Intervenciones para la prevención del delirio en pacientes hospitalizados no ingresados en una UCI
Resumen
Antecedentes
El delirio es un trastorno mental frecuente que es angustiante y tiene resultados adversos graves en los pacientes hospitalizados. La prevención del delirio es aconsejable desde la perspectiva de los pacientes y los cuidadores, así como de los profesionales sanitarios. Sin embargo, actualmente no está claro si las intervenciones para la prevención del delirio son eficaces.
Objetivos
Evaluar la efectividad de las intervenciones para la prevención del delirio en pacientes hospitalizados no ingresados en una Unidad de Cuidados Intensivos (UCI).
Métodos de búsqueda
Se hicieron búsquedas de todos los estudios aleatorios para prevenir el delirio en el registro especializado del Grupo Cochrane de Demencia y Trastornos Cognitivos (ALOIS ‐ the Cochrane Dementia and Cognitive Improvement Group) el 4 diciembre 2015. También se hicieron búsquedas en MEDLINE (Ovid SP), EMBASE (Ovid SP), PsycINFO (Ovid SP), Central (The Cochrane Library), CINAHL (EBSCOhost), LILACS (BIREME), Web of Science core collection (ISI Web of Science), ClinicalTrials.gov y en el WHO meta register of trials, ICTRP.
Criterios de selección
Se incluyeron los ensayos controlados aleatorios (ECA) de intervenciones farmacológicas y no farmacológicas con componentes únicos y múltiples, para prevenir el delirio en los pacientes hospitalizados no ingresados en una UCI.
Obtención y análisis de los datos
Dos autores de la revisión independientes examinaron los títulos y los resúmenes de las citas identificadas mediante la búsqueda en cuanto a la elegibilidad y extrajeron los datos; cualquier desacuerdo se resolvió por consenso. El resultado primario fue la incidencia de delirio; los resultados secundarios incluyeron duración y gravedad del delirio, atención institucional al momento del alta, calidad de vida y costos de la atención sanitaria. Se utilizaron los cocientes de riesgo (CR) como medidas del efecto del tratamiento para los resultados dicotómicos; y las diferencias de medias entre grupos y las desviaciones estándar para los resultados continuos.
Resultados principales
Se incluyeron 39 ensayos que reclutaron a 16 082 participantes y que evaluaron 22 intervenciones o comparaciones diferentes. Catorce ensayos fueron controlados con placebo, 15 evaluaron una intervención de prevención del delirio contra atención habitual y diez compararon dos intervenciones diferentes. Treinta y dos estudios se realizaron en pacientes sometidos a cirugía, la mayoría en contextos ortopédicos. Siete estudios se realizaron en contextos de medicina general o de medicina geriátrica.
Se encontró que las intervenciones con múltiples componentes redujeron la incidencia de delirio en comparación con la atención habitual (CR 0,69; IC del 95%: 0,59 a 0,81; siete estudios; 1950 participantes; pruebas de calidad moderada). Los tamaños del efecto fueron similares en los contextos médicos (CR 0,63; IC del 95%: 0,43 a 0,92; cuatro estudios; 1365 participantes) y quirúrgicos (CR 0,71; IC del 95%: 0,59 a 0,85; tres estudios; 585 participantes). En el subgrupo de pacientes con demencia preexistente, el efecto de las intervenciones con múltiples componentes aún no está claro (CR 0,90; IC del 95%: 0,59 a 1,36; un estudio (50 participantes; pruebas de baja calidad).
No hay pruebas claras de que los inhibidores de la colinesterasa sean eficaces para prevenir el delirio en comparación con placebo (CR 0,68; IC del 95%: 0,17 a 2,62; dos estudios, 113 participantes; pruebas de muy baja calidad).
Tres ensayos no proporcionan pruebas claras de un efecto de los fármacos antipsicóticos como grupo sobre la incidencia de delirio (CR 0,73; IC del 95%: 0,33 a 1,59; 916 participantes; pruebas de muy baja calidad). En un análisis de subgrupos preplanificado no hubo pruebas de la efectividad de un antipsicótico típico (haloperidol) (CR 1,05; IC del 95%: 0,69 a 1,60; dos estudios; 516 participantes, pruebas de calidad baja). Sin embargo, la incidencia de delirio fue menor (CR 0,36; IC del 95%: 0,24 a 0,52; un estudio; 400 participantes, pruebas de calidad moderada) en los pacientes tratados con un antipsicótico atípico (olanzapina) en comparación con placebo (pruebas de calidad moderada).
No hay pruebas claras de que la melatonina o los agonistas de la melatonina reduzcan la incidencia de delirio en comparación con placebo (CR 0,41; IC del 95%: 0,09 a 1,89; tres estudios, 529 participantes; pruebas de baja calidad).
Hay pruebas de calidad moderada de que la anestesia guiada por el índice biespectral (Bispectral Index [BIS]) reduce la incidencia de delirio en comparación con la anestesia sin el uso del BIS o la valoración clínica (CR 0,71; IC del 95%: 0,60 a 0,85; dos estudios; 2057 participantes).
No es posible generar afirmaciones de pruebas sólidas para un rango de intervenciones farmacológicas y anestésicas adicionales debido al escaso número de ensayos de calidad metodológica variable.
Conclusiones de los autores
Hay pruebas sólidas que apoyan las intervenciones con múltiples componentes para prevenir el delirio en los pacientes hospitalizados. No existen pruebas claras de que los inhibidores de la colinesterasa, los antipsicóticos o la melatonina reduzcan la incidencia de delirio. El uso del BIS para monitorizar y controlar la profundidad de la anestesia reduce la incidencia de delirio posoperatorio. La función de los fármacos y otras técnicas anestésicas para prevenir el delirio aún no está clara.
PICOs
Resumen en términos sencillos
Intervenciones para prevenir el delirio en los pacientes hospitalizados, sin incluir los que están en unidades de cuidados intensivos
Pregunta de la revisión
Se examinaron las pruebas de la efectividad de las intervenciones para la prevención del delirio en los pacientes hospitalizados, sin incluir los que están en unidades de cuidados intensivos (UCI) (salas especializadas para la atención de pacientes en estado crítico).
Antecedentes
El delirio es una enfermedad frecuente y grave en los pacientes ingresados en el hospital. Puede ser angustiante para los pacientes y sus familias. También aumenta las probabilidades de desarrollar otras complicaciones en el hospital, ser ingresados en una institución de atención o morir en el hospital. El delirio es una afección muy costosa para los servicios sanitarios. Por lo tanto, la prevención del delirio es aconsejable para los pacientes, las familias y los servicios sanitarios.
Hay muchos factores de riesgo para el desarrollo del delirio (p.ej. infección, deshidratación, ciertos fármacos). Por lo tanto, un enfoque para prevenir el delirio (llamado "intervenciones con múltiples componentes") está dirigido a estos múltiples factores de riesgo. Algunos fármacos tienen efectos sobre las sustancias químicas del cerebro implicadas en el desarrollo del delirio y pueden, por lo tanto, tener una función en la prevención. También hay algunas otras intervenciones que se dirigen a los factores de riesgo del delirio relacionados con la anestesia y el tratamiento médico alrededor del momento de la cirugía.
Características de los estudios
Las pruebas están actualizadas hasta 4 diciembre 2015. Se encontraron 39 ensayos que reclutaron a 16 082 participantes y probaron 22 intervenciones con múltiples componentes, farmacológicas o anestésicas diferentes, en comparación con atención habitual, placebo o diferentes intervenciones.
Hallazgos clave
Se encontraron pruebas sólidas de que las intervenciones con múltiples componentes pueden prevenir el delirio en los contextos médicos y quirúrgicos y pruebas menos sólidas de que reducen la gravedad del delirio. Las pruebas acerca de su efecto sobre la duración del delirio no son concluyentes.
Hay pruebas de que la monitorización de la profundidad de la anestesia puede reducir la aparición de delirio después de la anestesia general.
No se encontraron pruebas claras de que una variedad de fármacos u otras técnicas o procedimientos anestésicos sean eficaces para prevenir el delirio.
Calidad de la evidencia
Hay pruebas de calidad moderada que indican que las intervenciones con múltiples componentes reducen la incidencia de delirio. Las pruebas apoyan la implementación de intervenciones con múltiples componentes para la prevención del delirio en la atención habitual de los pacientes en el hospital.
Hay pruebas de calidad moderada de que la monitorización de la profundidad de la anestesia general se puede utilizar para prevenir el delirio en el posoperatorio.
La calidad de las pruebas para varios fármacos u otras técnicas o procedimientos anestésicos para la prevención del delirio es deficiente (debido al escaso número de ensayos y a la calidad variable de los métodos de los ensayos) y no se puede utilizar para informar los cambios en la práctica.
Financiación externa
Ninguna.
Conclusiones de los autores
Summary of findings
| Multi‐component delirium prevention intervention compared to usual care for hospitalised non‐ICU patients | ||||||
| Intervention: A multi‐component delirium prevention intervention versus usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| A multi‐component delirium prevention intervention | ||||||
| Incidence of delirium | 209 per 10002 | 144 per 1000 | RR 0.69 | 1950 | ⊕⊕⊕⊝ | |
| Duration of delirium | The mean duration of delirium in the control groups ranged from | The mean duration of delirium in the intervention groups was | 244 | ⊕⊝⊝⊝ | ||
| Severity of delirium | The standardised mean severity of delirium in the intervention groups was | 67 | ⊕⊕⊝⊝ | |||
| Length of admission | The mean length of admission in the control groups ranged from | The mean length of admission in the intervention groups was | 1920 | ⊕⊕⊕⊝ | ||
| Return to independent living | 682 per 10002 | 648 per 1000 | RR 0.95 | 1116 | ⊕⊕⊕⊝ | |
| Inpatient mortality | 81 per 10002 | 73 per 1000 | RR 0.90 | 859 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Three validated methods for delirium detection used ‐ the CAM, OBS and DRS 6 Higher baseline prevalence of dementia in the control groups of two studies compared to the intervention groups causing risk of bias 9 Downgraded because inconsistent results 10 Delirium Rating Scale‐Revised‐98 (0 to 46) and Confusion Assessment Method‐Severity (0 to 10) | ||||||
| Prophylactic cholinesterase inhibitor versus placebo for preventing delirium in hospitalised non‐ICU patients | ||||||
| Intervention: Prophylactic cholinesterase inhibitor versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prophylactic cholinesterase inhibitors | |||||
| Incidence of delirium | 218 per 10001 | 148 per 1000 | RR 0.68 | 113 | ⊕⊝⊝⊝ | |
| Duration of delirium ‐ not measured | N/A | N/A | N/A | N/A | ||
| Severity of delirium | The mean severity of delirium in the control groups was | The mean severity of delirium in the intervention groups was | 16 | ⊕⊕⊝⊝ | ||
| Length of admission | The mean length of admission ranged across control groups from | The mean length of admission in the intervention groups was | 128 | ⊕⊕⊝⊝ | ||
| Return to independent living ‐ not measured | N/A | N/A | N/A | N/A | ||
| Inpatient mortality ‐ not measured | N/A | N/A | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risk is the risk in the control group 2 Both studies are at high risk of attrition bias and have incomplete outcome data. 3 Downgraded because inconsistent results 4 Estimate of effect includes 'no benefit' and both appreciable benefit and appreciable harm. 5 Estimate of effect includes both 'no effect' and minimally important difference, downgraded two levels due to serious imprecision 6 Risk of bias unclear in all domains in one study (abstract only available). Remaining two studies have incomplete outcome reporting and are at risk of attrition bias 7 Downgraded due to imprecision in result | ||||||
| Prophylactic antipsychotic medications for preventing delirium in hospitalised non‐ICU patients | ||||||
| Intervention: Prophylactic antipsychotic medications versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prophylactic antipsychotic medications | |||||
| Incidence of delirium | 300 per 10001 | 165 per 1000 | RR 0.55 | 916 | ⊕⊝⊝⊝ | |
| Duration of delirium | The mean duration of delirium in the control groups ranged from 2.2 to 5.4 days | The mean duration of delirium in the intervention groups was | 178 | ⊕⊝⊝⊝ | ||
| Severity of delirium | The mean severity of delirium in the control groups ranged from 14.4 to 16.4 points | The mean severity of delirium in the intervention groups was | 178 | ⊕⊝⊝⊝ | ||
| Length of admission | The mean length of admission in the control group was 17.1 days | The mean length of admission in the intervention groups was | 68 | ⊕⊕⊝⊝ | ||
| Return to independent living ‐ not measured | N/A | N/A | N/A | N/A | ||
| Inpatient mortality ‐ not measured | N/A | N/A | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risk is the risk in the control group 2Downgraded because inconsistent results 3 Downgraded because of imprecision in results 4 Downgraded due to risk of bias 5 Downgraded two levels because very imprecise results | ||||||
| Prophylactic melatonin for preventing delirium in hospitalised non‐ICU patients | ||||||
| Intervention: Prophylactic melatonin versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prophylactic melatonin | |||||
| Incidence of delirium | 242 per 10001 | 128 per 1000 | RR 0.53 | 529 | ⊕⊝⊝⊝ | |
| Duration of delirium | The mean duration of delirium in the control group was 2 days | The mean duration of delirium in the intervention groups was | 104 | ⊕⊕⊕⊝ | ||
| Severity of delirium (binary severe vs. not severe) | 531 per 1000 | 457 per 1000 | RR 0.86 | 104 | ⊕⊕⊕⊝ | |
| Severity of delirium DRS‐R‐98 score | The mean severity of delirium in the control group was 6.3 points | The mean severity of delirium in the intervention group was 4.1 points lower (19.47 points lower to 11.27 points higher) | 6 (1 study) | ⊕⊕⊝⊝ | ||
| Length of admission | The mean length of admission in the control groups ranged from 11 to 18.5 days | The mean length of admission in the intervention groups was | 500 | ⊕⊕⊕⊝ | ||
| Return to independent living ‐ not measured | N/A | N/A | N/A | N/A | ||
| In‐hospital mortality | 47 per 10001 | 39 per 1000 | RR 0.84 | 543 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risk is the risk in the control group 2 Downgraded because inconsistent results 3 Downgraded because imprecise results 4 Downgraded due to risk of bias 5 Downgraded because imprecise results and very small number of events | ||||||
| Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement for preventing delirium in hospitalised non‐ICU patients | ||||||
| Intervention: Bispectral index (BIS)‐guided anaesthesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| BIS‐blinded/clinical judgement | BIS‐guided | |||||
| Incidence of delirium CAM, DSM‐IV | 226 per 10001 | 160 per 1000 | RR 0.71 | 2057 | ⊕⊕⊕⊝ | |
| Duration of delirium ‐ not measured | N/A | N/A | N/A | N/A | ||
| Severity of delirium ‐ not measured | N/A | N/A | N/A | N/A | ||
| Length of admission Days | The mean length of admission in the control groups ranged from 7 to 15.7 days | The mean length of admission in the intervention group was 0.94 days shorter (0.43 days shorter to 1.45 days shorter) | ‐ | 2057 | ⊕⊕⊕⊝ | |
| Return to independent living ‐ not measured | N/A | N/A | N/A | N/A | ||
| In‐hospital mortality ‐ not measured | N/A | N/A | N/A | N/A | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risk is the risk in the control group (BIS‐blinded/clinical judgement) | ||||||
Antecedentes
Descripción de la afección
El delirio es un trastorno de la conciencia y la cognición que generalmente tiene una aparición rápida y un curso variable. Se le ha llamado de varias maneras, como síndrome cerebral orgánico agudo, trastorno mental orgánico agudo y estado de confusión tóxico. Hasta el siglo XIX el delirio se utilizó para describir un trastorno del pensamiento, y las descripciones posteriores incluyeron trastornos de la percepción, a menudo con comportamiento hiperactivo, o pérdida de la conciencia. La publicación del Diagnostic and Statistical Manual (DSM) III (APA 1987) en 1987 agrupó estas ideas y combinó el trastorno de la conciencia con la deficiencia de la cognición. Las principales características del delirio (trastorno de la atención, cambios en la cognición y aparición aguda y curso variable) se han aclarado actualmente en la versión 10 de la International Classification of Diseases (ICD‐10) (WHO 1992), el DSM‐IV (APA 1994), y más recientemente en el DSM‐V (APA 2013). Este consenso ha permitido cierta estandarización de la investigación y una mayor comparabilidad entre los estudios, aunque se mantienen diferencias como la necesidad de pruebas de una causa subyacente en el DSM‐IV y el DSM‐V, pero no en la ICD‐10.
El delirio es frecuente en los pacientes hospitalizados. Del 10% al 30% de los ingresos en un hospital general desarrollan delirio (Levkoff 1991; Trzepacz 1996) y en los enfermos hospitalizados en medicina general se han informado tasas de aparición que varían del 11% al 42%(Siddiqi 2006). El delirio tiene una prevalencia de hasta el 60% en los pacientes de edad avanzada con discapacidades (Francis 1990), y del 7% al 9,6% en los pacientes de edad avanzada que se presentan a los departamentos de urgencias (Elie 2000; Hustey 2003). Después de la revascularización arterial coronaria en pacientes de edad avanzada se ha informado una incidencia del 33,6%(Santos 2004), y del 41% después de un reemplazo bilateral de rodilla (Williams‐Russo 1992). Después de una fractura de cadera la prevalencia general es del 43% al 61% (Holmes 2000). El cáncer también aumenta el riesgo de desarrollar delirio; el 18% de los pacientes ingresados en una sala de oncología y del 26% al 44% de los ingresados en un hospital o residencia para enfermos terminales con diagnóstico de cáncer avanzado desarrollaron delirio (Centeno 2004; Ljubisavljevic 2003). En los pacientes con SIDA suficientemente enfermos como para ser ingresados, la incidencia de delirio también es alta y se ha informado que es del 46% (Uldall 1997).
El delirio es grave, y sus resultados a corto y a largo plazo son significativos. La mortalidad aumenta (McCusker 2002), se reducen las capacidades funcionales (Moller 1998), aumenta el ingreso a la atención a largo plazo (Inouye 1998a), y aumenta la duración de la estancia hospitalaria (McCusker 2003a; Stevens 1998). La deficiencia de la función cognitiva puede persistir durante al menos un año (McCusker 2001), al igual que los síntomas de delirio, especialmente la falta de atención, la desorientación y el deterioro de la memoria (McCusker 2003b). Se reconoce cada vez más la angustia que produce un episodio de delirio para los enfermos y sus cuidadores (Breitbart 2002).
Los estudios de investigación en pacientes de edad avanzada han identificado varios factores de riesgo de delirio. La afección tiene claramente una etiología multifactorial y estos factores de riesgo interactúan (Inouye 1998b); a más factores de riesgo presentes, mayor probabilidad de que el paciente desarrolle delirio. Los factores de riesgo que se han identificado hasta ahora incluyen: aumento de la edad, déficit sensorial (deterioro visual o auditivo), falta de sueño, aislamiento social, limitación física, uso de un catéter vesical, eventos adversos iatrogénicos, tratamiento con fármacos múltiples (más de tres fármacos nuevos agregados), uso de fármacos psicoactivos, comorbilidades, enfermedad grave (especialmente infección, fractura o accidente cerebrovascular), deficiencia cognitiva previa, anomalía en la temperatura (fiebre o hipotermia), deshidratación, desnutrición y albúmina sérica baja (Inouye 1998b; Inouye 1999c; NICE 2010).
Los estudios en pacientes de oncología también han identificado varios factores de riesgo de delirio, por ejemplo metástasis óseas, presencia de neoplasia maligna hematológica, edad avanzada, deficiencia cognitiva y niveles de albúmina bajos (Ljubisavljevic 2003).
La identificación de esta lista variada de factores etiológicos indica varias cosas. Primero, es posible identificar a los pacientes con alto riesgo de desarrollar delirio y al modificar estos factores de riesgo se podría intentar prevenirlo; dichas estrategias de prevención se podrían dirigir a grupos específicos de pacientes.
Segundo, muchos de estos factores de riesgo se pueden considerar medidas de la "calidad de la atención" hospitalaria, p.ej. desnutrición, deshidratación, uso de limitaciones físicas, eventos iatrogénicos. La aparición del delirio puede, por lo tanto, considerarse una medida sustituta de la calidad de la atención durante la hospitalización (Inouye 1999b; Inouye 2014); y las intervenciones eficaces para prevenir el delirio se pueden considerar fundamentales para la mejoría de la calidad.
La mejoría de la calidad es un aspecto principal de la atención, en particular en los servicios para los pacientes de edad avanzada (Institute for Innovation 2006). Se conoce que los sistemas y servicios de atención sanitaria, internacionalmente, no han marchado al unísono con las transiciones demográficas y a menudo no logran satisfacer las necesidades complejas que requieren de una atención multidisciplinaria de números crecientes de personas de edad avanzada (Hubbard 2004). En realidad, con frecuencia los hospitales generales tienen características que de manera no intencional estimulan o agravan el delirio (Young 2007). Sin embargo, modificar estas características es desafiante y requiere cambios de amplio alcance en los sistemas de atención. Centrarse en la prevención del delirio puede ayudar a desarrollar las aptitudes profesionales necesarias, los aspectos culturales y el diseño de los servicios de manera que alcancen la calidad de la atención.
La prevención del delirio es claramente aconsejable para los pacientes y los cuidadores, y además puede reducir los costos de los servicios de salud. Los costos de la asistencia sanitaria en los pacientes que desarrollan delirio en las unidades de cuidados intensivos (UCI) fueron 31% mayores (USD 41 836 versus USD 27 106) (Milbrandt 2004). Un estudio no aleatorio de una intervención con múltiples componentes para el delirio también demostró una mejoría general en la relación entre costo y eficacia (Rizzo 2001).
Descripción de la intervención
Esta revisión evalúa la efectividad de las intervenciones farmacológicas y no farmacológicas para la prevención del delirio en los pacientes hospitalizados, con la exclusión del contexto de las UCI.
Se han desarrollado varias intervenciones no farmacológicas para la prevención del delirio en los pacientes hospitalizados. La mayoría ha seguido un enfoque multifactorial para la prevención del delirio y ha intentado prevenir varios factores de riesgo mediante protocolos, la educación o el rediseño de los sistemas, (Cole 2002; nouye 2000; IMilisen 2001), aunque algunas se dirigen a un único factor de riesgo solamente. Como ejemplos se incluyen los programas de educación para el personal de enfermería de las salas (Rockwood 1999), protocolos dirigidos a factores de riesgo específicos y que son implementados por un equipo interdisciplinario adiestrado (Inouye 1999a; Young 2015), e intervenciones de enfermería especializada para educar al personal de enfermería, evaluar y cambiar la medicación, estimular la movilización y mejorar el ambiente del paciente (Wanich 1992).
Las intervenciones farmacológicas se basan en una comprensión de las múltiples vías de los neurotransmisores involucrados en el desarrollo del delirio y las sustancias que potencialmente podrían modificarlos o modificar otros factores de riesgo importantes. Se incluyen, por ejemplo, los inhibidores de la colinesterasa, los antipsicóticos y los analgésicos. También hay algunas otras intervenciones dirigidas a los factores de riesgo del delirio relacionados con la cirugía y la atención perioperatoria, como la variación de los enfoques para la anestesia, la optimización de las transfusiones de sangre y el alivio del dolor posoperatorio.
De qué manera podría funcionar la intervención
El delirio tiene muchos factores de riesgo y factores precipitantes, y algunos pueden ser modificables. Trabajos anteriores han indicado que una combinación de factores de riesgo puede interactuar para aumentar la vulnerabilidad al delirio, y se han desarrollado y validado modelos para predecir este riesgo (Inouye 1993a). Las medidas para reducir el número o la gravedad de estos factores pueden ayudar a prevenir el delirio y pueden atenuar los resultados deficientes asociados.
Las intervenciones no farmacológicas de componentes únicos y múltiples se dirigen a uno o más de estos factores de riesgo.
Las intervenciones farmacológicas se dirigen a las vías importantes de los neurotransmisores que se han implicado en la fisiopatología compleja del delirio (p.ej. los inhibidores de la colinesterasa, los antipsicóticos) o su objetivo es abordar factores de riesgo importantes como el sueño y el dolor (p.ej. melatonina y gabapentinoides).
Diversos enfoques anestésicos y procedimientos perioperatorios también abordan los posibles factores de riesgo del delirio.
Por qué es importante realizar esta revisión
Como el delirio se asocia con resultados deficientes (Witlox 2010) que no parecen modificarse con el tratamiento (NICE 2010), las intervenciones para prevenir el delirio pueden ser particularmente importantes. Revisiones anteriores (Cole 1999; Milisen 2005) han indicado una función de las intervenciones con múltiples componentes para la prevención del delirio, pero no han sido sistemáticas o han utilizado criterios de selección menos rigurosos. Una revisión Cochrane anterior de prevención del delirio en pacientes hospitalizados publicada en 2007 encontró que las pruebas fueron escasas y recomendaron la necesidad de estudios de investigación adicionales (Siddiqi 2007). Actualmente no está claro si las intervenciones para la prevención del delirio son eficaces.
Objetivos
Evaluar la efectividad de las intervenciones diseñadas para prevenir el delirio en pacientes hospitalizados no ingresados en una unidad de cuidados intensivos.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
En esta revisión, se consideraron solamente ensayos controlados aleatorios.
Tipos de participantes
Se incluyeron los pacientes de 16 años de edad o más, ingresados en hospital generales para enfermedades agudas y con riesgo de desarrollar delirio. Se excluyeron los estudios realizados en la UCI porque es probable que las poblaciones e intervenciones en este contexto sean muy diferentes. También se excluyeron los contextos comunitarios, p.ej. las residencias geriátricas. Se excluyeron los estudios en contextos mixtos a menos que los datos se pudieran extraer por separado para los enfermos hospitalizados.
Tipos de intervenciones
Se consideraron todas las intervenciones farmacológicas y no farmacológicas diseñadas para prevenir el delirio. Se incluyeron los ensayos que tuvieron un grupo control que recibió atención habitual y los ensayos que compararon dos tipos de intervenciones. Se incluyeron los ensayos de iniciativas con estrategias múltiples coordinadas para prevenir el delirio (intervenciones con múltiples componentes). La atención estándar se definió como la atención habitual disponible en esa unidad.
Tipos de medida de resultado
Se identificaron medidas de resultado primarias, secundarias y adversas que son importantes para los pacientes, los cuidadores y para los sistemas de atención sanitaria y social.
Resultados primarios
-
Incidencia de delirio, mediante un método de diagnóstico validado
Resultados secundarios
-
Duración del delirio
-
Gravedad del delirio, medido con instrumentos validados que incluyen la Memorial Delirium Assessment Scale (MDAS) (Breitbart 1997), la Delirium Rating Scale (DRS) (Trzepacz 1988), y la DRS‐R‐98 (Trzepacz 2001)
-
Duración del ingreso
-
Estado cognitivo
-
Uso de medicación psicotrópica
-
Alteraciones conductuales
-
Actividades de la vida diaria
-
Retorno a la vida independiente
-
Atención institucional al momento del alta
-
Calidad de vida
-
Morbilidad psicológica de los cuidadores
-
Morbilidad psicológica del personal
-
Coste de la intervención
-
Costo para los servicios de atención sanitaria
-
Retiro de los protocolos por los pacientes
Eventos adversos
-
Eventos adversos (según la definición de los autores).
-
Complicaciones postoperatorias
-
Caídas
-
Úlceras por presión
-
Infecciones (específicamente infecciones de las heridas, infecciones urinarias, neumonía)
-
Eventos adversos cardíacos (específicamente infarto de miocardio e insuficiencia cardíaca)
-
Mortalidad
Para seleccionar los resultados secundarios se consideraron los que tenían probabilidades de influir para prevenir el delirio; y los resultados adversos se definieron como los efectos no favorables que se podrían asociar con la intervención o el comparador, aunque para algunos resultados la distinción entre ambos puede ser arbitraria.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
The search results are summarised in a PRISMA diagram (Figure 1). Of the 136 full‐text articles retrieved, 40 were considered eligible for inclusion; 69 were excluded (see Excluded studies); and 27 are ongoing (see Ongoing studies). Several articles identified as eligible reported outcome data for the same trial. Therefore, 33 new studies were eligible for inclusion and added to the six studies included in the original review (Siddiqi 2007), resulting in 39 included studies (see Included studies). Study authors were contacted for further information for six of these studies (Ashraf 2015; Bonaventura 2007; de Jonghe 2014; Hatta 2014; Jeffs 2013; Gauge 2014). However, unpublished data were only used for Hatta 2014, for which data for the subgroup of non‐ICU study participants were provided by the authors.

Study flow diagram
Included studies
The 39 studies included a total study population of 16,082 randomised participants, and assessed 22 different interventions or comparisons (Abizanda 2011; Aizawa 2002; Al‐Aama 2011; Ashraf 2015; Beaussier 2006; Berggren 1987; Bonaventura 2007; Boustani 2012; Chan 2013; de Jonghe 2014; Diaz 2001; Fukata 2014; Gauge 2014; Gruber‐Baldini 2013; Hatta 2014; Hempenius 2013; Jeffs 2013; Jia 2014; Kalisvaart 2005; Larsen 2010; Leung 2006; Li 2013; Liptzin 2005; Lundstrom 2007; Lurati 2012; Marcantonio 2001; Marcantonio 2011; Martinez 2012; Mouzopoulos 2009; Munger 2008; Papaioannou 2005; Pesonen 2011; Radtke 2013; Sampson 2007; Sieber 2010; Stoppe 2013; Urban 2008; Watne 2014; Whitlock 2015).
Study design
Fourteen studies were placebo‐controlled trials (Al‐Aama 2011; de Jonghe 2014; Diaz 2001; Hatta 2014; Kalisvaart 2005; Larsen 2010; Leung 2006; Liptzin 2005; Marcantonio 2011; Mouzopoulos 2009; Munger 2008; Pesonen 2011; Sampson 2007; Whitlock 2015). Fifteen studies evaluated a delirium prevention intervention against usual care (Abizanda 2011; Aizawa 2002; Ashraf 2015; Bonaventura 2007; Boustani 2012; Fukata 2014; Gauge 2014; Gruber‐Baldini 2013; Hempenius 2013; Jeffs 2013; Jia 2014; Lundstrom 2007; Marcantonio 2001; Martinez 2012; Urban 2008). Ten studies compared two different interventions (Beaussier 2006; Berggren 1987; Chan 2013; Li 2013; Lurati 2012; Papaioannou 2005; Radtke 2013; Sieber 2010; Stoppe 2013;Watne 2014).
Sample Size
The sample size of included studies was highly variable, ranging from 15 to 7507 randomised participants. Eighteen studies randomised less than 100 participants, of which eight randomised less than 50 (Aizawa 2002; Ashraf 2015; Hatta 2014; Leung 2006; Marcantonio 2011; Munger 2008; Stoppe 2013; Urban 2008).
Setting
Thirty‐ two studies were conducted in patients undergoing surgery or procedural interventions.
Orthopaedic practice was the most common setting (18 studies). Six of these evaluated interventions in patients undergoing elective arthroplasty or joint replacement (Kalisvaart 2005; Larsen 2010; Leung 2006; Liptzin 2005; Sampson 2007; Urban 2008); 11 included patients undergoing hip fracture repair Berggren 1987; de Jonghe 2014; Diaz 2001; Gruber‐Baldini 2013; Li 2013; Lundstrom 2007; Marcantonio 2001; Marcantonio 2011; Mouzopoulos 2009; Sieber 2010; Watne 2014), and one study was conducted in combined elective and emergency orthopaedic settings (Munger 2008).
Four studies were in patients undergoing cardiac surgery (Gauge 2014; Pesonen 2011; Stoppe 2013; Whitlock 2015); and one in patients undergoing inpatient cardiac catheterisation (Ashraf 2015).
Two studies were in patients undergoing surgery for cancer (Hempenius 2013 and Jia 2014), the latter specifically for colorectal cancer.
Two studies were in patients having general and colorectal surgery or colorectal surgery alone (Aizawa 2002; Beaussier 2006).
Five studies were in patients undergoing various other elective surgical procedures (Chan 2013; Fukata 2014; Lurati 2012; Papaioannou 2005; Radtke 2013). One of these included patients having abdominal surgery under general anaesthesia or orthopaedic surgery under general or spinal anaesthesia (Fukata 2014); and one study was in patients undergoing non‐cardiac surgery under general anaesthesia (Lurati 2012).
Only seven studies (2011 participants) evaluated interventions in a general medical or geriatric medical hospital environment (Abizanda 2011; Al‐Aama 2011; Bonaventura 2007; Boustani 2012; Hatta 2014; Jeffs 2013; Martinez 2012 ).
Participants
Age
In 29 studies, participants had a mean age in both allocation arms of more than 70 years. Six studies had a mean age of less than 70 years in one or both groups (Chan 2013; Liptzin 2005; Radtke 2013; Sampson 2007; Stoppe 2013; Whitlock 2015); and two studies had very low mean age of included participants, Urban 2008 (mean age 53 and 48 years in the intervention and control groups respectively) and Leung 2006 (overall mean age 59.6 years). Two studies did not present data on the mean age of participants (Bonaventura 2007; Papaioannou 2005).
Co‐morbidities
Eight studies used the Charlson Index (Charlson 1994) (Boustani 2012; de Jonghe 2014; Hatta 2014; Jeffs 2013; Leung 2006; Marcantonio 2001; Martinez 2012; Sieber 2010) to compare co‐morbidities between intervention and control groups. One study (Boustani 2012), reported higher Charlson Index scores in the usual care group.
Five studies presented the total number of co‐morbidities present for intervention and control groups (Abizanda 2011; Al‐Aama 2011; Bonaventura 2007; Diaz 2001; Hempenius 2013).
Nine studies presented the frequency of a range of specific co‐morbidities in both the intervention and control groups (Ashraf 2015; Berggren 1987; Chan 2013; Gruber‐Baldini 2013; Jia 2014; Lundstrom 2007; Lurati 2012; Pesonen 2011; Whitlock 2015). Lundstrom 2007 reported a difference between the intervention and control arms, with a higher rate of depression in the control group, and Ashraf 2015 had higher rates of coronary artery disease in the usual care group and higher rates of depression in the intervention group.
Seventeen studies did not report co‐morbidities at baseline (Aizawa 2002; Beaussier 2006; Fukata 2014; Gauge 2014; Kalisvaart 2005; Larsen 2010; Li 2013; Liptzin 2005; Marcantonio 2011; Mouzopoulos 2009; Munger 2008; Papaioannou 2005; Radtke 2013; Sampson 2007; Stoppe 2013; Urban 2008; Watne 2014).
Dementia
Eleven of the included studies excluded participants with dementia. This included using dementia diagnosis as an exclusion criteria (Diaz 2001; Jia 2014; Larsen 2010) or based on performance in cognitive testing (Ashraf 2015; Berggren 1987; Bonaventura 2007; Chan 2013; Li 2013; Papaioannou 2005; Radtke 2013; Stoppe 2013), most commonly using the Mini‐Mental State Examination (MMSE) score (Folstein 1975).
There were three studies where the proportion of participants with dementia differed between the intervention and control groups: in Gruber‐Baldini 2013, it was 27.3% in intervention versus 36.1% in control; in Lundstrom 2007, 27.5% in intervention versus 37.1% in control; and in Marcantonio 2001, 37% in intervention and 51% in control.
Interventions
Multi‐component interventions
Seven studies (2018 participants) evaluated non‐pharmacological multi‐component interventions (Abizanda 2011; Bonaventura 2007; Hempenius 2013; Jeffs 2013; Lundstrom 2007; Marcantonio 2001; Martinez 2012) in comparison to usual care. Individual components of each multi‐component intervention are summarised in Table 1. The number of components varied between two (Jeffs 2013) and 13 (Hempenius 2013) (Table 1). Most included individualised care, an educational component, reorientation, and early mobilisation. Many of the delirium risk factors targeted with multi‐component interventions relate to good basic care. The nature in which interventions were implemented varied between the studies: some relied on a protocol‐driven approach (Bonaventura 2007; Jeffs 2013; Marcantonio 2001), whilst others were more pragmatic in the delivery of the intervention (e.g. the family delivered the reorientation intervention in Martinez 2012). Two studies were based on therapist interventions (Abizanda 2011; Jeffs 2013), one was multidisciplinary including a Comprehensive Geriatric Assessment (Lundstrom 2007), and two were based on proactive perioperative input from a geriatrician (Hempenius 2013; Marcantonio 2001).
| Study | Intervention Components | |||||||||||||||||||
| Individualised care | Checklists/ protocols | Education/ training1 | Re‐orientation | Attention to sensory deprivation | Familiar objects | Cognitive stimulation | Nutrition/ hydration | Identification of infection | Mobilisation | Sleep hygiene | MDTcare2 | CGA3 | Oxygenation | Electrolytes | Pain control | Medication review | Mood4 | Bowel/ bladder care | Postoperative complications | |
| Abizanda 2011 | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||
| Bonaventura 2007 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||
| Jeffs 2013 | ✔ | ✔ | ||||||||||||||||||
| Martinez 2012 | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||
| Hempenius 2013 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||
| Lundstrom 2006 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||
| Marcantonio 2001 | ✔ | |||||||||||||||||||
1Education/training: structured education/training of staff or carers; 2MDT Multidisciplinary Team; 3CGA Comprehensive Geriatric Assessment; 4Mood: assessment for depression/anxiety
Pharmacological interventions
Thirteen studies assessed various pharmacological agents.
Although the pathophysiology of delirium remains unclear, acetylcholine is the neurotransmitter that has been most implicated in studies (Koponen 1999; Tune 1999), leading to suggestions that cholinesterase inhibitors may have a role in delirium management. Four studies tested the use of prophylactic cholinesterase inhibitors (Liptzin 2005; Marcantonio 2011; Munger 2008; Sampson 2007).
Three studies assessed antipsychotic medication (Fukata 2014; Kalisvaart 2005; Larsen 2010).
Melatonin is a hormone that has a role in sleep/wake regulation, and may be responsible for the disruption of the sleep/wake cycle seen in delirium (Figueroa‐Ramos 2009). This has led to suggestions that it could have a role in delirium prevention (Lewis 2004). Melatonin supplementation has been proposed as a treatment option for delirium (Bourne 2006), and there is case report evidence of its usefulness (Hanania 2002). Two studies investigated the use of melatonin (Al‐Aama 2011; de Jonghe 2014 ); and one used a melatonin agonist (Hatta 2014).
Citicoline (cytidine 5′‐diphosphocholine (CDP‐choline)), is a drug that has been implicated in cognitive impairment and memory, and therefore has been proposed as a treatment in traumatic brain injury, stroke, vascular dementia, Parkinson’s disease, and brain aging (Fioravanti 2006a). Citicoline has the function in the brain of stabilising cell membranes and reducing the presence of free radicals. In particular, there is some evidence that citicoline stimulates the release of dopamine neurotransmitters in the brain (Fioravanti 2005). One study tested citicoline (Diaz 2001).
Diazepam is a long‐acting benzodiazepine which is often used as an anxiolytic and has been used in the cardiac catheterisation setting with good effect (Woodhead 2007). Diphyenhydramine is an antihistamine medication which can cause sedation and has been used as an adjunct for individuals undergoing colonoscopy with good effect (Tu 2006). Evidence regarding premedication and postoperative delirium is unclear (Fines 2006) with concern that administering these medications may increase rates of post‐procedure or postoperative delirium. One study evaluated the combination of diazepam and diphenhydramine as premedication before cardiac catheterisation (Ashraf 2015).
Methylprednisolone is an intravenous steroid preparation with a wide range of clinical uses. Steroid use has been thought to be beneficial to individuals undergoing cardiopulmonary bypass, with evidence of reduction in new onset atrial fibrillation, postoperative bleeding and length of stay in the intensive care unit (ICU) (Whitlock 2008). A subsequent clinical trial failed to show benefit for the entire population undergoing cardiopulmonary bypass, but subgroup analysis suggested those at higher risk of adverse outcomes may benefit (Dieleman 2012). This formed the basis of the design of Whitlock 2015, evaluating methylprednisolone for those at high risk undergoing cardiopulmonary bypass, with incidence of delirium as a safety outcome measure.
Perioperative interventions
Postoperative delirium is a common complication of surgery in older people (Holmes 2000; Santos 2004; Williams‐Russo 1992), likely to be a consequence of the physiological and biochemical derangement induced by the underlying pathology, surgical trauma pain and anaesthesia. Perioperative care is, therefore, a potential focus for interventions to reduce postoperative delirium.
In surgical practice, there has been a move towards a concept of ‘enhanced recovery’ whereby surgical intervention, anaesthesia and postoperative care are modified in such a way as to minimise the overall impact of surgery, reducing postoperative complications and expediting recovery (Douglas 2001). Many postoperative complications (e.g. ileus, respiratory depression, chest infections, and myocardial ischaemia, all of which may predispose to delirium) could be reduced by the use of regional anaesthesia and opioid‐sparing analgesics (Bonnet 2005).
Eighteen studies tested various interventions addressing modifications to perioperative practice that might influence postoperative delirium. These are subdivided into five broad approaches; i) those that reduce opioid utilisation, ii) those that control/reduce depth of general anaesthesia, iii) those that consider alternative forms of general anaesthesia, iv) those which avoid general anaesthesia altogether and v) a miscellaneous group including studies investigating transfusion practice, fast track surgery and a 'delirium‐free protocol'.
i) Opioid‐sparing measures:
Techniques to reduce opioid utilisation include the administration of adjuvant analgesics; addition of intrathecal opioid to general anaesthesia; and peripheral local anaesthetic blockade. These were tested in six studies.
Gabapentinoids are commonly used for treatment of epilepsy, anxiety, and neuropathic pain, but also have a role as opioid‐sparing adjuncts for postoperative pain relief (Tippana 2007). Leung 2006 tested gabapentin and Pesonen 2011 tested pregabalin.
Ketamine is widely used as an adjuvant analgesic in a variety of perioperative pain settings (Bell 2006). Urban 2008 investigated the effect of adding ketamine at induction of anaesthesia as a postoperative infusion.
Parecoxib sodium is an intravenous analgesic preparation called a pro‐drug of another medication, valdecoxib, which is a selective cyclo‐oxygenase‐2 inhibitor (Cheer 2001). The use of non‐opioid adjuvant analgesia is a recognised approach to reduce the need for opiate medication and thus the associated side effects, particularly for older adults (Aubrun 2007). One study compared a regimen of regular intravenous parecoxib to a dose of morphine followed by administration of saline as postoperative analgesia, with morphine doses available to either group based on their pain scores.
The use of a ‘single shot spinal’ combined with general anaesthesia and patient controlled analgesia (PCA) is increasingly used as an alternative to continuous epidural infusions for intra and postoperative analgesia. The premise is that intrathecal opioid, with or without local anaesthetic adequately replaces an epidural regarding its intended benefits of reduced intraoperative and immediate postoperative opioid requirements, but without prolonged motor block or hypotension that would impede immediate postoperative mobilisation. Beaussier 2006 tested using a 'single shot spinal’ with general anaesthesia compared to general anaesthesia alone; and Mouzopoulos 2009 tested a fascia iliac compartment block performed every 24 hours from admission to discharge compared to treatment with paracetamol and intramuscular pethidine for patients with a fractured neck of femur.
ii) Controlling/reducing the depth of anaesthesia:
Finer titration of depth of anaesthesia could reduce delirium. Bispectral index (BIS), a number derived from analysis of the EEG, is increasingly used to monitor depth of anaesthesia. A BIS value of 100 is equivalent to full awareness and a value of 0 represents no electrical activity.
Sieber 2010 investigated light compared to deep sedation. Light sedation was represented by a BIS value of 80 and a patient responsive to vocal commands; and deep sedation by a BIS value of 50 and a patient unresponsive to noxious stimuli (i.e. equivalent to the effect of a general anaesthetic). Chan 2013 compared BIS‐guided anaesthesia to routine general anaesthesia with propofol. In the BIS‐guided group, the propofol infusion was titrated to maintain a BIS value of 40 to 60, whereas in the routine group anaesthesia was titrated according to clinical judgement. Radtke 2013 compared BIS‐guided and BIS‐blinded groups undergoing induction and maintenance of general anaesthesia and postoperative analgesia for a range of surgical interventions. Gauge 2014 compared targeted BIS and cerebral oxygenation monitoring for patients undergoing coronary bypass grafting compared to no BIS and oxygenation monitoring.
iii) Changing the mode of general anaesthesia:
Two studies explored the effect of mode of general anaesthesia, one using propofol (Stoppe 2013) and the other xenon (Lurati 2012), compared to sevoflurane.
iv) Avoiding general anaesthesia:
Two studies compared regional anaesthesia with general anaesthesia (Berggren 1987; Papaioannou 2005).
v) Miscellaneous perioperative interventions:
The remaining three studies each tested a different perioperative intervention.
Intraoperative blood transfusion has been implicated as a risk factor postoperative delirium (Carson 2011; Robinson 2009), although there are likely to be other aspects of the individual's condition or care which also influence the risk of developing delirium (Edelstein 2004). Gruber‐Baldini 2013 tested the use of liberal versus restrictive blood transfusion thresholds.
Jia 2014 tested fast‐track surgery compared to usual care; this approach as a means of reducing delirium and postoperative cognitive dysfunction has been suggested previously (Krenk 2012). The fast‐track approach tested by Jia 2014 included alterations in the preoperative preparation, anaesthesia, pain control and postoperative management compared to traditional care. This included: bowel preparation with oral purgatives rather than enemas, shorter period of fasting, avoidance of nasogastric tube, epidural rather than general anaesthesia and earlier removal of urinary catheter and mobilisation on the first postoperative day.
Aizawa 2002 tested a postoperative delirium‐free protocol (DFP), which contained benzodiazepines and pethidine compared to usual care. They administered intramuscular diazepam at 8 pm with a continuous infusion of flunitrazepam to maintain sleep and pethidine for analgesia, given for eight hours for the first three nights after surgery.
Computerised clinical decision support (CCDS)
Computerised clinical decision support software (CCDS) has been reported as an effective tool in prompting healthcare practitioners to comply with established protocols and preventive measures (Dexter 2001). It has also been trialled for improving the care of patients with delirium superimposed on dementia (Fick 2011). One study in our review (Boustani 2012), investigated the use of CCDS in medical inpatients.
Care in geriatric medicine unit versus orthopaedic unit following hip fracture
Individuals admitted following a fracture are typically placed under the care of an orthopaedic surgeon, pending operative intervention. However, the complex nature of the predominantly older adult population who experience a hip fracture has led to the emergence of orthogeriatric services, where input is also received from geriatricians. Comprehensive geriatric assessment (CGA) is an evidence‐based "multidimensional interdisciplinary diagnostic process used to determine the medical, psychological and functional capabilities of a frail older person to develop a coordinated and integrated plan for treatment and long‐term follow‐up" associated with improved outcomes, particularly when delivered in a dedicated ward (Ellis 2011). Watne 2014 designed their trial around their local service reconfiguration where older adults were admitted to their specialist geriatric medicine unit and received CGA comparing this to the care received in the orthopaedic unit.
Outcomes
Primary outcome
The incidence of delirium was recorded using several validated instruments, used singly or in combination.
In 15 studies, the Confusion Assessment Method (CAM) (Inouye 1990) alone was used to determine delirium incidence (Abizanda 2011; Ashraf 2015; Beaussier 2006; Boustani 2012; Chan 2013; Gauge 2014; Jeffs 2013; Leung 2006; Lurati 2012; Marcantonio 2001; Martinez 2012; Munger 2008; Sieber 2010; Urban 2008; Whitlock 2015). However, Munger 2008 presented data for the mean CAM score, rather than using the CAM score to determine delirium presence as a dichotomous outcome. The CAM‐ICU (Ely 2001) was used in two studies (Pesonen 2011; Stoppe 2013), although Pesonen 2011 used it as a continuous measure. Diagnostic and Statistical Manual (DSM‐III and DSM‐IV)criteria alone were used in five studies (Aizawa 2002; Li 2013; Lundstrom 2007; Papaioannou 2005; Radtke 2013). Jia 2014 used the DRS‐R‐98 (Trzepacz 2001) to diagnose incident delirium. Berggren 1987 used the Modified Organic Brain Syndrome Scale (OBS) (Gustafson 1985); Fukata 2014 used the NEECHAM confusion scale (Neelon 1996); and Sampson 2007 used the Delirium Symptom Interview (DSI) (Albert 1992).
Ten studies used multiple instruments for assessing delirium, some of which included measures to assess delirium severity. The CAM (Inouye 1990) and Memorial Delirium Assessment Scale (MDAS) (Breitbart 1997) were used by Al‐Aama 2011; Gruber‐Baldini 2013; Marcantonio 2011 and Watne 2014. However, Marcantonio 2011 only reported aggregated data for repeated CAM assessments within the same participant, which could not, therefore, be included in analysis of the primary outcome. Bonaventura 2007 used the CAM and DRS‐R‐98. DSM III‐R or IV were used in addition to the CAM by Kalisvaart 2005; to which Hatta 2014; Larsen 2010 and Mouzopoulos 2009 added the DRS‐R‐98; while Liptzin 2005 added the DSI. de Jonghe 2014 also used the Delirium Observation Screening Scale (DOSS) (Schuurmans 2003) in addition to DSM‐IV. Hempenius 2013 used the DOSS which, if positive, resulted in an assessment using DSM‐IV criteria and the DRS‐R‐98.
Frequency of primary outcome assessment
Nineteen studies assessed for delirium on a daily basis (Abizanda 2011; de Jonghe 2014; Diaz 2001; Fukata 2014; Hatta 2014; Hempenius 2013; Jia 2014; Kalisvaart 2005; Larsen 2010; Leung 2006; Liptzin 2005; Marcantonio 2001; Martinez 2012; Mouzopoulos 2009; Munger 2008; Papaioannou 2005; Pesonen 2011; Stoppe 2013;Watne 2014 ). Marcantonio 2011 assessed for delirium daily until discharge and again at two, four and six weeks after recruitment.
Three studies assessed delirium several times a day: Radtke 2013 and Aizawa 2002 conducted delirium assessments twice daily and Sampson 2007 assessed three times daily.
Delirium assessments were performed on days one, two, four and seven following admission by Bonaventura 2007, and on the first and seventh postoperative day by Berggren 1987. Al‐Aama 2011 assessed participants every 24 to 48 hours and Jeffs 2013 assessed every 48 hours. Boustani 2012 assessed participants every weekday. Urban 2008 assessed for delirium on postoperative day (POD) one; Lurati 2012 assessed on POD one, two and seven; and Sieber 2010 assessed on POD two and daily thereafter.
At the end of one study (Lundstrom 2007), a retrospective case notes review was performed by a blinded independent investigator to identify delirium according to DSM‐IV criteria for each postoperative day until discharge. A single delirium assessment with the OBS was also performed between the third and fifth postoperative day in this study. In Gauge 2014, delirium assessment was performed on day three +/‐ one day. Whitlock 2015 assessed only on postoperative day three, and Li 2013 assessed on postoperative day three and at one, three and six months. Ashraf 2015 assessed for delirium four hours post‐procedure and on the following day.
In three studies the specific frequency of delirium assessment was unclear (Beaussier 2006; Chan 2013; Gruber‐Baldini 2013), but described as 'regularly', 'throughout study period' or 'multiple times'.
Secondary outcomes
Duration of delirium was reported by 12 studies (de Jonghe 2014; Fukata 2014; Jeffs 2013; Kalisvaart 2005; Larsen 2010; Liptzin 2005; Lundstrom 2007; Marcantonio 2001; Martinez 2012; Mouzopoulos 2009; Sieber 2010; Watne 2014). Severity of delirium was reported by 11 studies (Al‐Aama 2011; de Jonghe 2014; Gruber‐Baldini 2013; Hatta 2014; Hempenius 2013; Jeffs 2013; Kalisvaart 2005; Larsen 2010; Marcantonio 2011; Mouzopoulos 2009; Watne 2014).
Fourteen studies reported data on cognitive outcomes (Ashraf 2015; Beaussier 2006; Bonaventura 2007; Chan 2013; de Jonghe 2014; Diaz 2001; Larsen 2010; Li 2013; Munger 2008; Papaioannou 2005; Pesonen 2011; Radtke 2013; Sieber 2010; Watne 2014). Mode of cognitive assessment varied: Ashraf 2015; Bonaventura 2007, Diaz 2001, Larsen 2010; Munger 2008; Papaioannou 2005 and Sieber 2010 used the Mini Mental State Examination (MMSE) (Folstein 1975); Beaussier 2006 assessed the number of days for MMSE to return to preoperative level; Chan 2013; Li 2013; Radtke 2013 and Watne 2014 assessed for postoperative cognitive dysfunction; Pesonen 2011 used the CAM‐ICU score on day five; and de Jonghe 2014 used IQCODE (Jorm 1989) and MMSE (Folstein 1975) assessment at three months follow‐up.
Length of hospital admission was a commonly used outcome measure, with only 11 of the included studies not reporting on this outcome (Bonaventura 2007; Diaz 2001; Fukata 2014; Gauge 2014; Hatta 2014; Larsen 2010; Leung 2006; Lurati 2012; Marcantonio 2011; Mouzopoulos 2009; Urban 2008).
Other secondary outcomes which were reported less frequently included: activities of daily living (Abizanda 2011; Watne 2014); behavioural disturbance (Aizawa 2002); activities of daily living performance (Abizanda 2011; de Jonghe 2014; Watne 2014); psychotropic medication use (Al‐Aama 2011; de Jonghe 2014; Gruber‐Baldini 2013; Pesonen 2011); return to previous residence or independent living (Hempenius 2013; Jeffs 2013; Lundstrom 2007; Marcantonio 2001); and institutionalisation (Watne 2014).
Adverse Outcomes
Only 15 of the included studies reported data on mortality, either in hospital or at follow‐up at three or 12 months (Abizanda 2011; Al‐Aama 2011; Beaussier 2006; Boustani 2012; Chan 2013; de Jonghe 2014; Hatta 2014; Lundstrom 2007; Lurati 2012; Mouzopoulos 2009; Radtke 2013; Sieber 2010; Stoppe 2013; Watne 2014; Whitlock 2015).
Other adverse outcomes reported include: adverse events (Abizanda 2011; Hatta 2014; Kalisvaart 2005; Marcantonio 2011; Sampson 2007); physical morbidity (Berggren 1987; Boustani 2012; Gruber‐Baldini 2013; Larsen 2010; Watne 2014); psychological morbidity (Berggren 1987; Chan 2013; Hempenius 2013; Lundstrom 2007); postoperative complications (Chan 2013; Hempenius 2013; Jia 2014; Papaioannou 2005; Sieber 2010; Whitlock 2015); falls (Boustani 2012; Hempenius 2013; Lundstrom 2007; Martinez 2012; Watne 2014); and pressure ulcers (Berggren 1987; Boustani 2012; Lundstrom 2007; Watne 2014).
Exclusion of prevalent delirium at baseline
Failure to exclude delirium at enrolment to the study was a common problem among included studies. Only 10 studies clearly excluded or accounted for prevalent cases of delirium at baseline (Abizanda 2011; Ashraf 2015; Boustani 2012; de Jonghe 2014; Hatta 2014; Gruber‐Baldini 2013; Jeffs 2013; Kalisvaart 2005; Martinez 2012; Sieber 2010).
Funding sources and declarations of interest
Most of the studies (24 out of 39) were funded via academic or governmental research institutions or grant funding schemes. Four studies were solely industry funded (Boustani 2012; Liptzin 2005; Munger 2008; Sampson 2007) and two received joint academic and industry funding (Lurati 2012; Radtke 2013). In nine studies the funding source was not reported (Aizawa 2002; Ashraf 2015; Bonaventura 2007; Diaz 2001; Gauge 2014; Jia 2014; Martinez 2012; Mouzopoulos 2009; Sieber 2010).
Eight studies reported there were potential interests to declare related to their publication (Boustani 2012; Gruber‐Baldini 2013; Hatta 2014; Larsen 2010; Leung 2006; Liptzin 2005; Lurati 2012; Stoppe 2013), which are listed in the Characteristics of included studies tables. Fourteen studies did not report on a declaration of interest (Aizawa 2002; Ashraf 2015; Beaussier 2006; Berggren 1987; Bonaventura 2007; Gauge 2014; Li 2013; Lundstrom 2007; Marcantonio 2001; Munger 2008; Papaioannou 2005; Sampson 2007; Sieber 2010;Urban 2008).
Excluded studies
We excluded 69 studies. Reasons for exclusion are given in Characteristics of excluded studies. Details of 27 studies identified as ongoing are given in Characteristics of ongoing studies.
Risk of bias in included studies
'Risk of bias' assessments are presented for each study in the 'Characteristics of included studies' table and are summarised in the text below and graphically in Figure 2. Only one study (Whitlock 2015) was assessed as at low risk of bias across all domains.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only one study (Bonaventura 2007) was assessed as high risk for selection bias considering both allocation concealment and random sequence generation. This was as a consequence of using the day of admission as the basis for their randomisation, which cannot be concealed. Nine studies (Aizawa 2002; Ashraf 2015; Berggren 1987; Gauge 2014; Liptzin 2005; Munger 2008; Radtke 2013; Sieber 2010; Stoppe 2013) were considered as unclear risk for selection bias on both criteria. This assessment was primarily made on the grounds of a lack of detail in the published report around the methods of generating the sequence and allocating participants to groups.
Blinding
Twenty‐three of the included studies (Abizanda 2011; Aizawa 2002; Ashraf 2015; Berggren 1987; Bonaventura 2007; Boustani 2012; Chan 2013; Fukata 2014; Gruber‐Baldini 2013; Hatta 2014; Hempenius 2013; Jeffs 2013; Jia 2014; Lundstrom 2007; Lurati 2012; Marcantonio 2001; Martinez 2012; Mouzopoulos 2009; Papaioannou 2005; Radtke 2013; Stoppe 2013; Urban 2008; Watne 2014) were assessed as high risk for performance bias as participants and personnel were not blinded to their allocation, often due to the nature of the intervention precluding such concealment. However, only six studies (Ashraf 2015; Fukata 2014; Gruber‐Baldini 2013; Lundstrom 2007; Martinez 2012; Watne 2014) were assessed at high risk of both performance and detection bias due to the assessors being unblinded in addition to participants and personnel. A further eight studies (Beaussier 2006; Bonaventura 2007; Boustani 2012; Gauge 2014; Jia 2014; Mouzopoulos 2009; Munger 2008; Papaioannou 2005) were assessed as unclear risk for detection bias due to a lack of reporting.
Incomplete outcome data
Ten studies were assessed as high risk for attrition bias (Al‐Aama 2011; Chan 2013; Larsen 2010; Liptzin 2005; Mouzopoulos 2009; Papaioannou 2005; Pesonen 2011; Radtke 2013; Sampson 2007; Urban 2008). This was due to incomplete reporting of losses or concerns about reasons for exclusion of participants. In particular, there were concerns about exclusions which may influence ascertainment of the primary outcome (delirium incidence) e.g. participants being too unwell to be assessed or developing postoperative complications. A further seven studies were considered at unclear risk for attrition bias (Aizawa 2002; Diaz 2001; Fukata 2014; Gauge 2014; Hempenius 2013; Kalisvaart 2005; Munger 2008. In these cases it was not possible to assess the potential bias associated with loss of participants due to a lack of detail in study reports.
Selective reporting
Three studies were assessed as high risk of reporting bias (Beaussier 2006; Berggren 1987; Lurati 2012). In all cases this was due to the reporting of outcomes not stated in the protocol or the methods for the study. Twelve studies were considered at low risk of reporting bias (Abizanda 2011; de Jonghe 2014; Gruber‐Baldini 2013; Hatta 2014; Hempenius 2013; Jeffs 2013; Larsen 2010; Marcantonio 2011; Radtke 2013; Stoppe 2013; Watne 2014; Whitlock 2015), with evidence of published protocols, formal trial registration or clear statement in relation to reporting contained in the published text. The remainder were assessed as unclear risk.
Other potential sources of bias
Seven studies were assessed as high risk of bias in this category (Aizawa 2002; Gruber‐Baldini 2013; Li 2013; Lundstrom 2007; Marcantonio 2001; Papaioannou 2005; Watne 2014).
In Aizawa 2002 no account was taken of how delirium assessment may have been affected by the sedating effects of the delirium‐free protocol. Similarly in Papaioannou 2005, there were concerns about unbalanced use of neuraxial analgesia between groups, affecting delirium assessment. Li 2013 administered supplementary morphine to both groups depending on pain scores, but use of this is significantly unbalanced and this is not accounted‐for in the interpretation of delirium findings. In Watne 2014, there are concerns about the integrity of the intervention delivered as the trial was conducted pragmatically and when beds were not available in the specialist unit, patients were cared‐for in the corridor, but are counted in the intervention group.
The proportion of included participants with dementia was imbalanced in three studies (Gruber‐Baldini 2013; Lundstrom 2007; Marcantonio 2001). In all cases there was a lower proportion of individuals with dementia in the intervention arm than the control arm. This has the potential to affect rates of incident delirium as delirium is known to be more common in individuals with dementia (Fong 2015).
Publication of two studies as abstracts (Gauge 2014; Munger 2008) gave insufficient information to allow for other sources of bias to be assessed, resulting in an assessment of unclear risk.
Effects of interventions
See: Summary of findings for the main comparison A multi‐component delirium prevention intervention compared to usual care for hospitalised non‐ICU patients; Summary of findings 2 Prophylactic cholinesterase inhibitor versus placebo for preventing delirium in hospitalised non‐ICU patients; Summary of findings 3 Prophylactic antipsychotic medications for preventing delirium in hospitalised non‐ICU patients; Summary of findings 4 Prophylactic melatonin for preventing delirium in hospitalised non‐ICU patients; Summary of findings 5 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement for preventing delirium in hospitalised non‐ICU patients
1. Multi‐component interventions versus usual care
Seven studies investigated the effectiveness of multi‐component interventions for the prevention of delirium (Abizanda 2011; Bonaventura 2007; Hempenius 2013; Jeffs 2013; Lundstrom 2007; Marcantonio 2001; Martinez 2012). A summary of findings for key outcomes is presented in summary of findings Table for the main comparison.
a. Primary outcome
Available case analysis was performed on 1950 of 2018 randomised participants, using data from all seven studies. Pooled analysis showed evidence of a reduction in the incidence of delirium for multi‐component interventions compared to usual care (risk ratio (RR) 0.69, 95% confidence interval (CI) 0.59 to 0.81, I2 = 0%; 1950 participants. We assessed this as moderate‐quality evidence (downgraded due to risk of bias) (Analysis 1.1; Figure 3).

Forest plot of comparison: 1 Multi‐component delirium prevention intervention (MCI) versus usual care, outcome: 1.1 Incident delirium.
b. Secondary outcomes
We pooled data on the duration of delirium from four trials (Jeffs 2013; Lundstrom 2007; Marcantonio 2001; Martinez 2012). The mean difference between groups was ‐1.16 days (shorter in the intervention group) but there was uncertainty about the size and direction of the effect (mean difference (MD) ‐1.16, 95% CI ‐2.96 to 0.64, I2 = 58%; 244 participants; assessed as very low‐quality evidence due to imprecision, risk of bias and inconsistency) (Analysis 1.3).
Delirium severity was reported as an outcome in only two multi‐component intervention trials, each of which used different measures of severity (Hempenius 2013; Jeffs 2013). Compared with usual care the standardised mean difference (SMD) in delirium severity was ‐1.04 (lower with multi‐component interventions) (SMD ‐1.04, 95% CI ‐1.65 to ‐0.43, I2 = 25%; 67 participants; low‐quality evidence due to risk of bias and imprecision) (Analysis 1.4).
We pooled data from six studies, which reported length of hospital admission (Abizanda 2011; Hempenius 2013; Jeffs 2013; Lundstrom 2007; Marcantonio 2001; Martinez 2012). The mean length of hospital admission was 0.01 days longer in the intervention compared to the usual care group (MD 0.01, 95% CI ‐0.48 to 0.51, I2 = 13%; 1920 participants; moderate‐quality evidence due to risk of bias) (Analysis 1.5).
One study assessed cognition (Bonaventura 2007); there was a clinically important difference in the mean MMSE score favouring those receiving multi‐component interventions compared to usual care (MD 9.10, 95% CI 7.20 to 11.00; 60 participants; very low‐quality evidence due to risk of bias and serious imprecision) (Analysis 1.6).
Abizanda 2011 reported on the number of participants whose Barthel Index score (Mahoney 1965) improved by 10 points during admission, comparing this between the groups. There was no evidence of effect of multi‐component interventions on improvements in activities of daily living compared to usual care (RR 1.15, 95% CI 0.91 to 1.47; 341 participants; low‐quality evidence, downgraded due to risk of bias and imprecision) (Analysis 1.7).
Four studies (Hempenius 2013; Jeffs 2013; Lundstrom 2007; Marcantonio 2001) reported on return to independent living. Again, there was no evidence of effect of multi‐component interventions compared to usual care (RR 0.95, 95% CI 0.85 to 1.06, I2 = 30%; 1116 participants; moderate‐quality evidence, downgraded due to risk of bias) (Analysis 1.8).
Lundstrom 2007 assessed depression with the Geriatric Depression Scale‐15 (GDS‐15) (Sheikh 1986), but found no evidence of any important effect of the intervention on this outcome (MD 0.70, 95% CI ‐0.44 to 1.84; 149 participants; low‐quality evidence, downgraded due to risk of bias and imprecision) (Analysis 1.9).
One study reported no withdrawals from 126 participants (Marcantonio 2001) (Analysis 1.10).
c. Adverse outcomes
Data on falls were only available from three studies (Hempenius 2013; Lundstrom 2007; Martinez 2012), there was no evidence of effect from multi‐component interventions compared to usual care (RR 0.57, 95% CI 0.16 to 2.01, I2 = 50%; 746 participants; very low‐quality evidence, downgraded due to risk of bias, serious imprecision and inconsistency) (Analysis 1.11).
Rates of pressure ulcers were only reported in two studies (Hempenius 2013; Lundstrom 2007) where there was evidence of a reduced risk of pressure ulcer formation in those receiving multi‐component interventions compared to usual care (RR 0.48, 95% CI 0.26 to 0.89, I2 = 0%; 457 participants; low‐quality evidence downgraded, due to risk of bias and imprecision) (Analysis 1.12).
Inpatient mortality was reported in three studies (Abizanda 2011; Hempenius 2013; Lundstrom 2007), with no evidence of effect of multi‐component interventions on inpatient mortality (RR 0.90, 95% CI 0.56 to 1.43, I2 = 57%; 859 participants; very low‐quality evidence, downgraded due to risk of bias, imprecision and inconsistency) (Analysis 1.13).
Lundstrom 2007 also reported on 12‐month mortality and found no evidence of effect of multi‐component interventions (RR 0.85, 95% CI 0.46 to 1.56; 199 participants; low‐quality evidence due to risk of bias and imprecision) (Analysis 1.14).
Hempenius 2013 reported on postoperative complications and there was no evidence of effect of multi‐component interventions on cardiovascular adverse events (RR 1.13, 95% CI 0.78 to 1.65; 260 participants; moderate‐quality evidence due to imprecision) or urinary tract infections (RR 1.20, 95% CI 0.45 to 3.20; 260 participants; low‐quality evidence due to serious imprecision) (Analysis 1.15; Analysis 1.16). Hempenius 2013 also reported on psychological morbidity, reporting SF‐36 scores for mental health (Ware 1992), dichotomized to having worsened versus improvement/stayed the same and there was no evidence of effect found (RR 0.88, 95% CI 0.64 to 1.20; 246 participants; moderate‐quality evidence due to imprecision) (Analysis 1.17).
Subgroup analysis by setting
The pre‐planned subgroup analysis assessed multi‐component delirium prevention trials in four medical (Abizanda 2011; Bonaventura 2007; Jeffs 2013; Martinez 2012) and three surgical (Hempenius 2013; Lundstrom 2007; Marcantonio 2001) settings. There were similar effect sizes in medical (RR 0.63, 95% CI 0.43 to 0.92; 1365 participants) and surgical (RR 0.71, 95% CI 0.59 to 0.85; 585 participants) settings in favour of the intervention reducing incident delirium (moderate‐quality evidence due to risk of bias for both) (Analysis 1.1; Figure 3).
Subgroup analysis by cognitive impairment
Only one trial (Marcantonio 2001) reported incident delirium in patients with pre‐existing dementia. In the intervention group 37% of participants were known to have dementia, compared to 51% of those in the control group. Delirium incidence was lower in patients receiving a multi‐component intervention in this subgroup also. However, the results are too imprecise to allow a conclusion to be drawn (RR 0.90, 95% CI 0.59 to 1.36; 50 participants; low‐quality evidence, downgraded due to risk of bias and imprecision) (Analysis 1.2).
2. Cholinesterase inhibitors versus placebo
Four studies investigated the effect of the cholinesterase inhibitor donepezil in the prevention of delirium (Liptzin 2005; Marcantonio 2011; Munger 2008; Sampson 2007). A 'Summary of findings' table for key outcomes is presented in summary of findings Table 2.
a. Primary outcome
Data from only two of these four studies (Liptzin 2005; Sampson 2007) could be used to estimate the primary outcome, delirium incidence, as Marcantonio 2011 reported repeated CAM measures within the same individuals, and Munger 2008 reported mean CAM scores only. There was no evidence of effect of cholinesterase inhibitors on incident delirium (RR 0.68, 95% CI 0.17 to 2.62, I2 = 60%; 113 participants; very low‐quality evidence due to risk of bias, serious imprecision and inconsistency) (Analysis 2.1; Figure 4).
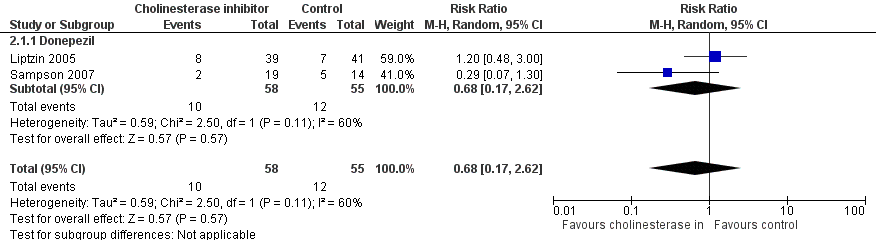
Forest plot of comparison: 2 Prophylactic cholinesterase inhibitor versus placebo, outcome: 2.1 Incident delirium.
b. Secondary outcomes
The effect of cholinesterase inhibitors on the duration of delirium episodes was assessed by Liptzin 2005, but no summary estimate was calculable due to the limited data available (Analysis 2.2).
The effect of cholinesterase inhibitors on the severity of delirium episodes was assessed by Marcantonio 2011 who reported no evidence of effect (MD ‐0.30, 95% CI ‐4.17 to 3.57; 16 participants; low‐quality evidence, downgraded two levels due to serious imprecision) (Analysis 2.3).
Pooled data from three studies reporting length of hospital admission (Liptzin 2005; Munger 2008; Sampson 2007) showed a mean difference of ‐0.34 days with cholinesterase inhibitor treatment compared to placebo (MD ‐0.34, 95% CI ‐1.54 to 0.86, I2 = 45%; 128 participants; low‐quality evidence downgraded due to risk of bias and imprecision) (Analysis 2.4).
One study examining the effect of cholinesterase inhibitor on cognition (Munger 2008) found no evidence of effect on MMSE (Folstein 1975) scores (MD ‐1.40, 95% CI ‐4.45 to 1.65; 15 participants; very low‐quality evidence, downgraded due to risk of bias and serious imprecision) (Analysis 2.5).
Two studies reported withdrawals from protocol (Liptzin 2005; Marcantonio 2011), finding no evidence of effect with cholinesterase inhibitor use compared to placebo (RR 0.95, 95% CI 0.49 to 1.87, I2 = 0%; 96 participants; low‐quality evidence, downgraded due to risk of bias and imprecision) (Analysis 2.6).
c. Adverse outcomes
Adverse events were reported in two studies in different formats. Sampson 2007 reported the mean adverse events in each group and found no evidence of difference in occurrence between groups (MD 0.13, 95% CI ‐0.26 to 0.52; 33 participants; low‐quality evidence due to risk of bias and imprecision) (Analysis 2.7). Marcantonio 2011 reported adverse events as a binary outcome and found a higher rate of adverse events in the cholinesterase inhibitor group compared to placebo (RR 6.25, 95% CI 0.35 to 112.52; 16 participants; low‐quality evidence downgraded two levels due to serious imprecision) (Analysis 2.8).
3. Antipsychotics versus placebo
Three studies investigated the effect of antipsychotic medication in the prevention of delirium (Fukata 2014; Kalisvaart 2005; Larsen 2010). A 'Summary of findings' table for key outcomes is presented in summary of findings Table 3.
a. Primary outcome
Two large studies evaluated antipsychotic medication versus placebo in elderly orthopaedic patients and one smaller study assessed those undergoing abdominal or orthopaedic surgery. Kalisvaart 2005 assessed oral haloperidol, a first generation (typical) antipsychotic preparation in 430 participants; data were available for 395 participants for available case analysis. Fukata 2014 administered prophylactic intravenous haloperidol to 121 patients from postoperative days one to three. Larsen 2010 tested oral olanzapine, a second generation (atypical) antipsychotic in 495 participants, with data for available case analysis for 400.
Pooled analysis of all three studies was inconclusive regarding an effect of antipsychotic treatment on incident delirium, but there was moderate heterogeneity between the studies (RR 0.73, 95% CI 0.33 to 1.59, I2= 90%; 916 participants; very low‐quality evidence due to risk of bias, imprecision and inconsistency) (Analysis 3.1; Figure 5).

Figure 5Forest plot of comparison: 3 Prophylactic antipsychotic versus control, outcome: 3.1 Incidence of delirium.
Subgroup analysis
The pre‐planned subgroup analysis assessed the effect of typical and atypical antipsychotics separately on delirium incidence. There was no evidence of effect of haloperidol on delirium incidence (RR 1.05, 95% CI 0.69 to 1.60, I2= 43%; two studies; 516 participants; low‐quality evidence downgraded due to risk of bias and inconsistency). However, the risk of incident delirium was lower with olanzapine than with placebo (RR 0.36, 95% CI 0.24 to 0.52; one study; 400 participants; moderate‐quality evidence due to risk of bias) (Figure 5).
b. Secondary outcomes
All three studies reported duration of delirium episodes. However, Fukata 2014 present mean duration data without a standard deviation so they could not be included in the quantitative analysis. Between the other two studies there was serious heterogeneity in duration findings. Haloperidol showed a large effect size, with a shorter duration of delirium in the intervention group compared to control (MD ‐6.40 days, 95% CI ‐9.38 to ‐3.42; one study; 68 participants). Olanzapine showed a longer duration for the intervention group (MD 0.60 days, 95% CI 0.10 to 1.10; one study; 110 participants). The pooled analysis of both showed a mean difference in delirium duration between intervention and control groups of ‐2.74 days (95% CI ‐9.59 to 4.11, I2 = 95%; 178 participants; very low‐quality evidence due to serious imprecision and inconsistency) (Analysis 3.2).
Both Kalisvaart 2005 and Larsen 2010 reported severity of delirium episodes, although there was serious heterogeneity between studies as before. Haloperidol showed a large effect size, with a reduction in severity of delirium in the intervention group compared to control (MD ‐4.00, 95% CI ‐5.86 to ‐2.14; 68 participants). Olanzapine showed an increased severity for the intervention group (MD 1.90, 95% CI 0.41 to 3.39; 110 participants). Pooled analysis showed no evidence of effect in delirium severity with antipsychotic treatment (MD ‐1.02, 95% CI ‐6.80 to 4.76, I2 = 96%; 178 participants; very low‐quality evidence due to serious imprecision and inconsistency) (Analysis 3.3).
Length of admission was only reported in one study (Kalisvaart 2005), which showed a mean difference of ‐5.50 days for haloperidol compared to placebo (95% CI ‐12.17 to 1.17; 68 participants; low‐quality evidence, downgraded two levels due to serious imprecision in results (Analysis 3.4).
Cognitive testing, using MMSE (Folstein 1975) was performed on the first day of the delirium episode by Larsen 2010. Those who received olanzapine had lower MMSE scores (poorer cognitive function) than those treated with placebo (MD ‐4.90, 95% CI ‐7.42 to ‐2.38; 110 participants; low‐quality evidence due to serious imprecision) (Analysis 3.5).
There was no evidence of effect of treatment allocation on withdrawal from protocol in pooled analysis including Kalisvaart 2005 & Larsen 2010 (RR 0.92, 95% CI 0.68 to 1.24, I2 = 0%; 925 participants; moderate‐quality evidence due to risk of bias) (Analysis 3.6).
c. Adverse outcomes
Adverse events were reported by Kalisvaart 2005; there was no evidence of effect of haloperidol on adverse events (RR 0.39, 95% CI 0.10 to 1.43; 430 participants; low‐quality evidence due to risk of bias and imprecision) (Analysis 3.7). Larsen 2010 report data on the occurrence of pneumonia (RR 7.28, 95% CI 0.38 to 140.11; 400 participants), urinary tract infection (RR 0.26, 95% CI 0.03 to 2.31; 400 participants) and congestive heart failure (RR 1.04, 95% CI 0.07 to 16.52; 400 participants) and there was no evidence of effect of olanzapine on the risk of developing these adverse events (Very low‐quality evidence, downgraded due to risk of bias and serious imprecision) (Analysis 3.8; Analysis 3.9; Analysis 3.10).
4. Melatonin or melatonin agonists versus placebo
Three studies investigated the effect of melatonin or melatonin agonists in the prevention of delirium (Al‐Aama 2011; de Jonghe 2014; Hatta 2014). Outcome data relevant to this review were obtained from the authors of Hatta 2014 for 43 participants who were cared for in acute medical wards rather than ICU. A 'Summary of findings' table for key outcomes is presented in summary of findings Table 4.
a. Primary outcome
All three studies reported the primary outcome, delirium incidence. The pooled analysis showed no evidence of effect of melatonin on incident delirium (RR 0.41, 95% CI 0.09 to 1.89 I2 = 78%; 529 participants; very low‐quality evidence due to risk of bias, imprecision and inconsistency) (Analysis 4.1; Figure 6).
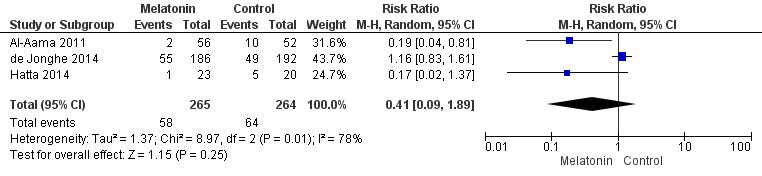
Forest plot of comparison: 4 Prophylactic melatonin versus placebo, outcome: 4.1 Incident delirium.
b. Secondary outcomes
Duration of delirium was only reported in one study (de Jonghe 2014). There was no evidence of a difference between melatonin and placebo groups in delirium duration (MD 0.00, 95% CI ‐0.57 to 0.57; 104 participants; moderate‐quality evidence downgraded due to imprecision) (Analysis 4.2) .
Severity of delirium was reported in all three studies but each in a different way. de Jonghe 2014 reported delirium severity as a binary outcome, severe or not severe (defined as >= 3 mg haloperidol administered during delirium episode). There was no evidence of a difference between melatonin and placebo groups in the occurrence of severe delirium (RR 0.86, 95% CI 0.58 to 1.27; 104 participants; moderate‐quality evidence due to imprecision) (Analysis 4.3) Al‐Aama 2011 reported delirium severity using MDAS (Breitbart 1997), however their results include those with prevalent as well as incident delirium and have not been included in the quantitative summary. Hatta 2014 reported delirium severity using the DRS‐R‐98 (Trzepacz 2001). There appeared to be a reduction in delirium severity in those receiving the melatonin agonist (RR ‐4.10, 95% CI ‐19.47 to 11.27; six participants), but the evidence was of low quality, downgraded two levels due to serious imprecision (Analysis 4.4).
Length of admission was reported in two studies, and there was no evidence of difference in admission duration between intervention and control groups (MD 0.09 days, 95% CI ‐1.20 to 1.39 days, I2 = 0%; 500 participants; moderate‐quality evidence due to imprecision) (Analysis 4.5).
de Jonghe 2014 assessed cognitive impairment using the Charlson index (Charlson 1994), IQCODE (Jorm 1989) and MMSE (Folstein 1975) at three‐month follow‐up. It appeared that those in the melatonin group had a lower risk of cognitive impairment, compared to those receiving placebo (RR 0.86, 95% CI 0.70 to 1.04; 378 participants). However, this evidence was of moderate quality due to imprecision of the result from a single study (Analysis 4.6).
There was no evidence of difference in performance of activities of daily living, using the Katz index (Katz 1970), in those receiving melatonin found by de Jonghe 2014 (MD 0.00, 95%CI ‐1.20 to 1.20; 369 participants; moderate‐quality evidence downgraded due to imprecision) (Analysis 4.7).
Al‐Aama 2011 examined rates of psychotropic medication use, and reported a high proportion of participants in both melatonin and control groups were prescribed these drugs (33/61 in melatonin group and 38/61 in the placebo group). There was no evidence of a difference in use, however, between groups (RR 0.87, 95% CI 0.64 to 1.18; 122 participants; moderate‐quality evidence due to imprecision) (Analysis 4.8). de Jonghe 2014 reported use of anti‐psychotic medications and benzodiazepines on a cumulative basis, looking at mean consumption of each drug class. There was evidence of reduced use of both anti‐psychotic medications (MD ‐1.00 mg, 95% CI ‐1.79 to ‐0.21 mg; 378 participants; moderate‐quality evidence downgraded as from a single study) and benzodiazepines (MD ‐11.60 mg, 95% CI ‐24.34 to 1.14 mg; 378 participants). However, in the case of benzodiazepine use the evidence was of low quality, downgraded due to serious imprecision (Analysis 4.9; Analysis 4.10).
Al‐Aama 2011 and Hatta 2014 also compared withdrawals from the study and found no evidence of a difference between melatonin and placebo groups (RR 1.00, 95% CI 0.15 to 6.87; 165 participants; low‐quality evidence, due to serious imprecision) (Analysis 4.11).
c. Adverse events
In‐hospital mortality was reported in all three studies and mortality at three months only by de Jonghe 2014. There was no evidence of effect on mortality rates with melatonin compared to placebo at either time‐period: In‐hospital mortality (RR 0.84, 95% CI 0.37 to 1.88, I2 = 0%; 543 participants; low‐quality evidence due to imprecision and low event rate) (Analysis 4.12) and three‐month mortality (RR 0.98, 95% CI 0.67 to 1.45; 378 participants; moderate‐quality evidence, downgraded due to imprecision) (Analysis 4.13).
Hatta 2014 reported adverse events and there were none reported in either group.
5. Citicoline versus placebo
One study tested the use of citicoline (Diaz 2001).
a. Primary outcome
The incidence of delirium was lower in the group treated with citicoline, but the results were too imprecise to allow a conclusion to be drawn (RR 0.68, 95% CI 0.22 to 2.06; 80 participants; moderate‐quality evidence) (Analysis 5.1).
b. Secondary outcomes
There was no clear evidence of effect on cognitive status with citicoline treatment using MMSE score (MD ‐1.47, CI ‐3.85 to 0.91; 81 participants; moderate‐quality evidence, downgraded due to imprecision) (Analysis 5.2).
c. Adverse outcomes
No data were reported for adverse outcomes.
6. Oral premedication with diazepam and diphenhydramine versus no premedication
One study of 49 participants undergoing inpatient elective cardiac catheterisation compared the effect of premedication with diazepam and diphenhydramine with no premedication (Ashraf 2015).
a. Primary outcome
There were no cases of incident delirium in either group (49 participants; low‐quality evidence, downgraded due to risk of bias and evidence from single small study).
b. Secondary outcomes
No data are reported on secondary outcomes.
c. Adverse outcomes
No data are reported on adverse outcomes.
7. Intravenous (IV) methylprednisolone versus placebo
One large multicentre study of 7507 participants undergoing cardiopulmonary bypass procedures who were at high risk of morbidity and mortality compared the effect of intravenous (IV) methylprednisolone versus placebo and incorporated incidence of delirium as a safety outcome (Whitlock 2015).
a. Primary outcome
IV Methylprednisolone has no effect on the incidence of delirium for patients undergoing high‐risk cardiopulmonary bypass procedures (RR 1.02, 95% CI 0.87 to 1.19; 7507 participants; high‐quality evidence) (Analysis 7.1).
b. Secondary outcomes
IV methylprednisolone has no effect on the length of stay for patients undergoing high‐risk cardiopulmonary bypass procedures (RR 0.00, 95% CI ‐0.20 to 0.20; 7507 participants; high‐quality evidence) (Analysis 7.2).
c. Adverse outcomes
IV methylprednisolone has no effect on 30‐day mortality for patients undergoing high‐risk cardiopulmonary bypass procedures (RR 0.87, 95% CI 0.70 to 1.07; 7507 participants; high‐quality evidence) (Analysis 7.3).
Evaluating postoperative complications, IV methylprednisolone appears to increase the risk of myocardial injury compared to placebo (RR 1.22, 95% CI 1.07 to 1.38; 7507 participants; high‐quality evidence) and has no effect on the risk of respiratory failure (RR 0.91, 95% CI 0.80 to 1.05; 7507 participants; high‐quality evidence) and infection (RR 0.94, 95% CI 0.84 to 1.06; 7507 participants; high‐quality evidence).
8. Gabapentinoids versus placebo
Two studies tested gabapentinoids agents. One assessed gabapentin in 21 patients (Leung 2006), and the other tested the more potent preparation, pregabalin, in 70 patients (Pesonen 2011). However, results for these studies could not be pooled as each measured different outcomes.
a. Primary outcome
In Leung 2006, the incidence of delirium was lower in the group treated with gabapentin, but the results were too imprecise to allow a conclusion to be drawn (RR 0.12, 95% CI 0.01 to 1.90; 21 participants; low‐quality evidence, downgraded due to risk of bias and imprecision) (Analysis 8.1).
Pesonen 2011 tested for postoperative delirium using a Finnish modified CAM‐ICU but reported only median scores, precluding use of these data in the analysis .
b. Secondary outcomes
Pesonen 2011 reported effect of pregabalin compared to placebo on length of hospital admission (MD ‐0.60 days 95% CI ‐2.12 to 0.92; 60 participants) (Analysis 8.2); cognition (measured with the CAM‐ICU on day five), (MD 1.00 95% CI ‐2.76 to 4.76; 60 participants) (Analysis 8.3); and use of psychotropic medication, (RR 0.53 95% CI 0.21 to 1.38; 60 participants) (Analysis 8.4). For all three outcomes, results were inconclusive and we judged the evidence to be low‐quality, downgraded due to imprecision and risk of bias.
Withdrawal from protocol appeared higher in the intervention group; however the results were too imprecise to allow a conclusion to be drawn (RR 9.0 95% CI 0.50 to 161.13; 70 participants; very low‐quality evidence downgraded due to risk of bias and serious imprecision) (Analysis 8.5).
c. Adverse outcomes
No data were reported for adverse outcomes.
9. Ketamine versus placebo
One study (Urban 2008) tested the use of ketamine in 26 patients undergoing lumbar spinal fusion.
a. Primary outcome
Rates of incident delirium appeared higher among those treated with ketamine compared to control. However, the results are too imprecise to allow a conclusion to be drawn (RR 2.00, 95% CI 0.21 to 19.23; 24 participants; very low‐quality evidence, downgraded due to risk of bias and serious imprecision) (Analysis 9.1).
b. Secondary outcomes
There was no evidence of effect of ketamine treatment on withdrawals from protocol (RR 1.00, 95% CI 0.07 to 14.34; 26 participants; very low‐quality evidence due to risk of bias and serious imprecision) (Analysis 9.2).
c. Adverse outcomes
No data were reported for adverse outcomes.
10. Intravenous (IV) parecoxib sodium analgesia versus morphine and saline
One study of 80 participants admitted as an emergency for femoral head replacement surgery compared administration of IV parecoxib 12‐hourly versus IV morphine (single dose) followed by IV saline (Li 2013).
a. Primary outcome
The incidence of delirium was lower in those receiving parecoxib compared to those receiving morphine and saline (RR 0.50, 95% CI 0.26 to 0.98; 80 participants; low‐quality evidence due to indirectness [as the comparison tests regular analgesia to one dose of analgesia then placebo], risk of bias and this being a single small study) (Analysis 10.1).
b. Secondary outcomes
Individuals receiving parecoxib had a shorter length of admission than those receiving morphine and saline (MD ‐0.90 days, 95% CI ‐1.58 to ‐0.22 days; 80 participants; low‐quality evidence due to indirectness and results from a single small study) (Analysis 10.2).
Data are presented for rates of postoperative cognitive dysfunction (POCD) at three days, one week, three months, and six months, with evidence of a reduction in the risk of POCD at one week (RR 0.38, 95% CI 0.15 to 0.98; 80 participants; low‐quality evidence downgraded due to indirectness, imprecision and results being from a single small study) (Analysis 10.4).
c. Adverse outcomes
No data were reported for adverse outcomes.
11. Intrathecal morphine and patient controlled analgesia (PCA) versus saline and PCA
One study (Beaussier 2006) tested the administration of intrathecal morphine preoperatively in addition to postoperative patient‐controlled intravenous morphine for pain control in 59 patients. Both groups received postoperative PCA, but the intervention group were given intrathecal morphine, and the control group, a similar volume of saline preoperatively.
a. Primary outcome
There was no evidence of effect on intrathecal and PCA morphine on rates of incident delirium (RR 0.90, 95% CI 0.44 to 1.85; 52 participants; low‐quality evidence due to risk of bias and imprecision) (Analysis 11.1).
b. Secondary outcomes
Data were presented on length of admission (MD ‐0.50 days, 95% CI ‐1.51 to 0.51; 52 participants) (Analysis 11.2); days for cognition to return to preoperative level (MD 0.20, 95% CI ‐1.03 to 1.43; 52 participants) (Analysis 11.3); and withdrawals from protocol (RR 0.78, 95% CI 0.19 to 3.17; 59 participants) (Analysis 11.4) for intrathecal PCA morphine compared to saline and PCA. For all these outcomes, there was no clear evidence of effect from the intervention. We judged the evidence to be of low quality, downgraded due to risk of bias and imprecision.
c. Adverse outcomes
Mortality appeared lower in those in the intrathecal and PCA morphine group, but the results were too imprecise for any conclusions to be drawn (RR 0.34, 95% CI 0.01 to 8.13; 59 participants; low‐quality evidence, downgraded two levels due to serious imprecision) (Analysis 11.5).
12. Fascia iliaca compartment block (FICB) versus placebo
One study (Mouzopoulos 2009) with 219 participants tested administration of fascia iliaca compartment block (FICB) to manage pain in hip fracture patients assessed as being at intermediate or high risk of delirium.
a. Primary outcome
Use of a FICB reduced the risk of incident delirium compared to placebo (RR 0.45, 95% CI 0.24 to 0.87; 207 participants; moderate‐quality evidence due to risk of bias) (Analysis 12.1).
b. Secondary outcomes
Use of a FICB reduced the severity of delirium episodes (MD ‐4.30, 95% CI ‐6.81 to ‐1.79; 36 participants) (Analysis 12.2) and duration of delirium episodes (MD ‐5.70 days, 95% CI ‐9.50 to ‐1.90; 36 participants) (Analysis 12.3). However, we judged the evidence to be of very low‐quality, downgraded due to risk of bias and serious imprecision.
c. Adverse outcomes
There was no evidence of effect of the intervention on risk of mortality (RR 0.51, 95% CI 0.05 to 5.58; 219 participants; low‐quality evidence downgraded two levels due to serious imprecision (Analysis 12.4).
13. Light versus deep propofol sedation
One study compared the effect of light and deep propofol sedation on the prevalence of postoperative delirium in 114 older adult patients who underwent hip fracture repair under spinal anaesthesia (Sieber 2010).
a. Primary outcome
The incidence of delirium was lower in those receiving light propofol sedation compared to deep propofol sedation (RR 0.48, 95% CI 0.26 to 0.89; 114 participants; moderate‐quality evidence due to risk of bias) (Analysis 13.1).
b. Secondary outcomes
There was no clear evidence of effect of level of sedation on delirium duration (MD ‐0.60 days, 95% CI ‐3.30 to 2.10; 34 participants; very low‐quality evidence due to risk of bias and serious imprecision) (Analysis 13.2).
There was no evidence of effect on level of sedation on length of admission (MD 0.20 days, 95% CI ‐0.80 to 1.20 days; 114 participants; moderate‐quality evidence, downgraded due to risk of bias) (Analysis 13.3).
Light propofol sedation improved cognitive performance (on day two postoperatively, assessed using MMSE score (Folstein 1975)) (MD 3.10, 95% CI 0.30 to 5.90; 114 participants; moderate‐quality evidence due to risk of bias) (Analysis 13.4).
c. Adverse outcomes
There was no evidence of effect of level of sedation on inpatient mortality (RR 0.50, 95% CI 0.05, to 5.36; 114 participants; low‐quality evidence downgraded two levels due to serious imprecision) (Analysis 13.5). There was no evidence of effect of the intervention on the risk of experiencing >=1 postoperative complication (RR 0.87, 95% CI 0.60 to 1.26; 114 participants; low‐quality evidence due to risk of bias and imprecision) (Analysis 13.6).
14. Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia or clinical judgement
Three studies Chan 2013 (925 participants), Radtke 2013 (1277 participants) and Gauge 2014 (81 participants) investigated the use of BIS in anaesthesia. Only two of these presented useable data for inclusion in the review (Chan 2013; Radtke 2013) as insufficient data were reported in Gauge 2014 (conference abstract). A summary of findings for key outcomes is presented in summary of findings Table 5.
a. Primary outcome
BIS‐guided anaesthesia was effective in reducing incident delirium (RR 0.71, 95% CI 0.60 to 0.85, I2 = 0%; 2057 participants; moderate‐quality evidence due to risk of bias) (Analysis 14.1; Figure 7).
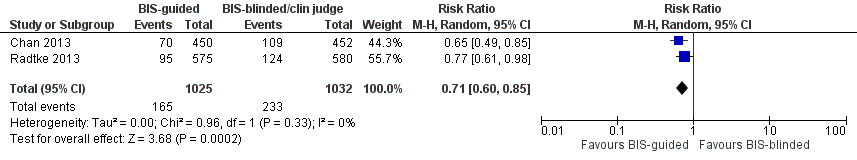
Forest plot of comparison: 11 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia, outcome: 11.1 Incident delirium.
b. Secondary outcomes
BIS‐guided anaesthesia resulted in a shorter length of admission than those receiving BIS‐blinded anaesthesia/clinical judgement (MD ‐0.94 days, 95% CI ‐1.45 to ‐0.43 days, I2 = 0%; 2057 participants; moderate‐quality evidence, downgraded due to risk of bias) (Analysis 14.2).
Use of BIS‐guided anaesthesia showed evidence of reducing rates of cognitive impairment at seven days (RR 0.87, 95% CI 0.71 to 1.05, I2 = 0%; 1938 participants) (Analysis 14.3) and at three months (RR 0.71, 95% CI 0.53 to 0.97; 1990 participants) (Analysis 14.4). However, we considered the evidence to be of low quality, downgraded due to risk of bias and imprecision.
c. Adverse outcomes
Chan 2013 reported SF‐36 mental summary scores (Ware 1992) at follow‐up and the BIS‐guided group had lower scores, indicating a poorer assessment of their own mental health (MD ‐1.90, 95% CI ‐3.40 to ‐0.40; 902 participants; moderate‐quality evidence downgraded as from a single study) (Analysis 14.5).
One study reported mortality at seven days (Chan 2013); there was no clear evidence of any effect on mortality (RR 1.49, 95% CI 0.42 to 5.25; 921 participants; low‐quality evidence, downgraded two levels due to serious imprecision) (Analysis 14.6).
Two studies reported mortality at three months (Chan 2013; Radtke 2013); there was no evidence of reduction in mortality (RR 1.10, 95% CI 0.77 to 1.59, I2 = 0%; 1938 participants; moderate‐quality evidence due to imprecision) (Analysis 14.7).
Chan 2013 reported rates of cardiac, respiratory and infectious adverse events. There was no evidence of a reduction in cardiac (RR 0.85, 95% CI 0.52 to 1.39; 902 participants) or respiratory adverse events (RR 0.79, 95% CI 0.59 to 1.07; 902 participants), but infectious adverse events were lower in the group receiving BIS‐guided anaesthesia (RR 0.72, 95% CI 0.55 to 0.95; 902 participants). However, the evidence was deemed of low quality due to risk of bias and being from a single study.
15. Sevoflurane versus propofol anaesthesia
Lurati 2012 compared sevoflurane, an inhalational anaesthetic versus propofol, an intravenous anaesthetic to reduce perioperative myocardial ischaemia in 385 patients undergoing noncardiac surgery.
a. Primary outcome
There was no evidence of effect on rates of incident delirium with sevoflurane anaesthesia compared to propofol anaesthesia (RR 0.79, 95% CI 0.47 to 1.34; 385 participants; low‐quality evidence downgraded due to risk of bias and imprecision) (Analysis 15.1).
b. Secondary outcomes
No data were reported for secondary outcomes.
c. Adverse outcomes
There was no evidence of a difference in mortality at 12 months between intervention and control groups (RR 1.19, 95% CI 0.70 to 2.02; 385 participants; low‐quality evidence downgraded two levels due to serious imprecision) (Analysis 15.2).
16. Xenon versus sevoflurane anaesthesia
Stoppe 2013 conducted a pilot trial to determine the feasibility and safety of xenon, a novel anaesthetic gas with neuroprotective and cardioprotective properties compared with sevoflurane a conventional inhalational anaesthetic in 30 patients undergoing elective coronary artery bypass grafting.
a. Primary outcome
There was no evidence of a difference in incidence of postoperative delirium between the xenon and sevoflurane groups. The highest incidence of delirium occurred on the second postoperative day (RR 0.75, 95% 0.20 to 2.79; 30 participants; very low‐quality evidence due to risk of bias and serious imprecision) (Analysis 16.1).
b. Secondary outcomes
Hospital admission appeared to be longer in those treated with xenon, but the results were too imprecise to allow conclusions to be drawn (MD 4.00 days, 95% CI ‐1.72 to 9.72 days; 30 participants; very low‐quality evidence, downgraded due to risk of bias and serious imprecision) (Analysis 16.2).
c. Adverse outcomes
There were no in‐hospital deaths amongst study participants (Analysis 16.3). There was no evidence of effect on adverse events (RR 0.75, 95% CI 0.34 to 1.64; 30 participants; low‐quality evidence downgraded due to risk of bias and imprecision) or the incidence of sepsis (RR 1.50, 95% CI 0.29 to 7.73; 30 participants; very low‐quality evidence downgraded due to risk of bias and serious imprecision) (Analysis 16.4; Analysis 16.5).
17. Epidural anaesthesia versus general anaesthesia
Two studies compared epidural versus general anaesthesia (Berggren 1987; Papaioannou 2005).
a. Primary outcome
We pooled data from both studies for the primary outcome of incident delirium, but the result was too imprecise to determine an effect (RR 1.19, 95% CI 0.69 to 2.03, I2 = 0%; 104 participants; very low‐quality evidence downgraded due to risk of bias and serious imprecision) (Analysis 17.1).
b. Secondary outcomes
There was no evidence of reduction in admission length, evaluated as those with a length of stay >10 days versus not (RR 0.59, 95% CI 0.28 to 1.24; 47 participants) (Analysis 17.2) and cognitive decline (MD 0.15, 95% CI 0.02 to 1.06; 47 participants) (Analysis 17.3) from one study (Papaioannou 2005). For both outcomes the result was inconclusive and we judged the evidence to be low quality, downgraded due to risk of bias and imprecision.
c. Adverse outcomes
Berggren 1987 examined physical morbidity and found no evidence of reduction in urinary tract infection (MD 1.33, 95% CI 0.57 to 3.09; 57 participants) and psychological morbidity (depression) (RR 1.04; 95% CI 0.23 to 4.71; 57 participants). The evidence for both outcomes was of low quality downgraded two levels due to serious imprecision of results) (Analysis 17.4; Analysis 17.5).
There was no evidence for reduction in postoperative complications using epidural versus general anaesthesia reported by Papaioannou 2005 (RR 0.92, 95% CI 0.35 to 2.39; 47 participants; very low‐quality evidence due to risk of bias and serious imprecision) (Analysis 17.6).
Berggren 1987 investigated the impact on pressure ulcers and reported no evidence of effect of reduction in pressure ulcer formation between epidural and general anaesthesia groups (RR 0.62, 95% CI 0.16 to 2.36; 57 participants; low‐quality evidence downgraded two levels due to serious imprecision) (Analysis 17.7).
18. Liberal versus restrictive blood transfusion thresholds
One study Gruber‐Baldini 2013 with 139 participants compared the use of liberal versus restrictive blood transfusion thresholds for individuals undergoing surgical repair of hip fracture. There was significant overlap in the volume of blood received by participants in the liberal and restrictive groups.
a. Primary outcome
There was no evidence to support liberal transfusion thresholds on rates of incident delirium (RR 0.75, 95% CI 0.45 to 1.27; 108 participants; moderate‐quality evidence due to risk of bias) (Analysis 18.1).
b. Secondary outcomes
There was no evidence that liberal transfusion thresholds affected the severity of delirium (MD ‐0.10 points, 95% CI ‐2.99 to 2.79; 38 participants; low‐quality evidence due to risk of bias and imprecision) or length of admission (MD ‐0.10 days, 95% CI ‐1.36 to 1.16 days; 138 participants; low‐quality evidence downgraded due to imprecision and risk of bias) (Analysis 18.2; Analysis 18.3). Use of psychoactive medication appeared balanced between the liberal and restrictive transfusion groups (RR 0.99, 95% CI 0.87 to 1.12; 138 participants; low‐quality evidence downgraded due to risk of bias and as results from a single small study) (Analysis 18.4).
c. Adverse outcomes
Data were reported on the occurrence of post‐randomisation adverse events, specifically infections and congestive heart failure. There was no evidence that liberal transfusions reduced the risk of infections (RR 1.09, 95% CI 0.23 to 5.22; 138 participants; very low‐quality evidence downgraded due to risk of bias and serious imprecision) or congestive heart failure (RR 0.55, 95% CI 0.05 to 5.88; 138 participants; very low‐quality evidence downgraded due to risk of bias and serious imprecision) (Analysis 18.5; Analysis 18.6).
19. Fast‐track surgery versus usual care
One study Jia 2014 with 240 participants evaluated the effects of fast‐track surgery for older adults with colorectal cancer compared to usual care.
a. Primary outcome
Evidence from this study supports fast‐track surgery as an intervention to reduce incident delirium (RR 0.26, 95% CI 0.09 to 0.77; 233 participants; low‐quality evidence, downgraded due to imprecision of results and risk of bias) (Analysis 19.1).
b. Secondary outcomes
There is evidence to support fast‐track surgery in reducing length of admission (MD ‐4.20 days, 95% CI ‐4.60 to ‐3.80 days; 233 participants; high‐quality evidence) (Analysis 19.2).
c. Adverse outcomes
The study reports on the occurrence of urinary tract infection and heart failure. It appeared that fast‐track surgery reduced the rate of urinary tract infection (RR 0.38, 95% CI 0.14 to 1.04), but this was low‐quality evidence as the result was too imprecise to draw a conclusion and there was risk of bias in outcome assessment (Analysis 19.3). There is evidence to support fast‐track surgery reducing the occurrence of heart failure compared to usual care (RR 0.31, 95% CI 0.10 to 0.91; 233 participants; low‐quality evidence downgraded due to risk of bias and imprecision) (Analysis 19.4)
20. Postoperative delirium‐free protocol (DFP) versus usual care
One small study Aizawa 2002 with 42 participants evaluated a 'delirium‐free protocol' which was comprised of overnight infusions of diazepam, flunitrazepam and pethidine to older postoperative surgical patients.
a. Primary outcome
DFP use was associated with a lower rate of incident delirium, but the result was imprecise (RR 0.14, 95% CI 0.02 to 1.06; 40 participants; low‐quality evidence downgraded due to risk of bias and imprecision) (Analysis 20.1).
b. Secondary outcomes
There was no evidence of effect of the DFP on length of admission (MD ‐4.30 days, 95% CI ‐12.51 to 3.91 days; 40 participants; very low‐quality evidence, downgraded due to risk of bias and serious imprecision) (Analysis 20.2).
There was no evidence of effect of the DFP on the risk of behavioural disturbance (RR 0.20, 95% CI 0.03 to 1.56; 40 participants; low‐quality evidence, downgraded due to risk of bias and imprecision) (Analysis 20.3).
c. Adverse outcomes
No data were reported for adverse outcomes.
21. Computerised clinical decision support system (CCDSS) versus usual care
One study Boustani 2012 assessed the use of a computerised clinical decision support system (CCDSS) on the management of 427 older adults with cognitive impairment compared to usual care.
a. Primary outcome
There was no evidence of the effect of CCDSS in reducing incident delirium (RR 1.08, 95% CI 0.82 to 1.43; 424 participants; moderate‐quality evidence due to risk of bias) (Analysis 21.1).
b. Secondary outcomes
There was no evidence of reduction in the length of admission (MD 0.90 days, 95% CI ‐0.35 to 2.15 days; 424 participants; low‐quality evidence, downgraded due to serious imprecision) (Analysis 21.2).
c. Adverse outcomes
There was no evidence of a change in rates of mortality within 30 days of discharge (RR 1.04, 95% CI 0.49 to 2.23; 424 participants; low‐quality evidence downgraded due to serious imprecision) (Analysis 21.3).
There was no evidence of effect on rates of falls (RR 0.93, 95% CI 0.39 to 2.19; 424 participants) or pressure ulcers (RR 1.09, 95% CI 0.64 to 1.84; 424 participants) with use of the CCDSS with moderate‐quality evidence downgraded due to imprecision. (Analysis 21.4; Analysis 21.5)
22. Geriatric unit care versus orthopaedic unit care
One trial of 329 older adults following hip fracture compared care in a specialist geriatric unit and comprehensive geriatric assessment to care in their orthopaedic unit (Watne 2014).
a. Primary outcome
There was no evidence that care in the geriatric unit reduced the incidence of delirium compared to care in the orthopaedic unit (RR 0.98, 95% CI 0.79 to 1.22; 329 participants; low‐quality evidence downgraded due to risk of bias and imprecision) (Analysis 22.1).
b. Secondary outcomes
There was no evidence that care in the geriatric unit reduced the duration (MD ‐1.00 days, 95% CI ‐2.04 to 0.04 days; 163 participants) (Analysis 22.2) or severity of delirium episodes (MD 1.50 points, 95% CI ‐1.00 to 4.00 points; 163 participants) (Analysis 22.3) compared to the orthopaedic unit, low‐quality evidence for both outcomes, downgraded due to risk of bias and imprecision.
Care in the geriatric unit increased length of hospital admission by a mean of three days (RR 3.00, 95% CI 1.94 to 4.06 days; moderate‐quality evidence downgraded due to risk of bias) compared to the orthopaedic unit (Analysis 22.4).
Outcome assessments at four and 12 months were conducted blinded to original allocation, unlike those conducted while in hospital.
There was no evidence that care in the geriatric unit affected cognitive function (using a composite score) at four months follow‐up (MD 1.80 points, 95% CI ‐5.92 to 9.52 points; 228 participants; low‐quality evidence downgraded two levels due to serious imprecision) (Analysis 22.5). Care in the geriatric unit appeared to increase the rate of incident dementia at 12 months (RR 2.26, 95% CI 0.60 to 8.49; 193 participants) (Analysis 22.6), however, the evidence was deemed to be of low quality and was downgraded two levels due to serious imprecision.
There was no evidence that activities of daily living (measured by Barthel Index (Mahoney 1965)) were affected by allocation to the geriatric unit or the orthopaedic unit (MD 1.00, 95% CI ‐0.70 to 2.70; moderate‐quality evidence downgraded due to imprecision) (Analysis 22.7).
There was no evidence that care in the geriatric unit affected risk of Institutionalisation at four (RR 1.06, 95% CI 0.58 to 1.91; 242 participants; moderate‐quality evidence downgraded due to imprecision) (Analysis 22.8) and 12 months (RR 0.86, 95% CI 0.47 to 1.59; 193 participants; moderate‐quality evidence downgraded due to imprecision) (Analysis 22.9).
c. Adverse outcomes
There was no evidence that care in the geriatric unit improved the rate of in‐hospital mortality (RR 0.56, 95% CI 0.21 to 1.47; 329 participants; moderate‐quality evidence downgraded due to imprecision) compared to the orthopaedic unit (Analysis 22.10).
Evaluating other adverse outcomes there was no evidence that care in the geriatric unit reduced the rate of falls (RR 1.30, 95% CI 0.61 to 2.77; 329 participants) (Analysis 22.11); pressure ulcer formation (RR 0.38, 95% CI 0.10 to 1.41; 329 participants) (Analysis 22.12); other medical adverse events (RR 0.96, 95% CI 0.76 to 1.23; 329 participants) (Analysis 22.13); or postoperative complications (RR 0.68, 95% CI 0.20 to 2.36; 329 participants) (Analysis 22.14) with low‐quality evidence for each comparison, downgraded due to risk of bias and imprecision.
Discusión
Resumen de los resultados principales
Las pruebas de la efectividad de la mayoría de las intervenciones para la prevención del delirio aún no están claras, con la excepción de las intervenciones con múltiples componentes.
Intervenciones con múltiples componentes
Hay pruebas de calidad moderada de siete ensayos controlados aleatorios de que las intervenciones con múltiples componentes reducen la incidencia de delirio, con una reducción general del riesgo de delirio de cerca del 30% en comparación con la atención habitual. Además, parecen tener tamaños similares del efecto en las poblaciones de estudio médicas y quirúrgicas.
A pesar del riesgo mayor de delirio en los pacientes con demencia, solamente un ensayo informó datos sobre la incidencia de delirio en este subgrupo (de 50 participantes); y en este estudio no hubo equilibrio en la prevalencia de demencia entre los grupos de intervención y control (Marcantonio 2001). La efectividad de estas intervenciones en los pacientes con demencia aún no está clara.
Los efectos sobre la duración del delirio, la duración del ingreso hospitalario, la institucionalización y la gravedad del delirio tampoco están claros. No existen pruebas claras del efecto sobre la mortalidad (durante el ingreso o a los 12 meses); la mortalidad a los 12 meses solamente se informó en un ensayo (Lundstrom 2007). Se informan diferencias clínicamente importantes para la cognición (en un estudio; 60 participantes, Bonaventura 2007) y las úlceras por presión (dos estudios; 457 participantes, Hempenius 2013; Lundstrom 2007), todas en una dirección que favorece a las intervenciones con múltiples componentes, aunque hay incertidumbre en estos resultados debido a la imprecisión.
Intervenciones farmacológicas
Inhibidores de la colinesterasa
No se encontraron pruebas claras del efecto beneficioso de un inhibidor de la colinesterasa, el donepezilo, para prevenir el delirio en una población ortopédica electiva sin deficiencia cognitiva. Se consideró que las pruebas disponibles fueron de muy baja calidad debido a la imprecisión y la considerable inconsistencia.
Antipsicóticos
En general no existen pruebas claras de la efectividad de los fármacos antipsicóticos como grupo en la prevención del delirio, aunque hay incertidumbre en este resultado debido a la imprecisión y la inconsistencia.
El análisis preplanificado de subgrupos indica que un fármaco antipsicótico atípico (olanzapina) puede reducir la incidencia de delirio, con un tamaño del efecto potencialmente grande, pero no hay pruebas claras que apoyen la efectividad del antipsicótico típico haloperidol. Sin embargo, es posible que en un estudio de haloperidol la optimización de la prevención no farmacológica del delirio en los brazos de intervención y control impidiera la detección de cualquier efecto beneficioso adicional de la medicación. En el otro estudio, el haloperidol se administró por tres días a partir del primer día del posoperatorio, lo que puede haber sido demasiado tarde para lograr algún efecto beneficioso preventivo, aunque este estudio también tuvo alto riesgo de sesgo debido a su naturaleza no cegada.
La repercusión sobre la gravedad y la duración del delirio también difirió entre dos estudios de haloperidol y olanzapina pero, paradójicamente, favoreció al grupo de intervención para el haloperidol y al grupo control para la olanzapina. No existen pruebas claras del efecto de los antipsicóticos sobre la duración del ingreso hospitalario.
Melatonina
No existen pruebas claras para apoyar la efectividad de la melatonina o los agonistas de la melatonina en la prevención del delirio. Sin embargo, hay considerable heterogeneidad en los resultados, lo que se puede haber debido a las diferentes poblaciones de estudio y las diferentes dosis. Al‐Aama 2011 informó un tamaño del efecto clínicamente importante en la reducción de la incidencia de delirio en los enfermos hospitalizados con afecciones médicas con la administración diaria de 0,5 mg de melatonina, (pruebas de baja calidad debido al seguimiento incompleto); aunque de Jonghe 2014 no informó efectos con la administración diaria de 3 mg de melatonina en pacientes con fractura de cadera a los que se les realizó cirugía aguda. Ramelteon, un agonista de la melatonina, se ha propuesto anteriormente como un tratamiento más seguro para el insomnio (Miyamoto 2009), pero no se encontraron pruebas de un efecto beneficioso sobre la prevención del delirio en un ensayo.
Otras intervenciones farmacológicas
No se encontraron pruebas que apoyaran la efectividad de la citicolina para reducir la incidencia de delirio.
La metilprednisolona no tuvo efectos sobre la incidencia de delirio.
En un ensayo pequeño de premedicación con diazepam y difenhidramina para el cateterismo cardíaco electivo en enfermos hospitalizados no hubo casos de delirio en ninguno de los grupos; por lo tanto, las pruebas de que la elección de la premedicación afecta la incidencia de delirio todavía no son concluyentes.
Intervenciones perioperatorias
Medidas economizadoras de opiáceos
Las pruebas acerca del efecto de la gabapentina, la ketamina o la morfina para la analgesia intratecal y controlada por el paciente (ACP) para la prevención del delirio no son concluyentes.
Hubo pruebas de que el parecoxib intravenoso (IV) redujo la incidencia de delirio en comparación con la morfina y la solución salina. Sin embargo, las pruebas fueron de baja calidad, provienen de un único estudio y están afectadas por posibles factores de confusión relacionados con la administración de morfina complementaria.
Hay pruebas de que el bloqueo compartimentado de la fascia iliaca (BCFI) para controlar el dolor en los pacientes con fractura de cadera es eficaz para reducir la incidencia de delirio. Pruebas de menor calidad también indicaron que podría reducir la gravedad y la duración de los episodios de delirio.
Reducción / control de la profundidad de la anestesia
La reducción de la profundidad de la anestesia general o el control de la profundidad es efectiva para prevenir el delirio. El uso de sedación ligera con propofol en comparación con la sedación profunda, y la anestesia guiada por el BIS en comparación con la anestesia sin el uso del BIS / valoración clínica fueron enfoques efectivos.
Cambio en la forma de anestesia
No existen pruebas de diferencias en el efecto sobre la incidencia de delirio del uso de propofol o xenón en comparación con la anestesia con sevoflurano.
Evitar la anestesia general
Las pruebas de la efectividad de la anestesia epidural en comparación con la anestesia general para la prevención del delirio no están claras.
Intervenciones perioperatorias variadas
No hubo pruebas de un estudio de que la transfusión de sangre liberal versus restrictiva fuera efectiva para prevenir el delirio.
Un estudio de cirugía con rehabilitación multimodal (fast‐track) en pacientes de edad avanzada con cáncer indicó que reduce la incidencia de delirio y la duración del ingreso hospitalario.
Un estudio que utilizó un "protocolo sin delirio" en pacientes de edad avanzada sometidos a laparotomía abierta es probable que diera lugar a la sedación de los participantes y no logró demostrar pruebas del efecto beneficioso sobre la incidencia de delirio.
Sistema computarizado de apoyo a las decisiones clínicas (SCADC)
Un estudio que utilizó un sistema computarizado de apoyo a las decisiones clínicas, aplicado a pacientes de medicina general y geriátrica no dio lugar a mejorías en la incidencia de delirio.
Atención en unidad geriátrica versus unidad ortopédica
No hubo pruebas de que la atención en la unidad de medicina geriátrica redujera la duración de la incidencia o la gravedad del delirio, ni otros resultados cognitivos y funcionales. Sin embargo, la atención en una unidad geriátrica aumentó la duración de la estancia hospitalaria en comparación con la atención en la unidad ortopédica.
Compleción y aplicabilidad general de las pruebas
Aunque se identificaron 39 ensayos para inclusión en esta revisión, el grupo de pruebas para la prevención del delirio en los pacientes hospitalizados no ingresados en una UCI aún es limitado, excepto para las intervenciones con múltiples componentes (siete ensayos). En su mayoría las demás intervenciones solamente se investigaron en uno o dos ensayos pequeños con considerable heterogeneidad en las intervenciones, los resultados, las poblaciones y los contextos estudiados, lo que impidió realizar metanálisis. Solamente un estudio (de una intervención con múltiples componentes en pacientes quirúrgicos) presentó resultados para los pacientes con demencia, un subgrupo importante a estudiar en la prevención del delirio. Se podría esperar que la efectividad de las intervenciones para el delirio difiera debido a la mayor prevalencia del delirio y los resultados más deficientes en la demencia.
Para las intervenciones con múltiples componentes, es probable que los ensayos y los metanálisis incluidos tuvieran poco poder estadístico para detectar la mortalidad y la institucionalización (ambos resultados relativamente poco frecuentes), lo que puede explicar la falta de repercusión observada sobre estas variables principales de evaluación, a pesar de la reducción del delirio incidente.
Aunque hubo pruebas que indican que el BCFI, el control de la profundidad de la anestesia y la cirugía con rehabilitación multimodal podrían reducir la incidencia de delirio posoperatorio, es importante señalar que en la práctica clínica habrá un rango de consideraciones aparte de la efectividad para la prevención del delirio (que incluyen las comorbilidades, el riesgo de caídas y la necesidad de rehabilitación) que guiarán la selección de los enfoques para la cirugía y la anestesia. Por lo tanto, según las pruebas de esta revisión solamente, no es posible hacer recomendaciones con respecto a la práctica quirúrgica y anestésica.
La mayoría de los estudios incluyó la incidencia de delirio como resultado, y la cognición y la duración del ingreso hospitalario también se informaron con frecuencia. Sin embargo, otros resultados importantes que incluyen la duración y la gravedad del delirio, la mortalidad, la institucionalización, el rendimiento en las actividades cotidianas y los resultados adversos no se informaron con frecuencia. Ningún estudio investigó la repercusión sobre la calidad de vida, la morbilidad psicológica de los cuidadores, la morbilidad psicológica del personal o los costos. Los estudios futuros deben abordar estas brechas en las intervenciones, los contextos y los resultados estudiados.
La imposibilidad de excluir el delirio prevalente al reclutamiento fue una limitación habitual en la mayoría de los estudios incluidos (29/39). Es posible que este hecho reduzca la precisión en los resultados porque las intervenciones no pueden prevenir los casos de delirio ya presentes en los participantes reclutados.
Calidad de la evidencia
Se utilizó el programa informático GRADEpro (GRADEpro 2014) para informar la generación de las afirmaciones sobre la calidad de las pruebas para cinco comparaciones: i) intervenciones con múltiples componentes versus atención habitual; ii) inhibidores de la colinesterasa versus placebo; iii) antipsicóticos versus placebo; iv) melatonina versus placebo y v) anestesia guiada por el BIS versus anestesia sin el uso del BIS / valoración clínica. Las tabulaciones completas para cada resultado están disponibles en: Resumen de los hallazgos para la comparación principal, Resumen de los hallazgos 2, Resumen de los hallazgos 3, Resumen de los hallazgos 4 y Resumen de los hallazgos 5.
Sobre la base de siete ensayos controlados aleatorios (ECA) (cuatro en pacientes con afecciones médicas y tres en pacientes con afecciones quirúrgicas), n = 1950 participantes, hay pruebas de calidad moderada de que las intervenciones con múltiples componentes para la prevención del delirio pueden reducir las tasas de delirio incidente; lo anterior es consistente entre los ensayos incluidos. Se disminuyó la calidad de las pruebas debido a la posibilidad de sesgo de realización (la naturaleza de la intervención impide el cegamiento de los participantes y los que administran la intervención). Los evaluadores de resultado no estaban cegados a la intervención en dos estudios, incluido el estudio con la ponderación más grande y la tasa de eventos más alta. Además, hay riesgo de otros sesgos en dos de los estudios incluidos debido a un desequilibrio entre los grupos intervención y control en cuanto a la prevalencia de demencia preexistente.
La heterogeneidad en las intervenciones con múltiples componentes estudiadas dificulta evaluar si los componentes específicos de las intervenciones son particularmente eficaces para la prevención del delirio.
Hay pruebas de calidad moderada de que las intervenciones con múltiples componentes no tienen efectos sobre la duración de la estancia hospitalaria (seis estudios, n = 1920 participantes) y pruebas de calidad moderada de ningún efecto sobre la probabilidad de retorno a la vida independiente (cuatro estudios, n = 1116). Hay incertidumbre considerable con respecto al efecto de las intervenciones con múltiples componentes sobre la duración del delirio debido a la evaluación de resultado no cegada en dos estudios, el desequilibrio en la prevalencia de la demencia en dos estudios y los resultados imprecisos.
Según dos ECA (n = 113 participantes), hay incertidumbre considerable con respecto al efecto de los inhibidores de la colinesterasa profilácticos para reducir la incidencia de delirio debido que las pruebas tienen muy baja calidad. Ambos estudios tienen datos de resultado faltantes; la calidad de las pruebas se disminuyó debido a la imprecisión y la inconsistencia en los resultados. Hay pruebas de baja calidad del efecto de los inhibidores de la colinesterasa profilácticos sobre el resultado gravedad del delirio (un estudio; n = 16 participantes) y la duración del ingreso (dos estudios; n = 128 participantes). La calidad de las pruebas se disminuyó debido a la importante imprecisión de los resultados de la gravedad del delirio y por la imprecisión y el riesgo de sesgo en la duración del ingreso.
Según tres ECA (n = 916 participantes), hay incertidumbre considerable con respecto al efecto de los fármacos antipsicóticos en la incidencia de delirio debido a pruebas de baja calidad, que se disminuyó debido al riesgo de sesgo, la inconsistencia y los resultados imprecisos. Hay pruebas de muy baja calidad del efecto de los fármacos antipsicóticos en la gravedad (dos estudios, n = 178 participantes) y la duración del delirio (dos estudios, n = 178 participantes), y pruebas de baja calidad de la estancia hospitalaria debido a los resultados inconsistentes y muy imprecisos (un estudio, n = 68 participantes).
Según tres ECA (n = 529 participantes), hay incertidumbre considerable con respecto al efecto de los agonistas de la melatonina / melatonina profilácticos sobre la incidencia de delirio debido a pruebas de muy baja calidad, que se disminuyó debido al riesgo de sesgo y a los resultados imprecisos e inconsistentes. Hay pruebas de calidad moderada de que la melatonina no afecta la duración del delirio, y la calidad se disminuyó porque los resultados provienen de un único estudio (n = 104). Hay incertidumbre con respecto al efecto de la melatonina sobre la gravedad del delirio debido a las pruebas de calidad moderada de un estudio que utilizó un resultado binario (n = 104) y a pruebas de baja calidad de un segundo estudio, que se disminuyó debido a imprecisión grave (n = 6). Hay pruebas de calidad moderada de que la melatonina no reduce la duración de la estancia hospitalaria (dos estudios; 500 participantes); la calidad de los resultados se disminuyó por la inconsistencia. Hay incertidumbre con respecto al efecto de la melatonina sobre la mortalidad hospitalaria debido a pruebas de baja calidad de tres estudios, que se disminuyó debido a los resultados imprecisos y al número muy pequeño de eventos (n = 543 participantes).
Según dos ECA (n = 2057 participantes), hay pruebas de calidad moderada de que la anestesia guiada por el BIS reduce la incidencia de delirio en comparación con la anestesia sin el uso del BIS / valoración clínica. Se disminuyó la calidad de las pruebas debido al riesgo de sesgo asociado con el hecho de que los participantes y el personal no estaban cegados y a la evaluación incompleta de los resultados. También hubo un riesgo incierto de sesgo de selección en Radtke 2013. También hay pruebas de calidad moderada de que la anestesia guiada por el BIS dio lugar a una duración más corta del ingreso hospitalario en comparación con la anestesia sin el uso del BIS / valoración clínica (dos estudios, n = 2057 participantes), que también se disminuyó debido al riesgo de sesgo.
Sesgos potenciales en el proceso de revisión
Esta revisión ha seguido los procedimientos Cochrane y solamente hubo un número pequeño de enmiendas al proceso de revisión que se esbozan en las Diferencias entre el protocolo y la revisión.
Acuerdos y desacuerdos con otros estudios o revisiones
La versión anterior de esta revisión (Siddiqi 2007) sólo incluyó seis estudios, ninguno de los cuales evaluó la misma intervención. La revisión destacó la posible función de una intervención con múltiples componentes (un Servicio de Consulta Geriátrica) y la administración de antipsicóticos atípicos, pero identificó la necesidad de un grupo grande de pruebas antes de establecer conclusiones o recomendaciones prácticas. La base de pruebas de las intervenciones con múltiples componentes para la prevención del delirio incidente en los pacientes hospitalizados no ingresados en una UCI ha aumentado considerablemente desde la versión anterior, y las pruebas resumidas en esta actualización apoyan la administración de intervenciones con múltiples componentes. Sin embargo, se encontró una falta continua de pruebas que apoyen la administración de antipsicóticos como grupo para la prevención del delirio.
El resultado principal de la revisión de la función positiva de las intervenciones con múltiples componentes para prevenir el delirio es consistente con la bibliografía más amplia publicada (Abraha 2015). El programa de intervención con múltiples componentes conocido como Hospital Elder Life Program (HELP) for Prevention of Delirium ha demostrado reducciones eficaces en la incidencia de delirio en ensayos no aleatorios (Inouye 1999a; Inouye 2000). Hshieh 2015 publicó un metanálisis de los estudios de intervención que utilizaron intervenciones con múltiples componentes no farmacológicas y, aunque identificó problemas similares con la heterogeneidad que limitaron el informe, encontró pruebas para apoyar reducciones en la incidencia de delirio y de las caídas. Dos revisiones sistemáticas recientes han llegado a conclusiones similares a las de esta revisión. Martinez 2015 identificó que las intervenciones con múltiples componentes fueron efectivas para reducir el delirio incidente y las caídas accidentales en los adultos hospitalizados. Zhang 2013 examinó específicamente la función de las intervenciones para prevenir el delirio posoperatorio e identificó que las intervenciones con múltiples componentes fueron beneficiosas, aunque la revisión también identificó efectos beneficiosos positivos de la sedación y los fármacos antipsicóticos que no se repitieron en los resultados de la presente revisión.
Las intervenciones con múltiples componentes para la prevención de delirio también se reconocen actualmente y se recomiendan en las guías prácticas. Las guías del National Institute for Health and Care Excellence (NICE) del Reino Unido para el delirio se publicaron en 2010 (NICE 2010). Identificaron que las intervenciones con múltiples componentes tienen una función fundamental para identificar y abordar los factores de riesgo clínicos modificables para la prevención del delirio. Se recomienda la evaluación y la intervención con múltiples componentes en el transcurso de las 24 horas posteriores al ingreso en los pacientes con riesgo; la intervención se debe personalizar según las necesidades del paciente y administrar por un equipo multidisciplinario (NICE 2010). Se ha identificado que se prevén ahorros en los costos, aunque en la presente revisión no se encontraron datos sobre este aspecto.
La falta de repercusión de las intervenciones con múltiples componentes sobre la mortalidad y la institucionalización, a pesar de la reducción del delirio, es un resultado sorprendente. Se considera que las caídas y la institucionalización se asocian con discapacidad y pueden representar complicaciones del síndrome de discapacidad (Clegg 2013; Eeles 2012; Fried 2001). Por lo tanto, la muerte y la institucionalización pueden, como variables de evaluación, representar manifestaciones no modificables de la discapacidad y ser relativamente poco sensibles a una reducción del delirio incidente, aunque un estudio reciente ha cuestionado la asociación del delirio con la discapacidad (Joosten 2014). El informe de la discapacidad inicial en los ensayos futuros (medida con un instrumento validado de evaluación de la discapacidad) ayudaría a aclarar esta relación.
Los hallazgos para los inhibidores de la colinesterasa son consistentes con estudios relacionados anteriores. Un ensayo grande de otro inhibidor de la colinesterasa, rivastigmina, para el tratamiento del delirio en los pacientes en cuidados intensivos se detuvo debido a inquietudes con la seguridad en 2010 y a que no hubo pruebas de efectividad (Sheldon 2010; van Eijk 2010).
Los resultados de los antipsicóticos también son consistentes con una revisión reciente publicada (Fok 2015).
La heterogeneidad de los resultados de la presente revisión para la melatonina también ha sido informada por Chen y colegas (Chen 2015). Dichos autores realizaron un análisis de subgrupos y concluyeron que la melatonina fue eficaz para prevenir el delirio en los pacientes con afecciones médicas, pero no quirúrgicas.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Multi‐component delirium prevention intervention (MCI) versus usual care, outcome: 1.1 Incident delirium.

Forest plot of comparison: 2 Prophylactic cholinesterase inhibitor versus placebo, outcome: 2.1 Incident delirium.

Figure 5Forest plot of comparison: 3 Prophylactic antipsychotic versus control, outcome: 3.1 Incidence of delirium.

Forest plot of comparison: 4 Prophylactic melatonin versus placebo, outcome: 4.1 Incident delirium.

Forest plot of comparison: 11 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia, outcome: 11.1 Incident delirium.

Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 1 Incident delirium.

Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 2 Incidence of delirium in patients with dementia.

Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 3 Duration of delirium.
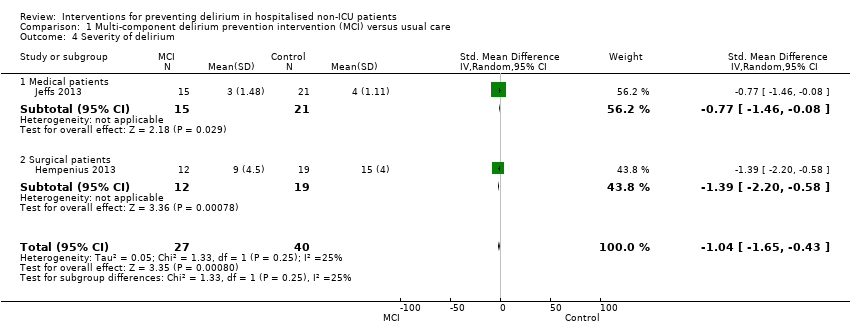
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 4 Severity of delirium.
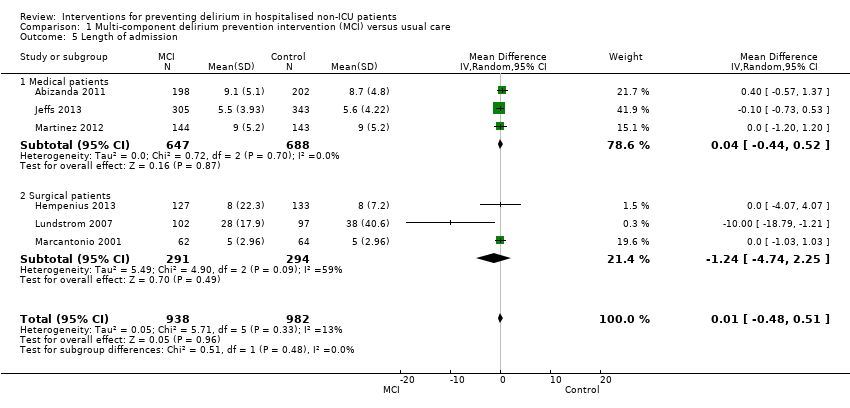
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 5 Length of admission.

Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 6 Cognition.
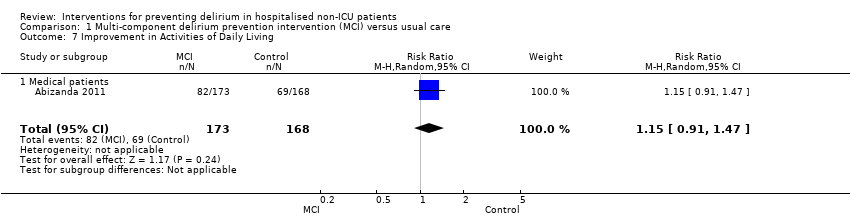
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 7 Improvement in Activities of Daily Living.
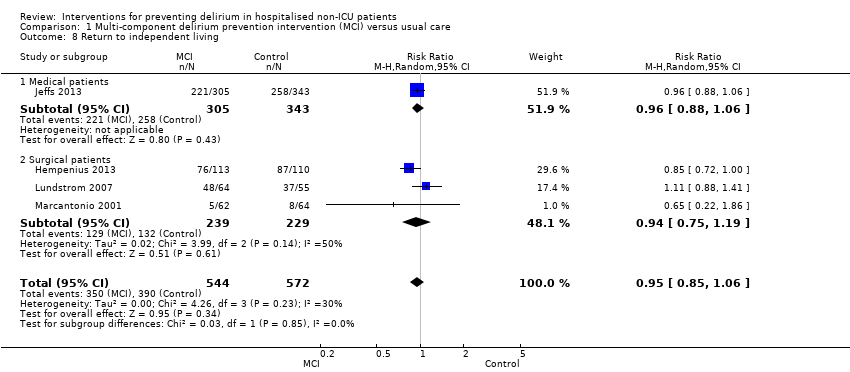
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 8 Return to independent living.
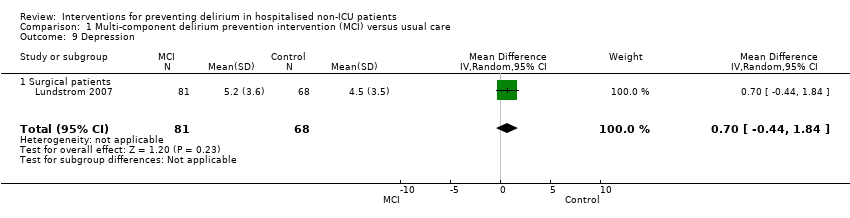
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 9 Depression.
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 10 Withdrawal from protocol.

Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 11 Falls.
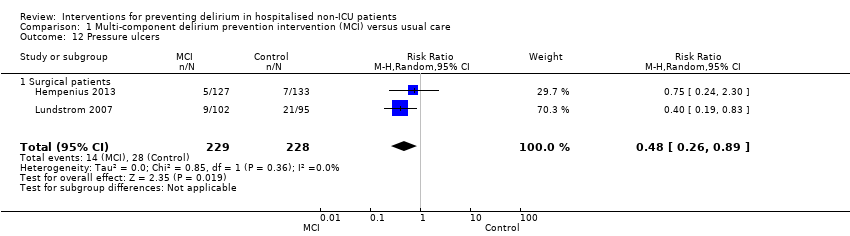
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 12 Pressure ulcers.
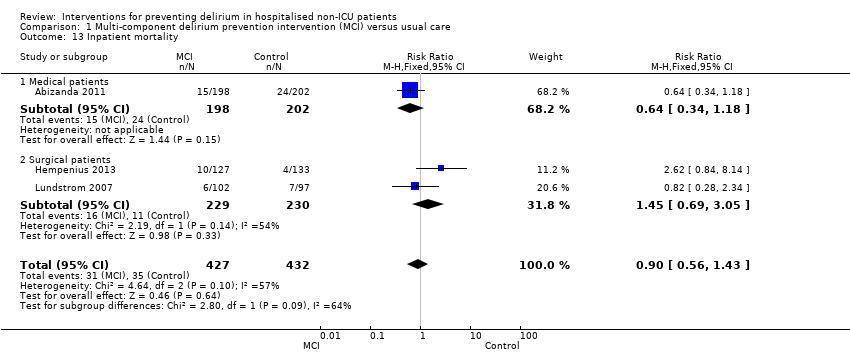
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 13 Inpatient mortality.
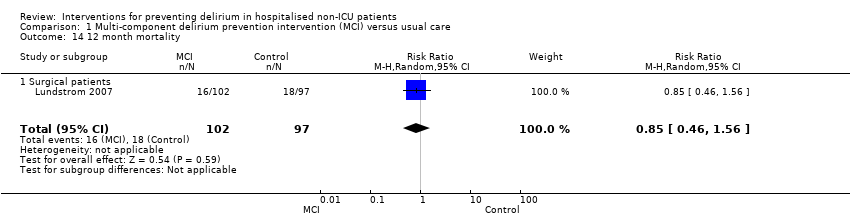
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 14 12 month mortality.

Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 15 Cardiovascular complication.

Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 16 Urinary tract infection.

Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 17 Mental health worsened.

Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 1 Incident delirium.

Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 2 Duration of delirium.
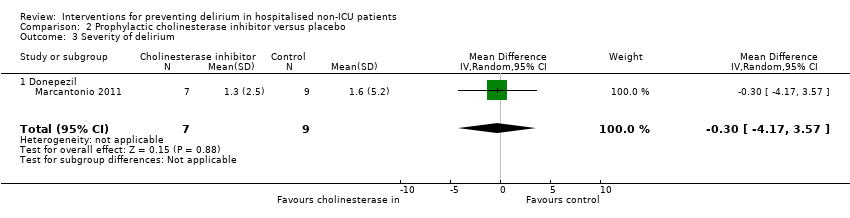
Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 3 Severity of delirium.
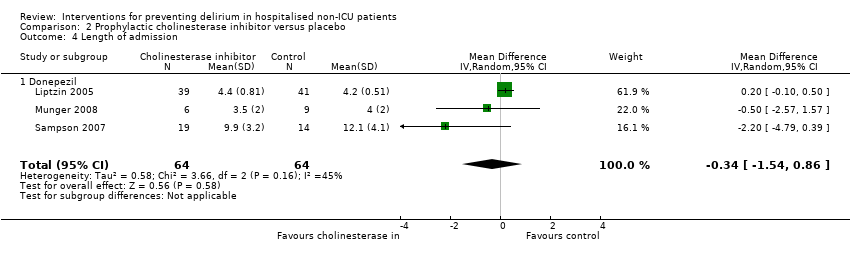
Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 4 Length of admission.
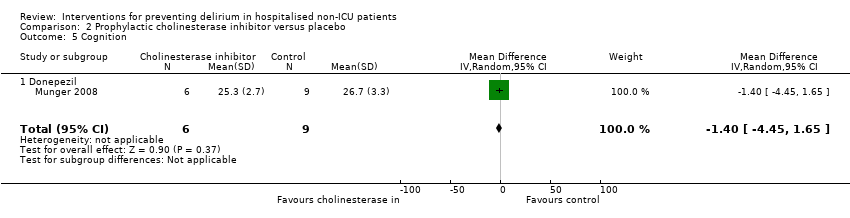
Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 5 Cognition.
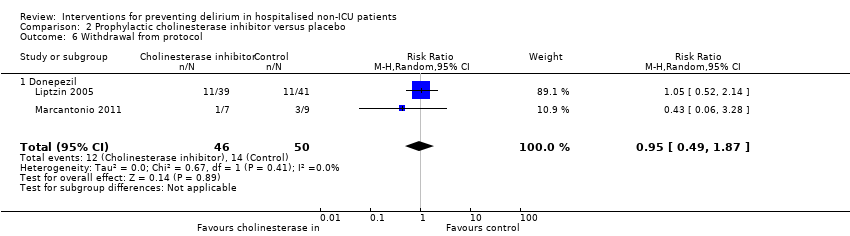
Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 6 Withdrawal from protocol.

Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 7 Adverse events (continuous).

Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 8 Adverse events (binary).
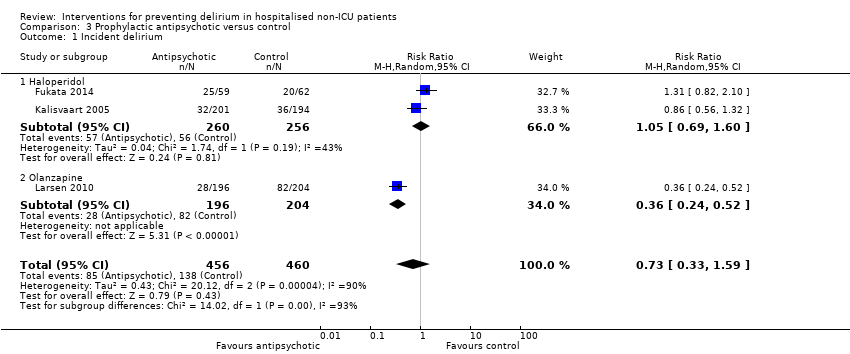
Comparison 3 Prophylactic antipsychotic versus control, Outcome 1 Incident delirium.

Comparison 3 Prophylactic antipsychotic versus control, Outcome 2 Duration of delirium.
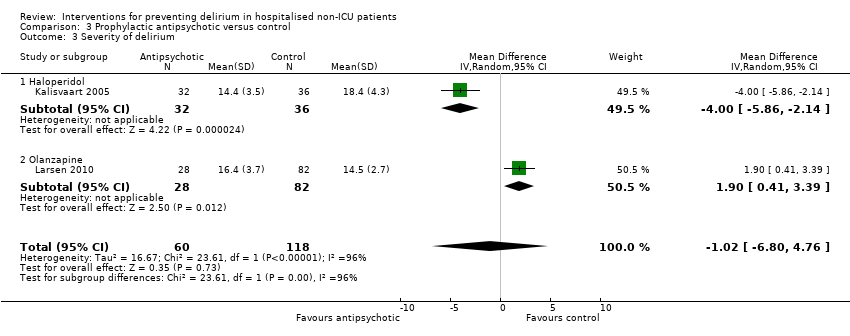
Comparison 3 Prophylactic antipsychotic versus control, Outcome 3 Severity of delirium.

Comparison 3 Prophylactic antipsychotic versus control, Outcome 4 Length of admission.
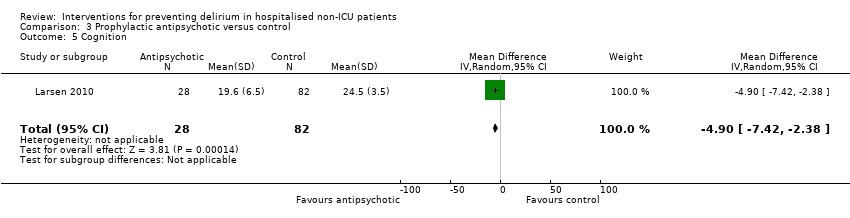
Comparison 3 Prophylactic antipsychotic versus control, Outcome 5 Cognition.
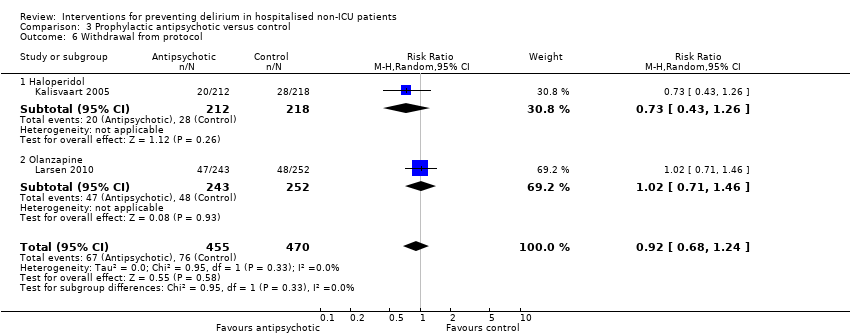
Comparison 3 Prophylactic antipsychotic versus control, Outcome 6 Withdrawal from protocol.
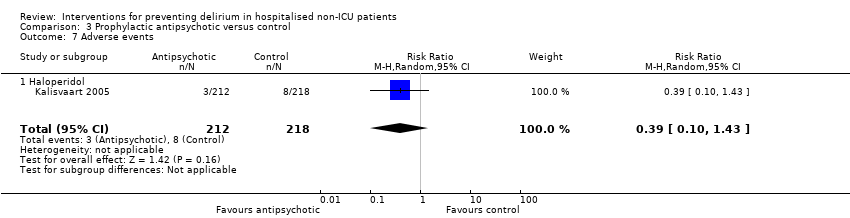
Comparison 3 Prophylactic antipsychotic versus control, Outcome 7 Adverse events.

Comparison 3 Prophylactic antipsychotic versus control, Outcome 8 Pneumonia.

Comparison 3 Prophylactic antipsychotic versus control, Outcome 9 Urinary tract infection.

Comparison 3 Prophylactic antipsychotic versus control, Outcome 10 Congestive heart failure.
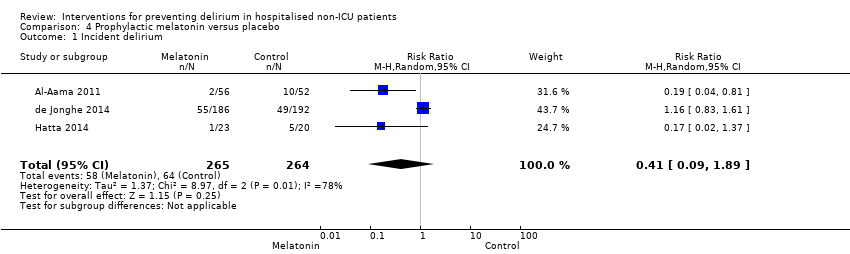
Comparison 4 Prophylactic melatonin versus placebo, Outcome 1 Incident delirium.
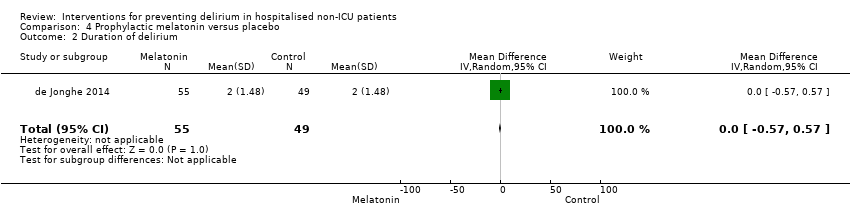
Comparison 4 Prophylactic melatonin versus placebo, Outcome 2 Duration of delirium.
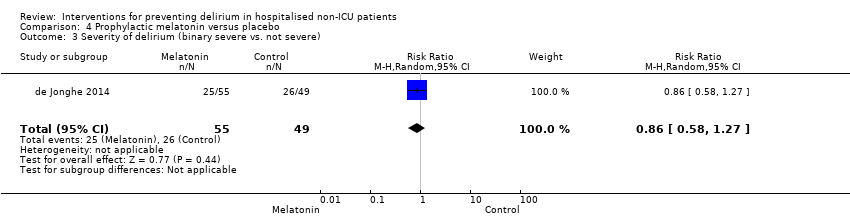
Comparison 4 Prophylactic melatonin versus placebo, Outcome 3 Severity of delirium (binary severe vs. not severe).
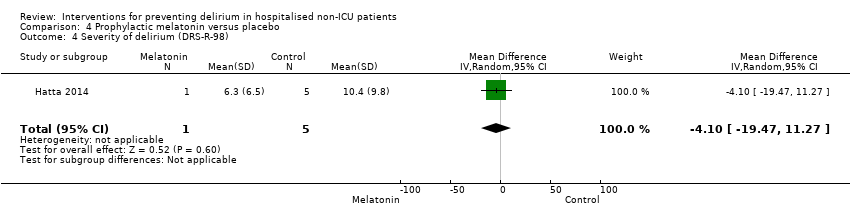
Comparison 4 Prophylactic melatonin versus placebo, Outcome 4 Severity of delirium (DRS‐R‐98).

Comparison 4 Prophylactic melatonin versus placebo, Outcome 5 Length of admission.
Comparison 4 Prophylactic melatonin versus placebo, Outcome 6 Cognitive impairment.
Comparison 4 Prophylactic melatonin versus placebo, Outcome 7 Activities of daily living.
Comparison 4 Prophylactic melatonin versus placebo, Outcome 8 Use of psychotropic medication (binary).
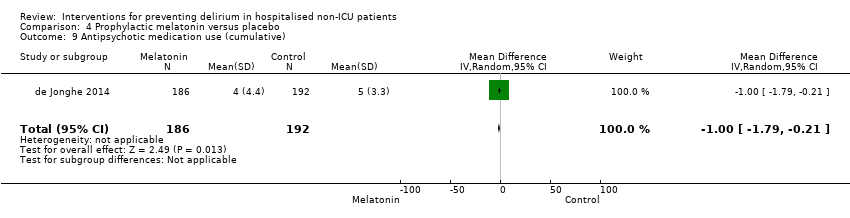
Comparison 4 Prophylactic melatonin versus placebo, Outcome 9 Antipsychotic medication use (cumulative).

Comparison 4 Prophylactic melatonin versus placebo, Outcome 10 Benzodiazepine use (cumulative).

Comparison 4 Prophylactic melatonin versus placebo, Outcome 11 Withdrawal from study.

Comparison 4 Prophylactic melatonin versus placebo, Outcome 12 In‐hospital mortality.

Comparison 4 Prophylactic melatonin versus placebo, Outcome 13 Mortality by 3 months.
Comparison 4 Prophylactic melatonin versus placebo, Outcome 14 Adverse events.

Comparison 5 Prophylactic citicoline versus placebo, Outcome 1 Incident delirium.

Comparison 5 Prophylactic citicoline versus placebo, Outcome 2 Cognitive status.

Comparison 6 Oral premedication with diazepam and diphenhydramine, Outcome 1 Incident delirium.
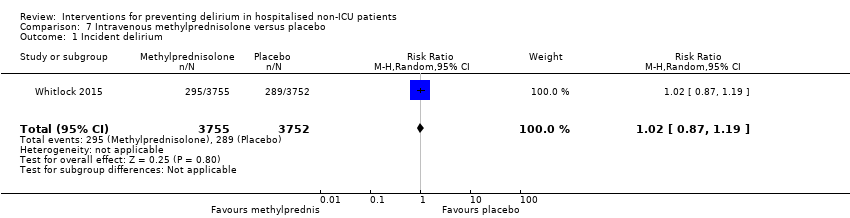
Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 1 Incident delirium.

Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 2 Length of admission.
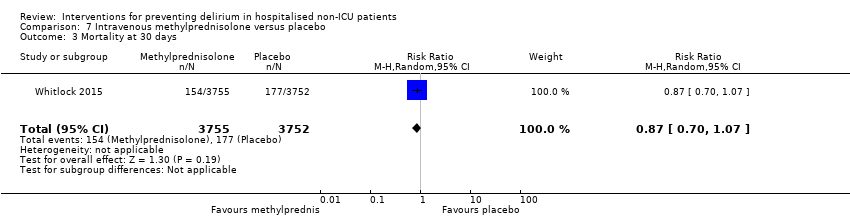
Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 3 Mortality at 30 days.

Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 4 Myocardial injury.
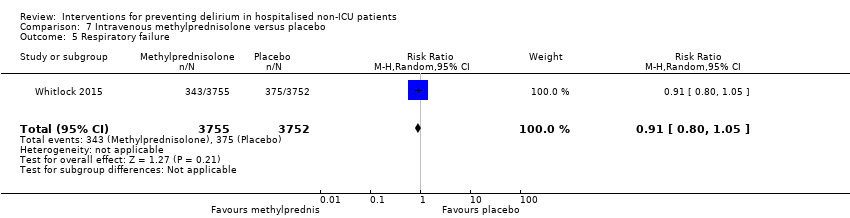
Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 5 Respiratory failure.
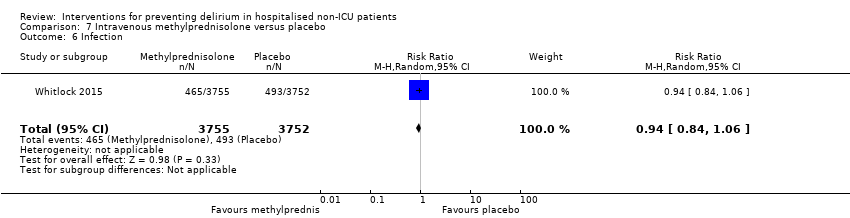
Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 6 Infection.

Comparison 8 Gabapentinoids versus placebo, Outcome 1 Incident delirium.
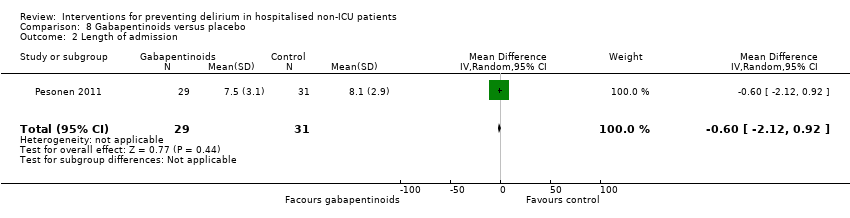
Comparison 8 Gabapentinoids versus placebo, Outcome 2 Length of admission.

Comparison 8 Gabapentinoids versus placebo, Outcome 3 Cognition.

Comparison 8 Gabapentinoids versus placebo, Outcome 4 Psychotropic Medication Use.

Comparison 8 Gabapentinoids versus placebo, Outcome 5 Withdrawal from protocol.

Comparison 9 Ketamine versus placebo, Outcome 1 Incident delirium.
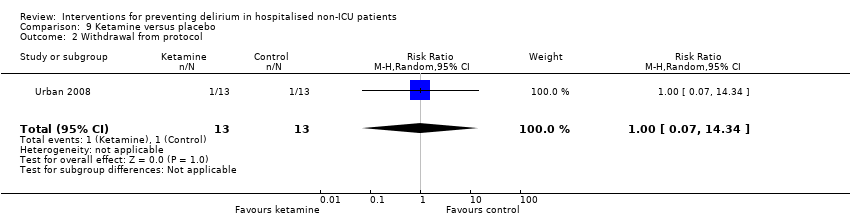
Comparison 9 Ketamine versus placebo, Outcome 2 Withdrawal from protocol.

Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 1 Incident delirium.
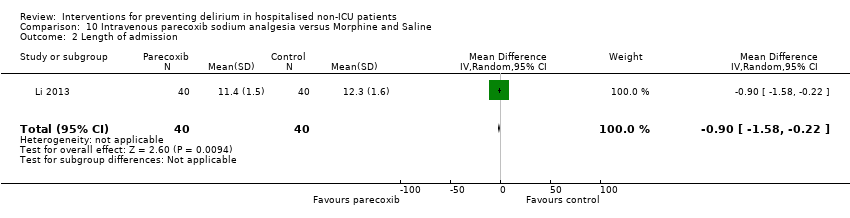
Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 2 Length of admission.

Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 3 Postoperative cognitive dysfunction at 3 days.

Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 4 Postoperative cognitive dysfunction at 1 week.
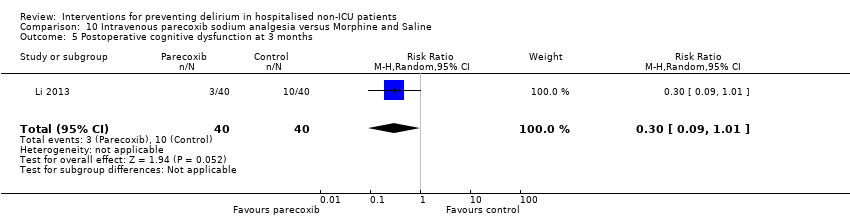
Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 5 Postoperative cognitive dysfunction at 3 months.

Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 6 Postoperative cognitive dysfunction at 6 months.

Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 1 Incident delirium.
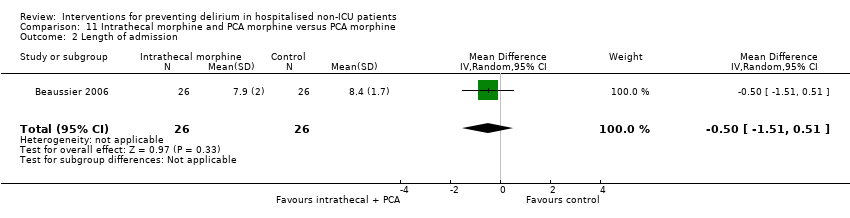
Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 2 Length of admission.

Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 3 Cognition ‐ days for MMSE to return to preoperative level.

Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 4 Withdrawal from protocol.

Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 5 Mortality.

Comparison 12 Fascia iliaca compartment block (FICB) versus placebo, Outcome 1 Incident delirium.
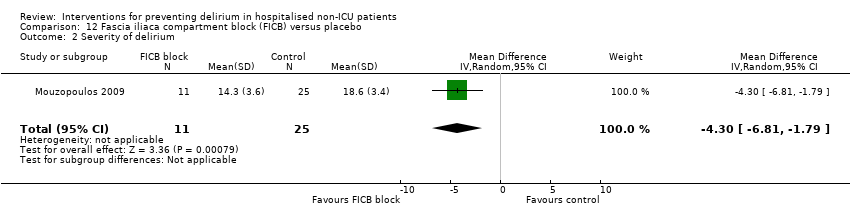
Comparison 12 Fascia iliaca compartment block (FICB) versus placebo, Outcome 2 Severity of delirium.

Comparison 12 Fascia iliaca compartment block (FICB) versus placebo, Outcome 3 Duration of delirium.
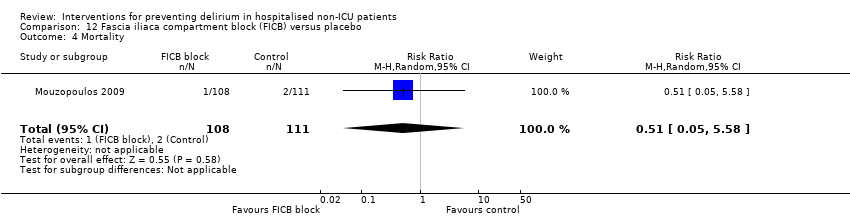
Comparison 12 Fascia iliaca compartment block (FICB) versus placebo, Outcome 4 Mortality.

Comparison 13 Light versus deep propofol sedation, Outcome 1 Incident delirium.
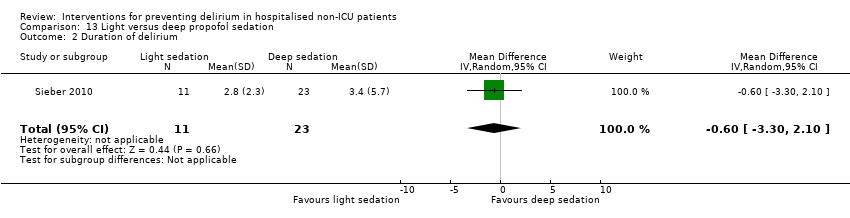
Comparison 13 Light versus deep propofol sedation, Outcome 2 Duration of delirium.

Comparison 13 Light versus deep propofol sedation, Outcome 3 Length of admission.
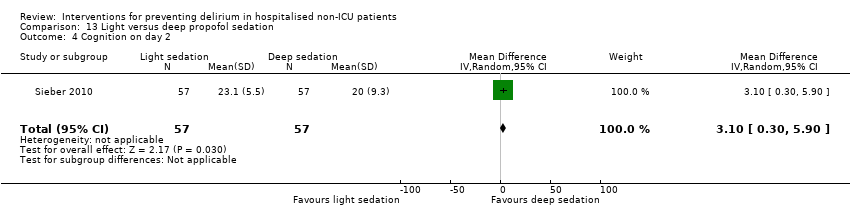
Comparison 13 Light versus deep propofol sedation, Outcome 4 Cognition on day 2.
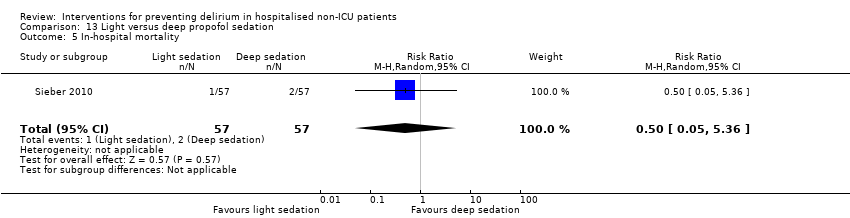
Comparison 13 Light versus deep propofol sedation, Outcome 5 In‐hospital mortality.

Comparison 13 Light versus deep propofol sedation, Outcome 6 Postoperative complications (>=1).

Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 1 Incident delirium.
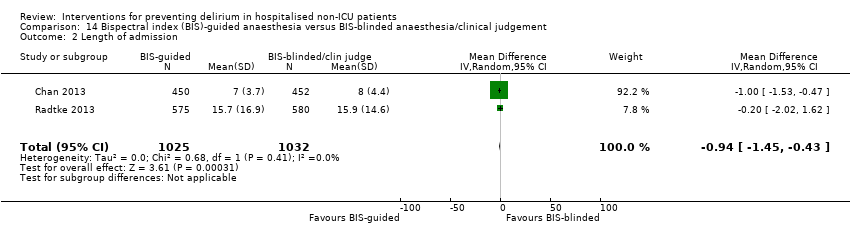
Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 2 Length of admission.
Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 3 Cognition at 7 days.

Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 4 Cognition at 3 months.
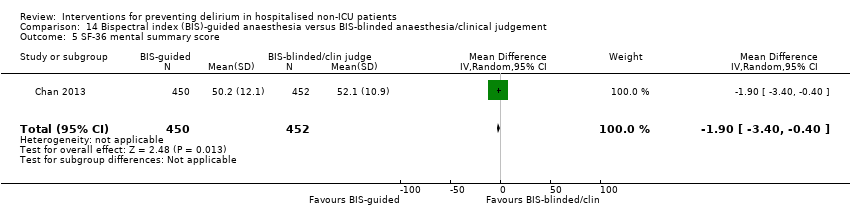
Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 5 SF‐36 mental summary score.

Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 6 Mortality at 7 days.

Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 7 Mortality at 3 months.

Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 8 Cardiac complications.

Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 9 Respiratory complications.

Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 10 Infective complications.
Comparison 15 Sevoflurane versus propofol anaesthesia, Outcome 1 Incident delirium.

Comparison 15 Sevoflurane versus propofol anaesthesia, Outcome 2 Mortality at 12 months.

Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 1 Incident delirium.
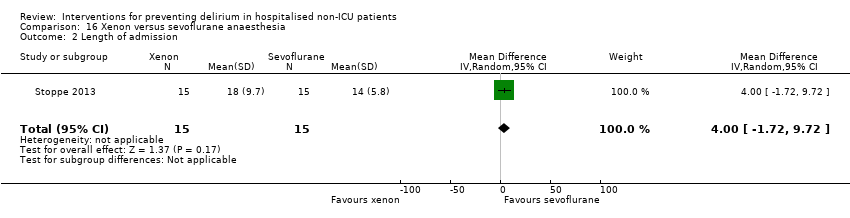
Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 2 Length of admission.

Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 3 In‐hospital mortality.

Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 4 Adverse events.

Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 5 Sepsis.

Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 1 Incident delirium.

Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 2 Length of admission > 10 days.

Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 3 Cognitive decline.
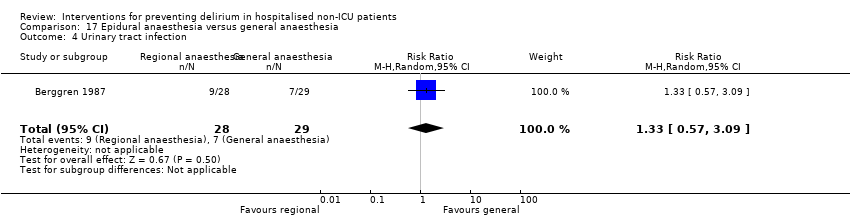
Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 4 Urinary tract infection.

Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 5 Psychological morbidity.

Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 6 Postoperative complications.

Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 7 Pressure ulcer.
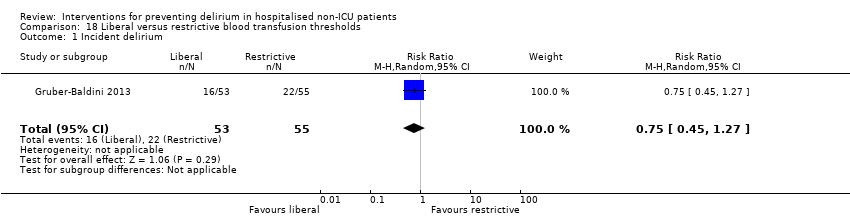
Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 1 Incident delirium.
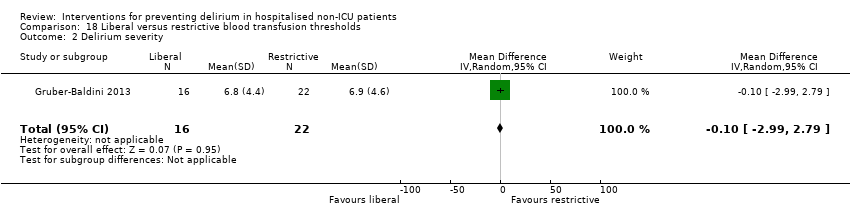
Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 2 Delirium severity.
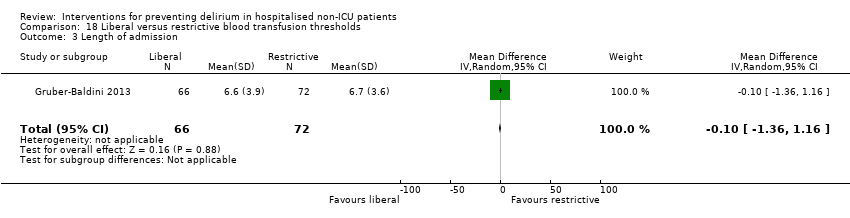
Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 3 Length of admission.

Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 4 Psychoactive medication use.

Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 5 Infection.

Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 6 Congestive heart failure.
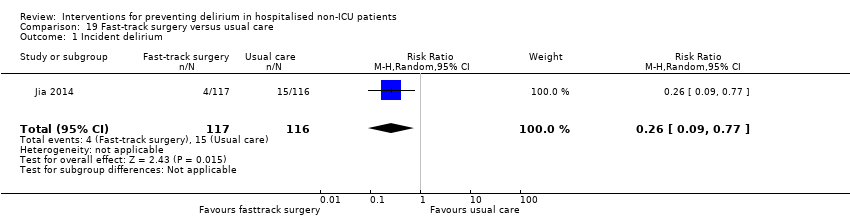
Comparison 19 Fast‐track surgery versus usual care, Outcome 1 Incident delirium.
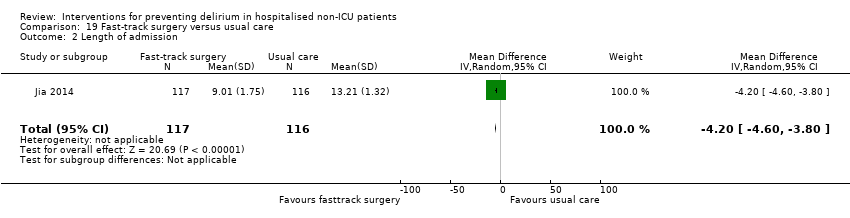
Comparison 19 Fast‐track surgery versus usual care, Outcome 2 Length of admission.
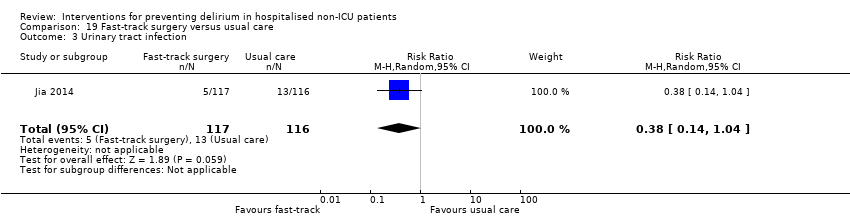
Comparison 19 Fast‐track surgery versus usual care, Outcome 3 Urinary tract infection.

Comparison 19 Fast‐track surgery versus usual care, Outcome 4 Heart failure.

Comparison 20 Postoperative delirium‐free protocol (DFP) versus usual care, Outcome 1 Incident delirium.

Comparison 20 Postoperative delirium‐free protocol (DFP) versus usual care, Outcome 2 Length of admission.

Comparison 20 Postoperative delirium‐free protocol (DFP) versus usual care, Outcome 3 Behavioural disturbance.

Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 1 Incident delirium.
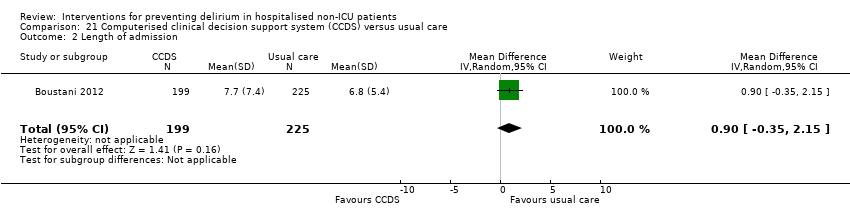
Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 2 Length of admission.

Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 3 Mortality within 30 days of discharge.
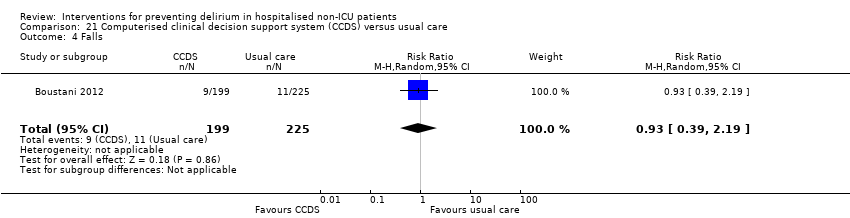
Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 4 Falls.

Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 5 Pressure ulcers.

Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 1 Incident delirium.
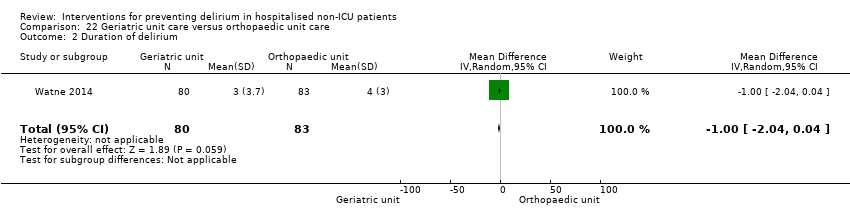
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 2 Duration of delirium.

Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 3 Severity of delirium.

Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 4 Length of admission.
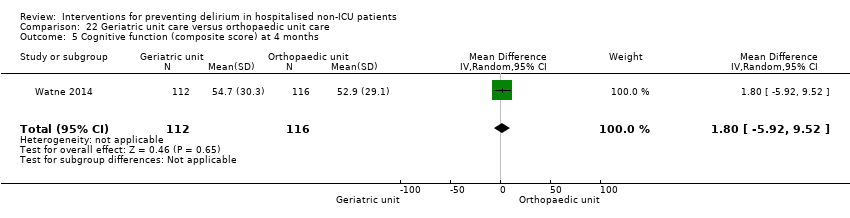
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 5 Cognitive function (composite score) at 4 months.
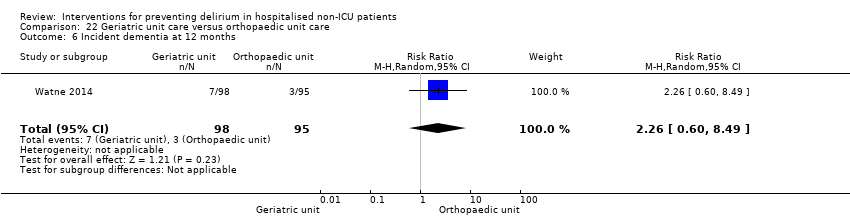
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 6 Incident dementia at 12 months.

Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 7 ADL function at 4 months.

Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 8 Institutionalisation at 4 months.

Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 9 Institutionalisation at 12 months.
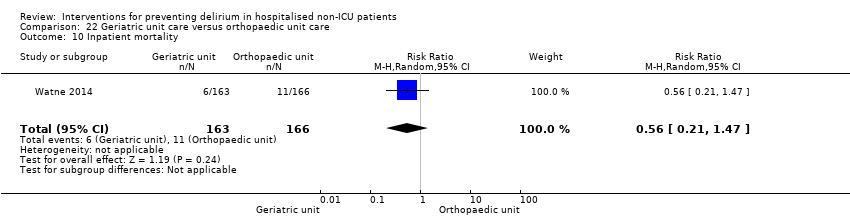
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 10 Inpatient mortality.

Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 11 Falls.
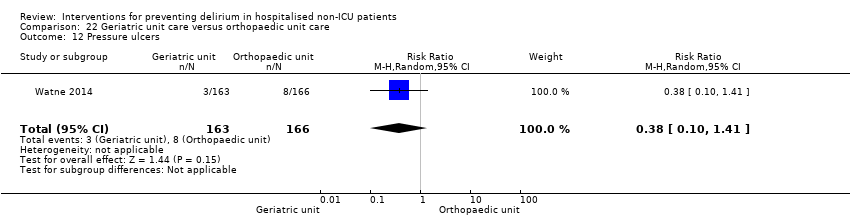
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 12 Pressure ulcers.
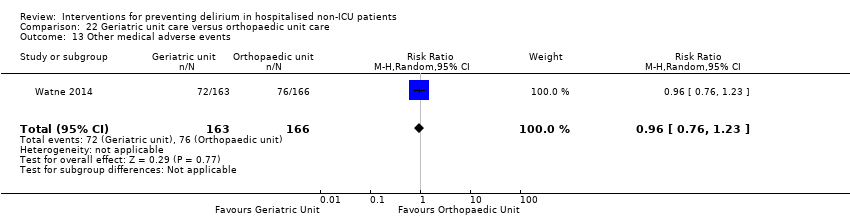
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 13 Other medical adverse events.

Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 14 Postoperative complications.
| Multi‐component delirium prevention intervention compared to usual care for hospitalised non‐ICU patients | ||||||
| Intervention: A multi‐component delirium prevention intervention versus usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| A multi‐component delirium prevention intervention | ||||||
| Incidence of delirium | 209 per 10002 | 144 per 1000 | RR 0.69 | 1950 | ⊕⊕⊕⊝ | |
| Duration of delirium | The mean duration of delirium in the control groups ranged from | The mean duration of delirium in the intervention groups was | 244 | ⊕⊝⊝⊝ | ||
| Severity of delirium | The standardised mean severity of delirium in the intervention groups was | 67 | ⊕⊕⊝⊝ | |||
| Length of admission | The mean length of admission in the control groups ranged from | The mean length of admission in the intervention groups was | 1920 | ⊕⊕⊕⊝ | ||
| Return to independent living | 682 per 10002 | 648 per 1000 | RR 0.95 | 1116 | ⊕⊕⊕⊝ | |
| Inpatient mortality | 81 per 10002 | 73 per 1000 | RR 0.90 | 859 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Three validated methods for delirium detection used ‐ the CAM, OBS and DRS 6 Higher baseline prevalence of dementia in the control groups of two studies compared to the intervention groups causing risk of bias 9 Downgraded because inconsistent results 10 Delirium Rating Scale‐Revised‐98 (0 to 46) and Confusion Assessment Method‐Severity (0 to 10) | ||||||
| Prophylactic cholinesterase inhibitor versus placebo for preventing delirium in hospitalised non‐ICU patients | ||||||
| Intervention: Prophylactic cholinesterase inhibitor versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prophylactic cholinesterase inhibitors | |||||
| Incidence of delirium | 218 per 10001 | 148 per 1000 | RR 0.68 | 113 | ⊕⊝⊝⊝ | |
| Duration of delirium ‐ not measured | N/A | N/A | N/A | N/A | ||
| Severity of delirium | The mean severity of delirium in the control groups was | The mean severity of delirium in the intervention groups was | 16 | ⊕⊕⊝⊝ | ||
| Length of admission | The mean length of admission ranged across control groups from | The mean length of admission in the intervention groups was | 128 | ⊕⊕⊝⊝ | ||
| Return to independent living ‐ not measured | N/A | N/A | N/A | N/A | ||
| Inpatient mortality ‐ not measured | N/A | N/A | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risk is the risk in the control group 2 Both studies are at high risk of attrition bias and have incomplete outcome data. 3 Downgraded because inconsistent results 4 Estimate of effect includes 'no benefit' and both appreciable benefit and appreciable harm. 5 Estimate of effect includes both 'no effect' and minimally important difference, downgraded two levels due to serious imprecision 6 Risk of bias unclear in all domains in one study (abstract only available). Remaining two studies have incomplete outcome reporting and are at risk of attrition bias 7 Downgraded due to imprecision in result | ||||||
| Prophylactic antipsychotic medications for preventing delirium in hospitalised non‐ICU patients | ||||||
| Intervention: Prophylactic antipsychotic medications versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prophylactic antipsychotic medications | |||||
| Incidence of delirium | 300 per 10001 | 165 per 1000 | RR 0.55 | 916 | ⊕⊝⊝⊝ | |
| Duration of delirium | The mean duration of delirium in the control groups ranged from 2.2 to 5.4 days | The mean duration of delirium in the intervention groups was | 178 | ⊕⊝⊝⊝ | ||
| Severity of delirium | The mean severity of delirium in the control groups ranged from 14.4 to 16.4 points | The mean severity of delirium in the intervention groups was | 178 | ⊕⊝⊝⊝ | ||
| Length of admission | The mean length of admission in the control group was 17.1 days | The mean length of admission in the intervention groups was | 68 | ⊕⊕⊝⊝ | ||
| Return to independent living ‐ not measured | N/A | N/A | N/A | N/A | ||
| Inpatient mortality ‐ not measured | N/A | N/A | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risk is the risk in the control group 2Downgraded because inconsistent results 3 Downgraded because of imprecision in results 4 Downgraded due to risk of bias 5 Downgraded two levels because very imprecise results | ||||||
| Prophylactic melatonin for preventing delirium in hospitalised non‐ICU patients | ||||||
| Intervention: Prophylactic melatonin versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prophylactic melatonin | |||||
| Incidence of delirium | 242 per 10001 | 128 per 1000 | RR 0.53 | 529 | ⊕⊝⊝⊝ | |
| Duration of delirium | The mean duration of delirium in the control group was 2 days | The mean duration of delirium in the intervention groups was | 104 | ⊕⊕⊕⊝ | ||
| Severity of delirium (binary severe vs. not severe) | 531 per 1000 | 457 per 1000 | RR 0.86 | 104 | ⊕⊕⊕⊝ | |
| Severity of delirium DRS‐R‐98 score | The mean severity of delirium in the control group was 6.3 points | The mean severity of delirium in the intervention group was 4.1 points lower (19.47 points lower to 11.27 points higher) | 6 (1 study) | ⊕⊕⊝⊝ | ||
| Length of admission | The mean length of admission in the control groups ranged from 11 to 18.5 days | The mean length of admission in the intervention groups was | 500 | ⊕⊕⊕⊝ | ||
| Return to independent living ‐ not measured | N/A | N/A | N/A | N/A | ||
| In‐hospital mortality | 47 per 10001 | 39 per 1000 | RR 0.84 | 543 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risk is the risk in the control group 2 Downgraded because inconsistent results 3 Downgraded because imprecise results 4 Downgraded due to risk of bias 5 Downgraded because imprecise results and very small number of events | ||||||
| Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement for preventing delirium in hospitalised non‐ICU patients | ||||||
| Intervention: Bispectral index (BIS)‐guided anaesthesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| BIS‐blinded/clinical judgement | BIS‐guided | |||||
| Incidence of delirium CAM, DSM‐IV | 226 per 10001 | 160 per 1000 | RR 0.71 | 2057 | ⊕⊕⊕⊝ | |
| Duration of delirium ‐ not measured | N/A | N/A | N/A | N/A | ||
| Severity of delirium ‐ not measured | N/A | N/A | N/A | N/A | ||
| Length of admission Days | The mean length of admission in the control groups ranged from 7 to 15.7 days | The mean length of admission in the intervention group was 0.94 days shorter (0.43 days shorter to 1.45 days shorter) | ‐ | 2057 | ⊕⊕⊕⊝ | |
| Return to independent living ‐ not measured | N/A | N/A | N/A | N/A | ||
| In‐hospital mortality ‐ not measured | N/A | N/A | N/A | N/A | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risk is the risk in the control group (BIS‐blinded/clinical judgement) | ||||||
| Study | Intervention Components | |||||||||||||||||||
| Individualised care | Checklists/ protocols | Education/ training1 | Re‐orientation | Attention to sensory deprivation | Familiar objects | Cognitive stimulation | Nutrition/ hydration | Identification of infection | Mobilisation | Sleep hygiene | MDTcare2 | CGA3 | Oxygenation | Electrolytes | Pain control | Medication review | Mood4 | Bowel/ bladder care | Postoperative complications | |
| Abizanda 2011 | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||
| Bonaventura 2007 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||
| Jeffs 2013 | ✔ | ✔ | ||||||||||||||||||
| Martinez 2012 | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||
| Hempenius 2013 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||
| Lundstrom 2006 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||
| Marcantonio 2001 | ✔ | |||||||||||||||||||
| 1Education/training: structured education/training of staff or carers; 2MDT Multidisciplinary Team; 3CGA Comprehensive Geriatric Assessment; 4Mood: assessment for depression/anxiety | ||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 7 | 1950 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.59, 0.81] |
| 1.1 Medical patients | 4 | 1365 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.43, 0.92] |
| 1.2 Surgical patients | 3 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.59, 0.85] |
| 2 Incidence of delirium in patients with dementia Show forest plot | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.59, 1.36] |
| 2.1 Surgical patients | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.59, 1.36] |
| 3 Duration of delirium Show forest plot | 4 | 244 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐2.96, 0.64] |
| 3.1 Medical patients | 2 | 63 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐2.43, 1.13] |
| 3.2 Surgical patients | 2 | 181 | Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐7.27, 2.46] |
| 4 Severity of delirium Show forest plot | 2 | 67 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.65, ‐0.43] |
| 4.1 Medical patients | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.46, ‐0.08] |
| 4.2 Surgical patients | 1 | 31 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐2.20, ‐0.58] |
| 5 Length of admission Show forest plot | 6 | 1920 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.48, 0.51] |
| 5.1 Medical patients | 3 | 1335 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.44, 0.52] |
| 5.2 Surgical patients | 3 | 585 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐4.74, 2.25] |
| 6 Cognition Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 9.10 [7.20, 11.00] |
| 6.1 Medical patients | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 9.10 [7.20, 11.00] |
| 7 Improvement in Activities of Daily Living Show forest plot | 1 | 341 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.91, 1.47] |
| 7.1 Medical patients | 1 | 341 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.91, 1.47] |
| 8 Return to independent living Show forest plot | 4 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.06] |
| 8.1 Medical patients | 1 | 648 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 8.2 Surgical patients | 3 | 468 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.75, 1.19] |
| 9 Depression Show forest plot | 1 | 149 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.44, 1.84] |
| 9.1 Surgical patients | 1 | 149 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.44, 1.84] |
| 10 Withdrawal from protocol Show forest plot | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Surgical patients | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Falls Show forest plot | 3 | 746 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.16, 2.01] |
| 11.1 Medical patients | 1 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.03] |
| 11.2 Surgical patients | 2 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.18, 3.46] |
| 12 Pressure ulcers Show forest plot | 2 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.89] |
| 12.1 Surgical patients | 2 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.89] |
| 13 Inpatient mortality Show forest plot | 3 | 859 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.56, 1.43] |
| 13.1 Medical patients | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.34, 1.18] |
| 13.2 Surgical patients | 2 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.69, 3.05] |
| 14 12 month mortality Show forest plot | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.56] |
| 14.1 Surgical patients | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.56] |
| 15 Cardiovascular complication Show forest plot | 1 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.78, 1.65] |
| 16 Urinary tract infection Show forest plot | 1 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.45, 3.20] |
| 17 Mental health worsened Show forest plot | 1 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.64, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.17, 2.62] |
| 1.1 Donepezil | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.17, 2.62] |
| 2 Duration of delirium Show forest plot | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Donepezil | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Severity of delirium Show forest plot | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐4.17, 3.57] |
| 3.1 Donepezil | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐4.17, 3.57] |
| 4 Length of admission Show forest plot | 3 | 128 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.54, 0.86] |
| 4.1 Donepezil | 3 | 128 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.54, 0.86] |
| 5 Cognition Show forest plot | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐4.45, 1.65] |
| 5.1 Donepezil | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐4.45, 1.65] |
| 6 Withdrawal from protocol Show forest plot | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.49, 1.87] |
| 6.1 Donepezil | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.49, 1.87] |
| 7 Adverse events (continuous) Show forest plot | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.26, 0.52] |
| 7.1 Donepezil | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.26, 0.52] |
| 8 Adverse events (binary) Show forest plot | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 6.25 [0.35, 112.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 3 | 916 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.33, 1.59] |
| 1.1 Haloperidol | 2 | 516 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.69, 1.60] |
| 1.2 Olanzapine | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.24, 0.52] |
| 2 Duration of delirium Show forest plot | 2 | 178 | Mean Difference (IV, Random, 95% CI) | ‐2.74 [‐9.59, 4.11] |
| 2.1 Haloperidol | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐6.4 [‐9.38, ‐3.42] |
| 2.2 Olanzapine | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 0.60 [0.10, 1.10] |
| 3 Severity of delirium Show forest plot | 2 | 178 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐6.80, 4.76] |
| 3.1 Haloperidol | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐5.86, ‐2.14] |
| 3.2 Olanzapine | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 1.90 [0.41, 3.39] |
| 4 Length of admission Show forest plot | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐5.5 [‐12.17, 1.17] |
| 4.1 Haloperidol | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐5.5 [‐12.17, 1.17] |
| 5 Cognition Show forest plot | 1 | 110 | Mean Difference (IV, Random, 95% CI) | ‐4.90 [‐7.42, ‐2.38] |
| 6 Withdrawal from protocol Show forest plot | 2 | 925 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.68, 1.24] |
| 6.1 Haloperidol | 1 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.43, 1.26] |
| 6.2 Olanzapine | 1 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.71, 1.46] |
| 7 Adverse events Show forest plot | 1 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.10, 1.43] |
| 7.1 Haloperidol | 1 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.10, 1.43] |
| 8 Pneumonia Show forest plot | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 7.28 [0.38, 140.11] |
| 9 Urinary tract infection Show forest plot | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.03, 2.31] |
| 10 Congestive heart failure Show forest plot | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.07, 16.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 3 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.09, 1.89] |
| 2 Duration of delirium Show forest plot | 1 | 104 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.57, 0.57] |
| 3 Severity of delirium (binary severe vs. not severe) Show forest plot | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.58, 1.27] |
| 4 Severity of delirium (DRS‐R‐98) Show forest plot | 1 | 6 | Mean Difference (IV, Random, 95% CI) | ‐4.10 [‐19.47, 11.27] |
| 5 Length of admission Show forest plot | 2 | 500 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐1.20, 1.39] |
| 6 Cognitive impairment Show forest plot | 1 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.04] |
| 7 Activities of daily living Show forest plot | 1 | 369 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.20, 1.20] |
| 8 Use of psychotropic medication (binary) Show forest plot | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.18] |
| 9 Antipsychotic medication use (cumulative) Show forest plot | 1 | 378 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐1.79, ‐0.21] |
| 10 Benzodiazepine use (cumulative) Show forest plot | 1 | 378 | Mean Difference (IV, Random, 95% CI) | ‐11.60 [‐24.34, 1.14] |
| 11 Withdrawal from study Show forest plot | 2 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.15, 6.87] |
| 12 In‐hospital mortality Show forest plot | 3 | 543 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.37, 1.88] |
| 13 Mortality by 3 months Show forest plot | 1 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.67, 1.45] |
| 14 Adverse events Show forest plot | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Incident delirium day 1 post surgery | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.22, 2.06] |
| 2 Cognitive status Show forest plot | 1 | 81 | Mean Difference (IV, Random, 95% CI) | ‐1.47 [‐3.85, 0.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.87, 1.19] |
| 2 Length of admission Show forest plot | 1 | 7507 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.20, 0.20] |
| 3 Mortality at 30 days Show forest plot | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.07] |
| 4 Myocardial injury Show forest plot | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.07, 1.38] |
| 5 Respiratory failure Show forest plot | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.80, 1.05] |
| 6 Infection Show forest plot | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 1.90] |
| 2 Length of admission Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐2.12, 0.92] |
| 3 Cognition Show forest plot | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐2.76, 4.76] |
| 4 Psychotropic Medication Use Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.21, 1.38] |
| 5 Withdrawal from protocol Show forest plot | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [0.50, 161.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.21, 19.23] |
| 2 Withdrawal from protocol Show forest plot | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.26, 0.98] |
| 2 Length of admission Show forest plot | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.58, ‐0.22] |
| 3 Postoperative cognitive dysfunction at 3 days Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.21, 1.02] |
| 4 Postoperative cognitive dysfunction at 1 week Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.15, 0.98] |
| 5 Postoperative cognitive dysfunction at 3 months Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.09, 1.01] |
| 6 Postoperative cognitive dysfunction at 6 months Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.9 [0.44, 1.85] |
| 2 Length of admission Show forest plot | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.51, 0.51] |
| 3 Cognition ‐ days for MMSE to return to preoperative level Show forest plot | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.03, 1.43] |
| 4 Withdrawal from protocol Show forest plot | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.19, 3.17] |
| 5 Mortality Show forest plot | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.24, 0.87] |
| 2 Severity of delirium Show forest plot | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐4.30 [‐6.81, ‐1.79] |
| 3 Duration of delirium Show forest plot | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐5.7 [‐9.50, ‐1.90] |
| 4 Mortality Show forest plot | 1 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.05, 5.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.89] |
| 2 Duration of delirium Show forest plot | 1 | 34 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐3.30, 2.10] |
| 3 Length of admission Show forest plot | 1 | 114 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.80, 1.20] |
| 4 Cognition on day 2 Show forest plot | 1 | 114 | Mean Difference (IV, Random, 95% CI) | 3.10 [0.30, 5.90] |
| 5 In‐hospital mortality Show forest plot | 1 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.36] |
| 6 Postoperative complications (>=1) Show forest plot | 1 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.60, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 2 | 2057 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.60, 0.85] |
| 2 Length of admission Show forest plot | 2 | 2057 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.45, ‐0.43] |
| 3 Cognition at 7 days Show forest plot | 2 | 1938 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.71, 1.05] |
| 4 Cognition at 3 months Show forest plot | 2 | 1990 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.53, 0.97] |
| 5 SF‐36 mental summary score Show forest plot | 1 | 902 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐3.40, ‐0.40] |
| 6 Mortality at 7 days Show forest plot | 1 | 921 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.42, 5.25] |
| 7 Mortality at 3 months Show forest plot | 2 | 1938 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.77, 1.59] |
| 8 Cardiac complications Show forest plot | 1 | 902 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.52, 1.39] |
| 9 Respiratory complications Show forest plot | 1 | 902 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.59, 1.07] |
| 10 Infective complications Show forest plot | 1 | 902 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.55, 0.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.47, 1.34] |
| 2 Mortality at 12 months Show forest plot | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.70, 2.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.20, 2.79] |
| 2 Length of admission Show forest plot | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐1.72, 9.72] |
| 3 In‐hospital mortality Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Adverse events Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.34, 1.64] |
| 5 Sepsis Show forest plot | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.29, 7.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.69, 2.03] |
| 2 Length of admission > 10 days Show forest plot | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.28, 1.24] |
| 3 Cognitive decline Show forest plot | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.02, 1.06] |
| 4 Urinary tract infection Show forest plot | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.57, 3.09] |
| 5 Psychological morbidity Show forest plot | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.23, 4.71] |
| 5.1 Depression | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.23, 4.71] |
| 6 Postoperative complications Show forest plot | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.35, 2.39] |
| 7 Pressure ulcer Show forest plot | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.16, 2.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.45, 1.27] |
| 2 Delirium severity Show forest plot | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐2.99, 2.79] |
| 3 Length of admission Show forest plot | 1 | 138 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.36, 1.16] |
| 4 Psychoactive medication use Show forest plot | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.12] |
| 5 Infection Show forest plot | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.23, 5.22] |
| 6 Congestive heart failure Show forest plot | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.05, 5.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.09, 0.77] |
| 2 Length of admission Show forest plot | 1 | 233 | Mean Difference (IV, Random, 95% CI) | ‐4.20 [‐4.60, ‐3.80] |
| 3 Urinary tract infection Show forest plot | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.14, 1.04] |
| 4 Heart failure Show forest plot | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.10, 0.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.06] |
| 2 Length of admission Show forest plot | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐4.30 [‐12.51, 3.91] |
| 3 Behavioural disturbance Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.03, 1.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.82, 1.43] |
| 2 Length of admission Show forest plot | 1 | 424 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐0.35, 2.15] |
| 3 Mortality within 30 days of discharge Show forest plot | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.49, 2.23] |
| 4 Falls Show forest plot | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.39, 2.19] |
| 5 Pressure ulcers Show forest plot | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident delirium Show forest plot | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.79, 1.22] |
| 2 Duration of delirium Show forest plot | 1 | 163 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐2.04, 0.04] |
| 3 Severity of delirium Show forest plot | 1 | 163 | Mean Difference (IV, Random, 95% CI) | 1.5 [1.00, 4.00] |
| 4 Length of admission Show forest plot | 1 | 329 | Mean Difference (IV, Random, 95% CI) | 3.0 [1.94, 4.06] |
| 5 Cognitive function (composite score) at 4 months Show forest plot | 1 | 228 | Mean Difference (IV, Random, 95% CI) | 1.80 [‐5.92, 9.52] |
| 6 Incident dementia at 12 months Show forest plot | 1 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [0.60, 8.49] |
| 7 ADL function at 4 months Show forest plot | 1 | 239 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐0.70, 2.70] |
| 8 Institutionalisation at 4 months Show forest plot | 1 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.58, 1.91] |
| 9 Institutionalisation at 12 months Show forest plot | 1 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.47, 1.59] |
| 10 Inpatient mortality Show forest plot | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.21, 1.47] |
| 11 Falls Show forest plot | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.61, 2.77] |
| 12 Pressure ulcers Show forest plot | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.10, 1.41] |
| 13 Other medical adverse events Show forest plot | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.76, 1.23] |
| 14 Postoperative complications Show forest plot | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.20, 2.36] |

