Rehabilitación vestibular para la disfunción vestibular periférica unilateral
Resumen
Antecedentes
Esta es una actualización de una revisión Cochrane publicada por primera vez en The Cochrane Library , en el número 4, 2007 y actualizada anteriormente en 2011.
La disfunción vestibular periférica unilateral (DVPU) puede ocurrir como resultado de una enfermedad, un traumatismo o tras una operación. La disfunción se caracteriza por dolencias por mareos, alteraciones visuales o de la mirada y alteraciones del equilibrio. El tratamiento actual incluye medicación, maniobras físicas y regímenes de ejercicio, estos últimos conocidos colectivamente como rehabilitación vestibular.
Objetivos
Evaluar la eficacia de la rehabilitación vestibular en la población adulta residente en la comunidad de personas con disfunción vestibular periférica unilateral sintomática.
Métodos de búsqueda
Se realizaron búsquedas de ensayos publicados y no publicados en el registro de ensayos del Grupo Cochrane de Enfermedades de Oído, Nariz y Garganta (Cochrane Ear, Nose and Throat Disorders Group Trials Register); Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL); PubMed; EMBASE; CINAHL; Web of Science; BIOSIS Previews; Cambridge Scientific Abstracts; ISRCTN y en otras fuentes adicionales. La búsqueda más reciente se realizó el 18 de enero de 2014.
Criterios de selección
Ensayos controlados aleatorizados de adultos que viven en la comunidad, diagnosticados con disfunción vestibular periférica unilateral sintomática. Se buscaron comparaciones de la rehabilitación vestibular versus el control (por ejemplo, placebo), otro tratamiento (rehabilitación no vestibular, por ejemplo, farmacológica) u otra forma de rehabilitación vestibular. La medida de resultado principal fue el cambio en la sintomatología especificada (por ejemplo, proporción con mareo resuelto, frecuencia o gravedad del mareo). Los resultados secundarios fueron las medidas de la función, la calidad de vida y/o las medidas del estado fisiológico, cuando se ha confirmado la reproducibilidad y se ha mostrado que es pertinente o está relacionada con el estado de salud (por ejemplo, la posturografía), y los efectos adversos.
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos estándar previstos por la Colaboración Cochrane.
Resultados principales
Se incluyeron en la revisión 39 estudios que incluían a 2441 participantes con trastornos vestibulares periféricos unilaterales. En los ensayos se abordó la eficacia de la rehabilitación vestibular frente a las intervenciones de control o simuladas, las intervenciones médicas u otras formas de rehabilitación vestibular. El hecho de no cegar a los evaluadores de resultados y la presentación de informes selectivos pueden haber sesgado los resultados en el 25% de los estudios, pero, por lo demás, hubo un bajo riesgo de sesgo de selección o de desgaste.
Los análisis individuales y agrupados de la medida de resultado primaria, la frecuencia de los mareos, mostraron un efecto estadísticamente significativo a favor de la rehabilitación vestibular sobre el control o la no intervención (odds‐ratio (OR) 2,67; intervalo de confianza (IC) del 95%: 1,85 a 3,86; cuatro estudios, 565 participantes). Las medidas de resultados secundarios relacionadas con los niveles de actividad o participación medidos, por ejemplo, con el Inventario de Mareos y Discapacidades, también mostraron una fuerte tendencia hacia las diferencias significativas entre los grupos (diferencia de medias estandarizada (DME) ‐0,83; IC del 95%: ‐1,02 a ‐0,64). La excepción fue cuando se comparó la rehabilitación vestibular basada en el movimiento con las maniobras físicas para el vértigo posicional paroxístico benigno (VPPB), en el que este último mostró ser superior en la tasa de curación a corto plazo (OR 0,19; IC del 95%: 0,07 a 0,49). No se informaron eventos adversos.
Conclusiones de los autores
Existe evidencia de calidad moderadas a alta de que la rehabilitación vestibular es un tratamiento seguro y eficaz de la disfunción vestibular periférica unilateral, basada en varios ensayos controlados aleatorizados de alta calidad. Hay evidencia moderada de que la rehabilitación vestibular resuelve los síntomas y mejora el funcionamiento a medio plazo. Sin embargo, hay evidencia de que para el grupo específico de diagnóstico de VPPB, las maniobras físicas (de reposicionamiento) son más eficaces a corto plazo que la rehabilitación vestibular basada en ejercicios; aunque una combinación de ambas es eficaz para la recuperación funcional a más largo plazo. No hay evidencia suficiente para discriminar entre las diferentes formas de rehabilitación vestibular.
PICO
Resumen en términos sencillos
Rehabilitación vestibular para mejorar el mareo, el equilibrio y la movilidad en pacientes con disfunción vestibular periférica unilateral
Antecedentes
Las personas con problemas vestibulares a menudo sufren mareos y dificultad con la visión, el equilibrio o la movilidad. Los trastornos vestibulares que se denominan unilaterales y periféricos (DVPU) son aquellos que afectan a un lado del sistema vestibular (unilateral) y sólo a la porción del sistema que está fuera del cerebro (periférico ‐ parte del oído interno). Entre los ejemplos de estos trastornos figuran el vértigo posicional paroxístico benigno (VPPB), la neuritis vestibular, la laberintitis, la enfermedad de Ménière unilateral o los problemas vestibulares después de procedimientos quirúrgicos como la laberintectomía o la extirpación de un neuroma acústico. La rehabilitación vestibular para estos trastornos se utiliza cada vez más e implica diversos regímenes basados en el movimiento. Los componentes de la rehabilitación vestibular pueden implicar aprender a provocar los síntomas para "desensibilizar" el sistema vestibular, aprender a coordinar los movimientos de los ojos y la cabeza, mejorar el equilibrio y las habilidades para caminar, y aprender sobre la afección y cómo afrontarla o ser más activo.
Características de los estudios
Se encontraron 39 ensayos controlados aleatorizados (con 2441 participantes) que investigaron el uso de la rehabilitación vestibular en este grupo de trastornos. Todos los estudios utilizaron una forma de rehabilitación vestibular e incluyeron adultos que vivían en la comunidad con DVPU sintomático y confirmado. Los estudios fueron variados en el sentido de que compararon la rehabilitación vestibular con otras formas de tratamiento (por ejemplo, medicación, atención habitual o maniobras pasivas), con intervenciones de control o de placebo o con otras formas de rehabilitación vestibular. Otra fuente de variación entre los estudios fue el uso de diferentes medidas de resultados (por ejemplo, informes de mareos, mejoras en el equilibrio, la visión o la marcha, o la capacidad de participar en la vida cotidiana).
Resultados clave
Debido a la variación entre los estudios, sólo fue posible una agrupación (combinación) limitada de datos. Se pudieron combinar los resultados de cuatro estudios, que demostraron que la rehabilitación vestibular era más eficaz que las intervenciones de control o simuladas para mejorar los informes subjetivos de mareo y para mejorar el desempeño de las funciones de la vida. Dos estudios dieron un resultado combinado a favor de la rehabilitación vestibular para mejorar la marcha. Todos los demás estudios individuales se mostraron favorables a la rehabilitación vestibular para mejorar aspectos como el equilibrio, la visión y las actividades de la vida diaria. La excepción a estos hallazgos fue para el grupo específico de personas con VPPB, donde las comparaciones de la rehabilitación vestibular con maniobras específicas de reposicionamiento físico mostraron que estas maniobras eran más efectivas en la reducción de los síntomas de mareo, particularmente a corto plazo. Sin embargo, otros estudios demostraron que la combinación de las maniobras con la rehabilitación vestibular era eficaz para mejorar la recuperación funcional a largo plazo. No hubo informes de efectos adversos después de ninguna rehabilitación vestibular. En los estudios con una evaluación de seguimiento (3 a 12 meses) se mantuvieron los efectos positivos. No hubo evidencia de que una forma de rehabilitación vestibular fuera superior a otra. Hay un creciente y consistente conjunto de evidencia que apoya el uso de la rehabilitación vestibular para las personas con mareos y pérdida funcional como resultado de la DVPU.
Calidad de la evidencia
Los estudios fueron en general de calidad moderada a alta, pero sus métodos fueron variados. Esta evidencia está actualizada hasta el 18 de enero de 2014.
Authors' conclusions
Background
This is an update of a Cochrane review first published in The Cochrane Library in Issue 4, 2007 and previously updated in 2011.
Description of the condition
People with dysfunction within the vestibular system (vestibulopathy) often complain of dizziness, visual or gaze disturbances, and balance disorders. Dizziness alone accounts for nearly seven million doctor visits per annum in the US (Gans 2002). These impairments lead to significant activity and participation restrictions for the person affected (Perez 2001). The cause of the dysfunction can be a disease‐related pathology or trauma and can be sited in the central (brain) or peripheral (inner ear) portions of the vestibular system. More specifically, because the vestibular system is replicated symmetrically in the periphery, many commonly presenting vestibulopathies involve unilateral (asymmetrical) peripheral vestibular dysfunction (UPVD). Examples of these disorders include benign paroxysmal positional vertigo (BPPV), vestibular neuritis, Ménière's disease (and endolymphatic hydrops) and perilymphatic fistula. Unilateral peripheral dysfunction can also occur after surgical interventions such as unilateral labyrinthectomy or neurectomy (acoustic or vestibular) (Curthoys 2000; Fetter 2000). This review will only address the management of these unilateral peripheral diagnoses.
Table 1 contrasts the incidence, aetiology, symptomatology, diagnosis and specific management of the most prevalent unilateral peripheral vestibulopathies. Whilst there are many aspects specific to each group, there are commonalities in terms of presentation of symptoms that have been reported to be amenable to interventions such as vestibular rehabilitation.
| Vestibulopathy | Incidence | Aetiology | Symptoms | Diagnosis | Treatment |
| Benign paroxysmal positional vertigo (BPPV) (idiopathic) | All age groups Peak 40 to 60 years 11 to 64 per 100,000 pa | Various: Canalithiasis (free‐floating debris in semicircular canals) Cupulolithiasis (debris attached to cupula) | Episodic vertigo after rapid head motion, lasting seconds to 1 minute; +/‐ nausea; some balance deficits; nystagmus (latency, fatigue, rotatory and beating) | Dix‐Hallpike test (post) (Dix 1952) Lateral head‐trunk tilt (Brandt 1999) etc. Use of ENG to record nystagmus | 1. Repositioning manoeuvre/s relative to semicircular canal (Cabrera Kang 2013; Epley 1992; Semont 1988) 2. VR 3. Vestibular suppressant medication for symptom relief 4. Vestibular neurectomy or post‐semicircular canal obliteration |
| Vestibular neuritis (Gans 2002)/neuronitis and labyrinthitis (Strupp 1998) | Unknown | Unclear Viral, autoimmune or vascular mechanisms Viral or bacterial infection of labyrinthine fluids (labyrinthitis) or CN VIII (neuritis) | Acute onset Distressing tonal imbalance producing: rotatory vertigo; spontaneous nystagmus (horizontal); falls to the affected side; nausea | From history and presentation ENG and caloric irrigation show reduced or no response in horizontal semicircular canal; ocular tilt reaction | Symptomatic medication (vestibular suppressants) Bacterial/viral management VR |
| Ménière's disease | Unknown Equal males and females Greatest in 3rd and 4th decades | Unclear Endolymphatic hydrops | Acute: unpredictable and episodic hearing loss, tinnitus and vertigo, +/‐ nausea, vomiting, visual disturbance, anxiety, motion sensitivity Chronic: UPVD or bilateral PVD | History and presentation Audiogram ENG with calorics Imaging the inner ear with high‐resolution MRI after tympanic gadolinium injection | Acute: medication (transtympanic glucocorticoids, antihistamines, suppressants) Chronic: VR, psychological support, surgery (see next row) |
| Postoperative: Neurectomy Intra‐tympanic injection of gentamycin | Unknown | For management of intractable UPVD, tumour removal, Ménière's | UPVD, i.e. spontaneous nystagmus, vertigo, disequilibrium, VOR gain, postural instability | — | VR Symptomatic medication (Dowdal‐Osborn 2002) |
| Perilymphatic fistula (Baloh 2003) | Unknown | History of head trauma, barotraumas or sudden strain; may be associated with chronic otitis or cholesteatoma; perforation of tympanic membrane | Unilateral hearing loss, vertigo, nystagmus | Induce symptoms by pressure in external ear canal Positive head thrust ENG Audiography | Symptomatic medication Surgical packing |
ENG: electronystagmography
MRI: magnetic resonance imaging
pa: per year
UPVD: unilateral peripheral vestibular disorder
VOR: vestibular ocular reflex
VR: vestibular rehabilitation
General treatment and management options
It has been reported that in many cases of chronic vestibular dysfunction, pharmacological and surgical interventions offer limited improvement (Smith‐Wheelock 1991). Medication is often directed at vestibular suppression and/or control of symptoms, such as nausea, or for specific disease processes, such as control of infection. Surgery has a limited role in the management of patients with vestibular dysfunction. It can be used as a 'last resort' in patients whose symptoms are attributable to episodic fluctuation in peripheral function. In such patients, a procedure may be undertaken to remove function from a peripheral vestibular structure (by, for example, labyrinthectomy) or to interrupt the central input of vestibular signals (by vestibular nerve section). Fluctuating vestibular function is thereby replaced with a fixed vestibular deficit. Surgery may also have a role in certain specific conditions, such as the repair of a perilymphatic fistula or removal of an acoustic neuroma.
Description of the intervention
There has been increasing interest in the use of vestibular rehabilitation for the treatment or management of patients with vestibular dysfunction (Chang 2008; Giray 2009; Hoffer 2011). Vestibular rehabilitation is an exercise‐based group of approaches that began with the aim of maximising central nervous system compensation for vestibular pathology (Hoffer 2011). The original protocols by Cooksey and Cawthorne used group activities in a hierarchy of difficulty to challenge the central nervous system (Cooksey 1946). More recently, specific components have been further defined in the vestibular rehabilitation armamentarium (Herdman 2000), each having differing physiological or behavioural rationales as summarised below:
-
Compensatory responses (for positional or motion‐provoked symptoms), based on the inherent plasticity of the central nervous system and using motion to habituate or reduce responsiveness to repetitive stimuli and to re‐balance tonic activity within the vestibular nuclei (Gans 2002). Whilst this process is often termed habituation it is more likely to be a compensatory or neuroplastic process (Hain 2011), rather than a physiological synaptic habituation response.
-
Adaptation for visual‐vestibular interaction (gaze stabilisation) and possibly eye/hand co‐ordination, using repetitive and provocative movements of the head and/or eyes to reduce error and restore vestibulo‐ocular reflex (VOR) gain (Balaban 2012; Cullen 2009).
-
Substitution promotes the use of individual or combinations of sensory inputs (such as visual or somatosensory) to bias use away from the dysfunctional vestibular input or conversely to strengthen use and drive compensation.
-
Postural control exercises, falls prevention, relaxation training, (re)conditioning activities and functional/occupational retraining are based on motor learning principles to change movement behaviour and/or to promote movement fitness.
In addition, there are specific repositioning manoeuvres that may be incorporated into the overall vestibular rehabilitation package for particular diagnostic groups of vestibular dysfunction (for example, benign paroxysmal positional vertigo) (Hilton 2014; Hunt 2012). These manoeuvres (e.g. canalith repositioning manoeuvres or Epley, Semont and Liberatory) are performed on the patient (rather than the patient performing exercises) and are based on a mechanical rationale to shift vestibular debris. Such techniques are not the focus of this review.
Why it is important to do this review
The symptoms and signs of vestibular dysfunction of varying aetiologies are frequent, and often chronic and disabling. Differential diagnosis between possible pathologies is often difficult, with many patients receiving a label of 'unilateral vestibulopathy of unknown cause' (Baloh 2003). Vestibular rehabilitation is a growing method used to reduce resultant impairments and drive adaptation, and is predominantly management‐based (in that it is not 'curative'). Furthermore, vestibular rehabilitation tends to be delivered, and investigated, as a package and prescription is based on the presence of symptoms rather than a specific diagnosis. This review updates the previous Cochrane reviews of 2011 and 2007 for vestibular rehabilitation and a second general review also published in 2007 for a broader range of vestibular disorders conducted by Hansson (Hansson 2007).
Objectives
To assess the effectiveness of vestibular rehabilitation in the adult, community‐dwelling population of people with symptomatic unilateral peripheral vestibular dysfunction.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
Community‐dwelling adults with vestibular dysfunction of unilateral peripheral origin, experiencing a combination of symptoms that may include one or all of the following: dizziness, vertigo, balance deficits (dysequilibrium), visual or gaze disturbances.
Participants with a diagnosis of a symptomatic unilateral, peripheral vestibular dysfunction, named as: peripheral vestibular hypofunction, vestibular neuritis, acoustic neuroma/schwannoma, perilymphatic fistula, Ménière's disease, benign paroxysmal positional vertigo or a combination of these. In the case of a diagnosis of Ménière's disease the participants are in the late stage with a fixed (non‐fluctuating) vestibular deficit. In some instances the authors reported including individuals with central or bilateral vestibular disorders. We contacted authors to obtain results separately for those with UPVD, and if this was not possible we included studies provided those with central and/or peripheral disorders numbered less than 10% of the sample size.
Types of interventions
Interventions described as 'vestibular rehabilitation' that are predominantly exercise and movement‐based, excluding specific (passive) repositioning manoeuvres.
Vestibular rehabilitation does not include medical, electrophysiological or pharmacological management.
Possible comparison interventions from the literature included:
-
vestibular rehabilitation versus control (placebo, sham or usual care);
-
vestibular rehabilitation versus other treatment (e.g. pharmacological or surgical); and
-
vestibular rehabilitation of one type versus another form of vestibular rehabilitation.
Types of outcome measures
Primary outcomes
Measure(s) of change in the specified symptomatology (for example, proportion with dizziness resolved, frequency or severity of dizziness). Symptomatic ratings must be reported and recorded pre‐ and post‐trial.
Secondary outcomes
Measure of function, quality of life and/or measure(s) of physiological status, where reproducibility has been confirmed and shown to be relevant or related to health status (for example, posturography). We also included adverse effects a secondary outcome.
Search methods for identification of studies
We conducted systematic searches for randomised controlled trials. There were no language, publication year or publication status restrictions. The date of the last search was 18 January 2014, following previous searches in July 2010 and March 2007.
Electronic searches
We searched the following databases from their inception for published, unpublished and ongoing trials: the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL 2013, Issue 12); PubMed; EMBASE; AMED; CINAHL; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; ISRCTN; ClinicalTrials.gov; ICTRP; Google Scholar and Google. In searches prior to 2013, we also searched BIOSIS Previews 1926 to 2012 and CNKI.
We modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by The Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in theCochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Handbook 2011)). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, we searched PubMed, TRIPdatabase and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials.
Data collection and analysis
Selection of studies
One of the authors retrieved papers from the identified lists on the basis of the title and abstract. The two authors then reviewed these in full against the established criteria and confirmed them as eligible for consideration. Where there was disagreement between the authors about the inclusion/exclusion criteria, we consulted a third expert and reached a consensus decision.
Data extraction and management
The two authors extracted data from the included studies independently using standardised data forms. Data included participant characteristics (number, age, gender), eligibility and exclusion criteria, setting, description of intervention/s and outcomes. Both authors independently extracted data and we resolved any differences in opinion by discussion and consensus, or by consulting a third expert if needed. In the event of unpublished studies, particularly those with published protocols and where data were incomplete in the published papers, we contacted the trial authors to obtain further details. We did not transform data for reproduction in figures or graphs.
Assessment of risk of bias in included studies
The two authors undertook assessment of the risk of bias of the included trials independently, with the following taken into consideration, as guided by theCochrane Handbook for Systematic Reviews of Interventions (Handbook 2011):
-
sequence generation;
-
allocation concealment;
-
blinding;
-
incomplete outcome data;
-
selective outcome reporting; and
-
other sources of bias.
We used the Cochrane 'Risk of bias' tool in RevMan 5.3 (RevMan 2014), which involves describing each of these domains as reported in the trial and then assigning a judgement about the adequacy of each entry: 'low', 'high' or 'unclear' risk of bias.
We also reported risk of bias as part of the analysis of findings.
Data synthesis
We extracted and analysed data to calculate odds ratios (OR) (fixed‐effect), 95% confidence intervals (CI) and individual and total effect sizes. This required the identification of the number of participants in each group in each trial and total number (for dichotomous data) and number of participants plus mean and standard deviations for each group (for continuous outcome data). We used the standardised mean difference (SMD) for continuous data, and the mean difference (MD) for outcomes from single studies.
There was considerable variation between trials with respect to clinical presentation, the types of exercises included in vestibular rehabilitation and the settings in which the trial was conducted (e.g. community with a booklet‐guided approach compared to a laboratory setting). We assessed heterogeneity between trials with the I2 statistic. Where significant heterogeneity was present, we attempted to explain the differences based on the patient clinical characteristics and interventions of the included studies. We performed neither sensitivity analysis nor subgroup analyses due to the small number of trials that could be pooled for the analysis of the primary outcome.
Results
Description of studies
Results of the search
The current search in January 2014 yielded 1184 titles: 1077 were removed in first‐level screening (i.e. removal of duplicates and clearly irrelevant references), leaving 107 studies which we retrieved in full, where possible. After excluding protocols and trials in progress, we reviewed 96 studies and 12 of these met the inclusion criteria (Basta 2011; Cakrt 2010; Foster 2012; Garcia 2013; Karanjai 2010; Marioni 2013; Morozetti 2011; Pavlou 2012; Rossi‐Izquierdo 2011; Rossi‐Izquierdo 2013; Winkler 2011; Yardley 2012). Five studies are currently in progress and we contacted all authors but results were not available for meta‐analysis. We excluded a further 13 studies (Amor‐Dorado 2012; Bielinska 2012; Cronin 2011; Gurkov 2012; Ipek 2011; Krueger 2010; Lauenroth 2012; Maciaszek J, Osinski 2012; Miranda 2010; Rossi‐Izquierdo 2013a; Sparrer 2013; Steenerson 1996; Wrisley 2011). The current review therefore includes a total of 39 studies (2441 participants). Figure 1 provides a summary of the search process.
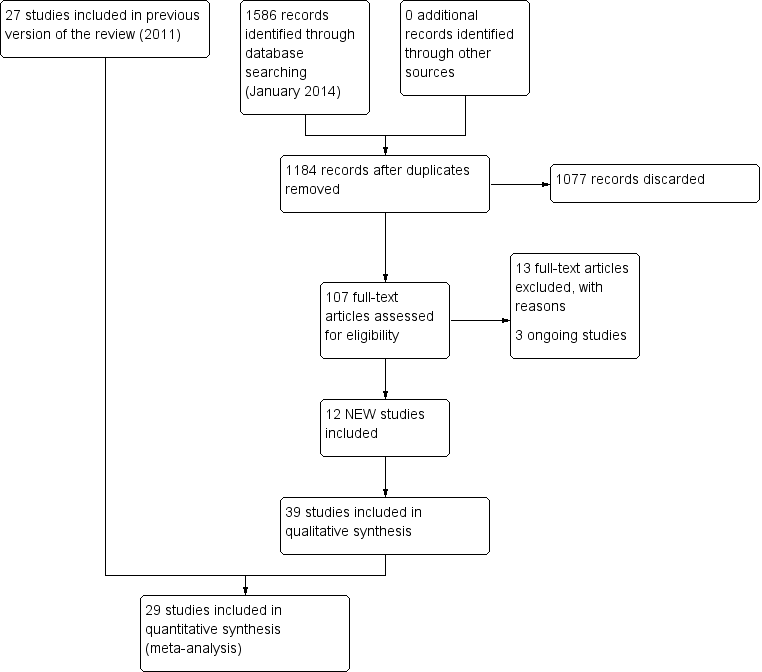
Study flow diagram for 2014 update.
From the 2011 update searches we retrieved a total of 802 references: we removed 652 of these after screening, leaving 150 references for further consideration. Of the 15 retrieved from this list, we ultimately included six studies and added these to the original 21 studies. We excluded a further 10 because they did not meet the review inclusion criteria (see Characteristics of excluded studies). A further four citations reported trial protocols, however the authors did not respond to our request for clarification of completion. The 2011 review therefore included a total of 27 studies (1668 participants) and we excluded a total of 21 studies.
In searches for the 2007 review, we retrieved a total of 232 papers and reviewed them against the inclusion criteria, with 32 being accepted for initial inclusion and quality assessment. After quality appraisal and full consideration we excluded a further 11 for reasons such as subject inclusion of mixed aetiology (e.g. unilateral and bilateral vestibular dysfunction, inclusion of vestibulopathy of central origin or of unknown aetiology), lack of clear intervention or lack of randomisation (see Characteristics of excluded studies table). We included several studies investigating patients with dizziness from a variety of aetiologies (unilateral and bilateral vestibular dysfunction) because they differentiated between the two groups in the analyses (Krebs 2003; Pavlou 2004; Scott 1994; Szturm 1994). This enabled the UPVD patients to be analysed separately. Yardley 1998 and Yardley 2004 also included subjects with dizziness of vestibular origin with mixed aetiology but stipulated that central pathology was excluded. We also decided that because these authors confirmed dizziness as the primary symptom that this would effectively confirm an asymmetrical pathology. We also noted that several papers reported the same trial but with differing outcome measures in each of the papers, notably Cohen 2003 and McGibbon 2004, although the two reports of the latter study were later excluded due to mixed aetiology.
Included studies
See Characteristics of included studies table.
Design
All studies were of parallel design and while they all reported randomisation the majority were unclear in their description of the method of allocation or generation (see Risk of bias in included studies).
The comparisons varied, with 16 investigating vestibular rehabilitation versus placebo or sham interventions. Seven studies compared vestibular rehabilitation with a non‐vestibular rehabilitation intervention. Eighteen studies compared a form of vestibular rehabilitation with one or more other forms of vestibular rehabilitation. Some studies involved multiple comparisons, for example vestibular rehabilitation versus control (sham) versus non‐vestibular rehabilitation (medication).
Sample sizes
A total of 2441 participants participated in the 39 studies, with a mean sample size of 64.7 and a range of 14 to 360. Sample size calculations were rarely reported and this omission (with probable poor statistical power) in the smaller studies was a frequent methodological flaw.
Settings
Five studies investigated vestibular rehabilitation in an acute hospital setting, with the remainder being conducted in community or outpatient environments. Some studies required the vestibular rehabilitation intervention to be performed in the outpatient clinic, others established programmes to be performed in the home or more frequently a combination of the two was administered.
Participants
Participants were all adults, living in the community under normal circumstances. The five studies investigating vestibular rehabilitation in the hospital setting recruited participants who were community dwellers pre‐ and postoperatively. Whilst the acute hospital inpatients were ultimately community dwellers, we separated these out in the final discussion. Age range varied, with most studies reporting a higher recruitment of people in the 65 plus range, reflecting the increasing incidence of dizziness with increasing age.
Eight studies investigated benign paroxysmal positional vertigo, six investigated acute unilateral vestibular loss, five investigated postoperative patients (either acoustic neuroma resection, removal of vestibular schwannoma or ablative vestibular surgery), three specifically investigated Ménière's (non‐acute phase) and the rest reported their sample variously as having chronic unilateral vestibular weakness, hypofunction, dysfunction or dizziness of vestibular origin (including labyrinthitis, neuronitis and other mixed or idiopathic unilateral peripheral vestibular dysfunction pathologies).
Interventions
As expected most studies included a mixture of the various components of vestibular rehabilitation, the most common combination being habituation (movement‐provoking) with gaze stabilising (adaptation), balance and gait/activity training (27). Other additions to this type of package included education (three), booklet‐based (three), sensory substitution (three) and relaxation (two). Five studies described single component vestibular rehabilitation: these included Varela 2001 that investigated Brandt‐Daroff exercises (a form of habituation), Cohen 2003 that investigated rapid versus slow head movements (habituation) and Scott 1994 that investigated relaxation. Two studies compared individualised vestibular rehabilitation with a generic vestibular rehabilitation programme (Szturm 1994; Zimbelman 1999).
Control or placebo interventions involved either usual care or some form of sham exercise that did not target compensatory or adaptation processes (e.g. sham manoeuvres, range of motion, general conditioning, general instructions or strength training).
Studies that compared vestibular rehabilitation with non‐vestibular rehabilitation interventions were also varied. Chang 2008, Cohen 2005, Toledo 2000 and Varela 2001 compared exercise‐based vestibular rehabilitation with repositioning manoeuvres; Kulcu 2008 and Horak 1992 compared vestibular rehabilitation with medication; Scott 1994 compared vestibular rehabilitation (relaxation) with electrical stimulation; and Barozzi 2006 compared oculomotor exercises (adaptation vestibular rehabilitation) with electrical stimulation.
Outcomes
There was considerable variation in the outcome measures used. We considered those that related to symptomatology (dizziness, dysequilibrium or visual disturbance) or functional status (gait, activities of daily living ‐ ADL). Secondary outcome measures that have previously been shown to relate to function, such as visual acuity or posturography (also described as computerised dynamic posturography or Equi‐test), were also considered (Balaguer Garcia 2012). Other reported physiological measures, such as electronystagmography (ENG) and tests for vestibular ocular reflex (VOR) and ocular torsion, subjective visual vertical or biomechanical tests of kinematic and kinetic parameters, were not considered because they have not been directly related to health or functional status.
The outcome measures included in the analyses were as follows.
Primary outcomes
Subjective measures of change in symptoms (impairments):
-
Dizziness cure rate ‐ 'cure' defined as the disappearance of the sensation of dizziness (Karanjai 2010; Varela 2001): dichotomous data of proportion cured.
-
Subjective improvement in dizziness ‐ subjects asked to nominate improvement (better) or no change/worsening in subjective experience of dizziness (dichotomous) (Foster 2012; Horak 1992; Karanjai 2010; Morozetti 2011; Yardley 1998; Yardley 2004; Yardley 2006; Zimbelman 1999).
-
Vertigo Symptom Scale (VSS) ‐ shortened version (14‐item), measuring frequency of dizziness/vertigo, imbalance and related autonomic symptoms during the past month, with a higher score indicating greater symptoms (score range 0 to 60) (Basta 2011; Pavlou 2004; Yardley 1998; Yardley 2004; Yardley 2006; Yardley 2012). (Component related to vertigo reported (VSS‐V), second component related to autonomic/somatic anxiety (VSS‐A)).
-
Vertigo visual analogue scale (VAS) ‐ subjective rating of vertigo on a closed VAS ranging from 0 mm (no symptoms) to 100 mm (worst possible symptoms) (Kammerlind 2005).
-
Vertigo intensity ‐ subjective rating of intensity of vertigo on a five‐point qualitative scale from 1 (no vertigo) to 5 (severe) (Chang 2008; Cohen 2002; Cohen 2003; Garcia 2013; Morozetti 2011).
-
Vertigo frequency ‐ subjective rating of frequency of vertigo experiences on a four‐point scale from 0 (no episodes per day) to 3 (more than 10 episodes per day or constantly) (Cohen 2003).
Secondary outcomes
Objective measures of change in impairment, activity or participation:
-
Repetitive head movement task ‐ measure of standard head movements and resultant provocation (or not) of symptoms, scored as time to perform and intensity of elicited vertigo. Reduction in time and intensity scores indicates improvement (intensity scores not analysed) (Cohen 2003).
-
Dynamic visual acuity ‐ tests for visual acuity during head movements either under predictable conditions (patient moved own head) or unpredictable (head moved by tester), related to oscillopsia and scored as number of errors during tests (Herdman 2003).
-
Romberg test ‐ a measure of standing balance, as dichotomous data, scored as number of pass or fail scores (Herdman 1995). Also (sharpened) Romberg test (scores) ‐ static standing balance tests, timed in seconds where a higher score indicates better (longer) balance (Kammerlind 2005; Yardley 1998).
-
Sway path ‐ measure of standing balance, recording the length of the path of the centre of force (in two planes) during a given time and potentially under differing stance conditions, giving a total sway path measured in metres per minute where the smaller path indicates greater balance proficiency (Strupp 1998).
-
Posturography ‐ (computerised dynamic posturography) a battery of standing balance tests under prescribed variable conditions (sensory organisation test), which can be scored as composite scores and sensory ratios (compared to normative data, other variables available) (Basta 2011; Cakrt 2010; Cohen 2002; Cohen 2003; Marioni 2013; Pavlou 2004; Rossi‐Izquierdo 2011; Rossi‐Izquierdo 2013).
-
Dynamic Gait Index (DGI) ‐ scores eight mobility tasks (ranging from straight walking through to stair ascent/descent) to give a total score of 24 points (Chang 2008; Pavlou 2012; Teggi 2009; Vereeck 2008; Winkler 2011).
-
Gait ataxia ‐ dichotomous data, scored as the presence or absence of abnormal co‐ordination during walking (Herdman 1995), or as continuous data from deviations along a lined walking task (Cohen 2003).
-
Tandem walk ‐ test of dynamic balance and gait proficiency where the patient walks 15 steps forward then backward along a line, scored as the number of correct steps (performed heel to toe and on line), with a higher score indicating greater proficiency (Kammerlind 2005).
-
Vestibular dysfunction in activities of daily living (VD‐ADL) ‐ questionnaire to rate the impact of dizziness or vestibular dysfunction on primary activities of daily life, with a higher score indicating greater functional loss (Cohen 2003; Yardley 1998).
-
Vertigo Handicap Questionnaire (VHQ) ‐ shortened version (14‐item), which measures restriction of activity caused by dizziness and the social effects of this activity restriction (score range 0 to 56) (Cohen 2003; Yardley 1998).
-
Dizziness Handicap Inventory (DHI) ‐ measures patient perception of handicap related to dizziness (an indication of the effect of the symptom on participation or quality of life), where a higher score indicates greater dysfunction (Barozzi 2006; Basta 2011; Garcia 2013; Giray 2009; Morozetti 2011; Rossi‐Izquierdo 2011; Rossi‐Izquierdo 2013; Teggi 2009; Winkler 2011; Yardley 2004; Yardley 2006; Zimbelman 1999).
-
Beck Anxiety Inventory ‐ a self report measure of anxiety state (Pavlou 2012).
-
Situational Vertigo Questionnaire ‐ a self report measure of visually induced vertigo (Pavlou 2012).
-
Subjective health ‐ self report of current health status with respect to dizziness (Yardley 2012).
Follow‐up assessment
Follow‐up was variable, from none (12 studies) to between two, three, six and 12 months for the remaining studies.
Excluded studies
We excluded a total of 34 studies from the review (see Characteristics of excluded studies table). We excluded the majority of these because the participants included mixed aetiologies without separate analysis for those with UPVD (19) or because the study was not randomised (seven).
Ongoing studies
Our search identified two published protocols and a further three trials, which were identified from clinical trial registries. We contacted the primary investigators to determine whether results were available for inclusion in this review. Results are not yet available (see Characteristics of ongoing studies table).
Risk of bias in included studies
The risk of bias for each of the six domains is reported for each trial in the individual 'Risk of bias' tables (see Characteristics of included studies). A summary is also illustrated in Figure 2 and Figure 3. These figures most significantly demonstrate a marked deficiency in the reporting of the methods used to generate and conceal the randomisation process across the majority of studies. The other domains were more clearly reported and we generally evaluated them as low risk of bias.
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
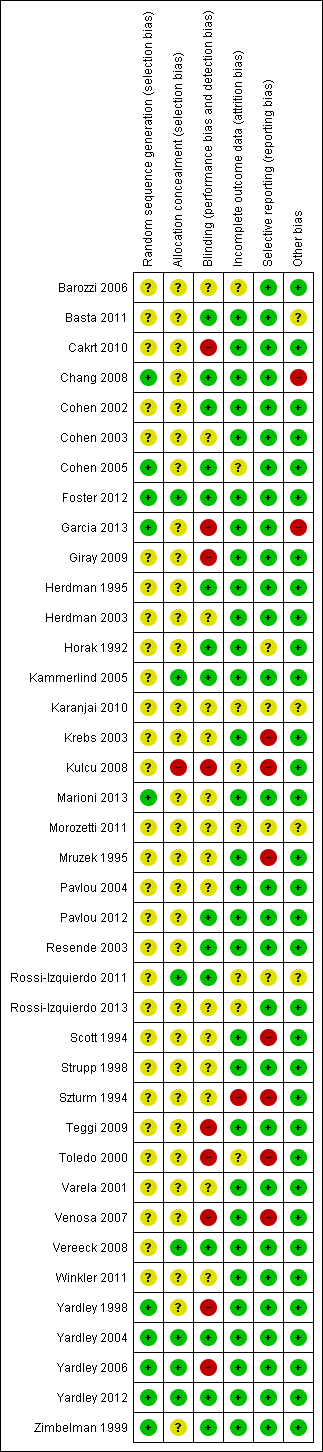
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
The majority of studies measured more than one aspect (symptomatology and/or function), therefore some participants appear in more than one section. Ten studies did not provide the necessary data to enable further analyses and therefore appear among the included studies but not in the meta‐analyses. The majority of all analyses contain data from only one study each, due to the heterogeneity of outcome measures within each comparison. Three studies potentially appear in more than one comparison as they had three‐way (or more) group comparisons (Cohen 2005; Horak 1992; Yardley 2006). Data from Vereeck 2008 appear twice in one analysis but this is reporting separate subgroups based on age (under 50 and over 50 years old).
A summary of individual study results can be found in Table 2.
| Study ID | Inclusion criteria | Intervention/comparator | Result |
| Unilateral peripheral vestibular deficit, 1 to 6 months after the acute phase, diagnosed by clinical examination, CDP, videonystagmography, rotatory chair and caloric tests demonstrating a canal paresis of at least 25% | Intervention groups (n not stated): oculomotor rehabilitation (adaptation) Comparator group (n not stated): vestibular electrical stimulation | No significant differences between groups | |
| Experienced balance disorder for more than 12 months due to the following conditions: canal paresis, otolith disorder, removal of an acoustic neuroma, microvascular compression syndrome, Parkinson's disease, presbyvertigo | Intervention group (n = 59): vibrotactile neurofeedback training and vestibular rehabilitation exercises performed daily (15 minutes) over 2 weeks with the Vertiguard system Comparator group (n = 9): sham Vertiguard device and vestibular rehabilitation exercises | Significant reduction in trunk and ankle sway and improved VSS scores on the Vertiguard group. No changes observed in the sham Vertiguard group | |
| Participants undergoing retrosigmoid microsurgical removal of vestibular schwannoma | Intervention group (n = 9): received visual feedback while performing VR using the BalanceMaster Comparator group (n = 8): control group received VR without feedback | 2‐week intervention post acoustic neuroma removal, significant improvement in 5 out of 7 centre of pressure parameters in quiet stance on foam in the visual feedback group only | |
| First ever attack of unilateral posterior canal BPPV, diagnosed by neurologist and clinical examination | Intervention group (n = 13): canalith repositioning technique (CRT) and vestibular exercises Comparator group (n = 13): CRT only | Intervention group demonstrated a significant improvement in single leg stance with eyes closed at the 2‐week assessment, and static balance and DGI at the 4‐week assessment | |
| Acoustic neuroma resection ‐ postoperative (1 week ‐ acute) diagnosed by history, audiometry, MRI | Intervention group (n = 16): VR (head exercises) Comparator group (n = 15): control (attention only) | No significant difference between groups | |
| Chronic vestibulopathy (labyrinthitis or neuronitis of more than 2 months) diagnosed by physician using posturography, calorics and oculomotor test battery | Intervention group (n = 13): VR (slow head exercises ‐ habituation) Comparator group 1 (n = 22): VR (rapid head exercises) Comparator group 2 (n = 18): VR (rapid plus attention) | All groups significantly improved for VI, VF, DHI, VSS VHQ no change | |
| Unilateral BPPV (post SC) diagnosed by physician (D‐H test), with dizziness for at least 1 week | Intervention group (n = 25): B‐D exercises Comparator group 1 (n = 25): habituation exercises Comparator group 2 (n = 24): CRM Comparator group 3 (n = 25): LM Comparator group 4 (n = 25): sham manoeuvre | Manoeuvres (CRM and LM) better results than exercises (B‐D, habituation), both better than sham | |
| Adults with a history suggestive of BPPV and Dix‐Hallpike manoeuvre consistent with unilateral posterior canal BPPV | Intervention group: (n = 33) half‐somersault manoeuvre was performed twice in the clinic and also given as a home exercise Comparator group: (n = 35) Epley manoeuvre was performed twice in the clinic and also given as a home exercise | Significantly less nystagmus observed after the initial half‐somersault manoeuvre, but no difference in recurrence over the 6‐month follow‐up period | |
| Participants were included if they had Ménière's disease diagnosed by an ENT specialist, and had complaints of dizziness between exacerbations of their disease | Intervention group (n = 23): 12 rehabilitation sessions (twice weekly for 45 minutes) with virtual reality stimuli in a Balance Rehabilitation Unit, plus diet and lifestyle advice and betahistine Intervention group (n = 21): 12 stimulus enriched exercise sessions (twice weekly) on the Balance Rehabilitation Unit, plus diet and lifestyle advice and betahistine | Intervention participants improved significantly on the DHI, dizziness analogue scale and had greater stability on posturography compared to control participants | |
| Participants were diagnosed by a neuro‐otologist or neurologist with chronic decompensated unilateral peripheral vestibular deficit, secondary to peripheral vestibular dysfunction. Diagnosed by ENG, bithermal caloric test, ocular motor testing and positional testing | Intervention group (n = 20): VR incorporating adaptation, substitution, visual desensitisation and balance exercises Comparator group (n = 21): control, no input | Significant improvements were seen in all parameters for the intervention group while there were no changes in the control group | |
| Participants post removal of acoustic neuroma. Diagnosed by MRI and surgically resected ‐ study performed in acute post period | Intervention group (n = 11): VR (adaptation to increase gain) plus ambulation exercises Comparator group (n = 8): smooth pursuit exercises (no head movement) plus ambulation exercises | Intervention group significant improvements for dysequilibrium VAS, VOR to slow head movements, gait and posturography on day 6 compared to control group | |
| Unilateral vestibular hypofunction with abnormal DVA, diagnosed by caloric, rotary chair, positive head thrust | Intervention group (n = 13): VR (adaptation to enhance VOR) Comparator group (n = 8): placebo exercises designed to be "vestibular neutral" | 12/13 improved DVA in intervention group Both improved VAS | |
| Peripheral vestibular dysfunction diagnosed by neuro‐otologist for BPPV, inner ear concussion syndrome, reduced unilateral vestibular function, 18 to 60 years of age | Intervention group (n = 14): VR Comparator group 1 (n = 4): general conditioning exercises Comparator group 2 (n = 8): medication (meclizine or Valium) | VR ‐ superior reduction in sway and increased SOOL DI decreased for both VR and medication 92% improvement rate with VR (75% with comparator group 1, 75% with comparator group 2) | |
| Acute unilateral vestibular loss confirmed by ENG with calorics | Intervention group (n = 28): VR (home exercises plus extra PT (habituation, adaptation, balance and gait) (extra PT included individualised instruction and further exercises) Comparator group (n = 26): VR (home exercises only) | No significant difference between groups ‐ intensity not supported | |
| Diagnosed with posterior canal BPPV through history and clinical examination (Dix‐Hallpike manoeuvre) | Intervention group: Brandt‐Daroff exercises 3 times a day for 2 weeks, n = 16 Comparator group 1: single Epley manoeuvre followed by post‐treatment instructions, n = 16 Comparator group 2: single Semont manoeuvre followed by post‐treatment instructions (sleep upright for 2 nights, then on the unaffected side for the next 5 nights), n = 16 | Statistical analysis of the differences between groups not performed; 73% of participants overall reported resolution of symptoms with no recurrence at 3 months follow‐up | |
| Mixed diagnoses ‐ unilateral and bilateral peripheral vestibular dysfunction. Diagnosed by VOR gain, calorics etc. | Intervention group (n = 42): VR (adaptation, balance) Comparator group (n = 44): control (strength exercises) | VR group significantly improved for gait speed and base of support measures UPVD and BVD groups improved equally though BVD were less functional at baseline | |
| Diagnosed with BPPV and has undergone repositioning techniques by their otorhinolaryngologists but were still complaining of vertigo and dysequilibrium | Intervention group (n = 19): VR (Cawthorne‐Cooksey exercises) Comparator group (n = 19): medication (betahistine) | The intervention group demonstrated significant improvements in the VSS and VDI at the end of the study (8 weeks) | |
| Adults aged 18 to 65 with acute unilateral peripheral vestibular disorder occurring within 2 weeks of entry into the study, with at least 50% weakness on videonystagmography with caloric testing | Intervention group (n = 15): posturography‐assisted VR Comparator group 1 (n = 15): group awaiting spontaneous compensation, no VR Comparator group 2 (controls, n = 10): healthy adults without a vestibular disorder | Both groups of participants with vestibular dysfunction improved over the 6‐week intervention but only the posturography‐assisted VR improved postural control, which approximated the healthy controls | |
| Adults with a chronic vestibular disorder diagnosed by otorhinolaryngologists | Intervention group (n = 10): home exercises based on vertical and horizontal vestibulo‐ocular reflex stimulation (VRS) Comparator group (n = 10): personalised VR home exercise programme | Both groups improved over time but the personalised VR group reported less dizziness on VAS and greater gains on the DHI | |
| Participants had been reviewed by a physician for acoustic neuroma or Ménière's disease and were referred for ablative surgery | Intervention group (n = 8): VR plus social reinforcement, 15 minutes, 2 x day plus a daily walk Comparator group 1 (n = 8): VR no social reinforcement Comparator group 2 (n = 8): general range of motion exercises plus social reinforcement | All the same at 4 weeks Intervention group and comparator group 1 significant improvement for MSQ at 7 weeks Intervention group significant improvement for DHI at 8 weeks CDP no difference between groups | |
| Peripheral vestibular disorder diagnosed by full vestibular examination | Intervention group (n = 20): VR (customised exercises, including gaze control and stability, balance training) Comparator group (n = 20): simulator (optokinetic disc to produce visual‐vestibular conflict plus above) | Both groups improved significantly on posturography: intervention group more than comparator group Subjective symptom reports reduced for both (? any difference) Visual‐vertigo symptoms improved for intervention comparator group Depression reduced significantly for both groups: intervention group more than comparator group Anxiety reduced for both BBS not sensitive | |
| Participants with a history of acute onset of vertigo and had a confirmed peripheral vestibular deficit on the basis of the caloric tests and/or rotational tests on ENG | Intervention group (n = 5): dynamic virtual reality, performed for 45 minutes twice weekly for 4 weeks plus home exercises and general conditioning programme (walking) Comparator group 1 (n = 11): static virtual reality image rehabilitation, performed for 45 minutes twice weekly for 4 weeks plus home exercises and general conditioning programme (walking) Comparator group 1 (n = 5): cross‐over of 5 group 1 participants who then received dynamic virtual reality (not included in our analysis) | After 4 weeks the dynamic groups reported significantly less visual vertigo, but depression improved in the static virtual reality VR group only | |
| Participants with BPPV diagnosed by ENT using history, ENT examination, ENG | Intervention group: VR (compensation, adaptation, sensory substitution, balance: C‐C) Comparator group: control (nil) | Intervention group significantly improved Comparator group no change | |
| Participants with instability due to chronic unilateral peripheral vestibular disorders, which had not spontaneously resolved after a month. Hypofunction was defined with caloric tests, at least 25% labyrinthic preponderance according to defined criteria | Intervention group (n = 12): computerised dynamic posturography (CDP), 5 sessions of approximately 15 to 20 minutes on consecutive days Comparator group (n = 12): optokinetic stimulation (OKN), 5 sessions lasting 5 to 15 minutes on consecutive days | Outcomes assessed 3 weeks after treatment. Both groups improved, with the CDP group showing greater gains in the visual and vestibular input and limits of stability, while the OKN group showed greater improvement in visual preference | |
| Participants with instability due to chronic unilateral peripheral vestibular disorders, which had not spontaneously resolved after a month | Intervention group (n = 13): 5 sessions of posturography‐assisted VR over a 2‐week period Comparator group (n = 13): 10 sessions of posturography‐assisted VR over a 2‐week period | Outcomes assessed 3 weeks after the intervention and both groups improved over time, with the 5‐session group reporting greater gains on the DHI, but some items of posturography improved to a greater extent in the 10‐session group | |
| Ménière's disease diagnosed by medical and audiological examination (5 were bilateral but had one "worse" ear) | Intervention group (n = 10): applied relaxation Comparator group (n = 10): transcutaneous nerve stimulation to the hand | No change in either group for relevant measures (dizziness etc.) Intervention group improved on hearing ability more than comparator group | |
| Vestibular neuritis (acute/sub‐acute). Diagnosed by history, examination ‐ nystagmus, postural imbalance, ENG, calorics, ocular tilt reaction | Intervention group (n = 19): VR (home exercises, based on Cooksey‐Cawthorne, Norre ‐ habituation, gaze exercises, sensory substitution, functional retraining) Comparator group (n = 20): control (nil exercise but encouragement to move) | For OT and SVV tests, intervention group equal to comparator group For SP, intervention group improved significantly more than comparator group, i.e. balance improved | |
| Clinical diagnosis of peripheral vestibular dysfunction, persistent dizziness, disorientation or imbalance for at least 1 year, and abnormal balance performance during CDP at baseline | Intervention group (n = 11): VR Comparator group (n = 12): VR (home, C‐C) | Intervention group had reduced falls, improved CDP values and reduced VOR asymmetry compared with comparator group | |
| Participants were recently hospitalised for an acute episode of rotational vertigo which lasted several days and were diagnosed with vestibular neuritis | Intervention group (n = 20): VR Comparator group (n = 20): control ("perform usual daily activities") | Significant improvement in DHI between groups and reduction in anxiety. For both groups, there was a significant correlation between change in anxiety and change in DHI/DGI | |
| BPPV diagnosed with clinical assessment and electronystagmography | Intervention group (n = 10): VR (PC, head‐eye and habituation) Comparator group 1 (n = 10): Semont manoeuvre Comparator group 2 (n = 20): Semont + VR | Intervention group 80% cure rate at day 15 versus comparator group 1 45% Intervention group 66% cure rate at 3 months versus comparator group 2 100% | |
| BPPV, diagnosed by history and D‐H test (nystagmus) | Intervention group (n = 29): VR (B‐D habituation exercises) Comparator group 1 (n = 35): Semont manoeuvre Comparator group 2 (n = 42): Epley manoeuvre | Comparator groups 1 and 2 had a similar cure rate at 1 week; by 3 months comparator group 2 were superior but comparator group 1 more stable CRM superior to habituation (B‐D) for BPPV | |
| Acute episode of rotational vertigo within the last 5 days | Intervention group (n = 45): VOR adaptation exercises (X1 and X2 viewing exercises) Comparator group (n = 42): placebo exercises (sham visual fixation task) | Intervention group recovered more quickly in all parameters measured and required significantly less medication by the end of the follow‐up period (21 days) | |
| Consecutive patients post removal of an acoustic neuroma | Intervention group (n = 31): customised VR (exercises for balance, head motion, mobility, gaze and treadmill walking) Comparator group (n = 22): general instructions | Participants were stratified according to age (above and below 50 years). Older participants performed significantly better than the control group for balance, TUG and tandem gait compared to the control group. There was no group effect for the younger participants | |
| Participants with chronic dizziness (greater than 6 months duration) who had completed a VR programme, functional range of motion and strength in the lower limbs and trunk, intact sensation in the lower limbs, ability to stand unassisted for 1 minute | Intervention group (n = 10): platform tilt perturbations only Comparator group 1 (n = 7): platform tilt perturbations and VR exercise programme Comparator group 2 (n = 12): VR only | Outcomes were assessed after the 3‐week intervention and a follow‐up at 2 months later. The VR group only demonstrated significant improvement on the DHI but the platform tilt groups improved activity and participation domain outcomes | |
| Dizziness of vestibular origin. Mixed aetiology ‐ diagnosed where possible by medical records (1/3) Possibility of central pathology | Intervention group (n = 67): VR (education, head and body movements, relaxation, breathing, encouragement to function) Comparator group (n = 76): control | Intervention group improved significantly on all measures more than comparator group, except VHQ (no difference) Overall intervention group 4 times more likely to report subjective improvement than comparator group | |
| Dizziness of vestibular origin diagnosed by case history and MPD | Intervention group (n = 83): VR (primary care: demonstration, booklet and follow‐up) Comparator group (n = 87): control, usual medical care | All measures improved significantly in VR group compared with control group Clinical improvement 67% VR; 38% control | |
| Participants with Ménière's disease (non‐acute phase) who had experienced dizziness of imbalance in the last 12 months, had consulted their GP regarding involvement in the study | Intervention group (n = 120): VR (booklet of exercises) Comparator group 1 (n = 120): SC (booklet for self management) | At 3 months intervention group had greater improvement on 5 measures compared with comparator group 1 (2 measures) compared with comparator group 2 (0 measures) At 6 months intervention group and comparator group 1 both reported significant improvement, more than comparator group 2 Correlation between adherence and outcome | |
| Chronic dizziness, as diagnosed by their GP | Intervention group (n = 112): VR (self management booklet with phone support from a vestibular therapist) | At 12 weeks all groups showed some improvement in the VSS, and at 1 year both intervention groups improved significantly compared to usual care | |
| Unilateral peripheral vestibular dysfunction diagnosed by neuro‐otological tests | Intervention group (n = 6): VR (individual with adaptation and postural control) Comparator group (n = 8): VR (general C‐C) | Intervention group improved dizziness over time, comparator group did not No change for either on the BBS (insensitive) No between‐group differences ‐ but 100% of intervention group reported improvement compared with 62.5% of comparator group Intervention group had more Ménière's disease |
BBS: Berg Balance Scale
B‐D: Brandt‐Daroff
BPPV: benign paroxysmal positional vertigo
BVD: bilateral vestibular dysfunction
C‐C: Cooksey‐Cawthorne
CDP: computerised dynamic posturography
CRM: canalith repositioning manoeuvre
CRT: canalith repositioning technique
DGI: Dynamic Gait Index
D‐H test: Dix‐Hallpike test
DHI: Dizziness Handicap Inventory
DI: dizziness intensity
DVA: dynamic visual acuity
ENG: electronystagmography
GP: general practitioner
LM: liberatory manoeuvre
MPD: motion‐provoked dizziness
MRI: magnetic resonance imaging
MSQ: motion sensitivity quotient
OKN: optokinetic reflex
OT: ocular tilt
PC: postural control
PT: physical therapy
SC: symptom control
SOOL: standing on one leg
SP: sway path
SVV: subjective visual vertical
TUG: Timed Up and Go
VAS: visual analogue scale
VDI: Vertigo Dizziness Imbalance questionnaire
VF: vertigo frequency
VHQ: Vestibular Handicap Questionnaire
VI: vertigo intensity
VOR: vestibular ocular reflex
VSS: Vertigo Symptom Scale
VR: vestibular rehabilitation
Comparison 1: Vestibular rehabilitation versus control (placebo, sham, usual care or no intervention)
We analysed 13 trials in this comparison (Cohen 2002; Cohen 2005; Giray 2009; Herdman 1995; Herdman 2003; Horak 1992; Resende 2003; Strupp 1998; Teggi 2009; Vereeck 2008; Yardley 1998; Yardley 2004; Yardley 2006). Three other studies performed this comparison (Krebs 2003; Marioni 2013; Venosa 2007), however they could not supply data to enable meta‐analysis.
We found statistically significant differences between vestibular rehabilitation and control/placebo interventions in favour of vestibular rehabilitation for the following outcomes.
Primary outcome
-
Subjective improvement in dizziness (odds ratio (OR) fixed‐effect 2.67, 95% confidence interval (CI) 1.85 to 3.86, P value < 0.0001; four studies, 565 participants) (Analysis 1.1).
-
Vertigo Symptom Scale (VSS) (standardised mean difference (SMD) fixed‐effect ‐0.68, 95% CI ‐0.87 to ‐0.49, P value < 0.00001; three studies, 553 participants) (Analysis 1.2).
Secondary outcomes
-
Gait ataxia (OR fixed‐effect 0.04, 95% CI 0.00 to 0.77, P value = 0.03; one study, 19 participants) (Analysis 1.3).
-
Vestibular disorders activities of daily living (VD‐ADL) (mean difference (MD) fixed‐effect ‐10.50, 95% CI ‐14.09 to ‐6.91, P value < 0.0001; one study, 16 participants) (Analysis 1.4).
-
Sway path (posturography data) (MD fixed‐effect ‐13.70, 95% CI ‐16.51 to ‐10.89, P value < 0.00001; one study, 39 participants) (Analysis 1.5).
-
Dynamic visual acuity (OR fixed 84.00, 95% CI 4.51 to 1564.26, P value = 0.003; one study, 21 participants) (Analysis 1.6).
-
Vertigo Handicap Questionnaire (VHQ) (MD fixed‐effect ‐3.40, 95% CI ‐6.76 to ‐0.04, P value = 0.05; one study, 143 participants) (Analysis 1.7).
-
Sharpened Romberg test scores (balance) (MD fixed‐effect 9.90, 95% CI 0.80 to 19.00, P value = 0.03; one study, 143 participants) (Analysis 1.8).
-
Dizziness Handicap Inventory (DHI) (SMD fixed‐effect ‐0.83, 95% CI ‐1.02 to ‐0.64, P value < 0.00001; five studies, 535 participants) (Analysis 1.9).
-
Dynamic Gait Index (DGI) (SMD fixed‐effect ‐0.92, 95% CI ‐1.38 to ‐0.46, P value < 0.0001; two studies, 93 participants) (Analysis 1.10) (Teggi 2009; Vereeck 2008, under 50 and over 50 years old).
Differences were non‐significant for the other four measures: Romberg test, vertigo intensity (two separate comparisons) and posturography.
The three studies that could not be included in the meta‐analysis, due to inadequate reporting of data, supported the positive findings of vestibular rehabilitation improving gait and reducing the duration of dizziness symptoms compared to a control group (Krebs 2003; Marioni 2013; Venosa 2007).
We calculated heterogeneity as being high in three analyses in this comparison. On visual inspection of Analysis 1.2 (Vertigo Symptom Scale) and Analysis 1.9 (Dizziness Handicap Inventory), we noted the same study to have markedly larger effects than the other pooled studies (Yardley 2004). Comparison of methods and clinical parameters did not reveal any clear reasons for the difference. Furthermore, removal of the study from each analysis still retained the statistically significant effects. In the third analysis (Analysis 1.10, Dynamic Gait Index) the Teggi 2009 study provided a higher effect size than the other pooled study results; again there were no obvious clinical or methodological differences to explain this, as all studies had acceptably low risk of bias and usual care control groups. However, in this instance removal of the study also removed the significant effect.
Comparison 2: Vestibular rehabilitation versus other treatment (non‐vestibular rehabilitation)
There were seven studies in this comparison (Barozzi 2006; Chang 2008; Cohen 2002; Cohen 2005; Horak 1992; Karanjai 2010; Varela 2001), with a further three studies with inadequate data (Kulcu 2008; Scott 1994; Toledo 2000).
Primary outcome
Statistically significant differences between vestibular rehabilitation and other interventions (manoeuvres) in favour of 'other' (where 'other' were physical manoeuvres for benign paroxysmal positional vertigo (BPPV)) were found for the following.
-
Dizziness cure rate (OR fixed 0.19, 95% CI 0.07 to 0.49, P value = 0.006; two studies, 119 participants) (Analysis 2.1).
Secondary outcomes
Statistically significant differences between vestibular rehabilitation plus canalith repositioning manoeuvres (physical manoeuvres for BPPV) and canalith repositioning manoeuvres (CRM) only, in favour of vestibular rehabilitation plus CRM were found for the following.
-
Dynamic Gait Index (MD fixed‐effect ‐1.00, 95% CI ‐1.85 to ‐0.15, P value = 0.02; one study, 26 participants) (Analysis 2.2).
Differences were non‐significant for all other measures (four): subjective improvement in dizziness, vertigo intensity (two) and Dizziness Handicap Inventory.
One study not included in the meta‐analysis compared a home‐based exercise programme with betahistine medication and found that the exercise programme improved dizziness symptoms and health‐related quality of life to a greater extent (Kulcu 2008). The second study compared relaxation with electrical stimulation and found no significant differences (Scott 1994). The third study not included in the meta‐analysis compared only the Semont manoeuvre with combined manoeuvre and vestibular rehabilitation for people with BPPV (Toledo 2000). The manoeuvre was found to be superior in cure rate in the short term (15 days), but the combination approach was superior in the longer term (three months). Details of the results of these studies are in the table Characteristics of included studies.
Comparison 3: Vestibular rehabilitation versus other form of vestibular rehabilitation
We included 12 studies in these analyses (Basta 2011; Cohen 2003; Kammerlind 2005; Morozetti 2011; Pavlou 2004; Pavlou 2012; Rossi‐Izquierdo 2011; Rossi‐Izquierdo 2013; Winkler 2011; Yardley 2006; Yardley 2012; Zimbelman 1999). Another four studies also performed this comparison but did not provide appropriate data (Cakrt 2010; Foster 2012; Mruzek 1995; Szturm 1994).
We found statistically significant differences between one form of vestibular rehabilitation and another form of vestibular rehabilitation for the following.
Primary outcome
-
Vertigo Symptom Scale ‐ vertigo component (VSS‐V) (SMD fixed‐effect ‐1.12, 95% CI ‐1.80 to ‐0.45, P value = 0.001; one study, 40 participants) (Analysis 3.1 section 2), in favour of the inclusion of simulator activities, however the overall vertigo symptom score was non‐significant (P value = 0.18).
Secondary outcomes
-
Dizziness Handicap Inventory (SMD fixed‐effect ‐0.96, 95% CI ‐1.78 to ‐0.14, P value = 0.02; one study, 26 participants) (Analysis 3.2 section 4), in favour of five sessions of balance training compared to 10.
Differences were non‐significant for all other measures (18) in these comparisons between different forms of vestibular rehabilitation: repetitive head movement task, vertigo visual analogue scale (VAS), tandem walk, posturography (five), VSS (four), DHI (seven), subjective improvement in dizziness, vertigo intensity, vertigo frequency, VHQ, ataxia, VD‐ADL and subjective health.
Four studies were not included in the meta‐analysis. One reported that after surgical removal of a schwannoma patients' recovered balance (as measured by posturography) was greater with visual feedback on training than without feedback (Cakrt 2010). Another found varying results when comparing a half‐somersault versus the Epley manoeuvre for BPPV, with the former superior in improving exercise‐induced dizziness (Foster 2012). One study reported similar results whether vestibular rehabilitation was performed with or without social support (Mruzek 1995). A final single study reported that a formal vestibular rehabilitation programme was more effective in improving balance/reducing falls than a home‐based Cooksey‐Cawthorne programme (Szturm 1994).
We evaluated heterogeneity as high, as indicated by the I2 statistic for two analyses. Visual inspection of the forest plot for Analysis 3.1 (Vertigo Symptom Scale) revealed that Pavlou 2004 had reported a larger effect size using the Vertigo Symptom Scale vertigo component ‐ this is to be expected clinically given that vertigo reduction is the primary goal and outcome of vestibular rehabilitation. The second analysis (Analysis 3.7) revealed that a larger effect size was produced by the Rossi‐Izquierdo 2011 study than other studies in the meta‐analysis. The overall effect was not significant and there was no obvious clinical or methodological explanation for the effect, other than that computerised dynamic posturography or posturography measures have multiple interpretations and parameters, which may not be appropriate for pooling.
Discussion
Summary of main results
If consideration is directed solely at the clinical question, 'Is vestibular rehabilitation effective in improving the symptoms of unilateral peripheral vestibular dysfunction?', then the evidence from this review is sufficient to answer yes, given the number of moderate to high quality studies reporting outcomes in favour of the vestibular rehabilitation intervention. This 2014 update has served to strengthen the original findings. The heterogeneity of the 39 studies still acts as a qualifier to this strong conclusion. The study variability lies in three domains: the varied comparators and the nature of the vestibular rehabilitation intervention, the sample characteristics (for example sub‐categories of unilateral peripheral vestibular dysfunction (UPVD), or acute versus chronic) and the outcome measures. In the following section we discuss the studies by grouping them in these three domains in turn, to answer the following subsidiary questions:
-
Is vestibular rehabilitation better than no or other interventions?
-
What form of vestibular rehabilitation is most effective?
-
Do different categories of unilateral peripheral vestibular dysfunction respond differently and what signs/symptoms are affected?
Unless otherwise indicated, we will only discuss the studies where data could be extracted.
Comparisons
Taken at the strictest level of evidence provided by meta‐analysis, the low risk of bias studies Giray 2009, Horak 1992, Teggi 2009, Vereeck 2008, Yardley 1998, Yardley 2004 and Yardley 2004 offer support for the use of vestibular rehabilitation to improve subjective measures of dizziness (including the Vertigo Symptom Scale (VSS)), level of participation (DHI) and gait performance (DGI) in people with chronic peripheral vestibulopathy, as compared to sham exercises or no vestibular rehabilitation/usual care. Individually the studies of Herdman 1995, Herdman 2003, Resende 2003 and Strupp 1998 also offer evidence of effectiveness in terms of improvement in measures of balance, activities of daily living and vision compared to no or sham interventions. These studies, as a body of evidence, therefore offer strong support for the effectiveness of vestibular rehabilitation across a broad range of outcomes in unilateral peripheral vestibular dysfunction as compared to placebo, sham or no intervention. It should be noted that a large degree of heterogeneity was found for the comparisons using the VSS and the DHI. We examined the studies that contributed to this finding, Yardley 2004 and Yardley 2006, and found that the only clinical source of heterogeneity was in the population, where one was general UPVD and the other Ménière's disease. However, these populations are both versions of chronic UPVD.
Studies that compared vestibular rehabilitation to other forms of unilateral peripheral vestibular dysfunction management (non‐vestibular rehabilitation) include Barozzi 2006 (electrical stimulation), Horak 1992 and Kulcu 2008 (medication), Chang 2008 (physical manoeuvres for benign paroxysmal positional vertigo (BPPV) (canalith repositioning manoeuvres (CRM)) plus vestibular rehabilitation versus CRM alone), Toledo 2000 (Semont manoeuvre), and Varela 2001 and Karanjai 2010 (Semont and Epley manoeuvres). Horak 1992 and Kulcu 2008 found that vestibular rehabilitation was superior to medication in improving subjective reports of dizziness in people with unilateral peripheral vestibular dysfunction. In contrast, Toledo 2000, Varela 2001 and Karanjai 2010 found in favour of manoeuvres over vestibular rehabilitation as defined for this review. The difference in findings can be explained by considering the different subject groups ‐ Horak recruited a pool of people with general peripheral vestibular dysfunction, whereas Varela and Karanjai investigated confirmed BPPV diagnoses only. This specific issue of BPPV will be discussed later. The studies by Cohen 2002 and Cohen 2005 failed to reach a sufficient effect size despite statistical significance in the original 2005 paper. Barozzi 2006 reported no difference in effect size between the vestibular rehabilitation and electrical stimulation groups.
Considering the comparative or relative effectiveness of different forms of vestibular rehabilitation, three studies reached statistical significance in our review. Pavlou 2004 compared customised home‐based vestibular rehabilitation exercises with the same programme plus simulator‐based visual and self motion stimulation, finding in favour of the supplemented programme. Therefore there is some evidence to support the addition of simulator‐based activities in a vestibular rehabilitation approach. A later study by Pavlou 2012 found that dynamic versus static virtual reality vestibular rehabilitation was superior in reducing visually induced dizziness. Rossi‐Izquierdo 2013 found that only five sessions of balance training (versus 10) were needed to improve dizziness experiences on the DHI, but that 10 were superior to five in improving balance. The lack of homogeneity means that it is not possible to draw strong conclusions about the other studies that compared different versions of vestibular rehabilitation. Further studies with a larger sample size are needed to clarify the questions of which exercises should be used, in what environment, administered by whom and for how long or how intensively (dosage).
Sub‐diagnoses of unilateral peripheral vestibular dysfunction
Acute UPVD
Five studies considered vestibular rehabilitation in the acute stage immediately post‐surgery for acoustic neuroma resection, removal of schwannoma or vestibular ablation. Vereeck 2008 reported that older participants in particular (over 50 years old) regained postural control more quickly with vestibular rehabilitation compared to general instructions, and that the greater benefits for postural control were maintained 12 months postoperatively. Herdman 1995 found a variable picture comparing vestibular rehabilitation that targeted vestibular gain versus eye movements that did not influence gain, reporting that balance and gait tests were superior in the vestibular rehabilitation group at day six postoperatively. Cohen 2002 found no difference between vestibular rehabilitation and sham interventions at day six; Cakrt 2010 found that patients post schwannoma removal, who received visual feedback as part of their vestibular rehabilitation, had greater improvement in balance parameters than those who did not receive feedback; and finally Mruzek 1995 found that vestibular rehabilitation (with or without social reinforcement) had better effects than a sham exercise for several dizziness and sensitivity quotients in the longer term (seven weeks post operation). Neither of the two latter studies could be included in a meta‐analysis.
Kammerlind 2005 investigated acute unilateral vestibular loss, comparing two forms of vestibular rehabilitation and finding them equally effective. Teggi 2009 (vestibular rehabilitation versus control) and Venosa 2007 (adaptation vestibular rehabilitation versus placebo) both reported greater benefits for people with acute vestibular presentations receiving vestibular rehabilitation, in terms of reduced symptom duration and medication use. Marioni 2013 found that posturography‐assisted vestibular rehabilitation compared to no vestibular rehabilitation had similar results but only the vestibular rehabilitation group improved to a level similar to healthy controls.
Benign paroxysmal positional vertigo
Eight studies investigated BPPV specifically. Resende 2003 investigated elderly patients with BPPV and compared vestibular rehabilitation (Cooksey‐Cawthorne type exercises) with no intervention ‐ both groups had received prior Ginkgo biloba. The vestibular rehabilitation group performed significantly better on measures of activities of daily living post‐intervention. In contrast, the study Varela 2001 also investigated participants with confirmed BPPV and found that manoeuvres (either Epley or Semont) were more effective in producing resolution than habituation exercises (Brandt 1999). They concluded that a hierarchy of interventions should be offered to people with BPPV, starting with a canalith repositioning manoeuvre. This suggestion has found favour in current clinical practice and is supported by the similar study of Cohen 2005 (though not in the meta‐analysis), who also found in favour of manoeuvres (canalith repositioning manoeuvre and modified Liberatory) compared to two versions of vestibular rehabilitation habituation exercise, noting that the exercises were also superior to a sham manoeuvre. Further, more recent, support is provided by Foster 2012 and Karanjai 2010, who both found in favour of the Epley manoeuvre compared to the Semont or Brandt‐Daroff manoeuvres. Similarly, Toledo 2000 found the Semont manoeuvre to be superior to vestibular rehabilitation alone at 15 days, however by three months a combination of Semont and vestibular rehabilitation was superior to either of the sole interventions. The Semont only group had a > 30% recurrence rate by this time leading these authors to suggest that vestibular rehabilitation has a preventative role. This result was confirmed more recently by Chang 2008, who compared canalith repositioning manoeuvres (CRM) with vestibular rehabilitation versus CRM alone. They reported that the combination promoted greater mobility skills (improved DGI) than the CRM alone. This body of evidence suggests that for people with BPPV the primary intervention should include manoeuvres to actually treat the condition and that this should be supported by vestibular rehabilitation to aid in longer‐term functional recovery. The evidence for the effectiveness of manoeuvres for BPPV is the subject of other Cochrane reviews (Hilton 2014; Hunt 2012).
Chronic and mixed forms of UPVD
The majority of studies investigated chronic dizziness of broad unilateral peripheral vestibular dysfunction origin and hence attract the general recommendations of this review.
More specifically vestibular neuritis was investigated firstly by Strupp 1998, who found postural control measures improved more in a group of patients with vestibular neuritis who performed vestibular rehabilitation (physical therapy and home‐based) compared to no specific intervention (other than encouragement to move). More recently Teggi 2009 also reported that vestibular rehabilitation significantly reduced anxiety in people with acute neuritis compared to the control group.
Scott 1994 investigated people with Ménière's disease but found no difference between applied relaxation training versus transcutaneous nerve stimulation on dizziness scores (could not be included in meta‐analysis). Yardley 2006 also investigated people in a non‐acute phase of Ménière's disease using booklet‐based forms of vestibular rehabilitation or symptom management and reported significant effects for subjective improvement in dizziness compared to control.
Outcome measures
Nineteen different measures were included in the results of this review, as summarised in the Results section. They covered impairments (dizziness and visual disturbances), activity restrictions (balance and gait parameters, activities of daily living) and participation restrictions (quality of life and social roles). As reported, the four common outcome measures available to pool were dizziness reduction scores and the vertigo symptom scale (measures of impairment), the Dizziness Handicap Inventory (measure of participation) and the Dynamic Gait Index (measure of activity). Future studies should consider evaluation at these three levels and should wherever possible use the vestibular‐specific scales.
Overall completeness and applicability of evidence
Clinical applicability of the evidence is impacted by the previously discussed areas of variance or heterogeneity. Clinicians are advised to read specifically for pertinent comparisons, outcomes and specific diagnostic groups. Another key aspect of applicability is the benefits over a longer time period and the existence of any mitigating adverse effects. Follow‐up was performed in the majority of studies and confirmed that any positive effects gained lasted for the three, six or 12‐month period. This lends further support to the conclusions in favour of the use of vestibular rehabilitation for unilateral peripheral vestibular dysfunction, as does the lack of reported adverse events. Studies also reported nil or low to moderate drop‐out rates and loss to follow‐up, although there was some suggestion that compliance may be an issue in some groups. Yardley 2006 reported a strong correlation between adherence and positive outcomes using booklet‐based vestibular rehabilitation, and again in 2012 confirmed superior outcomes for this intervention along with findings in favour of cost‐effectiveness (Yardley 2012). These issues warrant further investigation both within future randomised controlled trials and with qualitative methodology to establish individual experiences regarding patient acceptability of vestibular rehabilitation interventions.
Quality of the evidence
The overall quality of the evidence was acceptable. As can be seen in the 'Risk of bias' tables, there are few areas where there is a known high risk of bias that would cause readers to reconsider the strength of the evidence. However, there is a tendency for poor reporting particularly in the area of how the randomisation sequence was generated and to a lesser extent how randomisation was allocated. Although, it would be nice to see this as an historic problem and largely resolved in more recent studies with a higher awareness of trial conduct and reporting standards, this does not seem to be the case and therefore we make a strong plea for improved diligence by clinical researchers to improve both attention to trial conduct and to trial reporting.
There were isolated cases of high heterogeneity as assessed by the I2 statistic. Given the overall high level of clinical heterogeneity this was not unexpected but nevertheless again highlights the need for larger, standardised trials using consistent methods and outcomes.
Potential biases in the review process
We have applied a rigorous process of review and therefore expect minimal biases in extracting and reporting of data (both review authors selected studies for inclusion, and both independently extracted data and checked analyses with assistance from the editorial team). We have conducted extensive literature searches at each update of this review. The possibility of some publication bias cannot be ruled out, as our attempts to retrieve unpublished studies only included review of trial registries and contacting authors. Studies that were not registered nor published may therefore still exist.
Agreements and disagreements with other studies or reviews
There are still no alternate comprehensive systematic reviews covering the question of the effectiveness of vestibular rehabilitation for UPVD. There are many non‐systematic reviews and we have used these for their reference lists to ensure that we have found all known studies.

Study flow diagram for 2014 update.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 1 Subjective improvement in dizziness.

Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 2 Vertigo Symptom Scale.

Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 3 Gait ataxia.

Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 4 VD‐ADL (physical).

Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 5 Sway path.

Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 6 Dynamic visual acuity.

Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 7 Vestibular Handicap Questionnaire.

Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 8 Sharpened Romberg test (scores).

Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 9 Dizziness Handicap Inventory.

Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 10 Dynamic Gait Index.

Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 11 Romberg test.

Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 12 Vertigo intensity.
Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 13 Posturography.

Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 14 Vertigo intensity (BD versus sham).

Comparison 2 Vestibular rehabilitation versus other treatment, Outcome 1 Dizziness cure rate.

Comparison 2 Vestibular rehabilitation versus other treatment, Outcome 2 Dynamic Gait Index.

Comparison 2 Vestibular rehabilitation versus other treatment, Outcome 3 Subjective improvement in dizziness.

Comparison 2 Vestibular rehabilitation versus other treatment, Outcome 4 Vertigo intensity (BD versus CRM).

Comparison 2 Vestibular rehabilitation versus other treatment, Outcome 5 Vertigo intensity (XS versus CRM).

Comparison 2 Vestibular rehabilitation versus other treatment, Outcome 6 Dizziness Handicap Inventory.

Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 1 Vertigo Symptom Scale.

Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 2 Dizziness Handicap Inventory.

Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 3 Repetitive head movement task.

Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 4 Vertigo VAS.

Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 5 Romberg test (eyes closed).

Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 6 Tandem walk.

Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 7 Posturography.

Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 8 Subjective improvement in dizziness.

Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 9 Vertigo intensity.

Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 10 Vertigo frequency.

Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 11 Vertigo Handicap Questionnaire.

Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 12 Ataxia.

Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 13 Vestibular disorders ‐ activities of daily living scale.

Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 14 Dynamic Gait Index.

Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 15 Beck Depression Inventory.

Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 16 Subjective health.
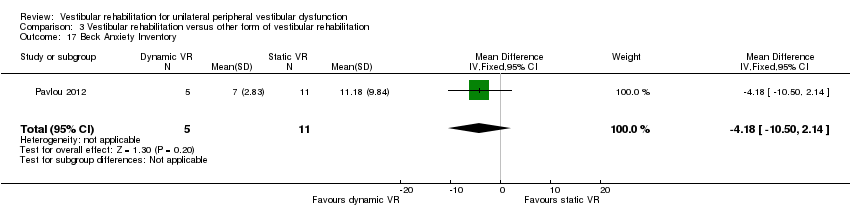
Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 17 Beck Anxiety Inventory.

Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 18 Situational vertigo questionnaire.
| Vestibulopathy | Incidence | Aetiology | Symptoms | Diagnosis | Treatment |
| Benign paroxysmal positional vertigo (BPPV) (idiopathic) | All age groups Peak 40 to 60 years 11 to 64 per 100,000 pa | Various: Canalithiasis (free‐floating debris in semicircular canals) Cupulolithiasis (debris attached to cupula) | Episodic vertigo after rapid head motion, lasting seconds to 1 minute; +/‐ nausea; some balance deficits; nystagmus (latency, fatigue, rotatory and beating) | Dix‐Hallpike test (post) (Dix 1952) Lateral head‐trunk tilt (Brandt 1999) etc. Use of ENG to record nystagmus | 1. Repositioning manoeuvre/s relative to semicircular canal (Cabrera Kang 2013; Epley 1992; Semont 1988) 2. VR 3. Vestibular suppressant medication for symptom relief 4. Vestibular neurectomy or post‐semicircular canal obliteration |
| Vestibular neuritis (Gans 2002)/neuronitis and labyrinthitis (Strupp 1998) | Unknown | Unclear Viral, autoimmune or vascular mechanisms Viral or bacterial infection of labyrinthine fluids (labyrinthitis) or CN VIII (neuritis) | Acute onset Distressing tonal imbalance producing: rotatory vertigo; spontaneous nystagmus (horizontal); falls to the affected side; nausea | From history and presentation ENG and caloric irrigation show reduced or no response in horizontal semicircular canal; ocular tilt reaction | Symptomatic medication (vestibular suppressants) Bacterial/viral management VR |
| Ménière's disease | Unknown Equal males and females Greatest in 3rd and 4th decades | Unclear Endolymphatic hydrops | Acute: unpredictable and episodic hearing loss, tinnitus and vertigo, +/‐ nausea, vomiting, visual disturbance, anxiety, motion sensitivity Chronic: UPVD or bilateral PVD | History and presentation Audiogram ENG with calorics Imaging the inner ear with high‐resolution MRI after tympanic gadolinium injection | Acute: medication (transtympanic glucocorticoids, antihistamines, suppressants) Chronic: VR, psychological support, surgery (see next row) |
| Postoperative: Neurectomy Intra‐tympanic injection of gentamycin | Unknown | For management of intractable UPVD, tumour removal, Ménière's | UPVD, i.e. spontaneous nystagmus, vertigo, disequilibrium, VOR gain, postural instability | — | VR Symptomatic medication (Dowdal‐Osborn 2002) |
| Perilymphatic fistula (Baloh 2003) | Unknown | History of head trauma, barotraumas or sudden strain; may be associated with chronic otitis or cholesteatoma; perforation of tympanic membrane | Unilateral hearing loss, vertigo, nystagmus | Induce symptoms by pressure in external ear canal Positive head thrust ENG Audiography | Symptomatic medication Surgical packing |
| ENG: electronystagmography | |||||
| Study ID | Inclusion criteria | Intervention/comparator | Result |
| Unilateral peripheral vestibular deficit, 1 to 6 months after the acute phase, diagnosed by clinical examination, CDP, videonystagmography, rotatory chair and caloric tests demonstrating a canal paresis of at least 25% | Intervention groups (n not stated): oculomotor rehabilitation (adaptation) Comparator group (n not stated): vestibular electrical stimulation | No significant differences between groups | |
| Experienced balance disorder for more than 12 months due to the following conditions: canal paresis, otolith disorder, removal of an acoustic neuroma, microvascular compression syndrome, Parkinson's disease, presbyvertigo | Intervention group (n = 59): vibrotactile neurofeedback training and vestibular rehabilitation exercises performed daily (15 minutes) over 2 weeks with the Vertiguard system Comparator group (n = 9): sham Vertiguard device and vestibular rehabilitation exercises | Significant reduction in trunk and ankle sway and improved VSS scores on the Vertiguard group. No changes observed in the sham Vertiguard group | |
| Participants undergoing retrosigmoid microsurgical removal of vestibular schwannoma | Intervention group (n = 9): received visual feedback while performing VR using the BalanceMaster Comparator group (n = 8): control group received VR without feedback | 2‐week intervention post acoustic neuroma removal, significant improvement in 5 out of 7 centre of pressure parameters in quiet stance on foam in the visual feedback group only | |
| First ever attack of unilateral posterior canal BPPV, diagnosed by neurologist and clinical examination | Intervention group (n = 13): canalith repositioning technique (CRT) and vestibular exercises Comparator group (n = 13): CRT only | Intervention group demonstrated a significant improvement in single leg stance with eyes closed at the 2‐week assessment, and static balance and DGI at the 4‐week assessment | |
| Acoustic neuroma resection ‐ postoperative (1 week ‐ acute) diagnosed by history, audiometry, MRI | Intervention group (n = 16): VR (head exercises) Comparator group (n = 15): control (attention only) | No significant difference between groups | |
| Chronic vestibulopathy (labyrinthitis or neuronitis of more than 2 months) diagnosed by physician using posturography, calorics and oculomotor test battery | Intervention group (n = 13): VR (slow head exercises ‐ habituation) Comparator group 1 (n = 22): VR (rapid head exercises) Comparator group 2 (n = 18): VR (rapid plus attention) | All groups significantly improved for VI, VF, DHI, VSS VHQ no change | |
| Unilateral BPPV (post SC) diagnosed by physician (D‐H test), with dizziness for at least 1 week | Intervention group (n = 25): B‐D exercises Comparator group 1 (n = 25): habituation exercises Comparator group 2 (n = 24): CRM Comparator group 3 (n = 25): LM Comparator group 4 (n = 25): sham manoeuvre | Manoeuvres (CRM and LM) better results than exercises (B‐D, habituation), both better than sham | |
| Adults with a history suggestive of BPPV and Dix‐Hallpike manoeuvre consistent with unilateral posterior canal BPPV | Intervention group: (n = 33) half‐somersault manoeuvre was performed twice in the clinic and also given as a home exercise Comparator group: (n = 35) Epley manoeuvre was performed twice in the clinic and also given as a home exercise | Significantly less nystagmus observed after the initial half‐somersault manoeuvre, but no difference in recurrence over the 6‐month follow‐up period | |
| Participants were included if they had Ménière's disease diagnosed by an ENT specialist, and had complaints of dizziness between exacerbations of their disease | Intervention group (n = 23): 12 rehabilitation sessions (twice weekly for 45 minutes) with virtual reality stimuli in a Balance Rehabilitation Unit, plus diet and lifestyle advice and betahistine Intervention group (n = 21): 12 stimulus enriched exercise sessions (twice weekly) on the Balance Rehabilitation Unit, plus diet and lifestyle advice and betahistine | Intervention participants improved significantly on the DHI, dizziness analogue scale and had greater stability on posturography compared to control participants | |
| Participants were diagnosed by a neuro‐otologist or neurologist with chronic decompensated unilateral peripheral vestibular deficit, secondary to peripheral vestibular dysfunction. Diagnosed by ENG, bithermal caloric test, ocular motor testing and positional testing | Intervention group (n = 20): VR incorporating adaptation, substitution, visual desensitisation and balance exercises Comparator group (n = 21): control, no input | Significant improvements were seen in all parameters for the intervention group while there were no changes in the control group | |
| Participants post removal of acoustic neuroma. Diagnosed by MRI and surgically resected ‐ study performed in acute post period | Intervention group (n = 11): VR (adaptation to increase gain) plus ambulation exercises Comparator group (n = 8): smooth pursuit exercises (no head movement) plus ambulation exercises | Intervention group significant improvements for dysequilibrium VAS, VOR to slow head movements, gait and posturography on day 6 compared to control group | |
| Unilateral vestibular hypofunction with abnormal DVA, diagnosed by caloric, rotary chair, positive head thrust | Intervention group (n = 13): VR (adaptation to enhance VOR) Comparator group (n = 8): placebo exercises designed to be "vestibular neutral" | 12/13 improved DVA in intervention group Both improved VAS | |
| Peripheral vestibular dysfunction diagnosed by neuro‐otologist for BPPV, inner ear concussion syndrome, reduced unilateral vestibular function, 18 to 60 years of age | Intervention group (n = 14): VR Comparator group 1 (n = 4): general conditioning exercises Comparator group 2 (n = 8): medication (meclizine or Valium) | VR ‐ superior reduction in sway and increased SOOL DI decreased for both VR and medication 92% improvement rate with VR (75% with comparator group 1, 75% with comparator group 2) | |
| Acute unilateral vestibular loss confirmed by ENG with calorics | Intervention group (n = 28): VR (home exercises plus extra PT (habituation, adaptation, balance and gait) (extra PT included individualised instruction and further exercises) Comparator group (n = 26): VR (home exercises only) | No significant difference between groups ‐ intensity not supported | |
| Diagnosed with posterior canal BPPV through history and clinical examination (Dix‐Hallpike manoeuvre) | Intervention group: Brandt‐Daroff exercises 3 times a day for 2 weeks, n = 16 Comparator group 1: single Epley manoeuvre followed by post‐treatment instructions, n = 16 Comparator group 2: single Semont manoeuvre followed by post‐treatment instructions (sleep upright for 2 nights, then on the unaffected side for the next 5 nights), n = 16 | Statistical analysis of the differences between groups not performed; 73% of participants overall reported resolution of symptoms with no recurrence at 3 months follow‐up | |
| Mixed diagnoses ‐ unilateral and bilateral peripheral vestibular dysfunction. Diagnosed by VOR gain, calorics etc. | Intervention group (n = 42): VR (adaptation, balance) Comparator group (n = 44): control (strength exercises) | VR group significantly improved for gait speed and base of support measures UPVD and BVD groups improved equally though BVD were less functional at baseline | |
| Diagnosed with BPPV and has undergone repositioning techniques by their otorhinolaryngologists but were still complaining of vertigo and dysequilibrium | Intervention group (n = 19): VR (Cawthorne‐Cooksey exercises) Comparator group (n = 19): medication (betahistine) | The intervention group demonstrated significant improvements in the VSS and VDI at the end of the study (8 weeks) | |
| Adults aged 18 to 65 with acute unilateral peripheral vestibular disorder occurring within 2 weeks of entry into the study, with at least 50% weakness on videonystagmography with caloric testing | Intervention group (n = 15): posturography‐assisted VR Comparator group 1 (n = 15): group awaiting spontaneous compensation, no VR Comparator group 2 (controls, n = 10): healthy adults without a vestibular disorder | Both groups of participants with vestibular dysfunction improved over the 6‐week intervention but only the posturography‐assisted VR improved postural control, which approximated the healthy controls | |
| Adults with a chronic vestibular disorder diagnosed by otorhinolaryngologists | Intervention group (n = 10): home exercises based on vertical and horizontal vestibulo‐ocular reflex stimulation (VRS) Comparator group (n = 10): personalised VR home exercise programme | Both groups improved over time but the personalised VR group reported less dizziness on VAS and greater gains on the DHI | |
| Participants had been reviewed by a physician for acoustic neuroma or Ménière's disease and were referred for ablative surgery | Intervention group (n = 8): VR plus social reinforcement, 15 minutes, 2 x day plus a daily walk Comparator group 1 (n = 8): VR no social reinforcement Comparator group 2 (n = 8): general range of motion exercises plus social reinforcement | All the same at 4 weeks Intervention group and comparator group 1 significant improvement for MSQ at 7 weeks Intervention group significant improvement for DHI at 8 weeks CDP no difference between groups | |
| Peripheral vestibular disorder diagnosed by full vestibular examination | Intervention group (n = 20): VR (customised exercises, including gaze control and stability, balance training) Comparator group (n = 20): simulator (optokinetic disc to produce visual‐vestibular conflict plus above) | Both groups improved significantly on posturography: intervention group more than comparator group Subjective symptom reports reduced for both (? any difference) Visual‐vertigo symptoms improved for intervention comparator group Depression reduced significantly for both groups: intervention group more than comparator group Anxiety reduced for both BBS not sensitive | |
| Participants with a history of acute onset of vertigo and had a confirmed peripheral vestibular deficit on the basis of the caloric tests and/or rotational tests on ENG | Intervention group (n = 5): dynamic virtual reality, performed for 45 minutes twice weekly for 4 weeks plus home exercises and general conditioning programme (walking) Comparator group 1 (n = 11): static virtual reality image rehabilitation, performed for 45 minutes twice weekly for 4 weeks plus home exercises and general conditioning programme (walking) Comparator group 1 (n = 5): cross‐over of 5 group 1 participants who then received dynamic virtual reality (not included in our analysis) | After 4 weeks the dynamic groups reported significantly less visual vertigo, but depression improved in the static virtual reality VR group only | |
| Participants with BPPV diagnosed by ENT using history, ENT examination, ENG | Intervention group: VR (compensation, adaptation, sensory substitution, balance: C‐C) Comparator group: control (nil) | Intervention group significantly improved Comparator group no change | |
| Participants with instability due to chronic unilateral peripheral vestibular disorders, which had not spontaneously resolved after a month. Hypofunction was defined with caloric tests, at least 25% labyrinthic preponderance according to defined criteria | Intervention group (n = 12): computerised dynamic posturography (CDP), 5 sessions of approximately 15 to 20 minutes on consecutive days Comparator group (n = 12): optokinetic stimulation (OKN), 5 sessions lasting 5 to 15 minutes on consecutive days | Outcomes assessed 3 weeks after treatment. Both groups improved, with the CDP group showing greater gains in the visual and vestibular input and limits of stability, while the OKN group showed greater improvement in visual preference | |
| Participants with instability due to chronic unilateral peripheral vestibular disorders, which had not spontaneously resolved after a month | Intervention group (n = 13): 5 sessions of posturography‐assisted VR over a 2‐week period Comparator group (n = 13): 10 sessions of posturography‐assisted VR over a 2‐week period | Outcomes assessed 3 weeks after the intervention and both groups improved over time, with the 5‐session group reporting greater gains on the DHI, but some items of posturography improved to a greater extent in the 10‐session group | |
| Ménière's disease diagnosed by medical and audiological examination (5 were bilateral but had one "worse" ear) | Intervention group (n = 10): applied relaxation Comparator group (n = 10): transcutaneous nerve stimulation to the hand | No change in either group for relevant measures (dizziness etc.) Intervention group improved on hearing ability more than comparator group | |
| Vestibular neuritis (acute/sub‐acute). Diagnosed by history, examination ‐ nystagmus, postural imbalance, ENG, calorics, ocular tilt reaction | Intervention group (n = 19): VR (home exercises, based on Cooksey‐Cawthorne, Norre ‐ habituation, gaze exercises, sensory substitution, functional retraining) Comparator group (n = 20): control (nil exercise but encouragement to move) | For OT and SVV tests, intervention group equal to comparator group For SP, intervention group improved significantly more than comparator group, i.e. balance improved | |
| Clinical diagnosis of peripheral vestibular dysfunction, persistent dizziness, disorientation or imbalance for at least 1 year, and abnormal balance performance during CDP at baseline | Intervention group (n = 11): VR Comparator group (n = 12): VR (home, C‐C) | Intervention group had reduced falls, improved CDP values and reduced VOR asymmetry compared with comparator group | |
| Participants were recently hospitalised for an acute episode of rotational vertigo which lasted several days and were diagnosed with vestibular neuritis | Intervention group (n = 20): VR Comparator group (n = 20): control ("perform usual daily activities") | Significant improvement in DHI between groups and reduction in anxiety. For both groups, there was a significant correlation between change in anxiety and change in DHI/DGI | |
| BPPV diagnosed with clinical assessment and electronystagmography | Intervention group (n = 10): VR (PC, head‐eye and habituation) Comparator group 1 (n = 10): Semont manoeuvre Comparator group 2 (n = 20): Semont + VR | Intervention group 80% cure rate at day 15 versus comparator group 1 45% Intervention group 66% cure rate at 3 months versus comparator group 2 100% | |
| BPPV, diagnosed by history and D‐H test (nystagmus) | Intervention group (n = 29): VR (B‐D habituation exercises) Comparator group 1 (n = 35): Semont manoeuvre Comparator group 2 (n = 42): Epley manoeuvre | Comparator groups 1 and 2 had a similar cure rate at 1 week; by 3 months comparator group 2 were superior but comparator group 1 more stable CRM superior to habituation (B‐D) for BPPV | |
| Acute episode of rotational vertigo within the last 5 days | Intervention group (n = 45): VOR adaptation exercises (X1 and X2 viewing exercises) Comparator group (n = 42): placebo exercises (sham visual fixation task) | Intervention group recovered more quickly in all parameters measured and required significantly less medication by the end of the follow‐up period (21 days) | |
| Consecutive patients post removal of an acoustic neuroma | Intervention group (n = 31): customised VR (exercises for balance, head motion, mobility, gaze and treadmill walking) Comparator group (n = 22): general instructions | Participants were stratified according to age (above and below 50 years). Older participants performed significantly better than the control group for balance, TUG and tandem gait compared to the control group. There was no group effect for the younger participants | |
| Participants with chronic dizziness (greater than 6 months duration) who had completed a VR programme, functional range of motion and strength in the lower limbs and trunk, intact sensation in the lower limbs, ability to stand unassisted for 1 minute | Intervention group (n = 10): platform tilt perturbations only Comparator group 1 (n = 7): platform tilt perturbations and VR exercise programme Comparator group 2 (n = 12): VR only | Outcomes were assessed after the 3‐week intervention and a follow‐up at 2 months later. The VR group only demonstrated significant improvement on the DHI but the platform tilt groups improved activity and participation domain outcomes | |
| Dizziness of vestibular origin. Mixed aetiology ‐ diagnosed where possible by medical records (1/3) Possibility of central pathology | Intervention group (n = 67): VR (education, head and body movements, relaxation, breathing, encouragement to function) Comparator group (n = 76): control | Intervention group improved significantly on all measures more than comparator group, except VHQ (no difference) Overall intervention group 4 times more likely to report subjective improvement than comparator group | |
| Dizziness of vestibular origin diagnosed by case history and MPD | Intervention group (n = 83): VR (primary care: demonstration, booklet and follow‐up) Comparator group (n = 87): control, usual medical care | All measures improved significantly in VR group compared with control group Clinical improvement 67% VR; 38% control | |
| Participants with Ménière's disease (non‐acute phase) who had experienced dizziness of imbalance in the last 12 months, had consulted their GP regarding involvement in the study | Intervention group (n = 120): VR (booklet of exercises) Comparator group 1 (n = 120): SC (booklet for self management) | At 3 months intervention group had greater improvement on 5 measures compared with comparator group 1 (2 measures) compared with comparator group 2 (0 measures) At 6 months intervention group and comparator group 1 both reported significant improvement, more than comparator group 2 Correlation between adherence and outcome | |
| Chronic dizziness, as diagnosed by their GP | Intervention group (n = 112): VR (self management booklet with phone support from a vestibular therapist) | At 12 weeks all groups showed some improvement in the VSS, and at 1 year both intervention groups improved significantly compared to usual care | |
| Unilateral peripheral vestibular dysfunction diagnosed by neuro‐otological tests | Intervention group (n = 6): VR (individual with adaptation and postural control) Comparator group (n = 8): VR (general C‐C) | Intervention group improved dizziness over time, comparator group did not No change for either on the BBS (insensitive) No between‐group differences ‐ but 100% of intervention group reported improvement compared with 62.5% of comparator group Intervention group had more Ménière's disease | |
| BBS: Berg Balance Scale | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Subjective improvement in dizziness Show forest plot | 4 | 565 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.67 [1.85, 3.86] |
| 2 Vertigo Symptom Scale Show forest plot | 3 | 553 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐0.87, ‐0.49] |
| 3 Gait ataxia Show forest plot | 1 | 19 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.77] |
| 4 VD‐ADL (physical) Show forest plot | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐10.5 [‐14.09, ‐6.91] |
| 5 Sway path Show forest plot | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐13.7 [‐16.51, ‐10.89] |
| 6 Dynamic visual acuity Show forest plot | 1 | 21 | Odds Ratio (M‐H, Fixed, 95% CI) | 84.0 [4.51, 1564.26] |
| 7 Vestibular Handicap Questionnaire Show forest plot | 1 | 143 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐6.76, ‐0.04] |
| 8 Sharpened Romberg test (scores) Show forest plot | 1 | 143 | Mean Difference (IV, Fixed, 95% CI) | 9.90 [0.80, 19.00] |
| 9 Dizziness Handicap Inventory Show forest plot | 5 | 535 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.83 [‐1.02, ‐0.64] |
| 10 Dynamic Gait Index Show forest plot | 2 | 93 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.92 [‐1.38, ‐0.46] |
| 11 Romberg test Show forest plot | 1 | 19 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.7 [0.33, 21.98] |
| 12 Vertigo intensity Show forest plot | 2 | 75 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐1.14, 0.26] |
| 13 Posturography Show forest plot | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐7.09, 9.29] |
| 14 Vertigo intensity (BD versus sham) Show forest plot | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐2.04, 0.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dizziness cure rate Show forest plot | 2 | 119 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.07, 0.49] |
| 2 Dynamic Gait Index Show forest plot | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐1.85, ‐0.15] |
| 3 Subjective improvement in dizziness Show forest plot | 1 | 21 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.30, 53.47] |
| 4 Vertigo intensity (BD versus CRM) Show forest plot | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.35, 0.95] |
| 5 Vertigo intensity (XS versus CRM) Show forest plot | 2 | 75 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.61, 0.30] |
| 6 Dizziness Handicap Inventory Show forest plot | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.85, 1.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vertigo Symptom Scale Show forest plot | 4 | 573 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.29, 0.05] |
| 1.1 Vertigo short‐form | 2 | 465 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.21, 0.15] |
| 1.2 Vertigo component | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.12 [‐1.80, ‐0.45] |
| 1.3 VSS total | 1 | 68 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐1.07, 0.34] |
| 2 Dizziness Handicap Inventory Show forest plot | 7 | 626 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.20, 0.12] |
| 2.1 Booklet plus | 2 | 465 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.14, 0.22] |
| 2.2 Individual | 1 | 14 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐1.72, 0.47] |
| 2.3 Vertiguard | 1 | 68 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.31, 0.11] |
| 2.4 Number of sessions | 1 | 26 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.78, ‐0.14] |
| 2.5 CDP‐assisted VR | 1 | 24 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.66, 0.94] |
| 2.6 Platform tilt | 1 | 29 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.64, 0.84] |
| 3 Repetitive head movement task Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 9.10 [0.12, 18.08] |
| 4 Vertigo VAS Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 4.5 [‐6.44, 15.44] |
| 5 Romberg test (eyes closed) Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐7.18, 0.58] |
| 6 Tandem walk Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.58, 1.58] |
| 7 Posturography Show forest plot | 5 | 193 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.55, 1.07] |
| 7.1 VR plus | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.69, 0.55] |
| 7.2 Vertiguard | 1 | 68 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.59, 0.81] |
| 7.3 CDP‐assisted VR | 2 | 50 | Std. Mean Difference (IV, Random, 95% CI) | 0.73 [‐2.48, 3.95] |
| 7.4 Speed of VR | 1 | 35 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.61, 0.76] |
| 8 Subjective improvement in dizziness Show forest plot | 1 | 14 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.27 [0.35, 197.61] |
| 9 Vertigo intensity Show forest plot | 2 | 55 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐1.03, 0.35] |
| 10 Vertigo frequency Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐2.05, 1.65] |
| 11 Vertigo Handicap Questionnaire Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 7.35 [‐4.94, 19.64] |
| 12 Ataxia Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.65, 0.19] |
| 13 Vestibular disorders ‐ activities of daily living scale Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.28, 0.68] |
| 14 Dynamic Gait Index Show forest plot | 2 | 45 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.41, 0.81] |
| 15 Beck Depression Inventory Show forest plot | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐8.01, 6.91] |
| 16 Subjective health Show forest plot | 2 | 435 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.65, 1.41] |
| 16.1 Booklet | 1 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.58, 1.71] |
| 16.2 Booklet plus | 1 | 205 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.53, 1.60] |
| 17 Beck Anxiety Inventory Show forest plot | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐4.18 [‐10.50, 2.14] |
| 18 Situational vertigo questionnaire Show forest plot | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐1.21, ‐0.05] |

