Duloxetina para el tratamiento de la neuropatía dolorosa, el dolor crónico o la fibromialgia
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, double‐blind, placebo‐controlled, parallel group trial of duloxetine in fibromyalgia | |
| Participants | 207 men or women over 18 years who fulfilled American College of Rheumatology criteria for fibromyalgia, and scoring 4 or more on the pain intensity item of the Fibromyalgia Impact Questionnaire (FIQ) | |
| Interventions | Duloxetine 60 mg twice daily versus placebo for 12 weeks with a 20‐day titration phase | |
| Outcomes | Follow‐up at 12 weeks Outcomes:
| |
| Notes | Greater use of antidepressants in the placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment to treatment groups was determined by a computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Used an interactive voice response system |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind for all assessments in 12‐week therapy phase, Investigators adjusted the number of placebo capsules similarly to maintain the blinding. Single‐blind in run‐in phase |
| Incomplete outcome data (attrition bias) | High risk | 46/104 (44%) in duloxetine and 37/103 (36%) in placebo group discontinued treatment but all dropouts accounted for and LOCF |
| Selective reporting (reporting bias) | Unclear risk | As above in incomplete outcome data |
| Other bias | Low risk | More use of antidepressants in the placebo group but this would bias against the treatment arm |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel group trial of duloxetine in fibromyalgia | |
| Participants | 354 participants Women only, ≥ 18 years of age who met criteria for primary fibromyalgia as defined by the American College of Rheumatology, and had a score of ≥ 4 on the average pain severity item of the Brief Pain Inventory (BPI) at randomisation | |
| Interventions | Duloxetine 60 mg daily, duloxetine 60 mg twice daily and placebo for 12 weeks | |
| Outcomes |
| |
| Notes | Company sponsored and run trial. Fibromyalgia Impact Questionnaire abandoned in favour of BPI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assignment of women who met entry criteria following the screening phase to one of three treatment groups: duloxetine 60 mg daily, duloxetine 60 mg twice daily (forced titration from 60 mg daily for 3 days to 60 mg twice daily), or placebo, with randomisation in a 1:1:1 ratio. Random assignment of the participants to treatment groups occurred within two stratified groups, those with and those without current major depressive disorder |
| Allocation concealment (selection bias) | Unclear risk | Probably low risk of bias as previous trial used an adequate method |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | High risk | High proportion of dropouts: 138 (39%) participants withdrew during the 12‐week therapy phase, 41 (35%) from the duloxetine 60 mg daily group, 45 (39%) from the duloxetine 60 mg twice daily group, and 52 (43%) from the placebo group (P = 0.407). Matched across groups but a high rate of loss "Partial intention to treat analysis". Efficacy analyses include all randomised participants with a baseline and at least one post‐baseline visit with efficacy data, while safety analyses included all randomised participants |
| Selective reporting (reporting bias) | Unclear risk | See incomplete outcome data above |
| Other bias | Low risk | Lilly study. No other bias identified |
| Methods | Phase IV randomised, double‐blind (subject, caregiver, investigator, outcomes assessor), placebo‐controlled, parallel assignment safety and efficacy study of duloxetine in fibromyalgia | |
| Participants | Men or women
| |
| Interventions | Duloxetine 60 to 120 mg daily for 24 weeks | |
| Outcomes | Time frame for all outcome measures 24 weeks Primary outcome
Secondary outcomes
| |
| Notes | Completed and published | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned 1:1 in a double‐blind fashion to duloxetine 60 mg once daily or placebo by a computer‐generated random sequence using an IVRS |
| Allocation concealment (selection bias) | Low risk | "Double blind". "Variable transition to active treatment strategy…thereby blinding the onset of active treatment to reduce the patient's expectation of experiencing side effects" |
| Blinding (performance bias and detection bias) | Unclear risk | No comment on formulation of drug or placebo but almost certainly double blinded both in up and down titration. However, significantly more participants on duloxetine withdrew with adverse effects |
| Incomplete outcome data (attrition bias) | Low risk | Accounted for as much as possible. High dropout rate (> 30%). Employs ITT ‐ use of a "restricted maximum likelihood‐based [mixed effects model repeated measures approach] analysis accounts for bias caused by non‐random missing data due to early discontinuation because of adverse events or lack of efficacy better than LOCF" |
| Selective reporting (reporting bias) | Low risk | "Patient Global Impression ‐ severity (PGI‐S) only assessed at baseline". Otherwise, paper reports all results |
| Other bias | Unclear risk | Lilly trial. 93.2% female participants, similar to all fibromyalgia studies |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel group study of duloxetine in fibromyalgia | |
| Participants | Women and men > 18 years of age who met the American College of Rheumatology 1990 criteria for primary fibromyalgia and had a score of > 4 on the average pain severity item of the Brief Pain Inventory (BPI)‐Modified Short Form. Patients with or without major depressive disorder or generalised anxiety disorder, as defined by the DSM‐IV and confirmed by the MINI were included. | |
| Interventions | Duloxetine 30 mg capsules or placebo for 12 weeks | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | Lilly study. No other bias identified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by a computer‐generated random sequence using an interactive voice response system (IVRS) |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Low risk | The duloxetine and placebo capsules were identical in appearance to maintain the blinding. Participants and investigators were kept blinded to the rescue criteria and dose increase; site personnel entered the major depressive disorder status at baseline and the CGI‐I for Depression scores through IVRS at every visit |
| Incomplete outcome data (attrition bias) | Low risk | Only 2 dropouts, both from duloxetine group and likely to have been of minimal significance |
| Selective reporting (reporting bias) | Low risk | Most outcomes presented except individual BPI severity items (worst pain, least pain, pain right now). However, no other outcomes with significant effect in completely negative trial |
| Other bias | Low risk | Lilly study. No other bias identified |
| Methods | 8‐week, randomised, double‐blind, placebo‐controlled, parallel‐group efficacy and safety study of duloxetine in the treatment of pain of unknown aetiology in people with major depressive disorder | |
| Participants | Women or men > 18 with major depressive disorder defined by DSM‐IV. At baseline, depression score of > 20 on the MADRS and at least moderate pain on Brief Pain Inventory Short Form (BPI‐SF) ‐ 3 or higher for "24 hour average pain". Participants were also devoid of any other diagnosed pain syndrome as per a medical history | |
| Interventions | Duloxetine 60 mg versus placebo for 8 weeks | |
| Outcomes | Primary outcome
Seconday outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" |
| Allocation concealment (selection bias) | Unclear risk | "Double blind" |
| Blinding (performance bias and detection bias) | Unclear risk | "Double blind" |
| Incomplete outcome data (attrition bias) | Low risk | ITT using all participants with 1 dose of drug. 25% dropout rate |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Low risk | Company sponsored trial |
| Methods | Six‐month, randomised, double‐blind, placebo‐controlled, clinical trial of duloxetine in fibromyalgia | |
| Participants | Male and female outpatients were eligible for the study if they were ≥ 18 years of age, met criteria for fibromyalgia as defined by the American College of Rheumatology, with or without major depressive disorder No criteria for pain level at entry | |
| Interventions | Duloxetine ‐ variable dose. Started at 60 mg (30 mg run in period over 1 week), randomised increase to 120 mg after 13 weeks if not > 50% reduction in pain on BPI average | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random sequence within each study centre stratified by major depressive disorder |
| Allocation concealment (selection bias) | Low risk | Double‐blind |
| Blinding (performance bias and detection bias) | Unclear risk | Participants "blinded", but not clear how the study managed dose escalations and decreases and whether blinding was maintained |
| Incomplete outcome data (attrition bias) | High risk | 37.6% to 38.6% discontinuations, significantly different in lack of efficacy only. Investigators used LOCF and MMRM to correct for dropouts |
| Selective reporting (reporting bias) | Low risk | 30% improvement in BPI‐average added post hoc |
| Other bias | Unclear risk | Lilly sponsored trial Significant unexplained treatment by investigator interaction |
| Methods | Phase III randomised, double‐blind (subject, caregiver, investigator, outcomes assessor), placebo‐controlled, parallel assignment safety and efficacy study of duloxetine in painful diabetic neuropathy | |
| Participants | 215 participants Men or women, aged 18 to 75, pain due to bilateral peripheral neuropathy caused by type I or type II diabetes with the pain beginning in the feet, and present for at least 6 months. Score of 4 or greater on the Brief Pain Inventory (BPI) on the 24‐hour average pain item | |
| Interventions | Duloxetine 60 mg daily for 12 weeks | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | Closed and completed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned |
| Allocation concealment (selection bias) | Low risk | "Double blind". Study medication in capsules…or matching placebo' |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | 15.6% to 17.9% dropout rate but ITT using LOCF and MMRM approach to minimise bias |
| Selective reporting (reporting bias) | Low risk | Selective use of MMRM or LOCF depending upon outcome. However, all measures reported |
| Other bias | Low risk | Lilly sponsored trial No adjustment for multiple comparisons |
| Methods | Randomised, double‐blind, placebo‐controlled trial of duloxetine in people with major depressive disorder and painful physical symptoms | |
| Participants | Adult (18 years of age) male or female outpatients were eligible ...if they met all of the following: a current episode of major depressive disorder according to the DSM‐IV‐TR and confirmed by the MINI with a history of at least one separate, previous episode of depression, and at both the screening and randomisation visits a MADRS total score of 20, and at least moderate pain with a score of 3 on the Brief Pain Inventory Short Form (BPI) average pain item, and a Clinical Global Impression of Severity (CGI‐S) score 4. Painful symptoms were not allowed to have an identifiable underlying cause | |
| Interventions | Duloxetine 60 mg once daily orally for 8 weeks vs placebo | |
| Outcomes |
| |
| Notes | Gaynor 2011b identical in design ‐ different patient group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | No concerns |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
| Methods | A randomised, double‐blind, placebo‐controlled trial of duloxetine in people with major depressive disorder and painful physical symptoms | |
| Participants | Adult (18 years of age) male or female outpatients were eligible ...if they met all of the following: a current episode of major depressive disorder according to the DSM‐IV‐TR and confirmed by the MINI with a history of at least one separate, previous episode of depression, and at both the screening and randomisation visits a MADRS total score of 20, and at least moderate pain with a score of 3 on the Brief Pain Inventory Short Form (BPI) average pain item, and a Clinical Global Impression of Severity (CGI‐S) score 4. Painful symptoms were not allowed to have an identifiable underlying cause | |
| Interventions | Duloxetine 60 mg once daily orally for 8 weeks vs placebo | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomised' |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | Unclear risk | 'Double Blind' |
| Incomplete outcome data (attrition bias) | Low risk | No concerns |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None identified |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel group, trial of duloxetine in painful diabetic neuropathy | |
| Participants | 457 participants Participants, at least 18 years of age, had daily pain due to polyneuropathy caused by type 1 or type 2 diabetes mellitus, which was present for a minimum of 6 months. This pain had to have begun in the feet with relatively symmetrical onset. The diagnosis was confirmed by a score of at least 3 on the Michigan Neuropathy Screening Instrument (MNSI). Participants were required to have a minimum score of 4 on the 24‐hour average pain score rated on an 11‐point (0 to 10) Likert scale. | |
| Interventions | Duloxetine 20 mg daily, 60 mg daily or 60 mg twice daily versus placebo for 8 weeks | |
| Outcomes |
| |
| Notes | Company sponsored and run trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned in a 1:1:1:1 ratio by a computer generated random sequence |
| Allocation concealment (selection bias) | Low risk | Participant numbers were assigned consecutively at each study site. The interactive voice response system was used to assign blister cards containing the study drug to each participant confirmed through interactive voice response system entry of a confirmation number on the card |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | High risk | All analyses were undertaken as an ITT analysis. All participants were analysed in the safety analysis and all participants with at least one post entry data point were analysed in an ITT analysis. Dropout rate was 25% with significantly more in the higher dose treatment groups |
| Selective reporting (reporting bias) | Unclear risk | See above |
| Other bias | Low risk | Company sponsored and run trial |
| Methods | Randomised, double‐blind, cross‐over clinical trial comparing amitriptyline and duloxetine in painful diabetic neuropathy | |
| Participants | 86 participants ‐ 65 randomised to treatment in 1st arm, 58 of whom completed both arms People of either sex with type 2 diabetes, aged between 18 and 75 years, who were on stable glucose‐lowering medications during the preceding month and who had painful diabetic neuropathy for at least 1 month were considered for the study. The study enrolled people who had a pain score of > 50%, as assessed by visual analogue scale (VAS). Painful diabetic neuropathy was confirmed by 1) medical history, 2) a diabetic neuropathy symptom (DNS) score of > 1 point (7), 3) a Diabetic Neuropathy Examination (DNE) score of > 3 points (8), 4) a modified neuropathy symptom score (mNSS) (9,10), and 5) increased thresholds on the vibration perception test and monofilament test | |
| Interventions | Amitiyptyline 10, 25 or 50 mg once daily at night or duloxetine, 20, 40 or 60 mg once daily at night Intervention only 6 weeks before 2 week washout and cross‐over to alternate arm. Participants commenced on lowest dose and then increased every 2 weeks to next dose if required by treating physician; thus potentially only 2 weeks on maximum dose. 48% of amitriptyline and 65% of duloxetine participants reached the highest dose of drug. 17% vs 5% of the participants preferred higher dose duloxetine to amitriptyline | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised (computer generated randomisation of blocks of 4) |
| Allocation concealment (selection bias) | Unclear risk | An independent person unrelated to the study carried out blinding and randomisation. Two separate companies provided medicines, so it is not clear that they were identical in appearance |
| Blinding (performance bias and detection bias) | Low risk | Blinded. Single physician assessment. "success of blinding was assessed by the accuracy of the physicians prediction at the end of the study" (34% correctly identified only) |
| Incomplete outcome data (attrition bias) | Low risk | No concerns |
| Selective reporting (reporting bias) | High risk | The primary end point of the study was the reduction in the median pain score from baseline, (patient’s global assessment of efficacy by VAS (0 to 100 points)). Secondary end points included the assessment of pain by the short‐form McGill Pain Questionnaire (11); an 11‐point Likert scale for pain (0 = no pain and 10 = excruciating pain); change in sleep pattern (increased, unchanged, or decreased); overall improvement by DNE score, DNS score, mNSS, and the 24‐point HAMD; and patient self evaluation of overall change on the basis of a 7‐point Patient Global Impression of Change (PGIC) scale not reported in analysis. |
| Other bias | High risk | Significant (but similar) carryover into period 2 despite 2 weeks' washout |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel group trial in diabetic peripheral neuropathic pain | |
| Participants | 348 participants Participants ≥ 18 years, with pain due to bilateral peripheral neuropathy caused by type 1 or type 2 diabetes mellitus. The pain had to begin in the feet with relatively symmetrical onset and be present for at least 6 months. Participants had to have a mean score of ≥ 4 when assessed for 24‐hour average pain severity on the Michigan Neuropathy Screening Instrument (MNSI) 11‐point Likert scale (from the patient diary prior to randomisation), and stable glycaemic control. Concomitant pain medications excluded. | |
| Interventions | Duloxetine 60 mg daily or duloxetine 60 mg twice daily versus placebo for 12 weeks | |
| Outcomes |
| |
| Notes | Company sponsored and run trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed at visit 3 in a 1:1:1 ratio. A computer‐generated random sequence determined assignment to treatment groups, using an IVRS |
| Allocation concealment (selection bias) | Low risk | Participants received either of (or a combination of, depending on their randomly assigned treatment) the following: 30 mg capsules of duloxetine hydrochloride or placebo capsules identical to duloxetine capsules. Participants randomly assigned to each treatment group were instructed to take two capsules (by mouth) every morning and every evening. Treatment was assigned using IVRS |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts were 52/340 (15%). Analysis was by ITT |
| Selective reporting (reporting bias) | Low risk | See above |
| Other bias | Low risk | Lilly study. No other bias identified |
| Methods | Phase II, randomised, double‐blind, placebo‐controlled, single group assignment, safety and efficacy study comparing duloxetine, ABT‐894 and placebo in diabetic neuropathic pain | |
| Participants | 108 participants Men and women 18 to 75 Inclusion criteria:
| |
| Interventions | Drug: ABT‐894, 1 mg, 2 mg, 4 mg twice daily Duration 8 weeks | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | Published 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants "were randomized 1:1 to each treatment arm via an interactive voice response system using a randomization schedule that was generated before study start" |
| Allocation concealment (selection bias) | Low risk | Careful attention to placebo and medication concealment noted |
| Blinding (performance bias and detection bias) | Low risk | No concerns |
| Incomplete outcome data (attrition bias) | Low risk | Almost 100% completed |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | None identified |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel group trial in fibromyalgia | |
| Participants | 520 participants Female and male outpatients ≥ 18 years of age who met criteria for fibromyalgia as defined by the American College of Rheumatology. Participants were required to have a score ≥ 4 on the average pain severity item (in the past 24 hours) of the Brief Pain Inventory (BPI‐modified Short Form at screening and at baseline. The study included people with or without current major depressive disorder and evaluated them for the presence of psychiatric disorders using the MINI. Prior to randomisation, the study required participants to discontinue any medications that might interfere with the evaluation of pain improvement, including analgesics (with the exception of up to 325 mg/day of aspirin for cardiac prophylaxis and paracetamol up to 2 g/day for pain), antidepressants, anticonvulsants, or other medications taken for fibromyalgia or pain | |
| Interventions | Duloxetine 20 mg daily, 60 mg daily or 60 mg twice daily versus placebo for 6 months | |
| Outcomes |
| |
| Notes | Company sponsored and run trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated random sequence determined assignment to treatment groups and the study randomly assigned each stratum (depressed and non‐depressed) within sites to achieve a relative balance across treatments |
| Allocation concealment (selection bias) | Unclear risk | Unclear although other trials from the same group have been adequate |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | High risk | 35% to 40% dropout at the 3 month interim analysis phase and up to 46% dropout for the 6 month phase. "Intention‐to‐treat unless otherwise specified". Safety analyses in all participants and others with data for at least 1 measure |
| Selective reporting (reporting bias) | Unclear risk | See above |
| Other bias | Low risk | Lilly study. No other bias identified |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel group enrichment trials with three phases comparing duloxetine to pregabalin in painful diabetic neuropathy | |
| Participants | 401 participants treated with duloxetine and 403 with pregabalin Included participants had pain due to bilateral peripheral neuropathy (caused by type 1 or type 2 diabetes mellitus. Pain must have begun in the feet, with relatively symmetrical onset. Daily pain should have been present for more than 3 months (assessed by questioning the patient). | |
| Interventions | Pregabalin titrated to 150 mg twice daily was compared to duloxetine titrated to 60 mg once daily (with placebo tablets to maintain blind between treatments) and treated in study phase II for 8 weeks. A third phase of non‐responding participants entered study phase III not included in this analysis | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised 1:1:1:1 in 4 parallel groups based on a computer generated sequence using IVRS |
| Allocation concealment (selection bias) | Unclear risk | Unclear ‐ although all drugs and placebo were similar and the allocation stratified by site, does not explicitly deal with concealment |
| Blinding (performance bias and detection bias) | Low risk | The trial maintained blinding by using over‐encapsulated duloxetine and pregabalin capsules, matching placebo and an identical dosing regimen for all groups in terms of numbers and timing of capsules |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropout in Phase II 17%, 9% with adverse events. All analyses performed on ITT (baseline + 1 measure for outcomes, all randomised for adverse events) with MMRM ‐ however no statement as to whether LOCF or BOCF used |
| Selective reporting (reporting bias) | High risk | Some partial reporting of outcomes (for example NPSI subscores not tabulated, PGI‐I and CGI‐I in figure form only and differences of reporting between phase II and phase III outcome reporting |
| Other bias | High risk | Lilly designed, interpreted, wrote and submitted. Ghost written by professional writer for company |
| Methods | Stratified, randomised, double‐blind, placebo‐controlled, parallel group study of patients with severe central neuropathic pain of more than 6 months duration from cerebrovascular or spinal cord lesions | |
| Participants | 48 participants aged 18 years or older with > 6 month severe neuropathic pain from cord or cerebrovascular cause, > 6 on visual analogue scale (VAS) (10 points), which started after sustaining the lesion and with the distribution of pain concomitant with the somatosensory system involvement. The trial allowed other medication if doses were stable for 6 weeks, except other antidepressants, which had to be stopped more than 30 days prior to receiving study medication | |
| Interventions | Duloxetine or placebo for 8 weeks. Duloxetine 60 mg at start. Increased if participants did not meet criteria of > 1.8 points improvement on VAS. At week 8 and study end 15 participants on 120 mg and 8 participants on 60 mg | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple computerised random sampling (clorandm.exe) assigned study codes N = 1 to the placebo or duloxetine arm. Consecutive participants who met inclusion criteria were randomly assigned to treatment with flexible dose placebo or flexible dose duloxetine |
| Allocation concealment (selection bias) | Low risk | The association between type of treatment and study code was only known to the Department of Epidemiology, Biostatistics and Bioinformatics and the hospital pharmacy department |
| Blinding (performance bias and detection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel group trial of duloxetine in diabetic peripheral neuropathic pain | |
| Participants | 334 participants Men or women ≥ 18 years and with > 6 months diabetic peripheral neuropathic pain secondary to type 1 or 2 diabetes (distal and symmetrical). At randomisation, score > 3 on Michigan Neuropathy Screening Instrument and average > 4 on 24 hour pain scale. Stable glucose control and HBA1c < 12. Multiple exclusions including other pain medications except paracetamol and aspirin | |
| Interventions | Duloxetine 60 mg daily, 60 mg twice daily or placebo for 12 weeks | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | Company sponsored and run trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed at the site level in that randomisation codes were assigned to sites in blocks, but there was no further stratification. Participants were randomly assigned to treatment in a 1:1:1 ratio. Assignment to a treatment group was determined by a computer‐generated random sequence using an IVRS |
| Allocation concealment (selection bias) | Low risk | The IVRS was used to assign blister cards containing study drug to each participant |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | High risk | Drop outs were 29/114 (25%) in duloxetine 60 mg daily, 34/112 (30%) in duloxetine 60 mg twice daily and 23/108 (21.3%) in the placebo group |
| Selective reporting (reporting bias) | Unclear risk | An ITT principle was used in the analyses of all efficacy variables. For each efficacy variable, the analysis included all randomised participants with a baseline and at least one non‐missing observation after baseline |
| Other bias | Low risk | Lilly study. No other bias identified |
| Methods | Phase III randomised, double‐blind (subject, caregiver, investigator, outcomes assessor), placebo‐controlled, parallel assignment, safety and efficacy study of duloxetine in diabetic peripheral neuropathic pain | |
| Participants | 339 participants randomised Male or female outpatients aged 20 years or older but less than 80 years at the time of consent:
| |
| Interventions | Duloxetine 40 mg or 60 mg orally daily versus placebo for 12 weeks | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | Recruiting | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assigning table was prepared using Create Key Code 3.3. Participants were randomly assigned…' Stratified for pain, duration of diabetic peripheral neuropathy, diabetes type, study centre |
| Allocation concealment (selection bias) | Unclear risk | No clear explanation of methodology |
| Blinding (performance bias and detection bias) | Unclear risk | "Double blind" |
| Incomplete outcome data (attrition bias) | Low risk | 10.2% to 16.9% dropout. All analyses using LOCF and MMRM |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Low risk | Lilly sponsored |
DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th ed
HbA1c: haemoglobin A1c
ITT: intention‐to‐treat
BOCF: best observation carried forward
LOCF: last observation carried forward
MADRS: Montgomery–Åsberg Depression Rating Scale
MINI: Mini International Neuropsychiatric Interview
MMRM: mixed‐effect model repeated measure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| 28 days only | |
| 6 weeks of treatment only | |
| Not randomised or controlled | |
| Osteoarthritis of the knee ‐ likely to cross over with Cochrane Musculoskeletal Group | |
| Osteoarthritis of the knee ‐ likely to cross over with Cochrane Musculoskeletal Group | |
| Trial of duloxetine in depression. Pain scales as secondary outcome measures only. It was not clear what sort of pain the participants had (for example musculoskeletal, neuropathic, headache) and the levels of pain at baseline were low compared to the included trials | |
| Four weeks treatment only in each group of 4 way crossover. Terminated early July 2013 | |
| Abstract publication of Smith 2013 | |
| Open and then double‐blind study comparing 2 doses of duloxetine 60 mg and 120 mg | |
| The first part of this cross‐over study was the only part of trial suitable for assessment (amitriptyline versus duloxetine) but only 4 weeks long ‐ thus excluded | |
| Open label | |
| Was registered in clinicaltrials.gov ‐ study terminated with no participants enrolled because no drug supplied | |
| Open label duloxetine vs no treatment or pre‐existing antidepressant | |
| Open label extension of Yasuda 2010 | |
| Pelvic pain | |
| Not a randomised controlled study but a report of 3 trials included in this review | |
| Not a double‐blind trial | |
| Open label study with dosage control only | |
| Summary report of 3 studies included in this review | |
| Back pain ‐ to be included in a Cochrane Back Group review ‐ Back Group informed. Published in full format in 2009 European Journal of Neurology 16: 1041‐8 | |
| Back pain ‐ to be included in a Cochrane Back Group review ‐ Back Group informed | |
| Open label extension | |
| Back pain ‐ to be included in a Cochrane Back Group review ‐ Back Group informed | |
| Back pain ‐ to be included in a Cochrane Back Group review ‐ Back Group informed | |
| Duration of treatment only 4 weeks | |
| Open label ‐ non blinded study | |
| Measured outcomes at durations of less than eight weeks | |
| Not double‐blind ‐ extension of Goldstein 2005 | |
| Open study, not blinded |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Phase II randomised, double blind, parallel assignment, safety and efficacy study |
| Participants | Male and female participants between 18 and 75 years of age Diabetes mellitus (type I or II) that is documented to be under stable glycaemic control over a period of at least 3 months, as indicated by a HbAIc of ≤ 12% and a stable dose of insulin or oral diabetic medication for 90 days prior to starting study medication. Evidence of symmetrical, bilateral pain in the lower extremities due to diabetic peripheral neuropathy. Presence of daily pain due to DPN for at least 3 months. Score ≥ 3 on the physical examination portion of the Michigan Neuropathy Screening Instrument (MNSI). Average weekly pain score of ≥ 4 on the numeric pain rating scale (NPRS) for symmetrical neuropathic pain in the feet and legs |
| Interventions | Drug: ADL5859 |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Notes | Completed ‐ no reference in Pubmed ‐ no information on clinicaltrials.gov‐ e‐mail written to company with request for information September 2012. |
HbA1c: haemoglobin A1c
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A randomised placebo controlled trial of duloxetine for central pain in multiple sclerosis |
| Methods | Randomised, double‐blind (caregiver, investigator), placebo‐controlled, parallel assignment safety/efficacy study |
| Participants | People with multiple sclerosis "who have central pain which is 4 or greater on a scale of 1‐10. Patients must have experienced pain for 2 months or longer prior to beginning the study." |
| Interventions | Duloxetine 30 mg (10 capsules) for 1 week, titrated up to 60 mg (40 capsules) for 5 weeks and titrated back down to 30 mg for 1 week Placebo for 7 weeks |
| Outcomes | Time frame for all outcomes, week 2 and week 6
|
| Starting date | January 2007 |
| Contact information | Brown, Theodore R., M.D., MPH Evergreen Healthcare Kirkland, Washington, United States, 98034 |
| Notes | NCT00457730 Lilly sponsored |
| Trial name or title | Three way interaction between gabapentin, duloxetine, and donepezil in patients with diabetic neuropathy |
| Methods | Randomised, double‐blind (subject, investigator, outcomes assessor), parallel assignment |
| Participants | Male or female. Diagnosis of diabetic neuropathy. Age 18 to 80 |
| Interventions | Group 1: donepezil 5 mg once per day for 12 weeks Group 2: duloxetine 30 mg twice a day for 12 weeks Group 3: combination of donepezil 2.5 mg and duloxetine 30 mg for 12 weeks Group 4: placebo pills. Gabapentin added to all groups at week 9 |
| Outcomes | Primary:
|
| Starting date | February 2008 to July 2010 |
| Contact information | Regina Curry, RN, CCRC 336‐716‐4294 Wake Forest University Baptist Medical Center Winston‐Salem, North Carolina, United States, 27157 |
| Notes | NCT00619983 Still recruiting 2013 ‐ estimated completion July 2013 |
| Trial name or title | Treatment of patients with diabetic peripheral neuropathic pain in China: duloxetine versus placebo |
| Methods | Randomized, double blind (subject, investigator), placebo‐controlled, parallel assignment, efficacy study |
| Participants | People over 18 years of age who present with pain due to bilateral diabetic peripheral neuropathy (type 1 or type 2 diabetes). Pain beginning in feet, relatively symmetrical onset, present daily for at least 6 months, confirmed by score of ≥ 3 on Michigan Neuropathy Screening Inventory |
| Interventions | Duloxetine 30 mg orally, once daily for 1 week; 60 mg once daily for next 11 weeks; 30 mg administered orally, once daily for 1 week during taper period Placebo once daily for 12 weeks, once daily for 1 week during taper period |
| Outcomes | Primary:
Secondary (changes measured from baseline to 12‐week endpoint):
|
| Starting date | April 2011 |
| Contact information | Eli Lilly and Company. Study director, tel: 1‐877‐CTLILLY (1‐877‐285‐4559) or 1‐317‐615‐4559 |
| Notes | NCT01179672 |
| Trial name or title | A study of duloxetine in adolescents with juvenile primary fibromyalgia syndrome |
| Methods | Phase III, randomised, double‐blind (subject, caregiver, investigator, outcomes assessor), parallel assignment, safety/efficacy study |
| Participants | Aged 13 to 17 years who meet criteria for primary juvenile primary fibromyalgia syndrome and have a score of greater than or equal to 4 on Brief Pain Inventory (BPI) average pain severity (Item 3) during screening |
| Interventions | Blinded period: 30 mg or 60 mg duloxetine or placebo once daily for 13 weeks Open label extension: 30 mg or 60 mg duloxetine once daily for 26 weeks |
| Outcomes | Primary:
Secondary:
|
| Starting date | February 2011 |
| Contact information | Eli Lilly and Company. Study Director: 1‐877‐CTLILLY (1‐877‐285‐4559) or 1‐317‐615‐4559 |
| Notes | NCT01237587 |
| Trial name or title | A phase III clinical trial of duloxetine in participants with fibromyalgia |
| Methods | Randomised, double‐blind (subject, investigator), placebo‐controlled, parallel assignment, safety/efficacy study |
| Participants | Participants with fibromyalgia aged 20 to 74 years Inclusion criteria:
|
| Interventions | Duloxetine hydrochloride orally 60 mg for 15 weeks or oral placebo for 15 weeks |
| Outcomes | Changes measured from baseline to 14 week endpoint Primary:
Secondary:
|
| Starting date | March 2012 |
| Contact information | Eli Lilly and Company, Shionogi. Tel: 1‐877‐CTLILLY (1‐877‐285‐4559) or 1‐317‐615‐4559 |
| Notes | NCT01552057 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with ≥ 50% improvement of pain at 12 weeks or less Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 1 Number of participants with ≥ 50% improvement of pain at 12 weeks or less. | ||||
| 1.1 Duloxetine 20 mg daily | 1 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.98, 2.09] |
| 1.2 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [1.26, 2.87] |
| 1.3 Duloxetine 60 mg daily | 4 | 908 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.44, 2.08] |
| 1.4 Duloxetine 120 mg daily | 4 | 870 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.08, 1.97] |
| 1.5 All doses | 5 | 1655 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [1.21, 1.92] |
| 2 Mean improvement in pain at 12 weeks or less Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 2 Mean improvement in pain at 12 weeks or less. | ||||
| 2.1 Duloxetine 20 mg daily | 1 | 179 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐1.05, 0.15] |
| 2.2 Duloxetine 60 mg daily | 4 | 722 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.26, ‐0.65] |
| 2.3 Duloxetine 120 mg daily | 4 | 828 | Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐1.21, ‐0.65] |
| 3 Number of participants with ≥ 30% improvement in pain at 12 weeks or less Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 3 Number of participants with ≥ 30% improvement in pain at 12 weeks or less. | ||||
| 3.1 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.18, 2.07] |
| 3.2 Duloxetine 60 mg daily | 4 | 799 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.33, 1.75] |
| 3.3 Duloxetine 120 mg daily | 3 | 659 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.21, 1.58] |
| 3.4 All doses | 4 | 1220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.30, 1.63] |
| 4 Mean improvement in SF‐36 Physical Subscore at 12 weeks or less Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 4 Mean improvement in SF‐36 Physical Subscore at 12 weeks or less. | ||||
| 4.1 Duloxetine 20 mg daily | 1 | 200 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐2.42, 1.88] |
| 4.2 Duloxetine 60 mg daily | 3 | 514 | Mean Difference (IV, Random, 95% CI) | 2.65 [1.38, 3.92] |
| 4.3 Duloxetine 120 mg daily | 2 | 409 | Mean Difference (IV, Random, 95% CI) | 2.80 [1.04, 4.55] |
| 5 Mean improvement in SF‐36 Mental Subscore at 12 weeks or less Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 5 Mean improvement in SF‐36 Mental Subscore at 12 weeks or less. | ||||
| 5.1 Duloxetine 20 mg daily | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.11 [‐0.98, 3.20] |
| 5.2 Duloxetine 60 mg daily | 3 | 514 | Mean Difference (IV, Fixed, 95% CI) | 1.08 [‐0.32, 2.48] |
| 5.3 Duloxetine 120 mg daily | 2 | 409 | Mean Difference (IV, Fixed, 95% CI) | 2.23 [0.69, 3.77] |
| 6 Mean improvement in SF‐36 Bodily Pain Subscore at 12 weeks or less Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 6 Mean improvement in SF‐36 Bodily Pain Subscore at 12 weeks or less. | ||||
| 6.1 Duloxetine 20 mg daily | 1 | 209 | Mean Difference (IV, Fixed, 95% CI) | 2.90 [‐2.37, 8.17] |
| 6.2 Duloxetine 60 mg daily | 2 | 421 | Mean Difference (IV, Fixed, 95% CI) | 5.58 [1.74, 9.42] |
| 6.3 Duloxetine 120 mg daily | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | 8.19 [4.33, 12.05] |
| 7 Mean improvement in Patient Reported Global Impression of Improvement at 12 weeks or less Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 7 Mean improvement in Patient Reported Global Impression of Improvement at 12 weeks or less. | ||||
| 7.1 Duloxetine 20 mg daily | 1 | 219 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.56, 0.10] |
| 7.2 Duloxetine 40 mg daily | 1 | 252 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.01, ‐0.29] |
| 7.3 Duloxetine 60 mg daily | 5 | 1018 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.77, ‐0.44] |
| 7.4 Duloxetine 120 mg daily | 4 | 870 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.73, ‐0.35] |
| 8 Mean improvement in BPI Severity ‐ average pain at 12 weeks or less Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 8 Mean improvement in BPI Severity ‐ average pain at 12 weeks or less. | ||||
| 8.1 Duloxetine 60 mg daily | 2 | 433 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.38, ‐0.57] |
| 8.2 Duloxetine 120 mg daily | 2 | 428 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐1.91, ‐0.41] |
| 9 Mean improvement in pain at rest (night pain) at 12 weeks or less Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 9 Mean improvement in pain at rest (night pain) at 12 weeks or less. | ||||
| 9.1 Duloxetine 20 mg daily | 1 | 222 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.90, 0.34] |
| 9.2 Duloxetine 60 mg daily | 3 | 664 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.27, ‐0.57] |
| 9.3 Duloxetine 120 mg daily | 3 | 664 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.45, ‐0.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with ≥ 50% improvement in pain at 12 weeks or less Show forest plot | 1 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.19, 1.80] |
| Analysis 2.1  Comparison 2 Duloxetine versus pregabalin in the treatment of painful diabetic neuropathy, Outcome 1 Number of participants with ≥ 50% improvement in pain at 12 weeks or less. | ||||
| 2 Mean improvement in pain at 12 weeks or less Show forest plot | 1 | 804 | Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐0.92, ‐0.32] |
| Analysis 2.2  Comparison 2 Duloxetine versus pregabalin in the treatment of painful diabetic neuropathy, Outcome 2 Mean improvement in pain at 12 weeks or less. | ||||
| 3 Number improved ≥ 30% at 12 weeks or less Show forest plot | 1 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.20, 1.68] |
| Analysis 2.3  Comparison 2 Duloxetine versus pregabalin in the treatment of painful diabetic neuropathy, Outcome 3 Number improved ≥ 30% at 12 weeks or less. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with ≥ 50% improvement of pain at 12 weeks or less Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 1 Number of participants with ≥ 50% improvement of pain at 12 weeks or less. | ||||
| 1.1 Duloxetine 20 mg daily | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.91, 2.14] |
| 1.2 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.75, 1.35] |
| 1.3 Duloxetine 60 mg daily | 2 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.20, 2.06] |
| 1.4 Duloxetine 120 mg daily | 4 | 1234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.40, 2.03] |
| 1.5 All doses | 5 | 1887 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.29, 1.75] |
| 2 Number of participants with ≥ 50% improvement of pain at more than 12 weeks Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 2 Number of participants with ≥ 50% improvement of pain at more than 12 weeks. | ||||
| 2.1 Duloxetine 60 mg daily | 1 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.10, 2.27] |
| 2.2 Duloxetine 120 mg daily | 2 | 616 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.07, 1.79] |
| 2.3 Duloxetine all doses | 2 | 845 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.09, 1.79] |
| 3 Number of participants with ≥ 30% improvement of pain at 12 weeks or less Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 3 Number of participants with ≥ 30% improvement of pain at 12 weeks or less. | ||||
| 3.1 Duloxetine 20 mg daily | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.94, 1.79] |
| 3.2 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.89, 1.45] |
| 3.3 Duloxetine 60 mg daily | 2 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.24, 1.85] |
| 3.4 Duloxetine 120 mg daily | 3 | 1020 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.26, 1.69] |
| 3.5 All doses | 4 | 1673 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.22, 1.56] |
| 4 Mean improvement in pain at 12 weeks or less Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 4 Mean improvement in pain at 12 weeks or less. | ||||
| 4.1 Duloxetine 30 mg daily | 1 | 308 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.86, 0.24] |
| 4.2 Duloxetine 120 mg daily | 1 | 507 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.35, ‐0.25] |
| 5 Mean improvement in the SF‐36 mental component summary subscore Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.5  Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 5 Mean improvement in the SF‐36 mental component summary subscore. | ||||
| 5.1 Duloxetine 20 mg daily | 1 | 223 | Mean Difference (IV, Random, 95% CI) | 0.81 [‐2.37, 3.99] |
| 5.2 Duloxetine 30 mg daily | 1 | 308 | Mean Difference (IV, Random, 95% CI) | 2.69 [0.31, 5.07] |
| 5.3 Duloxetine 60 mg daily | 2 | 515 | Mean Difference (IV, Random, 95% CI) | 3.31 [0.59, 6.02] |
| 5.4 Duloxetine 120 mg daily | 5 | 1531 | Mean Difference (IV, Random, 95% CI) | 4.22 [2.43, 6.02] |
| 6 Mean improvement in the SF‐36 physical component summary subscore Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.6  Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 6 Mean improvement in the SF‐36 physical component summary subscore. | ||||
| 6.1 Duloxetine 20 mg daily | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [‐1.92, 3.54] |
| 6.2 Duloxetine 30 mg daily | 1 | 308 | Mean Difference (IV, Fixed, 95% CI) | 0.84 [‐1.17, 2.85] |
| 6.3 Duloxetine 60 mg daily | 2 | 515 | Mean Difference (IV, Fixed, 95% CI) | 1.28 [‐0.33, 2.89] |
| 6.4 Duloxetine 120 mg daily | 5 | 1531 | Mean Difference (IV, Fixed, 95% CI) | 2.13 [0.95, 3.30] |
| 7 Mean improvement in the SF‐36 Bodily Pain Subscore Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.7  Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 7 Mean improvement in the SF‐36 Bodily Pain Subscore. | ||||
| 7.1 Duloxetine 60 mg daily | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | 8.2 [3.20, 13.20] |
| 7.2 Duloxetine 120 mg daily | 4 | 1243 | Mean Difference (IV, Fixed, 95% CI) | 5.96 [3.76, 8.16] |
| 8 Mean improvement in the Patient reported Global Impression of Change at completion of trial Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.8  Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 8 Mean improvement in the Patient reported Global Impression of Change at completion of trial. | ||||
| 8.1 Duloxetine 20 mg daily | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.96, ‐0.12] |
| 8.2 Duloxetine 30 mg daily | 1 | 308 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.71, ‐0.05] |
| 8.3 Duloxetine 60 mg daily | 2 | 519 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.73, ‐0.18] |
| 8.4 Duloxetine 120 mg daily | 3 | 826 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.66, ‐0.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with > 50% pain relief at 12 weeks or less Show forest plot | 2 | 1023 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.19, 1.59] |
| Analysis 4.1  Comparison 4 Duloxetine versus placebo for the treatment of pain in major depressive disorder, Outcome 1 Number of participants with > 50% pain relief at 12 weeks or less. | ||||
| 2 Participants with > 30% pain relief at 12 weeks or less Show forest plot | 3 | 1359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.15, 1.40] |
| Analysis 4.2  Comparison 4 Duloxetine versus placebo for the treatment of pain in major depressive disorder, Outcome 2 Participants with > 30% pain relief at 12 weeks or less. | ||||
| 3 Mean improvement in pain at 12 weeks or less Show forest plot | 3 | 1359 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐0.75, ‐0.35] |
| Analysis 4.3  Comparison 4 Duloxetine versus placebo for the treatment of pain in major depressive disorder, Outcome 3 Mean improvement in pain at 12 weeks or less. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean improvement in pain at 12 weeks or less Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐2.05, 0.05] |
| Analysis 5.1  Comparison 5 Duloxetine versus placebo in the treatment of central neuropathic pain, Outcome 1 Mean improvement in pain at 12 weeks or less. | ||||
| 2 Mean improvement in SF‐36 Physical Subscore Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐12.72, 16.72] |
| Analysis 5.2  Comparison 5 Duloxetine versus placebo in the treatment of central neuropathic pain, Outcome 2 Mean improvement in SF‐36 Physical Subscore. | ||||
| 3 Mean improvement in the SF‐36 Mental Subscore at 12 weeks Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐6.75, 14.75] |
| Analysis 5.3  Comparison 5 Duloxetine versus placebo in the treatment of central neuropathic pain, Outcome 3 Mean improvement in the SF‐36 Mental Subscore at 12 weeks. | ||||
| 4 Mean improvement in the SF‐36 Bodily Pain Subscore Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [‐0.81, 16.81] |
| Analysis 5.4  Comparison 5 Duloxetine versus placebo in the treatment of central neuropathic pain, Outcome 4 Mean improvement in the SF‐36 Bodily Pain Subscore. | ||||
| 5 Number of participants improved on PGI‐I (better or very much better) Show forest plot | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [1.02, 7.44] |
| Analysis 5.5  Comparison 5 Duloxetine versus placebo in the treatment of central neuropathic pain, Outcome 5 Number of participants improved on PGI‐I (better or very much better). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants with any adverse event Show forest plot | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.1  Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 1 Proportion of participants with any adverse event. | ||||
| 1.1 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.03, 1.52] |
| 1.2 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.01, 1.31] |
| 1.3 Duloxetine 60 mg daily | 13 | 4521 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.10, 1.20] |
| 1.4 Duloxetine 120 mg daily | 3 | 688 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.09, 1.30] |
| 1.5 Duloxetine all doses | 14 | 5258 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.11, 1.20] |
| 2 Nausea Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.2  Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 2 Nausea. | ||||
| 2.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.71, 3.00] |
| 2.2 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 5.43 [2.34, 12.58] |
| 2.3 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 6.55 [1.85, 23.17] |
| 2.4 Duloxetine 60 mg daily | 11 | 3642 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [2.14, 3.18] |
| 2.5 Duloxetine 120 mg daily | 4 | 787 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [2.06, 4.04] |
| 3 Dry mouth Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.3  Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 3 Dry mouth. | ||||
| 3.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.30, 2.47] |
| 3.2 Duloxetine 40 mg daily | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Duloxetine 60 mg daily | 6 | 2004 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.89, 3.67] |
| 3.4 Duloxetine 120 mg daily | 3 | 567 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.40 [1.94, 5.96] |
| 4 Dizziness Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.4  Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 4 Dizziness. | ||||
| 4.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.33, 2.33] |
| 4.2 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.89 [1.22, 28.58] |
| 4.3 Duloxetine 60 mg daily | 8 | 2257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.35, 2.51] |
| 4.4 Duloxetine 120 mg daily | 4 | 787 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.55, 3.83] |
| 5 Somnolence Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.5  Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 5 Somnolence. | ||||
| 5.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.41, 2.43] |
| 5.2 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [0.70, 7.06] |
| 5.3 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.15, 4.38] |
| 5.4 Duloxetine 60 mg daily | 8 | 2678 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.94 [2.17, 3.97] |
| 5.5 Duloxetine 120 mg daily | 4 | 787 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.76 [2.93, 7.74] |
| 6 Adverse event leading to cessation Show forest plot | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.6  Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 6 Adverse event leading to cessation. | ||||
| 6.1 Duloxetine 20 mg daily | 2 | 453 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.78, 2.39] |
| 6.2 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.69, 3.44] |
| 6.3 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.81, 4.77] |
| 6.4 Duloxetine 60 mg daily | 14 | 4837 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.60, 2.37] |
| 6.5 Duloxetine 120 mg daily | 7 | 1462 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [1.74, 3.04] |
| 6.6 All doses | 17 | 6285 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.67, 2.37] |
| 7 Serious adverse event Show forest plot | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.7  Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 7 Serious adverse event. | ||||
| 7.1 Duloxetine 20 mg daily | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.02] |
| 7.3 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [0.50, 17.30] |
| 7.4 Duloxetine 60 mg daily | 14 | 4842 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.60, 1.32] |
| 7.5 Duloxetine 120 mg daily | 6 | 1257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.25, 1.35] |
| 7.6 All doses | 14 | 4976 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.53, 1.25] |

Methodological quality summary: review authors' judgements about each methodological quality item for each included study. Green = low risk of bias; yellow = unclear risk of bias; red = high risk of bias

Duloxetine versus placebo in the treatment of painful neuropathy: Number of patients with >50% improvement of pain at <12 weeks.
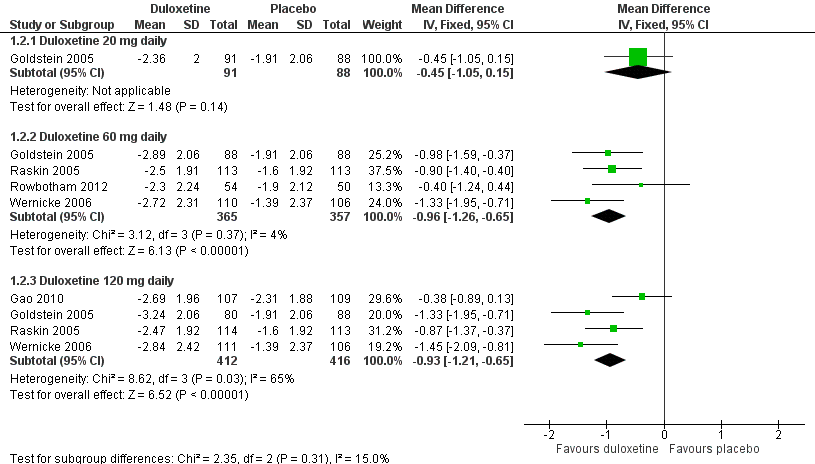
Duloxetine versus placebo in the treatment of pain: Mean improvement in pain at 12 weeks.

Duloxetine versus placebo in the treatment of pain: Number of patients with >30% improvement in pain at <12 weeks.
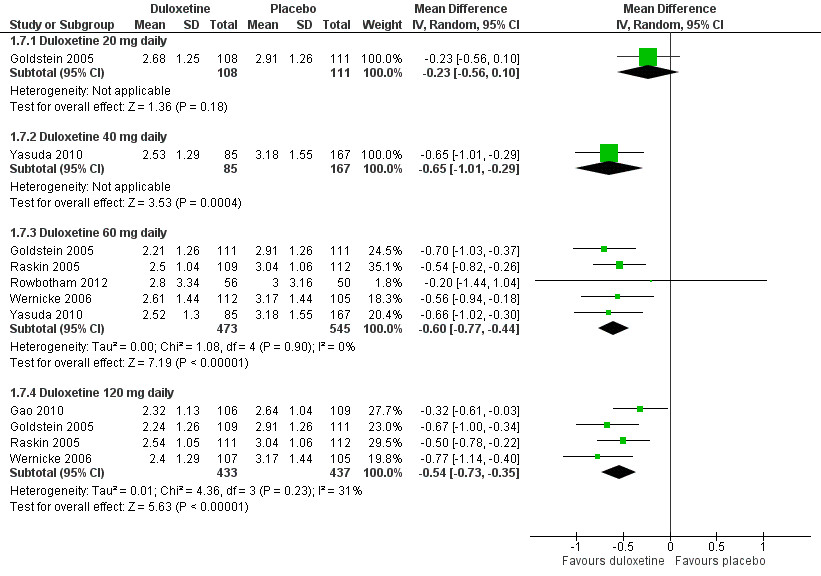
Duloxetine versus placebo in the treatment of pain: Patient reported global impression of change.

Duloxetine versus placebo in the treatment of pain: BPI severity ‐ average pain.
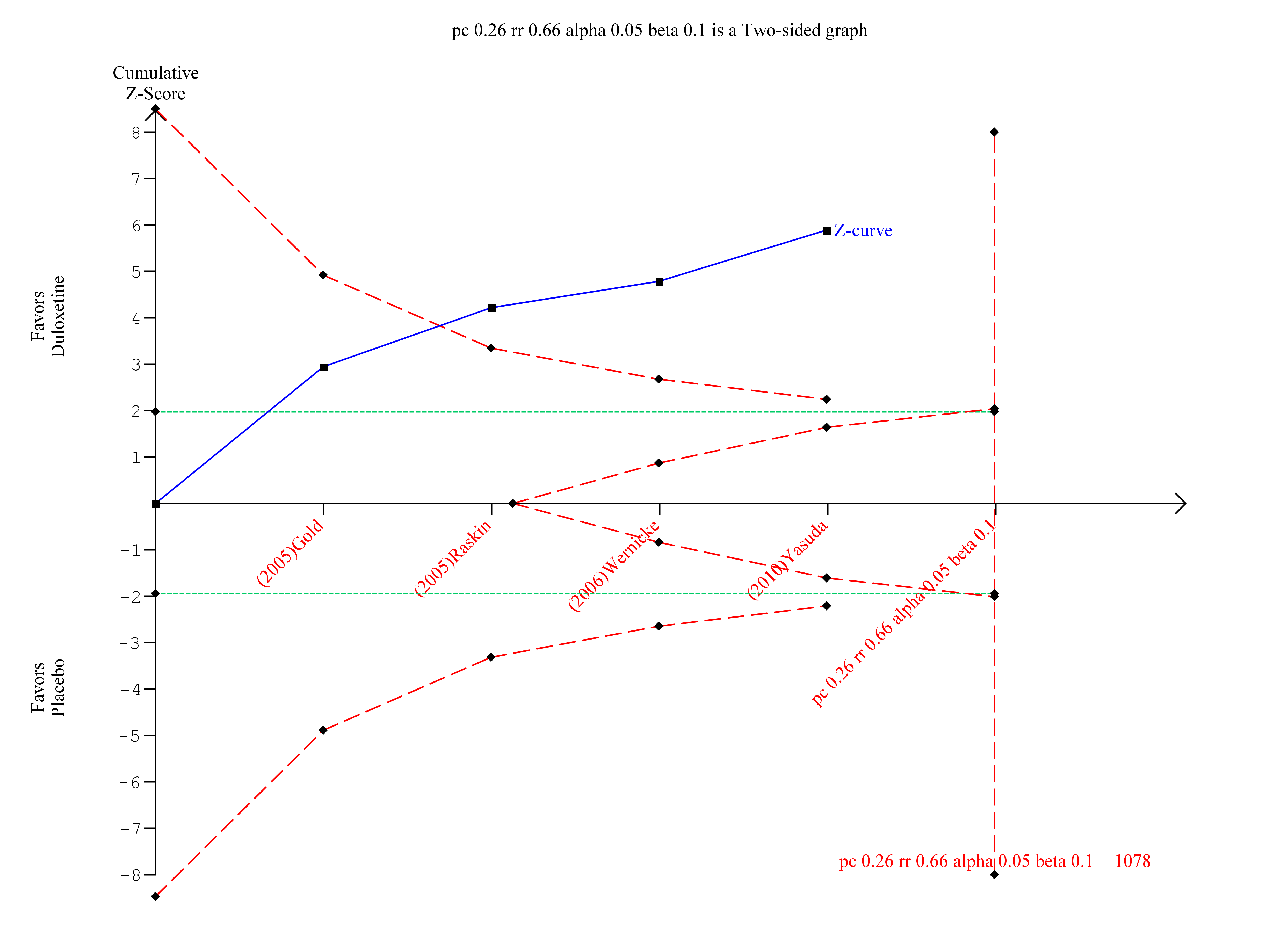
Trial sequential analysis of duloxetine versus placebo in the treatment of painful neuropathy ‐ 50% or more reduction in pain at 8‐12 weeks with at least 8 weeks of treatment
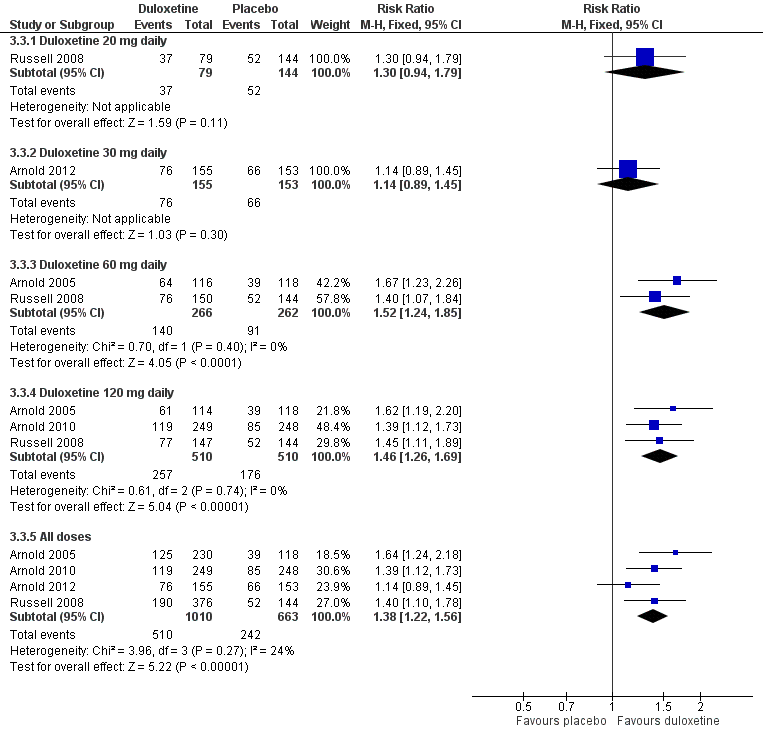
Duloxetine versus placebo in the treatment of fibromyalgia: >30% improvement <12 weeks.

Duloxetine versus placebo in the treatment of fibromyalgia: SF‐36 bodily pain.

Trial sequential analysis of duloxetine 60 mg versus placebo for the 50% reduction in pain in fibromyalgia with at least 8 weeks treatment at 8‐12 weeks

Trial Sequential Analysis of duloxetine 60 mg versus placebo in the treatment of painful physical symptoms in depression at less than 12 weeks with at least eight weeks of treatment

Adverse events leading to cessation of treatment.

Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 1 Number of participants with ≥ 50% improvement of pain at 12 weeks or less.

Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 2 Mean improvement in pain at 12 weeks or less.

Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 3 Number of participants with ≥ 30% improvement in pain at 12 weeks or less.

Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 4 Mean improvement in SF‐36 Physical Subscore at 12 weeks or less.

Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 5 Mean improvement in SF‐36 Mental Subscore at 12 weeks or less.
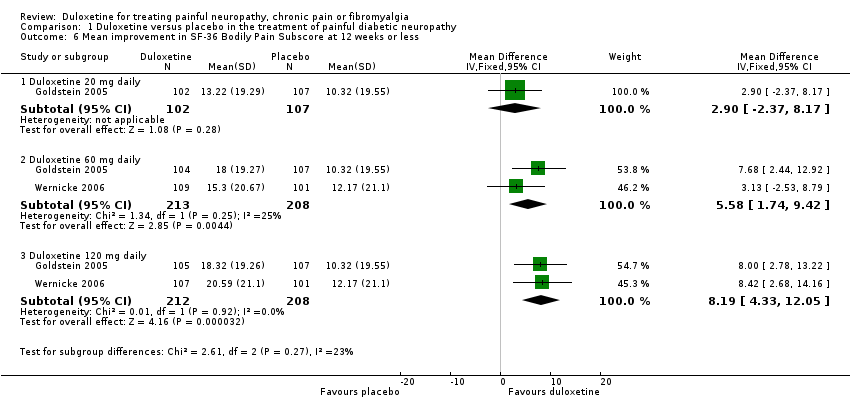
Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 6 Mean improvement in SF‐36 Bodily Pain Subscore at 12 weeks or less.

Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 7 Mean improvement in Patient Reported Global Impression of Improvement at 12 weeks or less.

Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 8 Mean improvement in BPI Severity ‐ average pain at 12 weeks or less.

Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 9 Mean improvement in pain at rest (night pain) at 12 weeks or less.

Comparison 2 Duloxetine versus pregabalin in the treatment of painful diabetic neuropathy, Outcome 1 Number of participants with ≥ 50% improvement in pain at 12 weeks or less.

Comparison 2 Duloxetine versus pregabalin in the treatment of painful diabetic neuropathy, Outcome 2 Mean improvement in pain at 12 weeks or less.
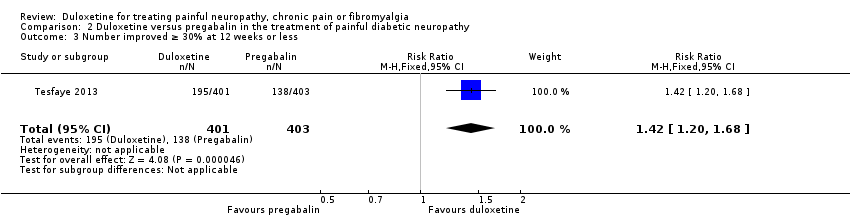
Comparison 2 Duloxetine versus pregabalin in the treatment of painful diabetic neuropathy, Outcome 3 Number improved ≥ 30% at 12 weeks or less.

Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 1 Number of participants with ≥ 50% improvement of pain at 12 weeks or less.

Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 2 Number of participants with ≥ 50% improvement of pain at more than 12 weeks.
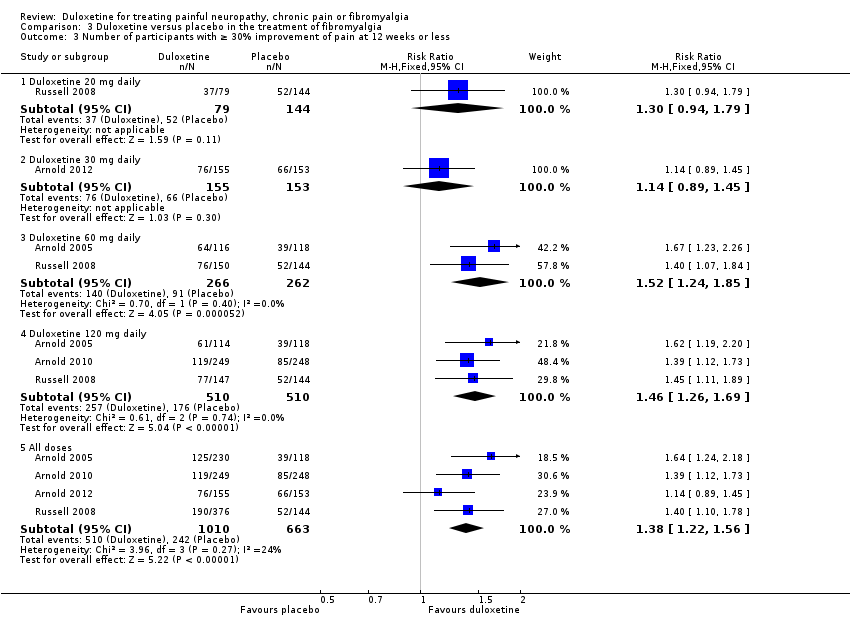
Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 3 Number of participants with ≥ 30% improvement of pain at 12 weeks or less.

Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 4 Mean improvement in pain at 12 weeks or less.
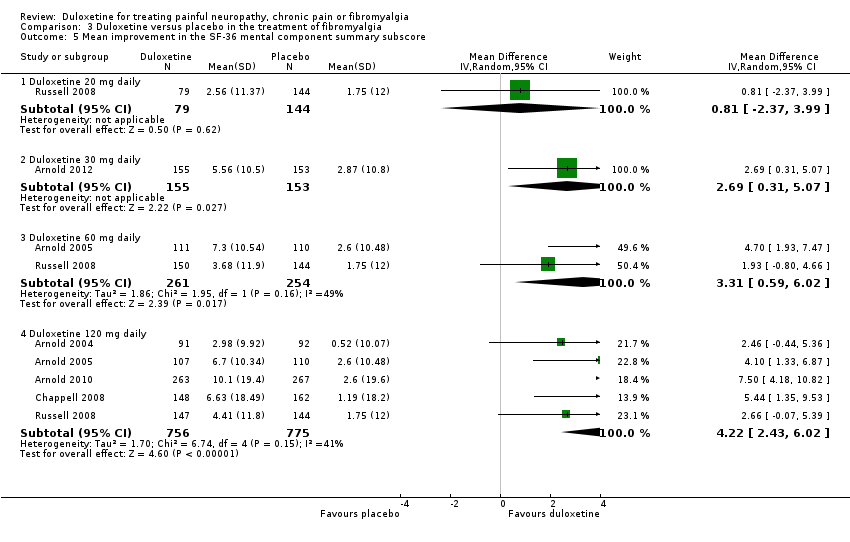
Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 5 Mean improvement in the SF‐36 mental component summary subscore.
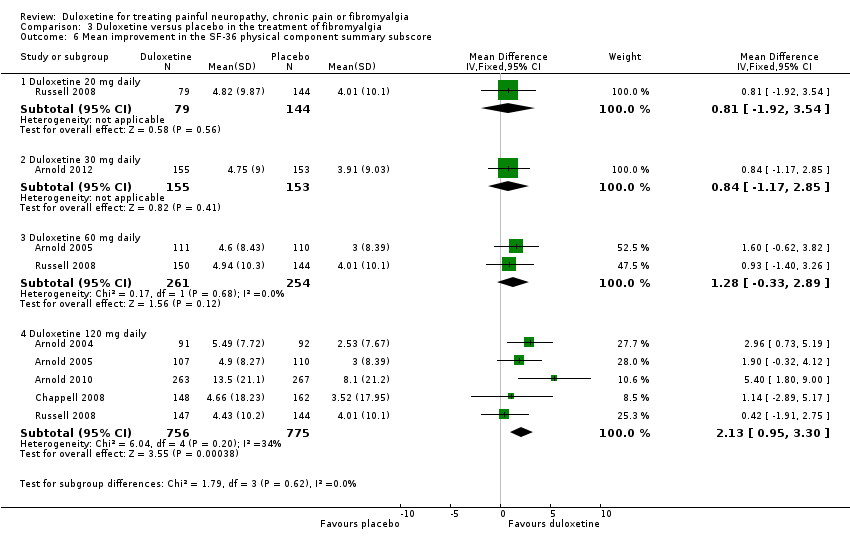
Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 6 Mean improvement in the SF‐36 physical component summary subscore.

Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 7 Mean improvement in the SF‐36 Bodily Pain Subscore.
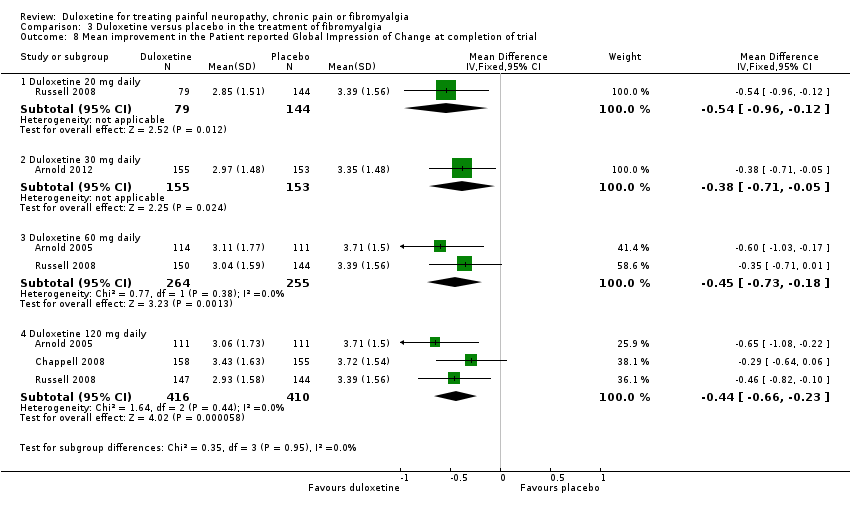
Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 8 Mean improvement in the Patient reported Global Impression of Change at completion of trial.

Comparison 4 Duloxetine versus placebo for the treatment of pain in major depressive disorder, Outcome 1 Number of participants with > 50% pain relief at 12 weeks or less.

Comparison 4 Duloxetine versus placebo for the treatment of pain in major depressive disorder, Outcome 2 Participants with > 30% pain relief at 12 weeks or less.
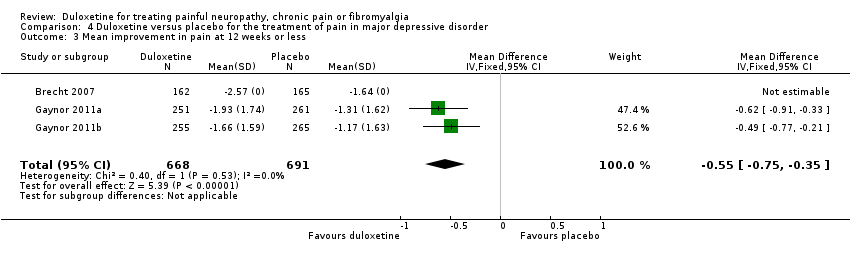
Comparison 4 Duloxetine versus placebo for the treatment of pain in major depressive disorder, Outcome 3 Mean improvement in pain at 12 weeks or less.

Comparison 5 Duloxetine versus placebo in the treatment of central neuropathic pain, Outcome 1 Mean improvement in pain at 12 weeks or less.

Comparison 5 Duloxetine versus placebo in the treatment of central neuropathic pain, Outcome 2 Mean improvement in SF‐36 Physical Subscore.

Comparison 5 Duloxetine versus placebo in the treatment of central neuropathic pain, Outcome 3 Mean improvement in the SF‐36 Mental Subscore at 12 weeks.

Comparison 5 Duloxetine versus placebo in the treatment of central neuropathic pain, Outcome 4 Mean improvement in the SF‐36 Bodily Pain Subscore.
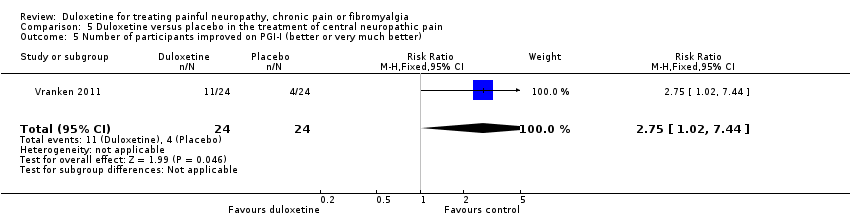
Comparison 5 Duloxetine versus placebo in the treatment of central neuropathic pain, Outcome 5 Number of participants improved on PGI‐I (better or very much better).

Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 1 Proportion of participants with any adverse event.

Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 2 Nausea.

Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 3 Dry mouth.

Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 4 Dizziness.

Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 5 Somnolence.

Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 6 Adverse event leading to cessation.

Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 7 Serious adverse event.
| Duloxetine for painful diabetic neuropathy | ||||||
| Patient or population: patients with painful neuropathy or chronic pain from diabetic peripheral neuropathy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Duloxetine | |||||
| Number of patients with ≥ 50% improvement of pain at 12 weeks or less Duloxetine 60 mg daily Follow‐up: 8 to 12 weeks | 257 per 1000 | 445 per 1000 | RR 1.73 | 908 | ⊕⊕⊕⊝ | NNTB for ≥ 50% reduction in pain at 60 mg daily: 5 (95% CI 4 to 7) |
| Mean improvement in pain at 12 weeks or less Duloxetine 60 mg daily Scale from: 0 to 10 | The mean mean improvement in pain at 12 weeks or less ‐ duloxetine 60 mg daily in the control groups was | The mean mean improvement in pain at 12 weeks or less ‐ duloxetine 60 mg daily in the intervention groups was | ‐ | 722 | ⊕⊕⊕⊝ | |
| Number of patients with ≥ 30% improvement in pain at 12 weeks or less Duloxetine 60 mg daily Follow‐up: 8 to 12 weeks | 411 per 1000 | 629 per 1000 | RR 1.53 | 799 | ⊕⊕⊕⊝ | NNTB for ≥ 30% reduction in pain at 60 mg duloxetine daily: 5 (95% CI 3 to 7) |
| Mean improvement in Patient Reported Global Impression of Change at 12 weeks or less Duloxetine 60 mg daily Scale from: 0 to 10 | The mean mean improvement in patient reported global impression of improvement change at 12 weeks or less ‐ duloxetine 60 mg daily in the control groups was | The mean mean improvement in Patient Reported Global Impression of Improvement Change at 12 weeks or less ‐ duloxetine 60 mg daily in the intervention groups was | ‐ | 1018 | ⊕⊕⊕⊝ | |
| Adverse event leading to cessation All neuropathic pain indications Duloxetine 60 mg daily | 56 per 1000 | 109 per 1000 | RR 1.95 | 4837 | ⊕⊕⊝⊝ | NNTH for duloxetine 60 mg daily, all indications, and all adverse effects leading to cessation: 18 (95% CI 13 to 30) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Four trials, all company sponsored and performed but all trials pre‐registered on ClinicalTrials.gov have been published. No publication bias detected. | ||||||
| Duloxetine for the chronic pain of fibromyalgia | |||||||
| Patient or population: patients with the chronic pain of fibromyalgia | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | ||
| Assumed risk | Corresponding risk | ||||||
| Control | Duloxetine | ||||||
| Number with ≥ 50% improvement of pain at 12 weeks or less Duloxetine 60 mg daily | 233 per 1000 | 366 per 1000 | RR 1.57 | 528 | ⊕⊕⊝⊝ | NNTB for ≥ 50% improvement of pain at duloxetine 60 mg daily: 8 (95% CI 4 to 21) | |
| Number with ≥ 30% improvement of pain at 12 weeks or less Duloxetine 60 mg daily Follow‐up: 8 to 12 weeks | 347 per 1000 | 527 per 1000 | RR 1.52 | 528 | ⊕⊕⊝⊝ | NNTB for ≥ 30% improvement of pain at duloxetine 60 mg daily: NNT 6 (95% CI 3 to 12) | |
| Mean improvement in the Patient Reported Global Impression of Change at completion of trial Duloxetine 60 mg daily Scale from: 0 to 10 | The mean mean improvement in the patient reported global impression of change at completion of trial ‐ duloxetine 60 mg daily in the control groups was | The mean mean improvement in the patient reported global impression of change at completion of trial ‐ duloxetine 60 mg daily in the intervention groups was | ‐ | 519 | ⊕⊕⊝⊝ | ||
| Mean improvement in pain at 12 weeks or less Duloxetine 120 mg daily LikertScale from: 0 to 10 | The mean mean improvement in pain at 12 weeks or less ‐ duloxetine 120 mg daily in the control groups was | The mean mean improvement in pain at 12 weeks or less ‐ duloxetine 120 mg daily in the intervention groups was | ‐ | 507 | ⊕⊕⊕⊝ | ||
| Adverse events | See comment | See comment | See comment | ‐ | See comment | See pooled adverse events in 'Summary of findings' table 1 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1 Substantial dropouts from all trials inform the outcomes. | |||||||
| Duloxetine for pain in major depressive disorder | ||||||
| Patient or population: patients with pain in major depressive disorder | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Duloxetine | |||||
| Number with ≥ 50% pain relief at 12 weeks or less | 360 per 1000 | 493 per 1000 | RR 1.37 | 1023 | ⊕⊕⊕⊝ | NNTB for ≥ 50% pain relief at < 12 weeks 60 mg duloxetine daily: 8 (95% CI 5 to 14) |
| Number with ≥ 30% pain relief at 12 weeks or less | 467 per 1000 | 593 per 1000 | RR 1.27 | 1359 | ⊕⊕⊝⊝ | NNTB for ≥ 30% pain relief at < 12 weeks 60 mg duloxetine: 8 (95% CI 4‐ to 14) |
| Mean improvement in pain at 12 weeks or less | The mean mean improvement in pain at 12 weeks or less in the control groups was | The mean mean improvement in pain at 12 weeks or less in the intervention groups was | 1359 | ⊕⊕⊝⊝ | ||
| Mean improvement in Patient Reported Global Impression of Change at 12 weeks or less | See comment | See comment | Not estimable | ‐ | See comment | Outcome not measured |
| Adverse events | See comment | See comment | Not estimable | ‐ | See comment | See pooled adverse events in 'Summary of findings' table 1 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Mixed causes for pain, not necessarily neuropathic. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with ≥ 50% improvement of pain at 12 weeks or less Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Duloxetine 20 mg daily | 1 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.98, 2.09] |
| 1.2 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [1.26, 2.87] |
| 1.3 Duloxetine 60 mg daily | 4 | 908 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.44, 2.08] |
| 1.4 Duloxetine 120 mg daily | 4 | 870 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.08, 1.97] |
| 1.5 All doses | 5 | 1655 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [1.21, 1.92] |
| 2 Mean improvement in pain at 12 weeks or less Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Duloxetine 20 mg daily | 1 | 179 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐1.05, 0.15] |
| 2.2 Duloxetine 60 mg daily | 4 | 722 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.26, ‐0.65] |
| 2.3 Duloxetine 120 mg daily | 4 | 828 | Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐1.21, ‐0.65] |
| 3 Number of participants with ≥ 30% improvement in pain at 12 weeks or less Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.18, 2.07] |
| 3.2 Duloxetine 60 mg daily | 4 | 799 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.33, 1.75] |
| 3.3 Duloxetine 120 mg daily | 3 | 659 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.21, 1.58] |
| 3.4 All doses | 4 | 1220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.30, 1.63] |
| 4 Mean improvement in SF‐36 Physical Subscore at 12 weeks or less Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Duloxetine 20 mg daily | 1 | 200 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐2.42, 1.88] |
| 4.2 Duloxetine 60 mg daily | 3 | 514 | Mean Difference (IV, Random, 95% CI) | 2.65 [1.38, 3.92] |
| 4.3 Duloxetine 120 mg daily | 2 | 409 | Mean Difference (IV, Random, 95% CI) | 2.80 [1.04, 4.55] |
| 5 Mean improvement in SF‐36 Mental Subscore at 12 weeks or less Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Duloxetine 20 mg daily | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.11 [‐0.98, 3.20] |
| 5.2 Duloxetine 60 mg daily | 3 | 514 | Mean Difference (IV, Fixed, 95% CI) | 1.08 [‐0.32, 2.48] |
| 5.3 Duloxetine 120 mg daily | 2 | 409 | Mean Difference (IV, Fixed, 95% CI) | 2.23 [0.69, 3.77] |
| 6 Mean improvement in SF‐36 Bodily Pain Subscore at 12 weeks or less Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Duloxetine 20 mg daily | 1 | 209 | Mean Difference (IV, Fixed, 95% CI) | 2.90 [‐2.37, 8.17] |
| 6.2 Duloxetine 60 mg daily | 2 | 421 | Mean Difference (IV, Fixed, 95% CI) | 5.58 [1.74, 9.42] |
| 6.3 Duloxetine 120 mg daily | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | 8.19 [4.33, 12.05] |
| 7 Mean improvement in Patient Reported Global Impression of Improvement at 12 weeks or less Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Duloxetine 20 mg daily | 1 | 219 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.56, 0.10] |
| 7.2 Duloxetine 40 mg daily | 1 | 252 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.01, ‐0.29] |
| 7.3 Duloxetine 60 mg daily | 5 | 1018 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.77, ‐0.44] |
| 7.4 Duloxetine 120 mg daily | 4 | 870 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.73, ‐0.35] |
| 8 Mean improvement in BPI Severity ‐ average pain at 12 weeks or less Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Duloxetine 60 mg daily | 2 | 433 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.38, ‐0.57] |
| 8.2 Duloxetine 120 mg daily | 2 | 428 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐1.91, ‐0.41] |
| 9 Mean improvement in pain at rest (night pain) at 12 weeks or less Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Duloxetine 20 mg daily | 1 | 222 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.90, 0.34] |
| 9.2 Duloxetine 60 mg daily | 3 | 664 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.27, ‐0.57] |
| 9.3 Duloxetine 120 mg daily | 3 | 664 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.45, ‐0.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with ≥ 50% improvement in pain at 12 weeks or less Show forest plot | 1 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.19, 1.80] |
| 2 Mean improvement in pain at 12 weeks or less Show forest plot | 1 | 804 | Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐0.92, ‐0.32] |
| 3 Number improved ≥ 30% at 12 weeks or less Show forest plot | 1 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.20, 1.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with ≥ 50% improvement of pain at 12 weeks or less Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Duloxetine 20 mg daily | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.91, 2.14] |
| 1.2 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.75, 1.35] |
| 1.3 Duloxetine 60 mg daily | 2 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.20, 2.06] |
| 1.4 Duloxetine 120 mg daily | 4 | 1234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.40, 2.03] |
| 1.5 All doses | 5 | 1887 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.29, 1.75] |
| 2 Number of participants with ≥ 50% improvement of pain at more than 12 weeks Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Duloxetine 60 mg daily | 1 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.10, 2.27] |
| 2.2 Duloxetine 120 mg daily | 2 | 616 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.07, 1.79] |
| 2.3 Duloxetine all doses | 2 | 845 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.09, 1.79] |
| 3 Number of participants with ≥ 30% improvement of pain at 12 weeks or less Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Duloxetine 20 mg daily | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.94, 1.79] |
| 3.2 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.89, 1.45] |
| 3.3 Duloxetine 60 mg daily | 2 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.24, 1.85] |
| 3.4 Duloxetine 120 mg daily | 3 | 1020 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.26, 1.69] |
| 3.5 All doses | 4 | 1673 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.22, 1.56] |
| 4 Mean improvement in pain at 12 weeks or less Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Duloxetine 30 mg daily | 1 | 308 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.86, 0.24] |
| 4.2 Duloxetine 120 mg daily | 1 | 507 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.35, ‐0.25] |
| 5 Mean improvement in the SF‐36 mental component summary subscore Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Duloxetine 20 mg daily | 1 | 223 | Mean Difference (IV, Random, 95% CI) | 0.81 [‐2.37, 3.99] |
| 5.2 Duloxetine 30 mg daily | 1 | 308 | Mean Difference (IV, Random, 95% CI) | 2.69 [0.31, 5.07] |
| 5.3 Duloxetine 60 mg daily | 2 | 515 | Mean Difference (IV, Random, 95% CI) | 3.31 [0.59, 6.02] |
| 5.4 Duloxetine 120 mg daily | 5 | 1531 | Mean Difference (IV, Random, 95% CI) | 4.22 [2.43, 6.02] |
| 6 Mean improvement in the SF‐36 physical component summary subscore Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Duloxetine 20 mg daily | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [‐1.92, 3.54] |
| 6.2 Duloxetine 30 mg daily | 1 | 308 | Mean Difference (IV, Fixed, 95% CI) | 0.84 [‐1.17, 2.85] |
| 6.3 Duloxetine 60 mg daily | 2 | 515 | Mean Difference (IV, Fixed, 95% CI) | 1.28 [‐0.33, 2.89] |
| 6.4 Duloxetine 120 mg daily | 5 | 1531 | Mean Difference (IV, Fixed, 95% CI) | 2.13 [0.95, 3.30] |
| 7 Mean improvement in the SF‐36 Bodily Pain Subscore Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Duloxetine 60 mg daily | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | 8.2 [3.20, 13.20] |
| 7.2 Duloxetine 120 mg daily | 4 | 1243 | Mean Difference (IV, Fixed, 95% CI) | 5.96 [3.76, 8.16] |
| 8 Mean improvement in the Patient reported Global Impression of Change at completion of trial Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Duloxetine 20 mg daily | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.96, ‐0.12] |
| 8.2 Duloxetine 30 mg daily | 1 | 308 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.71, ‐0.05] |
| 8.3 Duloxetine 60 mg daily | 2 | 519 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.73, ‐0.18] |
| 8.4 Duloxetine 120 mg daily | 3 | 826 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.66, ‐0.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with > 50% pain relief at 12 weeks or less Show forest plot | 2 | 1023 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.19, 1.59] |
| 2 Participants with > 30% pain relief at 12 weeks or less Show forest plot | 3 | 1359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.15, 1.40] |
| 3 Mean improvement in pain at 12 weeks or less Show forest plot | 3 | 1359 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐0.75, ‐0.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean improvement in pain at 12 weeks or less Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐2.05, 0.05] |
| 2 Mean improvement in SF‐36 Physical Subscore Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐12.72, 16.72] |
| 3 Mean improvement in the SF‐36 Mental Subscore at 12 weeks Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐6.75, 14.75] |
| 4 Mean improvement in the SF‐36 Bodily Pain Subscore Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [‐0.81, 16.81] |
| 5 Number of participants improved on PGI‐I (better or very much better) Show forest plot | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [1.02, 7.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants with any adverse event Show forest plot | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.03, 1.52] |
| 1.2 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.01, 1.31] |
| 1.3 Duloxetine 60 mg daily | 13 | 4521 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.10, 1.20] |
| 1.4 Duloxetine 120 mg daily | 3 | 688 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.09, 1.30] |
| 1.5 Duloxetine all doses | 14 | 5258 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.11, 1.20] |
| 2 Nausea Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.71, 3.00] |
| 2.2 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 5.43 [2.34, 12.58] |
| 2.3 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 6.55 [1.85, 23.17] |
| 2.4 Duloxetine 60 mg daily | 11 | 3642 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [2.14, 3.18] |
| 2.5 Duloxetine 120 mg daily | 4 | 787 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [2.06, 4.04] |
| 3 Dry mouth Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.30, 2.47] |
| 3.2 Duloxetine 40 mg daily | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Duloxetine 60 mg daily | 6 | 2004 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.89, 3.67] |
| 3.4 Duloxetine 120 mg daily | 3 | 567 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.40 [1.94, 5.96] |
| 4 Dizziness Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.33, 2.33] |
| 4.2 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.89 [1.22, 28.58] |
| 4.3 Duloxetine 60 mg daily | 8 | 2257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.35, 2.51] |
| 4.4 Duloxetine 120 mg daily | 4 | 787 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.55, 3.83] |
| 5 Somnolence Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.41, 2.43] |
| 5.2 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [0.70, 7.06] |
| 5.3 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.15, 4.38] |
| 5.4 Duloxetine 60 mg daily | 8 | 2678 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.94 [2.17, 3.97] |
| 5.5 Duloxetine 120 mg daily | 4 | 787 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.76 [2.93, 7.74] |
| 6 Adverse event leading to cessation Show forest plot | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Duloxetine 20 mg daily | 2 | 453 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.78, 2.39] |
| 6.2 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.69, 3.44] |
| 6.3 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.81, 4.77] |
| 6.4 Duloxetine 60 mg daily | 14 | 4837 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.60, 2.37] |
| 6.5 Duloxetine 120 mg daily | 7 | 1462 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [1.74, 3.04] |
| 6.6 All doses | 17 | 6285 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.67, 2.37] |
| 7 Serious adverse event Show forest plot | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Duloxetine 20 mg daily | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.02] |
| 7.3 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [0.50, 17.30] |
| 7.4 Duloxetine 60 mg daily | 14 | 4842 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.60, 1.32] |
| 7.5 Duloxetine 120 mg daily | 6 | 1257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.25, 1.35] |
| 7.6 All doses | 14 | 4976 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.53, 1.25] |

