Topical anaesthetics for pain control during repair of dermal laceration
Appendices
Appendix 1. Search strategy for CENTRAL, the Cochrane Library
#1 MeSH descriptor Lacerations, this term only
#2 MeSH descriptor Wounds and Injuries, this term only
#3 MeSH descriptor Facial Injuries explode all trees
#4 MeSH descriptor Finger Injuries explode all trees
#5 MeSH descriptor Wounds, Penetrating explode all trees
#6 MeSH descriptor Hand Injuries explode all trees
#7 MeSH descriptor Sutures explode all trees
#8 MeSH descriptor Surgical Stapling explode all trees
#9 (laceration* or wound* or suture or stapling or repair*):ti,ab
#10 ((facial or dermal or cutaneous or finger or hand or eyelid) near injur*):ti,ab
#11 (penetrat* near wound*)
#12 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11)
#13 (topical near (an?esthe* or lidocain* or lignocain* or tetracain* or amethocain* or benzocain* or butamben or dibucain* or pramoxin* or prilocain* or etidocian))
#14 emla* or (eutectic mixture of local an?esthe*) or (tetracaine?adrenaline?cocain*) or (tetracaine?epinephrine?cocain*) or (lidocaine?adrenaline?tetracain*) or (lidocaine?epinephrine?tetracain*) or (spray or ointment or gel or cream or lotion or jelly or balm):ti,ab
#15 MeSH descriptor Administration, Topical, this term only
#16 MeSH descriptor Ointments, this term only
#17 MeSH descriptor Gels, this term only
#18 (#13 OR #14 OR #15 OR #16 OR #17)
#19 (#12 AND #18)
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1. laceration.mp. or exp lacerations/ or exp facial Injuries/ or exp finger injuries/ or exp wounds, penetrating/ or exp hand injuries/ or exp sutures/ or exp surgical stapling/ or ((wounds.mp. or exp wounds/) and injuries/) or (injury adj3 (hand or eyelid or finger or facial or dermal)).mp. or cutaneous.mp. or staple.mp. or repair.mp.
2. (topical adj3 (an?esthe* or lidocaine or lignocaine or lidoderm or tetracaine or amethocaine or benzocaine or butamben or pramoxine or prilocaine or topical)).mp. or exp administration, topical/ or topical.ti,ab.or emla.mp.or eutectic mixture of local an?esthe*.mp. or tetracaine‐adrenaline‐cocaine.mp. or tetracaine‐epinephrine‐cocaine.mp. or lidocaine‐adrenaline‐tetracaine.mp. or lidocaine‐epinephrine‐tetracaine.mp. or spray.ti,ab. or ointment.mp. or exp ointments/ or gel.mp. or exp gels/ or cream.mp. or lotion.mp. or jelly.mp. or balm.mp.
3. 1 and 2
4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh.
5. 3 and 5
Appendix 3. Search strategy for CINAHL (EBSCO host)
S28 S20 and S27
S27 S21 or S22 or S23 or S24 or S25 or S26
S26 AB random* or controlled trial* or mulicenter or placebo*
S25 (MM 'Multicenter Studies')
S24 (MH 'Prospective Studies+')
S23 (MM 'Double‐Blind Studies') or (MM 'Single‐Blind Studies') or (MM 'Triple‐Blind Studies')
S22 (MM 'Placebos')
S21 (MM 'Random Assignment') or (MH 'Clinical Trials+')
S20 S12 and S19
S19 S13 or S14 or S15 or S16 or S17 or S18
S18 AB emla* or (eutectic mixture of local an?esthe*) or (tetracaine?adrenaline?cocain*) or (tetracaine?epinephrine?cocain*) or (lidocaine?adrenaline?tetracain*) or (lidocaine?epinephrine?tetracain*)
S17 AB spray or ointment or gel or cream or lotion or jelly or balm
S16 AB ( an?esthe* or lidocain* or lignocain* or tetracain* or amethocain* or benzocain* or butamben or dibucain* or pramoxin* or prilocain* or etidocian* ) and topical
S15 (MH 'Gels')
S14 (MH 'Ointments')
S13 (MH 'Administration, Topical')
S12 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11
S11 AB laceration* or wound* or injur* or stapl* or repairor or suture*
S10 AB penetrat* and AB wound*
S9 AB ( facial or dermal or cutaneous or finger or hand or eyelid ) and AB injur*
S8 (MH 'Surgical Stapling')
S7 (MH 'Sutures')
S6 (MH 'Hand Injuries')
S5 (MH 'Finger Injuries')
S4 (MH 'Arm Injuries')
S3 (MH 'Facial Injuries')
S2 (MH 'Wounds and Injuries+') or (MH 'Wounds, Penetrating+')
S1 (MH 'Tears and Lacerations')
Appendix 4. Search strategy for Embase (Ovid SP)
1. exp laceration/ or injury/ or exp face‐injury/ or exp finger‐injury/ or exp hand‐injury/ or exp suture/ (123072)
2. (laceration* or wound* or injur* or ((facial or dermal or cutaneous or finger or hand or eyelid) adj3 injur*)).ti,ab. or (penetrat* adj3 wound*).mp. or (stapl* or repairor or suture*).ti,ab.
3. 1 or 2
4. (topical adj3 (an?esthe* or lidocain* or lignocain* or tetracain* or amethocain* or benzocain* or butamben or dibucain* or pramoxin* or prilocain* or etidocian)).mp.
5. (emla* or eutectic mixture of local an?esthe* or tetracaine?adrenaline?cocain* or tetracaine?epinephrine?cocain* or lidocaine?adrenaline?tetracain* or lidocaine?epinephrine?tetracain*).mp.
6. (spray or ointment or gel or cream or lotion or jelly or balm).ti,ab.
7. topical‐drug‐administration/ or ointment/ or gel/
8. 6 or 4 or 7 or 5
9. 8 and 3
10. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab.) not (animals not (humans and animals)).sh.
11. 10 and 9
Appendix 5. Data extraction form
| First author | Journal/Conference proceedings, etc. | Year |
|
|
|
|
| Dates study conducted | Not reported |
| Country and setting (centres and departments) | |
| Source of funding | |
| Conflicts of interest overall | |
| Declaration of interests for each researcher |
| Trial characteristics | |
|
| Further details |
| RCT/Quasi |
|
| Study size (number of participants) |
|
| Setting of study (single‐centre vs multi‐centre, inpatient vs outpatient) |
|
| Participant characteristics | |
|
| Further details |
| Age (mean, median, range, etc.) |
|
| Sex of participants (numbers, percentages) |
|
| Wound characteristics (length, location of laceration, etc.) |
|
| Anaesthetic characteristics | |
|
| Further details |
| Infiltrated anaesthetic (agent, dosage) |
|
| Topical anaesthetic (agent, dosage, duration of application) |
|
| Were systemic analgesics or sedatives given? |
|
| Outcomes | |
|
| Further details |
| Primary measure of pain intensity (patient self‐report using pain scale such as VAS, numerical rating, etc.) |
|
| Secondary measure of pain intensity (incidence of topical anaesthetic failure, requirement of supplemental anaesthesia, participant behavioural responses, etc.) |
|
| Anaesthetic‐related toxicity or acute adverse events |
|
Methodological quality
| Sequence generation | |
| State here method used to generate sequence and reasons for grading | Grade (circle) |
|
| Low risk |
| High risk | |
| Unclear risk | |
| Allocation concealment | |
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
|
| Low risk |
| High risk | |
| Unclear risk | |
| Blinding | |
| State here method used to blind study and reasons for grading | Grade (circle) |
|
| Low risk |
| High risk | |
| Unclear risk | |
| Description of withdrawals and drop‐outs | |
| State here method used to address incomplete outcome data | Grade (circle) |
|
| Low risk |
| High risk | |
| Unclear risk | |
| Selection bias (selective outcome reporting) | |
| State here method used for selective reporting of outcomes, time points, subgroups or analyses. | Grade (circle) |
| Low risk | |
| High risk | |
| Unclear risk | |
| Sample size (per arm) Low risk ≥ 200 participants enrolled Unclear risk 50 to 199 participants High risk < 50 | Grade (circle) High risk Low risk Unclear risk |
Appendix 6. Local anaesthetics and vasoconstrictors including alternative names
Cocaine‐containing topical anaesthetics
AC = Epinephrine‐cocaine or adrenaline‐cocaine
C = Cocaine
MAC = Bupivacaine‐epinephrine‐cocaine or bupivacaine‐adrenaline‐cocaine
TAC = Tetracaine‐epinephrine‐cocaine or tetracaine adrenaline‐cocaine
TC = Tetracaine‐cocaine
Cocaine‐Free Topical Anaesthetics
Anaesthetic putty (containing 4.94% w/w lidocaine hydrochloride, equivalent to 4% w/w lidocaine base)
BN = Bupivacaine‐norepinephrine
EMLA = Eutectic mixture of local anaesthetics = lidocaine‐prilocaine
EN = Etidocaine‐norepinephrine
LAT = LET = Lidocaine‐epinephrine‐tetracaine or lidocaine‐adrenaline‐tetracaine
LE = Lidocaine‐epinephrine orlidocaine‐adrenaline
MN = Mepivacaine‐norepinephrine
PN = Prilocaine‐norepinephrine
PP = Prilocaine‐phenylephrine
T = Tetracaine
TE = Tetracaine‐epinephrine or tetracaine‐adrenaline
TLP = Tetracaine‐lidocaine‐phenylephrine
TP = Tetracaine‐phenylephrine
Alternative names for local anaesthetics and vasoconstrictors
Epinephrine is the same as adrenaline
Bupivacaine is also called marcaine or sensoricaine
Lidocaine is also called xylocaine.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
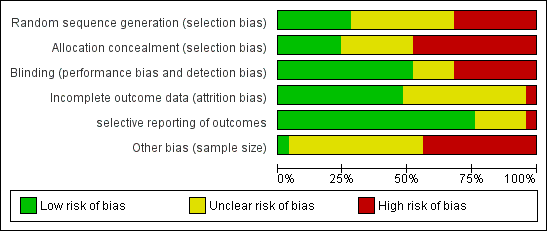
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
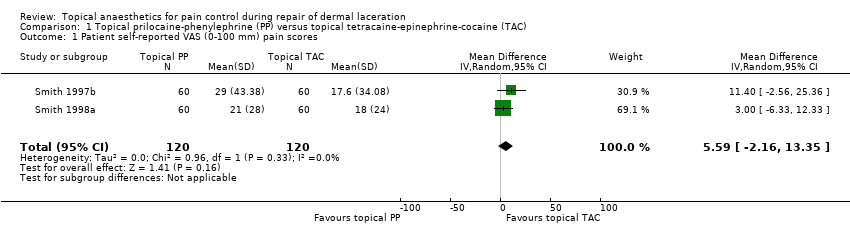
Comparison 1 Topical prilocaine‐phenylephrine (PP) versus topical tetracaine‐epinephrine‐cocaine (TAC), Outcome 1 Patient self‐reported VAS (0‐100 mm) pain scores.
| Pain control using topical local anaesthetics compared with infiltrated local anaesthetics or other topical agents for pain control during repair of dermal lacerations | ||||||
| Patient or population: adults and paediatric patients with dermal laceration Settings: any medical setting Intervention: topical local anaesthetics for pain control during repair of dermal laceration Comparison: infiltrated local anaesthetics or other topical agents for pain control during repair of dermal lacerations | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| (Infiltrated local anaesthetics or other topical agents) | (Topical local anaesthetics) | |||||
| Pain intensity measures Cocaine‐containing topical anaesthetics vs infiltrated local anaesthetics | See comment | See comment | Not estimable | 1006 | ⊕⊕⊝⊝ | Unable to mathematically combine results because of heterogeneity of outcome measures |
| Pain intensity measures Comparisons between different cocaine‐containing topical anaesthetics | See comment | See comment | Not estimable | 530 | ⊕⊕⊝⊝ | Unable to mathematically combine results because each topical anaesthetic comparison was limited to a single study |
| Pain intensity measures Cocaine‐free topical anaesthetics compared with infiltrated local anaesthetics | See comment | See comment | Not estimable | 543 | ⊕⊕⊝⊝ | Unable to mathematically combine results because of heterogeneity of outcome measures |
| Pain intensity measures Cocaine‐fee topical anaesthetics compared with cocaine‐containing topical anaesthetics | See comment | See comment | Not estimable | 1231 | ⊕⊕⊝⊝ | Two of the 11 trials studied a common topical anaesthetic and could be mathematically combined. |
| Pain intensity measures Comparisons between different cocaine‐free topical anaesthetics | See comment | See comment | Not estimable | 656 | ⊕⊕⊝⊝ | Trials could not be mathematically combined because each study compared a different cocaine‐free topical anaesthetic. |
| Anaesthetic‐related adverse effects | Study population | RR 0 (0 to 0) | 1686 | |||
| 1 per 1000 | 0 per 1000 | |||||
| Medium‐risk population | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aEach of the trials had high risk of bias in multiple domains or unclear risk of bias in three domains. bTwo of the four trials had at least one domain that was at high risk of bias. cTwo of the trials had unclear risk of bias in multiple domains, and the other two studies had high risk of bias in two domains. dSix of the studies had high risk of bias for at least one domain, and the other five studies had unclear risk of bias for one or more domains. eEach of the five trials had unclear risk of bias in one or more domains. However, no trials contained any domains that were clearly at high risk | ||||||
| Primary outcome subanalysis: pain intensity measures of topical prilocaine‐phenylephrine (PP) and topical tetracaine‐epinephrine‐cocaine (TAC) | ||||||
| Patient or population: treatment repair of dermal laceration Setting: any medical setting | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with topical tetracaine‐epinephrine‐cocaine (TAC) | Risk with topical prilocaine‐phenylephrine (PP) | |||||
| Participant self‐reported VAS (0‐100 mm) pain scores | Mean participant self‐reported VAS (0‐100 mm) pain score was 0. | Mean participant self‐reported VAS (0‐100 mm) pain scores in the intervention group was 5.59. | ‐ | 240 | Lowa | 5.59 (95% CI for effect estimate, 2.16 to 13.35) |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aEach of the trials had unclear risk of bias in one or more domains. However, no trials included any domains that were clearly at high risk. | ||||||
| Study | Anaesthetics | Participant self‐reported pain scores | Secondary outcome measures | Incidence of anaesthetic toxicity |
| Topical tetracaine‐epinephrine‐cocaine (TAC) vs infiltrated lidocaine | None | 1) Adequate initial anaesthesia (TAC = 89% vs infiltrated local anaesthetic = 79%; P = non‐significant) | Not reported | |
| Topical TAC vs infiltrated lidocaine | None | 1) Adequate initial anaesthesia for facial and scalp lacerations (topical TAC = 81% vs infiltrated local anaesthetic = 87%; P = 0.005). Adequate initial anaesthesia for extremity and trunk wounds (topical TAC = 43% vs infiltrated local anaesthetic = 89%; P < 0.0001) | 0/467 | |
| Topical TAC vs infiltrated lidocaine | None | 1) Verbal rating of anaesthetic efficacy (complete: TAC = 84% vs infiltrated local anaesthetic = 88%; P = not reported) | Not reported | |
| Topical TAC vs infiltrated lidocaine | Patient‐reported VAS (100 mm) pain scores (mean scores: topical TAC = 12.0 vs infiltrated local anaesthetic = 26.3; P = NS) | 1) Observer‐reported VAS pain scores | Not reported | |
| Topical TAC vs infiltrated lidocaine | None | 1) Observer‐reported VAS pain scores (suture technicians, research assistants, videotape reviewers) 2) Observer‐reported Lickert (1‐7) pain scores (parents, suture technicians) 3) Requirement for supplemental lidocaine infiltration (See Characteristics of included studies for data.) | Not reported | |
| Topical (epinephrine‐cocaine) AC vs infiltrated lidocaine | The study pooled patient‐reported VAS and Wong‐Baker Faces pain scores (mean score: topical AC = 4.50 vs infiltrated local anaesthetic = 4.40; P = NS) | 1) Physician‐rated VAS pain scores | 0/107 | |
| AC: epinephrine (adrenaline) and cocaine; BN: bupivacaine‐noradrenaline; BP: blood pressure; cm: centimetre; c/w:compared with; ED: emergency department; EMLA: Eutectic Mixture of Local Anesthetics (lidocaine and prilocaine); EN: etidocaine‐noradrenaline; LAT: lidocaine, epinephrine and tetracaine (same as LET); LE: lidocaine and epinephrine; LET: same as LAT; LG: local gel; LI: local infiltration; MAC: bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%; mm: milli‐metre; MN: mepivacaine‐noradrenaline; PN: prilocaine‐noradrenaline; N: number; NS: not significant; P = P value; PP: prilocaine, phenylephrine; RCT: randomized controlled trial; RICDRS: Restrained Infants and Children Distress Rating Scale; SD: standard deviation; SE: standard error; TA: tetracaine and epinephrine; TAC: tetracaine, epinephrine and cocaine; TLP: tetracaine, lidocaine and phenylephrine; TP: tetracaine and phenylephrine; VAS: visual analogue scale; vs: versus; w/w: weight per weight. | ||||
| Study | Topical Anaesthetics | Patient self‐reported pain scores | Secondary outcome measures | Incidence anaesthetic toxicity |
| Bupivacaine‐adrenaline‐cocaine (MAC) vs tetracaine‐epinephrine‐cocaine (TAC) | 1) In children < 12 years of age: Wong‐Baker Faces (1‐9) Scale (mean score ± SD: topical MAC = 2.35 ± .50 vs topical TAC = 2.46 ± 2.34; P = 0.96) 2) Participants 12 years of age or older: VAS (100 mm) pain scale (mean score ± SD: topical MAC = 6.9 ± 10.9 vs topical TAC = 12.0 ± 14.5; P = 0.16) | 1) Adequacy of initial anaesthesia 2) Participant preference for topical anaesthesia in the future | 0/180 | |
| TAC vs adrenaline‐cocaine (AC) | None | 1) Physician calculated total number of 'sutures eliciting pain' (topical AC = 7/103 (4%) vs topical TAC = 7/151 (7%); P = not reported) | 0/55 | |
| TAC vs cocaine (C) | None | 1) Incidence of 'poor anaesthesia' (topical cocaine = 20% vs topical TAC = 12%; P = not reported) 2) Physician numerical rating of anaesthetic effectiveness (0 = least effective to 10 = most effective) (mean scores ± SD: topical cocaine = 6.44 ± 3.48 vs topical TAC = 7.74 ± 3.03; P = 0.005) | 0/139 | |
| TAC (two different strengths) vs tetracaine‐cocaine (TC) | None | Topical TAC 1 vs topical TC: 2) Requirement for second dose of topical anaesthetic (TAC 1 = 30% vs topical TC = 66%; P < 0.003) 3) Requirement for supplemental lidocaine infiltration (TAC 1 = 6% vs topical TC = 9%; P = not reported) Topical TAC 2 vs topical TC: 2) Requirement for second dose of topical anaesthetic (TAC 2 = 46% vs TC = 66%; P < 0.003) 3) Requirement for supplemental lidocaine infiltration (TAC 2 = 2% vs TC = 9%; P = not reported) | 1/156 (erythematous rash 1 day after application of standard topical TAC) |
| Study | Anaesthetics | Participant self‐reported pain scores | Secondary outcome measures | Incidence of anaesthetic toxicity |
| Topical lidocaine‐epinephrine‐tetracaine (LAT) vs infiltrated lidocaine | VAS (100 mm) pain scores (median values: topical LAT = 0 vs infiltrated local anaesthetic = 0; P = 0.48) | 1) Physician‐rated VAS pain scores 2) Requirement for supplemental lidocaine infiltration 3) Percentage of painful sutures | Not reported | |
| Topical lidocaine‐epinephrine (LE) vs infiltrated lidocaine | VAS (100 mm) pain scores (mean score ± SD: topical TLE = 0.16 ± 0.46 vs infiltrated lidocaine = 0.20 ± 0.49; P = 0.59) | 1) Amount of lidocaine required (mg) 2) Total number of topical anaesthetic applications | Not reported | |
| Topical bupivacaine‐norepinephrine (BN), topical etidocaine‐norepinephrine (EN), topical mepivacaine‐norepinephrine (MN) and topical prilocaine‐norepinephrine (PN) vs infiltrated lidocaine | VAS (100 mm) pain scores (mean scores: BN = 18.3, EN = 46.5, MN = 27.0, PN = 36.0 vs infiltrated anaesthetic = 26.3, standard deviations not reported) (no significant difference between any of the cocaine‐free topical agents and infiltrated lidocaine) | 1) Observer‐reported VAS pain scores 2) Observer‐reported Likert pain scores 3) Oberver‐rated Restrained Infants and Children Disress Rating Scale 4) Suture technician‐rated anaesthetic effectiveness | Not reported | |
| Topical mepivacaine‐norepinephrine (MN) vs infiltrated lidocaine | None | 1) Observer‐reported VAS pain scale scores 2) Observer‐reported Lickert pain scores 3) Requirement for supplemental lidocaine infiltration (See characteristics of included studies for data) | Not reported | |
| Topical anaesthetic putty (containing 4.94% w/w lidocaine hydrochloride, equivalent to 4% w/w lidocaine base) vs lidocaine infiltration (1% w/v) | Mean pain score was 0.78 + 1.12 (SD) after lidocaine infiltration, 1.49 + 1.76 after topical anaesthetic putty. | 1) Need for rescue anaesthesia 2) Wound evaluation score 7‐10 days after treatment 3) Wound infection 4) Wound dehiscence 5) Adverse effects (inflamed wound or resuturing). | No anaesthetic toxicity reported | |
| Topical anaesthetic lidocaine, adrenaline and tetracaine (LAT) (4% lidocaine, 1:2 000 adrenaline, 1% tetracaine) vs lidocaine infiltration. Dosage of neither group was reported. | LAT gel group reported mean (± SE) pain intensity of 2.5 (0.52) vs 2.6 (0.58) for the lidocaine infiltration group. Pain during LAT application was 1.5 (0.40) vs 2.6 (0.58) during lidocaine infiltration (P ≤ 0.01). | 1) Pain score by parents or clinicians (intended to be gathered for children < 10 years old but such data were not reported) 2) Wound complications (infection, dehiscence, missing sutures) | None reported | |
| AC: epinephrine (adrenaline) and cocaine; BN: bupivacaine‐noradrenaline; BP: blood pressure; cm: centimetre; c/w: compared with; ED: emergency department; EMLA: Eutectic Mixture of Local Anesthetics (lidocaine and prilocaine); EN: etidocaine‐noradrenaline; LAT: lidocaine, epinephrine and tetracaine (same as LET); LE: lidocaine and epinephrine; LET: same as LAT; LG: local gel; LI: local infiltration; MAC: bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%; mm: milli‐metre; MN: mepivacaine‐noradrenaline; PN: prilocaine‐noradrenaline; N: number; NS: not significant; P = P value; PP: prilocaine, phenylephrine; RCT: randomized controlled trial; RICDRS: Restrained Infants and Children Distress Rating Scale; SD: standard deviation; SE: standard error; TA: tetracaine and epinephrine; TAC: tetracaine, epinephrine and cocaine; TLP: tetracaine, lidocaine and phenylephrine; TP: tetracaine and phenylephrine; VAS: visual analogue scale; vs: versus; w/w: weight per weight. | ||||
| Study | Topical anaesthetics | Participant self‐reported pain scores | Secondary outcome measures | Incidence of anaesthetic toxicity |
| Bupivacaine‐norepinephrine (BN), etidocaine‐norepinephrine (EN), mepivacaine‐norepinephrine (MN) and prilocaine‐norepinephrine (PN) vs tetracaine‐epinephrine‐cocaine (TAC) | Participant‐reported VAS (100 mm) pain scores (mean scores: BN = 18.3, EN = 46.5, MN, PN = 36.0 vs TAC = 12.0, standard deviations not reported) (TAC significantly outperformed EN; no significant differences between any other groups) | 1) Observer‐reported VAS and Likert pain scale scores 2) Observer‐rated Restrained Infants and Children Disress Rating Scale 3) Suture technician‐rated anaesthetic effectiveness | Not reported | |
| Mepivacaine‐norepinephrine (MN) vs | None | 1) Observer‐reported VAS pain scores (suture technicians, research assistants, videotape reviewers) 2) Observer‐reported Lickert (1‐7) pain scores (parents, suture technicians) 3) Requirement for supplemental lidocaine infiltration (See Characteristics of included studies for data.) | Not reported | |
| Prilocaine‐phenylephrine (PP), | VAS (100 mm) pain scores (mean score ± SD: PP = 29.0 ± 43.4, TP = 24.2 ± 37.2, TLP = 30.6 ± 40.3 vs TAC = 17.6 ± 34.1 (no significant differences between groups; P = 0.5) | 1) Oberver‐reported VAS (100 mm) pain scores 2) Oberver‐reported Likert (1‐7) pain scores 3) Suture technicians‐rated anaesthetic effectiveness | Not reported | |
| Prilocaine‐phenylephrine (PP) and bupivacaine‐phenylephrine (BP) vs TAC | VAS (100 mm) pain scores (mean score ± SD: PP = 21.0 ± 28.0 and BP = 41.0 ± 35.0 vs TAC = 18.0 ± 24.0) (no differences reported between groups; P = 0.07) | Observer‐reported VAS pain scores (suture technicians, research assistants and parents) | Not reported | |
| LAT vs TAC | Modified multi‐dimensional pain scale (0‐10) (mean ranked sum: LAT = 49.0 vs TAC = 46.9; P = 0.71) | 1) Physician‐rated modified multi‐dimensional pain scale (0‐10) 2) Percentage of sutures causing pain 3) Requirement for supplemental lidocaine infiltration | 0/95 | |
| LAT vs TAC | VAS (100 mm) pain scores (mean ranked sum: LET = 45.3 vs TAC = 50.8; P = 0.27) | 1) Physician‐reported VAS scores 2) Percentage of sutures causing pain | Not reported | |
| LAT vs TAC | None | 1) Adequacy of initial anaesthesia (LAT = 74.4% vs TAC = 79.5%; P = 0.46) 2) Anaesthetic effectiveness (complete anaesthesia: LAT = 82.4% vs topical TAC = 75.9%; P = 0.18) | 0/151 | |
| Lidocaine‐prilocaine (EMLA) vs TAC | VAS (100 mm) pain scores (mean score ± SD: EMLA = 46.0 ± 26.0 vs TAC = 40.0 ± 25.0; P = 0.50) | 1) Observer‐rated VAS pain scores 2) Requirement for supplemental lidocaine infiltration | Not reported | |
| Lidocaine‐epinephrine (LE) vs TAC | Faces pain scale (1‐9) scores (mean score ± SD: LE = 3.29 ± 1.92 vs TAC = 2.66 ± 1.78; P = 0.33) | Requirement for supplemental lidocaine infiltration | 0/35 | |
| Tetracaine‐epinephrine (TA) vs TAC | None | 1) Physician‐rating of anaesthetic effectiveness (complete anaesthesia: TA = 47.1% vs TAC = 75%' P < 0.05) 2) Requirement for rescue lidocaine infiltration (TA = 27.5% vs TAC = 8.9%; P = 0.01) | 0/107 | |
| Tetracaine (T) vs TAC | Numerical pain scale (0‐10) score (mean scores: tetracaine = 5.6 vs TAC = 3.53; P < 0.05; standard deviations not reported) | Requirement for supplemental lidocaine infiltration | Not reported | |
| AC: epinephrine (adrenaline) and cocaine; BN: bupivacaine‐noradrenaline; BP: blood pressure; cm: centimetre; c/w: compared with; ED: emergency department; EMLA: Eutectic Mixture of Local Anesthetics (lidocaine and prilocaine); EN: etidocaine‐noradrenaline; LAT: lidocaine, epinephrine and tetracaine (same as LET); LE: lidocaine and epinephrine; LET: same as LAT; LG: local gel; LI: local infiltration; MAC: bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%; mm: milli‐metre; MN: mepivacaine‐noradrenaline; PN: prilocaine‐noradrenaline; N: number; NS: not significant; P = P value; PP: prilocaine, phenylephrine; RCT: randomized controlled trial; RICDRS: Restrained Infants and Children Distress Rating Scale; SD: standard deviation; SE: standard error; TA: tetracaine and epinephrine; TAC: tetracaine, epinephrine and cocaine; TLP: tetracaine, lidocaine and phenylephrine; TP: tetracaine and phenylephrine; VAS: visual analogue scale; vs: versus; w/w: weight per weight | ||||
| Study | Topical anaesthetics | Participant self‐reported pain scores | Secondary outcome measures | Incidence of anaesthetic toxicity |
| Bupivacaine‐norepinephrine (BN) vs etidocaine‐norepinephrine (EN) vs mepivacaine‐norepinephrine (MN) vs prilocaine‐norepinephrine (PN) | Patient‐reported VAS (100 mm) pain scores (mean scores: topical BN = 18.3 vs topical EN = 46.5 vs topical MN = 27.0 vs topical PN = 36.0) (no significant differences between any cocaine‐free topical groups) | 1) Observer‐reported VAS and Likert pain scale scores 2) Observer‐rated Restrained Infants and Children Disress Rating Scale 3) Suture technician‐rated anaesthetic effectiveness | Not reported | |
| Prilocaine‐phenylephrine (PP) vs | VAS (100 mm) pain scores (mean score ± SD: PP = 29.0 ± 43.4 vs TP = 24.2 ± 37.2 vs TLP = 30.6 ± 40.3) (no significant differences between groups; P = 0.5) | 1) Oberver‐reported VAS (100 mm) pain scores 2) Oberver‐reported Likert (1‐7) pain scores 3) Suture technicians rated anaesthetic effectiveness | Not reported | |
| Prilocaine‐phenylephrine (PP) vs bupivacaine‐phenylephrine (BP) | VAS (100 mm) pain scores (mean score ± SD: topical PP = 21.0 ± 28.0 vs topical BP = 41.0 ± 35.0; P = 0.07) | Observer‐reported VAS pain scores (suture technicians, research assistants and parents) | Not reported | |
| Lidocaine‐prilocaine (EMLA) vs lidocaine‐epinephrine‐tetracaine (LAT) | VAS (100 mm) pain scores were not significantly different between the 2 groups (mean pain scores not provided; P > 0.05). | 1) Observer‐reported VAS pain scores (legal guardian and physician) 2) Requirement for supplemental lidocaine infiltration | Not reported | |
| Topical LAT gel vs LAT solution | None | 1) Adequacy of initial anaesthesia (adequate anaesthesia: LAT solution = 84% vs LAT gel = 82%; P > 0.05) 2) Effectiveness of anaesthesia (complete anaesthesia: LAT solution = 76% vs LAT gel = 85%; P = 0.007) | 0/194 | |
| AC: epinephrine (adrenaline) and cocaine; BN: bupivacaine‐noradrenaline; BP: blood pressure; cm: centimetre; c/w: compared with; ED: emergency department; EMLA: Eutectic Mixture of Local Anesthetics (lidocaine and prilocaine); EN: etidocaine‐noradrenaline; LAT: lidocaine, epinephrine and tetracaine (same as LET); LE: lidocaine and epinephrine; LET: same as LAT; LG: local gel; LI: local infiltration; MAC: bupivacaine 0.5%, epinephrine 1:2000, cocaine 10.0%; mm: milli‐metre; MN: mepivacaine‐noradrenaline; PN: prilocaine‐noradrenaline; N: number; NS: not significant; P = P value; PP: prilocaine, phenylephrine; RCT: randomized controlled trial; RICDRS: Restrained Infants and Children Distress Rating Scale; SD: standard deviation; SE: standard error; TA: tetracaine and epinephrine; TAC: tetracaine, epinephrine and cocaine; TLP: tetracaine, lidocaine and phenylephrine; TP: tetracaine and phenylephrine; VAS: visual analogue scale; vs: versus; w/w: weight per weight | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Patient self‐reported VAS (0‐100 mm) pain scores Show forest plot | 2 | 240 | Mean Difference (IV, Random, 95% CI) | 5.59 [‐2.16, 13.35] |


