Mohs micrographic surgery versus surgical excision for periocular basal cell carcinoma
Abstract
Background
Basal cell carcinoma (BCC) is the commonest skin cancer in the white population. It is traditionally treated by surgical excision (SE) or by Mohs micrographic surgery (MMS).
Objectives
The objective of this review was to compare the effectiveness, cost, complications and acceptability of periocular BCCs when operated by MMS or SE.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2014, Issue 1), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to February 2014), EMBASE (January 1980 to February 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 25 February 2014.
Selection criteria
We planned to include only randomised controlled trials (RCTs) comparing SE with MMS for treatment of periocular BCC.
Data collection and analysis
We did not find any studies that met the inclusion criteria for this review.
Main results
We did not find any studies that met the inclusion criteria for this review and hence none were included for analysis. Results of non‐randomised studies describing the individual techniques are reported.
Authors' conclusions
No reliable conclusions could be reached regarding which method of treatment (SE or MMS) resulted in a lower recurrence or complication rate for periocular BCC. No studies were found comparing the cost of either method directly.
High quality RCTs are therefore needed to improve the evidence base for the management of this condition.
Plain language summary
Mohs micrographic surgery versus surgical excision for periocular basal cell carcinoma
Review question
To compare the effectiveness, cost, complications and acceptability of two different surgical techniques for treating basal cell carcinoma (BCC) around the eyes.
Background
BCC is the commonest skin cancer and the most common cancer in people of white origin. It is usually seen on the sun exposed parts of the body like the face, neck, head and ears. Untreated lesions can slowly eat away the surrounding skin and hence they are also called "rodent ulcers".
The commonest treatment for BCC is surgery. This is traditionally achieved by surgical excision (SE) which involves cutting away the BCC, along with a margin of normal appearing skin around it to ensure complete removal of the cancer and to reduce the risk of recurrence.
Another type of surgery is Mohs micrographic surgery (MMS). This involves the removal of the skin tumour after colour coding the edges. This is then examined under the microscope to see if all the BCC has been removed. If any residual BCC is left at any particular edge further skin is removed from only that localised area by using the colour coding, and examined under the microscope. This process is continued until all the BCC is removed. This ensures complete tumour removal and spares normal tissue in the other directions.
MMS is considered the better alternative for treatment of certain types of BCC arising in the eyelids because it has the highest chance of curing the disease and minimises the size of the defect that needs to be repaired.
Unfortunately, this treatment is not available everywhere and not always employed because of practical limitations in the service delivery.
Surgical excision is thought to be a cheaper option as it does not require the special training, multiple procedures, and money involved in setting up and running a MMS service. However, longer duration of follow up, greater surgical morbidity and the cost of dealing with recurrences may over time significantly increase the cost of SE.
Study characteristics
We searched for studies where people with BCC had been randomly selected to be treated by one or the other method. The aim was to establish which treatment method is associated with lower recurrence rate. We also looked at the cost difference, complications and acceptability of the two procedures.
Key results
No studies were found that met the inclusion criteria.
Authors' conclusions
Background
Description of the condition
Basal cell carcinoma (BCC) is a skin cancer seen usually on the sun exposed parts of the body like the face, neck, head and ears. They are painless lesions which present commonly as scabs that bleed occasionally and do not heal completely. Untreated lesions can slowly eat away at the surrounding skin and hence they are also called "rodent ulcers". It is the commonest skin cancer and the most common cancer in people of white origin (Holme 2000). Though distant metastasis with mortality has been reported (Robinson 2003) this is rare and local spread is the norm.
Incidence of non‐melanoma skin cancers (including BCC and squamous cell carcinoma) in Wales has been reported as 265.4 per 100,000 population per annum (Holme 2000), while in Australia the age‐standardised incidence rate of BCC alone has been estimated to be as high as 884 per 100,000 population in the year 2002 (Staples 2006).
Description of the intervention
The treatment modalities for BCC include medical, radiotherapy, cryotherapy and surgical methods. Surgery is preferred to radiotherapy and cryotherapy for treatment of these lesions as surgery has been shown to have a lower failure rate and gives better cosmetic results (Avril 1997). Medical methods or treatment are generally considered experimental.
The most commonly employed surgical methods are:
1. Surgical excision (SE) which involves removing the visible parts of the tumour along with a margin of normal looking tissue around the tumour to remove all clinically invisible tumour extension.
2. Mohs micrographic surgery (MMS). Here the clinically apparent tumour is removed by curettage or excision. A thin layer of surrounding tissue is next removed after colour coding, thus dividing the specimen into different zones. The specimen is then examined under the microscope. The colour coding of the sides helps the surgeon to take additional sections from the defined zones where the tumour persists, thereby sparing normal tissue. This normal tissue (tumour free zones) would have been sacrificed in a simple surgical excision as described previously.
The standard method employed in looking at a histological specimen after it has been surgically excised, involves slicing the specimen like a loaf of bread. This looks at less than 2% of the specimen margin. In MMS, the whole face of the specimen is looked at. This allows 100% of the tumour margin to be examined.
The disadvantage of MMS is that it is time‐consuming and more than one procedure may be required to ensure complete tumour removal thereby increasing the cost of the procedure. Also the reconstruction is usually a separate procedure unlike SE when both removal and reconstruction are done as part of the same procedure.
How the intervention might work
The goals of SE are to remove all the tumour cells with the minimal excision of healthy tissue. Macroscopic (or outwardly visible) and microscopic tumour margins do not correlate directly. Studies have defined the chance of curing a BCC with various surgical margins; the smaller the surgical margin the greater the risk of not clearing the primary tumour. Bigger margins obviously leave behind a greater defect, which is more time consuming to repair and have a greater chance of being cosmetically or functionally inferior.
There are several clinical types of BCC. Macroscopic assessment of margins is usually sufficient for small nodular tumours. Pathology control of margins (frozen section or MMS) is required for sclerosing or recurrent lesions as the margins of the tumour may not be determinable. When pre‐determined margins are not used, there is a delay in closure until histological proof of clearance is available. Examination of surgically cleared margins often requires a second visit for closure.
Mohs micrographic surgery offers a method to combine histologically proved complete excisions with maximal tissue preservation. Its fundamental difference from conventional SE is that the excised specimen is looked at 'en face' (i.e. 100% of the tumour margin is examined histologically) and the tumour and specimen are marked to facilitate further excision of residual tumour from focal areas thereby preventing unnecessary removal of normal tissue.
Why it is important to do this review
Mohs micrographic surgery is considered the better alternative for treatment of certain types of periocular BCC because it has the highest chance of curing the disease and minimises the size of the defect that needs to be repaired. It was reported to have the lowest recurrence rate of any treatment modality in a large prospective series from Australia (Malhotra 2004a). A randomised controlled trial (RCT) of SE compared to MMS for BCC of the face found lower recurrence rates after MMS, though the differences were not statistically significant (Smeets 2004).
Unfortunately, this modality of therapy is a limited resource and not always employed because of practical limitations in the service delivery. The result is that SE with standard bread loaf examination of the surgical margins is employed. This does not require the money involved in setting up and running a MMS, and is perceived to be a cheaper option. Examination of surgically cleared margins sometimes requires a second visit for closure and has a higher recurrence rate than MMS. Extended follow up, greater surgical morbidity and the cost of dealing with recurrences may over time significantly increase the cost of SE.
Techniques other than MMS for primary tumour removal have been implicated as a factor responsible for the increased number of exenterations (removal of the entire contents of the orbit to remove locally spreading tumour) that have been seen in some units (Rahman 2005).
The RCT by Smeets et al. also commented that the total operative costs for MMS were higher than those of SE, but a cost comparison analysis from the United States found the cost comparable (Bialy 2004).
Objectives
The objective of this review was to compare the effectiveness, cost, complications and acceptability of periocular BCCs when operated by MMS or SE.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs. In the absence of RCTs, we discussed non‐randomised studies.
Types of participants
We included people with histology proven periocular BCC of any age or gender.
Types of interventions
We included studies that compared MMS to SE.
Types of outcome measures
Primary outcomes
The proportion of patients who recurred within five years ‐ ideal is five‐year recurrence rate.
Secondary outcomes
We looked for studies comparing the average cost per patient for excision of the tumour, reconstruction of defect and subsequent management in SE and MMS.
Adverse outcomes
We included studies comparing the complications of the two procedures.
Quality of life data
We included studies comparing the acceptability of MMS versus SE.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2014, Issue 1), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to February 2014), EMBASE (January 1980 to February 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 25 February 2014.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), mRCT (Appendix 4), ClinicalTrials.gov (Appendix 5) and the ICTRP (Appendix 6).
Searching other resources
When we find RCTs to include in the review we will do the following. We will manually search the reference lists of the trials included in the review for additional trials. We will use the Science Citation Index to find studies that have cited the included trials. The primary investigators of identified trials will be contacted for details of any additional trials.
We did not handsearch journals or conference proceedings for this review.
Data collection and analysis
Selection of studies
Two of the three authors (KN and OH) independently screened the titles and abstracts obtained from the searches to determine which studies fulfilled the eligibility criteria. The studies were graded into one of three headings ‐ included, excluded and uncertain. Full text copies of all studies in the included and uncertain groups were obtained and the same two authors ascertained if the studies met the inclusion criteria, again dividing the studies into included, excluded and uncertain. The studies labelled as uncertain were discussed by all three authors and if necessary further information was obtained by contacting the authors of the articles concerned. Any studies excluded at this stage were documented and a reason stated in the 'Characteristics of excluded studies' table.
No RCTs met our inclusion criteria, but if we do include trials in future updates, we will apply the following methods.
Data extraction and management
The data will be extracted independently by two authors and then checked before it is entered into RevMan (RevMan 2014) by one author.
The data extracted will include the following:
Methods: inclusion and exclusion criteria, method of allocation, and other aspects of study design.
Participants: age, sex, histological evidence of BCC, number randomised.
Interventions: studies comparing SE and MMS.
Outcomes:
‐ the time to recurrence of periocular BCCs when operated by MMS and SE;
‐ recurrence rates with the two procedures.
Economic data: the cost difference between the two procedures.
Adverse events and quality of life data: the complications and acceptability of the two procedures.
Assessment of risk of bias in included studies
The methodological quality of the studies meeting the inclusion criteria will be assessed according to the methods described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The following parameters will be assessed: sequence generation, allocation concealment, masking (blinding) of participants, personnel and outcome assessors, incomplete outcome data, selective outcome reporting and any other sources of bias identified. The other likely sources of bias could be if the study had a potential source of bias related to the specific study design used, if the study was stopped early due to some data‐dependent process (including a formal‐stopping rule), if it had extreme baseline imbalance, or if the study has been claimed to have been fraudulent.
We will classify each parameter as low risk of bias, high risk of bias and unclear. The studies graded as having a high risk of bias will be excluded from our analysis and further information may be sought from the authors of the studies graded as unclear.
We will also assess the impact of any assumptions made in this regard in a sensitivity analysis.
Measures of treatment effect
Data analysis will be done as directed in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). Odds ratio will be used for the primary outcome measure which is dichotomous data, and standardised mean difference for continuous data (cost difference).
Unit of analysis issues
It is unlikely that any cluster RCTs will be identified but if they are then analysis techniques appropriate to these will be used.
Dealing with missing data
We will contact the authors of the included studies for missing data wherever possible. A period of eight weeks will be allowed for the authors to respond and if no response is forthcoming after this period it will be recorded as missing data and the review will be conducted based on available information. We will attempt to extract data from the included studies to enable an available case analysis
Assessment of heterogeneity
We will initially check for heterogeneity in the studies by reading the trial reports to see if we can identify any methodological differences between studies that would make comparison unlikely. We will measure I2 value which estimates the amount of inconsistency between trials.
Assessment of reporting biases
If there are adequate numbers of trials to evaluate, a funnel plot will be used to examine publication bias.
Data synthesis
If there is sufficient homogeneity between trials we will combine the results in a meta‐analysis using a random‐effects model, unless the number of trials are three or less in which case a fixed‐effect model will be used. If substantial statistical or clinical heterogeneity is present we will not pool results but will present a descriptive overview of results. We will only conduct meta‐analysis for the primary outcome measure and one secondary outcome measure i.e. cost comparison.
Sensitivity analysis
We will conduct sensitivity analyses to determine the impact of exclusion of studies of lower methodological quality and unpublished studies.
Results
Description of studies
Results of the search
The electronic searches retrieved 229 titles and abstracts. The Trials Search Co‐ordinator scanned the search results and removed any references which were not relevant to the scope of the review. After independent review of the titles and abstracts by two review authors, we retrieved 46 full‐text articles of which only one was an RCT. As 45 of the full‐text articles were not trials, we did not document reasons for their exclusion. We found no randomised trials eligible for inclusion in the review.
An update search was done in November 2011. After deduplication the search identified a total of 80 references. We assessed these references which were made up of 15 abstracts from clinical trial registers and 65 abstracts from journals. The abstracts were independently assessed by two authors. We obtained a full‐text copy of one paper by Lagrange 2009 for potential inclusion in the review. However, after undertaking a partial translation we found that it was a discussion paper commenting on a five‐year follow‐up paper by Mosterd (Smeets 2004).
An update search run in February 2014 identified a further 103 references (Figure 1). The Trials Search Co‐ordinator removed nine duplicates and screened the remaining 94 references, of which 68 were not relevant to the scope of the review. We reviewed the remaining 26 reference but none met the inclusion criteria for the review.
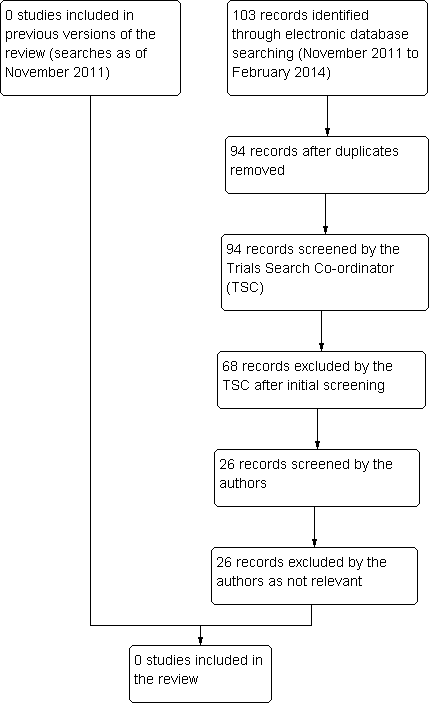
Results from searching for studies for inclusion in the review.
Included studies
No studies were found that met the inclusion criteria.
Excluded studies
Only one prospective RCT was found comparing SE to MMS in the management of BCC in the face (Smeets 2004). In this study only 8% (16 tumours) of the total primary BCCs and 5% (five tumours) of recurrent BCCs were from the periocular region. This study was excluded as we could not obtain data specific to the periocular area. See table: Characteristics of excluded studies. We also excluded a report by Lagrange 2009 as it was a discussion article of the previously published paper by Smeets 2004.
Risk of bias in included studies
No studies were found.
Effects of interventions
No studies were included in the review.
Discussion
We could not find any RCTs that compared MMS to SE directly. Hence this section will discuss the RCT that was excluded from the review and the bigger retrospective studies that look at each treatment modality.
One large, prospective, RCT looking at SE compared to MMS for BCC of the face, did not meet the inclusion criteria for this review as the results and outcomes did not differentiate periocular BCC from the rest of the facial tumours (Smeets 2004). In this study, 408 primary and 204 recurrent facial carcinomas (374 and 191 patients, respectively) were analysed separately. Patients were assigned SE or MMS (each 204 primary, 102 recurrent). The primary outcome for this study was the carcinoma recurrence rate. They also studied three secondary outcomes including incomplete excision, suboptimal aesthetic results and cost of treatment. This study demonstrated slightly fewer, but statistically insignificant, recurrences in the group who had MMS. Of the primary carcinomas five (3%) recurred after SE compared with three (2%) after MMS during 30 months of follow‐up. Of the recurrent carcinomas, three (3%) recurred after SE and none after MMS during 18 months of follow‐up. Although both differences favoured MMS, they were not statistically significant.
Smeets et al. concluded that treatment with MMS slightly, but not significantly, lowered the recurrence rate for both primary and recurrent tumours compared with SE.
In 2008, Mosterd et al. reported on the five‐year follow‐up findings of the same cohort of patients. They found that the difference in the number of recurrences between MMS and SE (2.5% in the MMS group and 4.1% in the SE group) was not statistically significant in primary BCCs at five years, but when the two treatments were compared in recurrent BCCs (2.4% in the MMS group and 12.1% in the SE group), the difference in the number of recurrences was statistically significant.
A similar conclusion was reached in a French discussion article by Lagrange who also noted the lower recurrence rate after MMS for recurrent BCC of the face when compared with SE (Lagrange 2009).
The largest study looking specifically into MMS for periocular BCC came from the Australian Mohs database, which is a prospective nationwide series of high‐risk periocular BCC managed by MMS (Malhotra 2004a). They looked at 1295 patients, in whom 47% of the tumours were in the lower lid and 48% were at the medial canthus. Recurrences accounted for 32% of the tumours and the rest were primary. The authors reported five‐year recurrence rates of 0% and 7.8% for primary and recurrent tumours respectively (Malhotra 2004b). They concluded that their study confirms that MMS is the treatment of choice for periocular BCC.
A Swedish group published a retrospective review of 66 BCCs excised with MMS. They reported a recurrence rate of 5% after a mean follow up of 49 months. The authors claim that the rate of recurrence in their series was less than reported with other modalities of treatment (Lindgren 2000).
In terms of long‐term follow up after MMS for periocular BCC, we found a few papers showing low five‐year recurrence rates after this method. Mohs himself reported a five‐year cure rate of 99% in 1,773 cases of BCC excised by his method (Mohs 1986). Nemet et al. found a higher recurrence rate after surgical re‐excision in incompletely excised eyelid BCCs as compared to MMS (Nemet 2006).
It is generally accepted that BCCs removed by MMS have the lowest recurrence rate, but similar cure rates have been reported with conventional frozen section control.
Hamada et al. looked at SE for 162 eyelid BCC cases and reported 1.6% five‐year recurrence in primary BCC and 4.35% recurrence for recurrent BCC. The authors removed a 4 mm margin and had 16% cases with incomplete primary excision (Hamada 2005).
One study comparing the five‐year recurrence rate of periocular BCC excised under frozen section control and without frozen section control reported a recurrence rate of 2.1% and 5% respectively (Wong 2002).
Cost
Mohs micrographic surgery has several advantages over SE including offering higher cure rates than SE and smaller defects. By far, the most important advantage is that by the MMS method 100% of the surgical margin is examined, while with the ‘bread loafing’ technique used to examine the specimens after SE a much smaller proportion of the margin is examined. Kimyai‐Asadi et al. have estimated that to detect 100% of positive margins by bread loafing, the entire specimen would have to be cut in 0.1 mm thick sections whereas normally the sections are 3 to 5 mm thick. However MMS is thought to be more expensive than SE due to the additional cost of the multiple visits and procedures involved to ensure tumour‐free margins before reconstruction (Kimyai‐Asadi 2007).
There were no studies directly comparing the cost difference between MMS and SE for periocular BCC.
Cook and Zitelli found MMS (USD 1243) to be 7% more expensive than office‐based SE with permanent sections (USD 1167) but 11% less expensive than office excision with frozen sections (USD 1400). This study involved 400 consecutive tumours of all types from all over the body. They also found MMS to be 37% less expensive than an ambulatory surgical facility‐based surgical excision (USD 1973). This difference persisted on analysing the costs by anatomic location (Cook 1998).
Bumstead and Ceilley, when studying 17 auricular malignant neoplasms, reported that on average SE removed 180% more tissue than excised by MMS in primary lesions and 347% more in recurrent lesions (Bumsted 1982). In a randomised trial involving 30 patients Muller et al. showed that the defect size after MMS was significantly smaller than that after SE (median area of 116.6 mm2 after MMS compared to 187.7 mm2 after SE) (Muller 2009).
Bentkover et al. reported that in their practice, excision of head and neck BCC with a rapid, cross‐sectional, frozen‐section technique was significantly cheaper than MMS with a comparable recurrence rate (Bentkover 2002).
In a study involving 98 patients with non‐melanoma facial and auricular skin cancer, Bialy et al. found the cost of MMS comparable to SE. They found 32% of margins positive after primary SE and assumed that a subsequent excision would clear the margins. The authors also assumed all deep margins to be clear after SE. In this study, MMS alone had a marginal cost saving over SE when a second procedure was needed to ensure complete tumour removal (Bialy 2004).
In a RCT comparing the cost‐effectiveness of MMS and SE for BCC of the face, Essers et al. found that the total treatment costs of MMS were significantly higher than SE for both primary and recurrent BCC. The cost difference persisted irrespective of histologic subtype and location of the tumour. They therefore recommended that it did not seem cost effective to introduce MMS on a large scale for both primary and recurrent BCC (Essers 2006).
It is therefore not conclusive whether MMS is more expensive than SE especially as the studies are unable to take into account the cost arising from the recurrences following both procedures which could tilt the balance in favour of MMS as the recurrence rate is considered to be lower with MMS.
Complications and acceptability of the two procedures
We were unable to find any study directly comparing the complications and acceptability of the two procedures for periocular BCC.
In the study by Smeets et al. the aesthetic outcomes did not significantly differ between MMS and SE in facial BCC (Smeets 2004). They reported no difference in postoperative complications between SE and MMS in primary carcinomas. More complications occurred after SE than after MMS in recurrent carcinomas. Complications included wound infections, graft necrosis, and/or postoperative bleeding.
Cook and Perone in a prospective study concluded that MMS is a very safe outpatient procedure when performed by properly trained surgeons, with an overall complication incidence of 1.64% (Cook 2003). Difficulties with haemostasis formed the bulk of the complications. None of the complications were significant enough to involve the assistance of another specialist or to require the hospitalisation of the patient.

Results from searching for studies for inclusion in the review.

