بیحسی ساب‐تنون در برابر بیحسی موضعی در جراحی کاتاراکت
چکیده
پیشینه
بیحسی لوکال در جراحی کاتاراکت میتواند با بیحسی ساب‐تنون (sub‐Tenon) یا بیحسی موضعی (topical) ایجاد شود. هر دو تکنیک، مزایای احتمالی دارند. این مرور، که در ابتدا در سال 2007 منتشر و در سال 2014 بهروز شد، به مقایسه این دو تکنیک بیحسی پرداخت.
اهداف
اهداف ما، مقایسه اثربخشی بیحسی موضعی (با یا بدون بیحسی لوکال اینتراکمرال (intracameral)) در برابر بیحسی ساب‐تنون در تخفیف درد طی جراحی کاتاراکت بود. درد را طی تجویز عامل بیحسی، درد پس از جراحی، رضایت از روند جراحی با در نظر گرفتن شرایط جراحی و رضایت بیمار را از میزان تسکین درد مرور کرده، و عوارض مرتبط را بررسی کردیم.
روشهای جستوجو
علاوه بر این، پایگاه مرکزی ثبت کارآزماییهای کنترل شده کاکرین؛ MEDLINE و EMBASE (آخرین جستوجو در نوامبر 2014) و فهرست منابع مقالات منتشر شده را جستوجو کردیم. به دنبال چکیدههای مقالات مطرح شده در کنفرانسها و کارآزماییهای در حال انجام بودیم و هیچ محدودیتی را برای وضعیت زبان نگارش مقاله یا انتشار آن قائل نشدیم.
معیارهای انتخاب
همه مطالعات تصادفیسازیشده را وارد کردیم که بیحسی ساب‐تنون را در برابر بیحسی موضعی در جراحی کاتاراکت مقایسه کردند.
گردآوری و تجزیهوتحلیل دادهها
کیفیت کارآزمایی را ارزیابی کرده و دادهها را در قالبی استخراج کردیم که امکان گنجاندن حداکثر دادهها را فراهم میکرد.
نتایج اصلی
هشت مطالعه را در این مرور بهروز شده گنجاندیم، اما فقط هفت مطالعه را با حضور 742 چشم جراحیشده از 617 شرکتکننده در تجزیهوتحلیل نگاه داشتیم. دو کارآزمایی متقاطع (cross‐over)، شامل 125 شرکتکننده، و پنج کارآزمایی موازی، شامل 492 شرکتکننده بودند. مطالعات بین سالهای 1997 و 2005 منتشر شدند. میانگین سنی شرکتکنندگان از 71.5 تا 83.5 سال متغیر بود. نسبت شرکتکنندگان زن از 54% تا 76% گزارش شد. بیحسی موضعی (با یا بدون تزریق داخل اینتراکمرال) در مقایسه با بیحسی ساب‐تنون، در جراحی کاتاراکت، درد حین جراحی را افزایش داده، اما درد پس از جراحی را طی 24 ساعت کاهش میدهد. دامنه اثر (معادل 1.1 در یک مقیاس 0 تا 10 برای درد حین جراحی و 0.2 در همان مقیاس برای درد پس از جراحی طی 24 ساعت)، اگرچه از نظر آماری معنیدار گزارش شد، احتمالا برای ارتباط بالینی بسیار کوچک بود. کیفیت شواهد برای درد طی جراحی در سطح بالا و برای درد طی 24 ساعت، در سطح متوسط ارزیابی شد. تفاوتهایی را برای درد طی تجویز بیحسی لوکال (سطح شواهد پائین) یافتیم، و نشانههایی به دست آمد مبنی بر اینکه رضایت جراح (سطح شواهد پائین) و رضایت شرکتکننده (سطح شواهد متوسط) با بیحسی موضعی کمتر بود. شواهد کافی وجود نداشت که بگوییم یک تکنیک در مقایسه با روش دیگر منجر به بروز عوارض حین جراحی بیشتر یا کمتری میشود.
نتیجهگیریهای نویسندگان
هر دو بیحسی موضعی و بیحسی ساب‐تنون، روشهای قابلقبول و بیخطری برای ارائه بیحسی در جراحی کاتاراکت هستند. درجه قابلقبولی از ناراحتی حین جراحی را باید با هر یک از این تکنیکها انتظار داشت. انجام کارآزماییهای تصادفیسازی و کنترل شده در زمینه بررسی تاثیرات استراتژیهای مختلف برای پیشگیری از بروز درد حین جراحی کاتاراکت میتواند مفید باشد.
PICOs
خلاصه به زبان ساده
بیحسی ساب‐تنون در برابر بیحسی موضعی در کنترل درد و ایجاد شرایط بهتر در جراحی کاتاراکت
کاتاراکت، شایعترین علت افت بینایی و نابینایی است؛ معمولا به دلیل روند طبیعی پیری رخ میدهد، اما ممکن است در افراد جوانتر نیز دیده شود. کاتاراکت به دنبال از دست رفتن شفافیت طبیعی لنز چشم رخ میدهد. این وضعیت با جراحی برداشتن لنز و جایگزینی آن با یک لنز صناعی درمان میشود. جراحی در حالت ایدهآل باید طی شش ماه پس از تشخیص انجام شود تا از اتلاف بیشتر بینایی جلوگیری شود. بحث در مورد این که کدام تکنیک بیحسی لوکال، تسکین بهتر درد را برای بیمار فراهم کرده و در عین حال، کار جراح را تسهیل میکند، ادامه دارد.
بیحسی موضعی، با قرار دادن قطرههای بیحس کننده لوکال یا ژل بیحس کننده لوکال، روی سطح چشم انجام میشود. بیحسی ساب‐تنون به این صورت است که ابتدا سطح چشم را با قطرههای بیحسی لوکال بیحس میکنند، بافت پوشاننده (ملتحمه و کپسول تنون) را با پنس بسیار باریکی در جلوی چشم نگه میدارند و با استفاده از قیچی سرگرد، یک برش کوچک در آن ایجاد میکنند. یک کانولای کوچک ساب‐تنون، از طریق این سوراخ عبور داده میشود تا بیحسکننده لوکال، به فضای ساب‐تنون تزریق شود. از مزایای بیحسی موضعی نسبت به بلوک ساب‐تنون، میتوان به کاهش زمان تجویز، درد کمتر طی تجویز و مدت زمان کوتاهتر اثر داروی بیحسی اشاره کرد، که به فرد اجازه میدهد به سرعت بینایی را پس از جراحی به دست آورد.
هشت کارآزمایی تصادفیسازی و کنترل شده را وارد مرور کردیم، و تجزیهوتحلیل خود را روی هفت مورد از آنها قرار دادیم: دو کارآزمایی متقاطع (cross‐over) شامل 125 شرکتکننده و پنج کارآزمایی موازی شامل 492 شرکتکننده. میانگین سنی شرکتکنندگان از 71.5 تا 83.5 سال متغیر بود. آرامبخش خوراکی فقط برای دو کارآزمایی استفاده شد. هیچ یک از کارآزماییها پیش از جراحی از آنالژزیکهای خوراکی استفاده نکرده، و هیچکدام منبع بودجه خود را ذکر نکردند. این مرور نشان داد که بیحسی ساب‐تنون نسبت به بیحسی موضعی حین جراحی کاتاراکت تسکین درد نسبتا بهتری را ارائه میدهد. این تفاوت، در مقیاس 0 تا 10 برابر با 1.1 بود. درد در روز پس از جراحی برای شرکتکنندگانی که بیحسی موضعی دریافت کردند کمی کمتر بود و این تفاوت در مقیاس 0 تا 10 معادل 0.2 گزارش شد. هم جراحان و هم شرکتکنندگان، بیحسی ساب‐تنون را ترجیح دادند. با این حال، تمام کارآزماییها در زمانی انجام شدند که جراحان تازه شروع به استفاده از بیحسی موضعی کرده بودند. شواهد کافی از کارآزماییهای وارد شده وجود نداشت تا مشخص شود که یک تکنیک بیحسی با بروز کمتر یا بیشتر عوارض مهم جراحی طی انجام جراحی (پارگی کپسول خلفی، پرولاپس عنبیه) وجود دارد که ممکن است منجر به عوارض پس از جراحی و در نهایت بینایی ضعیفتر شود یا خیر. هر دو روش بیحسی موضعی و بیحسی ساب‐تنون، روشهای قابلقبول و بیخطری برای ارائه بیحسی در جراحی کاتاراکت هستند.
Authors' conclusions
Summary of findings
| Topical anaesthesia compared with sub‐Tenon's anaesthesia for cataract surgery | ||||||
| Patient or population: patients with cataract surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sub‐Tenon's anaesthesia | Topical anaesthesia | |||||
| Intraoperative pain | Mean intraoperative pain in the intervention groups was | 501 | ⊕⊕⊕⊕ | Equivalent to 1.1 on a score from 0 to 10 | ||
| Pain during administration of anaesthesia | Mean pain during administration of anaesthesia in the intervention groups was | 184 | ⊕⊕⊝⊝ | |||
| Pain at 24 hours | Mean pain at 24 hours in the intervention groups was | 200 | ⊕⊕⊕⊝ | Equivalent to 0.2 on a score from 0 to 10 | ||
| Surgeon's satisfaction | Mean surgeon satisfaction in the intervention groups was | 200 | ⊕⊕⊝⊝ | |||
| Participant satisfaction | Mean participant satisfaction in the intervention groups was | 26 | ⊕⊕⊕⊝ | |||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aWe retained only studies in which the outcome assessor was blinded to the anaesthetic technique. | ||||||
Background
Description of the condition
The World Health Organization (WHO) defines normal vision as 20/25 or better, and near normal vision as 20/30 to 20/60. By this definition, the prevalence of low vision/blindness (worse than 20/60) in Canada would be 22 per 10,000 (Maberley 2006). The incidence increases with age: 52 per 10,000 population for individuals 65 to 74 years old; 416 per 10,000 population for those 75 to 84 years old; and 904 per 10,000 population for individuals 85 years of age and older (Maberley 2006). By the same definitions, cataract and cataract complications account for 29.9% (95% confidence interval (CI) 23.5% to 37.1%) of the causes of abnormal vision (Maberley 2006).
Cataract is defined as loss of transparency of the natural lens of the eye, usually due to the ageing process. It can be found in younger people secondary to congenital, drug‐induced, inflammatory or traumatic causes. Surgery is the only recognized treatment. Cataract surgery is actually the most commonly performed elective procedure (Malot 2011). Phacoemulsification with intraocular lens insertion is the technique of choice in the vast majority of countries (Behndig 2011). Surgery ideally should be performed within six months (possibly within six weeks) of diagnosis. Compared with those who waited six weeks or less, people who waited six months or longer experienced greater vision loss, reduced quality of life and an increased rate of falls (Hodge 2007).
Description of the intervention
Local anaesthesia (with or without sedation) is the anaesthetic technique used today for the vast majority of patients (Eichel 2005), and surgery is usually performed on an ambulatory basis (Malot 2011). Local anaesthesia may consist of retrobulbar blocks, peribulbar blocks, sub‐Tenon's blocks or topical anaesthesia (with or without intracameral injection) (Alhassan 2008; Ezra 2007; Nouvellon 2010). The ideal anaesthetic technique should provide excellent analgesia with a low rate of complications. In the light of the large number of surgeries performed each year ‐ a number that may increase with aging of the population ‐ the ideal technique should also be cost efficient.
Retrobulbar anaesthesia, which consists of injection of 3 to 5 mL of local anaesthetic inside the muscular cone, was the first block used for cataract surgery. However, it has been associated with rare but severe potential complications: retrobulbar haemorrhage, globe perforation, optic nerve injury, extraocular muscle damage and brain stem anaesthesia (Nouvellon 2010). For these reasons, retrobulbar anaesthesia has been replaced by peribulbar anaesthesia, defined as injection of local anaesthetic into the extraconal space (up to 12 mL). Although reduced risk of complications was expected with peribulbar anaesthesia as compared with retrobulbar anaesthesia, needle‐related complications were reported with peribulbar anaesthesia, and a clear advantage of one technique over the other was never formally demonstrated (Alhassan 2008). Sub‐Tenon's anaesthesia (episcleral or parabulbar anaesthesia) consists of the injection of 3 to 5 mL of local anaesthetic inside the episcleral space. The local anaesthetic can be injected through a blunt cannula after opening a small button hole into the conjunctiva and Tenon’s capsule with a blunt Wescott scissors, while the conjunctiva is grasped with a small forceps. As the technique avoids injection through a blindly inserted needle, it is believed to be safer than retrobulbar or peribulbar block.
Inroduced in the 1990s, topic anaesthesia has become the most commonly used form of anaesthesia for cataract surgery (Malot 2011). Local anaesthetic (in drop or gel form) is placed on the surface of the eye. This may be supplemented intraoperatively by injection of local anaesthetic into the anterior chamber of the eye with a blunt needle through the operative incision (intracameral) (Ezra 2007).
How the intervention might work
Advantages of topical anaesthesia over sub‐Tenon's block include reduced time in administration (≥ 12 patients per operating room per day) (Malot 2011), shorter duration of action, allowing the patient to rapidly regain sight after surgery, and less pain during administration of anaesthesia (Mathew 2003).
Why it is important to do this review
This is an update of a previous Cochrane review (Davison 2007). In the previous version, review authors concluded that topical anaesthesia was associated with a slight difference in pain experienced during the intraoperative period (mean difference (MD) 1.28, 95% confidence interval (CI) 0.83 to 1.72) and a greater incidence of posterior capsule tear and vitreous loss (4.3% vs 2.1%). This current version of the review was undertaken to look for new studies and to update methods.
Objectives
Our objectives were to compare the effectiveness of topical anaesthesia (with or without intracameral local anaesthetic) versus sub‐Tenon's anaesthesia in providing pain relief during cataract surgery. We reviewed pain during administration of anaesthesia, postoperative pain, surgical satisfaction with operating conditions and patient satisfaction with pain relief provided, and we looked at associated complications.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) comparing topical anaesthesia with or without intracameral injection versus sub‐Tenon's anaesthesia.
Types of participants
We included only studies performed on adult participants. We included studies in which participants underwent operation on one eye only or on both eyes, provided that the anaesthetic technique for the first eye was chosen at random.
Types of interventions
We included studies that compared sub‐Tenon's anaesthesia versus topical anaesthesia (eye drops or gel) with or without intracameral injection. We excluded studies in which participants received intravenous sedation, as clinical experience has shown that intravenous sedation can mask pain perceived by the person.
Types of outcome measures
Primary outcomes
-
Pain during surgery.
Secondary outcomes
-
Pain during administration of local anaesthetic.
-
Postoperative pain at 30 minutes and at 24 hours.
-
Surgical satisfaction with operating conditions.
-
Patient satisfaction with analgesia provided.
-
Complications that occurred as defined by study authors.
Search methods for identification of studies
Electronic searches
We searched MEDLINE (1990 to November 2014; Appendix 1), the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 11; Appendix 2) and EMBASE (1990 to November 2014; Appendix 3). The search was first run in 2006 (Davison 2007) and was updated for 2006 to 2011 in May 2011, and for 2011 to 2014 in November 2014.
Searching other resources
We searched reference lists of identified trials during data extraction to find additional trials. We looked at http://www.clinicaltrials.gov, http://isrctn.org,
http://www.umin.ac.jp/ctr/index.htm, http://www.anzctr.org.au/, http://www.trialregister.nl/ and https://eudract.ema.europa.eu/ for trials in progress in November 2014. We screened conference proceedings of anaesthesiology societies, as published in two major anaesthesiology journals, for 2012, 2013 and 2014: British Journal of Anaesthesiology and European Journal of Anaesthesiology. We also searched the website of the American Society of Anaesthesiologists in November 2014. We imposed no language restrictions in searching for publications, and we translated articles not published in English.
Data collection and analysis
Selection of studies
We reviewed the titles and abstracts identified by the searches. We obtained and assessed full‐text copies of possible relevant trials according to the definitions provided under Criteria for considering studies for this review. We assessed only trials meeting these criteria for methodological quality.
Data extraction and management
We selected studies, extracted data (Assessment of risk of bias in included studies; Types of outcome measures; Assessment of heterogeneity) and entered data into Review Manager. We entered first all details on the site where the study was performed and dates of data collection (to facilitate exclusion of duplicate publications), then that the study was included or the reason for rejection. Data and moderators for heterogeneity exploration were entered into the Comprehensive Meta‐Analysis programme. Evaluation of risk of bias was entered into RevMan. Data for analysis were then transferred within RevMan into the format required to include maximal numbers of studies (events and total numbers of participants for each group; means, standard deviations and numbers of participants included in each group; or generic inverse variance if necessary). When possible, we entered data into an intention‐to‐treat (ITT) analysis.
Assessment of risk of bias in included studies
We used the Cochrane tool to assess trial quality (Higgins 2011) for the following items.
-
Generation of the allocation sequence of interventions: Randomization was considered adequate if it was generated by a computer or a random number table algorithm. We judged other processes, such as tossing of a coin, to be adequate if the whole sequence was generated before the start of the trial. We considered the trial as quasi‐randomized if a non‐random system, such as dates, names or identification numbers, was used.
-
Concealment of allocation: We considered concealment adequate if the process that was used prevented participant recruiters, investigators and participants from knowing the intervention allocation of the next participant to be enrolled in the study. We considered concealment inadequate if the allocation method allowed participant recruiters, investigators or participants to know the treatment allocation of the next participant to be enrolled in the study.
-
Blinding of participants and personnel: We considered blinding adequate if both the participant and personnel taking care of the participant were blinded to the intervention. We considered blinding inadequate if both participants and personnel were not blinded to the intervention.
-
Blinding of outcome assessment: We considered blinding adequate if the outcome assessor was blinded to the intervention. We considered blinding inadequate if the outcome assessor was not blinded to the intervention.
-
Incomplete outcome data (attrition bias): We considered the trial adequate if all dropouts or withdrawals were accounted for, if the number of dropouts was small (< 20%) and was similar for both interventions and if reasons for dropping out seemed reasonable. We considered the trial inadequate for this specific item if reasons for dropping out were not stated or did not sound reasonable or if the number was high (≥ 20%) or was vastly different between groups.
-
Selective reporting (reporting bias): We considered the trial to have low risk of bias if all measurements stated in the Methods section were included in the Results section, and at high risk of bias if only some of the results mentioned in the Methods section were given in the Results section. Per‐protocol results (not ITT) were considered as selective reporting.
-
Any other risk of bias: We considered any other item that may have influenced study results. An apparent conflict of interest was considered to introduce risk of bias.
Measures of treatment effect
As much as was feasible, we reported results as risk ratios (RRs) and their 95% confidence intervals (95% CIs) for dichotomous data, and as mean differences and 95% CIs for continuous data. If some of the continuous data were given on different scales, we produced results as standardized mean differences (SMDs) and 95% CIs. For SMDs, we considered 0.2 a small effect, 0.5 a medium effect and ≥ 0.8 a large effect (Pace 2011). When an effect was noted, a number needed to treat for additional beneficial outcome (NNTB) or a number needed to treat for additional harmful outcome (NNTH) was calculated from the odds ratio.
We gave results for dichotomous data as RRs, as odds ratios are not easily understood by clinicians (Deeks 2002; McColl 1998). We used odds ratios for calculation of the NNTB and the NNTH (http://www.nntonline.net/visualrx/), as these values are less likely to be affected by the side (benefit or harm) on which data are entered (Cates 2002; Deeks 2002). When no effect was observed, we calculated the optimal information size to make sure that enough participants were included in the retained studies to justify a conclusion on the absence of effect (Pogue 1998) (http://www.stat.ubc.ca/˜rollin/stats/ssize/b2.html). A difference of 15% (increase or decrease) was considered as the minimal clinically relevant difference.
Unit of analysis issues
We included parallel and cross‐over trials (one eye compared with the other). For one study with more than two groups (Mathew 2003), the two subgroups (with and without intravenous cannula insertion) were fused (Analysis 1.1; Analysis 1.2).
Dealing with missing data
We did not use medians as equivalent to means. Instead, we used the P value and the numbers of participants included in each group to calculate the effect size. We did not used imputed results. We entered data as ITT data as much as was feasible. If we did not, we assigned the study to high risk of bias for selective reporting and entered data on a per‐protocol basis.
Assessment of heterogeneity
We considered clinical heterogeneity before pooling results and examined statistical heterogeneity before carrying out meta‐analyses. We quantified statistical heterogeneity by using the I2 statistic and entered data in the way (benefit or harm) that yielded the lowest value. We qualified the amount as low (< 25%), moderate (50%) or high (≥ 75%), depending on the value obtained for the I2 statistic (Higgins 2003).
Assessment of reporting biases
We examined publication bias with a funnel plot followed by Duval and Tweedie’s trim and fill technique for each outcome.
Data synthesis
We analysed data using RevMan 5.3.4 ((http://tech.cochrane.org/revman/download) and Comprehensive Meta‐Analysis, Version 2.2.044 (www.Meta‐Analysis.com) with a fixed‐effect model for comparisons with low heterogeneity as assessed by the I2 statistic (I2 < 25%) or a random‐effects model for comparisons with moderate or high heterogeneity (I2 ≥ 25%) (Higgins 2003). We presented the risk of bias assessment on a risk of bias graph. We presented results for each comparison as forests plots when appropriate. For comparisons when fewer than two studies were available, or when a moderate or high level of heterogeneity was observed, we provided results as a narrative review or in an additional table.
Subgroup analysis and investigation of heterogeneity
We explored any extent of heterogeneity but focused more specifically on comparisons with significant heterogeneity (I2 > 25%) (Higgins 2003) and explored heterogeneity by using Egger’s regression intercept (to assess the possibility of a small‐study effect; Rucker 2011), visual inspection of forest plots with studies placed in order according to a specific moderator, subgroupings (categorical moderators) or meta‐regressions (continuous moderators). We considered the following factors in the exploration of heterogeneity: cross‐over versus parallel trials, intracameral injection, oral sedation, year of publication, age, gender proportion, local anaesthetic used with topical anaesthesia, local anaesthetic used with sub‐Tenon's anaesthesia, volume used with sub‐Tenon's anaesthesia, jelly versus drops for topical anaesthesia and surgical technique.
Sensitivity analysis
We planned to perform a sensitivity analysis (based mainly on the risk of bias assessment: allocation concealment and blinding of the assessor). To allow the reader to be the judge on the adequacy of our decision to retain only studies at low risk of bias for intraoperative pain (Analysis 1.1), we left both types of studies (unclear and low risk of bias) in the analysis, but we retained only studies at low risk of bias for our assessment on the level of evidence and for determination of conclusions.
'Summary of findings'
We judged the quality of the body of evidence according to the system developed by the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) Working Group and presented our findings in a 'Summary of findings' table (http://ims.cochrane.org/revman/gradepro) for each outcome (Guyatt 2008; Guyatt 2011).
Results
Description of studies
Characteristics of all studies can be found under Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies.
Results of the search
We have included in Figure 1 the flow diagram of study selection for this review update.
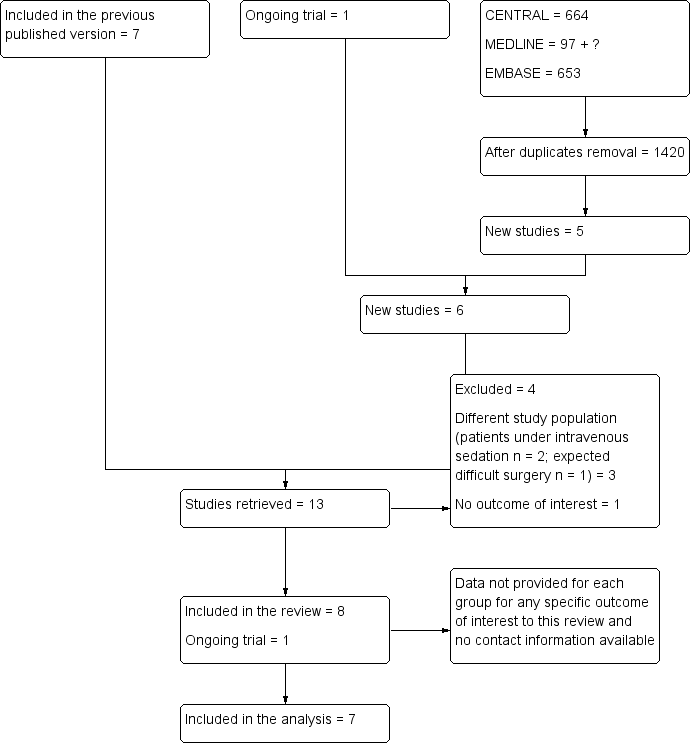
Flow diagram of study selection for the updated review.
Included studies
We included eight studies in this updated review but only seven studies with 742 operated eyes of 617 participants in the analysis. We could not extract the data for Adams 2008. Among the seven studies retained were two cross‐over trials (Vielpeau 1999; Zafirakis 2001) with 125 participants and five parallel trials (Chittenden 1997; Mathew 2003; Rüschen 2005; Sekundo 2004; Srinivasan 2004) with 492 participants (Characteristics of included studies). These studies were published between 1997 and 2005. The mean age of participants varied from 71.5 years to 83.5 years. The female proportion of participants varied from 54% to 76%. Oral sedation was used in only two studies (Sekundo 2004; Vielpeau 1999). No study used pre‐operative oral co‐analgesia. The local anaesthetic used for topical anaesthesia was lidocaine (Sekundo 2004), oxybuprocaine (Chittenden 1997), proparacaine (Mathew 2003; Zafirakis 2001), proxymethocaine (Srinivasan 2004) or tetracaine (Rüschen 2005; Vielpeau 1999). For topical anaesthesia, jelly was used in one study (Sekundo 2004) and drops in the other six studies. Sub‐Tenon's block was performed with 1.5 to 4 mL of lidocaine (Chittenden 1997; Rüschen 2005; Sekundo 2004), with lidocaine or carbocaine (Vielpeau 1999) or with a mixture of lidocaine and bupivacaine (Mathew 2003; Srinivasan 2004; Zafirakis 2001). Intracameral anaesthesia was added for one study only (Sekundo 2004).
Phacoemulsification was used for all studies. A clear corneal incision was used as the surgical technique in five studies (Mathew 2003; Rüschen 2005; Srinivasan 2004; Vielpeau 1999; Zafirakis 2001), although Vielpeau 1999 also placed a single corneal suture. The older technique of scleral tunnel was used by Chittenden 1997 (who also placed a superior rectus suture in the sub‐Tenon's group) and by Sekundo 2004.
Excluded studies
We excluded four studies (El Awady 2009; Huang 2014; Rodrigues 2008; Ryu 2009). One study was performed on a different population (potentially difficult cataract surgery) (El Awady 2009). One included no outcomes of interest (Huang 2014). In two studies, participants received intravenous midazolam and alfentanil (Rodrigues 2008) or propofol and remifentanil (Ryu 2009) (Characteristics of excluded studies).
Ongoing studies
One study is ongoing (NCT01344252). See Characteristics of ongoing studies for details.
Studies awaiting classification
No studies are awaiting classification.
Risk of bias in included studies
We presented risks of bias of included studies in Figure 2 and Figure 3.
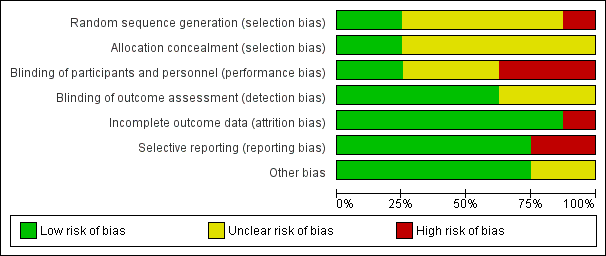
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged allocation concealment as appropriate for two studies (Rüschen 2005; Srinivasan 2004) and as unclear for the other studies (Figure 2; Figure 3).
Blinding
We judged blinding of participants and personnel as adequate for two studies (Rüschen 2005; Srinivasan 2004).
We judged blinding of the outcome assessor as adequate for five studies (Adams 2008; Rüschen 2005; Sekundo 2004; Srinivasan 2004; Zafirakis 2001).
Incomplete outcome data
We determined that attrition bias was addressed adequately by all studies except one (Rüschen 2005).
Selective reporting
We judged selective reporting to be a problem for two studies for which data were not analysed on an intention‐to‐treat basis (Mathew 2003; Rüschen 2005).
Other potential sources of bias
Characteristics of participants were not provided for two studies (Adams 2008; Chittenden 1997).
Effects of interventions
Primary outcome
Pain during surgery
This outcome was available for six studies (Chittenden 1997; Mathew 2003; Sekundo 2004; Srinivasan 2004; Vielpeau 1999; Zafirakis 2001), which included 605 participants. Pain during surgery was greater with topical anaesthesia than with sub‐Tenon's anaesthesia (SMD 0.64, 95% CI 0.43 to 0.84; I2 = 36%; Analysis 1.1). With one high‐quality trial used as a standard (Srinivasan 2004; standard deviation (SD) 2.3), this would correspond to a visual/verbal analogical pain scale (VAS) score of 1.5 on a scale from 0 to 10. Egger's regression intercept showed that a small‐study effect may partially explain the statistical heterogeneity (P value = 0.04 (two‐tailed)). Duval and Tweedie's trim and fill analysis showed that two studies might be missing to left of mean for an adjusted point of estimate of 0.55 (95% CI 0.33 to 0.77). When studies were subgrouped by risk of bias for blinding of the outcome assessor, the difference was significantly less in studies in which the outcome assessor was blinded to the anaesthetic technique used (I2 = 83% for heterogeneity between subgroups). The difference measured for this item in studies at low risk of bias (Sekundo 2004; Srinivasan 2004; Zafirakis 2001) would correspond to a difference in VAS scores of 1.1 on a scale from 0 to 10. We graded the evidence only on the basis of three studies at low risk of bias. In the light of results of one low risk of bias study (Srinivasan 2004; SD of sub‐Tenon's group 2.2), 120 participants (60 per group) were required to eliminate a difference of 1/10 in VAS score (from 3.4 to 2.4) (alpha 0.05; beta 0.2; one‐sided test).
Secondary outcomes
Pain during administration of local anaesthetic
This outcome was available for three studies, which included 520 participants (Mathew 2003; Srinivasan 2004; Zafirakis 2001). Heterogeneity in the pain occurring during administration of anaesthesia was high between these three studies and depended on whether the study was a cross‐over trial (Zafirakis 2001) (SMD ‐2.21, 95% CI ‐2.57 to ‐1.86) or a parallel trial (SMD ‐0.20, 95% CI ‐0.43, 0.04) (Mathew 2003; Srinivasan 2004) (I2 = 99% between subgroups) (Analysis 1.2). We found no evidence of small‐study effects or publication bias for this outcome. We graded the evidence on the basis of the two parallel trials only. Results of one study at low risk of bias (Srinivasan 2004; SD 2.0 in both groups) indicate that 100 participants (50 per group) were required to eliminate a difference of 1/10 in VAS score (from 3.6 to 2.6) (alpha 0.05; beta 0.2; one‐sided test).
Postoperative pain at 30 minutes and at 24 hours
This outcome was available at 30 minutes for two studies, which included 401 participants (Srinivasan 2004; Zafirakis 2001). Both studies showed that pain at 30 minutes was greater in the topical anaesthesia groups, but the amplitude of effect was higher in the cross‐over trial (Analysis 1.3). At 24 hours, results from one study, which included 200 participants (Zafirakis 2001), showed that pain was greater in the sub‐Tenon's group (SMD ‐0.47, 95% CI ‐0.75 to ‐0.19). This difference corresponds to 0.2 on a score from 0 to 10 (SD 0.4). On the basis of this study (Zafirakis 2001), 96 participants (48 per group) were required to eliminate a difference of 0.23 on a score from 0 to 10.
Surgical satisfaction with operating conditions
This outcome was available for only one study, which included 100 participants who underwent operations on both eyes with alternating anaesthetic techniques (Zafirakis 2001) (MD ‐0.7, 95% CI ‐0.30 to ‐1.10) on a score from 0 to 10. On the basis of results of Zafirakis 2001, 72 participants (36 per group) were required to eliminate a difference of 1 (from 9.6 to 8.6) on a scale from 0 to 10 (SD 1.7 in the topical group).
Participant satisfaction with analgesia provided
This outcome was available for only one study, which included 26 participants (Rüschen 2005) (SMD ‐1.13, 95% CI ‐0.30 to ‐1.96). On the basis of results of Rüschen 2005, with the median taken as the mean and with SD estimated from the interquartile value (1.35 × 0.96), 42 participants (21 per group) would be required to eliminate a difference of 1 on a score from ‐ 3 to + 3.
Complications that occurred as defined by study authors
Conversion from topical anaesthesia to sub‐Tenon's occurred in two studies (Rüschen 2005; Sekundo 2004) (Table 1). Subconjunctival haemorrhage and chemosis were seen with sub‐Tenon's blocks only (Table 1). Intraoperative complications were reported for three studies only. One study author (Srinivasan 2004) reported 3/65 posterior capsular tears compared with 2/136, and 0/65 cases of iris prolapsus compared with 1/136 for topical and sub‐Tenon's anaesthesia, respectively (Table 1). Two other study authors reported no intraoperative complications (Sekundo 2004; Vielpeau 1999); this information is not available for the other studies. Given an incidence of posterior capsular tear of 1%, 116,884 events (58,442 per group) would be required to eliminate an increase of 15% in incidence (alpha 0.05; beta 0.2; one‐sided test).
| Study ID | Subconjunctival haemorrhage T vs ST | Chemosis T vs ST | Transient increase in intraocular pressure T vs ST | Posterior capsular tear T vs ST | Iris prolapsus T vs ST | Conversion to ST | Iritis T vs ST | Comments |
| 0 | Complications not reported | |||||||
| 0 | Complications not reported | |||||||
| 1/14 | Complications not reported | |||||||
| 0/50 vs 0/50 | 0/50 vs 0/50 | 5/50 | 1/50 vs 1/50 | The topical anaesthesia group felt pain during cauterization of the episcleral vessels | ||||
| 3/65 vs 2/136 | 0/65 vs 1/136 | 0 | No participant required supplemental analgesia after surgery | |||||
| 25/25 vs 25/25a | 0/25 vs 15/25 | 0/25 vs 0/25 | 0/25 vs 0/25 | 0 | ||||
| 0/100 vs 22/100 | 0/100 vs 76/100 | 0 |
T: topical anaesthesia
ST: sub‐Tenon's anaesthesia
vs: versus
aAny amount
Level of evidence
For intraoperative pain, we included only three trials in our final assessment when the outcome assessor was blinded to the anaesthetic technique. We did not downgrade the evidence on the basis of the possibility of publication bias because correcting this would not lead to changes in the conclusion. We rated the evidence as high for this outcome.
For pain during administration of local anaesthetic, we retained only parallel trials in our final assessment. We downgraded the evidence on risk of bias and on imprecision because of the small numbers of participants/studies included (Guyatt 2011a). We rated the level of evidence as low for this outcome.
For pain at 24 hours, we downgraded the evidence on imprecision for the small numbers of participants/studies included. We rated the level of evidence as moderate.
For surgeon satisfaction, we downgraded the level of evidence on risk of bias and on imprecision because of the small numbers of participants/studies included. We rated the level of evidence as low.
For participant satisfaction, we downgraded the level of evidence by 2 for imprecision on the basis of a wide confidence interval and small numbers of included participants/studies. We upgraded the level of evidence on a large effect size (SMD > 0.8). We rated the level of evidence as moderate.
Discussion
We included in this updated review eight randomized controlled trials (RCTs) that met our inclusion criteria of evaluating topical anaesthesia against sub‐Tenon's anaesthesia for cataract surgery. Of these eight trials, we included seven in our analyses. We have described above the methodological shortcomings of these studies. Only two studies specified the method of randomization (Rüschen 2005; Srinivasan 2004), and the method of randomization was considered at high risk for one study (three groups with unequal numbers and different surgeons for different groups; Mathew 2003) (Figure 2; Figure 3).
All studies used different combinations of local anaesthetic agents in differing amounts and concentrations. For the purposes of this review, it was assumed that all were fit for the purpose for which they were used, that is, provision of anaesthesia by topical or sub‐Tenon's administration for a standard cataract surgery. Comparison of the effectiveness of local anaesthetic agents was outside the remit of this review. The same is true for the different types of operating techniques used (clear corneal incision and scleral tunnel with or without sutures). Throughout the world, different drugs and techniques are used for cataract surgery, so we included all studies in the review.
For the outcome of pain experienced during surgery, as compared with the previous version of this review (Davison 2007), we were able to enter six studies into this updated version (Analysis 1.1) (vs only four). In doing so, we found that the difference between anaesthetic techniques was overestimated in studies in which there was uncertainty around blinding of outcome assessor to the anaesthetic technique used (Analysis 1.1). If the outcome assessor was adequately blinded to the anaesthetic technique used, the difference in pain experienced during surgery was equivalent to 1.1 on a score from 0 to 10. It is notable that none of the investigators gave oral analgesics to participants before performing surgery. Pain during surgery was sometimes noted during cauterization of the episcleral vessels (Sekundo 2004) ‐ a step that can be avoided by making the incision slightly more anterior in the eye. The addition of intracameral local anaesthetic has been proposed to help decrease intraoperative pain among patients undergoing cataract surgery under topical anaesthesia (Ezra 2007). Intracameral local anaesthetic injection is a controversial issue. Intracameral local anaesthetics have been shown to be cytotoxic in animal studies, with some local anaesthetics possibly more harmful than others (Pescosolido 2011). In their review, Ezra et al. found that intracameral local anaesthetic injection decreases intraoperative VAS score (MD ‐0.27, 95% CI ‐0.43 to ‐0.11; I2 = 0%) on a scale from 0 to 10 (Ezra 2007). Although the difference was statistically significant, the clinical relevance of this difference is not obvious. In the present review, intracameral local anaesthetic injection was used in one study only (Sekundo 2004). Results from this study were not different (I2 = 0%; Analysis 1.1) from those of the other two studies using a similar method (outcome assessor blinded to the technique used) without intracameral injection (Srinivasan 2004; Zafirakis 2001). Pain perception may influence a person's co‐operation during surgery (Omulecki 2009). Pain may be perceived at various stages of surgery including at distention of the anterior chamber (Hou 2012), and prophylactic application of intraocular pressure‐lowering medications may alter pain sensation during phacoemulsification cataract surgery (Ulas 2013). Pre‐operative administration of 1 gram of oral acetaminophen reduces intraoperative and postoperative pain (Kaluzny 2010). Orally administered anxiolytics given before surgery may improve co‐operation of the patients (Khezri 2013).
In one cross‐over trial, participants found that pain related to administration of anaesthesia was significantly greater with sub‐Tenon's technique than with topical anaesthesia (SMD ‐2.21, 95% CI ‐2.57 to ‐1.86; in favour of topical anaesthesia; Analysis 1.2). However, the difference was not found when only parallel trials (SMD ‐0.20, 95% CI ‐0.43 to 0.04; I2 = 0%) were considered ‐ a situation that should be closer to everyday clinical practice. Slightly more pain at 24 hours was reported with sub‐Tenon's technique compared with topical anaesthesia (SMD ‐0.47, 95% CI ‐0.75 to ‐0.19; equivalent to a mean of 0.2 on a score from 0 to 10). Participant satisfaction and surgeon satisfaction were greater with sub‐Tenon's anaesthetic technique. Results for surgeon satisfaction with this technique reported in included studies may have been due to lack of practice with this "new" form of anaesthesia. Indeed, topical anaesthesia is now the preferred technique in the vast majority of cases (> 85%; Malot 2011), making it clear that with practice, surgeons can perform cataract surgery in most patients using topical anaesthesia only. Patient satisfaction may be different if those studies would be repeated including only surgeons who have a great deal of experience with topical anaesthesia.
In the previous version of this review (Davison 2007), concerns were raised about the fact that a non‐statistically significant higher percentage of posterior capsular tear was found in one study for the topical anaesthesia group (Table 1). Posterior capsular rupture may be associated with postoperative complications (Gonzalez 2014). The association of intraoperative and postoperative complications may lead to poorer postoperative visual acuity (Gonzalez 2014). Authors of the original review (Davison 2007) speculated that lack of akinesia could increase the chances of sudden inadvertent patient movement with subsequent inadvertent misplacement of a surgical tool by the surgeon. However, the number of participants included in the present updated meta‐analysis is insufficient to permit valid conclusions on the incidence of posterior capsular tear with one technique compared with the other (Effects of interventions). Lack of akinesia has not been identified as a risk factor for increasing intraoperative surgical complications during cataract surgery (Lee 2013). In their meta‐analysis, which included 2862 operated eyes, Lee et al. found a rate of 0.74% posterior capsular rupture with akinetic (sub‐Tenon's, peribulbar or retrobulbar) techniques (1494 eyes) compared with 0.80% with kinetic (topical with or without intracameral injection) anaesthetic techniques (1368 eyes) (P value = 0.84). Experienced surgeons can easily immobilize an eye by using two tools. Furthermore, certain surgical techniques (e.g. insertion of a multifocal lens; Hayashi 2015) may prove easier to execute with a kinetic technique, allowing patient collaboration during the procedure. Among factors associated with a higher rate of intraoperative complications, one can probably mention dense nuclear sclerosis and white cataracts (Briszi 2012). Age (Celebi 2014), surgical technique (phacoemulsification vs manual small‐incision; Zhang 2013) and use of topical anaesthesia in patients unable to lie flat (Injarie 2013) would not be risk factors for intraoperative complications. Allocation of cataract patients to surgeons matched for experience according to a pre‐operative assessment of their risk of complications would allow better surgical outcomes, especially for resident surgeons (Tsinopoulos 2013).
Summary of main results
Compared with sub‐Tenon's anaesthesia, topical anaesthesia (with or without intracameral injection) for cataract surgery increases intraoperative pain but decreases postoperative pain at 24 hours. The amplitude of the effect (equivalent to 1.1 on a score from 0 to 10 for intraoperative pain, and to 0.2 on the same scale for postoperative pain at 24 hours), although statistically significant, was probably too small to be of clinical relevance. Therefore, both techniques can be considered suitable for cataract surgery. There is not enough evidence from RCTs to say if one technique would have a higher or lower incidence of intraoperative complications compared with the other.
Overall completeness and applicability of evidence
Results of the present meta‐analysis suggest that both techniques can be used for cataract surgery. On the basis of three studies including 501 participants, in which the outcome assessor was blinded to the anaesthetic technique used, we found that the difference in intraoperative pain (equivalent to 1.1 on a score from 0 to 10, in favour of sub‐Tenon's technique) is probably too small to be of clinical relevance. This review does not include enough participants to exclude differences in intraoperative complications between these anaesthetic techniques.
Quality of the evidence
The quality of the evidence was rated as high for intraoperative pain and as moderate for pain at 24 hours and for patient satisfaction. The quality of the evidence was rated as low for pain during administration of anaesthesia and for surgeon satisfaction.
Potential biases in the review process
Studies were published between 1997 and 2005; therefore, results may not reflect the actual expertise of surgeons in topical anaesthesia.
Agreements and disagreements with other studies or reviews
Compared with the previous version of this review (Davison 2007), we found, in this updated version, that studies for which there was uncertainty about blinding of outcome assessors to the anaesthetic technique overestimated the amplitude of the difference in intraoperative pain scores between anaesthetic techniques. We found no evidence of a difference in complication rates between anaesthetic techniques, but the number of participants included in this review is clearly insufficient to allow conclusions on this.

Flow diagram of study selection for the updated review.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Topical anaesthesia versus sub‐Tenon's anaesthesia, Outcome 1 Pain during surgery.

Comparison 1 Topical anaesthesia versus sub‐Tenon's anaesthesia, Outcome 2 Pain during anaesthesia administration.

Comparison 1 Topical anaesthesia versus sub‐Tenon's anaesthesia, Outcome 3 Pain at 30 minutes after surgery.
| Topical anaesthesia compared with sub‐Tenon's anaesthesia for cataract surgery | ||||||
| Patient or population: patients with cataract surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sub‐Tenon's anaesthesia | Topical anaesthesia | |||||
| Intraoperative pain | Mean intraoperative pain in the intervention groups was | 501 | ⊕⊕⊕⊕ | Equivalent to 1.1 on a score from 0 to 10 | ||
| Pain during administration of anaesthesia | Mean pain during administration of anaesthesia in the intervention groups was | 184 | ⊕⊕⊝⊝ | |||
| Pain at 24 hours | Mean pain at 24 hours in the intervention groups was | 200 | ⊕⊕⊕⊝ | Equivalent to 0.2 on a score from 0 to 10 | ||
| Surgeon's satisfaction | Mean surgeon satisfaction in the intervention groups was | 200 | ⊕⊕⊝⊝ | |||
| Participant satisfaction | Mean participant satisfaction in the intervention groups was | 26 | ⊕⊕⊕⊝ | |||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aWe retained only studies in which the outcome assessor was blinded to the anaesthetic technique. | ||||||
| Study ID | Subconjunctival haemorrhage T vs ST | Chemosis T vs ST | Transient increase in intraocular pressure T vs ST | Posterior capsular tear T vs ST | Iris prolapsus T vs ST | Conversion to ST | Iritis T vs ST | Comments |
| 0 | Complications not reported | |||||||
| 0 | Complications not reported | |||||||
| 1/14 | Complications not reported | |||||||
| 0/50 vs 0/50 | 0/50 vs 0/50 | 5/50 | 1/50 vs 1/50 | The topical anaesthesia group felt pain during cauterization of the episcleral vessels | ||||
| 3/65 vs 2/136 | 0/65 vs 1/136 | 0 | No participant required supplemental analgesia after surgery | |||||
| 25/25 vs 25/25a | 0/25 vs 15/25 | 0/25 vs 0/25 | 0/25 vs 0/25 | 0 | ||||
| 0/100 vs 22/100 | 0/100 vs 76/100 | 0 | ||||||
| T: topical anaesthesia ST: sub‐Tenon's anaesthesia vs: versus aAny amount | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain during surgery Show forest plot | 6 | 705 | Std. Mean Difference (Random, 95% CI) | 0.64 [0.43, 0.84] |
| 1.1 Low risk for outcome assessor blindness | 3 | 501 | Std. Mean Difference (Random, 95% CI) | 0.47 [0.29, 0.66] |
| 1.2 Unclear risk for outcome assessor blindness | 3 | 204 | Std. Mean Difference (Random, 95% CI) | 0.90 [0.61, 1.20] |
| 2 Pain during anaesthesia administration Show forest plot | 3 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 2.1 Cross‐over trial | 1 | 200 | Std. Mean Difference (Random, 95% CI) | ‐2.21 [‐2.57, ‐1.86] |
| 2.2 Parallel trial | 2 | 320 | Std. Mean Difference (Random, 95% CI) | ‐0.20 [‐0.43, 0.04] |
| 3 Pain at 30 minutes after surgery Show forest plot | 2 | Std. Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 Cross‐over trial | 1 | 200 | Std. Mean Difference (Fixed, 95% CI) | 0.96 [0.67, 1.26] |
| 3.2 Parallel trial | 1 | 201 | Std. Mean Difference (Fixed, 95% CI) | 0.54 [0.24, 0.84] |

