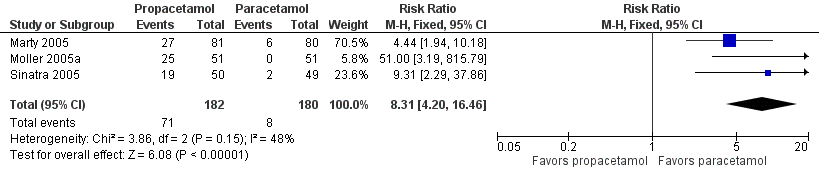
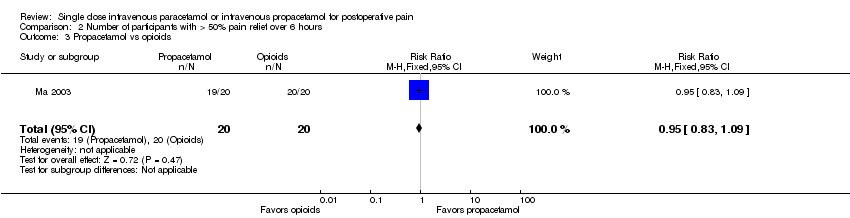
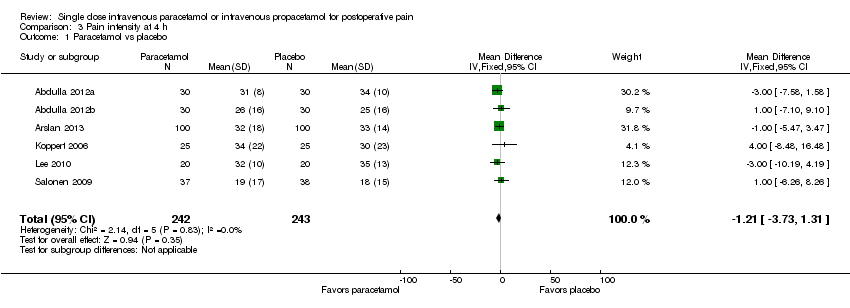
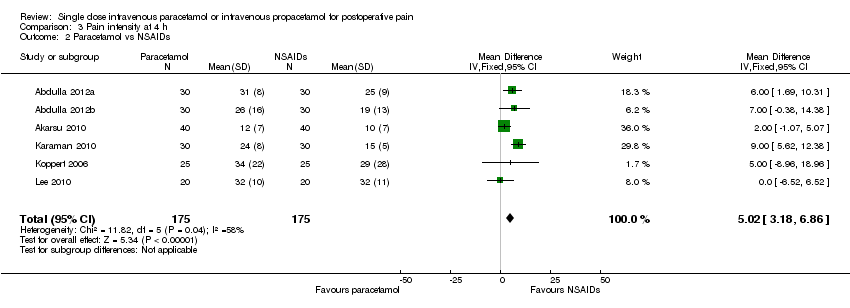
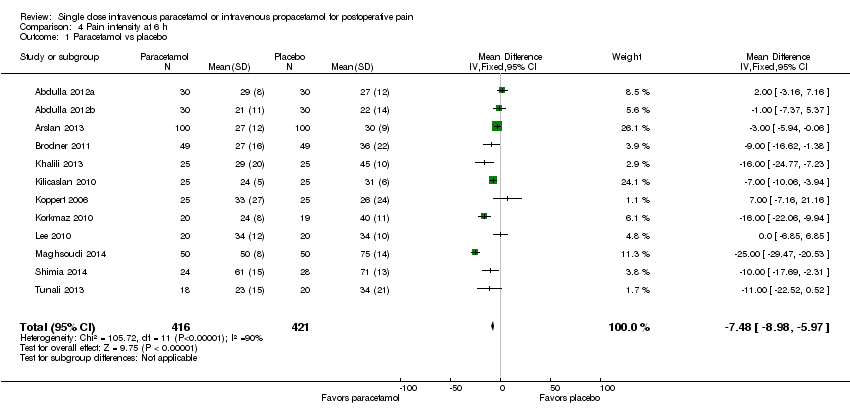
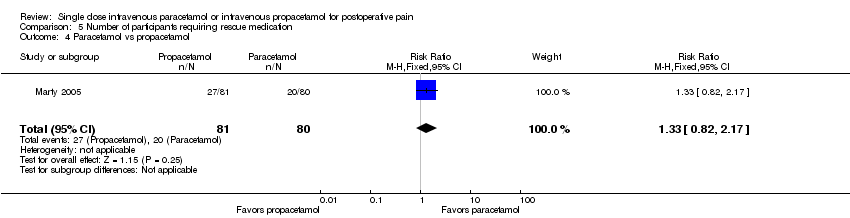
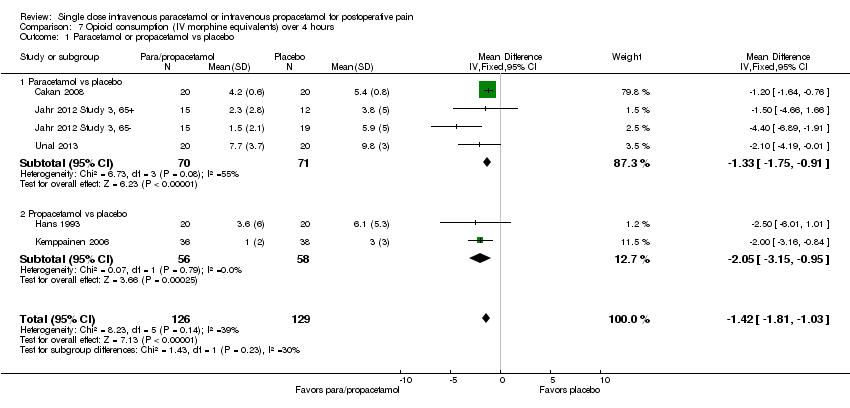
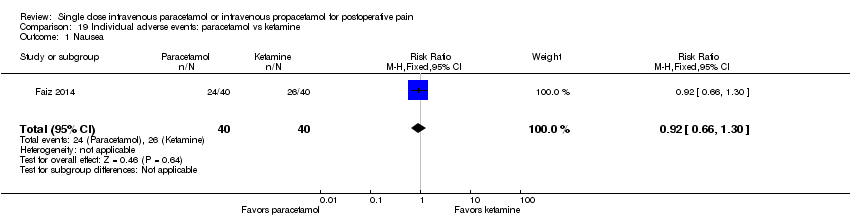
| 1 Nausea Show forest plot | 15 | 1267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.73, 0.98] |
|
| 1.1 Paracetamol vs placebo | 13 | 1037 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.66, 0.90] |
| 1.2 Propacetamol vs placebo | 3 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.98, 2.69] |
| 2 Vomiting Show forest plot | 15 | 1414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.57, 0.87] |
|
| 2.1 Paracetamol vs placebo | 13 | 1037 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.51, 0.80] |
| 2.2 Propacetamol vs placebo | 3 | 377 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.75, 3.48] |
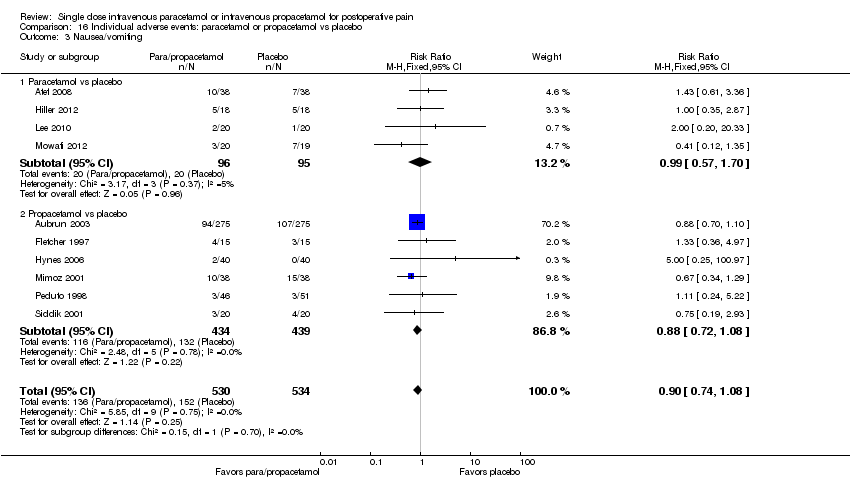
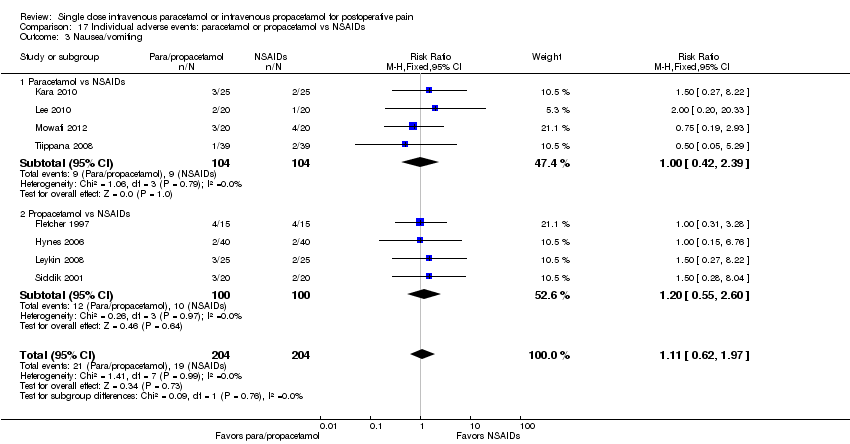
| 3 Nausea/vomiting Show forest plot | 10 | 1064 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.08] |
|
| 3.1 Paracetamol vs placebo | 4 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.57, 1.70] |
| 3.2 Propacetamol vs placebo | 6 | 873 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.72, 1.08] |
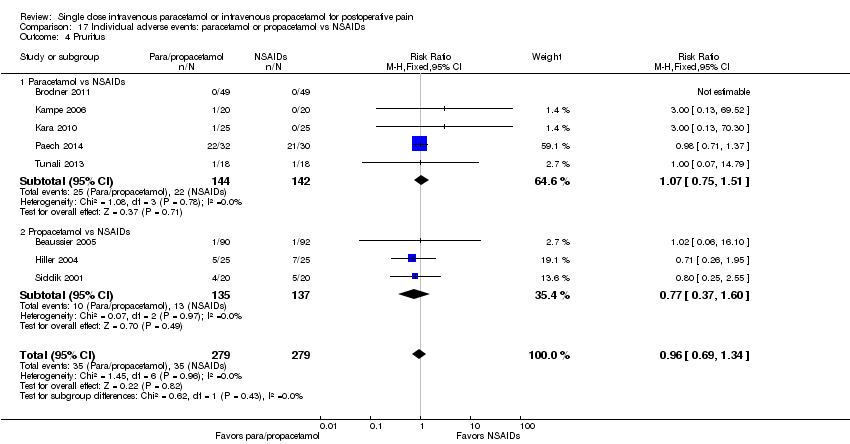
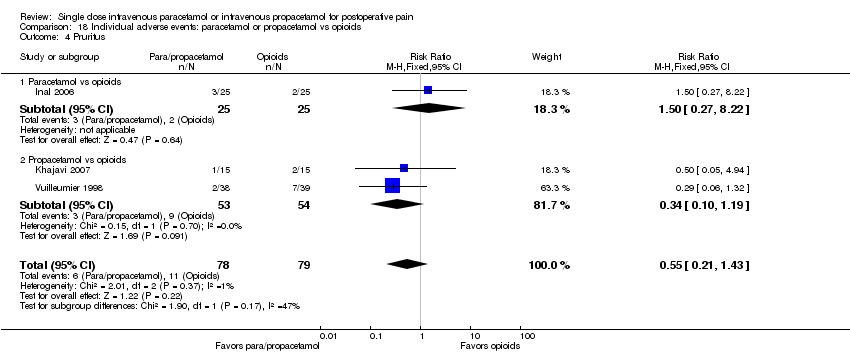
| 4 Pruritus Show forest plot | 7 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.60, 1.40] |
|
| 4.1 Paracetamol vs placebo | 5 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.64, 1.72] |
| 4.2 Propacetamol vs placebo | 3 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.29, 1.51] |
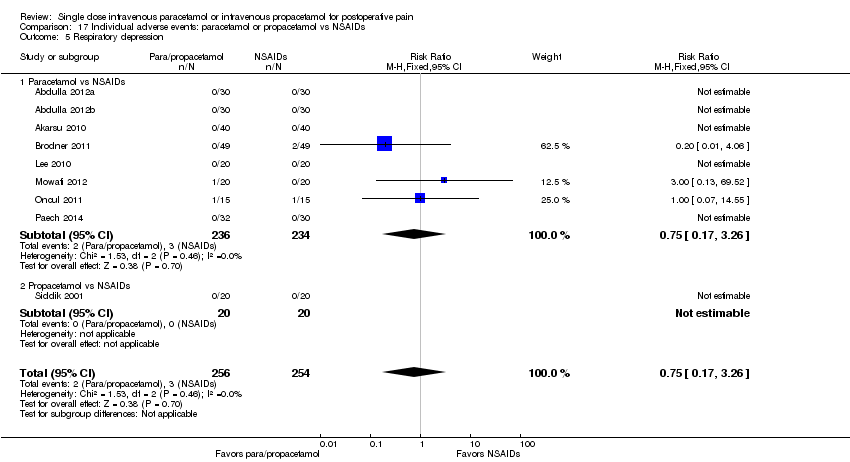
| 5 Respiratory depression Show forest plot | 11 | 1082 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.31, 1.92] |
|
| 5.1 Paracetamol vs placebo | 6 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.65] |
| 5.2 Propacetamol vs placebo | 5 | 719 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.35, 2.80] |
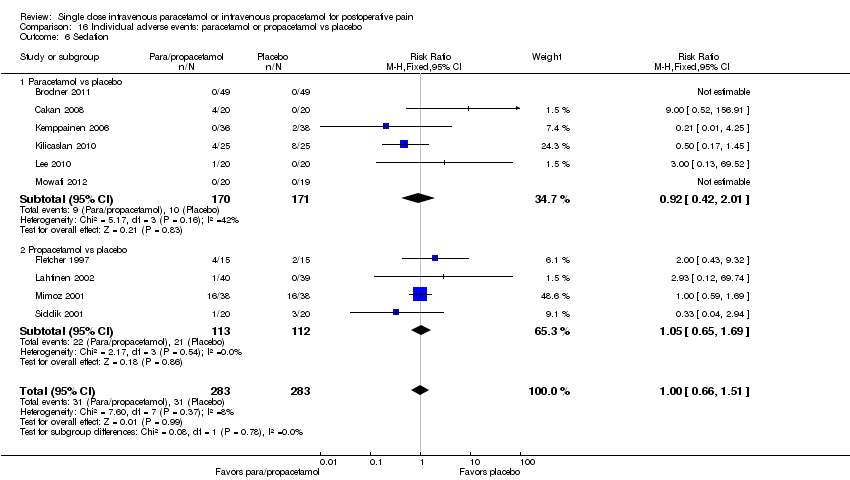
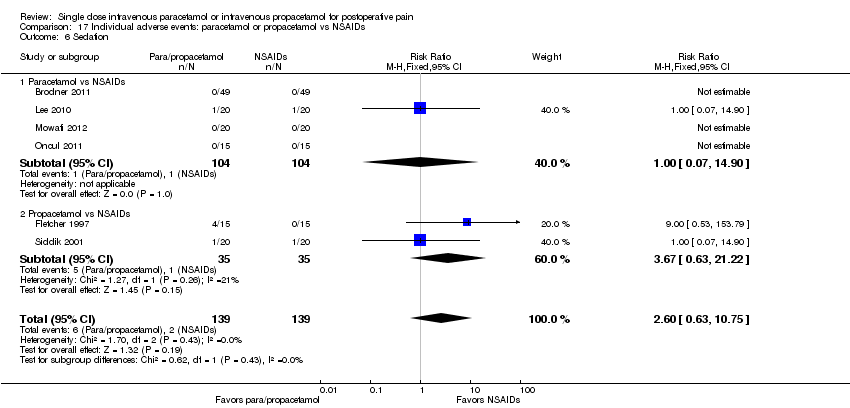
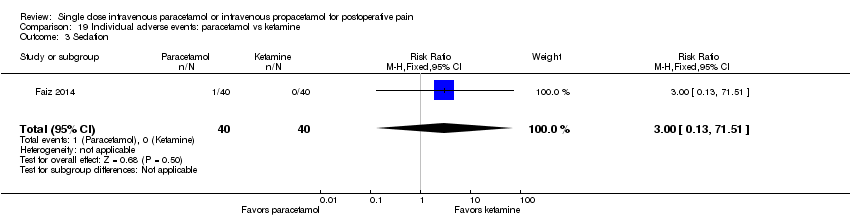
| 6 Sedation Show forest plot | 10 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.66, 1.51] |
|
| 6.1 Paracetamol vs placebo | 6 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.42, 2.01] |
| 6.2 Propacetamol vs placebo | 4 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.65, 1.69] |
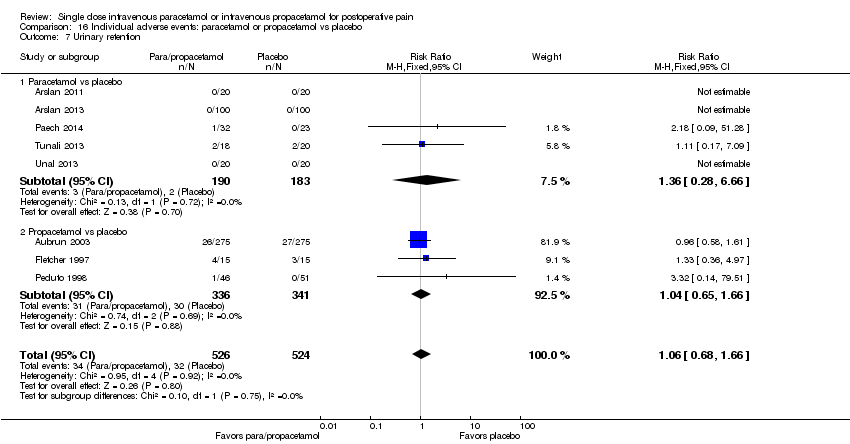
| 7 Urinary retention Show forest plot | 8 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.66] |
|
| 7.1 Paracetamol vs placebo | 5 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.28, 6.66] |
| 7.2 Propacetamol vs placebo | 3 | 677 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.65, 1.66] |
| 8 Allergy/skin rash/local reaction Show forest plot | 7 | 1131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.61, 3.91] |
|
| 8.1 Paracetamol vs placebo | 4 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.24, 4.34] |
| 8.2 Propacetamol vs placebo | 4 | 761 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.57, 6.73] |