Cirugía para la catarata posvitrectomía
Resumen
Antecedentes
La formación o aceleración de cataratas puede ocurrir después de una cirugía intraocular, especialmente después de la vitrectomía, una técnica quirúrgica para extraer el vítreo que se utiliza en el tratamiento de muchos trastornos que afectan al segmento posterior del ojo. El problema subyacente que llevó a la vitrectomía puede limitar el beneficio de la extracción del cristalino afectado por la catarata.
Objetivos
Evaluar la efectividad y la seguridad de la cirugía versus ninguna cirugía para la catarata posvitrectomía con respecto a la agudeza visual, la calidad de vida y otros resultados.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL) (que contiene el Registro Cochrane de Ensayos de Ojos y Visión) (2017, número 5), MEDLINE Ovid (1946 al 17 de mayo de 2017), Embase.com (1947 al 17 de mayo de 2017), PubMed (1946 al 17 de mayo de 2017), la base de datos de Literatura de Ciencias de la Salud de América Latina y el Caribe (LILACS) (enero de 1982 al 17 de mayo de 2017), el metaRegistro de Ensayos Controlados (mRCT) (www.controlled-trials.com); última búsqueda en mayo de 2013, ClinicalTrials.gov (www.clinicaltrials.gov); búsqueda el 17 de mayo de 2017, y la Plataforma de Registro Internacional de Ensayos Clínicos (ICTRP) de la Organización Mundial de la Salud (OMS) (www.who.int/ictrp/search/en); búsqueda el 17 de mayo de 2017. No se aplicaron restricciones de fecha o de idioma en las búsquedas electrónicas de ensayos.
Criterios de selección
Se planificó incluir ensayos controlados aleatorizados (ECA) y cuasialeatorizados que hubieran comparado la cirugía versus ninguna cirugía para extraer el cristalino de los ojos de los adultos en los que se habían desarrollado cataratas después de la vitrectomía.
Obtención y análisis de los datos
Dos autores de la revisión revisaron de forma independiente los resultados de la búsqueda según los procedimientos metodológicos estándar esperados por Cochrane.
Resultados principales
No se encontró ningún ECA o cuasi‐ECA que hubiera comparado la cirugía versus ninguna cirugía para extraer el cristalino de los ojos de los adultos en los que se habían desarrollado cataratas después de la vitrectomía.
Conclusiones de los autores
No hay ninguna evidencia proveniente de ECA o cuasi‐ECA para basar las recomendaciones clínicas del tratamiento quirúrgico de las cataratas posvitrectomía. Hay una clara necesidad de ECA para abordar este vacío de evidencia. En esos ensayos se debe estratificar a los participantes según su edad, el trastorno de la retina que da lugar a la vitrectomía y el estado del proceso de la enfermedad subyacente en el ojo contralateral. Los resultados que se evalúan en esos ensayos pueden incluir cambios (tanto ganancias como pérdidas) de la agudeza visual, la calidad de vida y eventos adversos como la ruptura capsular posterior y el desprendimiento de retina. Se deben examinar tanto los resultados a corto plazo (seis meses) como a largo plazo (uno o dos años).
PICO
Resumen en términos sencillos
Cirugía para eliminar las cataratas después de la vitrectomía
Objetivo de la revisión
El objetivo de esta revisión Cochrane fue evaluar la evidencia relativa al efecto de la cirugía para extraer el cristalino de los ojos en los que se desarrollan cataratas (opacidad del cristalino delante del ojo) después de la vitrectomía. La cirugía de vitrectomía se utiliza para extraer el vítreo (el gel transparente) en el centro del ojo durante la reparación o la mejora de una serie de trastornos de la retina (como el desprendimiento de retina y los orificios maculares o en casos de hemorragia vítrea).
¿Qué se estudió en esta revisión?
La vitrectomía puede resultar en la formación o aceleración de la catarata. El problema subyacente que llevó a la vitrectomía puede afectar a la agudeza visual, la calidad de vida y otros resultados después de la cirugía para extraer el cristalino afectado por la catarata.
Resultados principales
No se encontraron ensayos controlados aleatorizados (ensayos en los que los participantes habían sido asignados al azar a un grupo de tratamiento u otro) que evaluaran los beneficios o los riesgos (o ambos) de la cirugía de cataratas después de la vitrectomía. Dado que la cirugía de cataratas puede provocar la pérdida de la visión debido al empeoramiento o la recurrencia de la enfermedad que provocó la vitrectomía, su función en estos pacientes sigue siendo desconocida. Los ensayos futuros para abordar esta cuestión de la revisión deben separar a los participantes según la edad, el trastorno que da lugar a la vitrectomía y el estado del proceso de la enfermedad subyacente en el ojo opuesto. Los resultados pertinentes para los pacientes, como la mejora de la agudeza visual, otras medidas de la visión y la calidad de vida que normalmente se esperan de la cirugía de cataratas, y los daños deben examinarse tanto a corto plazo (seis meses después de la cirugía de cataratas) como a largo plazo (uno o dos años después de la cirugía de cataratas).
Mensajes clave
Existe una laguna de evidencia sobre si la cirugía para eliminar las cataratas en las personas en cuyos ojos se desarrollan cataratas después de la vitrectomía es mejor que ninguna cirugía.
¿Cuál es el grado de actualización de la revisión?
Se buscaron los estudios publicados hasta el 17 de mayo de 2017.
Authors' conclusions
Background
Description of the condition
Vitrectomy, commonly known as par plana vitrectomy (PPV), is a microsurgical procedure in which the vitreous gel is removed from an eye. Vitrectomy first was developed by Machemer in 1971 (Machemer 1971). Advances in surgical technique and instrumentation over the past 40 years have made vitrectomy a common surgical procedure for posterior segment disorders. Vitrectomy is indicated for numerous ocular conditions including vitreous loss in cataract surgery, subluxation of the lens, malignant glaucoma, dense pupillary membranes, non‐clearing vitreous hemorrhage due to diabetic retinopathy or vein occlusions, retinal detachment, macular hole, macular pucker, vitreo‐macular traction, and endophthalmitis. Specialized instruments and techniques are used to gain access to the vitreous cavity and retina. During vitrectomy surgery, three small incisions, each approximately 1.4 mm in length, are made in the eye in order to place instruments: a vitreous cutter, a fiber optic light source to illuminate the inside of the eye, and an infusion cannula to maintain proper intraocular pressure during the removal of the vitreous and other surgical maneuvers. At the end of surgery, the vitreous cavity may be filled with air, gas, silicone oil, or another type of tamponade to reduce the risk of retinal detachment or may be left unfilled.
Although vitrectomy has revolutionized the treatment of posterior segment disorders and improved visual outcomes in people with retinal diseases requiring surgical intervention, vitrectomy may compromise visual acuity by inducing cataract formation or progression in phakic eyes (Benson 1988). The three types of cataract are classified according to the location of the opacity: cortical, nuclear sclerosis, and posterior subcapsular. The type of cataract that forms or accelerates after vitrectomy is nuclear sclerotic cataract. In their 2004 review, Panozzo and Parolini noted that "Cataract formation is the most frequent complication of pars plana vitrectomy, even without the use of air, gas, or silicone oil". They reported that published proportions of eyes followed for at least 12 months in which nuclear sclerotic cataract developed after vitrectomy without vitreous tamponade ranged from 12% to 80% depending upon the length of follow‐up and age of patients at time of vitrectomy. Published proportions for eyes followed for at least 12 months after vitrectomy and gas tamponade ranged from 45% to 84% (Panozzo 2004).
The exact pathogenesis of cataract formation or acceleration after vitrectomy is unknown. Older studies have suggested that light toxicity, oxidation of lens proteins, use of intraocular gas, and length of operative time may be causative factors (Cherfan 1991; de Bustros 1988; Ogura 1991). Newer research suggests that vitrectomy surgery increases oxygen tension within the eye; oxygen exposure has been linked with progressive nuclear sclerotic cataract formation (Holekamp 2005; Palmquist 1988).
Epidemiology
As noted by Panozzo and Parolini, the studies they cited regarding cataract after vitrectomy typically were retrospective in design, varied in the method of diagnosing and classifying cataract, and reported the outcome after differing lengths of time after vitrectomy. The most reliable incidence data have been reported from multicenter randomized trials in which vitrectomy was an intervention because of their prospective design, detailed description of methods of cataract classification, and scheduled follow‐up examinations and procedures for study eyes.
The Vitrectomy for Macular Hole Study, a randomized controlled trial that evaluated vitrectomy for the treatment of macular holes, retrospectively examined the incidence of cataract progression from baseline scores among 74 phakic eyes of participants in the study who had only a single vitrectomy procedure (Cheng 2001). Investigators used a scoring system similar to the Lens Opacities Classification System II, which contains five grading categories for nuclear and posterior subcapsular opacities. Although duration of surgery did not increase the risk for cataract progression, vitrectomy itself was a risk factor for cataract acceleration; 60/74 (81%) eyes in the surgery cohort had nuclear sclerotic cataract progression at six months of follow‐up, compared with only 13/74 (18%) fellow eyes in the control group. Mean scores for nuclear sclerosis had increased from 1.08 ± 0.72 at baseline to 1.51 ± 0.59 by 12 months. By two years, 100% of eyes in the surgery cohort had cataract progression, compared with 8% of control eyes.
During the late 1990s, the Submacular Surgery Trials (SST) were initiated to evaluate surgical removal of subfoveal choroidal neovascularization (CNV) compared with observation in people with age‐related macular degeneration (AMD) (SST Group N and Group B studies), ocular histoplasmosis syndrome (OHS) (SST Group H study), and idiopathic CNV (SST Group H study) (SST 2004a; SST 2004b; SST 2004c). In these three randomized controlled trials, visually significant cataract was defined as either cataract surgery or lens opacity reported by the SST ophthalmologist to be sufficient to reduce visual acuity by two or more lines in a normal eye. Among the AMD participants in the SST Group N study, 80% of eyes assigned to vitrectomy and surgical removal of their subfoveal CNV had developed visually significant cataracts by two years of follow‐up. Sixty per cent of eyes had undergone cataract surgery by their last follow‐up examination two to four years after enrollment. Among the OHS participants in the SST Group H study, 39% of eyes assigned to vitrectomy developed visually significant cataracts, among which 24% underwent cataract removal. The difference between eyes with AMD and eyes with OHS developing postvitrectomy cataract was likely due to the median age of the participants. Patients under the age of 50 years have been reported rarely to develop postsurgical accelerated nuclear sclerosis (Melberg 1995).
Presentation and diagnosis
As with other cataract, eyes with postvitrectomy cataract typically present with decreased visual acuity despite anatomic and initial functional success of the vitrectomy surgery. Individuals who have undergone vitrectomy may have had lower levels of pre‐vitrectomy visual acuity or other vision deficits due to the underlying retinal pathology; therefore those with postvitrectomy cataract may present with poorer vision than individuals with typical age‐related cataracts. Diagnosis is based on ocular examination using slit‐lamp biomicroscopy, recognition of nuclear sclerosis, and the history of vitrectomy.
Description of the intervention
Cataract surgery, typically performed using phacoemulsification and intraocular lens implantation, commonly is recommended for individuals with visually significant lens opacities. Two features of postvitrectomy nuclear sclerosis make affected lenses especially challenging for cataract surgeons to remove. The nucleus tends to be harder than in age‐related nuclear sclerosis, requiring longer phacoemulsification time during the procedure. Also, the absence of vitreous in the posterior segment allows for more mobility of the posterior capsule, increasing the risk of capsular rupture. Surgery for postvitrectomy nuclear sclerotic cataract thus may be associated with a higher incidence of complications, although evidence from comparative studies is lacking (Ahfat 2003; Biro 2002). Thus, surgery to remove the lens of eyes with postvitrectomy cataract may result in no or less visual acuity improvement and more complications than usually anticipated following surgery for typical age‐related cataract.
How the intervention might work
While people who develop cataract after vitrectomy may undergo cataract extraction, visual acuity and other outcomes after cataract surgery may be poor due to the underlying retinal disorder. Most people who have vitrectomy surgery have serious underlying problems, as indicated by the reasons for vitrectomy. Furthermore, eyes with postvitrectomy cataract are at risk of the complications that can affect all eyes that undergo cataract surgery such as endophthalmitis, cystoid macular edema, etc. Vision is often already impaired before cataract surgery and may remain impaired after cataract surgery. Although cataract surgery in a normal eye typically improves vision, the visual prognosis after surgery for postvitrectomy cataract is uncertain. It likely depends on the success of treatment for the retinal disorder and avoidance of complications during cataract surgery.
Why it is important to do this review
The incidence of cataract formation after vitrectomy varies widely and has been reported to be between 6% and 100%. The majority of published studies confirm that a high rate of cataract formation occurs, but few data are available on visual acuity outcomes after cataract removal. The retinal problem that led to vitrectomy may progress or recur. However, peer‐reviewed data on outcomes after surgery for postvitrectomy cataract are scarce. Even in cases in which cataract formation is not due to vitrectomy, visual impairment can still exist despite cataract extraction. The Los Angeles Latino Eye Study published visual acuity outcomes after cataract extraction in adult Latinos and reported that 41% of eyes had visual impairment (defined as a best‐corrected visual acuity of 20/40 Snellen equivalent or less) (Barañano 2007). Age‐related macular degeneration and diabetic retinopathy accounted for approximately 57% of retinal pathology after cataract extraction. In addition, in eyes that have undergone vitrectomy surgery the absence of vitreous in the posterior segment allows for more mobility of the posterior capsule, increasing the risk of capsular rupture. Surgery for postvitrectomy nuclear sclerotic cataract may be associated with a higher incidence of complications. A prospective case series demonstrated postoperative complications such as posterior capsule rupture, lens subluxation, vitreous hemorrhage, and retinal redetachment (Pardo‐Muñoz 2006). A systematic review of outcomes from controlled clinical trials would therefore provide information for adequate counseling of patients and for guiding ophthalmologists' recommendations.
Objectives
To evaluate the effectiveness and safety of surgery versus no surgery for postvitrectomy cataract with respect to visual acuity, quality of life, and other outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include eligible randomized and quasi‐randomized controlled trials in this review as described in our review protocol (Do 2007). We considered quasi‐randomized trials to be trials that had adopted a method of allocation intended to allocate participants in a random fashion but were not strictly random. Examples include allocation by date of birth, Social Security number, etc. We planned to include trials with at least six months' follow‐up to allow for reporting of early adverse effects, even though our primary analysis would focus on one‐year follow‐up.
Types of participants
We planned to include trials that enrolled adult participants (age 18 years and over) with cataract that had developed after vitrectomy for any indication except for trauma. However, we planned to exclude trials that included both adult patients who had post‐traumatic vitrectomy and patients who had other indications for vitrectomy, except when outcomes were reported separately by indication. We planned to exclude trials that included only trauma cases, because these patients typically are younger and the pathogenesis of cataract formation is different.
Types of interventions
We planned to include trials that compared cataract surgery (of any type) with no surgery in such patients.
Types of outcome measures
Primary outcomes
Visual acuity improvement after cataract surgery of at least three letters on a logMAR chart, one line on the Snellen chart, or equivalent changes on other scales. While we planned to analyze the outcomes at one year, two years, and at longer time points of follow‐up as available from the included studies, our primary analysis was to focus on one‐year follow‐up.
Secondary outcomes
-
Quality of life measured by a validated scale.
-
Contrast sensitivity: improvement of at least one level, regardless of the manner in which it was measured in the included trials.
-
Progression of the condition that was the original indication for vitrectomy in people with diabetic retinopathy and age‐related macular degeneration (AMD) as defined by standard grading scales such as the Diabetic Retinopathy Scale for DR (ETDRS 1991) and the International Scale for AMD (Bird 1995).
Adverse outcomes
Specific adverse effects of interest included:
-
cystoid macular edema;
-
intraocular lens‐related complications, including dislocation, difficulty in placing the lens;
-
capsular opacification;
-
retinal detachment (new or recurrent).
We also planned to summarize all other adverse effects reported in the included studies.
Economic outcomes
We planned to tabulate or summarize data on the costs of procedures, consequences of complications, and any cost‐effectiveness data reported in the included studies in a narrative fashion.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following databases for randomized controlled trials and controlled clinical trials. There were no language or publication year restrictions. The date of the search was 17 May 2017.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 5) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 17 May 2017) (Appendix 1)
-
MEDLINE Ovid (1946 to 17 May 2017) (Appendix 2)
-
Embase.com (1947 to 17 May 2017) (Appendix 3)
-
PubMed (1946 to 17 May 2017) (Appendix 4)
-
LILACS (Latin American and Caribbean Health Science Information database) (1982 to 17 May 2017) (Appendix 5)
-
metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com; last searched May 2013) (Appendix 6)
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 17 May 2017) (Appendix 7)
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 17 May 2017) (Appendix 8)
We revised the searches of electronic databases in 2013 from the 2011 update. We searched PubMed and the WHO ICTRP, which we had not originally searched. We are no longer searching the UK Clinical Research Network Portfolio Database and the Australian New Zealand Clinical Trials Registry for this review.
Searching other resources
We planned to search the reference lists of included studies and the Science Citation Index‐Expanded database to identify any additional trials. We did not search any conference proceedings specifically for the purpose of this review.
Data collection and analysis
Selection of studies
Two review authors independently screened the titles and abstracts of all records identified in the electronic and manual searches. We labeled each record as 'yes ‐ relevant', 'maybe ‐ possibly relevant', or 'no ‐ definitely not relevant'. Two review authors then screened the full reports of records labeled 'yes' or 'maybe'; we re‐labeled each record as 'A ‐ include' or 'C ‐ exclude' based on consensus after review of the full reports. We listed studies reported in records labeled 'C ‐ exclude' after full‐text review in the 'Characteristics of excluded studies' table with reasons for exclusion. We planned to assess methodological quality for studies labeled as 'A ‐ include', however neither of the two review authors labeled a study as 'A ‐ include'.
We found no trials eligible for inclusion in either the original review or the updated review. The methods described below will be applicable to future updates of the review when trials eligible for inclusion have been conducted and reported.
Data extraction and management
Two review authors will independently extract data on basic characteristics of each study (including details of study design, characteristics of participants, interventions, and comparators) and the primary and secondary outcomes onto data collection forms developed in collaboration with Cochrane Eyes and Vision. Any discrepancies will be resolved by discussion. We will contact the authors of included studies for missing data. One review author will enter all data into Review Manager 5 (Review Manager 2014), and another review author will verify the data.
Assessment of risk of bias in included studies
Two review authors will independently assess the included studies for sources of systematic bias according to the guidelines in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We will evaluate studies for the following criteria: method of randomization and allocation concealment (selection bias), masking of outcome assessment (detection bias), completeness of outcome data and intention‐to‐treat analysis (attrition bias), and other potential sources of bias including source of trial funding. We will not assess masking of investigators, as the interventions to be compared preclude such efforts. Though an artificial lens placed in the eyes of participants in the intervention group may be recognized by the anatomic outcome assessor, visual acuity testing may have been performed by someone not responsible for examining the eye. Also, quality of life data may have been collected by some method that preserves masking of intervention or outcome assessment, or both. We will judge each criterion as either 'low risk of bias', 'high risk of bias', or 'unclear risk of bias'. The information in the Cochrane Handbook for Systematic Reviews of Interventions will guide our judgement for each criterion. We will contact authors of studies labeled 'unclear' for clarification. Differences between the two review authors will be resolved by discussion.
Measures of treatment effect
We will calculate a summary risk ratio for dichotomous outcomes (visual acuity improvement, progression of the condition that was the original indication for vitrectomy, and adverse events). We will calculate the mean difference for continuous outcomes (quality of life, cost‐effectiveness, and contrast sensitivity).
Unit of analysis issues
The unit of analysis will be the eye for vision‐related outcomes (visual acuity, contrast sensitivity, progression, and adverse events). The unit of analysis will be the person for quality of life and economic outcomes.
Dealing with missing data
We will attempt to contact the investigators of included trials for any missing data. If the investigators do not respond within four weeks, we will extract available data from the published report. We will refer to the guidelines in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions for handling missing data (Higgins 2011b).
Assessment of heterogeneity
We will examine the design and clinical heterogeneity among included studies by carefully analyzing their characteristics to determine whether the participants included, interventions compared, and outcomes assessed among individual trials are comparable. We will test for statistical heterogeneity formally using the Chi2 test. We also will use the I2 statistic value to determine the proportion of variation due to heterogeneity among studies, and will consider an I2 value greater than 50% to indicate substantial statistical heterogeneity. We will examine the degree of overlap in the confidence intervals of the studies. If there is poor overlap, we will take this to indicate the presence of statistical heterogeneity.
Assessment of reporting biases
We will examine a funnel plot to identify any evidence of publication bias when a sufficient number of studies are included, that is 10 or more. We will assess for selective outcome reporting at the individual trial level as part of the Assessment of risk of bias in included studies.
Data synthesis
In the presence of methodological or clinical heterogeneity, we will not perform meta‐analysis, and will describe the individual trial findings in the results section of the review.
If we detect no substantial methodological or clinical heterogeneity, we will combine trial results in meta‐analysis. If there is a small number of trials in the analysis (fewer than three), we will use a fixed‐effect model. If the number of trials is three or greater, we will use a random‐effects model. If substantial statistical heterogeneity is detected (I2 value greater than 50%), we will not present a summary effect measure and will report the individual trial findings narratively.
Subgroup analysis and investigation of heterogeneity
We will investigate heterogeneity, if present, through subgroup analyses. If sufficient data are available, we will conduct subgroup analyses based on the agents used to fill the vitreous space after vitrectomy, for example air, different gases, and by different indications for vitrectomy.
Sensitivity analysis
We will conduct sensitivity analyses to determine the impact of exclusion of studies of lower methodological quality, including quasi‐randomized trials, and exclusion of industry‐funded studies and unpublished studies.
Summary of findings
When sufficient data are available, we will present a 'Summary of findings' table of the main outcomes assessed in this review. The main outcomes, assessed at one‐year postrandomization, include visual acuity improvement after cataract surgery, quality of life measures, contrast sensitivity, progression of the condition that led to vitrectomy, economic outcomes, and adverse effects.
We will assess the certainty of evidence for each outcome using the GRADE approach (GRADEpro 2014). Two review authors will independently judge each outcome as providing very low, low, moderate, or high certainty of evidence. Any discrepancies will be resolved by discussion. We will base our judgements on the following five criteria.
-
Risk of bias in individual trials
-
Indirectness
-
Heterogeneity
-
Imprecision of estimate (wide confidence intervals)
-
Publication bias
Results
Description of studies
Results of the search
The original electronic searches retrieved a total of 1949 references and 29 additional records from clinical trials registers. After two review authors independently reviewed the titles and abstracts, we retrieved 36 full‐text articles. We found no randomized or quasi‐randomized trials eligible for inclusion in the review.
An updated search in April 2011 identified 785 references and 18 additional records from clinical trials registers. None of the records were eligible for inclusion in the review.
Another updated search in May 2013 identified 1009 references and 120 additional records from clinical trials registers. None of the records were eligible for inclusion in the review. During the process of this update, we reassessed the eligibility for all previously excluded studies and removed 22 out of 36 references from the list by reviewing titles and abstracts. All of the 22 references were either non‐human study or non‐randomized controlled trials. We kept the reasons for exclusion for the remaining 14 references from 10 studies in the Characteristics of excluded studies table.
The most recent updated search conducted in May 2017 yielded 891 references and 30 additional records from clinical trials registers. We assessed the records but none were randomized or quasi‐randomized clinical trials (Figure 1).

Study flow diagram.
Included studies
We did not identify any studies eligible for inclusion in this review.
Excluded studies
We excluded 10 studies after review of the full‐text report; none were relevant to the objective of this systematic review.
Risk of bias in included studies
We found no trials eligible for inclusion in the review to assess for risk of bias.
Effects of interventions
We found no trials eligible for inclusion in the review and therefore no information on effects of interventions.
Discussion
We found no RCT or quasi‐RCT that addressed the research question targeted for this review. The majority of the published literature on this subject is limited to retrospective case reports or non‐randomized prospective case series (Ahfat 2003; Pardo‐Muñoz 2006). The dearth of information on surgery for postvitrectomy cataract indicates that appropriate research on this topic is needed, as thousands of people undergo vitrectomy each year and are at risk of development of cataract and cataract surgery. Documentation of both the risks and benefits of surgery for postvitrectomy cataract from randomized trials is needed to inform patient counseling and clinical recommendations.
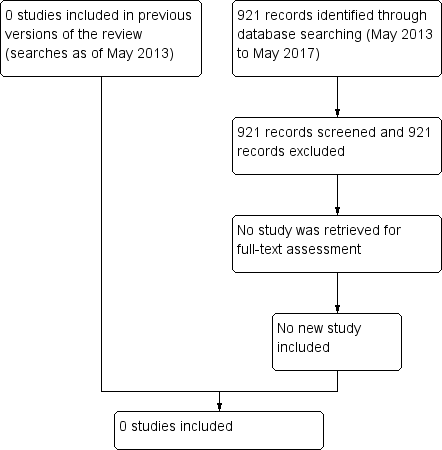
Study flow diagram.

