Intervenciones antivirales para pacientes con un trasplante hepático e infección recurrente del injerto debido al virus de la hepatitis C
Resumen
Antecedentes
El tratamiento antiviral para la infección recurrente por hepatitis C después del trasplante hepático es polémico debido al equilibrio no resuelto entre los efectos beneficiosos y perjudiciales.
Objetivos
Comparar los efectos beneficiosos terapéuticos y los efectos perjudiciales de diferentes regímenes antivirales en pacientes con injertos reinfectados por hepatitis C después del trasplante hepático.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Cochrane central de ensayos controlados (Cochrane Central Register of Controlled Trials, CENTRAL; número 1, 2013), MEDLINE, EMBASE y Science Citation Index Expanded hasta febrero de 2013.
Criterios de selección
Para la revisión sólo se consideraron los ensayos clínicos aleatorizados (independientemente del idioma, el cegamiento o el estado de publicación) que compararan diversos tratamientos antivirales (solos o en combinación) en el tratamiento de la recurrencia del virus de la hepatitis C en el trasplante hepático.
Obtención y análisis de los datos
Dos autores recopilaron los datos de forma independiente. Se calculó la razón de riesgos (RR) o la diferencia de medias (DM) con intervalos de confianza (IC) del 95%, usando los modelos de efectos fijos y de efectos aleatorios sobre la base del análisis de los casos disponibles. Cuando sólo hubo ensayos para un desenlace dicotómico, se realizó la prueba exacta de Fisher.
Resultados principales
En total, 17 ensayos con 736 pacientes cumplieron los criterios de inclusión para esta revisión. Todos los ensayos tenían un alto riesgo de sesgo. Once ensayos con 501 pacientes asignados al azar proporcionaron información para diversas comparaciones en esta revisión sistemática después de excluir los abandonos posteriores a la asignación al azar y los pacientes de los ensayos que no informaron ninguno de los desenlaces de interés para esta revisión. Las comparaciones para las que se disponía de desenlaces incluían interferón pegilado (peg) versus control; interferón peg más ribavirina versus control; ribavirina más interferón peg versus interferón peg; interferón peg (1,5 μg/kg/semana) más ribavirina versus interferón peg (0,5 μg/kg/semana) más ribavirina; amantadina más interferón peg más ribavirina versus interferón peg más ribavirina; interferón versus control; interferón más ribavirina versus control; ribavirina versus interferón; y ribavirina versus placebo. El seguimiento a largo plazo no estaba disponible en estos ensayos. No hubo diferencias significativas en la mortalidad, la necesidad de un nuevo trasplante, los rechazos del injerto que requieren un nuevo trasplante o tratamiento médico o el empeoramiento de la fibrosis, entre los grupos en ninguna de las comparaciones en las cuales se informaron estos desenlaces. La calidad de vida y la descompensación hepática no se informaron en los ensayos. Hubo una proporción significativamente mayor de participantes que desarrollaron eventos adversos graves en el grupo de tratamiento combinado de ribavirina más interferón peg que en el grupo de monoterapia con interferón peg (un ensayo; 56 participantes; 17/28 [60,7%] en el grupo de intervención versus 5/28 [17,9%] en el grupo control; RR 3,40; IC del 95%: 1,46 a 7,94). No hubo diferencias significativas en la proporción de participantes que desarrollaron eventos adversos graves ni en el número de eventos adversos graves entre los grupos de intervención y control en las otras comparaciones que informaron eventos adversos graves.
Conclusiones de los autores
Considerando la falta de un efecto clínico beneficioso, actualmente no hay evidencia para recomendar o refutar el tratamiento antiviral para la infección recurrente del injerto hepático por el virus de la hepatitis C. Se necesitan ensayos clínicos aleatorizados adicionales con bajo riesgo de sesgo y bajo riesgo de errores aleatorios y una duración adecuada del seguimiento.
Resumen en términos sencillos
Tratamiento antiviral para la infección recurrente del injerto hepático por el virus de la hepatitis C
Antecedentes
El hígado es un órgano importante del cuerpo y tiene varias funciones, entre ellas la generación de energía a partir de los alimentos; la producción del material necesario para la coagulación, el procesamiento y la excreción de fármacos y productos de desecho en la sangre; y el filtrado de las bacterias perjudiciales que entran en el cuerpo a través del intestino. El virus de la hepatitis C puede causar daño al hígado generalmente de forma insidiosa (infección crónica por hepatitis C). A veces, el daño hepático puede ser tan grave que el hígado no es capaz de realizar las funciones normales, lo que provoca insuficiencia hepática. El trasplante hepático es un tratamiento efectivo para la insuficiencia hepática causada por la infección crónica por hepatitis C. Sin embargo, el trasplante hepático no erradica el virus y éste puede afectar el injerto de hígado proveniente del donante. Una de las estrategias propuestas para tratar la recurrencia de la infección crónica por el virus de la hepatitis C en estos pacientes es la administración de tratamientos antivirales. No se conoce la efectividad de estos tratamientos. Se realizó una revisión detallada de la bibliografía médica (hasta febrero de 2013) para determinar los efectos beneficiosos y perjudiciales de diferentes tratamientos antivirales en pacientes con infección recurrente por virus de la hepatitis C después de ser sometidos a trasplante hepático debido a la infección crónica por el virus de la hepatitis C. Se buscó evidencia de ensayos clínicos aleatorizados solamente. Cuando se realizan de forma adecuada, dichos ensayos aportan la mejor evidencia. Dos autores identificaron de forma independiente los ensayos y obtuvieron la información de los mismos para minimizar el error.
Características de los estudios
Once ensayos con 501 receptores de trasplante hepático proporcionaron datos para esta revisión. En estos 11 ensayos los pacientes fueron asignados al azar a recibir diferentes tratamientos, incluido ningún tratamiento. El seguimiento a largo plazo no estaba disponible en estos ensayos.
Resultados clave
No hubo diferencias significativas en la proporción de pacientes que murieron, que requirieron un nuevo trasplante, que desarrollaron rechazo del injerto que requirió tratamiento o que tuvieron un aumento del daño hepático (según lo evaluado mediante un microscopio) entre los grupos en cualquiera de las comparaciones en las que se informaron estos desenlaces. La calidad de vida y la descompensación hepática no se informaron en los ensayos. Hubo una proporción significativamente mayor de participantes que desarrollaron complicaciones graves en el grupo de tratamiento combinado con ribavirina más interferón peg en comparación con el de monoterapia con interferón peg. No hubo diferencias significativas en la proporción de participantes que desarrollaron complicaciones graves ni en el número de episodios adversos graves entre los grupos de intervención y control en las otras comparaciones que informaron complicaciones graves. Actualmente no existe evidencia para recomendar el tratamiento antiviral en los pacientes con recurrencia de la infección crónica por el virus de la hepatitis C sometidos a trasplante hepático primario o a un nuevo trasplante.
Calidad de la evidencia
Todos los ensayos tuvieron un riesgo alto de errores sistemáticos (es decir, sesgo por el cual es posible establecer conclusiones equivocadas debido a la manera en que se realizaron los ensayos, sobrestimando los efectos beneficiosos y subestimando los efectos perjudiciales) y de errores aleatorios (existió la posibilidad de establecer conclusiones equivocadas debido a la intervención del azar). La calidad general de la evidencia fue muy baja.
Estudios de investigación futuros
Se necesitan más ensayos clínicos aleatorizados con bajo riesgo de errores aleatorios o sistemáticos para evaluar la supervivencia a largo plazo y otros efectos beneficiosos de diversas opciones de tratamiento en estos pacientes.
Authors' conclusions
Summary of findings
| Mortality | ||||||
| Patient or population: Participants with recurrent liver graft infection with hepatitis C virus. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Intervention | |||||
| Peg interferon vs.control | 62 per 1000 | 30 per 1000 | RR 0.48 | 65 | ⊕⊝⊝⊝ | The assumed risk was the control group risk. |
| Peg interferon plus ribavirin vs.control | 63 per 1000 | 189 per 1000 | RR 3 | 54 | ⊕⊝⊝⊝ | Since there were no deaths in the control group, the assumed risk was the control group risk in a different trial included in this review. |
| Ribavirin plus peg interferon vs.peg interferon | 41 per 1000 | 20 per 1000 | RR 0.5 | 98 | ⊕⊝⊝⊝ | The assumed risk was the control group risk. |
| Interferon vs.control | 63 per 1000 | 105 per 1000 | RR 1.67 | 12 | ⊕⊝⊝⊝ | Since there were no deaths in the control group, the assumed risk was the control group risk in a different trial included in this review. |
| Interferon plus ribavirin vs.control | 42 per 1000 | 12 per 1000 | RR 0.29 | 52 | ⊕⊝⊝⊝ | The assumed risk was the control group risk. |
| Ribavirin vs.interferon | There were no deaths in either group. | Not estimable | 30 | ⊕⊝⊝⊝ | ‐ | |
| Ribavirin vs.placebo | There were no deaths in either group. | Not estimable | 77 | ⊕⊝⊝⊝ | ‐ | |
| *The basis for the assumed risk is provided in the comments section. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The trial(s) was (were) of high risk of bias. | ||||||
| Retransplantation | ||||||
| Patient or population: Participants with recurrent liver graft infection with hepatitis C virus. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Intervention | |||||
| Retransplantation after start of therapy ‐ ribavirin vs.placebo | No retransplantation in either group | Not estimable | 77 | ⊕⊝⊝⊝ | ||
| Retransplantation after start of therapy ‐ interferon vs.control | 10 per 1000 | 17 per 1000 | RR 1.67 | 12 | ⊕⊝⊝⊝ | There was no retransplantation in the control group. So, we used an assumed risk of 1%. |
| *The basis for the assumed risk is provided in the comments section. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The trial(s) was (were) of high risk of bias. | ||||||
Background
In the UK, the annual incidence of liver transplantation is 13 per one million population (NHSBT). In the USA, the annual incidence of liver transplantation is 21 per one million population (OPTN/SRTR 2009). Liver transplant is performed mainly for end‐stage liver failure arising acutely (eg, viruses, drug overdose), or as a result of chronic liver disease (eg, cirrhosis due to alcohol consumption, viruses), or as a result of tumour (Lim 2006). Liver graft can be harvested from living donors (Bombuy 2004), or from cadavers (Koneru 2005; Cescon 2006). Liver transplant can be performed in adults or children (Lim 2006). Worldwide, there is a demand for liver transplants in surplus of supply. Split liver transplantation (using one cadaveric donor liver for two recipients) has been suggested as a way to decrease the organ shortage for liver transplant (Corno 2006).
Hepatitis C viral cirrhosis is one of the main causes for liver transplantation (Eason 2001). Re‐infection of the liver graft is virtually universal in patients who undergo liver transplantation for hepatitis C virus infection. Immunosuppressive regimens that avoid steroids are reported to have a lower rate of graft infection with hepatitis C virus than those that include steroids as part of immunosuppressive therapy (Eason 2001). Azathioprine and anti‐CD3 monoclonal antibody (OKT3) are other immunosuppressive agents that can influence the severity of fibrosis following hepatitis C viral recurrence after liver transplantation (Berenguer 2003). The recurrence rate with hepatitis C virus is also dependent on hepatitis C subtype (with subtype Ib showing a higher recurrence rate than other subtypes) (Sugo 2003); age of the donor (Cameron 2006); age of the recipient (Cameron 2006); model for end‐stage liver disease (MELD) score recipient (Cameron 2006); and warm ischaemic time (Cameron 2006). Antiviral prophylaxis to prevent the recurrence of chronic hepatitis C virus infection does not seem effective (Gurusamy 2013).
Antiviral agents such as ribavirin and interferon have been used to treat hepatitis C virus re‐infection in the liver grafts either alone or in combination (Gane 1998; Chalasani 2005; Duvoux 2006). However, concerns remain about the adverse effects of these agents such as anaemia (Chalasani 2005), haemolysis (Gane 1998), renal failure (Chalasani 2005; Duvoux 2006), depression (Chalasani 2005), and transplant rejection (Chalasani 2005; Duvoux 2006).
Previous reviews have not advocated routine therapy of patients with established recurrence of hepatitis C virus infection in liver transplant recipients (Triantos 2005; Arjal 2007). This is an update of the review published in The Cochrane Library Issue 1, 2010 (Gurusamy 2010), in which we did not recommend routine treatment of patients with established recurrence of hepatitis C virus.
Objectives
To compare the therapeutic benefits and harms of different antiviral regimens in patients with hepatitis C re‐infected grafts after liver transplantation.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised clinical trials that assessed antiviral intervention aimed at treatment of the hepatitis C virus re‐infected liver graft (irrespective of language, blinding, publication status, sample size, or whether the trials were adequately powered or not). We excluded quasi‐randomised trials (where the method of allocating participants to an intervention was not strictly random, eg, date of birth, hospital record number, alternation).
Types of participants
Patients with hepatitis C viral re‐infection of the liver graft (however defined by study authors) irrespective of age, cadaveric or living donor transplant, indication for liver transplantation, first or re‐transplantation, and the immunosuppressive therapy used.
Types of interventions
We included any antiviral treatment in patients with hepatitis C re‐infected liver grafts versus no intervention, placebo, or another antiviral treatment.
We did not include the following interventions:
-
Prophylactic treatment of hepatitis C virus in patients who do not have established re‐infection of the liver graft (ie, pre‐emptive therapy), as this was considered in another review (Gurusamy 2013).
-
Treatment for hepatitis C virus infection while waiting for liver transplant.
Types of outcome measures
Primary outcomes
-
Mortality (30‐days mortality and mortality at maximal follow‐up) after starting the treatment.
-
Re‐transplantation after the start of therapy.
-
Quality of life during and after treatment.
-
Serious adverse events were defined as any event that would increase mortality; were life‐threatening, required inpatient hospitalisation, resulted in a persistent or significant disability, or any important medical event that might have jeopardised the patient or required intervention to prevent it (ICH‐GCP 1997).
Secondary outcomes
-
Hepatic decompensation (bleeding varices, ascites, encephalopathy, coagulation disorders).
-
Rejection of liver transplant after the start of therapy (however defined by authors).
-
Worsening of fibrosis (however defined by authors).
We have provided the summary of findings table for mortality and retransplantation using GRADEpro (ims.cochrane.org/revman/other‐resources/gradepro) and planned to create a summary of findings table for quality of life.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; Issue 1, 2013), MEDLINE, EMBASE, and Science Citation Index Expanded (Royle 2003) to February 2013. We have given the search strategies in with the time spans for the searches in Appendix 1.
Searching other resources
We also searched the references of the identified trials to identify further relevant trials.
Data collection and analysis
Selection of studies
KSG and ET, CT, or EX identified the trials for inclusion independently of each other. The excluded studies with the reasons for the exclusion have been listed.
Data extraction and management
KSG and ET, CT, or EX independently extracted the following data.
-
Year and language of publication.
-
Country.
-
Year of conduct of trial.
-
Inclusion and exclusion criteria.
-
Adult or paediatric.
-
Population characteristics such as recipient age, sex ratio, interval between transplantation and treatment.
-
Number undergoing retransplantation.
-
Immunosuppressive therapy.
-
Other co‐existing viral diseases.
-
Co‐interventions.
-
Viral subtype.
-
Duration of follow‐up.
-
Outcomes (mentioned above).
-
Risk of bias (described below).
We sought any unclear or missing information clarified by contacting the authors of the individual trials. If there was any doubt whether the trials shared the same patients ‐ completely or partially (by identifying common study authors and centres) ‐ we intended to contact the authors of the trials to clarify whether the trial report had been duplicated. However, we had no such instances. It was clear from the multiple reports that they all reported on the same patients.
We resolved any differences in opinion through discussion and in case of unsettled disagreements, BRD adjudicated.
Assessment of risk of bias in included studies
KSG and CT or ET assessed the bias risk of the trials independently, without masking of the trial names. We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and the Cochrane Hepato‐Biliary Group Module (Gurusamy 2009b; Gluud 2013). Due to the risk of biased overestimation of intervention effects in randomised trials with high risk of bias (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Lundh 2012; Savović 2012; Savović 2012a), we assessed the following domains of risk of bias in the trials.
Sequence generation
-
Low risk of bias (the methods used was either adequate (eg, computer‐generated random numbers, table of random numbers) or unlikely to introduce confounding).
-
Uncertain risk of bias (there was insufficient information to assess whether the method used was likely to introduce confounding).
-
High risk of bias (the method used (eg, quasi‐randomised studies) was improper and likely to introduce confounding).
Allocation concealment
-
Low risk of bias (the method used (eg, central allocation) was unlikely to induce bias on the final observed effect).
-
Uncertain risk of bias (there was insufficient information to assess whether the method used was likely to induce bias on the estimate of effect).
-
High risk of bias (the method used (eg, open random allocation schedule) was likely to induce bias on the final observed effect).
Blinding of participants, personnel, and outcome assessors
-
Low risk of bias (blinding was performed adequately, or the outcome measurement was not likely to be influenced by lack of blinding).
-
Uncertain risk of bias (there was insufficient information to assess whether the type of blinding used was likely to induce bias on the estimate of effect).
-
High risk of bias (no blinding or incomplete blinding, and the outcome or the outcome measurement was likely to be influenced by lack of blinding).
Incomplete outcome data
-
Low risk of bias (the underlying reasons for missingness were unlikely to make treatment effects departure from plausible values, or proper methods were employed to handle missing data).
-
Uncertain risk of bias (there was insufficient information to assess whether the missing data mechanism in combination with the method used to handle missing data was likely to induce bias on the estimate of effect).
-
High risk of bias (the crude estimate of effects (eg, complete case estimate) was clearly biased due to the underlying reasons for missingness, and the methods used to handle missing data were unsatisfactory).
Selective outcome reporting
-
Low risk of bias (the trial protocol was available and all of the trial's pre‐specified outcomes that are of interest in the review had been reported or similar; if the trial protocol was not available, mortality and morbidity were reported).
-
Uncertain risk of bias (there was insufficient information to assess whether the magnitude and direction of the observed effect was related to selective outcome reporting).
-
High risk of bias (not all of the trial's pre‐specified primary outcomes had been reported or similar).
Vested interest bias
-
Low risk of bias (the trial was not performed or supported by any parties that might have conflicting interest, eg, drug manufacturer).
-
Uncertain risk of bias (any conflicts of interest of the trialist or trial funder were not clear).
-
High risk of bias (the trial was performed or supported by any parties that might have conflicting interest, eg, drug manufacturer).
We classified trials at low risk of bias in all domains to be at low risk of bias.
Measures of treatment effect
We performed the meta‐analyses according to the recommendations of The Cochrane Collaboration (Higgins 2011), and the Cochrane Hepato‐Biliary Group Module (Gluud 2013), using the software package Review Manager 5 (RevMan 2012). For dichotomous variables, we calculated the risk ratio (RR) with 95% confidence intervals (CI) in the presence of two or more trials for the outcomes. In the presence of only one trial for the outcome, we performed the Fisher's exact test using the statistical software StatsDirect 2.7. For continuous variables, we calculated the mean difference (MD) with 95% CI. For count data, outcomes such as serious adverse events, we calculated the rate ratio (RaR) with 95% CI using the methods shown in Section 9.4.8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For such a calculation, one needs the time that the patients were exposed to the risk of serious adverse events in each of the groups. We considered that both groups were exposed to the risk of serious adverse events for the same time period, which is a reasonable assumption considering that the patients were followed up for the same time in both groups. For time‐to‐event outcomes, we calculated the hazard ratio with 95% CI.
Unit of analysis issues
The units of analysis were the patients who had undergone liver transplantation and had developed recurrent hepatitis C virus infection.
Dealing with missing data
We performed the analysis using an intention‐to‐treat basis whenever possible (Newell 1992). Otherwise, we performed an available case analysis (Higgins 2011). In the absence of summary information such as mean and standard deviation for continuous outcomes, we planned to use the median for the meta‐analysis when the mean was not available and impute the standard deviation from P values according to the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011). If it was not possible to calculate the standard deviation from the P value or the CIs, we planned to impute the standard deviation as the highest standard deviation in the other trials included under that outcome, fully recognising that this form of imputation would decrease the weight of the study for calculation of MDs and bias the effect estimate to no effect in case of standardised mean difference (Higgins 2011). For time‐to‐event outcomes, we planned to calculate the natural logarithm of the hazard ratio and its standard error using methods suggested by Parmar et al (Parmar 1998).
Assessment of heterogeneity
We explored heterogeneity using the Chi2 test with significance set at P value 0.10, and we measured the quantity of heterogeneity using the I2 statistic (Higgins 2002). We also used overlapping of CI values on the forest plot to determine heterogeneity.
Assessment of reporting biases
We planned to use visual asymmetry on a funnel plot to explore reporting bias if 10 or more trials were identified (Egger 1997; Macaskill 2001). We also planned to perform the linear regression approach described by Egger 1997 to determine the funnel plot asymmetry.
Data synthesis
We performed the meta‐analyses using the software package Review Manager 5 (RevMan 2012), and following the recommendations of The Cochrane Collaboration (Higgins 2011), and the Cochrane Hepato‐Biliary Group Module (Gluud 2013). We used both random‐effects model (DerSimonian 1986), and fixed‐effect model (DeMets 1987), meta‐analyses. In case of discrepancy between the two models resulting in change of conclusions, we have reported both results; otherwise we have reported the results of the fixed‐effect model.
Trial sequential analysis
We planned to use trial sequential analysis to control for random errors due to sparse data and repetitive testing of the accumulating data for the primary outcomes (CTU 2011; Thorlund 2011). We planned to add the trials according to the year of publication, and if more than one trial was published in a year, add the trials in alphabetical order according to the surname of the first author. We planned to construct the trial sequential monitoring boundaries on the basis of the required information size (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010).
We planned to apply trial sequential analysis (CTU 2011; Thorlund 2011), using a required sample size calculated from an alpha error of 0.05, a beta error of 0.20, a control group proportion obtained from the results, and a relative risk reduction of 20% for binary outcomes when there were at least two trials to determine whether more trials are necessary on this topic (if the trial sequential alpha‐spending monitoring boundary and the required information size is reached or the futility zone is crossed, then more trials are unnecessary) (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010). We did not plan to perform trial sequential analysis for quality of life since trial sequential analysis cannot be performed for standardised mean differences.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses:
-
Trials with low risk of bias compared to trials with high risk of bias.
-
Adult compared to paediatric liver transplantation.
-
Different genotypes of virus.
-
Less than six months after liver transplant compared to more than six months after liver transplant.
These subgroup analysis were not performed because of the lack of trials of low risk of bias and because of the few trials included under each outcome.
Sensitivity analysis
We planned to perform a sensitivity analysis excluding the trials in which mean or standard deviation or both were imputed from the analysis.
Results
Description of studies
We identified 2411 references through electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (255 references), MEDLINE (536 references), EMBASE (820 references), and Science Citation Index Expanded (800 references). We excluded 629 duplicates and 1719 clearly irrelevant references through reading abstracts. Sixty‐three references were retrieved for further assessment. We identified two references through scanning reference lists of the identified randomised trials (Kizilisik 1997; Ghalib 2000). We excluded 28 references (25 studies) for the reasons listed in the Characteristics of excluded studies table. Seventeen randomised trials described in 37 references fulfilled the inclusion criteria. Of the 17 trials, only 11 trials could provide data for the review (Gane 1998; Cotler 2001; Samuel 2003; Chalasani 2005; Gordon 2005; Angelico 2007; Carrion 2007; Nair 2008; Gane 2009; Belli 2012; Calmus 2012). The reference flow is shown in Figure 1. Details about the sample size, patient characteristics, inclusion and exclusion criteria used in the trials, details of intervention and control, duration of treatment, and the risk of bias in the trials are shown in the Characteristics of included studies table.
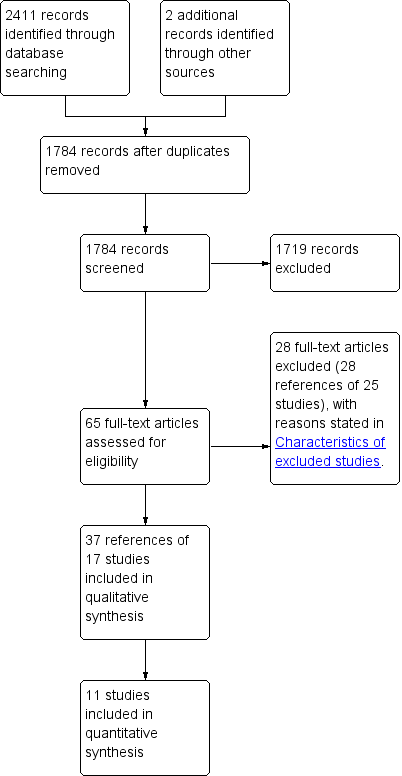
Study flow diagram.
Participants
A total of 736 liver transplant recipients with confirmed hepatitis C recurrence were randomised to various experimental interventions versus control interventions. The number of participants in each trial ranged from 5 to 78. A total of 37 participants were excluded from the trial after randomisation for various reasons as shown in the Characteristics of included studies table. The post‐randomisation drop‐outs ranged from 0% to 40%. We were unable to obtain the percentage of females and the mean age of participants in six trials (Crippin 1996; Ghalib 2000; Gordon 2005; Lodato 2008; Gane 2009; Aguilera 2011). The mean age of participants ranged between 51 and 60 years in the remaining trials. The proportion of females in these trials ranged between 11.1% and 37.0%. Eleven trials reported the proportion of patients belonging to viral genotype I (a subtype that is more difficult to treat than other subtypes) (Gane 1998; Cotler 2001; Samuel 2003; Chalasani 2005; Gordon 2005; Ghalib 2006; Angelico 2007; Carrion 2007; Lodato 2008; Yedibela 2011; Calmus 2012). The proportion of genotype I patients ranged between 50% and 100%. One trial included patients at least three months after liver transplantation (Crippin 1996). Ten trials included patients at least six months after liver transplantation (Gane 1998; Cotler 2001; Samuel 2003; Chalasani 2005; Angelico 2007; Carrion 2007; Gane 2009; Yedibela 2011; Belli 2012; Calmus 2012). The remaining six trials did not mention the minimum interval between the liver transplantation and the experimental intervention (Ghalib 2000; Gordon 2005; Ghalib 2006; Lodato 2008; Nair 2008; Aguilera 2011). Eleven trials included only patients with histological evidence of chronic viral hepatitis in the liver graft (Crippin 1996; Gane 1998; Samuel 2003; Chalasani 2005; Ghalib 2006; Angelico 2007; Carrion 2007; Lodato 2008; Nair 2008; Aguilera 2011; Belli 2012; Calmus 2012). Overall, we included 501 patients in 11 trials in the various comparisons in this systematic review (Gane 1998; Cotler 2001; Samuel 2003; Chalasani 2005; Gordon 2005; Angelico 2007; Carrion 2007; Nair 2008; Gane 2009; Belli 2012; Calmus 2012).
Comparisons
The trials included the following 13 comparisons. We chose the experimental intervention as the group that required an additional drug or a higher dosage (or both). We also considered 48 to 52 weeks as the standard duration of treatment and if there was a longer duration or shorter duration we considered that as the experimental intervention.
-
Pegylated (peg) interferon versus no intervention control (Chalasani 2005) ‐ 67 participants randomised to peg interferon (n = 32) versus no intervention control (n = 33) (two post‐randomisation drop‐outs).
-
Peg interferon plus ribavirin versus no intervention control (Carrion 2007; Belli 2012) ‐ 127 participants randomised to intervention (n = 63) versus no intervention control (n = 63) (one post‐randomisation drop‐out).
-
Ribavirin plus peg interferon versus peg interferon (Angelico 2007; Gane 2009) ‐ 98 participants randomised to Ribavirin plus peg interferon (n = 49) versus peg interferon control (n = 49).
-
Peg interferon (1.5 μg/kg/week; high dose) plus ribavirin versus peg interferon (0.5 μg/kg/week; low dose) plus ribavirin (Gordon 2005; Ghalib 2006) ‐ 72 participants randomised to high‐dose intervention (n = 41) versus low‐dose intervention control (n = 31).
-
Peg interferon alpha 2a versus peg interferon alpha 2b (Aguilera 2011) ‐ 68 participants randomised to peg interferon alpha 2a (n = 34) versus peg interferon alpha 2b (n = 34).
-
Amantadine plus peg interferon plus ribavirin versus peg interferon plus ribavirin (Nair 2008) ‐ 50 participants randomised to amantadine plus peg interferon plus ribavirin (n = 13) versus peg interferon plus ribavirin (n = 17) (20 post‐randomisation drop‐outs).
-
Interferon versus no intervention control (Crippin 1996; Cotler 2001) ‐ 47 participants randomised to interferon (n = 27) versus no intervention control (n = 13) (eight post‐randomisation drop‐outs).
-
Interferon plus ribavirin versus no intervention control (Samuel 2003) ‐ 52 participants randomised to interferon plus ribavirin (n = 28) versus no intervention control (n = 24).
-
Interferon plus ribavirin for 24 weeks versus interferon plus ribavirin for 48 weeks (Ghalib 2000) ‐ 5 participants randomised to short‐course experimental intervention (n = 3) versus standard course control (n = 2).
-
Ribavirin versus interferon (Gane 1998) ‐ 30 participants randomised to ribavirin (n = 40) versus interferon (n = 37) (one post‐randomisation drop‐out).
-
Ribavirin versus placebo (Calmus 2012) ‐ 78 participants randomised to ribavirin (n = 14) versus placebo control (n = 14) (two post‐randomisation drop‐outs).
-
Peg interferon plus ribavirin versus no intervention control in non‐responders (Lodato 2008) ‐ 18 participants who did not have virological response at 24 weeks of peg interferon plus ribavirin (non‐responders) were randomised to continued intervention (n = 9) versus no intervention (n = 9).
-
Ribavirin plus peg interferon versus peg interferon in relapsers and non‐responders to interferon and ribavirin therapy (Yedibela 2011) ‐ 24 participants randomised to Ribavirin plus peg interferon (n = 10) and versus peg interferon control (n = 11) (three post‐randomisation drop‐outs).
Peg interferon alpha 2a was used in four trials (Chalasani 2005; Angelico 2007; Gane 2009; Aguilera 2011), and peg interferon alpha 2b was used in seven trials (Gordon 2005; Ghalib 2006; Carrion 2007; Lodato 2008; Nair 2008; Aguilera 2011; Belli 2012). Interferon alpha 2a was used in two trials (Crippin 1996; Cotler 2001), and interferon alpha 2b was used in two trials (Ghalib 2000; Samuel 2003). One trial used pegylated interferon (Yedibela 2011). No details regarding whether this was alpha 2a or alpha 2b was stated in this trial (Yedibela 2011). One trial used interferon alpha (Gane 1998). No details regarding whether this was 2a or 2b were reported in this trial (Gane 1998).
Outcome measures
The outcomes reported in the trials were mortality (Gane 1998; Cotler 2001; Samuel 2003; Chalasani 2005; Angelico 2007; Carrion 2007; Gane 2009; Calmus 2012), retransplantation (Cotler 2001; Calmus 2012), serious adverse events (Chalasani 2005; Angelico 2007; Gane 2009; Calmus 2012), graft rejections after starting treatment (Cotler 2001; Samuel 2003; Chalasani 2005; Gordon 2005; Angelico 2007; Carrion 2007; Gane 2009; Belli 2012), and worsening of fibrosis (Gane 1998; Samuel 2003; Chalasani 2005; Angelico 2007; Carrion 2007; Nair 2008; Belli 2012). None of the trials reported quality of life or liver decompensation. Six trials did not report any outcomes of interest and so did not provide any data for the review (Crippin 1996; Ghalib 2000; Ghalib 2006; Lodato 2008; Aguilera 2011; Yedibela 2011). The other outcome measures reported by the individual trials are shown in the Characteristics of included studies table.
Risk of bias in included studies
The risk of bias is summarised in the 'Risk of bias' graph (Figure 2) and 'Risk of bias' summary (Figure 3). Eleven trials had adequate generation of allocation sequence (Crippin 1996; Cotler 2001; Samuel 2003; Chalasani 2005; Gordon 2005; Ghalib 2006; Angelico 2007; Carrion 2007; Lodato 2008; Belli 2012; Calmus 2012). Five trials had adequate allocation concealment (Cotler 2001; Samuel 2003; Chalasani 2005; Gordon 2005; Ghalib 2006; Belli 2012; Calmus 2012). Blinding of participants and healthcare providers is very difficult and may even be considered unethical by some in trials in which interferon or peg interferon was used in only one arm (Crippin 1996; Ghalib 2000; Cotler 2001; Samuel 2003; Chalasani 2005; Carrion 2007; Lodato 2008; Belli 2012), as the interferon had to be given subcutaneously weekly (peg interferon) or three time weekly (interferon). Understandably, no placebo was used in these trials. However, there is a potential bias in the effect estimate because of lack of blinding. In the remaining trials, a placebo could be used to blind the participants and healthcare providers/outcome assessors (Gane 1998; Gordon 2005; Ghalib 2006; Angelico 2007; Nair 2008; Gane 2009; Aguilera 2011; Yedibela 2011; Calmus 2012). However, only one of these nine trials used placebo (Calmus 2012), and the blinding was inadequate in the remaining eight trials (Gane 1998; Gordon 2005; Ghalib 2006; Angelico 2007; Nair 2008; Gane 2009; Aguilera 2011; Yedibela 2011). Seven trials were free from bias due to incomplete outcome data (Cotler 2001; Samuel 2003; Gordon 2005; Ghalib 2006; Angelico 2007; Carrion 2007; Lodato 2008). Only two trials reported both mortality and liver transplantation and were considered to be at low risk of selective reporting bias (Cotler 2001; Calmus 2012). The protocol was not available for any of the trials. Thus, all the trials were considered to be at risk of bias due to selective outcome reporting. None of the trials were free from source of funding bias. All the trials were considered to be of high risk of bias.
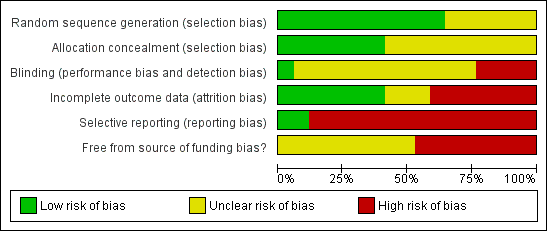
Methodological quality graph: Review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: Review authors' judgements about each methodological quality item for each included study.
Effects of interventions
See: Summary of findings for the main comparison Antiviral therapy for recurrent liver graft infection with hepatitis C virus (mortality); Summary of findings 2 Antiviral therapy for recurrent liver graft infection with hepatitis C virus (retransplantation)
Mortality
The following comparisons reported mortality (Analysis 1.1). The trials that contributed to the comparisons are shown along with the number of participants, RR, and P value (derived from the Fisher's exact test when there was only one trial and derived from Review Manager (RevMan 2012) when there were two or more trials). We have summarised the findings in summary of findings Table for the main comparison.
-
Peg interferon versus no intervention control (Chalasani 2005; 65 participants): RR 0.48; 95% CI 0.05 to 5.09; Fisher's exact test P value = 0.61.
-
Peg interferon plus ribavirin versus no intervention control (Carrion 2007; 54 participants): RR 3.00; 95% CI 0.13 to 70.53; Fisher's exact test P value = 1.00.
-
Ribavirin plus peg interferon versus peg interferon (Angelico 2007; Gane 2009; 98 participants): RR 0.50; 95% CI 0.05 to 5.20; test for overall effect P value = 0.56.
-
Interferon versus no intervention control (Cotler 2001; 12 participants): RR 1.67; 95% CI 0.08 to 33.75; Fisher's exact test P value = 1.00.
-
Interferon plus ribavirin versus no intervention control (Samuel 2003; 52 participants): RR 0.29; 95% CI 0.01 to 6.74; Fisher's exact test P value = 0.46.
-
Ribavirin versus interferon (Gane 1998; 30 participants): RR not estimable; Fisher's exact test P value = 1.00.
-
Ribavirin versus placebo (Calmus 2012; 77 participants): RR not estimable; Fisher's exact test P value = 1.00.
Overall, there was no significant difference in mortality between the intervention and control groups in any of the comparisons. There was no change in results when using the random‐effects model for the ribavirin plus peg interferon versus peg interferon comparison, the only comparison with at least two trials. The issue of random‐effects model versus fixed‐effect model did not arise in the other comparisons because of the presence of only one trial in these comparisons.
Trial sequential analysis was performed for the only comparison with at least two trials (ie, ribavirin plus peg interferon versus peg interferon). The proportion of patients recruited was less than 1% of the diversity‐adjusted required information size (DARIS) and so the trial sequential monitoring boundaries were not drawn. The conventional boundaries were not crossed (Figure 4.
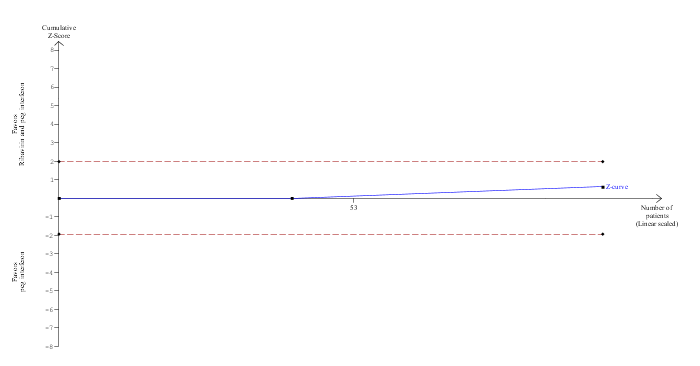
Trial Sequential Analysis of mortality (ribavirin plus peg interferon versus peg interferon)
The diversity‐adjusted required information size (DARIS) was calculated to 16,594 patients, based on the proportion of patients in the control group with the outcome of 4.1%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 0%. To account for zero event groups, a continuity correction of 0.01 was used in the calculation of the cumulative Z‐curve (blue line). After accruing 98 participants in two trials, only 0.59% of the DARIS has been reached. Accordingly, the Trial Sequential Analysis does not show the required information size and the trial sequential monitoring boundaries. As shown, the conventional boundaries (dotted red line) have also not been crossed by the cumulative Z‐curve.
Retransplantation
Two comparisons reported retransplantation (Analysis 1.2). The trials that contributed to the comparisons are shown along with the number of participants, RR, and P value derived from the Fisher's exact test as there was only one trial for each comparison. We have summarised the findings in summary of findings Table 2.
-
Interferon versus no intervention control (Cotler 2001; 12 participants): RR 1.67; 95% CI 0.08 to 33.75; Fisher's exact test P value = 1.00.
-
Ribavirin versus placebo (Calmus 2012; 77 participants): RR not estimable; Fisher's exact test P value = 1.00.
Overall, there was no significant difference in retransplantation between the intervention and control groups in the two comparisons. The issue of random‐effects model versus fixed‐effect model did not arise in the two comparisons because of the presence of only one trial in the two comparisons. Trial sequential analysis was not performed as there was only one trial for each comparison.
Quality of life
Quality of life was not reported in any of the trials.
Serious adverse events
Two comparisons reported the proportion of participants who developed serious adverse events (Analysis 1.3). The trials that contributed to the comparisons are shown along with the number of participants, RR, and P value derived from Fisher's exact test as there was only one trial for each comparison.
-
Ribavirin plus peg interferon versus peg interferon (Gane 2009; 56 participants): RR 3.40; 95% CI 1.46 to 7.94; Fisher's exact test P value = 0.002.
-
Ribavirin versus placebo (Calmus 2012; 77 participants): RR 5.55; 95% CI 0.70 to 43.95; Fisher's exact test P value = 0.11.
Overall, there was a significantly higher proportion of participants who developed serious adverse events in the ribavirin plus peg interferon combination therapy than peg interferon monotherapy and no significant difference in the proportion of participants who developed serious adverse events between the ribavirin and placebo groups as shown above. The issue of random‐effects model versus fixed‐effect model did not arise in the two comparisons because of the presence of only one trial in each of the two comparisons. Trial sequential analysis was not performed as there was only one trial for each comparison.
Two comparisons reported the number of serious adverse events in each group (Analysis 1.4). The trials that contributed to the comparisons are shown along with the number of participants, RaR, and P value derived from Review Manager (RevMan 2012).
-
Peg interferon versus no intervention control (Chalasani 2005; 65 participants): RaR 1.15; 95% CI 0.52 to 2.57; P value = 0.73.
-
Ribavirin plus peg interferon versus peg interferon (Angelico 2007; 42 participants): RaR 1.20; 95% CI 0.36 to 3.96; P value = 0.56.
Overall, there was no significant difference in number of serious adverse events between the intervention and control groups in the two comparisons. The issue of random‐effects model versus fixed‐effect model did not arise in the two comparisons because of the presence of only one trial in each of the two comparisons.
Liver decompensation
Liver decompensation was not reported in any of the trials.
Graft rejections
The following two comparisons reported graft rejections requiring retransplantation (Analysis 1.5). The trials that contributed to the comparisons are shown along with the number of participants, RR, and P value (derived from Fisher's exact test as there was only one trial for each comparison).
-
Peg interferon plus ribavirin versus no intervention control (Belli 2012; 72 participants): (RR 1.00; 95% CI 0.07 to 15.38); Fisher's exact test P value = 1.00.
-
Interferon versus no intervention control (Cotler 2001; 12 participants): RR 1.67; 95% CI 0.08 to 33.75; Fisher's exact test P value = 1.00.
Only one comparison reported graft rejections requiring medical treatment (Analysis 1.6). The trial that contributed to the comparison is shown along with the number of participants, RR, and P value (derived from Fisher's exact test as there was only one trial for each comparison).
-
Ribavirin plus peg interferon versus peg interferon (Angelico 2007; 42 participants): RR 0.33; 95% CI 0.04 to 2.95; Fisher's exact test P value = 0.061.
The following comparisons reported graft rejections without describing the treatment that these participants underwent for graft rejection (Analysis 1.7). The trials that contributed to the comparisons are shown along with the number of participants, RR, and P value (derived from Fisher's exact test when there was only one trial and derived from Review Manager when there were two or more trials) (RevMan 2012).
-
Peg interferon versus control (Chalasani 2005; 65 participants): RR 24.26; 95% CI 1.50 to 393.41; Fisher's exact test P value = 0.0001.
-
Peg interferon plus ribavirin versus control (Carrion 2007; 54 participants): RR 3.00; 95% CI 0.13 to 70.53; Fisher's exact test P value = 1.00.
-
Ribavirin plus peg interferon versus peg interferon (Gane 2009; 56 participants): RR 1.00; 95% CI 0.07 to 15.21; Fisher's exact test P value = 1.00.
-
Peg interferon (1.5 μg/kg/week) plus ribavirin versus peg interferon (0.5 μg/kg/week) plus ribavirin (Gordon 2005; 13 participants): RR not estimable; Fisher's exact test P value = 1.00.
-
Interferon plus ribavirin versus control (Samuel 2003; 52 participants): RR 2.59; 95% CI 0.11 to 60.69; Fisher's exact test P value = 1.00.
Overall there was no significant difference in the proportion of participants who developed graft rejection in any of the comparisons other than peg interferon versus control. In the comparison between peg interferon and control, the proportion of participants who developed graft rejection (of unknown treatment) was significantly higher in the peg interferon group than the control group as shown above. The issue of random‐effects model versus fixed‐effect model did not arise in any of these comparisons because of the presence of only one trial in these comparisons. Trial sequential analysis was not performed as there was only one trial for each comparison.
Fibrosis worsening
The following comparisons reported worsening of fibrosis (Analysis 1.8). The trials that contributed to the comparisons are shown along with the method used to measure the fibrosis, time of follow‐up measurement of fibrosis, number of participants, RR, and P value (derived from Fisher's exact test when there was only one trial and derived from Review Manager when there were two or more trials) (RevMan 2012).
-
Peg interferon versus no intervention control (Chalasani 2005; Ishak score, at the end of treatment; 45 participants): RR 0.82; 95% CI 0.30 to 2.19; Fisher's exact test P value = 0.75.
-
Peg interferon plus ribavirin versus no intervention control (Carrion 2007; Belli 2012; Sheur classification, six months after end of treatment (Carrion 2007) and Ishak score,12 months after end of treatment (Belli 2012); 126 participants): Fixed‐effect model = RR 0.71; 95% CI 0.51 to 0.98; test for overall effect P value = 0.04; random‐effects model = RR 0.63; 95% CI 0.23 to 1.74; test for overall effect P value = 0.38.
-
Ribavirin plus peg interferon versus peg interferon (Angelico 2007; Ishak score, 6 to 12 months after end of treatment; 42 participants): RR 2.00; 95% CI 0.20 to 20.41; Fisher's exact test P value = 1.00.
-
Amantadine plus peg interferon plus ribavirin versus peg interferon versus ribavirin (Nair 2008; METAVIR score, at the end of treatment; 30 participants): RR 0.75; 95% CI 0.28 to 2.02; Fisher's exact test P value = 0.71.
-
Interferon plus ribavirin versus no intervention control (Samuel 2003; METAVIR score, 24 weeks after end of treatment; 52 participants): RR 0.29; 95% CI 0.01 to 6.74; Fisher's exact test P value = 0.46.
-
Ribavirin versus interferon (Gane 1998; Knodell score, 24 weeks from start of treatment; 30 participants): RR 0.73; 95% CI 0.28 to 1.88; Fisher's exact test P value = 0.71.
Overall, there was no significant difference in fibrosis worsening between the intervention and control groups in any of the comparisons except for the fixed‐effect model for the comparison peg interferon plus ribavirin versus no intervention control, which showed significantly lower fibrosis worsening in the peg interferon plus ribavirin group as shown above. However, on using the random‐effects model, there was no significant difference between these groups as shown above. The issue of random‐effects model versus fixed‐effect model did not arise in the other comparisons because of the presence of only one trial in each of these comparisons. Trial sequential analysis was performed for the only comparison with at least two trials (ie, ribavirin plus peg interferon versus no intervention control). The proportion of patients recruited was only 3.1% of the DARIS and so trial sequential boundaries were not drawn. The conventional boundaries were not crossed (Figure 5).

Trial Sequential Analysis of fibrosis worsening (ribavirin plus peg interferon versus control)
The diversity‐adjusted required information size (DARIS) was calculated to 4066 patients, based on the proportion of patients in the control group with the outcome of 65.1%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 88.93%. After accruing 126 participants in two trials, only 3.1% of the DARIS has been reached. Accordingly, the Trial Sequential Analysis does not show the required information size and the trial sequential monitoring boundaries. As shown, the conventional boundaries (dotted red line) was no longer crossed by the cumulative Z‐curve after two trials although the conventional boundaries were crossed after the first trial.
Subgroup analysis
We did not perform any subgroup analyses because of the few trials included under each comparison.
Sensitivity analysis
Quality of life was not reported in any of the trials. So, we did not impute mean or standard deviation in any of the trials. For this reason, we did not perform any sensitivity analyses.
Reporting bias
We did not explore reporting bias using a funnel plot because of the few trials included under each comparison.
Discussion
This review has assessed the various therapies for hepatitis C virus recurrence in participants with liver transplantation. There were no significant differences in the mortality, retransplantation, or graft rejection requiring treatment in any comparison in the few trials that reported these outcomes. Quality of life and hepatic decompensation were not reported in any of the trials. The proportion of patients with serious adverse events was significantly higher in the ribavirin plus peg interferon combination therapy than peg interferon monotherapy. There was no significant difference in the proportion of patients who developed serious adverse events and the number of serious adverse events in the other comparisons in which serious adverse events were reported. These are the main clinical outcomes, which should determine whether antiviral therapy should be used for the treatment of recurrent liver graft infection with hepatitis C virus. However, the participants were followed up for only 24 to 26 weeks after the end of treatment (ie, around 17 to 18 months). Longer periods of follow‐up are necessary to determine any clinical benefit.
Anaemia, renal impairment, and other adverse effects (such as thrombocytopenia, neutropenia, headache, insomnia, and myalgia) are frequent with antiviral treatment (Gurusamy 2010). Considering the lack of clinical benefit and the frequent adverse effects, there is currently no evidence to recommend antiviral treatment for recurrent liver graft infection with hepatitis C virus. Having achieved the main objective, we decided to analyse the various factors that should be taken into account if a new trial assessing the role of antiviral treatment for recurrent liver graft infection with hepatitis C virus is performed.
One of the important issues that should be considered before a trial assessing the role of antiviral therapy for recurrent liver graft infection with hepatitis C virus is performed is the safety of the treatment. As mentioned previously, adverse effects such as thrombocytopenia, neutropenia, or anaemia may require reduction in dose or cessation of therapy. Evidence from a randomised clinical trial showed that granulocyte colony‐stimulating‐factor is effective in normalising neutropenia induced by interferon plus ribavirin therapy in participants with chronic viral hepatitis (Sharvadze 2007). Evidence from three randomised clinical trials showed that epoetin alpha (recombinant erythropoietin) is effective in 83% to 100% of participants (with chronic hepatitis C virus infection on interferon plus ribavirin therapy) in avoiding a reduction in ribavirin dose because of anaemia (Dieterich 2003; Afdhal 2004; Sharvadze 2006). Use of granulocyte colony‐stimulating‐factor and erythropoietin may help in achieving higher cumulative doses in participants with hepatitis C virus recurrence after liver transplantation also. Multivariate analysis of case series of interferon plus ribavirin therapy in liver transplantation participants showed that the participants who attained sustained virological response (SVR) had greater cumulative doses of interferon and ribavirin than those who did not attain sustained viral response (Sharma 2007). No serious adverse effects that preclude the use of antiviral therapy were reported in any trial. There was a significant difference in the proportion of participants who achieved SVR after combination therapy with peg interferon plus ribavirin when compared with no treatment (see comparison number 2 above). Observational studies have shown that SVR after treatment is associated with reduction in mortality (Picciotto 2007) compared with treatment failures. While this could be because of SVR after treatment being a prognostic marker, there is also a possibility of survival benefit (by decreasing the exposure of the liver to the viral insult) if SVR was achieved. Thus, perhaps it is worth carrying out further trials of low‐bias risk assessing the role of antiviral treatment for recurrent liver graft infection with hepatitis C virus. The main outcomes that need to be assessed in such trials would be mortality, retransplantation (particularly for graft failure), liver decompensation, and quality of life (to determine if the treatment improves the quality adjusted life years and to perform economic evaluation). In order to determine any difference in these outcomes, the duration and method of follow‐up should be appropriate. It is expected that any difference in survival is likely to be noted only after five years. Thus, the trial should be adequately powered; it should use the appropriate methodology and outcomes; and it should include a long period of follow‐up to determine the important outcomes.
The conductors of such trials are likely to face many problems. The first issue is the duration of the hepatitis C virus recurrence. The participants may have different stages of fibrosis in the graft. The effectiveness of the intervention may vary with the stage of fibrosis in the graft. So, the participants have to be stratified based on the stage of the fibrosis and a subgroup analysis based on the stage of fibrosis should be performed. Stratification may also have to be carried out for patient and viral factors such as genotype and the initial viral load, which may also influence the outcomes. The second issue is the choice of the experimental drug. From the trials included in this review, it appears that the combination therapy with peg interferon plus ribavirin (with the use of growth factors if necessary) offers maximum promise. However, considering the duration of recruitment (see below) and the long follow‐up required for the main outcomes to be assessed, it is possible that a much superior treatment becomes available. Protocols should be in place for such an eventuality. The third issue is that of blinding the participants. Since the duration of treatment is 48 to 52 weeks and weekly injections are required for peg interferon, the blinding of the participants may be difficult. This lack of blinding will result in bias in the quality of life measures. However, the main outcomes such as mortality, retransplantation, or liver decompensation may not be affected by lack of patient blinding. The healthcare provider can be blinded by requesting the patient or a third party not involved in the trial to give the subcutaneous injections. The outcome assessors can be blinded if adequate efforts are made to achieve this. Another issue is the bias arising due to missing outcomes. Because of the long duration of follow‐up required for the assessment of outcomes, adequate efforts must be made to minimise the proportion of participants lost to follow‐up. Another important issue is sample size calculations. In a study based on 11,036 liver transplant recipients in the United Network for Organ Sharing (UNOS) Scientific Registry (a database of liver transplant recipients in the USA) with a mean follow‐up of 2.1 years, the actuarial five‐year survival rate was 69.9% in liver transplants performed for hepatitis C virus infection as compared with that in non‐hepatitis C virus participants, which was 76.6% (Forman 2002). The actuarial five‐year graft survival rate was 56.8% in liver transplants performed for hepatitis C virus infections versus 67.7% in liver transplants performed for non‐hepatitis C virus participants. In another retrospective study (Ghobrial 1999), the five‐year retransplantation proportion was 76/374 (20.3%) after a median follow‐up of 22.7 months. The retransplantation proportion directly related to hepatitis C virus recurrence was 3.4%. However, retransplantation rate may be a difficult outcome as there is no uniform agreement among experts regarding the criteria for retransplantation and may not be a suitable objective outcome measure. If survival is chosen as the primary outcome, the presence of hepatocellular carcinoma in the removed liver may be a confounding factor (if a significant proportion of the participants have hepatocellular carcinoma). This may necessitate two different trials or one trial with a planned subgroup analysis of participants with and without hepatocellular carcinoma. This is because of the significantly lower survival rate in participants undergoing liver transplantation for malignancy (Forman 2002). The proportion of participants undergoing liver transplantation for malignancy who had hepatocellular carcinoma is not clear from the report by Forman 2002. However, the presence of hepatocellular carcinoma prior to liver transplantation for hepatitis C may influence the survival necessitating two different trials or one trial with a planned subgroup analysis.
None of the trials reported whether the donors consented for organ donation. Future trials should clearly state whether donors consented for organ donation.

Methodological quality graph: Review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: Review authors' judgements about each methodological quality item for each included study.

Trial Sequential Analysis of mortality (ribavirin plus peg interferon versus peg interferon)
The diversity‐adjusted required information size (DARIS) was calculated to 16,594 patients, based on the proportion of patients in the control group with the outcome of 4.1%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 0%. To account for zero event groups, a continuity correction of 0.01 was used in the calculation of the cumulative Z‐curve (blue line). After accruing 98 participants in two trials, only 0.59% of the DARIS has been reached. Accordingly, the Trial Sequential Analysis does not show the required information size and the trial sequential monitoring boundaries. As shown, the conventional boundaries (dotted red line) have also not been crossed by the cumulative Z‐curve.

Trial Sequential Analysis of fibrosis worsening (ribavirin plus peg interferon versus control)
The diversity‐adjusted required information size (DARIS) was calculated to 4066 patients, based on the proportion of patients in the control group with the outcome of 65.1%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 88.93%. After accruing 126 participants in two trials, only 3.1% of the DARIS has been reached. Accordingly, the Trial Sequential Analysis does not show the required information size and the trial sequential monitoring boundaries. As shown, the conventional boundaries (dotted red line) was no longer crossed by the cumulative Z‐curve after two trials although the conventional boundaries were crossed after the first trial.

Comparison 1 Intervention versus control, Outcome 1 Mortality.
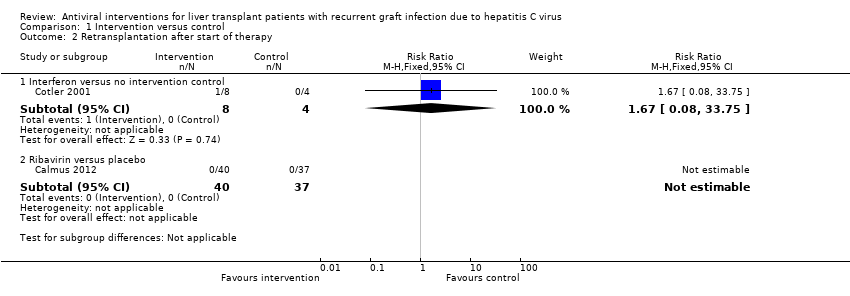
Comparison 1 Intervention versus control, Outcome 2 Retransplantation after start of therapy.
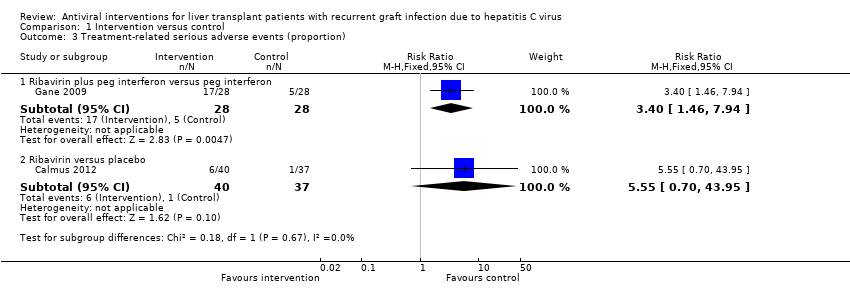
Comparison 1 Intervention versus control, Outcome 3 Treatment‐related serious adverse events (proportion).

Comparison 1 Intervention versus control, Outcome 4 Treatment‐related serious adverse events (number of serious adverse events).
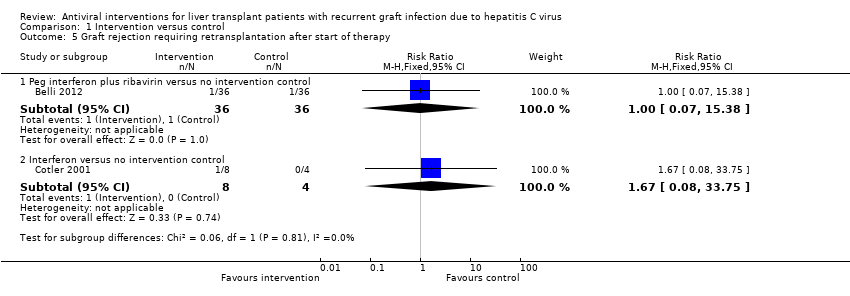
Comparison 1 Intervention versus control, Outcome 5 Graft rejection requiring retransplantation after start of therapy.

Comparison 1 Intervention versus control, Outcome 6 Graft rejection requiring medical treatment.

Comparison 1 Intervention versus control, Outcome 7 Graft rejection (others with unknown treatment).

Comparison 1 Intervention versus control, Outcome 8 Fibrosis worsening.
| Mortality | ||||||
| Patient or population: Participants with recurrent liver graft infection with hepatitis C virus. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Intervention | |||||
| Peg interferon vs.control | 62 per 1000 | 30 per 1000 | RR 0.48 | 65 | ⊕⊝⊝⊝ | The assumed risk was the control group risk. |
| Peg interferon plus ribavirin vs.control | 63 per 1000 | 189 per 1000 | RR 3 | 54 | ⊕⊝⊝⊝ | Since there were no deaths in the control group, the assumed risk was the control group risk in a different trial included in this review. |
| Ribavirin plus peg interferon vs.peg interferon | 41 per 1000 | 20 per 1000 | RR 0.5 | 98 | ⊕⊝⊝⊝ | The assumed risk was the control group risk. |
| Interferon vs.control | 63 per 1000 | 105 per 1000 | RR 1.67 | 12 | ⊕⊝⊝⊝ | Since there were no deaths in the control group, the assumed risk was the control group risk in a different trial included in this review. |
| Interferon plus ribavirin vs.control | 42 per 1000 | 12 per 1000 | RR 0.29 | 52 | ⊕⊝⊝⊝ | The assumed risk was the control group risk. |
| Ribavirin vs.interferon | There were no deaths in either group. | Not estimable | 30 | ⊕⊝⊝⊝ | ‐ | |
| Ribavirin vs.placebo | There were no deaths in either group. | Not estimable | 77 | ⊕⊝⊝⊝ | ‐ | |
| *The basis for the assumed risk is provided in the comments section. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The trial(s) was (were) of high risk of bias. | ||||||
| Retransplantation | ||||||
| Patient or population: Participants with recurrent liver graft infection with hepatitis C virus. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Intervention | |||||
| Retransplantation after start of therapy ‐ ribavirin vs.placebo | No retransplantation in either group | Not estimable | 77 | ⊕⊝⊝⊝ | ||
| Retransplantation after start of therapy ‐ interferon vs.control | 10 per 1000 | 17 per 1000 | RR 1.67 | 12 | ⊕⊝⊝⊝ | There was no retransplantation in the control group. So, we used an assumed risk of 1%. |
| *The basis for the assumed risk is provided in the comments section. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The trial(s) was (were) of high risk of bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Peg interferon versus no intervention control | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 5.09] |
| 1.2 Peg interferon plus ribavirin versus no intervention control | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.53] |
| 1.3 Ribavirin plus peg interferon versus peg interferon | 2 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.20] |
| 1.4 Interferon versus no intervention control | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.08, 33.75] |
| 1.5 Interferon plus ribavirin versus no intervention control | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.74] |
| 1.6 Ribavirin versus interferon | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 Ribavirin versus placebo | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Retransplantation after start of therapy Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Interferon versus no intervention control | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.08, 33.75] |
| 2.2 Ribavirin versus placebo | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Treatment‐related serious adverse events (proportion) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Ribavirin plus peg interferon versus peg interferon | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.4 [1.46, 7.94] |
| 3.2 Ribavirin versus placebo | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.55 [0.70, 43.95] |
| 4 Treatment‐related serious adverse events (number of serious adverse events) Show forest plot | 2 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 4.1 Peg interferon versus no intervention control | 1 | 65 | Rate Ratio (Fixed, 95% CI) | 1.15 [0.52, 2.57] |
| 4.2 Ribavirin plus peg interferon versus peg interferon | 1 | 42 | Rate Ratio (Fixed, 95% CI) | 1.20 [0.36, 3.96] |
| 5 Graft rejection requiring retransplantation after start of therapy Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Peg interferon plus ribavirin versus no intervention control | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.38] |
| 5.2 Interferon versus no intervention control | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.08, 33.75] |
| 6 Graft rejection requiring medical treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Ribavirin plus peg interferon versus peg interferon | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.95] |
| 7 Graft rejection (others with unknown treatment) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Peg interferon versus no intervention control | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 24.26 [1.50, 393.41] |
| 7.2 Peg interferon plus ribavirin versus no intervention control | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.53] |
| 7.3 Ribavirin plus peg interferon versus peg interferon | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.21] |
| 7.4 Peg interferon (1.5 μg/kg/week) plus ribavirin versus peg interferon (0.5 μg/kg/week) plus ribavirin | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.5 Interferon plus ribavirin versus no intervention control | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.59 [0.11, 60.69] |
| 8 Fibrosis worsening Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Peg interferon versus no intervention control | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.30, 2.19] |
| 8.2 Peg interferon plus ribavirin versus no intervention control | 2 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.51, 0.98] |
| 8.3 Ribavirin plus peg interferon versus peg interferon | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.20, 20.41] |
| 8.4 Amantadine plus peg interferon plus ribavirin versus peg interferon plus ribavirin | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.28, 2.02] |
| 8.5 Interferon plus ribavirin versus no intervention control | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.74] |
| 8.6 Ribavirin versus interferon | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.28, 1.88] |

