Antibióticos perioperatorios para la prevención de la endoftalmitis aguda después de la cirugía de cataratas
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: randomized controlled trial Exclusions and loss to follow‐up: none reported Study follow‐up: 1 week | |
| Participants | Setting: cataract surgery camp at Christian Hospital, Taxila, Pakistan Enrollment: 6618 people undergoing cataract surgery Age: not reported Gender: not reported Inclusion criteria: normal intraocular pressure; patent lacrimal drainage system Exclusion criteria: active signs of ocular infection or inflammation | |
| Interventions | Intervention 1: topical regimen alone (chloramphenicol‐sulfadimidine drops) Intervention 2: combined prophylaxis (topical regimen + periocular penicillin during surgery) General: all surgeries were performed by 1 surgeon; surgical technique, postoperative treatment, and follow‐up were identical for both groups. Preoperative treatment: on the day prior to surgery, all participants' faces were washed with soap and water, eyelashes were clipped, and antibiotic ointment was applied to the conjunctival sac. At the time of surgery, procaine 2% and retrobulbar blocks (lidocaine 2 mL of 2% with hyaluronidase 6 units/mL) were administered, participants' eyelids and surrounding face washed with sterile water, a lid speculum was inserted, and the conjunctival sac irrigated with sterile water. Surgical technique: the surgeon used intracapsular cataract extraction procedure and did not rescrub hands between cases or use gloves. All instruments were sterilized with a speed autoclave. Operative technique included a 180° von Graefe knife incision; 1 peripheral iridectomy; 1 to 3 virgin silk corneoscleral sutures placed after the iridectomy but before the lens extraction and forceps delivery of the lens. After the operation, 1 drop of medication (pilocarpine 4%, polymyxin B sulfate 5000 IU/mL, neomycin sulfate 2.5 mg/mL, and hydrocortisone acetate 5 mg/mL) was placed in the conjunctival sac and a sterile pad placed over the eye. Postoperative treatment: eyes were examined and dressed daily. Antibiotic ointment was instilled on the first day and on subsequent days a drop of sulfadimidine 5% and 1 drop of atropine 1% were instilled. Participants without complications were hospitalized for 1 week. | |
| Outcomes | Primary outcome: risk of clinical postoperative endophthalmitis within 1 week after surgery; diagnosis was determined by slit lamp evaluation showing significant inflammation in the anterior chamber; no bacterial cultures were taken. Unit of analysis: the participant (1 eye per person) | |
| Notes | Study dates: March to November 1977 Funding source: not reported Publication language: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants (performance bias) | Low risk | Although the study was reported to be masked, details of masking or the use of placebo were not reported. |
| Masking of physicians and clinical care providers (performance bias) | Low risk | Although the study was reported to be masked, details of masking or the use of placebo were not reported. |
| Masking of outcome assessment (detection bias) | Low risk | Although the study was reported to be masked, details of masking were not reported. |
| Incomplete outcome data (attrition bias) | Low risk | No exclusions or loss to follow‐up were reported; however, the study authors noted that data were limited to early postoperative infections occurring 1 week after surgery since most participants lived too far away for follow‐up visits once discharged. |
| Selective reporting (reporting bias) | Low risk | Results were reported for the primary outcome. |
| Other bias | Low risk | No other potential sources of bias identified. |
| Methods | Study design: randomized controlled trial Exclusions and loss to follow‐up: none reported Study follow‐up: 1 week | |
| Participants | Setting: cataract surgery camp at Christian Hospital, Taxila, Pakistan Enrolment: 77,015 people undergoing cataract surgery Age: not reported Gender: not reported Inclusion criteria: adults having nonimplant intracapsular cataract extractions Exclusion criteria: people receiving intraocular lenses and children with congenital or juvenile cataracts | |
| Interventions | Intervention 1: anterior sub‐Tenon injections (subconjunctival); given beside the limbus exactly subconjunctival or beneath the anterior part of Tenon's capsule Intervention 2: posterior sub‐Tenon injections (retrobulbar); given beside the eye behind the equator of the globe General: 2 types of antibiotics were used for the injections: benzyl penicillin 500,000 units/0.5 mL or ampicillin 200 mg/0.5 mL Preoperative treatment: all participants received 5 applications of 1 drop of sulfadimidine 10% and chloramphenicol 0.5% solution between the first preoperative examination and surgery (about a 13‐ to 22‐hour period). Surgical technique: the surgeons used intracapsular cataract extraction and did not rescrub hands between cases or use gloves. Surgeons were careful not to touch any needle, suture, or part of any instrument that would come into contact with the participants' eyes. Operations were performed quickly to keep the eye open for only 3 to 4 minutes. Most operations included a 180° von Graefe knife incision; 1 peripheral iridectomy; 3 to 5 virgin silk sutures placed after the incision but before the lens extraction; and intracapsular lens extraction performed with a simplified efficient cryoprobe. | |
| Outcomes | Primary outcome: risk of clinical postoperative endophthalmitis 1 week after surgery; diagnosis was determined by slit lamp evaluation showing significant inflammation in the anterior chamber, no bacterial cultures were taken Unit of analysis: the participant (1 eye per person) | |
| Notes | Study dates: January 1979 to June 1985 Funding source: not reported Publication language: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A deck of marked cards was used to randomize participants to treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | A deck of marked cards was shuffled daily and the top card at the time of surgery was used to allocate participants to treatment group. It is unclear whether the marks were concealed (face‐down) prior to allocation or whether the study personnel could preview the order of cards prior to allocation. |
| Masking of participants (performance bias) | Unclear risk | Masking of participants was not reported. |
| Masking of physicians and clinical care providers (performance bias) | Unclear risk | Surgeons could not be masked to the interventions. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) | Low risk | No exclusions or loss to follow‐up were reported; however, follow‐up was only for 1 week after surgery. |
| Selective reporting (reporting bias) | Unclear risk | 2 types of antibiotics were used for the injections depending on the surgeon doing the operation. The study authors reported that infection rates were similar between the 2 types of antibiotics and the 2 surgeons, but did not report infection rates by treatment group (anterior vs posterior injections) separately by type of antibiotic. |
| Other bias | Low risk | No other potential sources of bias identified. |
| Methods | Study design: randomized controlled trial Exclusions and loss to follow‐up: 21 (16%) participants were excluded; 20 because they missed a scheduled follow‐up visit and 1 due to ocular trauma requiring another surgery Study follow‐up: 20 days | |
| Participants | Setting: Hospital of the Medical School of the University of São Paulo, São Paulo, Brazil Enrolment: 129 people undergoing cataract surgery Age: group 1: 71 ± 10 years (range 44 to 88); group 2: 71 ± 10 years (range 41 to 88) Gender: 35/108 (32%) men and 73/108 (68%) women Inclusion criteria: participants undergoing phacoemulsification and intraocular lens implantation. Exclusion criteria: "history of uveitis or chronic ocular inflammation, pseudoexfoliation syndrome, history of ocular trauma, uncontrolled diabetes, pregnant and nursing women, allergy or sensitivity to any component of the medications, serious systemic diseases and perioperative complications, such as anterior capsule rupture and vitreous loss." | |
| Interventions | Intervention 1: fixed combination of gatifloxacin 0.3% and prednisolone acetate 1% (Zypred, Allergan) Intervention 2: individual instillation of gatifloxacin 0.3% and prednisolone acetate 1% (Zypred and Predfort) General: each participant received 2 bottles; drops instilled every 6 hours 1 day prior to surgery to 15 days postoperation Preoperative treatment: not reported Surgical technique: the surgeons performed phacoemulsification and intraocular lens implantation using the "phaco chop technique" under topical anesthesia. | |
| Outcomes | Outcomes assessed: best‐corrected visual acuity, tolerability (pain, photophobia, burning sensation, itching, foreign body sensation), signs of ocular inflammation (redness, edema, tearing, discharge), conjunctival hyperemia, central and incisional corneal edema, anterior chamber cells, intraocular pressure, presence of hypopyon, posterior capsule opacity, pigments or membrane in front of the intraocular lens, compliance, and adverse events; no bacterial cultures were taken Participants were seen on days 1, 7, 15, and 20 Unit of analysis: the participant (1 eye per person) | |
| Notes | Study dates: not reported Funding source: not reported, but drugs were provided by Allergan Laboratories, Inc Publication language: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned using the Research Randomizer software (site: www.randomizer.org); the value 1 was assigned to patients enrolled in Group I, and the value 2 was assigned to patients enrolled in Group II." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking of participants (performance bias) | Low risk | "The group assignment was masked from all patients and investigators. Each patient was given two identical bottles labeled according to their group assignment. All bottles were opaque and patients were instructed to apply one drop from each bottle in the operated eye every 6 h with a 5‐min interval between drops, beginning one day prior to the surgery until the 15th day." |
| Masking of physicians and clinical care providers (performance bias) | Low risk | "The group assignment was masked from all patients and investigators." |
| Masking of outcome assessment (detection bias) | Low risk | "The group assignment was masked from all patients and investigators." |
| Incomplete outcome data (attrition bias) | High risk | 21 (16%) participants were excluded from the analyses. |
| Selective reporting (reporting bias) | Low risk | Results were reported for the outcomes assessed. |
| Other bias | Low risk | No other potential sources of bias identified. |
| Methods | Study design: randomized controlled trial Exclusions and loss to follow‐up: 324 (2%) participants were lost to follow‐up; 68 participants were excluded because they did not undergo the planned surgery or they withdrew consent. Study follow‐up: 6 weeks | |
| Participants | Setting: 24 ophthalmology units in Austria, Belgium, Germany, Italy, Poland, Portugal, Spain, Turkey, and the UK Enrolment: 16,603 people undergoing phacoemulsification cataract surgery Age: median for men was 73 years; for women was 75 years Gender: 42% men and 58% women Inclusion criteria: participants having routine cataract surgery at any study unit. Exclusion criteria: participants allergic to penicillins and cephalosporins, people in long‐term nursing homes, pregnant, or < 18 years; people severely at risk of infection (i.e. atopic keratoconjunctivitis or active blepharitis). | |
| Interventions | Intervention 1: intracameral cefuroxime 0.9% (injected into the anterior chamber at the end of surgery) Intervention 2: topical levofloxacin 0.5% (instilled 1 drop 1 hour before surgery, 1 drop 30 minutes before surgery, and 3 more drops at 5‐minute intervals immediately after surgery) Intervention 3: combined intracameral cefuroxime and topical levofloxacin Intervention 4: placebo drops (no sham injection was given) General: all study centers used povidone iodine 5% for antisepsis. Some centers additionally performed skin cleansing procedures; no detergents were used. Postoperative treatment: all participants were given topical levofloxacin 0.5% starting the morning after surgery (approximately 18 hours after surgery) and 4 times daily for 6 days. | |
| Outcomes | Primary outcomes (at 6 weeks' postsurgery): Secondary outcomes: other risk factors for increased susceptibility, such as clear corneal incision or surgery during summer months, or decreased risk, such as foldable intraocular lenses inserted with sterile injector, etc. Unit of analysis: the participant (1 eye per person) | |
| Notes | Study dates: September 2003 to January 2006 Full study name: European Society of Cataract and Refractive Surgeons Study on the Antibiotic Prophylaxis of Post‐operative Endophthalmitis Funding source: European Society of Cataract and Refractive Surgeons and Santen GmbH, Germany Publication language: English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 12‐block computerized randomization stratified by study center was used. |
| Allocation concealment (selection bias) | Low risk | An electronic database was used to conceal the treatment assignments for each participant. Droppers were labeled with sequential subject IDs, which were entered into the database at the time of surgery to determine whether or not an injection should be given. Treatment allocation codes were held in a central randomization file. |
| Masking of participants (performance bias) | Low risk | Partial masking of participants was done with use of placebo drops. No sham injection was performed. |
| Masking of physicians and clinical care providers (performance bias) | Low risk | Partial masking of physicians was done by using identically labeled droppers. No sham injection was performed. |
| Masking of outcome assessment (detection bias) | Low risk | Physicians were partially masked and it was reported that clinical partners were masked throughout the study. |
| Incomplete outcome data (attrition bias) | Low risk | 324 (2%) participants who were lost to follow‐up and 68 (0.4%) participants who did not undergo the planned surgery or withdrew consent were excluded from the intention‐to‐treat analyses. |
| Selective reporting (reporting bias) | Low risk | Study outcomes were published in study protocols, trial registrations and methods papers prior to the study beginning. Results were reported for these primary and secondary outcomes. |
| Other bias | Low risk | Performed power calculations to enroll a study size to detect a 4‐fold reduction in risk at 5% significance level. The study chairman, coordinator, clinical partners, and data monitoring committee were masked while the study was running. |
| Methods | Study design: randomized controlled trial Exclusions and loss to follow‐up: eyes for which the surgical procedure was modified due to physician discretion at time of surgery were excluded from the study Study follow‐up: 6 weeks | |
| Participants | Setting: Gülhane Military Medical Academy and Medical School Hospital, Ankara, Turkey Enrolment: 644 eyes of 640 participants undergoing phacoemulsification cataract surgery Age: group 1: 64.2 ± 14.3 years (range 43 to 87); group 2: 61.2 ± 14.2 years (range 40 to 81) Gender: not reported Inclusion criteria: people scheduled to undergo phacoemulsification surgery Exclusion criteria: people with previous history of immunosuppressive treatment, diabetes mellitus, ocular surgery, recent infection, or inflammation | |
| Interventions | Intervention 1: balanced salt solution‐only irrigating infusion fluid (n = 322 eyes) Intervention 2: balanced salt solution with antibiotics (vancomycin 20 mg/mL and gentamicin 8 mg/mL; 322 eyes) General: interventions were given intraoperatively. Preoperative treatment, postoperative treatment, and follow‐up were identical for both groups. Preoperative treatment: 1‐day course of topical ofloxacin 0.3% and diclofenac sodium 1 mg/mL 4 times a day; conjunctival smears were obtained just before povidone iodine instillation at time of surgery. Surgical technique: phacoemulsification with a standard 3.2‐mm clear corneal incision, circular capsulotomy, and stop‐chop technique followed by foldable hydrophobic acrylic intraocular lens implantation; no sutures, subconjunctival antibiotics, or steroid injections were used. Postoperative treatment: eyes were treated with ofloxacin 0.3%, dexamethasone 1 mg/mL, and indomethacin 0.1% drops with a 4‐week tapering dose; participants were discharged the day after surgery. | |
| Outcomes | Primary outcomes: Participants were seen on days 2, 5, 10, 15, 30, and 45 Unit of analysis: the eye (both eyes of 4 participants were included separately in the analysis) | |
| Notes | Study dates: May 2000 to June 2002 Funding source: not reported Publication language: English The study authors reported the rate of postoperative endophthalmitis at their institution was 0.109%, but only 644 eyes were included in the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated to irrigating infusion fluid containing either balanced salt solution (BSS)‐only (group 1; 322 eyes of 320 patients) or BSS with antibiotics (20 mg/ml vancomycin and 8 mg/ml gentamicin) (group 2; 322 eyes of 320 patients), according to the scheduled day of surgery, which was performed one after another. (1:1)." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking of participants (performance bias) | Unclear risk | Masking of participants was not reported. |
| Masking of physicians and clinical care providers (performance bias) | Unclear risk | Masking of physicians was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) | High risk | Eyes for which the surgical procedure was modified due to physician discretion at time of surgery were excluded from the study. The number of excluded participants was not reported. |
| Selective reporting (reporting bias) | Low risk | Results were reported for both primary outcomes. |
| Other bias | Low risk | No other potential sources of bias identified. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Endophthalmitis was not an outcome of the study: 2 different postcataract surgery antibiotic/steroid therapeutic combinations were compared in an intra‐individual randomized controlled trial; 142 participants (284 eyes) completed the 15‐day study; the study outcomes were efficacy of treatment, frequency of complications, and participant satisfaction. | |
| Endophthalmitis was not an outcome of the study: topical ciprofloxacin 0.3% prior to cataract surgery was compared with no antibiotics in a randomized controlled trial; 46 participants completed the 1‐day study; the study outcomes were the presence of bacteria in cultures taken the day prior to surgery, the morning of surgery, immediately before surgery, and at the end of surgery. | |
| Not a randomized controlled trial: intracameral moxifloxacin was administered following standard cataract surgery in some eyes, but not others; all participants received topical moxifloxacin for 1 week after surgery; data were reviewed retrospectively and participants with intraoperative complications were excluded from analyses; the study outcomes were postoperative best‐corrected visual acuity, anterior chamber cell and flare, intraocular pressure, and corneal edema. | |
| Not a randomized controlled trial: subconjunctival injections of antibiotics were administered following intraocular surgical procedures (including cataract, glaucoma, corneal transplant, pupillary membrane needling, etc.) to alternate participants during the first phase of the study and to all participants subsequently; rates for postoperative endophthalmitis were not reported separately for people with cataract who did not receive antibiotics in the first phase of the study. | |
| Not a randomized controlled trial: topical neomycin/polymyxin‐B was administered either 1 day or 1 hour prior to cataract surgery; the authors reported the study as a prospective comparative case series; the study outcomes were the presence of bacteria in cultures taken prior to the application of povidone‐iodine before surgery, after the application of povidone‐iodine before surgery, and at the end of surgery. | |
| Not a randomized controlled trial: letter reporting changes made by a hospital following an increased rate of endophthalmitis; changes included reorganizing the layout of the operating theater and administering postoperative intracameral vancomycin. | |
| Endophthalmitis was not an outcome of the study: an intraoperative injection of triamcinolone and ciprofloxacin in a controlled‐release system (DuoCat) was compared with prednisolone and ciprofloxacin eye drops after cataract surgery in a randomized controlled trial; 135 participants completed the 4‐week study; the study outcomes were postoperative anterior chamber cell and flare, intraocular pressure, lack of anti‐inflammatory response, and presence of infection. | |
| Not a randomized controlled trial: South Indian eye camps were sequentially divided into 3 groups of treatment regimens: group 1: no prophylactic intracameral gentamicin was used, but oral and topical chloramphenicol was given to all participants; group 2: female participants received prophylactic intracameral gentamicin, but no chloramphenicol and male participants received oral and topical chloramphenicol, but no prophylactic intracameral gentamicin; group 3: all participants received prophylactic intracameral gentamicin, but no chloramphenicol or any other antibiotic was given. | |
| Not a randomized controlled trial: intracameral injections of vancomycin versus cefuroxime were administered at the end of cataract surgery; the authors reported the study as a prospective comparative case series; the study outcomes were postoperative uncorrected and corrected distance visual acuity, refraction, anterior chamber cell and flare, intraocular pressure, endothelial specular microscopy, and corneal edema and thickness. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Use of Intracameral Moxifloxacin for the Prevention of Acute Endophthalmitis Following Cataract Surgery: a Controlled and Randomized Clinical Trial |
| Methods | Study design: parallel group, randomized controlled trial Study follow‐up: 8 weeks |
| Participants | Setting: University of Campinas, Brazil Estimated enrolment: 6000 eyes of 6000 participants undergoing cataract surgery Inclusion criteria: people aged 50 to 100 years scheduled to undergo cataract surgery Exclusion criteria: vulnerable people; people with allergy to moxifloxacin; people with ocular or periocular infection, advanced glaucoma, or severe dry eye, or undergoing cataract surgery for traumatic cataract with ocular perforation or other reasons (e.g. glaucoma filtering surgery, vitreoretinal surgery, and cornea surgery |
| Interventions | Intervention 1: intracameral injection of moxifloxacin 0.5% at conclusion of cataract surgery Intervention 2: no intracameral injection |
| Outcomes | Primary outcome: |
| Starting date | Study dates: May 2016 to May 2018 |
| Contact information | Principal investigator: |
| Notes | Study sponsor: University of Campinas, Brazil |
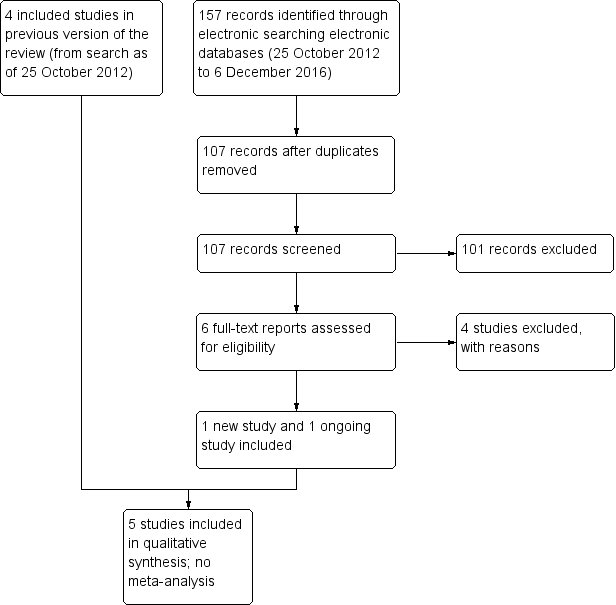
Study flow diagram.
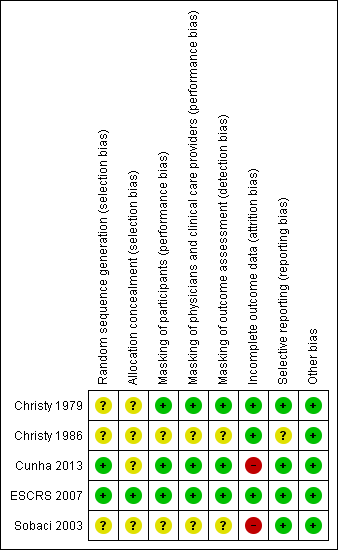
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Perioperative antibiotics for prevention of endophthalmitis after cataract surgery | ||||||||
| Population: participants undergoing cataract surgery Settings: eye hospital or clinic Outcome: risk of endophthalmitis after surgery | ||||||||
| Perioperative prophylaxis versus no prophylaxis | ||||||||
| Study ID | No. eyes and participants | Follow‐up | Comparison (intervention vs comparator) | Risk of endophthalmitis by study group | RR (95% CI) | Certainty of the evidence | ||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | |||||
| 644 eyes of 640 participants | 6 weeks | Treatment: BSS with antibiotics (vancomycin 20 mg/mL and gentamicin 8 mg/mL) | Not reported | 0/322 (0%) eyes | Not reported | 0.20 (0.01 to 4.15) | ⊕⊝⊝⊝ | |
| Control: BSS‐only irrigating infusion fluid | Not reported | 2/322 (0.62%) eyes | ||||||
| 16,603 eyes of 16,603 participants | 6 weeks | Treatment 1: combined intracameral cefuroxime and topical levofloxacin | 2/4052 (0.05%) eyes | 1/4052 (0.02%) eyes | 0.14 (0.03 to 0.63) | 0.10 (0.01 to 0.78) | ⊕⊕⊕⊕ | |
| Treatment 2: intracameral cefuroxime 0.9% | 3/4056 (0.07%) eyes | 2/4056 (0.05%) eyes | 0.21 (0.06 to 0.74) | 0.20 (0.04 to 0.91) | ⊕⊕⊕⊕ | |||
| Treatment 3: topical levofloxacin 0.5% | 10/4049 (0.25%) eyes | 7/4049 (0.17%) eyes | 0.72 (0.32 to 1.61) | 0.70 (0.27 to 1.84) | ⊕⊕⊕⊝ | |||
| Control: placebo drops | 14/4054 (0.35%) eyes | 10/4054 (0.25%) eyes | ||||||
| Comparisons of combinations of antibiotics with specific antibiotics | ||||||||
| Study ID | No. eyes and participants | Follow‐up | Interventions | Risk of endophthalmitis by study group | RR (95% CI) | Certainty of the evidence | ||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | |||||
| 6618 eyes of 6618 participants | 1 week | Treatment 1: combined prophylaxis (topical regimen + periocular penicillin at the time of surgery) | 5/3309 (0.15%) eyes | Not reported | 0.33 (0.12 to 0.92) | Not reported | ⊕⊕⊕⊝ | |
| Treatment 2: topical regimen alone (chloramphenicol‐sulfadimidine) | 15/3309 (0.45%) eyes | Not reported | ||||||
| 16,603 eyes of 16,603 participants | 6 weeks | Treatment 1: combined intracameral cefuroxime and topical levofloxacin | 2/4052 (0.05%) eyes | 1/4052 (0.02%) eyes | Treatment 1 vs treatment 2: 0.67 (0.11 to 3.99) | Treatment 1 vs treatment 2: 0.50 (0.05 to 5.52) | ⊕⊕⊕⊝ | |
| Treatment 2: intracameral cefuroxime 0.9% | 3/4056 (0.07%) eyes | 2/4056 (0.05%) eyes | Treatment 2 vs treatment 3: 0.30 (0.08 to 1.09) | Treatment 2 vs treatment 3: 0.29 (0.06 to 1.37) | ⊕⊕⊕⊝ | |||
| Treatment 3: topical levofloxacin 0.5% | 10/4049 (0.25%) eyes | 7/4049 (0.17%) eyes | Treatment 1 vs treatment 3: 0.20 (0.04 to 0.91) | Treatment 1 vs treatment 3: 0.14 (0.02 to 1.16) | ⊕⊕⊕⊕ | |||
| Mode of antibiotic delivery | ||||||||
| Study ID | No. eyes and patients | Follow‐up | Interventions | Risk of endophthalmitis by study group | RR (95% CI) | Certainty of the evidence | ||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | |||||
| 77,015 eyes of 77,015 participants | 1 week | Mode 1: Anterior sub‐Tenon injections (subconjunctival) | 38/39,752 (0.10%) eyes | Not reported | 0.85 (0.55 to 1.32) | Not reported | ⊕⊕⊕⊝ | |
| Mode 2: Posterior sub‐Tenon injections (retrobulbar) | 42/37,263 (0.11%) eyes | Not reported | ||||||
| 108 eyes of 108 participants | 3 weeks | Treatment 1: fixed combination of topical gatifloxacin 0.3% and prednisolone acetate 1% | 0/47 (0%) eyes | Not reported | 0.43 (0.02 to 10.34) | Not reported | ⊕⊝⊝⊝ | |
| Treatment 2: individual instillation of topical gatifloxacin 0.3% and prednisolone acetate 1% | 1/61 (2%) eyes | Not reported | ||||||
| GRADE Working Group grades of evidence | ||||||||
| BSS: balanced salt solution; CI: confidence interval; RR: risk ratio. *Presumed cases: includes both culture‐proven and clinically diagnosed cases of postoperative endophthalmitis. **Proven cases: cases confirmed by at least one of Gram stain, culture, or polymerase chain reaction (PCR) 1 Downgraded for imprecision (‐2) as the study did not enroll a sufficient number of participants to detect differences between groups. 2 Downgraded for high risk of attrition bias (‐1) as the study authors excluded participants at the time of surgery based on the surgeon's discretion (number excluded not reported). 3 Downgraded for imprecision (‐1) as the confidence interval of the effect estimate between groups was wide. 4 Downgraded for indirectness (‐1) as the study was conducted more than 30 years ago and the techniques for cataract surgery have since changed substantially. 5 Downgraded for high risk of attrition bias (‐1) as the study authors excluded participants who did not return for follow‐up (16% of study population). | ||||||||
| Comparisons of specific antibiotics or combinations of antibiotics | |||||||||
| Study ID | Groups | Proportion of eyes with final VA > 20/40 following endophthalmitis | RR (95% CI) | Proportion of eyes with final VA < 20/200 following endophthalmitis | RR (95% CI) | ||||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | ||
| Group 1: intracameral cefuroxime injection, with or without topical levofloxacin drops | 2/5 (40%) eyes | 1/3 (33.3%) eyes | 0.69 (0.22 to 2.11) | 0.57 (0.11 to 2.95) | 0/5 (0%) eyes | 0/3 (0%) eyes | 0.46 (0.03 to 7.48) | 0.50 (0.03 to 7.54) | |
| Group 2: no injection, with or without topical levofloxacin drops | 14/24 (58.3%) eyes | 10/17 (58.1%) eyes | 4/24 (16.7%) eyes | 4/17 (23.5%) eyes | |||||
| CI: confidence interval; final VA: visual acuity at time of last follow‐up visit (range 3 weeks to 8 months); VA: visual acuity. *Presumed cases: includes both culture‐proven and clinically diagnosed cases of postoperative endophthalmitis. **Proven cases: cases confirmed by at least one of Gram stain, culture, or polymerase chain reaction (PCR). | |||||||||

