Miel para la tos aguda en niños
Appendices
Appendix 1. AMED (Ovid) search strategy
1 cough/
2 cough*.tw.
3 1 or 2
4 honey/
5 honey*.tw.
6 4 or 5
7 3 and 6
Appendix 2. CAB Abstracts (Thomson Reuters) search strategy
Topic=(cough*) AND Topic=(honey*)Timespan=2009‐2011. Databases=CAB Abstracts.
Appendix 3. Previous searches
For the 2014 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 10), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (January 2012 to October week 4, 2014), EMBASE (January 2012 to October 2014), CINAHL (January 2012 to October 2014), Web of Science (2011 to October 2014), AMED (2011 to October 2014), LILACS (2011 to October 2014) and CAB abstracts (2011 to January 2014).
Our initial 2009 search covered CENTRAL (The Cochrane Library 2009, Issue 2), MEDLINE (1950 to April Week 2, 2009), Embase (1990 to April 2009), CINAHL (1982 to April 2009), Web of Science (2000 to April 2009), AMED (1985 April 2009) and LILACS (1982 to April 2009). The search was then updated in January 2012 to cover the period April 2009 to January 2012.
Appendix 4. CENTRAL and MEDLINE (Ovid) search strategy
1 Cough/
2 cough*.tw
3 cough*.tw
4 Honey/
5 honey*.tw.
6 4 or 5
7 3 and 6
Appendix 5. Embase (Elsevier) search strategy
#7. #3 AND #6
#6. #4 OR #5
#5. honey*:ab,ti
#4. 'honey'/de
#3. #1 OR #2
#2. cough*:ab,ti
#1. 'coughing'/exp
Appendix 6. CINAHL (EBSCO) search strategy
S7 S3 and S6
S6 S4 or S5
S5 TI honey* or AB honey*
S4 (MH "Honey")
S3 S1 or S2
S2 TI cough* or AB cough*
S1 (MH "Cough")
Appendix 7. Web of Science (Clarivate Analytics) search strategy
Topic=(cough*) AND Topic=(honey*)
Appendix 8. LILACS (BIREME) search strategy
(mh:cough OR cough* OR tos OR tosse) AND (mh:honey OR honey* OR miel OR mel) AND db:("LILACS")
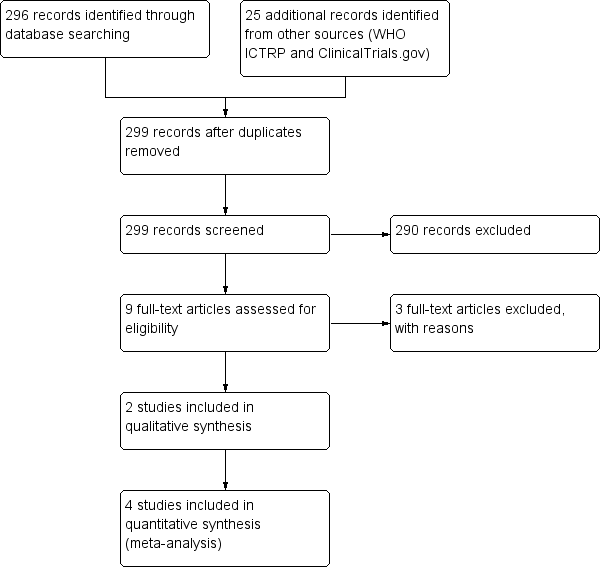
Study flow diagram (2018 update).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
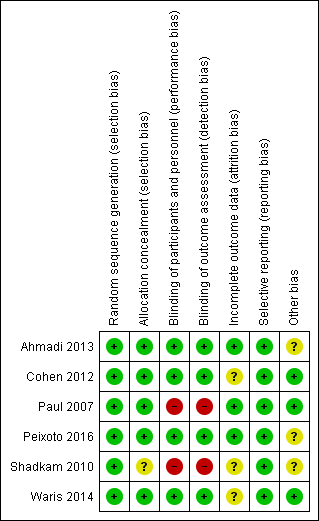
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
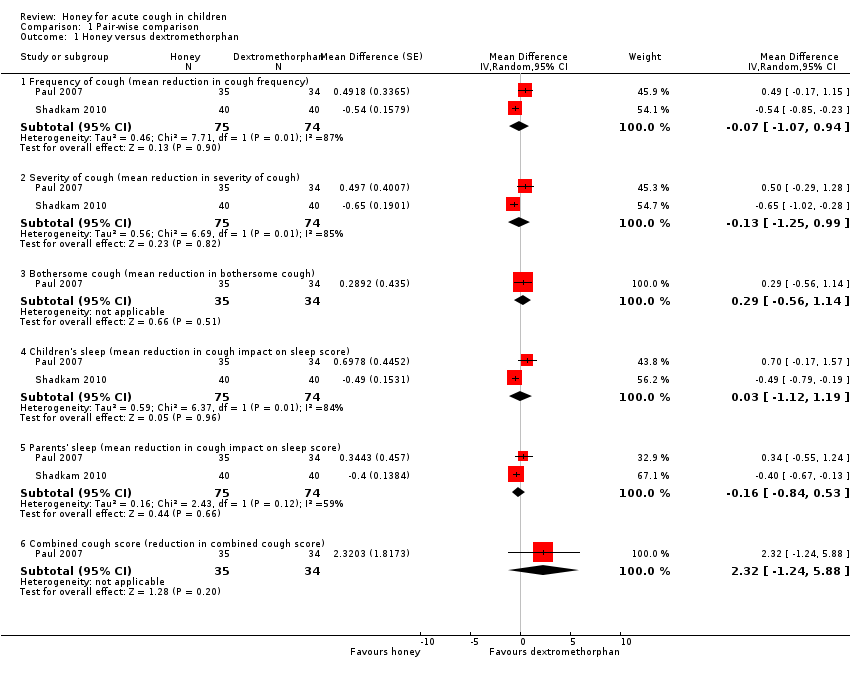
Comparison 1 Pair‐wise comparison, Outcome 1 Honey versus dextromethorphan.

Comparison 1 Pair‐wise comparison, Outcome 2 Honey versus diphenhydramine.
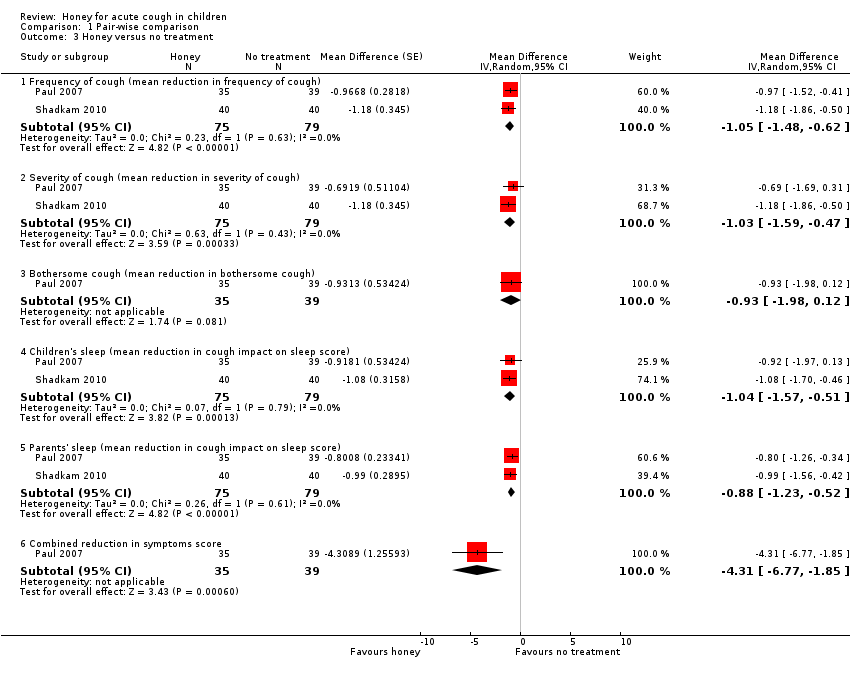
Comparison 1 Pair‐wise comparison, Outcome 3 Honey versus no treatment.
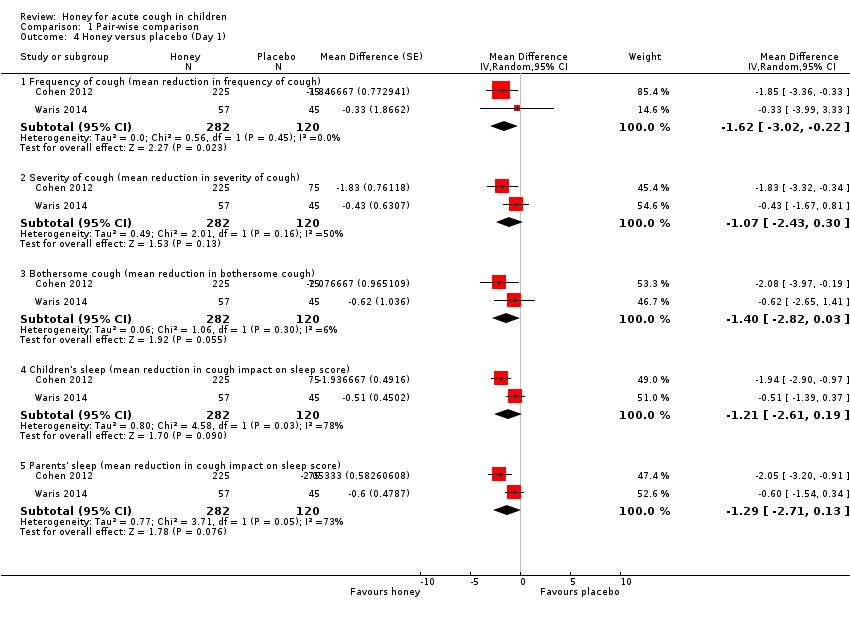
Comparison 1 Pair‐wise comparison, Outcome 4 Honey versus placebo (Day 1).
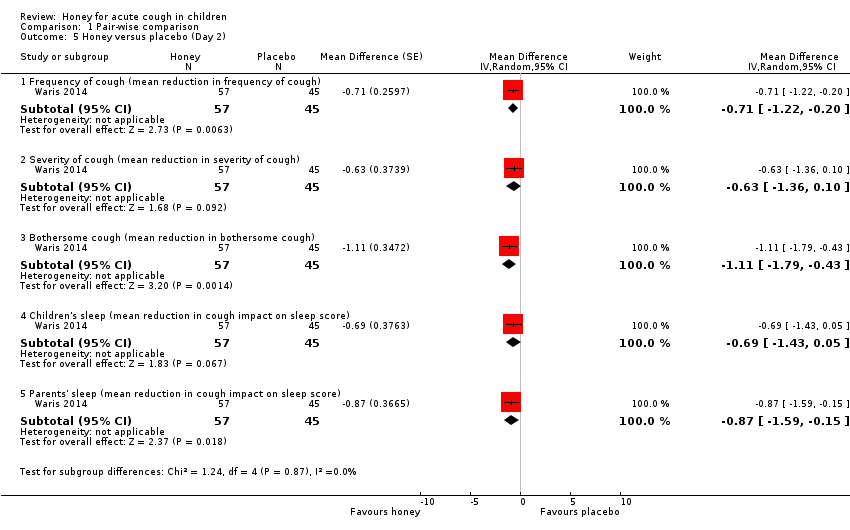
Comparison 1 Pair‐wise comparison, Outcome 5 Honey versus placebo (Day 2).
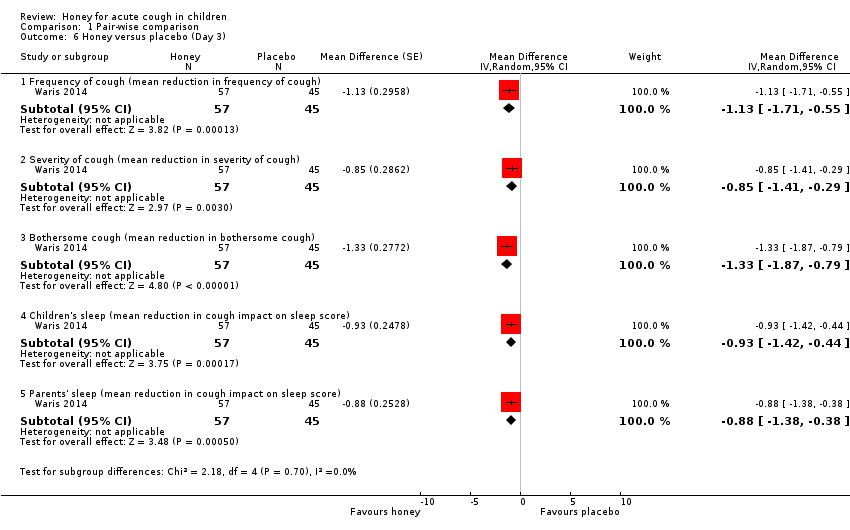
Comparison 1 Pair‐wise comparison, Outcome 6 Honey versus placebo (Day 3).

Comparison 1 Pair‐wise comparison, Outcome 7 Honey versus placebo (Day 4).
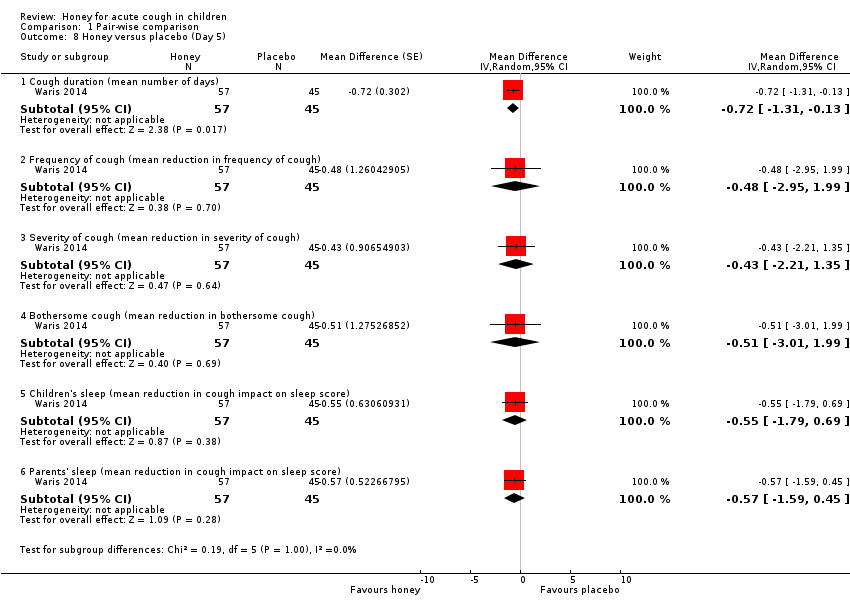
Comparison 1 Pair‐wise comparison, Outcome 8 Honey versus placebo (Day 5).

Comparison 1 Pair‐wise comparison, Outcome 9 Honey versus salbutamol (Day 1).
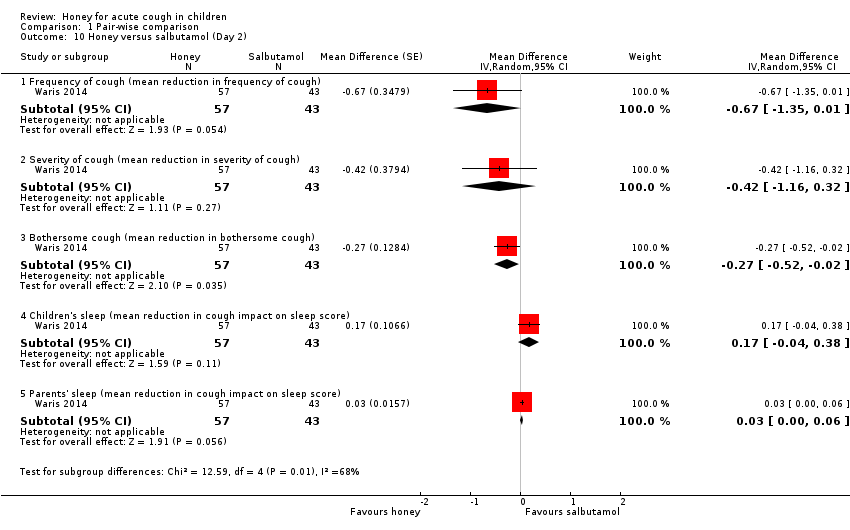
Comparison 1 Pair‐wise comparison, Outcome 10 Honey versus salbutamol (Day 2).

Comparison 1 Pair‐wise comparison, Outcome 11 Honey versus salbutamol (Day 3).
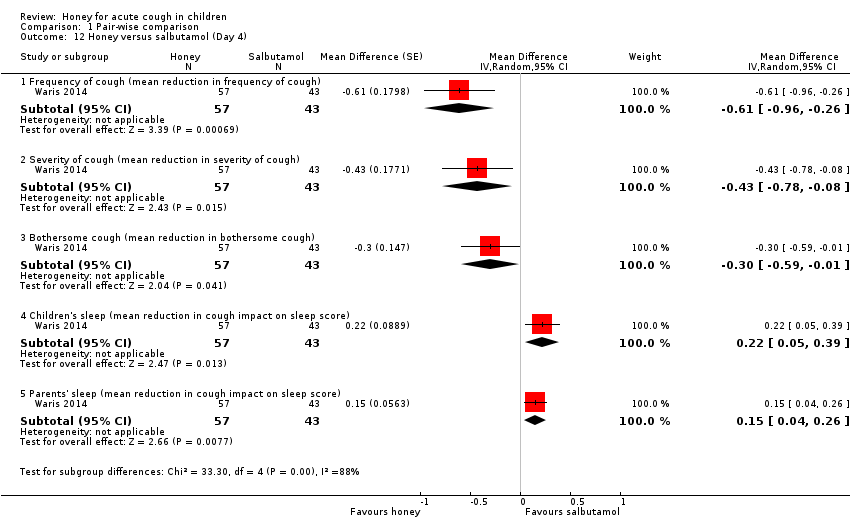
Comparison 1 Pair‐wise comparison, Outcome 12 Honey versus salbutamol (Day 4).

Comparison 1 Pair‐wise comparison, Outcome 13 Honey versus salbutamol (Day 5).

Comparison 2 Pre‐ and postintervention comparison, Outcome 1 Cough frequency (mean reduction in frequency).
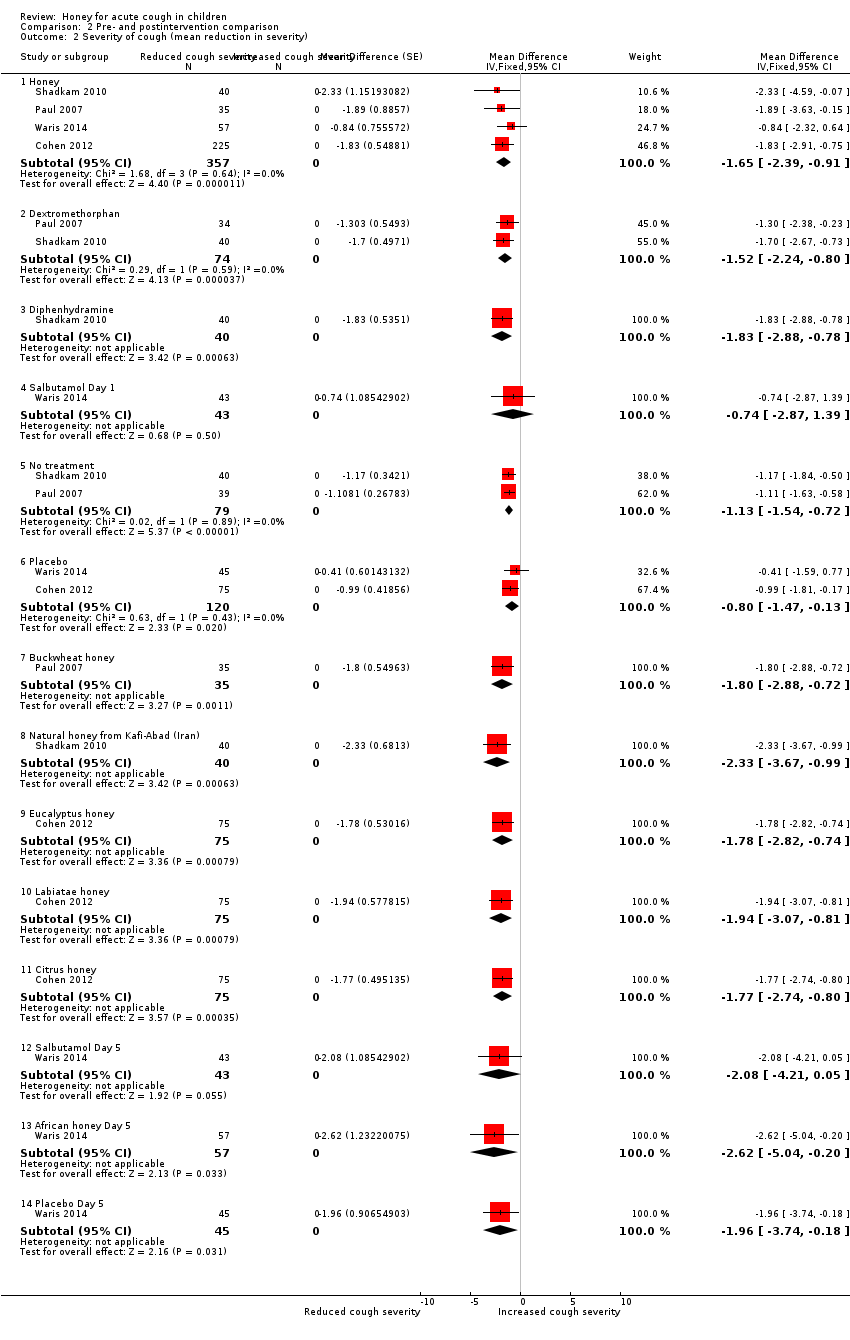
Comparison 2 Pre‐ and postintervention comparison, Outcome 2 Severity of cough (mean reduction in severity).
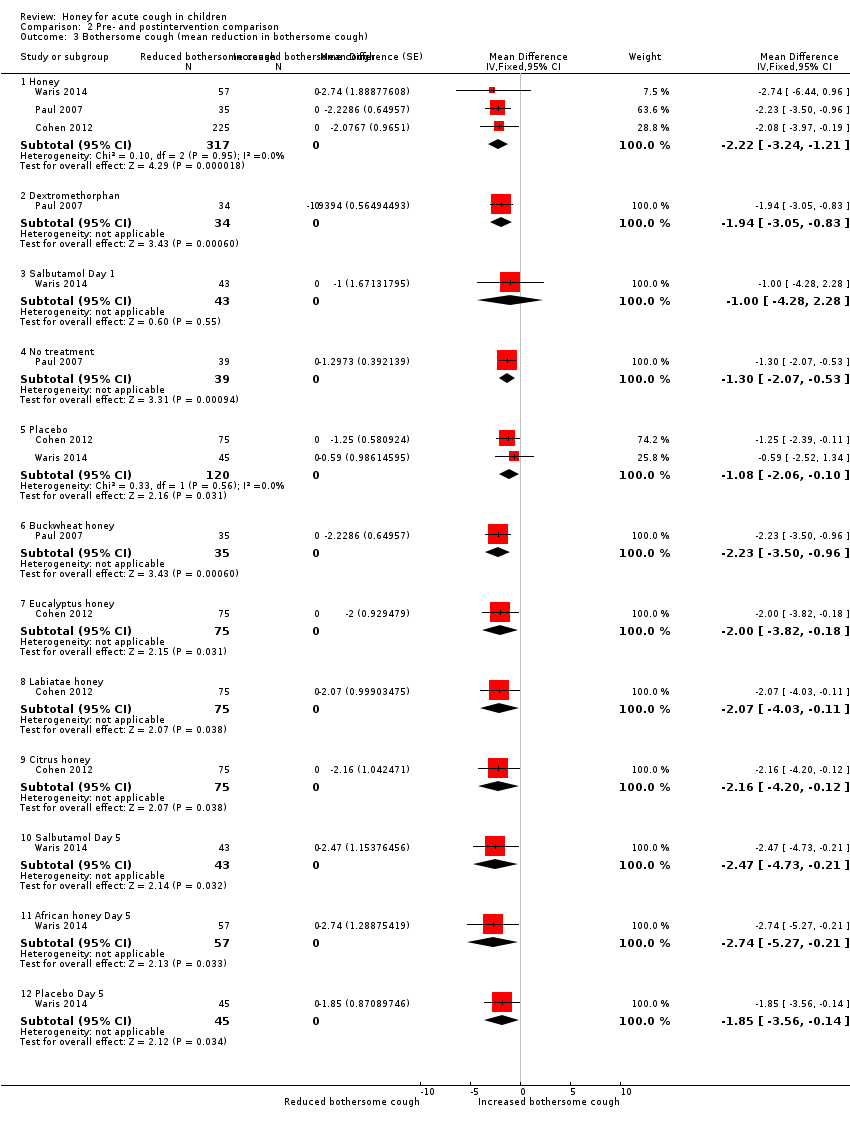
Comparison 2 Pre‐ and postintervention comparison, Outcome 3 Bothersome cough (mean reduction in bothersome cough).

Comparison 2 Pre‐ and postintervention comparison, Outcome 4 Children's sleep (mean reduction in cough impact on sleep score).
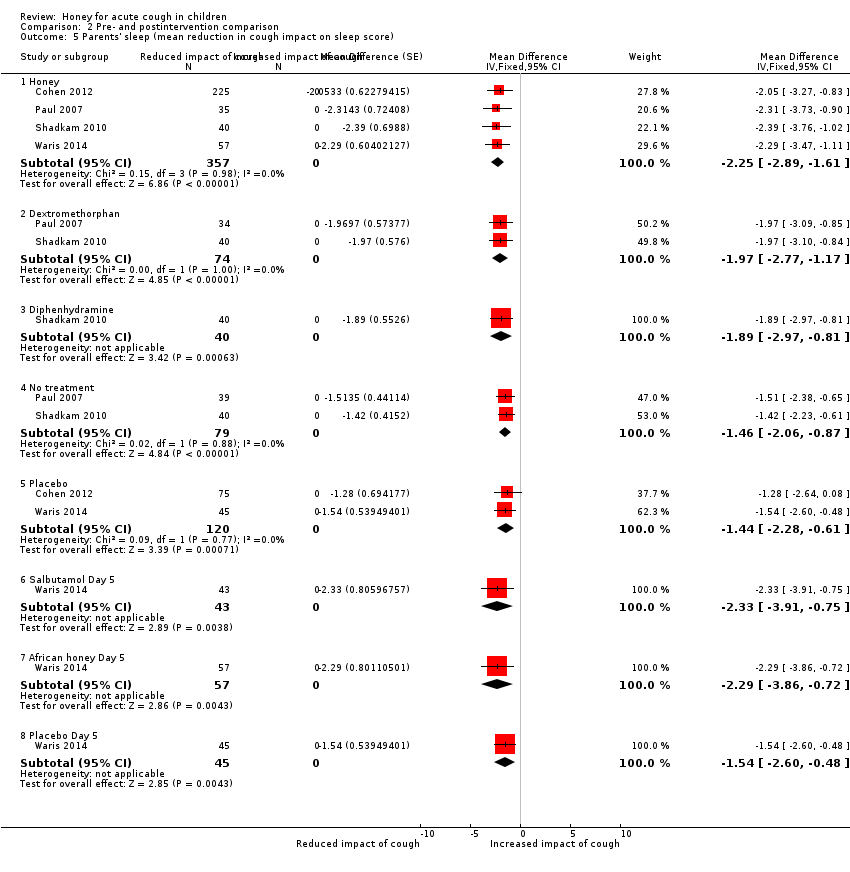
Comparison 2 Pre‐ and postintervention comparison, Outcome 5 Parents' sleep (mean reduction in cough impact on sleep score).
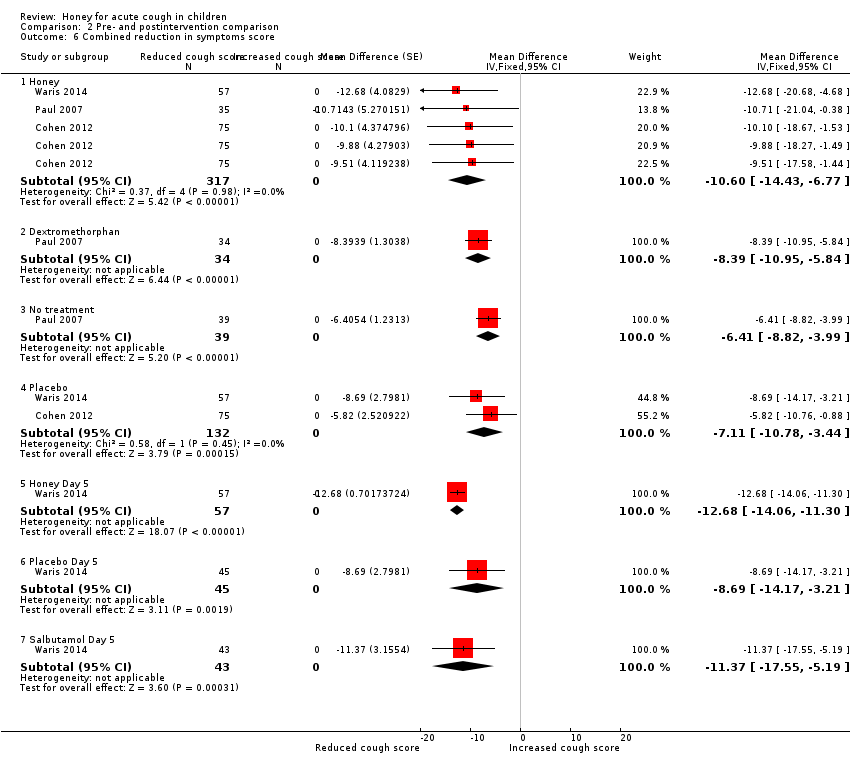
Comparison 2 Pre‐ and postintervention comparison, Outcome 6 Combined reduction in symptoms score.
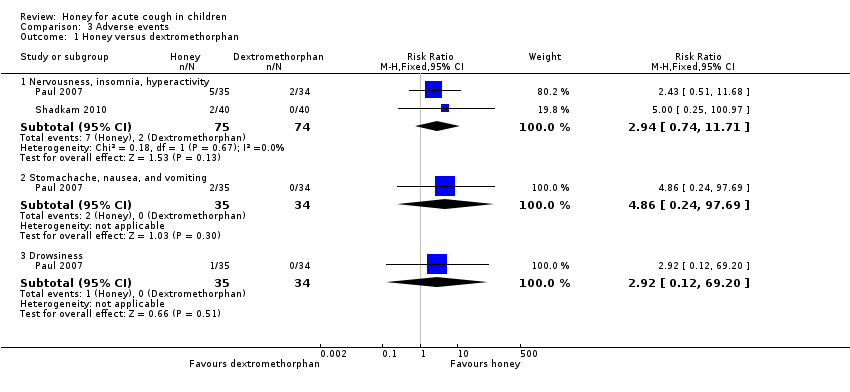
Comparison 3 Adverse events, Outcome 1 Honey versus dextromethorphan.
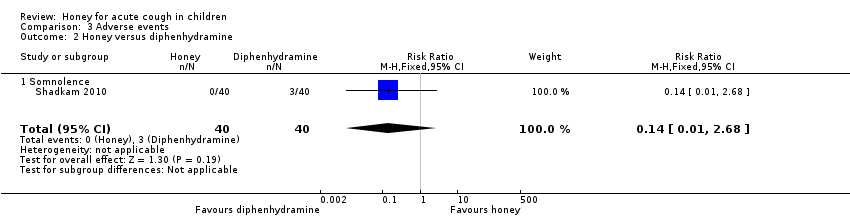
Comparison 3 Adverse events, Outcome 2 Honey versus diphenhydramine.

Comparison 3 Adverse events, Outcome 3 Honey versus placebo.

Comparison 3 Adverse events, Outcome 4 Honey versus salbutamol.

Comparison 3 Adverse events, Outcome 5 Honey versus no treatment.
| Honey compared to dextromethorphan for acute cough in children | ||||||
| Patient or population: acute cough in children | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with dextromethorphan | Risk with honey | |||||
| Duration of cough | ‐ | ‐ | ‐ | ‐ | ‐ | Not assessed |
| Frequency of cough1 | The mean frequency of cough (reduction in frequency of cough score) was ‐1.54. | MD 0.07 score lower | ‐ | 149 | ⊕⊕⊝⊝ | Follow‐up: mean 1 day |
| Severity of cough1 | The mean severity of cough (reduction in severity of cough score) was ‐1.52. | MD 0.13 score lower | ‐ | 149 | ⊕⊕⊝⊝ | Follow‐up: mean 1 day |
| Bothersome cough1 | The mean bothersome cough (reduction in bothersome nature of cough score) was ‐1.94. | MD 0.29 score higher | ‐ | 69 | ⊕⊕⊕⊝ | Follow‐up: mean 1 day |
| Cough impact on children's sleep1 | The mean cough impact on children's sleep (cough impact on children' sleep score) was ‐1.75. | MD 0.03 score higher | ‐ | 149 | ⊕⊕⊝⊝ | Follow‐up: mean 6 days |
| Cough impact on parents' sleep1 | The mean cough impact on parents' sleep (cough impact on parents' sleep score) was ‐1.97. | MD 0.16 score lower | ‐ | 149 | ⊕⊕⊝⊝ | Follow‐up: mean 1 day |
| Adverse events | Population | |||||
| Nervousness, insomnia, hyperactivity | 3 per 100 | 8 per 100 (2 to 32) | RR 2.94 | 149 | ⊕⊕⊝⊝ | Follow‐up: mean 1 day |
| Stomachache, nausea, and vomiting | 1 per 100 | 7 per 100 (0 to 100) | RR 4.86 | 69 | ⊕⊕⊝⊝ | |
| Drowsiness | 1 per 100 | 4 per 100 | RR 2.92 | 69 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Assessed on a 7‐point Likert scale from 0 to 6; lower score is better. | ||||||
| Honey compared to diphenhydramine for acute cough in children | ||||||
| Patient or population: acute cough in children | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with diphenhydramine | Risk with honey | |||||
| Cough duration | ‐ | ‐ | ‐ | ‐ | ‐ | Not assessed |
| Frequency of cough1 | The mean frequency of cough (reduction in cough frequency score) was ‐1.73. | MD 0.57 lower | ‐ | 80 | ⊕⊕⊝⊝ | Follow‐up: mean 1 day |
| Severity of cough1 | The mean severity of cough (reduction in cough severity score) was ‐1.83. | MD 0.6 lower | ‐ | 80 | ⊕⊕⊝⊝ | Follow‐up: mean 1 day |
| Cough impact on children's sleep1 | The mean cough impact on children's sleep (cough impact on children' sleep score) was ‐1.64. | MD 0.55 score lower | ‐ | 80 | ⊕⊕⊝⊝ | Follow‐up: mean 6 days |
| Cough impact on parents' sleep1 | The mean cough impact on parents' sleep (cough impact on parents' sleep score) was ‐1.89. | MD 0.48 lower | ‐ | 80 | ⊕⊕⊝⊝ | Follow‐up: mean 1 day |
| Adverse event: Somnolence | Population | 1 per 100 | RR 0.14 | 80 | ⊕⊕⊝⊝ | Follow‐up: mean 1 day |
| 8 per 100 | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Assessed on a 7‐point Likert scale from 0 to 6; lower score is better. | ||||||
| Honey compared to no treatment for acute cough in children | ||||||
| Patient or population: acute cough in children | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with no treatment | Risk with honey | |||||
| Cough duration | ‐ | ‐ | ‐ | ‐ | ‐ | Not assessed |
| Frequency of cough1 | The mean frequency of cough (reduction in cough frequency score) was ‐0.98. | MD 1.05 lower | ‐ | 154 | ⊕⊕⊕⊝ | Follow‐up: mean 1 day |
| Severity of cough1 assessed with: 7‐point Likert scale | The mean severity of cough (reduction in severity of cough score) was ‐1.13. | MD 1.03 score lower | ‐ | 154 | ⊕⊕⊕⊝ | Follow‐up: mean 1 day |
| Bothersome cough1 | The mean bothersome cough (reduction in bothersome nature of cough score) was ‐1.30. | MD 0.93 score lower | ‐ | 74 | ⊕⊕⊝⊝ | Follow‐up: mean 1 day |
| Cough impact on children's sleep1 | The mean cough impact on children's sleep (cough impact on children' sleep score) was ‐1.28. | MD 1.04 score lower | ‐ | 154 | ⊕⊕⊕⊝ | Follow‐up: mean 6 days |
| Cough impact on parents' sleep1 assessed with: 7‐point Likert scale | The mean cough impact on parents' sleep (cough impact on parents' sleep score) was ‐1.46. | MD 0.88 score lower | ‐ | 154 | ⊕⊕⊕⊝ | Follow‐up: mean 1 day |
| Adverse events | Population | |||||
| Nervousness, insomnia, hyperactivity | 1 per 100 | 6 per 100 | RR 9.40 (1.16 to 76.20) | 154 | ⊕⊕⊝⊝ | Follow‐up: mean 1 day |
| Stomachache, nausea, and vomiting | 1 per 100 | 7 per 100 | RR 5.90 (0.27 to 127.14) | 74 | ⊕⊕⊝⊝ | |
| Drowsiness | 1 per 100 | 4 per 100 | RR 3.43 (0.14 to 87.09) | 74 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Assessed on a 7‐point Likert scale from 0 to 6; lower score is better. | ||||||
| Honey compared to placebo for acute cough in children | ||||||
| Patient or population: acute cough in children | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with honey | |||||
| Day 1 | ||||||
| Frequency of cough1 | The mean frequency of cough (reduction in cough frequency score) was ‐0.99. | MD 1.62 score lower | ‐ | 402 | ⊕⊕⊕⊝ | Follow‐up: mean 1 day |
| Severity of cough1 | The mean severity of cough (reduction in severity of cough score) was ‐0.80. | MD 1.07 score lower | ‐ | 402 | ⊕⊕⊕⊝ | |
| Bothersome cough (mean improvement score)1 | The mean bothersome cough (reduction in bothersome nature of cough) was ‐1.08. | MD 1.4 score lower | ‐ | 402 | ⊕⊕⊕⊝ | |
| Cough impact on children's sleep1 | The mean cough impact on children's sleep (cough impact on children' sleep score) was ‐1.03. | MD 1.21 score lower | ‐ | 402 | ⊕⊕⊕⊝ | Follow‐up: mean 6 days |
| Cough impact on parents' sleep1 | The mean cough impact on parents' sleep (cough impact on parents' sleep score) was ‐1.44. | MD 1.29 score lower | ‐ | 402 | ⊕⊕⊕⊝ | Follow‐up: mean 1 day |
| Day 3 | ||||||
| Frequency of cough1 | The mean frequency of cough (reduction in frequency of cough score) was ‐0.9. | MD 1.13 score lower | ‐ | 102 | ⊕⊕⊕⊝ | Follow‐up: mean 6 days |
| Severity of cough1 | The mean severity of cough (reduction in severity of cough score) was ‐1.08. | MD 0.85 score lower | ‐ | 102 | ⊕⊕⊕⊝ | |
| Bothersome cough1 | The mean bothersome cough (reduction in bothersome nature of cough score) was ‐0.99. | MD 1.33 score lower | ‐ | 102 | ⊕⊕⊕⊝ | |
| Cough impact on children's sleep1 | The mean cough impact on children's sleep (cough impact on children's sleep score) was ‐0.46. | MD 0.93 score lower | ‐ | 102 | ⊕⊕⊕⊝ | |
| Cough impact on parents' sleep3 | The mean cough impact on parents' sleep (cough impact on parents' sleep score) was ‐1.04. | MD 0.88 score lower | ‐ | 102 | ⊕⊕⊕⊝ | |
| Day 5 | ||||||
| Cough duration | The mean cough duration was 5.18 days. | MD 0.72 days lower | ‐ | 102 | ⊕⊕⊕⊝ | Follow‐up: mean 6 days; assessed with: 7‐point Likert scale |
| Frequency of cough1 | The mean frequency of cough (reduction in frequency of cough score) was ‐1.95. | MD 0.48 score lower | ‐ | 102 | ⊕⊕⊕⊝ | |
| Severity of cough1 | The mean severity of cough (reduction in severity of cough score) was ‐1.96. | MD 0.43 score lower | ‐ | 102 | ⊕⊕⊕⊝ | |
| Bothersome cough1 | The mean bothersome cough (reduction in bothersome nature of cough score) was ‐1.85. | MD 0.51 score lower | ‐ | 102 | ⊕⊕⊕⊝ | |
| Cough impact on children's sleep1 | The mean cough impact on children's sleep (cough impact on children' sleep score) was ‐1.68. | MD 0.55 score lower | ‐ | 102 | ⊕⊕⊕⊝ | |
| Cough impact on parents' sleep | The mean cough impact on parents' sleep (cough impact on parents' sleep score) was ‐1.54. | MD 0.57 score lower | ‐ | 102 | ⊕⊕⊕⊝ | |
| Adverse events | Population | |||||
| Stomachache, nausea, and vomiting | 11 per 100 | 21 per 100 | RR 1.91 | 402 | ⊕⊕⊕⊝ | Follow‐up: mean 6 days |
| Diarrhoea | 13 per 100 | 12 per 100 | RR 0.92 | 102 | ⊕⊕⊝⊝ | |
| Tachycardia | 2 per 100 | 4 per 100 | RR 1.58 | 102 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Assessed on a 7‐point Likert scale from 0 to 6; lower score is better. | ||||||
| Honey compared to salbutamol for acute cough in children | ||||||
| Patient or population: acute cough in children | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with salbutamol | Risk with honey | |||||
| Day 1 | ||||||
| Frequency of cough (mean improvement score)1 | The mean frequency of cough (reduction in frequency of cough score) was ‐0.52. | MD 0.26 lower | ‐ | 100 | ⊕⊕⊕⊝ | Follow‐up: mean 6 days |
| Severity of cough (mean improvement score)1 | The mean severity of cough (reduction in severity of cough score) was ‐0.74. | MD 0.1 lower | ‐ | 100 | ⊕⊕⊕⊝ | |
| Bothersome cough (mean improvement score)1 | The mean bothersome cough (reduction in bothersome nature of cough score) was ‐1.00. | MD 0.21 lower | ‐ | 100 | ⊕⊕⊕⊝ | |
| Cough impact on children's sleep1 | The mean cough impact on children's sleep (cough impact on children' sleep score) was ‐1.24. | MD 0.09 higher | ‐ | 100 | ⊕⊕⊕⊝ | |
| Cough impact on parents' sleep1 | The mean cough impact on parents' sleep (cough impact on parents' sleep score) was ‐1.22. | MD 0.05 higher | ‐ | 100 | ⊕⊕⊕⊝ | |
| Day 3 | ||||||
| Frequency of cough1 | The mean frequency of cough (reduction in frequency of cough score) was ‐1.34. | MD 0.69 lower | ‐ | 100 | ⊕⊕⊕⊝ | Follow‐up: mean 4 days |
| Severity of cough1 | The mean severity of cough (reduction in severity of cough score) was ‐1.59. | MD 0.34 lower | ‐ | 100 | ⊕⊕⊕⊝ | |
| Bothersome cough1 | The mean bothersome cough (reduction in bothersome nature of cough score) was ‐2.08. | MD 0.24 lower | ‐ | 100 | ⊕⊕⊕⊝ | |
| Cough impact on children's sleep1 | The mean cough impact on children's sleep (cough impact on children' sleep score) was ‐2.25. | MD 0.31 higher | ‐ | 100 | ⊕⊕⊕⊝ | Follow‐up: mean 6 days |
| Cough impact on parents' sleep1 | The mean cough impact on parents' sleep (cough impact on parents' sleep score) was ‐2.13. | MD 0.21 higher | ‐ | 100 | ⊕⊕⊕⊝ | Follow‐up: mean 4 days |
| Day 5 | ||||||
| Cough duration | The mean cough duration was 5 days. | MD 0.54 days lower | ‐ | 100 | ⊕⊕⊕⊝ | Follow‐up: mean 6 days |
| Frequency of cough (mean improvement score)1 | The mean frequency of cough (reduction in frequency of cough score) was ‐2.19. | MD 0.54 lower | ‐ | 100 | ⊕⊕⊕⊝ | |
| Severity of cough (mean improvement score)1 | The mean severity of cough (reduction in severity of cough score) was ‐2.08. | MD 0.41 lower | ‐ | 100 | ⊕⊕⊕⊝ | |
| Bothersome cough (mean improvement score)1 | The mean bothersome cough (reduction in bothersome nature of cough score) was ‐2.47. | MD 0.27 lower | ‐ | 100 | ⊕⊕⊕⊝ | |
| Cough impact on children's sleep1 | The mean cough impact on children's sleep (cough impact on children's sleep score) was ‐2.47. | MD 0.15 higher | ‐ | 100 | ⊕⊕⊕⊝ | |
| Cough impact on parents' sleep1 | The mean cough impact on parents' sleep (cough impact on parents' sleep score) was ‐2.33. | MD 0.04 higher | ‐ | 100 | ⊕⊕⊕⊝ | |
| Adverse events | Population | |||||
| Stomachache, nausea, and vomiting | 30 per 100 | 53 per 100 | RR 1.74 | 100 | ⊕⊕⊕⊝ | Follow‐up: mean 6 days |
| Rash | 9 per 100 | 2 per 100 | RR 0.19 | 100 | ⊕⊕⊕⊝ | |
| Tachycardia | 2 per 100 | 4 per 100 | RR 1.51 (0.14 to 16.10) | 100 | ⊕⊕⊝⊝ | |
| Diarrhoea | 21 per 100 | 12 per 100 | RR 0.59 | 100 | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Assessed on a 7‐point Likert scale from 0 to 6; lower score is better. | ||||||
| Study ID | Cough | Honey (N = 29) | Bromelin (N = 31) | P value | Certainty of the evidence |
| Frequency of cough1 | |||||
| Before, median (P25 to P75) After, median (P25 to P75) Mean ± SD | 3 (2 to 4) 1 (1 to 1) 1.76 ± 0.87 | 3 (2 to 3) 1 (1 to 1) 1.71 ± 0.78 | 0.832 0.943 | ⊕⊕⊕⊝ | |
| Severity of cough1 | |||||
| Mean ± SD assessed with: unvalidated 5‐point cough scale from 0 to 4 | ‐0.86 ± 0.45 | ‐0.97 ± 0.62 | 0.322 0.223 | ⊕⊕⊕⊝ | |
| Honey (N = 63) | Diphenhydramine (N = 63) | ||||
| Proportion of children with reduction in frequency and severity of daytime cough5 | 84.1% (N = 53) | 58.7% (N = 37) | "< 0.02" | ⊕⊕⊕⊝ | |
| Proportion of children with reduction in frequency and severity of nighttime cough5 | 79.4% (N = 50) | 58.7% (N = 37) | "< 0.02" | ⊕⊕⊕⊝ | |
| SD: standard deviation 1Assessed on an unvalidated 5‐point cough scale from 0 to 4; lower score is better. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Honey versus dextromethorphan Show forest plot | 2 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 Frequency of cough (mean reduction in cough frequency) | 2 | 149 | Mean Difference (Random, 95% CI) | ‐0.07 [‐1.07, 0.94] |
| 1.2 Severity of cough (mean reduction in severity of cough) | 2 | 149 | Mean Difference (Random, 95% CI) | ‐0.13 [‐1.25, 0.99] |
| 1.3 Bothersome cough (mean reduction in bothersome cough) | 1 | 69 | Mean Difference (Random, 95% CI) | 0.29 [‐0.56, 1.14] |
| 1.4 Children's sleep (mean reduction in cough impact on sleep score) | 2 | 149 | Mean Difference (Random, 95% CI) | 0.03 [‐1.12, 1.19] |
| 1.5 Parents' sleep (mean reduction in cough impact on sleep score) | 2 | 149 | Mean Difference (Random, 95% CI) | ‐0.16 [‐0.84, 0.53] |
| 1.6 Combined cough score (reduction in combined cough score) | 1 | 69 | Mean Difference (Random, 95% CI) | 2.32 [‐1.24, 5.88] |
| 2 Honey versus diphenhydramine Show forest plot | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 2.1 Frequency of cough (mean reduction in cough frequency) | 1 | 80 | Mean Difference (Random, 95% CI) | ‐0.57 [‐0.90, ‐0.24] |
| 2.2 Severity of cough (mean reduction in severity of cough) | 1 | 80 | Mean Difference (Random, 95% CI) | ‐0.6 [‐0.94, ‐0.26] |
| 2.3 Children's sleep (mean reduction in cough impact on sleep score) | 1 | 80 | Mean Difference (Random, 95% CI) | ‐0.55 [‐0.87, ‐0.23] |
| 2.4 Parents' sleep (mean reduction in cough impact on sleep score) | 1 | 80 | Mean Difference (Random, 95% CI) | ‐0.48 [‐0.76, ‐0.20] |
| 3 Honey versus no treatment Show forest plot | 2 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 Frequency of cough (mean reduction in frequency of cough) | 2 | 154 | Mean Difference (Random, 95% CI) | ‐1.05 [‐1.48, ‐0.62] |
| 3.2 Severity of cough (mean reduction in severity of cough) | 2 | 154 | Mean Difference (Random, 95% CI) | ‐1.03 [‐1.59, ‐0.47] |
| 3.3 Bothersome cough (mean reduction in bothersome cough) | 1 | 74 | Mean Difference (Random, 95% CI) | ‐0.93 [‐1.98, 0.12] |
| 3.4 Children's sleep (mean reduction in cough impact on sleep score) | 2 | 154 | Mean Difference (Random, 95% CI) | ‐1.04 [‐1.57, ‐0.51] |
| 3.5 Parents' sleep (mean reduction in cough impact on sleep score) | 2 | 154 | Mean Difference (Random, 95% CI) | ‐0.88 [‐1.23, ‐0.52] |
| 3.6 Combined reduction in symptoms score | 1 | 74 | Mean Difference (Random, 95% CI) | ‐4.31 [‐6.77, ‐1.85] |
| 4 Honey versus placebo (Day 1) Show forest plot | 2 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 4.1 Frequency of cough (mean reduction in frequency of cough) | 2 | 402 | Mean Difference (Random, 95% CI) | ‐1.62 [‐3.02, ‐0.22] |
| 4.2 Severity of cough (mean reduction in severity of cough) | 2 | 402 | Mean Difference (Random, 95% CI) | ‐1.07 [‐2.43, 0.30] |
| 4.3 Bothersome cough (mean reduction in bothersome cough) | 2 | 402 | Mean Difference (Random, 95% CI) | ‐1.40 [‐2.82, 0.03] |
| 4.4 Children's sleep (mean reduction in cough impact on sleep score) | 2 | 402 | Mean Difference (Random, 95% CI) | ‐1.21 [‐2.61, 0.19] |
| 4.5 Parents' sleep (mean reduction in cough impact on sleep score) | 2 | 402 | Mean Difference (Random, 95% CI) | ‐1.29 [‐2.71, 0.13] |
| 5 Honey versus placebo (Day 2) Show forest plot | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 5.1 Frequency of cough (mean reduction in frequency of cough) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐0.71 [‐1.22, ‐0.20] |
| 5.2 Severity of cough (mean reduction in severity of cough) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐0.63 [‐1.36, 0.10] |
| 5.3 Bothersome cough (mean reduction in bothersome cough) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐1.11 [‐1.79, ‐0.43] |
| 5.4 Children's sleep (mean reduction in cough impact on sleep score) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐0.69 [‐1.43, 0.05] |
| 5.5 Parents' sleep (mean reduction in cough impact on sleep score) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐0.87 [‐1.59, ‐0.15] |
| 6 Honey versus placebo (Day 3) Show forest plot | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 6.1 Frequency of cough (mean reduction in frequency of cough) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐1.13 [‐1.71, ‐0.55] |
| 6.2 Severity of cough (mean reduction in severity of cough) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐0.85 [‐1.41, ‐0.29] |
| 6.3 Bothersome cough (mean reduction in bothersome cough) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐1.33 [‐1.87, ‐0.79] |
| 6.4 Children's sleep (mean reduction in cough impact on sleep score) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐0.93 [‐1.42, ‐0.44] |
| 6.5 Parents' sleep (mean reduction in cough impact on sleep score) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐0.88 [‐1.38, ‐0.38] |
| 7 Honey versus placebo (Day 4) Show forest plot | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 7.1 Frequency of cough (mean reduction in frequency of cough) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐1.16 [‐1.83, ‐0.49] |
| 7.2 Severity of cough (mean reduction in severity of cough) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐0.88 [‐1.59, ‐0.17] |
| 7.3 Bothersome cough (mean reduction in bothersome cough) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐0.90 [‐1.76, ‐0.04] |
| 7.4 Children's sleep (mean reduction in cough impact on sleep score) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐0.7 [‐1.25, ‐0.15] |
| 7.5 Parents' sleep (mean reduction in cough impact on sleep score) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐0.90 [‐1.51, ‐0.29] |
| 8 Honey versus placebo (Day 5) Show forest plot | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 8.1 Cough duration (mean number of days) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐0.72 [‐1.31, ‐0.13] |
| 8.2 Frequency of cough (mean reduction in frequency of cough) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐0.48 [‐2.95, 1.99] |
| 8.3 Severity of cough (mean reduction in severity of cough) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐0.43 [‐2.21, 1.35] |
| 8.4 Bothersome cough (mean reduction in bothersome cough) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐0.51 [‐3.01, 1.99] |
| 8.5 Children's sleep (mean reduction in cough impact on sleep score) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐0.55 [‐1.79, 0.69] |
| 8.6 Parents' sleep (mean reduction in cough impact on sleep score) | 1 | 102 | Mean Difference (Random, 95% CI) | ‐0.57 [‐1.59, 0.45] |
| 9 Honey versus salbutamol (Day 1) Show forest plot | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 9.1 Frequency of cough (mean reduction in frequency of cough) | 1 | 100 | Mean Difference (Random, 95% CI) | ‐0.26 [‐3.14, 2.62] |
| 9.2 Severity of cough (mean reduction in severity of cough) | 1 | 100 | Mean Difference (Random, 95% CI) | ‐0.1 [‐0.39, 0.19] |
| 9.3 Bothersome cough (mean reduction in bothersome cough) | 1 | 100 | Mean Difference (Random, 95% CI) | ‐0.21 [‐0.90, 0.48] |
| 9.4 Children's sleep (mean reduction in cough impact on sleep score) | 1 | 100 | Mean Difference (Random, 95% CI) | 0.09 [‐0.05, 0.23] |
| 9.5 Parents' sleep (mean reduction in cough impact on sleep score) | 1 | 100 | Mean Difference (Random, 95% CI) | 0.05 [‐0.03, 0.13] |
| 10 Honey versus salbutamol (Day 2) Show forest plot | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 10.1 Frequency of cough (mean reduction in frequency of cough) | 1 | 100 | Mean Difference (Random, 95% CI) | ‐0.67 [‐1.35, 0.01] |
| 10.2 Severity of cough (mean reduction in severity of cough) | 1 | 100 | Mean Difference (Random, 95% CI) | ‐0.42 [‐1.16, 0.32] |
| 10.3 Bothersome cough (mean reduction in bothersome cough) | 1 | 100 | Mean Difference (Random, 95% CI) | ‐0.27 [‐0.52, ‐0.02] |
| 10.4 Children's sleep (mean reduction in cough impact on sleep score) | 1 | 100 | Mean Difference (Random, 95% CI) | 0.17 [‐0.04, 0.38] |
| 10.5 Parents' sleep (mean reduction in cough impact on sleep score) | 1 | 100 | Mean Difference (Random, 95% CI) | 0.03 [‐0.00, 0.06] |
| 11 Honey versus salbutamol (Day 3) Show forest plot | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 11.1 Frequency of cough (mean reduction in frequency of cough) | 1 | 100 | Mean Difference (Random, 95% CI) | ‐0.69 [‐1.13, ‐0.25] |
| 11.2 Severity of cough (mean reduction in severity of cough) | 1 | 100 | Mean Difference (Random, 95% CI) | ‐0.34 [‐0.64, ‐0.04] |
| 11.3 Bothersome cough (mean reduction in bothersome cough) | 1 | 100 | Mean Difference (Random, 95% CI) | ‐0.24 [‐0.38, ‐0.10] |
| 11.4 Children's sleep (mean reduction in cough impact on sleep score) | 1 | 100 | Mean Difference (Random, 95% CI) | 0.31 [0.13, 0.49] |
| 11.5 Parents' sleep (mean reduction in cough impact on sleep score) | 1 | 100 | Mean Difference (Random, 95% CI) | 0.21 [0.06, 0.36] |
| 12 Honey versus salbutamol (Day 4) Show forest plot | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 12.1 Frequency of cough (mean reduction in frequency of cough) | 1 | 100 | Mean Difference (Random, 95% CI) | ‐0.61 [‐0.96, ‐0.26] |
| 12.2 Severity of cough (mean reduction in severity of cough) | 1 | 100 | Mean Difference (Random, 95% CI) | ‐0.43 [‐0.78, ‐0.08] |
| 12.3 Bothersome cough (mean reduction in bothersome cough) | 1 | 100 | Mean Difference (Random, 95% CI) | ‐0.3 [‐0.59, ‐0.01] |
| 12.4 Children's sleep (mean reduction in cough impact on sleep score) | 1 | 100 | Mean Difference (Random, 95% CI) | 0.22 [0.05, 0.39] |
| 12.5 Parents' sleep (mean reduction in cough impact on sleep score) | 1 | 100 | Mean Difference (Random, 95% CI) | 0.15 [0.04, 0.26] |
| 13 Honey versus salbutamol (Day 5) Show forest plot | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 13.1 Cough duration (mean number of days) | 1 | 100 | Mean Difference (Random, 95% CI) | ‐0.54 [‐0.98, ‐0.10] |
| 13.2 Frequency of cough (mean reduction in frequency of cough) | 1 | 100 | Mean Difference (Random, 95% CI) | ‐0.54 [‐1.03, ‐0.05] |
| 13.3 Severity of cough (mean reduction in severity of cough) | 1 | 100 | Mean Difference (Random, 95% CI) | ‐0.41 [‐0.78, ‐0.04] |
| 13.4 Bothersome cough (mean reduction in bothersome cough) | 1 | 100 | Mean Difference (Random, 95% CI) | ‐0.27 [‐0.48, ‐0.06] |
| 13.5 Children's sleep (mean reduction in cough impact on sleep score) | 1 | 100 | Mean Difference (Random, 95% CI) | 0.15 [0.04, 0.26] |
| 13.6 Parents' sleep (mean reduction in cough impact on sleep score) | 1 | 100 | Mean Difference (Random, 95% CI) | 0.04 [0.01, 0.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cough frequency (mean reduction in frequency) Show forest plot | 4 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 Honey | 4 | 357 | Mean Difference (Fixed, 95% CI) | ‐1.71 [‐2.28, ‐1.13] |
| 1.2 Dextromethorphan | 2 | 74 | Mean Difference (Fixed, 95% CI) | ‐1.54 [‐2.30, ‐0.78] |
| 1.3 Diphenhydramine | 1 | 40 | Mean Difference (Fixed, 95% CI) | ‐1.73 [‐2.72, ‐0.74] |
| 1.4 Placebo | 2 | 120 | Mean Difference (Fixed, 95% CI) | ‐0.99 [‐1.79, ‐0.18] |
| 1.5 Salbutamol Day 1 | 1 | 43 | Mean Difference (Fixed, 95% CI) | ‐0.52 [‐6.28, 5.24] |
| 1.6 No treatment | 2 | 79 | Mean Difference (Fixed, 95% CI) | ‐0.98 [‐1.38, ‐0.59] |
| 1.7 Buckwheat honey | 1 | 35 | Mean Difference (Fixed, 95% CI) | ‐1.89 [‐2.96, ‐0.81] |
| 1.8 Natural honey from Kafi‐Abad (Iran) | 1 | 40 | Mean Difference (Fixed, 95% CI) | ‐2.16 [‐3.40, ‐0.92] |
| 1.9 Eucalyptus honey | 1 | 75 | Mean Difference (Fixed, 95% CI) | ‐1.77 [‐3.22, ‐0.32] |
| 1.10 Labiatae honey | 1 | 75 | Mean Difference (Fixed, 95% CI) | ‐1.82 [‐3.30, ‐0.34] |
| 1.11 Citrus honey | 1 | 75 | Mean Difference (Fixed, 95% CI) | ‐1.95 [‐3.55, ‐0.35] |
| 1.12 Salbutamol Day 5 | 1 | 43 | Mean Difference (Fixed, 95% CI) | ‐2.19 [‐3.55, ‐0.83] |
| 1.13 African honey Day 5 | 1 | 57 | Mean Difference (Fixed, 95% CI) | ‐2.65 [‐4.32, ‐0.98] |
| 1.14 Placebo Day 5 | 1 | 45 | Mean Difference (Fixed, 95% CI) | ‐1.95 [‐4.42, 0.52] |
| 2 Severity of cough (mean reduction in severity) Show forest plot | 4 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 Honey | 4 | 357 | Mean Difference (Fixed, 95% CI) | ‐1.65 [‐2.39, ‐0.91] |
| 2.2 Dextromethorphan | 2 | 74 | Mean Difference (Fixed, 95% CI) | ‐1.52 [‐2.24, ‐0.80] |
| 2.3 Diphenhydramine | 1 | 40 | Mean Difference (Fixed, 95% CI) | ‐1.83 [‐2.88, ‐0.78] |
| 2.4 Salbutamol Day 1 | 1 | 43 | Mean Difference (Fixed, 95% CI) | ‐0.74 [‐2.87, 1.39] |
| 2.5 No treatment | 2 | 79 | Mean Difference (Fixed, 95% CI) | ‐1.13 [‐1.54, ‐0.72] |
| 2.6 Placebo | 2 | 120 | Mean Difference (Fixed, 95% CI) | ‐0.80 [‐1.47, ‐0.13] |
| 2.7 Buckwheat honey | 1 | 35 | Mean Difference (Fixed, 95% CI) | ‐1.80 [‐2.88, ‐0.72] |
| 2.8 Natural honey from Kafi‐Abad (Iran) | 1 | 40 | Mean Difference (Fixed, 95% CI) | ‐2.33 [‐3.67, ‐0.99] |
| 2.9 Eucalyptus honey | 1 | 75 | Mean Difference (Fixed, 95% CI) | ‐1.78 [‐2.82, ‐0.74] |
| 2.10 Labiatae honey | 1 | 75 | Mean Difference (Fixed, 95% CI) | ‐1.94 [‐3.07, ‐0.81] |
| 2.11 Citrus honey | 1 | 75 | Mean Difference (Fixed, 95% CI) | ‐1.77 [‐2.74, ‐0.80] |
| 2.12 Salbutamol Day 5 | 1 | 43 | Mean Difference (Fixed, 95% CI) | ‐2.08 [‐4.21, 0.05] |
| 2.13 African honey Day 5 | 1 | 57 | Mean Difference (Fixed, 95% CI) | ‐2.62 [‐5.04, ‐0.20] |
| 2.14 Placebo Day 5 | 1 | 45 | Mean Difference (Fixed, 95% CI) | ‐1.96 [‐3.74, ‐0.18] |
| 3 Bothersome cough (mean reduction in bothersome cough) Show forest plot | 3 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 Honey | 3 | 317 | Mean Difference (Fixed, 95% CI) | ‐2.22 [‐3.24, ‐1.21] |
| 3.2 Dextromethorphan | 1 | 34 | Mean Difference (Fixed, 95% CI) | ‐1.94 [‐3.05, ‐0.83] |
| 3.3 Salbutamol Day 1 | 1 | 43 | Mean Difference (Fixed, 95% CI) | ‐1.0 [‐4.28, 2.28] |
| 3.4 No treatment | 1 | 39 | Mean Difference (Fixed, 95% CI) | ‐1.30 [‐2.07, ‐0.53] |
| 3.5 Placebo | 2 | 120 | Mean Difference (Fixed, 95% CI) | ‐1.08 [‐2.06, ‐0.10] |
| 3.6 Buckwheat honey | 1 | 35 | Mean Difference (Fixed, 95% CI) | ‐2.23 [‐3.50, ‐0.96] |
| 3.7 Eucalyptus honey | 1 | 75 | Mean Difference (Fixed, 95% CI) | ‐2.0 [‐3.82, ‐0.18] |
| 3.8 Labiatae honey | 1 | 75 | Mean Difference (Fixed, 95% CI) | ‐2.07 [‐4.03, ‐0.11] |
| 3.9 Citrus honey | 1 | 75 | Mean Difference (Fixed, 95% CI) | ‐2.16 [‐4.20, ‐0.12] |
| 3.10 Salbutamol Day 5 | 1 | 43 | Mean Difference (Fixed, 95% CI) | ‐2.47 [‐4.73, ‐0.21] |
| 3.11 African honey Day 5 | 1 | 57 | Mean Difference (Fixed, 95% CI) | ‐2.74 [‐5.27, ‐0.21] |
| 3.12 Placebo Day 5 | 1 | 45 | Mean Difference (Fixed, 95% CI) | ‐1.85 [‐3.56, ‐0.14] |
| 4 Children's sleep (mean reduction in cough impact on sleep score) Show forest plot | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 4.1 Honey | 4 | 357 | Mean Difference (Random, 95% CI) | ‐2.23 [‐2.87, ‐1.59] |
| 4.2 Dextromethorphan | 2 | 74 | Mean Difference (Random, 95% CI) | ‐1.75 [‐2.46, ‐1.04] |
| 4.3 Diphenhydramine | 1 | 40 | Mean Difference (Random, 95% CI) | ‐1.64 [‐2.58, ‐0.70] |
| 4.4 No treatment | 2 | 79 | Mean Difference (Random, 95% CI) | ‐1.28 [‐1.81, ‐0.76] |
| 4.5 Placebo | 2 | 120 | Mean Difference (Random, 95% CI) | ‐1.03 [‐2.05, 0.00] |
| 4.6 Salbutamol Day 5 | 1 | 43 | Mean Difference (Random, 95% CI) | ‐2.47 [‐3.84, ‐1.10] |
| 4.7 African honey Day 5 | 1 | 57 | Mean Difference (Random, 95% CI) | ‐2.32 [‐3.63, ‐1.01] |
| 4.8 Placebo Day 5 | 1 | 45 | Mean Difference (Random, 95% CI) | ‐1.68 [‐2.63, ‐0.73] |
| 5 Parents' sleep (mean reduction in cough impact on sleep score) Show forest plot | 4 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 5.1 Honey | 4 | 357 | Mean Difference (Fixed, 95% CI) | ‐2.25 [‐2.89, ‐1.61] |
| 5.2 Dextromethorphan | 2 | 74 | Mean Difference (Fixed, 95% CI) | ‐1.97 [‐2.77, ‐1.17] |
| 5.3 Diphenhydramine | 1 | 40 | Mean Difference (Fixed, 95% CI) | ‐1.89 [‐2.97, ‐0.81] |
| 5.4 No treatment | 2 | 79 | Mean Difference (Fixed, 95% CI) | ‐1.46 [‐2.06, ‐0.87] |
| 5.5 Placebo | 2 | 120 | Mean Difference (Fixed, 95% CI) | ‐1.44 [‐2.28, ‐0.61] |
| 5.6 Salbutamol Day 5 | 1 | 43 | Mean Difference (Fixed, 95% CI) | ‐2.33 [‐3.91, ‐0.75] |
| 5.7 African honey Day 5 | 1 | 57 | Mean Difference (Fixed, 95% CI) | ‐2.29 [‐3.86, ‐0.72] |
| 5.8 Placebo Day 5 | 1 | 45 | Mean Difference (Fixed, 95% CI) | ‐1.54 [‐2.60, ‐0.48] |
| 6 Combined reduction in symptoms score Show forest plot | 3 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 6.1 Honey | 3 | 317 | Mean Difference (Fixed, 95% CI) | ‐10.60 [‐14.43, ‐6.77] |
| 6.2 Dextromethorphan | 1 | 34 | Mean Difference (Fixed, 95% CI) | ‐8.39 [‐10.95, ‐5.84] |
| 6.3 No treatment | 1 | 39 | Mean Difference (Fixed, 95% CI) | ‐6.41 [‐8.82, ‐3.99] |
| 6.4 Placebo | 2 | 132 | Mean Difference (Fixed, 95% CI) | ‐7.11 [‐10.78, ‐3.44] |
| 6.5 Honey Day 5 | 1 | 57 | Mean Difference (Fixed, 95% CI) | ‐12.68 [‐14.06, ‐11.30] |
| 6.6 Placebo Day 5 | 1 | 45 | Mean Difference (Fixed, 95% CI) | ‐8.69 [‐14.17, ‐3.21] |
| 6.7 Salbutamol Day 5 | 1 | 43 | Mean Difference (Fixed, 95% CI) | ‐11.37 [‐17.55, ‐5.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Honey versus dextromethorphan Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Nervousness, insomnia, hyperactivity | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.94 [0.74, 11.71] |
| 1.2 Stomachache, nausea, and vomiting | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.86 [0.24, 97.69] |
| 1.3 Drowsiness | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.12, 69.20] |
| 2 Honey versus diphenhydramine Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.68] |
| 2.1 Somnolence | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.68] |
| 3 Honey versus placebo Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Stomachache, nausea, and vomiting | 2 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.12, 3.24] |
| 3.2 Diarrhoea | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.33, 2.55] |
| 3.3 Tachycardia | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.15, 16.86] |
| 4 Honey versus salbutamol Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Stomachache, nausea, and vomiting | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [1.04, 2.92] |
| 4.2 Rash | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.02, 1.63] |
| 4.3 Tachycardia | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.14, 16.10] |
| 4.4 Diarrhoea | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.24, 1.45] |
| 5 Honey versus no treatment Show forest plot | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.99 [1.55, 31.58] |
| 5.1 Nervousness, insomnia, hyperactivity | 2 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.40 [1.16, 76.20] |
| 5.2 Stomachache, nausea, and vomiting | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.90 [0.27, 127.14] |
| 5.3 Drowsiness | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.43 [0.14, 87.09] |

