Movilización inmediata versus tardía después del accidente cerebrovascular
Appendices
Appendix 1. CENTRAL and DARE search strategy (the Cochrane Library)
#1 [mh ^"cerebrovascular disorders"] or [mh "basal ganglia cerebrovascular disease"] or [mh "brain ischemia"] or [mh "carotid artery diseases"] or [mh "cerebral small vessel diseases"] or [mh "intracranial arterial diseases"] or [mh "intracranial embolism and thrombosis"] or [mh "intracranial hemorrhages"] or [mh ^stroke] or [mh "brain infarction"] or [mh ^"stroke, lacunar"] or [mh ^"vasospasm, intracranial"] or [mh ^"vertebral artery dissection"]
#2 (stroke* or poststroke or apoplex* or cerebral next vasc* or brain next vasc* or cerebrovasc* or cva* or SAH):ti,ab
#3 ((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or "middle cerebral artery" or MCA* or "anterior circulation" or "posterior circulation" or "basilar artery" or "vertebral artery" or "space‐occupying") near/5 (isch*emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*)):ti,ab
#4 ((brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal next gangli* or putaminal or putamen or "posterior fossa" or hemispher* or subarachnoid) near/5 (hemorrhag* or haemorrhage* or hematoma* or haematoma* or bleed*)):ti,ab
#5 [mh ^hemiplegia] or [mh paresis]
#6 (hemipleg* or hemipar* or paresis or paretic):ti,ab
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 [mh ^"bed rest"] or [mh ^immobilization] or [mh ^rest]
#9 (bed rest or bed‐rest or bedrest or bed bound or bed‐bound or bedbound):ti,ab
#10 ((confined or restrict* or immobili*) near/5 bed):ti,ab
#11 [mh ^"early ambulation"]
#12 [mh ^"Physical Therapy Modalities"] or [mh ^"Physical Therapy (Specialty)"]
#13 [mh ^rehabilitation] or [mh ^"activities of daily living"] or [mh ^"recovery of function"]
#14 [mh ^movement] or [mh ^locomotion] or [mh ^walking] or [mh ^"motor activity"]
#15 [mh ^"exercise movement techniques"] or [mh ^exercise] or [mh ^"exercise therapy"]
#16 (stroke unit* or "mobility protocol"):ti,ab
#17 #12 or #13 or #14 or #15 or #16
#18 [mh ^"time factors"] or [mh ^time] or early:ti,ab
#19 #17 and #18
#20 ((early or earlie* or accelerat* or immediat* or "fast‐track" or timing or rapid*) near/5 (mobil* or ambulat* or rehab* or physiotherapy or "physical therapy" or "physical activity" or movement or sitting or standing or walking or semi next recumb* or "out of bed")):ti,ab
#21 #8 or #9 or #10 or #11 or #19 or #20
#22 #7 and #21
#23 [mh ^"cerebrovascular disorders" [mj]/NU,RH,TH] or [mh "basal ganglia cerebrovascular disease" [mj]/NU,RH,TH] or [mh "brain ischemia" [mj]/NU,RH,TH] or [mh "carotid artery diseases" [mj]/NU,RH,TH] or [mh "cerebral small vessel diseases" [mj]/NU,RH,TH] or [mh "intracranial arterial diseases" [mj]/NU,RH,TH] or [mh "intracranial embolism and thrombosis" [mj]/NU,RH,TH] or [mh "intracranial hemorrhages" [mj]/NU,RH,TH] or [mh ^stroke [mj]/NU,RH,TH] or [mh "brain infarction" [mj]/NU,RH,TH] or [mh "stroke, lacunar" [mj]/NU,RH,TH] or [mh ^"vasospasm, intracranial" [mj]/NU,RH,TH] or [mh ^"vertebral artery dissection" [mj]/NU,RH,TH]
#24 #18 and #23
#25 #22 or #24
Appendix 2. MEDLINE search strategy
MEDLINE 2016
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp cerebral small vessel diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or stroke, lacunar/ or vasospasm, intracranial/ or vertebral artery dissection/
2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
5. hemiplegia/ or exp paresis/
6. (hemipleg$ or hemipar$ or paresis or paretic).tw.
7. or/1‐6
8. bed rest/ or immobilization/ or rest/
9. (bed rest or bed‐rest or bedrest or bed bound or bed‐bound or bedbound).tw.
10. ((confined or restricted or immobili$) adj5 bed).tw.
11. early ambulation/
12. Physical Therapy Modalities/ or "Physical Therapy (Specialty)"/
13. rehabilitation/ or "activities of daily living"/ or recovery of function/
14. movement/ or locomotion/ or walking/ or motor activity/
15. exercise movement techniques/ or exercise/ or exercise therapy/
16. (stroke unit$ or mobility protocol).tw.
17. 12 or 13 or 14 or 15 or 16
18. time factors/ or time/ or early.tw.
19. 17 and 18
20. ((early or earlie$ or accelerat$ or immediat$ or fast‐track or timing or rapid$) adj5 (mobil$ or ambulat$ or rehab$ or physiotherapy or physical therapy or physical activity or movement or sitting or standing or walking or semi‐recumb$ or out of bed)).tw.
21. 8 or 9 or 10 or 11 or 19 or 20
22. 7 and 21
23. *cerebrovascular disorders/nu, rh or exp *basal ganglia cerebrovascular disease/nu, rh or exp *brain ischemia/nu, rh or exp *carotid artery diseases/nu, rh or exp *cerebral small vessel diseases/nu, rh or exp *intracranial arterial diseases/nu, rh or exp *"intracranial embolism and thrombosis"/nu, rh or exp *intracranial hemorrhages/nu, rh or *stroke/nu, rh or exp *brain infarction/nu, rh or *stroke, lacunar/nu, rh or *vasospasm, intracranial/nu, rh or *vertebral artery dissection/nu, rh
24. 18 and 23
25. 22 or 24
26. Randomized Controlled Trials as Topic/
27. random allocation/
28. Controlled Clinical Trials as Topic/
29. control groups/
30. clinical trials as topic/
31. double‐blind method/
32. single‐blind method/
33. randomized controlled trial.pt.
34. controlled clinical trial.pt.
35. clinical trial.pt.
36. (random$ or RCT or RCTs).tw.
37. (controlled adj5 (trial$ or stud$)).tw.
38. (clinical$ adj5 trial$).tw.
39. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
40. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
41. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
42. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
43. trial.ti.
44. (assign$ or allocat$).tw.
45. controls.tw.
46. or/26‐45
47. 25 and 46
48. exp animals/ not humans.sh.
49. 47 not 48
Appendix 3. Embase Ovid search strategy
1. cerebrovascular disease/ or exp basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or cerebral artery disease/ or exp cerebrovascular accident/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or stroke unit/ or stroke patient/
2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
5. paralysis/ or hemiparesis/ or hemiplegia/ or paresis/
6. (hemipleg$ or hemipar$ or paresis or paretic).tw.
7. 1 or 2 or 3 or 4 or 5 or 6
8. bed rest/ or immobilization/ or rest/
9. (bed rest or bed‐rest or bedrest or bed bound or bed‐bound or bedbound).tw.
10. ((confined or restrict$ or immobili$) adj5 bed).tw.
11. mobilization/ or patient mobility/ or physical mobility/
12. exp physiotherapy/ or physiotherapist/ or exp rehabilitation/ or daily life activity/ or convalescence/ or "movement (physiology)"/ or exp locomotion/ or motor activity/ or exp exercise/ or exp kinesiotherapy/
13. (stroke unit$ or mobility protocol).tw.
14. 11 or 12 or 13
15. early intervention/ or time/ or early.tw.
16. 14 and 15
17. ((early or earlie$ or accelerat$ or immediat$ or fast‐track or timing or rapid$) adj5 (mobil$ or ambulat$ or rehab$ or physiotherapy or physical therapy or physical activity or movement or sitting or standing or walking or semi‐recumb$ or out of bed)).tw.
18. 8 or 9 or 10 or 16 or 17
19. 7 and 18
20. *cerebrovascular disease/rh or exp *basal ganglion hemorrhage/rh or exp *brain hematoma/rh or exp *brain hemorrhage/rh or exp *brain infarction/rh or exp *brain ischemia/rh or exp *carotid artery disease/rh or *cerebral artery disease/rh or exp *cerebrovascular accident/rh or exp *intracranial aneurysm/rh or exp *occlusive cerebrovascular disease/rh
21. 15 and 20
22. 19 or 21
23. Randomized Controlled Trial/ or "randomized controlled trial (topic)"/
24. Randomization/
25. Controlled clinical trial/ or "controlled clinical trial (topic)"/
26. control group/ or controlled study/
27. clinical trial/ or "clinical trial (topic)"/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/
28. Double Blind Procedure/
29. Single Blind Procedure/ or triple blind procedure/
30. (random$ or RCT or RCTs).tw.
31. (controlled adj5 (trial$ or stud$)).tw.
32. (clinical$ adj5 trial$).tw.
33. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
34. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
35. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
36. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
37. trial.ti.
38. (assign$ or allocat$).tw.
39. controls.tw.
40. or/23‐39
41. 22 and 40
42. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/)
43. 41 not 42
Appendix 4. CINHAL EBSCO search strategy
S1 .(MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR (MH "Intracranial Embolism and Thrombosis") OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections")
S2 .(MH "Stroke Patients") OR (MH "Stroke Units")
S3 .TI ( stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH ) or AB ( stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH )
S4 .TI ( brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying ) or AB ( brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying )
S5 .TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypoxi* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypox* )
S6 .S4 and S5
S7 .TI ( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid )
S8 .TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )
S9 .S7 and S8
S10 .(MH "Hemiplegia")
S11 .TI ( hemipleg* or hemipar* or paresis or paretic ) or AB ( hemipleg* or hemipar* or paresis or paretic )
S12 .S1 OR S2 OR S3 OR S6 OR S9 OR S10 OR S11
S13 .(MH "Early Ambulation")
S14 .(MH "Bed Rest") OR (MH "Bed Rest Care (Iowa NIC)") OR (MH "Rest (Iowa NOC)")
S15 .(MH "Immobilization")
S16 .TI ( bed rest or bed‐rest or bedrest or bed bound or bed‐bound or bedbound) OR AB ( bed rest or bed‐rest or bedrest or bed bound or bed‐bound or bedbound)
S17 .TI ((confined or restricted or immobili*) N5 bed) OR AB ((confined or restricted or immobili*) N5 bed)
S18 .(MH "Physical Mobility") OR (MH "Mobility Therapy (Saba CCC)")
S19 .(MH "Ambulation Aids+") OR (MH "Ambulation Therapy (Saba CCC)") OR (MH "Exercise Therapy: Ambulation (Iowa NIC)") OR (MH "Ambulation: Walking (Iowa NOC)") OR (MH "Walking+")
S20 .(MH "Activities of Daily Living+") or (MH "Physical Therapy+") or (MH "Rehabilitation") or (MH "Movement+")
S21 .TI ( stroke unit* or mobility protocol ) OR AB ( stroke unit* or mobility protocol )
S22 .S18 OR S19 OR S20 OR S21
S23 .(MH "Time+") OR (MH "Early Intervention") OR TI ( early ) OR AB ( early )
S24 .S22 AND S23
S25 .TI ( (early or earlie* or accelerat* or immediate* or fast‐track or timing or rapid) N10 (mobil* or ambulat* or rehab* or physiotherapy or physical therapy or physical activity or movement or sitting or standing or walking or semi‐recumb* or out of bed) ) OR AB ( (early or earlie* or accelerat* or immediate or fast‐track or timing or rapid) N10 (mobil* or ambulat* or rehab* or physiotherapy or physical therapy or physical activity or movement or sitting or standing or walking or semi‐recumb* or out of bed) )
S26 .S13 OR S14 OR S15 OR S16 OR S17 OR S24 OR S25
S27 .S12 AND S26
S28 .(MH "Randomized Controlled Trials") or (MH "Random Assignment") or (MH "Random Sample+")
S29 .(MH "Clinical Trials") or (MH "Intervention Trials") or (MH "Therapeutic Trials")
S30 .(MH "Double‐Blind Studies") or (MH "Single‐Blind Studies") or (MH "Triple‐Blind Studies")
S31 .(MH "Control (Research)") or (MH "Control Group")
S32 .(MH "Quasi‐Experimental Studies")
S33 .PT (clinical trial or randomized controlled trial)
S34 .TI (random* or RCT or RCTs) or AB (random* or RCT or RCTs)
S35 .TI (controlled N5 (trial* or stud*)) or AB (controlled N5 (trial* or stud*))
S36 .TI (clinical* N5 trial*) or AB (clinical* N5 trial*)
S37 .TI ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*)) or AB ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*))
S38 .TI ((control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*)) or AB ((control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*))
S39 .TI ((singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*)) or AB ((singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*))
S40 .TI trial
S41 .TI (assign* or allocat*) or AB (assign* or allocat*)
S42 .TI controls or AB controls
S43 .TI (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*) or AB (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*)
S44 .S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43
S45 .S27 AND S44
Appendix 5. PsycINFO Ovid search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or exp cerebral ischemia/ or cerebral small vessel disease/ or cerebrovascular accidents/ or subarachnoid hemorrhage/
2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
5. hemiparesis/ or hemiplegia/
6. (hemipleg$ or hemipar$ or paresis or paretic).tw.
7. or/1‐6
8. (bed rest or bed‐rest or bedrest or bed bound or bed‐bound or bedbound).tw.
9. ((confined or restricted or immobili$) adj5 bed).tw.
10. physical mobility/ or physical therapy/ or physical activity/ or physical therapists/
11. rehabilitation/ or "activities of daily living"/
12. motor processes/ or locomotion/ or walking/
13. exercise/
14. (stroke unit$ or mobility protocol).tw.
15. 10 or 11 or 12 or 13 or 14
16. early intervention/ or time/ or early.tw.
17. 15 and 16
18. ((early or earlie$ or accelerat$ or immediat$ or fast‐track or timing or rapid$) adj5 (mobil$ or ambulat$ or rehab$ or physiotherapy or physical therapy or physical activity or movement or sitting or standing or walking or semi‐recumb$ or out of bed)).tw.
19. 8 or 9 or 17 or 18
20. 7 and 19
21. clinical trials/ or treatment effectiveness evaluation/ or placebo/
22. (random$ or RCT or RCTs).tw.
23. (controlled adj5 (trial$ or stud$)).tw.
24. (clinical$ adj5 trial$).tw.
25. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
26. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
27. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
28. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
29. trial.ti.
30. (assign$ or allocat$).tw.
31. controls.tw.
32. or/21‐31
33. 20 and 32
Appendix 6. AMED Ovid search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/
2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
5. hemiplegia/ or gait disorders/
6. (hemipleg$ or hemipar$ or paresis or paretic).tw.
7. 1 or 2 or 3 or 4 or 5 or 6
8. bed rest/ or immobilization/ or rest/
9. (bed rest or bed‐rest or bedrest or bed bound or bed‐bound or bedbound).tw.
10. ((confined or restricted or immobili$) adj5 bed).tw.
11. mobilisation/
12. physical therapy modalities/ or physiotherapists/ or exp exercise therapy/ or rehabilitation/ or exp locomotion/ or movement/ or motor activity/ or "activities of daily living"/
13. (stroke unit$ or mobility protocol).tw.
14. 11 or 12 or 13
15. time/ or early.tw.
16. 14 and 15
17. ((early or earlie$ or accelerat$ or immediat$ or fast‐track or timing or rapid$) adj5 (mobil$ or ambulat$ or rehab$ or physiotherapy or physical therapy or physical activity or movement or sitting or standing or walking or semi‐recumb$ or out of bed)).tw.
18. 8 or 9 or 10 or 16 or 17
19. 7 and 18
20. clinical trials/ or randomized controlled trials/ or random allocation/
21. research design/ or comparative study/
22. double blind method/ or single blind method/
23. (random$ or RCT or RCTs).tw.
24. (controlled adj5 (trial$ or stud$)).tw.
25. (clinical$ adj5 trial$).tw.
26. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
27. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
28. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
29. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
30. trial.ti.
31. (assign$ or allocat$).tw.
32. controls.tw.
33. or/20‐32
34. 19 and 33
Appendix 7. SPORTDiscus EBSCO search strategy
S1 .DE "CEREBROVASCULAR disease" OR DE "BRAIN ‐‐ Hemorrhage" OR DE "CEREBRAL embolism & thrombosis" OR DE "STROKE" OR DE "BRAIN ‐‐ Wounds & injuries" OR DE "BRAIN damage"
S2 .DE "CEREBROVASCULAR disease ‐‐ Patients"
S3 .TI ( stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH ) or AB ( stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH )
S4 .TI ( brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying ) or AB ( brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying )
S5 .TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypoxi* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypox* )
S6 .S4 and S5
S7 .TI ( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid )
S8 .TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )
S9 .S7 and S8
S10 .DE "HEMIPLEGIA" OR DE "HEMIPLEGICS" OR DE "GAIT disorders"
S11 .TI ( hemipleg* or hemipar* or paresis or paretic ) or AB ( hemipleg* or hemipar* or paresis or paretic )
S12 .S1 OR S2 OR S3 OR S6 OR S9 OR S10 OR S11
S13 .DE "REST" OR DE "IMMOBILIZATION (Therapeutics)"
S14 .TI ( bed rest or bed‐rest or bedrest or bed bound or bed‐bound or bedbound) OR AB ( bed rest or bed‐rest or bedrest or bed bound or bed‐bound or bedbound)
S15 .TI ((confined or restricted or immobili*) N5 bed) OR AB ((confined or restricted or immobili*) N5 bed)
S16 .DE "PHYSICAL activity” OR DE "PHYSICAL therapists"
S17 .DE "REHABILITATION" OR DE "MEDICAL rehabilitation" OR DE "RECOVERY training" OR DE "MOVEMENT therapy"
S18 .DE "ACTIVITIES of daily living training" OR DE "ACTIVITIES of daily living" OR DE "FUNCTIONAL training"
S19 .DE "BODY movement" OR DE "MOVEMENT therapy" OR DE "LOCOMOTION" OR DE "WALKING" OR DE "EXERCISE" OR DE "ARM exercises" OR DE "CHAIR exercises" OR DE "LEG exercises" OR DE "STRENGTH training" OR DE "STRETCHING exercises"
S20 .DE "EXERCISE therapy"
S21 .TI ( stroke unit* or mobility protocol ) OR AB ( stroke unit* or mobility protocol )
S22 .S16 OR S17 OR S18 OR S19 OR S20 OR S21
S23 .TI ( early ) OR AB ( early )
S24 .S22 AND S23
S25 .TI ( (early or earlie* or accelerat* or immediat* or fast‐track or timing or rapid) N10 (mobil* or ambulat* or rehab* or physiotherapy or physical therapy or physical activity or movement or sitting or standing or walking or semi‐recumb* or out of bed) ) OR AB ( (early or earlie* or accelerat* or immediate or fast‐track or timing or rapid) N10 (mobil* or ambulat* or rehab* or physiotherapy or physical therapy or physical activity or movement or sitting or standing or walking or semi‐recumb* or out of bed) )
S26 .S13 OR S14 OR S15 OR S24 OR S25
S27 .S12 AND S26
Appendix 8. Web of Science search strategy
Web of Science: Core Collection 1900‐2016 (Science Citation Index Expanded (SCI‐EXPANDED), Social Sciences Citation Index (SSCI), and Arts & Humanities Citation Index (A&HCI)).
# 26. #25 AND #12 AND #6 (Indexes=SCI‐EXPANDED, SSCI, A&HCI Timespan=2006‐2016)
# 25. #24 OR #23 OR #22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13
# 24. TS=controls
# 23. TS=(assign* or allocat*)
# 22. TI=trial
# 21. TS=(placebo* or sham)
# 20. TS=(cross‐over or cross over or crossover)
# 19 .TS=((singl* or doubl* or tripl* or trebl*) NEAR/5 (blind* or mask*))
# 18. TS=((control or experiment* or conservative) NEAR/5 (treatment or therapy or procedure or manage*))
# 17. TS=(quasi‐random* or quasi random* or pseudo‐random* or pseudo random*)
# 16. TS=((control or treatment or experiment* or intervention) NEAR/5 (group* or subject* or patient*))
# 15. TS=(clinical* NEAR/5 trial*)
# 14. TS=(controlled NEAR/5 (trial* or stud*))
# 13. TS=(random* or RCT or RCTs)
# 12. #7 OR #8 OR #11
# 11. #9 AND #10
# 10. TS=(mobil* or ambulat* or rehab* or physiotherapy or physical therapy or physical activity or movement or sitting or standing or walking or semi‐recumb* or out of bed)
# 9. TS=(early or earlie* or accelerat* or immediat* or fast‐track or timing or rapid*)
# 8. TS=((confined or restricted or immobili*) NEAR/5 bed).
# 7. TS=(bed rest or bed‐rest or bedrest or bed bound or bed‐bound or bedbound).
# 6. #5 OR #4 OR #3 OR #2 OR #1
# 5. TS=((unilateral or spatial or hemi$spatial or visual) NEAR/5 neglect)
# 4. TS=(hemipleg* or hemipar* or paresis or paretic or hemineglect or hemi‐neglect)
# 3. TS=((brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) NEAR/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*))
# 2. TS=((brain* or cerebr* or cerebell* or intracran* or intracerebral) NEAR/5 (isch$emi* or infarct* or thrombo* or emboli* or occlus*))
# 1. TS=(stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva* or apoplex* or SAH)
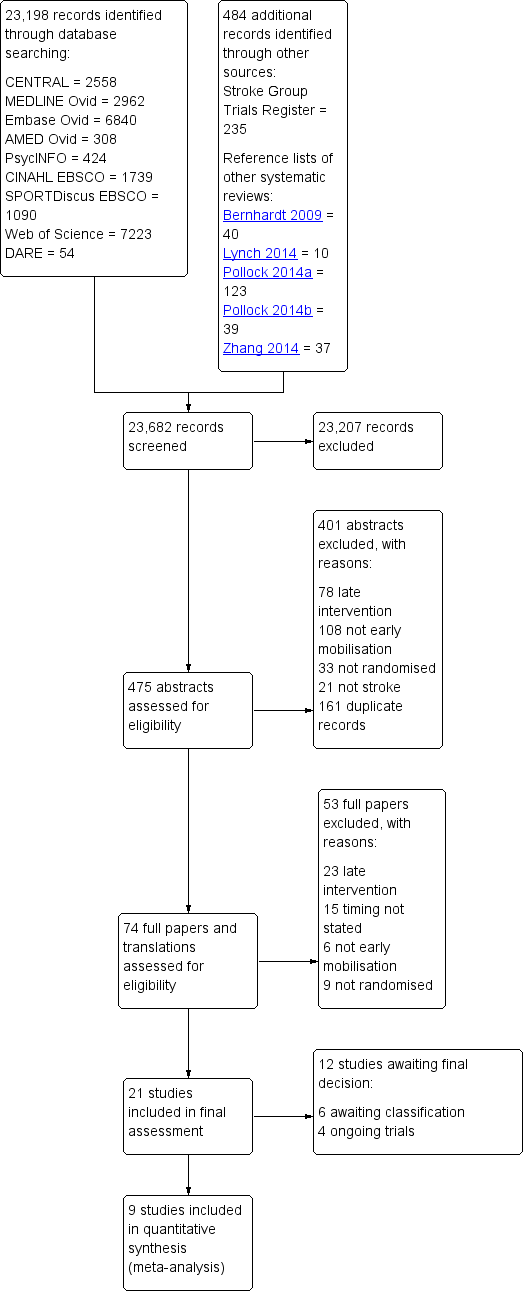
Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Network plot of all trials. Each point shows the time‐to‐first mobilisation (TTFM) classifications. The lines show the number of trials directly comparing each TTFM category.
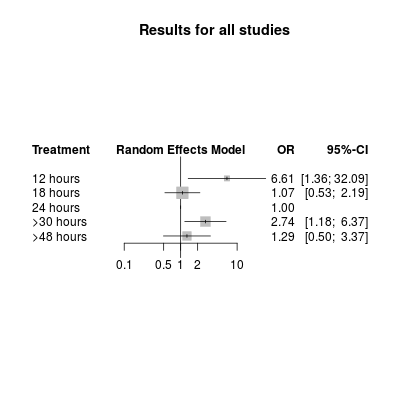
Network meta‐analysis plot for poor outcome (death or dependency at 3 months). The treatment column shows the time‐to‐first mobilisation (TTFM) categories. The results are the odds ratio (95% confidence interval) for the odds of a poor outcome with TTFM of 24 hours as the reference (OR = 1.0).

Network meta‐analysis plot for death at 3 months. The treatment column shows the time‐to‐first mobilisation (TTFM) categories. The results are the odds ratio (95% confidence interval) for the odds of death with TTFM of 24 hours as the reference (OR = 1.0).

Comparison 1 Very early mobilisation (VEM) versus standard care (measured at end of scheduled follow‐up), Outcome 1 Death or poor outcome.
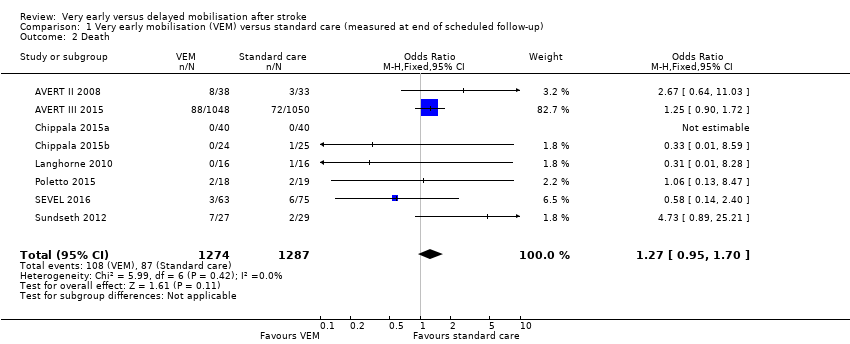
Comparison 1 Very early mobilisation (VEM) versus standard care (measured at end of scheduled follow‐up), Outcome 2 Death.

Comparison 1 Very early mobilisation (VEM) versus standard care (measured at end of scheduled follow‐up), Outcome 3 Death or dependence (modified Rankin score 3 to 6).
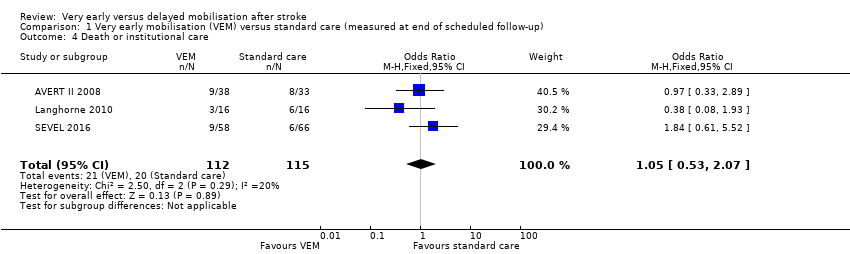
Comparison 1 Very early mobilisation (VEM) versus standard care (measured at end of scheduled follow‐up), Outcome 4 Death or institutional care.
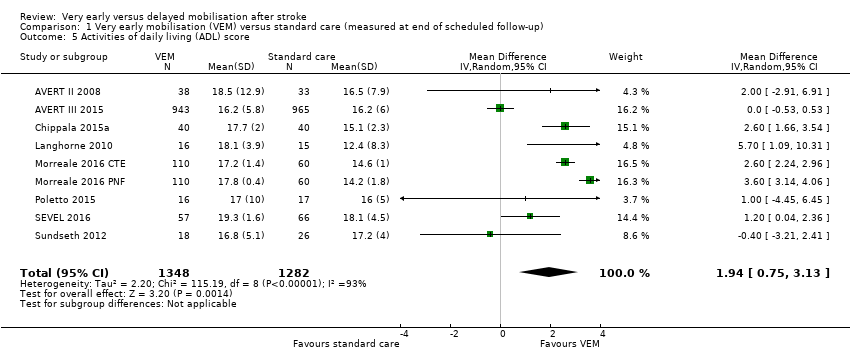
Comparison 1 Very early mobilisation (VEM) versus standard care (measured at end of scheduled follow‐up), Outcome 5 Activities of daily living (ADL) score.
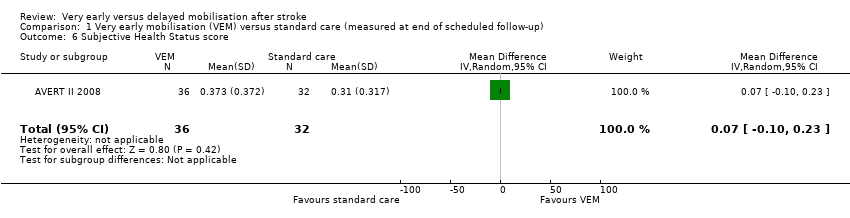
Comparison 1 Very early mobilisation (VEM) versus standard care (measured at end of scheduled follow‐up), Outcome 6 Subjective Health Status score.

Comparison 1 Very early mobilisation (VEM) versus standard care (measured at end of scheduled follow‐up), Outcome 7 Able to walk.
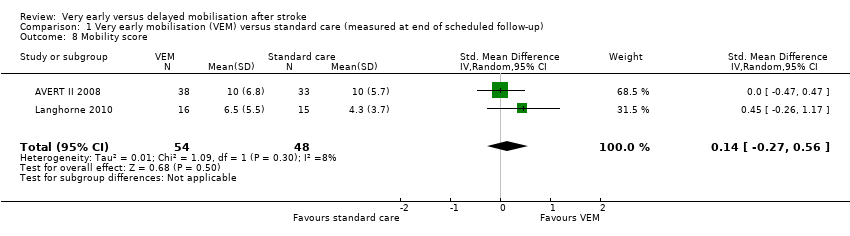
Comparison 1 Very early mobilisation (VEM) versus standard care (measured at end of scheduled follow‐up), Outcome 8 Mobility score.

Comparison 1 Very early mobilisation (VEM) versus standard care (measured at end of scheduled follow‐up), Outcome 9 Any complication: participants who experienced at least one complication.

Comparison 1 Very early mobilisation (VEM) versus standard care (measured at end of scheduled follow‐up), Outcome 10 Type of complication: participants who experienced at least one complication.

Comparison 1 Very early mobilisation (VEM) versus standard care (measured at end of scheduled follow‐up), Outcome 11 Mood score.

Comparison 1 Very early mobilisation (VEM) versus standard care (measured at end of scheduled follow‐up), Outcome 12 Length of acute hospital stay (days).
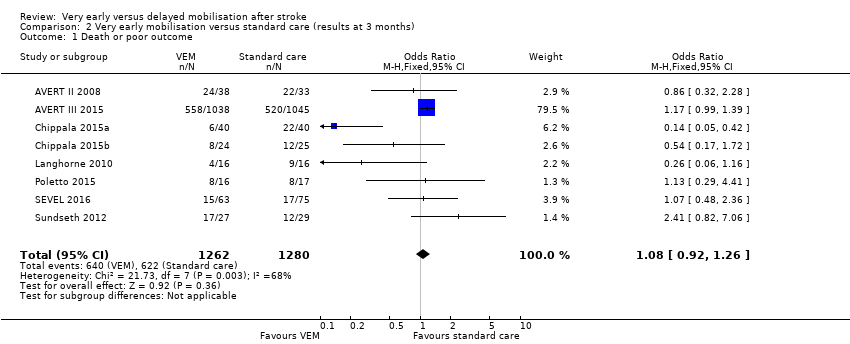
Comparison 2 Very early mobilisation versus standard care (results at 3 months), Outcome 1 Death or poor outcome.
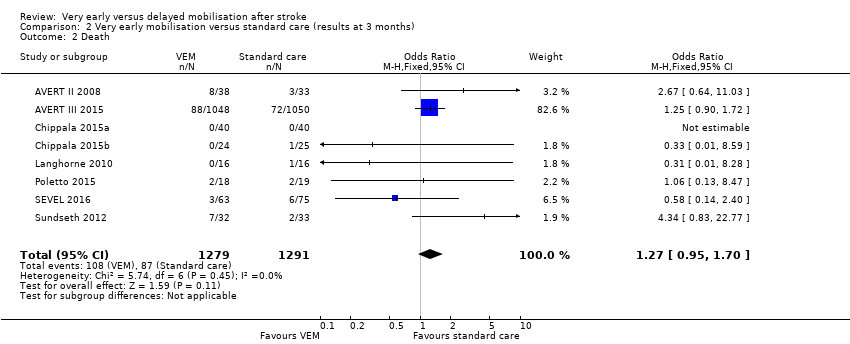
Comparison 2 Very early mobilisation versus standard care (results at 3 months), Outcome 2 Death.

Comparison 2 Very early mobilisation versus standard care (results at 3 months), Outcome 3 Death or dependence (modified Rankin score 3 to 6).
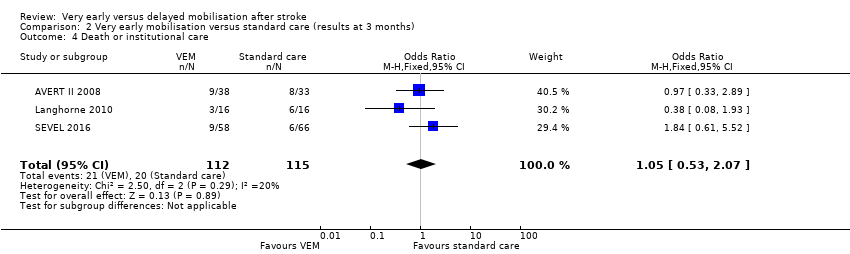
Comparison 2 Very early mobilisation versus standard care (results at 3 months), Outcome 4 Death or institutional care.
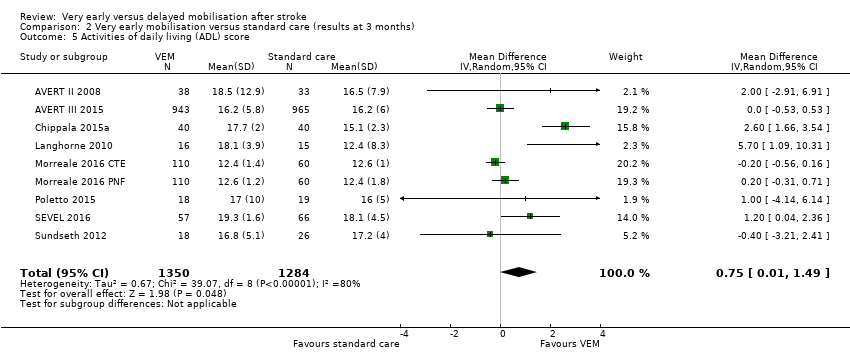
Comparison 2 Very early mobilisation versus standard care (results at 3 months), Outcome 5 Activities of daily living (ADL) score.
| Very early mobilisation versus delayed mobilisation | ||||||
| Patient or population: adults with acute stroke Settings: stroke unit or acute ward Intervention: very early mobilisation (VEM) Comparison: delayed mobilisation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Delayed mobilisation | Very early mobilisation | |||||
| Death or a poor outcome (median 3‐month follow‐up) | Medium risk population | OR 1.08 (0.92 to 1.26) | 2542 (8) | ⊕⊕⊕⊝ | Largest trial (2104 participants) found increased risk of death or poor outcome with VEM | |
| 486 per 1000 | 507 per 1000 | |||||
| Death (median 3‐month follow‐up) | Medium risk population | OR 1.27 (0.95 to 1.70) | 2561 (8) | ⊕⊕⊕⊝ | Sensitivity analysis suggested increased risk of death in trials with earlier onset of VEM | |
| 68 per 1000 | 85 per 1000 | |||||
| Death or dependence (modified Rankin score 3 to 6; median 3‐month follow‐up) | Medium risk population | OR 1.08 (0.92 to 1.26) | 2542 (8) | ⊕⊕⊕⊝ | Largest trial found increased risk of death or dependency with VEM | |
| 486 per 1000 | 507 per 1000 | |||||
| Activities of daily living (ADL) (Barthel Index score 0 to 20; lower = 0; median 3‐month follow‐up) | The mean Barthel Index scores across control groups ranged from 14.2 to 18.1. | The mean Barthel Index score in the intervention groups was on average 1.94 points higher (0.75 higher to 3.13 higher). | MD 1.94 higher (0.75 to 3.13 higher) | 2630 (8) | ⊕⊕⊝⊝ | Higher rate of missing data |
| Subjective Health Status score (Assessment of Quality of Life Score 0 to 1; lower = 0; end of scheduled follow‐up) | The mean Assessment of Quality of Life (AQoL) score in the control group was 0.306 | The mean AQoL score in the intervention group was on average 0.07 points higher (0.1 lower to 0.23 higher) | MD 0.07 higher (0.1 lower to 0.23 higher) | 68 (1) | ⊕⊝⊝⊝ | Higher rate of missing data Data from one trial only |
| Any complication: participants who experience at least one complication (median 3‐month follow‐up) | Medium risk population | OR 0.88 (0.73 to 1.06) | 2778 (6) | ⊕⊕⊕⊝ | Uncertain blinding at follow‐up | |
| 224 per 1000 | 200 per 1000 | |||||
| Length of acute hospital stay (days) | The mean length of stay across control groups ranged from 9.8 to 14.9 days. | The mean length of stay in the intervention groups was, on average, 1.44 days less (2.28 days less to 0.60 day less) | MD 1.44 lower (2.28 lower to 0.60 lower) | 2551 (8) | ⊕⊕⊝⊝ | Different definitions and imprecise measures of length of stay were reported Result largely depends on two small trials with small SDs |
| *The basis for the assumed risk (e.g. the mean control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Downgraded for potential risk of performance bias. b Downgraded for unexplained heterogeneity. c Downgraded for imprecision. | ||||||
| Trial | Stated aim for mobilisation activity | Participant median age (% male) | Stroke severity (moderate or severe stroke) | Early mobilisation TTFM (hours; median; IQR) | Usual care TTFM (hours; median; IQR) | Average frequency of mobilisation events per day (early vs usual care) | Average amount of mobilisation activity (early vs usual care) |
| Earlier and more | 75 yrs (54%) | 57% | 18.1 (12.8 to 21.5) | 30.8 (23.0 to 39.9) | 2 vs 0 | 167 vs 69 mins/admission mobilisation activity | |
| Earlier and more | 73 yrs (39%) | 45% | 18.5 (12.8 to 22.3) | 22.4 (16.5 to 29.3) | 6.5 vs 3 | 31 vs 10 mins/day mobilisation activity | |
| Earlier and more | 60 yrs (52%) | 68% | 18 (16.6 to 19.8) | 30.5 (29.0 to 35.0) | Not stated | Extra 5 to 30 mins/day out‐of‐bed activity | |
| Earlier and more | 64 yrs (57%) | 44% | 18 (16.6 to 19.8) | 30.5 (29.0 to 35.0) | Not stated | Extra 5 to 30 mins/day out‐of‐bed activity | |
| Earlier and more | 68 yrs (50%) | 28% | 27.3 (26.0 to 29.0) | 32.0 (22.5 to 47.3) | Not stated | More early mobilisation, standing or walking, recorded using activity monitors (P = 0.02) | |
| Earlier | 64 yrs (72%) | < 89% | < 24 | 96 | Not stated | 60 mins/day more early mobilisation group for first 4 days | |
| Earlier and more | 65 yrs (35%) | < 70% | 43 | 72 | 0.54 vs 0.03 | Extra 30 mins/day out‐of‐bed activity | |
| Earlier | 70 yrs (64%) | 44% | 25.9 (22.5 to 29.3) | 71.5 (68.1 to 74.9) | Not stated | 83.7 vs 56.6 mins/day | |
| Earlier | 77 yrs (45%) | 34% | 13.1 (8.5 to 25.6) | 33.3 (26.0 to 39.0) | Not stated | Not stated | |
| IQR: interquartile range | |||||||
| Usual care group TTFM characteristics | Very early mobilisation group TTFM characteristics | ||||
| 12 hours | 18 hours | 24 hours | > 30 hours | > 48 hours | |
| 12 hours | ‐ | ‐ | ‐ | ‐ | ‐ |
| 18 hours | ‐ | ‐ | ‐ | ‐ | ‐ |
| 24 hours | ‐ | AVERT III 2015 (2014 ppts) | ‐ | ‐ | ‐ |
| > 30 hours | Sundseth 2012 (65 ppts) | AVEAVERT II 2008; Chippala 2015a; Chippala 2015b (211 ppts) | Langhorne 2010 (32 ppts) | ‐ | ‐ |
| > 48 hours | SEVEL 2016 167 ppts) | Poletto 2015 (39 ppts) | ‐ | ||
| ppts = participants Table shows Time‐to‐first mobilisation (TTFM) in each trial with the very early mobilisation TTFM group in the columns and usual care TTFM in the rows. The number of trials (participants) in each direct comparison of TTFM are also shown. For example Sundseth 2012 compared TTFM of approximately 12 hours with > 30 hours and included 65 participants. We did not include data from Morreale 2016 in this analysis as we did not have access to dichotomous data on poor outcome or death. | |||||
| TTFM category | TTFM recorded in the trials (median; IQR) | Direct comparison (OR) | Indirect comparison (OR) | Log difference (95% CI) between direct and indirect comparisons | P value of difference between direct and indirect comparisons | Network meta‐analysis (OR and 95% CI) |
| 12 hours | 13 (9 to 26) | NA | 6.62 | NA | NA | 6.61 (1.36 to 32.09) |
| 18 hours | 18 (13 to 21) | 1.17 | 0.80 | ‐0.39 (‐2.09 to 1.31) | 0.65 | 1.07 (0.53 to 2.19) |
| 24 hours | 26 (22 to 29) | 1.00 (reference) | Reference | Reference | Reference | Reference |
| > 30 hours | 32 (26 to 40) | 3.86 | 2.46 | 0.45 (‐1.50 to 2.41) | 0.65 | 2.74 (1.18 to 6.37) |
| > 48 hours | 72 (68 to 75) | 0.94 | 3.03 | ‐1.18 (‐3.33 to 0.98) | 0.28 | 1.29 (0.50 to 3.37) |
| The first two columns show the TTFM category plus the actual recorded TTFM for that category. The next two columns show the odds ratio of a poor outcome for the direct and indirect comparison of the TTFM category, with 24 hours as the reference category. The fifth column shows the log difference, and the sixth shows the P value, between the two odds ratio estimates. The final column shows the network meta‐analysis results, which combine the direct and indirect evidence. CI: confidence interval | ||||||
| TTFM category | TTFM recorded in the trials (median; IQR) | Direct comparison (OR) | Indirect comparison (OR) | Log difference (95% CI) between direct and indirect comparisons | P value of difference between direct and indirect comparisons | Network meta‐analysis (OR and 95% CI) |
| 12 hours | 13 (9 to 26) | NA | 4.18 | NA | NA | 4.17 (0.57 to 30.7) |
| 18 hours | 18 (13 to 21) | 1.25 | 4.35 | 1.25 (‐1.16 to 3.66) | 0.31 | 1.27 (0.92 to 1.76) |
| 24 hours | 26 (22 to 29) | 1.00 (reference) | ‐ | ‐ | ‐ | ‐ |
| > 30 hours | 32 (26 to 40) | 3.19 | 0.82 | 1.36 (‐2.12 to 4.84) | 0.44 | 0.96 (0.32 to 2.92) |
| > 48 hours | 72 (68 to 75) | 1.73 | 0.77 | 0.81 (‐1.99 to 3.62) | 0.57 | 1.41 (0.41 to 4.82) |
| The first two columns show the TTFM category plus the actual recorded TTFM for that category. The next two columns show the odds ratio of a poor outcome for the direct and indirect comparison of the TTFM category, with 24 hours as the reference. The fifth column shows the log difference, and the sixth shows the P value, between the two odds ratio estimates. The final column shows the network meta‐analysis results, which combine the direct and indirect evidence. CI: confidence interval | ||||||
| Intervention TTFM | Comparison TTFM (reference treatment) | No. of studies (participants) with direct comparison evidence | Direct comparison evidence OR (95% CI) | Quality of the evidence (GRADE) for direct comparisons | Direct plus indirect evidence (NMA) OR (95% CI) | Quality of the evidence (GRADE) for NMA |
| Poor outcome | ||||||
| 12 hours | 24 hours | 0 | NA | NA | 6.61 (1.36 to 32.1) | Low a, b |
| 18 hours | 24 hours | 1 (2104) | 1.17 (0.99 to 1.39) | Moderate c | 1.07 (0.53 to 2.19) | Low b, c |
| 30 to 48 hours | 24 hours | 1 (32) | 3.85 (0.86 to 16.7) | Low b, e | 2.74 (1.18 to 6.37) | Low b, e |
| More than 48 hours | 24 hours | 1 (167) | 0.94 (0.42 to 2.08) | Low d, e | 1.29 (0.50 to 3.37) | Low b, e |
| Death | ||||||
| 12 hours | 24 hours | 0 | NA | NA | 4.17 (0.57 to 30.7) | Low a, b |
| 18 hours | 24 hours | 1 (2104) | 1.25 (0.90 to 1.72) | Moderate c | 1.27 (0.92 to 1.76) | Low b, c |
| 30 to 48 hours | 24 hours | 1 (32) | 3.03 (0.12 to 100) | Low b, e | 0.96 (0.32 to 2.92) | Low b, e |
| More than 48 hours | 24 hours | 1 (167) | 1.75 (0.42 to 7.14) | Low b, e | 1.41 (0.41 to 4.82) | Low b, e |
| a Main trial in the loop was small and had missing data b Downgraded for imprecision c Based on a single large trial d Based on single small trial e Uncertain blinding of follow up | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or poor outcome Show forest plot | 8 | 2542 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.92, 1.26] |
| 2 Death Show forest plot | 8 | 2561 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.95, 1.70] |
| 3 Death or dependence (modified Rankin score 3 to 6) Show forest plot | 8 | 2542 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.92, 1.26] |
| 4 Death or institutional care Show forest plot | 3 | 227 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.53, 2.07] |
| 5 Activities of daily living (ADL) score Show forest plot | 9 | 2630 | Mean Difference (IV, Random, 95% CI) | 1.94 [0.75, 3.13] |
| 6 Subjective Health Status score Show forest plot | 1 | 68 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.10, 0.23] |
| 7 Able to walk Show forest plot | 4 | 2255 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.83, 1.21] |
| 8 Mobility score Show forest plot | 2 | 102 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.27, 0.56] |
| 9 Any complication: participants who experienced at least one complication Show forest plot | 7 | 2778 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.73, 1.06] |
| 10 Type of complication: participants who experienced at least one complication Show forest plot | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Complications of immobility | 7 | 2778 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.60, 1.03] |
| 10.2 Other complications | 6 | 2435 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.78, 1.23] |
| 11 Mood score Show forest plot | 2 | 100 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.33, 0.46] |
| 12 Length of acute hospital stay (days) Show forest plot | 8 | 2551 | Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐2.28, ‐0.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or poor outcome Show forest plot | 8 | 2542 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.92, 1.26] |
| 2 Death Show forest plot | 8 | 2570 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.95, 1.70] |
| 3 Death or dependence (modified Rankin score 3 to 6) Show forest plot | 8 | 2542 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.92, 1.26] |
| 4 Death or institutional care Show forest plot | 3 | 227 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.53, 2.07] |
| 5 Activities of daily living (ADL) score Show forest plot | 9 | 2634 | Mean Difference (IV, Random, 95% CI) | 0.75 [0.01, 1.49] |

