Sistemas de recordatorio para mejorar la adherencia de los pacientes a las citas médicas para el diagnóstico y el tratamiento de la tuberculosis
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Trial design: Quasi‐RCT | |
| Participants | Number of participants: 627 randomized Inclusion criteria: consecutive children ages 1 to 12 years due for a TB test in an urban children's hospital outpatient department; 1 child per family enrolled Exclusion criteria: not stated | |
| Interventions | All patients received a written information sheet with the times to return; skin tests were circled in permanent marker and date of return stamped on mother's and child's hands All families received education regarding the importance of skin testing for TB and the need for follow‐up to read the results. Instructions were given to return to the clinic in 48 to 72 hours Intervention of interest:
Control:
Other interventions not included in this review:
| |
| Outcomes | Outcomes included in this review:
Outcomes not included in this review :
| |
| Notes | Location: USA Trial dates: not specified Baseline data: comparable Funding: Ambulatory Pediatrics Association | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomized by day of the week. |
| Allocation concealment (selection bias) | High risk | Sequential allocation. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) | Low risk | 627/627 (100%), no missing data. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | None identified. |
| Methods | Trial design: RCT | |
| Participants | Number of participants: 170 randomized; 150 analysed Inclusion criteria: patients with symptoms reporting at the Institute of Tuberculosis and Chest Diseases in Madras; with radiographic evidence of TB but negative smears; aged ≥ 12 years; prescribed national TB programme recommended regimen; living within a radius of about 5 km from the clinic; bona fide residents of Madras city and regarded as stable (expected to remain in the city for at least 1 year) Exclusion criteria: not stated | |
| Interventions | Intervention of interest:
| |
| Outcomes | Outcomes included in this review:
Outcomes not included in this review:
| |
| Notes | Location: South India Trial dates: not specified Funding: Indian Council of Medical Research Baseline data: comparable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only described as "randomised". |
| Allocation concealment (selection bias) | Unclear risk | Only described as "randomised". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) | Low risk | 150/170 (89%); 20 participants excluded from main analysis because of death (8), lost to follow‐up (6), chemotherapy change (3), or transfer to more accessible clinics (3), but missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | None identified. |
| Methods | Trial design: RCT | |
| Participants | Number of participants: 98 randomized Inclusion criteria: patients aged > 15 years diagnosed with MTB who had never been treated with second line TB drugs, patients in whom DST and HIV testing were performed and whose liver function tests were lower than 2 times the upper limits of normal. Exclusion criteria: pregnant patients, MDR‐TB patients resistant to 3 or more of 6 classes of second‐line drugs, patients with history of epilepsy or alcoholism, patients who could not answer questions by the researcher and patients who could not complete the treatment. | |
| Interventions | All patients had DOTS Intervention of interest:
Control:
| |
| Outcomes | Outcomes included in this review:
Outcomes not included in this review:
| |
| Notes | Location: Northern Thailand Trial dates: April 2008 to December 2009 Baseline data: comparable Funding: Graduate School of Chulalongkorn University, and the Department of Disease Control, MInistry of Public Health, Thailand | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only described as "randomised". |
| Allocation concealment (selection bias) | Unclear risk | Only described as "randomised". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) | Low risk | No missing data, 98/98 (100%). |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | None identified. |
| Methods | Trial design: RCT | |
| Participants | Number of participants: 480 randomized Inclusion criteria: new smear‐positive PTB; never been treated previously; delayed coming to collect drugs at the health centre for at least 3 days after scheduled appointment; identified from official patient record cards Exclusion criteria: re‐treatment patients | |
| Interventions | Intervention of interest:
Control:
| |
| Outcomes | Outcomes included in this review:
Outcomes not included in this review:
| |
| Notes | Location: Iraq Trial dates: May 2001 to May 2002 Baseline data: not reported Funding: the EMRO/DCD/TDR Small Grants Scheme for Operational Research in Tropical and Communicable Diseases | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By random‐numbers table (confirmed by the trial authors). |
| Allocation concealment (selection bias) | Low risk | Using sequentially numbered and sealed opaque envelopes (confirmed by the trial authors). |
| Blinding of outcome assessment (detection bias) | Low risk | The evaluation was blind as the information about outcome was collected by a field worker who did not know which group the patients were assigned to. |
| Incomplete outcome data (attrition bias) | Low risk | No missing data, 480/480 (100%). |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | None identified. |
| Methods | Trial design: RCT | |
| Participants | Number of participants: 200 randomized Inclusion criteria: newly diagnosed adult PTB patients; sputum positive for acid‐fast bacilli (AFB); no treatment or < 15 days previous treatment; not in moribund condition or suffering from disorders like diabetes, cardiac failure, or renal failure; willing to stay in the hospital for the initial 1‐month intensive phase of treatment Exclusion criteria: not stated | |
| Interventions | Intervention of interest:
Control:
| |
| Outcomes | Outcomes included in this review:
Outcomes not included in this review:
| |
| Notes | Location: South India Trial dates: not specified Baseline data: not reported Funding: the Scientific Committee of Anti‐tuberculosis Association of Tamilnadu | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐numbers table (confirmed by the trial authors). |
| Allocation concealment (selection bias) | Low risk | Centralized randomization by a third party (confirmed by the trial authors). |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | 200/200 (100%), no missing data. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | None identified. |
| Methods | Trial design: RCT | |
| Participants | Number of participants: 200 randomized Inclusion criteria: volunteers who participated in a university‐sponsored TB detection drive; mostly college students Exclusion criteria: not stated | |
| Interventions | Intervention of interest:
Control:
| |
| Outcomes | Outcomes included in this review:
Outcomes not included in this review : None. | |
| Notes | Location: USA Trial dates: not specified Baseline data: comparable Funding: Research Grants Committee, University of Alabama | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only described as "randomised". |
| Allocation concealment (selection bias) | Unclear risk | Only described as "randomised". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) | Low risk | 200/200 (100%), no missing data. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | None identified. |
| Methods | Trial design: RCT | |
| Participants | Number of participants: 553 randomized Inclusion criteria: volunteers who participated in a university‐sponsored TB detection drive Exclusion criteria: not stated | |
| Interventions | Intervention of interest:
Control:
| |
| Outcomes | Outcomes included in this review:
Outcomes not included in this review : None. | |
| Notes | Location: USA Trial dates: not specified Baseline data: comparable Funding: Research Grants Committee, University of Alabama | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only described as "randomised". |
| Allocation concealment (selection bias) | Unclear risk | Only described as "randomised". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) | Low risk | 553/553 (100%), no missing data. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | None identified. |
| Methods | Trial design: RCT | |
| Participants | Number of participants: 318 randomized Inclusion criteria: school children of both sexes in the first year of primary school in state‐run and private schools in the provinces of Barcelona, on anti‐TB chemoprophylaxis Exclusion criteria: children with active TB confirmed by medical examination and chest x‐ray | |
| Interventions | Intervention of interest:
Control:
| |
| Outcomes | Outcomes included in this review:
Outcomes not included in this review:
| |
| Notes | Location: Spain Trial dates: academic year 1985 to 1986 Baseline data: not reported Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only described as "randomised". |
| Allocation concealment (selection bias) | Unclear risk | Only described as "randomised". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) | Unclear risk | 275/318 (86.5%); 43/318 (13.5%) withdrew from treatment, but number withdrew from each group not stated, nor reasons for missing data provided. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | None identified. |
| Methods | Trial design: Quasi‐RCT | |
| Participants | Number of participants: 2008 randomized Inclusion criteria: patients with scheduled appointments in the Tuberculosis Control Program of Santa Clara County Health Department over a period of 6 months Exclusion criteria: not stated | |
| Interventions | Intervention of interest:
Control:
Appropriate recorded message was sent to patients between 18.00 and 21.00 the evening before the scheduled appointment. The system allows a message to be left on answering machines and to call back up to 5 times at half‐hour intervals if patients' lines were busy or there was no answer after 8 rings. For households whose primary language was English, Spanish, Vietnamese, or Tagalog, the message was sent in that language. | |
| Outcomes | Outcomes included in this review:
Outcomes not included in this review :
| |
| Notes | Location: USA Trial dates: not specified Baseline data: not reported Funding: SBIR grants #2 R44 AI31750‐02 from the National Institute of Allergy and Infectious Diseases and #1 R43 AG10659‐01 from the National Institute on Aging | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Within each 5‐week period each message variation was used once on each weekday, different variations were used each day of a given week by a computer‐generated system. |
| Allocation concealment (selection bias) | High risk | Sequential allocation. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified. |
| Incomplete outcome data (attrition bias) | Low risk | 2008/2008 (100%), no missing data. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | None identified. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Pre‐experimental design with historical comparison, cultural intervention with no reminder. | |
| Clinic DOT versus family DOT, did not mention the intervention of interest. | |
| Case‐control study design. | |
| Intervention did not include reminders. | |
| Intervention did not include reminders. | |
| Enhanced Tuberculosis Adherence (ETA) model versus DOT, ETA is a complex intervention contains treatment supporter visits but the results cannot be disaggregated. | |
| Report. | |
| Most participants did not have need for screening, prophylaxis or treatment for TB, and results for the individuals in these categories were not presented separately. | |
| A retrospective study using routinely collected data from the South African national database for TB surveillance. | |
| Description on community education and mobilization of a TB preventive programme, reminder is not a main component of the integrated intervention package. | |
| Intervention did not include reminders. | |
| The study evaluated case management that includes in‐hospital direct supervision plus a home visit on discharge. | |
| Conference research abstracts. | |
| Treatment outcomes from the mobile phone intervention were derived from a case study. | |
| A pilot randomized trial evaluating the acceptance, feasibility and initial efficacy of a text messaging intervention to support TB treatment adherence. The intervention was more of a notification system (by the patient) of drug intake and an educational intervention rather than a reminder system. | |
| Intervention did not include reminders. | |
| Review article. | |
| Cohort study design. | |
| Intervention did not include reminders, except for those routinely provided and also applied to the control group. | |
| Intervention did not include reminders. | |
| Process of reminders not described and the main objective was to assess predictors of latent TB infection completion by using structural equation modelling among homeless adults. | |
| Cross‐sectional study. | |
| A RCT compared a pre‐recorded telephone reminder message (TeleMinder system) twice with no reminder message. It only reported the percentages of participants returned for skin test reading without the events and numbers of each groups. We contacted with the authors but got no feedback. | |
| Reminders not adequately described or systematically applied. | |
| Review article. | |
| Conference research abstracts. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The development and evaluation of m‐Health service in the control of tuberculosis (TB) in India ‐ TIMTAM trial |
| Methods | Study design: randomized; sequentially numbered, sealed, opaque envelopes; participant and outcome assessor blinded Inclusion criteria:
Exclusion criteria: Not able to sign the informed consent document. |
| Participants | Target sample size: 500 |
| Interventions | DOTS Plus mHealth: Patients in arm A (DOTS plus m‐Health) will receive three text (SMS) messages every week for the duration of their treatment as a part of the trial. Patients will be provided with a card containing contact details for a telephone help line (24‐hour help line), with clear instructions that this can be used, free of charge, when access to face to face consultation is not available and medical advice is required. |
| Outcomes | Primary:
Secondary:
Timepoint: Baseline, 3, and 6 months |
| Starting date | Date of registration: 14 July 2011 Date of first enrolment: 1 September 2011 Last refreshed on: 3 February 2014 Recruitment status: Not yet recruiting |
| Contact information | URL: http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=2883 Dilip Mathai IDTRC IV Floor SP Complex Ida Scudder Road Vellore Vellore, TAMIL NADU, 632004, India |
| Notes | Study ID: CTRI/2011/07/001889 Location: India |
| Trial name or title | Cluster randomized trial of using mobile text messaging and a medication monitor in tuberculosis (TB) case management |
| Methods | Study design: Cluster randomized non‐blinded controlled trial Inclusion criteria:
Exclusion criteria:
|
| Participants | Target sample size: 4176 participants (116 per cluster; 9 clusters per arm; 4 arms); age minimum: N/A; age maximum: N/A; gender: N/A |
| Interventions |
|
| Outcomes | Primary:
Secondary:
|
| Starting date | Date of registration: 21 July 2011 Last refreshed on: 20 January 2014 Date of first enrolment: 1 June 2011 Recruitment status: Completed/not recruiting |
| Contact information | URL:http://isrctn.org/ISRCTN46846388 Shiwen Jiang China Center for Disease Control and Prevention No. 155 Changbai Road Changping District 102206 Beijing China |
| Notes | Study ID: ISRCTN46846388 Register: ISRCTN Location: China |
| Trial name or title | Interventions to promote adherence to tuberculosis treatment among patients attending basic medical unit of Taluka Gambat, Pakistan |
| Methods | Study design: Non‐randomized, single group assignment, open label Inclusion criteria:
|
| Participants | Target sample size: 1280 participants; age minimum: 18 years; age maximum: N/A; gender: both |
| Interventions | Education, counselling, default tracers, quality of care |
| Outcomes | Primary:
|
| Starting date | Date of registration: 4 November 2011 Date of first enrolment: January 2004 Last refreshed on: 17 October 2012 Recruitment status: Completed |
| Contact information | URL:http://clinicaltrials.gov/show/NCT01471977 Nisar Sheikh Gambat Institute of Medical Sciences |
| Notes | Study ID: NCT01471977 Location: Pakistan |
| Trial name or title | A randomized controlled trial to examine the effectiveness of use of mobile phones and text messaging to improve adherence to treatment of latent TB |
| Methods | Study design: Randomized, single group assignment, open label Inclusion criteria:
Exclusion criteria:
|
| Participants | Target sample size: 486 participants; age minimum: 19 years; age maximum: N/A; gender: both |
| Interventions | Cell phone text messages |
| Outcomes | Primary:
|
| Starting date | Date of registration: 6 March 2012 Last refreshed on: 10 February 2014 Date of first enrolment: April 2012 Recruitment status: Recruiting |
| Contact information | URL:http://clinicaltrials.gov/show/NCT01549457 Dr. Richard Lester BC Centre for Disease Control Canada |
| Notes | Study ID: NCT01549457 Register: ClinicalTrials.gov Location: Canada |
| Trial name or title | Evaluating the effectiveness of interactive SMS reminders on TB drug compliance and treatment |
| Methods | Study design: Randomized, parallel assignment, open label Inclusion criteria:
Exclusion criteria:
|
| Participants | Target sample size: 2200 participants; age minimum: 15 years; age maximum: N/A; gender: both |
| Interventions | Interactive reminders |
| Outcomes | Primary:
Secondary:
|
| Starting date | Date of registration: 13 September 2012 Date of first enrolment: March 2011 Last refreshed on: 17 October 2012 Recruitment status: Recruiting |
| Contact information | URL:http://clinicaltrials.gov/show/NCT01690754 Shama Mohammed Interactive Research and Development |
| Notes | Study ID: NCT01690754 Location: Pakistan |
| Trial name or title | Innovative approach in tuberculosis care in Armenia |
| Methods | Study design: Randomized, efficacy study, parallel assignment, open label Inclusion criteria:
Exclusion criteria:
|
| Participants | Target sample size: 400 participants; age minimum: 18 years; age maximum: N/A; gender: both |
| Interventions | Self‐administered drug intake strategy, TB knowledge and socio‐psychological counselling session, SMS text messages, phone calls, educational leaflet |
| Outcomes | Primary:
Secondary:
Time frame: At baseline, 1, and 3 months after starting the ambulatory phase of the treatment and upon completion of the treatment (an expected average of 4 months after starting the ambulatory phase of the treatment) |
| Starting date | Date of registration: 4 March 2014 Date of first enrolment: March 2014 Last refreshed on: 31 March 2014 Recruitment status: Active, not recruiting |
| Contact information | URL:http://clinicaltrials.gov/show/NCT02082340 Varduhi Petrosyan American University of Armenia Fund |
| Notes | Study ID: NCT02082340 Location: Armenia |
| Trial name or title | Evaluation of therapeutic adherence support by SMS on the cure rate of tuberculosis: a protocol of a randomized control study |
| Methods | Study design: Randomized, parallel assignment Inclusion criteria:
Exclusion criteria
|
| Participants | Target sample size: 260 participants; age minimum: 18 years; age maximum: 60 years; gender: both |
| Interventions | SMS |
| Outcomes | Primary:
Secondary:
|
| Starting date | Date of registration: 5 July 2013 Last refreshed on: 3 February 2014 Date of first enrolment: 21 February 2013 Recruitment status: Open to recruitment: actively recruiting participants |
| Contact information | URL: http://www.pactr.org/ATMWeb/appmanager/atm/atmregistry?dar=true&tNo=PACTR201307000583416 Jean‐Louis Abena Programme, Ministry of Public Health, Cameroon |
| Notes | Study ID: PACTR201307000583416 Register: PACTR Location: South Africa |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Attendance at single clinic appointment Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 TB treatment: reminder versus none, Outcome 1 Attendance at single clinic appointment. | ||||
| 1.1 Pre‐appointment phone call | 1 | 615 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.10, 1.59] |
| 1.2 Defaulter reminder letter | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 5.04 [1.61, 15.78] |
| 2 TB cure or treatment completion Show forest plot | 3 | 778 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.11, 1.23] |
| Analysis 1.2  Comparison 1 TB treatment: reminder versus none, Outcome 2 TB cure or treatment completion. | ||||
| 2.1 Pre‐appointment phone call | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.02, 1.27] |
| 2.2 Defaulter reminder letter or home visit | 2 | 680 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.11, 1.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Attendance at single clinic appointment Show forest plot | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.87, 1.45] |
| Analysis 2.1  Comparison 2 TB treatment: comparison of different reminders, Outcome 1 Attendance at single clinic appointment. | ||||
| 2 TB cure or treatment completion Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.95, 1.51] |
| Analysis 2.2  Comparison 2 TB treatment: comparison of different reminders, Outcome 2 TB cure or treatment completion. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Attendance at single clinic appointment Show forest plot | 1 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.07, 1.59] |
| Analysis 3.1  Comparison 3 TB prophylaxis: reminder versus none, Outcome 1 Attendance at single clinic appointment. | ||||
| 2 Attendance at final clinic appointment Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 TB prophylaxis: reminder versus none, Outcome 2 Attendance at final clinic appointment. | ||||
| 2.1 Routine phone call every three months | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.21, 1.72] |
| 2.2 Routine nurse home visit every three months | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.23, 1.74] |
| 2.3 Routine doctor clinic every three months | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.98, 1.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Attendance at single clinic appointment Show forest plot | 4 | 1900 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.92, 1.10] |
| Analysis 4.1  Comparison 4 Skin test reading: reminder versus none, Outcome 1 Attendance at single clinic appointment. | ||||
| 1.1 Take home reminder card | 2 | 711 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.04] |
| 1.2 Pre‐appointment phone call | 3 | 1189 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.92, 1.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Attendance at single clinic appointment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Skin test reading: comparison of different reminders, Outcome 1 Attendance at single clinic appointment. | ||||
| 1.1 Take‐home card versus postcard | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Take‐home card versus telephone call | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Postcard versus telephone call | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
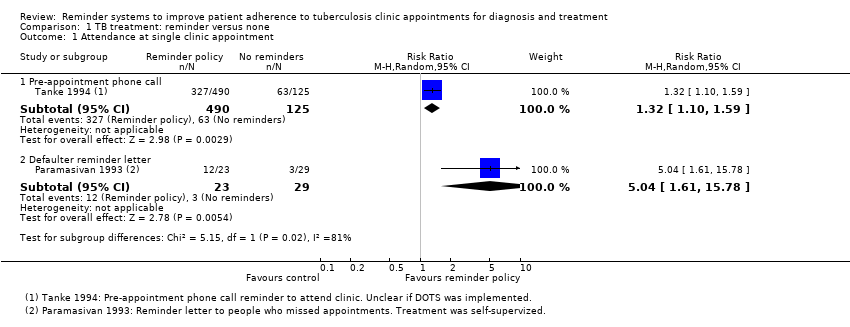
Comparison 1 TB treatment: reminder versus none, Outcome 1 Attendance at single clinic appointment.

Comparison 1 TB treatment: reminder versus none, Outcome 2 TB cure or treatment completion.

Comparison 2 TB treatment: comparison of different reminders, Outcome 1 Attendance at single clinic appointment.

Comparison 2 TB treatment: comparison of different reminders, Outcome 2 TB cure or treatment completion.

Comparison 3 TB prophylaxis: reminder versus none, Outcome 1 Attendance at single clinic appointment.
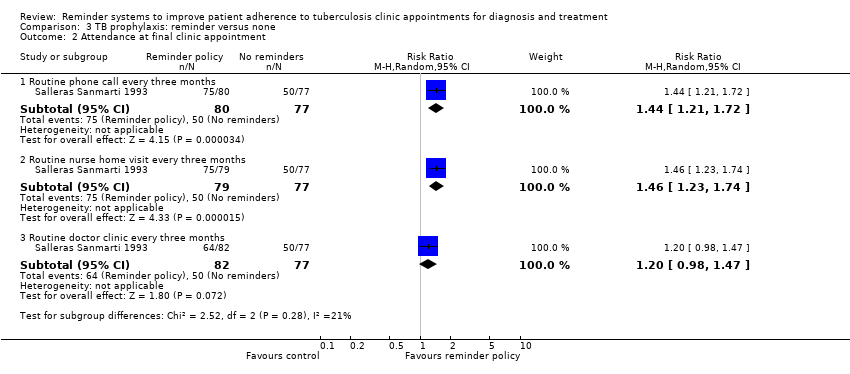
Comparison 3 TB prophylaxis: reminder versus none, Outcome 2 Attendance at final clinic appointment.

Comparison 4 Skin test reading: reminder versus none, Outcome 1 Attendance at single clinic appointment.

Comparison 5 Skin test reading: comparison of different reminders, Outcome 1 Attendance at single clinic appointment.
| TB treatment: pre‐appointment reminder versus no reminder | |||||
| Patient or population: People on TB treatment | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No reminder | Pre‐appointment reminder | ||||
| Attendance at single clinic appointment | 50 per 100 | 66 per 100 (55 to 80) | RR 1.32 (1.10 to 1.59) | 615 | ⊕⊕⊝⊝ low1,2 |
| Completion of TB treatment | 88 per 100 | 100 per 100 (90 to 100) | RR 1.14 (1.02 to 1.27) | 92 | ⊕⊕⊝⊝ low3,4,5 |
| The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 for serious risk of bias: This trial was quasi‐randomized and is at high risk of selection bias. | |||||
| TB treatment: defaulter reminder versus no reminder | |||||
| Patient or population: People on TB treatment | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No reminder | Defaulter reminder | ||||
| Attendance at single clinic appointment | 10 per 100 | 52 per 100 (17 to 100) | RR 5.04 | 52 (1 trial) | ⊕⊕⊝⊝ |
| Completion of TB treatment | 78 per 100 | 91 per 100 | RR 1.17 | 680 | ⊕⊕⊕⊝ |
| The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1No serious risk of bias: This trial was at low risk of selection bias, but was unblinded. | |||||
| TB skin testing: pre‐appointment reminder versus no reminder | |||||
| Patient or population: People at risk of TB | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No reminder | Pre‐appointment reminder | ||||
| Attendance at clinic | 60 per 100 | 63 per 100 | RR 1.06 | 1189 | ⊕⊕⊝⊝ |
| The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by 1 for serious risk of bias: Two trials are quasi‐RCTs and at high risk of selection bias. The third provides few details of randomization and is at unclear risk. | |||||
| Search set | Cochrane SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb | SCI‐EXPANDED & SSCI | CINAHL | |
| 1 | tuberculosis | tuberculosis | tuberculosis | tuberculosis | tuberculosis | tuberculosis | tuberculosis | |
| 2 | adherence | PATIENT COMPLIANCE | TUBERCULOSIS/DRUG THERAPY/PREVENTION AND CONTROL | TUBERCULOSIS | adherence | adherence | adherence | |
| 3 | compliance | PATIENT DROPOUTS | PATIENT COMPLIANCE | PATIENT‐COMPLIANCE | compliance | compliance | compliance | |
| 4 | monitor* | REMINDER SYSTEMS | PATIENT DROPOUTS | medication adherence | Monitor$ | monitor* | monitor* | |
| 5 | reminder* | TREATMENT REFUSAL | COOPERATIVE BEHAVIOUR | REMINDER‐SYSTEM | Reminder$ | reminder* | reminder* | |
| 6 | phone or SMS* or text or messaging | DIRECTLY OBSERVED THERAPY | TREATMENT REFUSAL | TREATMENT‐REFUSAL | phone or SMS$ or text or messaging | non‐adherence | non‐adherence | |
| 7 | 2 or 3 or 4 or 5 or 6 | medication adherence | medication adherence | DIRECTLY‐OBSERVED‐THERAPY | 2 or 3 or 4 or 5 or 6 | late patient tracer | late patient tracer | |
| 8 | 1 and 7 | electronic monitoring | REMINDER SYSTEMS | electronic monitoring | 1 and 7 | phone or SMS* or text or messaging | phone or SMS* or text or messaging | |
| 9 | — | nonadherence | electronic monitoring | nonadherence | — | 2‐8/OR | 2‐8/OR | |
| 10 | — | non‐adherence | nonadherence | non‐adherence | — | 1 AND 9 | 1 AND 9 | |
| 11 | — | late patient tracer | non‐adherence | late patient tracer | — | — | — | |
| 12 | — | phone or SMS* or text or messaging | DIRECTLY OBSERVED THERAPY | phone or SMS* or text or messaging | — | — | — | |
| 13 | — | 2‐12 | late patient tracer | 1 or 2 | — | — | — | |
| 14 | — | 1 AND 13 | phone or SMS* or text or messaging | 3‐12/OR | — | — | — | |
| 15 | — | — | 1 or 2 | 13 and 14 | — | — | — | |
| 16 | — | — | 3‐14/OR | — | — | — | — | |
| 17 | — | — | 15 and 16 | — | — | — | — | |
| aCochrane Infectious Diseases Group Specialized Register and the Cochrane Effective Practice and Organisation of Care Group Specialized Register. | ||||||||
| Trial ID | Country | Age group | TB status | TB intervention | Supervision of treatment | Type of reminder | Timing of reminder | Pre/post appointment | Control |
| USA | Adults | At risk of TB | Test | N/A | Take home reminder card1 | N/A | N/A | Verbal statement in clinic | |
| USA | Adults | At risk of TB | Test | N/A | Take home reminder card2 | N/A | N/A | Verbal statement in clinic | |
| N/A | Postcard | 1 day | Pre‐appointment | Verbal statement in clinic | |||||
| N/A | Phone call | 1 day | Pre‐appointment | Verbal statement in clinic | |||||
| USA | All | At risk of TB | Test | N/A | Phone call3 | 1 day | Pre‐appointment | No phone call | |
| USA | Children | At risk of TB | Test | N/A | Phone call | 1 day | Pre‐appointment | Take home reminder card | |
| Spain | Children | Asymptomatic | Prophylaxis | Parents | A routine phone call every 3 months | N/A | N/A | One‐off advice to take treatment for 12 months | |
| A routine nurse home visit every 3 months | N/A | N/A | One‐off advice to take treatment for 12 months | ||||||
| A routine doctor clinic appointment every 3 months | N/A | N/A | One‐off advice to take treatment for 12 months | ||||||
| USA | All | Asymptomatic | Prophylaxis | Unclear | Phone call3 | 1 day | Pre‐appointment | No phone call | |
| USA | All | Symptomatic | Treatment | Unclear | Phone call3 | 1 day | Pre‐appointment | No phone call | |
| Thailand | > 15 years | Symptomatic | Treatment | DOTS | Phone call | 1 day | Pre‐appointment | DOTS alone | |
| Iraq | Not stated | Symptomatic | Treatment | DOTS | Home visit | 3 days | Post‐appointment | DOTS alone | |
| India | > 12 years | Symptomatic | Treatment | Self‐monthly pick‐up of meds | Home visit | 4 days | Post‐appointment | Reminder letter | |
| India | Adult | Symptomatic | Treatment | Self‐monthly pick‐up of meds | Reminder card | 3 days | Post‐appointment | No reminder card | |
| 1Roberts 1983b also evaluated the effects of three types of participant commitment to return (no commitment, verbal, verbal plus written), and two types of verbal messaging on the importance of returning (enhanced versus standard). | |||||||||
| Outcome | Hypothesis | Power | α error | Proportion in control group | Proportion in intervention group | Total sample size required |
| Attendance at clinic appointment | Superiority | 80% | 5% | 50% | 75% | 110 |
| 80% | 90% | 394 | ||||
| TB cure or treatment completion | Superiority | 80% | 5% | 50% | 75% | 110 |
| 80% | 90% | 394 | ||||
| We performed calculations using http://www.sealedenvelope.com | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Attendance at single clinic appointment Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Pre‐appointment phone call | 1 | 615 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.10, 1.59] |
| 1.2 Defaulter reminder letter | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 5.04 [1.61, 15.78] |
| 2 TB cure or treatment completion Show forest plot | 3 | 778 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.11, 1.23] |
| 2.1 Pre‐appointment phone call | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.02, 1.27] |
| 2.2 Defaulter reminder letter or home visit | 2 | 680 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.11, 1.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Attendance at single clinic appointment Show forest plot | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.87, 1.45] |
| 2 TB cure or treatment completion Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.95, 1.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Attendance at single clinic appointment Show forest plot | 1 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.07, 1.59] |
| 2 Attendance at final clinic appointment Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Routine phone call every three months | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.21, 1.72] |
| 2.2 Routine nurse home visit every three months | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.23, 1.74] |
| 2.3 Routine doctor clinic every three months | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.98, 1.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Attendance at single clinic appointment Show forest plot | 4 | 1900 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.92, 1.10] |
| 1.1 Take home reminder card | 2 | 711 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.04] |
| 1.2 Pre‐appointment phone call | 3 | 1189 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.92, 1.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Attendance at single clinic appointment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Take‐home card versus postcard | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Take‐home card versus telephone call | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Postcard versus telephone call | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

