérance de la capsaïcine pour le traitement des neuropathies chroniques douloureuses ne sont pas clairement établies.
Objectifs
Examiner les preuves issues d'essais contrôlés portant sur l'efficacité et la tolérance de la capsaïcine en application topique pour la douleur neuropathique chronique chez l'adulte.
Stratégie de recherche documentaire
Les bases de données Cochrane CENTRAL, MEDLINE, EMBASE et l'Oxford Pain Relief Database ont été consultées en mai 2009.
Critères de sélection
Les essais randomisés en double aveugle et contrôlés par placebo d'une durée d'au moins six semaines utilisant la capsaïcine topique pour traiter la douleur neuropathique.
Recueil et analyse des données
Deux auteurs de revue ont évalué la qualité et la validité des essais et extrait les données de manière indépendante. Des informations ont été extraites concernant le nombre de participants ressentant un soulagement de la douleur (amélioration clinique) après au moins six semaines et présentant des réactions cutanées locales ; ces données ont été utilisées pour calculer le risque relatif et le nombre de sujets à traiter pour observer un bénéfice du traitement (NST) ou pour nuire (NNN). Des informations supplémentaires concernant la définition du soulagement de la douleur et les événements indésirables spécifiques ont été recherchées.
Résultats principaux
Six études (389 participants) comparaient l'application régulière d'une crème contenant une faible dose de capsaïcine (0,075 %) à une crème placebo ; le NST pour tout soulagement de la douleur pendant six à huit semaines était de 6,6 (4,1 à 17). Deux études (709 participants) comparaient une seule application d'un patch contenant une dose élevée de capsaïcine (8 %) à un patch de placebo ; le NST pour un soulagement de la douleur ≥ 30 % pendant douze semaines était de 12 (6,4 à 70). Les réactions cutanées locales étaient plus fréquentes avec la capsaïcine, étaient généralement tolérables et s'atténuaient avec le temps ; le NNN pour une application répétée à faible dose était de 2,5 (2,1 à 3,1). Les données étaient insuffisantes pour analyser l'un ou l'autre de ces ensembles de données par affection ou définition du résultat. Toutes les études étaient conformes aux critères minimums de qualité et de validité, mais le maintien de l'assignation en aveugle posait un problème potentiel.
Conclusions des auteurs
La capsaïcine, sous forme d'application répétée d'une crème à faible dose (0,075 %) ou d'application unique d'un patch à dose élevée (8 %), pourrait apporter un certain soulagement de la douleur chez certains patients atteints de neuropathie douloureuse. L'irritation cutanée locale est fréquente. Elle est souvent légère et passagère mais pourrait entraîner un arrêt prématuré. Les effets systémiques indésirables sont rares. Les estimations des bénéfices et effets délétères ne sont pas solides car les données disponibles concernant les différents troubles neuropathiques sont insuffisantes et que la définition des résultats est variable.
PICOs
Résumé simplifié
Capsaïcine appliquée sur la peau pour la douleur neuropathique chronique chez l'adulte
La capsaïcine topique (appliquée sur la peau) peut permettre de soulager la douleur associée à plusieurs affections neuropathiques douloureuses mais peut causer une irritation locale de la peau entraînant une sensation de brûlure ou de démangeaison. Le niveau de soulagement de la douleur ressenti par les patients est incertain en raison des différentes définitions utilisées dans ces études. La fiabilité de l'estimation du nombre d'individus pouvant potentiellement bénéficier de ce traitement est limitée car le nombre de participants était insuffisant et que les réponses variaient probablement pour les différentes affections neuropathiques. La capsaïcine seule ou combinée à un autre traitement pourrait s'avérer utile pour soulager la douleur des patients qui ne répondent pas aux autres traitements disponibles ou ne les tolèrent pas.
Authors' conclusions
Background
This is an update of a review that considered all strengths and formulations of capsaicin and was first published in 2009 (Derry 2009). At that time there were few studies of the high‐concentration (8%) patch; more studies of that formulation are now available. We decided to split the review into low (< 1%) and high (8%) concentration formulations because the conditions of their use are completely different, and because of the 100‐fold difference in concentration of capsaicin between them. This review will consider use of high‐concentration capsaicin; low‐concentration is considered in a separate review (Derry 2012).
Data for the incidence of neuropathic pain are difficult to obtain, but one systematic review of prevalence and incidence in the Oxford region of the UK indicates prevalence rates per 100,000 of 34 for postherpetic neuralgia (PHN), 400 for diabetic neuropathy and trigeminal neuropathy, and 2000 for fibromyalgia (McQuay 2007). Different estimates in the UK indicate incidences per 100,000 person‐years observation of 40 (95% confidence intervals (CI) 39 to 41) for PHN, 27 (26 to 27) for trigeminal neuralgia, 1 (1 to 2) for phantom limb pain, and 15 (15 to 16) for painful diabetic neuropathy (PDN), with rates decreasing in recent years for phantom limb pain and PHN and increasing for PDN (Hall 2006). The prevalence of neuropathic pain in Austria was reported as being 3.3% (Gustorff 2008). More recent surveys tend to agree that around 15% to 25% of patients with chronic pain (at least moderate pain lasting three months or longer) have neuropathic symptoms (Ohayon 2012; Toth 2009; Yawn 2009), and one systematic review of prevalence and incidence studies showed that the percentage with neuropathic symptoms varies with painful condition (Sadosky 2008). Many patients with neuropathic pain experience marked long‐term reduction in health‐related quality of life, as for example, with herpes zoster (Bouhassira 2012).
Description of the condition
Neuropathic pain occurs as a consequence of damage to the central nervous system (CNS) (e.g. cerebrovascular accident, multiple sclerosis or spinal cord injury) or peripheral nervous system (PNS) (e.g. PDN, PHN, surgery). Topical agents are most likely to be used for localised, peripheral neuropathies. This review includes studies in which participants had experienced neuropathic pain for at least three months. The high‐concentration formulation of capsaicin has been used to treat postherpetic neuralgia and painful HIV‐neuropathy.
Description of the intervention
Topical medications are applied externally and are taken up through the skin. They exert their effects close to the site of application, and there is no substantial systemic uptake or distribution. This compares with transdermal application, where the medication is applied externally and is taken up through the skin, but relies on systemic distribution for its effect.
Low‐concentration capsaicin creams have not convincingly been shown to be effective for neuropathic pain (Derry 2012). The initial burning sensation felt on application of capsaicin limits the amount of active substance that can be applied at one time, which necessitates frequent (four times per day) application, and reduces patient compliance. The high‐concentration (8%) patch was developed to increase the amount of capsaicin delivered to the skin, and improve tolerability. Rapid delivery is thought to improve tolerability because cutaneous nociceptors are 'defunctionalised' quickly, and the single application avoids both non‐compliance and contamination of the home environment with particles of dried capsaicin cream (Anand 2011). At the time of this review, the 8% patch is the only high strength formulation of capsaicin available.
The treatment is applied as a single application dermal patch over the area where painful symptoms are felt. Each patch (280 cm2) contains 640 micrograms of capsaicin/cm2, and can be cut to treat smaller areas and irregular shapes, or up to four patches can be used simultaneously to treat large areas, such as the back (EMC 2012). The skin to which patches are applied should not be broken or irritated. The skin is treated with a topical local anaesthetic (e.g. topical lidocaine 4% for 60 minutes) before application because the capsaicin may cause an intense burning sensation, and the anaesthetic is then washed off thoroughly, and the skin dried, before the patch is applied. The patch is left in place for 30 minutes when applied to the feet, or 60 minutes for other areas, before removal and careful cleansing of the skin, using a specially formulated cleanser, to remove any residual capsaicin. Application must be carried out in a healthcare centre by trained personnel, and patients are usually monitored for up to two hours after treatment. Stringent conditions are required, and as well as using trained healthcare professionals, the treatment setting needs to be well ventilated and spacious due to the vapour of the capsaicin, and cough due to inhalation of capsaicin particles/dust is a hazard for both the healthcare professionals and the patients. Treatment can be repeated after 12 weeks if necessary.
High‐concentration capsaicin is licensed in the EU to treat neuropathic pain in non‐diabetic patients, and in the US to treat peripheral herpetic neuralgia. It is available on prescription only; it was licensed in 2009 in Europe and the USA. The FDA refused a license for neuropathic pain in HIV in 2012. We could find no information about how many patients have been prescribed the treatment.
How the intervention might work
Capsaicin is the active compound present in chilli peppers, responsible for making them hot when eaten. It binds to nociceptors (sensory receptors responsible for sending signals that cause the perception of pain) in the skin, and specifically to the TRVP1 receptor, which controls movement of sodium and calcium ions across the cell membrane. Initially, binding opens the ion channel (influx of sodium and calcium ions), causing depolarisation and the production of action potentials, which are usually perceived as itching, pricking or burning sensations. Repeated applications or high concentrations give rise to a long‐lasting effect, which has been termed 'defunctionalisation', probably owing to a number of different effects that together overwhelm the cell's normal functions, and can lead to reversible degeneration of nerve terminals (Anand 2011).
Adverse events from capsaicin are mainly at the application site (burning, stinging, erythema), and systemic events are rare. Achieving double‐blind conditions in placebo‐controlled trials using capsaicin can therefore be difficult.
Why it is important to do this review
The earlier review (Derry 2009) identified only two studies using the high‐concentration patch. Since then more studies have become available for review, and use of the high‐concentration product has been licensed in Europe and the USA (and probably elsewhere). Recent revisions to guidelines (AUREF 2012) also require that the data are re‐analysed so that the evidence presented meets new, more stringent, evidence standards. A review of the current evidence for the efficacy of topical capsaicin for chronic neuropathic pain in adults is needed for purchasers of healthcare, prescribers and consumers to make informed choices about their use.
Objectives
To review the evidence from controlled trials on the efficacy and tolerability of topically applied, high‐concentration (8%) capsaicin for neuropathic pain in adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, controlled, double‐blind trials comparing high‐concentration (8%) topical capsaicin with placebo or other active treatment for neuropathic pain, with at least 10 participants per treatment arm. Studies published only as abstracts or studying experimentally induced pain were excluded.
Types of participants
Adult participants (16 years or more) with neuropathic pain of at least moderate intensity (Collins 1997) resulting from any cause, with a duration of at least three months and as defined in the study using accepted diagnostic criteria.
Types of interventions
Included studies had at least one treatment arm using a single application of high‐concentration (8%) topical capsaicin, and a comparator arm using placebo or other active treatment.
Types of outcome measures
Information was sought on participant characteristics: age, sex, and condition treated.
Primary outcomes
Analyses of all primary and secondary efficacy outcomes were conducted according to type of painful condition, because interventions are known to have different effects in different types of neuropathic pain (Moore 2009). For adverse events, we combined all conditions.
The primary efficacy outcome was 'clinical improvement', defined as at least a 50% reduction in pain, or equivalent measure such as a 'very good' or 'excellent' global assessment of treatment, or 'none' or 'slight' pain on rest or movement, measured on a categorical scale (Moore 1998a). We used the following hierarchy of outcomes, in order of preference, to extract data for the primary outcome:
-
patient‐reported reduction in pain of at least 50%;
-
patient‐reported global assessment of treatment of 'very good' or 'excellent';
-
patient‐reported global assessment of treatment 'much' or 'very much' improved;
-
'none' or 'slight' pain on movement;
-
'none' or 'slight' pain on rest or spontaneous pain.
We intended to use the first three categories of the hierarchy as part of our definition of highest level of evidence using outcomes 8 to 12 weeks after treatment initiation, and where results reported were 'true' responders without use of imputation methods for treatment withdrawal (AUREF 2012; Moore 2012).
Physician or investigator‐reported outcomes of efficacy were not used.
Secondary outcomes
Secondary outcomes sought were:
-
numbers of participants with adverse events: local and systemic, and cough;
-
numbers of withdrawals: all cause, lack of efficacy, and adverse events.
We anticipated that outcomes would be reported after different durations of treatment, and extracted data reported around 8 to 12 weeks as this is the expected duration of a single‐dose administration of high‐concentration topical capsaicin, but not generally to examine outcomes at less than six weeks because that would be considered an inadequate duration of effect. Where longer duration outcomes were available we also extracted these. We also anticipated that reporting of adverse events would vary between trials with regard to the terminology used, method of ascertainment, and categories that are reported (e.g. occurring in at least 5% of patients or where there is a statistically significant difference between treatment groups). Care was taken to identify these details.
Search methods for identification of studies
Electronic searches
We searched the following databases:
-
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 10);
-
MEDLINE via Ovid (10 December 2012);
-
EMBASE via Ovid (10 December 2012).
See Appendix 1 for the MEDLINE search strategy, Appendix 2 for the EMBASE search strategy and Appendix 3 for the CENTRAL search strategy.
There was no language restriction.
Searching other resources
We also searched the reference lists of review articles and included studies, together with a clinical trials database (www.clinicaltrials.gov). We did not search grey literature and short abstracts. Manufacturers and license holders were not contacted for unpublished clinical trial data.
Data collection and analysis
Two review authors independently selected the studies for inclusion, assessed methodological quality and study validity, and extracted data. Disagreements were resolved through discussion.
Selection of studies
We reviewed the titles and abstracts of studies identified by the searches on screen to eliminate those that clearly did not satisfy the inclusion criteria. We obtained full reports of the remaining studies to determine inclusion in the review. We considered cross‐over studies only if data from the first treatment period were reported separately. Studies in oral, ocular or buccal diseases, and in musculoskeletal conditions were excluded.
Data extraction and management
We abstracted information on participants, interventions, and outcomes from the original reports into a standard data extraction form. We did not contact authors for further information.
Assessment of risk of bias in included studies
We assessed the included studies for methodological quality using the five‐point Oxford Quality Scale (Jadad 1996) that considers randomisation, blinding, and study withdrawals and dropouts.
The authors independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion. We assessed the following for each study:
-
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, e.g. random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). Studies using a non‐random process (e.g. odd or even date of birth; hospital or clinic record number) were excluded.
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). Studies that did not conceal allocation (e.g. open list) were excluded.
-
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study states that it was blinded and describes the method used to achieve blinding, e.g. identical tablets; matched in appearance and smell); unclear risk of bias (study states that it was blinded but does not provide an adequate description of how it was achieved). Studies that were not double‐blind were excluded.
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (< 10% of participants did not complete the study and/or used ‘baseline observation carried forward’ analysis); unclear risk of bias (used 'last observation carried forward' analysis); high risk of bias (used 'completer' analysis).
-
Size of study (checking for possible biases confounded by small size). We assessed studies as being at low risk of bias (≥ 200 participants per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); high risk of bias (< 50 participants per treatment arm).
Measures of treatment effect
We used relative risk (or 'risk ratio', RR) to establish statistical difference. We used numbers needed to treat (NNT) and pooled percentages as absolute measures of benefit or harm.
We used the following terms to describe adverse outcomes in terms of harm or prevention of harm:
-
When significantly fewer adverse outcomes occur with capsaicin than with control (placebo or active) we used the term the number needed to treat to prevent one event (NNTp).
-
When significantly more adverse outcomes occur with capsaicin compared with control (placebo or active) we used the term the number needed to harm or cause one event (NNH).
Unit of analysis issues
We accepted randomisation by individual patient only.
Dealing with missing data
The most likely source of missing data was expected to be from participants dropping out from the studies. We looked specifically for evidence of 'last observation carried forward' (LOCF) and used a dichotomous responder analysis, where a responder is defined as a participant who experienced the predefined outcome and remained in the study (e.g. did not withdraw due to adverse events). Last observation carried forward is a potential source of major bias in chronic pain studies (Moore 2012).
For all outcomes we carried out analyses, as far as possible, on a modified intention‐to‐treat (ITT) basis, i.e. we included all participants who were randomised and received an intervention. Where sufficient information was reported, we added back missing data in the analyses we undertook.
Assessment of heterogeneity
We assessed heterogeneity of studies visually (L'Abbé 1987). Where data could be pooled, we would report the I2 statistic.
Assessment of reporting biases
We planned to assess publication bias by examining the number of participants in trials with zero effect (relative risk of 1.0) needed for the point estimate of the NNT to increase beyond a clinically useful level (Moore 2008a). In this case, we specified a clinically useful level as a NNT of 10 for clinical improvement at eight weeks.
Data synthesis
We undertook meta‐analysis using a fixed‐effect model. A random‐effects model for meta‐analysis would have been used if there was significant clinical heterogeneity and it was considered appropriate to combine studies.
We determined that we would analyse data for each painful condition in two tiers, according to outcome and freedom from known sources of bias.
-
The first tier used data meeting current best standards, where studies report the outcome of at least 50% pain intensity reduction over baseline (or its equivalent), without the use of LOCF or other imputation method for dropouts, an ITT analysis, in studies lasting 8 to 12 weeks or longer in parallel‐group studies, and where there were at least 200 participants (preferably at least 400) in the comparison. These top‐tier results are reported first.
-
The second tier used any available data, but where one or more of these conditions were not met, for example with at least 30% pain intensity reduction, use of LOCF or a completer analysis, studies lasting 4 to 6 weeks, and where the numbers of participants and studies were small.
Where appropriate we planned to calculate relative benefit (RB) and risk (RR) estimates with 95% confidence intervals (CI) using a fixed‐effect model (Morris 1995). NNT and number needed to treat to harm (NNH) and 95% CIs would be calculated using the pooled number of events, using the method devised by Cook and Sackett (Cook 1995). We assumed a statistically significant difference from control when the 95% CI of the RR or RB did not include the number one.
Subgroup analysis and investigation of heterogeneity
All analyses were planned to be according to individual painful conditions, because placebo response rates with the same outcome can vary between conditions, as can the drug‐specific effects (Moore 2009). Subgroup analyses were not planned since experience of previous reviews indicated that there would be too few data for any meaningful subgroup analysis.
Sensitivity analysis
We planned to consider sensitivity analyses for study outcome (top‐tier evidence versus second‐tier), study size, and methodological quality (2 versus ≥ 3).
At least 200 participants had to be available in any of these different contexts before information was pooled (Moore 1998b).
Results
Description of studies
Types of efficacy outcomes reported
In these studies, patients with chronic pain were given a single 30 to 90‐minute intervention with high‐concentration topical capsaicin, and their pain was then measured over the following 8 to 12 weeks. Because the intervention itself was painful and could cause localised pain at the application site, no pain measurements were generally made in the first post‐treatment week. The outcomes then reported were of two distinct types:
-
Assessment of the longevity of benefit is obtained from a patient global impression of change (PGIC) made at specific points, usually eight and 12 weeks after drug administration. These outcomes are regarded as first‐tier evidence. The expected pattern would be early, but not later, differences between active and control interventions.
-
Average pain scores over weeks 2 to 8 and 2 to 12 were calculated, and the number and/or percentage of participants with pain intensity reduction of at least 30% or at least 50% over baseline recorded. These outcomes are regarded as second‐tier evidence. These outcomes might be regarded as assessing whether the intervention 'worked' in providing a larger proportion of participants with adequate pain relief with the intervention than with control. Because the largest difference between active treatment and control typically occurred in the first 4 to 6 weeks after treatment, these measures do not adequately address for how long the benefits lasted.
Not all studies reported all of these outcomes, so data were inconsistently available for pooling and analysis.
Results of the search
The search identified eight studies, one of which was an ongoing study examining a short (five‐minute) application of high‐concentration capsaicin (NCT01228838).
Neuropathic pain conditions studied were postherpetic neuropathy (Backonja 2008; Backonja 2010; Irving 2011; Webster 2010a; Webster 2010b) and HIV‐neuropathy (Clifford 2012; Simpson 2008). In all studies pain was of at least moderate severity and was frequently unresponsive to, or poorly controlled by, conventional therapy. In studies of postherpetic neuropathy the mean age of participants was 70 to 71 years and men and women were enrolled in approximately equal numbers. In studies of HIV‐neuropathy the mean age of participants was 48 and 50 years and about 90% were men.
The duration of application of high‐concentration topical capsaicin varied between 30 and 90 minutes, with the bulk of patients treated for 60 minutes. Thirty‐minute application was used in one group of participants in Clifford 2012, and 30 and 90‐minute duration of administration was used in Simpson 2008 and Webster 2010a.
Because application of capsaicin to the skin, particularly at this high concentration, initially causes erythema (redness) and a burning or stinging sensation in many individuals, maintaining the double‐blind status of studies is problematic. Studies used a low dose (0.04%) of capsaicin in the control patch to produce some degree of skin irritation without effective analgesia, in an attempt to prevent participants form guessing their treatment allocation.
All studies permitted concomitant oral or transdermal drugs (opioids ≤ 60 mg morphine/day equivalent) to be continued for neuropathic pain without change in dose or frequency, but all topical medications were discontinued at least seven days before the study.
Included studies
Six studies, with 2073 participants, fulfilled the entry criteria. Two (Backonja 2008; Simpson 2008) had been included in the original review, and four (Clifford 2012; Irving 2011; Webster 2010a; Webster 2010b) were new studies. Details of included studies are in the 'Characteristics of included studies' table.
Excluded studies
One study (Backonja 2010) was excluded after reading the full report, because study duration was only four weeks (see the 'Characteristics of excluded studies' table).
Risk of bias in included studies
We scored each study for methodological quality using the Oxford Quality Score; all studies scored 4/5 except Simpson 2008, which scored 3/5. No study clearly reported the method of randomisation, but since these studies were carried out under rigorous conditions by the pharmaceutical company it is likely that the schedule was computer‐generated.
The 'Risk of bias' table has been completed for all studies for sequence generation, allocation concealment, blinding, incomplete outcome data, and size. No studies were considered at high risk of bias from any of these criteria. In most cases where the risk was assessed as 'unknown' it is likely that the methods were rigorous, but the reporting inadequate (e.g. randomisation, allocation concealment). Where there was incomplete outcome data due to missing values, the most relevant outcomes were reported without last observation carried forward imputation.
Full details can be found in the 'Characteristics of included studies' table and Figure 1.
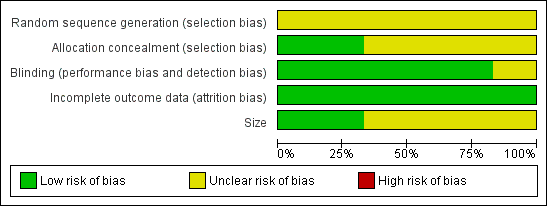
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Allocation
None of the studies adequately described the method of randomisation.
Blinding
Studies were all described as double‐blind, and this was generally well described.
Incomplete outcome data
The nature of the studies was that all participants received the single application of topical high‐concentration capsaicin at the start of the study, but there were some withdrawals or losses to follow‐up thereafter, though these were generally small. Modified LOCF analysis was used for some efficacy outcomes, but no imputation was used for weekly pain scores or patient global assessment of treatment, where non‐reporting was regarded as non‐response. All participants were included for safety analyses.
Selective reporting
There were considerable differences between studies in how results were reported. For example, the number of participants with at least 30% or 50% reduction in average pain intensity over baseline over 2 to 8 or 2 to 12 weeks were not all reported in all studies, though there were references to statistical difference between active treatment and control. PGIC scores were not always reported at both eight and 12 weeks, and sometimes were reported as 'much or very much' improved, and others as 'slightly, much or very much' improved. For no single efficacy outcome was there consistent reporting in all studies.
Other potential sources of bias
Studies were generally large and apparently well conducted, so there were no other obvious sources of bias.
Effects of interventions
Details of study efficacy outcomes are in Appendix 4, adverse events and withdrawals in Appendix 5, and patch tolerability in Appendix 6. Preliminary analyses demonstrated that duration of administration of high‐concentration topical capsaicin of 30 and 90 minutes resulted in no discernable difference in efficacy from 60 minutes (Analysis 1.2; Analysis 1.4), so in the following analyses results for different duration of patch application have been combined.
Number of participants achieving clinical improvement at 8 to 12 weeks
Postherpetic neuralgia
Postherpetic neuralgia (PHN) was the neuropathic pain condition in four studies (Backonja 2008; Irving 2011; Webster 2010a; Webster 2010b) involving 1272 participants (742 exposed to high‐concentration topical capsaicin, 530 to low‐concentration 0.04% capsaicin control). Not all outcomes were reported in all studies, with the exception of at least 30% pain intensity reduction over 2 to 8 weeks compared with baseline pain, which was reported in all four studies.
First‐tier evidence
First‐tier evidence on the efficacy of high‐concentration topical capsaicin in painful postherpetic neuralgia came from patient global impression of change (PGIC) outcomes of much or very much improved at eight and 12 weeks in only two of the four studies (Irving 2011; Webster 2010b). Results are shown in Summary of Results table A. At both eight and 12 weeks there was a significant benefit for high‐concentration over low‐concentration topical capsaicin, with point estimates of the numbers needed to treat (NNTs) of 8.8 and 7.0 respectively (Analysis 1.1; Figure 2).

Forest plot of comparison: 1 8% capsaicin versus control (single dose), outcome: 1.1 PGIC much or very much improved at 8 and 12 weeks.
Second‐tier evidence
Second‐tier evidence results are shown in Summary of Results A. The magnitude of the treatment effect was similar for ≥ 30% reduction and ≥ 50% reduction over baseline for the average weekly pain intensity over 2 to 8 (≥ 30% PIR 2 to 8 weeks; ≥ 50% PIR 2 to 8 weeks) and 2 to 12 weeks (≥ 30% PIR 2 to 12 weeks; ≥ 50% PIR 2 to 12 weeks), with NNT point estimates of between 10 and 12 (Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Figure 3).

Forest plot of comparison: 1 8% capsaicin versus control (single dose), outcome: 1.2 PHN ‐ at least 50% pain intensity reduction over weeks 2 to 8.
However, it is worth noting that one study (Backonja 2008) reported that there was no significant difference between active therapy and control for the 50% pain intensity reduction. Another (Webster 2010a) presented data for 12 weeks that appeared to support its claim that 12‐week and 8‐week results were similarly effective (for 30% and 50% pain intensity reduction), without providing information for analysis.
Summary of Results A
| Number of | Percent with outcome | |||||
| Outcome | Trials | Participants | 8% capsaicin | control | Relative benefit | NNT |
| Postherpetic neuralgia | ||||||
| PGIC much/very much 8 weeks | 2 | 571 | 36 | 25 | 1.4 (1.1 to 1.8) | 8.8 (5.3 to 26) |
| PGIC much/very much 12 weeks | 2 | 571 | 39 | 25 | 1.6 (1.2 to 2.0) | 7.0 (4.6 to 15) |
| ≥ 30% PIR 2 to 8 weeks | 4 | 1272 | 43 | 34 | 1.3 (1.1 to 1.5) | 11 (6.8 to 26) |
| ≥ 30% PIR 2 to 12 weeks | 3 | 973 | 46 | 37 | 1.3 (1.1 to 1.5) | 10 (6.3 to 28) |
| ≥ 50% PIR 2 to 8 weeks | 3 | 870 | 29 | 20 | 1.4 (1.1 to 1.9) | 12 (7.2 to 41) |
| ≥ 50% PIR 2 to 12 weeks | 2 | 571 | 33 | 24 | 1.3 (1.0 to 1.7) | 11 (6.1 to 62) |
| HIV‐neuropathy | ||||||
| PGIC much/very much 12 weeks | 1 | 307 | 27 | 10 | 2.8 (1.4 to 5.6) | 5.8 (3.8 to 12) |
| ≥30% PIR 2 to 12 weeks | 2 | 801 | 39 | 30 | 1.4 (1.1 to 1.7) | 11 (6.2 to 47) |
HIV‐neuropathy
Painful HIV‐neuropathy was the neuropathic pain condition in two studies (Clifford 2012; Simpson 2008) involving 801 participants (557 exposed to high‐concentration topical capsaicin, 244 to low‐concentration 0.04% capsaicin control). Not all outcomes were reported in both studies, with the exception of at least 30% pain intensity reduction over 2 to 12 weeks compared with baseline pain.
First‐tier evidence
First‐tier evidence on the efficacy of high‐concentration topical capsaicin in HIV‐neuropathy came from PGIC outcomes of much or very much improved at 12 weeks in only one study (Simpson 2008). Results are shown in Summary of Results table A. There was a significant benefit for high‐concentration over low‐concentration topical capsaicin, with a point estimate of the NNTs of 5.8.
Second‐tier evidence
Only the outcome of at least 30% pain intensity reduction over two to 12 weeks compared with baseline was reported (Summary of Results A) (Analysis 1.6; Figure 4). The point estimate of the NNT was 11.
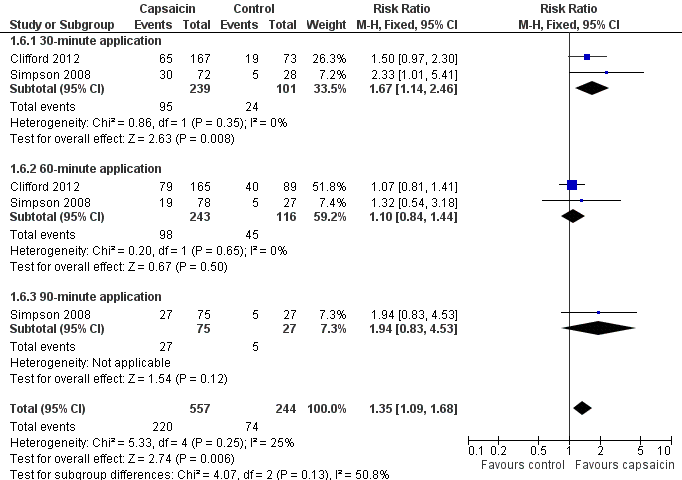
Forest plot of comparison: 1 8% capsaicin versus control (single dose), outcome: 1.6 HIVN ‐ at least 30% pain intensity reduction over weeks 2 to 12.
Subgroup analysis
Dose and condition
Analysis by dose (duration of application) and pain condition has been carried out in the primary analysis above.
Sensitivity analysis of the primary outcome
Study size
Only one study reporting top‐level evidence in any one pain condition had ≥ 200 participants in both treatment arms so no analysis by study size was possible.
Study quality
All studies had quality scores > 2/5, so no analysis by study quality was possible.
Adverse events
Reporting of adverse events was inconsistent and incomplete (Appendix 5). Most studies did not report the precise methods used to collect adverse event data, such as use of direct/indirect questioning or patient diaries, or the timing of data collection, but they did consistently classify adverse events and serious adverse events according to the Medical Dictionary for Regulatory Activities. The majority of adverse events were transient and mild to moderate in intensity. Five studies reported adverse events occurring in ≥ 3% (Backonja 2008; Clifford 2012; Irving 2011; Webster 2010a; Webster 2010b) and one in > 2% (Simpson 2008) of patients in any treatment arm, together with any serious adverse events. The most common events were application site (skin) reactions
Local skin reactions
All included studies reported on local skin reactions. The control patches contained a low concentration (0.04%) of capsaicin to mimic the burning sensation of capsaicin without providing effective pain relief. It was not possible to determine the number of participants experiencing any kind of local skin reaction since more than one symptom may appear in an individual participant. We chose to analyse 'erythema, pain, papules and pruritus' as these were fairly consistently reported in individual studies. For analysis we have combined studies in PHN and HIV‐neuropathy and all durations of application since there were no obvious differences or trends and the number of events was small.
Some studies captured all adverse events following application. These we defined as Group 1 studies, which comprised Backonja 2008; Clifford 2012; Irving 2011 (Analysis 1.7). The other studies reported adverse events differently; these Group 2 studies comprised Simpson 2008; Webster 2010a; Webster 2010b (Summary of results B; Analysis 1.8). Two (Webster 2010a; Webster 2010b) specifically stated that "treatment associated erythema, discomfort and pain on the day of treatment were not captured as adverse events but reported as dermal assessment scores or 'Pain Now' NRPS scores". They reported very much lower rates of skin adverse events, presumably because events in the first day were not included. One other study (Simpson 2008) did not specify whether it included skin reactions on the first day as adverse events, but it also had a very much lower rate and is analysed with the Webster studies. Figure 5 shows the proportion of participants in active and control arms for each comparison, for the most common skin adverse events reported.
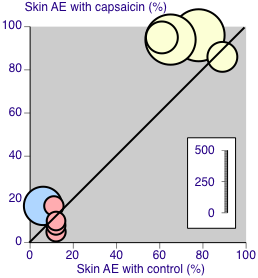
Skin adverse event rates with capsaicin and control. Yellow symbols are studies recording all events (Group 1). Pink symbols are studies specifying that events are not recorded on the first day after treatment (Group 2). The blue symbol did not specify the period over which events were recorded (Group 2). Size of symbol is proportional to the size of the study
Summary of results B
| Outcome | Studies | Participants | Relative Risk (95% CI) | NNH (95% CI) |
| Group 1 | ||||
| Erythema | 3 | 1312 | 1.4 (1.3 to 1.5) | 5.9 (4.5 to 8.4) |
| Pain | 3 | 1312 | 2.3 (2.0 to 2.6) | 2.4 (2.2 to 2.8) |
| Papules | 3 | 1312 | 3.6 (1.9 to 6.9) | 23 (16 to 46) |
| Pruritus | 3 | 1312 | 2.0 (0.98 to 4.0) | not calculated |
| Oedema | 3 | 1312 | 3.0 (1.4 to 6.2) | 38 (23 to 110) |
| Group 2 | ||||
| Erythema | 1 | 129 | nsd | not calculated |
| Pain | 3 | 735 | 1.9 (0.99 to 3.5) | not calculated |
| Papules | 3 | 735 | 1.6 (0.59 to 4.2) | not calculated |
| Pruritus | 3 | 735 | 1.6 (0.98 to 2.5) | not calculated |
| Oedema | 3 | 735 | 1.3 (0.75 to 2.4) | not calculated |
| NNH ‐ number needed to harm; nsd ‐ no significant difference | ||||
Patch tolerability
Use of local anaesthetic before application, local cooling, and availability of short‐acting opioids for pain relief in the first few days following treatment, all help to increase tolerability of the treatment. All studies assessed tolerability by the number of patients able to complete at least 90% of the intended application time, the degree of dermal irritation two hours after application (FDA 1999), and the numbers of patients using medication for treatment‐related discomfort on days 0 to 5 (Summary of results C). For analysis we have again combined studies in PHN and HIV‐neuropathy and all durations of application since there were no obvious differences or trends and the number of events was small for most outcomes (Analysis 1.9).
Summary of results C
| Outcome | Studies | Participants | Relative Risk (95% CI) | NNH (95% CI) |
| < 90% application time | 6 | 2074 | 3.3 (1.2 to 9.2) | 77 (45 to 260) |
| DIS > 2 at 2 h | 3 | 1065 | 12 (4.0 to 34) | 9.6 (7.7 to 13) |
| DIS > 0 at 2 h | 2 | 606 | 2.3 (1.6 to 3.2) | 4.5 (3.3 to 6.7) |
| Pain medication 0 to 5 d | 5 | 2073 | 2.5 (2.1 to 2.9) | 3.7 (3.2 to 4.3) |
| DIS ‐ dermal irritation score; NNH ‐ number needed to harm | ||||
The estimate for NNH for achieving less than 90% of the scheduled patch application time should be interpreted with caution since the numbers of participants with this outcome were very small; 22/1300 (1.9%) with capsaicin and 2/774 (0.58%) with control.
Systemic adverse events
Systemic adverse events included diarrhoea, nausea, vomiting, fatigue, infections, musculoskeletal disorders, hypertension, dizziness, and headache. They occurred in fewer than 5% of participants in each treatment arm, with no obvious differences between different doses and control arms (Appendix 5). Three studies (Simpson 2008; Webster 2010a; Webster 2010b) specifically reported on cough, which occurred in 2% to 3% of participants treated with high‐concentration capsaicin and 0% to 4% of those treated with control. No further analysis of systemic adverse events was carried out.
Serious adverse events
Serious adverse events were uncommon. Five studies provided data for analysis (Backonja 2008; Irving 2011; Simpson 2008; Webster 2010a; Webster 2010b); 39/966 (4.1%) of participants treated with capsaicin, and 19/613 (3.2%) treated with control experienced serious adverse events, giving a RR of 1.4 (0.82 to 2.4) (Analysis 1.10; Figure 6). The NNH was not calculated. The remaining study (Clifford 2012) reported that serious adverse events occurred with similar frequency in both treatment groups (6%), and none were judged treatment‐related.
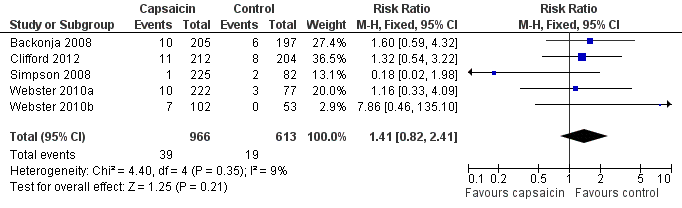
Forest plot of comparison: 1 8% capsaicin versus control (single dose), outcome: 1.10 Serious adverse events.
One event was judged possibly related to study medication. This participant experienced increased blood pressure on the day of treatment following treatment with capsaicin (Backonja 2008).
There were six deaths, four following treatment with capsaicin (one each in Clifford 2012; Irving 2011; Simpson 2008; Webster 2010a) and two following control (both in Simpson 2008). None were judged related to study medication.
Withdrawals
Adverse events
A total of 12 withdrawals due to adverse events were reported in 1298 participants (0.92%) treated with capsaicin and eight in 775 participants (1.03%) treated with control, giving a relative risk of 0.87 (0.37 to 2.0); the NNH was not calculated (Analysis 1.11).
Lack of efficacy
A total of 20 withdrawals due to lack of efficacy were reported in 1298 participants (1.5%) treated with capsaicin and 24 in 775 participants (3.1%) treated with control, giving a relative risk of 0.58 (0.32 to 1.04); the NNTp was 64 (34 to 610) (Analysis 1.11).
Withdrawals for other reasons (e.g. lost to follow‐up) were generally below 10% and evenly distributed between treatment arms (Appendix 5). No further statistical analysis of withdrawals was carried out.
Discussion
Summary of main results
A single application of a high‐concentration (8%) patch for 30 to 90 minutes provides significant pain relief for up to 12 weeks in some people with chronic pain arising from postherpetic neuralgia (PHN) or HIV‐neuropathy. Top‐tier evidence that can be regarded as reliable and trustworthy generated numbers needed to treat (NNTs) of between about 6 and 9 measured at eight or 12 weeks; for every seven to nine people treated, one will experience improvement in pain over 12 weeks who would not have done with control. These results for an outcome at a specific point in time were supported by positive benefits with a similar order of magnitude for second‐tier outcomes of people with average pain intensity reductions of at least 50% or at least 30% measured over periods of time between two and 12 weeks.
These results might be compared with an NNT of 5.4 (3.9 to 9.2) over 12 weeks for 600 mg pregabalin in 702 participants with postherpetic neuralgia (Moore 2009). The NNT for much or very much improved in 1121 participants treated with gabapentin (any dose) or control yielded an NNT of 5.5 (4.3 to 7.7) (Moore 2011a). No other drug therapies have comparable data sets for estimation of efficacy in postherpetic neuralgia.
Painful HIV‐neuropathy is a condition in which there are no large comparable data sets, but where few therapies appear to demonstrate any benefit (Phillips 2010); topical high‐concentration capsaicin is therefore notable for providing some evidence of effective pain relief.
Treatments for chronic pain are characterised by the proportion of people obtaining high degrees of treatment‐specific pain relief being small. However, the benefits go way beyond pain itself, with associated benefits in terms of improved sleep, reduced fatigue and depression, an overall improvement in quality of life, and even the ability to spend more time in employment or looking after the family (Gülfe 2010; Hoffman 2010; Ikenberg 2012; Moore 2009; Straube 2011).
Use of capsaicin at the high concentration of 8% is associated with increased local skin reactions, primarily burning, stinging and erythema that affects many patients, whether or not they obtain good pain relief, but these effects resolve quickly after the single application.
Overall completeness and applicability of evidence
The decision to exclude studies of less than six weeks' duration reduced the amount of evidence available to us; we excluded a single study with only 38 participants (Backonja 2010) amounting to only about 3% of the total number of participants. We feel this is justified because benefits extending to only four weeks are unlikely to outweigh the considerable efforts associated with high‐concentration capsaicin use, at least at the moment.
The largest deficiencies resulted from inconsistent reporting, especially of efficacy outcomes. For example, for five of the six efficacy outcomes reported from the four PHN studies, complete data were available for analysis for only one outcome, and for the other five the amount available varied between 45% and 76% of the total participants. Importantly, both top‐tier outcomes for benefit at eight or 12 weeks after application were calculated using only 45% of participants. For HIV‐neuropathy, only two of the six outcomes were reported, and only one second‐tier outcome reported in both studies.
This represents a considerable loss of evidence from otherwise high‐quality and well‐conducted large studies. It is a deficiency that could affect the applicability of the results we have, and probably reflects the difficulties in reporting large, detailed, and complex clinical trials within the severe constraints of the allowable size of papers for publication. The deficiency should be rectified. Rectification would require no more studies, but rather a better access to trial data, perhaps in the form of clinical trial reports, as has been done before (Moore 2005; Moore 2008b; Moore 2011b; Straube 2010).
Adverse event reporting was also limited by different ways of reporting data. This is a problem that has been commented upon previously in pain studies (Edwards 1999; Loke 2001). Moreover, the studies reviewed provided no useful information relevant to safety concerning long‐term repeated applications.
One aspect of the therapy that has not been examined specifically is a one‐hour treatment with lidocaine alone, in the absence of capsaicin, though this was also used with low‐concentration 0.04% capsaicin as part of the control group treatment.
Quality of the evidence
All six included studies were of generally high quality, with deficiencies in describing the process of randomisation and allocation concealment. Data handling with missing data did not adversely affect quality or involve any possible biases. Because topical capsaicin is associated with erythema, burning, and local pain, a 'true' placebo would have led to immediate unblinding. Instead, 0.04% topical capsaicin was used as an 'active' placebo control, one that would mimic the local adverse effects of capsaicin without longer‐term pain relief. Responses to various outcomes with control were of the order of 25% of participants benefiting for the outcome of much or very much improved on the PGIC in postherpetic neuralgia, compared with 15% to 20% for the same outcome with placebo in trials of pregabalin (Moore 2009) and gabapentin (Moore 2011a). That could be taken to suggest that the low‐concentration control had some very small longer‐term benefit that would work to diminish the apparent efficacy of high‐concentration topical capsaicin, but this should not be considered more than speculation with the evidence available, especially relating to benefits from low‐concentration capsaicin creams (Derry 2012).
Potential biases in the review process
We used an extensive search strategy, which was based on a previous Cochrane review, and examined bibliographies, reference lists, and clinical trial registries. High‐concentration capsaicin is a relatively recent therapy and it is unlikely that relevant high‐quality large studies have been overlooked. However, the relatively high NNTs for high‐concentration topical capsaicin combined with incomplete reporting of the patient global impression of change (PGIC) outcomes means that null effect data from only about 240 to 220 participants would be needed to raise the NNT above 10 (Moore 2008a), at which point the efficacy of the therapy might be regarded as very limited.
Agreements and disagreements with other studies or reviews
We know of no other systematic reviews or meta‐analyses of high‐concentration topical capsaicin. The previous version of this review had too few data for a satisfactory review and an even earlier version had no information on high‐concentration capsaicin (Mason 2004). A review based on only some of the studies (Jones 2011) came to broadly similar conclusions while not performing any pooled analysis. A review on drugs used to treat postherpetic neuralgia seemed not to include many studies because it had a search only up to 2009 (Edelsberg 2009). Another review of interventions for postherpetic neuralgia contained so many methodological problems that no safe conclusions can be made about its findings (Wolff 2011).
This review is in broad agreement with a review of interventions for painful HIV‐associated neuropathy (Phillips 2010), though that review did not include one study included here (Clifford 2012).

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Forest plot of comparison: 1 8% capsaicin versus control (single dose), outcome: 1.1 PGIC much or very much improved at 8 and 12 weeks.

Forest plot of comparison: 1 8% capsaicin versus control (single dose), outcome: 1.2 PHN ‐ at least 50% pain intensity reduction over weeks 2 to 8.

Forest plot of comparison: 1 8% capsaicin versus control (single dose), outcome: 1.6 HIVN ‐ at least 30% pain intensity reduction over weeks 2 to 12.

Skin adverse event rates with capsaicin and control. Yellow symbols are studies recording all events (Group 1). Pink symbols are studies specifying that events are not recorded on the first day after treatment (Group 2). The blue symbol did not specify the period over which events were recorded (Group 2). Size of symbol is proportional to the size of the study

Forest plot of comparison: 1 8% capsaicin versus control (single dose), outcome: 1.10 Serious adverse events.

Comparison 1 8% capsaicin versus control (single dose), Outcome 1 PGIC much or very much improved at 8 and 12 weeks.

Comparison 1 8% capsaicin versus control (single dose), Outcome 2 PHN ‐ at least 50% pain intensity reduction over weeks 2 to 8.
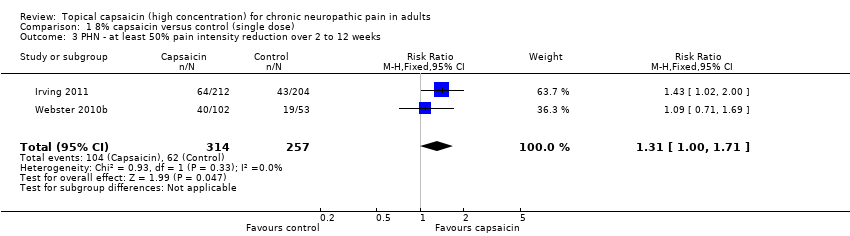
Comparison 1 8% capsaicin versus control (single dose), Outcome 3 PHN ‐ at least 50% pain intensity reduction over 2 to 12 weeks.
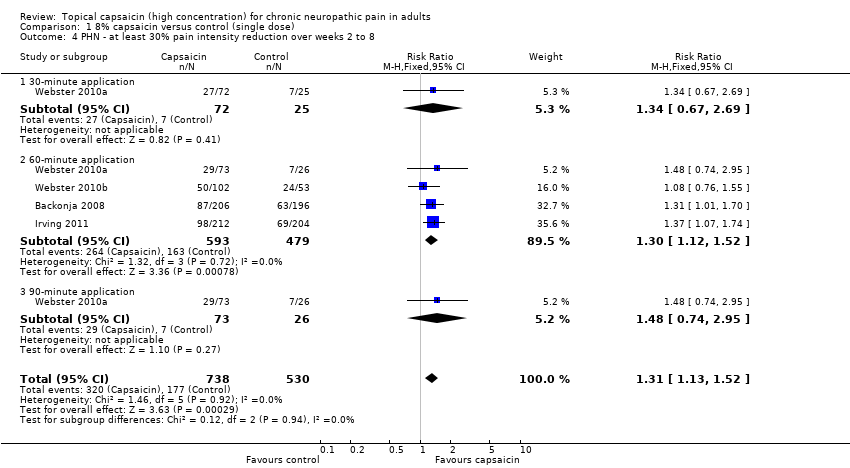
Comparison 1 8% capsaicin versus control (single dose), Outcome 4 PHN ‐ at least 30% pain intensity reduction over weeks 2 to 8.

Comparison 1 8% capsaicin versus control (single dose), Outcome 5 PHN ‐ at least 30% pain intensity reduction over weeks 2 to 12.

Comparison 1 8% capsaicin versus control (single dose), Outcome 6 HIVN ‐ at least 30% pain intensity reduction over weeks 2 to 12.
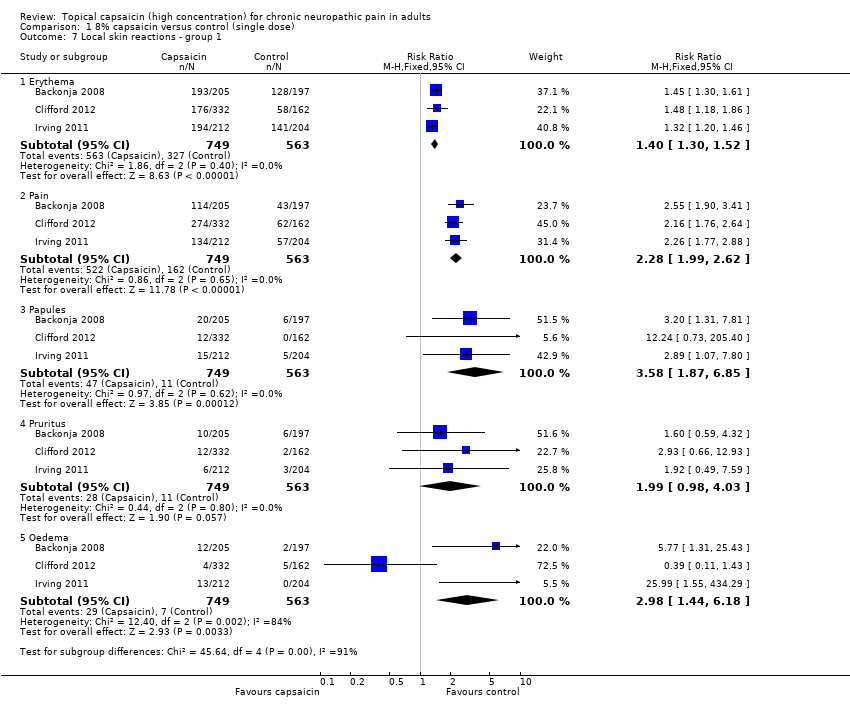
Comparison 1 8% capsaicin versus control (single dose), Outcome 7 Local skin reactions ‐ group 1.

Comparison 1 8% capsaicin versus control (single dose), Outcome 8 Local skin reactions ‐ group 2.

Comparison 1 8% capsaicin versus control (single dose), Outcome 9 Patch tolerability.
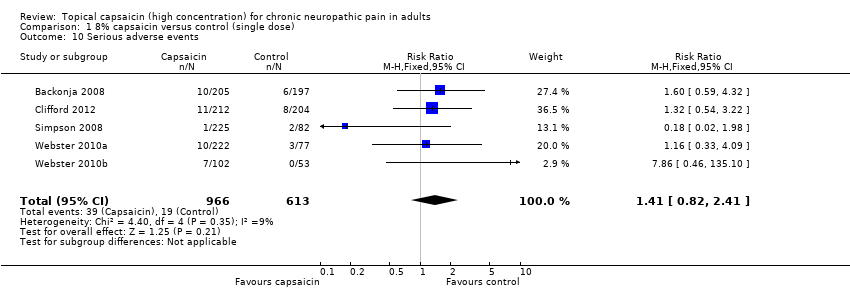
Comparison 1 8% capsaicin versus control (single dose), Outcome 10 Serious adverse events.

Comparison 1 8% capsaicin versus control (single dose), Outcome 11 Withdrawals.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PGIC much or very much improved at 8 and 12 weeks Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 8 weeks | 2 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.10, 1.84] |
| 1.2 12 weeks | 2 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.20, 1.99] |
| 2 PHN ‐ at least 50% pain intensity reduction over weeks 2 to 8 Show forest plot | 3 | 870 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.12, 1.86] |
| 2.1 30‐minute application | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [0.73, 11.88] |
| 2.2 60‐minute application | 3 | 674 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.03, 1.75] |
| 2.3 90‐minute application | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.64, 6.33] |
| 3 PHN ‐ at least 50% pain intensity reduction over 2 to 12 weeks Show forest plot | 2 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.00, 1.71] |
| 4 PHN ‐ at least 30% pain intensity reduction over weeks 2 to 8 Show forest plot | 4 | 1268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.13, 1.52] |
| 4.1 30‐minute application | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.67, 2.69] |
| 4.2 60‐minute application | 4 | 1072 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.12, 1.52] |
| 4.3 90‐minute application | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.74, 2.95] |
| 5 PHN ‐ at least 30% pain intensity reduction over weeks 2 to 12 Show forest plot | 3 | 973 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.07, 1.45] |
| 6 HIVN ‐ at least 30% pain intensity reduction over weeks 2 to 12 Show forest plot | 2 | 801 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.09, 1.68] |
| 6.1 30‐minute application | 2 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.14, 2.46] |
| 6.2 60‐minute application | 2 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.84, 1.44] |
| 6.3 90‐minute application | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.83, 4.53] |
| 7 Local skin reactions ‐ group 1 Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Erythema | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.30, 1.52] |
| 7.2 Pain | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.99, 2.62] |
| 7.3 Papules | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.58 [1.87, 6.85] |
| 7.4 Pruritus | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.98, 4.03] |
| 7.5 Oedema | 3 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [1.44, 6.18] |
| 8 Local skin reactions ‐ group 2 Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Erythema | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.31 [0.35, 114.82] |
| 8.2 Pain | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.99, 3.47] |
| 8.3 Papules | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.59, 4.24] |
| 8.4 Pruritus | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.98, 2.50] |
| 8.5 Oedema | 3 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.75, 2.39] |
| 9 Patch tolerability Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 < 90% of application time | 6 | 2074 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [1.17, 9.15] |
| 9.2 Dermal irritation score > 2 at 2 h | 3 | 1065 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.80 [4.04, 34.48] |
| 9.3 Dermal irritation score > 0 at 2 h | 2 | 606 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.60, 3.26] |
| 9.4 Rescue medication 0 to 5 d | 6 | 2073 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [2.13, 2.87] |
| 10 Serious adverse events Show forest plot | 5 | 1579 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.82, 2.41] |
| 11 Withdrawals Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Adverse events | 6 | 2073 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.37, 2.00] |
| 11.2 Lack of efficacy | 6 | 2073 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.32, 1.02] |

