Carbamazepina de liberación inmediata versus carbamazepina de liberación controlada en el tratamiento de la epilepsia
Resumen
Antecedentes
La carbamazepina (CBZ) es un fármaco de uso común para la epilepsia que se asocia con eventos adversos problemáticos como mareos, diplopía, somnolencia, mala coordinación e inestabilidad. Estos eventos adversos suelen ocurrir durante los picos de concentración plasmática del fármaco. Estos eventos adversos pueden limitar la dosis diaria de CBZ que puede ser tolerada y reducir las posibilidades de control de las crisis epilépticas en pacientes que requieren dosis altas. Una formulación de liberación controlada de CBZ proporciona la misma dosis durante un período de tiempo más largo en comparación con una formulación estándar de liberación inmediata, reduciendo así los picos posteriores a la dosis en la concentración plasmática de CBZ y reduciendo potencialmente los eventos adversos.
Ésta es una versión actualizada de la revisión Cochrane original publicada en el número 12, 2014.
Objetivos
Determinar la eficacia de la CBZ de liberación inmediata (CBZ‐LI) versus CBZ de liberación controlada (CBZ‐LC) en pacientes con diagnóstico de epilepsia.
Se investigaron las siguientes preguntas de revisión.
(1) Para los pacientes recién diagnosticados que comienzan a tomar CBZ, ¿cómo se comparan las formulaciones LI y LC en cuanto a eficacia y tolerabilidad?
(2) Para los pacientes tratados con CBZ‐LI que presentan eventos adversos inaceptables, ¿qué efecto se consigue en el control de las crisis epilépticas y la tolerabilidad con un cambio a una formulación de LC frente a continuar con la formulación de LI?
Métodos de búsqueda
Se realizaron búsquedas en el Registro Especializado del Grupo Cochrane de Epilepsia (Cochrane Epilepsy Group), CENTRAL y MEDLINE (Ovid) desde su inicio hasta el 30 de agosto de 2016.
Criterios de selección
Ensayos controlados aleatorizados que comparan la CBZ‐LI con la CBZ‐LC en pacientes que comienzan la monoterapia y en pacientes que actualmente reciben tratamiento con CBZ‐LI pero que presentan eventos adversos inaceptables.
Las medidas de resultados principales incluían medidas de la frecuencia de las crisis epilépticas, la incidencia de los eventos adversos, la proporción de pacientes con fracaso del tratamiento y medidas de la calidad de vida.
Obtención y análisis de los datos
Dos autores de la revisión evaluaron de forma independiente los ensayos para su inclusión, extrajeron los datos y registraron la información pertinente en un formulario estandarizado de extracción de datos. La herramienta Cochrane de riesgo de sesgo se utilizó para evaluar la calidad metodológica de los estudios incluidos.
La heterogeneidad de los ensayos incluidos con respecto a la notificación de los resultados dio lugar a que sólo fuera posible un análisis narrativo y descriptivo de los datos categóricos y del tiempo transcurrido hasta el evento.
Resultados principales
Diez ensayos (296 participantes) cumplieron los criterios de inclusión en esta revisión. Sólo un estudio tuvo un bajo riesgo de sesgo. Dos estudios tuvieron un alto riesgo de sesgo y el resto de los estudios se calificaron como de riesgo de sesgo poco claro. Un ensayo incluyó a pacientes con epilepsia recientemente diagnosticada y nueve incluyeron a pacientes tratados con CBZ‐LI.
Ocho ensayos informaron medidas heterogéneas de la frecuencia de crisis epilépticas con resultados contradictorios. Se observó una diferencia estadísticamente significativa en un solo ensayo, donde los pacientes que recibieron CBZ‐LC presentaron menos crisis epilépticas que los pacientes que recibieron CBZ‐LI.
Nueve ensayos informaron las medidas de eventos adversos. Hubo una tendencia a favor de la CBZ‐LC con cuatro ensayos que informaron una reducción estadísticamente significativa de los eventos adversos en comparación con la CBZ‐LI. Otros dos ensayos informaron un menor número de eventos adversos con CBZ‐LC, pero la reducción no fue estadísticamente significativa. Un ensayo no encontró diferencias en los eventos adversos, y otro ensayo informó más eventos adversos en el grupo de CBZ LC que en el grupo de CBZ LI, aunque el aumento no fue estadísticamente significativo.
Conclusiones de los autores
Para esta actualización no se identificaron nuevos estudios elegibles y las conclusiones extraídas de la revisión inicial no han cambiado.
Actualmente, los datos de los ensayos no confirman ni refutan que la CBZ‐LC sea mejor que la CBZ‐LI para la frecuencia de crisis epilépticas ni para los eventos adversos en pacientes con epilepsia recientemente diagnosticada.
Para los ensayos que incluyen a pacientes con epilepsia bajo CBZ‐LI, no se pueden establecer conclusiones sobre la superioridad de la CBZ‐LC con respecto a la frecuencia de las crisis epilépticas.
La CBZ‐LC tiende a asociarse con menos eventos adversos en comparación con la CBZ‐LI. Por lo tanto, un cambio a la CBZ‐LC puede ser una estrategia beneficiosa en pacientes con un control aceptable de las crisis epilépticas con la CBZ‐LI pero que presentan eventos adversos inaceptables. Los ensayos incluidos eran de pequeño tamaño y de calidad metodológica deficiente, lo que limita la validez de esta conclusión.
Se requieren ensayos controlados aleatorizados que comparen la CBZ‐LC con la CBZ‐LI y que utilicen resultados clínicamente relevantes para informar la elección del preparado de CBZ para los pacientes con epilepsia recientemente diagnosticada.
PICO
Resumen en términos sencillos
Carbamazepina de liberación rápida versus carbamazepina de liberación lenta como medicación para pacientes con epilepsia
Antecedentes
La epilepsia es un trastorno cerebral común que suele tratarse con carbamazepina. Con el tratamiento, las personas suelen tener menos crisis epilépticas, pero muchas personas experimentan efectos secundarios. Cuando se ingiere la carbamazepina, ésta se introduce rápidamente en el torrente sanguíneo y se produce un fuerte aumento de los niveles del fármaco en la sangre. Estos "picos" pueden estar asociados a efectos secundarios como mareos, visión doble, somnolencia, inestabilidad y mala coordinación. Un tipo de carbamazepina que libera el medicamento en el cuerpo lentamente puede disminuir estos "picos" en los niveles sanguíneos, lo que posiblemente signifique menos efectos secundarios.
Esta revisión comparó estudios que analizaron las diferencias entre una carbamazepina de "liberación rápida" y una carbamazepina de "liberación lenta".
Participantes
Para ser incluidos en esta revisión, todos los participantes tenían que ser diagnosticados con epilepsia y estar recién comenzando el tratamiento con carbamazepina o estar ya en él pero con efectos secundarios intolerables. Los participantes podían ser de cualquier edad o sexo.
Estudios
En la revisión se incluyeron diez ensayos con un total de 296 personas con epilepsia. Los 10 ensayos fueron ensayos controlados aleatorizados (los pacientes se compararon en grupos asignados al azar). En todos los estudios había por lo menos dos grupos, uno que tomaba carbamazepina de liberación rápida y otro que tomaba carbamazepina de liberación lenta, y algunos también tenían un grupo de control (un grupo de personas no epilépticas). La evidencia está actualizada hasta agosto 2016.
Resultados
Sólo uno de 10 estudios encontró una diferencia significativa entre los dos tipos de carbamazepina en el número de convulsiones experimentadas, ya que los pacientes a los que se les prescribió carbamazepina de liberación lenta tuvieron menos crisis epilépticas que los pacientes a los que se les prescribió el fármaco de liberación rápida. Los pacientes que recibían carbamazepina de liberación lenta tendían a presentar menos efectos secundarios.
Calidad de la evidencia
De los diez ensayos de la revisión, sólo un estudio se consideró de "buena" calidad, por lo que la evidencia de esta revisión se calificó como de baja calidad. Cabe destacar que existen pocos estudios que evalúen las diferencias entre ambos tipos de carbamazepina y que se necesitan estudios adicionales antes de poder establecer una conclusión definitiva sobre uno de ellos sobre el otro.
Authors' conclusions
Background
Description of the condition
Epilepsy is defined as the tendency to spontaneous, excessive neuronal discharge manifesting as seizures. It is a common disorder with an incidence of 50 per 100,000 per year and a prevalence of 0.5% to 1% in the developed world (Hauser 1993).
Description of the intervention
In the treatment of epilepsy, carbamazepine (CBZ) is a first‐line antiepileptic drugs of proven efficacy when compared to other standard drugs such as valproate (Marson 2000; NICE 2012). However, CBZ is associated with a number of adverse events including dose‐related events such as dizziness, double vision and unsteadiness.
How the intervention might work
Adverse events can occur during peaks in plasma concentration of CBZ following ingestion of a dose. The occurrence of such events may limit the daily dose that can be tolerated and reduce the chances of seizure control for patients requiring higher doses (Vojvodic 2002). This problem may be compounded by unpredictable fluctuations of CBZ serum concentrations due to its poor water solubility, which causes slow and irregular absorption. Such problems might be alleviated by prescribing a controlled‐release formulation, which delivers the same dose over a longer period of time when compared to a standard formulation, thereby reducing post‐dose peaks and potentially reducing adverse events.
CBZ has numerous qualities that may make it a good candidate for a controlled‐release preparation. These qualities include a short half‐life, lack of first‐pass metabolism, a narrow therapeutic index and efficient absorption throughout the gastrointestinal tract (Collins 2000). Several controlled‐release preparations are currently available.
Why it is important to do this review
In this review we have summarised evidence from randomised controlled trials assessing immediate‐release and controlled‐release CBZ in patients with epilepsy. Through assessing the intended effects, including reduction in seizure frequency, and unintended effects, including adverse drug reactions, we aimed to inform clinical decision making in this population. This review is an update of a previously published Cochrane review on immediate‐release versus controlled‐release carbamazepine for the treatment of epilepsy (Powell 2010; Powell 2014a; Powell 2014b).
Objectives
To determine the efficacy of immediate‐release CBZ (IR CBZ) versus controlled‐release CBZ (CR CBZ) in patients diagnosed with epilepsy.
The following review questions were investigated.
(1) For newly diagnosed patients commencing CBZ, how do IR and CR formulations compare for efficacy and tolerability?
(2) For patients on established treatment with IR CBZ but experiencing unacceptable adverse events, what is the effect on seizure control and tolerability of a switch to a CR formulation versus remaining on the IR formulation?
Methods
Criteria for considering studies for this review
Types of studies
(1) Randomised controlled trials (RCTs) comparing IR CBZ to CR CBZ. Our initial intention was to only include studies with an adequate method of allocation concealment. However, due to the small number of studies identified by the literature searches, studies where the method of randomisation was not clearly stated have also been included.
(2) Studies could be double blind, single blind or unblinded.
Types of participants
Patients of any age and either gender with a diagnosis of epilepsy who were either:
(1) commencing monotherapy with IR CBZ or commencing monotherapy with CR CBZ; or
(2) currently prescribed monotherapy with IR CBZ but experiencing unacceptable adverse events and were being switched to a CR CBZ formulation.
Types of interventions
The intervention group should have received a CR formulation of CBZ and the control group a standard, IR formulation of CBZ.
Types of outcome measures
The outcome measures of interest to this review are listed below for studies addressing each objective.
Primary outcomes
Objective 1: analysis of newly diagnosed patients
(1) Time to 12‐month remission
Objective 2: analysis of patients with established epilepsy
(1) Proportion seizure free at six months
Secondary outcomes
For objective 1: analysis of newly diagnosed patients
(1) Proportion seizure free at six months;
(2) Proportion seizure free at 12 months;
(3) A 50% or greater reduction in seizure frequency;
(4) Proportion with treatment failure (inadequate seizure control, adverse events, or both) at six months;
(5) Proportion with treatment failure (inadequate seizure control, adverse events, or both) at 12 months;
(6) Incidence of adverse events; and
(7) Quality of life measures.
For objective 2: analysis of patients with established epilepsy
(1) Proportion seizure free at 12 months;
(2) A 50% or greater reduction in seizure frequency;
(3) Proportion with treatment failure (inadequate seizure control, adverse events, or both) at six months;
(4) Proportion with treatment failure (inadequate seizure control, adverse events, or both) at 12 months;
(5) Incidence of adverse events; and
(6) Quality of life measures.
Search methods for identification of studies
We carried out searches as follows.
Electronic searches
Searches were run for the original review in September 2009 and subsequent searches were run in July 2011, September 2013, and November 2014. For the latest update we searched the following databases. There were no language restrictions.
(a) Cochrane Epilepsy Group Specialized Register (30 August 2016) using the search strategy outlined in Appendix 1.
(b) Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO), 30 August 2016, using the search strategy outlined in Appendix 2.
(c) MEDLINE (Ovid, 1946 to 30 August 2016) using the strategy outlined in Appendix 3.
Searching other resources
We did not contact pharmaceutical companies or researchers in the field as a result of the time period the majority of studies were published. However, contact may be made prior to a future update of this review.
Data collection and analysis
Selection of studies
Two review authors (GP, MS) screened all the titles, abstracts and keywords of publications identified by the searches to assess eligibility for inclusion. Publications that clearly did not meet the inclusion criteria were excluded at this stage. A paper copy of the full publication of each relevant study was obtained. Both review authors assessed these studies according to pre‐specified selection criteria. Any disagreement concerning eligibility for inclusion was resolved by discussion and a consensus decision was made.
Data extraction and management
Two review authors (GP, MS) independently extracted the data and recorded relevant information on a standardised data extraction form. The extracted results were compared to assess agreement. The data reported by published sources were used for analysis in this review. Both review authors pilot tested the data collection form on a sample study and found it to be suitable. Disagreement or uncertainty concerning extracted data was resolved by discussion and a consensus decision was made.
Assessment of risk of bias in included studies
Three review authors (GP, MS and AR) independently assessed the risk of bias for each trial using the Cochrane risk of bias tool (Higgins 2011). We discussed and resolved any disagreements. We rated studies as high, low or unclear for six domains applicable to RCTs: randomisation method, allocation concealment, blinding methods, incomplete outcome data, selective outcome reporting and other sources of bias.
We intended to create 'summary of findings' tables and use the GRADE approach for assessing quality of evidence. However, this was not appropriate due to the discrepancy between the outcomes of interest in the protocol and the reviewed studies.
Measures of treatment effect
For categorical outcomes, we planned to express relative treatment effects as the risk ratio with corresponding 95% confidence intervals (CI). For time‐to‐event data we did not plan to undertake a meta‐analysis using aggregate data, rather we planned to summarise the trial results in text tables. Due to the small number of trials identified, we did not explore the possibility of obtaining individual patient data to include in a meta‐analysis using inverse variance methods. This may be considered in an update of the review. Similarly, quality of life data were summarised in the text and tables.
Unit of analysis issues
We included crossover trials as well as parallel group trials in the review. However, there were no unit of analysis issues as we could not combine any of the data from the included studies in a meta‐analysis. All findings from the included studies were reported narratively.
Dealing with missing data
We did not seek missing data from the study authors due to the time period during which the majority of studies were published (i.e. from 1987 through 1998). However, contact may be made prior to an update of this review.
Assessment of heterogeneity
Clinical heterogeneity was assessed by comparing study designs and the recruited patient populations among the trials. Where appropriate, the degree of heterogeneity was to be assessed using the I2 statistic. A value of 25% would indicate low heterogeneity, 50% moderate heterogeneity and 75% high heterogeneity. If heterogeneity was present, its significance was considered and a decision made as to whether meta‐analysis was appropriate. If so, a random‐effects model would be used.
Assessment of reporting biases
Due to the age of the included studies (published between 1987 and 1998), protocols were not requested from the authors. To assess outcome reporting bias we used the ORBIT tool (Kirkham 2010). We originally intended to examine funnel plots to investigate potential publication bias, however this was not possible.
Data synthesis
No meta‐analyses were carried out, all results were discussed narratively. Comparisons we expected to investigate included:
-
IR versus CR on time to 12‐month remission;
-
IR versus CR on proportion seizure free at six months.
Other comparisons included IR versus CR for all secondary outcomes (see Types of outcome measures).
Subgroup analysis and investigation of heterogeneity
We stratified the comparisons made by type of participant, that is newly diagnosed patients and established epilepsy patients.
Sensitivity analysis
No sensitivity analyses were planned.
Results
Description of studies
Results of the search
Searching of the databases as described in Search methods for identification of studies yielded 1973 records, and two additional records were identified from other sources. After the removal of duplicates (865), the remaining 1110 records were screened for potential inclusion: 1087 were excluded for irrelevance. The following assessment for eligibility excluded another 13 studies (see Figure 1 and Characteristics of excluded studies for reasons of exclusion) leaving a total of 10 studies to be included in the review. None of these studies were included in a meta‐analysis. No new studies were identified by the current literature search carried out on 30 August 2016.

Study flow diagram.
Included studies
Ten trials fulfilled the criteria for inclusion in this review. One trial concerned patients with newly diagnosed epilepsy and was included under the first objective (Nag 1998); 20 patients with ages ranging from 16 to 35 years were recruited. The remaining nine trials included patients currently treated with IR CBZ and were included under the second objective. In total, 296 patients with ages ranging from 6 to 69 year were recruited into these 10 trials. One trial included children in addition to adult patients (Kaski 1991). Summary information on the trials included in this review can be found in the Characteristics of included studies table. In addition, more detailed information, particularly concerning interventions and the results of each study, can be found in Appendix 4.
Included trials were primarily concerned with the pharmacokinetic parameters of both CBZ and its predominant metabolite, CBZ epoxide. The clinical parameters that were of interest in this review were generally considered as secondary outcomes within the included studies and there was significant heterogeneity with respect to the outcomes reported. The clinical outcomes that were reported included mean seizure frequency, total number of seizures experienced in each study arm, mean total number of seizures per patient in each study arm, total incidence of adverse events reported in each study arm, total number of patients reporting adverse events, and total scores of inventories designed to quantify adverse event occurrence.
Excluded studies
Two trials were not published in English and could not be included due to restrictions that prevented translation (Dam 1980; Remy 1990). It is likely one study (Dam 1980) is subsequently included (Dam 1981). The remaining study may be included in an update of this review. Thirteen trials were excluded as they did not meet the eligibility criteria. Four trials did not include CR formulations of CBZ (Dam 1981; Ghose 1983; Monaco 1984; Thakker 1991), two of which were concerned with the dose frequency of IR CBZ (Ghose 1983; Monaco 1984). Three trials did not make the required treatment comparison: two trials involved a comparison of two CR CBZ formulations (Jensen 1990; Scheuch 1992), and one trial compared CR CBZ to sodium valproate (Sobaniec 2004). There was no mention of randomisation in two trials (Bojinova 1997; Pieters 1992). One trial was observational in design, involving a treatment cohort with no control group (Mirza 1998), and a further trial did not report any relevant outcome measures (Ramsay 1989). Additional details can be found in the table Characteristics of excluded studies.
Risk of bias in included studies
See Figure 2 for a summary of the risk of bias in each included study. For each study, we allocated an overall rating for the risk of bias. One study was rated as low risk of bias (Persson 1990), two as high risk of bias (Nag 1998; Sivenius 1988) and the remainder as unclear risk of bias. See below for specific domain ratings. Across all studies we rated the evidence as unclear risk of bias.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We rated allocation concealment as unclear risk of bias for all of the studies as the trials did not report the methods used for allocation concealment. Persson 1990 was rated as low risk of bias for sequence generation due to the use of a computer program for patient randomisation. All of the other studies were rated as unclear risk of bias for sequence generation as the method used for randomisation was not described, although all stated that randomisation had been performed.
Blinding
Four studies were rated as high risk of bias for the blinding domain due to the fact that they were open label trials (Nag 1998; Sivenius 1988) or single‐blind (Aldenkamp 1987; Reunanen 1990). The remaining eight trials were adequately blinded, involving identical tablets and packaging.
Incomplete outcome data
All of the studies in this review were rated as low risk of attrition bias because the proportion of patients who did not complete the studies was low in each study. Thus the number of patients randomised but not included in the analysis was determined not to be significant. Therefore, despite some studies not reporting missing data, or not carrying out intention‐to‐treat analysis, there were deemed to be no issues with attrition bias.
Selective reporting
Study protocols were not requested due to the time period in which the majority of the studies were published. All but one study (Sivenius 1988), was rated as low risk of bias for this domain. We applied the ORBIT classification system to this study and rated it 'A' due to its lack of reporting analysed adverse effect data; therefore this study was rated as high risk of bias for selective reporting.
Other potential sources of bias
Four studies were rated as unclear risk of bias in this category due to quoting financial support from Ciba‐Geigy Pharmaceuticals, Intas Pharmaceuticals, Shire Pharmaceuticals, all of which produce CR carbamazepine based medications (Anonymous 1995; Garnett 1998; McKee 1991; Nag 1998).
Effects of interventions
With the exception of adverse events, none of the included studies reported on our pre‐specified primary and secondary outcomes. A narrative summary follows concerning the results for each relevant outcome. More detailed results can be found in the Additional tables section.
Objective 1: analysis of newly diagnosed patients
Seizure frequency
No data relating to seizure frequency were reported in the one trial involving patients with newly diagnosed epilepsy (Nag 1998).
Incidence of adverse events
One unblinded parallel trial was identified that involved 20 adult patients with newly diagnosed epilepsy (Nag 1998). During the 20‐day study period, a total of four adverse events were reported in patients prescribed IR CBZ: diplopia, rash, and two reports of sedation. Two adverse events, sedation and diplopia, were reported in patients prescribed CR CBZ. The data is presented in a forest plot, together with other studies where this is possible (Figure 3) The statistical significance of the difference between groups was not reported.

Forest plot of comparison: 1 Adverse Events, outcome: 1.1 Adverse Events.
Objective 2: analysis of patients with established epilepsy
Seizure frequency
The occurrence of seizures during each treatment period was reported heterogeneously. The majority of studies where seizures were recorded reported the mean number of seizures per patient during each study period.
-
Garnett 1998 reported an increased mean number of seizures per patient during CR CBZ treatment. A mean 2.8 seizures per patient (range 0 to 29) occurred in patients prescribed CR CBZ compared to 1.6 (range 0 to 18) in patients prescribed IR CBZ in each two‐week treatment arm. This difference was not statistically significant. The CBZ dose remained stable throughout the study period.
-
Persson 1990 reported an increased mean number of seizures per patient for the IR CBZ treatment group: 2.2 compared to 1.2 during CR CBZ treatment. These figures were only derived from the first month of the three‐month treatment period. This was not statistically significant. There were no statistically significant differences between the mean number of seizures per month per patient for IR CBZ (1.34) and CR CBZ (1.24). The CBZ dosage remained the same throughout the study period.
-
McKee 1991 reported an increased mean number of seizures per patient during treatment with CR CBZ: 3.8 (SD 0.9) compared to 2.8 (SD 1.2) during treatment with IR CBZ (95% CI ‐0.7 to 2.8). The CBZ dose remained stable during the four‐week study period. Seizure frequency was not statistically significantly different for either CBZ formulation when compared to baseline.
-
Anonymous 1995 reported a lower mean monthly seizure rate during IR CBZ treatment: 0.41 compared to 0.53 during treatment with CR CBZ. SDs or CIs were not given for these estimates. The CBZ dose remained stable during each 56‐day study arm. The difference was not statistically significant.
Seizure frequency was reported in the following studies.
-
Canger 1990 reported a statistically significant reduction in mean monthly seizure frequency during treatment with CR CBZ: 6.3 (SD 9.8) compared to 9.3 (SD 15.6) during treatment with IR CBZ. The CBZ dose remained stable during the one‐month study period.
-
Kaski 1991 reported the total number of seizures during the 10‐week study period: 44 (range 9 to 133) in patients prescribed CR CBZ compared to 42.7 (range 4 to 107) in patients prescribed IR CBZ. This difference was not statistically significant. The CBZ dose remained stable throughout the study period.
The total numbers of seizures that occurred in each study group was reported in the following studies.
-
Reunanen 1990 reported an increased total number of seizures during treatment with IR CBZ: 56 seizures compared to 31 during treatment with CR CBZ in each two‐week treatment arm. The CBZ dose remained stable throughout the study period. This difference was not statistically significant.
-
Sivenius 1988 reported identical total numbers of seizures during each treatment period. Nine seizures occurred during treatment with both CBZ formulations in each two‐week study period. The CBZ dose remained stable throughout the study period.
Incidence of adverse events
Adverse events were reported heterogeneously in the studies included in this review. The following studies used various inventories designed to assess adverse events as a result of antiepileptic drugs. Total scale scores were calculated following completion of the studies to allow the comparison of these psychometric outcomes between groups.
-
McKee 1991 reported significantly lower cognitive adverse event scores at one hour with CR CBZ compared to IR CBZ. In addition, reaction times were significantly shorter at one and four hours with CR CBZ compared to IR CBZ.
-
Aldenkamp 1987 reported increased performance in various tests of cognitive function in patients taking CR CBZ. The statistical significance of this result was not reported.
-
Persson 1990 reported lower scores on a combined systemic toxicity and neurotoxicity scale in patients taking CR CBZ compared to those on IR CBZ. The difference was statistically significant.
Numerous studies reported the individual numbers of adverse events reported.
-
Anonymous 1995 reported that four patients experienced six adverse events when prescribed CR CBZ: dizziness (2 patients), diplopia (1), headache (1), nausea (1) and vomiting (1). Five patients experienced five adverse events when prescribed IR CBZ: dizziness, drowsiness, hand tremor, stomach cramps and vomiting. These differences were not statistically significant.
-
Reunanen 1990 reported 19 adverse events during IR CBZ treatment compared to 12 with CR CBZ. Dizziness (7 patients), fatigue (4), visual disturbance (4), headache (1) and difficulty with co‐ordination (3) were experienced during IR CBZ treatment. Dizziness (1), fatigue (4), visual disturbance (2), headache (2), difficulty with co‐ordination (1), nausea (1) and gastric discomfort (1) were experienced during CR CBZ treatment. The difference was statistically significant for dizziness, reported seven times during IR CBZ treatment and just once during CR CBZ treatment.
-
Garnett 1998 reported one adverse event: somnolence during IR CBZ treatment.
The following studies reported the number of patients experiencing adverse events.
-
Sivenius 1988 reported that four patients in each treatment group experienced adverse events. No further details concerning the individual adverse events were reported.
-
Canger 1990 stated that 26 patients reported intermittent adverse events with IR CBZ whereas six patients reported adverse events with CR CBZ. No further details concerning the individual adverse events were reported. The difference was statistically significant.
Discussion
Summary of main results
Ten trials were included in this review. All were primarily concerned with comparisons of the pharmacokinetic parameters of IR CBZ and CR CBZ.
Only one trial involving patients with newly diagnosed epilepsy was identified (Nag 1998). This trial involved only 20 patients and reported a total of six adverse events, four occurring during IR CBZ treatment. Given the small number of patients and low number of events, no conclusions can be drawn regarding the comparative tolerability of IR CBZ and CR CBZ in patients with newly diagnosed epilepsy. Measures of seizure frequency, quality of life measures and time‐to‐event data were not reported.
Eight of the included trials reported measures of seizure frequency in patients with an established diagnosis of epilepsy and currently treated with IR CBZ. The reported outcome measures were heterogeneous and we have described general trends. Three trials reported a reduced occurrence of seizures in patients taking CR CBZ, which was statistically significant in one trial. In addition, three trials reported a reduced occurrence of seizures in patients taking IR CBZ, which was statistically significant in one trial. The remaining two trials reported no difference in seizure occurrence between the two formulations. There appears to be no difference between formulations in controlling the occurrence of seizures. However, the absence of further statistical analyses, methodological limitations and risk of bias limit the accuracy of these narrative conclusions.
Eight of the included trials reported data concerning adverse events in patients with an established diagnosis of epilepsy and already prescribed IR CBZ. Although methods of reporting differed greatly, and outcomes could not be statistically combined, four of the trials found a significantly reduced incidence of adverse events in patients taking CR CBZ compared to IR CBZ. A further two trials reported a lower incidence of adverse events with CR CBZ, which was not statistically significant. Of the remaining two trials, one found no difference in adverse event rates between the two CBZ formulations and one reported a reduced incidence of adverse events in patients taking IR CBZ (Anonymous 1995). Interestingly this crossover trial included the largest study sample, 101 patients, and had one of the longest study periods, with 56 days in each treatment arm. Our narrative analysis suggests a reduced incidence of adverse events and therefore superiority of CR CBZ compared to IR CBZ when considering adverse events. These results do not, however, provide robust evidence due to the heterogenous methods of reporting that prevented further statistical analysis of the results, inherent limitations in methodological quality, and the risk of bias present in many of the trials included in this review.
Overall completeness and applicability of evidence
For the first objective of this review, the comparison in newly diagnosed patients, only one study met the inclusion criteria specified in our protocol. Therefore, the evidence for this conclusion is lacking without other studies to support it.
For the second objective, the comparison in patients with established epilepsy, only one study showed any significant difference in seizure frequency between the two groups, with patients in the CR group experiencing a lower mean monthly seizure frequency. In the remaining seven studies that reported seizure frequency there was no significant difference between the two groups. For adverse events, however, five studies reported a significant reduction in named or all adverse events for those patients on CR CBZ, with a further two studies reporting similar results but without reporting statistical significance. The remaining three studies noted no significant differences in adverse events between the two groups.
Quality of the evidence
Few of the clinical outcomes pre‐specified in the protocol of this review were measured in these trials. The overall methodological quality of the trials included in this review was poor, as was the reporting of important methodological factors. The risk of bias was low in only one of the 10 trials included in this review (Persson 1990). The methods of randomisation and allocation concealment were unclear in the remaining nine trials, whilst eight trials were adequately blinded.
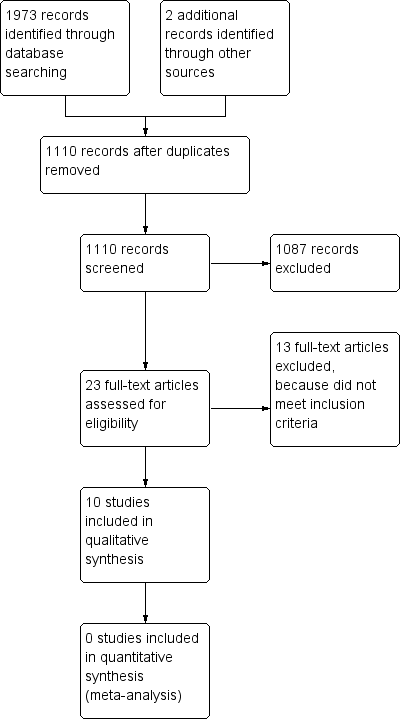
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Adverse Events, outcome: 1.1 Adverse Events.

Comparison 1 Adverse Events, Outcome 1 Adverse Events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse Events Show forest plot | 2 | 107 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.30, 2.43] |

