Duloxetina para el tratamiento de la neuropatía dolorosa, el dolor crónico o la fibromialgia
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007115.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 03 enero 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Neuromuscular
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
MPTL and RACH screened references, selected trials and extracted data independently. The first draft was written by MPTL and then revised and agreed by all three authors. The authors undertook the 2013 update in the same manner.
Sources of support
Internal sources
-
Institute of Neurology, University College London, UK.
-
National Institute for Health Research University College LondonBiomedical Research Centre, UK.
This Systematic Review Update was supported by researchers at the National Institute for Biomedical Research University College London Hospitals Biomedical Research Centre
External sources
-
None, UK.
Declarations of interest
RACH has no competing interests which affect his impartiality in preparing this review.
PJW: none known.
MPL has received honoraria for consultation from Baxter Pharmaceuticals, CSL Behring and LfB and a travel support grant from Grifols, all manufacturers of IVIg. He was a blinded investigator in the study of Comi et al. 2002.
MPL is one of two Joint Co‐ordinating Editors of the Cochrane Neuromuscular Disease Group and RACH is a member of the group's editorial board. Editorial decisions regarding the review were handled by other members of the editorial board without the influence of the review authors.
Acknowledgements
MPTL is supported by the Biomedical Reseach Centre of UCLH Foundation Trust. The MRC Centre for Neuromuscular Disease hosts the Cochrane Neuromuscular Disease Group.
The Cochrane Neuromuscular Disease Group Trials Search Co‐ordinator, Angela Gunn, developed and ran searches.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Jan 03 | Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia | Review | Michael PT Lunn, Richard AC Hughes, Philip J Wiffen | |
| 2009 Oct 07 | Duloxetine for treating painful neuropathy or chronic pain | Review | Michael PT Lunn, Richard AC Hughes, Philip J Wiffen | |
| 2009 Jul 08 | Duloxetine for treating painful neuropathy or chronic pain | Protocol | Michael PT Lunn, Richard AC Hughes, Philip J Wiffen | |
Differences between protocol and review
The review used the 'Risk of bias' table in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions instead of the previous methodological quality assessment and incorporated a 'Risk of bias' table. These methods were not available when the protocol was written.
We also included 'Summary of findings' tables for each comparison.
For this update we included trial sequential analyses.
We changed the title from 'Duloxetine for treating painful neuropathy or chronic pain' to 'Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia'
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Analgesics [*administration & dosage, adverse effects];
- Chronic Pain [*drug therapy];
- Diabetic Neuropathies [*drug therapy];
- Duloxetine Hydrochloride;
- Fibromyalgia [*drug therapy];
- Neuralgia [*drug therapy];
- Randomized Controlled Trials as Topic;
- Thiophenes [*administration & dosage, adverse effects];
Medical Subject Headings Check Words
Adult; Humans;
PICO

Methodological quality summary: review authors' judgements about each methodological quality item for each included study. Green = low risk of bias; yellow = unclear risk of bias; red = high risk of bias

Duloxetine versus placebo in the treatment of painful neuropathy: Number of patients with >50% improvement of pain at <12 weeks.
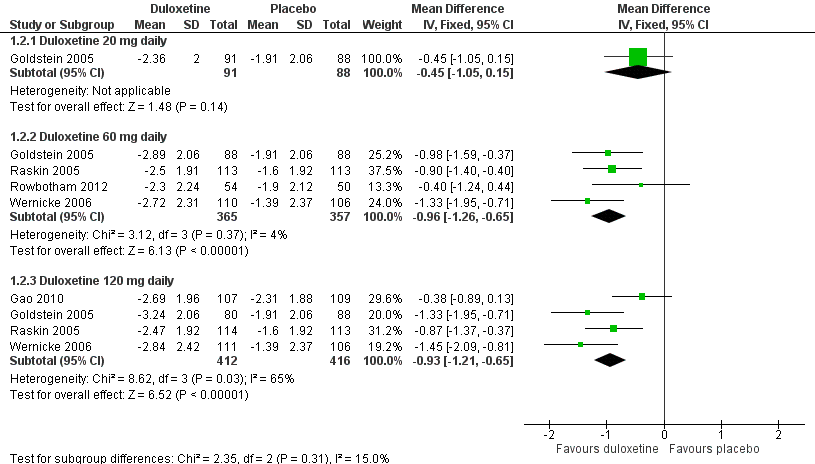
Duloxetine versus placebo in the treatment of pain: Mean improvement in pain at 12 weeks.

Duloxetine versus placebo in the treatment of pain: Number of patients with >30% improvement in pain at <12 weeks.
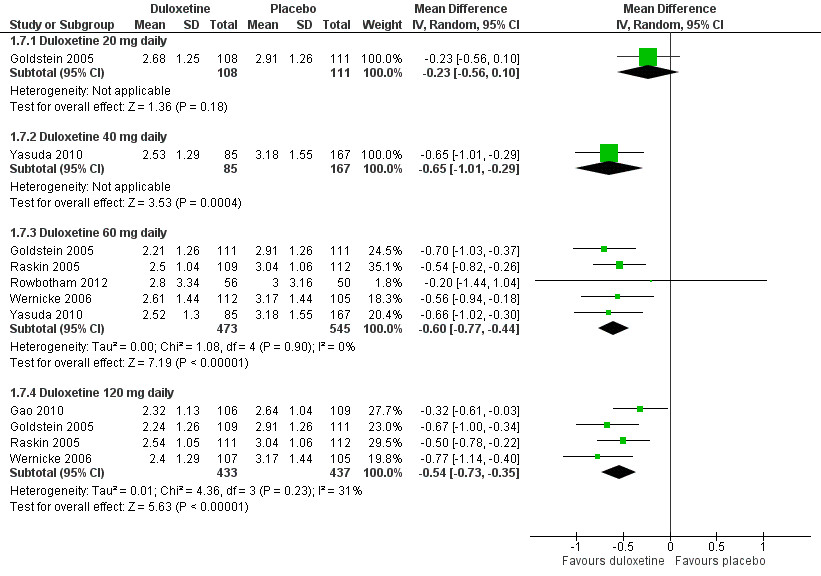
Duloxetine versus placebo in the treatment of pain: Patient reported global impression of change.

Duloxetine versus placebo in the treatment of pain: BPI severity ‐ average pain.
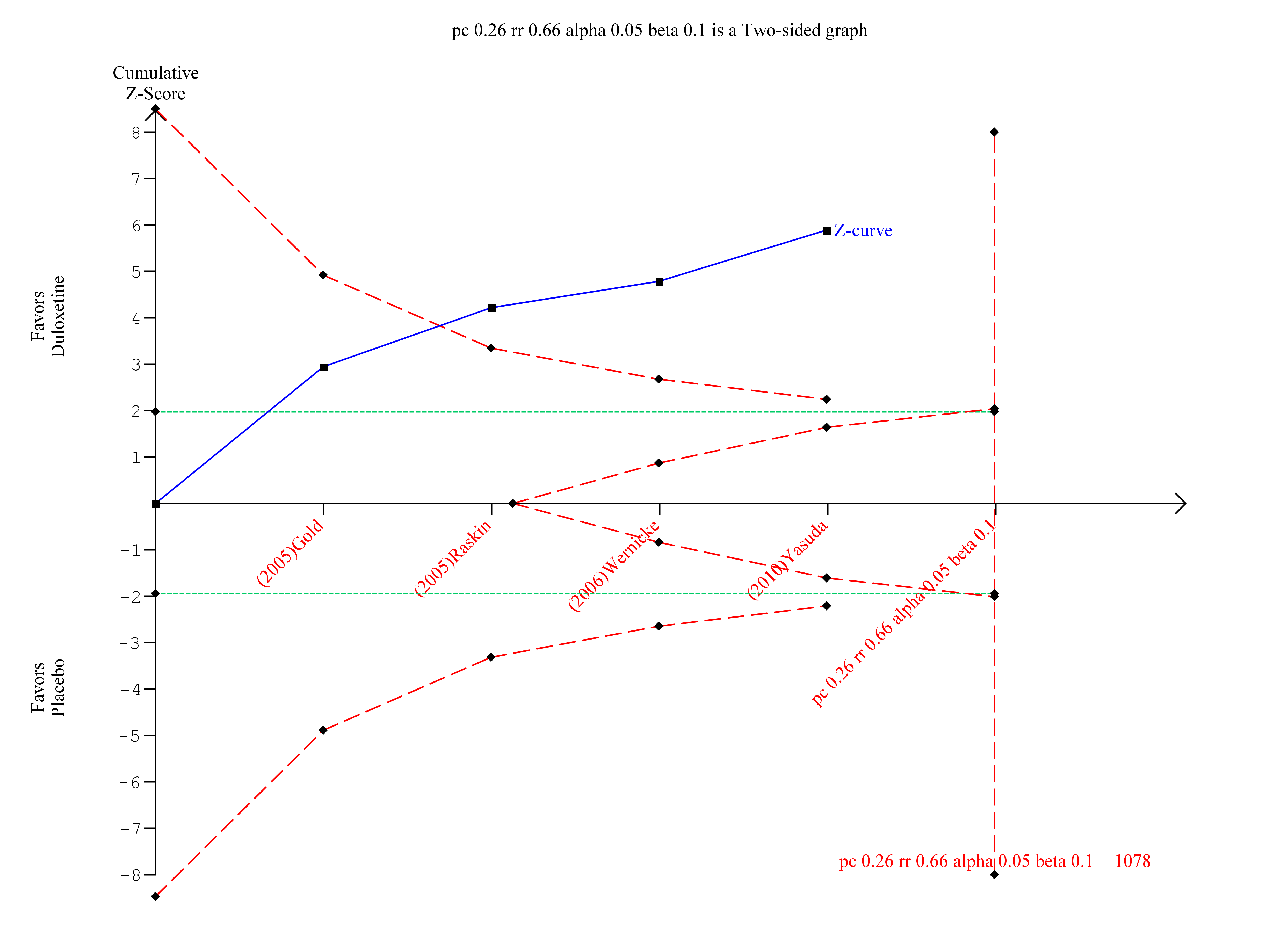
Trial sequential analysis of duloxetine versus placebo in the treatment of painful neuropathy ‐ 50% or more reduction in pain at 8‐12 weeks with at least 8 weeks of treatment
Duloxetine versus placebo in the treatment of fibromyalgia: >30% improvement <12 weeks.

Duloxetine versus placebo in the treatment of fibromyalgia: SF‐36 bodily pain.

Trial sequential analysis of duloxetine 60 mg versus placebo for the 50% reduction in pain in fibromyalgia with at least 8 weeks treatment at 8‐12 weeks

Trial Sequential Analysis of duloxetine 60 mg versus placebo in the treatment of painful physical symptoms in depression at less than 12 weeks with at least eight weeks of treatment

Adverse events leading to cessation of treatment.

Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 1 Number of participants with ≥ 50% improvement of pain at 12 weeks or less.

Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 2 Mean improvement in pain at 12 weeks or less.

Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 3 Number of participants with ≥ 30% improvement in pain at 12 weeks or less.

Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 4 Mean improvement in SF‐36 Physical Subscore at 12 weeks or less.

Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 5 Mean improvement in SF‐36 Mental Subscore at 12 weeks or less.
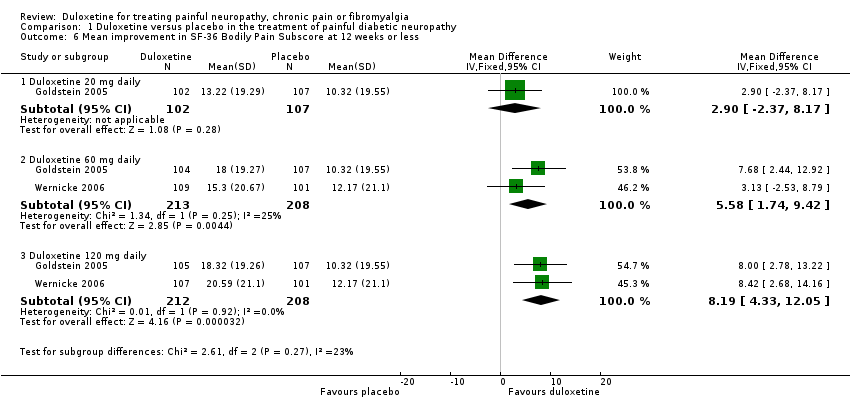
Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 6 Mean improvement in SF‐36 Bodily Pain Subscore at 12 weeks or less.

Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 7 Mean improvement in Patient Reported Global Impression of Improvement at 12 weeks or less.

Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 8 Mean improvement in BPI Severity ‐ average pain at 12 weeks or less.

Comparison 1 Duloxetine versus placebo in the treatment of painful diabetic neuropathy, Outcome 9 Mean improvement in pain at rest (night pain) at 12 weeks or less.

Comparison 2 Duloxetine versus pregabalin in the treatment of painful diabetic neuropathy, Outcome 1 Number of participants with ≥ 50% improvement in pain at 12 weeks or less.

Comparison 2 Duloxetine versus pregabalin in the treatment of painful diabetic neuropathy, Outcome 2 Mean improvement in pain at 12 weeks or less.
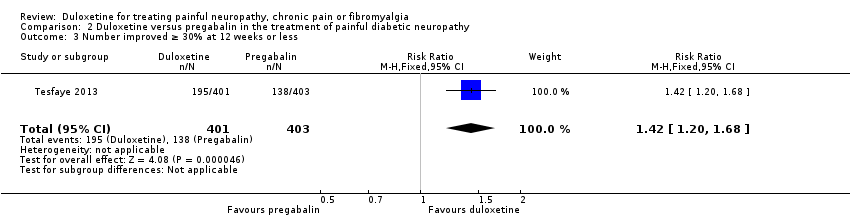
Comparison 2 Duloxetine versus pregabalin in the treatment of painful diabetic neuropathy, Outcome 3 Number improved ≥ 30% at 12 weeks or less.

Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 1 Number of participants with ≥ 50% improvement of pain at 12 weeks or less.

Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 2 Number of participants with ≥ 50% improvement of pain at more than 12 weeks.
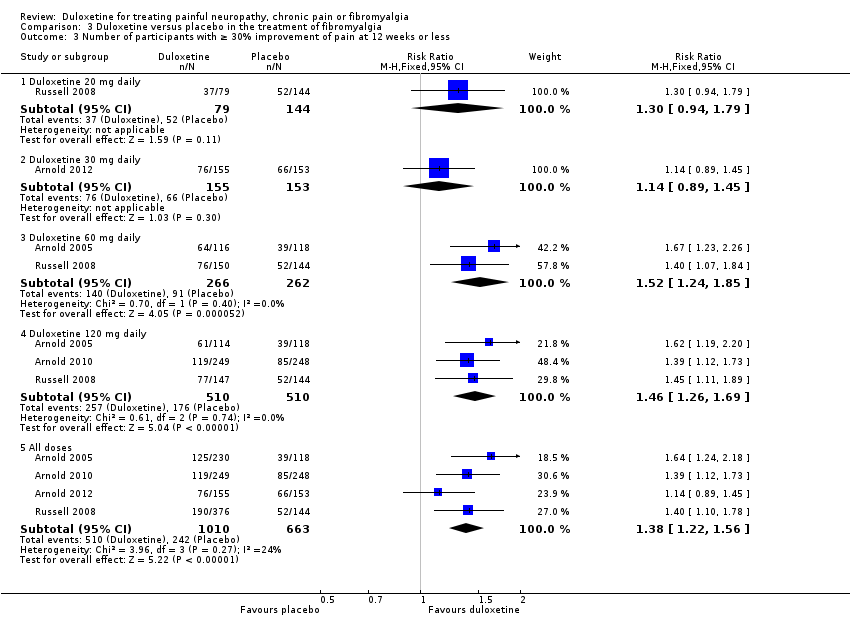
Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 3 Number of participants with ≥ 30% improvement of pain at 12 weeks or less.

Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 4 Mean improvement in pain at 12 weeks or less.
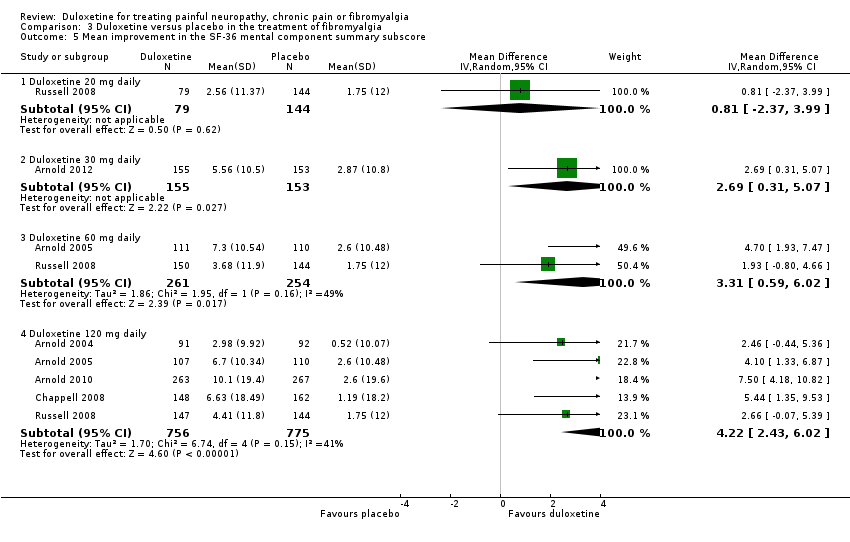
Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 5 Mean improvement in the SF‐36 mental component summary subscore.
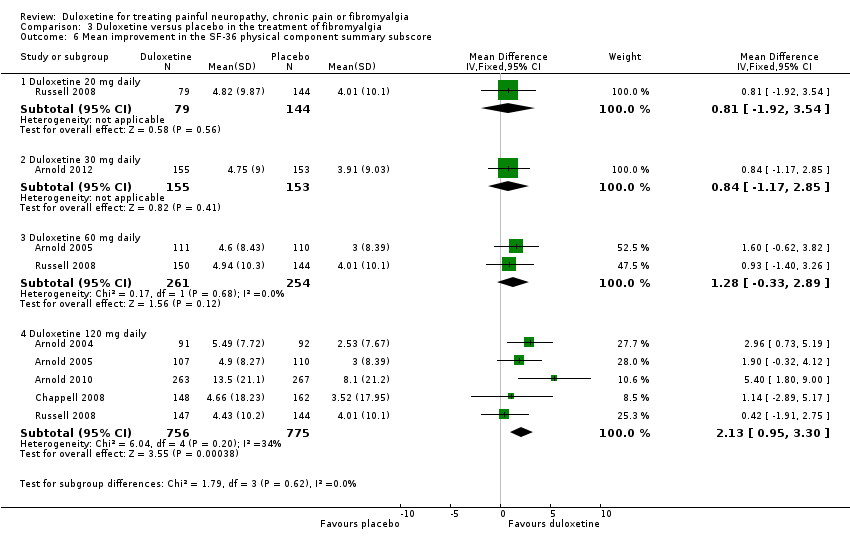
Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 6 Mean improvement in the SF‐36 physical component summary subscore.

Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 7 Mean improvement in the SF‐36 Bodily Pain Subscore.
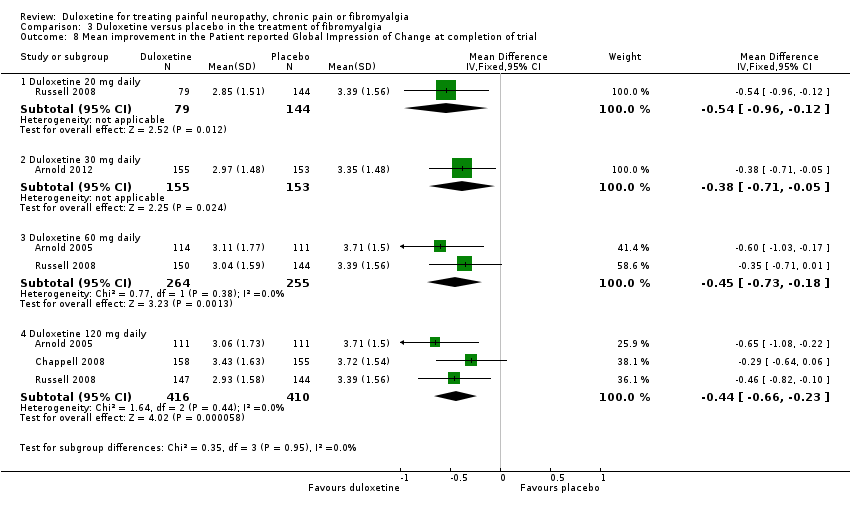
Comparison 3 Duloxetine versus placebo in the treatment of fibromyalgia, Outcome 8 Mean improvement in the Patient reported Global Impression of Change at completion of trial.

Comparison 4 Duloxetine versus placebo for the treatment of pain in major depressive disorder, Outcome 1 Number of participants with > 50% pain relief at 12 weeks or less.

Comparison 4 Duloxetine versus placebo for the treatment of pain in major depressive disorder, Outcome 2 Participants with > 30% pain relief at 12 weeks or less.
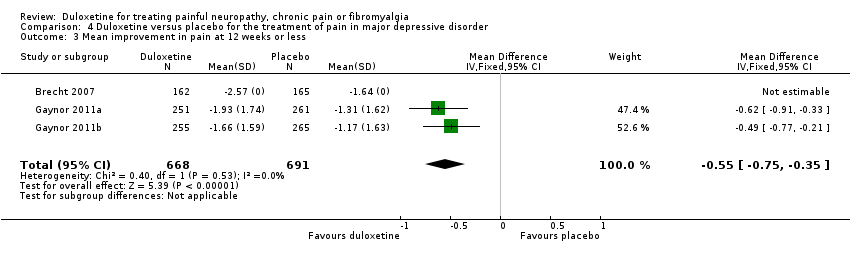
Comparison 4 Duloxetine versus placebo for the treatment of pain in major depressive disorder, Outcome 3 Mean improvement in pain at 12 weeks or less.

Comparison 5 Duloxetine versus placebo in the treatment of central neuropathic pain, Outcome 1 Mean improvement in pain at 12 weeks or less.

Comparison 5 Duloxetine versus placebo in the treatment of central neuropathic pain, Outcome 2 Mean improvement in SF‐36 Physical Subscore.

Comparison 5 Duloxetine versus placebo in the treatment of central neuropathic pain, Outcome 3 Mean improvement in the SF‐36 Mental Subscore at 12 weeks.

Comparison 5 Duloxetine versus placebo in the treatment of central neuropathic pain, Outcome 4 Mean improvement in the SF‐36 Bodily Pain Subscore.
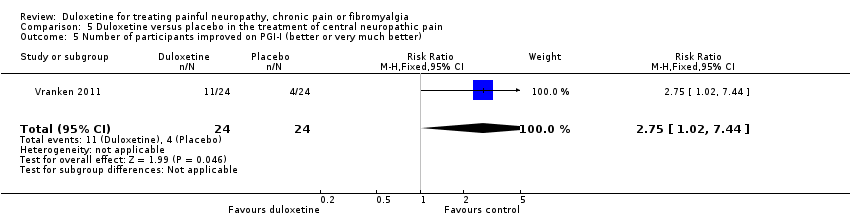
Comparison 5 Duloxetine versus placebo in the treatment of central neuropathic pain, Outcome 5 Number of participants improved on PGI‐I (better or very much better).

Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 1 Proportion of participants with any adverse event.

Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 2 Nausea.

Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 3 Dry mouth.

Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 4 Dizziness.

Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 5 Somnolence.

Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 6 Adverse event leading to cessation.

Comparison 6 Duloxetine versus placebo: adverse events during first 12 weeks of treatment for painful neuropathy or fibromyalgia, Outcome 7 Serious adverse event.
| Duloxetine for painful diabetic neuropathy | ||||||
| Patient or population: patients with painful neuropathy or chronic pain from diabetic peripheral neuropathy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Duloxetine | |||||
| Number of patients with ≥ 50% improvement of pain at 12 weeks or less Duloxetine 60 mg daily Follow‐up: 8 to 12 weeks | 257 per 1000 | 445 per 1000 | RR 1.73 | 908 | ⊕⊕⊕⊝ | NNTB for ≥ 50% reduction in pain at 60 mg daily: 5 (95% CI 4 to 7) |
| Mean improvement in pain at 12 weeks or less Duloxetine 60 mg daily Scale from: 0 to 10 | The mean mean improvement in pain at 12 weeks or less ‐ duloxetine 60 mg daily in the control groups was | The mean mean improvement in pain at 12 weeks or less ‐ duloxetine 60 mg daily in the intervention groups was | ‐ | 722 | ⊕⊕⊕⊝ | |
| Number of patients with ≥ 30% improvement in pain at 12 weeks or less Duloxetine 60 mg daily Follow‐up: 8 to 12 weeks | 411 per 1000 | 629 per 1000 | RR 1.53 | 799 | ⊕⊕⊕⊝ | NNTB for ≥ 30% reduction in pain at 60 mg duloxetine daily: 5 (95% CI 3 to 7) |
| Mean improvement in Patient Reported Global Impression of Change at 12 weeks or less Duloxetine 60 mg daily Scale from: 0 to 10 | The mean mean improvement in patient reported global impression of improvement change at 12 weeks or less ‐ duloxetine 60 mg daily in the control groups was | The mean mean improvement in Patient Reported Global Impression of Improvement Change at 12 weeks or less ‐ duloxetine 60 mg daily in the intervention groups was | ‐ | 1018 | ⊕⊕⊕⊝ | |
| Adverse event leading to cessation All neuropathic pain indications Duloxetine 60 mg daily | 56 per 1000 | 109 per 1000 | RR 1.95 | 4837 | ⊕⊕⊝⊝ | NNTH for duloxetine 60 mg daily, all indications, and all adverse effects leading to cessation: 18 (95% CI 13 to 30) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Four trials, all company sponsored and performed but all trials pre‐registered on ClinicalTrials.gov have been published. No publication bias detected. | ||||||
| Duloxetine for the chronic pain of fibromyalgia | |||||||
| Patient or population: patients with the chronic pain of fibromyalgia | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | ||
| Assumed risk | Corresponding risk | ||||||
| Control | Duloxetine | ||||||
| Number with ≥ 50% improvement of pain at 12 weeks or less Duloxetine 60 mg daily | 233 per 1000 | 366 per 1000 | RR 1.57 | 528 | ⊕⊕⊝⊝ | NNTB for ≥ 50% improvement of pain at duloxetine 60 mg daily: 8 (95% CI 4 to 21) | |
| Number with ≥ 30% improvement of pain at 12 weeks or less Duloxetine 60 mg daily Follow‐up: 8 to 12 weeks | 347 per 1000 | 527 per 1000 | RR 1.52 | 528 | ⊕⊕⊝⊝ | NNTB for ≥ 30% improvement of pain at duloxetine 60 mg daily: NNT 6 (95% CI 3 to 12) | |
| Mean improvement in the Patient Reported Global Impression of Change at completion of trial Duloxetine 60 mg daily Scale from: 0 to 10 | The mean mean improvement in the patient reported global impression of change at completion of trial ‐ duloxetine 60 mg daily in the control groups was | The mean mean improvement in the patient reported global impression of change at completion of trial ‐ duloxetine 60 mg daily in the intervention groups was | ‐ | 519 | ⊕⊕⊝⊝ | ||
| Mean improvement in pain at 12 weeks or less Duloxetine 120 mg daily LikertScale from: 0 to 10 | The mean mean improvement in pain at 12 weeks or less ‐ duloxetine 120 mg daily in the control groups was | The mean mean improvement in pain at 12 weeks or less ‐ duloxetine 120 mg daily in the intervention groups was | ‐ | 507 | ⊕⊕⊕⊝ | ||
| Adverse events | See comment | See comment | See comment | ‐ | See comment | See pooled adverse events in 'Summary of findings' table 1 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1 Substantial dropouts from all trials inform the outcomes. | |||||||
| Duloxetine for pain in major depressive disorder | ||||||
| Patient or population: patients with pain in major depressive disorder | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Duloxetine | |||||
| Number with ≥ 50% pain relief at 12 weeks or less | 360 per 1000 | 493 per 1000 | RR 1.37 | 1023 | ⊕⊕⊕⊝ | NNTB for ≥ 50% pain relief at < 12 weeks 60 mg duloxetine daily: 8 (95% CI 5 to 14) |
| Number with ≥ 30% pain relief at 12 weeks or less | 467 per 1000 | 593 per 1000 | RR 1.27 | 1359 | ⊕⊕⊝⊝ | NNTB for ≥ 30% pain relief at < 12 weeks 60 mg duloxetine: 8 (95% CI 4‐ to 14) |
| Mean improvement in pain at 12 weeks or less | The mean mean improvement in pain at 12 weeks or less in the control groups was | The mean mean improvement in pain at 12 weeks or less in the intervention groups was | 1359 | ⊕⊕⊝⊝ | ||
| Mean improvement in Patient Reported Global Impression of Change at 12 weeks or less | See comment | See comment | Not estimable | ‐ | See comment | Outcome not measured |
| Adverse events | See comment | See comment | Not estimable | ‐ | See comment | See pooled adverse events in 'Summary of findings' table 1 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Mixed causes for pain, not necessarily neuropathic. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with ≥ 50% improvement of pain at 12 weeks or less Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Duloxetine 20 mg daily | 1 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.98, 2.09] |
| 1.2 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [1.26, 2.87] |
| 1.3 Duloxetine 60 mg daily | 4 | 908 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.44, 2.08] |
| 1.4 Duloxetine 120 mg daily | 4 | 870 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.08, 1.97] |
| 1.5 All doses | 5 | 1655 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [1.21, 1.92] |
| 2 Mean improvement in pain at 12 weeks or less Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Duloxetine 20 mg daily | 1 | 179 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐1.05, 0.15] |
| 2.2 Duloxetine 60 mg daily | 4 | 722 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.26, ‐0.65] |
| 2.3 Duloxetine 120 mg daily | 4 | 828 | Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐1.21, ‐0.65] |
| 3 Number of participants with ≥ 30% improvement in pain at 12 weeks or less Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.18, 2.07] |
| 3.2 Duloxetine 60 mg daily | 4 | 799 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.33, 1.75] |
| 3.3 Duloxetine 120 mg daily | 3 | 659 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.21, 1.58] |
| 3.4 All doses | 4 | 1220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.30, 1.63] |
| 4 Mean improvement in SF‐36 Physical Subscore at 12 weeks or less Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Duloxetine 20 mg daily | 1 | 200 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐2.42, 1.88] |
| 4.2 Duloxetine 60 mg daily | 3 | 514 | Mean Difference (IV, Random, 95% CI) | 2.65 [1.38, 3.92] |
| 4.3 Duloxetine 120 mg daily | 2 | 409 | Mean Difference (IV, Random, 95% CI) | 2.80 [1.04, 4.55] |
| 5 Mean improvement in SF‐36 Mental Subscore at 12 weeks or less Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Duloxetine 20 mg daily | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.11 [‐0.98, 3.20] |
| 5.2 Duloxetine 60 mg daily | 3 | 514 | Mean Difference (IV, Fixed, 95% CI) | 1.08 [‐0.32, 2.48] |
| 5.3 Duloxetine 120 mg daily | 2 | 409 | Mean Difference (IV, Fixed, 95% CI) | 2.23 [0.69, 3.77] |
| 6 Mean improvement in SF‐36 Bodily Pain Subscore at 12 weeks or less Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Duloxetine 20 mg daily | 1 | 209 | Mean Difference (IV, Fixed, 95% CI) | 2.90 [‐2.37, 8.17] |
| 6.2 Duloxetine 60 mg daily | 2 | 421 | Mean Difference (IV, Fixed, 95% CI) | 5.58 [1.74, 9.42] |
| 6.3 Duloxetine 120 mg daily | 2 | 420 | Mean Difference (IV, Fixed, 95% CI) | 8.19 [4.33, 12.05] |
| 7 Mean improvement in Patient Reported Global Impression of Improvement at 12 weeks or less Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Duloxetine 20 mg daily | 1 | 219 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.56, 0.10] |
| 7.2 Duloxetine 40 mg daily | 1 | 252 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.01, ‐0.29] |
| 7.3 Duloxetine 60 mg daily | 5 | 1018 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.77, ‐0.44] |
| 7.4 Duloxetine 120 mg daily | 4 | 870 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.73, ‐0.35] |
| 8 Mean improvement in BPI Severity ‐ average pain at 12 weeks or less Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Duloxetine 60 mg daily | 2 | 433 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.38, ‐0.57] |
| 8.2 Duloxetine 120 mg daily | 2 | 428 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐1.91, ‐0.41] |
| 9 Mean improvement in pain at rest (night pain) at 12 weeks or less Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Duloxetine 20 mg daily | 1 | 222 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.90, 0.34] |
| 9.2 Duloxetine 60 mg daily | 3 | 664 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.27, ‐0.57] |
| 9.3 Duloxetine 120 mg daily | 3 | 664 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.45, ‐0.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with ≥ 50% improvement in pain at 12 weeks or less Show forest plot | 1 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.19, 1.80] |
| 2 Mean improvement in pain at 12 weeks or less Show forest plot | 1 | 804 | Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐0.92, ‐0.32] |
| 3 Number improved ≥ 30% at 12 weeks or less Show forest plot | 1 | 804 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.20, 1.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with ≥ 50% improvement of pain at 12 weeks or less Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Duloxetine 20 mg daily | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.91, 2.14] |
| 1.2 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.75, 1.35] |
| 1.3 Duloxetine 60 mg daily | 2 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.20, 2.06] |
| 1.4 Duloxetine 120 mg daily | 4 | 1234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.40, 2.03] |
| 1.5 All doses | 5 | 1887 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.29, 1.75] |
| 2 Number of participants with ≥ 50% improvement of pain at more than 12 weeks Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Duloxetine 60 mg daily | 1 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.10, 2.27] |
| 2.2 Duloxetine 120 mg daily | 2 | 616 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.07, 1.79] |
| 2.3 Duloxetine all doses | 2 | 845 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.09, 1.79] |
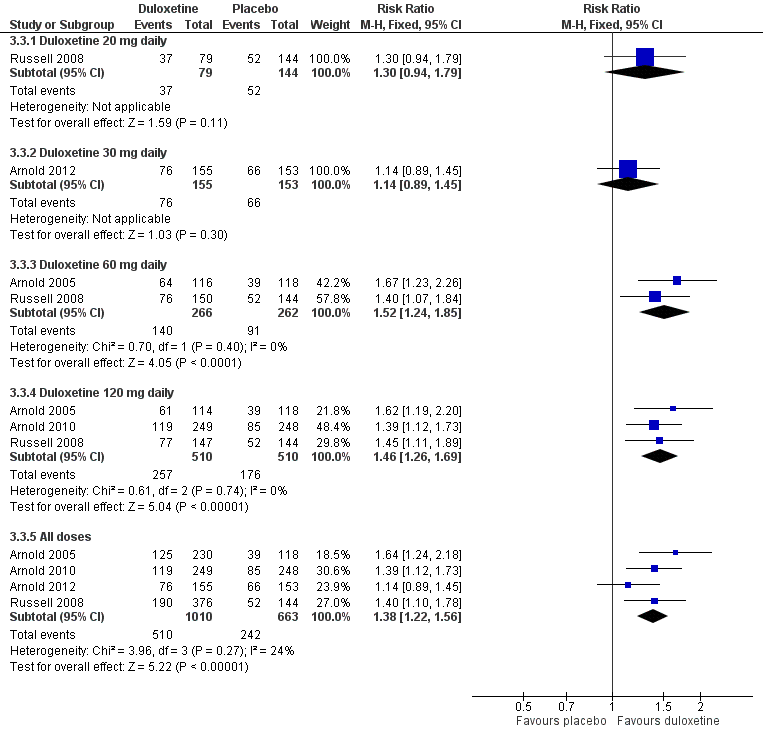
| 3 Number of participants with ≥ 30% improvement of pain at 12 weeks or less Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Duloxetine 20 mg daily | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.94, 1.79] |
| 3.2 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.89, 1.45] |
| 3.3 Duloxetine 60 mg daily | 2 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.24, 1.85] |
| 3.4 Duloxetine 120 mg daily | 3 | 1020 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.26, 1.69] |
| 3.5 All doses | 4 | 1673 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.22, 1.56] |
| 4 Mean improvement in pain at 12 weeks or less Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Duloxetine 30 mg daily | 1 | 308 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.86, 0.24] |
| 4.2 Duloxetine 120 mg daily | 1 | 507 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.35, ‐0.25] |
| 5 Mean improvement in the SF‐36 mental component summary subscore Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Duloxetine 20 mg daily | 1 | 223 | Mean Difference (IV, Random, 95% CI) | 0.81 [‐2.37, 3.99] |
| 5.2 Duloxetine 30 mg daily | 1 | 308 | Mean Difference (IV, Random, 95% CI) | 2.69 [0.31, 5.07] |
| 5.3 Duloxetine 60 mg daily | 2 | 515 | Mean Difference (IV, Random, 95% CI) | 3.31 [0.59, 6.02] |
| 5.4 Duloxetine 120 mg daily | 5 | 1531 | Mean Difference (IV, Random, 95% CI) | 4.22 [2.43, 6.02] |
| 6 Mean improvement in the SF‐36 physical component summary subscore Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Duloxetine 20 mg daily | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [‐1.92, 3.54] |
| 6.2 Duloxetine 30 mg daily | 1 | 308 | Mean Difference (IV, Fixed, 95% CI) | 0.84 [‐1.17, 2.85] |
| 6.3 Duloxetine 60 mg daily | 2 | 515 | Mean Difference (IV, Fixed, 95% CI) | 1.28 [‐0.33, 2.89] |
| 6.4 Duloxetine 120 mg daily | 5 | 1531 | Mean Difference (IV, Fixed, 95% CI) | 2.13 [0.95, 3.30] |
| 7 Mean improvement in the SF‐36 Bodily Pain Subscore Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Duloxetine 60 mg daily | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | 8.2 [3.20, 13.20] |
| 7.2 Duloxetine 120 mg daily | 4 | 1243 | Mean Difference (IV, Fixed, 95% CI) | 5.96 [3.76, 8.16] |
| 8 Mean improvement in the Patient reported Global Impression of Change at completion of trial Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Duloxetine 20 mg daily | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.96, ‐0.12] |
| 8.2 Duloxetine 30 mg daily | 1 | 308 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.71, ‐0.05] |
| 8.3 Duloxetine 60 mg daily | 2 | 519 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.73, ‐0.18] |
| 8.4 Duloxetine 120 mg daily | 3 | 826 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.66, ‐0.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with > 50% pain relief at 12 weeks or less Show forest plot | 2 | 1023 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.19, 1.59] |
| 2 Participants with > 30% pain relief at 12 weeks or less Show forest plot | 3 | 1359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.15, 1.40] |
| 3 Mean improvement in pain at 12 weeks or less Show forest plot | 3 | 1359 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐0.75, ‐0.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean improvement in pain at 12 weeks or less Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐2.05, 0.05] |
| 2 Mean improvement in SF‐36 Physical Subscore Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐12.72, 16.72] |
| 3 Mean improvement in the SF‐36 Mental Subscore at 12 weeks Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐6.75, 14.75] |
| 4 Mean improvement in the SF‐36 Bodily Pain Subscore Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [‐0.81, 16.81] |
| 5 Number of participants improved on PGI‐I (better or very much better) Show forest plot | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [1.02, 7.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants with any adverse event Show forest plot | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.03, 1.52] |
| 1.2 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.01, 1.31] |
| 1.3 Duloxetine 60 mg daily | 13 | 4521 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.10, 1.20] |
| 1.4 Duloxetine 120 mg daily | 3 | 688 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.09, 1.30] |
| 1.5 Duloxetine all doses | 14 | 5258 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.11, 1.20] |
| 2 Nausea Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.71, 3.00] |
| 2.2 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 5.43 [2.34, 12.58] |
| 2.3 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 6.55 [1.85, 23.17] |
| 2.4 Duloxetine 60 mg daily | 11 | 3642 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [2.14, 3.18] |
| 2.5 Duloxetine 120 mg daily | 4 | 787 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [2.06, 4.04] |
| 3 Dry mouth Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.30, 2.47] |
| 3.2 Duloxetine 40 mg daily | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Duloxetine 60 mg daily | 6 | 2004 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.89, 3.67] |
| 3.4 Duloxetine 120 mg daily | 3 | 567 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.40 [1.94, 5.96] |
| 4 Dizziness Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.33, 2.33] |
| 4.2 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.89 [1.22, 28.58] |
| 4.3 Duloxetine 60 mg daily | 8 | 2257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.35, 2.51] |
| 4.4 Duloxetine 120 mg daily | 4 | 787 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.55, 3.83] |
| 5 Somnolence Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Duloxetine 20 mg daily | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.41, 2.43] |
| 5.2 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [0.70, 7.06] |
| 5.3 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.15, 4.38] |
| 5.4 Duloxetine 60 mg daily | 8 | 2678 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.94 [2.17, 3.97] |
| 5.5 Duloxetine 120 mg daily | 4 | 787 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.76 [2.93, 7.74] |
| 6 Adverse event leading to cessation Show forest plot | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Duloxetine 20 mg daily | 2 | 453 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.78, 2.39] |
| 6.2 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.69, 3.44] |
| 6.3 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.81, 4.77] |
| 6.4 Duloxetine 60 mg daily | 14 | 4837 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.60, 2.37] |
| 6.5 Duloxetine 120 mg daily | 7 | 1462 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [1.74, 3.04] |
| 6.6 All doses | 17 | 6285 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.67, 2.37] |
| 7 Serious adverse event Show forest plot | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Duloxetine 20 mg daily | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Duloxetine 30 mg daily | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.02] |
| 7.3 Duloxetine 40 mg daily | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [0.50, 17.30] |
| 7.4 Duloxetine 60 mg daily | 14 | 4842 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.60, 1.32] |
| 7.5 Duloxetine 120 mg daily | 6 | 1257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.25, 1.35] |
| 7.6 All doses | 14 | 4976 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.53, 1.25] |

