نقش مکمل زینک برای پیشگیری از پنومونی در کودکان 2 تا 59 ماهه
چکیده
پیشینه
پنومونی (pneumonia) یک علت اصلی برای موربیدیتی و مورتالیتی در کودکان زیر پنج سال است. اکثر مرگومیرها در طول دوران طفولیت و در کشورهای با سطح درآمد پائین اتفاق میافتد. گزارش شده که مکمل زینک به صورت روزانه از بروز عفونت حاد دستگاه تنفسی تحتانی (acute lower respiratory tract infection; LRTI) پیشگیری کرده و احتمال مورتالیتی کودک را کاهش میدهد. این یک نسخه بهروز از مروری است که اولین بار در سال 2010 منتشر شد.
اهداف
ارزیابی اثربخشی مکمل زینک در پیشگیری از پنومونی در کودکان دو تا 59 ماهه.
روشهای جستوجو
ما در CENTRAL؛ (شماره 21؛ اکتبر 2016)، MEDLINE (از 1966 تا اکتبر 2016)، Embase (از 1974 تا اکتبر 2016)، LILACS (از 1982 تا اکتبر 2016)، CINAHL (از 1981 تا اکتبر 2016)، Web of Science (از 1985 تا اکتبر 2016) و IMSEAR (از 1980 تا اکتبر 2016) به جستوجو پرداختیم.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) که به ارزیابی مکمل زینک برای پیشگیری از پنومونی در کودکان 2 تا 59 ماهه پرداخته بودند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم به بررسی کیفیت کارآزماییها و استخراج دادهها پرداختند.
نتایج اصلی
ما هیچ مطالعه جدیدی را برای ورود به این نسخه بهروز شده شناسایی نکردیم. شش مطالعه را شامل 5193 شرکتکننده وارد مرور کردیم.
تجزیهوتحلیلها نشان دادند که مکمل زینک بروز پنومونی را تا 13% (خطر نسبی (RR) اثر‐ثابت: 0.87؛ 95% فاصله اطمینان (CI): 0.81 تا 0.94؛ شش مطالعه، شواهد با کیفیت پائین) و شیوع پنومونی را تا 41% (RR اثرات‐تصادفی: 0.59؛ 95% CI؛ 0.35 تا 0.99؛ یک مطالعه، n = 609؛ شواهد با کیفیت پائین) کاهش میدهد. در تجزیهوتحلیل زیر‐گروه، ما دریافتیم که زینک بروز پنومونی تعریف شده بر اساس معیارهای بالینی خاص را تا 21% کاهش میدهد (یعنی تایید شده توسط معاینه قفسه سینه یا پرتونگاری قفسه سینه) (RR اثر‐ثابت: 0.79؛ 95% CI؛ 0.71 تا 0.88؛ چهار مطالعه، n = 3261)، اما هیچ تاثیری بر تعریف موردی پنومونی با ویژگی پائینتر (یعنی تنفس سریع مختص سن با یا بدون تورفتگی قسمت تحتانی قفسه سینه) نداشت (RR اثر‐ثابت: 0.95؛ 95% CI؛ 0.86 تا 1.06؛ چهار مطالعه، n = 1932).
نتیجهگیریهای نویسندگان
بین مکمل زینک در کودکان و کاهش بروز و شیوع پنومونی رابطه وجود دارد.
PICOs
خلاصه به زبان ساده
مکمل زینک برای پیشگیری از پنومونی در کودکان دو تا 59 ماهه
سوال مطالعه مروری
ما اثربخشی مکمل زینک را در پیشگیری از پنومونی (pneumonia) در کودکان دو تا 59 ماهه مورد ارزیابی قرار دادیم.
پیشینه
زینک یک عنصر حیاتی برای رشدونمو کودکان است. بین مقادیر بسیار کم زینک و خطر بالای ابتلا به عفونت، به ویژه اسهال و پنومونی رابطه وجود دارد. کودکان بیشتر مستعد ابتلا به کمبود زینک هستند، زیرا آنها کمتر قادر به جذب زینک از رژیم غذایی روزانه هستند و برخی کودکان، به ویژه در کشورهای با سطح درآمد پائین، ممکن است در دوران پیش از تولد مقادیر کافی زینک از مادرانشان دریافت نکرده باشند. گزارش شده که مکمل زینک در کودکان از بروز پنومونی پیشگیری کرده است.
تاریخ جستوجو
ما تا اکتبر 2016 در منابع علمی به جستوجو پرداختیم. این یک نسخه بهروز از مرور منتشر شده در سال 2010 است. ما هیچ مطالعه جدیدی برای این نسخه بهروز شده نیافتیم.
ویژگیهای مطالعه
شش مطالعه را که به تحقیق و بررسی درباره نقش مکمل زینک در پیشگیری از پنومونی پرداخته بودند، وارد مرور کردیم. مطالعات در بنگلادش، هند، پرو و آفریقای جنوبی به اجرا درآمده و 5193 کودک دو تا 59 ماهه را دربرمیگرفتند. کودکان حاضر در مطالعه، یا زینک یا یک درمان به ظاهر مشابه اما بدون زینک دریافت کردند. در دو مطالعه، به کودکان ویتامین A نیز داده شد.
منابع تامین مالی مطالعه
تمامی مطالعات وارد شده از حمایت مالی برخوردار شده بودند. البته سه مورد از آنها به طور واضح عنوان کرده بودند که ارگانهای تامین کننده منابع مالی هیچ نقشی در طرحریزی و نتایج به دست آمده از مطالعه نداشتند.
نتایج کلیدی
مکمل زینک به طور معناداری با کاهش بروز و شیوع پنومونی میان کودکان دو تا 59 ماهه رابطه داشت. در تجزیهوتحلیل زیر‐گروه، ما دریافتیم که تشخیص دقیقتر (معاینه از طریق رادیولوژی) کاهش بروز پنومونی را افزایش میدهد.
کیفیت شواهد
در مجموع کیفیت شواهد بر مبنای رویکرد درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE) در سطح پائین ارزیابی شد.
Authors' conclusions
Summary of findings
| Zinc supplementation compared with placebo for the prevention of pneumonia in children aged 2 months to 59 months | ||||||
| Patient or population: children aged 2 months to 59 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Zinc supplementation | |||||
| Pneumonia incidence | 343 per 1000 | 299 per 1000 | RR 0.87 | 5193 | ⊕⊕⊝⊝ | |
| Pneumonia prevalence | 22 per 1000 | 13 per 1000 | RR 0.59 | 609 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Studies have unclear information on allocation concealment, blinding and reporting biases. | ||||||
Background
Description of the condition
Pneumonia is the largest cause of childhood mortality, accounting for 15% of all childhood deaths under five years (WHO 2015), and 19% of all childhood deaths in low‐income countries (Bryce 2005; Rudan 2008). Pneumonia is diagnosed by the presence of either fast breathing or lower chest wall indrawing (WHO 2015) in children aged under five years. Presenting features in viral or bacterial pneumonia are similar, however wheezing is more common in children with viral pneumonia.
Pneumonia can be prevented by immunising against Haemophilus influenzae type B (Hib), pneumococcus, measles and pertussis (whooping cough) (WHO 2015). Nutrition including breastfeeding for the first six months of life plays a major role by boosting immunity against causative organisms of pneumonia (WHO 2015). Good hygiene and clean indoor air also help in preventing pneumonia (WHO 2015).
Rudan 2013 identified five risk factors for pneumonia: malnutrition, low birth weight, nonexclusive breast feeding, solid fuel use and overcrowding. By 2010, pneumonia episodes for children aged under four years in low‐ and middle‐income countries (LMICs) decreased by 25% over the past decade to about 0.22 episodes per child per year when exposure to the five risk factors decreased by 20% to 30% (Rudan 2013). Therefore, any interventions that can improve child survival from pneumonia are important.
According to the World Health Organization (WHO), zinc deficiency accounts for 13% of all lower respiratory tract infection (LRTIs) (WHO 2009). Acute LRTI is a leading cause of mortality among children aged under five years (Bryce 2005; Rudan 2008), and which is responsible for nearly two million deaths annually, mainly in low‐income countries.
Description of the intervention
In 2005, zinc intake was inadequate among 17.3% of the world's population; rates of zinc adequacy are lower in sub‐Saharan Africa and South Asia (Wessells 2012). The World Bank described multiple factors such as low animal food intake, less bioavailability of dietary zinc, and zinc loss during events of diarrhoea (Bhutta 1999; Black 1998) that could potentially lead to zinc deficiency in low‐income settings (World Bank 2016). The WHO estimated that zinc deficiency may be related to 800,000 deaths a year, with more than 50% occurring in children under five years of age. Children are more susceptible to zinc deficiency because of lower rates of intestinal absorption and from starting life with low body stores due to intrapartum under‐nutrition (Krebs 2014).
Mild‐to‐moderate zinc deficiency is associated with impaired physical growth, delayed sexual maturity, behavioural disturbances, anorexia, affected permeability of the intestinal tract and decreased immunocompetence as well as subclinical inflammation; moderate‐to‐severe deficiency can lead to diarrhoea; and severe deficiency produces dermatitis and alopecia (Krebs 2014).
There is evidence that preventative zinc supplementation reduces pneumonia morbidity (Bhutta 1999; Yakoob 2011) and is beneficial in reducing diarrhoeal episodes in children with acute and persistent diarrhoea (Bhandari 1994; Zinc Group 2000). However, there is insufficient evidence to support preventive zinc supplementation or adjunctive zinc supplementation with antibiotics to reduce pneumonia‐specific mortality (Haider 2011; Yakoob 2011). Zinc supplements (zinc sulphate, zinc gluconate, zinc acetate) can be administered as tablets, syrup or powder. However, zinc supplements need to be administered with caution because excess levels may cause toxicity.
How the intervention might work
Zinc plays an important role in cell regeneration, immunity and growth (Krebs 2014; Shankar 1998). Zinc deficiency decreases T‐lymphocytes and T‐helper, impairs macrophage function and reduced killer cells (Ibs 2003; Ravaglia 2000), and adversely impacts innate immunity affecting interferon (IFN) gamma production, interleukin‐2 (IL‐2) and tumour necrosis factor‐α (TNF‐α) (Krebs 2014). Zinc supplementation in children increases levels of complement in the blood that modulate the function of T‐lymphocytes, T‐helper, macrophages and neutrophils and hence improves the ability to fight infection. Zinc supplementation improves circulating levels of T‐lymphocytes and other macrophages that enhance ability to fight infection (Fraker 1993). Zinc is not stored in the body, but repletion is straightforward. Children with diarrhoea can become zinc deficient quickly. Furthermore, if zinc deficiency occurs during periods of growth, it can be conducive to growth faltering as is shown by a review that found correlation between dietary zinc inadequacy and stunting among children aged under four years (Krebs 2014; Wessells 2012).
Why it is important to do this review
Bhutta 1999 showed that zinc was associated with a reduced incidence of pneumonia. Aggarwal 2007 included more recent studies, but included children aged between birth and 59 months who were given zinc supplements for at least a few months. Roth 2010 calculated the effect size by case definition of pneumonia, and included studies that administered zinc supplements to children aged between birth and 59 months. Yakoob 2011 focused on zinc supplementation for more than three months in children aged under five years and found evidence to support the positive impact of zinc supplementation on diarrhoeal and pneumonia morbidity and mortality to include it in the LiST tool. However Yakoob 2011 did not include low birth weight or small‐for‐gestational age infants, nor provide a specific case definition for pneumonia. Similarly, a review by Bhutta 2013 of diarrhoea and pneumonia interventions reported a non‐significant impact of preventive zinc supplementation on acute lower respiratory infection (ALRI)‐related mortality.
We investigated zinc supplementation for at least three months in children aged from two months to 59 months and conducted a meta‐analysis of data published up to 2011. However, with new and upcoming trials, this review required updating.
Objectives
To evaluate the effectiveness of zinc supplementation in the prevention of pneumonia in children aged two to 59 months.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) evaluating supplementation of zinc for the prevention of pneumonia in children aged from two to 59 months. We included studies that defined an episode of pneumonia in the following ways.
-
Reported cough or difficulty in breathing with a respiratory rate above the WHO‐defined age‐specific values (respiratory rate of 50 breaths per minute or more for children aged two to 11 months, or respiratory rate of 40 breaths per minute or more for children aged 12 to 59 months), and either documented fever over 38°C or chest in‐drawing (WHO 1990).
-
A diagnosis of pneumonia based on chest examination by a physician.
-
A diagnosis of pneumonia based on a chest radiograph.
We included trials published in languages other than English. Quasi‐RCTs were not eligible for inclusion.
Types of participants
We included children aged from two months to 59 months.
Types of interventions
Oral supplement containing at least the USA's recommended daily allowance of zinc versus either an oral supplement without zinc or placebo. We excluded trials in which children were given additional supplements unless the co‐interventions other than zinc were the same in both groups. The recommended daily allowance for infants is 5 mg of elemental zinc per day and 10 mg per day for children aged from one to five years (RDA 1989). We included trials in which supplements were administered for at least three months and outcome surveillance was carried out for at least four weeks.
Types of outcome measures
Primary outcomes
-
Numbers of new episodes of pneumonia in children aged from two months to 59 months.
Secondary outcomes
-
Prevalence (number of cases of pneumonia at a given time per total days of observation) of pneumonia in children aged from two months to 59 months.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Library (www.thecochranelibrary.com, accessed 21 October 2016), which contains the Acute Respiratory Infections Group's Specialised Register, MEDLINE (November 2009 to 21 October 2016) and Embase (January 2010 to 21 October 2016). We searched LILACS (1982 to 21 October 2016) using a new search strategy. We also searched CINAHL (1981 to 21 October 2016) and Web of Science (1985 to 21 October 2016). Details of previous searches are presented in Appendix 1.
We used the search strategy described in Appendix 2 to search MEDLINE and CENTRAL. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011) . We adapted the search strategy to search Embase (Appendix 3), LILACS (Virtual Health Library) (Appendix 4), CINAHL (Appendix 5) and Web of Science (Appendix 6). We searched IMSEAR using the search terms "pneumonia" and "zinc". We imposed no language or publication restrictions.
Figure 1 illustrates the search and study selection processes.

Study flow diagram
Searching other resources
We searched the WHO ICTRP (www.who.int/ictrp) and ClinicalTrials.gov trials registries (latest search 8 July 2016). We also searched related conference proceedings for relevant abstracts. We also checked the reference lists of all trials identified.
Data collection and analysis
Selection of studies
Two review authors independently assessed all potential studies identified for inclusion. We resolved any disagreement through discussion, and if required, consulted the third review author.
Data extraction and management
We designed a data extraction form. Two review authors extracted data. We resolved discrepancies through discussion, and if required, we consulted the third review author. We entered data into Review Manager software (RevMan 2014) and checked for accuracy. There were no new studies identified for inclusion in this update.
Assessment of risk of bias in included studies
There were no new studies identified for inclusion for this update. For the 2010 version of this review (Lassi 2010), two review authors (ZSL, BAH) independently assessed risk of bias using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion with the third review author (ZAB).
Measures of treatment effect
For dichotomous outcomes, we extracted the total number of participants for each group and numbers of participants experiencing an event. We used risk ratio (RR) and 95% confidence intervals (CIs) to describe effect sizes.
Unit of analysis issues
We carried out statistical analysis using RevMan 2014). Initially, we used fixed‐effect inverse variance meta‐analysis to combine data where trials examined the same intervention, and study populations and methods were judged sufficiently similar. Where we suspected clinical or methodological heterogeneity between studies sufficient to suggest that treatment effects may differ between trials, we conducted random‐effects meta‐analysis. When we identified substantial heterogeneity in a fixed‐effect meta‐analysis, we noted this and repeated the analysis using the random‐effects method.
Dealing with missing data
We noted levels of attrition among included studies. As far as possible, we performed analyses on an intention‐to‐treat (ITT) basis. We attempted to include all participants randomised to each group in the analyses.
Assessment of heterogeneity
We measured heterogeneity among the trials by calculating the I² statistic, and Chi² P value. If the I² statistic exceeded 50%, and the Chi² P value was less than 0.1, we would have considered heterogeneity to be substantial. We did not find any heterogeneity; therefore, we did not attempt subgroup analyses based on differences in zinc dosage, participants' age, supplementation in healthy children compared with children recovering from an episode of illness, and pre‐intervention zinc levels. However, we attempted to look for the differential effect of zinc supplementation on the case definition of ALRI.
Assessment of reporting biases
We aimed to assess reporting bias by comparing published study reports with study protocols. We planned to assess for the presence of publication bias by looking for funnel plot asymmetry; however, the low number of included studies meant this was not feasible.
Data synthesis
We carried out statistical analysis using RevMan 2014 software. In the absence of significant heterogeneity, we used the fixed‐effect meta‐analysis model for combining data.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis based on diagnosis of pneumonia.
Sensitivity analysis
We planned to carry out a sensitivity analysis to explore the effect of trial quality (assessed by concealment of allocation), by excluding studies with clearly inadequate allocation concealment. However, only two studies had adequate allocation concealment, therefore we did not run the sensitivity analysis.
Results
Description of studies
See Characteristics of included studies, Table 1 and Characteristics of excluded studies tables.
| Study | Supplement | Schedule | Duration | Surveillance | |
| Zinc | Control | ||||
| Zinc gluconate 10 mg | Both groups vitamin A | Daily | 4 months | Once weekly | |
| Zinc sulphate 10 mg | Placebo | Daily | 6 months | Every 2 weeks | |
| Zinc acetate 35 mg to infants 70 mg to children aged > 12 months | Placebo | Weekly | 12 months | Once weekly | |
| Zinc gluconate 10 mg | Both groups vitamin A | Daily | (Continued until 24 months of age) | Once weekly | |
| Zinc gluconate 10 mg | Placebo | Daily | 6 months | Once weekly | |
| Zinc gluconate 10 mg | Placebo | Daily | 4 months | Every 5th day | |
Results of the search
This is an update of a review previously published in 2010 (Lassi 2010). The most recent search (to October 2016) did not identify any new studies for inclusion.
Included studies
We included six trials. These studies were from Bangladesh (Brooks 2005), India (Bhandari 2002; Sazawal 1998), Peru (Penny 2004), and South Africa (Bobat 2005; Luabeya 2007).
Penny 2004 conducted a randomised, double‐blind, placebo‐controlled community‐based trial in Lima, Peru that involved 238 children with diarrhoea for more than 14 days who were aged from six to 36 months. Children were randomised to receive zinc alone, zinc plus vitamins or placebo for two weeks. Childrens' characteristics at baseline were similar except for sex and length‐for‐age Z scores.
Brooks 2005 conducted a study in a low‐income population in an urban setting of Dhaka, Bangladesh. The study included 1665 children aged from 60 days to 12 months who were randomly allocated to receive oral zinc (70 mg) or placebo once weekly. Children were assessed weekly in the children's homes. Children with suggestive respiratory disease or diarrhoea were referred to clinics where they were assessed and diagnoses made based on the WHO criteria.
Bhandari 2002 included 2482 children aged six to 30 months who were from urban slums in New Delhi, India. Children received daily elemental zinc (10 mg for infants; 20 mg for older children) or placebo for four months. Both groups received single dose vitamin A (100,000 IU for infants and 200,000 IU for older children) at enrolment. Both groups were comparable on socio‐demographic profile, anthropometry, child feeding practices, morbidity in the previous 24 hours, and plasma zinc concentration.
Luabeya 2007 included children aged from four to six months from the KwaZulu‐Natal Province of South Africa. Children were randomised to two intervention arms and one control arm. Children in the control arm received vitamin A only; in the first intervention arm they were given vitamin A plus zinc; and in the second intervention arm children received vitamin A, zinc and multiple micronutrients. Children received supplements until 24 months of age, thus each child received zinc supplements for 18 to 20 months. Among the participants, 32 children were born with HIV positive status, and 154 were born without HIV infection to HIV positive mothers. Groups differed on weight‐for‐length scores.
The double‐blind controlled trial by Sazawal 1998 was conducted in a low socioeconomic population of 609 children aged between six and 35 months in urban India. Children were assigned to zinc supplementation (n = 298) and placebo (n = 311) groups. Children in the treatment group received 10 mg elemental zinc and placebo group children received a substance similar in colour and taste. The daily fixed dose of 5 mL per child was given to all enrolled children for six months, but this was increased to 10 mL for children with diarrhoeal illnesses. The baseline characteristics of included children were similar for age, sex, nutritional status and baseline plasma zinc.
Bobat 2005 conducted a randomised, double‐blind, placebo‐controlled equivalence trial of zinc supplementation at Grey's Hospital in Pietermaritzburg, South Africa. The study included 96 children aged from six to 60 months with HIV 1 infection who were randomly assigned to receive 10 mg of elemental zinc sulphate or placebo daily for six months. Baseline characteristics of included children were similar.
Excluded studies
We excluded 30 studies that did not satisfy inclusion criteria. Seven studies included children outside the age limits of our review criteria (Lira 1998; McDonald 2015; Osendarp 2002; Sur 2003; Taneja 2009; Tielsch 2007 (also reported a different response to intervention definition than we used); Vakili 2009). We excluded seven studies that provided supplements for less than three months (Baqui 2002; Castillo‐Duran 1987; Chandyo 2010; Feiken 2014; Rahman 2001; Roy 1999; Sempértegui 1996). We excluded 14 studies that applied different case definitions for ALRI/pneumonia, which were not based on WHO criteria, radiograph or chest examination by physician (Baqui 2003; Larson 2010; Long 2006; Lind 2004; Malik 2014; Mazoomar 2010; Ninh 1996; Reul 1997; Richard 2006; Sampaio 2013; Sanchez 2014; Soofi 2013; Rosado 1997; Umeta 2000). We excluded one study because children received zinc supplement in a fortified drink (Bates 1993). We also excluded one study (Adhikari 2016) because it had included children with recurrent infections.
Please refer to Characteristics of excluded studies for further details.
Risk of bias in included studies
Figure 2 and Figure 3 provide graphical summaries of the 'Risk of bias' assessment for the six included studies. With one exception (Penny 2004), studies were assessed as providing low or unclear risk of bias in relation to methods. Luabeya 2007 had the lowest risk of bias; Penny 2004 was assessed at unclear risk of bias.
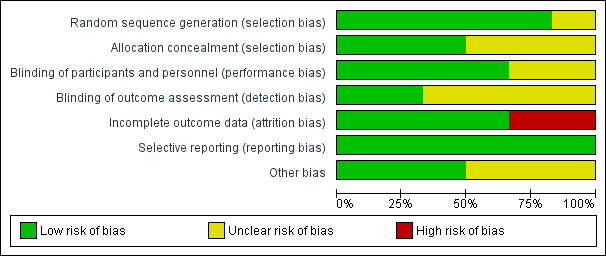
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
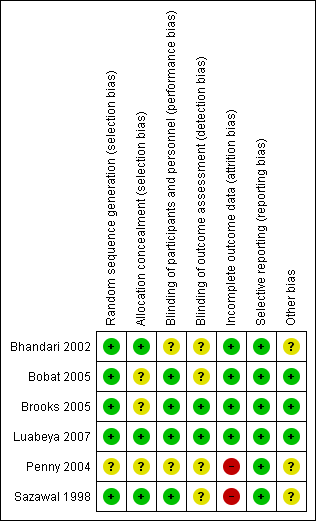
Methodological quality summary: review authors' judgements about each methodological quality item for each included study
Allocation
Most included studies were assessed as demonstrating adequate sequence generation and used computer‐generated sequencing techniques (Bhandari 2002; Bobat 2005; Brooks 2005; Luabeya 2007; Sazawal 1998). Allocation concealment was adequate three studies (Bhandari 2002; Luabeya 2007; Sazawal 1998); the remainder did not report in sufficient detail to enable assessment (Bobat 2005; Brooks 2005; Penny 2004).
Blinding
Blinding of participants and study personnel was achieved in four studies (Bobat 2005; Brooks 2005; Luabeya 2007; Sazawal 1998). The remaining studies reported insufficient information to permit judgement (Bhandari 2002; Penny 2004).
Blinding of outcome assessors was achieved in Brooks 2005 and Luabeya 2007. Brooks 2005 reported that blinding was not affected because a proportion of children in both the zinc and placebo groups reacted to the taste such that treatment could not be distinguished; Luabeya 2007 reported that outcomes assessors were blinded to assignment.
Incomplete outcome data
Attrition and exclusions were described in all except studies Penny 2004 and Sazawal 1998. The other four studies (Bhandari 2002; Bobat 2005; Brooks 2005; Luabeya 2007) reported reasons for exclusions; these included refusal to participate, moved outside the study site, taste of the syrup, and death.
Selective reporting
Only Luabeya 2007 was registered with a trials register. None of the other studies had published protocols (Bhandari 2002; Bobat 2005; Brooks 2005; Penny 2004; Sazawal 1998). However, all proposed outcomes were reported.
Other potential sources of bias
The funding agencies were reported to have had no input to design or study results in three studies (Bobat 2005; Brooks 2005; Luabeya 2007). Bhandari 2002; Penny 2004 and Sazawal 1998 did not mention the role of funding agencies explicitly.
Effects of interventions
All six studies reported the incidence of pneumonia (Bhandari 2002; Bobat 2005; Brooks 2005; Luabeya 2007; Penny 2004; Sazawal 1998), while one study (Sazawal 1998) also reported prevalence rates.
Incidence of pneumonia
Administration of zinc supplementation showed a statistically significant impact on reducing pneumonia incidence by 13% (risk ratio (RR) 0.87, 95% confidence interval (CI) 0.81 to 0.94, fixed‐effect model, six studies, n = 5193, low‐quality evidence). There was no heterogeneity (I² statistic = 28%, Chi² test P = 0.20; Analysis 1.1).
We pooled studies that used similar case definitions. Studies that used clinical definitions of age‐specific fast‐breathing, with or without lower chest indrawing did not exhibit impact from zinc supplementation on reducing pneumonia (RR 0.95, 95% CI 0.86 to 1.06, fixed‐effect model, four studies, n = 1932). There was no heterogeneity (I² statistic = 0%, Chi² test P = 0.74).
Studies that applied case definition for pneumonia based on chest examination or chest radiograph exhibited significant impact from zinc supplementation on reducing incidence of pneumonia by 21% (RR 0.79, 95% CI 0.71 to 0.88, fixed‐effect model, four studies, n = 3261). There was no heterogeneity (I² statistic = 0%, Chi² test P = 0.44). Bobat 2005 included children with HIV. When this study was removed from the analysis there was no difference in results (RR 0.88, 95% CI 0.81 to 0.95, fixed‐effect model, three studies, n = 3165). Evidence was low quality (summary of findings Table for the main comparison).
Prevalence of pneumonia
Administration of zinc supplementation showed a statistically significant impact on reducing the pneumonia prevalence by 41% among children aged from two to 59 months (RR 0.59, 95% CI 0.35 to 0.99, fixed‐effect model, one study, n = 609, low‐quality evidence; Analysis 1.2). (summary of findings Table for the main comparison).
Discussion
Summary of main results
This is an update of a review first published in 2010 and included no new studies. Previous conclusions are unchanged.
We included six randomised controlled trials (RCTs) evaluating the impact of zinc supplementation in children aged from two to 59 months. Our meta‐analysis indicated that zinc supplementation led to reductions in the incidence of pneumonia by 13% and pneumonia prevalence by 41%. The greater overall reduction of pneumonia prevalence result is due to prevalence data originating from one study.
We found that zinc reduced pneumonia incidence (defined by specific clinical criteria and confirmed by chest examination or chest radiograph) by 21%. We found an expected association of acute lower respiratory infection (ALRI) case definition with an effect size (P = 0.02). We found benefits of zinc supplementation when ALRI was diagnosed following clinical examination or chest radiography suggesting infection.
Reduction in pneumonia incidence supports the use of zinc supplements among children two to 59 months of age. Because zinc is not stored in the body, adequate zinc needs to be available in daily diet (Sanstead 1995). Children, particularly those from low‐income countries who have inadequate intake of food that contains zinc (mainly foods of animal origin) should receive supplements to address deficiency.
Overall completeness and applicability of evidence
Our findings from can be extrapolated for children living in low‐ and middle‐income countries because most included studies were from disadvantaged urban areas of low‐income countries (Bangladesh, India, Peru, and South Africa). In two studies, either children or their mothers had HIV positive status (Bobat 2005; Luabeya 2007). In most studies, children were excluded if they had co‐morbidities such as tuberculosis, congenital heart disease, less than 60% median weight‐for‐age Z score, and nutritional oedema. Penny 2004 recruited children on their breastfeeding status. The use of consistent and accurate definitions of pneumonia, with emphasis on clinical documentation of key signs or radiographic diagnosis, would avoid misclassification and lead to greater confidence in study findings.
Quality of the evidence
We assessed that review outcomes were low according to GRADE criteria. We downgraded evidence quality because outcomes were ascertained using different criteria and 'Risk of bias' assessment. Of note, half of the included studies reported pneumonia according to the WHO definition (WHO 1990); the remainder relied on clinical examination and chest radiographs. We therefore looked for differential effects on outcome estimates with respect to case definition and reported their impact separately.
Allocation concealment was adequately described overall. All included studies were deemed to be adequately blinded for treatment assignment. Completion rates were greater than 90% in four included studies; Penny 2004 reported completion by 16% of participants and Sazawal 1998 did not report numbers of participants who dropped out. The low levels of missing data meant that we chose not to assess the impact on overall estimates because it was not anticipated to cause significant bias in the study results.
Potential biases in the review process
We undertook a systematic search of the literature to identify all studies that met our inclusion criteria. Study selection and data extraction decisions were cross‐checked independently by the review authors. Included studies were not free from bias; only one study was assessed at low risk of bias for all domains. We could not assess reporting bias because of the limited numbers of included studies.
Agreements and disagreements with other studies or reviews
We found RCT evidence from both high‐ and low‐income countries showing an effect of zinc in decreasing morbidity and mortality in children due to respiratory and gastro‐intestinal infections (Hambidge 1999; Sazawal 1998). The effect of zinc against infectious diseases is therapeutic as well as preventive.
Findings reported by the Zinc Investigators’ Collaborative Group in (Bhutta 1999) and Aggarwal 2007 were consistent with our review. Bhutta 1999 reported that children who received zinc supplements had positive effects for pneumonia (odds ratio (OR) 0.59, 95% CI 0.41 to 0.83). Moreover, Bhutta 1999 included trials that administered zinc supplements with therapeutic intent, which might have led to an overestimation of the potential preventive effects of zinc. Aggarwal 2007 included trials that recruited children aged from birth to 59 months who were provided zinc supplementation for at least three months; analysis showed 20% decreased incidence of respiratory illness among children supplemented with zinc compared with placebo. Similar findings were reported by Roth 2010 who also assessed specific case definitions for impact evaluation of children aged from birth to five years. Roth 2010 reported that zinc reduced ALRI incidence defined by specific clinical criteria (incidence rate ratio (IRR) 0.65, 95% CI 0.52 to 0.82), compared with no effect on lower‐specificity ALRI case definitions based on caregiver reports (IRR 1.01, 95% CI 0.91 to 1.12) or WHO non‐severe pneumonia (IRR 0.96, 95% CI 0.86 to 1.08).

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies

Methodological quality summary: review authors' judgements about each methodological quality item for each included study

Comparison 1 Zinc supplementation vs placebo, Outcome 1 Pneumonia incidence.

Comparison 1 Zinc supplementation vs placebo, Outcome 2 Pneumonia prevalence.
| Zinc supplementation compared with placebo for the prevention of pneumonia in children aged 2 months to 59 months | ||||||
| Patient or population: children aged 2 months to 59 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Zinc supplementation | |||||
| Pneumonia incidence | 343 per 1000 | 299 per 1000 | RR 0.87 | 5193 | ⊕⊕⊝⊝ | |
| Pneumonia prevalence | 22 per 1000 | 13 per 1000 | RR 0.59 | 609 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Studies have unclear information on allocation concealment, blinding and reporting biases. | ||||||
| Study | Supplement | Schedule | Duration | Surveillance | |
| Zinc | Control | ||||
| Zinc gluconate 10 mg | Both groups vitamin A | Daily | 4 months | Once weekly | |
| Zinc sulphate 10 mg | Placebo | Daily | 6 months | Every 2 weeks | |
| Zinc acetate 35 mg to infants 70 mg to children aged > 12 months | Placebo | Weekly | 12 months | Once weekly | |
| Zinc gluconate 10 mg | Both groups vitamin A | Daily | (Continued until 24 months of age) | Once weekly | |
| Zinc gluconate 10 mg | Placebo | Daily | 6 months | Once weekly | |
| Zinc gluconate 10 mg | Placebo | Daily | 4 months | Every 5th day | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pneumonia incidence Show forest plot | 6 | 5193 | Risk Ratio (Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 1.1 Diagnosis based on age‐specific fast breathing with or without lower chest indrawing | 4 | 1932 | Risk Ratio (Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| 1.2 Diagnosis based age‐specific fast breathing and confirmed by chest examination or chest radiograph | 4 | 3261 | Risk Ratio (Fixed, 95% CI) | 0.79 [0.71, 0.88] |
| 2 Pneumonia prevalence Show forest plot | 1 | 609 | Risk Ratio (Fixed, 95% CI) | 0.59 [0.35, 0.99] |

