Corticosteroides prenatales antes de la cesárea programada a término para mejorar los desenlaces neonatales
Resumen
Antecedentes
Los neonatos a término que nacen por cesárea electiva tienen más probabilidades de desarrollar morbilidad respiratoria que los que nacen por vía vaginal. Los corticosteroides profilácticos en los embarazos pretérmino de feto único aceleran la maduración pulmonar y reducen la incidencia de complicaciones respiratorias. No está claro si la administración en gestaciones a término, antes de la cesárea, mejora los desenlaces respiratorios de estos neonatos sin causar morbilidades innecesarias a la madre o al neonato.
Objetivos
El objetivo de esta revisión fue evaluar el efecto de la administración profiláctica de corticosteroides antes de la cesárea programada a término, en comparación con la atención habitual (que podía ser placebo o ningún tratamiento), sobre la morbilidad fetal, neonatal y materna. También se evaluó el impacto del tratamiento en el niño más adelante en la vida.
Métodos de búsqueda
Para esta actualización se realizaron búsquedas en el Registro de ensayos del Grupo Cochrane de Embarazo y parto (Cochrane Pregnancy and Childbirth), en ClinicalTrials.gov (20 de enero de 2021) y en las listas de referencias de los estudios identificados.
Criterios de selección
Se incluyeron los ensayos controlados aleatorizados que compararon la administración profiláctica de corticosteroides prenatales (betametasona o dexametasona) con placebo o con ningún tratamiento, administrados antes de la cesárea programada a término (en la semana 37 de gestación o después). Los ensayos controlados cuasialeatorizados y aleatorizados por conglomerados también fueron elegibles para inclusión.
Obtención y análisis de los datos
Para la obtención y el análisis de los datos se utilizaron los métodos estándar del Grupo Cochrane de Embarazo y parto. Dos autores de la revisión de forma independiente evaluaron los ensayos para inclusión, evaluaron el riesgo de sesgo, evaluaron la confiabilidad (en base a criterios predefinidos desarrollados por el Grupo Cochrane de Embarazo y parto), extrajeron los datos y comprobaron su exactitud y evaluaron la certeza de la evidencia mediante el método GRADE. Los desenlaces principales de esta revisión fueron el síndrome de dificultad respiratoria (SDR), la taquipnea transitoria del neonato (TTN), el ingreso en cuidados especiales neonatales por morbilidad respiratoria y la necesidad de ventilación mecánica.
Se planificó realizar análisis de subgrupos para los desenlaces principales según la edad gestacional en el momento de la asignación al azar y el tipo de corticosteroide (betametasona o dexametasona). También se planificó realizar un análisis de sensibilidad, incluyendo sólo los estudios con bajo riesgo de sesgo.
Resultados principales
Se incluyó un ensayo en el que los participantes se asignaron al azar a recibir betametasona o atención habitual. El ensayo incluyó a 942 mujeres y 942 neonatos reclutados en diez hospitales del Reino Unido entre 1995 y 2002. Esta revisión incluye sólo los ensayos que cumplieron los criterios predefinidos de fiabilidad. Se eliminaron del análisis tres ensayos que se incluyeron en la versión anterior de esta revisión.
El riesgo de sesgo fue bajo para la generación de la secuencia aleatoria, el ocultamiento de la asignación y los datos incompletos de desenlaces. El riesgo de sesgo de informe selectivo de los desenlaces fue incierto porque no hubo un protocolo de ensayo publicado y, por lo tanto, no está claro si todos los desenlaces planificados se informaron por completo. Debido a la falta de cegamiento, se consideró que existía un alto riesgo de sesgo de realización y de detección. Se disminuyó la certeza de la evidencia debido a la preocupación por el riesgo de sesgo y por la imprecisión debido a las bajas tasas de eventos y a los amplios intervalos de confianza (IC) del 95%, que son compatibles con un posible efecto beneficioso y un posible efecto perjudicial.
En comparación con la atención habitual, no está claro si los corticosteroides prenatales reducen el riesgo de SDR (riesgo relativo [RR] 0,34; IC del 95%: 0,07 a 1,65; un estudio; 942 neonatos) o de TTN (RR 0,52; IC del 95%: 0,25 a 1,11; un estudio; 938 recién nacidos) porque la certeza de la evidencia es baja y los IC del 95% son compatibles con un posible efecto beneficioso y un posible efecto perjudicial.
Los corticosteroides prenatales probablemente reducen el riesgo de ingreso en cuidados especiales neonatales por complicaciones respiratorias, en comparación con la atención habitual (RR 0,45; IC del 95%: 0,22 a 0,90; un estudio; 942 neonatos; evidencia de certeza moderada). La proporción de neonatos ingresados en cuidados especiales neonatales por morbilidad respiratoria tras el tratamiento con corticosteroides prenatales fue del 2,3%, en comparación con el 5,1% en el grupo de atención habitual.
No está claro si los corticosteroides prenatales tienen algún efecto sobre el riesgo de necesitar ventilación mecánica, en comparación con la atención habitual (RR 4,07; IC del 95%: 0,46 a 36,27; un estudio; 942 neonatos; evidencia de certeza muy baja). El efecto de los corticosteroides prenatales sobre el desarrollo materno de infección posparto/pirexia en las primeras 72 horas no está claro debido a la certeza muy baja de la evidencia; un estudio (942 mujeres) informó que no hubo casos. Los estudios incluidos no proporcionaron datos sobre la hipoglucemia neonatal ni la mortalidad/morbilidad grave materna.
Conclusiones de los autores
La evidencia de un ensayo controlado aleatorizado indica que los corticosteroides profilácticos antes de la cesárea programada a término probablemente reducen el ingreso en la unidad de cuidados intensivos neonatales por morbilidad respiratoria. No se sabe con certeza si la administración de corticosteroides prenatales reduce las tasas de síndrome de dificultad respiratoria (SDR) o taquipnea transitoria del neonato (TTN). La certeza general de la evidencia para los desenlaces principales fue baja o muy baja, aparte del desenlace de ingreso en cuidados especiales neonatales (todos los niveles) por morbilidad respiratoria, para el que la evidencia fue de certeza moderada. Por lo tanto, actualmente no hay datos suficientes para establecer conclusiones firmes.
Se necesita más evidencia para investigar el efecto de los corticosteroides prenatales profilácticos en la incidencia de morbilidad respiratoria reconocida, como el SDR. Cualquier ensayo futuro debe evaluar el equilibrio entre el efecto beneficioso en términos respiratorios y los posibles efectos adversos inmediatos (p.ej., hipoglucemia), así como los efectos adversos a largo plazo (p.ej., el rendimiento académico) en el lactante. Hay muy poca información sobre los desenlaces de la salud materna que permita asegurar que los corticosteroides no suponen un mayor riesgo de efectos perjudiciales para la madre.
Los estudios de investigación futuros deberían considerar la posibilidad de investigar la efectividad de los corticosteroides prenatales en diferentes edades gestacionales antes de la cesárea. Hay nueve estudios potencialmente elegibles que están actualmente en curso y se podrían incluir en futuras actualizaciones de esta revisión.
PICO
Resumen en términos sencillos
Corticosteroides para la prevención de problemas respiratorios graves en el recién nacido tras una cesárea a término
¿Cuál es el problema?
Los recién nacidos a término (a partir de la semana 37 de embarazo) que nacen por cesárea programada, antes del inicio del parto, tienen más probabilidades de desarrollar complicaciones respiratorias que los nacidos por vía vaginal. Se ha demostrado que administrar a la madre unas inyecciones denominadas corticosteroides reduce el riesgo de problemas respiratorios en los que nacen antes de las 34 semanas de embarazo, pero no está claro si también son útiles para los que nacen por cesárea a término.
¿Por qué es esto importante?
La cesárea aumenta el riesgo de que el recién nacido a término presente problemas respiratorios, como una respiración rápida durante los primeros días (conocida como taquipnea transitoria del neonato) y el, más grave, síndrome de dificultad respiratoria (SDR). Los recién nacidos afectados pueden necesitar tratamiento en unidades de cuidados especiales. Este riesgo disminuye desde la semana 37 hasta la 39 de gestación, momento en el que es bajo. La mayoría de las cesáreas se realizan después de las 39 semanas de gestación, pero hay algunos casos en los que el parto se debe realizar antes. El objetivo de esta revisión fue investigar si los corticosteroides pueden reducir las tasas de problemas respiratorios antes de la cesárea, sin causar problemas para la madre ni el recién nacido.
¿Cómo se identificó y evaluó la evidencia?
Se buscó en la literatura médica estudios controlados aleatorizados que cumplieran con los criterios de fiabilidad y que compararan los efectos de los corticosteroides con un tratamiento placebo (ficticio) o con la atención habitual. La confianza en los hallazgos se calificó sobre la base de factores como el número de estudios, los métodos de estudio, el número de mujeres y recién nacidos implicados, el número de episodios y la variabilidad de los hallazgos.
¿Qué evidencia se encontró?
Se incorporó evidencia hasta el 20 de enero de 2021. Se incluyó un ensayo en el que participaron 942 mujeres y 942 recién nacidos reclutados en diez hospitales del Reino Unido. Las mujeres del grupo de tratamiento recibieron dos dosis del corticosteroide betametasona mediante una inyección en el músculo. Las mujeres del grupo control recibieron la atención habitual. No se utilizaron procedimientos de cegamiento, por lo que todas las mujeres, los cuidadores y los investigadores sabían quiénes recibieron corticosteroides y quiénes recibieron la atención habitual.
No se sabe si los corticosteroides reducen el riesgo de taquipnea transitoria del neonato (un problema respiratorio leve) o síndrome de dificultad respiratoria (es decir, problemas respiratorios graves) en comparación con la atención habitual. Los corticosteroides prenatales probablemente reducen el riesgo de ingreso en cuidados especiales neonatales por complicaciones respiratorias en comparación con la atención habitual.
No se sabe si los corticosteroides tienen algún efecto sobre el riesgo de que el recién nacido necesite asistencia respiratoria adicional (ventilación mecánica) en comparación con la atención habitual. No se sabe con certeza si los corticosteroides prenatales tienen algún efecto sobre las mujeres que desarrollan una infección o una temperatura elevada en las 72 horas siguientes al parto (no hubo casos en el único estudio en el que participaron 942 mujeres).
No se encontró evidencia del riesgo de que el recién nacido presente baja glucosa en sangre ni sobre el riesgo de que la mujer sufra una enfermedad grave, infección de la herida o muera.
Certeza de la evidencia
La certeza de la evidencia del ensayo aleatorizado incluido fue de muy baja a moderada. Esto significa que no es posible estar completamente seguros de que los futuros ensayos establezcan las mismas conclusiones sobre los efectos beneficiosos del tratamiento en los recién nacidos de madres que reciben un ciclo de corticosteroides prenatales antes de la cesárea a término.
¿Qué significa esto?
En un estudio se redujo el riesgo de ingreso en cuidados especiales neonatales por problemas respiratorios. No se sabe si los corticosteroides tienen algún efecto sobre el riesgo de problemas respiratorios graves (síndrome de dificultad respiratoria) o de respiración rápida (taquipnea transitoria) en el neonato, en comparación con la atención habitual. Se necesitan más estudios para investigar si los corticosteroides prenatales reducen el riesgo de problemas respiratorios graves (como el síndrome de dificultad respiratoria). Los ensayos futuros deben asegurarse de evaluar los posibles efectos perjudiciales a corto y largo plazo para la madre y el recién nacido después de recibir un ciclo de corticosteroides prenatales antes de la cesárea a término. Otros estudios de investigación podrían considerar la posibilidad de evaluar si los efectos beneficiosos o perjudiciales identificados por la administración de un ciclo de corticosteroides prenatales se ven afectados por la edad gestacional en la que se realiza la cesárea planificada.
Hay nueve estudios potencialmente elegibles que están actualmente en curso y se podrían incluir en futuras actualizaciones de esta revisión.
Authors' conclusions
Summary of findings
| Antenatal corticosteroids compared to usual care prior to planned caesarean at term on maternal and neonatal outcomes | ||||||
| Patient or population: women undergoing planned elective caesarean section at 37 weeks' gestation and beyond for singleton pregnancy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with usual care | Risk with Antenatal corticosteroids | |||||
| Respiratory distress syndrome (RDS) | Study population | RR 0.34 | 942 | ⊕⊕⊝⊝ | It is uncertain if antenatal corticosteroids have any effect on risk of RDS compared with usual care. | |
| 11 per 1000 | 4 per 1000 | |||||
| Transient tachypnoea of the neonate (TTN) | Study population | RR 0.52 | 942 | ⊕⊕⊝⊝ | It is uncertain antenatal corticosteroids have any effect on risk of TTN compared with usual care. | |
| 40 per 1000 | 21 per 1000 | |||||
| Admission to neonatal special care (all levels) for respiratory morbidity | Study population | RR 0.45 | 942 | ⊕⊕⊕⊝ | Antenatal corticosteroids probably reduce the risk of admission to neonatal special care for respiratory complications compared with usual care. | |
| 51 per 1000 | 23 per 1000 | |||||
| Need for mechanical ventilation | Study population | RR 4.07 | 942 | ⊕⊝⊝⊝ | It is uncertain if antenatal steroids have any effect on the risk of needing mechanical ventilation compared with usual care. | |
| 2 per 1000 | 9 per 1000 | |||||
| Neonatal hypoglycaemia | Study population | Not estimable | 0 studies | ‐ | Outcome not reported in included trial | |
| 0 per 1000 | 0 per 1000 | |||||
| Maternal mortality and severe morbidity | Study population | Not estimable | 0 studies | ‐ | Outcome not reported in included trial | |
| 0 per 1000 | 0 per 1000 | |||||
| Maternal development of postpartum infection/pyrexia in the first 72 hours | Study population | Not estimable | 942 (1 RCT) | ⊕⊝⊝⊝ | It is uncertain if antenatal steroids have any effect on the risk of maternal development of postpartum infection/pyrexia. One trial reported zero cases of postpartum infection/pyrexia in the first 72 hours. | |
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level for serious risk of bias: lack of blinding could influence outcomes 2Downgraded one level for serious imprecision: low event rate and 95% confidence interval that spans possible benefit and possible harm 3Downgraded two levels for very serious imprecision: very low event rate and wide 95% confidence interval that spans possible benefit and possible harm 4Downgraded two levels for very serious imprecision: zero events | ||||||
Background
Description of the condition
The rate of babies born by caesarean section is increasing globally, particularly in high‐ and middle‐income countries (Betrān 2016). Caesarean section is a risk factor for the development of neonatal respiratory complications, mostly respiratory distress syndrome (RDS) and transient tachypnoea of the neonate (TTN), both in term and preterm infants (Dani 1999; Gerten 2005; Levine 2001; Morrison 1995; Nielsen 1984; Reed 1978; White 1985). Neonates born at term by caesarean delivery are more likely to develop respiratory morbidity than those born vaginally; this risk increases further for the subgroup of neonates born after elective caesarean section, i.e. before the onset of labour (Bowers 1982; Gerten 2005; Hansen 2007; Morrison 1995), with potentially severe implications (Roth‐Kleiner 2003). The risk decreases with advancing gestational age, and neonates born between 37 weeks and zero days' gestation (37 + 0) and 37 + 6 are at 1.7 times greater risk for respiratory complications than those born between 38 + 0 and 38 + 6, which in turn are at 2.4 times greater risk than those born between 39 + 0 and 39 + 6 (Morrison 1995). This trend is particularly pronounced for RDS, where the risk decreases from about 39/1000 for the period between 37 + 0 to 37 + 6 to about 8/1000 for the period between 39 + 0 to 39 + 6, with the odds ratio in comparison to vaginal delivery similarly decreasing from 12.9 before 39 weeks to 1.1 from 39 + 0 onwards (Zanardo 2004). There is little evidence on how mode of anaesthesia (Nielsen 1984; Van den Berg 2001) and fetal gender (Dani 1999; Van den Berg 2001) further affect this risk.
In view of this evidence, it is currently recommended that elective caesarean section should be deferred to 39 weeks' gestation (NICE 2004). However, approximately 10% to 15% of women planned for caesarean may deliver before 39 weeks, and there may be concerns about waiting in the presence of specific clinical indications or previous history. Prophylactic corticosteroids in singleton preterm pregnancies accelerate lung maturation and reduce the incidence of RDS, and administration of steroids is currently recommended between 24 and 34 weeks in cases of threatened preterm labour, antepartum haemorrhage, preterm rupture of membranes or in any condition requiring elective preterm delivery (NICE 2017; RCOG 2004; WHO 2015).
Description of the intervention
In 1972, Liggins and Howie (Liggins 1972) were the first to demonstrate the efficacy of a single course of antenatal corticosteroids in reducing the incidence of RDS in the preterm infants enrolled in their randomised control trial. Subsequently, a number of systematic reviews have been completed and have demonstrated the benefits associated with administration of single (McGoldrick 2020) and repeat course/s (Crowther 2015) of antenatal corticosteroids in women at risk of preterm birth.
The evidence for administration of corticosteroids after 35 weeks' gestation is more controversial. The most recent version of the Cochrane Review on prophylactic corticosteroids for accelerating fetal lung maturation found, in subgroup analyses, no evidence that gestational age at trial entry led to different rates of death, RDS, intraventricular haemorrhage (IVH) or birthweight. This subgroup analysis compared women whose gestational age at trial entry was less than or equal to 35 weeks + 0 days or more than 34 weeks and 0 days (McGoldrick 2020). However, there was some overlap in the gestational age subgroups and the infants included in these randomised trials were born vaginally or by caesarean section.
It remains unclear whether antenatal corticosteroid use at later gestational ages, and prior to planned caesarean section, provide discernable benefit without causing unnecessary harm. One of the randomised controlled trials included in the review on antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth (McGoldrick 2020) included a proportion of babies born at term (Gyamfi‐Bannerman 2017). This trial demonstrated a reduced rate of neonatal respiratory complications in infants who had been administered a course of antenatal corticosteroids prior to preterm delivery between 34 and 36 weeks of gestation (Gyamfi‐Bannerman 2017). However, the authors reported an increased rate of neonatal hypoglycaemia in the infants exposed to betamethasone compared to controls. The potential implications of this are concerning, given the association between prolonged symptomatic neonatal hypoglycaemia and brain injury (Burns 2008; Kerstjens 2012). There has also been some concern about the potential neurodevelopmental harms associated with antenatal corticosteroid exposure at late preterm and early term gestations (Jobe 2018; Jobe 2021; Melamed 2019; Raikkőnen 2020).
How the intervention might work
Respiratory morbidity in cases of term elective caesarean births appears to have a different pathophysiology than in preterm birth, the most likely reasons being fluid retention in the lungs (Avery 1966) and, especially, lack of the physiological catecholamine surge (Brown 1983; Irestedt 1984). Interestingly, recent evidence indicates that, apart from the traditional mechanical concept of 'vaginal squeeze', molecular mechanisms (predominantly lung epithelial sodium channels) promote alveolar fluid drainage, and these channels may be underactive in fetuses not exposed to the process of labour (Jain 2006). Glucocorticoids (a class of corticosteroids) appear to increase the number and function of sodium channels, as well as the responsiveness to catecholamines and thyroid hormones (Jain 2006), providing a rationale for their exogenous administration in cases of elective caesarean.
Why it is important to do this review
The practical impact of routine steroid administration in scheduled caesarean delivery at term is important to assess, given that caesarean births represent 30% to 40% of all births in some countries (McClure 2007), and approximately half of these are elective at term. After the publication of the Antenatal Steroids for Term Elective Caesarean Section (ASTECS) trial (Stutchfield 2005) and the original version of this Cochrane Review (Sotiriadis 2009), a recommendation for prophylactic corticosteroids before elective cesarean section at term was introduced in the now archived Royal College of Obstetricians and Gynaecologists (RCOG) Green‐top Guideline No. 7 (RCOG 2010). The recommendation was not repeated in the currently active National Institute for Health and Care Excellence (NICE) guidelines on preterm birth (NICE 2017) or caesarean section (NICE 2021). The only scientific body that currently considers this practice is the International Federation of Gynaecology and Obstetrics (FIGO) (FIGO 2019). Meanwhile, a follow‐up of the ASTECS study reported that children exposed to antenatal corticosteroids were slightly more likely to be in the lower quartile of academic ability at school than non‐exposed children (Stutchfield 2013), and much attention has been drawn to the potential of antenatal corticosteroids to cause neonatal hypoglycaemia in late‐preterm neonates (Gyamfi‐Bannerman 2017). Therefore, it is timely to update the evidence and reassess the overall effect of antenatal steroids before elective caesarean section at term.
Objectives
The objective of this review was to assess the effect of prophylactic corticosteroid administration before elective caesarean section at term, as compared to usual management (which includes placebo or no treatment), on fetal and neonatal morbidity and maternal morbidity. We also assessed the impact of the treatment on the child in later life.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials comparing prophylactic antenatal corticosteroid administration with placebo or with no treatment, given before elective caesarean section at term (at or after 37 + 0 weeks of gestation). Quasi‐randomised and cluster‐randomised controlled trials were also eligible for inclusion. We did not consider emergency caesarean deliveries at term, as steroid administration is not an appropriate intervention for these cases. Cross‐over trials were not eligible for inclusion.
Types of participants
We included women with singleton or twin pregnancies at term (37 + 0 weeks or more) who underwent elective caesarean section under general or regional anaesthesia. We did not consider triplet pregnancies due to their low prevalence and low likelihood to reach term.
Types of interventions
We included prophylactic maternal corticosteroid administration compared with placebo or no treatment.
Types of outcome measures
For the fetus/neonate
-
Respiratory distress syndrome (RDS) (as defined by the authors of primary reports)
-
Transient tachypnoea of the neonate (TTN) (as defined by the authors of primary reports)
-
Admission to neonatal special care for respiratory morbidity (all levels of care or neonatal intensive care unit (NICU))
-
Need for mechanical ventilation
-
Neonatal hypoglycaemia (defined as a blood glucose of less than 2.6 millimoles per litre (mmol/L))
For the woman
-
Maternal mortality and severe morbidity
-
Maternal development of postpartum infection/pyrexia in the first 72 hours
For the fetus/neonate
-
Admission to neonatal special care for any indication (all levels of special care or NICU)
-
Development of neonatal respiratory complications (pneumonia, air leak syndrome)
-
Neonatal infectious morbidity (e.g. systemic infection in the first 48 hours of life or whilst on the NICU)
-
Surfactant use
-
Perinatal death
-
Chronic lung disease (need for oxygen supplementation beyond 28 days of life)
-
Length of stay in the NICU
-
Duration of mechanical ventilation
-
Readmission for respiratory problems after initial discharge
-
Long‐term infantile morbidity
-
Survival free of neurodevelopmental disability (defined as one or more of the following: cerebral palsy, deafness, blindness, developmental delays/intellectual impairment (Mental Developmental Index (MDI) or Psychomotor Development Index (PDI) less than 70))
-
Cognitive impairment (as defined by authors)
-
Emotional and behavioural problems
For the woman
-
Adverse maternal effects of therapy
Different levels of neonatal special care may not be directly comparable in different reports. In the context of this review, we considered the definitions of the American Pediatric Association (specialty neonatal care ‐ level II (resuscitation and stabilisation of preterm and/or ill infants before transfer to a facility at which newborn intensive care is provided) versus subspecialty neonatal care ‐ level III (NICU)) (Stark 2004) and the British Association of Perinatal Medicine (special care versus high dependency and intensive care) (BAPM 2001).
Search methods for identification of studies
The following section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (20 January 2021). The Register is a database containing over 27,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register — including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings; and the list of journals reviewed via the current awareness service — please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (which includes the results of the centralised search of the WHO International Clinical Trials Registry Platform (ICTRP));
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections ('Included studies', 'Excluded studies', 'Studies awaiting classification' or 'Ongoing studies').
In addition, we searched ClinicalTrials.gov for unpublished, planned and ongoing trial reports (20 January 2021), using the search methods described in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies. We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see Sotiriadis 2018. For this update, the following methods were used for assessing the new studies that were identified as a result of the updated search. The following section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third review author.
Screening eligible studies for trustworthiness
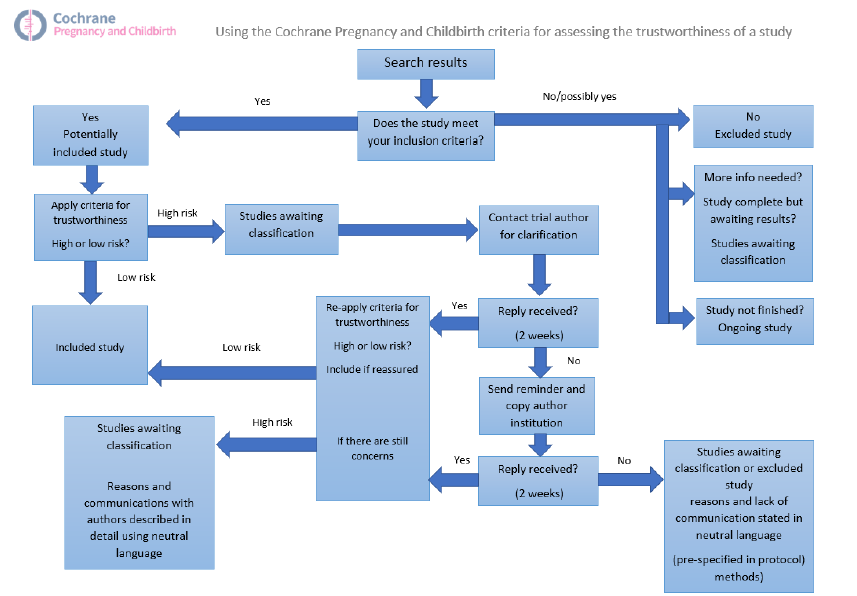
All studies meeting our inclusion criteria were evaluated by at least two review authors against predefined criteria to select studies that, based on available information, were deemed to be sufficiently trustworthy to be included in the analysis. The trustworthiness screening tool was developed by Cochrane Pregnancy and Childbirth and contains the following criteria.
Research governance
-
Was the study prospectively registered (for those studies published after 2010) If not, was there a plausible reason?
-
When requested, did the trial authors provide/share the protocol and/or ethics approval letter?
-
Did the trial authors engage in communication with the Cochrane Review authors within the agreed timelines?
-
Did the trial authors provide individual participant data upon request? If not, was there a plausible reason?.
Baseline characteristics
-
Is the study free from characteristics of the study participants that appear too similar (e.g. distribution of the mean and standard deviation (SD) excessively narrow or excessively wide, as noted by Carlisle 2017)?
Feasibility
-
Is the study free from characteristics that could be implausible (e.g. large numbers of women with a rare condition (such as severe cholestasis in pregnancy) recruited within 12 months)?
-
In cases with (close to) zero losses to follow‐up, is there a plausible explanation?
Results
-
Is the study free from results that could be implausible (e.g. massive risk reduction for main outcomes with small sample size)?
-
Do the numbers randomised to each group suggest that adequate randomisation methods were used (e.g. is the study free from issues such as unexpectedly even numbers of women ‘randomised’ including a mismatch between the numbers and the methods, if the authors say ‘no blocking was used’ but still end up with equal numbers, or if the authors say they used ‘blocks of 4’ but the final numbers differ by 6)?
Studies assessed as being potentially ‘high risk’ were not included in the review. Where a study was classified as ‘high risk’ we attempted to contact the study authors to address any possible lack of information/concerns. In cases where we could not obtain contact details for the study authors, or where adequate information remained unavailable, the study was categorised as ‘awaiting classification’ and the reasons and communications with the author (or lack of) were described in detail.
Abstracts
Data from abstracts will only be included if, in addition to the trustworthiness assessment, the study authors have confirmed in writing that the data to be included in the review have come from the final analysis and will not change. If such information is not available/provided, the study will remain in ‘awaiting classification’ (as above).
See Figure 1 for details of how we applied the trustworthiness screening criteria.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors independently extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy. When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
For each included study we assessed the method as being at:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as being at:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as being at:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes. We assessed methods used to blind outcome assessment as being at low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described the completeness of data, including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses that we undertook.
We assessed methods as being at:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation); or
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as being at:
-
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review had been reported);
-
high risk of bias (where not all the study’s prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported); or
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For each included study we described any important concerns we had about other possible sources of bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CIs).
Continuous data
We used the mean difference (MD) if outcomes were measured in the same way between trials. In future updates, should different instruments have been used to measure the same continuous outcome in different ways we will use the standardised mean difference (SMD) with 95% CIs, with the following interpretations.
-
SMD 0.8 or greater = large effect.
-
SMD greater than 0.49 and less than 0.8 = medium effect.
-
SMD greater than 0.19 and less than 0.5 = small effect.
-
SMD less than 0.2 = trivial or no effect.
Unit of analysis issues
The unit randomisation was per woman. In future updates, if we identify trials with twin pregnancies, we will use the number of babies as the denominator.
If we identify trials with more than two arms in future updates, we will take appropriate steps to include all possible pairwise comparisons in the analysis. For example, in a trial with two corticosteroids groups and one control group we would add the two intervention arms together to compare against the control arm for binary outcomes. To avoid double‐counting participants in an analysis of continuous data we would divide the denominator in the control arm by the number of different intervention arms and compare each control group to the separate intervention groups.
Cluster‐randomised trials
No cluster‐randomised trials were identified for inclusion. In future updates, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials, in accordance with the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). We will adjust their standard errors (SEs) using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible) or from a similar trial. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Dealing with missing data
In cases where trial data were missing we contacted the trial authors to ask for missing data. For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We did not identify enough studies to combine in a meta‐analysis, but in future updates where more data are available we will assess heterogeneity in each meta‐analysis using the I² and Chi² statistics and visual inspection of forest plots. We will use the following guidance from the Cochrane Handbook of Systematic Reviewsof Interventions to interpret the I² statistic (Higgins 2020).
-
0% to 40%: might not be important.
-
30% to 60%: may represent moderate heterogeneity.
-
50% to 90%: may represent substantial heterogeneity.
-
75% to 100%: considerable heterogeneity.
If we identify substantial heterogeneity we will use a random‐effects model to conduct the analysis and attempt to explain possible sources of heterogeneity.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it using formal statistical tests.
Data synthesis
We carried out statistical analysis using Review Manager 5 software (RevMan 2014). We did not identify enough studies to carry out meta‐analysis, but in future updates we will use fixed‐effect analysis for combining data where it is reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged to be sufficiently similar.
If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average of the range of possible treatment effects and the results will be presented as the average treatment effect with 95% CIs and the estimate of I².
Subgroup analysis and investigation of heterogeneity
We defined an a priori subgroup analysis to investigate if there are differences between different types of corticosteroids (betamethasone and dexamethasone) and gestational age at birth (37 + 0 to 37 + 6 weeks; 38 + 0 to 38 + 6 weeks; 39 + 0 weeks or later) in neonatal respiratory outcomes because of their different biologic mode of action. In this review, the antenatal corticosteroids were specifically administered 24 to 48 hours before scheduled birth. As the allocation to treatment or placebo was random throughout 37 to 39 weeks, we have no reason to expect bias in allocation according to gestational age. In this context, this subgroup comparison was not postrandomisation.
We did not identify enough studies to undertake subgroup analysis, but in future updates we will assess the following outcomes in subgroup analyses.
-
Respiratory distress syndrome (as defined by the authors of primary reports).
-
Transient tachypnoea of the neonate (as defined by the authors of primary reports).
-
Admission to neonatal special care for respiratory morbidity (all levels of care or NICU).
-
Need for mechanical ventilation.
We will assess subgroup differences by interaction tests available within Review Manager 5 (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analyses by removing studies from the analysis which had high risk of bias in terms of random sequence generation, allocation concealment or incomplete outcome data. However, there were too few studies included in this update to carry out any meaningful sensitivity analysis.
Summary of findings and assessment of the certainty of the evidence
For this update we used the GRADE approach, as outlined in the GRADE handbook, in order to assess the certainty of the body of evidence relating to the following outcomes for the main comparison, antenatal corticosteroids versus no steroids.
-
Respiratory distress syndrome (as defined by the authors of primary reports).
-
Transient tachypnoea of the neonate (as defined by the authors of primary reports).
-
Admission to neonatal special care for respiratory morbidity (all levels of care or NICU).
-
Need for mechanical ventilation.
-
Neonatal hypoglycaemia.
-
Maternal mortality and severe morbidity.
-
Maternal development of postpartum infection/pyrexia in the first 72 hours.
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5 (RevMan 2014) in order to create a summary of findings table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Results of the search
See Figure 2 for a full description of the study identification process.
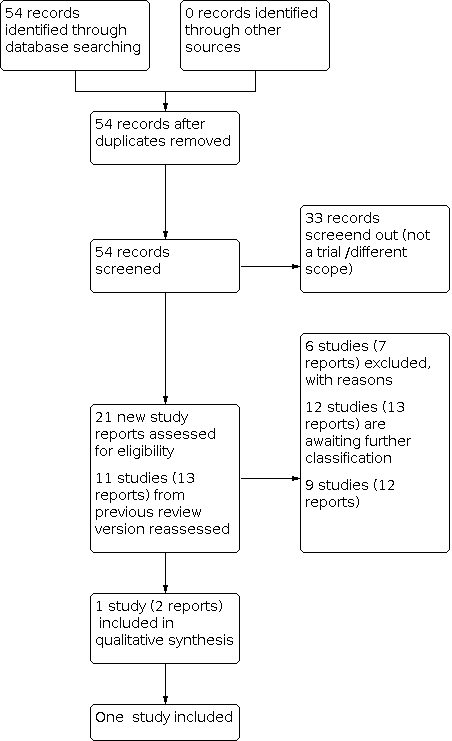
Study flow diagram
For this review update, we retrieved 21 new study reports to assess. We also reassesed all 11 studies (13 reports) in the previous version of the review. We included one study (two reports) and excluded six (seven reports). Nine studies (12 reports) are ongoing and 12 studies (13 reports) are awaiting classification.
Screening eligible studies for trustworthiness
We categorised 12 studies as awaiting classification because they did not fulfil our trustworthiness criteria (see Characteristics of studies awaiting classification). From the 13 eligible studies identified from our search and the four included studies in the previous version of the review, we judged that 12 studies did not meet our trustworthiness criteria for the following reasons.
-
Ten studies published since 2010 did not provide a plausible reason for not having prospective trial registration (Ahmed 2015; Afzal 2019; Ammar 2013; Elewa 2020; Ismail 2017; Kurt 2019; Nada 2016; Nabil 2020; Nooh 2018; Sadiq 2019).
-
One study had authors who have not responded to our queries seeking clarification about their outcome data (Elbohoty 2020).
-
One study was published only as an abstract and the authors have not confirmed that the data were from the final analysis (Kholeif 2010).
Included studies
One study met our inclusion criteria and our trustworthiness criteria (Stutchfield 2005). In this study 998 women with singleton pregnancies were randomised; 942 women and 942 infants were included in the analysis. For further details see Characteristics of included studies.
Design
The study was a two‐arm, parallel‐group randomised controlled trial.
Setting
The study took place in ten hospitals the UK.
Participants
The study included women with singleton pregnancies, undergoing a planned elective cesarean section at or after 37 weeks' gestation.
Interventions and comparators
The women in the treatment group received two doses of 12 mg of betamethasone, administered intramuscularly 24 hours apart, 48 hours before delivery. The women in the comparator group received treatment as usual without antenatal steroids.
Outcomes
The study reported all four of our primary outcomes and several of our secondary outcomes. We did not identify any evidence relating to chronic lung disease, duration of mechanical ventilation, maternal development of postpartum infection/pyrexia, trauma infection, long‐term infantile morbidity or survival free of neurodevelopmental disability.
Dates of study
The study took place from February 1995 to December 2002.
Funding sources
The study received funding from the UK's National Health Service.
Declarations of interest
The authors of Stutchfield 2005 declared that they had no competing interests.
Ongoing studies
We identified nine ongoing studies (see Characteristics of ongoing studies).
Excluded studies
We excluded six studies (see Characteristics of excluded studies). In two of these, the women undergoing caesarean section were not at term gestations (Christofori 2011; Ontela 2018). Three studies had differences in gestational ages of participants among the treatment and control groups (Koch 2016; Sananes 2017). One study was a comparison between two different corticosteroids (Issa 2019). One study (Jain 2005) was terminated due to slow enrolment and lack of funding. No trial data were available to include in this review.
Risk of bias in included studies
Figure 3 illustrates the risks of bias, which are explained in more detail below.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We judged Stutchfield 2005 to be at low risk of bias for random sequence generation because it used a random number generator to allocate women to treatment groups.
Allocation concealment
We judged the included study to be at low risk of bias for allocation concealment because the list of treatment allocation was kept centrally and was concealed from all participants.
Blinding
Performance bias
We judged the included study to be at high risk of bias for blinding of participants and personnel because no placebo was used in the control group, therefore women and the people involved in their care were aware of their treatment allocation, which could have had an influence on outcomes.
Detection bias
We judged the study to be high risk of bias for blinding of outcome assessment because the assessors were not blinded to treatment allocation, which could have had an influence on outcomes.
Incomplete outcome data
We judged Stutchfield 2005 to be at low risk of bias for incomplete outcome data because attrition was low and non‐differential.
Selective reporting
We judged the included study to be at unclear risk of bias for selective reporting because there was no published trial protocol, and it is not clear which outcomes were prespecified and whether all the planned outcomes were reported in full.
Other potential sources of bias
We judged the study to be at low risk of other potential sources of bias because there was nothing in the trial report to suggest any other potential biases.
Effects of interventions
This review includes one study and therefore meta‐analysis was not possible.
Antenatal corticosteroids (betamethasone) versus usual care
Primary outcomes
Respiratory distress syndrome (RDS) (as defined by the authors of primary reports)
It is uncertain if antenatal corticosteroids have any effect on the risk of RDS compared with usual care (risk ration (RR) 0.34, 95% CI 0.07 to 1.65; 1 study; 942 infants; low‐certainty evidence; Analysis 1.1; summary of findings Table 1). The certainty of evidence is low and the 95% CI is consistent with possible benefit and possible harm.
Transient tachypnoea of the neonate (TTN) (as defined by the authors of primary reports)
It is uncertain if antenatal corticosteroids reduce the risk of TTN compared with usual care (RR 0.52, 95% CI 0.25 to 1.11; 1 study; 942 infants; low‐certainty evidence; Analysis 1.2; summary of findings Table 1) because the certainty of evidence is low and the 95% CI is consistent with possible benefit and possible harm. The proportion of infants with TTN after treatment with antenatal corticosteroids was 2.1%, compared with 4% in the usual care group.
Admission to neonatal special care for respiratory morbidity (all levels of care or neonatal intensive care unit (NICU))
Antenatal corticosteroids probably reduce the risk of admission to neonatal special care for respiratory complications compared with usual care (RR 0.45, 95% CI 0.22 to 0.90; 1 study; 942 infants; moderate‐certainty evidence; Analysis 1.3; summary of findings Table 1). The proportion of infants admitted to neonatal special care for respiratory morbidity after treatment with antenatal corticosteroids was 2.3%, compared with 5.1% in the usual care group.
The included study (Stutchfield 2005) also reported a lower risk of admission to the NICU (RR 0.15, 95% CI 0.03 to 0.64, 1 study; 942 infants; Analysis 1.4).
Need for mechanical ventilation
It is uncertain if antenatal steroids have any effect on the risk of needing mechanical ventilation compared with usual care (RR 4.07, 95% CI 0.46 to 36.27; 1 study; 942 infants; very low‐certainty evidence; Analysis 1.5; summary of findings Table 1), because the certainty of evidence is very low and the 95% CI is consistent with possible benefit and possible harm.
Hypoglycaemia (blood glucose less than 2.6 mmol/L)
This outcome was not reported.
Maternal mortality and severe morbidity
This outcome was not reported.
Maternal development of postpartum infection/pyrexia in the first 72 hours
We are uncertain about the effect of antenatal corticosteroids on the maternal development of postpartum infection/pyrexia in the first 72 hours; Stutchfield 2005 reported zero cases (942 women, very low‐certainty evidence; Analysis 1.6, summary of findings Table 1).
Secondary outcomes
Admission to neonatal special care for any indication (all levels of special care or NICU)
It is uncertain if antenatal steroids have any effect on the risk of admission to neonatal special care for any indication (RR 0.81, 95% CI 0.49 to 1.33; 1 study; 942 infants; Analysis 1.7, summary of findings Table 1).
Development of neonatal respiratory complications (pneumonia, air leak syndrome)
This outcome was not reported.
Neonatal infectious morbidity
There were no cases of sepsis in either the corticosteroids group (467 infants) or the usual care group (475 infants); Analysis 1.8.
Surfactant use
This outcome was not reported.
Perinatal death
There were no perinatal deaths in either the corticosteroids group (467 infants) or the usual care group (475 infants); Analysis 1.9.
Chronic lung disease (need for oxygen supplementation beyond 28 days of life)
This outcome was not reported.
Length of stay in the neonatal intensive care unit
Antenatal corticosteroids may lead to a decrease in length of stay in the NICU (MD ‐2.14 days, 95% CI ‐2.50 to ‐1.78 days; 1 study; 942 infants; Analysis 1.10).
Duration of mechanical ventilation
This outcome was not reported.
Readmission for respiratory problems after initial discharge
It is uncertain if antenatal corticosteroids have any effect on the risk of readmission for respiratory problems (RR 0.66, 95% CI 0.35 to 1.25; 1 study; 407 children; Analysis 1.11). These data are based on follow‐up questionnaires completed when the children in Stutchfield 2005 were between eight and 15 years old.
Long‐term infantile morbidity
This outcome was not reported.
Survival free of neurodevelopmental disability (defined as one or more of the following: cerebral palsy, deafness, blindness, developmental delays/intellectual impairment (Mental Developmental Index or Psychomotor Development Index less than 70))
This outcome was not reported.
Cognitive impairment (as defined by authors)
It is uncertain if antenatal corticosteroids have any effect on cognitive impairment, measured as learning difficulties (RR 0.81, 95% CI 0.49 to 1.35; Analysis 1.12; 1 study; 407 children). The learning difficulties measured were dyslexia, attention deficit hyperactivity disorder, severe learning difficulties, Asperger’s syndrome, autism, X‐linked mental retardation, global developmental delay with dysmorphic features, and Down's syndrome. These data are based on follow‐up questionnaires completed when the children in Stutchfield 2005 were between eight and 15 years old.
Emotional and behavioural problems
It is uncertain if antenatal corticosteroids have any effect on emotional and behavioural problems, measured with the Strengths and Difficulties questionnaire (SDQ) score (ranging from zero to 25, where a higher score indicates greater difficulties) (MD 0.18, 95% CI ‐1.12 to 1.48; 1 study; 407 children; Analysis 1.13). These data are based on follow‐up questionnaires completed when the children in Stutchfield 2005 were between eight and 15 years old.
Adverse maternal effects of therapy
There were seven reports of adverse effects in the corticosteroid group compared to zero in the usual care group (RR 15.26, 95% CI 0.87 to 266.36; 1 study; 942 participants; Analysis 1.14). The adverse effects included generalised flushing, nausea, tenderness at the injection site and difficulty sleeping.
Discussion
Summary of main results
In this update we included only one trial, involving 942 women and 942 neonates. There are fewer trials in this update compared to the previous version of the review (Sotiriadis 2018) because we have assessed all the previously included trials against the Cochrane Pregnancy and Childbirth trustworthiness tool. Our results are therefore based only on data that we have judged to be trustworthy.
Antenatal corticosteroids probably reduce the risk of admission to neonatal special care for respiratory complications compared with usual care (summary of findings Table 1).
Compared with usual care, it is uncertain if antenatal corticosteroids have any effect on the risk of RDS, TTN or the risk of needing mechanical ventilation because the certainty of the evidence is low (for the outcomes of RDS and TTN) or very low (for the outcome of mechanical ventilation) and the 95% CI is consistent with possible benefit and possible harm. It is uncertain if antenatal steroids have any effect on the risk of maternal development of postpartum infection/pyrexia (there were zero events) (summary of findings Table 1).
In terms of our secondary outcomes, it is uncertain if antenatal corticosteroids have any effect on the risk of admission to neonatal special care, sepsis, perinatal death, readmission for respiratory problems, cognitive impairment, or emotional and behavioural problems. Antenatal corticosteroids may reduce the length of stay in the NICU. There is limited to no outcome data on substantial maternal health outcomes.
Overall completeness and applicability of evidence
Every effort was made to identify all published and unpublished randomised trial data for the use of prophylactic corticosteroids prior to elective caesarean section at term. Prophylactic antenatal corticosteroids may reduce the rates of admission to special care or NICU for respiratory complications after elective caesarean section (Stutchfield 2005). It is unclear if antenatal corticosteroids have any effect on the risk of RDS, TTN or mechanical ventilation. There is insufficient evidence to investigate if there are differences for gestational age at birth (37 + 0 to 37 + 6 weeks; 38 + 0 to 38 + 6 weeks; 39 + 0 weeks or later) in neonatal respiratory outcomes.
Currently, there are only a limited number of trials that evaluate the effects of prophylactic antenatal corticosteroid administration prior to caesarean section at term. Only one trial is included in this review (Stutchfield 2005). Therefore, there are insufficient data to draw any firm conclusions. Furthermore, this trial was undertaken over 16 years ago, and neonatal practice is likely to have changed, which could impact upon the clinical outcomes included in this review. This trial was a multicentre pragmatic study, and therefore the favourable results demonstrated may also reflect the true effect in regular clinical practice. However, as participants and outcome assessors were not blinded, and admission to a special care or intensive care unit is largely a subjective choice and may be affected by knowledge of treatment assignment, this outcome should be interpreted with caution.
Another systematic review published in 2016 included six trials comparing the use of antenatal corticosteroids with placebo or no treatment in women with a singleton pregnancy at 34 or more weeks' gestation (Saccone 2016). The population included both women expected to deliver in the late preterm period (34 + 0 to 36 + 6 weeks' gestation) and women before planned caesarean delivery at term (37 weeks' gestation or more). The authors found that administration of corticosteroids reduced neonatal respiratory morbidity. Interestingly, two of the three trials (which included women at 37 weeks' gestation or more) included in Saccone 2016 were considered for inclusion in our review, but unfortunately we were unable to confirm trustworthiness, therefore they remain in Studies awaiting classification.
Much larger numbers of participants are needed to adequately power clinical trials to evaluate the true impact of prophylactic antenatal corticosteroid administration on respiratory outcomes with associated morbidity (e.g. RDS). There is also a need to adequately assess for any maternal or neonatal harm associated with corticosteroid administration at term gestations. This is particularly important in view of the low rates of RDS and admission to NICU in this population, and therefore clinicians need to carefully consider the balance of statistical significance versus the clinical significance of the outcomes and the impact on the mother or baby.
The balance of risk is particularly important, as currently there is only limited evidence on any long‐term follow‐up of these infants. In the cohort of infants from the ASTECS trial (Stutchfield 2005), nearly twice as many betamethasone‐exposed children did not attain English proficiency (13% versus 7%), and they were twice as likely to be ranked in the lower quartile of academic ability by teachers (18% versus 9%) (Stutchfield 2013). Although this follow‐up study suffered from a high attrition rate (49%), it highlights the need for caution and for future trials to consider potential harms, both short‐ and long‐term, in their study outcomes. Further data on maternal health outcomes are also needed to provide assertions that the treatment is not harmful to women in terms of adverse effects or increased infectious morbidity.
There are currently nine studies that are ongoing and will be considered for inclusion in future updates. Encouragingly, one of the registered trials includes key clinical outcomes for both the infant and mother and has potential plans for long‐term follow‐up (Groom 2020).
Quality of the evidence
Current evidence comes from only one study. In an effort to ensure that all studies included in the review are sufficiently credible we have applied the trustworthy screening tool developed by the Cochrane Pregnancy and Childbirth Group (Figure 1). Therefore, three of the studies (Ahmed 2015; Nada 2016; Nooh 2018) included in the last version of this systematic review (Sotiriadis 2018) are awaiting classification and are not included in this update. We are awaiting further information from the trial authors particularly in relation to prospective registration, a requirement for all trials published after 2010.
The data we present here come from the only trial that met our prespecified criteria for trial trustworthiness; therefore we can be confident that our findings are based on rigorous clinical trial evidence. Our decision not to include data from three trials that were included in the previous version of this review is based on concerns about the rigour and conduct of those trials. Similar concerns were raised in a recent article specifically examining the trustworthiness of the same trials (Mol 2021). Mol and colleagues have approached the investigators and their institutions and have received no documentation to confirm the findings presented in the published reports of the trials. Our own approaches to the study investigators have also failed to produce a response.
Furthermore, we decided not to include a further nine studies that met our inclusion criteria in terms of population, intervention, comparator and study design. The investigators of these studies have not yet responded to our attempts to seek clarification regarding various aspects of study conduct.
Unresolved queries about data integrity and lack of prospective trial registration are our main concerns about the trials whose data we have not used in this review. Where we have asked for further information about data presented in a conference abstract or in a full trial report, for example the cause of death of infants who died in a study, we are still waiting for a response. In the absence of adequate assurance about data provenance, we deemed it appropriate not to use these data in our analysis. Similarly, data from trials that were not registered before the investigators began to enrol participants may be problematic. Despite the long‐standing recognition of the important role of prospective trial registration in reducing the risk of selective outcome reporting (De Angelis 2004; Simes 1986), compliance with prospective registration remains at only 41.7% according to a recent analysis of 10,500 randomised controlled trials (Al‐Durra 2020). Without prospective registration it is very difficult to assess whether completed, published trials have reported their results in full. Systematic reviews and clinical guidelines that include evidence from trials whose integrity is uncertain can in turn lead to patients being put at risk if interventions are implemented without reliable evidence of their effectiveness.
The overall certainty of the evidence for the primary outcomes was found to be low and very low (summary of findings Table 1), apart from the outcome of admission to neonatal special care (all levels) for respiratory morbidity, which was of moderate certainty. The main concern with the included trial is the high risk of performance bias, as both participants and health professionals were aware of their group allocation. Although all outcomes may be affected, it is likely that more subjective management decisions or assessments of outcome are more susceptible to bias arising from lack of blinding, as compared to more objective ones (Higgins 2008). Admission of neonates to special care is one such example; clinicians potentially biased in favour of the intervention may have been more likely to organise admission to NICU for babies not exposed to corticosteroids. Such bias was possible, but less likely, for the radiological diagnosis of RDS or TTN by independent specialists. Similarly, admission to lower levels of special care may be more prone to bias than referral to NICU, as the latter would require more clinical and laboratory findings.
The limitations of the evidence are reflected in its assessment according to GRADE. As shown in summary of findings Table 1, for the outcomes of RDS and TTN, the certainty of the evidence was downgraded by one level for study limitations and one level for imprecision. Therefore, the certainty of the evidence for these outcomes was judged as low. For the outcome of NICU admission, we downgraded by one level for study limitations, therefore the certainty of the evidence is judged as moderate. We additionally downgraded by one level for imprecision for the outcome of need for mechanical ventilation, therefore the certainty for this outcome was judged as very low. In practical terms, this means that the true effect may be (or it is likely to be, for the outcome of need for mechanical ventilation) substantially different from our estimates, and the results should therefore be interpreted with caution.
A further potential concern is the lack of long‐term outcomes in the studies. This could result in preferentially favouring antenatal corticosteroid administration, when potential long‐term harms remain unknown.
Potential biases in the review process
We endeavoured to minimise the potential bias of the review process by ensuring an up‐to‐date search was undertaken to try and identify all relevant trials pertinent to the review. Two or more review authors independently appraised and extracted the trial data required and the data extraction process was complete and without missing data that would potentially exclude eligible studies. In the rare case where data were missing, or in order to adequately assess the risk of bias of the trial, we sought additional data from the trial authors who responded on average in a timely and clear manner.
Two of the review authors independently conducted the GRADE assessment of the certainty of evidence and four of the review authors independently applied the trustworthiness criteria to the studies that met our inclusion criteria. We acknowledge that there may be an element of subjectivity in both the GRADE assessment and in assessing trustworthiness, but we made every effort to minimise any risk of bias in this respect by ensuring that we carried out these steps independently and with referral to a Cochrane Pregnancy and Childbirth editor, where necessary, in order to reach consensus.
Agreements and disagreements with other studies or reviews
The favourable effect of antenatal corticosteroids on fetuses at risk for preterm birth has been reported in multiple trials and systematic reviews. Steroids were first tried as a means to accelerate fetal lung maturation and reduce perinatal respiratory morbidity; the latest update of the relevant Cochrane Review reaffirms that antenatal steroids reduce the incidence of RDS (RR 0.71, 95% CI 0.65 to 0.78) and moderate/severe RDS (RR 0.70, 95% CI 0.59 to 0.83) for preterm birth. In contrast, the evidence is uncertain with regard to the risk of chronic lung disease (RR 0.86, 95% CI 0.41 to 1.79) (McGoldrick 2020). It remains uncertain whether antenatal corticosteroids are equally effective in reducing neonatal respiratory morbidity in term infants delivered by elective caesarean section.
Apart from respiratory morbidity, antenatal corticosteroids in fetuses at risk for preterm birth also reduce neonatal mortality and severe neonatal morbidity, including necrotising enterocolitis and intraventricular haemorrhage), without increasing maternal and perinatal complications (McGoldrick 2020; Sotiriadis 2015). Moreover, there is some evidence, partly derived from non‐randomised trials, that steroids in fetuses at risk for preterm birth can also reduce severe neurological morbidity in childhood (Sotiriadis 2015; McGoldrick 2020).
In contrast, a recent population‐based cohort study has raised some concern that administration of antenatal corticosteroids at term gestations is associated with mental and behavioural disorders in the exposed children (Raikkőnen 2020). Despite some inherent limitations with this study (Raikkőnen 2020), this concurs with the cohort of infants from the ASTECS trial (Stutchfield 2005), who were followed up into childhood and were more likely to be ranked in the lower quartile of academic ability by their teachers (Stutchfield 2013). This evidence is certainly not conclusive and merits further investigation and consideration in any future randomised trials.
A common clinical question is whether either betamethasone or dexamethasone is superior to the other regarding their effectiveness and safety profile. The paradigm of fetuses at risk for preterm birth shows that there are no substantial differences between the two agents and the sample sizes for dexamethasone are usually much smaller than those for betamethasone, resulting in wide confidence intervals for the former drug (ASTEROID 2019). As only betamethasone was tested in our review, we cannot draw conclusions for comparative effectiveness. This comparison is also included in another Cochrane Review (Brownfoot 2013).

Study flow diagram

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 1: Respiratory distress syndrome

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 2: Transient tachypnoea of the neonate

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 3: Admission to neonatal special care (all levels) for respiratory morbidity

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 4: Admission to neonatal intensive care unit for respiratory morbidity

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 5: Need for mechanical ventilation

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 6: Maternal development of postpartum infection

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 7: Admission to neonatal special care (all levels) for any indication

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 8: Neonatal infectious morbidity

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 9: Perinatal death

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 10: Length of stay in neonatal intensive care unit (days)

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 11: Readmission for respiratory problems after initial discharge

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 12: Cognitive impairment

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 13: Emotional and behavioural problems: measured with Strengths and difficulties questionnaire

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 14: Adverse maternal effects of therapy
| Antenatal corticosteroids compared to usual care prior to planned caesarean at term on maternal and neonatal outcomes | ||||||
| Patient or population: women undergoing planned elective caesarean section at 37 weeks' gestation and beyond for singleton pregnancy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with usual care | Risk with Antenatal corticosteroids | |||||
| Respiratory distress syndrome (RDS) | Study population | RR 0.34 | 942 | ⊕⊕⊝⊝ | It is uncertain if antenatal corticosteroids have any effect on risk of RDS compared with usual care. | |
| 11 per 1000 | 4 per 1000 | |||||
| Transient tachypnoea of the neonate (TTN) | Study population | RR 0.52 | 942 | ⊕⊕⊝⊝ | It is uncertain antenatal corticosteroids have any effect on risk of TTN compared with usual care. | |
| 40 per 1000 | 21 per 1000 | |||||
| Admission to neonatal special care (all levels) for respiratory morbidity | Study population | RR 0.45 | 942 | ⊕⊕⊕⊝ | Antenatal corticosteroids probably reduce the risk of admission to neonatal special care for respiratory complications compared with usual care. | |
| 51 per 1000 | 23 per 1000 | |||||
| Need for mechanical ventilation | Study population | RR 4.07 | 942 | ⊕⊝⊝⊝ | It is uncertain if antenatal steroids have any effect on the risk of needing mechanical ventilation compared with usual care. | |
| 2 per 1000 | 9 per 1000 | |||||
| Neonatal hypoglycaemia | Study population | Not estimable | 0 studies | ‐ | Outcome not reported in included trial | |
| 0 per 1000 | 0 per 1000 | |||||
| Maternal mortality and severe morbidity | Study population | Not estimable | 0 studies | ‐ | Outcome not reported in included trial | |
| 0 per 1000 | 0 per 1000 | |||||
| Maternal development of postpartum infection/pyrexia in the first 72 hours | Study population | Not estimable | 942 (1 RCT) | ⊕⊝⊝⊝ | It is uncertain if antenatal steroids have any effect on the risk of maternal development of postpartum infection/pyrexia. One trial reported zero cases of postpartum infection/pyrexia in the first 72 hours. | |
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level for serious risk of bias: lack of blinding could influence outcomes 2Downgraded one level for serious imprecision: low event rate and 95% confidence interval that spans possible benefit and possible harm 3Downgraded two levels for very serious imprecision: very low event rate and wide 95% confidence interval that spans possible benefit and possible harm 4Downgraded two levels for very serious imprecision: zero events | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Respiratory distress syndrome Show forest plot | 1 | 942 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.07, 1.65] |
| 1.1.1 Birth before 38 + 0 weeks | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.09] |
| 1.1.2 Birth 38 + 0 to 38 + 6 weeks | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.80] |
| 1.1.3 Birth at or after 39 + 0 weeks | 1 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.02, 8.96] |
| 1.2 Transient tachypnoea of the neonate Show forest plot | 1 | 942 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.25, 1.11] |
| 1.2.1 Birth before 38 + 0 weeks | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.95 [0.45, 34.45] |
| 1.2.2 Birth 38 + 0 to 38 + 6 weeks | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.05, 0.61] |
| 1.2.3 Birth at or after 39 + 0 weeks | 1 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.28, 9.78] |
| 1.3 Admission to neonatal special care (all levels) for respiratory morbidity Show forest plot | 1 | 942 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.22, 0.90] |
| 1.3.1 Birth before 38 + 0 weeks | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.16, 1.57] |
| 1.3.2 Birth 38 + 0 to 38 + 6 weeks | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.17, 1.14] |
| 1.3.3 Birth at or after 39 + 0 weeks | 1 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.04, 3.50] |
| 1.4 Admission to neonatal intensive care unit for respiratory morbidity Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5 Need for mechanical ventilation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.6 Maternal development of postpartum infection Show forest plot | 1 | 942 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.7 Admission to neonatal special care (all levels) for any indication Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.7.1 Birth before 38 + 0 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.7.2 Birth 38 + 0 to 38 + 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.7.3 Birth at or after 39 + 0 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.8 Neonatal infectious morbidity Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.9 Perinatal death Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.10 Length of stay in neonatal intensive care unit (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.11 Readmission for respiratory problems after initial discharge Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.12 Cognitive impairment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.13 Emotional and behavioural problems: measured with Strengths and difficulties questionnaire Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.14 Adverse maternal effects of therapy Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |


