Пероральные антикоагулянты у людей с раком без терапевтических и профилактических показаний для антикоагуляции (разжижения крови)
Abstract
Background
Oral anticoagulants may improve the survival of people with cancer through both an antitumor effect and antithrombotic effect, yet increase the risk of bleeding.
Objectives
To evaluate the efficacy and safety of oral anticoagulants in ambulatory people with cancer undergoing chemotherapy, hormonal therapy, immunotherapy or radiotherapy, but otherwise have no standard therapeutic or prophylactic indication for anticoagulation.
Search methods
We conducted a comprehensive literature search in February 2016 that included a major electronic search of Cochrane Central Register of Controlled Trials (CENTRAL) (2016, Issue 1), MEDLINE (Ovid) and Embase (Ovid); handsearching of conference proceedings; checking of references of included studies; a search for ongoing studies; and using the 'related citation' feature in PubMed. As part of the living systematic review approach, we are running continual searches and will incorporate new evidence rapidly after it is identified. This update of the systematic review is based on the findings of a literature search conducted on 14 December 2017.
Selection criteria
Randomized controlled trials (RCTs) assessing the benefits and harms of vitamin K antagonist (VKA) or direct oral anticoagulants (DOAC) in ambulatory people with cancer. These participants are typically undergoing systemic anticancer therapy, possibly including chemotherapy, target therapy, immunotherapy or radiotherapy, but otherwise have no standard therapeutic or prophylactic indication for anticoagulation.
Data collection and analysis
Using a standardized form, we extracted data in duplicate on study design, participants, intervention outcomes of interest and risk of bias. Outcomes of interest included all‐cause mortality, symptomatic venous thromboembolism (VTE), symptomatic deep vein thrombosis (DVT), pulmonary embolism (PE), major bleeding, minor bleeding and health‐related quality of life (HRQoL). We assessed the certainty of evidence for each outcome using the GRADE approach (GRADE Handbook).
Main results
Of 8545 identified citations, including 7668 unique citations, 16 papers reporting on 7 RCTs fulfilled the inclusion criteria. These trials enrolled 1486 participants. The oral anticoagulant was warfarin in six of these RCTs and apixaban in the seventh RCT. The comparator was either placebo or no intervention. The meta‐analysis of the studies comparing VKA to no VKA did not rule out a clinically significant increase or decrease in mortality at one year (risk ratio (RR) 0.95, 95% confidence interval (CI) 0.87 to 1.03; risk difference (RD) 29 fewer per 1000, 95% CI 75 fewer to 17 more; moderate certainty evidence). One study assessed the effect of VKA on thrombotic outcomes. The study did not rule out a clinically significant increase or decrease in PE when comparing VKA to no VKA (RR 1.05, 95% CI 0.07 to 16.58; RD 0 fewer per 1000, 95% CI 6 fewer to 98 more; very low certainty evidence), but found that VKA compared to no VKA likely decreases the incidence of DVT (RR 0.08, 95% CI 0.00 to 1.42; RD 35 fewer per 1000, 95% CI 38 fewer to 16 more; low certainty evidence). VKA increased both major bleeding (RR 2.93, 95% CI 1.86 to 4.62; RD 107 more per 1000, 95% CI 48 more to 201 more; moderate certainty evidence) and minor bleeding (RR 3.14, 95% CI 1.85 to 5.32; RD 167 more per 1000, 95% CI 66 more to 337 more; moderate certainty evidence).
The study assessing the effect of DOAC compared to no DOAC did not rule out a clinically significant increase or decrease in mortality at three months (RR 0.24, 95% CI 0.02 to 2.56; RD 51 fewer per 1000, 95% CI 65 fewer to 104 more; low certainty evidence), PE (RR 0.16, 95% CI 0.01 to 3.91; RD 28 fewer per 1000, 95% CI 33 fewer to 97 more; low certainty evidence), symptomatic DVT (RR 0.07, 95% CI 0.00 to 1.32; RD 93 fewer per 1000, 95% CI 100 fewer to 32 more; low certainty evidence), major bleeding (RR 0.16, 95% CI 0.01 to 3.91; RD 28 fewer per 1000, 95% CI 33 fewer to 97 more; low certainty evidence); and minor bleeding (RR 4.43, 95% CI 0.25 to 79.68; RD 0 fewer per 1000, 95% CI 0 fewer to 8 more; low certainty evidence).
Authors' conclusions
The existing evidence does not show a mortality benefit from oral anticoagulation in people with cancer but suggests an increased risk for bleeding.
Editorial note: this is a living systematic review. Living systematic reviews offer a new approach to review updating in which the review is continually updated, incorporating relevant new evidence, as it becomes available. Please refer to the Cochrane Database of Systematic Reviews for the current status of this review.
PICOs
Резюме на простом языке
Пероральные антикоагулянты у онкологических пациентов
Актуальность
Доказательства из исследований позволяют предположить, что средства, разжижающие кровь (называемые антикоагулянтами), могут не только предотвратить образование жизнеугрожающих тромбов, но и оказывать прямое противораковое действие. Однако антикоагулянты могут также увеличить риск серьёзного и смертельного кровотечения. Поэтому важно понимать плюсы и минусы лечения, чтобы пациенты и их врачи были осведомлены о балансе рисков и пользы.
Характеристика исследований
Мы провели поиск научной литературы на предмет исследований антикоагулянтов у людей с раком. Доказательства актуальны на декабрь 2017 года.
Основные результаты
Мы включили семь испытаний с участием 1486 человек, страдающих раком. Большинство испытаний включали участников с различными типами рака, в особенности раком лёгкого и поджелудочной железы. Мы нашли шесть исследований использования варфарина и одно исследование использования апиксабана. При рассмотрении людей с онкологическими заболеваниями в целом варфарин не влиял на смертность (частоту смертей) или риск образования тромбов. Однако, он повысил риск большого кровотечения на 107 человек на 1000 населения и малого кровотечения на 167 человек на 1000 населения. Апиксабан не повлиял на смертность, рецидив тромбообразования в кровеносных сосудах, большое или малое кровотечение; однако эти выводы были основаны только на одном исследовании.
Определенность доказательств
Сравнивая результаты лечения с варфарином и без него, мы пришли к выводу, что определенность доказательств (степень нашей уверенности в результатах) умеренная в отношении смертности в течение одного года, а также большого и малого кровотечения, низкая в отношении симптоматического тромбоза глубоких вен (тромб в глубокой вене, чаще всего в ногах) и очень низкая в отношении тромбоэмболии лёгких (тромб в кровеносных сосудах лёгких).
Сравнивая результаты лечения с апиксабаном и без него, мы оценили определенность доказательств как низкую в отношении смертности, большого и малого кровотечения, тромбоэмболии лёгких и симптоматического тромбоза глубоких вен.
Примечание редактора: это живой систематический обзор. Живые систематические обзоры предлагают новый подход к обновлениям обзоров, при котором обзор постоянно обновляется, внедряя соответствующие новые доказательства по мере их появления. Пожалуйста, обратитесь к Базе Данных Систематических Обзоров Кокрейн, чтобы узнать текущий статус этого обзора.
Authors' conclusions
Summary of findings
| VKA prophylaxis compared to No prophylaxis in ambulatory patients with cancer without VTE receiving systemic therapy | |||||
| Patient or population: ambulatory people with cancer without VTE receiving systemic therapy Setting: outpatient Intervention: VKA prophylaxis Control: no prophylaxis | |||||
| Outcomes | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
|---|---|---|---|---|---|
| Risk with No prophylaxis | Risk difference with VKA prophylaxis | ||||
| Mortality | 1281 | ⊕⊕⊕⊝ | RR 0.95 | Study population | |
| 574 per 1,000 | 29 fewer per 1,000 | ||||
| PE | 311 | ⊕⊝⊝⊝ | RR 1.05 | Study population | |
| 6 per 1,000 | 0 fewer per 1,000 | ||||
| Symptomatic DVT | 311 | ⊕⊕⊝⊝ | RR 0.08 | Study population | |
| 38 per 1,000 | 35 fewer per 1,000 | ||||
| Major bleeding | 1281 | ⊕⊕⊕⊝ | RR 2.93 | Study population | |
| 55 per 1,000 | 107 more per 1,000 | ||||
| Minor bleeding | 863 | ⊕⊕⊕⊝ | RR 3.14 | Study population | |
| 78 per 1,000 | 167 more per 1,000 | ||||
| HRQoL ‐ not reported | ‐ | ‐ | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded by one level due to concern about both risk of bias (lack of blinding in patients and personnel and unclear allocation concealment in 4 out of 5 studies) and imprecision (95% CI is consistent with the possibility for important benefit (75 per 1000 absolute reduction) and the possibility of important harm (17 per 1000 absolute increase), large event rate) 2 Downgraded by one level due to indirectness. Levine 1994 used fixed dose of VKA instead of adjusted dose which is not representative of the current practice. This study was the only trial that reported on PE and symptomatic DVT. 3 Downgraded by two levels due to very serious imprecision. 95% CI is consistent with the possibility for important benefit (6 per 1000 absolute reduction) and the possibility of important harm (98 per 1000 absolute increase), including only 2 events. 4Levine 1994 used fixed dose of VKA instead of adjusted dose which is not representative of the current practice. This study was the only trial that reported on PE and symptomatic DVT. We do not think that this indirectness has underestimated the effect on symptomatic DVT (RR 0.08) 5 Downgraded by two levels due to very serious imprecision. Only 6 events among 311 participants. 6 Downgraded by one level due to concern about risk of bias (lack of blinding in patients and personnel and unclear allocation concealment in 4 out of 5 studies) 7 Downgraded by one level due to concern about risk of bias (lack of blinding in patients and personnel and unclear allocation concealment in 3 out of 4 studies) | |||||
| DOAC prophylaxis compared to No prophylaxis in ambulatory patients with cancer without VTE receiving systemic therapy | |||||
| Patient or population: ambulatory people with cancer without VTE receiving systemic therapy Setting: outpatient Intervention: DOAC prophylaxis Control: no prophylaxis | |||||
| Outcomes | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
|---|---|---|---|---|---|
| Risk with No prophylaxis | Risk difference with DOAC prophylaxis | ||||
| Mortality | 92 | ⊕⊕⊝⊝ | RR 0.24 | Study population | |
| 67 per 1,000 | 51 fewer per 1,000 | ||||
| PE | 92 | ⊕⊕⊝⊝ | RR 0.16 | Study population | |
| 33 per 1,000 | 28 fewer per 1,000 | ||||
| Symptomatic DVT | 92 | ⊕⊕⊝⊝ | RR 0.07 | Study population | |
| 100 per 1,000 | 93 fewer per 1,000 | ||||
| Major bleeding | 92 | ⊕⊕⊝⊝ | RR 0.16 | Study population | |
| 33 per 1,000 | 28 fewer per 1,000 | ||||
| Minor bleeding | 92 | ⊕⊕⊝⊝ | RR 4.43 | Low | |
| 0 per 1,000 | 0 fewer per 1,000 | ||||
| HRQoL ‐ not reported | ‐ | ‐ | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Concern due to unclear allocation concealment 2 Downgraded by two levels due to very serious imprecision: 95% CI is consistent with the possibility for important benefit (65 per 1000 absolute reduction) and the possibility of important harm (104 per 1000 absolute increase), including only 3 events among 92 participants. 3 Downgraded by two levels due to very serious imprecision: 95% CI is consistent with the possibility for important benefit (33 per 1000 absolute reduction) and the possibility of important harm (97 per 1000 absolute increase), including only 1 events among 92 participants. 4 Downgraded by two levels due to very serious imprecision: Including only 3 events among 92 participants. 5 Downgraded by two levels due to very serious imprecision: Including only 4 events among 92 participants. | |||||
Background
Please refer to the glossary for the definitions of technical terms (Table 1).
| Term | Meaning |
|---|---|
| Adjuvant therapy | Assisting in the amelioration or cure of disease. |
| Anticoagulation | Process of hindering the clotting of blood especially by treatment with an anticoagulant. |
| Antithrombotic | Used against or tending to prevent thrombosis (clotting). |
| Apixaban | Oral direct factor Xa inhibitor used for anticoagulation. |
| Coagulation | Clotting. |
| Direct factor Xa inhibitor | Anticoagulant medications used for anticoagulation. Apixaban is an oral direct factor Xa inhibitor. |
| Deep vein thrombosis (DVT) | Condition marked by the formation of a thrombus within a deep vein (e.g. leg or pelvis) that may be asymptomatic or symptomatic (as swelling and pain) and that is potentially life‐threatening if dislodgment of the thrombus results in pulmonary embolism. |
| Fibrin | White insoluble fibrous protein formed from fibrinogen by the action of thrombin especially in the clotting of blood. |
| Fondaparinux | An anticoagulant medication. |
| Hemostatic system | The system that shortens the clotting time of blood and stops bleeding. |
| Heparin | Enzyme occurring especially in the liver and lungs that prolongs the clotting time of blood by preventing the formation of fibrin. 2 forms of heparin that are used as anticoagulant medications are: unfractionated heparin (UFH) and low‐molecular‐weight heparins (LMWH). |
| Major bleeding | Bleeding that is intracranial or retroperitoneal, if it leads directly to death, or if results in hospitalization or transfusion. |
| Metastasis | Spread of a cancer cells from the initial or primary site of disease to another part of the body. |
| Minor bleeding | Any bleeding not classified as major bleeding. |
| Oncogene | Gene having the potential to cause a normal cell to become cancerous. |
| Osteoporosis | Condition that affects mainly older women and is characterized by decrease in bone mass with decreased density and enlargement of bone spaces producing porosity and brittleness. |
| Pulmonary embolism (PE) | Embolism of a pulmonary artery or 1 of its branches that is produced by foreign matter and most often a blood clot originating in a vein of the leg or pelvis and that is marked by labored breathing, chest pain, fainting, rapid heart rate, cyanosis, shock and sometimes death. |
| Stroma | The supporting framework of an organ typically consisting of connective tissue. |
| Thrombin | Proteolytic enzyme formed from prothrombin that facilitates the clotting of blood by catalyzing conversion of fibrinogen to fibrin. |
| Thrombocytopenia | Persistent decrease in the number of blood platelets that is often associated with hemorrhagic conditions. |
| Vitamin K antagonist (VKA) | Anticoagulant medications. Warfarin is a vitamin K antagonist. |
| Warfarin | Anticoagulant medication that is a vitamin K antagonist. |
| Ximelagatran | Anticoagulant medication. |
Description of the condition
Studies have implicated the tumor‐mediated activation of the hemostatic system in both the formation of tumor stroma and in tumor metastasis (Dvorak 1986; Francis 1998; Levine 2003). In one cohort study of over 3000 healthy participants with 15 years' follow‐up, cancer mortality was three times more common in participants who were hypercoagulable at baseline than in participants who were not (Miller 2004).
Description of the intervention
Vitamin K antagonists (VKA) have been the mainstay of oral anticoagulant therapy since the mid‐1950s. Well‐designed clinical trials have shown their effectiveness for the primary and secondary prevention of several venous and arterial thrombotic diseases (Ansell 2008).
Apixaban belongs to the family of oral activated factor X inhibitors, which is currently approved in Europe for venous thromboembolism (VTE) prevention following major orthopedic surgery (King 2013).
How the intervention might work
Since the 1930s, scientists have been exploring the effects of anticoagulation on cancer (Smorenburg 2001), and there is evidence that warfarin has an inhibitory effect on tumor growth and metastasis. Schulman 2000 showed that in people with a first episode of VTE, cancer incidence was lower when treated with oral anticoagulants for six months rather than for six weeks. These observations led to the hypothesis that the antitumor effect of oral anticoagulants, in addition to their antithrombotic effect, may improve outcomes of people with cancer.
Why it is important to do this review
In the early 1980s, one large US Veterans Administration Cooperative Study suggested that warfarin, as a single anticoagulant agent, may favourably modify the course of some types of human malignancy such as small cell lung cancer (SCLC) (Zacharski 1981). Conversely, in another trial, warfarin did not improve the outcomes of people with SCLC receiving chemotherapy and radiotherapy (Maurer 1997). The last update of this Cochrane systematic review, published in 2014, identified six trials enrolling 1770 participants (Chahinian 1989; Levine 1994; Levine 2012; Maurer 1997; Stanford 1979; Zacharski 1984), concluded that the existing evidence did not suggest a mortality benefit from oral anticoagulation in people with cancer, while the risk for bleeding was increased (Akl 2014a). There have also been publications on the use of newer oral anticoagulants in people with cancer (Levine 2012). See Table 1 for glossary.
Living review approach: following the publication of this current 2017 update of the review, we will maintain it as a living systematic review: we will be continually running the searches and incorporating newly identified studies (for more information about the living systematic review approach being piloted by Cochrane, see Appendix 1). We consider that a living systematic review approach is appropriate for this review for four reasons. First, the review addresses an important subject for clinical practice; people with cancer have a high rate of VTE, up to 17.7% (Ay 2010). In addition, VTE is associated with a 2.3 times increased risk of death in people with non‐small cell lung cancer (NSCLC) and breast cancer, 2.5 times lengthening of hospital stay among people with lung cancer, and 50% increased total costs for people with lung cancer (Chew 2007; Chew 2008; Connolly 2012). Second, there is uncertainty in the existing evidence; the 2014 update of this systematic review did not provide definitive results about suspected subgroup effects on all‐cause mortality. Third, there are several ongoing trials in this area that will be important to incorporate in a timely manner. Fourth, we are planning to use this living systematic review as the basis of a living recommendation in a clinical practice guideline with the American Society of Hematology (Akl 2017). For more information about the living systematic review approach being piloted by Cochrane, see Appendix 2.
Objectives
To evaluate the efficacy and safety of oral anticoagulants in ambulatory people with cancer undergoing chemotherapy, hormonal therapy, immunotherapy or radiotherapy, but otherwise have no standard therapeutic or prophylactic indication for anticoagulation.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
Participants of any age (including children) with cancer with no standard indication for prophylactic anticoagulation (e.g. for acute illness, for central venous line placement, perioperatively) or for therapeutic anticoagulation (e.g. for the treatment of deep vein thrombosis (DVT) or pulmonary embolism (PE)). Typically, these participants are undergoing chemotherapy, target therapy, immunotherapy or radiotherapy.
Types of interventions
Intervention:
-
oral pharmacological thromboprophylaxis with VKA;
-
oral pharmacological thromboprophylaxis with DOAC.
Control:
-
no pharmacological thromboprophylaxis.
We included any comparison of a combination of the three management options listed above. The protocol from original studies should have planned to provide all other co interventions (e.g. chemotherapy) similarly.
Types of outcome measures
Primary outcomes
-
All‐cause mortality.
Secondary outcomes
-
Symptomatic DVT: events had to be suspected clinically, and diagnosed using an objective diagnostic test such as: venography, 125I‐fibrinogen‐uptake test, impedance plethysmography or compression ultrasound.
-
PE: events had to be suspected clinically, and diagnosed using an objective diagnostic test such as: pulmonary perfusion/ventilation scans, computed tomography, pulmonary angiography or autopsy.
-
Major bleeding: we accepted the authors' definitions of major bleeding.
-
Minor bleeding: we accepted the authors' definitions of minor bleeding.
-
Health‐related quality of life (HRQoL): had to be measured using a validated tool.
Search methods for identification of studies
Electronic searches
The search was part of a comprehensive search for studies of anticoagulation in people with cancer. We did not use language restrictions. We conducted comprehensive searches on 14 December 2017, following the original electronic searches performed in January 2007, February 2010, February 2013 and February 2016 (last major search). We electronically searched the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (starting 1946) and Embase (starting 1980; accessed via Ovid). For each database, the search strategies combined terms for anticoagulants, terms for cancer and a search filter for RCTs. We list the full search strategies for each of the electronic databases in Appendix 3, Appendix 4, and Appendix 5.
Living review approach: since the last major search in February 2016, we have been running searches monthly, using auto‐alerts to deliver the monthly yield by email. We will incorporate new evidence rapidly after it is identified. This update of the systematic review is based on the findings of a literature search conducted on 14 December 2017. We will review search methods and strategies approximately yearly, to ensure they reflect any terminology changes in the topic area, or in the databases.
Searching other resources
We handsearched the conference proceedings of the American Society of Clinical Oncology (ASCO, starting with its first volume, 1982 up to November 2017) and of the American Society of Hematology (ASH, starting with its 2003 issue up to November 2017). We also searched ClinicalTrials.gov and WHO International Clinical Trials Registry Platform for ongoing studies. We reviewed the reference lists of papers included in this review and of other relevant systematic reviews. We used the 'related citation' feature in PubMed to identify additional articles and 'citation tracking' of included studies in Web of Science Core Collection. In addition, we contacted experts in the field to check for unpublished and ongoing trials.
Living systematic review approach: we will search on a monthly basis the conference proceedings of ASCO and ASH soon after their publications, ClinicalTrials.gov, and WHO International Clinical Trials Registry Platform. As an additional step, we will contact corresponding authors of ongoing studies as they are identified and ask them to advise when results are available. We will continue to review the reference lists for any prospectively identified studies, with running the 'related citation' feature for all included studies on a monthly basis. Also, we will contact the corresponding authors of any newly included studies for advice as to other relevant studies. Using citation alerts, we will conduct citation tracking of included studies in Web of Science Core Collection on an ongoing basis.
Data collection and analysis
Selection of studies
Two review authors independently screened the titles and abstracts of identified articles for eligibility. We retrieved the full text of articles judged as potentially eligible by at least one review author. Two review authors then independently screened the full‐text articles for eligibility using a standardized form with explicit inclusion and exclusion criteria. The two review authors resolved their disagreements by discussion or by consulting a third review author.
Living systematic review approach: for the monthly searches, we will immediately screen any new citations retrieved each month. As the first step of monthly screening, we will apply the machine learning classifier (RCT model) available in the Cochrane Register of Studies (CSR‐Web; Wallace 2017). The classifier assigns a probability (from 0 to 100) to each citation for being a true RCT. For citations that are assigned a probability score of less than 10, the machine learning classifier currently has a specificity/recall of 99.987% (Wallace 2017). For citations assigned a score from 10 to 100, we will screen them in duplicate and independently. Citations that score nine or less will be screened by Cochrane Crowd (Cochrane Crowd). Any citations that are deemed to be potential RCTs by Cochrane Crowd will be returned to the authors for screening.
Data extraction and management
Two review authors independently extracted data from each included study and resolved any disagreements by discussion. We aimed at collecting data related to the following.
Participants
-
Number of participants randomized to each study arm.
-
Number of participants followed up in each study arm.
-
Number of withdrawals from each study arm.
-
Population characteristics (e.g. age, gender, co morbidities, co interventions).
-
History of VTE.
-
Type of cancer.
-
Stage of cancer.
-
Time since cancer diagnosis.
Interventions
-
Type of anticoagulant: VKA, DOAC, other.
-
Intensity of VKA therapy (international normalized ratio (INR) target) or dose.
-
Duration of treatment.
-
Control: placebo or no intervention.
-
Cointerventions including chemotherapy, target therapy, immunotherapy or radiotherapy (type and duration).
Outcomes
For dichotomous variables, we extracted data necessary to conduct complete case analysis as the primary analysis. We collected all‐cause mortality at one year (time point defined a priori in the protocol), six months, two years and five years (time point defined post hoc based upon results reported in the individual RCTs). When we could not obtain the number of events at the time points of interest from the paper or from the authors, two review authors calculated these numbers in duplicate and independently from survival curves, if available (Zacharski 1984). We used the mean of the two estimates when they differed. We assessed agreement between the two review authors for each estimated value by calculating the percentage difference, which is the difference between the two estimates divided by the denominator (number of people at risk for the event) and multiplied by 100. For some studies, where VTE was not reported as a separate outcome, we added the number of events of DVT and PE.
We attempted to contact study authors for incompletely reported data. We decided a priori to consider abstracts in the main analysis only if study authors supplied us with full reports of their methods and results; otherwise abstracts were included only in the sensitivity analysis.
Other
-
Source of funding.
-
Ethical approval.
-
Conflict of interest.
Assessment of risk of bias in included studies
We assessed risk of bias at the study level using Cochrane's 'Risk of bias' tool. Two review authors independently assessed the methodological quality of each included study and resolved their disagreements by discussion. Methodological criteria included:
-
adequate sequence generation;
-
allocation concealment;
-
blinding of participants and personnel;
-
blinding of outcome assessment;
-
percentage of follow‐up and whether incomplete outcome data were addressed;
-
whether the study was free of selective reporting;
-
whether the study was stopped early for benefit.
See Dealing with missing data section for information on assessing risk of bias associated with participants with missing data per outcome and across studies.
Measures of treatment effect
We collected and analyzed risk ratios (RRs) with 95% confidence intervals (CI) for dichotomous data. None of the outcomes of interest was meta‐analyzed as a continuous variable.
Unit of analysis issues
The unit of analysis was the participant.
Dealing with missing data
Determining participants with missing data
It was unclear whether certain participant categories (e.g. those described as 'withdrew consent' or 'experienced adverse events') were followed up by the trial authors (versus had missing participant data) (Akl 2016). To deal with this issue, we made the following considerations:
-
'ineligible participants' and 'did not receive the first dose' participant categories, which are defined prior to the initiation of the study intervention, most likely have missing participant data;
-
'withdrew consent', 'lost to follow‐up' (LTFU) and 'outcome not assessable' participant categories and any other category explicitly reported as not being followed up, which are defined after the initiation of the study intervention, most likely have missing participant data;
-
'dead,' 'experienced adverse events,' 'non‐compliant' and 'discontinued prematurely' (and similarly described) participant categories, less likely have missing participant data.
Dealing with participants with missing data in the primary meta‐analysis
In the primary meta‐analysis, we used a complete case analysis approach, that is, we excluded participants considered to have missing data (Guyatt 2017).
For categorical data, we used the following calculations for each study arm:
-
denominator: (number of participants randomized) ‐ (number of participants most likely with missing data, both pre‐ and postintervention initiation);
-
numerator: number of participants with observed events (i.e. participants who had at least one event for the outcome of interest during their available follow‐up time).
For continuous data, we planned to use for each study arm, the reported mean and standard deviation (SD) for participants followed up by the trial authors.
Assessing the risk of bias associated with participants with missing data
When the primary meta‐analysis of a specific outcome found a statistically significant effect, we conducted sensitivity meta‐analyses to assess the risk of bias associated with missing participant data. Those sensitivity meta‐analyses used a priori plausible assumptions about the outcomes of participants considered to have missing data. The assumptions we used in the sensitivity meta‐analyses were increasingly stringent to challenge the statistical significance of the results of the primary analysis progressively (Akl 2013; Ebrahim 2013).
For categorical data, and for RR showing a reduction in effect (RR less than 1), we used the following increasingly stringent but plausible assumptions (Akl 2013).
-
For the control arm, relative incidence (RI) among participants with missing data (LTFU) compared to participants with available data (followed up, FU) in the same arm (RILTFU/FU) = 1; for the intervention arm, RILTFU/FU = 1.5.
-
For the control arm, RILTFU/FU = 1; for the intervention arm, RILTFU/FU = 2.
-
For the control arm, RILTFU/FU = 1; for the intervention arm, RILTFU/FU = 3.
-
For the control arm, RILTFU/FU = 1; for the intervention arm, RILTFU/FU = 5.
For RR showing an increase in effect (RR greater than 1), we switched the above assumptions between the control and interventions arms (i.e. used RILTFU/FU = 1 for the intervention arm).
Specifically, we used the following calculations for each study arm:
-
denominator: (number of participants randomized) ‐ (number of participants most likely with missing data, pre intervention initiation);
-
numerator: (number of participants with observed events) + (number of participants most likely with missing data postintervention initiation, with assumed events).
Assumed events were calculated by applying the a priori plausible assumptions to the participants considered most likely with missing data postintervention initiation.
For continuous data, we planned to use the four strategies suggested by Ebrahim and colleagues (Ebrahim 2013). The strategies imputed the means for participants with missing data based on the means of participants followed up in individual trials included in the systematic review. To impute SD, we used the median SD from the control arms of all included trials (Ebrahim 2013).
Assessment of heterogeneity
We assessed heterogeneity between trials by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials that could not be ascribed to sampling variation (I2 test; Higgins 2003), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). If there was evidence of substantial heterogeneity, we attempted to investigate the possible reasons for this (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
We assessed selective outcome reporting bias by trying to identify whether the study was included in a trial registry, whether a protocol was available and whether the methods section provided a list of outcomes. We compared the list of outcomes from those sources to the outcomes reported in the published paper. We planned to create funnel plots for outcomes including 10 or more trials.
Data synthesis
For dichotomous data, we calculated the RR separately for each study (DerSimonian 1986; RevMan 2014). When analyzing data related to participants who were reported as not compliant, we attempted to adhere to the principles of intention‐to‐treat (ITT) analysis. We approached the issue of non‐compliance independently from that of missing data (Alshurafa 2012). We then pooled the results of the different studies using a random‐effects model. We assessed the certainty of evidence at the outcome level using the GRADE approach (GRADE handbook).
Living systematic review approach: whenever new evidence (studies, data or information) that meets the review inclusion criteria is identified, we will immediately assess risk of bias and extract the data and incorporate it in the synthesis, as appropriate. We will not adjust the meta‐analyses to account for multiple testing given the methods related to frequent updating of meta‐analyses are under development (Simmonds 2017).
Subgroup analysis and investigation of heterogeneity
We planned to explore substantial heterogeneity by conducting subgroup analyses based on the type of oral anticoagulant and the characteristics of participants (type and stage of cancer, and whether participants were on cancer treatment or not). In particular, we conducted subgroup analyses for participants with lung cancer (either SCLC or NSCLC) versus participants with non‐lung cancer. We included in the lung versus non‐lung subgroup analysis data from:
-
studies that recruited only participants with lung cancer (either SCLC or NSCLC) and studies that recruited only participants with non‐lung cancer;
-
studies that recruited both lung and non‐lung cancer if they provided data for subgroups of participants with lung cancer AND data for subgroups of participants with non‐lung cancer;
-
studies that recruited both participants with lung and non‐lung cancer but did not provide subgroup data, if more than 75% of participants had lung cancer or more than 75% of participants had non‐lung cancer.
Sensitivity analysis
Unlike the 2014 update of this review, we included in the sensitivity analysis the studies published as abstracts only. As described above, we also planned for sensitivity meta‐analyses to assess the risk of bias associated with missing participant data when the primary meta‐analysis of a specific outcome found a statistically significant effect.
Results
Description of studies
Results of the search
Figure 1 shows the study flow diagram. As of November 2017, the search strategy identified 7668 unique citations. The title and abstract screening identified 155 potentially eligible citations. The full‐text screening of the full texts of these 155 citations identified six eligible RCTs published as full reports (Chahinian 1989; Levine 1994; Levine 2012; Maurer 1997; Stanford 1979; Zacharski 1984). We had also identified one eligible study published as an abstract but for which we were unable to obtain the necessary data from the authors (Ciftci 2012). In addition, we identified three ongoing studies (Bristol‐Myers Squibb 2006; Janssen Research & Development, LLC 2015; Rodriguez 2015).
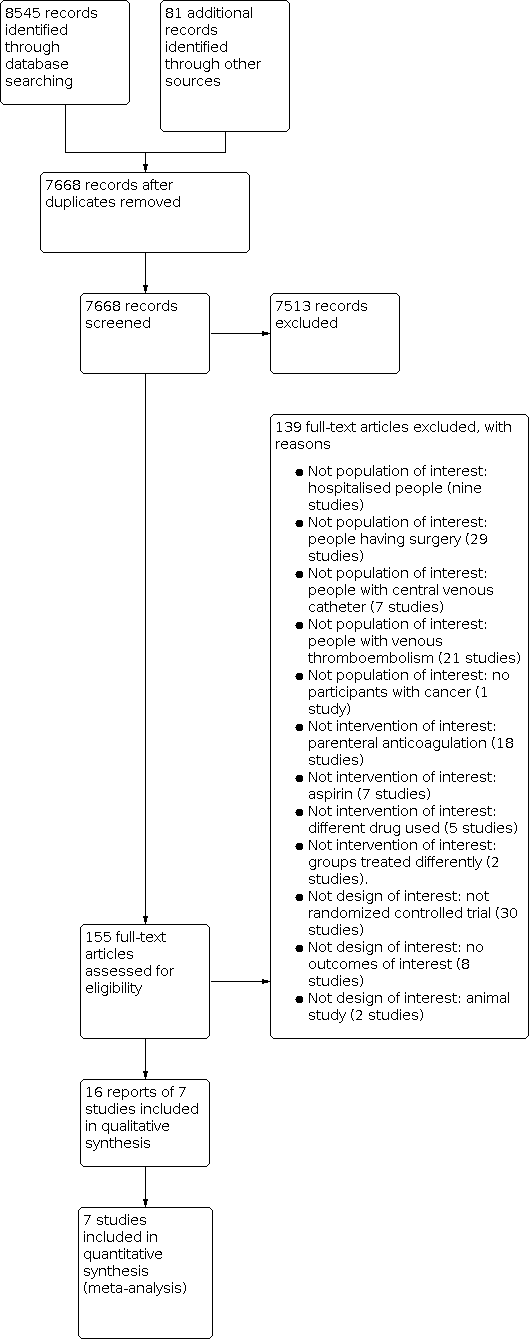
Study flow diagram.
Included studies
Six included RCTs used warfarin as the intervention and one RCT used apixaban as the intervention (Levine 2012). A total of 1486 participants were recruited and follow‐up data were available for 1464 participants (Chahinian 1989; Ciftci 2012; Levine 1994; Levine 2012; Maurer 1997; Stanford 1979; Zacharski 1984).
Chahinian and colleagues recruited 189 participants with extensive SCLC undergoing chemotherapy and with a Cancer and Leukemia Group B (CALGB) performance status of 0 to 3 (Chahinian 1989). Participants were randomized to receive either warfarin (to maintain prothrombin time (PT) between 1.5 and 2) or no warfarin. Therapy was started on the first day of chemotherapy and continued throughout the chemotherapy course. Assessed outcomes included mortality, major bleeding and minor bleeding. Follow‐up rate was 97.3%.
Ciftci and colleagues recruited 91 participants with lung cancer undergoing chemotherapy (Ciftci 2012). Participants were randomized to receive warfarin 5 mg daily or no warfarin starting day one of chemotherapy. Assessed outcomes included mortality and bleeding. Participants were followed up for six months. Information about the follow‐up of participants was not reported.
Levine and colleagues recruited 315 participants with stage IV breast cancer, undergoing chemotherapy, with a minimum life expectancy of three months and with a good performance status based on the Eastern Cooperative Oncology Group (ECOG less than 3) assessment (Levine 1994). Participants were randomized to receive either warfarin at a "therapeutic dose" (to maintain INR between 1.3 and 1.9) or placebo. Treatment began either at the start of chemotherapy or within four weeks and continued until one week after termination of chemotherapy. Assessed outcomes included mortality, DVT, PE, major bleeding and minor bleeding. Follow‐up rate was 99%.
Levine and colleagues recruited 125 participants with advanced or metastatic lung, breast, gastrointestinal, bladder, ovarian or prostate cancers; cancer of unknown origin; myeloma; or selected lymphomas receiving either first‐line or second‐line chemotherapy. Half of the participants had ECOG of 0, and 30% had central venous catheter (CVC) (VTE risk factor) (Levine 2012). Participants were recruited from six sites in Canada and eight sites in the USA. Participants were randomized to receive placebo, or apixaban 5 mg, 10 mg or 20 mg once daily for 12 weeks beginning within four weeks of the date on which the first‐line or second‐line chemotherapy was begun. Assessed outcomes were mortality, major bleed, clinically relevant non‐major bleed, VTE, symptomatic DVT and symptomatic PE. For this review, we only included the dosages 5 mg and 10 mg. The study reported complete follow‐up.
Maurer and colleagues recruited 369 participants with limited‐stage SCLC undergoing chemotherapy and radiotherapy, with a minimum life expectancy of two months and a CALGB performance status less than 3 (Maurer 1997). Participants were randomized to receive either warfarin at a "therapeutic dose" (to maintain PT between 1.4 and 1.6) or no warfarin. Treatment was started on the first day of chemotherapy and continued three weeks after the last cycle of chemotherapy. Assessed outcomes included mortality, major bleeding and minor bleeding. The study reported complete follow‐up.
Stanford and colleagues recruited 24 participants with a small cell carcinoma (at least stage T3 disease) of the bronchus receiving chemotherapy; 75% of participants were males and 79% had extrathoracic metastases (Stanford 1979). Participants were randomized to receive heparin or warfarin or dextran at different time intervals during chemotherapy or no anticoagulant. Assessed outcomes were mortality and bleeding. The study reported complete follow‐up.
Zacharski and colleagues recruited 431 participants with different types of cancer undergoing chemotherapy and with a minimum life expectancy of two months (Zacharski 1984). Participants were randomized to receive either warfarin (to approximately double PT) or no warfarin. Treatment was given until death or the end of the study. Assessed outcomes included mortality and major bleeding. The authors reported data on 418 participants omitting 13 participants who had resection with curative intent for Duke's C carcinoma of the colon because "no conclusions could be reached for this category." The authors had reported earlier on a subgroup of 50 participants with SCLC (Zacharski 1981). Study reported 97% follow‐up.
Excluded studies
We excluded 139 studies for the following reasons: not population of interest: hospitalized people (nine studies); people having surgery (29 studies); people with CVC (seven studies); people with VTE (21 studies); no participants with cancer (one study); not intervention of interest: parenteral anticoagulation (18 studies); aspirin (seven studies); different drug used (five studies). Groups treated differently (two studies). Not design of interest: not RCT (30 studies); no outcomes of interest (eight studies), animal study (two studies).
Risk of bias in included studies
The judgments for the risk of bias are summarized in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
The method of sequence generation was unclear for one study (Chahinian 1989), but adequate for the remaining six (Ciftci 2012; Levine 1994; Levine 2012; Maurer 1997; Stanford 1979; Zacharski 1984). Allocation was adequately concealed in one of the seven included studies (Maurer 1997). It was unclear whether it was adequately concealed in six studies (Chahinian 1989; Ciftci 2012; Levine 1994 ; Levine 2012; Stanford 1979; Zacharski 1984).
Blinding
Blinding of participants and personnel (performance bias)
We judged participants and personnel to be blinded in two studies (Levine 1994; Levine 2012), and not blinded in five studies (Chahinian 1989; Ciftci 2012; Maurer 1997; Stanford 1979; Zacharski 1984).
Blinding of outcome assessment (detection bias)
We judged outcome assessors to be blinded in two studies (Levine 1994; Levine 2012), and probably not blinded in five studies (Chahinian 1989; Ciftci 2012; Maurer 1997; Stanford 1979; Zacharski 1984).
Incomplete outcome data
Two studies reported a complete follow‐up rate (Levine 2012; Stanford 1979). Only one study reported follow‐up data per outcome and not per participant (Chahinian 1989); the follow‐up rate for mortality was 97.4% and major bleeding was 97.3%. Levine 1994 reported a follow‐up rate of 97% and Zacharski 1984 reported a follow‐up rate of 98.7%. Two studies, Maurer 1997 and Ciftci 2012, did not report on follow‐up rates.
Selective reporting
None of the seven studies was registered or had a published protocol. Six studies reported the outcomes listed in the methods section in the results section (Chahinian 1989; Levine 1994; Levine 2012; Maurer 1997; Stanford 1979; Zacharski 1984). Reporting bias was unclear in one study (Ciftci 2012).
Other potential sources of bias
None noted.
Effects of interventions
See: Summary of findings 1 VKA prophylaxis compared to No prophylaxis in ambulatory patients with cancer without VTE receiving systemic therapy; Summary of findings 2 DOAC prophylaxis compared to No prophylaxis in ambulatory patients with cancer without VTE receiving systemic therapy (Q6b‐ Oral)
Agreement between the two biostatisticians who extracted data from survival curves was high with a mean percentage difference in measured survival of 1.5%. In a sensitivity analysis using either biostatistician estimates or the mean of their estimates, we noted no difference in the statistical significance of the results. Heterogeneity was low to moderate in all but one analysis.
Comparison 1: vitamin K antagonist versus no vitamin K antagonist
All‐cause mortality
Mortality at six months: meta‐analysis of three RCTs, including 964 participants, and comparing VKA to no VKA, did not rule out a clinically significant increase or decrease in mortality at six months (RR 0.93, 95% CI 0.77 to 1.13) (Analysis 1.1) (Chahinian 1989; Maurer 1997; Zacharski 1984). The I2 value indicated that the percentage of the variability in effect estimates that was due to heterogeneity rather than sampling error (chance) was low (I2 = 6%).
Mortality at one year: meta‐analysis of five RCTs including 1281 participants, and comparing VKA to no VKA, did not rule out a clinically significant increase or decrease in mortality at one year (RR 0.95, 95% CI 0.87 to 1.03; risk difference (RD) 29 fewer per 1000, 95% CI 75 fewer to 17 more; moderate certainty evidence) (Analysis 1.3) (Chahinian 1989; Levine 1994; Maurer 1997; Stanford 1979: Zacharski 1984). The I2 value indicated that the percentage of the variability in effect estimates that was due to heterogeneity rather than sampling error (chance) was absent (I2 = 0%). The certainty of evidence for mortality at one year was moderate due to imprecision (Summary of findings table 1). Appendix 6 includes the Evidence Profile (a more detailed version of the Summary of findings table 1).
Mortality at two years: meta‐analysis of two RCTs including 528 participants, and comparing VKA to no VKA, did not rule out a clinically significant increase or decrease in mortality at two years (RR 0.95, 95% CI 0.70 to 1.30) (Analysis 1.5) (Chahinian 1989; Maurer 1997). The I2 value indicated that the percentage of the variability in effect estimates that was due to heterogeneity rather than sampling error (chance) was high (I2 = 93%).
Mortality at five years: one RCT including 344 participants, and comparing VKA to no VKA did not rule out a clinically significant increase or decrease in mortality at five years (RR 0.93, 95% CI 0.83 to 1.03) (Analysis 1.6) (Maurer 1997).
Inverted funnel plot was not done due to the relatively few trials to permit an accurate assessment.
In a subgroup analysis of participants with lung cancer (SCLC and NSCLC) versus non‐lung cancer, the test for subgroup effect was not statistically significant for mortality at any follow‐up time (six months: P = 0.14 (Analysis 1.2); one year: P = 1.00 (see Analysis 1.4)) (Chahinian 1989; Maurer 1997; Zacharski 1984). Of note, Maurer 1997 recruited participants with limited SCLC, while Chahinian 1989 recruited participants with extensive SCLC.
Symptomatic venous thromboembolism
One study reported on the incidence of DVT and PE (Levine 1994). The study found that the use of VKA compared to no VKA likely decreased the incidence of DVT (RR 0.08, 95% CI 0.00 to 1.42; RD 35 fewer per 1000, 95% CI 38 fewer to 16 more; low certainty evidence) and did not rule out a clinically significant increase or decrease in PE (RR 1.05, 95% CI 0.07 to 16.58; RD 0 fewer per 1000, 95% CI 6 fewer to 98 more; low certainty evidence). The certainty of evidence for symptomatic DVT due to imprecision and very low for PE due to indirectness and imprecision (see Summary of findings table 1). Appendix 6 includes the Evidence Profile (a more detailed version of the Summary of findings table 1).
Major bleeding
Meta‐analysis of five RCTs including 1281 participants showed that VKA increased the risk of major bleeding compared to no VKA (RR 2.93, 95% CI 1.86 to 4.62; RD 107 more per 1000, 95% CI 48 more to 201 more; moderate certainty evidence) (Analysis 1.9) (Chahinian 1989; Levine 1994; Maurer 1997; Stanford 1979; Zacharsky 1985). The I2 value indicated that the percentage of the variability in effect estimates that was due to heterogeneity rather than sampling error (chance) was low (I2 = 6%). Since the primary meta‐analysis found a statistically significant effect, and in order to assess the risk of bias associated with missing participant data, we conducted sensitivity meta‐analyses using the a priori plausible assumptions detailed in the Methods section. The effect estimate remained statistically significant even when using the most stringent plausible assumption (RR 2.70. 95% CI 1.92 to 3.79). We judged the quality of evidence as moderate (Summary of findings table 1). These results did not change in a meta‐analysis including the study published as an abstract (RR 2.89, CI 2.07 to 4.04; RD 1.06 more per 1000, 95% CI 60 more to 170 more) (Ciftci 2012).
In subgroup analyses of participants with lung cancer (SCLC and NSCLC) versus non‐lung cancer, the test for subgroup effect was not statistically significant (P = 0.16) (Analysis 1.10) (Chahinian 1989; Maurer 1997; Stanford 1979; Zacharski 1984). Of note, Maurer 1997 recruited participants with limited SCLC while Chahinian 1989 recruited participants with extensive SCLC.
Minor bleeding
Meta‐analysis of four RCTs including 863 participants showed that VKA increased the risk of minor bleeding compared to no VKA (RR 3.14, 95% CI 1.85 to 5.32; RD 167 more per 1000, 95% CI 66 more to 337 more; moderate certainty evidence) (Analysis 1.11) (Chahinian 1989; Levine 1994; Maurer 1997; Stanford 1979). The I2 value indicated that the percentage of the variability in effect estimates that was due to heterogeneity rather than sampling error (chance) may represent low heterogeneity (I2 = 21%). Since the primary meta‐analysis found a statistically significant effect, and in order to assess the risk of bias associated with missing participant data, we conducted sensitivity meta‐analyses using the a priori plausible assumptions detailed in the Methods section. The effect estimate remained statistically significant even when using the most stringent plausible assumption (RR 2.89, 95% CI 1.96 to 4.27). We judged the quality of evidence as moderate (see Summary of findings table 1).
In subgroup analyses of participants with lung cancer (SCLC and NSCLC) versus non‐lung cancer the test for subgroup effect was not statistically significant (P = 0.59) (Analysis 1.12) (Chahinian 1989; Maurer 1997; Stanford 1979; Zacharski 1984). Of note, Maurer 1997 recruited participants with limited SCLC, while Chahinian 1989 recruited participants with extensive SCLC.
Health‐related quality of life
We found no data for health‐related QoL.
Comparison 2: direct oral anticoagulant versus no direct oral anticoagulant
Mortality
One study with 92 participants comparing apixaban with placebo did not rule out a clinically significant decrease or increase in mortality at three months (RR 0.24, 95% CI 0.02 to 2.56; RD 51 fewer per 1000, 95% CI 65 fewer to 104 more; low certainty evidence) (Levine 2012). The certainty of evidence was low due to imprecision.
Symptomatic venous thromboembolism
One study with 92 participants comparing apixaban with placebo did not rule out a clinically significant decrease or increase in rate of symptomatic DVT (RR 0.07, 95% CI 0.00 to 1.32; RD 93 fewer per 1000, 95% CI 100 fewer to 32 more; low certainty evidence) or rate of PE (RR 0.16, 95% CI 0.01 to 3.91; RD 28 fewer per 1000, 95% CI 33 fewer to 97 more; low certainty evidence) (Levine 2012). There were no missing participant data for this outcome. The certainty of evidence was low due to imprecision (see Summary of findings table 2).
Major bleeding
One study with 92 participants comparing apixaban with placebo did not rule out a clinically significant decrease or increase in major bleeding (RR 0.16, 95% CI 0.01 to 3.91; RD 28 fewer per 1000, 95% CI 33 fewer to 97 more; low certainty evidence) (Levine 2012). The certainty of evidence was low due to imprecision (see Summary of findings table 2).
Minor bleeding
One study with 92 participants comparing apixaban with placebo did not rule out a clinically significant decrease or increase in minor bleeding (RR 4.43, 95% CI 0.25 to 79.68; RD 0 fewer per 1000, 95% CI 0 fewer to 8 more; low certainty evidence) (Levine 2012). The certainty of evidence was low due to imprecision (see Summary of findings table 2).
Health‐related quality of life
We found no data for health‐related QoL.
Discussion
Summary of main results
VKA appear to have no effect on mortality and VTE in people with cancer who have no therapeutic or prophylactic indication for anticoagulation; however, VKA increase major bleeding and minor bleeding. A subgroup analysis suggested no difference in mortality or risk of bleeding in people with lung cancer compared to people with non‐lung cancer.
The systematic review identified only one trial comparing apixaban with placebo. The study did not confirm or exclude a clinically important effect of apixaban on mortality, symptomatic DVT and PE, major bleeding and minor bleeding.
Overall completeness and applicability of evidence
Unfortunately, the available data were insufficient to assess the statistical significance of potentially clinically significant benefit in different types of cancer such as SCLC and NSCLC. The study results apply directly to the types of cancer the eligible studies have focused on, that is, mostly lung cancer.
Only one included study reported excluding people with a requirement for long‐term oral anticoagulation without indicating how many such participants were excluded (Levine 1994); this could potentially limit the generalizability of the findings to similar populations. In fact, people with other indications for anticoagulation might experience greater effects on survival compared with the ones included in that study.
Quality of the evidence
For the comparison of VKA versus no VKA, we judged the quality of the evidence as moderate for mortality at one year, and for major and minor bleeding, very low for PE and low for symptomatic DVT. We downgraded the quality of evidence for mortality due to imprecision, and for bleeding outcomes due to risk of bias (study limitations). We downgraded the quality of evidence for PE due to indirectness (the study used fixed‐dose VKA which is not representative of current practice) and for imprecision (low number of events) and DVT due to imprecision.
For the comparison of DOAC versus no DOAC, we judged the quality of evidence as low for all outcomes due to imprecision related to the small number of events, expressed for most outcomes as very wide CIs including both values suggesting major benefit and values suggesting major harm.
Potential biases in the review process
Our systematic approach to searching, study selection and data extraction should have minimized the likelihood of missing relevant studies or relevant data. The inclusion of different types of cancer in the same study precluded us from conducting the subgroup analyses to explore effect modifiers such as stage of cancer. The interpretation of findings was also limited by not including data from the trials published as abstracts. We had to calculate the number of mortality events at six, 12 and 24 months from the survival curves for only one study (Zacharski 1984). Also, there might be potential bias associated with multiple testing in the planned meta‐analyses and currently there are no plans to adjust meta‐analyses for multiple testing.
Agreements and disagreements with other studies or reviews
One systematic review by Zhang and colleagues assessed the effects of anticoagulants on the health outcomes of people with lung cancer with no indication for anticoagulation (Zhang 2013). The meta‐analysis included nine RCTs: five RCTs assessed VKA as the intervention, while the other four RCTs assessed low‐molecular‐weight heparin as the intervention. The meta‐analysis found a long‐term survival benefit and a reduction in the incidence of VTE, in addition to a significant increase in risk of bleeding. This analysis had the problem of clinical heterogeneity, something we tried to avoid by separating the analysis of oral and parenteral anticoagulation (Akl 2011a).
The systematic review performed by Di Nisio and colleagues assessed the efficacy of primary VTE thromboprophylaxis in ambulatory people with cancer receiving chemotherapy (Di Nisio 2016). Consistent with our findings, their study found no effect on mortality, or incidence of DVT and PE when comparing VKA to no VKA. They found a four‐fold increase in major bleeding episodes in participants given VKA versus no VKA (RR 3.82, 95% CI 0.97 to 15.04) compared to a three‐fold increase in our study (RR 2.93, 95% CI 1.86 to 4.62). This might be due to the different reports from which the data were abstracted. Di Nisio and colleagues abstracted data from an older version of Zacharsky's study that included 50 participants (Zacharski 1981), while in our study, we used data from a newer report that included 418 participants (Zacharsky 1985).

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Comparison 1: Vitamin K antagonist (VKA) versus no VKA, Outcome 1: Mortality at 6 months: main analysis

Comparison 1: Vitamin K antagonist (VKA) versus no VKA, Outcome 2: Mortality at 6 months: subgroup analysis (lung cancer)

Comparison 1: Vitamin K antagonist (VKA) versus no VKA, Outcome 3: Mortality at 12 months: main analysis

Comparison 1: Vitamin K antagonist (VKA) versus no VKA, Outcome 4: Mortality at 12 months: subgroup analysis (lung cancer)

Comparison 1: Vitamin K antagonist (VKA) versus no VKA, Outcome 5: Mortality at 2 years

Comparison 1: Vitamin K antagonist (VKA) versus no VKA, Outcome 6: Mortality at 5 years

Comparison 1: Vitamin K antagonist (VKA) versus no VKA, Outcome 7: Symptomatic deep vein thrombosis

Comparison 1: Vitamin K antagonist (VKA) versus no VKA, Outcome 8: Pulmonary embolism

Comparison 1: Vitamin K antagonist (VKA) versus no VKA, Outcome 9: Major bleeding: main analysis

Comparison 1: Vitamin K antagonist (VKA) versus no VKA, Outcome 10: Major bleeding: subgroup analysis (lung cancer)

Comparison 1: Vitamin K antagonist (VKA) versus no VKA, Outcome 11: Minor bleeding: main analysis

Comparison 1: Vitamin K antagonist (VKA) versus no VKA, Outcome 12: Minor bleeding: subgroup analysis (lung cancer)

Comparison 2: Direct oral anticoagulants (DOAC) versus no DOAC, Outcome 1: Mortality at 3 months

Comparison 2: Direct oral anticoagulants (DOAC) versus no DOAC, Outcome 2: Symptomatic deep vein thrombosis

Comparison 2: Direct oral anticoagulants (DOAC) versus no DOAC, Outcome 3: Pulmonary embolism

Comparison 2: Direct oral anticoagulants (DOAC) versus no DOAC, Outcome 4: Major bleeding

Comparison 2: Direct oral anticoagulants (DOAC) versus no DOAC, Outcome 5: Minor bleeding
| VKA prophylaxis compared to No prophylaxis in ambulatory patients with cancer without VTE receiving systemic therapy | |||||
| Patient or population: ambulatory people with cancer without VTE receiving systemic therapy Setting: outpatient Intervention: VKA prophylaxis Control: no prophylaxis | |||||
| Outcomes | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
|---|---|---|---|---|---|
| Risk with No prophylaxis | Risk difference with VKA prophylaxis | ||||
| Mortality | 1281 | ⊕⊕⊕⊝ | RR 0.95 | Study population | |
| 574 per 1,000 | 29 fewer per 1,000 | ||||
| PE | 311 | ⊕⊝⊝⊝ | RR 1.05 | Study population | |
| 6 per 1,000 | 0 fewer per 1,000 | ||||
| Symptomatic DVT | 311 | ⊕⊕⊝⊝ | RR 0.08 | Study population | |
| 38 per 1,000 | 35 fewer per 1,000 | ||||
| Major bleeding | 1281 | ⊕⊕⊕⊝ | RR 2.93 | Study population | |
| 55 per 1,000 | 107 more per 1,000 | ||||
| Minor bleeding | 863 | ⊕⊕⊕⊝ | RR 3.14 | Study population | |
| 78 per 1,000 | 167 more per 1,000 | ||||
| HRQoL ‐ not reported | ‐ | ‐ | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded by one level due to concern about both risk of bias (lack of blinding in patients and personnel and unclear allocation concealment in 4 out of 5 studies) and imprecision (95% CI is consistent with the possibility for important benefit (75 per 1000 absolute reduction) and the possibility of important harm (17 per 1000 absolute increase), large event rate) 2 Downgraded by one level due to indirectness. Levine 1994 used fixed dose of VKA instead of adjusted dose which is not representative of the current practice. This study was the only trial that reported on PE and symptomatic DVT. 3 Downgraded by two levels due to very serious imprecision. 95% CI is consistent with the possibility for important benefit (6 per 1000 absolute reduction) and the possibility of important harm (98 per 1000 absolute increase), including only 2 events. 4Levine 1994 used fixed dose of VKA instead of adjusted dose which is not representative of the current practice. This study was the only trial that reported on PE and symptomatic DVT. We do not think that this indirectness has underestimated the effect on symptomatic DVT (RR 0.08) 5 Downgraded by two levels due to very serious imprecision. Only 6 events among 311 participants. 6 Downgraded by one level due to concern about risk of bias (lack of blinding in patients and personnel and unclear allocation concealment in 4 out of 5 studies) 7 Downgraded by one level due to concern about risk of bias (lack of blinding in patients and personnel and unclear allocation concealment in 3 out of 4 studies) | |||||
| DOAC prophylaxis compared to No prophylaxis in ambulatory patients with cancer without VTE receiving systemic therapy | |||||
| Patient or population: ambulatory people with cancer without VTE receiving systemic therapy Setting: outpatient Intervention: DOAC prophylaxis Control: no prophylaxis | |||||
| Outcomes | № of participants | Certainty of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
|---|---|---|---|---|---|
| Risk with No prophylaxis | Risk difference with DOAC prophylaxis | ||||
| Mortality | 92 | ⊕⊕⊝⊝ | RR 0.24 | Study population | |
| 67 per 1,000 | 51 fewer per 1,000 | ||||
| PE | 92 | ⊕⊕⊝⊝ | RR 0.16 | Study population | |
| 33 per 1,000 | 28 fewer per 1,000 | ||||
| Symptomatic DVT | 92 | ⊕⊕⊝⊝ | RR 0.07 | Study population | |
| 100 per 1,000 | 93 fewer per 1,000 | ||||
| Major bleeding | 92 | ⊕⊕⊝⊝ | RR 0.16 | Study population | |
| 33 per 1,000 | 28 fewer per 1,000 | ||||
| Minor bleeding | 92 | ⊕⊕⊝⊝ | RR 4.43 | Low | |
| 0 per 1,000 | 0 fewer per 1,000 | ||||
| HRQoL ‐ not reported | ‐ | ‐ | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Concern due to unclear allocation concealment 2 Downgraded by two levels due to very serious imprecision: 95% CI is consistent with the possibility for important benefit (65 per 1000 absolute reduction) and the possibility of important harm (104 per 1000 absolute increase), including only 3 events among 92 participants. 3 Downgraded by two levels due to very serious imprecision: 95% CI is consistent with the possibility for important benefit (33 per 1000 absolute reduction) and the possibility of important harm (97 per 1000 absolute increase), including only 1 events among 92 participants. 4 Downgraded by two levels due to very serious imprecision: Including only 3 events among 92 participants. 5 Downgraded by two levels due to very serious imprecision: Including only 4 events among 92 participants. | |||||
| Term | Meaning |
|---|---|
| Adjuvant therapy | Assisting in the amelioration or cure of disease. |
| Anticoagulation | Process of hindering the clotting of blood especially by treatment with an anticoagulant. |
| Antithrombotic | Used against or tending to prevent thrombosis (clotting). |
| Apixaban | Oral direct factor Xa inhibitor used for anticoagulation. |
| Coagulation | Clotting. |
| Direct factor Xa inhibitor | Anticoagulant medications used for anticoagulation. Apixaban is an oral direct factor Xa inhibitor. |
| Deep vein thrombosis (DVT) | Condition marked by the formation of a thrombus within a deep vein (e.g. leg or pelvis) that may be asymptomatic or symptomatic (as swelling and pain) and that is potentially life‐threatening if dislodgment of the thrombus results in pulmonary embolism. |
| Fibrin | White insoluble fibrous protein formed from fibrinogen by the action of thrombin especially in the clotting of blood. |
| Fondaparinux | An anticoagulant medication. |
| Hemostatic system | The system that shortens the clotting time of blood and stops bleeding. |
| Heparin | Enzyme occurring especially in the liver and lungs that prolongs the clotting time of blood by preventing the formation of fibrin. 2 forms of heparin that are used as anticoagulant medications are: unfractionated heparin (UFH) and low‐molecular‐weight heparins (LMWH). |
| Major bleeding | Bleeding that is intracranial or retroperitoneal, if it leads directly to death, or if results in hospitalization or transfusion. |
| Metastasis | Spread of a cancer cells from the initial or primary site of disease to another part of the body. |
| Minor bleeding | Any bleeding not classified as major bleeding. |
| Oncogene | Gene having the potential to cause a normal cell to become cancerous. |
| Osteoporosis | Condition that affects mainly older women and is characterized by decrease in bone mass with decreased density and enlargement of bone spaces producing porosity and brittleness. |
| Pulmonary embolism (PE) | Embolism of a pulmonary artery or 1 of its branches that is produced by foreign matter and most often a blood clot originating in a vein of the leg or pelvis and that is marked by labored breathing, chest pain, fainting, rapid heart rate, cyanosis, shock and sometimes death. |
| Stroma | The supporting framework of an organ typically consisting of connective tissue. |
| Thrombin | Proteolytic enzyme formed from prothrombin that facilitates the clotting of blood by catalyzing conversion of fibrinogen to fibrin. |
| Thrombocytopenia | Persistent decrease in the number of blood platelets that is often associated with hemorrhagic conditions. |
| Vitamin K antagonist (VKA) | Anticoagulant medications. Warfarin is a vitamin K antagonist. |
| Warfarin | Anticoagulant medication that is a vitamin K antagonist. |
| Ximelagatran | Anticoagulant medication. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Mortality at 6 months: main analysis Show forest plot | 3 | 946 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.77, 1.13] |
| 1.2 Mortality at 6 months: subgroup analysis (lung cancer) Show forest plot | 3 | 946 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.77, 1.14] |
| 1.2.1 Lung cancer (small cell and non‐small cell) | 3 | 813 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 1.2.2 Non‐lung cancer | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.82, 1.82] |
| 1.3 Mortality at 12 months: main analysis Show forest plot | 5 | 1281 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.03] |
| 1.4 Mortality at 12 months: subgroup analysis (lung cancer) Show forest plot | 5 | 1281 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.03] |
| 1.4.1 Lung cancer (small cell and non‐small cell) | 4 | 837 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.05] |
| 1.4.2 Non‐lung cancer | 2 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.81, 1.10] |
| 1.5 Mortality at 2 years Show forest plot | 2 | 528 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.70, 1.30] |
| 1.6 Mortality at 5 years Show forest plot | 1 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.83, 1.03] |
| 1.7 Symptomatic deep vein thrombosis Show forest plot | 1 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 1.42] |
| 1.8 Pulmonary embolism Show forest plot | 1 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.07, 16.58] |
| 1.9 Major bleeding: main analysis Show forest plot | 5 | 1281 | Risk Ratio (M‐H, Random, 95% CI) | 2.93 [1.86, 4.62] |
| 1.10 Major bleeding: subgroup analysis (lung cancer) Show forest plot | 5 | 1281 | Risk Ratio (M‐H, Random, 95% CI) | 2.85 [1.76, 4.62] |
| 1.10.1 Lung cancer (small cell and non‐small cell) | 4 | 837 | Risk Ratio (M‐H, Random, 95% CI) | 3.95 [2.38, 6.55] |
| 1.10.2 Non‐lung cancer | 2 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.63, 4.89] |
| 1.11 Minor bleeding: main analysis Show forest plot | 4 | 863 | Risk Ratio (M‐H, Random, 95% CI) | 3.14 [1.85, 5.32] |
| 1.12 Minor bleeding: subgroup analysis (lung cancer) Show forest plot | 4 | 865 | Risk Ratio (M‐H, Random, 95% CI) | 3.19 [1.83, 5.55] |
| 1.12.1 Lung cancer (small cell and non‐small cell) | 3 | 554 | Risk Ratio (M‐H, Random, 95% CI) | 3.79 [1.55, 9.24] |
| 1.12.2 Non‐lung cancer | 1 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [0.64, 9.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Mortality at 3 months Show forest plot | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.02, 2.56] |
| 2.2 Symptomatic deep vein thrombosis Show forest plot | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.32] |
| 2.3 Pulmonary embolism Show forest plot | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.01, 3.91] |
| 2.4 Major bleeding Show forest plot | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.01, 3.91] |
| 2.5 Minor bleeding Show forest plot | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 4.43 [0.25, 79.68] |

