Tratamiento de la enfermedad periodontal para la prevención de resultados adversos durante el parto en embarazadas
Resumen
Antecedentes
La enfermedad periodontal ha estado vinculada con varios trastornos, como enfermedades cardiovasculares, accidente cerebrovascular, diabetes y resultados adversos en el embarazo, todos probablemente a través de las vías inflamatorias sistémicas. Es frecuente en las mujeres en edad fecunda, y las enfermedades de las encías tienden a empeorar durante el embarazo. Alguna evidencia de los estudios observacionales indica que la intervención periodóntica puede reducir los resultados adversos durante el embarazo. Se necesita una revisión Cochrane integral de ensayos aleatorizados para evaluar el efecto del tratamiento periodóntico sobre la salud perinatal y materna.
Objetivos
Evaluar los efectos del tratamiento de la enfermedad periodontal en pacientes embarazadas con objeto de prevenir o reducir la morbilidad y la mortalidad perinatal y materna.
Métodos de búsqueda
El especialista en información del Grupo Cochrane de Salud Oral (Cochrane Oral Health Group) realizó una búsqueda en las siguientes bases de datos: registro de ensayos del Grupo Cochrane de Salud Oral (Cochrane Oral Health's Trials Register) (hasta el 6 de octubre de 2016), el registro de ensayos del Grupo Cochrane de Embarazo y Parto (Cochrane Pregnancy and Childbirth's Trials Register) (hasta el 7 de octubre de 2016), el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL; 2016, número 9) en la Cochrane Library, MEDLINE Ovid (1946 a 6 de octubre de 2016), Embase Ovid (1980 a 6 de octubre de 2016) y en LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database; 1982 a 6 de octubre de 2016). Se hicieron búsquedas de ensayos en curso en ClinicalTrials.gov y en la World Health Organization International Clinical Trials Registry Platform el 6 octubre 2016. No se impusieron restricciones de idioma ni fecha de publicación en la búsqueda en las bases de datos electrónicas.
Criterios de selección
Se incluyeron todos los ensayos controlados aleatorizados (ECA) que investigaban los efectos del tratamiento periodóntico en la prevención o la reducción de la morbilidad y la mortalidad perinatal y materna. Se excluyeron los estudios en los que no se informaban los resultados obstétricos.
Obtención y análisis de los datos
Dos autores de la revisión examinaron los títulos y los resúmenes de forma independiente y extrajeron los datos mediante un formulario de extracción de datos probado previamente. Los datos que faltaban se obtuvieron mediante contacto con los autores y se evaluó el riesgo de sesgo con la herramienta Cochrane de riesgo de sesgo. Cuando fue apropiado, se agruparon los resultados de los ensayos comparables y se expresaron como riesgos relativos (RR) o diferencias de medias (DM) con intervalos de confianza (IC) del 95%. Se utilizó el modelo de efectos aleatorios para el agrupamiento, excepto cuando hubo un número insuficiente de estudios. La calidad de la evidencia se evaluó con los criterios GRADE.
Resultados principales
Hubo 15 ECA (n = 7161 participantes) que cumplieron con los criterios de inclusión. Todos los estudios incluidos estuvieron en riesgo alto de sesgo, principalmente debido a la falta de cegamiento y el desequilibrio en las características iniciales de los participantes. Los estudios reclutaron a mujeres embarazadas de centros de atención prenatal que padecían periodontitis (14 estudios) o gingivitis (un estudio). Las dos comparaciones principales fueron: el tratamiento periodontal frente a la ausencia de tratamiento durante el embarazo y el tratamiento periodontal frente a un tratamiento periodontal alternativo. La comparación directa entre los tratamientos periodónticos evaluó un tratamiento más intensivo versus uno menos intensivo.
Once estudios compararon el tratamiento periodóntico con ningún tratamiento durante el embarazo. El metanálisis no muestra una diferencia clara en el nacimiento prematuro < 37 semanas (RR 0,87; IC del 95%: 0,70 a 1,10; 5671 participantes; 11 estudios; evidencia de baja calidad) entre el tratamiento periodontal y la ausencia de tratamiento. Hay evidencia de baja calidad de que el tratamiento periodóntico puede reducir el bajo peso al nacer < 2500 g (9,70% con tratamiento periodóntico versus 12,60% sin tratamiento; RR 0,67, IC del 95%: 0,48 a 0,95; 3470 participantes; siete estudios).
No está claro si el tratamiento periodontal produce una diferencia en el nacimiento prematuro < 35 semanas (RR 1,19; IC del 95%: 0,81 a 1,76; 2557 participantes; dos estudios) y < 32 semanas (RR 1,35; IC del 95%: 0,78 a 2,32; 2755 participantes; tres estudios), el bajo peso al nacer < 1500 g (RR 0,80; IC del 95%: 0,38 a 1,70; 2550 participantes; dos estudios), mortalidad perinatal (incluidas las muertes fetales y neonatales hasta los primeros 28 días después del nacimiento) (RR 0,85; IC del 95%: 0,51 a 1,43; 5320 participantes; siete estudios; evidencia de muy baja calidad) y preeclampsia (RR 1,10; IC del 95%: 0,74 a 1,62; 2946 participantes; tres estudios; evidencia de muy baja calidad). No hay evidencia de una diferencia en pequeño para la edad gestacional (RR 0,97; IC del 95%: 0,81 a 1,16; 3610 participantes; tres estudios; evidencia de baja calidad) cuando se compara el tratamiento periodontal con ningún tratamiento.
Cuatro estudios compararon el tratamiento periodóntico con el tratamiento periodóntico alternativo. El agrupamiento de los datos no fue posible debido a la heterogeneidad. Los resultados informados fueron el parto prematuro < 37 semanas, el parto prematuro < 35 semanas, el peso al nacer< 2500 g, el peso al nacer < 1500 g y la mortalidad perinatal (evidencia de muy baja calidad). No está claro si hay una diferencia en el parto prematuro < 37 semanas, el parto prematuro < 35 semanas, el peso al nacer < 2500 g, el peso al nacer < 1500 g y la mortalidad perinatal al comparar diferentes tratamientos periodónticos debido a que la calidad de la evidencia es muy baja.
La mortalidad materna y los efectos adversos de la intervención no ocurrieron en ninguno de los estudios que informaron cualquiera de los resultados.
Conclusiones de los autores
No está claro si el tratamiento periodóntico durante el embarazo tiene un impacto sobre el parto prematuro (evidencia de baja calidad). Hay evidencia de baja calidad de que el tratamiento periodóntico puede reducir el bajo peso al nacer (< 2500 g), sin embargo, la confianza en el cálculo del efecto es limitada. Hay evidencia insuficiente para determinar qué tratamiento periodóntico es mejor para prevenir los resultados obstétricos adversos. La investigación futura debe procurar informar sobre los resultados periodónticos junto con los resultados obstétricos.
PICOs
Resumen en términos sencillos
Tratamiento de las enfermedades de las encías para la prevención de los resultados adversos durante el parto en embarazadas
¿Cuál es el objetivo de esta revisión?
El objetivo de esta revisión Cochrane era determinar si el tratamiento de las enfermedades de las encías puede prevenir los resultados adversos durante el parto en las pacientes embarazadas. Los investigadores Cochrane recopilaron y analizan todos los estudios pertinentes para responder a esta pregunta y encontraron 15 estudios relevantes .
Mensajes clave
No existe evidencia de que el tratamiento de las enfermedades de las encías reduzca el número de neonatos nacidos antes de las 37 semanas de embarazo, sin embargo, puede reducir el número de neonatos que nacen con un peso menor que 2500 g. No se conoce si hay una diferencia en los resultados adversos durante el parto al comparar diferentes métodos de tratamiento de las enfermedades de las encías.
¿Qué se estudió en la revisión?
La salud de las encías tiende a empeorar durante el embarazo. Ha habido alguna investigación que asocia las enfermedades de las encías con resultados adversos durante el parto. La revisión evaluó los estudios en los que las pacientes embarazadas con enfermedades de las encías fueron tratadas mediante una combinación de diferentes técnicas mecánicas con o sin antibióticos.
¿Cuáles son los principales resultados de la revisión?
Los autores de la revisión encontraron 15 estudios pertinentes. Cinco eran de América del Norte, cuatro de América del Sur, tres de Europa, dos de Asia y uno de Austalia. Once estudios compararon el raspado y alisado radicular o el raspado y el pulido con ningún tratamiento mientras que los otros cuatro estudios compararon el raspado y alisado radicular con tratamientos mecánicos alternativos.
Cuando las pacientes embarazadas con enfermedades de las encías que reciben tratamiento periodóntico se comparan con las que reciben ningún tratamiento:
‐ no hay ninguna diferencia clara en el número de neonatos nacidos antes de las 37 semanas (evidencia de baja calidad);
‐ puede haber menos neonatos con un peso al nacer menor que 2500 g (evidencia de baja calidad).
No está claro si un tratamiento periodóntico es mejor que los tratamientos periodónticos alternativos en cuanto a la prevención de los resultados adversos durante el parto.
¿Cuál es el grado de actualización de esta revisión?
Los autores de la revisión buscaron estudios que se habían publicado hasta octubre 2016.
Authors' conclusions
Summary of findings
| Periodontal treatment compared to no treatment for preventing adverse birth outcomes in pregnant women | ||||||
| Patient or population: pregnant women considered to have periodontal disease after dental examination | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | Periodontal treatment | |||||
| Gestational age (preterm birth < 37 weeks) | Study population | RR 0.87 | 5671 | ⊕⊕⊝⊝ | Preterm birth < 35 weeks and < 32 weeks were also reported. There was no evidence of a difference in preterm birth < 35 weeks (RR 1.19 (0.81 to 1.76), 2 studies; 2557 participants) and < 32 weeks (RR 1.35 (0.78 to 2.32), 3 studies; 2755 participants) (VERY LOW2 quality evidence) | |
| 131 per 1000 | 114 per 1000 | |||||
| Birth weight (low birth weight < 2500 g) | Study population | RR 0.67 | 3470 | ⊕⊕⊝⊝ | Low birth weight < 1500 g was reported in 2 studies. There was no evidence of a difference in low birth weight < 1500 g (RR 0.80 (0.38 to 1.70); 2550 participants) (VERY LOW2 quality evidence) | |
| 126 per 1000 | 84 per 1000 | |||||
| Small for gestational age | Study population | RR 0.97 (0.81 to 1.16) | 3610 | ⊕⊕⊝⊝ | ||
| 115 per 1000 | 111 per 1000 | |||||
| Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth) | Study population | RR 0.85 (0.51 to 1.43) | 5320 | ⊕⊝⊝⊝ | ||
| 18 per 1000 | 16 per 1000 (9 to 26) | |||||
| Maternal mortality | 0% in both groups | Not estimated | 2134 (4 RCTs) | ‐ | ||
| Pre‐eclampsia | Study population | RR 1.10 | 2946 | ⊕⊝⊝⊝ | ||
| 64 per 1000 | 70 per 1000 | |||||
| Adverse effects of therapy | 0% in both groups | Not estimated | 2389 (4 RCTs) | ‐ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded 2 levels: serious limitation ‐ high risk of bias due to other bias (imbalance in baseline characteristics); serious inconsistency ‐ substantial heterogeneity (I2 = 66%). | ||||||
Background
Description of the condition
Periodontal disease is a disease of the supporting tissues of the teeth that may affect the gums, periodontal ligament membrane, cementum and bone around the tooth socket. It can present as two main types.
-
Gingivitis ‐ an inflammation of the gums (gingivae) around the teeth which does not cause loss of periodontal attachment (Int Workshop 1999).
-
Periodontitis ‐ in susceptible patients, gingivitis can progress to periodontal disease, with inflammation and destruction of the supporting tissues around the teeth.
Periodontal disease is related to low socioeconomic status (OSG 2000) and lower educational achievement (Machuca 1999). Periodontal disease has been linked to microbial infections which lead to systemic increase in proinflammatory prostaglandins and cytokines (Kim 2006). These have been hypothesised, through systemic inflammatory pathways, to lead to a number of conditions, such as cardiovascular disease, stroke, diabetes and adverse pregnancy outcomes (Papapanou 2015). While periodontal disease is common in women of reproductive age overall (e.g. 19% of Australian females 15 and over (Chrisopoulos 2012)), it is believed that gum conditions tend to worsen during pregnancy due to hormonal changes (Figuero 2013; Krejci 2002).
Observational studies in humans have shown associations between periodontal disease and adverse pregnancy outcomes including preterm birth (Ide 2013; Jeffcoat 2001; Jeffcoat 2002; Offenbacher 1996a), preterm premature rupture of the membranes (PPROM) (Offenbacher 1996b), pre‐eclampsia (Boggess 2003), pregnancy loss (Xiong 2007), and postcaesarean endometritis (Swamy 2002).
Not all observational studies, however, have found an association between preterm birth or low birth weight and periodontal disease. Davenport and colleagues in London, UK, in a case‐control study of 236 preterm infants and 507 term controls, using clinical periodontal indices measured on the labour ward, found the risk of preterm low birth weight decreased with increasing pocket depth (adjusted odds ratio (OR) 0.78, 95% confidence interval (CI) 0.64 to 0.99). The authors concluded that their results did "not support a specific drive to improve periodontal health of pregnant women as a means of improving pregnancy outcomes" (Davenport 2002). Moore and colleagues failed to find an association between preterm birth and periodontal disease in a large prospective cohort study and a smaller case‐control study (Moore 2004; Moore 2005).
Description of the intervention
Periodontal treatment may involve nonsurgical and surgical therapies, used alone or in combination. The most common periodontal therapy involves counselling on oral hygiene to educate patients on how to prevent the accumulation of dental plaque and calculus. In nonsurgical approaches, a dental hygienist or dentist removes plaque and calculus by using either hand instruments (scalers and curettes), ultrasound equipment (mechanical debridement), or polishing (Worthington 2013). When patients do not respond favourably to the initial nonsurgical treatment, surgical intervention may be required.
Antiseptic mouthwashes such as chlorhexidine can be used as a short‐term adjunct to oral hygiene measures, particularly after surgery when the patient cannot brush the area that has been operated on. Sometimes patients are given gels aimed at reducing oral bacterial load, and oral or topical antimicrobials (doxycycline, metronidazole) (Ciancio 2002). Local or systemic antibiotics may be limited to severe or aggressive periodontitis cases where symptoms persist after debridement and where good oral hygiene is evident.
How the intervention might work
The mechanism of action of periodontal treatment in preventing adverse birth outcomes is not fully understood. The general aims of treatment of periodontal disease are to resolve the inflammation by bringing the amount of plaque and calculus down to minimal levels; and to prevent or limit the tissue destruction to preserve dentition, maintain appearance and minimise discomfort (Pilot 1980; Sheiham 2002; Wennström 1990). Periodontal treatment must be followed by good oral hygiene in order for the inflammation to remain under control; resolution of this inflammation/infection may be an important outcome for preventing preterm birth. Thus instructing and motivating individuals to clean their teeth properly is an important component of periodontal treatment.
Dental care providers may be concerned that commonly used drugs such as anaesthetics, antibiotics and analgesics may harm the foetus. There may also be concern that bacteraemia caused by some dental procedures may lead to uterine infections, spontaneous abortions or preterm labour (Michalowicz 2009). Experts have recommended that dental treatment be avoided early in pregnancy during organogenesis and also late in pregnancy to avoid supine hypotension although such advice is regarded to be overly cautious by some obstetricians (Michalowicz 2008).
Why it is important to do this review
Adverse birth outcomes are traumatic and also have huge cost implications. Due to a World Health Organization (WHO) report showing that preterm birth is the second leading cause of death in children under five, addressing preterm birth has become a priority for achieving the Millennium Development Goal on infant mortality (WHO 2012). Some observational studies have stimulated interest in the treatment of women with periodontal disease in pregnancy for the possible prevention of preterm birth and other adverse birth outcomes. A systematic review of nine observational studies and three intervention studies concluded that there is some preliminary evidence to suggest that periodontal intervention may reduce adverse pregnancy outcomes (Scannapieco 2003). Many more recent reviews have since been published focusing mostly on foetal/neonatal outcomes (e.g. Fogacci 2011; Polyzos 2010). There is need for a comprehensive systematic review of randomised controlled trials assessing the effects of periodontal treatment on mothers as well as their babies.
Objectives
To assess the effects of treating periodontal disease in pregnant women in order to prevent or reduce perinatal and maternal morbidity and mortality.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished and ongoing randomised controlled trials that compare treatments for periodontal disease during pregnancy with control treatment, or treatment with alternative interventions during pregnancy or no treatment.
Types of participants
Pregnant women considered to have periodontal disease after dental examination. The types of periodontal disease included diagnoses of gingivitis and periodontitis (Int Workshop 1999).
Types of interventions
Treatment during pregnancy for periodontal disease, performed by a dentist, dental hygienist or therapist (including mechanical debridement using scaling and root planing, polishing, or surgery), either singly or in combination with counselling on oral hygiene, antiseptic oral agents, topical or systemic antimicrobial therapies compared with either placebo (for adjunctive treatment), no treatment or alternative treatments.
Types of outcome measures
Primary outcomes
Primary outcomes were chosen to be most representative of the clinically important measures of effectiveness and safety.
-
Perinatal outcomes:
1. gestational age at birth (preterm birth less than 37 weeks, very preterm birth less than 34 weeks, extremely preterm birth less than 28 weeks);
2. birth weight;
3. small for gestational age (variously defined);
4. perinatal mortality.
-
Maternal outcomes:
5. mortality;
6. pre‐eclampsia (variously defined);
7. adverse effects of therapy.
Secondary outcomes
These include other measures of effectiveness, complications, satisfaction with care and health service use.
-
Maternal outcomes:
1. plaque levels measured using any appropriate scale;
2. gingival health measured using any appropriate scale;
3. changes in probing depth;
4. changes in clinical attachment levels.
We have included periodontal outcomes as secondary outcomes to establish whether or not periodontal treatment in this population results in improvements in periodontal health. This is needed in order to investigate possible sources of heterogeneity and thus to interpret findings about preterm morbidity outcomes. However, periodontal outcomes are not the main focus of the review, therefore we excluded studies not reporting any of the primary outcomes.
We collated and reported any other outcomes recorded in the studies in an appendix (Additional Table 1) to be used for developing a core outcome set in trials on pregnancy and childbirth.
| Outcome | Study ID |
| Birth weight ≥ 2500 g | |
| Small for gestational age (10th percentile) | |
| Preterm/low birth weight | López 2002; López 2005; Oliveira 2011; Radnai 2009; Sadatmansouri 2006; Pirie 2013 |
| Birth length | Michalowicz 2006; Newnham 2009; Offenbacher 2009; Pirie 2013 |
| Head circumference | |
| Amniotic fluid index (< 5 cm, > 25 cm) | |
| Umbilical artery S/D ratios | |
| Umbilical cord artery/vein blood (number, pH, PCO2, PO2, base excess) | |
| Meconium in amniotic fluid | |
| Decision on delivery based on electronic fetal heart rate monitoring | |
| Scalp pH measured in labour | |
| Nonreassuring fetal heart rate pattern | |
| Caesarean delivery for nonreassuring fetal heart rate | |
| Electronic fetal heart rate monitoring in labour | |
| Ventilation | |
| Continuous Positive Airway Pressure (CPAP) | |
| Oxygen | |
| Special care nursery admission | |
| 1‐min Apgar score | |
| 5‐min Apgar score (0‐3, 4‐7, 8‐10) | |
| Apgar score (< 7 at 1 min, < 7 at 5 min) | |
| Admission to neonatal intensive care unit (number admitted, length of stay > 2 days, discharged alive) | |
| Sepsis necessitating antibiotics | |
| Composite neonatal morbidity/mortality | |
| HELLP syndrome, severe pre‐eclampsia | |
| Prenatal visits | |
| Onset of labour (spontaneous, induced, augmented, no labour) | |
| Mode of delivery (spontaneous vaginal, assisted vaginal, elective caesarean, emergency caesarean) | |
| Fever > 37o C in labour | |
| Postpartum haemorrhage (> 1000 mL) | |
| Retained placenta | |
| Fraction of expected birth weight | |
| Urinary tract infection | |
| Vaginosis, underweight, onset prenatal care after 20 weeks of gestation |
S/D = systolic/diastolic ratio.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions:
-
Cochrane Oral Health's Trials Register (searched 6 October 2016) (Appendix 1);
-
Cochrane Pregnancy and Childbirth's Trials Register (searched 7 October 2016) (Appendix 2);
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 9) in the Cochrane Library (searched 6 October 2016) (Appendix 3);
-
MEDLINE Ovid (1946 to 6 October 2016) (Appendix 4);
-
Embase Ovid (1980 to 6 October 2016) (Appendix 5);
-
LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database; 1982 to 6 October 2016) (Appendix 6).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6 (Lefebvre 2011).
Searching other resources
The following trial registries were searched for ongoing studies:
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 6 October 2016) (Appendix 7);
-
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 6 October 2016) (Appendix 7).
We searched the reference lists of included studies and relevant systematic reviews for further studies.
We did not perform a separate search for adverse effects of interventions used, we considered adverse effects described in included studies only.
Data collection and analysis
We carried out data collection and analysis according to the methods stated in the published protocol (Middleton 2015), which are based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)
Selection of studies
Two review authors independently screened the titles and abstracts identified from the literature search. We discarded studies not meeting the inclusion criteria. For studies appearing to meet the inclusion criteria, or where there was insufficient information to make a clear decision, we obtained full reports and two review authors independently assessed them to establish whether the studies met the inclusion criteria. We resolved disagreements by discussion, with a third review author consulted if resolution was not possible. We entered studies rejected at this or subsequent stages in Characteristics of excluded studies tables and recorded the main reason for exclusion.
Data extraction and management
Data extraction was done independently and in duplicate into data extraction forms. We extracted relevant data from full‐text articles that met the inclusion criteria. If reported, information was collected on.
-
Trial setting: country and number of trial centres.
-
Methods: study design, total study duration and date.
-
Participant characteristics: age, sociodemographics, ethnicity, diagnostic criteria and total number.
-
Eligibility criteria: inclusion and exclusion criteria.
-
Intervention and comparator.
-
Outcomes: outcome definition, unit of measurement and time of collection.
-
Results: number of participants allocated to each group, missing participants, sample size.
-
Funding source.
We compared completed data extraction forms to check for discrepancies and made clarifications by referring to the relevant study paper. After checking data for accuracy, we entered them into the Characteristics of included studies tables.
Assessment of risk of bias in included studies
Two review authors independently assessed all studies meeting the inclusion criteria for their risk of bias using criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The following domains were assessed.
-
Sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Blinding of participants and personnel (performance bias).
-
Blinding of outcome assessment (detection bias).
-
Incomplete outcome data (attrition bias).
-
Selective reporting (reporting bias).
-
Other bias.
We judged the studies to be at either low, high or unclear risk of bias for each domain assessed, based on the guidance in Higgins 2011. The different judgements on risk of bias were interpreted as follows.
-
Low risk of bias: plausible bias unlikely to seriously alter the results if all domains were at low risk of bias.
-
Unclear risk of bias: plausible bias that raises some doubt about the results if one or more domains were at unclear risk of bias.
-
High risk of bias: plausible bias that seriously weakens confidence in the results if one or more domains were at high risk of bias.
Measures of treatment effect
For dichotomous outcomes, we expressed the treatment effect as risk ratios (RR) with corresponding 95% confidence intervals (CI). For continuous outcomes, we expressed the treatment effect as mean difference (MD) with 95% CI. However, if the studies assessed the same continuous outcome in different ways, we planned to estimate the treatment effect using the standardised mean difference (SMD). Time to birth was expressed as hazard ratio (HR) with corresponding 95% CI.
Unit of analysis issues
The participant was the unit of analysis. For studies comparing more than two intervention groups, we made multiple pair‐wise comparisons between all possible pairs of intervention groups.
Dealing with missing data
We contacted authors where there was missing data or studies had not reported data in sufficient detail. We attempted to derive the data using relevant statistical tools and calculators. Missing standard deviations would be estimated by calculating a correlation coefficient from a study reported in considerable detail using methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions Section 16.1.3.2 (Higgins 2011). Where we were unable to get missing data from authors, we presented the study results only as a narrative summary.
Assessment of heterogeneity
We tested for heterogeneity using a Chi2 test and P < 0.1 gave an indication of the presence of heterogeneity. Inconsistency was quantified and represented by the I2 statistic. The thresholds were interpreted as follows:
-
0% to 40%: might not be important;
-
30% to 60%: may represent moderate heterogeneity;
-
50% to 90%; may represent substantial heterogeneity;
-
75% to 100%: considerable heterogeneity.
Where heterogeneity was detected, we investigated possible causes and addressed them using methods described in Higgins 2011.
Assessment of reporting biases
Most reporting biases were avoided by not restricting the literature search to published literature or by language and date. We investigated publication bias for the preterm birth < 37 weeks outcome using a funnel plot. The magnitude of publication bias was to be determined by visual inspection of the asymmetry of the funnel plot. In addition, we were to test funnel plot asymmetry by performing a linear regression of intervention effect estimate against its standard error, weighted by the inverse of the variance of the intervention effect estimate (Egger 1997).
Data synthesis
We carried out a meta‐analysis where there was sufficient number of studies that assessed similar populations, interventions and outcomes. Study data were synthesised using the random‐effects model if there were more than three studies in the meta‐analysis; otherwise we used the fixed‐effect model. The random‐effects model gives wider confidence intervals for the intervention effects, resulting in a more conservative estimate of effect. We combined effect estimates of studies we considered appropriate for inclusion in the meta‐analysis. We pooled RRs for dichotomous outcomes and MDs for continuous outcomes. The primary analyses were limited to the prespecified outcomes. Studies reporting mean birth weight and gestational age were not pooled. The skewness of the data due to the rarity of the events precluded our pooling the data in a meta‐analysis. We presented study data not included in the meta‐analyses in additional tables. We presented a narrative summary of the included studies where we were unable to carry out a meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We planned to undertake subgroup analyses of potential effect modifiers to investigate their influence on the effect size of the intervention if there were 10 studies or more. We identified several potential modifiers of effect: the severity of periodontal disease; who gave the treatment (periodontist, dental hygienist or general dental practitioner); the number of treatment sessions given; the gestational age at which the treatment was started; and the following prognostic factors: maternal age, smokers versus nonsmokers, and socioeconomic class. However, we were unable to undertake these analyses due to the insufficient number of studies.
Sensitivity analysis
We were to undertake a sensitivity analysis if we had a sufficient number of studies, to assess whether the findings of the review were robust to the decisions made during the review process. In particular, we planned to exclude studies at high or unclear risk of bias from analysis, as well as those with estimated standard deviations, to assess whether this affected the findings of the review.
Presentation of main results
We presented the main results in a 'Summary of findings' table. The main comparison (periodontal treatment versus no treatment) and primary outcomes were exported to GRADEprofiler software (GRADEpro GDT 2014) for quality assessment. Based on risk of bias, inconsistency, imprecision, indirectness and publication bias, we rated the quality of the evidence for each outcome as high, moderate, low or very low. These ratings have been defined as follows.
-
High: further research is very unlikely to change our confidence in the estimate of effect.
-
Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
-
Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
-
Very low: any estimate of effect is very uncertain.
Results
Description of studies
From the literature search, 786 records were retrieved after deduplication and 1 unpublished study was obtained from a study author. Titles and abstracts of these 787 records were screened by two members of the review team independently. After the screening, 752 records were discarded and we attempted to obtain 35 full‐text articles for further scrutiny. 15 studies (19 published articles) were included and nine studies (13 published articles) were excluded for different reasons. There are two trials awaiting classification and one ongoing trial. Data extraction of the 15 studies was done and all 15 studies were included in the meta‐analysis (Figure 1).
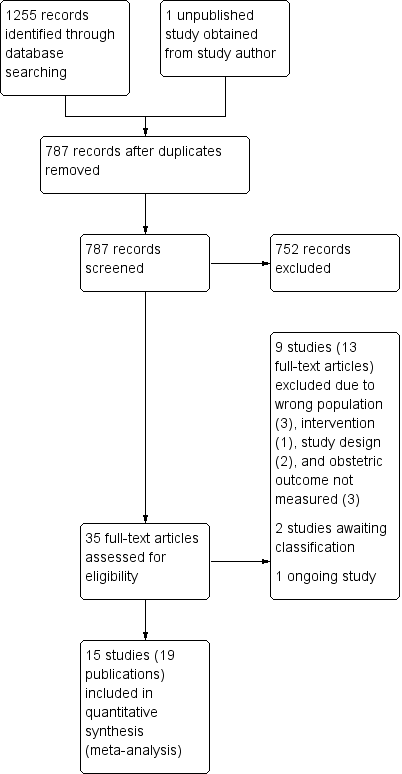
Study flow diagram.
Included studies
Fifteen studies (Farrell 2003; Herrera 2009; Jeffcoat 2003; López 2002; López 2005; Macones 2010; Michalowicz 2006; Newnham 2009; Offenbacher 2006; Offenbacher 2009; Oliveira 2011; Pirie 2013; Radnai 2009; Sadatmansouri 2006; Tarannum 2007) were included in the review. The details of these studies are reported in the Characteristics of included studies section.
Participants
The studies included 7161 pregnant women recruited from prenatal care facilities who had either periodontitis (mostly mild) or gingivitis. Participants were mostly in their first or second trimester except in two studies (Herrera 2009; Radnai 2009) where some women in the third trimester of their pregnancy were included. Mean gestational age (± standard deviation) of the participants at entry was between 14.0 ± 1.5 weeks to 39.6 ± 1.2 weeks in six studies (López 2002; Michalowicz 2006; Newnham 2009; Offenbacher 2009; Pirie 2013; Radnai 2009). Seven studies reported gestational age ranging from 9 to 34 weeks (Farrell 2003; Herrera 2009; Jeffcoat 2003; Macones 2010; Oliveira 2011; Sadatmansouri 2006; Tarannum 2007). Two studies reportedly included women at ≤ 22 weeks (López 2005) and < 22 weeks (Offenbacher 2006) gestation. Mean age of participants was reported in all but one study (Farrell 2003) and ranged between 22.2 ± 4.3 and 30.5 ± 5.5 years.
Nine studies reported baseline data on the proportion of participants with previous history of various adverse obstetric outcomes and it ranged from 3% to 55% (Jeffcoat 2003; López 2002; López 2005; Macones 2010; Michalowicz 2006; Newnham 2009; Offenbacher 2006; Offenbacher 2009; Pirie 2013). Adverse obstetric outcomes previously experienced by participants in these nine studies were preterm birth, preterm low birth weight, spontaneous abortion and stillbirth. However, there is no indication as to whether the estimates also account for participants who had experienced multiple adverse obstetric outcomes. In one study (Herrera 2009) all the participants had mild pre‐eclampsia at recruitment stage. None of the studies excluded participants on the basis of previous history of adverse obstetric outcomes except Radnai 2009. Offenbacher 2009 excluded women with "any obstetric finding that precluded enrolment in the study", however, these obstetric findings referred to were not clearly stated and some participants had a history of preterm delivery.
Severity of periodontitis ranged from moderate to severe and there was variation in definition of periodontitis across the studies. Periodontal disease was defined as four or more teeth with one or more sites with probing depth of 4 mm or more and clinical attachment level as 3 mm or more in three studies (López 2002; Oliveira 2011; Sadatmansouri 2006). In two studies (Jeffcoat 2003; Offenbacher 2009), periodontal disease was defined as three or more sites with attachment level of 3 mm or more. The other 10 studies had no common definition for periodontal disease (Additional Table 2).
| Study ID | Case definition |
| ≥ 3 sites with CAL ≥ 3 mm | |
| ≥ 4 teeth with ≥ 1 sites with PD ≥ 4 mm and with CAL ≥ 3 mm | |
| ≥ 6 sites with ≥ 5 mm probing depth and ≥ 3 sites with ≥ 3 mm loss of periodontal attachment | |
| AAP criteria ‐ PPD up to 6 mm with CAL up to 4 mm | |
| Gingival inflammation with over ≥ 25% of sites with BOP and no sites with CAL > 2 mm | |
| Attachment loss ≥ 3 mm on ≥ 3 teeth | |
| ≥ 4 teeth with ≥ 1 sites with PD ≥ 4 mm and with CAL ≥ 2 mm | |
| PPD ≥ 4 mm at ≥ 12 probing sites in fully erupted teeth | |
| ≥ 2 sites measuring ≥ 5 mm probing depths plus periodontal attachment loss of 1‐2 mm at ≥ 1 sites with probing depths ≥ 5 mm | |
| ≥ 4 sites with PD ≥ 4 mm and ≥ 4 sites with CAL ≥ 4 mm | |
| Chronic: ≥ 4 mm probing depth, at least 1 site, and BOP for ≥ 50% of teeth | |
| ≥ 2 mm attachment loss at ≥ 50% of examined sites |
AAP = American Academy of Periodontology; BOP = bleeding on probing; CAL = clinical attachment level; PD = pocket depth; PPD = periodontal pocket depth.
Study design and setting
All the included studies were published between 2003 and 2013. The studies were single‐centre (Farrell 2003; Herrera 2009; Jeffcoat 2003; López 2002; López 2005; Pirie 2013; Radnai 2009; Sadatmansouri 2006; Tarannum 2007) and multicentre (Macones 2010; Michalowicz 2006; Newnham 2009; Offenbacher 2006; Offenbacher 2009; Oliveira 2011) randomised controlled trials (RCTs) conducted in either university hospitals, public hospitals, public health clinics, antenatal clinics, maternity hospitals or a combination of university and antenatal clinics (for the multicentre trials).
Thirty‐three per cent of the studies were conducted in North America (Jeffcoat 2003; Macones 2010; Michalowicz 2006; Offenbacher 2006; Offenbacher 2009), 27% in South America (Herrera 2009; López 2002; López 2005; Oliveira 2011), 13% in Asia (Sadatmansouri 2006; Tarannum 2007), 20% in Europe (Farrell 2003; Pirie 2013; Radnai 2009), and 7% in Australia (Newnham 2009).
The source of funding was not stated in half of the studies (Farrell 2003; Herrera 2009; Jeffcoat 2003; López 2005; Macones 2010; Newnham 2009; Sadatmansouri 2006; Tarannum 2007) while the other half were funded by research institutes (Michalowicz 2006; Offenbacher 2009), the government (Pirie 2013), scientific research fund (López 2002), a university (Oliveira 2011), "institutional support" (Radnai 2009), and a manufacturer of oral healthcare products (Offenbacher 2006).
Interventions
The intervention arm in all the studies included a combination of multiple subcomponents (Additional Table 3). Apart from two studies (López 2002; Sadatmansouri 2006), none of the studies had common intervention subcomponents. The studies were split into two comparisons. Eleven studies compared periodontal treatment provided during pregnancy with no treatment and four studies did a head‐to‐head comparison of different periodontal treatments.
| Study | Number of visits | When | Intervention | Comparator | |
| Periodontal treatment versus no treatment | |||||
| 5 visits | 12 weeks | Plaque assessment Oral hygiene instruction | None | ||
| 1 session lasting 1‐2 hours | Supragingival and subgingival cleaning | None | |||
| Maintenance therapy every 2‐3 weeks till delivery | Plaque control instruction | None | |||
| Maintenance therapy every 2‐3 weeks till delivery | Plaque control instruction | None | |||
| Up to 4 visits | SRP | None | |||
| 3 treatments over 3 weeks | 20 weeks | Nonsurgical debridement of subgingival and supragingival plaque | None | ||
| Up to 4 sessions (mean 1.3 ± 0.4) | Supragingival and subgingival SRP | None | |||
| Maintenance therapy every 3 weeks till delivery | Dental prophylaxis | Tooth cleaning kit | |||
| Not stated | 32 weeks | Supragingival and subgingival scaling and polishing | None | ||
| Not reported | 28 weeks | SRP | None | ||
| 4‐5 sessions with a 1‐week interval between each appointment | Unclear | SRP | Plaque control instruction (toothbrushing) | ||
| Periodontal treatment versus alternative periodontal treatment | |||||
| Antibiotics 3 times daily for 1 week | SRP | SRP | Dental prophylaxis | ||
| Not stated | SRP | Superficial tooth cleaning | |||
| 4‐6 weeks follow‐up visit | SRP | Supragingival debridement | |||
| Performed over 2 1‐hour sessions | Completed by end of 24 weeks | Supragingival and subgingival SRP | Supragingival cleaning | ||
CHX = chlorhexidine; SRP = scaling and root planing.
-
Periodontal treatment (any combination of mechanical treatment) versus no treatment (Farrell 2003; Herrera 2009; López 2002; López 2005; Michalowicz 2006; Newnham 2009; Offenbacher 2009; Oliveira 2011; Radnai 2009; Sadatmansouri 2006; Tarannum 2007).
-
Periodontal treatment versus alternative periodontal treatment (Jeffcoat 2003; Macones 2010; Offenbacher 2006; Pirie 2013).
For the head‐to‐head comparison, the least intensive or complex intervention was regarded as the control group or 'alternative periodontal treatment'. Participants received between 1 to 5 periodontal treatment sessions. All studies except Pirie 2013 indicated that maintenance therapy was provided till delivery and this involved chlorhexidine rinse, oral hygiene instructions or dental prophylaxis. The studies rarely provided sufficient details on the number of sessions, time of treatment and maintenance and these intervention regimens varied from study to study. Periodontal treatment was administered by periodontists in three studies (Herrera 2009; Radnai 2009; Tarannum 2007) and hygienists/therapists in two studies (Macones 2010; Offenbacher 2009). Four studies referred to the professionals as 'clinicians' (Jeffcoat 2003), 'hygienists or periodontists' (Newnham 2009), 'trained personnel' (Oliveira 2011), and in one study participants were examined by periodontists, but it is not clear whether periodontists also administered the intervention (Offenbacher 2006). Six studies made no reference to the expertise of the professional who administered the intervention (Farrell 2003; López 2002; López 2005; Michalowicz 2006; Pirie 2013; Sadatmansouri 2006).
All the studies were two‐arm trials involving an intervention and a control arm except for a three‐arm trial (Jeffcoat 2003) which compared SRP (scaling and root planing) + placebo versus SRP + antimicrobial versus alternative mechanical treatment + placebo. The comparisons were divided as follows:
-
SRP + placebo versus alternative mechanical treatment + placebo;
-
SRP + antimicrobial versus alternative mechanical treatment + placebo;
-
SRP + antimicrobial versus SRP + placebo.
Outcomes
All the studies had to report at least one obstetric outcome to be included in the review. Ten studies (Herrera 2009; López 2002; López 2005; Michalowicz 2006; Newnham 2009; Offenbacher 2006; Offenbacher 2009; Oliveira 2011; Pirie 2013; Sadatmansouri 2006) also reported periodontal outcomes. Obstetric outcomes not specified in our protocol were collated and reported in Additional Table 2. Outcomes of interest reported in the studies are as follows.
-
Gestational age ‐ preterm birth < 37 weeks, < 35 weeks and < 32 weeks were reported. All 15 studies reported preterm birth < 37 weeks. Preterm birth < 35 weeks was reported in four studies (Jeffcoat 2003; Macones 2010; Michalowicz 2006; Offenbacher 2009) and preterm birth < 32 weeks was reported in three studies (Farrell 2003; Michalowicz 2006; Offenbacher 2009). Gestational age was reported as time‐to‐event data in two studies (Michalowicz 2006; Newnham 2009) and mean gestational age (weeks) was reported in six studies (López 2002; López 2005; Newnham 2009; Radnai 2009; Sadatmansouri 2006; Tarannum 2007).
-
Birth weight: low birth weight < 2500 g was reported in eight studies (López 2002; Macones 2010; Michalowicz 2006; Offenbacher 2009; Oliveira 2011; Radnai 2009; Sadatmansouri 2006; Tarannum 2007) and low birth weight < 1500 g was reported in three studies (Macones 2010; Michalowicz 2006; Offenbacher 2009). Mean birth weight was reported in eight studies (López 2002; López 2005; Michalowicz 2006; Newnham 2009; Offenbacher 2009; Radnai 2009; Sadatmansouri 2006; Tarannum 2007).
-
Small for gestational age (10th percentile) was reported in three studies (Michalowicz 2006; Newnham 2009; Offenbacher 2009).
-
Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth) was reported in nine studies. Fetal deaths were reported in six studies (López 2002; López 2005; Macones 2010; Oliveira 2011; Pirie 2013; Tarannum 2007), however, the gestational age of pregnancy loss was rarely reported. Neonatal death was reported in three studies (Michalowicz 2006; Newnham 2009; Offenbacher 2009).
-
Maternal mortality was reported in one study (Michalowicz 2006) and data for three other studies (López 2002; López 2005; Radnai 2009) were provided through personal communication with study authors.
-
Pre‐eclampsia was reported in three studies (López 2002; Michalowicz 2006; Offenbacher 2009), however, one additional study (Herrera 2009) which recruited women who already had mild pre‐eclampsia reported a "progression from mild to severe pre‐eclampsia" as one of its outcomes.
-
Adverse effects of therapy: there were no adverse effects in López 2002; López 2005; Newnham 2009; Radnai 2009 (personal communication).
-
Periodontal outcomes: the periodontal outcomes probing depth, clinical attachment level, bleeding on probing, gingival index, and plaque index were reported in 10 studies (Herrera 2009; López 2002; López 2005; Michalowicz 2006; Newnham 2009; Offenbacher 2006; Offenbacher 2009; Oliveira 2011; Pirie 2013; Sadatmansouri 2006) and reported properly for inclusion in a meta‐analysis in seven of the 10 studies.
Excluded studies
We excluded nine trials and stated the reasons for exclusion (Characteristics of excluded studies table). Studies were excluded due to non‐randomisation (Gazolla 2007; Geisinger 2014), failure to report any obstetric outcome (Pack 1980; Thomson 1982), and the inclusion of participants regardless of periodontal status (Moreira 2014; Weidlich 2013). Two studies seem to have assessed the same study populations as those assessed in some included studies and lacked additional information to supplement the primary studies (Jeffcoat 2011; Penova‐Veselinovic 2015). One study assessed a single intervention which is not normally used as standalone treatment for periodontal disease (Jiang 2016).
Risk of bias in included studies
The risk of bias assessment within the included studies and across the domains is reported in the Characteristics of included studies table and summarised in Figure 2; Figure 3. All included studies were at high risk of bias.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The studies were assessed for selection bias based on the adequacy of randomisation as well as allocation concealment. Ten studies were all at unclear risk of bias and five studies were at low risk of bias for this domain (Herrera 2009; Jeffcoat 2003; Macones 2010; Michalowicz 2006; Pirie 2013).
The studies at unclear risk of bias did not provide sufficient information on allocation concealment (Farrell 2003; López 2002; López 2005; Newnham 2009; Offenbacher 2006; Offenbacher 2009; Oliveira 2011; Radnai 2009; Sadatmansouri 2006; Tarannum 2007) or randomisation (Offenbacher 2006; Oliveira 2011; Sadatmansouri 2006) or both.
For the five studies at low risk of selection bias, randomisation code was centrally generated by a research pharmacist who also provided a double packet with coding information for each participant. Allocation was concealed in the second packet and was to be revealed only in the event of an emergency (Jeffcoat 2003). Michalowicz 2006 used permuted randomised blocks made available by telephone call to the co‐ordinating centre and for Macones 2010 permuted block randomisation was accomplished centrally. Herrera 2009 reportedly "randomised by block". This information was not sufficient but considered adequate given that allocation concealment was achieved using "...closed envelopes prepared by professionals external to the research group." One study (Pirie 2013) avoided selection bias by using computer generated allocations which were concealed by labelled opaque sealed envelopes.
Blinding
Performance bias
There was only one study (Jeffcoat 2003) at low risk of performance bias. It was placebo‐controlled and code breaking seems to have occurred only at the end of the study. All the other studies did not state whether blinding was carried out, however, they were considered to be at high risk of performance bias as the mechanical nature of the interventions made the blinding of the participants impossible.
Detection bias
Nine studies (Farrell 2003; Jeffcoat 2003; López 2002; López 2005; Macones 2010; Michalowicz 2006; Newnham 2009; Offenbacher 2009; Pirie 2013) clearly indicated that obstetric outcomes were assessed blindly or assessed independent of the caregiver. Given that obstetric outcomes are likely to have been assessed independent of the caregivers (dental professional), we had considered marking all studies 'low' for detection bias. However, there were concerns regarding whether independent assessment was sufficient to prevent detection bias, which led to marking the other six studies (Herrera 2009; Offenbacher 2006; Oliveira 2011; Radnai 2009; Sadatmansouri 2006; Tarannum 2007) with insufficient information 'unclear' .
We were not concerned as to whether periodontal outcomes were assessed blindly or not as periodontal outcomes are not the primary focus of the review.
Incomplete outcome data
Most of the studies had excluded participants who had experienced certain adverse pregnancy outcomes of relevance to this review such as eligible or indicated preterm birth (preterm births foreseen to occur due to complications), stillbirth and spontaneous abortion (pregnancy loss). These data were collected and added to the results of this review and the denominators were adjusted accordingly.
Eleven studies judged to be at low risk of bias either reported that there was no attrition (Herrera 2009; Pirie 2013; Sadatmansouri 2006) or had similarly low attrition rates across groups (Jeffcoat 2003; López 2005; Macones 2010; Michalowicz 2006; Newnham 2009; Oliveira 2011; Radnai 2009; Tarannum 2007).
Four studies were at high risk of bias due to high attrition rates of > 20% (Farrell 2003; Offenbacher 2006; Offenbacher 2009) and imbalance in attrition rates across groups (López 2002).
Selective reporting
Five studies were judged to be at high risk of reporting bias due to failure to report periodontal outcomes (Farrell 2003; Jeffcoat 2003; Macones 2010; Radnai 2009) and poor reporting of periodontal outcome follow‐up data (Tarannum 2007).
Ten studies were at low risk of reporting bias (Herrera 2009; López 2002; López 2005; Michalowicz 2006; Newnham 2009; Offenbacher 2006; Offenbacher 2009; Oliveira 2011; Pirie 2013; Sadatmansouri 2006).
Other potential sources of bias
Seven studies (Jeffcoat 2003; Michalowicz 2006; Newnham 2009; Pirie 2013; Radnai 2009; Sadatmansouri 2006; Tarannum 2007) with no other apparent source of bias were marked 'low'. Two studies reported an imbalance in the number of participants allocated to the groups (Farrell 2003), number of nulliparous women and alcohol use among participants (Offenbacher 2009) and were both marked 'unclear' as there was lack of clarity on whether these impacted on the validity of the results. Six studies (Herrera 2009; López 2002; López 2005; Macones 2010; Offenbacher 2006; Oliveira 2011) were at high risk of other bias due to an imbalance in participant characteristics across groups.
Effects of interventions
Comparison 1: periodontal treatment versus no treatment
See summary of findings Table for the main comparison.
Primary outcome 1: gestational age/preterm birth
Preterm birth < 37 weeks was reported in all 11 studies which compared periodontal treatment with no treatment. Three studies additionally reported on preterm birth < 35 weeks and < 32 weeks (Analysis 1.1). There is no clear difference in preterm birth < 37 weeks (risk ratio (RR) 0.87, 95% confidence interval (CI) 0.70 to 1.10; participants = 5671; studies = 11; I2 = 66%) between periodontal treatment and no treatment. This is due to low‐quality evidence downgraded for risk of bias and inconsistency. It is uncertain whether periodontal treatment leads to a difference in preterm birth < 35 weeks (RR 1.19, 95% CI 0.81 to 1.76; participants = 2557; studies = 2; I2 = 0%) and < 32 weeks (RR 1.35, 95% CI 0.78 to 2.32; participants = 2755; studies = 3; I2 = 0%) because the quality of evidence is very low. The evidence was downgraded due to high risk of bias and very serious imprecision.
Some studies had excluded 'indicated preterm' births (preterm births foreseen to occur due to complications) from their analyses. We decided to include these data in the 'preterm birth' analysis of this review. Given that the studies had not specified the gestational age (in weeks) when these indicated preterm births had occurred, we included them in the < 37 weeks analyses only. Therefore the < 35 weeks and < 32 weeks preterm birth results could be underestimated.
Six studies analysing 2573 participants reported on mean gestational age in weeks and presented sufficient data for inclusion in a meta‐analysis. However, the data were not suitable for pooling in a meta‐analysis due to the skewed nature of the data. Mean difference ranged between ‐0.1 and 1.4 weeks (Additional Table 4). Two studies (Michalowicz 2006: hazard ratio 0.93, 95% CI 0.63 to 1.37; and Newnham 2009: hazard ratio 1.02, 95% CI 0.91 to 1.15) which reported gestational age as time‐to‐event data both show that there is probably no clear difference in gestational age at delivery between periodontal treatment and no treatment. The evidence was downgraded for serious imprecision due to wide confidence intervals.
| Mean gestational age (weeks) | ||||||
| Periodontal treatment | No periodontal treatment | |||||
| Study ID | Mean | SD | Participants | Mean | SD | Participants |
| 39.6 | 1.2 | 163 | 39 | 2 | 188 | |
| 39.26 | 1.5 | 560 | 38.9 | 1.7 | 283 | |
| 39.1 | 2.1 | 538 | 39.2 | 2.1 | 540 | |
| 37.5 | 1.7 | 41 | 36.1 | 2.8 | 42 | |
| 38.5 | 0.8 | 15 | 37.9 | 1.3 | 15 | |
| 33.8 | 2.8 | 99 | 32.7 | 2.8 | 89 | |
| Mean birth weight (grams) | ||||||
| Periodontal treatment | No periodontal treatment | |||||
| Study ID | Mean | SD | Participants | Mean | SD | Participants |
| 3501 | 429 | 163 | 3344 | 598 | 188 | |
| 3426 | 477 | 560 | 3325 | 535 | 283 | |
| 3239 | 586 | 406 | 3258 | 575 | 403 | |
| 3370.6 | 613.4 | 538 | 3423.4 | 597.3 | 540 | |
| 3227 | 612 | 872 | 3241 | 590 | 866 | |
| 3079 | 592.3 | 41 | 2602.4 | 668.3 | 42 | |
| 3371 | 394.2 | 15 | 3059 | 389 | 15 | |
| 2565.3 | 331.2 | 99 | 2459.6 | 380.7 | 89 | |
SD = standard deviation.
Primary outcome 2: birth weight
Seven studies analysing 3470 participants reported on birth weight (low birth weight < 2500 g). Of the seven studies reporting on low birth weight < 2500 g, two studies (n = 2550) also reported on low birth weight < 1500 g. Periodontal treatment may reduce the incidence of low birth weight < 2500 g (RR 0.67, 95% CI 0.48 to 0.95; I2 = 59%; Analysis 1.2). We downgraded the evidence from high to low as a result of high risk of bias and serious inconsistency. It is uncertain whether periodontal treatment leads to a difference in low birth weight < 1500 g (RR 0.80, 95% CI 0.38 to 1.70; I2 = 45%; Analysis 1.2) compared to no treatment. The evidence was downgraded to very low as a result of high risk of bias in the studies and very serious imprecision of the results.
Eight studies analysing 5120 participants reported on birth weight (grams). However, data were not suitable for pooling in a meta‐analysis due to the skewed nature of the data. Mean difference ranged between ‐52.8 and 476.6 grams (Additional Table 4).
Primary outcome 3: small for gestational age
Three studies analysing 3610 participants reported outcome data on small for gestational age. Periodontal treatment may lead to no clear difference in births of babies which are small for gestational age when compared with no treatment (RR 0.97, 95% CI 0.81 to 1.16; Analysis 1.3). We used the fixed‐effect model and moderate heterogeneity was evident (Chi2 = 4.39, degrees of freedom (df) = 2 (P = 0.11); I2 = 54%). Due to risk of bias and serious inconsistency, we downgraded the evidence to low quality.
Primary outcome 4: perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth)
Fetal (spontaneous abortions and stillbirths) and neonatal deaths were reported in seven studies (n = 5320 participants); only two studies reported the exact gestational age at which mortality occurred (Michalowicz 2006; Newnham 2009). It is uncertain whether periodontal treatment increases or decreases perinatal mortality (RR 0.85, 95% CI 0.51 to 1.43; I2 = 21%; Analysis 1.4) because the quality of the evidence is very low. The evidence was downgraded for very serious limitation and very serious imprecision.
Primary outcome 5: maternal mortality
Four studies (López 2002; López 2005; Michalowicz 2006; Radnai 2009) reported 0% maternal mortality rate.
Primary outcome 6: pre‐eclampsia
Three studies analysing 2946 participants reported on pre‐eclampsia. It is uncertain whether periodontal treatment results in a difference in pre‐eclampsia when compared to no treatment (RR 1.10, 95% CI 0.74 to 1.62) because the quality of evidence is very low. We used the random‐effects model and heterogeneity was assessed as not important (Chi2 = 2.72, df = 2 (P = 0.26); I2 = 27%; Analysis 1.5). The evidence was downgraded due to high risk of bias and very serious imprecision. An additional study which evaluated 60 participants that had mild pre‐eclampsia reported on progression to severe pre‐eclampsia. Due to the quality of evidence (very low), it is uncertain whether periodontal treatment results in a difference in severe pre‐eclampsia when compared to no treatment (RR 1.21, 95% CI 0.77 to 1.92; very low quality ‐ downgraded for high risk of bias and very serious imprecision).
Primary outcome 7: adverse effects of therapy
There were no adverse effects (0%) in four studies (López 2002; López 2005; Newnham 2009; Radnai 2009).
Secondary outcomes
Periodontal outcomes reported in the studies were probing depth, bleeding on probing, plaque index, and clinical attachment level. All the studies reported baseline and final scores except Offenbacher 2009 which reported mean change score. This study was included in the meta‐analysis as a subgroup. All four periodontal indices showed a reduction in favour of periodontal treatment. However due to considerable heterogeneity (91% to 100%), the results were not meta‐analysed (Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9).
Periodontal indices were also reported in seven studies (López 2002; López 2005; Michalowicz 2006; Newnham 2009; Offenbacher 2009; Oliveira 2011; Sadatmansouri 2006) in various measures that could not be incorporated into a meta‐analysis. Periodontal measures were improved in women who underwent periodontal treatment compared to no treatment in all the studies and all the outcome measures reported. These results are reported in detail in additional tables (Additional Table 5).
| Study ID | Outcome | Periodontal treatment | Number of participants | Alternative periodontal/no treatment | Number of participants | P value |
| % sites with PD 4‐6 mm (mean ± SD) | 2.9 ± 3.9 | 163 | 27 ± 14 | 188 | 0.001 | |
| % sites with CAL ≥ 3 mm (mean ± SD) | 6.1 ± 7.8 | 163 | 25.4 ± 17.2 | 188 | 0.001 | |
| % sites with PD > 4 mm (mean ± SD) | 1.8 ± 2.9 | 573 | 14.5 ± 2.8 | 287 | 0.0001 | |
| Change PD at sites initially 4‐6 mm (mean ± SE) | 0.38 ± 0.02 | 405 | 0.88 ± 0.02 | 407 | < 0.001 | |
| Change PD at sites initially ≥ 7 mm (mean ± SE) | 1.07 ± 0.14 | 405 | 1.84 ± 0.14 | 407 | < 0.001 | |
| Change % sites with CAL ≥ 2 mm (mean ± SD) | 0.84 ± 0.85 | 405 | 9.72 ± 0.87 | 407 | < 0.001 | |
| % sites with PD > 4 mm (median (IQR)) | 3.3 (1.2‐7) | 354 | Not reported | Not reported | < 0.001 | |
| % sites BOP (median (IQR)) | 28.7 (17.9‐42.5) | 354 | Not reported | Not reported | < 0.001 | |
| Extent of PD ≥ 4 mm (mean ± SE) | 13.7 ± 1.5 | 25 | 10.5 ± 1.2 | 28 | < 0.0001 | |
| PI ≥ 1 (mean ± SE) | 67.8 ± 5.6 | 25 | 87 ± 5.3 | 28 | 0.02 | |
| Change PD at sites initially ≥ 4 mm (mean ± SD) | 1.47 ± 0.574 | 689 | 7.81 ± 0.559 | 728 | Not reported | |
| Sites with PD ≥ 4 mm (% (95% CI)) | 1.19 (1‐1.39) | 113 | 6.36 (5.92‐6.81) | 112 | < 0.0001 | |
| Sites with CAL ≥ 3 mm (% (95% CI)) | 5.72 (5.3‐6.14) | 113 | 6.58 (6.13‐7.03) | 112 | 0.0069 | |
| Number of sites PD ≥ 4 mm (median (IQR)) | 10 (6‐22) | 45 | Not reported | 45 | Not reported | |
| Number of sites PD ≥ 5 mm (median (IQR)) | 1 (0‐4) | 45 | Not reported | 45 | Not reported | |
| Number of sites AL ≥ 4 mm (median (IQR)) | 10 (5‐19) | 45 | Not reported | 45 | Not reported | |
| Number of sites AL ≥ 5 mm (median (IQR)) | 0 (0‐2) | 45 | Not reported | 45 | Not reported | |
| Number of sites plaque present (median (IQR)) | 57 (40‐82.5) | 45 | Not reported | 45 | Not reported | |
| Number of sites BOP present (median (IQR)) | 78 (63.5‐90) | 45 | Not reported | 45 | Not reported | |
| % of sites plaque present | 37 (28‐54.8) | 45 | Not reported | 45 | Not reported | |
| % of sites BOP present | 50 (42.9‐54.1) | 45 | Not reported | 45 | Not reported | |
| % of sites PD ≥ 4 mm | 78 (63.5‐90) | 45 | Not reported | 45 | Not reported | |
| % sites with PD 4 mm (mean ± SD) | 53.31 ± 18.5 | 15 | 68.6 ± 20.2 | 15 | 0.04 | |
| % sites with CAL 3 mm (mean ± SD) | 41.4 ± 18.4 | 15 | 67.1 ± 15.6 | 15 | 0.000 |
AL = attachment loss; BOP = bleeding on probing; CAL = clinical attachment level; IQR = interquartile range; PD = probing depth; PPD = periodontal pocket depth; SD = standard deviation; SE = standard error.
Comparison 2: periodontal treatment versus alternative periodontal treatment
For gestational age (preterm birth < 37 weeks and preterm birth < 35 weeks), we had three subcomparisons: SRP (scaling and root planing) versus alternative mechanical treatment; SRP + antimicrobial versus SRP + placebo; and SRP + antimicrobial versus alternative mechanical treatment + placebo. We did not pool the data due to clinical heterogeneity.
Primary outcome 1: gestational age/preterm birth
Preterm births < 37 weeks and < 35 weeks were reported in four studies. For all three subcomparisons made, it is uncertain whether there is a difference between periodontal treatment and alternative periodontal treatment in preterm birth < 37 weeks (Analysis 2.1) except for SRP + antimicrobials which may increase preterm births < 37 weeks compared to SRP + placebo (RR 3.08, 95% CI 1.15 to 8.20; participants = 243; studies = 1). With the results there is very low certainty due to very serious imprecision and high risk of bias.
-
SRP versus alternative mechanical treatment (RR 0.87, 95% CI 0.46 to 1.67; participants = 1168; studies = 4; I2 = 61%; very low quality).
-
SRP + antimicrobial versus alternative mechanical treatment + placebo (RR 1.40, 95% CI 0.67 to 2.92; participants = 243; studies = 1; very low quality).
None of the subcomparisons showed a difference in preterm births < 35 weeks (Analysis 2.2) and the quality of evidence was similarly very low in all cases.
-
SRP versus alternative mechanical treatment (not pooled due to considerable heterogeneity).
-
SRP + antimicrobial versus SRP + placebo.
-
SRP + antimicrobial versus alternative mechanical treatment + placebo.
Mean gestational age (weeks) was reported in only two studies which analysed 855 participants. Mean gestational age ranged between ‐0.6 and ‐0.2 weeks (Additional Table 6).
| Mean gestational age (weeks) | ||||||
| Periodontal treatment | Alternative periodontal treatment | |||||
| Study ID | Mean | SD | Participants | Mean | SD | Participants |
| 38.6 | 2.8 | 376 | 38.8 | 2.3 | 380 | |
| 39.4 | 2.3 | 49 | 40 | 2.5 | 50 | |
| Mean birth weight (grams) | ||||||
| 3076.1 | Not reported | 376 | 3143.8 | Not reported | 380 | |
| 3510 | 650 | 49 | 3580 | 630 | 50 | |
SD = standard deviation.
Primary outcome 2: birth weight
One study (Macones 2010) with 756 participants reported on low birth weight < 2500 g and < 1500 g. It is uncertain whether there is a difference in low birth weight < 2500 g (RR 1.39, 95% CI 0.92 to 2.09; participants = 756; I2 = 0%; Analysis 2.3) and low birth weight < 1500 g (RR 1.85, 95% CI 0.69 to 4.96; participants = 756; I2 = 0%; Analysis 2.3) between the periodontal treatments. In both cases we downgraded the evidence three levels to very low due to high risk of bias and very serious imprecision.
One study (Pirie 2013) analysing 99 participants reported on mean birth weight (kilograms). The study reported a mean difference in birth weight between groups of ‐0.07 kilograms. An additional study (Macones 2010) analysing 756 participants reported on mean birth weight (grams). The study reported a difference in mean birth weight of ‐67.7 grams (Additional Table 6).
Primary outcome 3: small for gestational age
Not reported.
Primary outcome 4: perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth)
This outcome was reported in two studies (n = 855 participants). It is uncertain whether there is a difference in perinatal mortality between the periodontal treatments (RR 1.06, 95% CI 0.60 to 1.85; I2 = 0%; Analysis 2.4). The evidence was downgraded to low as result of high risk of bias and very serious imprecision.
Primary outcome 5: maternal mortality
Not reported.
Primary outcome 6: pre‐eclampsia
Not reported.
Primary outcome 7: adverse effects of therapy
Not reported.
Secondary outcomes
Periodontal indices reported were probing depth, clinical attachment level, bleeding on probing and gingival index. SRP may slightly improve probing depth, attachment loss, bleeding on probing and gingival index (Analysis 2.5; Analysis 2.6; Analysis 2.7; Analysis 2.8).
-
Probing depth: mean difference (MD) ‐0.93, 95% CI ‐1.12 to ‐0.74; participants = 53; studies = 1; I2 = 0%.
-
Clinical attachment level: MD ‐0.13, 95% CI ‐0.23 to ‐0.03; participants = 53; studies = 1; I2 = 0%.
-
Bleeding on probing: MD ‐28.00, 95% CI ‐38.54 to ‐17.46; participants = 53; studies = 1; I2 = 0%.
-
Gingival index: MD ‐28.00, 95% CI ‐144.13 to 88.13; participants = 53; studies = 1; I2 = 0%.
Periodontal indices were also reported in two studies (Offenbacher 2006; Pirie 2013) in various measures that could not be incorporated in the meta‐analysis. Offenbacher 2006 showed that SRP resulted in a reduction in plaque index and on the other hand an increase in extent of probing depth ≥ 4 mm (mean ± standard error) compared to the alternative periodontal treatment. Pirie 2013 made no comparison between the two groups rather it compared baseline and postintervention results in the SRP group which was not as important for this review. These results are reported in detail in additional tables (Additional Table 5).
Discussion
Summary of main results
The main results of the primary outcomes are summarised in summary of findings Table for the main comparison. Fifteen randomised controlled trials provided sufficient data for inclusion in the meta‐analysis. The trials were grouped under two broad comparisons: periodontal treatment versus no treatment; periodontal treatment versus alternative periodontal treatment.
-
Eleven studies compared periodontal treatment and no treatment. There is no evidence of a difference in preterm birth < 37 weeks.
-
Periodontal treatment may reduce low birth weight (< 2500 g) (33% reduction) in pregnant women. The quality of evidence is low. The broader literature suggests that most low birth weights in high‐income countries are related to preterm births, however, this is unexpectedly not reflected in the results of this review (WHO 2012).
-
Periodontal treatment improves periodontal health.
-
For primary outcomes small for gestational age and pre‐eclampsia there is no clear difference between periodontal treatment and no treatment.
-
It is not clear if there is a difference in perinatal mortality outcomes (including fetal and neonatal deaths up to the first 28 days after birth) when periodontal treatment is compared with no treatment.
-
There were no adverse effects of the therapy or maternal mortality.
-
There were four studies comparing periodontal treatment with alternative periodontal treatment and it is uncertain whether there is a difference in adverse birth outcomes when periodontal treatments are compared. Periodontal data for this comparison were not pooled due to considerable heterogeneity.
We were unable to pool data on mean gestational age (weeks), mean birth weight (grams/kilograms) and periodontal data due to the skewness of the data.
Overall completeness and applicability of evidence
The studies recruited pregnant women who had different severities of periodontal disease ranging from mild to severe (mostly mild). The participants were women at various stages of pregnancy, different ages, ethnicity and socioeconomic status except two studies (Sadatmansouri 2006; Tarannum 2007) which made no reference to ethnicity. There was variation in periodontal treatment procedures across studies. This correctly reflects current disagreements in clinical practice with regards to periodontal treatment planning (John 2013). The review compares the effect of periodontal treatment versus no treatment and goes further to compare different periodontal treatments. All but one study (Jeffcoat 2003) assessed a combination of mechanical treatments compared to no treatment or in a head‐to‐head comparison. The different interventions assessed cover the range of periodontal treatments that would be given to pregnant women in practice making the results generalisable. All the primary and secondary outcomes were mostly reported. Maternal mortality and adverse effects of the therapy were rarely reported and did not occur in any of the studies that reported them (personal communication with trial authors). Both outcomes may not have been reported due to the fact that no events occurred. Five studies (Farrell 2003; Jeffcoat 2003; Macones 2010; Radnai 2009; Tarannum 2007) failed to report outcome data on periodontal health. We are aware of the fact that the efficacy of periodontal treatment on periodontal health is the basis of its theoretical effect on obstetric outcomes, therefore the absence of periodontal outcome data in the previously mentioned studies puts any potential benefits on obstetric outcomes in doubt. We also acknowledge that the Hawthorne effect (McCambridge 2014) may have resulted in an overestimation of the results by improving participant behaviour in response to their awareness of being part of the trial. This review covers a wide range of participants which would make the evidence applicable to similar population in low and middle‐income countries except that standard antenatal care in these settings may not include an oral health component.
Quality of the evidence
The quality of evidence ranged from low to very low. This was due to high risk of bias, serious imprecision and serious inconsistency. All included studies were at high risk of bias due to the lack of blinding of participants in 13 studies (93%), imbalance in baseline characteristics in seven studies (46%), failure to report periodontal data at follow‐up in four studies (26%) and attrition in four studies (26%). Imprecision was mostly due to very low number of events and for rare outcomes with insufficient sample sizes.
For the main comparison (periodontal treatment versus no treatment), effect estimates were mostly inconsistent across studies. Therefore we downgraded once for moderate to substantial heterogeneity. Heterogeneity may have resulted from variations in the average risk of the outcome events in participants. Clinical heterogeneity due to differences in severities of periodontitis, gestational age and history of preterm birth was evident. The baseline risk of adverse birth outcomes is an aggregate measure of case‐mix factors such as age, severity of periodontitis, previous history of adverse birth outcomes and participants' potential to benefit from an intervention would depend largely on their risk status. Periodontal treatment also varied greatly with studies including different combinations of interventions as part of a care package (Additional Table 3).
The evidence is relevant to the review question and was not downgraded for indirectness. We generated a funnel plot (Figure 4), however, due to the small number of studies we were unable to draw any meaningful conclusion on asymmetry. Since we had taken other measures to prevent publication bias, the evidence was not downgraded for it.

Funnel plot of Comparison 1 Periodontal treatment versus no treatment, Outcome 1.1 Gestational age (preterm birth).
Potential biases in the review process
Data extraction and risk of bias assessment had previously been carried out by a different set of review authors. To ensure consistency and avoid bias, Zipporah Iheozor‐Ejiofor and Anne‐Marie Glenny arbitrated the ratings. Studies not reporting pregnancy outcomes were excluded from the review. Studies would not normally be excluded on the basis of outcome (Higgins 2011). However, given that the primary aim of the review was to assess the efficacy of periodontal treatment in preventing adverse birth outcomes, the inclusion of studies that did not assess obstetric outcomes would have detracted from the focus of the review. Less than half of the included studies provided contact details of authors and most of the emails provided were not valid at the time we attempted to contact the authors. Only three study authors replied to our request for additional information. Therefore it was neither possible to obtain missing information nor clarify partially reported information from most of the study authors. One of the 15 included studies was not published and study data were retrieved from the author via email correspondence. The inclusion of this study meant that some study information remained unclear as we could only get partial information from one of the researchers before the research group was dissolved. This would have introduced reporting bias, however, we carried out a sensitivity analysis by removing this trial from the meta‐analyses and this did not change the direction of effect nor did the effect size change significantly. Therefore this was not considered a source of bias. Before the data synthesis, we assessed studies to assess whether there was sufficient homogeneity. Attempts to contact a study author (Macones 2010) that did not report standard deviation of birth weight were unsuccessful. This may have introduced reporting bias, however, bias was prevented by including the study results as a narrative summary. The studies were not assessed for periodontal outcome detection bias because periodontal outcomes were not the main focus of the review. Birth weight and perinatal mortality were not defined at the protocol stage. We acknowledge that the data analyses for these outcomes were mostly determined by the reporting in individual studies. We considered splitting outcome data on perinatal mortality into miscarriage, stillbirth, neonatal mortality. Given that it was not in the original protocol to split these outcome data, this would have resulted in bias (it should be noted that only two studies reported data for gestational age at which mortality occurred). Three studies (López 2002; López 2005; Tarannum 2007) involved ongoing periodontal care, plaque control, and reinforcement of oral hygiene instructions throughout pregnancy while other studies involved periodontal therapy that occurred as a one‐time (or over several appointments) event. There could be implications for reduction of inflammation that accompany ongoing therapy which may have implications for pregnancy outcomes. However, we did not assess whether duration of treatment influenced the results in a subgroup analysis. We intend to investigate this in future updates.
Agreements and disagreements with other studies or reviews
We identified some previously published reviews that had aimed to assess the effect of periodontal treatment on adverse birth outcomes. These reviews used widely similar methodology with variations in study selection, choice of outcome and data analysis. We carried out two meta‐analyses to compare periodontal treatment versus no treatment and periodontal treatment versus alternative periodontal treatment. Contrary to our approach all the published systematic reviews we identified combined all their studies in a single meta‐analysis irrespective of study comparison. The three reviews (Fogacci 2011; Polyzos 2010; Schwendicke 2015) we identified assessed the effect of periodontal treatment on preterm birth < 37 weeks of gestation, low birth weight and spontaneous abortion/stillbirths (perinatal mortality) and all concluded that periodontal treatment did not confer any advantage in pregnant women with periodontitis.
Fogacci 2011 had evaluated the effect of periodontal treatment on preterm birth and low birth weight. The results on preterm birth are in agreement with the results of our review. The authors had controlled for a number of confounding factors by carrying out separate meta‐analyses. Obtaining similar results from a review with consistent results reinforces the validity of our findings. Another review published in 2010 (Polyzos 2010) had evaluated 12 of the 14 studies included in this review. The review differed from our review in that it excluded participants whose pregnancies resulted in spontaneous abortions or stillbirths. These outcomes were considered important in our review and were extracted from the individual studies if the information was reported. For Polyzos 2010, 'high' quality trials and 'low' quality trials were analysed separately for preterm birth < 37 weeks and low birth weight. Low quality trials, unlike the high quality trials, showed statistically significant results in favour of periodontal treatment. However, there are uncertainties regarding the validity of the overall risk of bias assessment. There was only one assessment for blinding but it is not clear whether this assessment was for performance bias, detection bias or both. Again, contrary to our risk of bias assessment, Polyzos 2010 had assessed all studies including López 2002; Offenbacher 2006; Offenbacher 2009, which we had assessed as high risk, as being at low risk of attrition bias. Schwendicke 2015 stratified the results using arbitrary cut‐offs categorising studies based on high (≥ 20% for preterm birth, ≥ 20% for low birth weight, ≥ 1% for perinatal mortality) or moderate (< 20% preterm birth, < 20% low birth weight, < 1% for perinatal mortality) control group event proportions. Overall the combined results ('high' + 'low' quality in Polyzos 2010; high + moderate control group event proportions in Schwendicke 2015) of all the trials for preterm birth < 37weeks of gestation and spontaneous abortions/stillbirths reported in Polyzos 2010 and Schwendicke 2015 are in agreement with our review. Our review found that periodontal treatment may reduce low birth weight, however, this finding is in disagreement with all three systematic reviews. This variation might have resulted from the reviews combining studies that compared periodontal treatment versus no treatment together with those that did a head‐to‐head comparison between periodontal treatment and alternative periodontal treatment, thereby shifting the results towards no effect.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of Comparison 1 Periodontal treatment versus no treatment, Outcome 1.1 Gestational age (preterm birth).
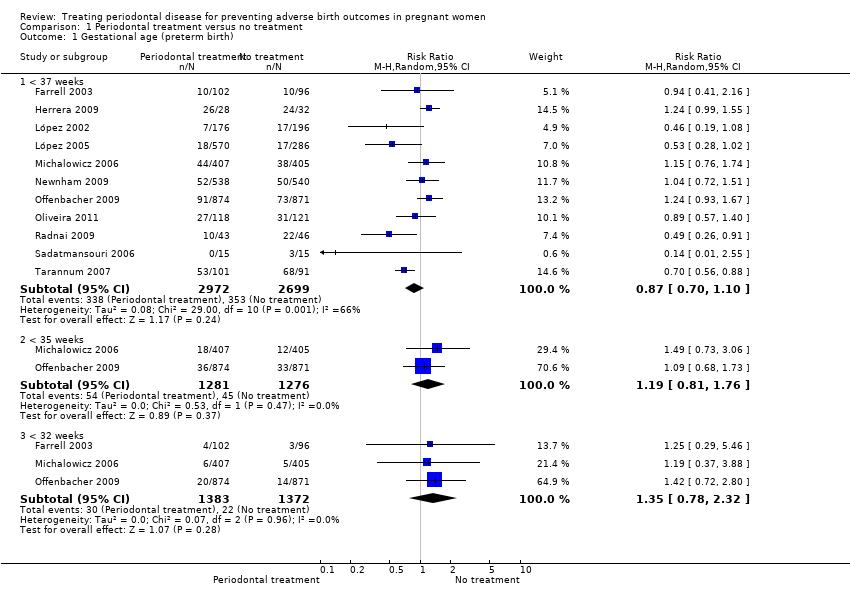
Comparison 1 Periodontal treatment versus no treatment, Outcome 1 Gestational age (preterm birth).

Comparison 1 Periodontal treatment versus no treatment, Outcome 2 Birth weight (low birth weight).

Comparison 1 Periodontal treatment versus no treatment, Outcome 3 Small for gestational age.

Comparison 1 Periodontal treatment versus no treatment, Outcome 4 Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth).

Comparison 1 Periodontal treatment versus no treatment, Outcome 5 Pre‐eclampsia.
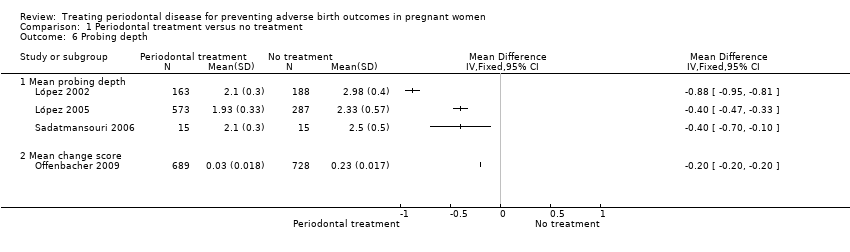
Comparison 1 Periodontal treatment versus no treatment, Outcome 6 Probing depth.

Comparison 1 Periodontal treatment versus no treatment, Outcome 7 Bleeding on probing.
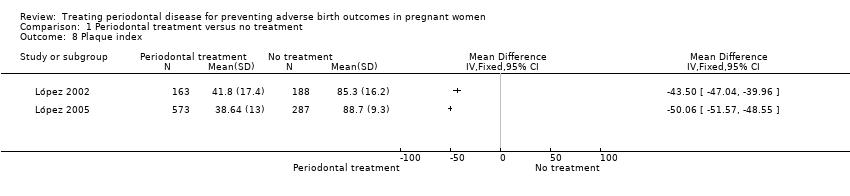
Comparison 1 Periodontal treatment versus no treatment, Outcome 8 Plaque index.
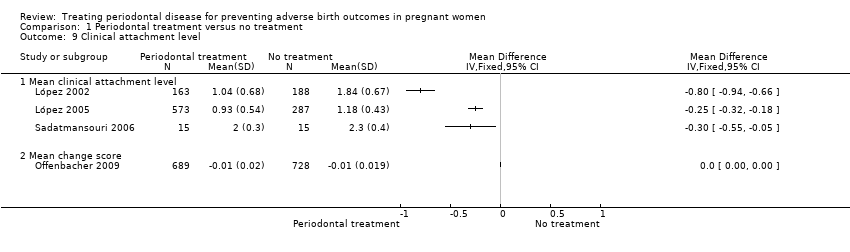
Comparison 1 Periodontal treatment versus no treatment, Outcome 9 Clinical attachment level.
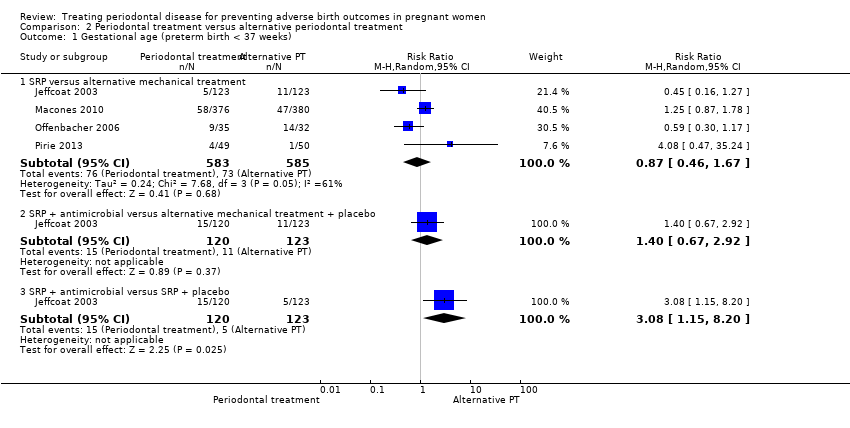
Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 1 Gestational age (preterm birth < 37 weeks).
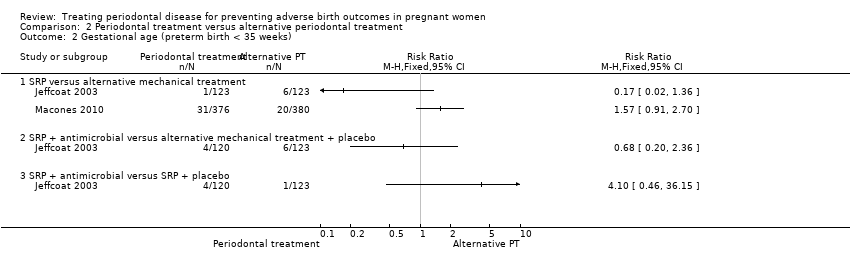
Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 2 Gestational age (preterm birth < 35 weeks).
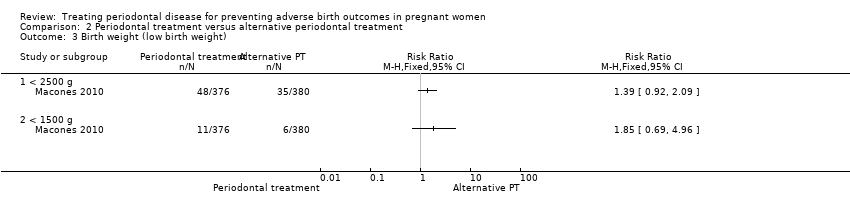
Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 3 Birth weight (low birth weight).

Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 4 Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth).
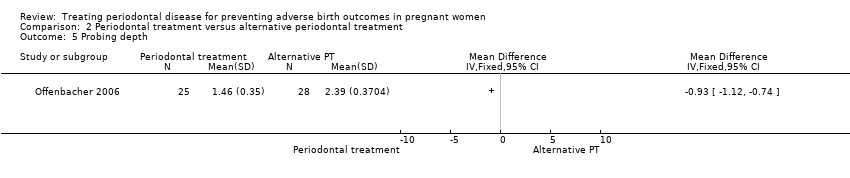
Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 5 Probing depth.

Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 6 Clinical attachment level.

Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 7 Bleeding on probing.
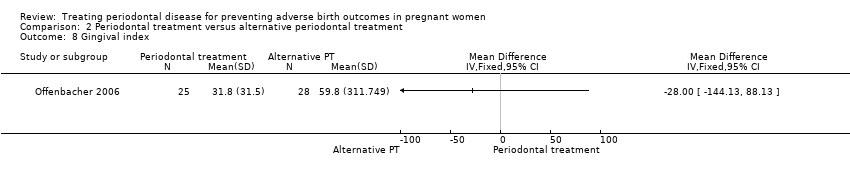
Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 8 Gingival index.
| Periodontal treatment compared to no treatment for preventing adverse birth outcomes in pregnant women | ||||||
| Patient or population: pregnant women considered to have periodontal disease after dental examination | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | Periodontal treatment | |||||
| Gestational age (preterm birth < 37 weeks) | Study population | RR 0.87 | 5671 | ⊕⊕⊝⊝ | Preterm birth < 35 weeks and < 32 weeks were also reported. There was no evidence of a difference in preterm birth < 35 weeks (RR 1.19 (0.81 to 1.76), 2 studies; 2557 participants) and < 32 weeks (RR 1.35 (0.78 to 2.32), 3 studies; 2755 participants) (VERY LOW2 quality evidence) | |
| 131 per 1000 | 114 per 1000 | |||||
| Birth weight (low birth weight < 2500 g) | Study population | RR 0.67 | 3470 | ⊕⊕⊝⊝ | Low birth weight < 1500 g was reported in 2 studies. There was no evidence of a difference in low birth weight < 1500 g (RR 0.80 (0.38 to 1.70); 2550 participants) (VERY LOW2 quality evidence) | |
| 126 per 1000 | 84 per 1000 | |||||
| Small for gestational age | Study population | RR 0.97 (0.81 to 1.16) | 3610 | ⊕⊕⊝⊝ | ||
| 115 per 1000 | 111 per 1000 | |||||
| Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth) | Study population | RR 0.85 (0.51 to 1.43) | 5320 | ⊕⊝⊝⊝ | ||
| 18 per 1000 | 16 per 1000 (9 to 26) | |||||
| Maternal mortality | 0% in both groups | Not estimated | 2134 (4 RCTs) | ‐ | ||
| Pre‐eclampsia | Study population | RR 1.10 | 2946 | ⊕⊝⊝⊝ | ||
| 64 per 1000 | 70 per 1000 | |||||
| Adverse effects of therapy | 0% in both groups | Not estimated | 2389 (4 RCTs) | ‐ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded 2 levels: serious limitation ‐ high risk of bias due to other bias (imbalance in baseline characteristics); serious inconsistency ‐ substantial heterogeneity (I2 = 66%). | ||||||
| Outcome | Study ID |
| Birth weight ≥ 2500 g | |
| Small for gestational age (10th percentile) | |
| Preterm/low birth weight | López 2002; López 2005; Oliveira 2011; Radnai 2009; Sadatmansouri 2006; Pirie 2013 |
| Birth length | Michalowicz 2006; Newnham 2009; Offenbacher 2009; Pirie 2013 |
| Head circumference | |
| Amniotic fluid index (< 5 cm, > 25 cm) | |
| Umbilical artery S/D ratios | |
| Umbilical cord artery/vein blood (number, pH, PCO2, PO2, base excess) | |
| Meconium in amniotic fluid | |
| Decision on delivery based on electronic fetal heart rate monitoring | |
| Scalp pH measured in labour | |
| Nonreassuring fetal heart rate pattern | |
| Caesarean delivery for nonreassuring fetal heart rate | |
| Electronic fetal heart rate monitoring in labour | |
| Ventilation | |
| Continuous Positive Airway Pressure (CPAP) | |
| Oxygen | |
| Special care nursery admission | |
| 1‐min Apgar score | |
| 5‐min Apgar score (0‐3, 4‐7, 8‐10) | |
| Apgar score (< 7 at 1 min, < 7 at 5 min) | |
| Admission to neonatal intensive care unit (number admitted, length of stay > 2 days, discharged alive) | |
| Sepsis necessitating antibiotics | |
| Composite neonatal morbidity/mortality | |
| HELLP syndrome, severe pre‐eclampsia | |
| Prenatal visits | |
| Onset of labour (spontaneous, induced, augmented, no labour) | |
| Mode of delivery (spontaneous vaginal, assisted vaginal, elective caesarean, emergency caesarean) | |
| Fever > 37o C in labour | |
| Postpartum haemorrhage (> 1000 mL) | |
| Retained placenta | |
| Fraction of expected birth weight | |
| Urinary tract infection | |
| Vaginosis, underweight, onset prenatal care after 20 weeks of gestation | |
| S/D = systolic/diastolic ratio. | |
| Study ID | Case definition |
| ≥ 3 sites with CAL ≥ 3 mm | |
| ≥ 4 teeth with ≥ 1 sites with PD ≥ 4 mm and with CAL ≥ 3 mm | |
| ≥ 6 sites with ≥ 5 mm probing depth and ≥ 3 sites with ≥ 3 mm loss of periodontal attachment | |
| AAP criteria ‐ PPD up to 6 mm with CAL up to 4 mm | |
| Gingival inflammation with over ≥ 25% of sites with BOP and no sites with CAL > 2 mm | |
| Attachment loss ≥ 3 mm on ≥ 3 teeth | |
| ≥ 4 teeth with ≥ 1 sites with PD ≥ 4 mm and with CAL ≥ 2 mm | |
| PPD ≥ 4 mm at ≥ 12 probing sites in fully erupted teeth | |
| ≥ 2 sites measuring ≥ 5 mm probing depths plus periodontal attachment loss of 1‐2 mm at ≥ 1 sites with probing depths ≥ 5 mm | |
| ≥ 4 sites with PD ≥ 4 mm and ≥ 4 sites with CAL ≥ 4 mm | |
| Chronic: ≥ 4 mm probing depth, at least 1 site, and BOP for ≥ 50% of teeth | |
| ≥ 2 mm attachment loss at ≥ 50% of examined sites | |
| AAP = American Academy of Periodontology; BOP = bleeding on probing; CAL = clinical attachment level; PD = pocket depth; PPD = periodontal pocket depth. | |
| Study | Number of visits | When | Intervention | Comparator | |
| Periodontal treatment versus no treatment | |||||
| 5 visits | 12 weeks | Plaque assessment Oral hygiene instruction | None | ||
| 1 session lasting 1‐2 hours | Supragingival and subgingival cleaning | None | |||
| Maintenance therapy every 2‐3 weeks till delivery | Plaque control instruction | None | |||
| Maintenance therapy every 2‐3 weeks till delivery | Plaque control instruction | None | |||
| Up to 4 visits | SRP | None | |||
| 3 treatments over 3 weeks | 20 weeks | Nonsurgical debridement of subgingival and supragingival plaque | None | ||
| Up to 4 sessions (mean 1.3 ± 0.4) | Supragingival and subgingival SRP | None | |||
| Maintenance therapy every 3 weeks till delivery | Dental prophylaxis | Tooth cleaning kit | |||
| Not stated | 32 weeks | Supragingival and subgingival scaling and polishing | None | ||
| Not reported | 28 weeks | SRP | None | ||
| 4‐5 sessions with a 1‐week interval between each appointment | Unclear | SRP | Plaque control instruction (toothbrushing) | ||
| Periodontal treatment versus alternative periodontal treatment | |||||
| Antibiotics 3 times daily for 1 week | SRP | SRP | Dental prophylaxis | ||
| Not stated | SRP | Superficial tooth cleaning | |||
| 4‐6 weeks follow‐up visit | SRP | Supragingival debridement | |||
| Performed over 2 1‐hour sessions | Completed by end of 24 weeks | Supragingival and subgingival SRP | Supragingival cleaning | ||
| CHX = chlorhexidine; SRP = scaling and root planing. | |||||
| Mean gestational age (weeks) | ||||||
| Periodontal treatment | No periodontal treatment | |||||
| Study ID | Mean | SD | Participants | Mean | SD | Participants |
| 39.6 | 1.2 | 163 | 39 | 2 | 188 | |
| 39.26 | 1.5 | 560 | 38.9 | 1.7 | 283 | |
| 39.1 | 2.1 | 538 | 39.2 | 2.1 | 540 | |
| 37.5 | 1.7 | 41 | 36.1 | 2.8 | 42 | |
| 38.5 | 0.8 | 15 | 37.9 | 1.3 | 15 | |
| 33.8 | 2.8 | 99 | 32.7 | 2.8 | 89 | |
| Mean birth weight (grams) | ||||||
| Periodontal treatment | No periodontal treatment | |||||
| Study ID | Mean | SD | Participants | Mean | SD | Participants |
| 3501 | 429 | 163 | 3344 | 598 | 188 | |
| 3426 | 477 | 560 | 3325 | 535 | 283 | |
| 3239 | 586 | 406 | 3258 | 575 | 403 | |
| 3370.6 | 613.4 | 538 | 3423.4 | 597.3 | 540 | |
| 3227 | 612 | 872 | 3241 | 590 | 866 | |
| 3079 | 592.3 | 41 | 2602.4 | 668.3 | 42 | |
| 3371 | 394.2 | 15 | 3059 | 389 | 15 | |
| 2565.3 | 331.2 | 99 | 2459.6 | 380.7 | 89 | |
| SD = standard deviation. | ||||||
| Study ID | Outcome | Periodontal treatment | Number of participants | Alternative periodontal/no treatment | Number of participants | P value |
| % sites with PD 4‐6 mm (mean ± SD) | 2.9 ± 3.9 | 163 | 27 ± 14 | 188 | 0.001 | |
| % sites with CAL ≥ 3 mm (mean ± SD) | 6.1 ± 7.8 | 163 | 25.4 ± 17.2 | 188 | 0.001 | |
| % sites with PD > 4 mm (mean ± SD) | 1.8 ± 2.9 | 573 | 14.5 ± 2.8 | 287 | 0.0001 | |
| Change PD at sites initially 4‐6 mm (mean ± SE) | 0.38 ± 0.02 | 405 | 0.88 ± 0.02 | 407 | < 0.001 | |
| Change PD at sites initially ≥ 7 mm (mean ± SE) | 1.07 ± 0.14 | 405 | 1.84 ± 0.14 | 407 | < 0.001 | |
| Change % sites with CAL ≥ 2 mm (mean ± SD) | 0.84 ± 0.85 | 405 | 9.72 ± 0.87 | 407 | < 0.001 | |
| % sites with PD > 4 mm (median (IQR)) | 3.3 (1.2‐7) | 354 | Not reported | Not reported | < 0.001 | |
| % sites BOP (median (IQR)) | 28.7 (17.9‐42.5) | 354 | Not reported | Not reported | < 0.001 | |
| Extent of PD ≥ 4 mm (mean ± SE) | 13.7 ± 1.5 | 25 | 10.5 ± 1.2 | 28 | < 0.0001 | |
| PI ≥ 1 (mean ± SE) | 67.8 ± 5.6 | 25 | 87 ± 5.3 | 28 | 0.02 | |
| Change PD at sites initially ≥ 4 mm (mean ± SD) | 1.47 ± 0.574 | 689 | 7.81 ± 0.559 | 728 | Not reported | |
| Sites with PD ≥ 4 mm (% (95% CI)) | 1.19 (1‐1.39) | 113 | 6.36 (5.92‐6.81) | 112 | < 0.0001 | |
| Sites with CAL ≥ 3 mm (% (95% CI)) | 5.72 (5.3‐6.14) | 113 | 6.58 (6.13‐7.03) | 112 | 0.0069 | |
| Number of sites PD ≥ 4 mm (median (IQR)) | 10 (6‐22) | 45 | Not reported | 45 | Not reported | |
| Number of sites PD ≥ 5 mm (median (IQR)) | 1 (0‐4) | 45 | Not reported | 45 | Not reported | |
| Number of sites AL ≥ 4 mm (median (IQR)) | 10 (5‐19) | 45 | Not reported | 45 | Not reported | |
| Number of sites AL ≥ 5 mm (median (IQR)) | 0 (0‐2) | 45 | Not reported | 45 | Not reported | |
| Number of sites plaque present (median (IQR)) | 57 (40‐82.5) | 45 | Not reported | 45 | Not reported | |
| Number of sites BOP present (median (IQR)) | 78 (63.5‐90) | 45 | Not reported | 45 | Not reported | |
| % of sites plaque present | 37 (28‐54.8) | 45 | Not reported | 45 | Not reported | |
| % of sites BOP present | 50 (42.9‐54.1) | 45 | Not reported | 45 | Not reported | |
| % of sites PD ≥ 4 mm | 78 (63.5‐90) | 45 | Not reported | 45 | Not reported | |
| % sites with PD 4 mm (mean ± SD) | 53.31 ± 18.5 | 15 | 68.6 ± 20.2 | 15 | 0.04 | |
| % sites with CAL 3 mm (mean ± SD) | 41.4 ± 18.4 | 15 | 67.1 ± 15.6 | 15 | 0.000 | |
| AL = attachment loss; BOP = bleeding on probing; CAL = clinical attachment level; IQR = interquartile range; PD = probing depth; PPD = periodontal pocket depth; SD = standard deviation; SE = standard error. | ||||||
| Mean gestational age (weeks) | ||||||
| Periodontal treatment | Alternative periodontal treatment | |||||
| Study ID | Mean | SD | Participants | Mean | SD | Participants |
| 38.6 | 2.8 | 376 | 38.8 | 2.3 | 380 | |
| 39.4 | 2.3 | 49 | 40 | 2.5 | 50 | |
| Mean birth weight (grams) | ||||||
| 3076.1 | Not reported | 376 | 3143.8 | Not reported | 380 | |
| 3510 | 650 | 49 | 3580 | 630 | 50 | |
| SD = standard deviation. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gestational age (preterm birth) Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 < 37 weeks | 11 | 5671 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.10] |
| 1.2 < 35 weeks | 2 | 2557 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.81, 1.76] |
| 1.3 < 32 weeks | 3 | 2755 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.78, 2.32] |
| 2 Birth weight (low birth weight) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 < 2500 g | 7 | 3470 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.48, 0.95] |
| 2.2 < 1500 g | 2 | 2550 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.38, 1.70] |
| 3 Small for gestational age Show forest plot | 3 | 3610 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.81, 1.16] |
| 4 Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth) Show forest plot | 7 | 5320 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.51, 1.43] |
| 5 Pre‐eclampsia Show forest plot | 3 | 2946 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.74, 1.62] |
| 6 Probing depth Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Mean probing depth | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Mean change score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Bleeding on probing Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Mean bleeding on probing | 5 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Mean change score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Plaque index Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Clinical attachment level Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Mean clinical attachment level | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Mean change score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gestational age (preterm birth < 37 weeks) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 SRP versus alternative mechanical treatment | 4 | 1168 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.46, 1.67] |
| 1.2 SRP + antimicrobial versus alternative mechanical treatment + placebo | 1 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.67, 2.92] |
| 1.3 SRP + antimicrobial versus SRP + placebo | 1 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 3.08 [1.15, 8.20] |
| 2 Gestational age (preterm birth < 35 weeks) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 SRP versus alternative mechanical treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 SRP + antimicrobial versus alternative mechanical treatment + placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 SRP + antimicrobial versus SRP + placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Birth weight (low birth weight) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 < 2500 g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 < 1500 g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth) Show forest plot | 2 | 855 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.60, 1.85] |
| 5 Probing depth Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Clinical attachment level Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Bleeding on probing Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Gingival index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

