Combinación de agentes tocolíticos para la inhibición del trabajo de parto prematuro
Resumen
Antecedentes
El parto prematuro representa la causa más importante por sí sola de mortalidad y morbilidad en los recién nacidos y una causa principal de morbilidad en las embarazadas. Los agentes tocolíticos incluyen una variedad amplia de fármacos que pueden inhibir el trabajo de parto para prolongar el embarazo. La administración de estos agentes puede hacer que se gane tiempo para permitir que el feto madure más antes de nacer, permitir la administración de corticosteroides prenatales para la maduración pulmonar y proporcionar tiempo para el traslado intrauterino a un hospital con unidad de cuidados intensivos neonatales. Sin embargo, algunos fármacos tocolíticos se asocian con efectos secundarios graves. Las combinaciones de fármacos tocolíticos pueden ser más eficaces que los agentes tocolíticos solos o ninguna intervención, sin afectar negativamente a la madre ni al recién nacido.
Objetivos
Evaluar los efectos sobre los resultados maternos, fetales y neonatales de cualquier combinación de fármacos tocolíticos para el tratamiento del trabajo de parto prematuro en comparación con otro tratamiento, ningún tratamiento o placebo.
Métodos de búsqueda
Se hicieron búsquedas en el registro de ensayos del Grupo Cochrane de Embarazo y Parto (Cochrane Pregnancy and Childbirth Group) (31 de enero de 2014) y en las listas de referencias de estudios recuperados.
Criterios de selección
Se incluyeron los ensayos controlados aleatorios que compararon una combinación de agentes tocolíticos, administrados por cualquier vía o cualquier dosis, para inhibir el trabajo de parto prematuro versus otro tratamiento (que incluye otras combinaciones de tocolíticos o tocolíticos únicos), ninguna intervención o placebo.
Obtención y análisis de los datos
Dos autores de la revisión de manera independiente evaluaron los informes de los estudios para ver su elegibilidad, extrajeron los datos y evaluaron el riesgo de sesgo.
Resultados principales
Once estudios cumplieron los criterios de inclusión. Dos estudios no informaron datos de resultados relevantes para la revisión, por lo que los resultados de la revisión se basan en nueve ensayos que contribuyeron con datos. Los resultados primarios fueron mortalidad perinatal, resultados maternos o infantiles graves, reacciones farmacológicas adversas, parto antes de las 48 horas de ingreso al ensayo, parto antes de las 34 semanas de gestación y parto prematuro sin completar un ciclo completo de esteroides prenatales antes de las 24 horas de que se produzca el parto. La calidad de las pruebas de los ensayos incluidos fue mixta; solamente tres ensayos fueron controlados con placebo.
Los ensayos incluidos examinaron siete comparaciones diferentes: ritodrina intravenosa más magnesio oral o intravenoso (sulfato o gluconato) versus ritodrina intravenosa sola (tres ensayos, 231 embarazadas); ritodrina intravenosa más supositorios de indometacina versus ritodrina intravenosa sola (un ensayo, 208 embarazadas); ritodrina intravenosa más progesterona vaginal versus ritodrina intravenosa sola (un ensayo, 83 embarazadas); sulfato de hexoprenalina intravenoso más clorhidrato de magnesio intravenoso versus sulfato de hexoprenalina intravenoso solo (un ensayo, 24 embarazadas); fenoterol intravenoso más naproxeno oral versus fenoterol intravenoso solo (un ensayo, 72 embarazadas); pentoxifilina oral más sulfato de magnesio intravenoso más fenoterol intravenoso versus sulfato de magnesio intravenoso más fenoterol intravenoso (un ensayo, 125 embarazadas); y terbutalina intravenosa más metoprolol oral versus terbutalina intravenosa sola (un ensayo, 17 embarazadas). Pocos estudios con números pequeños de embarazadas estuvieron disponibles para cada comparación, por lo que en el metanálisis se agruparon datos muy escasos. Los ensayos no informaron muchos de los resultados primarios.
Tres ensayos examinaron ritodrina intravenosa más magnesio oral o intravenoso (sulfato o gluconato) en comparación con ritodrina intravenosa sola. Un estudio con 41 embarazadas informó más reacciones farmacológicas adversas en los grupos que recibieron tocolíticos combinados (cociente de riesgos [CR] 7,79; intervalo de confianza [IC] del 95%: 1,11 a 54,80). Dos ensayos informaron la interrupción del tratamiento debido a efectos secundarios graves (los resultados no se combinaron debido a heterogeneidad estadística alta, I² = 83%); un ensayo informó un aumento en los efectos secundarios graves en el grupo que recibió ritodrina intravenosa sola (CR 7,79; IC del 95%: 1,11 a 54,80; 41 embarazadas); en el otro ensayo, no hubo diferencias claras entre los grupos (CR 0,23; IC del 95%: 0,03 a 1,97; 107 embarazadas). No se informaron otros resultados primarios.
Un ensayo evaluó ritodrina intravenosa más supositorios de indometacina versus ritodrina intravenosa sola. No hubo diferencias significativas entre los grupos para la mortalidad perinatal o la morbilidad neonatal grave. No se informaron resultados de otros resultados primarios.
No hubo diferencias significativas entre los grupos que recibieron ritodrina intravenosa más progesterona vaginal en comparación con ritodrina intravenosa sola en la mayoría de los resultados informados, aunque el período de latencia (tiempo desde el reclutamiento hasta el parto) aumentó en el grupo que recibió la combinación de tocolíticos.
Para otras combinaciones de agentes tocolíticos, los resultados primarios se informaron pocas veces y en los resultados secundarios no hubo diferencias entre los grupos.
Conclusiones de los autores
No está claro si una combinación de fármacos tocolíticos para el trabajo de parto prematuro es más ventajosa para las embarazadas y los recién nacidos, debido a la falta de ensayos grandes bien diseñados que incluyan los resultados de interés. No hay ensayos de regímenes de combinación que utilicen los agentes tocolíticos usados con gran frecuencia como los bloqueantes de los canales de calcio (nifedipino) y los antagonistas de los receptores de oxitocina (atosiban). Se necesitan ensayos adicionales antes de establecer conclusiones específicas sobre el uso del tratamiento con tocolíticos combinados para el trabajo de parto prematuro.
PICO
Resumen en términos sencillos
Combinación de fármacos tocolíticos para la inhibición del trabajo de parto prematuro
El parto prematuro (parto antes de las 37 semanas) es la causa más importante por sí sola de muertes y enfermedad en los recién nacidos y la causa principal de complicaciones en las embarazadas. Los agentes tocolíticos incluyen una variedad amplia de fármacos que pueden desacelerar o detener las contracciones del trabajo de parto para prolongar el embarazo y mejorar potencialmente los resultados de salud del recién nacido. El uso de una combinación de dos o más fármacos tocolíticos puede prolongar la duración del embarazo en comparación con administrar un fármaco tocolítico único o ninguna intervención, sin afectar negativamente a la madre o al recién nacido ni empeorar los efectos secundarios del fármaco.
Esta revisión examinó los efectos de cualquier combinación de fármacos tocolíticos para el tratamiento del trabajo de parto prematuro en comparación con otro tratamiento, ningún tratamiento o placebo. Los resultados de la revisión se basan en los datos de nueve ensayos controlados aleatorios que examinaron siete comparaciones diferentes de fármacos.
Tres ensayos examinaron el fármaco betamimético ritodrina más magnesio en comparación con ritodrina sola. Los ensayos informaron los efectos secundarios adversos, pero hubo inconsistencias entre los ensayos sobre qué tratamiento provocó menos efectos secundarios graves. Otros resultados no se informaron o fueron claramente diferentes entre los grupos de tratamiento.
Un ensayo analizó ritodrina más indometacina versus ritodrina sola. No hubo diferencias claras entre los grupos en cuanto a los recién nacidos gravemente enfermos. Los hallazgos para otros resultados no fueron claramente diferentes. No hubo diferencias claras entre los grupos que recibieron ritodrina más progesterona en comparación con ritodrina sola en la mayoría de los resultados informados, aunque el tiempo entre la administración de los fármacos y el parto aumentó en el grupo que recibió la combinación de tocolíticos. Para otras combinaciones de agentes tocolíticos, los resultados no demostraron diferencias entre los grupos. No hubo ensayos de regímenes de combinación que utilizaran agentes tocolíticos usados con gran frecuencia como los bloqueantes de los canales de calcio (nifedipino) y los antagonistas de los receptores de oxitocina (atosiban).
Debido a que las pruebas no son suficientes, no está claro si los regímenes tocolíticos de combinación son más o menos eficaces que utilizar un fármaco tocolítico único, o si tienen más efectos adversos. Algunos de los fármacos tocolíticos utilizados con gran frecuencia no se han examinado en ensayos como parte de regímenes de combinación, por lo que se necesitan estudios de investigación adicionales.
Authors' conclusions
Background
Description of the condition
Preterm birth is defined as birth before 37 completed weeks of gestation (Bryce 2005). It occurs in 11.1% of births globally, affecting an estimated 14.9 million babies every year (Blencowe 2012; Born Too Soon Report 2012). In an analysis of 65 countries with reliable trend data on preterm births (1990 to 2010), 62 countries had stable or rising preterm birth rates (Blencowe 2012). This has been attributed at least in part to rising rates of provider‐initiated preterm birth and improved registration of extremely preterm births (Blencowe 2012; Davidoff 2006). It is generally accepted that approximately 30% to 35% of preterm births are provider‐initiated (by induction or caesarean section) for maternal and/or fetal indications, while 65% to 70% are spontaneous preterm births. Spontaneous preterm births includes those due to spontaneous preterm labour (40% to 45%) and those following preterm rupture of membranes (25% to 30%) (Goldenberg 2008). The precise cause of spontaneous preterm birth is often unknown, however several maternal factors are known to increase risk, including maternal age (adolescence and advanced age), maternal history of preterm birth, race, multiple pregnancy, short inter‐pregnancy interval, infections, medical conditions, poor nutrition, lifestyle factors, psychological factors and genetic predisposition (Goldenberg 2008; Plunkett 2008).
Preterm birth is the single largest cause of newborn mortality and is estimated to account for 29% of deaths in the first four weeks of life (Lawn 2009). Preterm newborns have higher rates of a range of neonatal morbidities, including respiratory (respiratory distress syndrome, persistent pulmonary hypertension) infectious (pneumonia, meningitis, sepsis, necrotizing enterocolitis), central nervous system (intraventricular haemorrhage, seizures) and metabolic (hypoglycaemia, jaundice and hyperbilirubinaemia) morbidities (Teune 2011). Administering antenatal corticosteroids to women at risk of preterm birth has been shown to reduce the overall risk of neonatal death, respiratory distress syndrome, cerebroventricular haemorrhage, necrotising enterocolitis, the need for respiratory support or intensive care admissions and systemic infections in the first 48 hours of life (Roberts 2006).
In the post‐neonatal period, preterm infants are at continued risk of mortality and morbidity (Katz 2013; Teune 2011). In the longer term, they experience more illnesses, hospital admissions and educational and behavioral problems in childhood and early adulthood, (Boyle 2011; Ekeus 2010; Talge 2010; van Baar 2009) as well as higher rates of visual and hearing impairments, cerebral palsy and neuro‐developmental and behavioural impairments (Hagberg 2001; Marlow 2005; O'Connor 2007).
Description of the intervention
Tocolytic therapy
Tocolytic agents include a wide range of drugs that can slow or suppress uterine contractions. Tocolytics are considered advantageous in spontaneous preterm labour to (a) allow time for the fetus to mature, potentially avoiding the deleterious effects of prematurity; (b) allow time for antenatal corticosteroids (ACS) to be administered and have a clinical effect; and (c) allow time for intrauterine transfer to a higher‐care centre, where neonatal intensive care facilities (or other services) are available (Anotayanonth 2004).
The ideal tocolytic agent should be effective, easy to administer, without significant maternal, fetal or neonatal side effects and permit time for antenatal corticosteroids to be administered and take effect (Crowther 2002). A variety of tocolytic treatments have been used to inhibit uterine activity in women in spontaneous preterm labour, including beta‐adrenergic receptor agonists (betamimetics), prostaglandin inhibitors, calcium channel blockers, oxytocin receptor antagonists and magnesium sulphate (Anotayanonth 2004; Crowther 2002; Duckitt 2002; Flenady 2014; Flenady 2014a; Gaunekar 2004; King 2005). However, there is considerable global variation in types, doses and regimens of tocolytic agents used to manage preterm labour.
How the intervention might work
Uterine contractility is stimulated by increases in intracellular calcium ion concentrations in myometrial cells (Gáspár 2013). Intracellular calcium ion concentration is mediated by both voltage‐gated calcium channels (for influx of calcium ions) and release of calcium ions from sarcoplasmic reticulum. Calcium ions bind to calmodulin and activate myosin light chain kinase (MLCK) in myometrial cells, leading to the phosphorylation of myosin light chains, initiating cross‐bridging in contractile proteins and ultimately myometrial contraction (Gáspár 2013; Noble 2009; Wray 2003).
Tocolytic agents inhibit uterine contractions through one of two pathways: Firstly, by affecting the contractile proteins in the myometrium (by blocking myosin phosphorylation through intracellular messengers). Betamimetics, nitric oxide donors, magnesium sulphate and calcium channel blockers affect contractile proteins. Betamimetics bind to beta‐adrenergic receptors (increasing intracellular cyclic adenosine monophosphate, that inhibits myosin light chain kinase) preventing myosin phosphorylation. Nitric oxide donors produce a similar response through cyclic guanosine monophosphate. Conversely, magnesium sulphate and calcium channel blockers prevent intracellular calcium ion influx, also reducing myosin light chain kinase activity (Caritis 2005; Gáspár 2013).
The second pathway to preventing uterine contractility is through inhibiting synthesis or blocking the activity of myometrial stimulants (such as oxytocin and prostaglandins). By binding oxytocin receptors, oxytocin receptor antagonists (such as atosiban) prevent the oxytocin‐mediated intracellular increase of inositol triphosphate, which normally increases intracellular calcium levels. Comparatively, cyclo‐oxygenase (COX) enzymes that catalyse prostaglandin production can be inhibited by general (COX) and selective (COX‐2) enzyme inhibitors (Caritis 2005; Gáspár 2013).
A combination of two or more tocolytic agents could be used to reduce uterine contractions by targeting different pathways in myometrial contraction, potentially improving the overall tocolytic effect (either additively or synergistically). Furthermore, combination therapy could permit reductions in dosage and/or frequency of individual tocolytic agents, potentially decreasing the rate of adverse effects while maintaining efficacy.
Why it is important to do this review
There is evidence that some tocolytic agents decrease the number of women in preterm labour giving birth within 48 hours when compared with placebo. This can increase the likelihood of receiving a full course of antenatal corticosteroids, as well as increase the time for antenatal corticosteroids to have a clinical effect. However, due to the lack of systematic reviews examining maternal and newborn morbidity and mortality outcomes following combination tocolytic use, inhibiting preterm labour with more than one tocolytic agent is currently based on an incomplete understanding of risks and benefits. Furthermore, combinations of tocolytic agents have the potential for greater efficacy compared to single agents, as well as the potential for reduced dosage and/or frequency of administration, that could reduce the rate of adverse effects.
Objectives
To assess the effects on maternal, fetal and neonatal outcomes of any combination of tocolytic drugs for the treatment of preterm labour compared with any other treatment, no treatment or placebo.
Methods
Criteria for considering studies for this review
Types of studies
Any adequate published, unpublished or ongoing randomised controlled trial that compares a combination of tocolytic agents, administered by any route or any dose, for inhibiting preterm labour versus any other treatment (including other combinations of tocolytics, or single tocolytic), no intervention or placebo. We excluded quasi‐randomised controlled trials.
Types of participants
Pregnant women assessed as being in spontaneous preterm labour (see definition below) and considered suitable for tocolytic agents.
Types of interventions
The following groups of comparisons were assessed for inclusion.
-
Combination of tocolytic drugs versus any other combination of tocolytic agents.
-
Combination of tocolytic drugs versus any other tocolytic agent alone.
-
Combination of tocolytic drugs versus any other intervention.
-
Combination of tocolytic drugs versus no intervention.
-
Combination of tocolytic drugs versus placebo.
Types of outcome measures
Clinically relevant outcomes for trials of tocolysis for inhibiting preterm labour have been prespecified following consultation with the editors and authors of the individual reviews.
Consensus was reached on a set of six ‘core’ outcomes, which are highlighted below. These will be included in all tocolysis reviews. In addition to these core outcomes, individual teams may include other outcomes as necessary.
Primary outcomes
Seven primary outcomes were chosen as being most representative of the clinically important measures of ineffectiveness and complications.
-
Serious maternal outcomes (see definition below).
-
Short‐term and long‐term serious infant outcome (see definition below).
-
Birth before 48 hours of trial entry.
-
Preterm neonate delivered without full course of antenatal steroids (see definition below) completed at least 24 hours before birth.
-
Perinatal death after trial entry (see definition below).
-
Birth before 34 completed weeks.
-
Adverse drug reactions (including discontinuation of therapy because of adverse effects).
Definitions
Preterm labour
The presence of regular uterine contractions on a preterm pregnancy (with intact or ruptured membranes) with or without cervical dilatation.
Serious maternal outcomes
Death, cardiac arrest, respiratory arrest, admission to intensive care unit.
Short‐term and long‐term serious infant outcome
Determined by the presence of any of the following: death; chronic lung disease (use of supplemental oxygen therapy at 36 weeks' postmenstrual age or at 28 days of life or later); grade three or four intraventricular haemorrhage or periventricular leukomalacia; major sensorineural disability at two years of age defined as any one or more of the following: severe or profound vision impairment, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy or developmental delay/intellectual impairment (defined as developmental quotient or intelligence quotient less than two standard deviations below the mean).
Perinatal death after trial entry
Fetal death or neonatal death up to 28 days of life.
Full course of corticosteroids
Betamethasone (24 mg) intramuscularly (IM) divided in two to four doses, given in 24 hours, 20 to 24 mg of dexamethasone IM divided in four to six doses given in 24 hours, or 2 g of hydrocortisone intravenously divided in four doses given in 24 hours.
Secondary outcomes
Maternal
-
Need for additional tocolytics.
-
Recurrence of labour.
-
Caesarean section birth.
-
Antepartum haemorrhage.
-
Postpartum haemorrhage.
-
Length of hospital stay.
-
Breastfeeding.
-
Satisfaction with treatment.
-
Quality of life at 12 to 24 months after the birth (measured by validated instruments).
-
Psychological aspects of mother and family.
-
Maternal oedema (non‐prespecified outcome).
Infant/child
-
Preterm neonate delivered without full course of antenatal steroids (see definition below) completed at least 24 hours before birth.
-
Birth before seven days of trial entry.
-
Birth before 28 completed weeks.
-
Birth before 37 completed weeks.
-
Pregnancy prolongation (latency: interval between randomisation and birth).
-
Gestational age at birth.
-
Birthweight.
-
Apgar score less than seven at five minutes.
-
Respiratory distress syndrome.
-
Use of mechanical ventilation.
-
Duration of mechanical ventilation.
-
Persistent pulmonary hypertension of the neonate.
-
Intraventricular haemorrhage.
-
Intraventricular haemorrhage ‐ grade three or four.
-
Periventricular leukomalacia.
-
Chronic lung disease.
-
Necrotising enterocolitis.
-
Retinopathy of prematurity.
-
Neonatal jaundice.
-
Neonatal sepsis.
-
Fetal death.
-
Neonatal death.
-
Infant death.
-
Arterial pH < 7.1 (non pre‐specified).
Health service use
-
Admission to neonatal intensive care unit.
-
Neonatal length of hospital stay.
-
Treatment associated costs.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 January 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE;
-
weekly searches of Embase;
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched reference lists of retrieved studies.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identify as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, review authors (J Nardin (JN), H West (HW), T Dowswell (TD)) extracted the data using the agreed form. We resolved discrepancies through discussion. We entered data into Review Manager software (RevMan 2012) and checked them for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (HW, TD) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect the results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or was supplied by the trial authors, we re‐included missing data in the analyses which we undertook. We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting bias (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
-
low risk of other bias;
-
high risk of other bias;
-
unclear whether there is risk of other bias.
(7) Overall risk of bias
We have made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we consider it likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes are measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome using different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion in this review. If we identify cluster‐randomised trials for inclusion in a subsequent update of this review, we will include them in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Handbook (Higgins 2011) using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation on the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit.
We planned to use methods used for the analysis of cluster‐randomised trials to analyse data from trials which included more than 10% multiple pregnancies provided information was available (in the trial reports or from authors) on outcomes for twins or higher multiples. In this version of the review no such trials were included; trials either explicitly excluded women with multiple pregnancies or there was insufficient information to carry out planned analysis.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial is the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2012). We use fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials examined the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we planned to use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. If the average treatment effect was not clinically meaningful, we did not combine trials.
Subgroup analysis and investigation of heterogeneity
If we had identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses.
We planned to carry out the following subgroup analyses.
-
Gestational age (less than 28 weeks of gestation versus 28 weeks and above).
-
Intact versus ruptured membranes.
-
Single versus multiple pregnancy.
We planned to restrict subgroup analyses to the primary outcomes.
However, only one trial reported data that could be analysed based on the status of the membranes and in view of heterogeneity, we have presented results for these subgroups separately (Gamissans 1982). Planned subgroup analyses will be carried out in future updates of this review if more data become available.
We planned to assess differences between subgroups by interaction tests available in RevMan 2012. We would have reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analysis to explore the effect of trial quality. This would involve analysis based on the rating of selection bias and attrition bias. Studies of poor quality (those rated as 'unclear' or 'high risk of bias') would be excluded in the analysis in order to assess for any substantive difference to the overall result. However, all of the included trials were rated as unclear or high risk of bias for selection and/or attrition bias, therefore, no sensitivity analyses were performed
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
Our search strategy identified 31 citations corresponding to 27 studies that were assessed for inclusion. While 11 studies (14 citations) met our inclusion criteria, two of these did not report any outcome data relevant to the review (Castillo 1988; Schauf 2005). Further information about both of these trials is set out in the Characteristics of included studies tables, but these studies do not contribute any data to the analyses, and are not otherwise mentioned in the results below. Sixteen studies (17 citations) were excluded (see: Figure 1).
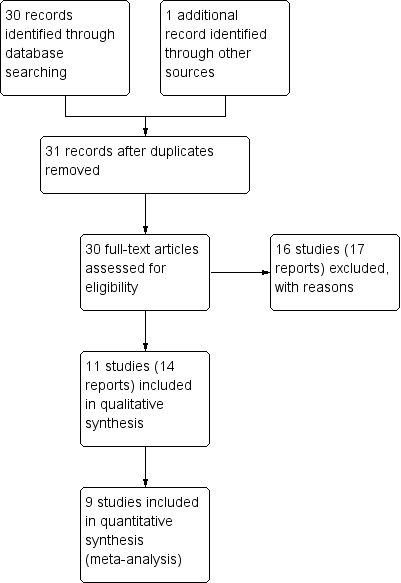
Study flow diagram.
Results are based on nine included trials that contributed data. Some of these studies reported on very few of our pre‐specified primary and secondary outcomes, and for most outcomes only one study contributed data so, overall, very few data were pooled in meta‐analysis.
Included studies
The included studies were categorised according to the type of drugs being compared.
-
Intravenous (IV) ritodrine plus oral or IV magnesium (gluconate or sulphate) versus IV ritodrine alone: three trials, including a total of 231 women (Ally 1992; Ferguson 1984; Hatjis 1987).
-
IV ritodrine plus indomethacin suppositories versus IV ritodrine alone: one trial, including 208 women (Gamissans 1982).
-
IV ritodrine plus vaginal progesterone versus IV ritodrine alone: one trial with 83 women (Arikan 2011).
-
IV hexoprenaline sulphate plus IV magnesium hydrochloride versus IV hexoprenaline sulphate alone: one trial, including a total of 24 women (Francioli 1988).
-
IV fenoterol plus oral naproxen versus IV fenoterol alone: one trial, including 72 women (Rios‐Anez 2001).
-
Oral pentoxifylline plus IV magnesium sulphate plus IV fenoterol versus IV magnesium sulphate plus IV fenoterol: one trial with 125 women (Lauterbach 2012).
-
IV terbutaline plus oral metoprolol versus IV terbutaline alone: one trial with data for 17 women (Ross 1983).
Settings
The studies were reported between 1982 and 2012 in six countries (USA (Ferguson 1984; Hatjis 1987; Ross 1983); France (Ally 1992; Francioli 1988); Turkey (Arikan 2011); Spain (Gamissans 1982); Poland (Lauterbach 2012); and Venezuela (Rios‐Anez 2001).
Participants
All of the studies recruited women in preterm labour. All studies recruited women with a gestational age less than 37 weeks, but the criteria relating to gestation varied particularly with regard to the lower gestational age (22 to 35 weeks (Ally 1992); 24 to 34 weeks (Arikan 2011); up to 36 weeks (Ferguson 1984); 28 to 36 weeks (Francioli 1988); 20 to 36 weeks (Gamissans 1982); 20 to 35 weeks (Hatjis 1987); 23 to 34 weeks (Lauterbach 2012); 31 to 36 weeks (Rios‐Anez 2001) and 26 to 36 weeks (Ross 1983).
In one trial it was specifically stated that women with both intact and ruptured amniotic membranes were included (Gamissans 1982); however, four studies specifically mentioned excluding women with preterm prelabour rupture of membranes (Ally 1992; Arikan 2011; Francioli 1988; Ross 1983) and in the remaining studies, it was not clear whether inclusion was confined to women with intact membranes. Five studies specifically mentioned exclusion of women with multiple pregnancies (Arikan 2011; Francioli 1988; Gamissans 1982; Lauterbach 2012; Rios‐Anez 2001). Women with serious complications or disease (including infection and renal problems) were specifically excluded by Ally 1992; Arikan 2011; Gamissans 1982; Lauterbach 2012; and Ross 1983. Other exclusion criteria included previous uterine or cervical surgery, fetal anomaly and deteriorating condition of the mother or fetus.
Interventions
All of the trials included therapy through intravenous infusion in at least one of the groups, and in most trials there was maintenance therapy with oral drugs following successful tocolysis. Dosage and duration of initial tocolysis varied, and several trials mentioned adjusting infusion rates depending on side effects and the effect of tocolysis on uterine contractions. Co‐interventions were not always well described. Use of corticosteroids (betamethasone or dexamethasone) for fetal lung maturation were mentioned by Arikan 2011; Ferguson 1984; Gamissans 1982; Lauterbach 2012; and Ross 1983. In the remaining studies it was not clear whether or not women received corticosteroids during the first 24 hours of tocolytic therapy.
Outcomes
All of the studies included some measure of pregnancy prolongation, although this was not measured consistently in different studies. Other pre‐specified outcomes were not consistently reported across studies. Side effects were reported in a minority of trials, and fewer than half of the included studies reported infant mortality and serious morbidity.
Excluded studies
We excluded 16 studies. The main reason for exclusion was that the method of allocation was not random; this applied to 11 studies (Caballero 1979; How 2006; Ieda 1991; Illia 1993; Katz 1983; Ogburn 1985; Reynolds 1978; Richter 1975; Richter 1979; Spearing 1979; Wischnik 1989). In three studies the intervention was not relevant to the review; either the women did not receive a combination of tocolytic drugs (Bedoya 1972; Dunlop 1986; Morales 2013 ) or women in both arms received the same drugs in different sequences (Kawagoe 2011). Finally, in the study by Herzog 1999, women were randomised to receive the same combination of tocolytic drugs but the way drugs were administered varied.
Risk of bias in included studies
Assessment of the methodological quality of the included studies was based on risk of bias in relation to selection bias (method of randomisation and allocation concealment), performance bias, detection bias, attrition bias (loss of participants from the analyses) and reporting bias. A summary of 'Risk of bias' assessments for each study, and for included trials overall, are set out in Figure 2 and Figure 3.
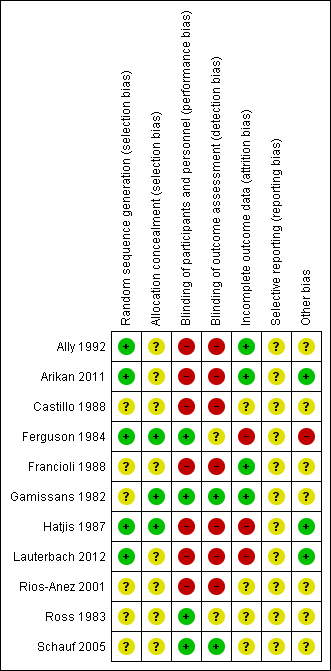
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
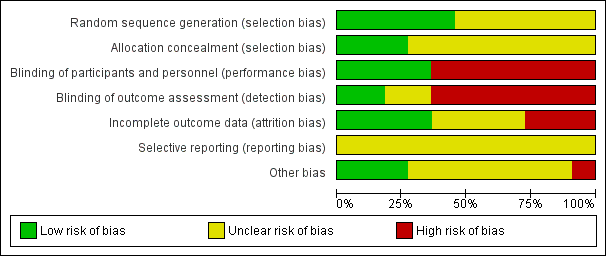
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Generation of the randomisation sequence
Five studies described methods for generating the randomisation sequence, which we judged were at low risk of bias; Ally 1992; Ferguson 1984; Hatjis 1987; and Lauterbach 2012 described using random number tables or pre‐prepared random lists, while Arikan 2011 reported using a computerised random‐number generator. In the remaining studies, the method for determining the randomisation sequence was either not clear, or not mentioned at all (Castillo 1988; Francioli 1988; Gamissans 1982; Rios‐Anez 2001; Ross 1983; Schauf 2005).
Allocation concealment
In eight of the studies, the method for concealing group allocation at the point of randomisation was not clear (Ally 1992; Arikan 2011; Castillo 1988; Francioli 1988; Lauterbach 2012; Rios‐Anez 2001; Ross 1983; Schauf 2005). Gamissans 1982 described a double‐blind design and Ferguson 1984 and Hatjis 1987 concealed allocations in sealed envelopes.
Blinding
Four of the studies were placebo‐controlled and were assessed as being at low risk of bias for blinding (Ferguson 1984; Gamissans 1982; Ross 1983; Schauf 2005). In two of these studies, outcome assessors were also blinded (Gamissans 1982; Schauf 2005), while this was not mentioned in Ferguson 1984 or Ross 1983. The remaining studies either did not attempt, or did not mention blinding. As the regimens in different arms of the trials were different, it is likely that staff providing care and evaluating outcomes would be aware of treatment allocation, and these studies have been assessed as high risk of bias due to lack of blinding (Ally 1992; Arikan 2011; Castillo 1988; Francioli 1988; Hatjis 1987; Lauterbach 2012; Rios‐Anez 2001).
Incomplete outcome data
There was no serious sample attrition in the trials by Ally 1992, Arikan 2011, Francioli 1988 and Gamissans 1982, and loss to follow‐up was not clear for Castillo 1988, Rios‐Anez 2001, Ross 1983 and Schauf 2005. In three trials, sample attrition was a serious source of bias. In these studies a significant proportion of the women were excluded from the study after randomisation or were lost to follow‐up for reasons that may have related to treatment, and this may have affected outcomes (e.g. delivered early or withdrew due to side effects) (Ferguson 1984; Hatjis 1987; Lauterbach 2012).
Selective reporting
Although the assumption that if something was not reported it was not done could be true for most of the trials that are currently published, the inadequate reporting for most of the trials included in this review could be also related to the period during which the majority of them were published. The lack of information for those published during the 1980s made methodological quality evaluation difficult (Castillo 1988; Ferguson 1984, Francioli 1988; Gamissans 1982; Hatjis 1987; Ross 1983). In addition, two studies were assessed using translated notes (Ally 1992; Francioli 1988) and, again, this made assessment of selective reporting more difficult.
Other potential sources of bias
Most studies described the characteristics of women in each of the study groups, and there was no clear evidence of baseline imbalance. The gestational age was slightly different between groups in the Ally 1992 trial, and in the Ferguson 1984 trial, recruitment was stopped early due to the high incidence of side effects, and this may have introduced bias.
Effects of interventions
This systematic review included 11 studies with a total of 895 women. Data from nine randomised controlled trials with a total of 776 women reported outcome data relevant to this review.
The sample sizes of all the trials that fulfilled the inclusion criteria were small, most comparisons used data from single studies only and none of them had sufficient clinical power to identify differences between groups for many outcomes. The biggest trial included 208 women and was further subgrouped by state of membranes to deal with clinical heterogeneity (Gamissans 1982).
It was not feasible to compare all combinations together versus single drugs, as the mechanisms of action among different drug families are not the same and combining data in meta‐analysis might have led to heterogeneous and spurious results. Therefore, we examined different combinations of drugs separately. For most of the comparisons and outcomes analysed, only one or few trials reported findings.
Comparison 1: IV ritodrine plus IV or oral magnesium (gluconate or sulphate) versus IV ritodrine alone
Three trials compared IV ritodrine plus IV or oral magnesium (gluconate or sulphate) versus IV ritodrine alone.
Primary outcomes
Only one trial, including 41 women, evaluated differences in 'adverse drug reactions', showing a higher incidence in the combination group (risk ratio (RR) 7.79, 95% confidence interval (CI) 1.11 to 54.80) (Ferguson 1984) (Analysis 1.1).
'Discontinuation of therapy' was reported in two trials (Ally 1992; Ferguson 1984); we have not pooled results due to the high heterogeneity observed between the included trials (I² = 83%); one trial reported increased side effects in the group receiving ritodrine alone (RR 7.79, 95% CI 1.11 to 54.80, 41 women); in the other trial there were no clear differences between groups (RR 0.23, 95% CI 0.03 to 1.97, 107 women), (Analysis 1.2).
None of the studies included in this comparison reported on our other primary outcomes.
Secondary outcomes
Only one trial (Hatjis 1987), including 64 women, reported the outcome 'birth before seven days after trial entry'; the difference between groups did not reach statistical significance (RR 0.62, 95% CI 0.38 to 1.01), (Analysis 1.3). One trial of 107 women (Ally 1992) assessed birthweight, showing no clear difference between treatment groups (mean difference (MD) 77 g, 95% CI ‐87.86 to 241.86) (Analysis 1.4).
Other secondary outcomes were not reported.
Comparison 2: IV ritodrine plus indomethacin suppositories versus IV ritodrine alone
For the second comparison, only one trial fulfilled the inclusion criteria (Gamissans 1982). The trial involved 208 women, and the results were reported separately according to the state of the membranes (intact versus ruptured), to handle an evident clinical cause of heterogeneity. Due to significant heterogeneity for the results of some outcomes, we are presenting the results separately for women with intact membranes (153 women) or ruptured membranes (55 women) reflecting the approach used in the trial report.
Primary outcomes
There was no clear difference between groups for perinatal mortality in women with intact membranes (RR 0.46, 95% CI 0.17 to 1.26), or ruptured membranes (RR 0.96, 95% CI 0.15 to 6.37) (Analysis 2.1).
Data on respiratory distress syndrome (RDS) were reported for the women with intact amniotic membranes; there were very few events and no strong evidence of difference between groups (RR 0.51, 95% CI 0.05 to 5.47) (Analysis 2.2).
For maternal adverse drug reactions there was no clear difference between groups receiving the combined therapy versus ritodrine alone in either the intact or ruptured membranes groups (RR 1.11, 95% CI 0.67 to 1.87; RR 0.77, 95% CI 0.36 to 1.66, respectively) (Analysis 2.3).
Other primary outcomes were not reported.
Secondary outcomes
Results showed a statistically significant difference in the number of women giving birth before 37 weeks of gestation and recurrence of labour favouring the combination group in those women with intact membranes (RR 0.70, 95% CI 0.50 to 0.97; RR 0.59, 95% CI 0.36 to 0.99, respectively) (Analysis 2.4; Analysis 2.5).
For women with ruptured membranes the evidence did not demonstrate a difference between groups for birth before 37 weeks (RR 0.92, 95% CI 0.70 to 1.22) (Analysis 2.4), and frequency of labour recurrence was very similar in the two treatment groups (RR 1.02, 95% CI 0.68 to 1.52) (Analysis 2.5).
No differences were found for infant Apgar score less than seven at five minutes irrespective of membrane status (intact: RR 1.01, 95% CI 0.31 to 3.36; ruptured: RR 1.29, 95% CI 0.32 to 5.22) (Analysis 2.6).
Other outcomes were not reported.
Comparison 3: IV ritodrine plus vaginal progesterone versus IV ritodrine alone
One study with data for 83 women is included in this comparison (Arikan 2011). The study did not have sufficient statistical power to identify differences between groups for most of the outcomes reported.
Primary outcomes
There was only one infant death, no significant differences between groups, and very wide 95% CIs around the effect estimate for this outcome (RR 0.31, 95% CI 0.01 to 7.41) (Analysis 3.1).
Event rates were low for serious morbidity in newborns, and there were no significant differences between groups for infant sepsis (RR 0.93, 95% CI 0.14 to 6.30) (Analysis 3.2), RDS (RR 0.93, 95% CI 0.06 to 14.38) (Analysis 3.3), or for need for mechanical ventilation (RR 1.86, 95% CI 0.18 to 19.73) (Analysis 3.4). There were no estimable data for infant necrotising enterocolitis or intraventricular haemorrhage.
Secondary outcomes
There were low event rates for poor infant outcomes at birth and no apparent differences between groups for low infant Apgar score at five minutes (RR 0.23, 95% CI 0.03 to 1.99) (Analysis 3.7), arterial blood pH less than 7.1 (non pre‐specified outcome) (RR 0.47, 95% CI 0.09 to 2.40) (Analysis 3.8), infant admission to neonatal intensive care unit (NICU) (RR 0.93, 95% CI 0.20 to 4.34) (Analysis 3.9), or mean length of NICU stay (days) (MD ‐7.90, 95% CI ‐48.95 to 33.15) (the denominator for this last non‐prespecified outcome was not clear, and in the analysis we have used the number of babies admitted to NICU rather than the whole sample) (Analysis 3.10).
Infants whose mothers were randomised to receive the combination of tocolytics were less likely to have a low birthweight (less than 2500 g) (RR 0.41, 95% CI 0.20 to 0.84) (Analysis 3.12), and a higher mean birthweight (MD 398.0 g, 95% CI 86.37 to 709.63) (Analysis 3.11).
The period between randomisation and birth (latency) was increased for women in the combined group by a mean of almost 11 days (MD 10.90, 95% CI 3.56 to 18.24) (Analysis 3.14). This finding was reflected in fewer women giving birth before 37 weeks in the combined tocolytic group (RR 0.66, 95% CI 0.45 to 0.97) (Analysis 3.13), and an increase in the mean gestational age at delivery (MD 1.20 weeks, 95% CI 0.08 to 2.32) (Analysis 3.15).
There was no significant difference between groups for caesarean section (RR 0.75, 95% CI 0.51 to 1.10) (Analysis 3.16). Other outcomes were not reported.
Comparison 4: IV hexoprenaline sulphate plus IV magnesium hydrochloride versus IV hexoprenaline sulphate alone
Only 24 women were recruited in the only trial included in this comparison (Francioli 1988), with no difference observed in the only outcome analysed by the authors: birth before seven days of trial entry (RR 0.85, 95% CI 0.14 to 5.06) (Analysis 4.1).
Comparison 5: IV fenoterol plus oral naproxen versus IV fenoterol alone
For the comparison IV fenoterol plus oral naproxen versus IV fenoterol alone, only one trial was included (Rios‐Anez 2001). In this trial only 72 women were recruited and results did not show any difference in the only outcome relevant to the review that was reported: 'birth before 37 weeks of gestation' (RR 0.50, 95% CI 0.23 to 1.09) (Analysis 5.1).
Comparison 6: Oral pentoxifylline plus IV magnesium sulphate plus IV fenoterol versus IV magnesium sulphate plus IV fenoterol
One trial examined this comparison (Lauterbach 2012). One‐hundred and forty‐eight women were randomised; however, there was considerable loss to follow‐up following randomisation.
Data for perinatal death and delivery within seven days were available for 125 women. There was no significant difference between groups for perinatal death (RR 0.19, 95% CI 0.02 to 1.55) (Analysis 6.1), or delivery within seven days (RR 0.77, 95% CI 0.29 to 2.03) (Analysis 6.2).
Other outcomes were reported in this trial but analyses excluded women who either delivered within the first week of tocolysis, or withdrew from the trial, or changed the place of birth, or were intolerant to pentoxifylline. Of the 148 women randomised, data for pregnancy outcomes and serious infant morbidity were reported for only 96 of the original sample: 39% of the intervention group and 31% of the control group were not included in the analysis of these outcomes. In view of the high risk of bias for such outcomes, we have not, therefore, reported them in this review.
Comparison 7: IV terbutaline plus oral metoprolol versus IV terbutaline alone
One trial with data for 17 women is included in this comparison (Ross 1983). Only two outcomes relevant to the review were reported. There was no clear difference between groups for mean time to delivery and the standard deviations for this outcome suggest considerable variation within groups (MD ‐6.80 days, 95% CI ‐14.92 to 1.32) (Analysis 7.2). No women in either arm of this trial were reported to have oedema (non pre‐specified outcome).
Discussion
This systematic review included 11 studies with a total of 895 women. Data from nine randomised controlled trials with a total of 776 women reported outcome data relevant to this review. Overall, seven different combinations of tocolytic drugs were examined with only one or few small trials per comparison, so very few data were pooled in meta‐analysis. While most of the included studies reported some measure of pregnancy prolongation, other outcomes were infrequently reported.
Summary of main results
Three trials examined intravenous (IV) ritodrine plus oral or IV magnesium (sulphate or gluconate) compared with IV ritodrine alone. One study with 41 women reported more adverse drug reactions in the group receiving the combination of tocolytic drugs. Two trials reported discontinuation of therapy due to severe side effects, with one trial reporting increased side effects in the group receiving ritodrine alone, while in the other trial there was no clear difference between groups. Other outcomes were either not reported, or not statistically significant.
One trial compared IV ritodrine plus indomethacin suppositories versus IV ritodrine alone. There were no significant differences between groups for perinatal death serious neonatal morbidity. Results for other primary outcomes were not reported..
There were no significant differences between groups receiving IV ritodrine plus vaginal progesterone compared with IV ritodrine alone for most outcomes reported, although the latency period (time from randomisation to delivery) was increased in the group receiving the combination of tocolytics.
For other combinations of tocolytic agents, results did not demonstrate differences between groups.
Overall completeness and applicability of evidence
Although the aim of this systematic review was to evaluate combinations of tocolytic agents compared with any other treatment on maternal and neonatal outcomes, we pre‐specified outcomes chosen as the most representative clinical measures of effectiveness and complications. Unfortunately, some of these outcomes, particularly those to which the tocolytic treatment would be most relevant (e.g. 'birth before 48 hours of trial entry' to allow the administration of a complete course of corticosteroids), were not assessed by any of the included studies.
The samples sizes of the individual trials were generally small. Moreover, the analysis of the largest study (which included only 208 women) was stratified into two groups because of a clinical source of heterogeneity (state of membranes). This and the poor quality of reporting in the majority of the included trials made conclusions difficult, and in most cases studies were underpowered to identify differences between groups. Antenatal corticosteroid use has an important effect on neonatal outcomes, however, its use was reported in only five of the included studies. Thus review findings must to be interpreted with considerable caution.
The most serious practical limitation of this systematic review is that none of the identified studies (included and excluded) evaluated two of the most commonly used tocolytic agents in high‐resource settings, atosiban and nifedipine, as part of a combination or single tocolytic therapy. Many settings have abandoned the use of betamimetics for tocolysis due to their unfavourable side‐effect profile in comparison with other agents (Flenady 2014). These results are therefore not clinically relevant in settings where betamimetics are no longer used.
A theoretically effective combination of tocolytics would include agents that act through different mechanisms, affecting contractile proteins in the myometrial cell and also blocking the action of myometrial stimulants. The latter action is only known to be performed by cyclo‐oxygenase (COX) inhibitors (indomethacin) and oxytocin antagonists (atosiban). Therefore, future trials of combinations of tocolytic agents should, where appropriate, include at least one of these two agents, as well as considering the need to evaluate calcium channel blockers in combination regimens.
Quality of the evidence
It was difficult to assess the methodological quality of most of the trials included in the review because of poor reporting. In most of the trials, methods for generating the randomisation sequence and concealing allocation at the point of randomisation were not clear or presented high risk of bias. Only four of the trials reported that blinding had been attempted. Lack of blinding may mean findings are at high risk of bias, as care would have been provided, and many of the outcomes assessed, by staff who would have been aware of what treatment a woman had received. In three trials, sample attrition was a serious source of bias because women were excluded from the study after randomisation or were lost to follow‐up for reasons that may have related to treatment and this may have affected outcomes.
Potential biases in the review process
We are aware that the review process itself is subject to bias, and we took steps to minimise bias. At least two review authors carried out data extraction and assessed risk of bias independently; however, a different review team may not have made identical decisions.
Agreements and disagreements with other studies or reviews
As with previous Cochrane reviews of tocolytic drugs, this review showed that betamimetics are the most commonly studied tocolytic agent. However, one review has demonstrated that (when used alone) calcium channel blockers are preferred to other single tocolytic drugs, particularly betamimetics (mostly ritodrine), due to comparable efficacy and fewer side effects (Flenady 2014). Despite this, we found no trial that included calcium channel blockers in a combination tocolytic regimen. A review of the use of COX inhibitors alone (including indomethacin) for treating preterm labour could not recommend COX inhibitor use due to insufficient data (King 2005). However, in a small number of participants COX inhibitors reduced the proportion of women delivering preterm. The risks associated with magnesium sulphate alone in threatened preterm labour (Crowther 2002) should be carefully considered in tocolytic combination regimens.

Study flow diagram.
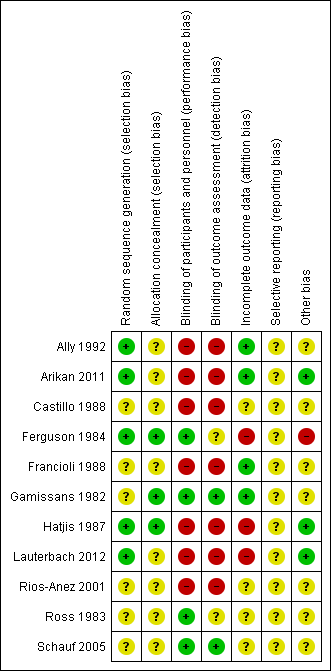
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 IV ritodrine plus oral/IV magnesium (gluconate or sulphate) versus IV ritodrine, Outcome 1 Adverse drug reaction.

Comparison 1 IV ritodrine plus oral/IV magnesium (gluconate or sulphate) versus IV ritodrine, Outcome 2 Discontinuation of therapy because of maternal side effects.

Comparison 1 IV ritodrine plus oral/IV magnesium (gluconate or sulphate) versus IV ritodrine, Outcome 3 Birth before 7 days of trial entry.

Comparison 1 IV ritodrine plus oral/IV magnesium (gluconate or sulphate) versus IV ritodrine, Outcome 4 Birthweight.
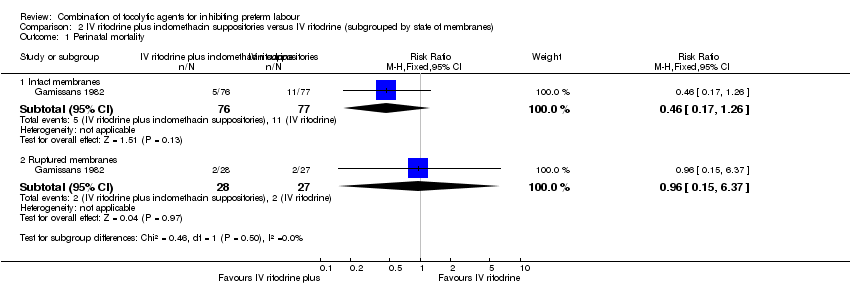
Comparison 2 IV ritodrine plus indomethacin suppositories versus IV ritodrine (subgrouped by state of membranes), Outcome 1 Perinatal mortality.

Comparison 2 IV ritodrine plus indomethacin suppositories versus IV ritodrine (subgrouped by state of membranes), Outcome 2 Respiratory distress syndrome.

Comparison 2 IV ritodrine plus indomethacin suppositories versus IV ritodrine (subgrouped by state of membranes), Outcome 3 Adverse drug reaction.
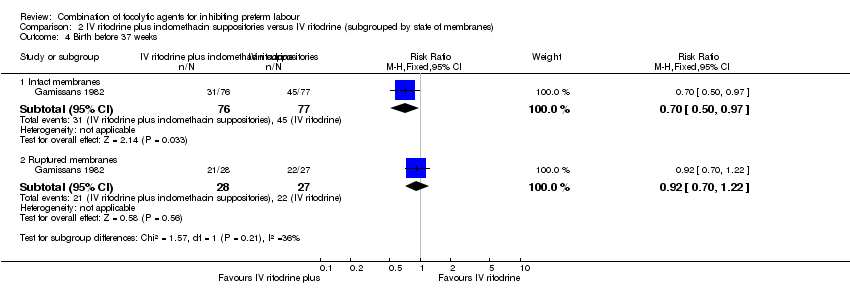
Comparison 2 IV ritodrine plus indomethacin suppositories versus IV ritodrine (subgrouped by state of membranes), Outcome 4 Birth before 37 weeks.

Comparison 2 IV ritodrine plus indomethacin suppositories versus IV ritodrine (subgrouped by state of membranes), Outcome 5 Recurrence of labour.

Comparison 2 IV ritodrine plus indomethacin suppositories versus IV ritodrine (subgrouped by state of membranes), Outcome 6 Apgar score < 7 at 5 minutes.

Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 1 Infant mortality.

Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 2 Infant sepsis.
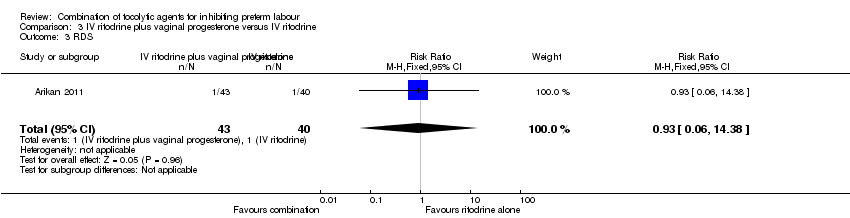
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 3 RDS.
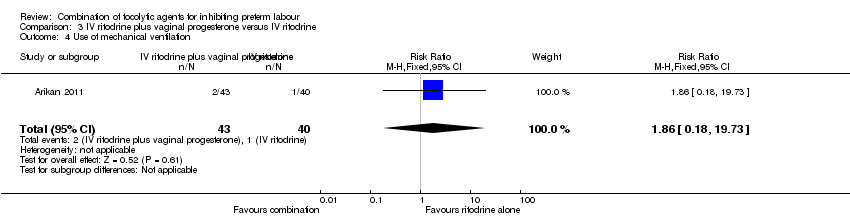
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 4 Use of mechanical ventilation.
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 5 Necrotising enterocolitis.

Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 6 Intraventricular haemorrhage.

Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 7 Apgar less than 7 at 5 minutes.

Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 8 (Non pre‐specified) Arterial pH < 7.1.

Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 9 NICU admission.

Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 10 Mean NICU stay (days).

Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 11 Mean birthweight (g).
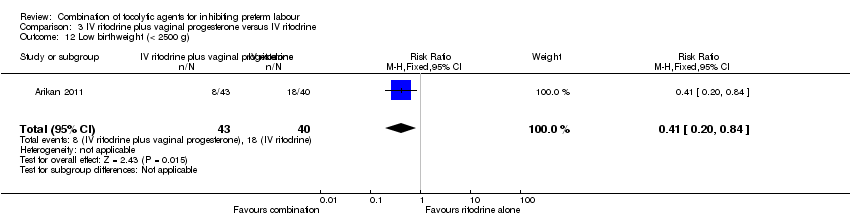
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 12 Low birthweight (< 2500 g).

Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 13 Delivery before 37 weeks.

Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 14 Latency ‐ days.

Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 15 Mean GA at delivery.

Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 16 Caesarean section.

Comparison 4 IV hexoprenaline plus IV magnesium sulphate versus IV hexoprenaline, Outcome 1 Birth before 7 days of trial entry.

Comparison 5 IV fenoterol plus oral naproxen versus IV fenoterol, Outcome 1 Birth before 37 weeks.

Comparison 6 Oral pentoxifylline plus IV magnesium sulphate plus IV fenoterol versus IV magnesium sulphate plus IV fenoterol, Outcome 1 Perinatal death.

Comparison 6 Oral pentoxifylline plus IV magnesium sulphate plus IV fenoterol versus IV magnesium sulphate plus IV fenoterol, Outcome 2 Delivery with 7 days.

Comparison 7 IV terbutaline plus oral metoprolol versus IV terbutaline, Outcome 1 (Non‐prespecified) Maternal oedema.

Comparison 7 IV terbutaline plus oral metoprolol versus IV terbutaline, Outcome 2 Mean time to delivery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse drug reaction Show forest plot | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.79 [1.11, 54.80] |
| 2 Discontinuation of therapy because of maternal side effects Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Birth before 7 days of trial entry Show forest plot | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 1.01] |
| 4 Birthweight Show forest plot | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | 77.0 [‐87.86, 241.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Intact membranes | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.17, 1.26] |
| 1.2 Ruptured membranes | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.15, 6.37] |
| 2 Respiratory distress syndrome Show forest plot | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.47] |
| 2.1 Intact membranes | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.47] |
| 3 Adverse drug reaction Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Intact membranes | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.67, 1.87] |
| 3.2 Ruptured membranes | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.36, 1.66] |
| 4 Birth before 37 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Intact membranes | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.50, 0.97] |
| 4.2 Ruptured membranes | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.70, 1.22] |
| 5 Recurrence of labour Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Intact membranes | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.36, 0.99] |
| 5.2 Ruptured membranes | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.68, 1.52] |
| 6 Apgar score < 7 at 5 minutes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Intact membranes | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.31, 3.36] |
| 6.2 Ruptured membranes | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.32, 5.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infant mortality Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.41] |
| 2 Infant sepsis Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.14, 6.30] |
| 3 RDS Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.06, 14.38] |
| 4 Use of mechanical ventilation Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.18, 19.73] |
| 5 Necrotising enterocolitis Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Intraventricular haemorrhage Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Apgar less than 7 at 5 minutes Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.03, 1.99] |
| 8 (Non pre‐specified) Arterial pH < 7.1 Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.09, 2.40] |
| 9 NICU admission Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.20, 4.34] |
| 10 Mean NICU stay (days) Show forest plot | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | ‐7.90 [‐48.95, 33.15] |
| 11 Mean birthweight (g) Show forest plot | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | 398.00 [86.37, 709.63] |
| 12 Low birthweight (< 2500 g) Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.20, 0.84] |
| 13 Delivery before 37 weeks Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.45, 0.97] |
| 14 Latency ‐ days Show forest plot | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | 10.90 [3.56, 18.24] |
| 15 Mean GA at delivery Show forest plot | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [0.08, 2.32] |
| 16 Caesarean section Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.51, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Birth before 7 days of trial entry Show forest plot | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.14, 5.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Birth before 37 weeks Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.23, 1.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal death Show forest plot | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.55] |
| 2 Delivery with 7 days Show forest plot | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.29, 2.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 (Non‐prespecified) Maternal oedema Show forest plot | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Mean time to delivery Show forest plot | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐6.80 [‐14.92, 1.32] |

