Régimen dietético con bajo contenido de bacterias versus régimen dietético de control para la prevención de la infección en pacientes con cáncer tratados con quimioterapia que causa episodios de neutropenia
Resumen
Antecedentes
La neutropenia es un efecto secundario potencialmente grave de la quimioterapia y un factor de riesgo principal de infecciones que puede ser potencialmente mortal. Se ha formulado la hipótesis de que un régimen dietético con bajo contenido de bacterias (LBD, por sus siglas en inglés) puede prevenir la aparición de infecciones y la mortalidad (relacionada con la infección) en los pacientes con cáncer tratados con quimioterapia que causa episodios de neutropenia, aunque aún existe mucha incertidumbre. Esta revisión es una actualización de una revisión Cochrane publicada anteriormente.
Objetivos
El objetivo primario de esta revisión fue determinar la eficacia del LBD versus un régimen dietético de control para la prevención de la aparición de infección y la disminución de la mortalidad (relacionada con la infección) en pacientes adultos y pediátricos con cáncer tratados con quimioterapia que causa episodios de neutropenia. Los objetivos secundarios fueron evaluar el tiempo hasta el primer episodio febril, la necesidad de tratamiento empírico con antibióticos, la aceptabilidad del régimen dietético y la calidad de vida.
Métodos de búsqueda
Se hicieron búsquedas en las siguientes bases de datos electrónicas: Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials; CENTRAL) (2015, número 4), Database of Abstracts of Reviews of Effects (DARE) (2015, número 4), PubMed (desde 1946 hasta el 4 de mayo de 2015), EMBASE (desde 1980 hasta el 4 de mayo de 2015) y el Cumulative Index to Nursing and Allied Health Literature (CINAHL) (desde 1981 hasta el 4 de mayo de 2015).
Además, se realizaron búsquedas en las listas de referencias de artículos y actas de congresos relevantes de la American Society of Hematology (ASH; de 2000 a 2015), la European Bone Marrow Transplantation (EBMT; de 2000 a 2015), la Oncology Nurses Society (ONS; de 2000 a 2015), la International Society for Paediatric Oncology (SIOP; de 2000 a 2014), la Multinational Association of Supportive Care in Cancer (MASCC; de 2000 a 2015), la American Society of Clinical Oncology (ASCO; de 2000 a 2015), la Interscience Conference of Antimicrobial Agents and Chemotherapy (ICAAC; de 2000 a 2015), la European Society for Clinical Nutrition and Metabolism (ESPEN; de 2000 a 2015), la American Society for Parenteral and Enteral Nutrition (ASPEN; de 2000 a 2015) y la European Hematology Association (EHA; de 2000 a 2015). En mayo de 2015 se examinaron el National Institutes of Health Register mediante clinicaltrials.gov y el International Standard Randomised Controlled Trial Number (ISRCTN) Register (www.controlled‐trials.com).
Criterios de selección
Ensayos controlados aleatorios (ECA) que compararan el uso de un LBD frente a un régimen dietético de control con respecto a la tasa de infección, la mortalidad (relacionada con la infección), el tiempo hasta el primer episodio febril, la necesidad de tratamiento empírico con antibióticos, la aceptabilidad del régimen dietético y la calidad de vida en pacientes con cáncer adultos y pediátricos tratados con quimioterapia que causa episodios de neutropenia.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, seleccionaron los estudios, evaluaron el riesgo de sesgo y extrajeron los datos. Los análisis se realizaron según las guías del Manual Cochrane para Revisiones Sistemáticas de Intervenciones (Cochrane Handbook for Systematic Reviews of Interventions).
Resultados principales
En la versión original de esta revisión se identificaron tres ECA que evaluaban diferentes regímenes dietéticos de intervención y de control en 192 pacientes (97 asignados al azar al régimen dietético de intervención; 95 al régimen dietético de control) con diferentes tipos de neoplasias. En la actualización, no se identificaron nuevos estudios elegibles. Las cointervenciones (p.ej. ambiente protector, profilaxis antimicrobiana, cuidados del catéter venoso central, cuidado bucal, prácticas de higiene, factores estimulantes de colonias) y las definiciones de resultado también difirieron entre los estudios. En todos los estudios incluidos la política estándar fue la administración de antibióticos empíricos (y a veces también antimicóticos) a (algunos de) los pacientes diagnosticados una infección. Dos estudios incluyeron adultos y un estudio incluyó niños. En todos los estudios sólo se proporcionó una descripción escasa de los regímenes de tratamiento. Todos los estudios tuvieron limitaciones metodológicas. No fue posible combinar los resultados de los estudios incluidos. En dos estudios individuales no se identificaron diferencias estadísticamente significativas en la tasa de infección entre el régimen dietético de intervención y el de control; otro estudio no mostró diferencias significativas entre los grupos de tratamiento en el número de ciclos de quimioterapia con una infección. Ninguno de los estudios mencionó la mortalidad relacionada con la infección, aunque en un estudio no se observaron diferencias significativas en la supervivencia general entre los grupos de tratamiento. El tiempo desde la aparición de la neutropenia hasta la fiebre, la duración de los antibióticos empíricos y los antimicóticos, la aceptabilidad del régimen dietético (es decir, seguir el régimen dietético fácilmente y seguir el régimen dietético en todos ciclos de quimioterapia) y la calidad de vida fueron evaluados por sólo un estudio; no se identificaron diferencias estadísticamente significativas entre los grupos de tratamiento para ninguno de los desenlaces.
Conclusiones de los autores
Actualmente ninguna prueba de ECA individuales en niños y adultos con diferentes neoplasias malignas resalta el uso de un LBD para la prevención de la infección y los resultados relacionados. Todos los estudios difirieron con respecto a las cointervenciones, las definiciones de resultado y los regímenes dietéticos de intervención y de control. Como no pudieron agruparse los resultados y todos los estudios tenían limitaciones metodológicas graves, no pudieron establecerse conclusiones definitivas. Sin embargo, cabe destacar que "ninguna prueba de efecto", como se identificó en esta revisión, no es lo mismo que "pruebas de ausencia de efecto": Según las pruebas actualmente disponibles, no se pueden proporcionar recomendaciones para la práctica clínica. Se necesita investigación adicional de alta calidad.
PICO
Resumen en términos sencillos
Régimen dietético con bajo contenido de bacterias versus régimen dietético de control para la prevención de la infección en pacientes con cáncer tratados con quimioterapia que causa episodios de neutropenia
La neutropenia es un efecto secundario potencialmente grave de la quimioterapia y un factor de riesgo principal de infecciones que puede ser potencialmente mortal. Se ha argumentado que un régimen dietético con bajo contenido de bacterias (es decir, alimentos y bebidas con niveles bajos de bacterias) puede prevenir la aparición de infecciones y la muerte (relacionada con la infección) en pacientes con cáncer tratados con quimioterapia que causa episodios de neutropenia.
Los autores de la revisión identificaron tres estudios aleatorios que comparaban diferentes dietas en 192 niños y adultos con diferentes tipos de cáncer. Otras intervenciones, como la profilaxis antimicrobiana (es decir, la prevención de infecciones mediante un tratamiento antimicrobiano como antibióticos) y las prácticas de higiene, y las definiciones de los resultados de estudio también difirieron entre los estudios y se proporcionó información muy limitada sobre el tratamiento anticanceroso. Todos los estudios tuvieron problemas metodológicos. Lamentablemente, no fue posible la combinación de los resultados de los estudios incluidos, aunque actualmente no existen pruebas de estudios individuales que sugieran que el uso de un régimen dietético con bajo contenido de bacterias previene las infecciones. Los datos sobre la supervivencia, el tiempo desde la aparición de la neutropenia hasta el comienzo de la fiebre, la duración de los antibióticos y los antimicóticos (es decir, agentes dirigidos a la micosis) empíricos (es decir, comenzar el tratamiento antes de la determinación de un diagnóstico definitivo), la aceptabilidad del régimen dietético y la calidad de vida fueron evaluados por sólo un estudio; para ningún resultado se observaron diferencias estadísticamente significativas entre los grupos de tratamiento. Ninguno de los estudios evaluó la mortalidad relacionada con la infección. Sin embargo, cabe destacar que "ninguna prueba de efecto", como se identificó en esta revisión, no es lo mismo que "pruebas de ausencia de efecto": No se identificaron diferencias entre las dietas, posiblemente porque se incluyeron menos pacientes en estos estudios. Según las pruebas disponibles en la actualidad, los autores de la revisión no pudieron hacer recomendaciones para la práctica clínica. Se necesita investigación adicional de alta calidad.
Authors' conclusions
Background
Neutropenia, defined as an absolute neutrophil count (ANC) < 0.5 × 109/L, is a potentially serious side effect of chemotherapy and high‐dose irradiation (MacVittie 1997) and a major risk factor for infection and sepsis. Neutrophils, constituting 55% to 70% of circulating white blood cells, have the ability to identify, ingest and destroy most foreign invaders (Candell 1991). When the ANC falls to < 1.0 × 109/L, susceptibility to infection is increased. The frequency and severity of infections are inversely proportional to the neutrophil count and are directly proportional to the duration of neutropenia (Hughes 2002). Patients with both solid tumours and haematological malignancies treated with high‐dose chemotherapy have a significantly increased risk of developing life‐threatening infection.
Infection‐related mortality in patients with severe neutropenia is approximately 4% to 6% in adult patients and 0.4% to 1.0% in paediatric patients (Hughes 2002; Pizzo 1999; Roguin 1996). At least 50% of neutropenic patients who become febrile have an established or occult infection, and at least 20% with a neutrophil count < 0.1 × 109/L have bacteraemia (Hughes 2002).
Approximately 80% of the organisms that cause infection in neutropenic patients arise from endogenous microbial florae colonising the skin and respiratory, genitourinary and gastrointestinal tracts (Barber 2001). Currently, coagulase‐negative staphylococci are the most common blood isolates; Enterobacteriaceae (i.e. Enterobacter species, Escherichia coli and Klebsiella species) and non‐fermenting gram‐negative rods (i.e. Pseudomonas aeruginosa and Stenotrophomonas species) are isolated less often (Freifeld 2011). Invasive fungal infection is another important cause of morbidity and mortality. Predisposing factors for fungal infection include use of broad‐spectrum antibiotics, corticosteroids, parenteral nutrition and indwelling intravenous catheters, along with graft‐versus‐host disease after an allogeneic stem cell transplantation. The most commonly isolated fungal pathogens are Aspergillus and Candida species (Barber 2001).
Significant advances in supportive care for neutropenic patients have been made since the mid‐1990s. Nowadays, supportive care management for neutropenia is directed by risk assessment in adults (Klastersky 2000; Talcott 1992) and by evidence‐based guidelines for management of neutropenia and prevention of opportunistic infection developed by the Centers for Disease Control and Prevention (CDC, USA) for both adults and children (Dykewicz 2001; Hughes 2002). These recommendations to prevent healthcare‐associated infection involve antimicrobial prophylaxis, colony‐stimulating factors, a protective environment, oral care, central venous catheter (CVC) care, hand washing, personal hygiene practices, dietary restrictions and outpatient treatment (Dykewicz 2001). However, despite these achievements, infection continues to be a major cause of morbidity and mortality among neutropenic patients.
With regard to dietary restrictions, it has been hypothesised that a diet for neutropenic patients should reduce pathogens in the gastrointestinal tract by excluding specific foods that may act as a vector for bacteria. The first such diet was developed in the 1960s with the intention of providing a completely germ‐free diet (Reimer 1966). Since that time, foods have been sterilised by autoclaving, prolonged baking, gamma irradiation or canning (Aker 1983). Germ‐free diets were considered unpalatable; therefore, the US National Institutes of Health, Department of Dietary and Environmental Sanitation, designed the 'cooked‐food' diet. Although not germ‐free, this diet was aimed at eliminating foods with high bacterial counts (Preisler 1970). In a randomised trial, the National Cancer Institute demonstrated that within a decontaminated environment, a germ‐free diet offered little advantage over a cooked‐food diet with reference to bacterial stool cultures (Preisler 1970). Although the cooked‐food diet was more acceptable to patients than the germ‐free diet, patients who adhered to this diet for longer than four to six weeks often became frustrated with the food selection (Moody 2002). Occasionally, this diet affected their acceptance of other medical therapies as well, which led clinicians to investigate liberalisation of the diet (Pizzo 1982). Pizzo et al cultured 236 commercially available foods and identified fewer than 500 colony‐forming units per gram in 66% of these foods. Investigators proposed that these foods were acceptable for neutropenic patients; this liberalised diet became known as the low bacterial diet (LBD) (Pizzo 1982).
The role of diet in the risk of infection among patients with neutropenia remains controversial (French 2001). Dietary restrictions vary in the literature and among institutions. Recommendations range from no dietary restrictions to extensive restrictions. Two surveys (French 2001; Smith 2000) revealed several differences among LBDs used by hospitals in the USA. Furthermore, much variation was reported regarding initiation and discontinuation of the LBD. Few clinical studies have been undertaken to assess the efficacy of the LBD in reducing infection among neutropenic patients, and currently no substantial evidence is available to prove the benefit of the LBD (Larson 2004). As the LBD may pose an unnecessary burden for patients who already have problems with maintaining adequate oral intake because of complications of high‐dose chemotherapy (e.g. mucositis), it would be beneficial to expand our knowledge regarding the efficacy of this diet. This review serves as an update of the first systematic review (Mank 2012) conducted to evaluate the state of evidence on low bacterial diet versus control diet for preventing infection in cancer patients treated with chemotherapy that causes episodes of neutropenia.
Objectives
The primary objective of this review was to determine the efficacy of an LBD versus a control diet in preventing infection and in decreasing (infection‐related) mortality in adult and paediatric cancer patients receiving chemotherapy that causes episodes of neutropenia. Secondary objectives were to assess time to first febrile episode, need for empirical antibiotic therapy, diet acceptability and quality of life.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing use of an LBD versus a control diet.
Types of participants
Cancer patients who received chemotherapy causing episodes of neutropenia. Both adults and children one year of age and older were eligible for inclusion. Children younger than one year of age were excluded because of large differences in metabolism and feeding patterns.
Types of interventions
An LBD versus a control diet.
LBD was defined as any diet intended to reduce ingestion of bacterial and fungal contaminants through exclusion of foods such as uncooked fruits and vegetables, cold cuts, undercooked eggs and meat, unsterilised water, unpasteurised milk products and soft cheeses. The control diet could be any other diet.
Types of outcome measures
Primary outcomes
-
Infection rate (as defined by authors of the original studies).
-
(Infection‐related) mortality (as defined by authors of the original studies).
Secondary outcomes
-
Time to first febrile episode (as defined by authors of the original studies).
-
Need for empirical antibiotic therapy (as defined by authors of the original studies).
-
Diet acceptability (as defined by authors of the original studies).
-
Quality of life (as defined by authors of the original studies).
Search methods for identification of studies
See Cochrane Childhood Cancer Group (CCG) and Cochrane Gynaecological Cancer Group (GCG) methods used in reviews (Module CCG 2010; Module GCG 2010).
Electronic searches
In the original version of the review, review authors searched the following electronic databases: the Central Register of Controlled Trials (CENTRAL) (2011, Issue 3; including earlier searches in 2008 and 2010), the Database of Abstracts of Reviews of Effects (DARE) (2011, Issue 3; including earlier searches in 2008 and 2010), PubMed (from 1946 to 20 October 2011; including earlier searches in 2008 and 2010), EMBASE (from 1980 to 20 October 2011; including earlier searches in 2008 and 2010) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (from 1981 to 20 October 2011; including earlier searches in 2008 and 2010). Search strategies used for the different electronic databases (using a combination of controlled vocabulary and text word terms) are stated in the appendices (Appendix 1; Appendix 2; Appendix 3; Appendix 4).
For the update, we repeated the same searches in CENTRAL and DARE (2015, Issue 4), PubMed (2011 to 4 May 2015), EMBASE (from 2011 to 4 May 2015) and CINAHL (from 2011 to 4 May 2015).
Searching other resources
In the original version of the review, we found information about trials not registered in The Cochrane Library, PubMed, EMBASE or CINAHL, published or unpublished, by searching the reference lists of relevant articles and review articles. We searched the following conference proceedings electronically: American Society of Hematology (ASH; from 2000 to 2011), European Bone Marrow Transplantation (EBMT; from 2000 to 2010), Oncology Nurses Society (ONS; from 2000 to 2011), International Society for Paediatric Oncology (SIOP; from 2000 to 2010), Multinational Association of Supportive Care in Cancer (MASCC; from 2000 to 2010), American Society of Clinical Oncology (ASCO; from 2000 to 2011), Interscience Conference of Antimicrobial Agents and Chemotherapy (ICAAC; from 2000 to 2011), European Society for Clinical Nutrition and Metabolism (ESPEN; from 2000 to 2011), American Society for Parenteral and Enteral Nutrition (ASPEN; from 2000 to 2011) and European Hematology Association (EHA; from 2000 to 2011) (see Appendix 5 for search terms). We searched for ongoing trials in the register of the National Institutes of Health (via clinicaltrials.gov) and the International Standard Randomised Controlled Trial Number (ISRCTN) Register (via controlled‐trials.com; see Appendix 5 for search terms; we screened both in June 2010, October 2011 and May 2012). We contacted researchers working in this area to identify ongoing trials. We imposed no language restrictions.
For the update, we searched the above mentioned conference proceedings from 2011 or 2012 until 2015, except for SIOP abstracts (until 2014), and we searched ongoing trial registries in May 2015, all by using the same keywords as mentioned in the appendices. In addition, we searched the reference lists of relevant articles and review articles.
Data collection and analysis
Selection of studies
After employing the search strategy described previously, two review authors independently identified studies meeting the eligibility criteria of this review. We obtained in full text for closer inspection any study that seemed to meet the inclusion criteria upon review of the title, the abstract or both. We clearly stated reasons for exclusion of any study considered for the review. We resolved disagreements between review authors by consensus, with no need for a third party arbiter.
Data extraction and management
Two review authors independently extracted data by using standardised forms. We extracted data on study design, characteristics of participants (e.g. age, sex, disease, treatment, antimicrobial prophylaxis, colony‐stimulating factors, protective environment, oral care, CVC care, hand washing, hygiene practices), interventions (description of diet in intervention and control group), outcome measures (as described previously) and length of follow‐up. We resolved disagreements between review authors by consensus, with no need for a third party arbiter.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias in included studies (i.e. selection bias, performance bias, detection bias (for each outcome separately, with the exception of overall mortality, because for that outcome, blinding was not relevant), attrition bias (for each outcome separately), reporting bias and other bias). We used 'Risk of bias' items as described in the module of the CCG (Module CCG 2010), which are based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed reporting bias by comparing the methods and results sections of the manuscript; we did not obtain protocols. We resolved disagreements between review authors by consensus, with no need for a third party arbiter. We considered the risk of bias in included studies when interpreting results of the review.
Measures of treatment effect
We analysed dichotomous variables by using risk ratios (RRs). We presented all results with corresponding 95% confidence intervals (CIs).
Dealing with missing data
When relevant data were missing with regards to study selection, we contacted the principal investigator of the study. Only researchers of Van 't Veer 1987 were able to provide additional information. We extracted data by the allocated intervention, irrespective of compliance with treatment, to allow an intention‐to‐treat analysis. If this was not possible, we stated this and performed an 'as treated' analysis.
Assessment of heterogeneity
Pooling of results for this review was not possible; therefore, assessment of heterogeneity (both by visual inspection of the forest plot and by formal statistical testing for heterogeneity, i.e. the I2 statistic (Higgins 2003; Higgins 2011)) was not applicable.
Assessment of reporting biases
In addition to evaluating reporting bias as described in the Assessment of risk of bias in included studies section, we planned to assess reporting bias by constructing a funnel plot when we had identified a sufficient number of included studies (i.e. at least 10 studies included in a meta‐analysis) because otherwise the power of the tests would be too low to allow us to distinguish chance from real asymmetry (Higgins 2011). As pooling of results was not possible, this was not applicable.
Data synthesis
We entered data into Review Manager software as provided by The Cochrane Collaboration (RevMan 2014); we performed analyses according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used a fixed‐effect model throughout the review and pooled results only when study groups were comparable, including definitions of LBD and control diet. We summarised descriptively studies for which pooling of results was not possible.
Sensitivity analysis
As pooling of results was not possible, sensitivity analyses for 'Risk of bias' items (i.e. excluding studies with high risk of bias and studies for which risk of bias was unclear and comparing results of studies with low risk of bias vs results of all available studies) were not applicable.
Results
Description of studies
Results of the search
Searches conducted for the original version of this review using the electronic databases of CENTRAL, DARE, PubMed, EMBASE and CINAHL yielded a total of 619 references (n = 373 in 2008, n = 75 in 2010 and n = 171 in 2011). After initial screening of titles or abstracts, or both, we excluded 612 references that clearly did not meet all criteria for inclusion of studies in this review. We assessed the seven remaining references in full; three fulfilled all criteria and thus were deemed eligible for inclusion (Gardner 2008; Moody 2006; Van Tiel 2007). We excluded the other four references for the reasons described in the Characteristics of excluded studies table (DeMille 2006; Fopp 1975; Wilson 2002; Ziegler 1992).
A scan of the reference lists of included articles and reviews revealed no additional eligible studies. By scanning the ongoing trials databases, we identified two ongoing trials (see the Characteristics of ongoing studies table). Researchers working in this area were not aware of any ongoing trials. By scanning conference proceedings, we identified one possibly eligible study that has not been published in full yet and thus is awaiting further classification (Van 't Veer 1987; for more information, see the Characteristics of studies awaiting classification table), and we added one study to the Characteristics of excluded studies table (Veber 2010).
Searches of CENTRAL and DARE, PubMed, EMBASE/Ovid and CINAHL/EBSCO for this update yielded a total of 558 references; 495 records remained after deduplication. After screening titles and abstracts, we excluded 489 studies, and we assessed six studies as full text. We excluded all six studies for reasons described in the Characteristics of excluded studies table (Carr 2014; Foster 2014; Brown 2014; Lund 2014; Fox 2012; Trifilio 2012). We identified no studies by searching conference proceedings, ongoing trial registries and reference lists of the six assessed studies and reviews identified in the update.
In summary, the total number of included studies was three. We also identified one study that has not been published in full yet and is awaiting further classification and two ongoing trials. See Figure 1 for a flow diagram of studies selected for this update.

Flow diagram of study selection.
Included studies
We have summarised characteristics of the included studies below. For more detailed information, see the Characteristics of included studies table.
We identified three RCTs (Gardner 2008; Moody 2006; Van Tiel 2007) conducted to assess different intervention and control diets (see Characteristics of included studies table for more detailed information). The total number of participants included in these three RCTs was 192: 97 were randomised to intervention groups and 95 to control groups. Two studies included adults (Gardner 2008; Van Tiel 2007), and one study included children (Moody 2006); participants had different types of haematological malignancies or solid tumours (all studies provided only a scant description of treatment regimens).
Supportive care measures differed between studies. Investigators in one study treated participants in high‐efficiency particulate air‐filtered rooms (Gardner 2008); in the other studies, use of a protective environment was unclear (Moody 2006; Van Tiel 2007). Investigators in two studies provided antimicrobial prophylaxis for participants, but types of agents differed between and within studies (Gardner 2008; Van Tiel 2007); in the other study, this treatment was unclear (Moody 2006). Two studies used granulocyte colony‐stimulating factors for some participants (Gardner 2008; Moody 2006); in the other study, this treatment was unclear (Van Tiel 2007). Two studies used central lines for all participants (Gardner 2008; Moody 2006); in the other study, this treatment was unclear (Van Tiel 2007). No studies mentioned CVC care, and no studies mentioned oral care. Two studies did not mention hygiene practices (including hand washing) (Gardner 2008; Van Tiel 2007), whereas investigators in the other study provided hygiene instructions for all participants (Moody 2006).
Risk of bias in included studies
See the 'Risk of bias' table under Characteristics of included studies and Figure 2 for exact scores per study and support for judgements made.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
For evaluation of selection bias, we assessed random sequence generation and allocation concealment. Both of these items, and thus the risk of selection bias, were unclear in one study (Gardner 2008). The other two studies (Moody 2006; Van Tiel 2007) had low risk of selection bias.
Blinding
For evaluation of performance bias, we assessed blinding of participants and healthcare providers. In two studies, risk of performance bias was unclear: In Van Tiel 2007, blinding of both participants and healthcare providers was unclear, and in Moody 2006, participants were blinded, but blinding of healthcare providers was unclear. In the other study, risk of performance bias was high (Gardner 2008).
For evaluation of detection bias, we assessed blinding of outcome assessors for all outcomes separately, with the exception of mortality, because for that outcome, blinding was not relevant. Three studies evaluated the infection rate: In two studies, risk of detection bias was unclear (Gardner 2008; Van Tiel 2007), and in one study, risk of detection bias was high (Moody 2006). Time to first febrile episode (Moody 2006), need for empirical antibiotic therapy (Van Tiel 2007), diet acceptability (Moody 2006) and quality of life (Moody 2006) were evaluated in only one study; for all of these outcomes, risk of detection bias was unclear.
Incomplete outcome data
For evaluation of attrition bias, we assessed incomplete outcome data for all outcomes separately. Three studies evaluated the infection rate: In two studies, risk of attrition bias was low (Gardner 2008; Moody 2006), and in the other study, this risk was unclear (Van Tiel 2007). The following outcomes were evaluated in only one study: overall survival (Gardner 2008; low risk of attrition bias), time to first febrile episode (Moody 2006; low risk of attrition bias), need for empirical antibiotic therapy (Van Tiel 2007; unclear risk of attrition bias), diet acceptability (Moody 2006; unclear risk of attrition bias) and quality of life (Moody 2006; unclear risk of attrition bias).
Selective reporting
For evaluation of reporting bias, we assessed selective reporting. In all included studies, the risk was low.
Other potential sources of bias
For evaluation of other potential sources of bias, we assessed differences between treatment groups for the following items: received anticancer treatment more likely to cause neutropenia, co‐interventions (i.e. protective environment, antimicrobial prophylaxis, CVC care, oral care, hygiene practices and colony‐stimulating factors) and other (as reported in the original study).
In two studies, the risk of other potential sources of bias was unclear (Gardner 2008; Van Tiel 2007), and in the other study, we could not rule out the presence of this type of bias (Moody 2006). For a more detailed description of all items, see the 'Risk of bias' section of the Characteristics of included studies table.
Effects of interventions
None of the articles did allow data extraction for all end points (see the Characteristics of included studies table for a more detailed description of extractable end points for each article). We calculated all RRs, 95% CIs and P values mentioned below in RevMan 2014, unless stated otherwise.
Unfortunately, because of differences in co‐interventions (i.e. protective environment, antimicrobial prophylaxis, CVC care, oral care, hygiene practices, colony‐stimulating factors), outcome definitions used and intervention and control diets provided, it was not possible to pool the results of included studies. Also, Van Tiel 2007 did not present the data that we needed to perform adequate analyses (for further information, see below).
Infection rate
All included studies used different definitions of infection rate.
Gardner 2008 assessed the rate of infection (i.e. major infections, minor infections and fevers of unknown origin; see Characteristics of included studies table for the exact definitions) and identified no statistically significant differences between treatment groups: 68 out of 78 participants (87%) in the cooked‐diet group and 57 out of 75 (76%) in the raw‐diet group developed an infection (RR 1.15, 95% CI 0.98 to 1.34; P value = 0.08; see Figure 3). Among 68 infections in the cooked‐diet group were 23 major infections (34%), five minor infections (7%) and 40 fevers of unknown origin (59%). The raw‐diet group had 26 major infections (46%), four (7%) minor infections and 27 (47%) fevers of unknown origin. Among the 23 major infections in the cooked‐diet group were 12 microbiologically documented infections (52%), and among the 26 major infections in the raw‐diet group were 22 (85%); this information was not reported for minor infections. Although not explicitly stated, we assumed that investigators performed tests to determine pathogenic organisms in all participants. Among the 23 major infections in the cooked‐diet group were 12 cases of pneumonia (52%), seven cases of bacteraemia or fungaemia (31%) and four cases of pneumonia and bacteraemia or fungaemia combined (17%), whereas among the 26 major infections in the raw‐diet group were four cases of pneumonia (16%), 17 cases of bacteraemia or fungaemia (65%) and five cases of pneumonia and bacteraemia or fungaemia combined (19%); this information was not reported for minor infections.

Forest plot of comparison: 1 Cooked diet vs raw diet, outcome: 1.1 Infections (i.e. major infection, minor infection and fever of unknown origin).
Moody 2006 assessed the rate of neutropenic infection (see Characteristics of included studies table for the exact definition) and identified no statistically significant differences between treatment groups: Four out of nine children (44%) in the US Food and Drug Administration (FDA)‐approved food safety guidelines and neutropenic diet guidelines group and four out of 10 children (40%) in the FDA‐approved food safety guidelines‐only group developed a neutropenic infection (RR 1.11, 95% CI 0.39 to 3.19; P value = 0.84; see Figure 4). None of the four neutropenic infections (0%) in the FDA‐approved food safety guidelines and neutropenic diet guidelines group were documented (see Characteristics of included studies table for the exact definition), whereas two out of four neutropenic infections (50%) in the FDA‐approved safety guidelines‐only group were documented (i.e. one case of Pseudomonas sepsis and one of respiratory virus pneumonia).
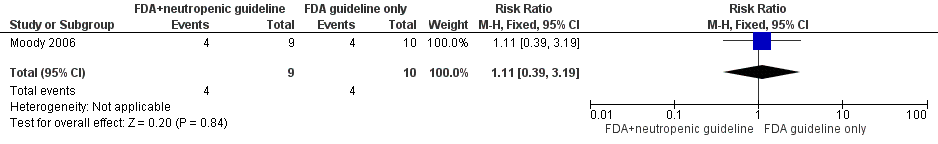
Forest plot of comparison: 2 FDA food safety guidelines and neutropenic diet guidelines vs FDA food safety guidelines only, outcome: 2.1 Neutropenic infection.
Van Tiel 2007 did not report the infection rate as the number of participants with an infection (defined as a temperature ≥ 38.5°C or < 36°C with a single measurement, for which empirical antibiotics were administered), but as the number of chemotherapy cycles with infection present. As a result, we could not adequately analyse the infection rate in this study, but we have provided descriptive results: The LBD group had 14 chemotherapy cycles with infection (of which seven were microbiologically confirmed (50%)) among 20 chemotherapy cycles given (70%), and the normal hospital‐diet group had 17 chemotherapy cycles with infection (of which seven were microbiologically confirmed (41%)) among 21 chemotherapy cycles given (81%). Researchers observed no significant differences (P value = 0.48, as reported in the original article). Please note that investigators assessed this outcome for all available chemotherapy cycles, but it was unclear whether they evaluated all participants within these cycles; therefore, we cannot be certain that an intention‐to‐treat analysis was performed.
(Infection‐related) mortality
None of the included studies mentioned infection‐related mortality.
Gardner 2008 stated that overall survival (no definition provided) in both treatment groups was as expected for newly diagnosed acute myeloid leukaemia and high‐risk myelodysplastic syndrome; investigators observed no significant differences (P value = 0.36, as reported in the original article).
Time to first febrile episode
One study evaluated time to fever. Moody 2006 identified no significant differences in time to fever (defined as time from onset of neutropenia to start of fever) between both treatment groups (no further information and no significance level provided).
Need for empirical antibiotic therapy
In all included studies, it was standard policy to give empirical antibiotics (and sometimes also antimycotics) to (some of) the participants diagnosed with an infection (for more information, see the Characteristics of included studies table). Only one study provided explicit data on the use of empirical antibiotics and antimycotics (Van Tiel 2007). In the LBD group, the median number of days per chemotherapy cycle with empirical antibiotics and antimycotics was 11 days (range 0 to 22 days) and 0 days (range 0 to 9 days), respectively, whereas in the normal hospital‐diet group, the median number was 14.5 days (range 0 to 28 days) and 0 days (0 to 20 days), respectively. Investigators detected no significant differences between treatment groups for duration of empirical antibiotics (P value = 0.09, as reported in the original article) or duration of empirical antimycotics (P value = 0.96, as reported in the original article). Please note that it was unclear whether this outcome was assessed in all participants; therefore, we cannot be certain that intention‐to‐treat analyses were performed.
Diet acceptability
One study evaluated diet acceptability (Moody 2006). In the FDA‐approved food safety guidelines and neutropenic diet guidelines group, all nine children (100%) reported that they were easily able to follow the guidelines, and seven out of nine children (78%) felt they could follow the guidelines through all chemotherapy cycles. All children (100%) reported some difficulty with the food restrictions, especially avoidance of fast foods and raw fruits. In the FDA‐approved guidelines‐only group, nine out of 10 children (90%) reported that they were easily able to follow the guidelines, and that they could follow them through all chemotherapy cycles. No significant differences were identified between treatment groups for following the diet easily (RR 1.10, 95% CI 0.84 to 1.45; P value = 0.50) and for following the diet throughout all chemotherapy cycles (RR 0.86, 95% CI 0.58 to 1.30; P value = 0.48) (see Figure 5). Please note that it was not stated whether all participants were evaluated for this outcome, although it is likely that they were (it was stated that no participants discontinued the study). An intention‐to‐treat analysis was performed.

Forest plot of comparison: 2 FDA food safety guidelines and neutropenic diet guidelines vs FDA food safety guidelines only, outcome: 2.2 Diet acceptability.
Quality of life
One study evaluated quality of life. Moody 2006 used the Peds QL Pediatric Quality of Life Inventory Core Module and Cancer Module by self reports or parent proxy reports, or both (Varni 2002), and identified no statistically significant changes in score from baseline to follow‐up for either arm by child self report or parent proxy report (for both Core and Cancer Modules; no further information provided). Please note that it was not stated whether all participants were evaluated for this outcome, although it is likely that they were (it was stated that no participants discontinued the study). However, we are not certain that an intention‐to‐treat analysis was performed.
Discussion
Neutropenia is a potentially serious side effect of chemotherapy and a major risk factor for infection, which can be life‐threatening. It has been argued that a low bacterial diet (LBD) can prevent infection and (infection‐related) mortality in cancer patients receiving chemotherapy that causes episodes of neutropenia, but much remains unclear. This is an update of the the first systematic review conducted to evaluate this important topic in both adults and children.
To adequately ascertain the efficacy of a dietary intervention, the best study design, provided that design and execution are correct, is a randomised controlled trial (RCT) in which the only difference between intervention and control groups is the diet used.
In the original version of the review, we identified three RCTs including a total of 192 participants with different types of haematological malignancies and solid tumours, in which investigators evaluated different intervention and control diets; in the update, we identified no new eligible studies. Investigators in all studies provided only a scant description of treatment regimens. In all included studies, it was standard policy to give empirical antibiotics (and sometimes also antimycotics) to (some of) the participants diagnosed with an infection. The first study (Gardner 2008) included adults and defined infection as major infections, minor infections and fevers of unknown origin. Investigators randomised participants to a diet that contained only cooked fruits and vegetables versus a diet that permitted fresh (i.e. raw) fruits and vegetables. Participants were treated in high‐efficiency particulate air‐filtered rooms, and they received antimicrobial prophylaxis. Granulocyte colony‐stimulating factors were used in some participants. All participants had central lines, but neither central venous catheter (CVC) care nor oral care and hygiene practices were mentioned. The second study (Moody 2006) included children and evaluated neutropenic infections. Participants were randomised between FDA‐approved food safety guidelines and neutropenic diet guidelines versus FDA‐approved food safety guidelines only. Granulocyte colony‐stimulating factors were used in some participants. All received hygiene instructions. All participants had central lines, but CVC care was not mentioned. Use of a protective environment, antimicrobial prophylaxis and oral care was not mentioned. The third study (Van Tiel 2007) included adults and defined infection as a temperature ≥ 38.5°C or < 36°C, with a single measurement for which empirical antibiotics were administered. Participants were randomised between an LBD and a normal hospital diet. Use of a protective environment, granulocyte colony‐stimulating factors, oral care and hygiene practices were not mentioned, but participants did receive antimicrobial prophylaxis. It was unclear whether participants had central lines; CVC care was not mentioned.
Differences in co‐interventions (e.g. protective environment, antimicrobial prophylaxis, CVC care, oral care, hygiene practices, colony‐stimulating factors), outcome definitions used and intervention and control diets provided precluded pooling of the results of included studies. This should be kept in mind when the results of individual studies are interpreted. Also, one study did not present the data (i.e. the number of participants with an infection) that we needed to perform adequate analyses (Van Tiel 2007).
Two individual studies identified no statistically significant differences in infection rate between participants receiving intervention and control diets (Gardner 2008; Moody 2006); the study that did not present the data needed for adequate analyses reported no significant differences between treatment groups in the number of chemotherapy cycles with an infection (Van Tiel 2007). Infection‐related mortality was not mentioned in any of the included studies, but investigators in one study (Gardner 2008) observed no significant differences in overall survival (no definition provided) between treatment groups. One study (Moody 2006) evaluated time to fever (defined as time from onset of neutropenia to start of fever) and identified no significant differences between treatment groups. One study provided data on use of empirical antibiotics and antimycotics apart from infection rate (Van Tiel 2007). Again, researchers identified no significant differences in duration of empirical antibiotics and antimycotics between treatment groups. One study evaluated diet acceptability (Moody 2006) and no significant differences were identified between treatment groups for following the diet easily and for following the diet throughout all chemotherapy cycles. Finally, one study evaluated quality of life (Moody 2006) and identified no statistically significant changes in score from baseline to follow‐up for either treatment arm by child self report or by parent proxy report.
In this review, we tried to perform intention‐to‐treat analyses, because they provide the most realistic and unbiased answer to the question of clinical effectiveness (Lachin 2000). However, for assessment of infection rate and use of empirical antibiotics by Van Tiel 2007 and quality of life by Moody 2006, it was unclear whether these outcomes were assessed in all participants, so we cannot be certain that an intention‐to‐treat analysis has been performed.
'No evidence of effect', as identified in this review, is not the same as 'evidence of no effect'. It is possible that no significant differences between treatment groups were identified because participants included in these studies were too few to reveal a difference (i.e. low power). Also, baseline imbalances between treatment groups (as included in the other potential sources of bias assessment) might have played a role.
Risk of bias in included studies varied. Often, bias could not be ruled out because of lack of reporting. However, at the moment, this is the best available evidence from RCTs comparing an LBD versus a control diet.
Even though RCTs provide the highest level of evidence, it should be recognised that data from non‐randomised studies are available. Trifilio 2012 compared the neutropenic diet (ND) versus a general hospital diet (GD) in participants with adult hematopoetic stem cell transplantation at one centre. As far as we are aware, this is the only non‐randomised study that compared ND versus GD. Researchers analysed the electronic medical records of 726 consecutive recipients; 363 participants received ND (from October 2004 to August 2006), and the next 363 participants received GD (September 2006 to August 2008). In the GD group, significantly fewer microbiological infections were confirmed compared with the ND group. No differences between groups were detected for length of hospital stay, days to engraftment, neutropenic fever, duration of antibiotic therapy and overall mortality. No information was given on quality of life and diet adherence, although the change to GD was received well by participants. Results of this non‐randomised study seem to be in accordance with results of the three included randomised studies. The design of the study and possible confounders require caution in deriving conclusions.
We are awaiting the results of two ongoing studies (NCT00726934; NCT00947648).

Flow diagram of study selection.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.

Forest plot of comparison: 1 Cooked diet vs raw diet, outcome: 1.1 Infections (i.e. major infection, minor infection and fever of unknown origin).

Forest plot of comparison: 2 FDA food safety guidelines and neutropenic diet guidelines vs FDA food safety guidelines only, outcome: 2.1 Neutropenic infection.
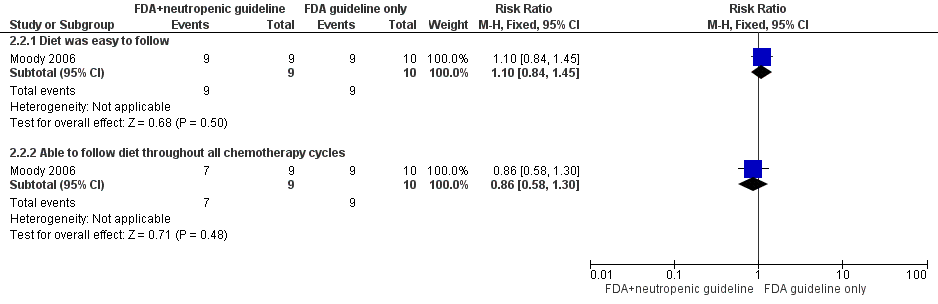
Forest plot of comparison: 2 FDA food safety guidelines and neutropenic diet guidelines vs FDA food safety guidelines only, outcome: 2.2 Diet acceptability.

Comparison 1 Cooked diet versus raw diet, Outcome 1 Infections (i.e. major infection, minor infection and fever of unknown origin).

Comparison 2 FDA food safety guidelines and neutropenic diet guidelines versus FDA food safety guidelines only, Outcome 1 Neutropenic infection.

Comparison 2 FDA food safety guidelines and neutropenic diet guidelines versus FDA food safety guidelines only, Outcome 2 Diet acceptability.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infections (i.e. major infection, minor infection and fever of unknown origin) Show forest plot | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.98, 1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neutropenic infection Show forest plot | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.39, 3.19] |
| 2 Diet acceptability Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Diet was easy to follow | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.84, 1.45] |
| 2.2 Able to follow diet throughout all chemotherapy cycles | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.58, 1.30] |

